Abstract
Forceful, unilateral contractions modulate corticomotor paths targeting the resting, contralateral hand. However, it is unknown whether mirror-viewing of a slowly moving but forcefully contracting hand would additionally affect these paths. Here we examined corticospinal excitability and short-interval intracortical inhibition (SICI) of the right-ipsilateral primary motor cortex (M1) in healthy young adults under no-mirror and mirror conditions at rest and during right wrist flexion at 60% maximal voluntary contraction (MVC). During the no-mirror conditions neither hand was visible, whereas in the mirror conditions participants looked at the right hand's reflection in the mirror. Corticospinal excitability increased during contractions in the left flexor carpi radialis (FCR) (contraction 0.41 mV vs. rest 0.21 mV) and extensor carpi radialis (ECR) (contraction 0.56 mV vs. rest 0.39 mV), but there was no mirror effect (FCR: P = 0.743, ηp2 = 0.005; ECR: P = 0.712, ηp2 = 0.005). However, mirror-viewing of the contracting and moving wrist attenuated SICI relative to test pulse in the left FCR by ∼9% compared with the other conditions (P < 0.05, d ≥ 0.62). Electromyographic activity in the resting left hand prior to stimulation was not affected by the mirror (FCR: P = 0.255, ηp2 = 0.049; ECR: P = 0.343, ηp2 = 0.035) but increased twofold during contractions. Thus viewing the moving hand in the mirror and not just the mirror image of the nonmoving hand seems to affect motor cortical inhibitory networks in the M1 associated with the mirror image. Future studies should determine whether the use of a mirror could increase interlimb transfer produced by cross-education, especially in patient groups with unilateral orthopedic and neurological conditions.
Keywords: cross-education, strength training, mirror training, primary motor cortex, transcranial magnetic stimulation
action observation generates an internal replica of that action in the observer's motor system without causing overt motor actions (Buccino et al. 2001, 2004). Observation of a motor act performed by oneself, observation of a motor act performed by someone else, and viewing a motor act in a mirror (which is often the case in dance and sport practice) all activate the same neural structures as the actual movement execution, producing subliminal facilitation of neurons forming the motor neural network (Caspers et al. 2010; Cook et al. 2014; Molenberghs et al. 2012). The subliminal engagement of neurons might have an adaptive role in motor learning (Jeannerod 2001), and therefore action observation seems to be a potential tool to facilitate motor learning.
A specific form of motor practice that makes use of action observation is mirror training. In mirror training, the practicing limb's image is superimposed over the resting limb (Matthys et al. 2009; Nojima et al. 2012), creating the illusion in the mirror that the resting limb is moving. Mirror training is known to reduce phantom limb pain (Ramachandran et al. 1995; Ramachandran and Rogers-Ramachandran 1996) and enhance recovery of motor function of the paretic lower (Sutbeyaz et al. 2007) and upper (Michielsen et al. 2011; Yavuzer et al. 2008) extremity after a stroke and can also facilitate skill acquisition of the nontrained hand in healthy participants (Hamzei et al. 2012; Lappchen et al. 2012; Nojima et al. 2012). The benefits of mirror training are widely accepted, but the mechanisms responsible for these beneficial effects are unclear. Although viewing a movement in the form of action observation can activate, for example, the primary motor cortex (M1), whether or not and how such activation serves as a neural contribution for the beneficial effects of mirror training has not yet been verified (Lappchen et al. 2012; Nojima et al. 2012).
Mirror training exerts a strong influence on the motor network, mainly through the increased activation of areas involved in the allocation of attention and cognitive control (Deconinck et al. 2014). There is evidence that mirror-viewing of hand and finger movements performed at a fraction of the maximal voluntary force can facilitate ipsilateral corticospinal excitability (Garry et al. 2005) and corticomotor activity (Shinoura et al. 2008) compared with a no-vision condition. The increased activation of the ipsilateral M1 (Newton et al. 2002; Sehm et al. 2010) and the increased excitability of the corticospinal path targeting the resting hand (Foltys et al. 2003; Hortobagyi et al. 2003; Howatson et al. 2011; Hoy et al. 2007; Muellbacher et al. 2000; Perez and Cohen 2008, 2009) are also observed for forceful unilateral contractions without a mirror; however, it is unknown whether the visual illusion of a slowly moving, forcefully contracting wrist in the mirror can additionally affect corticospinal excitability and motor cortical activity in the hemisphere ipsilateral to the moving hand. Such information is needed as a first step to explain how mirror-viewing could augment interlimb strength transfer, a viable treatment option for patients with unilateral orthopedic and neurological impairments (Farthing and Zehr 2014).
The purpose of the present study was to determine the effects of mirror-viewing of the resting and contracting right wrist on corticospinal excitability and short-interval intracortical inhibition (SICI), assessed with transcranial magnetic stimulation (TMS) in the resting left flexor carpi radialis (FCR) and extensor carpi radialis (ECR). The ECR was measured to determine whether the observed responses to TMS would provide evidence for a directional specificity of excitability related to the mirror illusion. We suspect that mirror-viewing of the right wrist's movement—however monotonic, slow, and low-skill—creates the illusion in the ipsilateral M1 that the resting left wrist is actually moving and this illusion, a surrogate for actual movement, triggers the increase in ipsilateral M1 excitability. If this assumption is correct then we predict a mirror effect to increase neuronal excitability during a contraction that is caused by the illusion of the left hand moving but no mirror effect at rest because the trigger, i.e., movement illusion, for modulating excitability, is absent.
MATERIALS AND METHODS
Participants.
Twenty-seven right-handed [average handedness score 95% (Oldfield 1971)] healthy volunteers (22 men, 5 women) with mean (±SD) age, height, mass, and body mass index of 27 (±7) yr, 1.76 (±0.07) m, 76.0 (±13.0) kg, and 24.4 (±2.9) kg/m2, respectively, participated in the study. Prior to testing, participants completed a comprehensive screening questionnaire to determine medical [screening standard questionnaire for TMS (Rossi et al. 2009)] and experimental (i.e., previous fracture in arm or hand, pain in arm or hand) contraindications to the protocol. All participants provided written informed consent to the experimental procedure, which was approved by the University's Research Ethics Committee and in accordance with the Declaration of Helsinki.
Experimental setup.
One week before the main experiment, participants visited the laboratory for a 30-min familiarization trial to become accustomed with the laboratory setting and TMS. During the experiment, which lasted ∼1.5 h, the participant sat comfortably in a chair with both forearms resting on a custom-built table. The lever arm of an isokinetic dynamometer (Biodex Medical Systems, Shirley, NY) was aligned and configured so that the participant was able to perform shortening contractions of the right wrist flexors in the transversal plane over the table surface. Contractions were performed at 20°/s, started with the wrist at 20° extension, and ended with the wrist at 20° flexion (ensuring a total range of motion of 40°). The participant touched the lever arm in the sagittal plane with the thumb uppermost and the fingers extended to avoid finger flexion during wrist flexion. Participants performed shortening wrist flexion contractions with the right hand by pressing at the metacarpophalangeal joint on a plastic-covered manipulandum that projected vertically downward toward the table surface. The distance between the axis of rotation and the metacarpophalangeal joint position on the manipulandum was held at a constant length between conditions for each participant but was adjusted between participants to account for anatomical differences. For the resting conditions, the participant touched the lever arm in neutral position, meaning that the right wrist was in anatomical zero (0°) position.
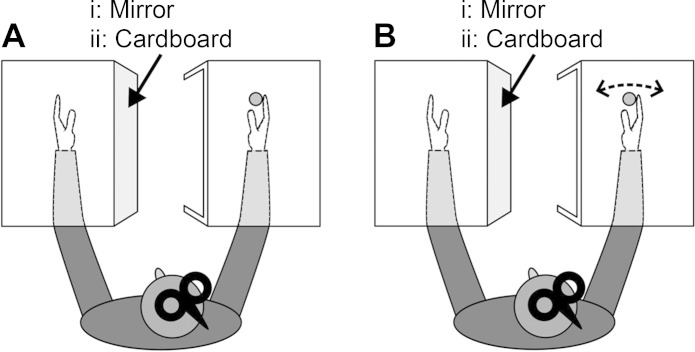
The experiment started with recording of the torque produced during a shortening maximal voluntary contraction (MVC) of the right wrist flexors. Thereafter, participants placed the left and right forearms inside two different boxes. The right box was open on the left side but was positioned in a way that prevented the participant from seeing the right hand directly. Depending on the experimental condition, a cardboard wall (no-mirror condition) or a mirror (mirror condition) was mounted on the central vertical wall of the left box and aligned in the sagittal plane in front of the participant. The cardboard and the mirror were used either to prevent seeing or to create a mirror image of the right hand, thereby giving the illusion that the left hand was being observed (Fig. 1). To maintain a constant position of the head, participants focused on a dot placed on the cardboard wall at a position that equated to the gaze of the participant when viewing the mirror image of his/her right hand.
Fig. 1.
Experimental setup at rest (A) and during a forceful shortening contraction of the right wrist flexors (B). Both forearms were rested on a custom-built table and placed inside 2 different boxes that blocked the view of the participant. i: The mirror mounted on the central vertical wall of the left box created the illusion of the left hand moving by mirror-viewing the right hand. ii: The no-mirror condition had a cardboard wall mounted on the central vertical wall of the left box.
Approximately 20 min after the MVCs, TMS was delivered to measure corticospinal excitability and SICI of the right M1 in four different conditions, namely, the mirror and no-mirror conditions at rest and during a forceful shortening contraction of the dominant-right wrist flexors at 60% MVC. TMS was delivered when the right wrist was in anatomical zero (0°) position (no-mirror and mirror resting conditions) or when the right wrist passed anatomical zero position (no-mirror and mirror contraction conditions). The left arm was placed in the same anatomical position as the right arm during all conditions, and any adornments (e.g., jewelry, watches) were removed for the duration of the experiment. The order of conditions was randomized between participants. Participants received verbal feedback from one of the researchers to reach the target torque that appeared on the dynamometer's monitor, but visual feedback was not provided at any point. Data acquisition was initiated 30 ms before the TMS stimulus was delivered. The TMS protocol adhered to current safety and ethical guidelines (Rossi et al. 2009), and all items on the methodology checklist that pertain to paired-pulse TMS have been reported and controlled (Chipchase et al. 2012). It remains unclear whether corticospinal excitability and SICI are affected by associated activity [i.e., the electromyogram (EMG) activity of the contralateral resting muscles during a unilateral muscle contraction], and because participants were less able to prevent associated activity at higher force levels (Zijdewind and Kernell 2001), we used 60% MVC as the target contraction intensity to minimize the influence of associated activity on corticospinal excitability and SICI. During the experimental conditions, participants were frequently reminded to completely relax the left arm when performing shortening right wrist flexion movements. Trials in which the associated left FCR or left ECR activity exceeded the background noise level of 25 μV were excluded from the analyses (Howatson et al. 2011; Muellbacher et al. 2000; Perez and Cohen 2008). Thereafter and for all variables, outliers were identified with a modified and more stringent version of the interquartile range method, marking values below Q1 − 1.5 × (Q2 − Q1) and values above Q3 + 1.5 × (Q3 − Q2) as outliers. All outliers were excluded from further analysis.
Maximum voluntary contraction.
After a warm-up consisting of one set of 10 shortening muscle contractions at individually estimated 50% MVC, participants performed a further three shortening right wrist flexion MVCs followed by three shortening left wrist flexion MVCs. MVCs were recorded at the same movement speed (20°/s) and range of motion (20° wrist extension to 20° wrist flexion) as during the task. The torque was recorded when the wrist passed anatomical zero for each MVC; the highest of the three contractions was recorded as the MVC. After completion of the experiment we measured shortening right wrist flexion MVC in a subsample of participants (n = 5) to examine the potential existence of fatigue.
Magnetic stimulation of primary motor cortex.
To evoke motor-evoked potentials (MEPs), TMS was delivered from a magnetic stimulator (Magstim 2002; Magstim, Spring Gardens, UK) through a figure-of-eight remote control coil (loop diameter 9 cm; Magstim) with a monophasic current waveform. Paired pulses were produced with the addition of a second Magstim 2002 stimulator equipped with a BiStim2 timing module, and pulses were delivered through the same figure-of-eight coil. The coil was placed over the M1 and was moved in 0.5-cm steps over the M1 to identify the optimal scalp position, i.e., hot spot, for activation of the left FCR overlying right M1. The hot spot targeting the left FCR is also able to produce stable MEPs in the left ECR (Carson and Ruddy 2012; MacKinnon and Rothwell 2000). The hot spot correlates well with the stimulation of Brodmann's area 4 (Mills et al. 1992). The coil was held with the handle pointing backward and 45° away from the midline so the direction of the current induced in the brain was from posterior to anterior. Initially the “hot spot” was located on each participant. The hot spot was defined as the optimal position of the coil on the scalp where the lowest threshold is capable of evoking the biggest potential in the targeted muscle (Rossini et al. 1994). The hot spot was marked with a marker pen to ensure constant positioning throughout the experiment. After the hot spot had been identified, resting motor threshold (rMT) was determined as the lowest stimulator intensity to produce an MEP of ≥50 μV in the target muscle in 5 of 10 trials (Rossini et al. 1994).
Corticospinal excitability and SICI of right M1.
To determine the effect of mirror-viewing on corticospinal excitability and SICI of the right M1 during rest and shortening right wrist flexion, single-pulse (to measure corticospinal excitability) and paired-pulse (to measure SICI) TMS were presented in random order for the mirror and no-mirror conditions. During all conditions, the MEP amplitude determining corticospinal excitability and SICI was measured in the resting left FCR and ECR. We measured corticospinal excitability by a single TMS pulse delivered at a suprathreshold intensity of 120% rMT, as part of the SICI measurement. For measuring SICI a subthreshold conditioning pulse at 80% rMT, an intensity sufficient to produce intracortical inhibition (Howatson et al. 2011; Perez and Cohen 2008), preceded the suprathreshold test pulse of 120% rMT with an interstimulus interval of 2 ms (Kujirai et al. 1993). The 2-ms interstimulus interval was used to create a deep amount of inhibition (Kujirai et al. 1993) and to avoid a mixture of the two distinct phases of inhibition (Fisher et al. 2002). A total of 20 MEPs were evoked in each condition, 10 MEPs for measuring corticospinal excitability and 10 MEPs for measuring SICI, with an interval of ∼5 s between stimuli. For determining SICI the conditioned MEPs were expressed relative to the MEPs from the unconditioned test pulse.
Surface EMG.
Surface EMG was recorded from the left and right FCR and ECR to quantify voluntary muscle activity during the experimental conditions and evoked responses (MEPs) from TMS. After the skin surface was shaved and cleaned with an alcohol wipe, electrodes (model 1041PTS; Kendall, Tyco Healthcare Group, Mansfield, MA) were placed on the muscle belly (interelectrode distance 2 cm) with the ground electrode fixed on the distal styloid process of the left radius. Surface EMG was band-pass filtered at 20–2,000 Hz, amplified ×1,000 (CED 1902; Cambridge Electronic Design, Cambridge, UK; Digitimer, Welwyn Garden City, UK), sampled at 5 kHz (CED Power 1401; Cambridge Electronic Design) and recorded on a personal computer. MEPs were analyzed off-line for peak-to-peak amplitude (Signal, v.5.04; Cambridge Electronic Design). The mean surface EMG, expressed relative to the EMG activity during shortening wrist flexion MVC, was rectified and computed over a 30-ms period prior to the stimulation artifact.
Statistical analyses.
Data are presented as means ± SD. The normal distribution for each variable was tested with the Kolmogorov-Smirnov test. For all variables except for torque, a log transformation was applied to correct for a positively skewed distribution of the data.
The main analysis addressing the hypothesis that mirror-viewing of a moving and forcefully contracting hand increases ipsilateral M1 excitability was a State (rest, contraction) × Condition (no-mirror, mirror) ANOVA with repeated measures on both factors. We performed this main analysis for each of the following variables: corticospinal excitability, SICI, and surface EMG activity in the left and right FCR and ECR, respectively. We also used a one-way repeated-measures ANOVA with five levels to determine whether wrist flexion torque of 60% MVC was similar during the mirror and no-mirror conditions in which we measured corticospinal excitability and SICI. We performed Tukey honestly significant difference (HSD) post hoc pairwise comparison to determine the means that were different.
To verify that fatigue did not affect the results, a paired-samples t-test was used to determine whether the maximal torque was similar at the start and end of the experiment. For the mirror and no-mirror conditions, a Pearson's correlation analysis was used to determine whether the change in corticospinal excitability and SICI relative to rest was correlated with the associated activity measured in the left (“resting”) FCR. For all four conditions, an additional Pearson's correlation analysis was performed to test whether surface EMGs recorded from the right and left wrist were correlated. For Pearson's product correlations we used the nontransformed data. Significance was accepted as P < 0.05. For main effects partial eta squared (ηp2) was calculated as a measure of effect size with cutoffs ≥0.01 (small), ≥0.06 (medium), and ≥0.14 (large) (Cohen 1988).
RESULTS
Table 1 shows the descriptive data for the four conditions. The main results were that viewing the mirror at rest did not affect TMS metrics but viewing the mirror while contracting the right wrist flexors reduced SICI in the left wrist flexors but not in the antagonist wrist extensors. These results were obtained under experimental conditions that were well controlled for muscle EMG activity and the level of torque subjects generated.
Table 1.
Descriptive data for the four experimental conditions
| Condition | Torquea, Nm | Torqueb, Nm | CSE Left FCR, mV | CSE Left ECR, mV | SICIc Left FCR, % of control | SICIc Left ECR, % of control | EMG Left FCR, mV | EMG Left ECR, mV | EMG Right FCR, mV | EMG Right ECR, mV |
|---|---|---|---|---|---|---|---|---|---|---|
| No-mirror, rest | N/A | N/A | 0.20 (0.15) | 0.40 (0.44) | 39.1 (23.3) | 57.0 (25.5) | 0.0010 (0.0003) | 0.0035 (0.0034) | 0.0017 (0.0023) | 0.0015 (0.0012) |
| Mirror, rest | N/A | N/A | 0.21 (0.14) | 0.37 (0.33) | 38.4 (24.4) | 56.2 (21.8) | 0.0011 (0.0004) | 0.0031 (0.00265) | 0.0019 (0.0030) | 0.0027 (0.0019)‡ |
| No-mirror, contraction | 7.8 (2.3) | 7.8 (2.3) | 0.43 (0.29)* | 0.58 (0.44)* | 37.8 (16.2) | 58.8 (22.0) | 0.0021 (0.0021)* | 0.0054 (0.0040)* | 0.1159 (0.0494)* | 0.0270 (0.0137)* |
| Mirror, contraction | 7.9 (2.4) | 7.8 (2.3) | 0.41 (0.26)* | 0.55 (0.32)* | 46.9 (18.9)† | 58.9 (17.4) | 0.0021 (0.0018)* | 0.0042 (0.0025)* | 0.1227 (0.0601)* | 0.0245 (0.0128)* |
Values are means (SD).
CSE, corticospinal excitability; ECR, extensor carpi radialis; EMG, electromyogram; FCR, flexor carpi radialis; N/A, not applicable; SICI, short-interval intracortical inhibition.
Torque recorded at the moment of stimulation for measuring corticospinal excitability;
torque recorded at the moment of stimulation for measuring SICI;
higher value means less inhibition.
Compared with the resting conditions (P < 0.001);
compared with all other conditions (P < 0.05);
compared with the no-mirror resting condition (P < 0.05).
Torque.
The torque produced during right wrist shortening contractions successfully attained the 60% MVC target torque and was similar for corticospinal excitability and SICI measured with and without the mirror (F3,26 = 0.8, P = 0.513). Also, the maximal torque production at the start (12.6 ± 3.9 Nm) was not different from the maximal torque produced at the end of the experiment [13.1 ± 4.5 Nm; t(4) = −0.845, P = 0.446], indicating that the protocol did not induce fatigue.
Corticospinal excitability.
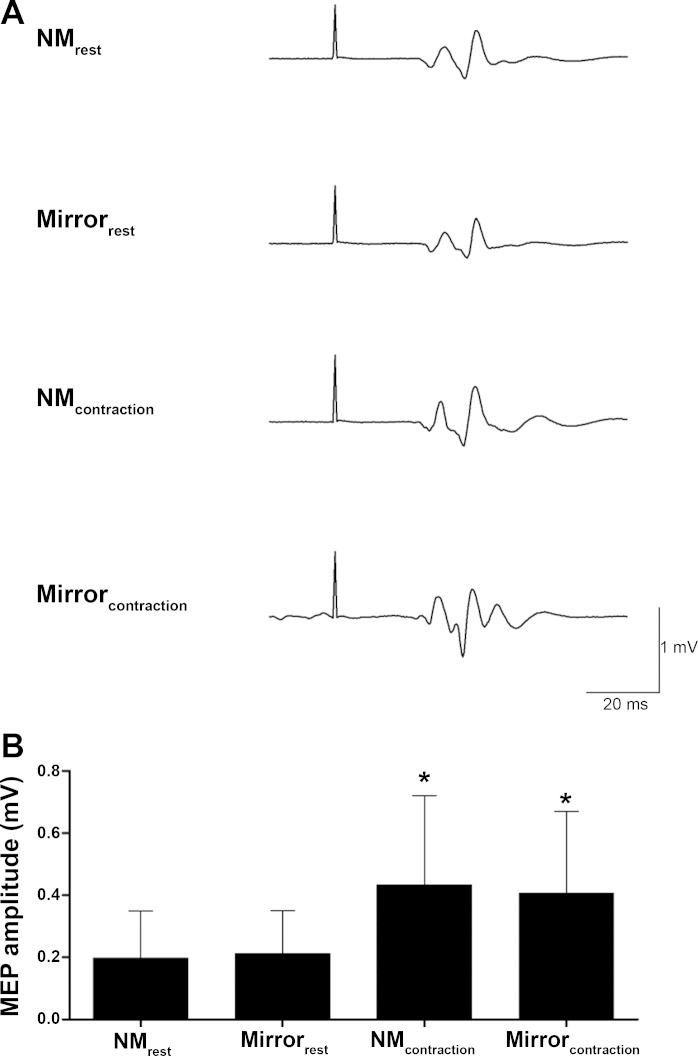
Figure 2A shows a representative trace of MEPs for a single participant, and Fig. 2B shows the group data illustrating corticospinal excitability of the right M1 recorded from the left FCR for the mirror and no-mirror conditions when both hands were at rest and during contraction. The State (rest, contraction) × Condition (no-mirror, mirror) repeated-measures ANOVA showed that corticospinal excitability was higher in both FCR (F1,26 = 77.5, P < 0.001, ηp2 = 0.749) and ECR (F1,26 = 27.0, P < 0.001, ηp2 = 0.510) during contraction compared with rest (FCR +105%, ECR +47%), but there was no effect of mirror for either muscle (FCR: F1,26 = 0.1, P = 0.734, ηp2 = 0.005; ECR: F1,26 = 0.1, P = 0.712, ηp2 = 0.005).
Fig. 2.
Corticospinal excitability of the right primary motor cortex recorded from the left flexor carpi radialis (FCR). A: representative trace of motor-evoked potentials (MEPs) from a single participant. B: mean (±SD) MEP size for the 4 different conditions. NMrest, both hands at rest with vision of both hands blocked; Mirrorrest, both hands at rest while mirror-viewing the right hand; NMcontraction, left hand at rest while the right hand performed shortening wrist flexion contractions with vision of both hands blocked; Mirrorcontraction, left hand at rest while mirror-viewing the shortening right wrist flexion contractions. *Significantly different from corticospinal excitability in resting conditions (P < 0.001; n = 27).
SICI.
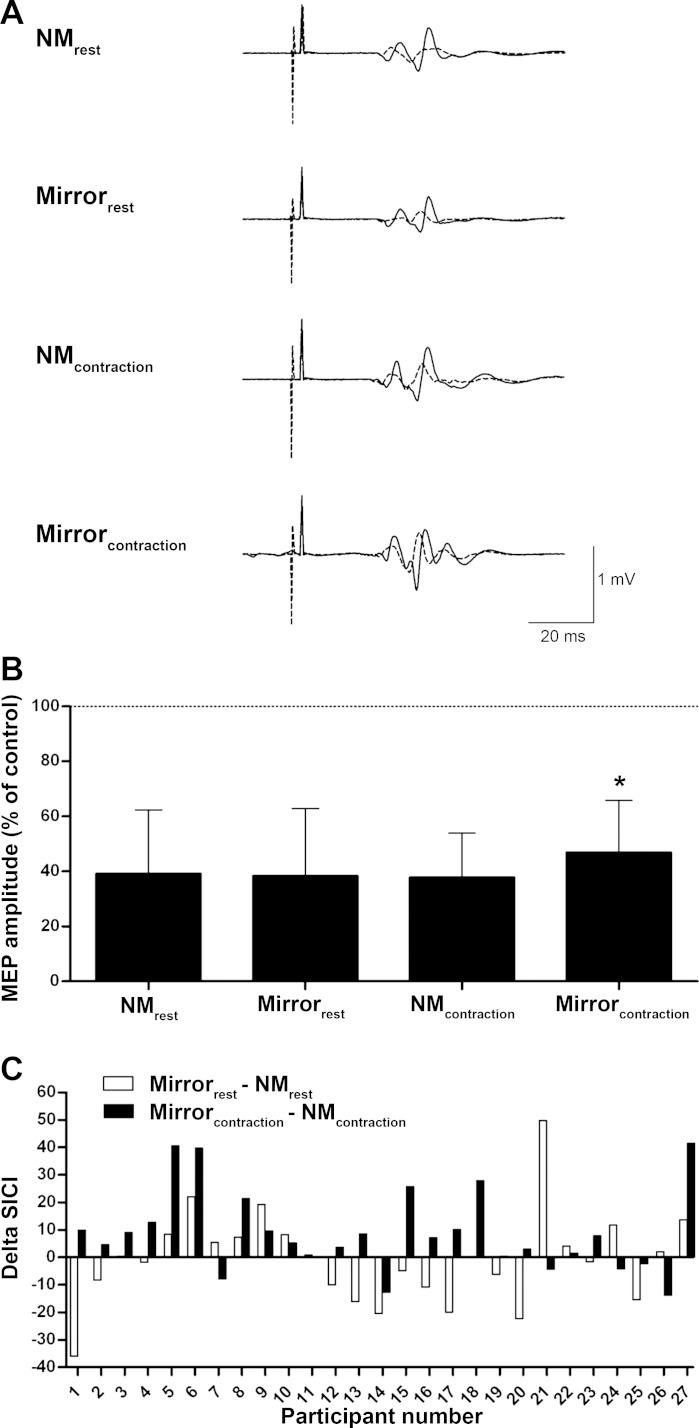
Figure 3A illustrates a representative trace of MEPs illustrating SICI for a single participant, and Fig. 3, B and C, show the SICI group data, evoked in the right M1 and recorded from the left FCR, for the four different conditions. There was no State (F1,26 = 3.6, P = 0.070, ηp2 = 0.120) or Condition (F1,26 = 2.9, P = 0.101, ηp2 = 0.100) main effect, but there was a State × Condition interaction (F1,26 = 6.9, P = 0.014, ηp2 = 0.209) for SICI recorded from the left FCR. Post hoc analysis revealed that there was ∼9% less SICI only when subjects contracted the right wrist flexors while viewing the wrist flexion movement in the mirror (P < 0.05, d ≥ 0.62). No State (F1,26 = 0.9, P = 0.347, ηp2 = 0.034) or Condition (F1,26 = 0.1, P = 0.782, ηp2 = 0.003) effect or State × Condition interaction (F1,26 = 0.2, P = 0.676, ηp2 = 0.007) was observed for SICI recorded from the left ECR.
Fig. 3.
Short-interval intracortical inhibition (SICI) in the right primary motor cortex recorded from the left FCR. A higher value means less SICI. A: representative trace of MEPs of a single participant; each tracing comprises 1 trial. Solid line, control MEP; dotted line, conditioned MEP illustrating SICI. B: mean (±SD) % of SICI relative to control. Horizontal dashed line at 100% represents the control value, i.e., absence of inhibition or facilitation. C: individual % difference of SICI between the mirror and no-mirror condition at rest (white bars) and during contraction (black bars). A positive value means a mirror image induced reduction of SICI, whereas a negative value means a mirror image induced increase of SICI. *Significantly different from SICI in all other conditions (P < 0.05; n = 27).
EMG responses in resting left limb.
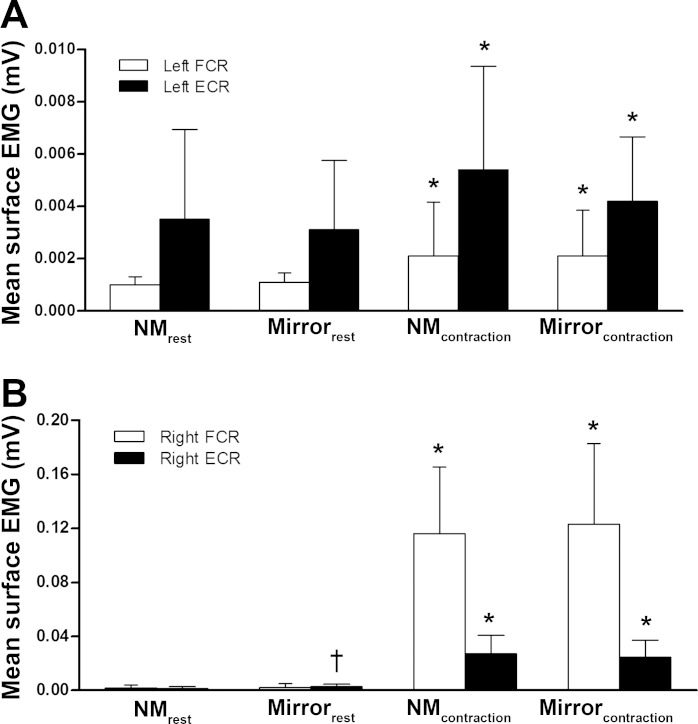
The ongoing EMG activity in the “resting” left FCR and ECR prior to stimulation was 43% higher during contraction of the contralateral limb compared with at rest (FCR: F1,26 = 32.4, P < 0.001, ηp2 = 0.555; ECR: F1,26 = 15.1, P = 0.001, ηp2= 0.368, Fig. 4A). No effect of viewing the limb in the mirror (FCR: F1,26 = 1.4, P = 0.255, ηp2 = 0.049; ECR: F1,26 = 0.9, P = 0.343, ηp2 = 0.035) or State × Condition interaction (FCR: F1,26 = 0.4, P = 0.521, ηp2 = 0.016; ECR: F1,26 = 0.9, P = 0.343, ηp2 = 0.035) was observed.
Fig. 4.
Mean (±SD) surface electromyogram (EMG), expressed relative to the EMG activity of a maximal shortening wrist flexion contraction. A: mean surface EMG for the left FCR and left extensor carpi radialis (ECR) for the 4 different conditions (n = 27). B: surface EMG for the right FCR and right ECR for the 4 different conditions (n = 27). Significant difference from surface EMG in the resting conditions (*P < 0.001) and from the no-mirror resting condition (†P < 0.05).
EMG responses in right limb.
The EMG activity present in the right FCR (0.119 ± 0.055 mV) was substantially greater than the EMG activity in the right ECR (0.026 ± 0.013 mV) during shortening right wrist flexion contractions. Mean surface EMG of the right FCR was higher during contractions compared with rest (F1,26 = 1,030.9, P < 0.001, ηp2 = 0.975) but was not affected by the mirror (F1,26 = 0.290, P = 0.595, ηp2 = 0.011). For the mean surface EMG of the right ECR, State (F1,26 = 440.6, P < 0.001, ηp2 = 0.944), Condition (F1,26 = 13.4, P = 0.001, ηp2 = 0.341), and State × Condition interaction (F1,26 = 23.4, P < 0.001, ηp2 = 0.473) effects were observed. Post hoc analysis revealed that EMG activity of the right ECR was not different for the mirror and no-mirror contraction conditions (P > 0.05) but was 80% higher for the mirror condition compared with the no-mirror condition at rest (P < 0.05; Fig. 4B).
Relationships between TMS responses and EMG activity in resting left limb.
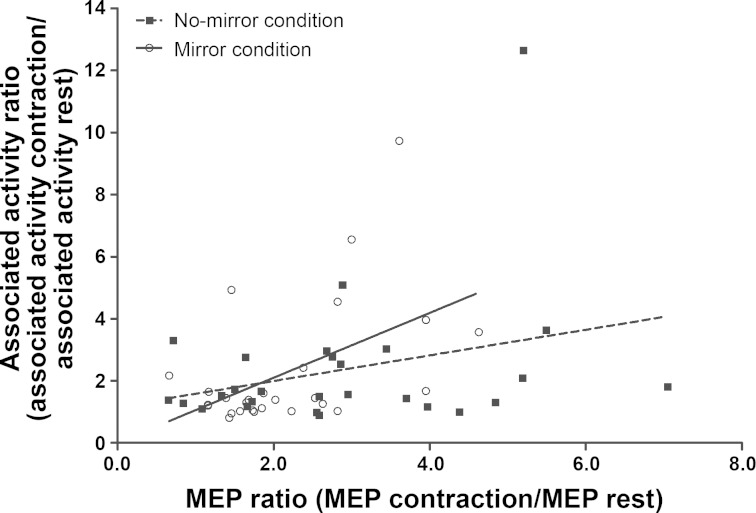
Figure 5 shows the relationship for the mirror and no-mirror viewing conditions between the change in corticospinal excitability relative to rest and the change in surface EMG of the left (noncontracting) FCR relative to rest. The change in corticospinal excitability was positively correlated to the change in surface EMG activity for the mirror condition but not for the no-mirror condition (mirror: r = 0.496, P = 0.009; no-mirror: r = 0.297, P = 0.132). No correlation was found between the change in SICI relative to rest and the change in surface EMG activity relative to rest for the mirror and no-mirror conditions (mirror: r = 0.042, P = 0.833; no-mirror: r = 0.175, P = 0.383).
Fig. 5.
Relationship for the mirror and no-mirror conditions between the change in corticospinal excitability relative to rest and the change in associated activity of the left FCR relative to rest. The change in corticospinal excitability was positively correlated to the change in surface EMG activity for the mirror condition but not for the no-mirror condition (mirror: r = 0.496, P = 0.009; no-mirror: r = 0.297, P = 0.132; n = 27).
Relationships between EMG activity in left and right limb.
The amount of EMG activity of the resting left limb was unrelated to the amount of surface EMG of the right limb for both FCR (no-mirror, rest: r = −0.075, P = 0.711; mirror, rest: r = 0.135, P = 0.501; no-mirror, contraction: r = 0.121, P = 0.548; mirror, contraction: r = 0.378, P = 0.052) and ECR (no-mirror, rest: r = 0.070, P = 0.728; mirror, rest: r = 0.318, P = 0.106; no-mirror, contraction: r = −0.061, P = 0.762; mirror, contraction: r = 0.291, P = 0.140).
DISCUSSION
We tested the hypothesis that mirror-viewing of the right wrist's flexion movement creates the illusion in the ipsilateral M1 that the resting left wrist is actually moving and this illusion changes neuronal excitability in healthy young adults. We demonstrate for the first time that performing slow, monotonic, and effortful wrist flexion while looking at the mirror image of the moving right hand reduces inhibition in the left FCR, but not ECR, compared with the no-mirror contraction and resting conditions with and without a mirror. The data are consistent with the idea that the illusion of the left hand moving and not the mirror image of the resting hand triggered the reduction in motor cortical excitability in the right-ipsilateral M1. The absence of an effect in the ECR indicates that the mirror seems to affect only the homologous agonist but not the antagonist projections. Mirror-viewing did not affect corticospinal excitability during contraction and at rest.
The results of the present study are consistent with the preponderance of data showing that mirror-viewing has little or no effect on corticospinal excitability during motor activity (Carson and Ruddy 2012; Funase et al. 2007; Reissig et al. 2014). For example, the use of a mirror does not seem to interact with contraction intensity or the nature of the contraction (static: Reissig et al. 2014; dynamic: Carson and Ruddy 2012; Funase et al. 2007). However, there is also evidence for an ∼25% increase in ipsilateral M1 corticospinal excitability in conjunction with viewing the isometrically contracting index finger (∼20% MVC) in a mirror (Garry et al. 2005). The cause of the discrepant data is unclear, considering that the experimental and recording conditions were similar in two studies, one showing an increase (Garry et al. 2005) and the other showing no effect (Reissig et al. 2014). The insensitivity of corticospinal excitability to mirror-viewing in the present study may be related to a saturation effect. Conceivably, the strong (60% MVC) muscle contraction produced a perimaximal level of excitation in the ipsilateral corticospinal path so that mirror-viewing of the contracting hand could not further increase excitability compared with the no-mirror condition.
The present data are the first to document that SICI in the right-ipsilateral M1 is modulated when a forceful right-handed unilateral contraction is performed while viewing the slowly moving wrist in the mirror. Previous studies have shown that SICI in the right-ipsilateral M1 decreased with increasing isometric right wrist flexion force (Perez and Cohen 2008), decreased during shortening wrist flexion contractions compared with rest (Howatson et al. 2011), and decreased during forceful lengthening compared with shortening wrist flexion contractions (Howatson et al. 2011). SICI in the no-mirror condition showed that contractions at 60% MVC did not affect SICI compared with rest. However, we demonstrate uniquely that mirror-viewing of the slowly moving and contracting hand decreased SICI in the right-ipsilateral M1, suggesting that it is not the contraction itself but the visual illusion of a moving left hand that modulates SICI. In support of this, a previous study showed that mirror-viewing of isometric index finger abductions did not change ipsilateral SICI compared with no-vision and other visual feedback conditions (Reissig et al. 2014); hence to create a mirror illusion and modulate SICI, it would seem the viewed image must be moving.
The premotor cortex, an area engaged in the modulation of M1 interneuron activity (Munchau et al. 2002), plays a significant role in the visual guidance of upper limb movements (Wise et al. 1997) and is therefore involved in mirror training (Hamzei et al. 2012). Thus it is possible that the modulatory effects of the premotor cortex on M1 interneurons caused the mirror-induced effect on SICI. In addition to the increased activation of the right-ipsilateral dorsal premotor cortex, Hamzei and colleagues (Hamzei et al. 2012) observed an increased activation of the left supplementary motor area after mirror training, an area known to be important in bimanual coordination (Donchin et al. 1998; Stephan et al. 1999). The present study focused on the M1, an area also known to be involved in the control of bimanual coordination (Donchin et al. 1998). There is evidence that SICI contributes to the regulation of bimanual coordination (Stinear and Byblow 2002, 2004). Therefore, this collective evidence of attenuated SICI together with the increased activation of the supplementary motor areas (Hamzei et al. 2012) after mirror training suggests that mirror-viewing of the exercising hand creates the illusion of a synchronous bimanual movement (i.e., wrist flexion with the right hand and an illusionary wrist flexion movement observed in the left hand).
An additional cortical structure that responds to the mirror image of a moving limb, but not measured in the present study, is the superior temporal gyrus. Visual information is processed differently when unilateral motor practice is performed with and without viewing a mirror (Matthys et al. 2009; Mehnert et al. 2013; Wang et al. 2013). During mirror training with the right arm, visual input is directed toward both occipital lobes with the concomitant activation of the right-ipsilateral precuneus (Mehnert et al. 2013; Wang et al. 2013) and superior temporal gyrus (Matthys et al. 2009). The superior temporal sulcus has coordinates similar to the superior temporal gyrus (Matthys et al. 2009), which is a core element of the mirror-neuron system involved in the processing of visual information (Iacoboni 2005; Iacoboni et al. 2001), whereas the precuneus seems to be involved in mediating visuomotor transformations (Dohle et al. 2011). The fact that visual information is solely processed in the ipsilateral hemisphere corresponding to the mirror image implies that the mirror creates a visual illusion as if participants exercised the left hand. Although not measured in the present experiment, there is evidence that the anterior portion of the corpus callosum, involved in interhemispheric inhibition (IHI), contributes to the integration of perception and action within a subcortico-cortical network creating a unified experience of how we perceive the visual world and prepare our actions (Schulte and Muller-Oehring 2010). It is suggested that stimulus-driven activity in one hemisphere suppresses activity in the opposite hemisphere by increasing the amount of IHI (Avanzino et al. 2014; Chiarello and Maxfield 1996). The illusion of a moving left hand while mirror-viewing the moving right hand might cause a shift in attention to the ipsilateral hemisphere to process the visual information associated with the mirror image.
During a unilateral contraction there is normally some inadvertent, so-called associated activity in the resting contralateral muscle (Sehm et al. 2010; van Duinen et al. 2008; Zijdewind et al. 2006; Zijdewind and Kernell 2001). Viewing the mirror did not affect the magnitude of associated activity in the left FCR and antagonist ECR. Although we repeated the instruction to the participant to keep his/her left hand relaxed, the magnitude of EMG activity was twofold during contractions compared with rest and was higher for the ECR than for the FCR. The associated activity, relative to the EMG activity at rest, was slightly higher than in some previous work examining unilateral wrist contractions (Sehm et al. 2010), but the absolute values were still low compared with other unilateral contraction studies (Heetkamp et al. 2014; Zijdewind et al. 2006; Zijdewind and Kernell 2001). The source of this associated activity is unclear, but bilateral M1 activation (Zijdewind et al. 2006) together with the bilateral activation of the supplementary motor area and cerebellum (Sehm et al. 2010) are thought to give rise to associated activity. Our data favor the idea that associated activity comes from the concomitant activation of both hemispheres, both M1s in particular. We found a strong and significant correlation (r = 0.496) between the associated activity and the increase in corticospinal excitability of the right-ipsilateral M1 compared with rest for the mirror condition and a moderate but nonsignificant correlation (r = 0.297) for the no-mirror condition (Fig. 5). This correlation implies that there is a link between the magnitude of corticospinal excitability and the amount of associated activity and that this link is strengthened when the contracting right hand is viewed in the mirror. Thereby, mirror-viewing of the contracting right hand resulted in a borderline significant correlation between EMG activity of the left (i.e., associated activity) and right agonist FCR. Altogether, mirror-viewing of the contracting right hand strengthens the connectivity between the contracting agonist and contralateral homologous muscle, possibly via a mirror-induced modulation of the link between bilateral M1 activation and amount of associated activity.
Mirror-viewing of a unilateral muscle contraction affected SICI but not associated activity in the present study. Thus a lack of change in associated activity strengthens the idea that the activity that modulates SICI in response to mirror-viewing arises in the ipsilateral M1. However, without measuring IHI, we cannot specifically ascertain whether this modulation occurs as a process intrinsic to ipsilateral M1, through IHI, or both. Future studies will have to disentangle the effects of mirror-viewing on associated activity and IHI to better understand the mechanism of how mirror-viewing works and could be applied to clinical conditions.
Limitations.
The anterior corticospinal tract, which does not cross the medulla and occupies 5–15% of the entire corticospinal tract, has been proposed as a motor recovery pathway from the unaffected M1 to the affected extremities (Jang 2014). It is hypothesized that this ipsilateral motor pathway might be facilitated by mirror training (Deconinck et al. 2014), so for our study this would mean that mirror-viewing not only affected the right-ipsilateral but also the left-contralateral M1, an area we did not examine. Another interesting aspect that is missing is the comparison between an active vision condition, where participants directly viewed the contracting right hand, and the mirror condition where participants observed the contracting right hand in the mirror. Previous work showed that ipsilateral M1 corticospinal excitability was not different between these two conditions during a static movement (Garry et al. 2005; Reissig et al. 2014), but during a dynamic movement ipsilateral corticospinal excitability (Kang et al. 2011) and ipsilateral M1 activity (Touzalin-Chretien et al. 2010) were significantly higher for the mirror condition. This again underpins the notion that the observed image must be dynamic to induce a mirror effect, and although we have not tested the hypothesis, we expect that mirror-viewing of a wrist flexion increases corticospinal excitability compared with an active vision condition.
Implications for practice.
Mirror training is used in the treatment of chronic pain conditions (Bowering et al. 2013) and to improve motor function after stroke (Thieme et al. 2012). Somewhat surprisingly, recent work without a mirror showed that strength training of the unaffected limb is beneficial for the recovery of the impaired limb after stroke (Clark and Patten 2013; Dragert and Zehr 2011, 2013), wrist fractures (Magnus et al. 2013), and anterior cruciate ligament reconstructive surgery (Papandreou et al. 2013). The performance improvement in the contralateral homologous muscle of the nontrained limb following a period of effortful unilateral motor practice is referred to as cross-education (Farthing et al. 2007; Hortobagyi 2005; Munn et al. 2004; Zhou 2000), but there may be additional clinical benefits from the hypothesis that unilateral strength training with a mirror could augment the cross-education of muscle strength (Howatson et al. 2013; Zult et al. 2014). Reduction in SICI observed in the present study could be one mechanism to explain how the use of a mirror increases the transfer effect reported in cross-education studies.
In summary, viewing one's own right hand in a mirror, appearing as the left hand, during a slow but forceful muscle contraction reduces one form of intracortical inhibition (SICI) in the right-ipsilateral M1. This modulation of SICI was specific to the left FCR, the contralateral homolog of the task muscle on the right side. The use of a mirror, however, did not affect corticospinal excitability of the right M1 and the associated activity in the homolog FCR and nonhomolog ECR. Thus viewing the moving hand and not just the mirror image of the nonmoving hand seems to affect motor cortical inhibitory networks in the hemisphere associated with the mirror image. These acute mirror-induced changes support the idea that mirror-aided unilateral strength training might be more effective than unilateral strength training without a mirror for accelerating functional recovery from unilateral impairments. Future studies should determine whether the use of a mirror could increase interlimb transfer produced by cross-education, especially in patient populations with unilateral orthopedic and neurological conditions.
GRANTS
This work was supported by a start-up fund from the University Medical Center Groningen.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.Z., S.G., T.H., and G.H. conception and design of research; T.Z., S.G., K.T., and G.H. performed experiments; T.Z. analyzed data; T.Z., S.G., K.T., T.H., and G.H. interpreted results of experiments; T.Z. and S.G. prepared figures; T.Z., S.G., K.T., T.H., and G.H. drafted manuscript.
ACKNOWLEDGMENTS
The authors thank Koen Huits for creating the artwork for Fig. 1.
REFERENCES
- Avanzino L, Raffo A, Pelosin E, Ogliastro C, Marchese R, Ruggeri P, Abbruzzese G. Training based on mirror visual feedback influences transcallosal communication. Eur J Neurosci 40: 2581–2585, 2014. [DOI] [PubMed] [Google Scholar]
- Bowering KJ, O'Connell NE, Tabor A, Catley MJ, Leake HB, Moseley GL, Stanton TR. The effects of graded motor imagery and its components on chronic pain: a systematic review and meta-analysis. J Pain 14: 3–13, 2013. [DOI] [PubMed] [Google Scholar]
- Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, Seitz RJ, Zilles K, Rizzolatti G, Freund HJ. Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. Eur J Neurosci 13: 400–404, 2001. [PubMed] [Google Scholar]
- Buccino G, Lui F, Canessa N, Patteri I, Lagravinese G, Benuzzi F, Porro CA, Rizzolatti G. Neural circuits involved in the recognition of actions performed by nonconspecifics: an FMRI study. J Cogn Neurosci 16: 114–126, 2004. [DOI] [PubMed] [Google Scholar]
- Carson RG, Ruddy KL. Vision modulates corticospinal suppression in a functionally specific manner during movement of the opposite limb. J Neurosci 32: 646–652, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspers S, Zilles K, Laird AR, Eickhoff SB. ALE meta-analysis of action observation and imitation in the human brain. Neuroimage 50: 1148–1167, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarello C, Maxfield L. Varieties of interhemispheric inhibition, or how to keep a good hemisphere down. Brain Cogn 30: 81–108, 1996. [DOI] [PubMed] [Google Scholar]
- Chipchase L, Schabrun S, Cohen L, Hodges P, Ridding M, Rothwell J, Taylor J, Ziemann U. A checklist for assessing the methodological quality of studies using transcranial magnetic stimulation to study the motor system: an international consensus study. Clin Neurophysiol 123: 1698–1704, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DJ, Patten C. Eccentric versus concentric resistance training to enhance neuromuscular activation and walking speed following stroke. Neurorehabil Neural Repair 27: 335–344, 2013. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. New York: Academic, 1988. [Google Scholar]
- Cook R, Bird G, Catmur C, Press C, Heyes C. Mirror neurons: from origin to function. Behav Brain Sci 37: 177–192, 2014. [DOI] [PubMed] [Google Scholar]
- Deconinck FJ, Smorenburg AR, Benham A, Ledebt A, Feltham MG, Savelsbergh GJ. Reflections on mirror therapy: a systematic review of the effect of mirror visual feedback on the brain. Neurorehabil Neural Repair (August 26, 2014). doi: 10.1177/1545968314546134. [DOI] [PubMed] [Google Scholar]
- Dohle C, Stephan KM, Valvoda JT, Hosseiny O, Tellmann L, Kuhlen T, Seitz RJ, Freund HJ. Representation of virtual arm movements in precuneus. Exp Brain Res 208: 543–555, 2011. [DOI] [PubMed] [Google Scholar]
- Donchin O, Gribova A, Steinberg O, Bergman H, Vaadia E. Primary motor cortex is involved in bimanual coordination. Nature 395: 274–278, 1998. [DOI] [PubMed] [Google Scholar]
- Dragert K, Zehr EP. Bilateral neuromuscular plasticity from unilateral training of the ankle dorsiflexors. Exp Brain Res 208: 217–227, 2011. [DOI] [PubMed] [Google Scholar]
- Dragert K, Zehr EP. High-intensity unilateral dorsiflexor resistance training results in bilateral neuromuscular plasticity after stroke. Exp Brain Res 225: 93–104, 2013. [DOI] [PubMed] [Google Scholar]
- Farthing JP, Borowsky R, Chilibeck PD, Binsted G, Sarty GE. Neuro-physiological adaptations associated with cross-education of strength. Brain Topogr 20: 77–88, 2007. [DOI] [PubMed] [Google Scholar]
- Farthing JP, Zehr EP. Restoring symmetry: clinical applications of cross-education. Exerc Sport Sci Rev 42: 70–75, 2014. [DOI] [PubMed] [Google Scholar]
- Fisher RJ, Nakamura Y, Bestmann S, Rothwell JC, Bostock H. Two phases of intracortical inhibition revealed by transcranial magnetic threshold tracking. Exp Brain Res 143: 240–248, 2002. [DOI] [PubMed] [Google Scholar]
- Foltys H, Meister IG, Weidemann J, Sparing R, Thron A, Willmes K, Topper R, Hallett M, Boroojerdi B. Power grip disinhibits the ipsilateral sensorimotor cortex: a TMS and fMRI study. Neuroimage 19: 332–340, 2003. [DOI] [PubMed] [Google Scholar]
- Funase K, Tabira T, Higashi T, Liang N, Kasai T. Increased corticospinal excitability during direct observation of self-movement and indirect observation with a mirror box. Neurosci Lett 419: 108–112, 2007. [DOI] [PubMed] [Google Scholar]
- Garry MI, Loftus A, Summers JJ. Mirror, mirror on the wall: viewing a mirror reflection of unilateral hand movements facilitates ipsilateral M1 excitability. Exp Brain Res 163: 118–122, 2005. [DOI] [PubMed] [Google Scholar]
- Hamzei F, Lappchen CH, Glauche V, Mader I, Rijntjes M, Weiller C. Functional plasticity induced by mirror training: the mirror as the element connecting both hands to one hemisphere. Neurorehabil Neural Repair 26: 484–496, 2012. [DOI] [PubMed] [Google Scholar]
- Heetkamp J, Hortobagyi T, Zijdewind I. Increased bilateral interactions in middle-aged subjects. Front Aging Neurosci 6: 5, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortobagyi T. Cross education and the human central nervous system. IEEE Eng Med Biol Mag 24: 22–28, 2005. [DOI] [PubMed] [Google Scholar]
- Hortobagyi T, Taylor JL, Petersen NT, Russell G, Gandevia SC. Changes in segmental and motor cortical output with contralateral muscle contractions and altered sensory inputs in humans. J Neurophysiol 90: 2451–2459, 2003. [DOI] [PubMed] [Google Scholar]
- Howatson G, Taylor MB, Rider P, Motawar BR, McNally MP, Solnik S, DeVita P, Hortobagyi T. Ipsilateral motor cortical responses to TMS during lengthening and shortening of the contralateral wrist flexors. Eur J Neurosci 33: 978–990, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howatson G, Zult T, Farthing JP, Zijdewind I, Hortobagyi T. Mirror training to augment cross-education during resistance training: a hypothesis. Front Hum Neurosci 7: 396, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy KE, Georgiou-Karistianis N, Laycock R, Fitzgerald PB. Using transcranial magnetic stimulation to investigate the cortical origins of motor overflow: a study in schizophrenia and healthy controls. Psychol Med 37: 583–594, 2007. [DOI] [PubMed] [Google Scholar]
- Iacoboni M. Neural mechanisms of imitation. Curr Opin Neurobiol 15: 632–637, 2005. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Koski LM, Brass M, Bekkering H, Woods RP, Dubeau MC, Mazziotta JC, Rizzolatti G. Reafferent copies of imitated actions in the right superior temporal cortex. Proc Natl Acad Sci USA 98: 13995–13999, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang SH. The corticospinal tract from the viewpoint of brain rehabilitation. J Rehabil Med 46: 193–199, 2014. [DOI] [PubMed] [Google Scholar]
- Jeannerod M. Neural simulation of action: a unifying mechanism for motor cognition. Neuroimage 14: S103–S109, 2001. [DOI] [PubMed] [Google Scholar]
- Kang YJ, Ku J, Kim HJ, Park HK. Facilitation of corticospinal excitability according to motor imagery and mirror therapy in healthy subjects and stroke patients. Ann Rehabil Med 35: 747–758, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol 471: 501–519, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappchen CH, Ringer T, Blessin J, Seidel G, Grieshammer S, Lange R, Hamzei F. Optical illusion alters M1 excitability after mirror therapy: a TMS study. J Neurophysiol 108: 2857–2861, 2012. [DOI] [PubMed] [Google Scholar]
- MacKinnon CD, Rothwell JC. Time-varying changes in corticospinal excitability accompanying the triphasic EMG pattern in humans. J Physiol 528: 633–645, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnus CR, Arnold CM, Johnston G, Dal-Bello Haas V, Basran J, Krentz JR, Farthing JP. Cross-education for improving strength and mobility after distal radius fractures: a randomized controlled trial. Arch Phys Med Rehabil 94: 1247–1255, 2013. [DOI] [PubMed] [Google Scholar]
- Matthys K, Smits M, Van der Geest JN, Van der Lugt A, Seurinck R, Stam HJ, Selles RW. Mirror-induced visual illusion of hand movements: a functional magnetic resonance imaging study. Arch Phys Med Rehabil 90: 675–681, 2009. [DOI] [PubMed] [Google Scholar]
- Mehnert J, Brunetti M, Steinbrink J, Niedeggen M, Dohle C. Effect of a mirror-like illusion on activation in the precuneus assessed with functional near-infrared spectroscopy. J Biomed Opt 18: 066001, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michielsen ME, Selles RW, van der Geest JN, Eckhardt M, Yavuzer G, Stam HJ, Smits M, Ribbers GM, Bussmann JB. Motor recovery and cortical reorganization after mirror therapy in chronic stroke patients: a phase II randomized controlled trial. Neurorehabil Neural Repair 25: 223–233, 2011. [DOI] [PubMed] [Google Scholar]
- Mills KR, Boniface SJ, Schubert M. Magnetic brain stimulation with a double coil: the importance of coil orientation. Electroencephalogr Clin Neurophysiol 85: 17–21, 1992. [DOI] [PubMed] [Google Scholar]
- Molenberghs P, Cunnington R, Mattingley JB. Brain regions with mirror properties: a meta-analysis of 125 human fMRI studies. Neurosci Biobehav Rev 36: 341–349, 2012. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Facchini S, Boroojerdi B, Hallett M. Changes in motor cortex excitability during ipsilateral hand muscle activation in humans. Clin Neurophysiol 111: 344–349, 2000. [DOI] [PubMed] [Google Scholar]
- Munchau A, Bloem BR, Irlbacher K, Trimble MR, Rothwell JC. Functional connectivity of human premotor and motor cortex explored with repetitive transcranial magnetic stimulation. J Neurosci 22: 554–561, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn J, Herbert RD, Gandevia SC. Contralateral effects of unilateral resistance training: a meta-analysis. J Appl Physiol 96: 1861–1866, 2004. [DOI] [PubMed] [Google Scholar]
- Newton J, Sunderland A, Butterworth SE, Peters AM, Peck KK, Gowland PA. A pilot study of event-related functional magnetic resonance imaging of monitored wrist movements in patients with partial recovery. Stroke 33: 2881–2887, 2002. [DOI] [PubMed] [Google Scholar]
- Nojima I, Mima T, Koganemaru S, Thabit MN, Fukuyama H, Kawamata T. Human motor plasticity induced by mirror visual feedback. J Neurosci 32: 1293–1300, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113, 1971. [DOI] [PubMed] [Google Scholar]
- Papandreou M, Billis E, Papathanasiou G, Spyropoulos P, Papaioannou N. Cross-exercise on quadriceps deficit after ACL reconstruction. J Knee Surg 26: 51–58, 2013. [DOI] [PubMed] [Google Scholar]
- Perez MA, Cohen LG. Mechanisms underlying functional changes in the primary motor cortex ipsilateral to an active hand. J Neurosci 28: 5631–5640, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez MA, Cohen LG. Scaling of motor cortical excitability during unimanual force generation. Cortex 45: 1065–1071, 2009. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS, Rogers-Ramachandran D. Synaesthesia in phantom limbs induced with mirrors. Proc Biol Sci 263: 377–386, 1996. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS, Rogers-Ramachandran D, Cobb S. Touching the phantom limb. Nature 377: 489–490, 1995. [DOI] [PubMed] [Google Scholar]
- Reissig P, Garry MI, Summers JJ, Hinder MR. Visual feedback-related changes in ipsilateral cortical excitability during unimanual movement: implications for mirror therapy. Neuropsychol Rehabil 24: 936–957, 2014. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A, Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 120: 2008–2039, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijevic MR, Hallett M, Katayama Y, Lucking CH. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol 91: 79–92, 1994. [DOI] [PubMed] [Google Scholar]
- Schulte T, Muller-Oehring EM. Contribution of callosal connections to the interhemispheric integration of visuomotor and cognitive processes. Neuropsychol Rev 20: 174–190, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehm B, Perez MA, Xu B, Hidler J, Cohen LG. Functional neuroanatomy of mirroring during a unimanual force generation task. Cereb Cortex 20: 34–45, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoura N, Suzuki Y, Watanabe Y, Yamada R, Tabei Y, Saito K, Yagi K. Mirror therapy activates outside of cerebellum and ipsilateral M1. NeuroRehabilitation 23: 245–252, 2008. [PubMed] [Google Scholar]
- Stephan KM, Binkofski F, Halsband U, Dohle C, Wunderlich G, Schnitzler A, Tass P, Posse S, Herzog H, Sturm V, Zilles K, Seitz RJ, Freund HJ. The role of ventral medial wall motor areas in bimanual co-ordination. A combined lesion and activation study. Brain 122: 351–368, 1999. [DOI] [PubMed] [Google Scholar]
- Stinear JW, Byblow WD. Disinhibition in the human motor cortex is enhanced by synchronous upper limb movements. J Physiol 543: 307–316, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinear JW, Byblow WD. An interhemispheric asymmetry in motor cortex disinhibition during bimanual movement. Brain Res 1022: 81–87, 2004. [DOI] [PubMed] [Google Scholar]
- Sutbeyaz S, Yavuzer G, Sezer N, Koseoglu BF. Mirror therapy enhances lower-extremity motor recovery and motor functioning after stroke: a randomized controlled trial. Arch Phys Med Rehabil 88: 555–559, 2007. [DOI] [PubMed] [Google Scholar]
- Thieme H, Mehrholz J, Pohl M, Behrens J, Dohle C. Mirror therapy for improving motor function after stroke. Cochrane Database Syst Rev 3: CD008449, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touzalin-Chretien P, Ehrler S, Dufour A. Dominance of vision over proprioception on motor programming: evidence from ERP. Cereb Cortex 20: 2007–2016, 2010. [DOI] [PubMed] [Google Scholar]
- van Duinen H, Renken R, Maurits NM, Zijdewind I. Relation between muscle and brain activity during isometric contractions of the first dorsal interosseus muscle. Hum Brain Mapp 29: 281–299, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Fritzsch C, Bernarding J, Holtze S, Mauritz KH, Brunetti M, Dohle C. A comparison of neural mechanisms in mirror therapy and movement observation therapy. J Rehabil Med 45: 410–413, 2013. [DOI] [PubMed] [Google Scholar]
- Wise SP, Boussaoud D, Johnson PB, Caminiti R. Premotor and parietal cortex: corticocortical connectivity and combinatorial computations. Annu Rev Neurosci 20: 25–42, 1997. [DOI] [PubMed] [Google Scholar]
- Yavuzer G, Selles R, Sezer N, Sutbeyaz S, Bussmann JB, Koseoglu F, Atay MB, Stam HJ. Mirror therapy improves hand function in subacute stroke: a randomized controlled trial. Arch Phys Med Rehabil 89: 393–398, 2008. [DOI] [PubMed] [Google Scholar]
- Zhou S. Chronic neural adaptations to unilateral exercise: mechanisms of cross education. Exerc Sport Sci Rev 28: 177–184, 2000. [PubMed] [Google Scholar]
- Zijdewind I, Butler JE, Gandevia SC, Taylor JL. The origin of activity in the biceps brachii muscle during voluntary contractions of the contralateral elbow flexor muscles. Exp Brain Res 175: 526–535, 2006. [DOI] [PubMed] [Google Scholar]
- Zijdewind I, Kernell D. Bilateral interactions during contractions of intrinsic hand muscles. J Neurophysiol 85: 1907–1913, 2001. [DOI] [PubMed] [Google Scholar]
- Zult T, Howatson G, Kadar EE, Farthing JP, Hortobagyi T. Role of the mirror-neuron system in cross-education. Sports Med 44: 159–178, 2014. [DOI] [PubMed] [Google Scholar]