Abstract
The frequent jumps of the eyeballs—called saccades—imply the need for a constant correction of motor errors. If systematic errors are detected in saccade landing, the saccade amplitude adapts to compensate for the error. In the laboratory, saccade adaptation can be studied by displacing the saccade target. Functional selectivity of adaptation for different saccade types suggests that adaptation occurs at multiple sites in the oculomotor system. Saccade motor learning might be the result of a comparison between a prediction of the saccade landing position and its actual postsaccadic location. To investigate whether a thalamic feedback pathway might carry such a prediction signal, we studied a patient with a lesion in the posterior ventrolateral thalamic nucleus. Saccade adaptation was tested for reactive saccades, which are performed to suddenly appearing targets, and for scanning saccades, which are performed to stationary targets. For reactive saccades, we found a clear impairment in adaptation retention ipsilateral to the lesioned side and a larger-than-normal adaptation on the contralesional side. For scanning saccades, adaptation was intact on both sides and not different from the control group. Our results provide the first lesion evidence that adaptation of reactive and scanning saccades relies on distinct feedback pathways from cerebellum to cortex. They further demonstrate that saccade adaptation in humans is not restricted to the cerebellum but also involves cortical areas. The paradoxically strong adaptation for outward target steps can be explained by stronger reliance on visual targeting errors when prediction error signaling is impaired.
Keywords: reactive saccades, saccade adaptation, scanning saccades, thalamic lesion
the thalamus is a central relay station for feedback pathways from subcortical motor areas to the frontal cortex (Sommer 2003). These pathways carry information about ongoing motor commands upstream to inform cortical areas about motor actions and their perceptual consequences. Such internal monitoring of saccadic eye movements may critically support stable visual perception and adaptive motor performance. The subjective impression of visual stability appears to be mediated—at least partially—by information transfer from the superior colliculus via central thalamus to the frontal eye fields (FEF) (Sommer and Wurtz 2002). In humans, lesions of thalamic nuclei involved in this pathway lead to impairments in visual stability across eye movements (Ostendorf et al. 2010, 2013). Motor stability may be achieved by a separate pathway that ascends from the cerebellum and contributes to the mapping of sensory cues to appropriate motor responses (Middleton and Strick 2000; Mushiake and Strick 1993). Indeed, lesions of thalamic nuclei within this cerebello-frontal pathway lead to impairments in oculomotor learning in humans (Gaymard et al. 2001). Such oculomotor learning is required to adapt saccade amplitude when saccades systematically fail to reach intended landing positions (Hopp and Fuchs 2004; Pelisson et al. 2010). This process can be studied in the laboratory by consistently displacing the saccade target while the saccade is in flight (McLaughlin 1967). Recent studies suggest that saccade adaptation is driven by a prediction error, i.e., a comparison between the predicted and the actual postsaccadic retinal error (Bahcall and Kowler 2000; Chen-Harris et al. 2008; Collins and Wallman 2012; Herman et al. 2013; Wong and Shelhamer 2012).
Saccade adaptation is not a unitary phenomenon but involves several mechanisms, depending on how it is elicited. First, saccades can be either shortened (inward adaptation) or lengthened (outward adaptation) depending on the direction of the intrasaccadic target displacement. A number of studies have reported substantial differences in the dynamics and the perceptual consequences of inward vs. outward adaptation in humans (Cecala and Freedman 2008; Ethier et al. 2008; Miller et al. 1981; Schnier et al. 2010; Straube and Deubel 1995; Zimmermann and Lappe 2010) and monkeys (Straube et al. 1997).
Second, saccade adaptation is selective for the type of saccade: Adaptation of saccades performed to suddenly appearing targets (reactive saccades) does not transfer to saccades performed when scanning a set of stationary targets (scanning saccades) (Alahyane et al. 2007; Collins and Doré-Mazars 2006; Cotti et al. 2007; Deubel 1995; Erkelens and Hulleman 1993; Fujita et al. 2002; Gaveau et al. 2005; Hopp and Fuchs 2010). Differences between these saccade types and adaptation directions are also reflected in the dependence of adaptation on eye position (Havermann et al. 2011; Zimmermann 2013; Zimmermann et al. 2011; Zimmermann and Lappe 2011).
Recent studies on motor and perceptual changes induced by saccade adaptation have suggested that visual localization and saccade targeting share a common coordinate system: Targets flashed briefly before saccade execution were mislocalized in the direction of adaptation (Awater et al. 2005; Collins et al. 2007; Schnier et al. 2010; Zimmermann and Lappe 2009), and even mislocalization of the saccade target itself has been observed (Bahcall and Kowler 1999; Collins et al. 2009; Klingenhoefer and Bremmer 2011). These perceptual effects can occur even when the eye does not move and therefore demonstrate plasticity in the perception of visual space (Hernandez et al. 2008; Zimmermann and Lappe 2010). Changes in spatial localization of visual objects after saccade adaptation also depend on the presentation duration of a visual target: Reactive saccade adaptation changes only the perception of briefly flashed objects, whereas scanning saccade adaptation also changes the spatial localization of stationary visual objects (Zimmermann and Lappe 2009).
For reactive saccades, electrophysiological studies in monkeys (Barash et al. 1999; Catz et al. 2008; Desmurget et al. 1998; Kojima et al. 2010; Prsa and Thier 2011; Soetedjo et al. 2008; Soetedjo and Fuchs 2006; Straube et al. 2001; Takagi et al. 1998), lesion studies in humans (Golla et al. 2008; Xu-Wilson et al. 2009), and brain imaging studies (Desmurget et al. 1998; Gerardin et al. 2012) suggest an important role of the cerebellar vermis in oculomotor adaptation. Movement fields of neurons in the superior colliculus, on the other hand, show no changes during adaptation of reactive saccades (Frens and Van Opstal 1997; Quessy et al. 2010), although changes in firing rates have been observed (Takeichi et al. 2007). Similarly, movement fields in lateral intraparietal cortex are unchanged after inward adaptation of reactive saccades (Steenrod et al. 2013).
So far, the question of distinct neural substrates for different types of saccade adaptation has only been addressed in a single patient study on cerebellar patients (Alahyane et al. 2008). This study described a double dissociation, with deficient adaptation of reactive but not scanning saccades in patients with medial cerebellar lesions and impaired scanning but not reactive saccade adaptation in patients with lateral cerebellar lesions. Similarly, a recent fMRI study suggested that scanning saccade adaptation involves dorsal areas of the frontal and parietal cortex whereas reactive saccade adaptation involves more ventral parts of the frontal and parietal cortex (Gerardin et al. 2012). This would fit well with a transthalamic feedback pathway from the cerebellum to the frontal cortex that is involved in reactive saccade adaptation (Gaymard et al. 2001). Possible impairments of scanning saccade adaptation, however, have not been investigated with thalamic lesions so far. Here we tested whether adaptation of reactive and scanning saccades indeed relies on distinct feedback pathways to the cortex. A patient with a highly selective ischemic lesion of the right ventrolateral (VL) nucleus was tested with a set of newly developed reactive and scanning paradigms.
METHODS
Patient
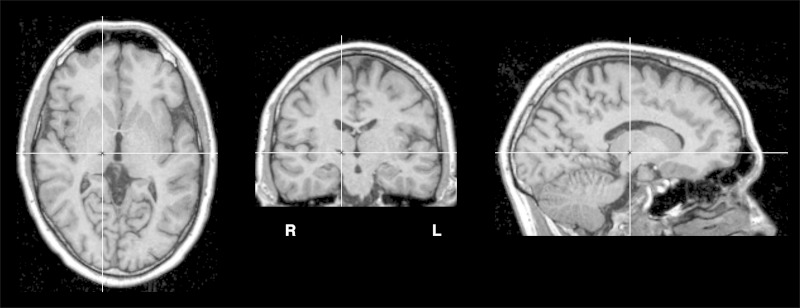
Patient RK is a 31-year-old, right-handed man who sustained a stroke in the right thalamus 18 mo before testing in the present study. On initial neurological examination, left-sided hemiataxia with a slight concomitant hemiparesis was noted. In addition, the patient reported pricking paresthesia of the left arm and left side of the face. Neurological deficits disappeared completely within a few weeks. At testing, neurological examination was normal. MR imaging (1.5-T Magnetom Vision, Siemens, MPRAGE, 1-mm isotropic resolution) revealed a small ischemic lesion in the right lateral thalamus (see Fig. 1). No other thalamic or extrathalamic lesions were detected. Coronal reconstructions from this MRI data set were evaluated against corresponding plates of an established atlas of the human thalamus (Morel 2007). This assessment clearly suggests affection of the ventral lateral posterior nucleus (ventral division, VLpv) and of the ventral medial (VM) nucleus of the right thalamus, according to the anatomical nomenclature proposed by Morel (2007).
Fig. 1.
Structural MRI scan of patient RK (T1 weighted). Horizontal (left), coronal (center), and sagittal (right) reconstructions at the level of maximum lesion volume are shown. Figure follows radiological convention, i.e., right side of brain (R) is shown on left and vice versa.
Control Group
Five subjects (all male; 1 author, 4 naive subjects; mean age 30 yr) participated as control subjects in all experiments. All subjects (except for the author) were students from the Institute of Psychology in Münster, Germany and had normal or corrected-to-normal vision. Subjects gave informed consent. The experiments were performed according to the principles laid down in the Declaration of Helsinki and were approved by the local ethics committee.
Apparatus
The participant was seated 57 cm in front of a 22-in. computer monitor (Eizo FlexScan F930) with the head stabilized by a chin rest. The visible screen diagonal was 20 in., resulting in a visual field of 40° × 30°. Stimuli were presented at a frame rate of 120 Hz and a resolution of 800 × 600 pixels. The room was otherwise completely dark. To avoid visibility of the screen borders, the display monitor was covered with a transparent foil that reduced luminance by ∼2 log units. Eye movements were monitored by an Eyelink 1000 system (SR Research), which samples gaze positions at a frequency of 1,000 Hz. Viewing was binocular, but only the dominant eye was recorded. The system detected start and end of a saccade when eye velocity exceeded or fell below 22°/s and acceleration was above or below 4,000°/s2.
Procedure
Adaptation of reactive and scanning saccades was tested for rightward and leftward saccade directions in separate sessions, requiring a total of four sessions. Each session contained four phases in the following order: preadaptation (40 trials), adaptation (80 trials), test (40 trials), and deadaptation (20 trials).
Reactive Saccade Adaptation
Reactive saccade trials started with the presentation of a fixation point (1 × 1°; luminance 0.06 cd/m2; red color) on the horizontal midline of the screen (Fig. 2A). For testing rightward (leftward) saccades, the fixation point was placed 17.5° left (right) of the screen center. The subject was instructed to first direct gaze to the fixation point. After 1,000 ms of stable fixation, a saccade target (1 × 1°; luminance 0.06 cd/m2; red color) appeared in 66% of all trials 30° away from the fixation point on the other side of the screen. Subjects were instructed to perform a saccade to the target as soon as it appeared. In preadaptation and deadaptation trials the saccade target remained on in its initial position after execution of the saccade or was extinguished during the saccade in the case in which localization judgments had to be performed (see below). In adaptation trials, the saccade target was displaced by 6° during execution of the saccade, either inward toward the position of the fixation point or outward away from the fixation point. The remaining 33% of the trials served to introduce unpredictability of the saccade target location to avoid stereotyping of the saccade. In these trials, the target was presented either 20° above or below the fixation point. These trials were not entered into data analysis. Each trial ended 1,000 ms after saccade onset. Thereafter, a complete white screen was shown for 1,000 ms after each trial to prevent dark adaptation of the subject.
Fig. 2.
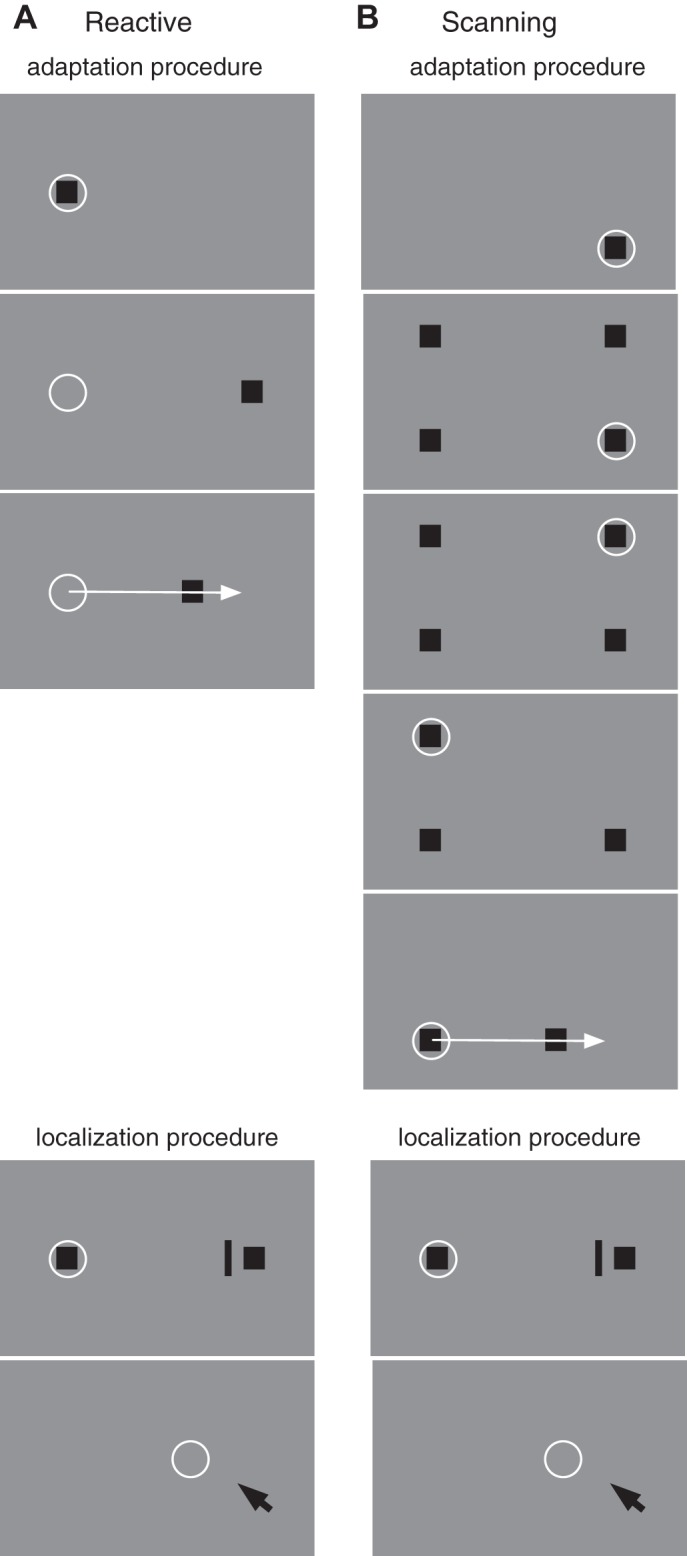
A: experimental procedure for reactive saccade adaptation. At the beginning of the trial (top) a fixation point (square) is presented near the left screen border. The subject's gaze (circle) is directed to the fixation point. After 1,000 ms (middle) the fixation point disappears and a saccade target appears 30° to the right of the fixation point. The subject initiates the saccade to the target. When the saccade onset is detected (bottom) the saccade target is displaced, inducing a visual error after the saccade. In localization trials a probe bar was shown at least 150 ms before saccade execution for 20 ms. After saccade execution, a mouse pointer was shown with which the subject had to localize the position of the probe bar. B: experimental procedure for scanning saccade adaptation. At trial onset (top), 4 squares are presented. The subject fixates the bottom right square (circle). At voluntary pace, the subject scans the squares in a counterclockwise manner. As the subject executes a saccade, the previously inspected squares are extinguished. Adaptation takes place during the saccade from the bottom left to the bottom right square (bottom). When the onset of the saccade is detected, the bottom right square is displaced to the left, inducing a visual error after the saccade. Localization was measured with the same procedure as in reactive saccades.
Scanning Saccade Adaptation
Scanning saccade trials also started with the presentation of a fixation point (Fig. 2B). For testing rightward (leftward) saccades, the fixation point was located in the bottom right (left) corner of the screen [−17.5 to the right (left) of screen center]. After 500 ms, three additional targets appeared in the remaining corners of the screen. Each target contained either one or two small dots that could be discriminated with foveal vision only. The subject was instructed to scan the four targets one by one counterclockwise (in rightward trials) or clockwise (in leftward trials) in a self-paced manner and count the number of targets that contained two dots. This secondary task was included to ensure that subjects truly fixated each target. During the execution of the scanning saccades, previously fixated saccade targets were turned off as soon as the eye tracker detected that the eye moved >2.5° away from the target. The fixation point turned into a fourth scanning target as soon as the subject started the first saccade away from the fixation point. Adaptation was induced for the last saccade of the sequence (Fig. 2B), i.e., the one that brought the eye back to the initial fixation location. The final target was displaced by 6° inward during execution of the saccade. As in reactive saccade sessions, the initial amplitude of the adapted saccade was thus 30°.
The subject ended the trial by pressing the computer mouse when the complete scan path was finished and the last saccade target was fixated. Then a white response screen appeared on which the subject had to indicate the observed number of dot pairs contained in the saccade targets by a mouse click. After the subject gave the response, the next trial started automatically.
Test Trials
Test trials were run before and after adaptation to measure adaptation strength in conditions without postsaccadic target feedback and potential effects on localization of briefly flashed visual probes in conditions without postsaccadic target feedback. Previously we have shown that saccade adaptation produces changes in the perception visual space (Zimmermann and Lappe 2010). The subject had to perform a horizontal saccade toward the visual target as in all other trials. In addition, a bar (0.3 × 4°; luminance 0.2 cd/m2) was presented for 20 ms at a randomly chosen horizontal position in a range of 2° around the saccade target (i.e., between 28° and 32°). After the subject had performed the saccade a mouse cursor appeared, which the subject used to indicate the perceived position of the bar. The cursor appeared 1,000 ms after the saccade near the bottom of the screen at a randomly assigned horizontal position between 35° and 40°. The saccade target was turned off during execution of the saccade as soon as the eye tracker detected that the eye had moved >2.5° in direction of the saccade target.
In reactive saccade trials, the bar was flashed 50 ms after the appearance of the saccade target [i.e., ∼150 ms (depending on saccade latency) before the reactive saccade]. In scanning saccade trials, the bar was flashed when the eye tracker detected that the eye position was on the bottom left saccade target (i.e., before the saccade that was adapted). In both cases, trials in which the bar was flashed <100 ms before saccade onset were omitted from analysis to exclude a potentially confounding interference from perisaccadic mislocalization (Ross et al. 2001). Subjects were instructed to click in the bottom right corner of the monitor screen in the case in which they had not seen the target. These trials were omitted from further analysis.
Before and after adaptation 20 such test trials were performed, alternating with 20 regular preadaptation or adaptation trials.
RESULTS
Reactive Saccade Adaptation
Adaptation curves.
Figure 3 shows the saccadic gain change over the course of the session for patient RK and the control group. Patient RK and each control subject ran each of the four conditions (inward and outward adaptation for leftward and rightward saccade direction) twice. Session data were pooled within subjects. To check whether the patient's variability across the two sessions differed from the control group's variability, we calculated the absolute difference in saccade gain for the preadaptation and the test trials. For inward adaptation sessions the variability of the patient did not differ more than 2 standard deviations from the control group, either for leftward saccades [pretrials: RK 1.03%, control group 0.22% (SD: 0.76%); test trials: RK 0.29%, control group 0.59% (SD: 0.56%)] or for rightward saccades [pretrials: RK 1.46%, control group 0.24% (SD: 0.65%); test trials: RK 0.53%, control group 0.69 (SD: 1.41%)]. Similarly, for outward adaptation the patient was within 2 standard deviations of the control group's variability for leftward saccades [pretrials: RK 1.05%, control group 0.44 (SD: 0.53); test trials: RK 0.71%, control group 1.19% (SD: 2.64%)] and for rightward saccades [pretrials: RK 0.71%, control group 0.03% (SD: 0.79%); test trials: RK 0.01%, control group 2.48% (SD: 1.47%)].
Fig. 3.
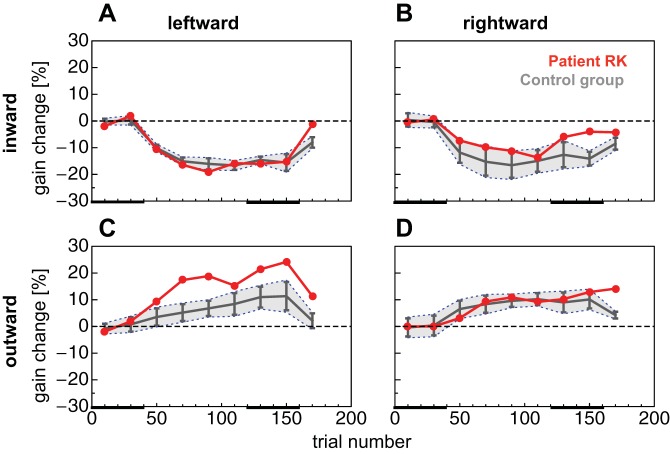
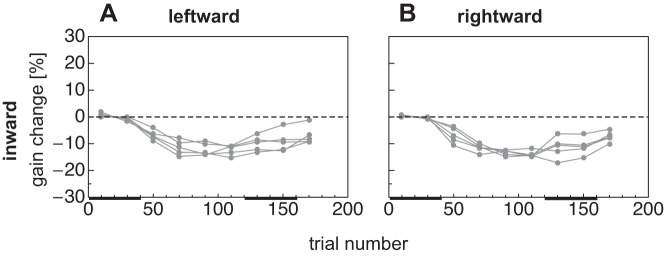
A: adaptation curve for leftward reactive saccade inward adaptation sessions. Saccade landing positions from the control group are shown in gray, and those from patient RK are shown in red. Data are binned with a bin width of 20 trials. Error bars represent 95% confidence intervals and gray shaded areas SDs. Black bars at bottom represent test trial epochs in preadapted and adapted states, respectively. B–D: adaptation curves for rightward reactive saccade inward adaptation (B), contraversive (leftward) reactive saccade outward adaptation (C), and ipsiversive (rightward) reactive saccade outward adaptation (D). Same conventions as in A.
Saccade gain change was calculated by dividing all saccade landing positions by the mean landing position from the preadaptation phase (trials 1–40). The saccade landing data are binned into bins with a width of 20 trials. Each point in Fig. 3 indicates the mean gain change of a bin containing 20 trials. Error bars represent the 95% confidence intervals across subjects in the control group (cf. Fig. 4 for individual adaptation curves of control subjects).
Fig. 4.
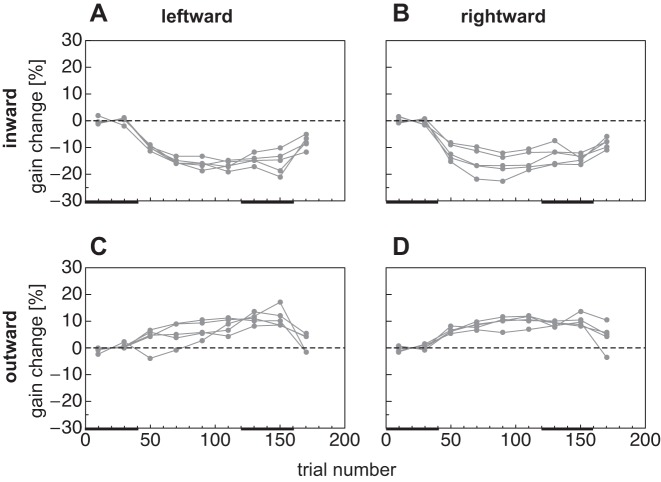
Adaptation curve for leftward (A and C) and rightward (B and D) reactive saccade inward (A and B) and outward (C and D) adaptation sessions. Saccade landing positions are shown for each participant of the control group. Data are binned with a bin width of 20 trials. Black bars at bottom represent test trial epochs in preadapted and adapted states, respectively.
Figure 3A shows the adaptation curve from patient RK and the control group for inward adaptation of leftward saccades. The preadaptation phase (first 40 trials) contained trials in which the saccade target remained in its initial position and test trials in which the target was extinguished during the saccade. From trial 40 to trial 120, the saccade target was displaced during the saccade. Between trial 120 and trial 160, adaptation trials in which the target was displaced during the saccade were intermixed with test trials in which the target was simply extinguished during the saccade. In these inward adaptation sessions, gain decrease of patient RK reached a maximum gain change of −19.05% after 60 adaptation trials. The control group average reached a maximum adaptation level on average after 80 adaptation trials (maximum gain change, −16.66%). From trial 160 on, the experiment finished with 20 deadaptation trials with the saccade target remaining in its initial position. Leftward saccades showed strong deadaptation back to their preadaptation amplitude. For leftward saccades, adaptation curves of the patient and the control group were almost identical. Figure 3B shows average adaptation curves for inward adaptation of rightward saccades. Although the patient reaches an adaptation level almost identical to the control group (see bin at 120 trials), he shows a rapid deadaptation in test trials. In half of these trials, the saccade target disappeared during execution of the saccade to measure the localization performance. While in the control group the adaptation state remained unchanged, patient RK was apparently unable to keep the adaptation level. Patient RK's maximum gain change was −14.22% after 80 adaptation trials. The control group, however, adapted −16.57% after 60 adaptation trials.
Remarkably, in outward adaptation sessions for leftward saccades, the patient adapted much stronger than the control group (shown in Fig. 3C). He reached a maximum gain change of 24.08% after 120 adaptation trials. The control group, however, had a maximum gain change of 11.34% after 120 adaptation trials. In rightward outward adaptation patient and control group adapted similarly (shown in Fig. 3D). The patient had a maximum gain change of 14.34% after 140 adaptation trials, and the control group had a maximum gain change of 10.21% after 80 adaptation trials.
In summary, we can state that with respect to the site of his (rightward) thalamic lesion, patient RK 1) showed stronger adaptation in the contraversive compared with the ipsiversive direction, 2) showed weaker inward adaptation than control subjects for the ipsiversive direction and stronger outward adaptation than control subjects for the contraversive direction, and 3) showed altered deadaptation in the ipsiversive direction.
Average adaptation.
To compare adaptation between the patient and the control group statistically, we estimate average gain from the test trials before and after adaptation. Table 1 summarizes average saccade gain from before and after reactive saccade adaptation together with the respective gain change values and an asymmetry index for patient and control group. Gain changes in the patient that were two standard deviations outside of the control group's mean were considered abnormal (see Table 1). For patient RK, an inability of adaptation retention for rightward (ipsiversive) saccades was observed. Outward gain change, however, was abnormally increased for leftward (contraversive) saccades. Moreover, gain change for RK was weaker for rightward (ipsiversive) saccades than for leftward (contraversive) saccades.
Table 1.
Reactive saccade adaptation gain values for control group and patient RK
| Initial Gain |
Final Gain |
Gain Change |
|||||
|---|---|---|---|---|---|---|---|
| L | R | L | R | L | R | Asymmetry Index | |
| Reactive inward adaptation gain | |||||||
| Patient RK | 0.91 | 0.93 | 0.77 | 0.87 | −15.59 | −5.11* | −50.62* |
| Control group | 0.96 ± 0.008 | 0.92 ± 0.02 | 0.81 ± 0.02 | 0.8 ± 0.03 | −14.93 ± 2.56 | −13.37 ± 2 | −5.3 ± 11.9 |
| Reactive outward adaptation gain | |||||||
| Patient RK | 0.91 | 0.9 | 1.11 | 1.04 | 21.79* | 11.61* | −30.479 |
| Control group | 0.95 ± 0.02 | 0.91 ± 0.03 | 1.03 ± 0.07 | 0.99 ± 0.03 | −8.87 ± 5.23 | 8.17 ± 2.63 | −1.3 ± 30.3 |
Values are means ± SD.
Gain change values and asymmetry indexes of patient RK that are >2 SD intervals outside the control group's mean.
Mislocalization.
We also looked at potential changes in visual localization between the pre- and postadaptation phases. However, it turned out that the data could not be analyzed because many of RK's location reports touched the border of the screen. The reason for this was a strong and unexpected overestimation of positional eccentricities in RK's visual localization that existed not only during saccades but also during fixation (gathered in a separate experiment). He overestimated the eccentricities of flashed bars by >15% for leftward saccades and ∼5% for rightward saccades. This is quite remarkable, since normal subjects typically underestimate eccentricities of flashed probes by ∼10% (Awater et al. 2005; Müsseler et al. 1999). It is also worth noting that, despite this overestimation of flashed bars, his preadaptation amplitudes for reactive saccades showed the typical hypometry observed in normal subjects (see Table 1).
Scanning Saccade Adaptation
Adaptation curves.
As for reactive saccade adaptation, both patient and control group performed each scanning saccade adaptation session twice. We first checked again whether the variability across the two sessions was comparable between patient and control group. The variability of the patient did not differ more than 2 standard deviations from the control group, either for leftward saccades [pretrials: RK 0.72%, control group 0.14% (SD: 0.76%); test trials: RK 1.53%, control group 0.09% (SD: 1.53%)] or for rightward saccades [pretrials: RK 1.42%, control group 0.28% (SD: 0.69%); test trials: RK 2.86%, control group 0.57 (SD 1.17%)].
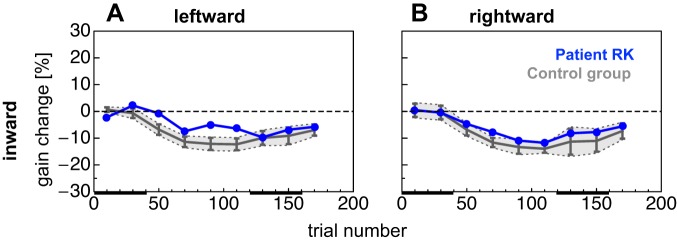
Changes in saccade gain in scanning saccade adaptation sessions for RK and control subjects are shown in Fig. 5 (cf. Fig. 6 for individual adaptation curves of control subjects). Because of time constraints of the patient and a higher interindividual variability for outward adaptation observed in previous studies, we decided to only probe inward adaptation in the patient and the control group. The time course of adaptation in contraversive (leftward) saccades differed between patient and control group. The control group adapted quicker to the target displacement and reached the maximum gain change of −13.96 after 80 adaptation trials. The patient, however, reached his maximum gain change of −9.87% after 100 adaptation trials. Figure 5B shows average changes in saccade gain in adaptation of ipsiversive (rightward) saccades. In these trials, the patient adapted comparably too but slightly less than the control group. He reached the maximum gain change of −11.75% after 80 adaptation trials, similarly to the control group with a maximum gain change of −12.30% after 80 adaptation trials. Leftward and rightward saccades deadapted only slightly in the deadaptation trials.
Fig. 5.
Adaptation curve for leftward (A) and rightward (B) scanning saccade inward adaptation sessions. Saccade landing positions from the control group are shown in gray, and those from patient RK are shown in blue. Data are binned with a bin width of 20 trials. Error bars represent 95% confidence intervals and gray shaded areas SDs. Black bars at bottom represent test trial epochs in preadapted and adapted states, respectively.
Fig. 6.
Adaptation curve for leftward (A) and rightward (B) scanning saccade inward adaptation sessions. Saccade landing positions are shown for each participant of the control group. Data are binned with a bin width of 20 trials. Black bars at bottom represent test trial epochs in preadapted and adapted states, respectively.
Average adaptation results.
Saccade gains from the preadaptation and the test phases together with the respective gain change values and the asymmetry index for the patient and the control group are shown in Table 2. For none of these values and both saccade directions was patient RK outside two standard deviations of the control group's mean. Moreover, his gain change between preadaptation and the postadaptation test phase was of similar strength for leftward (−6.96%) and rightward (−6.95%) saccades. Also, the control subjects showed almost no difference in gain increase for leftward (−9.49%) and rightward (−11.04%) saccades. Saccade gains of patient RK in scanning adaptation were perfectly within the distribution of the control group. The lesion in the cerebello-thalamic tract thus left the adaptation of scanning saccades unaffected.
Table 2.
Scanning saccade inward adaptation gain values for control group and patient RK
| Initial Gain |
Final Gain |
Gain Change |
|||||
|---|---|---|---|---|---|---|---|
| L | R | L | R | L | R | Asymmetry Index | |
| Patient RK | 0.92 ± 0.08 | 0.93 ± 0.05 | 0.84 ± 0.1 | 0.86 ± 0.07 | −8.82 | −7.75 | −6.46 |
| Control group | 0.95 ± 0.01 | 0.95 ± 0.02 | 0.86 ± 0.03 | 0.85 ± 0.04 | −9.49 ± 2.81 | −11.05 ± 3.00 | −7.66 ± 20.55 |
Values are means ± SD. No gain change values and asymmetry indexes of patient RK were >2 SD outside the control group's mean.
DISCUSSION
We tested a patient with a focal lesion in the thalamic VL nucleus in different saccade adaptation paradigms. For inward adaptation of reactive, but not scanning, saccades the patient showed a deficit in adaptation retention for saccades into the ipsilesional hemifield compared with saccades into the contralesional hemifield. Our results provide the first lesion evidence in human patients that the functional separation between reactive and scanning saccade adaptation is paralleled by structurally distinct pathways from the cerebellum through the thalamus to the cerebral cortex. The data further suggest that inward adaptation in patients with lesions to the VL nucleus may depend on an exposure of the visual motor error after every trial, thus suggesting that for retention of adaptation across saccades without visual feedback, access to cerebral cortex via thalamic pathways is mandatory.
The results reported here confirm and elaborate on a previous study, where thalamic lesions within the cerebello-frontal pathway led to reduced reactive saccade adaptation for ipsiversive saccade directions (Gaymard et al. 2001). Patient RK showed no impairment in scanning saccade adaptation. Apparently, the lesion in the VL nucleus selectively disrupted oculomotor plasticity for reactive saccades. The cerebello-thalamic tract carries oculomotor signals and sends them to cortical areas (Gallay et al. 2008). It has also been suggested that a pathway from the superior colliculus to the FEF passes partly through the VL nucleus in humans (Bellebaum et al. 2005; Tehovnik et al. 2000). In line with Gaymard et al. (2001), our data suggest that the VL nucleus is an essential relay station for motor learning. This result highlights the involvement of higher cortical areas in human saccade adaptation. Scanning saccade adaptation seems to rely on a mechanism independent of the VL nucleus of the cerebello-thalamic tract. Previous studies have already gathered evidence for a structural separation of scanning and reactive saccade adaptation (Pelisson et al. 2010): Patients with lesions in the medial cerebellum had impairments in reactive but not voluntary saccade adaptation, whereas patients with lateral lesions showed reduced adaptation in voluntary but not reactive saccade adaptation (Alahyane et al. 2008). Different cortical activation patterns for reactive and scanning saccade adaptation were found in a recent fMRI study (Gerardin et al. 2012): Reactive saccade adaptation was associated with activation in the cerebellum, middle temporal, temporo-parietal, and frontal areas. Scanning saccades involved the same cerebellar and frontal areas but in addition also parietal areas. Finally, single-pulse TMS over the parietal cortex selectively disturbed scanning saccade adaptation (Panouillères et al. 2014). Our data are consistent with these findings in that a lesion in a lateral portion of cerebellar-recipient thalamus likely projecting to frontal cortex selectively impaired reactive saccade adaptation. Separate transthalamic pathways that were spared in our patient may carry information about scanning saccade adaptation to the FEF: Central cerebellar-recipient thalamic portions have been shown to target both parietal cortex and the FEF and may qualify as candidate regions (for a recent review, see Prevosto and Sommer 2013). A structural separation of both adaptation modes is also consistent with the idea that reactive and scanning saccades are coded in different reference frames (Zimmermann 2013; Zimmermann et al. 2011).
For outward adaptation of reactive saccades, the gain change for saccades into the contralesional hemifield of patient RK was even stronger than that of the control group. Similar asymmetric adaptation results have been described by Gaymard et al. (2001). Thus the lesion in the thalamus seems to increase saccade adaptation in this case. We speculate that this seemingly paradoxical finding could hint at the type of signal that is transmitted from the cerebellum via thalamus to frontal cortex. Recent studies have suggested that saccade adaptation is driven by a mismatch between the predicted and actually observed saccade landing position (Bahcall and Kowler 2000; Chen-Harris et al. 2008; Collins and Wallman 2012; Herman et al. 2013; Wong and Shelhamer 2012). Such a prediction may for instance indicate that a saccade will slightly undershoot its visual target. For rightward saccades this means that the saccade is predicted to land to the left of the target. This prediction is then compared with the actual reafferent visual error, i.e., the distance between the saccade landing site and target location. A systematic error would indicate a need to adapt saccade amplitude to compensate for this consistent mismatch. We speculate that the adaptation pattern we observe in patient RK may be explained by impaired transmission of such a prediction error. Estimates of saccade landing accuracy should then increasingly be based on the retinal postsaccadic error only. If the retinal error instead of the prediction error is used as a learning signal, the relative strength of inward and outward adaptation should change. Specifically, if the lesion in the right thalamus disrupts the prediction error transmission only for reafferent targets in the left visual hemifield (i.e., contralateral to the lesion), adaptation changes are expected in exactly the conditions that were considered abnormal in our patient: For both inward adaptation in rightward saccades and outward adaptation in leftward saccades, the target will be in the left visual hemifield after saccade execution (see Fig. 7). For rightward saccades (Fig. 7A), the predicted landing for a typical (Kapoula 1985), slightly hypometric saccade is to the left of the initial target T1. Because of the intrasaccadic target displacement, however, the retinal error indicates that the eye landed to the right of the final target T2. The prediction error comprises the distance of the target to its predicted location, i.e., the distance between T1 and T2. Hence, the retinal error will be smaller than the prediction error. Therefore, if processing of the prediction error is impaired, inward adaptation will be smaller. The opposite is the case for outward adaptation and leftward saccades (Fig. 7B): When a leftward saccade undershoots the target, the retinal error is larger than the prediction error because the intrasaccadic target step shifts the target T2 even further away from the landing side. Thus if the prediction error cannot properly be processed and the system therefore relies on the pure retinal error only, outward adaptation will be larger. The hypothesis of a deficient prediction error transmission would be consistent with the adaptation data of patient RK: For ipsiversive saccades inward adaptation was weaker and for contraversive saccades outward adaptation was stronger than in control subjects. However, such a possible explanation would critically rest on two requirements: a systematic saccade undershoot that leads to different error magnitudes for retinal vs. prediction errors and a separate transmission of prediction errors for the two hemifields, at least on the thalamic level.
Fig. 7.
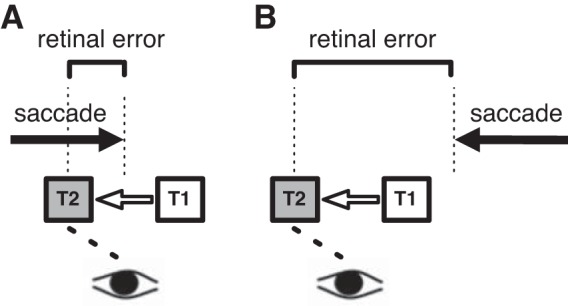
Graphical illustration of the proposed mechanisms that drive adaptation in patient RK. In the model it is assumed that RK's oculomotor system is impaired in processing mismatches between predicted and actual saccade landing errors for postsaccadic targets appearing in the left visual hemifield. A: a rightward saccade will typically undershoot the initial target at position T1. When the target is displaced inward to position T2, it will be seen in the left hemifield after the saccade has been executed. If the lesion in the VL nucleus disturbs error prediction, only the retinal error between postsaccade landing and the physical position T2 can be used for adaptation. Since the undershoot already brought the saccade closer to position T2, the retinal error is smaller than the mismatch derived from error prediction. Adaptation magnitude therefore should be decreased compared with healthy subjects. B: following the same logic, for a leftward saccade to a target T1, which will then be displaced in the outward direction, the typical saccade undershoot will lead to an increase in adaptation magnitude if the oculomotor system cannot rely on the error prediction mechanism.
In conclusion, reactive saccade adaptation depends on cerebellar signals sent through the thalamus to frontal cortex. Disruptions of this tract impair motor learning, possibly reflecting a deficient transmission of a prediction error signal through this pathway. Scanning saccade adaptation is not affected by a lesion in the VL nucleus of the cerebello-thalamic tract and therefore seems to rely on separate neural mechanisms.
GRANTS
This work was supported by BMBF Visuo-Spatial Cognition 01GW0651-4, DFG La 952/3-2, and DFG La 952/6.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: E.Z., F.O., C.J.P., and M.L. conception and design of research; E.Z. and M.L. performed experiments; E.Z. and M.L. analyzed data; E.Z., F.O., C.J.P., and M.L. interpreted results of experiments; E.Z. and F.O. prepared figures; E.Z. drafted manuscript; E.Z., F.O., C.J.P., and M.L. edited and revised manuscript; E.Z., F.O., C.J.P., and M.L. approved final version of manuscript.
REFERENCES
- Alahyane N, Fonteille V, Urquizar C, Salemme R, Nighoghossian N, Pelisson D, Tilikete C. Separate neural substrates in the human cerebellum for sensory-motor adaptation of reactive and of scanning voluntary saccades. Cerebellum 7: 595–601, 2008. [DOI] [PubMed] [Google Scholar]
- Alahyane N, Salemme R, Urquizar C, Cotti J, Guillaume A, Vercher JL, Pélisson D. Oculomotor plasticity: are mechanisms of adaptation for reactive and voluntary saccades separate? Brain Res 1135: 107–121, 2007. [DOI] [PubMed] [Google Scholar]
- Awater H, Burr D, Lappe M, Morrone MC, Goldberg ME. Effect of saccadic adaptation on localization of visual targets. J Neurophysiol 93: 3605–3614, 2005. [DOI] [PubMed] [Google Scholar]
- Bahcall DO, Kowler E. Illusory shifts in visual direction accompany adaptation of saccadic eye movements. Nature 400: 864–866, 1999. [DOI] [PubMed] [Google Scholar]
- Bahcall DO, Kowler E. The control of saccadic adaptation: implications for the scanning of natural visual scenes. Vision Res 40: 2779–2796, 2000. [DOI] [PubMed] [Google Scholar]
- Barash S, Melikyan A, Sivakov A, Zhang M, Glickstein M, Thier P. Saccadic dysmetria and adaptation after lesions of the cerebellar cortex. J Neurosci 19: 10931–10939, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellebaum C, Daum I, Koch B, Schwarz M, Hoffmann KP. The role of the human thalamus in processing corollary discharge. Brain 128: 1139–1154, 2005. [DOI] [PubMed] [Google Scholar]
- Bruno A, Morrone MC. Influence of saccadic adaptation on spatial localization: comparison of verbal and pointing reports. J Vis 7: 16.1–16.13, 2007. [DOI] [PubMed] [Google Scholar]
- Catz N, Dicke PW, Thier P. Cerebellar-dependent motor learning is based on pruning a Purkinje cell population response. Proc Natl Acad Sci USA 105: 7309–7314, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecala AL, Freedman EG. Amplitude changes in response to target displacements during human eye-head movements. Vision Res 48: 149–166, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Harris H, Joiner WM, Ethier V, Zee DS, Shadmehr R. Adaptive control of saccades via internal feedback. J Neurosci 28: 2804–2813, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins T, Doré-Mazars K. Eye movement signals influence perception: evidence from the adaptation of reactive and volitional saccades. Vision Res 46: 3659–3673, 2006. [DOI] [PubMed] [Google Scholar]
- Collins T, Doré-Mazars K, Lappe M. Motor space structures perceptual space: evidence from human saccadic adaptation. Brain Res 1172: 32–39, 2007. [DOI] [PubMed] [Google Scholar]
- Collins T, Rolfs M, Deubel H, Cavanagh P. Post-saccadic location judgments reveal remapping of saccade targets to non-foveal locations. J Vis 9: 29.1–29.9, 2009. [DOI] [PubMed] [Google Scholar]
- Collins T, Wallman J. The relative importance of retinal error and prediction in saccadic adaptation. J Neurophysiol 107: 3342–3348, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotti J, Guillaume A, Alahyane N, Pelisson D, Vercher JL. Adaptation of voluntary saccades, but not of reactive saccades, transfers to hand pointing movements. J Neurophysiol 98: 602–612, 2007. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Pélisson D, Urquizar C, Prablanc C, Alexander GE, Grafton ST. Functional anatomy of saccadic adaptation in humans. Nat Neurosci 1: 524–528, 1998. [DOI] [PubMed] [Google Scholar]
- Deubel H. Separate adaptive mechanisms for the control of reactive and volitional saccadic eye movements. Vision Res 35: 3529–3540, 1995. [DOI] [PubMed] [Google Scholar]
- Erkelens CJ, Hulleman J. Selective adaptation of internally triggered saccades made to visual targets. Exp Brain Res 93: 157–164, 1993. [DOI] [PubMed] [Google Scholar]
- Ethier V, Zee DS, Shadmehr R. Changes in control of saccades during gain adaptation. J Neurosci 28: 13929–13937, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frens MA, Van Opstal AJ. Monkey superior colliculus activity during short-term saccadic adaptation. Brain Res Bull 43: 473–483, 1997. [DOI] [PubMed] [Google Scholar]
- Fujita M, Amagai A, Minakawa F, Aoki M. Selective and delay adaptation of human saccades. Brain Res Cogn Brain Res 13: 41–52, 2002. [DOI] [PubMed] [Google Scholar]
- Gallay MN, Jeanmonod D, Liu J, Morel A. Human pallidothalamic and cerebellothalamic tracts: anatomical basis for functional stereotactic neurosurgery. Brain Struct Funct 212: 443–463, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaveau V, Alahyane N, Salemme R, Desmurget M. Self-generated saccades do not modify the gain of adapted reactive saccades. Exp Brain Res 162: 526–531, 2005. [DOI] [PubMed] [Google Scholar]
- Gaymard B, Rivaud-Péchoux S, Yelnik J, Pidoux B, Ploner CJ. Involvement of the cerebellar thalamus in human saccade adaptation. Eur J Neurosci 14: 554–560, 2001. [DOI] [PubMed] [Google Scholar]
- Georg K, Lappe M. Effects of saccadic adaptation on visual localization before and during saccades. Exp Brain Res 192: 9–23, 2009. [DOI] [PubMed] [Google Scholar]
- Gerardin P, Miquée A, Urquizar C, Pélisson D. Functional activation of the cerebral cortex related to sensorimotor adaptation of reactive and voluntary saccades. Neuroimage 61: 1100–1112, 2012. [DOI] [PubMed] [Google Scholar]
- Golla H, Tziridis K, Haarmeier T, Catz N, Barash S, Thier P. Reduced saccadic resilience and impaired saccadic adaptation due to cerebellar disease. Eur J Neurosci 27: 132–144, 2008. [DOI] [PubMed] [Google Scholar]
- Havermann K, Zimmermann E, Lappe M. Eye position effects in saccadic adaptation. J Neurophysiol 106: 2536–2545, 2011. [DOI] [PubMed] [Google Scholar]
- Herman JP, Blangero A, Madelain L, Khan A, Harwood MR. Saccade adaptation as a model of flexible and general motor learning. Exp Eye Res 114: 6–15, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez TD, Levitan CA, Banks MS, Schor CM. How does saccade adaptation affect visual perception? J Vis 8: 3.1–3.16, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp JJ, Fuchs AF. The characteristics and neuronal substrate of saccadic eye movement plasticity. Prog Neurobiol 72: 27–53, 2004. [DOI] [PubMed] [Google Scholar]
- Hopp JJ, Fuchs AF. Identifying sites of saccade amplitude plasticity in humans: transfer of adaptation between different types of saccade. Exp Brain Res 202: 129–145, 2010. [DOI] [PubMed] [Google Scholar]
- Kapoula Z. Evidence for a range effect in the saccadic system. Vision Res 25: 1155–1157, 1985. [DOI] [PubMed] [Google Scholar]
- Klingenhoefer S, Bremmer F. Saccadic suppression of displacement in face of saccade adaptation. Vision Res 51: 881–889, 2011. [DOI] [PubMed] [Google Scholar]
- Kojima Y, Soetedjo R, Fuchs AF. Changes in simple spike activity of some Purkinje cells in the oculomotor vermis during saccade adaptation are appropriate to participate in motor learning. J Neurosci 30: 3715–3727, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin SC. Parametric adjustment in saccadic eye movements. Percept Psychophys 2: 359–362, 1967. [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev 31: 236–250, 2000. [DOI] [PubMed] [Google Scholar]
- Miller JM, Anstis T, Templeton WB. Saccadic plasticity: parametric adaptive control by retinal feedback. J Exp Psychol Hum Percept Perform 7: 356–366, 1981. [DOI] [PubMed] [Google Scholar]
- Morel A. Stereotactic Atlas of the Human Thalamus and Basal Ganglia. New York: Informa Healthcare, 2007. [Google Scholar]
- Mushiake H, Strick PL. Preferential activity of dentate neurons during limb movements guided by vision. J Neurophysiol 70: 2660–2664, 1993. [DOI] [PubMed] [Google Scholar]
- Müsseler J, Hejden AH, Mahmud SH, Deubel H, Ertsey S. Relative mislocalization of briefly presented stimuli in the retinal periphery. Percept Psychophys 61: 1646–1661, 1999. [DOI] [PubMed] [Google Scholar]
- Ostendorf F, Liebermann D, Ploner CJ. Human thalamus contributes to perceptual stability across eye movements. Proc Natl Acad Sci USA 107: 1229–1234, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostendorf F, Liebermann D, Ploner CJ. A role of the human thalamus in predicting the perceptual consequences of eye movements. Front Syst Neurosci 7: 10, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panouillères M, Habchi O, Gerardin P, Salemme R, Urquizar C, Farne A, Pélisson D. A role for the parietal cortex in sensorimotor adaptation of saccades. Cereb Cortex 24: 304–314, 2014. [DOI] [PubMed] [Google Scholar]
- Pelisson D, Alahyane N, Panouillères M, Tilikete C. Sensorimotor adaptation of saccadic eye movements. Neurosci Biobehav Rev 34: 1103–1120, 2010. [DOI] [PubMed] [Google Scholar]
- Prevosto V, Sommer MA. Cognitive control of movement via the cerebellar-recipient thalamus. Front Syst Neurosci 7: 56, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prsa M, Thier P. The role of the cerebellum in saccadic adaptation as a window into neural mechanisms of motor learning. Eur J Neurosci 33: 2114–2128, 2011. [DOI] [PubMed] [Google Scholar]
- Quessy S, Quinet J, Freedman E. The locus of motor activity in the superior colliculus of the rhesus monkey is unaltered during saccadic adaptation. J Neurosci 30: 14235–14244, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J, Morrone MC, Goldberg ME, Burr DC. Changes in visual perception at the time of saccades. Trends Neurosci 24: 113–121, 2001. [DOI] [PubMed] [Google Scholar]
- Schnier F, Zimmermann E, Lappe M. Adaptation and mislocalization fields for saccadic outward adaptation in humans. J Eye Mov Res 3: 1–18, 2010. [Google Scholar]
- Soetedjo R, Fuchs AF. Complex spike activity of Purkinje cells in the oculomotor vermis during behavioral adaptation of monkey saccades. J Neurosci 26: 7741–7755, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soetedjo R, Kojima Y, Fuchs AF. Complex spike activity in the oculomotor vermis of the cerebellum: a vectorial error signal for saccade motor learning? J Neurophysiol 100: 1949–1966, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer MA. The role of the thalamus in motor control. Curr Opin Neurobiol 13: 663–670, 2003. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. A pathway in primate brain for internal monitoring of movements. Science 296: 1480–1482, 2002. [DOI] [PubMed] [Google Scholar]
- Steenrod SC, Phillips MH, Goldberg ME. The lateral intraparietal area codes the location of saccade targets and not the dimension of the saccades that will be made to acquire them. J Neurophysiol 109: 2596–2605, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube A, Deubel H. Rapid gain adaptation affects the dynamics of saccadic eye movements in humans. Vision Res 35: 3451–3458, 1995. [DOI] [PubMed] [Google Scholar]
- Straube A, Deubel H, Ditterich J, Eggert T. Cerebellar lesions impair rapid saccade amplitude adaptation. Neurology 57: 2105–2108, 2001. [DOI] [PubMed] [Google Scholar]
- Straube A, Fuchs AF, Usher S, Robinson FR. Characteristics of saccadic gain adaptation in rhesus macaques. J Neurophysiol 77: 874–895, 1997. [DOI] [PubMed] [Google Scholar]
- Takagi M, Zee DS, Tamargo RJ. Effects of lesions of the oculomotor vermis on eye movements in primate: saccades. J Neurophysiol 80: 1911–1931, 1998. [DOI] [PubMed] [Google Scholar]
- Takeichi N, Kaneko CR, Fuchs AF. Activity changes in monkey superior colliculus during saccade adaptation. J Neurophysiol 97: 4096–4107, 2007. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Sommer MA, Chou IH, Slocum WM, Schiller PH. Eye fields in the frontal lobes of primates. Brain Res Brain Res Rev 32: 413–448, 2000. [DOI] [PubMed] [Google Scholar]
- Wong AL, Shelhamer M. Using prediction errors to drive saccade adaptation: the implicit double-step task. Exp Brain Res 222: 55–64, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu-Wilson M, Chen-Harris H, Zee DS, Shadmehr R. Cerebellar contributions to adaptive control of saccades in humans. J Neurosci 29: 12930–12939, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann E. The reference frames in saccade adaptation. J Neurophysiol 109: 1815–1823, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann E, Burr D, Morrone MC. Spatiotopic visual maps revealed by saccadic adaptation in humans. Curr Biol 21: 1380–1384, 2011. [DOI] [PubMed] [Google Scholar]
- Zimmermann E, Lappe M. Mislocalization of flashed and stationary visual stimuli after adaptation of reactive and scanning saccades. J Neurosci 29: 11055–11064, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann E, Lappe M. Motor signals in visual localization. J Vis 6: 11, 2010. [DOI] [PubMed] [Google Scholar]
- Zimmermann E, Lappe M. Eye position effects in oculomotor plasticity and visual localization. J Neurosci 31: 7341–7348, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]