Abstract
Previous studies have demonstrated that, as naive murine CD4+ cells differentiate into Th1 cells, they lose expression of the second chain of IFN-γR (IFN-γR2). Hence, the IFN-γ-producing subset of Th cells is unresponsive to IFN-γ. Analysis of IFN-γ-producing CD8+ T cells demonstrates that, like Th1 cells, these cells do not express IFN-γR2. To define the importance of IFN-γ signaling for the development of functional CD8+ T cells, mice either lacking IFN-γR2 or overexpressing this protein were examined. While CD8+ T cell development and function appear normal in IFN-γR2−/− mice, CD8+ T cell function in IFN-γR2 transgenic is altered. IFN-γR2 transgenic CD8+ T cells are unable to lyse target cells in vitro. However, these cells produce Fas ligand, perforin, and granzyme B, the effector molecules required for killing. Interestingly, TG CD8+ T cells proliferate normally and produce cytokines, such as IFN-γ in response to antigenic stimulation. Therefore, although IFN-γ signaling is not required for the generation of normal cytotoxic T cells, constitutive IFN-γ signaling can selectively impair the cytotoxic function of CD8+ T cells.
Effector CD8+ T cells, also known as CTLs, are important mediators of immune responses against certain pathogens, transformed cells, and foreign organ grafts (1, 2). These T cells perform their functions by elaborating a number of distinct effector mechanisms. CD8+ T cells produce cytokines such as TNF-α and IFN-γ. TNF-α is a proinflammatory cytokine that functions to inhibit viral gene expression and replication and can initiate apoptotic signaling (3). IFN-γ has been shown to activate effector cells such as macrophages and neutrophils, regulate CD4+ Th cell differentiation, as well as metabolically suppress infected or transformed cells (4). CD8+ T cells can also directly attack and induce cytolysis of their target cells by two distinct pathways, both of which are activated in response to signaling through the TCR (5). One of these pathways involves the release of soluble cytotoxic factors such as the pore-forming molecule perforin and the granzymes, which are stored within cytoplasmic granules (6, 7). These molecules are released shortly following TCR triggering and lead to the perforation of the membrane and the activation of caspases in target cells, resulting in their eventual lysis. Following activation and de novo protein synthesis, CD8+ T cells also up-regulate the expression of Fas ligand (FasL)3 (CD95 ligand) on their cell surface that, through interaction with Fas (CD95) on the target cell, triggers the apoptotic pathway (3, 7). In addition to these two pathways, 24–48 h postactivation CD8+ T cells begin producing TNF-α, which can also lead to killing of target cells (8).
The differentiation of naive Th cells into mature effector cells is regulated by a number of factors, including the strength of the activating stimulus (peptide affinity, Ag dose, and level of costimulation), the route of immunization, and the cytokines present in their immediate microenvironment (9-11). Like Th cells, the activation and maturation of naive CD8+ T cells into CTLs require TCR signaling (7, 12). The quality of the TCR signal can also regulate the effector function of the CTL (13, 14). For example, it has been shown that different epitopes of a particular viral Ag can lead to functionally heterogeneous effector CD8+ T cells (15, 16).
Extensive work has demonstrated that cytokines present at the time of TCR triggering are critical in regulating the course of Th differentiation, leading to the generation of distinct, mature effector Th subsets, such as Th1 and Th2 cells (17, 18). Moreover, during their differentiation, Th1 and Th2 cells acquire differential responsiveness to cytokines. For instance, while Th2 cells can signal through the IFN-γR, Th1 cells are unresponsive to IFN-γ because they do not express the second chain of its receptor (IFN-γR2) (19-21). Transgenic (TG) overexpression of this protein, and the resultant responsiveness to IFN-γ in Th1 cells, profoundly impairs the effector function of these cells, indicating that the regulation of responsiveness to this cytokine is critical for normal Th1-dependent immunity (22).
The role that cytokines play in the thymic development, activation of CD8+ T cells, and the acquisition of mature CTL phenotypes is less clear. The regulation of responsiveness to cytokines by CD8+ T cells is also virtually unexplored. IFN-γ-producing CD8+ T cells, like Th1 cells, may potentially regulate their ability to signal through IFN-γR during their differentiation. It is also possible that IFN-γ signaling participates in, or affects, certain stages in the development of the mature effector phenotype in CD8+ T cells. To explore this possibility, IFN-γ signaling in CD8+ T cells was investigated.
In this study, IFN-γ-producing CD8+ T cells are shown to be incapable of signaling through IFN-γR because they lack expression of IFN-γR2, an integral component of the IFN-γR complex. As a result, these cells are insensitive to the biologic functions of this cytokine, such as up-regulation of class I MHC molecules. In TG mice, in which IFN-γR2 expression is dysregulated, CD8+ T cells are responsive to IFN-γ. In response to antigenic stimulation, these T cells are activated, and proliferate and produce cytokines normally. However, these cells are functionally impaired, as they are unable to lyse allogeneic target cells. In contrast, CTL function in CD8+ T cells isolated from IFN-γR2−/− mice, which lack this receptor chain, is normal. These findings suggest that the regulation of responsiveness to IFN-γ in CD8+ T cells somehow participates in their maturation into CTLs. Therefore, in addition to the quality of the TCR signal, cytokines may regulate the acquisition of mature effector functions by CD8+ T cells.
Materials and Methods
Mice
IFN-γR2 TG mice were originally described by Tau et al. (22). TG mice were bred to the C57BL/6 genetic background for at least 10 generations. All TG mice used were heterozygous for the IFN-γR2 transgene. IFN-γR2 TG litters were screened by PCR using the following primers: 5′-GCACGTGGTTAAGCTCTCG (located in the CD2 promoter) and 5′-TGTCTCTGTGATGTCCGTACA. Appropriate controls (sex-matched littermates or age- and sex-matched mice) were used where normal or wild-type mice are indicated. IFN-γR2-deficient mice were originally generated and described by Lu et al. (23). These mice were bred into the 129 genetic background for at least 10 generations. Where normal or wild-type mice are indicated, age- and sex-matched pure bred 129 mice were used (The Jackson Laboratory, Bar Harbor, ME). BALB/c mice were purchased from Jackson Laboratory. All mice were 4–6 wk of age at the start of a given experiment. All animal experiments conformed to Columbia University’s Institutional Animal Care and Use Protocols.
Tumor cell lines and tissue culture
Unless otherwise noted, cells were grown in complete RPMI containing 10 μg/ml penicillin-streptomycin, 2 mM l-glutamine, 0.1 mM nonessential amino acids, 0.1 mM 2-ME, 1 mM sodium pyruvate, 10 mM HEPES, and 10% FBS. IL-2 media is complete RPMI containing IL-2 (20 U/ml). The EL-4 and S49.1 tumor cell lines were purchased from the American Type Culture Collection (Manassas, VA).
Cytokines and Abs
The mAbs 145-2C11 (anti-CD3; unconjugated, FITC), GK1.5 (anti-CD4; unconjugated), RM4-4 (anti-CD4; biotin, PE, FITC), 53-6.7 (anti-CD8; biotin, PE, FITC), M1/70 (anti-CD11b/Mac-1α; unconjugated), RA3-6B2 (anti-CD45R/B220; unconjugated), 57.51 (anti-CD28; unconjugated), 74D (anti-CD25; biotin), H1.2F3 (anti-CD69; FITC), MFL3 (anti-CD95L/FasL; biotin), AF6-88.4 (anti-H-2Kb; FITC), and XMG1.2 (anti-IFN-γ; PE, FITC) were purchased from BD PharMingen (San Diego, CA). GR-20 (anti-IFN-γR1; FITC) and RA3-6B2 (anti-CD45R/B220; biotin) were a gift of M. Nawijn. Streptavidin-conjugated fluochromes (FITC, PE, and CyChrome) were purchased from BD PharMingen. Human rIL-2 was provided by the National Cancer Institute Biological Research Branch (Frederick, MD). Recombinant murine IFN-γ and human IFN-α A/D were purchased from Genzyme (Cambridge, MA).
CD8+ T cell purification
CD8+ T cells were purified by negative selection similarly to previously described CD4+ T cell purification (22). Briefly, single cell suspensions from lymph nodes and/or spleens containing no RBCs were first incubated with rat anti-mouse mAbs against B cells (anti-B220/CD45R), monocytes (anti-CD11b), and CD4+ T cells (anti-CD4) at 20 μg/ml each, washed, and then incubated with anti-rat IgG Dynabeads (Dynal, Lake Success, NY). Ab-coated cells were removed using a magnetic concentrator (Dynal). The purity of CD8+ T cells was at least 80%, as determined by flow cytometry.
CD8+ T cell lines and clones
Allo-specific CD8+ T cells were derived based on published protocol (24). Accordingly, mice were primed by i.p. injection of 2–4 × 107 irradiated (17 Gy; 60Co Gammacell), allogeneic (BALB/c-derived) splenocytes (25). Two weeks later, CD8+ cells were purified from spleens and mesenteric lymph nodes, as described above. To generated cell lines, CD8+ T cells (1–3 × 106/well in a 24-well plate)) were stimulated with 7 × 106 APCs (irradiated BALB/c splenocytes)/well. Cultures were expanded in IL-2-containing media 24–48 h after stimulation. CD8+ T cells were cultured in IL-2 media for 10 days following stimulation, at which point they were restimulated with APCs, as above. Cultures were maintained by serial 9- to 14-day cycles of stimulation with APCs and expansion in IL-2 media. Allo-specific CD8+ T cell clones were generated as follows: 96 multiples of 8, 40, 200, and 1000 CD8+ T cells purified from allo-primed mice (as above) were aliquoted into U-bottom microtiter plates in 100 μl complete RPMI. Cells were stimulated with 5 × 104 allogeneic APCs/well. Every 2–4 days, a portion of the supernatant was aspirated and replaced with IL-2 media. Cells were restimulated at days 10 and 20 by adding 5 × 104 APCs/well. Only clones from plates in which fewer than 30% of the wells contained live cells were expanded and propagated (as above) and used in experiments.
Analysis of Stat1 activation
Stat1 activation was detected by EMSA, as previously described (26, 27). Briefly, cells were treated with either IFN-α (15 ng/ml) or IFN-γ (10 ng/ml) for 30 min, and whole cell protein extracts were prepared (28). The extracts were incubated with a radiolabeled probe derived from the IFN-regulatory factor-1 (IRF-1) γ-activated site (GAS) element (5′-GATCGATTTCCCCGAAAT). The shift in electromobility imparted by the binding of activated STATs to the probe was detected by resolving the complexes on a polyacrylamide gel and visualized by autoradiography.
RNA isolation and Northern analysis
Total RNA was prepared by using QiaShredder, followed by RNeasy Mini Kit, according to the instructions of the manufacturer (Qiagen, Valencia, CA). RNA (10 μg) from each sample was fractionated on a formaldehyde agarose gel and transferred to a nylon membrane, which was then hybridized with a radiolabeled probe and visualized by autoradiography, all according to standard protocol (29). cDNA probes were radiolabeled with [α-32P]dCTP and [α-32P]dATP using the Readyprime II random prime labeling system (Amersham Pharmacia Biotech, Piscataway, NJ). The IRF-1 cDNA was a kind gift of C. Schindler (30). The IFN-γR2 cDNA was previously described (20, 31). RT-PCR using total RNA isolated from wild-type (WT) CTLs was performed with primers 5′-CTCCACGTGCTT TCACCAAA and 5′-GGAAAATAGTACAGAGAGGCA for granzyme B, and 5′-TGCTACACTGCCACTCGGTCA and 5′-TTGGCTACCTTG GAGTGGGAG for perforin cDNA, according to previously described primers and conditions (32). The suppressors of cytokine signaling-1 (SOCS)-1 and SOCS-3 cDNAs were previously described (33).
Intracytoplasmic cytokine analysis and flow cytometry
All Ab staining, unless otherwise indicated, was performed in PBS containing 1% BSA and 0.1% sodium azide based on standard protocol. Following staining, cells were fixed in PBS containing 2% paraformaldehyde and analyzed using a FACScan flow cytometer (BD Biosciences, Mountain View, CA). Data analysis was performed using CellQuest software (BD Biosciences). Intracellular cytokine staining was performed based on recommended manufacturer’s protocol (BD PharMingen). In brief, 106 freshly purified or day 4–9 CD8+ T cells in IL-2 media were either stimulated with 0.5 μg/ml PMA (Sigma-Aldrich, St. Louis, MO) and 5 μg/ml ionomycin (Sigma-Aldrich) or left unstimulated for 2–4 h at 37°C. At this point, brefeldin A (Sigma-Aldrich) was added to 10 μg/ml for an additional 2 h. Alternatively, 3 × 105 freshly purified or day 5 CD8+ T cells were either stimulated with 7 × 105 APCs, or left unstimulated for 2 h, at which point brefeldin A was added for an additional 10 h. Cells were then stained for cell surface markers, fixed in 2% paraformaldehyde, permeabilized with 0.5% saponin (Sigma-Aldrich), and stained for intracytoplasmic IFN-γ or with an isotype-matched Ab as a control.
Cytotoxicity assays
Cytotoxicity assays were performed based on standard protocol (34). In brief, 1–2 × 106 target cells (S49 or EL-4) were labeled with 0.1–0.2 mCi51Cr, washed three times, and plated in 96-well microtiter plates at 5 × 103/well in 100 μl complete RPMI with or without 5 μg/ml Con A (Amersham Pharmacia Biotech). Effector cells (CD8+ T cell lines or clones 4–7 days poststimulation) were added in triplicate and in equal volume of media at the indicated ratios. In one set of experiments, CD8+ T cells freshly purified from primed mice (as above) were used as effectors 7 days following in vitro stimulation. In place of effectors, media alone (spontaneous lysis) or 1% Triton X (maximum lysis) was added to control wells. After incubation for 4 h at 37°C, 100 μl of culture supernatant was removed from each well and counted for radioactivity. Values for percentage of specific lysis were calculated as follows:
Net Con A-induced lysis was determined by calculating the difference in percent specific lysis values at each E:T ratio between the Con A-treated and untreated assays.
Cell division cycle profile analysis using CFSE
CD8+ cells (freshly purified clones or lines, described elsewhere) were labeled with CFSE (Molecular Probes, Eugene, OR) in a modification of a previously described technique (22, 35). Briefly, cells were washed with serum-free RPMI. Cells (107 cells/ml) were then labeled with 10 μM CFSE in serum-free RPMI at 37°C for 10 min, and CFSE was then neutralized with complete RPMI. CFSE-labeled CD8+ T cells were cultured either on anti-CD3 mAb + anti-CD28 mAb-coated plates (5 μg/ml each Ab in PBS overnight at 4°C) or stimulated with APCs as above. Initial CFSE-labeling efficiency and the fluorescein intensities at the end of each experiment were detected using a FACScan flow cytometer. Histogram overlays and peak distribution analyses were performed using CellQuest software.
Results
CD8+ T cells do not express IFN-γR2 mRNA and are unresponsive to IFN-γ
It has previously been demonstrated that IFN-γ-producing CD4+ T cells (Th1) are unresponsive to IFN-γ because they do not express IFN-γR2 (19-21). Other IFN-γ-producing lymphocytes, such as CD8+ T cells, may, like Th1 cells, modulate their ability to respond to this cytokine during their differentiation. To test this possibility, allo-specific CD8+ T lines and clones were generated. The ability of these cell lines to respond to IFNs was assessed.
Signaling by IFNs leads to the phosphorylation of STAT monomers, allowing the formation of homo- and heterodimers that can bind specific elements in the promoters of IFN-responsive genes and ultimately regulate their expression (36). Type II IFN (IFN-γ) stimulates the phosphorylation of Stat1 and the formation of Stat1 homodimers, which bind GAS elements in the promoters of IFN-γ-responsive genes. The type I IFNs (IFN-α/β) stimulate the phosphorylation of Stat1 and Stat2 and activate the expression of genes whose promoters contain either GAS elements (which bind Stat1 homodimers) or IFN-stimulated response elements (which bind a complex of Stat1, Stat2, and IRF-9).
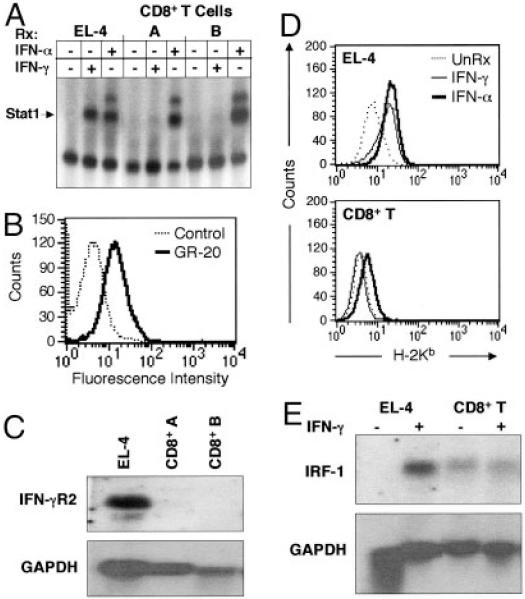
To determine whether CD8+ T cells are responsive to IFN-γ, protein extracts from IFN-γ- and IFN-α-treated cells were examined for GAS-binding activity (activated Stat1 complexes) by EMSA. Activated Stat1 complexes were detected in extracts prepared from control EL-4 cells treated with either IFN-γ or IFN-α (Fig. 1A). Activated Stat1 complexes were also detected in CD8+ T cells cultured with IFN-α, but strikingly, CD8+ T cells cultured with IFN-γ were unable to activate Stat1 (Fig. 1A). This suggests that one or more components of the IFN-γ signaling pathway are either absent, defective, or inhibited in CD8+ T cells. The observation that Stat1 activation is detected following treatment with IFN-α indicates that Stat1, as well as Janus kinase 1 are present and functional in CD8+ T cells. To specifically identify the signaling defect in CD8+ T cells, the integrity of the IFN-γR complex was examined. Similarly to Th1 cells (20, 21), IFN-γR1 protein was detected on the surface of CD8+ T cells using the GR-20 Ab (Fig. 1B). However, mRNA encoding the IFN-γR2 chain was not detected in these cells (Fig. 1C).
FIGURE 1.
CD8+ T cells do not express IFN-γR2 and are not responsive to IFN-γ. A, EMSA of protein extracts from untreated or IFN-γ- or IFN-α-treated (Rx) CD8+ T cell clones or control cells using GAS site as probe. B, Flow cytometric analysis of IFN-γR2 expression on CD8+ T cell clones with GR-20 Ab with isotype-matched control (73). C, Northern blot analysis of IFN-γR2 mRNA expression using mRNA by CD8+ T cell clones and control, with GAPDH probe used as loading control. D, FACS analysis of class I MHC (H-2Kb) expression by CD8+ T cell clones and control cells (UnRx) treated with either IFN-α (15 ng/ml) or IFN-γ (10 ng/ml) for 72 h. E, Northern blot analysis of irf-1 mRNA expression using mRNA isolated from CD8+ T cell clones and control cells either untreated or treated with IFN-γ for 12 h, with GAPDH probe used as loading control.
Downstream of Stat1 activation, IFNs induce the expression of many genes, including the class I MHC genes and irf-1 (37). As expected, treatment of control T cells with either IFN-α or IFN-γ resulted in increased levels of cell surface H-2Kb (Fig. 1D). In CD8+ T cells, however, while IFN-α had an inductive effect, IFN-γ had no apparent effect on cell surface H-2Kb levels (Fig. 1D). Furthermore, IFN-γ was unable to induce irf-1 gene expression in CD8+ T cells (Fig. 1E). Together, these data suggest that CD8+ T cells are unable to respond to IFN-γ because they may lack IFN-γR2, the signal-transducing component of the IFN-γR (38).
CD8+ T cells from IFN-γR2−/− mice develop and function normally
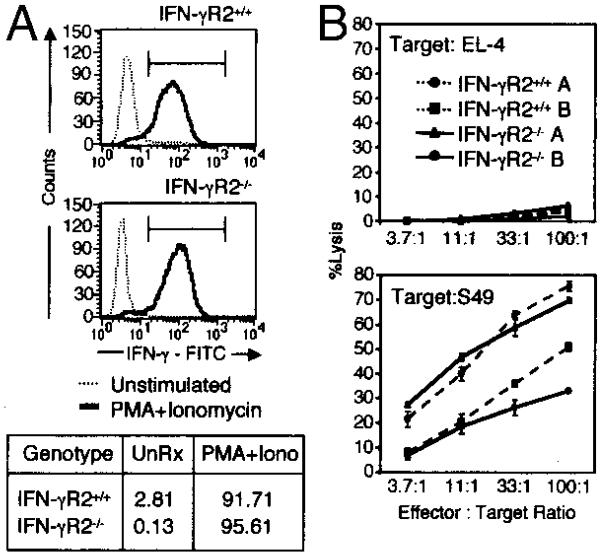
Because CD8+ T cells do not appear to express IFN-γR2, it may be that this receptor, and perhaps IFN-γ responsiveness in general, are dispensable for the development, differentiation, and the function of CD8+ T cells. To examine this possibility, CD8+ T cells isolated from mice that are unable to respond to IFN-γ were analyzed. Prior studies have shown that CD8+ T cells from mice deficient in IFN-γR1 can elaborate normal lytic and proliferative memory responses against virally infected targets (39). IFN-γR1-deficient mice are also resistant to most viral infections, suggesting that IFN-γ signaling may not be required for the development and function of CD8+ T cells. To directly examine the requirement for IFN-γR2 in the CD8+ T cell system, CD8+ T cells were isolated from IFN-γR2-deficient mice and were analyzed. Prior studies have demonstrated normal numbers of CD8+ T cells in the lymphoid organs of IFN-γR2−/− mice (23), which is similar to findings in other IFN-γ-insensitive or IFN-γ-deficient systems. IFN-γR2−/− allo-specific CD8+ T cells proliferated and produced normal levels of IFN-γ in response to a number of activating stimuli, such as phorbol ester + calcium ionophore or allogeneic APCs (Fig. 2A and data not shown). Furthermore, these cells exhibited equivalent levels of specific allogeneic target lysis as compared with allo-specific CD8+ T cells derived from WT littermate controls (Fig. 2B). IFN-γR2−/− CD8+ T cells therefore appear indistinguishable from their WT counterparts despite their inability to transduce the IFN-γ signal at all points during their development and maturation. These data suggest that IFN-γ signaling is not essential either for the development of CD8+ T cells or for their function.
FIGURE 2.
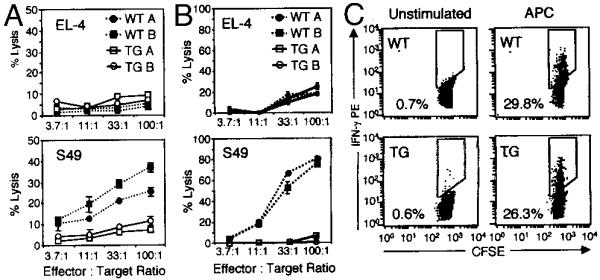
Signaling through IFN-γR is not required for the normal cytokine production and killing by CD8+ T cells. A, IFN-γ production of allo-specific CD8+ T cell lines derived from WT and IFN-γR2−/− mice. Six days postallostimulation, untreated (- - - -) or PMA + ionomycin (IONO)-treated (—) cells were stained for intracytoplasmic IFN-γ and analyzed by flow cytometry. Chart below indicates the percentages of events within the indicated IFN-γ-positive gate. B, 51Cr release assay measuring the specific cytotoxicity of allo-specific IFN-γR2−/− (—) and WT (- - - -) CD8+ T cell lines toward syngeneic (EL-4, top) or allogeneic (S49, bottom) tumor cell lines.
TG expression of IFN-γR2 endows CD8+ T cells with IFN-γ responsiveness
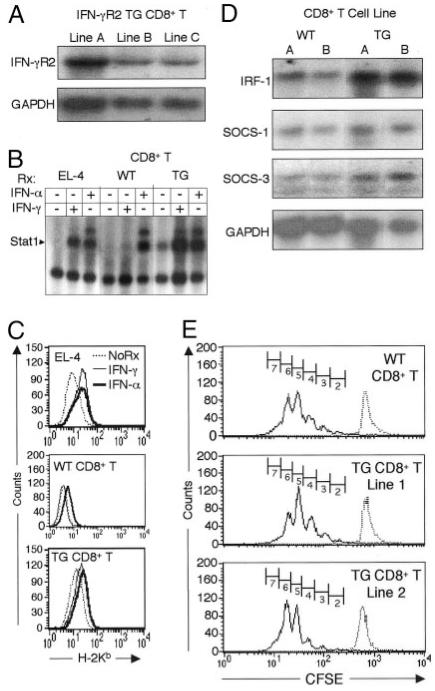
The experiments above suggest that CD8+ T cells lack expression of IFN-γR2 and are insensitive to IFN-γ. To examine whether these two findings are directly linked, CD8+ T cells were isolated from IFN-γR2 TG mice, and their ability to signal through IFN-γR was analyzed. In these mice, TG expression of IFN-γR2 is driven by the CD2 promoter/enhancer. This transgene mRNA, which had been previously shown to be expressed in CD4+ T cells (22), is also detected in CD8+ T cells isolated from IFN-γR2 TG mice (Fig. 3A). In addition, IFN-γR2 TG CD8+ T cells are able to activate Stat1 in response to treatment with IFN-γ, in contrast to WT CD8+ T cells (Fig. 3B). This indicates overexpression of IFN-γR2 is sufficient to restore IFN-γ signaling in CD8+ T cells, and suggests that IFN-γR2 deficiency may be the sole locus of the defect in IFN-γ signaling seen in WT CD8+ T cells.
FIGURE 3.
TG expression of IFN-γR2 restores responsiveness to IFN-γ in CD8+ T cells and does not affect their ability to proliferate, but alters their gene expression. A, Detection of IFN-γR2 mRNA expression in IFN-γR2 TG CD8+ T cell clones and control cells by Northern blot as in Fig. 1C. B, EMSA of protein extracts from IFN-γ- and IFN-α-treated (Rx) IFN-γR2 TG CD8+ T cell clones and control cells as in Fig. 1a. C, FACS analysis of class I MHC (H-2Kb) expression by IFN-α- or IFN-γ-treated IFN-γR2 TG CD8+ T cell clones and control cells, as in Fig. 1D. NoRx, Untreated. D, mRNA was isolated from WT and TG CD8+ T cells (two cell lines each) 5 days following stimulation with allogeneic APCs. IRF-1, SOCS-1, SOCS-3, and GAPDH expression was visualized by Northern blotting. E, Analysis of the distribution of CFSE label by flow cytometry. Freshly isolated WT and CD8+ IFN-γR2 TG CD8+ T cells from spleens and mesenteric lymph nodes were labeled with CFSE and stimulated with anti-CD3 + anti-CD28 Abs in the presence of IL-2. Cells were analyzed by FACS on day 3. Cell divisions undergone by CD8+ cells are represented by numbered gates.
The altered IFN-γ signaling potential observed in TG CD8+ T cells suggests that their biologic responses to IFN-γ may differ from those of WT CD8+ T cells. As expected, TG CD8+ T cells up-regulate levels of cell surface class I MHC in response to treatment with IFN-α, like WT CD8+ T cells (Fig. 3C). However, unlike their WT counterparts, IFN-γR2 TG CD8+ T cells can increase cell surface expression of class I MHC molecules in response to IFN-γ (Fig. 3C). Interestingly, the basal level of class I MHC molecules is higher in TG than WT CD8+ T cells, which may be the reason for the more modest effect of IFNs on the up-regulation of these molecules in TG cells (Fig. 3C). Nevertheless, the up-regulation or class I MHC molecules in response to IFN-γ in TG cells is both significant and reproducible.
As previously reported, IFN-γR2 TG mice appear healthy and thrive when housed in a conventional facility (nonbarrier), and have normal proportions of CD8+ T cells in their spleens and lymph nodes (22). Furthermore, FACS analysis of thymocytes (CD4 vs CD8 vs CD3, and CD4 vs CD8 vs CD69) has revealed no differences between WT and TG mice. TG CD8+ T cells proliferate and can be propagated in vitro similarly to their WT counterparts. However, because IFN-γ has been reported to have antiproliferative effects (4), it is possible that TG CD8+ T cells have a proliferative disadvantage secondary to autocrine effects of this cytokine. To examine the effects of IFN-γ signaling on the mitotic profiles of CD8+ T cells, CD8+ T cells were purified from WT and TG mice and were labeled with CFSE, an amine-reactive, membrane-permeant, fluorescein-based dye that does not interfere with the biologic function of CD8+ T cells (40). The decay of the CFSE label was followed under a number of stimulation and proliferative conditions. WT and TG CD8+ T cells proliferate equally well when stimulated with either anti-CD3 plus anti-CD28 mAbs (Fig. 3E and Table I) or allo-APCs (data not shown). These experiments were also performed using standard [3H]thymidine incorporation techniques, which yielded similar results (data not shown). Together, these data demonstrate that the IFN-γR2 transgene uniquely endows CD8+ T cells with IFN-γ responsiveness, but does not appear to affect their in vivo development or in vitro proliferation.
Table I. Distribution of the CFSE label in CD8+ T cells.
| No. of Cycles |
||||||
|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | 7 | |
| WT | 2.27 | 6.48 | 17.37 | 33.61 | 32.26 | 8.4 |
| TG line 1 | 0.79 | 2.58 | 9.91 | 32.86 | 45.01 | 9.41 |
| TG line 2 | 2.63 | 9.25 | 23.75 | 38.84 | 22.36 | 3.57 |
Altered gene expression in IFN-γR2 TG CD8+ T cells
IFN-γ has been shown to regulate the expression of over 200 genes, including transcription factors, such as IRF family members, c-fos, and c-jun, and signaling molecules such as SOCS family members, csk, lyn, and lck (37, 41-43). Stat1 is constitutively activated in TG CD8+ cells (Fig. 3B), most likely as a result of autocrine effects of IFN-γ. Furthermore, the basal levels of cell surface class I MHC molecules are elevated on IFN-γR2 TG CD8+ T cells. It is likely that autocrine effects of IFN-γ (Fig. 3B) induce the higher baseline of cell surface MHC molecules (Fig. 3C). To examine whether the IFN-γR2 transgene affects the expression of other IFN-γ-regulated genes, mRNA was isolated from activated CD8+ T cell lines derived from WT and TG mice. The mRNA levels of irf-1 and, more modestly, SOCS-1 and SOCS-3, were found to be elevated in TG cells (Fig. 3D). This suggests that the pattern of expression of these, and potentially other, IFN-γ-regulated genes is altered in IFN-γR2 TG CD8+ T cells.
IFN-γ signaling does not affect CD8+ T cell cytokine and proliferative memory responses ex vivo
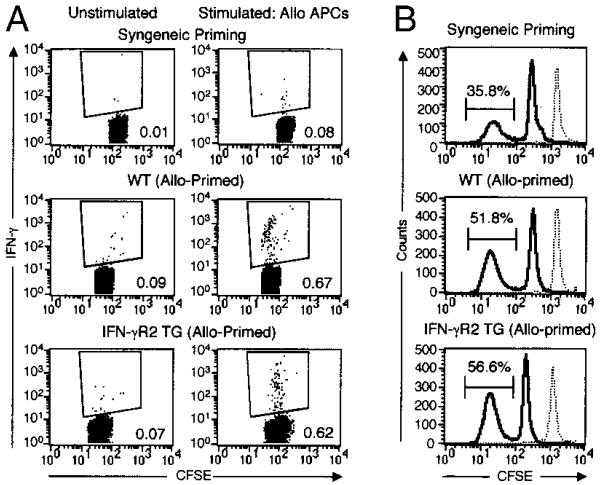
To examine whether the signals transduced through IFN-γR affect CD8+ T cell responses in vivo, Ag-specific memory responses, such as proliferation and cytokine production, were examined in IFN-γR2 TG mice. CD8+ T cells were purified from primed mice, labeled with CFSE, and stimulated with allogeneic APCs. Both the fraction of CD8+ T that are alloreactive in the primed mice, and the proportion of these alloreactive cells that are IFN-γ producers, were determined by flow cytometry. Approximately 0.1% of isolated CD8+ T cells from mice primed with syngeneic APCs produced IFN-γ in response to ex vivo allogeneic stimulation (Fig. 4A). In contrast, at least 6-fold greater numbers of allo-primed WT and TG CD8+ T cells produced IFN-γ following ex vivo stimulation with allogeneic APCs (Fig. 4A). These results corroborate the in vitro data showing no difference in IFN-γ production between WT and TG cells (see below).
FIGURE 4.
IFN-γR2 TG mice elaborate normal CD8+ T cell proliferative and cytokine memory responses ex vivo. A and B, Ex vivo IFN-γ production and alloresponder frequency following syngeneic or allogeneic priming. WT and IFN-γR2 TG mice were primed as indicated. At day 10, CD8+ T cells were purified, labeled with CFSE, and stimulated with allo-APCs or not. A, At 12 h, cells were stained for intracytoplasmic IFN-γ and analyzed by flow cytometry. Plots represent CD8-positive, CFSEbright (non-APC) events. The values in each panel represent the percentages of IFN-γ-positive events within the indicated gates. B, On day 3, the CFSE label distribution of the allostimulated cells analyzed by flow cytometry. Plots represent CD8+, CFSEbright (non-APC) events. CFSE intensity at day 0 (dashed curve) is overlaid with the CFSE distribution at day 3 (solid curve). Of two clusters of CFSEbright cells in each histogram, the high intensity peak corresponds to the nonalloreactive cell population, whereas the low intensity peak represents the responder population that has undergone four to six cell division cycles. Values represent the percentages of CD8+ T cells responding to allogeneic stimulation within the indicated gates.
Three days following allostimulation, the distribution of the CFSE label in the population was analyzed by flow cytometry to determine the proportion of allo-responding CD8+ T cells. Both allo-primed WT and TG mice had comparable proportions of alloresponding CD8+ T cells (51.8% and 56.6%, respectively), which were consistently higher than the proportion of alloresponders from mice primed with syngeneic cells (35%) (Fig. 4B). Moreover, WT and TG alloresponder populations exhibited similar rates of CFSE decay (Fig. 4B, and data not shown). Therefore, in response to ex vivo antigenic challenge, primed TG mice generate equivalent proportions of CD8+ responders that proliferate and produce IFN-γ similarly to their WT counterparts.
IFN-γ signaling specifically impairs the effector killing function of CD8+ T cells
Although CD8+ T cells are resistant to IFN-γ, and signaling through IFN-γR appears to be dispensable for the normal development and function of these cells, IFN-γR2 TG CD8+ T cells appear to proliferate normally (Figs. 1–3). This raises the question of why CD8+ T cells become unresponsive to IFN-γ. It is possible that unresponsiveness to IFN-γ is not a mere phenomenon, but is an essential feature of CD8+ T cells intimately associated with the some element of their function. To test this possibility, two effector functions of CD8+ T cells, cytokine production and target cell lysis, were examined in vitro. Allo-specific CD8+ T cells derived from WT and IFN-γR2 TG mice were assayed for their ability to lyse 51Cr-labeled syngeneic (S49) and allogeneic (EL-4) tumor cell lines. WT CD8+ T cells specifically lysed allogeneic targets efficiently, but could not lyse syngeneic target cells (Fig. 5B). Strikingly, IFN-γR2 TG cells were unable to lyse allogeneic or syngeneic target cells (Fig. 5B). This defect in killing was confirmed multiple times with both CD8+ T cell lines and clones, as well as with freshly isolated CD8+ T cells from primed WT and TG mice (Fig. 5A). In contrast, both WT and TG CD8+ T cells produced equivalent amounts of IFN-γ in response to a number of stimuli, including plate-bound anti-CD3 + anti-CD28 mAbs, allo-APCs, and PMA + ionomycin, as assayed by both intracytoplas-mic staining and ELISA (Fig. 5C and data not shown). Together, these data suggest that IFN-γ responsiveness selectively alters the target cell lysis effector function of TG CD8+ T cells, but not their ability to produce IFN-γ.
FIGURE 5.
IFN-γ signaling impairs CD8+ T cell cytotoxicity, but not cytokine production. A and B, 51Cr release assay measuring the specific cytotoxicity of allo-specific IFN-γR2 TG (—) and WT (- - - -) CD8+ T cell clones (B) or CD8+ T cells freshly isolated from allo-primed mice (A) toward syngeneic (EL-4, top) or allogeneic (S49, bottom) tumor cell lines. C, IFN-γ production of allo-specific CD8+ T cell lines derived from IFN-γR2 mice. Six days postallostimulation, CFSE-labeled CD8+ T cells were stimulated with APCs or not. Twelve hours later, cells were stained for intracytoplasmic IFN-γ and analyzed by flow cytometry. Plots represent CFSEbright (non-APC) events. Values represent the percentages of IFN-γ-positive events within the indicated gates.
IFN-γ signaling does not affect the ability of CD8+ T cells to produce effector molecules
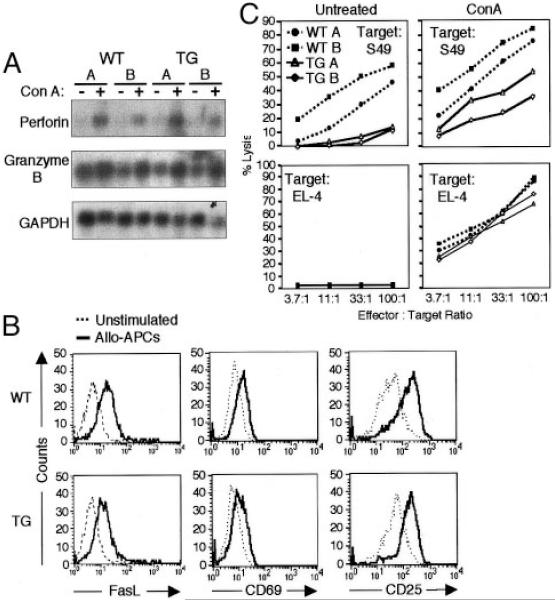
Allo-specific IFN-γR2 TG CD8+ T cells are unable to lyse allogeneic target cells, but are capable of normal proliferation and IFN-γ production. To identify the mechanism responsible for the observed defect in cytotoxicity, the ability of TG cells to produce effector molecules involved in killing was examined. WT and TG CD8+ T cells express equivalent levels of both perforin- and granzyme B-encoding mRNA at baseline, and these mRNA species are similarly up-regulated following treatment with Con A in WT and TG CD8+ T cells (Fig. 6A). Furthermore, cell surface levels of FasL are up-regulated in both WT and TG CD8+ T cells in response to stimulation with either allo-APCs or polyclonal stimuli (Fig. 6B and data not shown). The extent of increase in cell surface levels of FasL in TG cells (median fluorescence intensity rising from 4.5 to 11.4 upon stimulation) is consistently more modest than that seen in WT cells (5.3 rising to 16.3). These data suggest that the effector molecules required for the cytotoxic function of CTLs are expressed by TG CD8+ T.
FIGURE 6.
IFN-γR2 TG CD8+ T cells are normally activated, produce effector killing molecules, and exhibit cytotoxic activity following chemical stimulus. A, Detection of perforin, granzyme B, and GAPDH mRNA expression in unstimulated (5 days postallostimulation) or Con A-stimulated (5 days) WT and IFN-γR2 TG CD8+ T cell lines by Northern blot. B, Flow cytometric analysis of cell surface FasL, CD69, and CD25 expression on WT and IFN-γR2 TG CD8+ T cell clones that were either stimulated with APCs for 12 h (—) or left unstimulated (- - - -). Histogram represents CD8-positive events. C, Redirected killing assay measuring 51Cr release by labeled targets incubated with WT and IFN-γR2 TG CD8+ T cell lines in the presence or absence of Con A.
The defect in killing may potentially be caused by an inability of IFN-γ-responsive CD8+ T cells to either normally recognize, or be properly activated by, allogeneic targets. To address this question, the effect of allogeneic stimulation on TG CD8+ T cell activation was examined. Following incubation with allo-APCs or stimulation with anti-CD3, TG cells can up-regulate expression of the activation markers CD69 and CD25 (IL-2Rα) equally well as their WT counterparts, suggesting that these cells recognize and are normally activated by allogeneic stimuli (Fig. 6B and data not shown). These data, together with the observation that TG CD8+ T cells proliferate normally (Fig. 3E and Table I), argue that IFN-γ signaling does not affect the ability of CD8+ T cells to be specifically activated through TCR-MHC contact, or through polyclonal TCR triggering.
It appears that TCR contact with physiologically appropriate MHC molecules results in the activation of IFN-γR2 TG CD8+ T cells and induction of cytokine production. Although TCR triggering in WT cells results in activation of their cytotoxic pathways, this type of stimulus may be insufficient to induce the exocytosis of cytotoxic granules in TG cells. It is possible that a particularly strong activating stimulus, such as Con A, may be able to induce exocytosis in TG CD8+ T cells, resulting in target cell lysis. Con A aggregates cell surface glycoproteins, thereby supernormally activating T cells independently of allo-MHC molecules (44). The addition of Con A to a standard killing assay (redirected killing) enhanced the cytotoxicity of both WT and TG CD8+ T cells (Fig. 6C). TG cells did not achieve the same absolute levels of S49 target cell lysis as did their WT counterparts in this redirected killing assay (Fig. 6C). However, the relative increase in cytotoxicity at each E:T ratio was equivalent among all WT and TG CD8+ T cell lines (Table II). Moreover, redirected killing using the syngeneic cell line EL-4 as a target resulted in equivalent increases in both relative and absolute values of lysis among all effector cell lines tested (Fig. 6D).
Table II. Net Con A-mediated lysis of allogeneic targets (S49).
| 3.7:1 | 11:1 | 33:1 | 100:1 | |
|---|---|---|---|---|
| WT A | 18.5 | 28.2 | 31.1 | 30.2 |
| WT B | 21.2 | 19.4 | 23.9 | 26.8 |
| TG A | 12.4 | 30.0 | 32.2 | 40.6 |
| TG B | 8.2 | 18.5 | 21.2 | 24.1 |
Together, these data suggest that IFN-γR2 TG CD8+ T cells produce the effector molecules that are required for killing, but are, nevertheless, unable to lyse their targets under normal conditions. The killing defect in TG cells can be circumvented through the addition of Con A, a strong activating stimulus. This suggests that signaling through the IFN-γR causes a subtle, selective defect in CD8+ T cell activation that affects the elaboration of cytotoxicity rather than the production of the components required for this effector function.
Discussion
It is established that cytokines play a pivotal role in the development of mature, effector Th phenotypes (9). Previous studies have demonstrated that Th cells acquire differential responsiveness to cytokines during their differentiation (19-21). For instance, Th1 cells, the IFN-γ-producing CD4+ T cell subset, do not respond to IFN-γ because they lack expression of IFN-γR2 (20, 21). The present study investigates cytokine responsiveness in CD8+ T cells and its potential effects on the acquisition of a mature CTL phenotype. The experiments in this study demonstrate that, like their CD4+ counterparts, IFN-γ-producing CD8+ T cells express IFN-γR1, but not IFN-γR2, and are unable to transduce the IFN-γ signal, or respond to the biologic effects of this cytokine. There is no effective Ab to IFN-γR2, and standard binding assays may not be sufficiently sensitive to determine whether IFN-γR2 is present on the surface of CD8+ T cells, because this chain is not required for binding IFN-γ (45). Therefore, our data demonstrating that CD8+ T cells lack IFN-γR2 mRNA, are able to activate Stat1 in response to treatment with IFN-α but not with IFN-γ, and that TG expression of IFN-γR2 confers IFN-γ responsiveness to these cells, is the best available set of experiments to demonstrate this causal relationship. In support of this, our original report describing the lack of IFN-γ signaling in Th1 cells demonstrated that transfection of cDNA encoding IFN-γR2 into Th1 clones allows them to respond to IFN-γ (20).
Experiments using CD8+ T cells derived from IFN-γR2−/− mice demonstrate that IFN-γR is not required for the development of a mature CTL phenotype. Furthermore, TG overexpression of IFN-γR2 not only restores IFN-γ responsiveness in CD8+ T cells, but results in CTLs with altered function. This establishes an interesting paradigm, that IFN-γ-producing T cells down-modulate expression of IFN-γR2 and consequently acquire an IFN-γ-unresponsive phenotype. Moreover, unresponsiveness to IFN-γ appears to be essential for the development of the normal effector phenotypes of these cells.
Much is known about how IFN-γ and other cytokines affect CD4+ Th subset differentiation and function. However, the current understanding of the role of cytokines in the development, differentiation, and function of CD8+ T cells is still rudimentary. The finding that CD8+ T cells are insensitive to IFN-γ suggests that the modulation of responsiveness to this cytokine by these cells may be an event integral to their development. Remarkably, forced responsiveness to IFN-γ in CD8+ T cells appears to specifically impair their ability to kill, but leaves their other functions intact. It is interesting that the function of both CD4+ and CD8+ T cells is impaired in IFN-γR2 TG mice, but the effects of transgene expression on these cells are qualitatively different. TG Th1 cells elaborate defective IFN-γ-dependent responses, whereas production of this cytokine by TG CD8+ T cells is intact (22). It may be that these two cell types have innate differences in their “hard wiring” for IFN-γ production, as suggested by the dependence of Th1, but not CD8+ T cells on Stat4 for production of this cytokine (46).
A number of groups have described CTLs whose function is altered, with a phenotype similar to that of IFN-γR2 TG CD8+ T cells. In these studies, CTLs stimulated by superantigen (47), subdominant viral epitopes (16), or altered peptides (altered peptide ligand phenomenon) (48, 49), and naturally occurring virus-specific CTLs (15, 50) appear to elaborate defective killing mechanisms, but can be activated to proliferate and produce IFN-γ normally. It seems that, depending on the nature of the TCR signal, CTLs that appear to be normally activated can be selectively unable to elaborate one or more effector mechanisms. One group has shown that CTLs that cannot generate an appropriate Ca2+ signal in response to TCR triggering are unable to kill through the perforin/granzyme pathway (51-53). Degranulation can be induced by treatment of these cells with phorbol ester plus calcium ionophore, which circumvents the TCR to induce a large spike in intracellular Ca2+.
Like previously described CTLs with altered function, IFN-γR2 TG CD8+ T cells are activated, produce IFN-γ, and up-regulate FasL in response to antigenic stimulation. Although the extent of FasL up-regulation is more modest on TG than on WT CD8+ T cells, it is unlikely that this is the locus of the defect in killing in IFN-γR2 TG CD8+ T cells, because our cytotoxicity assay system does not measure FasL-mediated killing, which typically requires a longer assay time (54, 55). Furthermore, S49 cells, the allogeneic targets used in the killing assay, may be resistant to FasL-mediated killing (56). Remarkably, TG cells are unable to kill via the exocytotic pathway, although they produce the effector molecules required for this function. In contrast, in the presence of Con A, TG cells are able to lyse allogeneic and syngeneic targets. One effect of Con A is to promote cell-cell contact, which raises the possibility that TG CTLs may be unable to form the effector-target cell contacts that are required for killing (57). This potential dysfunction could be due to altered expression of adhesion molecules in these cells. However, since the typical net effect of IFN-γ is the up-regulation of cell surface levels of adhesion molecules, such as selectins (e.g., CD62L and CD62E) and integrins (e.g., CD49a-f and CD11a-c) on a host of cell lines, TG CTLs are probably able to bind with target cells (37). Con A can also induce multiple intracellular events, including protein tyrosine kinase activation, leading to increase in tyrosine-phosphorylated proteins, phosphatidylinositol 3-kinase activation, activation of pathways downstream of G proteins, rise of intracellular Ca2+, and the nuclear translocation of NF-ATp (58-62). These events correlate with the commonly accepted role of Con A as a polyclonal activator of T cells. It is likely that this lectin promotes killing by TG cells by triggering intracellular pathways in these cells that are not activated, or are differently activated, by TCR-MHC contact alone. Since many pathways activated by Con A are normally downstream of the TCR, these findings raise the possibility that the quality of the TCR signal in IFN-γR2 TG CTLs is altered.
IFN-γ is a pleiotropic cytokine that is known to regulate the expression of a vast array of genes, and consequently a host of cellular and immunological processes (37). In IFN-γR2 TG CD8+ T cells, Stat1 is constitutively active, and baseline levels of IFN-γ-inducible proteins are elevated. We believe this occurs because of autocrine effects of IFN-γ rather than an artifact of overexpression of IFN-γR2 because we have previously reported that Stat1 is inactive in TG Th2 cell clones, which by definition do not produce IFN-γ (22). This suggests that the constitutive activation of the IFN-γ pathway seen in TG CD8+ T cells is ligand dependent. Furthermore, altered pattern of gene expression in CD8+ T cells derived from IFN-γR2 TG mice can potentially create an environment that is incompatible with the acquisition of killing function or the generation of an appropriate TCR signal, leading to a selective defect in perforin/granzyme-mediated cytotoxicity in these cells. One potential mechanism for this defect may involve the SOCS-1 and SOCS-3 proteins, whose expression is known to be induced by IFN-γ, and is up-regulated in IFN-γR2 CD8+ T cells (42). These SOCS proteins may affect signaling by cytokines that are important in the function of CD8+ T cells. Both SOCS-1 and SOCS-3 have been implicated in the attenuation of the IFN-α signal (63-65). CTLs from mice that are deficient in the IFN-αβR were unable to elaborate Ag-specific killing functions, but were able to produce IFN-γ (66, 67). SOCS-3 has also been shown to inhibit Janus kinase 2 activation, and therefore, the activation of Stat5 by GM-CSF and perhaps by IL-5 (68, 69). Defects in IL-5 and GM-CSF have been shown to affect killing by CD8+ T cells (70, 71). Therefore, through the induction of SOCS expression, IFN-γ may potentially initiate an inhibitory cross-talk with the IFN-α, IL-5, and/or GM-CSF pathways, all of which have been implicated in CTL cytotoxicity. Beyond their role as suppressors of cytokine signaling, these members of the SOCS family have been implicated in inhibiting the activation of signaling pathways downstream of the TCR (72) (A. Banerjee, personal communication). In addition to the SOCS, there may be other IFN-γ-inducible factors that mediate this inhibitory cross-talk between the IFN-γR and TCR pathways, or that regulate pathways linking T cell activation to granule exocytosis. These factors may also be dysregulated in IFN-γR2 TG CD8+ T cells, leading to the observed phenotype.
This study has demonstrated that, during their development or differentiation, CD8+ T cells down-modulate expression of IFN-γR2, thereby extinguishing their ability to respond to IFN-γ. This event appears to be crucial for the acquisition of a fully functional, mature, CTL phenotype, since signaling through IFN-γR blocks the ability to utilize the perforin/granzyme cytotoxic pathway by CD8+ T cells that are stimulated by their cognate Ag. However, it seems that IFN-γ signaling is not required for either the thymic development of CD8+ T cells or their differentiation into mature CTLs. What, then, is the biological importance of the control of IFN-γR2 expression? This phenomenon may be a self-protective mechanism invoked by CD8+ T cells to avoid the potential deleterious effects of IFN-γ. Therefore, down-modulation of IFN-γR2 would occur automatically, either concomitantly with their acquisition of the capacity to produce IFN-γ, following the initial triggering of signaling through IFN-γR, or in response to another signal delivered during their differentiation. On the other hand, the modulation of IFN-γR2 expression could potentially be used as a self-regulatory switch by CD8+ T cells. Continued IFN-γR2 expression in naive CD8+ T, which may, in fact, be normally responsive to IFN-γ, would delay their maturation or prevent it entirely. These cells would not be able to acquire a mature cytotoxic phenotype until down-modulation of IFN-γR2 expression is induced. Furthermore, by modulating IFN-γR2 expression, mature CTLs may be able to actively regulate their capacity to kill using the perforin/granzyme pathway. These scenarios represent an IFN-γ-mediated mechanism by which the immune system can self-limit its responses, thereby controlling the supply of new CTLs or modulating the function of existing CTLs. Whether the IFN-γ responsiveness phenotype becomes fixed or remains flexible, the regulation of IFN-γR2 expression is an integral event in CD8+ T cell development and mature phenotype acquisition. IFN-γ-producing T cells, and potentially all IFN-γ-producing cells, may need to extinguish their ability to respond to IFN-γ by down-regulating the expression of IFN-γR2 to develop into mature effector cells.
Acknowledgments
We thank Binfeng Lu for providing the IFN-γR2−/− mice; Benvenuto Pernis and Julie Losman for their critical reading of the manuscript; and Eric Pamer for useful discussions.
Footnotes
This work was supported by American Cancer Society Grant IM783 and National Institutes of Health Grant PO1AI39675. This work was supported by ACS-IM783 and by NIH-PO1AI39675.
Abbreviations used in this paper: FasL, Fas ligand; GAS, γ-activated site; IRF, IFN-regulatory factor; SOCS, suppressors of cytokine signaling; TG, transgenic; WT, wild type.
References
- 1.Harty JT, Tvinnereim AR, White DW. CD8+ T cell effector mechanisms in resistance to infection. Annu. Rev. Immunol. 2000;18:275. doi: 10.1146/annurev.immunol.18.1.275. [DOI] [PubMed] [Google Scholar]
- 2.Doherty PC, Allan W, Eichelberger M, Carding SR. Roles of αβ and γδ T cell subsets in viral immunity. Annu. Rev. Immunol. 1992;10:123. doi: 10.1146/annurev.iy.10.040192.001011. [DOI] [PubMed] [Google Scholar]
- 3.Wallach D, Varfolomeev EE, Malinin NL, Goltsef YV, Kovalenko AV, Boldin MP. Tumor necrosis factor receptor and Fas signaling mechanisms. Annu. Rev. Immunol. 1999;17:331. doi: 10.1146/annurev.immunol.17.1.331. [DOI] [PubMed] [Google Scholar]
- 4.Billiau A. Interferon-γ: biology and role in pathogenesis. Adv. Immunol. 1996;62:61. doi: 10.1016/s0065-2776(08)60428-9. [DOI] [PubMed] [Google Scholar]
- 5.Henkart PA, Sitkovsky MV. Two ways to kill target cells. Curr. Biol. 1994;4:923. doi: 10.1016/s0960-9822(00)00207-4. [DOI] [PubMed] [Google Scholar]
- 6.Trapani JA, Davis J, Sutton VR, Smyth MJ. Proapoptotic functions of cytotoxic lymphocyte granule constituents in vitro and in vivo. Curr. Opin. Immunol. 2000;12:323. doi: 10.1016/s0952-7915(00)00094-7. [DOI] [PubMed] [Google Scholar]
- 7.Kagi D, Ledermann B, Burki K, Zinkernagel RM, Hengartner H. Molecular mechanisms of lymphocyte-mediated cytotoxicity and their role in immunological protection and pathogenesis in vivo. Annu. Rev. Immunol. 1996;14:207. doi: 10.1146/annurev.immunol.14.1.207. [DOI] [PubMed] [Google Scholar]
- 8.Zheng L, Fisher G, Miller RE, Peschon MJ, Lynch DH, Lenardo MJ. Induction of apoptosis in mature T cells by tumor necrosis factor. Nature. 1995;377:348. doi: 10.1038/377348a0. [DOI] [PubMed] [Google Scholar]
- 9.O’Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8:275. doi: 10.1016/s1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- 10.Constant S, Pfeiffer C, Woodard A, Pasqualini T, Bottomly K. Extent of T cell receptor ligation can determine the functional differentiation of naive CD4+ T cells. J. Exp. Med. 1995;182:1591. doi: 10.1084/jem.182.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Constant SL, Bottomly K. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu. Rev. Immunol. 1997;15:297. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 12.Zinkernagel RM, Doherty PC. Restriction of in vitro T cellmediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature. 1974;248:701. doi: 10.1038/248701a0. [DOI] [PubMed] [Google Scholar]
- 13.Sercacs EE, Lehman PV, Ametani A, Benichou G, Miller A, Moudgil K. Dominance and crypticity of T cell antigenic determinants. Annu. Rev. Immunol. 1993;11:729. doi: 10.1146/annurev.iy.11.040193.003501. [DOI] [PubMed] [Google Scholar]
- 14.Yewdell JW, Bennink JR. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu. Rev. Immunol. 1999;17:51. doi: 10.1146/annurev.immunol.17.1.51. [DOI] [PubMed] [Google Scholar]
- 15.Klenerman P, Zindernagel RM. Original antigenic sin impairs cytotoxic T lymphocyte responses to viruses bearing variant epitopes. Nature. 1998;394:482. doi: 10.1038/28860. [DOI] [PubMed] [Google Scholar]
- 16.Spencer JV, Braciale TJ. Incomplete CD8+ T lymphocyte differentiation as a mechanism for subdominant cytotoxic T lymphocyte responses to a viral antigen. J. Exp. Med. 2000;191:1687. doi: 10.1084/jem.191.10.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 1989;7:145. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 18.Paul WE, Seder RA. Lymphocyte responses and cytokines. Cell. 1994;76:241. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 19.Szabo SJ, Jacobson NG, Dighe AS, Gubler U, Murphy DM. Developmental commitment to the Th2 lineage by extinction of IL-12 signaling. Immunity. 1995;2:665. doi: 10.1016/1074-7613(95)90011-x. [DOI] [PubMed] [Google Scholar]
- 20.Pernis A, Gupta S, Gollob KJ, Garfein E, Coffman RL, Schindler C, Rothman P. Lack of interferon γ receptor β chain and the prevention of interferon γ signaling in TH1 cells. Science. 1995;269:245. doi: 10.1126/science.7618088. [DOI] [PubMed] [Google Scholar]
- 21.Bach EA, Szabo SJ, Dighe AS, Ashkenazi A, Aguet M, Murphy KM, Schreiber RD. Ligand-induced autoregulation of IFN-γ receptor β chain expression in T helper cell subsets. Science. 1995;270:1215. doi: 10.1126/science.270.5239.1215. [DOI] [PubMed] [Google Scholar]
- 22.Tau GZ, von der Weid T, Lu B, Cowan S, Kvatyuk M, Pernis A, Cattoretti G, Braunstein NS, Coffman RL, Rothman PB. Interferon γ signaling alters the function of T helper type 1 cells. J. Exp. Med. 2000;192:977. doi: 10.1084/jem.192.7.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu B, Ebensperger C, Dembic Z, Wang Y, Kvatyuk M, Lu T, Coffman RL, Pestka S, Rothman PB. Targeted disruption of the interferon-γ receptor 2 gene results in severe immune defects in mice. Proc. Natl. Acad. Sci. USA. 1998;95:8233. doi: 10.1073/pnas.95.14.8233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fitch FW, Gajewski TF. Production of T cell clones. In: Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W, editors. Current Protocols in Immunology. Vol. 1. John Wiley & Sons; New York: 1992. p. 3.13.1.. [Google Scholar]
- 25.Kruisbeek AM. Proliferative assays for T cell function. In: Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W, editors. Current Protocols in Immunology. Vol. 1. John Wiley & Sons; New York: 1991. p. 3.12.1.. [DOI] [PubMed] [Google Scholar]
- 26.Eilers A, Seegert D, Schindler C, Baccarini M, Decker T. The response of γ interferon activation factor is under developmental control in cells of the macrophage lineage. Mol. Cell. Biol. 1993;13:3245. doi: 10.1128/mcb.13.6.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pernis A, Gupta S, Yopp J, Garfein E, Kashleva H, Schindler C, Rothman P. γ Chain-associated cytokine receptors signal through distinct transducing factors. J. Biol. Chem. 1995;270:14517. doi: 10.1074/jbc.270.24.14517. [DOI] [PubMed] [Google Scholar]
- 28.Rothman P, Kreider B, Azam M, Levy D, Wegenka U, Eilers A, Decker T, Horn F, Kashleva H, Ihle J. Cytokines and growth factors signal through tyrosine phosphorylation of a family of related transcription factors. Immunity. 1994;1:457. doi: 10.1016/1074-7613(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning, A Laboratory Manual. Cold Spring Harbor Press; New York: 1989. [Google Scholar]
- 30.Pine R, Canova A, Schindler C. Tyrosine phosphorylated p91 binds to a single element in the ISGF/IRF-1 promoter to mediate induction by IFNα and IFNγ, and is likely to autoregulate the p91 gene. EMBO J. 1994;13:158. doi: 10.1002/j.1460-2075.1994.tb06245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hemmi S, Bohni R, Stark G, Di Marco F, Aguet M. A novel member of the interferon receptor family complements functionality of the murine interferon-γ receptor in human cells. Cell. 1994;76:803. doi: 10.1016/0092-8674(94)90355-7. [DOI] [PubMed] [Google Scholar]
- 32.Hyodo Y, Matsui K, Hayashi N, Tsutsui H, Kashiwamura S, Yamauchi H, Hiroishi K, Takeda K, Tagawa Y, Iwakura Y, et al. IL-18 up-regulates perforin-mediated NK activity without increasing perforin messenger RNA expression by binding to constitutively expressed IL-18 receptor. J. Immunol. 1999;162:1662. [PubMed] [Google Scholar]
- 33.Losman JA, Chen XP, Hilton D, Rothman P. SOCS-1 is a potent inhibitor of IL-4 signal transduction. J. Immunol. 1999;162:3770. [PMC free article] [PubMed] [Google Scholar]
- 34.Wunderlich J, Shearer G. Induction and measurement of cytotoxic T lymphocyte activity. In: Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W, editors. Current Protocols in Immunology. Vol. 1. John Wiley & Sons; New York: 1991. p. 3.11.1.. [Google Scholar]
- 35.Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J. Immunol. Methods. 1994;171:131. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 36.Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu. Rev. Biochem. 1998;67:227. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 37.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-γ. Annu. Rev. Immunol. 1997;15:749. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 38.Tau G, Rothman P. Biologic functions of the IFN-γ receptors. Allergy. 1999;54:1233. doi: 10.1034/j.1398-9995.1999.00099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang S, Hendriks W, Althage A. Immune response in mice that lack the interferon-γ receptor. Science. 1993;259:1693. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 40.Oehen S, Bruduscha-Riem K, Oxenius A, Odermatt B. A simple method for evaluating the rejection of grafted spleen cells by flow cytometry and tracing adoptively transferred cells by light microscopy. J. Immunol. Methods. 1997;207:33. doi: 10.1016/s0022-1759(97)00089-6. [DOI] [PubMed] [Google Scholar]
- 41.Sakamoto H, Yasukawa H, Masuhara M, Tanimura S, Sasaki A, Yuge K, Ohtsubo M, Ohtsuka A, Fujita T, Ohta T, et al. A Janus kinase inhibitor, JAB, is an interferon-γ-inducible gene and confers resistance to interferons. Blood. 1998;92:1668. [PubMed] [Google Scholar]
- 42.Starr R, Willson TA, Viney EM, Murray LJ, Rayner JR, Jenkins BJ, Gonda TJ, Alexander WS, Metcalf D, Nicola NA, Hilton DJ. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387:917. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki R, Sakamoto H, Yasukawa H, Masuhara M, Wakioka T, Sasaki A, Yuge K, Komiya S, Inoue A, Yoshimura A. CIS3 and JAB have different regulatory roles in interleukin-6 mediated differentiation and STAT3 activation in M1 leukemia cells. Oncogene. 1998;17:2271. doi: 10.1038/sj.onc.1202143. [DOI] [PubMed] [Google Scholar]
- 44.Douglas SD, Kamin RM, Fudenberg HH. Human lymphocyte response to phytomitogens in vitro: normal, agammaglobulinemic and paraproteinemic individuals. J. Immunol. 1969;103:1185. [PubMed] [Google Scholar]
- 45.Bach EA, Aguet M, Schreiber RD. The IFNγ receptor: a paradigm for cytokine receptor signaling. Annu. Rev. Immunol. 1997;15:563. doi: 10.1146/annurev.immunol.15.1.563. [DOI] [PubMed] [Google Scholar]
- 46.Carter LL, K M. Murphy. Lineage-specific requirement for signal transducer and activator of transcription (Stat)4 in interferon γ production from CD4+ versus CD8+ T cells. J. Exp. Med. 1999;189:1355. doi: 10.1084/jem.189.8.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fuller CL, Braciale VL. Selective induction of CD8+ cytotoxic T lymphocyte effector function by Staphylococcus enterotoxin B. J. Immunol. 1998;161:5179. [PubMed] [Google Scholar]
- 48.Kessler B, Hudrisier D, Schroeter M, Tschopp J, Crettini JC, Luescher IF. Peptide modification of blocking of CD8, resulting in weak TCR signaling, can activate CTL for Fasbut not perforin-dependent cytotoxicity or cytokine production. J. Immunol. 1998;161:6939. [PubMed] [Google Scholar]
- 49.Cao W, Tykodi SS, Esser MT, Braciale VL, Braciale TJ. Partial activation of CD8+ T cells by self-derived peptides. Nature. 1995;378:295. doi: 10.1038/378295a0. [DOI] [PubMed] [Google Scholar]
- 50.Appay V, Nixon DF, Donahoe SM, Gillespie GMA, Dong T, King A, Ogg GS, Spiegel HML, Conlon C, Spina CA, et al. HIV-specific CD8+ T cells produce antiviral cytokines but are impaired in cytolytic function. J. Exp. Med. 2000;192:63. doi: 10.1084/jem.192.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fuller CL, Ravichandran KS, Braciale VL. Phosphatidylinositol 3-kinase-dependent and -independent cytolytic effector functions. J. Immunol. 1999;163:6337. [PubMed] [Google Scholar]
- 52.Esser MT, Krishnamurthy B, Vraciale VL. Distinct T cell receptor signaling requirements for perforin- or FasL-mediated cytotoxicity. J. Exp. Med. 1996;183:1697. doi: 10.1084/jem.183.4.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Esser MT, Haverstik DM, Fuller CL, Gullo CA, Braciale VL. Ca2+ signaling modulates cytolytic T lymphocyte effector functions. J. Exp. Med. 1998;187:1057. doi: 10.1084/jem.187.7.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Esser MT, Dinglasan RD, Krishnamurthy B, Gullo CA, Graham MB, Braciale VL. IL-2 induces Fas ligand/Fas (CD95L/CD95) cytotoxicity in CD8+ and CD4+ T lymphocyte clones. J. Immunol. 1997;158:5612. [PubMed] [Google Scholar]
- 55.Aung S, Graham BS. IL-4 diminishes perforin-mediated and increases Fas ligand-mediated cytotoxicity in vivo. J. Immunol. 2000;164:3487. doi: 10.4049/jimmunol.164.7.3487. [DOI] [PubMed] [Google Scholar]
- 56.Broome HE, Dargan CM, Brunner T, Green DR. Sensitivity of S49.1 cells to anti-CD95 (Fas/Apo-1)-induced apoptosis: effects of CD95, Bcl-2 or Bcl-x transduction. Cell Death Differ. 1998;5:200. doi: 10.1038/sj.cdd.4400329. [DOI] [PubMed] [Google Scholar]
- 57.Phondke GP, Madyastha KR, Madyastha PR, Barth RF. Quantitative assay for lectin-induced cytoagglutination by means of an electronic particle-counting technique. J. Natl. Cancer Inst. 1981;66:637. doi: 10.1093/jnci/66.4.637. [DOI] [PubMed] [Google Scholar]
- 58.Takeuchi F, Taniguchi T, Maeda H, Fujii C, Takeuchi N, Yamamura H. The lectin concanavalin A stimulates a protein-tyrosine kinase p72syk in peripheral blood lymphocytes. Biochim. Biophys. Acta. 1993;194:91. doi: 10.1006/bbrc.1993.1789. [DOI] [PubMed] [Google Scholar]
- 59.Stanley JB, Gorczynski R, Huang CK, Love J, Mills GB. Tyrosine phosphorylation is an obligatory event in IL-2 secretion. J. Immunol. 1990;145:2189. [PubMed] [Google Scholar]
- 60.Matsuo T, Hazeki K, Hazeki O, Katada T, Ui M. Activation of phosphatidylinositol 3-kinase by concanavalin A through dual signaling pathways, G-protein-coupled and phosphotyrosine-related, and an essential role of the G-protein-coupled signals for the lectin-induced respiratory burst in human monocytic THP-1 cells. Biochem. J. 1996;315:505. doi: 10.1042/bj3150505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lad PM, Olson CV, Grewal IS. Role of a pertussis toxin substrate in the control of lectin-induced cap formation in human neutrophils. Biochem. J. 1986;238:29. doi: 10.1042/bj2380029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Komada H, Nakabayashi H, Hara M, Izutsu K. Early calcium signaling and calcium requirements for the IL-2 receptor expression and IL-2 production in stimulated lymphocytes. Cell. Immunol. 1996;173:215. doi: 10.1006/cimm.1996.0270. [DOI] [PubMed] [Google Scholar]
- 63.Shen X, Hong F, Nguyen VA, Gao B. IL-10 attenuates IFN-α-activated STAT1 in the liver: involvement of SOCS2 and SOCS3. FEBS Lett. 2000;480:132. doi: 10.1016/s0014-5793(00)01905-0. [DOI] [PubMed] [Google Scholar]
- 64.Magrangeas F, Boisteau O, Denis S, Jacques Y, Minvielle S. Negative cross-talk between interleukin-3 and interleukin-11 is mediated by suppressor of cytokine signalling-3 (SOCS-3) Biochem. J. 2001;353:223. doi: 10.1042/0264-6021:3530223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Song MM, Shuai K. The suppressor of cytokine signaling (SOCS) 1 and SOCS3 but not SOCS2 proteins inhibit interferon-mediated antiviral and antiproliferative activities. J. Biol. Chem. 1998;273:35056. doi: 10.1074/jbc.273.52.35056. [DOI] [PubMed] [Google Scholar]
- 66.Van den Broek MF, Muller U, Huang S, Aguet M, Zinkernagel RM. Antiviral defense in mice lacking both α/β and γ interferon receptors. J. Virol. 1995;69:4792. doi: 10.1128/jvi.69.8.4792-4796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cousens LP, Peterson R, Hsu S, Dorner A, Altman JD, Ahmed R, Biron CA. Two roads diverged: interferon α/β- and interleukin 12-mediated pathways in promoting T cell interferon γ responses during viral infection. J. Exp. Med. 1999;189:1315. doi: 10.1084/jem.189.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cohney SJ, Sanden D, Cacalano NA, Yoshimura A, Mui A, Migone TS, Johnston JA. SOCS-3 is tyrosine phosphorylated in response to interleukin-2 and suppresses STAT5 phosphorylation and lymphocyte proliferation. Mol. Cell. Biol. 1999;19:4980. doi: 10.1128/mcb.19.7.4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stoiber D, Kovarik P, Cohney S, Johnston JA, Steinlein P, Decker T. Lipopolysaccharide induces in macrophages the synthesis of the suppressor of cytokine signaling 3 and suppresses signal transduction in response to the activating factor IFN-γ. J. Immunol. 1999;163:2640. [PubMed] [Google Scholar]
- 70.Wada H, Noguchi Y, Marino MW, Dunn AR, Old LJ. T cell functions in granulocyte/macrophage colony-stimulating factor deficient mice. Proc. Natl. Acad. Sci. USA. 1997;94:12557. doi: 10.1073/pnas.94.23.12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Apostolopoulos V, McKenzie IF, Lees C, Matthaei KI, Young IG. A role for IL-5 in the induction of cytotoxic T lymphocytes in vivo. Eur. J. Immunol. 2000;30:1733. doi: 10.1002/1521-4141(200006)30:6<1733::AID-IMMU1733>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 72.Matsuda T, Yamamoto T, Kishi H, Yoshimura A, Muraguchi A. SOCS-1 can suppress CD3ζ- and Syk-mediated NF-AT activation in a non-lymphoid cell line. FEBS Lett. 2000;472:235. doi: 10.1016/s0014-5793(00)01444-7. [DOI] [PubMed] [Google Scholar]
- 73.LeClaire RD, Basu M, Pinson DM, Redick ML, Hunt JS, Zavodny PJ, Pace JL, Russell SW. Characterization and use of monoclonal and polyclonal antibodies against the mouse interferon-γ receptor. J. Leukocyte Biol. 1992;51:507. doi: 10.1002/jlb.51.5.507. [DOI] [PubMed] [Google Scholar]