Abstract
Dopamine is now well established as a modulator of locomotor rhythms in a variety of developing and adult vertebrates. However, in mice, while all five dopamine receptor subtypes are present in the spinal cord, it is unclear which receptor subtypes modulate the rhythm. Dopamine receptors can be grouped into two families—the D1/5 receptor group and the D2/3/4 group, which have excitatory and inhibitory effects, respectively. Our data suggest that dopamine exerts contrasting dose-dependent modulatory effects via the two receptor families. Our data show that administration of dopamine at concentrations >35 μM slowed and increased the regularity of a locomotor rhythm evoked by bath application of 5-hydroxytryptamine (5-HT) and N-methyl-d(l)-aspartic acid (NMA). This effect was independent of the baseline frequency of the rhythm that was manipulated by altering the NMA concentration. We next examined the contribution of the D1- and D2-like receptor families on the rhythm. Our data suggest that the D1-like receptor contributes to enhancement of the stability of the rhythm. Overall, the D2-like family had a pronounced slowing effect on the rhythm; however, quinpirole, the D2-like agonist, also enhanced rhythm stability. These data indicate a receptor-dependent delegation of the modulatory effects of dopamine on the spinal locomotor pattern generator.
Keywords: dopamine, locomotion, monoamine, spinal cord
neural circuits that produce basic rhythmic motor patterns of locomotion, in vertebrates, reside primarily in the spinal cord and are subject to neuromodulation from a wide range of sources both intrinsic and extrinsic to the spinal cord (Dunbar et al. 2010; Gordon and Whelan 2008; Jankowska et al. 1967a, 1967b; Kiehn et al. 1999; Kiehn and Kjaerulff 1996; Madriaga et al. 2004; Schmidt and Jordan 2000; Sharples et al. 2014). Monoamines are an important group of neuromodulators that are released onto spinal cord circuits and are critical for the expression of locomotion (Sharples et al. 2014). Additionally, monoamines also endow these circuits with the necessary flexibility to control the precision, timing, and constancy of locomotor behaviors in order to adapt to different internal and external demands, essential for survival (Grillner 2003; Miles and Sillar 2011).
In contrast with the other monoamines, the role of dopamine in controlling spinal locomotor circuits has been largely neglected in the mammal. What we do know is that dopamine is released within the spinal cord during stepping activity (Gerin and Privat 1998; Jordan and Schmidt 2002) to alter motor output (Barbeau and Rossignol 1991; Clemens et al. 2012; Han et al. 2007; Han and Whelan 2009; Lapointe et al. 2009; Madriaga et al. 2004; McCrea et al. 1997) and modulate sensory transmission in a variety of species. Dopaminergic fibers project from the diencephalon (A11 area), and all five dopamine receptors are present in the ventral horn of the adult mouse spinal cord (Holstege et al. 1996; Qu et al. 2006; Ridet et al. 1992; Weil-Fugazza and Godefroy 1993; Yoshida and Tanaka 1988; Zhu et al. 2007), where motor circuits are located. The five dopamine receptor types are divided into two dopamine receptor families that have been distinguished on the basis biochemical studies. D1 and D5 receptors belonging to the D1-like family elicit excitatory postsynaptic effects, and D2, D3, and D4 receptors belonging to the D2-like family elicit inhibitory effects on both presynaptic and postsynaptic terminals (Missale et al. 1998; Neve et al. 2004).
The key role for dopaminergic transmission in locomotion is highlighted by the fact that l-DOPA-elicited air stepping in intact neonatal rats is blocked by D1-like and D2-like receptor antagonists (McCrea et al. 1997) and that D1 agonists can promote stepping in adult mice (Lapointe et al. 2009). Additionally, previous work has emphasized that dopamine can modulate ongoing drug-evoked locomotor rhythms and in rats can even evoke locomotor-like activity (Barrière et al. 2004; Humphreys and Whelan 2012; Jiang et al. 1999; Madriaga et al. 2004; Milan et al. 2014; Schotland et al. 1995; Whelan et al. 2000). Collectively, these studies emphasize an important role for dopamine in the modulation of locomotor function in the developing and adult spinal cord.
An important step in understanding how dopamine exerts sustained neuromodulatory actions on central pattern generating (CPG) networks is to identify the receptors activated by dopamine. Previous reports in the neonatal mouse have investigated the underlying dopamine receptors that contribute to ongoing locomotor rhythms evoked by either pharmacological application of 5-hydroxytryptamine (5-HT) (Madriaga et al. 2004) or electrical stimulation of the afferents that reside in the cauda equina (Gordon and Whelan 2006). These studies, as well as others in the rat, provide evidence that dopamine's excitatory effects are mainly mediated via a D1-like receptor-based pathway (Barrière et al. 2004; Seth et al. 1993). Similar roles for the D1-like receptor systems are also found to modulate locomotor activity in Xenopus tadpoles (Clemens et al. 2012) and lamprey (Schotland et al. 1995). In contrast, the role of the D2-like receptor subfamily in motor control at the level of the spinal cord of mammals is less understood.
The aim of the present investigation was to build on and extend previous observations of the dopaminergic modulation of rhythmic activity in the neonatal mouse spinal cord, by investigating the effects of dopamine and the D1-like and D2-like agonists on a preexisting locomotor-like rhythm evoked by pharmacological application of 5-HT and N-methyl-d(l)-aspartic acid (NMA). In the rat spinal cord, application of 5-HT and NMA is sufficient to induce stable, rhythmic activity (Cazalets et al. 1992; Cowley and Schmidt 1995, 1997; Kjaerulff and Kiehn 1996; Kremer and Lev-Tov 1997). However, in the mouse, application of 5-HT and NMA can at certain concentrations generate less coordinated rhythmic activity between the left and right sides of the spinal cord (Jiang et al. 1999; Whelan et al. 2000). Therefore, we specifically used this relatively unstable locomotor-like rhythm to highlight which aspects of rhythmic activity were altered by dopamine application. Our results demonstrate that dopamine application as low as 35 μM stabilizes a preexisting 5-HT, NMA-evoked locomotor-like rhythm and slows the rhythm frequency. The underlying boost in excitation leading to a stabilization of rhythmic output was found to be both D1 and D2 dependent; however, in other studies a D1 effect was noted although D2 effects were not ruled out (Barrière et al. 2004). Our results with a combination of pharmacology and transgenic D3 receptor knockouts point to dopamine's ability to slow down the frequency of the rhythm to be mediated by D2-dependent signaling mechanisms. Our data highlight an important contribution for dopamine receptor activation in the maintenance and stability of ongoing CPG network activity. A portion of our data has been published in abstract form (Humphreys and Whelan 2011).
METHODS
Ethical approval and animals.
Experiments were performed on neonatal Swiss Webster mice at 0–3 days old (P0–P2) (n = 142). Experiments were also conducted on D3 receptor knockout mice (D3KO; strain B6.129S4-Drd3tm1dac/J; stock no. 002958, Jackson Laboratory, Bar Harbor, ME; n = 10) and appropriate associated wild-type (WT) control mice (C57BL/6, n = 6). All procedures used were approved by the University of Calgary Health Sciences Animal Care Committee and the East Carolina University Institutional Care and Use Committee, respectively.
Tissue preparation: spinal cord isolation.
Animals were anesthetized by cooling or intraperitoneal injection of ketamine (90 mg/ml)-xylazine (10 mg/ml), decapitated, and eviscerated to expose the vertebral column. The remaining tissue was placed ventral side up in a dissection chamber filled with room-temperature oxygenated (95% O2-5% CO2) artificial cerebrospinal fluid (aCSF) (in mM: 128 NaCl, 4 KCl, 1.5 CaCl2, 1 MgSO4, 0.5 Na2HPO4, 21 NaHCO3, 30 d-glucose), spinal cords were exposed via a ventral laminectomy, and dorsal and ventral roots were cut. The spinal cord was removed and left to stabilize for 15–20 min before transfer to a recording chamber, ventral side up, with oxygenated aCSF and gradually heated from room temperature to 27°C. The spinal cord was allowed another 20 min to further stabilize before the ventral roots of the second and fifth lumbar segments (L2 and L5) were attached with tight-fitting suction electrodes. D3KO experiments followed the same dissection procedure; however, ventral root recordings were made at room temperature (∼21–22°C) from D3KO and WT cords. In these experiments, a WT and a D3KO preparation were tested in parallel in the same recording chamber to ensure identical experimental conditions.
Pharmacology.
Fictive locomotion was elicited in isolated spinal cord and intact hindlimb preparations by bath application of NMA (5 μM; Sigma-Aldrich) and 5-HT (10 μM; Sigma-Aldrich). To measure the modulatory effects of dopamine on the rhythm, most experiments involved bath application of 50 μM dopamine; however, a subset of experiments also involved bath application of dopamine at 1 μM, 10 μM, 30 μM, 35 μM, and 40 μM (Sigma-Aldrich). In a subset of experiments we targeted the D1-like (D1/D5) and D2-like (D2/D3/D4) subfamilies. Receptor-selective dopamine agonists used were the D1-like agonist SKF-81297 (20 μM; Tocris) and the D2-like agonist quinpirole (20 μM; Sigma-Aldrich). Dopamine receptor-selective antagonists used were the D1-like antagonist LE 300 (1–4 μM; Sigma-Aldrich), the D2-like receptor antagonists L-741,626 (6–12 μM; Sigma-Aldrich) and sulpiride (20 μM; Sigma-Aldrich), and the D3-preferring antagonist SB-277,011-A (1 μM; Tocris). In all experiments a wash of 500–800 ml of aCSF containing the baseline drugs was performed to restore baseline conditions after drug testing.
Electrophysiological recordings.
Neurograms were obtained from isolated spinal cord preparations by drawing segmental ventral roots from both the right and left ventral roots in the second lumbar (L2) segment and one or both left and right ventral roots in the fifth lumbar (L5) segment into tight-fitting suction electrodes filled with bath aCSF. Neurograms were amplified (100–10,000 times), filtered (low pass 1 kHz), digitized (Digidata 1440; Molecular Devices, Sunnyvale, CA), acquired with Clampex 10 software (Molecular Devices), and saved on a laboratory computer for off-line analysis. All data obtained during isolated spinal cord experiments were analyzed with custom-written programs (MATLAB, MathWorks, Natick, MA; Spinalcore, A. Lev-Tov) and commercially available programs (Spike2, CED, Cambridge, UK).
Data analyses.
Continuous recordings of drug-evoked locomotor activity during isolated spinal cord experiments were high-pass filtered (100 Hz) and rectified before a Morlet cross-wavelet spectrogram analysis of ventral root bursting pairs in Spinal Core (Mor and Lev-Tov 2007) producing coherent power spectrograms was performed. For convenience, nonsignificant regions are omitted from the spectrogram; colors indicate the logarithmic power of the signal, which shows the power of the signal at a given frequency. High-power regions are assigned “warm” colors (i.e., red and orange), and low-power regions are assigned “cool” colors (i.e., green and blue). The high-power band representing the locomotor activity was then identified and selected as a region of interest for analysis. Regions of interest were segmented into 20 bins over the course of a 10-min trial from rhythms evoked under different drug conditions and analyzed to extract three main features including rhythm frequency, coherent power, and phase of the pattern. Data from each of the 20 bins of the respective 10-min trial were normalized to the average of the 20 bins from the control or baseline condition. Normalized bins were then averaged across 10-min trials of the respective experimental conditions.
Separate dose-response experiments were conducted for the D1-like agonist SKF-81297 and the D2-like agonist quinpirole (1, 3, 10, 30, 100 μM) by recording spontaneous ventral root activity from single L2 roots. This permitted us to record from up to four separate isolated spinal cords simultaneously to ensure equivalent experimental conditions between preparations. Data over a 10-min period of time were high-pass filtered (100 Hz) and the root mean square (RMS) of bursting activity measured. A response ratio was calculated between the RMS of bursting activity between 10 and 20 min after addition of SKF-81297 to the bath relative to a 10-min baseline recording.
Statistics.
All statistical tests were conducted on the raw, unnormalized data. Paired t-tests were conducted on experiments involving repeated-measures assessment at two points in time, and repeated-measures analyses of variance (ANOVA) were conducted on experiments involving assessment at three or more points in time. Tukey post hoc analysis was conducted when significant effects were detected with ANOVAs with a significance level of P < 0.05. Unpaired t-tests were conducted for between-group comparisons of two groups and a one-way ANOVA for comparisons of more than two groups. Circular statistics were conducted on phase analyses of rhythmic locomotor activity to test for directionality (Rayleigh's test, P < 0.05).
RESULTS
Dopamine promotes stability and reduces frequency of ongoing locomotor activity.
Bath application of monoamines such as 5-HT (rat/mouse) or dopamine (rat) to neonatal isolated spinal cord preparations is capable of eliciting a robust locomotor-like pattern of activity (Barrière et al. 2004; Madriaga et al. 2004; Nishimaru et al. 2000). In contrast, our work demonstrates that in the mouse bath application of dopamine alone did not elicit a strong locomotor pattern of activity but rather low-frequency rhythmic bursting activity that does not show the left-right alternation of a basic locomotor pattern (Fig. 1, Bi and Bii). Our work and that of others demonstrates that bath-applied dopamine does play an important role in rodents in the stabilization of fictive locomotor activity. What is not known are the receptor mechanisms that mediate these effects in mice. To study the mechanisms of dopaminergic modulation of locomotion, we made use of an unstable preexisting locomotor rhythm evoked solely by bath application of 5-HT and NMA in the neonatal mouse spinal cord isolation preparation. This provides the advantage of being able to ascertain the stabilizing effects of dopamine on rhythms coupled with an analysis of effects on frequency.
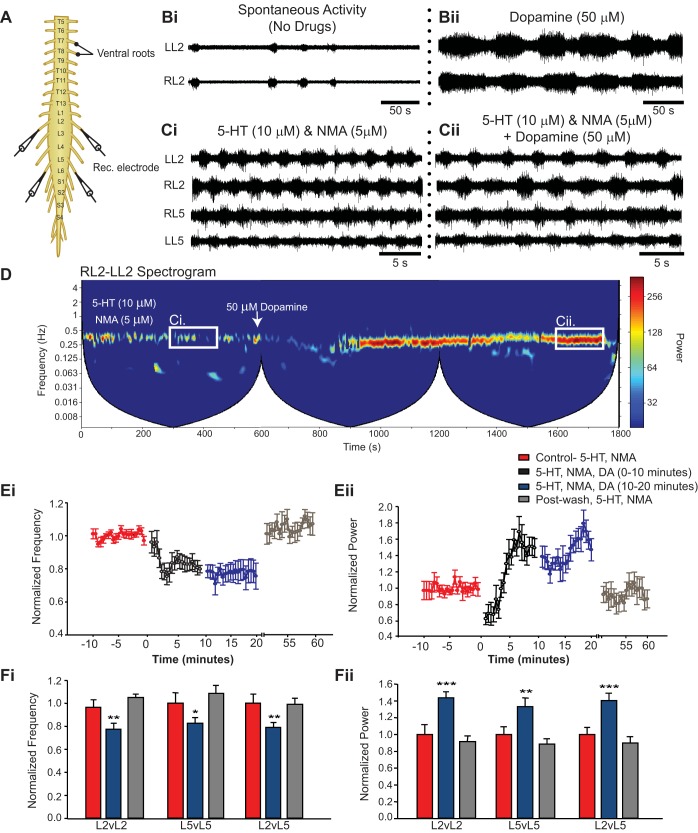
Fig. 1.
Dopamine reduces locomotor rhythm frequency and increases rhythm stability. A: ventral root neurogram recordings from an isolated spinal cord of a neonatal mouse. Bi and Bii: bath application of dopamine alone (50 μM) evokes low-frequency rhythmic bursting activity in ventral root pairs that does not resemble a locomotor pattern. When dopamine is applied during a preexisting fictive locomotor rhythm evoked by 5-hydroxytryptamine (5-HT, 10 μM) and N-methyl-d(l)-aspartic acid (NMA, 5 μM) (Ci) it reduces the frequency of the rhythm (Cii, Ei, Fi) and increases the stability of the rhythm indicated by an increase in power (Cii, Eii, Fii). D: spectrogram depicts a cross-wavelet analysis of a locomotor rhythm recorded from left and right L2 ventral root neurograms evoked by 5-HT and NMA in the first 600 s with subsequent addition of dopamine for up to 20 min after addition of dopamine. Rhythm frequency is displayed on the y-axis and rhythm power displayed as warm or cool colors, with warmer colors representing higher power. Line and bar graphs depict the average normalized frequency and power of the locomotor rhythm (±SE) from neurograms recorded in the left and right L2 and L5 and ipsilateral L2-L5 bursting activity. Bar graphs depict normalized data averaged over 10-min time intervals during respective drug conditions. *P < 0.05; **P < 0.01; ***P < 0.001.
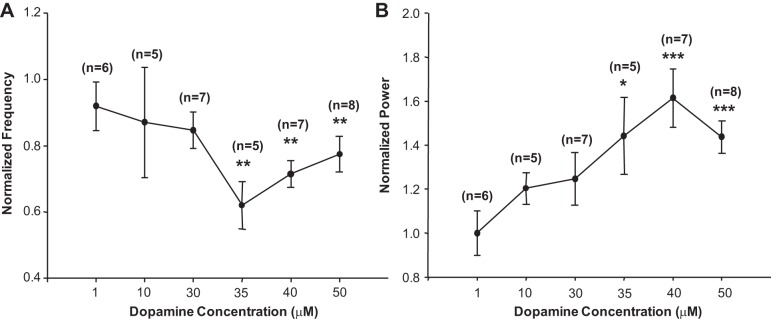
The baseline rhythm evoked by 5-HT and NMA consisted of bursting activity that alternated between left and right ventral root neurograms within the same segment and alternating bursts between L2 and L5 neurograms on the ipsilateral side of the cord (Fig. 1Ci). In the spectrogram this was apparent as a locomotor frequency band around 0.4 Hz (Fig. 1D). Addition of 50 μM dopamine to this rhythm resulted in an initial degradation of stability of the rhythm during the first 3 min. After ∼3 min, a more stable [power: n = 8, F(2,14) = 22.6, P < 0.001; Fig. 1, D and Eii] and lower-frequency [n = 8, F(2,14) = 17.8, P < 0.001; Fig. 1, D and Ei] alternating rhythm between left and right L2 ventral root bursts emerged (Fig. 1Cii) comparable to that previously reported (Humphreys and Whelan 2012). Similar effects were observed when comparing left-right alternation of L5 neurograms [n = 7; frequency: F(2,12) = 9.2, P < 0.01, Fig. 1Fi; power: F(2,12) = 19.9, P < 0.001, Fig. 1Fii] and ipsilateral alternating activity between L2 and L5 ventral roots [n = 8; frequency: F(2,14) = 12.6, P < 0.001, Fig. 1Fi; power: F(2,14) = 20.0, P < 0.0001, Fig. 1Fii]. There was no change in the phase or vector length of the alternating pattern with the addition of dopamine to the preexisting locomotor rhythm (data not shown). In a separate set of experiments we determined that 35 μM dopamine was the lowest concentration required to significantly stabilize and reduce the frequency of an ongoing 5-HT, NMA-evoked locomotor rhythm (n = 35; Fig. 2).
Fig. 2.
Dose-dependent modulatory effects of dopamine on the locomotor rhythm. Concentrations of dopamine as low as 35 μM are sufficient to reduce the frequency (A) and increase the power (B) of preexisting 5-HT- and NMA-evoked locomotor activity. Data are presented as mean ± SE frequency (A) and power (B) normalized to baseline rhythm evoked by 5-HT and NMA alone. Asterisks denote significant differences from baseline 5-HT-, NMA-evoked rhythm (not shown) from a 10-min time window between 10 and 20 min after dopamine application (repeated-measures ANOVA, Tukey post hoc, P < 0.05). *P < 0.05; **P < 0.01; ***P < 0.001.
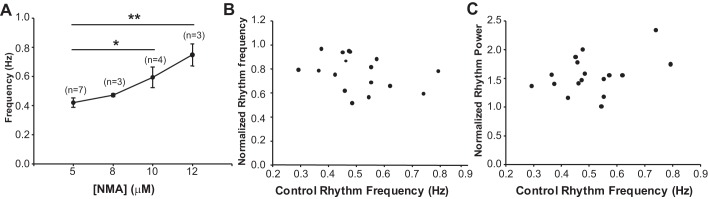
Given that different neuronal populations are recruited at different frequencies of locomotor activity (Zhong et al. 2011), this could alter the modulatory effects of dopamine. To address this we tested the ability of dopamine to modulate ongoing locomotor activity at different locomotor frequencies by varying the concentration of NMA (5–12 μM) (Talpalar and Kiehn 2010) in the bath (n = 17, frequency range = 0.29–0.79 Hz; Fig. 3A). As expected, increasing the concentration of NMA in the bath significantly increased the frequency of the rhythm [1-way ANOVA: F(3,13) = 7.9, P = 0.003; Fig. 3A]. However, the ability of dopamine to modulate the rhythm frequency and power remained true independent of the induced locomotor frequency (Fig. 3, B and C).
Fig. 3.
Locomotor rhythm frequency evoked by 5-HT and NMA was manipulated by modifying the concentration of NMA in the bath. A: higher concentrations of NMA combined with 10 μM 5-HT increase rhythm frequency. Data are presented as mean ± SE rhythm frequency, with the number of preparations in parentheses above each point. Asterisks denote significant differences (Tukey post hoc *P < 0.05, **P < 0.01). B and C: degree of modulation by dopamine on locomotor rhythm frequency (B) and power (C) is not dependent on the preexisting rhythm frequency. Control rhythm frequency is displayed on x-axis, with the degree of modulation on the y-axis for each experiment (black dots) normalized to baseline control rhythm frequency (B) and power (C).
D1-like and D2-like receptor mechanisms of modulation.
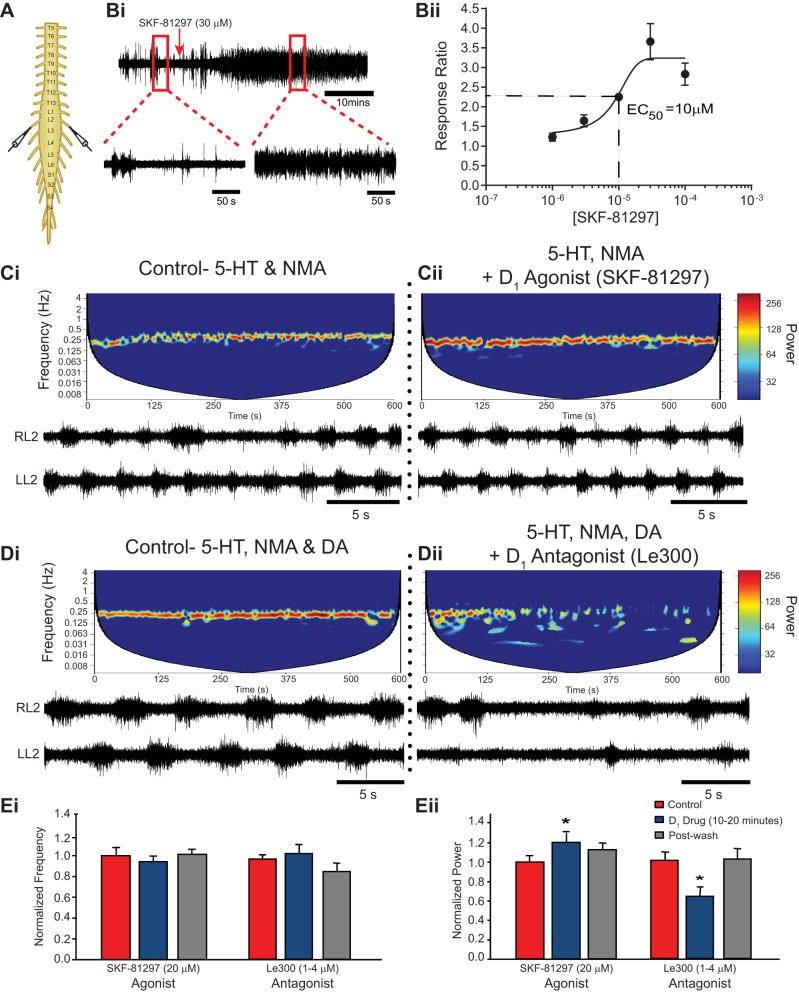
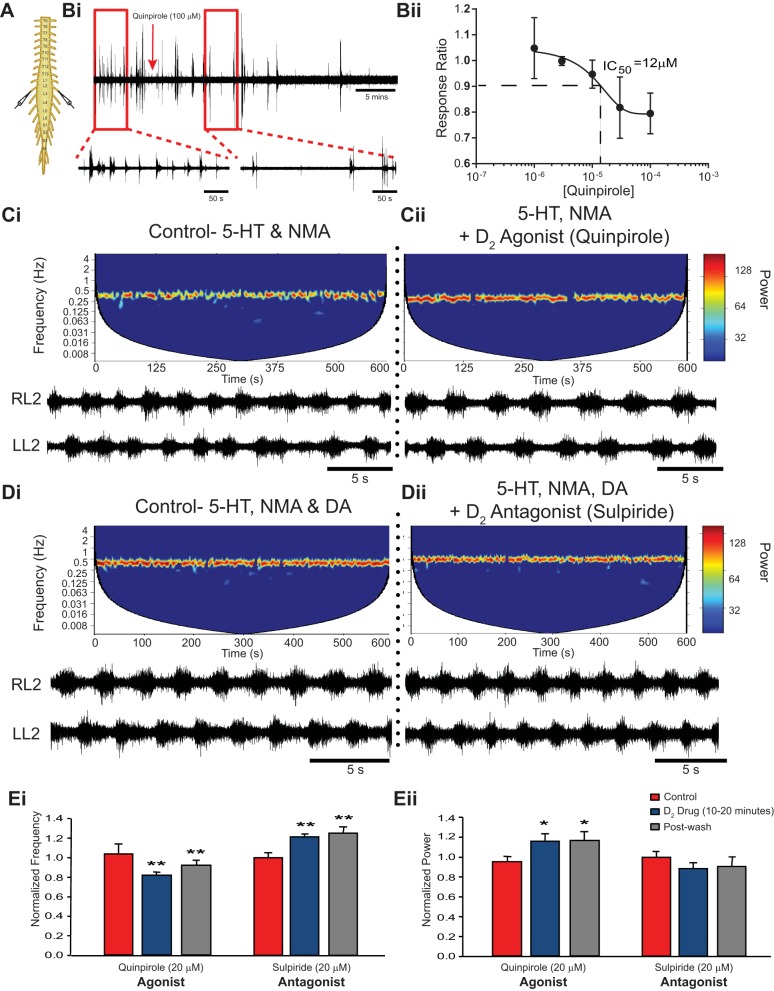
To test the different dopamine receptor family contributions to the modulation of an ongoing 5-HT and NMA-evoked rhythm, we focused specifically on the left-right alternating pattern of the L2 neurograms. Application of the D1-like agonist SKF-81297 (20 μM) increased the stability of the ongoing 5-HT and NMA-evoked rhythm (Fig. 4Ci) evident by a significant increase in normalized power [n = 11; F(2,20) = 6.59, P = 0.006; Fig. 4, Cii and Eii] but had no effect on frequency [n = 11; F(2,20) = 1.5, P = 0.25; Fig. 4Ei]. A 500-ml wash of aCSF containing 5-HT and NMA restored the rhythm to control conditions. Twenty micromolar D1-like agonist SKF-81297 was utilized based on a dose-response experiment using spontaneous ventral root bursting as a probe of motor circuit excitability (n = 20). These experiments determined the EC50 to be 10 μM (Fig. 4, Bi and Bii).
Fig. 4.
D1-like receptor activation increases rhythm stability with no effect on frequency. A and B: spontaneous activity from single ventral root neurograms of the isolated spinal cord (A) were used to determine the EC50 for D1-mediated excitation of motor output (B). The EC50 for SKF-81297 was calculated as 10 μM by generating a response ratio between the root mean square of activity in a 10–20 min window after drug application compared with a 10-min baseline. Data are presented as mean ± SE response ratio as a function of SKF-81297 concentration. Bath application of the D1-like receptor agonist SKF-81297 during a 5-HT-, NMA-evoked rhythm (Ci) increased stability (Cii and Eii) but had no effect on frequency (Cii and Ei). Similarly, the D1-like antagonist LE 300 (1–4 μM) destabilized the locomotor rhythm (Dii and Eii) but had no effect on frequency (Dii and Ei) of a rhythm evoked by 5-HT, NMA, and dopamine (Di). Spectrograms depict cross-wavelet analysis of neurogram activity from left and right L2 ventral roots over time, with the rhythm frequency on the y-axis and rhythm stability indicated by the power as color bands. A more stable rhythm is displayed as warmer colors (C and D). Data are presented as mean ± SE normalized rhythm frequency (Ei) and power (Eii) over a 10-min window between 10 and 20 min after dopamine application and are normalized to a 10-min window of the control rhythm evoked by 5-HT and NMA. *Significant differences from baseline control rhythm (repeated-measures ANOVA, Tukey post hoc) with P < 0.05.
To further explore the role of dopamine during ongoing locomotor activity evoked by combined application of 5-HT, NMA, and dopamine (50 μM), D1-like antagonists were bath applied in separate experiments to perturb the rhythm. In four of five preparations, bath application of the D1-preferring antagonist LE 300 (1–4 μM) resulted in degradation of the rhythm within 5–10 min [n = 5; F(2,8) = 6.14, P = 0.02; Fig. 4, Dii and Eii], which was restored to original conditions after a wash with 500–700 ml of aCSF containing 5-HT, NMA, and dopamine.
In a separate set of experiments, addition of the D2-like agonist quinpirole (20 μM) to the 5-HT and NMA-evoked rhythm (Fig. 5Ci) increased the stability indicated by a significant increase in power [n = 7; F(2,12) = 5.8, P = 0.02; Fig. 5, Cii and Eii] and reduction in frequency [n = 7; F(2,12) = 8.9, P < 0.004; Fig. 5, Cii and Ei]. The D2-like agonist activity persisted after a wash with 500–700 ml of aCSF containing 5-HT and NMA, indicating the possibility for long-lasting effects of D2-like receptor activation on rhythm stability. Dose-response experiments (n = 18) determined the IC50 of quinpirole's ability to reduce spontaneous motor activity to be 12 μM (Fig. 5, Bi and Bii). We then tested the ability of the D2 antagonist L-741,626 to reduce the quinpirole-mediated effects. Similar to previous experiments, bath application of the D2-like agonist quinpirole (20 μM) to a locomotor rhythm evoked by 5-HT and NMA resulted in a significant reduction in frequency (P < 0.01) and increase in stability (P < 0.05) of the rhythm. Subsequent bath application of the D2 receptor antagonist L-741,626 (12 μM) offset the quinpirole-induced effects, increasing the frequency [F(3,12) = 7.03, P = 0.005] and reducing the stability [F(3,12) = 4.23, P = 0.029] back to that of the baseline 5-HT and NMA-evoked rhythm (data not shown).
Fig. 5.
D2-like receptor activation increases rhythm stability and decreases rhythm frequency. A and B: spontaneous activity from single ventral root neurograms of the isolated spinal cord (A) were used to determine the IC50 for D2-mediated inhibition of motor output (B). The IC50 for quinpirole was calculated as 12 μM by generating a response ratio between the root mean square of activity in a 10–20 min window after drug application compared with a 10-min baseline. Bath application of the D2-like agonist quinpirole (20 μM) decreased the frequency (Cii and Ei) and increased stability (Cii and Eii) of a preexisting locomotor rhythm evoked by 5-HT and NMA (Ci). Similarly, bath application of the D2-like antagonist sulpiride (20 μM) increased the frequency (Dii and Ei) but had no effect on stability (Dii and Eii) of a rhythm evoked by 5-HT, NMA, and dopamine (Di). Ei and Eii: bar graphs represent mean ± SE rhythm frequency (Ei) and power (Eii) normalized to baseline control rhythm conditions. Spectrograms depict cross-wavelet analysis of neurogram activity from left and right L2 ventral roots over time, with the rhythm frequency on the y-axis and rhythm stability indicated by the power as the color bands. A more stable rhythm is displayed as warmer colors (C and D). Data are presented as mean ± SE normalized rhythm frequency (Ei) and power (Eii) over a 10-min window between 10 and 20 min after dopamine application and are normalized to a 10-min window of the control rhythm evoked by 5-HT and NMA. Significant differences from baseline control rhythm (repeated-measures ANOVA, Tukey post hoc) with *P < 0.05 and **P < 0.01.
Surprisingly, bath application of the D2-like antagonist L-741,626 (6 μM) had no effect on the 5-HT-, NMA-, and dopamine-evoked locomotor rhythm [n = 5; frequency: F(2,8) = 1.36, P = 0.31; power: F(2,8) = 4.5, P = 0.049; Tukey post hoc P > 0.05]. To test for the possibility of competitive binding of L-741,626 and dopamine on D2 receptors, the concentration of the antagonist was doubled and bath applied to a rhythm evoked by 5-HT, NMA, and a lower concentration of dopamine (35 μM); however, still no effect was observed [n = 5; frequency: F(2,8) = 1.2, P = 0.36; power: F(2,8) = 1.5, P = 0.29; data not shown].
In an additional set of experiments, we bath-applied the D2-preferring antagonist sulpiride (20 μM) to an ongoing locomotor rhythm evoked by 5-HT, NMA, and 35 μM dopamine, which resulted in a significant increase in rhythm frequency [n = 4; F(2,6) = 17.0, P < 0.001; Fig. 5, Dii and Eii] with no change in stability [F(2,6) = 1.53, P = 0.29; Fig. 5, Dii and Ei]. Given the comparative binding affinities of L-741,626 for D2 receptors, sulpiride being a more general D2-like antagonist (D2, D3, D4), and the fact that the D3 receptor has the highest affinity to dopamine, we considered the possibility that the frequency modulation of dopamine may be in part mediated by D3 receptors.
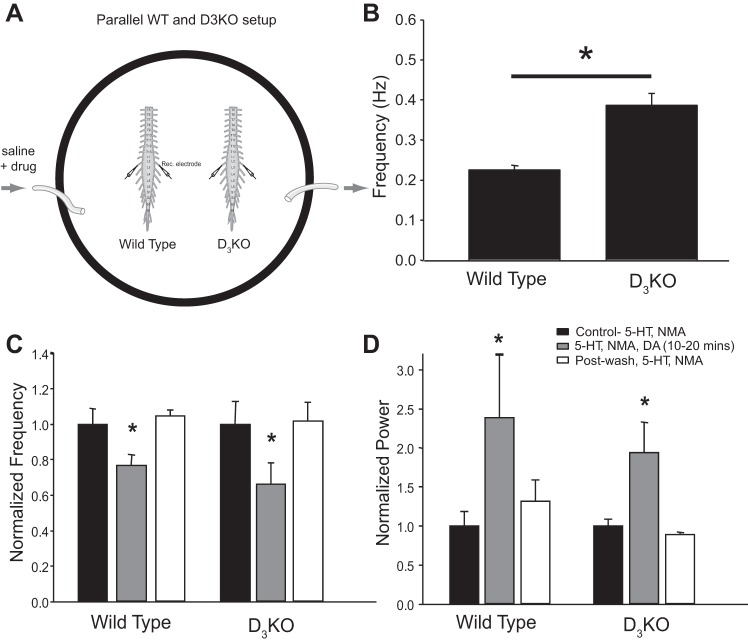
With this in mind, experiments were conducted on D3KO mice. We found that the baseline locomotor rhythm (evoked by only 5-HT and NMA) frequency was significantly higher in the D3KO isolated spinal cords compared with WT [n = 12; WT, n = 6, frequency = 0.23 ± 0.03 Hz; D3KO, n = 6, frequency = 0.39 ± 0.08 Hz; unpaired t-test: t(10) = −3.07, P = 0.017; Fig. 6B]. Note that in these experiments, the WT and D3KO animals were always side by side in the same chamber—subject to the same conditions (Fig. 6A; cf. methods). Dopamine (50 μM) elicited a similar robust reduction in frequency and increase in stability of the 5-HT- and NMA-evoked locomotor rhythm in both WT and D3KO [n = 12: WT, n = 6; D3KO, n = 6; frequency: F(2,10) = 5.97, P = 0.02; power: F(2,10) = 15.06, P = 0.0003; Fig. 6, C and D]. As these data were obtained at 21–22°C, we specifically tested the possible role of temperature. While we did observe a temperature effect on initial rhythm frequency, we found that the respective WT data obtained at 21°C and 27°C could be normalized to each other (data not shown). Therefore, the direct comparison between WT and D3KO in the same dish and at the same temperature suggests that temperature did not contribute to the impact of dopamine's modulatory actions.
Fig. 6.
A: 5-HT- and NMA-evoked locomotor activity was recorded from left and right L2 ventral root of wild-type (WT) and D3 knockout (D3KO) mice in parallel and in the same recording chamber to ensure identical experimental conditions. B: baseline rhythm frequency was higher in D3KO compared with WT. Data are presented as mean ± SE rhythm frequency (*significant difference between groups, P < 0.05). C and D: subsequent application of dopamine resulted in a significant reduction in locomotor rhythm frequency (C) and increase in stability (D). Data are presented as mean ± SE normalized rhythm frequency (C) and power (D) over a 10-min window between 10 and 20 min after dopamine application and are normalized to a 10-min window of the control rhythm evoked by 5-HT and NMA. *Significant differences from baseline control rhythm (repeated-measures ANOVA, Tukey post hoc) with P < 0.05.
DISCUSSION
The ability for spinal dopamine to modulate ongoing locomotor activity is of growing interest in the field of motor control. Over the past decade, work in vivo has implicated an important role for dopamine in generating and maintaining stepping behavior (Gerin and Privat 1998; Jordan and Schmidt 2002; Lapointe et al. 2009; McCrea et al. 1997). Additionally, in vitro work has highlighted the ability of dopamine to modulate ongoing locomotor rhythms in several vertebrate species (Barrière et al. 2004; Clemens et al. 2012; Gordon and Whelan 2006; Jiang et al. 1999; Madriaga et al. 2004; Schotland et al. 1995; Whelan et al. 2000). However, experimental protocols in the mouse have often relied upon stable control rhythms induced either pharmacologically or by electrical stimulation, to test the modulatory effectiveness of dopamine. This has raised some questions regarding whether the effects of dopamine are masked by the presence of a stable control rhythm. Therefore in the present study we sought to expand upon an existing body of research (Barrière et al. 2004; Gordon and Whelan 2006; Kjaerulff and Kiehn 1996; Madriaga et al. 2004; Whelan et al. 2000) and test which aspects of a less coordinated, pharmacologically induced rhythm were modulated by the presence of dopamine. The results indicate that the presence of dopamine induced a more stable, slower rhythm. Indeed, our work suggests that D1-like and D2-like receptors have distinct roles in controlling rhythm stability, with the D2 receptors playing an important role in the modulation of rhythm frequency.
The potential for dopamine to modulate ongoing locomotor rhythms in vitro was recognized first in the lamprey (Schotland et al. 1995) and later in other vertebrate systems (Barrière et al. 2004; Gordon and Whelan 2006; Kiehn and Kjaerulff 1996; Madriaga et al. 2004; Smith et al. 1988). Initial experiments described dopamine's ability to dose-dependently slow down the cycle frequency of swimming behavior (Schotland et al. 1995), with lower concentrations increasing rhythm frequency. Similar dose-dependent modulatory effects on spontaneous locomotor activity have been reported in the premetamorphic Xenopus tadpole such that low concentrations of dopamine activate D2-like receptors and reduce spontaneous locomotor activity whereas higher concentrations activate D1-like receptors and facilitate spontaneous locomotor activity (Clemens et al. 2012). More recent work in the isolated mouse has reported an excitatory role for dopamine in network output (Gordon and Whelan 2006; Madriaga et al. 2004; Whelan et al. 2000). Our present work adds to this growing body of research in the following important ways: First, the number of studies investigating the dopaminergic modulation of ongoing locomotor rhythms in the neonatal mouse is limited. Although a report in the neonatal rat documenting dopamine's role during ongoing locomotor activity exists, we cannot assume that the underlying receptor subtypes activated during fictive locomotion are the same. Second, of the limited studies that do exist in the mouse, two have relied upon evoking robust control rhythms prior to investigating dopamine's modulatory effects (Gordon and Whelan 2006; Madriaga et al. 2004). Therefore, it is arguable that the magnitude of dopamine's effect on a preexisting stable rhythm was not fully revealed. The remaining study that showed dopamine's modulatory effects on an uncoordinated control rhythm (Whelan et al. 2000) did not ascribe distinct roles for the underlying dopamine receptor families responsible for mediating dopamine's effects. We present data demonstrating that D1-like receptor pathways may act in parallel with D2-like receptor pathways to increase the stability of a preexisting 5-HT-, NMA-induced rhythm. Evidence in the brain and spinal cord supports the finding that a synergy between D1 and D2 receptor-based signaling pathways exists and functions to produce appropriate behavioral output in the brain and spinal cord (Barrière et al. 2004; Braun and Chase 1986; LaHoste et al. 2000; Missale et al. 1998). However, it is important to note that application of the D1-like antagonist LE 300 resulted in degradation of the stable 5-HT-, NMA-, dopamine-evoked locomotor-like rhythm, while the D2-like antagonist L-741,626 with preferential binding affinity for D2 only degraded rhythm stability in the presence of the D2-like agonist quinpirole, and not when dopamine was present. This points to the possibility that at the D2-mediated effects may be masked by stronger D1-mediated effects at the higher concentrations (50 μM and 35 μM) of dopamine used in these experiments.
In the present investigation we show that dopamine does not demonstrate a concentration-dependent bidirectional influence on locomotor rhythm frequency in the mouse spinal cord as reported in the lamprey (Schotland et al. 1995) and Xenopus (Clemens et al. 2012). Additionally, L-741,626 exerted no effects on rhythm frequency in the presence of dopamine. This was unexpected given that application of the D2-like receptor agonist quinpirole decreased rhythm frequency (Fig. 5) and the broader D2/D3 antagonist sulpiride selectively increased the rhythm frequency with dopamine on board. Based on this, we considered the possibility that the frequency modulation may be mediated by the D3 receptor subtype. To overcome the challenges of selectively targeting the D3 receptor population with traditional pharmacological approaches, we utilized a transgenic D3KO mouse to test this hypothesis. In contrast to our hypothesis, dopamine still reduced rhythm frequency of both WT and D3KO. Interestingly, when testing D3KO and WT animal spinal cord preparations in parallel and at the same ambient temperature, we found that the baseline frequency of locomotor rhythms evoked by 5-HT and NMA was significantly higher in D3KO than in WT. These data suggest that D3 receptors exert an inhibitory influence on the locomotor-generating circuitry, and that the lack of this receptor in the D3KO releases the CPG in the transgenic animals from the D3 receptor-mediated inhibition. While the mechanism underlying this phenotypical switch remains speculative, it is conceivable that the dysfunction of the D3 receptor gives rise to an increase in spinal D1 receptor protein levels that in turn might additionally fasten the locomotor rhythm observed in the D3KO, similar to recent findings that suggested a D1 receptor upregulation when testing pain-related sensorimotor pathways in D3KO animals (Brewer et al. 2014).
We made use of pharmacological and transgenic approaches to examine the role of the D2 receptors in the modulation of rhythm frequency. Given that dopamine was capable of reducing rhythm frequency in the D3KO spinal cords and the more selective D2 antagonist (L-741,626) of increasing rhythm frequency in the absence of D1 receptor activity in WT cords, it is likely that D2 receptors mediate this effect. These findings point to the fact that if we wish to fully understand the role that dopamine plays in the modulation of spinal motor circuits, we must not consider just the receptor families (i.e., D1-like and D2-like) but also the individual receptor types (i.e., D1–D5), which are all expressed nonuniformly across the lumbar spinal cord, with all five receptor subtypes expressed in motor neurons (Zhu et al. 2007). It should be noted that this receptor characterization was conducted in P14-age mice, and therefore consideration must be given to the potential developmental receptor expression profile in the first 5 postnatal days. Furthermore, consideration must also be given to the possibility of cross-family heterodimers that form, for example, between D1 and D2 (George and O'Dowd 2007) or D1–D3 (Cruz-Trujillo et al. 2013; Surmeier et al. 1996).
What is clear is that dopamine is a potent modulator of locomotor network activity; however, if we wish to more fully understand the role of spinal dopamine in motor control we must further examine how dopamine shapes different components of the network. This includes the modulation of intrinsic properties and synaptic inputs to motor neurons and other populations of neurons that make up the pattern formation elements and rhythm-generating components of locomotor CPGs. Indeed, dopamine does increase the excitability of motor neurons by reducing potassium conductances (IA, SKCa) (Han et al. 2007) and increasing excitatory glutamatergic transmission onto motor neurons (Han and Whelan 2009) that could contribute to dopamine's excitatory increase on burst amplitude. However, further work is required to probe how dopamine modulates other components of the locomotor network. For example, in the lamprey the frequency modulation of the rhythm by dopamine has been attributed to modulation of inhibitory commissural interneurons that participate in the generation of the left-right alternating pattern of fictive swimming (Hill et al. 2003a; Wang et al. 2011). Furthermore, dopamine's reduction in rhythm frequency has also been suggested to be due in part to a decrease in descending excitatory reticulospinal input to motor neurons (Svensson et al. 2003) and also increasing activity of inhibitory commissural interneurons that participate in the generation of the left-right alternating pattern of fictive swimming (Hill et al. 2003b; Kemnitz 1997; McPherson and Kemnitz 1994; Wang et al. 2011). Until recently, targeting similar circuitry in the mammalian spinal cord was highly inaccessible compared with that in the lamprey. Recent advances in optogenetic methods will serve as a valuable tool to interrogate circuit function in the control of locomotion and provide further insight into the mechanisms through which dopamine acts to shape locomotor activity.
Conclusions.
We have demonstrated that dopamine modulates ongoing spinally generated locomotor activity but is insufficient to evoke coordinated patterns of locomotor-like activity when applied on its own. Specifically, dopamine stabilizes ongoing locomotor activity through both D1-like and D2-like receptor mechanisms. We provide evidence that activation of the D2 receptors plays a role in dopamine's ability to reduce the frequency of the rhythm. Our experiments with D3KO mice suggest that the D3 receptors may be constitutively involved in the regulation of rhythm frequency in the absence of dopamine; however, our data do not exclude a role for D4 receptors that are also highly expressed within the ventral horn of the spinal cord (Zhu et al. 2007). Collectively, these data provide a receptor-based understanding of dopamine's modulation of spinal cord CPGs.
GRANTS
We gratefully acknowledge funding from the Canadian Institutes of Health Research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.A.S., J.M.H., A.M.J., S.D., N.D., and S.C. performed experiments; S.A.S., J.M.H., A.M.J., S.D., and N.D. analyzed data; S.A.S., J.M.H., S.C., and P.J.W. interpreted results of experiments; S.A.S., J.M.H., A.M.J., and S.D. prepared figures; S.A.S. and P.J.W. drafted manuscript; S.A.S., J.M.H., A.M.J., S.D., N.D., S.C., and P.J.W. edited and revised manuscript; S.A.S., J.M.H., A.M.J., S.D., N.D., S.C., and P.J.W. approved final version of manuscript; S.C. and P.J.W. conception and design of research.
ACKNOWLEDGMENTS
We acknowledge technical assistance from Jill Ejdrygiewicz.
REFERENCES
- Accili D, Fishburn CS, Drago J, Steiner H, Lachowicz JE, Park BH, Gauda EB, Lee EJ, Cool MH, Sibley DR, Gerfen CR, Westphal H, Fuchs S. A targeted mutation of the D3 dopamine receptor gene is associated with hyperactivity in mice. Proc Natl Acad Sci USA 93: 1945–1949, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbeau H, Rossignol S. Initiation and modulation of the locomotor pattern in the adult chronic spinal cat by noradrenergic, serotonergic and dopaminergic drugs. Brain Res 546: 250–260, 1991. [DOI] [PubMed] [Google Scholar]
- Barrière G, Mellen N, Cazalets JR. Neuromodulation of the locomotor network by dopamine in the isolated spinal cord of newborn rat. Eur J Neurosci 19: 1325–1335, 2004. [DOI] [PubMed] [Google Scholar]
- Braun AR, Chase TN. Obligatory D-1/D-2 receptor interaction in the generation of dopamine agonist related behaviors. Eur J Pharmacol 131: 301–306, 1986. [DOI] [PubMed] [Google Scholar]
- Brewer KL, Baran CA, Whitfield BR, Jensen AM, Clemens S. Dopamine D3 receptor dysfunction prevents anti-nociceptive effects of morphine in the spinal cord. Front Neural Circuits 8: 62, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazalets JR, Sqalli-Houssaini Y, Clarac F. Activation of the central pattern generators for locomotion by serotonin and excitatory amino acids in neonatal rat. J Physiol 455: 187–204, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens S, Belin-Rauscent A, Simmers J, Combes D. Opposing modulatory effects of D1- and D2-like receptor activation on a spinal central pattern generator. J Neurophysiol 107: 2250–2259, 2012. [DOI] [PubMed] [Google Scholar]
- Cowley KC, Schmidt BJ. Effects of inhibitory amino acid antagonists on reciprocal inhibitory interactions during rhythmic motor activity in the in vitro neonatal rat spinal cord. J Neurophysiol 74: 1109–1117, 1995. [DOI] [PubMed] [Google Scholar]
- Cowley KC, Schmidt BJ. Regional distribution of the locomotor pattern-generating network in the neonatal rat spinal cord. J Neurophysiol 77: 247–259, 1997. [DOI] [PubMed] [Google Scholar]
- Cruz-Trujillo R, Avalos-Fuentes A, Rangel-Barajas C, Paz-Bermúdez F, Sierra A, Escartín-Perez E, Aceves J, Erlij D, Florán B. D3 dopamine receptors interact with dopamine D1 but not D4 receptors in the GABAergic terminals of the SNr of the rat. Neuropharmacology 67: 370–378, 2013. [DOI] [PubMed] [Google Scholar]
- Dunbar MJ, Tran MA, Whelan PJ. Endogenous extracellular serotonin modulates the spinal locomotor network of the neonatal mouse. J Physiol 588: 139–156, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George SR, O'Dowd BF. A novel dopamine receptor signaling unit in brain: heterooligomers of D1 and D2 dopamine receptors. ScientificWorldJournal 7: 58–63, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerin C, Privat A. Direct evidence for the link between monoaminergic descending pathways and motor activity. II. A study with microdialysis probes implanted in the ventral horn of the spinal cord. Brain Res 794: 169–173, 1998. [DOI] [PubMed] [Google Scholar]
- Gordon IT, Whelan PJ. Monoaminergic control of cauda-equina-evoked locomotion in the neonatal mouse spinal cord. J Neurophysiol 96: 3122–3129, 2006. [DOI] [PubMed] [Google Scholar]
- Gordon IT, Whelan PJ. Brainstem modulation of locomotion in the neonatal mouse spinal cord. J Physiol 586: 2487–2497, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S. The motor infrastructure: from ion channels to neuronal networks. Nat Rev Neurosci 4: 573–586, 2003. [DOI] [PubMed] [Google Scholar]
- Han P, Nakanishi ST, Tran MA, Whelan PJ. Dopaminergic modulation of spinal neuronal excitability. J Neurosci 27: 13192–13204, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han P, Whelan PJ. Modulation of AMPA currents by D1-like but not D2-like receptors in spinal motoneurons. Neuroscience 158: 1699–1707, 2009. [DOI] [PubMed] [Google Scholar]
- Hill AA, Masino MA, Calabrese RL. Intersegmental coordination of rhythmic motor patterns. J Neurophysiol 90: 531–538, 2003a. [DOI] [PubMed] [Google Scholar]
- Hill RH, Svensson E, Dewael Y, Grillner S. 5-HT inhibits N-type but not L-type Ca2+ channels via 5-HT1A receptors in lamprey spinal neurons. Eur J Neurosci 18: 2919–2924, 2003b. [DOI] [PubMed] [Google Scholar]
- Holstege JC, van Dijken H, Buijs RM, Goedknegt H, Gosens T, Bongers CM. Distribution of dopamine immunoreactivity in the rat, cat and monkey spinal cord. J Comp Neurol 376: 631–652, 1996. [DOI] [PubMed] [Google Scholar]
- Humphreys J, Whelan P. Dopamine exerts activation dependent modulation of spinal locomotor circuits in the mouse (Abstract). Soc Neurosci Abstr 2011: 471.08, 2011. [DOI] [PubMed] [Google Scholar]
- Humphreys JM, Whelan PJ. Dopamine exerts activation-dependent modulation of spinal locomotor circuits in the neonatal mouse. J Neurophysiol 108: 3370–3381, 2012. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Jukes MG, Lund S, Lundberg A. The effect of DOPA on the spinal cord. 5. Reciprocal organization of pathways transmitting excitatory action to alpha motoneurones of flexors and extensors. Acta Physiol Scand 70: 369–388, 1967a. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Jukes MG, Lund S, Lundberg A. The effect of DOPA on the spinal cord. 6. Half-centre organization of interneurones transmitting effects from the flexor reflex afferents. Acta Physiol Scand 70: 389–402, 1967b. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Carlin KP, Brownstone RM. An in vitro functionally mature mouse spinal cord preparation for the study of spinal motor networks. Brain Res 816: 493–499, 1999. [DOI] [PubMed] [Google Scholar]
- Jordan LM, Schmidt BJ. Propriospinal neurons involved in the control of locomotion: potential targets for repair strategies? Prog Brain Res 137: 125–139, 2002. [DOI] [PubMed] [Google Scholar]
- Kemnitz CP. Dopaminergic modulation of spinal neurons and synaptic potentials in the lamprey spinal cord. J Neurophysiol 77: 289–298, 1997. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Kjaerulff O. Spatiotemporal characteristics of 5-HT and dopamine-induced rhythmic hindlimb activity in the in vitro neonatal rat. J Neurophysiol 75: 1472–1482, 1996. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Sillar KT, Kjaerulff O, McDearmid JR. Effects of noradrenaline on locomotor rhythm-generating networks in the isolated neonatal rat spinal cord. J Neurophysiol 82: 741–746, 1999. [DOI] [PubMed] [Google Scholar]
- Kjaerulff O, Kiehn O. Distribution of networks generating and coordinating locomotor activity in the neonatal rat spinal cord in vitro: a lesion study. J Neurosci 16: 5777–5794, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer E, Lev-Tov A. Localization of the spinal network associated with generation of hindlimb locomotion in the neonatal rat and organization of its transverse coupling system. J Neurophysiol 77: 1155–1170, 1997. [DOI] [PubMed] [Google Scholar]
- LaHoste GJ, Henry BL, Marshall JF. Dopamine D1 receptors synergize with D2, but not D3 or D4, receptors in the striatum without the involvement of action potentials. J Neurosci 20: 6666–6671, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapointe NP, Rouleau P, Ung RV, Guertin PA. Specific role of dopamine D1 receptors in spinal network activation and rhythmic movement induction in vertebrates. J Physiol 587: 1499–1511, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madriaga MA, McPhee LC, Chersa T, Christie KJ, Whelan PJ. Modulation of locomotor activity by multiple 5-HT and dopaminergic receptor subtypes in the neonatal mouse spinal cord. J Neurophysiol 92: 1566–1576, 2004. [DOI] [PubMed] [Google Scholar]
- McCrea AE, Stehouwer DJ, van Hartesveldt C. Dopamine D1 and D2 antagonists block l-DOPA-induced air-stepping in decerebrate neonatal rats. Brain Res Dev Brain Res 100: 130–132, 1997. [DOI] [PubMed] [Google Scholar]
- McPherson DR, Kemnitz CP. Modulation of lamprey fictive swimming and motoneuron physiology by dopamine, and its immunocytochemical localization in the spinal cord. Neurosci Lett 166: 23–26, 1994. [DOI] [PubMed] [Google Scholar]
- Milan L, Barrière G, De Deurwaerdère P, Cazalets JR, Bertrand SS. Monoaminergic control of spinal locomotor networks in SOD1G93A newborn mice. Front Neural Circuits 8: 77, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles GB, Sillar KT. Neuromodulation of vertebrate locomotor control networks. Physiology (Bethesda) 26: 393–411, 2011. [DOI] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev 78: 189–225, 1998. [DOI] [PubMed] [Google Scholar]
- Mor Y, Lev-Tov A. Analysis of rhythmic patterns produced by spinal neural networks. J Neurophysiol 98: 2807–2817, 2007. [DOI] [PubMed] [Google Scholar]
- Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. J Recept Signal Transduct Res 24: 165–205, 2004. [DOI] [PubMed] [Google Scholar]
- Nishimaru H, Takizawa H, Kudo N. 5-Hydroxytryptamine-induced locomotor rhythm in the neonatal mouse spinal cord in vitro. Neurosci Lett 280: 187–190, 2000. [DOI] [PubMed] [Google Scholar]
- Qu S, Ondo WG, Zhang X, Xie WJ, Pan TH, Le WD. Projections of diencephalic dopamine neurons into the spinal cord in mice. Exp Brain Res 168: 152–156, 2006. [DOI] [PubMed] [Google Scholar]
- Ridet JL, Sandillon F, Rajaofetra N, Geffard M, Privat A. Spinal dopaminergic system of the rat: light and electron microscopic study using an antiserum against dopamine, with particular emphasis on synaptic incidence. Brain Res 598: 233–241, 1992. [DOI] [PubMed] [Google Scholar]
- Schmidt BJ, Jordan LM. The role of serotonin in reflex modulation and locomotor rhythm production in the mammalian spinal cord. Brain Res Bull 53: 689–710, 2000. [DOI] [PubMed] [Google Scholar]
- Schotland J, Shupliakov O, Wikström MA, Brodin L, Srinivasan M, You ZB, Herrera-Marschitz M, Zhang W, Hokfelt T, Grillner S. Control of lamprey locomotor neurons by colocalized monoamine transmitters. Nature 374: 266–268, 1995. [DOI] [PubMed] [Google Scholar]
- Seth P, Gajendiran M, Maitra KK, Ross HG, Ganguly DK. Evidence for D1 dopamine receptor-mediated modulation of the synaptic transmission from motor axon collaterals to Renshaw cells in the rat spinal cord. Neurosci Lett 158: 217–220, 1993. [DOI] [PubMed] [Google Scholar]
- Sharples SA, Koblinger K, Humphreys JM, Whelan PJ. Dopamine: a parallel pathway for the modulation of spinal locomotor networks. Front Neural Circuits 8: 55, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Feldman JL, Schmidt BJ. Neural mechanisms generating locomotion studied in mammalian brain stem-spinal cord in vitro. FASEB J 2: 2283–2288, 1988. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Song WJ, Yan Z. Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J Neurosci 16: 6579–6591, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson E, Woolley J, Wikström MA, Grillner S. Endogenous dopaminergic modulation of the lamprey spinal locomotor network. Brain Res 970: 1–8, 2003. [DOI] [PubMed] [Google Scholar]
- Talpalar AE, Kiehn O. Glutamatergic mechanisms for speed control and network operation in the rodent locomotor CpG. Front Neural Circuits 4: 19, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Grillner S, Wallén P. 5-HT and dopamine modulates CaV1.3 calcium channels involved in postinhibitory rebound in the spinal network for locomotion in lamprey. J Neurophysiol 105: 1212–1224, 2011. [DOI] [PubMed] [Google Scholar]
- Weil-Fugazza J, Godefroy F. Dorsal and ventral dopaminergic innervation of the spinal cord: functional implications. Brain Res Bull 30: 319–324, 1993. [DOI] [PubMed] [Google Scholar]
- Whelan P, Bonnot A, O'Donovan MJ. Properties of rhythmic activity generated by the isolated spinal cord of the neonatal mouse. J Neurophysiol 84: 2821–2833, 2000. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Tanaka M. Existence of new dopaminergic terminal plexus in the rat spinal cord: assessment by immunohistochemistry using anti-dopamine serum. Neurosci Lett 94: 5–9, 1988. [DOI] [PubMed] [Google Scholar]
- Zhong G, Sharma K, Harris-Warrick RM. Frequency-dependent recruitment of V2a interneurons during fictive locomotion in the mouse spinal cord. Nat Commun 2: 274, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Clemens S, Sawchuk MA, Hochman S. Expression and distribution of all dopamine receptor subtypes (D1–D5) in the mouse lumbar spinal cord: a real-time polymerase chain reaction and non-autoradiographic in situ hybridization study. Neuroscience 149: 885–897, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]