Abstract
The utricle provides critical information about spatiotemporal properties of head movement. It comprises multiple subdivisions whose functional roles are poorly understood. We previously identified four subdivisions in turtle utricle, based on hair bundle structure and mechanics, otoconial membrane structure and hair bundle coupling, and immunoreactivity to calcium-binding proteins. Here we ask whether these macular subdivisions are innervated by distinctive populations of afferents to help us understand the role each subdivision plays in signaling head movements. We quantified the morphology of 173 afferents and identified six afferent classes, which differ in structure and macular locus. Calyceal and dimorphic afferents innervate one striolar band. Bouton afferents innervate a second striolar band; they have elongated terminals and the thickest processes and axons of all bouton units. Bouton afferents in lateral (LES) and medial (MES) extrastriolae have small-diameter axons but differ in collecting area, bouton number, and hair cell contacts (LES >> MES). A fourth, distinctive population of bouton afferents supplies the juxtastriola. These results, combined with our earlier findings on utricular hair cells and the otoconial membrane, suggest the hypotheses that MES and calyceal afferents encode head movement direction with high spatial resolution and that MES afferents are well suited to signal three-dimensional head orientation and striolar afferents to signal head movement onset.
Keywords: hair cells, turtle, utricle, vestibular afferents
the vestibular labyrinth is poorly understood compared, for example, to the auditory periphery and the retina. This is especially true of the major otoconial organs, the utricle and saccule, which encode linear head accelerations, including head position in gravity space, and transmit this information to the CNS. One important question is how the sensory surface of otoconial organs is organized to transduce the full range of signals generated by head movement. There are two broad possibilities: 1) all regions are equipotential in their ability to encode temporal properties of head movement (e.g., different frequencies, accelerations) or 2) different regions of the macula play distinctive functional roles.
Considerable evidence supports the second of these alternatives. The best-known example of regional specializations in otoconial maculae is the ubiquitous division between striola and extrastriola: the striolar region of vertebrate maculae differs from the extrastriola in the structure and molecular composition of the otoconial membrane (OM) (e.g., Goodyear et al. 1994; Lim 1979; Lindeman 1969; Werner 1933; reviewed in Fermin et al. 1998; Lewis et al. 1985), which transmits the mechanical stimuli arising from head movements to receptors (hair cells), and in the structure and physiology of its hair cells (e.g., Baird 1994a, 1994b; Goodyear and Richardson 1992; Li et al. 2008; Schweizer et al. 2009; Weng and Correia 1999; reviews in Eatock and Lysakowski 2006; Eatock and Songer 2011; Lewis et al. 1985; Platt 1983) and their postsynaptic primary afferents (e.g., Baird and Lewis 1986; Baird and Schuff 1994; Desai et al. 2005; Fernandez et al. 1990; Si et al. 2003; reviews in Eatock and Songer 2011; Lysakowski and Goldberg 2004). For example, there are three broad classes of primary afferents in amniotes: all-calyx afferents (C units), which contact type I hair cells exclusively; bouton afferents (B units), which contact only type II hair cells; and dimorphic afferents (D units), which contact both hair cell types. In mammals, C units and B units are restricted to the striola and extrastriola, respectively (Fernandez et al. 1990), and the physiological properties of D units differ significantly as a function of macular locus (striola vs. extrastriola; Goldberg et al. 1990b).
There is also evidence for further macular differentiation beyond the striola: extrastriola dichotomy, including subdivisions within the striola (well documented in nonmammalian amniotes; Jorgensen 1989; Si et al. 2003; Xue and Peterson 2006), differences between the medial (MES) and lateral (LES) extrastriolae (Baird and Schuff 1994; Li et al. 2008; Rowe and Peterson 2006; Xue and Peterson 2006), and a possible juxtastriolar region between the striola and the MES (Chang et al. 1992; Baird and Schuff 1994; Fernandez et al. 1990; Goldberg et al. 1990b; Saidel and Crowder 1997). Thus the available data indicate that otoconial maculae may comprise multiple regions with distinctive properties. We need to characterize these macular regions in order to understand how they encode information about head movement and transmit it to the CNS. In support of this goal, the present study sought to identify and characterize regional differences in the morphology of primary afferents innervating the utricular macula of a turtle.
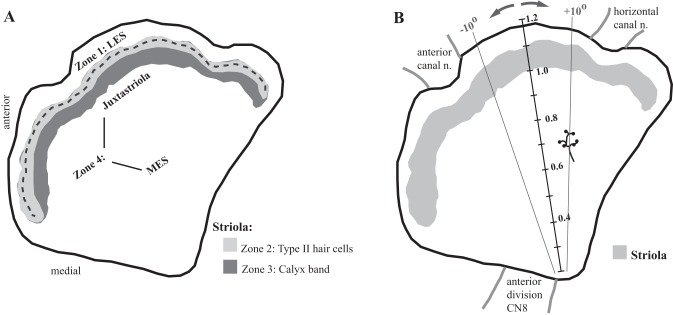
We focused on turtle utricle for two reasons. First, the utricle is generally considered to be a dedicated vestibular organ (but see Zhu et al. 2014); other otoconial organs such as the saccule and lagena may also function in hearing and seismic detection (Narins and Lewis 1984), which can complicate functional interpretations. Second, more is known about the structure (Moravec and Peterson 2004; Rowe and Peterson 2006; Severinsen et al. 2003; Xue et al. 2007; Xue and Peterson 2003, 2006), physiology (Meyer and Eatock 2011), and mechanical properties (Davis et al. 2007; Davis and Grant 2014; Dunlap et al. 2012; Dunlap and Grant 2014; Nam et al. 2005, 2006, 2007a, 2007b; Silber et al. 2004; Spoon et al. 2011; Spoon and Grant 2011) of hair bundles and the OM in turtle utricle than in any other species. These data have revealed four structurally and mechanically distinct macular zones (Fig. 1A). Zone 1 corresponds to the LES, and Zone 4 corresponds to the MES. These two extrastriolar zones have similarly compliant hair bundles (Spoon et al. 2011), but they differ significantly in the structure of their hair bundles (Rowe and Peterson 2006; Severinsen et al. 2003; Xue and Peterson 2006) and overlying OMs (Davis et al. 2007; Xue et al. 2007; Xue and Peterson 2003). The remaining two zones constitute the striola. Zone 2 is a band of type II hair cells with structurally distinct hair bundles (Rowe and Peterson 2006; Xue and Peterson 2006) that are the stiffest in the macula (Spoon et al. 2011). Zone 3 is a band of type I hair cells with their postsynaptic calyces and a small number of intercalated type II hair cells (Xue and Peterson 2006); thus, in turtle utricle, type I hair cells and calyx-bearing afferents are restricted to Zone 3. The type I hair cells have significantly more stereocilia than all other macular hair cells (Moravec and Peterson 2004).
Fig. 1.
Afferent terminal location. A: macular subdivisions. We assigned each reconstructed terminal to a macular subdivision as follows. The striola comprises 2 parallel bands. The more lateral band (light gray; Zone 2) contains type II hair cells only. The line of hair cell polarity reversal (LPR; dashed line) runs near the lateral margin of Zone 2. The more medial band (dark gray; Zone 3) is defined by the presence of calyces; all type I hair cells and all C and D units are confined to Zone 3. Lateral to the striola is Zone 1 [lateral extrastriola (LES)], which contains type II hair cells only. Medial to the striola is Zone 4. Most of Zone 4 is occupied by type II hair cells with distinctive hair bundles (Xue and Peterson 2006) and afferent terminals (present results); we refer to this region as the medial extrastriola (MES). Finally, we identified an ∼150-μm-wide band medial to striolar Zone 3 where afferent structure differs significantly from that in the rest of Zone 4: the juxtastriola. B: terminal location. To assign each reconstructed terminal a standardized location in x-y space, we anchored a radial grid on a transect line (solid black line) that runs from the posterior margin of the anterior nerve (anterior division CN8) to a point midway between the anterior and horizontal canal nerves. We used this radial grid to assign each terminal a radial location, where negative and positive angles are anterior and posterior to the transect line, respectively. The figure shows example radial lines (for ±10°). Distance along each radial line was expressed as a % distance along the transect, where 1.0 represents the medial margin of the calyx band (Zone 3; gray profile). It is readily visible with differential interference contrast (DIC) optics and therefore serves as a consistent starting point for radial distance measurements. Because the macula is ∼1 mm mediolaterally, tick marks along the transect are ∼100 μm apart. In the example shown, the terminal is roughly 10° posterior to the transect line and 300 μm from the medial margin of the striola (% distance = 0.7).
These differences in structure and mechanics suggest that macular zones in turtle utricle will play different roles in encoding head movement. They also raise the question of whether distinctive populations of afferents may innervate the four macular zones. Such distinctive afferent populations would be important because they could contribute to the specialized signaling capabilities of different macular loci. Here we test this hypothesis: we use morphological approaches to ask whether distinct afferent populations transmit signals from structurally and mechanically distinct subdivisions of the utricular macula.
MATERIALS AND METHODS
Twenty-six juvenile turtles, Trachemys (Pseudemys) scripta elegans, of both sexes (3.5- to 5.75-in. carapace length; Kons Scientific, Germantown, WI) provided useful data for reconstruction. The animals were kept in 100-gallon Rubbermaid stock tanks with homemade Plexiglas resting platforms. Room and basking lights (spot light 12–15 in. above resting platform) were on a 12:12-h on-off light cycle. Room temperature was kept at 85–88°F, which kept the water temperature at 77°F. The animals were fed daily with Wardley Reptile Ten-Total Essential Nutrition food sticks (That Fish Place, Lancaster, PA). All experiments were conducted on the utricle and the anterior branch of the VIIIth nerve. In earlier experiments animals were killed via an overdose of Brevital (0.4–0.6 ml im; followed by decapitation). In subsequent experiments animals were killed via an overdose of Euthasol (390 mg pentobarbital sodium and 50 mg phenytoin sodium per ml; 0.5–0.8 ml im). Six additional experiments were conducted in conjunction with a separate physiology study; these animals were killed by decapitation. All experiments followed protocols reviewed and approved by the Ohio University Animal Care and Use Committee.
Terminology
In this paper, the term “unit” refers to a utricular primary afferent. C units are all-calyx afferents; D units are dimorphic afferents; B units are bouton afferents. The term “CD units” refers, collectively, to C units and D units combined, i.e., all calyx-bearing units. “Terminal” refers to all the endings of an afferent within the macular epithelium, i.e., all the endings arising from a single parent axon. These endings can bear calyces (C units), boutons (B units), or both (D units). The term “calyx” refers to a single cup. Calyx endings on CD units terminate as simple (1 calyx) or complex (2 or more calyces) calyces. Spines are very fine processes that emerge from the apical half of calyces and terminate, often as small swellings, near the apical surface of the macula.
Tissue Processing
Peroxidase labeling.
For labeling of utricular primary afferents, we killed turtles by one of the methods described above and removed lower jaw and neck viscera to expose the right VIIIth cranial nerve (CNVIII) for injection. We secured the preparation with Impregum F impression material (3M ESPE, no. 31710) and supported CNVIII with agarose gel (4% low melt) and oxygenated the tissue with turtle Ringer solution (95% O2-5% CO2). We made small (10- to 20-μm tip electrode) injections of biotinylated dextran amine (BDA), 3,000 (Molecular Probes, D7135) or 10,000 (Molecular Probes, D1956) molecular weight, into the anterior branch of CNVIII (positive current, 50% duty cycle, 1–3 μA for 35–45 min). After tracer delivery, we incubated the tissue in iced, bubbling oxygenated turtle Ringer solution for 5–7 h and then fixed the tissue by immersion in 10% buffered formalin phosphate (Fisher, SF100-4) for 12 h at 4°C. After initial fixation, we removed the utricle and attached nerve from the whole head, cut away the membranous labyrinth overlying the utricle to improve reagent access to the tissue, and used a fine stream of bath solution to separate the OM from the underlying neuroepithelium. The resulting macular preparation was fixed in 10% buffered formalin phosphate for an additional 6–7 h (room temperature, with rotation). After a rinse in phosphate buffer (PB, 0.1 M; 16–18 h; 4°C, with rotation), we visualized the BDA by rinsing the tissue in 0.1 M PB (4 × 10 min); defatted in alcohol (10 min each at 50%, 70%, 95%, 100% EtOH, followed by 3 min each at 100%, 95%, 70%, 50% EtOH); blocked endogenous peroxidase [44.5 ml distilled H2O (dH2O) 0.5 ml 30% H2O2, 5 ml methanol; 20 min]; visualized the BDA with an ABC kit (Vector Laboratories, Vectastain PK-6100) as follows: wash 3 × 10 min each in 0.1 M PB, incubate 24–48 h in ABC solution at 4°C, rinse 6 × 5 min each in 0.1 M PB, incubate for 15 min in cobalt-enhanced diaminobenzidine tetrahydrochloride (DAB; 10-mg tablet dissolved in 10 ml dH2O plus 10 ml 0.2 M PB; Sigma, D5905-50); and visualized the DAB by incubating the maculae for ≤4 min with DAB plus H2O2 (0.05%), followed by three rinses with 1 M PB, 5 min each. After the ABC reaction, we trimmed the macula with attached nerve, dehydrated it with EtOH (15 min each at 50%, 70%, 80%, 90%, 2 × 30 min each at 95% and 100%), cleared it with xylene (3 × 1 h each), and mounted it under a coverslip with Permount.
In three additional cases, we labeled all afferent terminals in the maculae with an antibody against β-III tubulin (Covance, PRB-435P), followed by a biotinylated secondary antibody (Invitrogen, B2770), and we visualized the labeled terminals via a peroxidase reaction as described above. We used these cases to assess calyx complexity in CD units.
Fluorescence labeling.
We used utricular whole mounts labeled with fluorescent probes to address two questions raised by our BDA material: Do spines contact type I and type II hair cells? And how many hair cells, on average, are contacted by different afferent types?
Examination of BDA-labeled afferents revealed that some calyces emit very fine processes that terminate, often as small swellings, near the apical surface of the epithelium; we call these processes spines because of their resemblance to the well-known spines on CNS neurons (Harris 1999). To further investigate these spines, we labeled five utricles with a cocktail of primary antibodies against the calcium-binding proteins (CBPs) calretinin (CR; Swant, 6B3) and calbindin (CB; Swant, CB38a), followed by the appropriate anti-mouse and anti-rabbit secondary antibodies (Invitrogen, A11029 and A21071). This double labeling procedure maximizes the number of strongly labeled calyces, which facilitates visualization of spines in fluorescence material, and it labels most type II hair cells in Zone 3, which enabled us to estimate the fraction of spine terminations on type II hair cells. We examined fluorescence material with a Zeiss 510 laser scanning confocal microscope.
To estimate the number of hair cells contacted by different afferent types, we labeled two maculae with phalloidin (Invitrogen, A12379), which identifies hair cells by labeling the actin in their hair bundles, and we processed one of these maculae as for the BDA-labeled afferents (to equate shrinkage with that in dehydrated materials used to measure afferent terminal collecting areas). We created a montage of the central transect (Fig. 1B) using overlapping confocal stacks (stacks collected with a Zeiss ×100 alpha-Fluar objective, NA 1.45). This transect was 100 μm wide and extended from medial to lateral margins of the macula (i.e., it included Zones 1–4). We used this material to estimate the number of hair cells within the median collecting area of afferents in each macular zone (see Number of hair cells contacted by afferents at different macular loci below).
Data Analysis
Identification and reconstruction of afferents.
We reconstructed the BDA-labeled terminals of 173 utricular afferents that were densely filled and well isolated: 43 C units, 25 D units, and 105 B units (Fig. 2). We scored each afferent as a C unit, D unit, or B unit on the basis of its terminal structure. All terminals with calyces and one or more boutonlike processes were scored as D units; for five D units (20%), the only boutonlike process was a spine. As in turtle posterior canal (Brichta and Peterson 1994), D units are much less numerous than C units, and a special effort was needed to collect enough D unit terminals for statistical analysis.
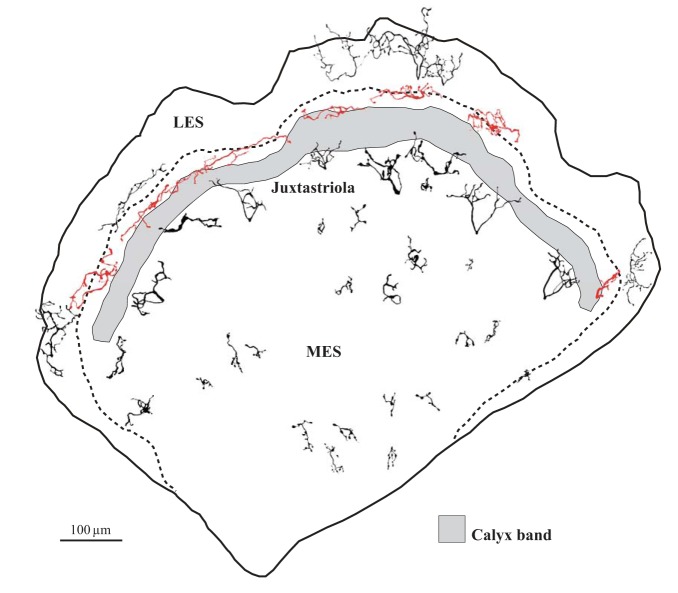
Fig. 2.
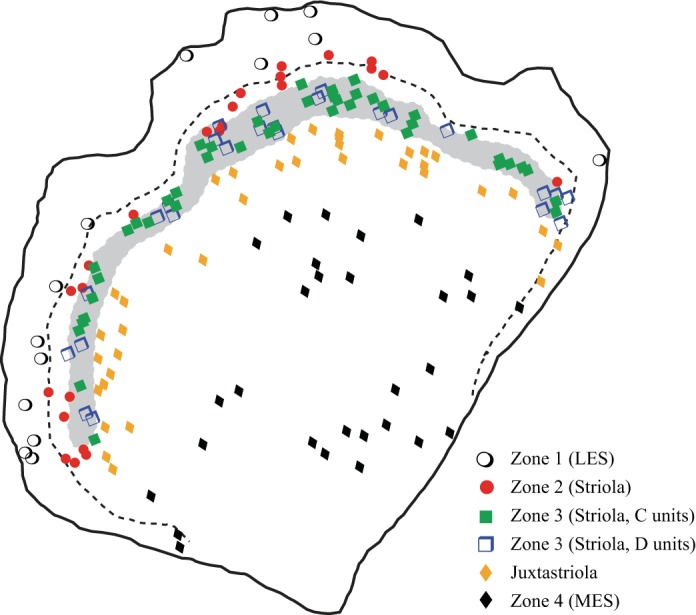
Macular location of reconstructed afferent terminals. Schematic of right turtle utricular macula. Gray profile represents the calyx band (Zone 3); C and D units are restricted to this band. Dashed line represents the line of hair cell polarity reversal (LPR). The location of the calyx band and the trajectory of the LPR are based on a map of an in situ utricle labeled with phalloidin and α-tubulin to show hair cell orientation (they label stereocilia and kinocilia, respectively) and with β-tubulin to show calyces (Graydon et al. 2006). Symbols represent approximate location of all afferent terminals reconstructed in this study. Terminal classes are indicated by different symbols (see key).
We scored the macular location of each afferent as follows. We created a mapping image of each macula with Axiovision (Zeiss), and we scored each well-filled and isolated afferent terminal by type (bouton, calyx, dimorph) and by location (Zones 1–4; Fig. 1A). Then we assigned each unit a position on a radial grid, which was anchored along a transect from the posterior margin of the utricular nerve to the midpoint between the outer edges of the nerves to anterior and horizontal canals (at the lateral edge of the epithelium; Fig. 1B). We assigned each unit a radial position (angle relative to the transect), where 0 = transect and negative and positive angles indicate radial positions toward the anterior and posterior ends of utricle, respectively. The unit was also assigned a percent distance along its radial line, from the anchor point to the medial edge of the calyx band [Zone 3; identifiable with differential interference contrast (DIC) optics and because injections labeled multiple CD units in all experiments], where 100% = medial margin of calyx band, <100% = more medial locations, and >100% = more lateral locations (Fig. 1B).
Finally, we reconstructed afferent terminals in three dimensions and quantified their morphology with Neurolucida computerized morphometry software (MicroBrightField, Williston, VT) coupled to a Zeiss Axioplan microscope. We reconstructed terminals with a Zeiss ×100 oil immersion objective (NA 1.45) and reconstructed their axons a with a Zeiss ×63 Immersol objective (NA 1.2). We followed afferent axons to their somata, or as far centrally as possible. Table 1 lists the measures recorded or calculated for each afferent terminal and used in our analysis. All measures were normalized to the average macular area of the 26 utricles used in this study. We conducted exploratory and inferential statistical analyses with S+ (version 8.2; Tibco Spotfire), and we compared differences between afferent groups with t1way and lincon (robust analogs of 1-way ANOVA and multiple comparisons, respectively; Wilcox 2005; see discussion in Xue and Peterson 2006). Additional statistical tests are described in results.
Table 1.
Morphological variables
| Variable | Definition |
|---|---|
| Axon diameter, μm | Average diameter from central end of axon to first branch point within epithelium (axons were followed as far centrally as possible, to soma if possible) |
| Surface area, μm2 | Total surface area of all processes from entry into epithelium |
| Volume, μm3 | Total volume of all processes from entry into epithelium |
| Total process length, μm | Total length of all processes from entry into epithelium |
| Nodes | Number of branch points; can be bi- or trifurcations |
| Ends | Number of end points (calyx or bouton) on terminal |
| Collecting area, μm2 | Area of convex polygon drawn around tips of distal dendrites |
| Boutons | Total number of varicosities on terminal (en passant and terminal) |
| Bouton diameter, μm | Average diameter of all varicosities on terminal |
| Bouton density | Boutons per collecting area |
| Distance between boutons, μm | Average (crow-fly) distance between all pairs of varicosities on terminal |
| Calyces | Total number of calyces (cups) on terminal |
| Distance between calyces, μm | Average (crow-fly) distance between all pairs of calyces on terminal |
| Calyces and boutons | Total of all calyces (cups) and varicosities on terminal |
Number of hair cells contacted by afferents at different macular loci.
We followed earlier authors in calculating the number of hair cells contacted by individual afferents by comparing hair cell density and afferent collecting area at the same macular locus (e.g., Fernandez et al. 1990). First, we used Neurolucida to map the location of all hair cells in a central transect (Fig. 1B) through one phalloidin-labeled macula. Next we divided this transect into bins (100 μm wide, i.e., the width of the confocal image, and 25 μm tall, i.e., along the mediolateral axis), and we counted the number of hair cells in each bin. Then we assigned each bin to a macular zone; we were able to determine zone boundaries by their distance from the line of hair cell polarity reversal (LPR; visible in this phalloidin-labeled material as changes in the orientation of the cuticular plate notch that marks the location of the kinocilium) and from changes in hair bundle arrays, because bundles in each zone have a characteristic array structure (Moravec and Peterson 2004; Rowe and Peterson 2006). In this way we were able to estimate hair cell density in each macular zone (see Fig. 11). To relate these hair cell densities to dimensions in the BDA material used to reconstruct terminals, we dehydrated and cleared additional phalloidin-labeled maculae. This processing severely compromised the phalloidin label, but in one macula we were able to count hair cells across most of the transect. We calculated a shrinkage factor by comparing hair cell densities in dehydrated and undehydrated maculae at comparable locations (3 samples in each macula), and we applied this shrinkage factor when calculating the approximate number of hair cells within the collecting areas of afferents supplying each macular subdivision (see Table 7).
Fig. 11.
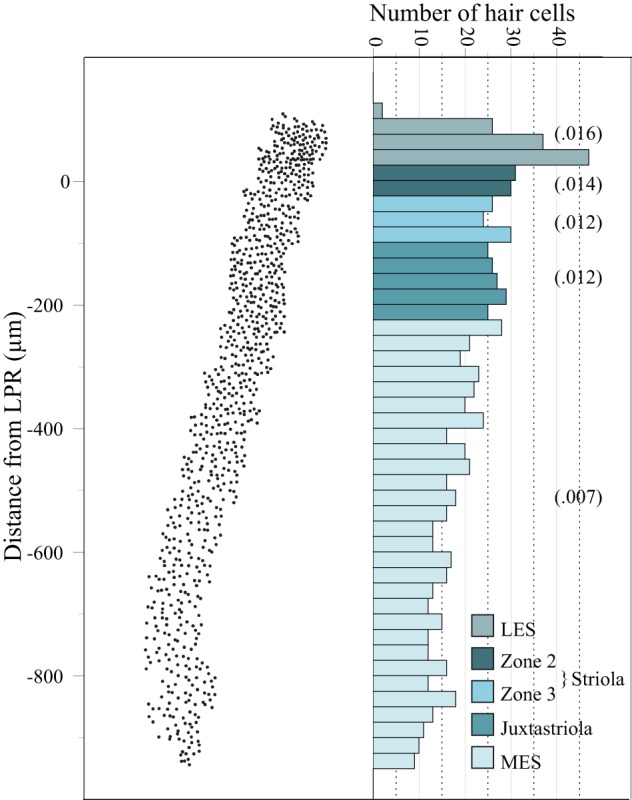
Regional differences in hair cell density. Left: x-y plot showing the coordinates of all hair cells in a 100-μm-wide mediolateral transect through the macula (black line with tick marks in Fig. 1B). The macula was treated with phalloidin to label hair cell bundles and cuticular plates, which allowed us to distinguish the line of hair cell polarity reversal (LPR). y-Axis, distance from the LPR, where 0 = LPR (x-axis shows approximate anterior-posterior spread of hair cells in the transect). In macular regions supplied by bouton afferents (LES, Zone 2, juxtastriola, MES) hair cell density decreases from lateral (top) to medial (bottom); there is also a slight density decrease in striolar Zone 3 (the calyx band). Right: histogram showing lateral-to-medial decrease in hair cell density. The histogram is aligned as closely as possible with the x-y plot (left) on which it is based. Each bar represents ∼25 μm along the mediolateral axis. Approximate boundaries of each macular subdivision are indicated by different colors (see key). Numbers in parentheses represent the average number of hair cells per zone in each region of the macula. Note that these density numbers are lower than those in Table 7 because macular area in this histogram is not corrected for shrinkage as it is in Table 7 (see legend of Table 7).
Table 7.
Estimated hair cell/afferent contacts
| Macular Subdivision | Hair Cell Density | Collecting Area, μm2 | Hair Cells per Afferent | Number of Boutons | Boutons per Hair Cell |
|---|---|---|---|---|---|
| LES | 0.0217 | 3,978 | 86.3 | 37.5 | 0.43 |
| Striolar Zone 2 | 0.0181 | 2,131 | 38.6 | 27.0 | 0.70 |
| Juxtastriola | 0.0156 | 2,248 | 35.1 | 17.5 | 0.50 |
| MES | 0.0097 | 556 | 5.4 | 12.0 | 2.22 |
Hair cell density (hair cells/μm2) is calculated from Fig. 11 with an additional 25% correction (decrease in area used to calculate hair cell density) to equalize shrinkage for hair cell counts and afferent reconstructions. Collecting areas and number of boutons are medians from Neurolucida reconstructions. Hair cells per afferent (hair cell density × collecting area) is the estimated number of hair cells that fall within the median afferent collecting area in each zone. Boutons per hair cell (number of boutons/hair cells per afferent) is the estimated number of bouton contacts made by an afferent on each hair cell within its collecting area.
Spines.
To determine spine frequency, we counted calyces and spine terminals in four utricles (4 turtles), using macular regions where BDA label of calyces was especially dense because such dense label optimizes visualization of spines. We used these counts to estimate the percentage of calyces that bear spines. We analyzed each utricle twice and averaged the results.
To help identify the cells contacted by spines, we used confocal scans of three maculae (3 turtles) that had been labeled with the CR + CB cocktail described above, because this double label facilitates visualization of spines and also labels many hair cells in Zone 3. We scored each spine based on whether it contacted a calyx, a type I hair cell, a type II hair cell, or something else (i.e., either made no contact or contacted an unstained profile; these categories could not be distinguished in our fluorescent material, so they were combined into one group).
RESULTS
Calyx-Bearing Units
In juvenile turtle utricle, all C and D units (collectively, CD units) are restricted to a subdivision of the striola that we call Zone 3 (Fig. 1A; Moravec and Peterson 2004; Rowe and Peterson 2006; Xue and Peterson 2006). They form a crescent-shaped band, which is 75–100 μm wide; it is innervated by ∼120–160 CD units that bear ∼270–350 calyces (Table 2).
Table 2.
Calyx complexity of CD units
| No. of Calyces |
|||||||
|---|---|---|---|---|---|---|---|
| One | Two | Three | Four | Five | Total Units | Total Calyces | |
| Case 1 | 48 | 52 | 48 | 11 | 1 | 160 | 345 |
| Case 2 | 29 | 49 | 33 | 10 | 1 | 122 | 271 |
| Case 3 | 32 | 46 | 35 | 10 | 2 | 125 | 279 |
| Average % | 0.27 | 0.36 | 0.29 | 0.08 | 0.01 | ||
Values are number of calyceal terminals on C and D units, subdivided by the number of calyces (cups) in the terminal. Average %, % of total calyx terminals with 1, 2, etc. calyces, averaged across 3 cases.
Terminal structure.
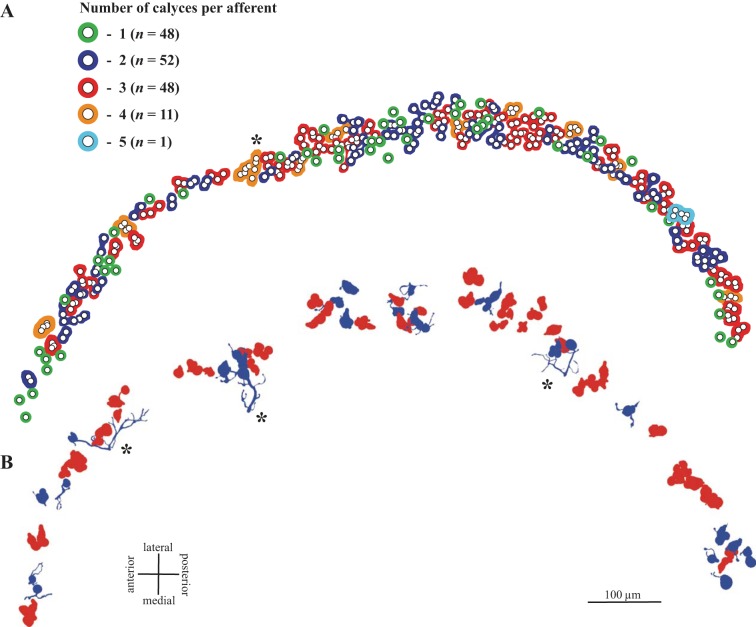
Within this band, we observed CD units with one to five calyces (Table 2). Figure 3A illustrates calyx complexity for one case in which all calyces were labeled with β-III tubulin. Each open circle represents a calyx (cup); colored profiles around one or more calyces demarcate all calyces arising from a single parent axon. Profiles are colored according to the number of calyces in the unit. The calyces on most CD units form a tight cluster; with rare exceptions (Fig. 3A), terminals do not span the width of Zone 3 or extend longitudinally along the striola. Thus the calyces associated with each afferent collect signals from type I hair cells at restricted locations within Zone 3.
Fig. 3.
CD units in turtle macula. A: calyx complexity. Calyces from all CD units in 1 β-III tubulin-peroxidase-labeled case are indicated by small circles (corresponds to case 1 in Table 2). Colored profiles surrounding 1 or more circles demarcate all calyces arising from a single afferent; colors indicate the number of calyces arising from that afferent (1–5; see key). Note that units supply a restricted region of the striola; they do not extend longitudinally along the striola or, with rare exceptions (*), span the mediolateral width of the striola. B: reconstructed CD units: approximate locations of the 25 D units (blue) and 43 C units (red) reconstructed in this study. Note that most D units, like C units, have restricted collecting areas. Asterisks mark 3 exceptions; these are the only D units with >10 boutons.
Because of the high terminal density in the β-III-labeled whole mounts we used to count calyces, we were unable to distinguish between C and D units with confidence, but analysis of individually reconstructed, BDA-labeled afferents reveals differences in the calyx-bearing endings of C and D units (Fig. 3B, Fig. 4, Fig. 5). Most C units (77%) bear two or three calyces (range 1–5), whereas most terminals on D units (52%) are simple (Fig. 5). Thus C and D units differ significantly in the average number of type I hair cells they contact (2.7 for C units vs. 1.7 for D units; Mann-Whitney U = 245.5, P < 0.001) and in the distributions of calyx complexity (Fig. 5; Kolmogorov-Smirnov test, P < 0.005).
Fig. 4.
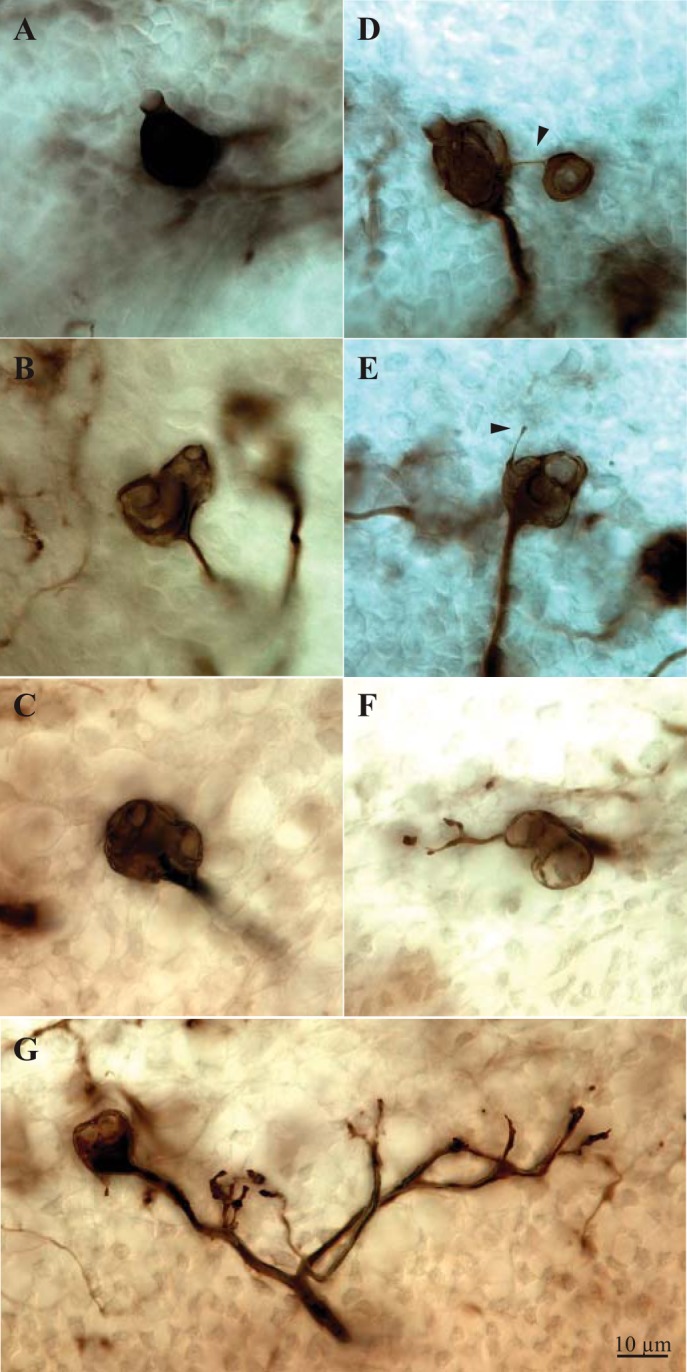
Terminals of C and D units. A–D: C units with simple (A), double (B), triple (C), and quadruple (D) calyces. D shows a rare example of a calyx terminal with calyces separated by a well-defined branch (arrowhead); typically, calyces in a terminal form a tight cluster. E–G: D units with a spine (E; arrowhead) and simple (F) and extensive (G) bouton terminals. Terminal in G corresponds to the leftmost asterisk in Fig. 3B.
Fig. 5.
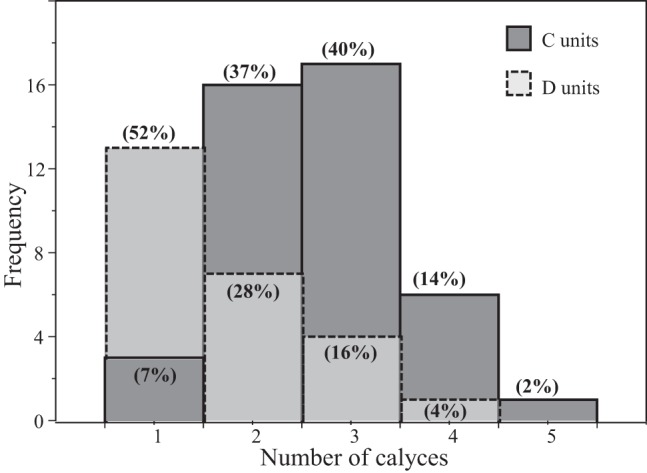
Calyx complexity in C and D units. Histogram compares calyx complexity in reconstructed C (solid lines) and D (dashed lines) units. x-Axis, number of calyces in the terminal; y-axis, number of afferents. Numbers in parentheses for each bar indicate % of C or D units bearing the specified number of calyces in a terminal. For example, 52% of D units and 7% of C units are simple (1 calyx); 40% of C units and 16% of D units end in triple calyces.
There are also significant differences between C and D units in number of nodes (P < 0.001), number of ends (P < 0.005), total process length (P < 0.001), and two-dimensional collecting area (P < 0.001) (D > C in all cases; Table 3) because D unit terminals bear bouton clusters as well as calyces. However, these bouton clusters are small, with 1–22 boutons (Fig. 3B). Most (72%) have 1–5 boutons; only three (12% of total D units) have >10 boutons (Fig. 3B). Thus most D units, like C units, collect signals from a restricted region of Zone 3.
Table 3.
Morphology of CD units
| Property | C Units | D Units |
|---|---|---|
| Axon diameter, μm | 2.6 | 2.5 |
| 2.39–2.77 | 2.17–2.66 | |
| 1.76–3.46 | 1.66–3.48 | |
| Nodes | 1 | 3 |
| 1–1 | 2–4 | |
| 0–3 | 1–14 | |
| Ends | na | 4 |
| 3–5 | ||
| 2–15 | ||
| Boutons | na | 4 |
| 2–5 | ||
| 1–23 | ||
| Bouton diameter, μm | na | 1.57 |
| 1.45–1.75 | ||
| 1.04–2.44 | ||
| Calyces | 3 | 1 |
| 2–3 | 1–2 | |
| 1–5 | 1–4 | |
| Collecting area, μm2 | 89.1 | 217.0 |
| 72.54–116.07 | 187.22–299.66 | |
| 15.4–367.4 | 73.8–1,996.5 | |
| Total process length, μm | 47.8 | 77.0 |
| 41.52–58.7 | 62.75–89.92 | |
| 20.9–95.6 | 40.3–306.7 | |
| Distance between boutons, μm | na | 11.2 |
| 5.54–18.45 | ||
| 0–33.3 | ||
| Distance between calyces, μm | 8.8 | 0 |
| 8.15–10.08 | 0–7.89 | |
| 0–15.6 | 0–12.1 | |
| Calyces + boutons | na | 6 |
| 4–6 | ||
| 2–25 | ||
| Calyces + boutons/collecting area | 0.03 | 0.02 |
| 0.025–0.034 | 0.018–0.027 | |
| 0.01–0.07 | 0.01–0.06 |
Values are medians, 95% confidence intervals of median (Zar 1999), and range for morphological properties of C and D units. All measures of size are normalized. For C and D units, n = 43 and n = 25, respectively. na, Not applicable.
Spines.
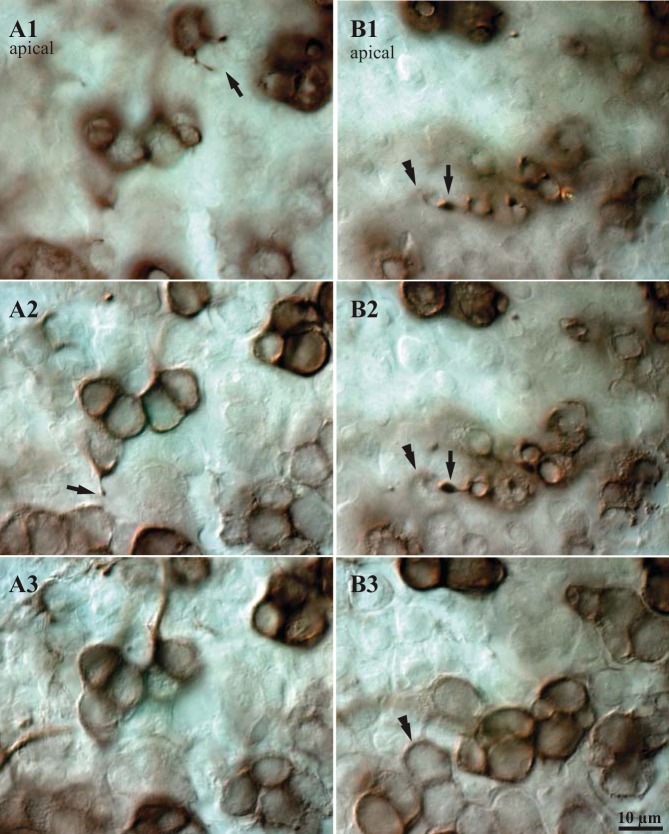
We occasionally observed one or two fine, short processes emerging from the upper half of a calyx and extending toward the apical surface of the epithelium; often these processes end in a tiny swelling (Fig. 6). Such small spines can be detected most reliably in tissue with very dense BDA-peroxidase label. In four cases with patches of strongly labeled CD units, we determined that slightly less than 10% of calyces bear spines (Table 4). Some spines appeared to contact the necks of type I hair cells (identified by their labeled calyces; Fig. 6), above the calyx (compare Fig. 6, A and B), but in BDA material it was impossible to determine whether the spines contacted type II hair cells because their somata are not labeled. To estimate the relative frequency of type I and type II hair cells contacted by spines, we labeled utricular whole mounts with a cocktail of two CBPs, CR and CB (see materials and methods). We found that approximately one-third of the spines contacted type I hair cells and the remaining two-thirds contacted type II hair cells (Table 5). Spines did not appear to contact afferents or supporting cells. Since the number of type II hair cells in Zone 3 is small relative to the number of type I hair cells, the density of spine contacts on type II hair cells is high relative to those on type I hair cells.
Fig. 6.
Spines on CD units. A and B: DIC micrographs showing examples of spines that arise from BDA-peroxidase-labeled calyces and terminate near the apical surface of the macula. The 3 images in each series (A1–A3, B1–B3) show 3 focal planes from most apical (1) to most basal (3); vertical steps between images are 3–10 μm. A1: 2 spines arise from a labeled calyx and terminate near the apical surface of the epithelium (arrow); under DIC optics they appear to contact the necks of unlabeled hair cells. A2: a third spine arises from the neck of a labeled calyx (arrow); the base of this calyx is visible in A3. B1: a spine with a relatively large terminal swelling (arrow) terminates just above the neck of a calyx (double arrowhead). B2: the same contact seen slightly deeper in the epithelium; the body of the calyx (double arrowhead) is clearer than in B1. B3: base of the same calyx. Such images suggest that spines terminate near the apices of type I hair cells.
Table 4.
Frequency of calyceal spines
| Case | Calyces | Spines | Percentage |
|---|---|---|---|
| 1 | 148 | 16 | 10.8 |
| 2 | 220 | 18 | 8.2 |
| 3 | 243 | 20 | 8.2 |
| 4 | 83 | 8 | 9.6 |
| Average | 173.5 | 15.5 | 9.2 |
Values are numbers and % of calyces that bear spines in heavily biotinylated dextran amine (BDA)-labeled regions of the macula in 4 utricles (4 turtles).
Table 5.
Targets of calyceal spines
| Case | Spines | Type 1 Contacts | Percentage |
|---|---|---|---|
| 1 | 13 | 3 | 23.1 |
| 2 | 6 | 2 | 33.0 |
| 3 | 14 | 5 | 35.7 |
| Average | 11 | 3.3 | 30.6 |
Values are numbers and % of spines visualized by calretinin (CR) + calbindin (CB) immunofluorescence that contact type I hair cells in 3 utricles (3 turtles). Type I hair cells were identified by their labeled calyces. Percentages may be slightly underestimated because a small number of calyces are not CR+ or CB+; their associated type I hair cells would have been misidentified as type II hair cells.
Bouton Units
All remaining subdivisions of the turtle macula (Zones 1, 2, and 4) are innervated by B units exclusively, but there are striking differences in their terminals. Furthermore, afferent terminals just medial to the calyx band differ sufficiently from those in the remainder of Zone 4 that they define a previously unsuspected band, which we call the juxtastriola.
Figure 7 shows examples of different B unit types at their approximate locations on a schematic macula. In the LES (Zone 1), B unit terminals have large collecting areas with numerous fine processes and small boutons, giving them a characteristic “lacy” appearance (Fig. 7, Fig. 8A, Fig. 9). In striolar Zone 2, terminals have thick processes and relatively large boutons (Fig. 7, Fig. 8B, Fig. 9); terminal field shape is generally narrow and elongated parallel to the striola. Medial to the calyx band (i.e., in Zone 4), the macula can be further subdivided on the basis of terminal structure. Within 150 μm of the calyx band, terminals have coarse processes and collecting areas comparable in size to those in striolar Zone 2 but typically rounded rather than elongated (Fig. 7, Fig. 8C, Fig. 9). The remainder of Zone 4 is supplied by afferents with very small collecting areas, which are often radially elongated (Fig. 7, Fig. 8D, Fig. 9). To reflect these afferent differences in Zone 4, we refer to the two macular subdivisions as the juxtastriola and the MES, respectively. Table 6 summarizes morphological differences between B units in the four macular subdivisions.
Fig. 7.
Reconstructed B units. Examples of reconstructed B units shown at their approximate location on the same schematic macula depicted in Fig. 2. Afferents supplying striolar Zone 2 are shown in red. Dashed line is line of hair cell polarity reversal. Note that in Zone 4 terminals adjacent to the calyx band (juxtastriola) have larger collecting areas and thicker processes than more medial terminals (MES). Some LES terminals appear to extend beyond the macular epithelium because they come from maculae with slightly different boundaries than that shown here.
Fig. 8.
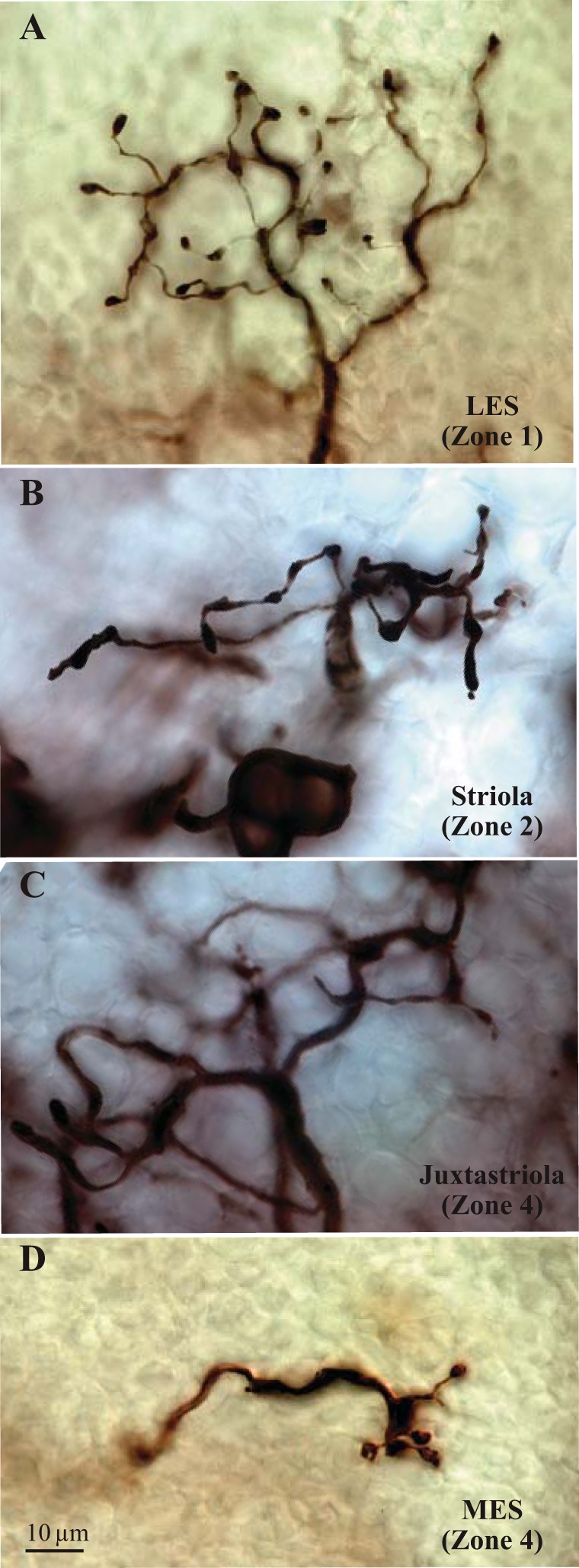
Terminals of B units. Photomicrographs of BDA-filled terminals of B units in different macular subdivisions. Units supplying the LES (A) tend to have extensive terminals with fine processes and numerous small boutons, giving them a characteristic “lacy” appearance. B unit terminals in striolar Zone 2 (B) are generally elongated parallel to the striola except at its anterior and posterior ends, where these terminals can be round. In Zone 4 (C and D), bouton terminals adjacent to the calyx band (C, juxtastriola) have significantly larger axons and collecting areas than more medial B units (D). Because of this marked difference in afferent structure, we recognize a juxtastriolar band (C) that is distinct from the MES (D).
Fig. 9.
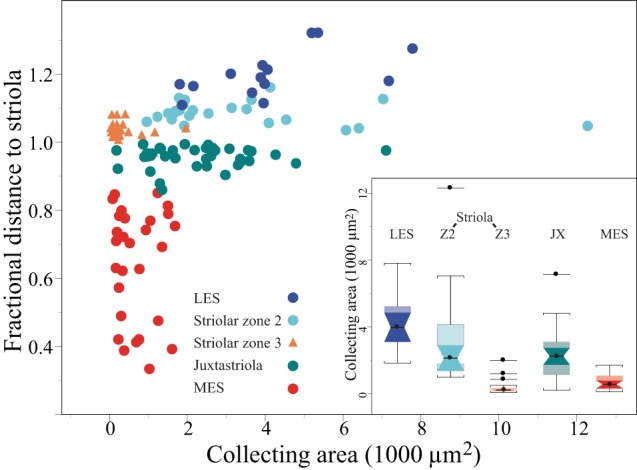
Regional differences in B unit collecting areas. Distance plot showing that collecting area varies significantly with position along a mediolateral transect through the macula. In this plot, 1.0 on y-axis represents the medial margin of the calyx band (Zone 3), which was readily visible in our material and therefore served as a reliable starting point when comparing terminal location across animals. Each division represents 10% of the distance along a radial line that transects the macula (see Fig. 1B). Because the macula is roughly 1 mm in diameter, each division represents ∼100 μm. Terminals of B units in different macular subdivisions are represented by different colors (see key). Collecting areas of D units in striolar Zone 3 are shown for comparison (orange triangles). The 3 D units with relatively large collecting areas correspond to the D units marked by asterisks in Fig. 3B. Inset: box plots compare collecting areas of terminals at different macular loci. Color code corresponds to symbol color in the distance plot. All B unit terminals are significantly larger than D unit terminals in Zone 3 (Z3). B unit terminals are largest in the LES and smallest in the MES. B units flanking the calyx band (Z3) have similar collecting areas: striolar Zone 2 (Z2) and the juxtastriola (JX). Box plot conventions: circles, medians; boxes, interquartile range; notched boxes, confidence intervals of the median; whiskers, 1.5 × interquartile range; isolated points, outliers.
Table 6.
Morphology of B units
| Property | LES (n = 14) | Striolar Zone 2 (n = 24) | Juxtastriola (n = 38) | MES (n = 29) |
|---|---|---|---|---|
| Axon diameter, μm | 1.43 | 2.25 | 1.66 | 1.21 |
| 1.341–1.812 | 1.973–2.384 | 1.557–1.719 | 1.064–1.330 | |
| 1.08–2.39 | 1.30–4.36 | 1.16–2.63 | 0.77–1.85 | |
| Surface area, μm2 | 1,508.9 | 1,662.7 | 1,235.2 | 508.5 |
| 1,199.02–2,499.55 | 1,338.73–2,455.03 | 882.15–1,504.06 | 378.70–873.85 | |
| 892.8–3,579.2 | 692.7–3,303.3 | 362.5–2,943.0 | 192.8–1,412.1 | |
| Volume, μm3 | 468.6 | 774.3 | 459.9 | 197.8 |
| 348.33–1,064.43 | 408.44–1,207.29 | 364.26–826.24 | 146.59–344.85 | |
| 250.3–2,333.5 | 175.4–2,023.1 | 138.7–2,125.9 | 41.0–856.3 | |
| Total process length, μm | 471.7 | 359.4 | 274.6 | 121.8 |
| 371.43–575.82 | 290.96–449.97 | 214.52–340.60 | 81.80–175.25 | |
| 289.4–835.1 | 181.6–965.5 | 87.3–495.2 | 56.9–237.8 | |
| Nodes | 14.5 | 10 | 7 | 6 |
| 9–17 | 8–14 | 6–9 | 4–7 | |
| 7.0–22.0 | 5–38 | 4–15 | 2–15 | |
| Ends | 16 | 12.5 | 8 | 7 |
| 10–20 | 10–15 | 7–11 | 5–8 | |
| 8–23 | 7–44 | 5–17 | 4–16 | |
| Collecting area, μm2 | 3,977.9 | 2,131.2 | 2,248.4 | 556.0 |
| 2,189.08–5,389.57 | 1,824.52–4,124.70 | 1,352.23–2,686.09 | 337.43–1,062.00 | |
| 1,840.3–7,816.5 | 993.9–12,315.1 | 215.1–7,135.5 | 119.6–1,720.2 | |
| Boutons | 37.5 | 27 | 17.5 | 12 |
| 24–50 | 20–33 | 14–22 | 10–18 | |
| 19–63 | 10–126 | 7–33 | 8–41 | |
| Bouton diameter, μm | 1.7 | 2.0 | 1.9 | 1.8 |
| 1.52–1.90 | 1.62–2.42 | 1.65–2.19 | 1.74–2.22 | |
| 1.3–2.9 | 1.1–3.4 | 1.2–4.4 | 1.1–3.5 | |
| Boutons/collecting area | 0.010 | 0.013 | 0.009 | 0.027 |
| 0.005–0.014 | 0.0096–0.0169 | 0.0072–0.0110 | 0.0143–0.0356 | |
| 0.003–0.026 | 0.001–0.032 | 0.004–0.060 | 0.006–0.076 | |
| Distance between boutons, μm | 45.6 | 37.9 | 32.7 | 19.0 |
| 33.18–57.65 | 31.56–42.67 | 26.64–36.79 | 16.34–25.17 | |
| 31.1–68.3 | 23.3–91.5 | 11.6–61.6 | 9.2–35.7 |
Values are median, 95% confidence intervals of median (Zar 1999), and range for morphological properties of B units in 4 macular subdivisions; n = 105. All measures of size are normalized. LES, lateral extrastriola; MES, medial extrastriola.
Axon diameter.
Axon diameters of bouton units vary as a function of macular location (Fig. 10). LES and MES axon diameters are comparable, but both are smaller than B unit axons in striolar Zone 2 (P < 0.001). Juxtastriolar axons are intermediate (comparable to LES axons but larger than axons in the MES; P < 0.001). For comparison, Fig. 10 also shows axon diameters for C and D units. They do not differ from each other, but both have larger axon diameters than B units (P < 0.005), except for B units in striolar Zone 2, where axon diameters are comparable to those of D units. Tables 3 and 6 summarize axon diameters for CD and B units, respectively.
Fig. 10.
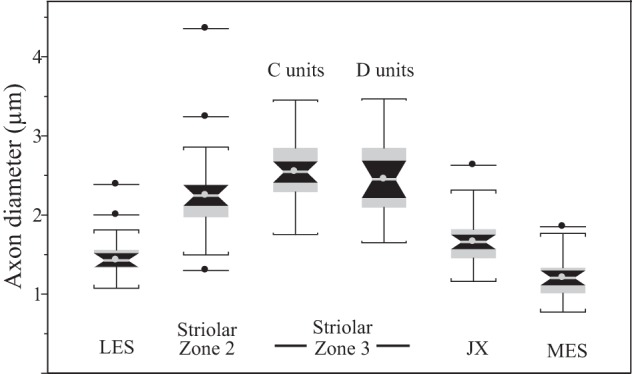
Regional differences in parent axon diameter. Box plots showing differences in axon diameter of utricular afferents supplying different macular subdivisions. C and D units are confined to striolar Zone 3. Their axon diameters are not significantly different. Afferents supplying the other macular regions are all B units. They differ significantly in axon diameter: striolar Zone 2 axons are the largest of all B units, and MES axons are smallest. Box plot conventions as in Fig. 9.
Hair cell-to afferent-convergence.
There are significant zonal differences in the collecting areas of bouton afferents (Fig. 9). Median collecting areas are largest in the LES and smallest in the MES; afferents in striolar Zone 2 and the juxtastriola have similar, intermediate collecting areas. For comparison, the collecting areas of dimorphic afferents are also shown in Fig. 9. They are significantly smaller than those of the smallest bouton afferents (MES: P < 0.05). The collecting areas of C units (not shown) are consistently small (Table 3).
In parallel with this lateral-to-medial decrease in collecting area there is a lateral-to-medial decrease in hair cell density in regions supplied by bouton afferents (LES, Zone 2, juxtastriola, LES; Fig. 11, Table 7). As a result, there are striking regional differences in the number of hair cells per afferent (i.e., number of hair cells within an afferent's collecting area): from >80 hair cells per afferent in the LES to ∼5 hair cells per afferent in the MES (Table 7). Table 7 also shows the median number of boutons per afferent type and an estimate of the number of bouton contacts made by an afferent onto each hair cell within its collecting area. An MES afferent makes an estimated 2.2 contacts on each hair cell in its collecting area, whereas the number of contacts in other macular zones ranges from 0.43 to 0.7.
DISCUSSION
In this study, we quantified the terminal structure of turtle utricular afferents and estimated hair cell-to-afferent convergence. We find that distinct populations of afferents supply the macular subdivisions we previously identified on the basis of hair bundle structure (Rowe and Peterson 2006; Xue and Peterson 2006) and mechanics (Spoon et al. 2011), OM structure (Davis et al. 2007; Xue and Peterson 2003), OM-bundle coupling (Xue et al. 2007), immunoreactivity to CBPs (Miller et al. 2009), and hair cell physiology (Meyer and Eatock 2011).
Methodological Issues in Afferent Morphology
Afferent axons form a tight bundle as they enter the macula so that it can be difficult to follow individual labeled axons past this point, even if their terminals are well separated in the neuroepithelium. We were able to follow ∼25% of the 173 reconstructed afferents (43/173) back to their somata with confidence. One potential concern is that bouton terminals might belong to efferent neurons rather than afferents. We believe this is not likely for three reasons. First, we followed ∼25% of bouton terminals (26/105) back to their somata, including 17–40% of our sample from each of the four subdivisions innervated by B units (LES, striolar Zone 2, juxtastriola, MES). These undoubted afferents provide characteristic terminals to each subdivision. Second, considerable evidence suggests that efferent terminals have a distinctive structure, with widely branching, fine processes studded with numerous, tiny varicosities (gerbil: Purcell and Perachio 1997; pigeon: Si et al. 2003; review: Lysakowski and Goldberg 2004). B unit terminals supplying turtle utricle clearly have different structures (Fig. 7, Fig. 8), and they are restricted to one macular subdivision. Third, we occasionally observed labeled terminals with characteristic efferent morphology, including numerous branches that covered most of the macula. In one case, we reconstructed two branches of one terminal. These two branches alone had a normalized terminal area of >470,000 μm2 with 458 boutons. Terminals in the LES, which have the most efferent-like terminals, have median collecting areas more than two orders of magnitude smaller (3,977.9 μm2) with a median of 37.5 boutons (Table 6).
Zone assignment is a potential source of error. Each of the macular subdivisions has a distinctive bundle structure (Moravec and Peterson 2004; Rowe and Peterson 2006; Spoon et al. 2011; Xue and Peterson 2006), but we could not visualize hair bundles reliably in our material (even under DIC optics). Instead, we took advantage of the fact that every injection labeled a significant number of CD units and that calyces are generally visible under DIC. We then used the location of the calyx band together with our earlier data on distances from the calyx band to other macular subdivisions (Moravec and Peterson 2004; Rowe and Peterson 2006; Xue and Peterson 2006) to assign bouton terminals to a zone (LES, Zone 2, juxtastriola, MES). These distances, as well as subdivision boundaries themselves, are not precise. Thus it is possible that some terminals were misassigned. The most likely error is between striolar Zone 2 and adjacent LES (Zone 1) afferents; similarly, the boundary between the MES and the juxtastriola is not clearly defined. These uncertainties about zone assignment would tend to obscure differences between macular subdivisions, and it is thus noteworthy that many morphological differences between terminals in each region are still statistically significant.
Six Classes of Utricular Afferents Have Distinctive Morphologies and Macular Locations
We have identified six afferent classes on the basis of their terminal structure and macular terminations. Three of these correspond to the previously documented types in amniote utricles: C units, D units, and B units (Fernandez et al. 1990; Si et al. 2003; reviews: Goldberg 2000; Goldberg et al. 2012; Lysakowski and Goldberg 2004). In addition, we find that B unit terminals differ significantly depending on macular locus.
CD units.
CD units are found exclusively in a narrow band within the striola, which we call Zone 3 (Moravec and Peterson 2004; Rowe and Peterson 2006; Xue and Peterson 2006). The most striking difference between C and D units was in calyx complexity. Over half the calyces on D units are simple. In contrast, simple calyces on C units are rare (7%); most are complex, contacting two to five hair cells (Fig. 5).
We were surprised to find that some (∼10%) of calyces extended fine spines toward the apical surface of the epithelium (Fig. 6). These tiny processes are easiest to see in densely labeled peroxidase material. We based our counts on four such densely labeled patches of utricular epithelium, but we suspect that 10% is an underestimate of spine frequency. Some spines appear to contact the necks of type I hair cells where the necks extend above the calyx (e.g., Fig. 6B), but we cannot exclude the possibility that some contacts are made with the upper margin of the calyx, especially if it is lightly labeled in our BDA or fluorescence material. Ultrastructural data will be necessary to resolve this and to determine whether, in fact, the spines form synaptic contacts. Interestingly, spines appear to contact hair cells well above the basolateral region where synaptic ribbons are found (Lysakowski and Goldberg 2008; Wersäll 1954; review: Eatock and Lysakowski 2006). Preliminary data using an antibody against CtBP2 as a synaptic ribbon marker suggest that at least some spine-hair cell contacts involve no synaptic ribbons (Peterson EH, unpublished observation). Ultrastructural analyses of rat utricular maculae have reported spinelike processes from calyces that make nonribbon synapses on neighboring hair cells (Ross 1997). These nonribbon synapses add to the growing body of evidence [e.g., basolateral processes on type II hair cells (Pujol et al. 2014) and the well-known ribbon contacts of type II hair cells onto the outer face of calyces (Lysakowski and Goldberg 2004)] that circuitry in the vestibular periphery may be more complex than previously supposed (e.g., Ross 1997). Fine processes terminating near the apical surface of the utricular macula have been reported in pigeon (Si et al. 2003). Spinelike contacts between hair cells and afferents have also been reported in amphibian papilla (Graydon et al. 2014).
B units.
B units innervate the entire utricular macula outside striolar Zone 3. They comprise four morphologically distinct subpopulations, which supply distinct macular loci. B units supplying the LES (Zone 1) have small-diameter axons (Fig. 10), and distinctive “lacy” terminals (Fig. 7, Fig. 8), with fine processes, numerous boutons, and the largest collecting areas of any afferent type (Fig. 9). In striolar Zone 2, B unit terminals are elongated parallel to the striola (Fig. 7, Fig. 8) with thick processes, often resembling claws. Axon diameters are larger than those of other B units, comparable to those of dimorphs (Fig. 10). Medial to the calyx band (Zone 4), two populations of B units supply the macula. In most of this region (the MES) collecting areas (Fig. 9) and axon diameters (Fig. 10) are smaller than those of any other B units, but near the calyx band terminal processes are noticeably thicker than those in the MES (Fig. 7) and both collecting areas (Fig. 9) and axon diameters (Fig. 10) are significantly larger. In recognition of these structural differences, we assign this region of Zone 4 its own name, the juxtastriola.
Hair Cell-to-Afferent Convergence Varies with Macular Locus
In addition to their structural differences, B units also differ in estimated connectivity with their presynaptic hair cells. As noted previously (Rowe and Peterson 2006), hair cell density declines from lateral to medial across the macula (Fig. 11; Table 7); this trend is interrupted by a slight density decrease in the calyx band (Zone 3; Fig. 11) as reported in turtle (Rowe and Peterson 2006; Severinsen et al. 2003) and rodent (Desai et al. 2005) utricle. Collecting areas also decrease from lateral to medial (Table 7). Thus there is a lateral-to-medial gradient in the number of hair cells potentially contacted by each afferent, from >80 in the LES to <10 in the MES (Table 7). Using the median number of boutons per afferent (Table 7), we calculate that MES afferents make over three times more bouton contacts on each presynaptic hair cell than B units in other regions of the macula (Table 7).
Relation to Previous Work on Utricular Afferents
Utricular afferent terminal morphology has been quantified in chinchilla (Fernandez et al. 1990; Goldberg et al. 1990b), bullfrog (Baird and Schuff 1994), pigeon (Si et al. 2003), and turtle (present results). One striking difference between these species is in the presence and distribution of calyces. They are absent in bullfrog (Baird and Schuff 1994), confined to one or two striolar bands in turtle (Jorgensen 1974, 1988; Moravec and Peterson 2004; Xue and Peterson 2006; present results) and birds (Jorgensen 1989; Jorgensen and Anderson 1973; Rosenhall 1970; Si et al. 2003), or found throughout the macula in chinchilla and other mammals (Fernandez et al. 1990; Goldberg et al. 1990b; Lindeman 1969). The significance of these differences is not known. A second striking difference is the complex striolar lamination seen in bullfrog (Baird 1994a, 1994b), turtles (Jorgensen 1974, 1988; Miller et al. 2009; Moravec and Peterson 2004; Spoon et al. 2011; Xue and Peterson 2006; present results), and birds (Jorgensen 1989; Jorgensen and Anderson 1973; Rosenhall 1970; Si et al. 2003) but apparently absent in mammals (Desai et al. 2005; Fernandez et al. 1990; Goldberg et al. 1990b; Leonard and Kevetter 2002; Li et al. 2008; Lindeman 1969; Simmons et al. 2010). The clearest similarities between these species are the tendencies for the striola to be supplied by large-diameter afferents and for striolar hair cells to have significantly lower KS ratios (the ratio between the height of the kinocilium and the tallest stereocilia; Xue and Peterson 2006), features that they share with fish (review: Platt 1983).
Detailed comparison of afferent terminal structure in species for which data exist is problematic because of differences in methodologies used to collect and analyze structural data, but some generalizations are possible. C units are restricted to the striola in all three species; they are least complex in mammals, most complex in pigeon, and intermediate in turtle. For example, in mammals roughly half of C units are simple and they rarely contact more than three type I hair cells (Desai et al. 2005; Fernandez et al. 1990). In contrast, C units in pigeon are never simple; they contact 2–15 type I hair cells (mean 7.1; Si et al. 2003). C units in turtle contact one to five hair cells (median: 3) in our material. Severinsen et al. (2003) report that turtle utricular calyces can contact up to eight hair cells but that two to four is most common. Finally, calyx clusters on C units are significantly more complex than those on D units in pigeon (Table 2 in Si et al. 2003) and turtle (present results, Fig. 5) but not in chinchilla (Table 1 in Fernandez et al. 1990). The role of calyx complexity is not known, but one effect of increasing complexity will be to average the directional preferences of their presynaptic hair cells. The excitation axes of closely adjacent hair cells can vary 20–40° (Rowe and Peterson 2006), so averaging signals from these hair cells will reduce the noise in head direction signaling.
B units in turtle are strikingly similar to those in bullfrog (Baird and Schuff 1994), including a cotillus (the region medial to the striola) dominated by afferents with small collecting areas (their Fig. 7) except for those adjacent to the striola (their Fig. 8). B units in pigeon also differ structurally with macular locus, but MES collecting areas are not especially small as they are in frog and turtle. B units in chinchilla have large collecting areas and are restricted to the macular periphery, but bouton sprays on dimorphs, which supply the entire chinchilla macula, show significant striola-to-periphery increases in terminal field diameter and bouton number (Table 2 in Fernandez et al. 1990). Thus bouton terminal structure on B and D units depends on macular locus in all species studied, but very small MES terminals are restricted to bullfrog and turtle.
Organization of the Macula
Our results on utricular afferents add to growing evidence that vertebrate maculae are composed of distinct subdivisions, which differ from each other in structure, mechanics, immunoreactivity, and physiology. Such differences make it likely that macular subdivisions play different behavioral roles. These roles have not been established, but together the macular subdivisions must encode 1) head movement direction, including head orientation in gravity space, and 2) a range of temporal characteristics, from tonic head postures to head transients with a frequency content that can reach 50 Hz (squirrel monkey: Armand and Minor 2001; turtle: Rivera et al. 2012). Finally, relaying these spatiotemporal properties of head movement to the CNS must occur with accuracy and speed sufficient to trigger appropriate postural, visual, and vegetative compensatory reflexes and to continually update the moving animal's subjective sense of its orientation in space. The available data suggest hypotheses about the way some macular subdivisions might contribute to this task.
Extrastriola.
It is clear that the MES and the LES are not simple mirror images of each other, differing only in hair cell polarity. In turtle hair bundles in both regions have large KS ratios, suggesting that both will have wide operating ranges (see discussion in Spoon et al. 2011), but other bundle features and hair cell density differ in the two regions (Rowe and Peterson 2006; Severinsen et al. 2003; Xue and Peterson 2006; present results; also in mice: Li et al. 2008), as does afferent morphology (present results; also in bullfrog: Baird and Schuff 1994), and in the structure of the OM and the coupling between hair bundles and the OM (Davis et al. 2007; Xue et al. 2007; Xue and Peterson 2003). The functional role of the LES is unclear, but several lines of evidence suggest that turtle MES may be well suited to providing an ongoing readout of head orientation in gravity space. The otoconial layer over the MES is very thick (Davis et al. 2007; Xue and Peterson 2003), and the long kinocilia of MES hair cells are tightly tethered to it (Xue et al. 2007). This coupling, and their large operating range (Meyer and Eatock 2011; Spoon et al. 2011), mean that MES hair cells will closely track the otoconial layer during a wide range of head tilts. MES hair cells either do not adapt or adapt slowly (Meyer and Eatock 2011), and their signals are relayed to the CNS by small-diameter (and presumably slowly conducting) axons (present results), features that are suitable for monitoring tonic or slowly changing head position. Finally, the very small collecting areas of MES afferents (present results) suggest that they will sample a small range of hair bundle excitation axes and therefore may monitor the direction of head tilt with high spatial resolution.
Data from other species are consistent with the suggestion that the MES monitors head posture. Bullfrog type B hair cells closely resemble MES hair cells in turtle, and they dominate the bullfrog MES (Lewis and Li 1975). Their responses to injected current and slow adaptation suggest that they may monitor head tilt (Baird 1994a, 1994b). These hair cells are innervated by tonic afferents that, as in turtle, have small-diameter axons (Baird and Lewis 1986). In mammals, extrastriolar afferents show relatively tonic responses to head tilts (compared with striolar afferents), and these afferent differences depend, inter alia, on differences in the properties of their presynaptic hair cells (reviews: Eatock and Songer 2011; Goldberg et al. 2012; Lysakowski and Goldberg 2004).
Striola.
Hair bundle structure and mechanics suggest that turtle striola is well suited to encode high-frequency head transients and/or the onset of head movement. Striolar hair bundles have short kinocilia and low KS ratios, suggesting that they will have a short operating range (see discussion in Spoon et al. 2011). They also present a broad, steeply sloped face to endolymph flow caused by OM displacement (Rowe and Peterson 2006; Severinsen et al. 2003; Xue and Peterson 2006), and our models suggest that this produces near-total MET channel opening with 100-nm bundle displacement (compared with MES bundles, which require 500-nm displacement; Nam et al. 2005). Both of these features will tend to generate maximal transduction currents at head movement onset and help striolar hair cells encode high-frequency stimuli. Our present results suggest that these fast signals are relayed to the CNS over the largest-diameter (presumably fastest conducting) axons supplying the macula. Hair cell and afferent data from other species also support the suggestion that the striola is specialized for encoding fast, phasic head movements including frog (Baird 1994a, 1994b; Baird and Lewis 1986) and rodents (Goldberg et al. 1990b; Songer and Eatock 2013; reviews: Dimiccoli et al. 2013; Eatock and Songer 2011; Goldberg et al. 2012; Lysakowski and Goldberg 2004).
C units (all-calyx afferents) are confined to the striola in all species for which data are available (rodents: Desai et al. 2005; Fernandez et al. 1990; pigeon: Si et al. 2003; turtle: present results). Three features of C units suggest that they may provide especially accurate information about head movement direction. First, their small collecting areas sample a limited range of hair cell excitation angles. Second, their tendency to form complex calyces means that individual C units will tend to average out local irregularities in hair cell directional preferences and so reduce the noise in signals about head movement direction. Finally, individual type I hair cells provide inputs to only one C unit, which reduces redundancy and increases statistical independence in C unit signals to the CNS and so increases the coding efficiency of the C unit ensemble (Schwartz and Simoncelli 2001).
The significance of striolar Zone 2 is unclear. A similar striolar band of type II hair cells is present in birds (Jorgensen 1989; Jorgensen and Anderson 1973; Rosenhall 1970; Si et al. 2003) and, based on bundle structure, in bullfrog (Baird 1994a, 1994b) but not in mammals. B units in Zone 2 resemble B units supplying the torus region in turtle posterior canal (BT units; Brichta and Goldberg 2000a). Both often have thick processes, their axon diameters are comparable to those of CD units and larger than those of other B units, and they are immunoreactive to CR (Brichta and Goldberg 2000a; Brichta and Peterson 1994; Desai et al. 2005; Miller et al. 2009; Monk and Peterson 1995). Response-intensity functions of BT units suggest that they have a narrow operating range and thus may be well suited to detect small postural adjustments of the head (Brichta and Goldberg 2000b). Interestingly, hair bundle structure and mechanics predict that Zone 2 hair cells will have a shorter operating range than any other macular hair cells (Spoon et al. 2011), suggesting that B units in utricular Zone 2 may play a role similar to that suggested for canal BT units.
GRANTS
This work was supported by National Institute on Deafness and Other Communication Disorders Grant DC-05063.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.A.H., G.J.L., B.W., M.H.R., and E.H.P. conception and design of research; J.A.H., G.J.L., B.W., and M.H.R. performed experiments; J.A.H., G.J.L., M.H.R., and E.H.P. analyzed data; J.A.H., G.J.L., B.W., M.H.R., and E.H.P. interpreted results of experiments; J.A.H. and E.H.P. drafted manuscript; J.A.H., B.W., M.H.R., and E.H.P. edited and revised manuscript; G.J.L., B.W., and E.H.P. prepared figures; M.H.R. and E.H.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Iain Miller for important help with fluorescence material and Dr. J. W. Grant for many valuable discussions of utricular mechanics.
REFERENCES
- Armand M, Minor LB. Relationship between time- and frequency-domain analyses of angular head movements in the squirrel monkey. J Comput Neurosci 11: 217–239, 2001. [DOI] [PubMed] [Google Scholar]
- Baird RA. Comparative transduction mechanisms of hair cells in the bullfrog utriculus. I. Responses to intracellular current. J Neurophysiol 71: 666–684, 1994a. [DOI] [PubMed] [Google Scholar]
- Baird RA. Comparative transduction mechanisms of hair cells in the bullfrog utriculus. II. Sensitivity and response dynamics to hair bundle displacement. J Neurophysiol 71: 685–705, 1994b. [DOI] [PubMed] [Google Scholar]
- Baird RA, Lewis ER. Correspondences between afferent innervation patterns and response dynamics in the bullfrog utricle and lagena. Brain Res 369: 48–64, 1986. [DOI] [PubMed] [Google Scholar]
- Baird RA, Schuff NR. Peripheral innervation patterns of vestibular nerve afferents in the bullfrog utriculus. J Comp Neurol 342: 279–298, 1994. [DOI] [PubMed] [Google Scholar]
- Brichta AM, Goldberg JM. Morphological identification of physiologically characterized afferents innervating the turtle posterior crista. J Neurophysiol 83: 1202–1223, 2000a. [DOI] [PubMed] [Google Scholar]
- Brichta AM, Goldberg JM. Responses to efferent activation and excitatory response-intensity relations of turtle posterior-crista afferents. J Neurophysiol 83: 1224–1242, 2000b. [DOI] [PubMed] [Google Scholar]
- Brichta AM, Peterson EH. Functional architecture of vestibular primary afferents from the posterior semicircular canal of a turtle, Pseudemys (Trachemys) scripta elegans. J Comp Neurol 344: 481–507, 1994. [DOI] [PubMed] [Google Scholar]
- Chang JS, Popper AN, Saidel WM. Heterogeneity of sensory hair cells in a fish ear. J Comp Neurol 324: 621–640, 1992. [DOI] [PubMed] [Google Scholar]
- Davis JL, Grant JW. Turtle utricle dynamic behavior using a combined anatomically accurate model and experimentally measured hair bundle stiffness. Hear Res 318: 37–44, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JL, Xue J, Peterson EH, Grant JW. Layer thickness and curvature effects on utricle deformation in the red ear slider turtle: static and modal analysis. J Vestib Res 17: 145–162, 2007. [PMC free article] [PubMed] [Google Scholar]
- Desai SS, Zeh C, Lysakowski A. Comparative morphology of rodent vestibular periphery. I. Saccular and utricular maculae. J Neurophysiol 93: 251–266, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimiccoli M, Girard B, Berthoz A, Bennequin D. Striola magica. A functional explanation of otolith geometry. J Comput Neurosci 35: 125–154, 2013. [DOI] [PubMed] [Google Scholar]
- Dunlap MD, Grant JW. Experimental measurement of utricle system dynamic response to inertial stimuli. J Assoc Res Otolaryngol 15: 511–528, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap MD, Spoon CE, Grant JW. Experimental measurement of utricle dynamic response. J Vestib Res 22: 57–68, 2012. [DOI] [PubMed] [Google Scholar]
- Eatock RA, Lysakowski A. Mammalian vestibular hair cells. In: Vertebrate Hair Cells, edited by Eatock RA, Fay PR, Popper AN. New York: Springer, 2006, p. 348–442. [Google Scholar]
- Eatock RA, Songer JE. Vestibular hair cells and afferents: two channels for head motion signals. Annu Rev Neurosci 34: 501–534, 2011. [DOI] [PubMed] [Google Scholar]
- Fermin CD, Lychakov DV, Campos A, Hara H, Sondag E, Jones T, Jones S, Taylor M, Meza-Ruiz G, Martin DS. Otoconia biogenesis, phylogeny, composition and functional attributes. Histol Histopathol 13: 1103–1154, 1998. [DOI] [PubMed] [Google Scholar]
- Fernandez C, Goldberg JM, Baird RA. The vestibular nerve of the chinchilla. III. Peripheral innervation patterns in the utricular macula. J Neurophysiol 63: 767–780, 1990. [DOI] [PubMed] [Google Scholar]
- Goldberg JM. Afferent diversity and the organization of central vestibular pathways. Exp Brain Res 130: 277–297, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JM, Desmadryl G, Baird RA, Fernández C. The vestibular nerve of the chinchilla. IV. Discharge properties of utricular afferents. J Neurophysiol 63: 781–790, 1990a. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Desmadryl G, Baird RA, Fernandez C. The vestibular nerve of the chinchilla. V. Relation between afferent discharge properties and peripheral innervation patterns in the utricular macula. J Neurophysiol 63: 791–804, 1990b. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Wilson VJ, Cullen KE, Angelaki DE, Buttner-Ennever JA, Minor LB, Broussard DM, Fukushima K. The Vestibular System. New York: Oxford Univ. Press, 2012. [Google Scholar]
- Goodyear R, Holley M, Richardson G. Visualisation of domains in the avian tectorial and otolithic membranes with monoclonal antibodies. Hear Res 80: 93–104, 1994. [DOI] [PubMed] [Google Scholar]
- Goodyear R, Richardson G. Distribution of 275 kD hair cell antigen and cell surface specialisations on auditory and vestibular hair bundles in the chicken inner ear. J Comp Neurol 325: 243–256, 1992. [DOI] [PubMed] [Google Scholar]
- Graydon C, Xue J, Peterson EH. Longitudinal variation in the striola of a turtle, Trachemys scripta: direction is more important than you think (Abstract). Assoc Res Otolaryngol Abstr 29: 151–152, 2006. [Google Scholar]
- Graydon CW, Cho S, Diamond JS, Kachar B, von Gersdorff H, Grimes WN. Specialized postsynaptic morphology enhances neurotransmitter dilution and high-frequency signaling at an auditory synapse. J Neurosci 34: 8358–8372, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM. Structure, development, and plasticity of dendritic spines. Curr Opin Neurobiol 9: 343–348, 1999. [DOI] [PubMed] [Google Scholar]
- Jorgensen JM. The sensory epithelia of the inner ear of two turtles, Testudo graeca L. and Pseudemys scripta. Acta Zool 55: 289–298, 1974. [Google Scholar]
- Jorgensen JM. The number and distribution of calyceal hair cells in the inner ear utricular macula of some reptiles. Acta Zool 69: 169–175, 1988. [Google Scholar]
- Jorgensen JM. Number and distribution of hair cells in the utricular macula of some avian species. J Morphol 201: 187–204, 1989. [DOI] [PubMed] [Google Scholar]
- Jorgensen JM, Anderson T. On the structure of the avian maculae. Acta Zool 54: 121–130, 1973. [Google Scholar]
- Leonard RB, Kevetter GA. Molecular probes of the vestibular nerve. I. Peripheral termination patterns of calretinin, calbindin and peripherin containing fibers. Brain Res 928: 8–17, 2002. [DOI] [PubMed] [Google Scholar]
- Lewis ER, Leverenz EL, Bialek WS. The Vertebrate Inner Ear. Boca Raton, FL: CRC, 1985. [Google Scholar]
- Lewis ER, Li CW. Hair cell types and distributions in the otolithic and auditory organs of the bullfrog. Brain Res 83: 35–50, 1975. [Google Scholar]
- Li A, Xue J, Peterson EH. Architecture of the mouse utricle: macular organization and hair bundle heights. J Neurophysiol 99: 718–733, 2008. [DOI] [PubMed] [Google Scholar]
- Lim DJ. Fine morphology of the otoconial membrane and its relationship to the sensory epithelium. Scan Electron Microsc 3: 929–938, 1979. [PubMed] [Google Scholar]
- Lindeman HH. Regional differences in structure of the vestibular sensory regions. J Laryngol Otol 83: 1–17, 1969. [DOI] [PubMed] [Google Scholar]
- Lysakowski A, Goldberg JM. Morphophysiology of the vestibular periphery. In: The Vestibular System, edited by Highstein SM, Fay RR, Popper AN. New York: Springer, 2004, p. 57–152. [Google Scholar]
- Lysakowski A, Goldberg JM. Ultrastructural analysis of the cristae ampullares in the squirrel monkey (Saimiri sciureus). J Comp Neurol 511: 47–64, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M, Eatock RA. Transduction in the turtle utricle (Abstract). Assoc Res Otolaryngol Abstr 34: 86, 2011. [Google Scholar]
- Miller I, Price B, Berryman M, Peterson EH. Calretinin stains bouton terminals in turtle utricular striola (Abstract). Assoc Res Otolaryngol Abstr 32: 320, 2009. [Google Scholar]
- Monk G, Peterson EH. Calretinin is not specific for calyceal afferents in the semicircular canals of Pseudemys scripta (Abstract). Soc Neurosci Abstr 21: 918, 1995. [Google Scholar]
- Moravec WJ, Peterson EH. Differences between stereocilia numbers on type I and type II vestibular hair cells. J Neurophysiol 92: 3153–3160, 2004. [DOI] [PubMed] [Google Scholar]
- Nam JH, Cotton JR, Grant JW. Effects of fluid forcing on vestibular hair bundles. J Vestib Res 15: 263–278, 2005. [PubMed] [Google Scholar]
- Nam JH, Cotton JR, Grant W. A virtual hair cell. I. Addition of gating spring theory into a 3-D bundle mechanical model. Biophys J 92: 1918–1928, 2007a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam JH, Cotton JR, Grant W. A virtual hair cell. II. Evaluation of mechanoelectric transduction parameters. Biophys J 92: 1929–1937, 2007b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam JH, Cotton JR, Peterson EH, Grant W. Mechanical properties and consequences of stereocilia and extracellular links in vestibular hair bundles. Biophys J 90: 2786–2795, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narins PM, Lewis ER. The vertebrate ear as an exquisite seismic sensor. J Acoust Soc Am 76: 1384–1387, 1984. [DOI] [PubMed] [Google Scholar]
- Platt C. The peripheral vestibular system of fishes. In: Fish Neurobiology, edited by Northcutt RG, Davis RE. Ann Arbor, MI: Univ. of Michigan Press, 1983, p. 89–123. [Google Scholar]
- Pujol R, Pickett SB, Nguyen TB, Stone JS. Large basolateral processes on type II hair cells are novel processing units in mammalian vestibular organs. J Comp Neurol 522: 3141–3159, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell IM, Perachio AA. Three-dimensional analysis of vestibular efferent neurons innervating semicircular canals of the gerbil. J Neurophysiol 78: 3234–3248, 1997. [DOI] [PubMed] [Google Scholar]
- Rivera AR, Davis J, Grant W, Blob RW, Peterson E, Neiman AB, Rowe M. Quantifying utricular stimulation during natural behavior. J Exp Zool A Ecol Genet Physiol 317: 467–480, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenhall U. Some morphological principles of the vestibular maculae in birds. Arch Klin Exp Ohren Nasen Kehlkopfheilkd 197: 154–182, 1970. [DOI] [PubMed] [Google Scholar]
- Ross MD. Morphological evidence for local microcircuits in rat vestibular maculae. J Comp Neurol 379: 333–346, 1997. [DOI] [PubMed] [Google Scholar]
- Rowe MH, Peterson EH. Autocorrelation analysis of hair bundle structure in the utricle. J Neurophysiol 96: 2653–2669, 2006. [DOI] [PubMed] [Google Scholar]
- Saidel WM, Crowder JA. Expression of cytochrome oxidase in hair cells of the teleost utricle. Hear Res 109: 63–77, 1997. [DOI] [PubMed] [Google Scholar]
- Schwartz O, Simoncelli EP. Natural signal statistics and sensory gain control. Nat Neurosci 4: 819–825, 2001. [DOI] [PubMed] [Google Scholar]
- Schweizer FE, Savin D, Luu C, Sultemeier DR, Hoffman LF. Distribution of high-conductance calcium-activated potassium channels in rat vestibular epithelia. J Comp Neurol 517: 134–145, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severinsen SA, Jorgensen JM, Nyengaard JR. Structure and growth of the utricular macula in the inner ear of the slider turtle Trachemys scripta. J Assoc Res Otolaryngol 4: 505–520, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si X, Zakir MM, Dickman JD. Afferent innervation of the utricular macula in pigeons. J Neurophysiol 89: 1660–1677, 2003. [DOI] [PubMed] [Google Scholar]
- Silber J, Cotton J, Nam JH, Peterson EH, Grant W. Computational models of hair cell bundle mechanics. III. 3-D utricular bundles. Hear Res 197: 112–130, 2004. [DOI] [PubMed] [Google Scholar]
- Simmons DD, Tong B, Schrader AD, Hornak AJ. Oncomodulin identifies different hair cell types in the mammalian inner ear. J Comp Neurol 518: 3785–3802, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songer JE, Eatock RA. Tuning and timing in mammalian type I hair cells and calyceal synapses. J Neurosci 33: 3706–3724, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoon C, Grant W. Biomechanics of hair cell kinocilia: experimental measurement of kinocilium shaft stiffness and base rotational stiffness with Euler-Bernoulli and Timoshenko beam analysis. J Exp Biol 214: 862–870, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoon C, Moravec WJ, Rowe MH, Grant JW, Peterson EH. Steady-state stiffness of utricular hair cells depends on macular location and hair bundle structure. J Neurophysiol 106: 2950–2963, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng T, Correia MJ. Regional distribution of ionic currents and membrane voltage response of type II hair cells in the vestibular neuroepithelium. J Neurophysiol 82: 2451–2461, 1999. [DOI] [PubMed] [Google Scholar]
- Werner CF. Die Differenzierung der Maculae im Labyrinth, insbesondere bei Säugetieren. Z Anat Entwicklungsgesch 99: 696–709, 1933. [Google Scholar]
- Wersäll J. The minute structure of the crista ampullaris in the guinea pig as revealed by the electron microscope. Acta Otolaryngol 44: 359–369, 1954. [DOI] [PubMed] [Google Scholar]
- Wilcox RR. Introduction to Robust Estimation and Hypothesis Testing. Burlington, MA: Elsevier, 2005. [Google Scholar]
- Xue J, Miller I, Peterson EH. Coupling between hair bundles and otoconial membrane in turtle utricle (Abstract). Assoc Res Otolaryngol Abstr 30: 326, 2007. [Google Scholar]
- Xue J, Peterson EH. Spatial patterns in the structure of the otolithic membranes (Abstract). Assoc Res Otolaryngol Abstr 26: 32, 2003. [Google Scholar]
- Xue J, Peterson EH. Hair bundle heights in the utricle: differences between macular locations and hair cell types. J Neurophysiol 95: 171–186, 2006. [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostatistical Analysis. Upper Saddle River, NJ: Prentice-Hall, 1999. [Google Scholar]
- Zhu H, Tang X, Wei W, Maklad A, Mustain W, Rabbitt R, Highstein S, Allison J, Zhou W. Input-output functions of vestibular afferent responses to air-conducted clicks in rats. J Assoc Res Otolaryngol 15: 73–86, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]