Abstract
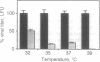
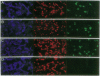
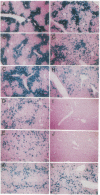
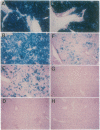
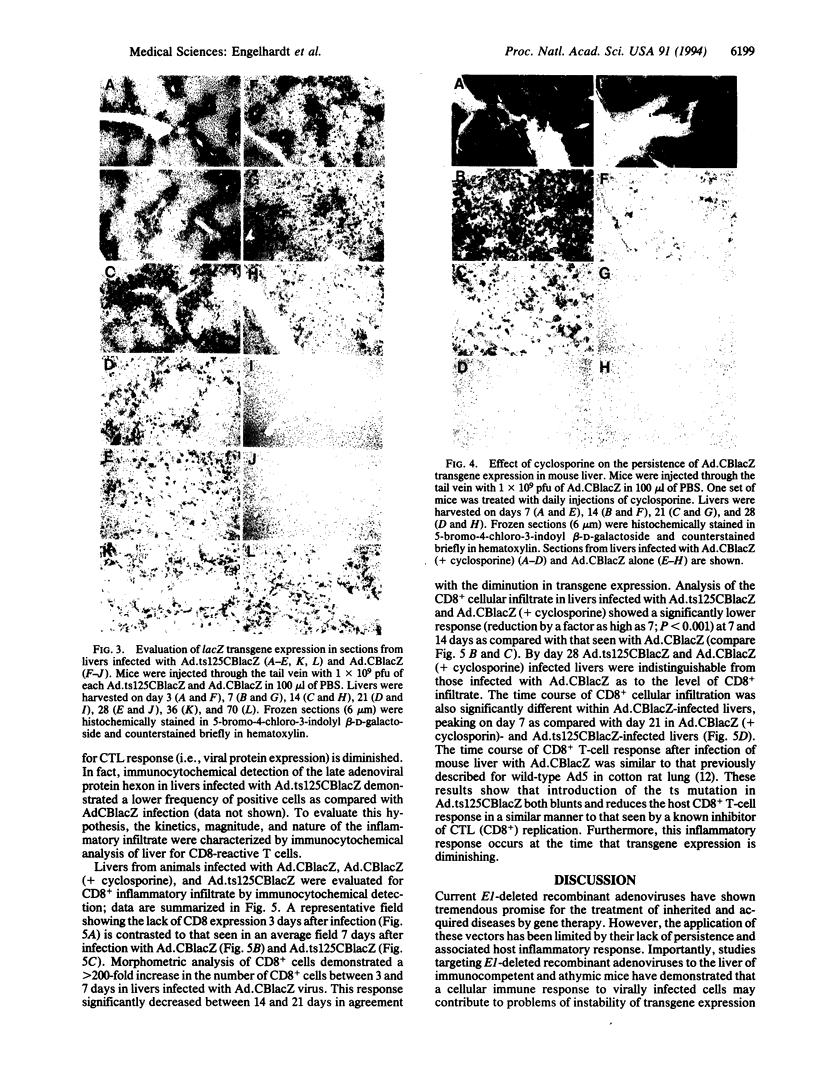
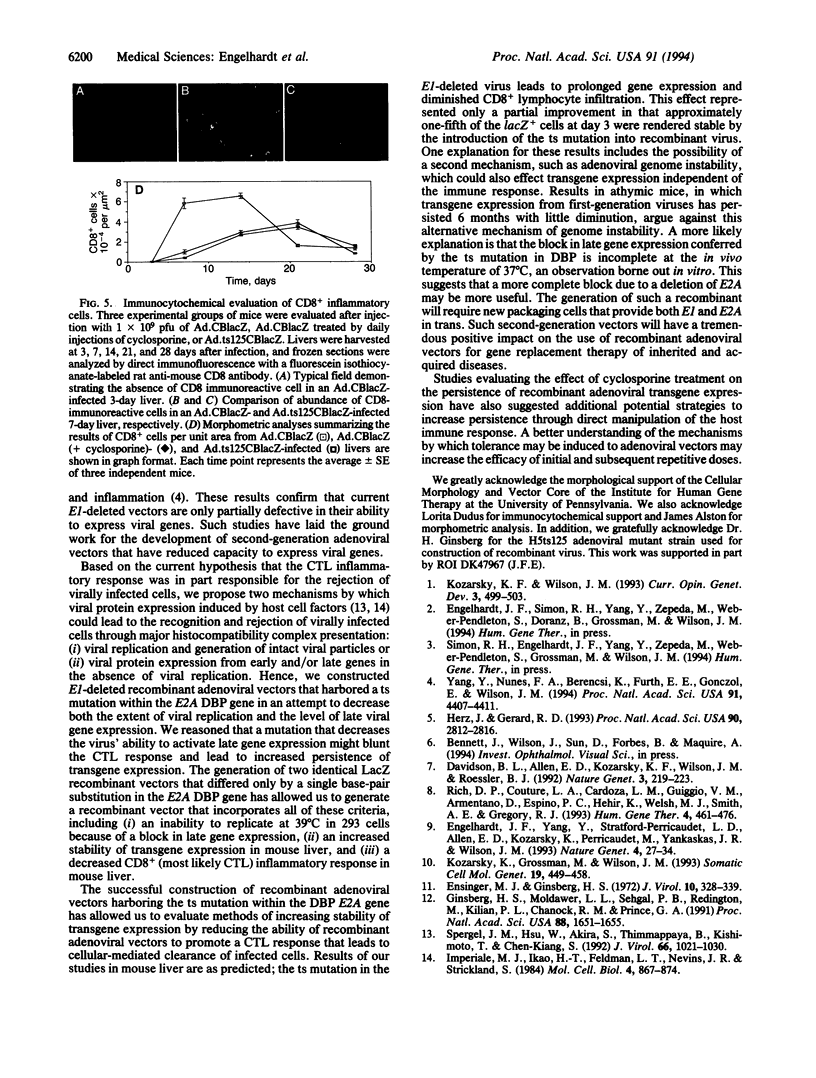
First-generation recombinant adenoviruses that lack E1 sequences have shown tremendous promise in animal and human models of gene therapy. Important limitations of these vectors are that recombinant gene expression is transient and inflammation occurs at the site of gene transfer. Our hypothesis for generating vectors with increased persistence is that present recombinant adenoviruses express viral proteins that stimulate cellular immune responses leading to destruction of the infected cells and repopulation of the organ with non-transgene-containing cells. This model predicts that further crippling of the virus will improve persistence and diminish pathology. We describe in this report second-generation recombinant adenoviruses harboring a beta-galactosidase-expressing transgene in which a temperature-sensitive mutation has been introduced into the E2A gene of an E1-deleted recombinant. At nonpermissive temperature, this virus fails to express late gene products, even when E1 is expressed in trans. The biology of this recombinant was studied in vivo in the context of mouse liver, a setting that is permissive for adenovirus type 5 replication. Animals that received the second-generation virus expressed the transgene for at least 70 days, whereas expression of the first-generation virus was no longer than 14 days. In addition, the inflammatory response, as measured by infiltration of CD8+ T cells, was blunted and delayed in livers infected with second-generation virus. These studies illustrate that modifications that disrupt structural protein expression in recombinant adenoviruses may be useful in enhancing their utility for gene therapy.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davidson B. L., Allen E. D., Kozarsky K. F., Wilson J. M., Roessler B. J. A model system for in vivo gene transfer into the central nervous system using an adenoviral vector. Nat Genet. 1993 Mar;3(3):219–223. doi: 10.1038/ng0393-219. [DOI] [PubMed] [Google Scholar]
- Engelhardt J. F., Yang Y., Stratford-Perricaudet L. D., Allen E. D., Kozarsky K., Perricaudet M., Yankaskas J. R., Wilson J. M. Direct gene transfer of human CFTR into human bronchial epithelia of xenografts with E1-deleted adenoviruses. Nat Genet. 1993 May;4(1):27–34. doi: 10.1038/ng0593-27. [DOI] [PubMed] [Google Scholar]
- Ensinger M. J., Ginsberg H. S. Selection and preliminary characterization of temperature-sensitive mutants of type 5 adenovirus. J Virol. 1972 Sep;10(3):328–339. doi: 10.1128/jvi.10.3.328-339.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg H. S., Moldawer L. L., Sehgal P. B., Redington M., Kilian P. L., Chanock R. M., Prince G. A. A mouse model for investigating the molecular pathogenesis of adenovirus pneumonia. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1651–1655. doi: 10.1073/pnas.88.5.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz J., Gerard R. D. Adenovirus-mediated transfer of low density lipoprotein receptor gene acutely accelerates cholesterol clearance in normal mice. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2812–2816. doi: 10.1073/pnas.90.7.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperiale M. J., Kao H. T., Feldman L. T., Nevins J. R., Strickland S. Common control of the heat shock gene and early adenovirus genes: evidence for a cellular E1A-like activity. Mol Cell Biol. 1984 May;4(5):867–874. doi: 10.1128/mcb.4.5.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozarsky K. F., Wilson J. M. Gene therapy: adenovirus vectors. Curr Opin Genet Dev. 1993 Jun;3(3):499–503. doi: 10.1016/0959-437x(93)90126-a. [DOI] [PubMed] [Google Scholar]
- Kozarsky K., Grossman M., Wilson J. M. Adenovirus-mediated correction of the genetic defect in hepatocytes from patients with familial hypercholesterolemia. Somat Cell Mol Genet. 1993 Sep;19(5):449–458. doi: 10.1007/BF01233250. [DOI] [PubMed] [Google Scholar]
- Rich D. P., Couture L. A., Cardoza L. M., Guiggio V. M., Armentano D., Espino P. C., Hehir K., Welsh M. J., Smith A. E., Gregory R. J. Development and analysis of recombinant adenoviruses for gene therapy of cystic fibrosis. Hum Gene Ther. 1993 Aug;4(4):461–476. doi: 10.1089/hum.1993.4.4-461. [DOI] [PubMed] [Google Scholar]
- Spergel J. M., Hsu W., Akira S., Thimmappaya B., Kishimoto T., Chen-Kiang S. NF-IL6, a member of the C/EBP family, regulates E1A-responsive promoters in the absence of E1A. J Virol. 1992 Feb;66(2):1021–1030. doi: 10.1128/jvi.66.2.1021-1030.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Nunes F. A., Berencsi K., Furth E. E., Gönczöl E., Wilson J. M. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]