Abstract
The quantitative relationship between presynaptic calcium influx and transmitter release critically depends on the spatial coupling of presynaptic calcium channels to synaptic vesicles. When there is a close association between calcium channels and synaptic vesicles, the flux through a single open calcium channel may be sufficient to trigger transmitter release. With increasing spatial distance, however, a larger number of open calcium channels might be required to contribute sufficient calcium ions to trigger vesicle fusion. Here we used a combination of pharmacological calcium channel block, high-resolution calcium imaging, postsynaptic recording, and 3D Monte Carlo reaction-diffusion simulations in the adult frog neuromuscular junction, to show that release of individual synaptic vesicles is predominately triggered by calcium ions entering the nerve terminal through the nearest open calcium channel. Furthermore, calcium ion flux through this channel has a low probability of triggering synaptic vesicle fusion (∼6%), even when multiple channels open in a single active zone. These mechanisms work to control the rare triggering of vesicle fusion in the frog neuromuscular junction from each of the tens of thousands of individual release sites at this large model synapse.
Keywords: neuromuscular junction, calcium channels, active zone, synapse, MCell
neurotransmitter release is triggered by action potential-evoked Ca2+ influx through voltage-gated Ca2+ channels. The magnitude and time course of transmitter release is variable and tightly dependent on the coupling between Ca2+ channels and synaptic vesicles (Augustine and Neher 1992; Meinrenken et al. 2002). At some synapses, the summed Ca2+ flux through many open channels appears to be required for synaptic vesicle fusion, suggesting a loose coupling between Ca2+ channels and vesicle fusion machinery (Borst and Sakmann 1996; Meinrenken et al. 2002; Wu et al. 1999). However, at other synapses a small number of Ca2+ channels are often sufficient to trigger vesicle fusion (Brandt et al. 2005; Bucurenciu et al. 2010; Jarsky et al. 2010; Schmidt et al. 2013). In fact, it has been argued that the Ca2+ flux through only one or two open Ca2+ channels triggers vesicle fusion at some synapses (Scimemi and Diamond 2012; Stanley 1993; Yoshikami et al. 1989). Such variable stoichiometric relationships are likely determined by the spatial organization of Ca2+ channels and synaptic vesicles into either microdomain- or nanodomain-controlled release sites (Eggermann et al. 2012; Tarr et al. 2013).
The frog neuromuscular junction (NMJ) has been commonly used to study presynaptic mechanisms that control transmitter release (Bennett 1996; Grinnell 1995; Harlow et al. 2001; Meriney and Dittrich 2013). This synapse features hundreds of linear active zones that are each characterized by double rows of presynaptic ion channels and synaptic vesicles (Heuser et al. 1974; Pawson et al. 1998). Experimentally, it has been estimated that individual active zones release on average a single vesicle following every other action potential stimulus (Dittrich et al. 2013). An earlier study by Shahrezaei et al. (2006) assumed 200 Ca2+ channels per active zone. However, a recent statistical analysis of high spatial and temporal resolution Ca2+ imaging data (Luo et al. 2011) showed that active zones contain relatively few Ca2+ channels (20–40) among the total of 200–250 presynaptic transmembrane protein particles observed in freeze fracture electron microscopy, and that during an action potential these Ca2+ channels open with relatively low probability (∼0.2). These data suggest that the number of Ca2+ channels in each active zone is roughly equivalent to the number of docked synaptic vesicles (Luo et al. 2011; Wachman et al. 2004). Consistent with this hypothesis, previous studies have suggested that transmitter release at the frog NMJ is controlled by Ca2+ influx through very few, perhaps only one or two channels (Shahrezaei et al. 2006; Yoshikami et al. 1989). The resulting approximate 1:1 Ca2+ channel-vesicle stoichiometry significantly impacts how we think about the structure-function relationship in this system. Here, using a combination of high-resolution Ca2+ imaging, postsynaptic recording, pharmacological channel block, and 3D Monte Carlo reaction-diffusion simulations of a realistic active zone model, we present evidence that vesicle fusion is a rare event triggered primarily by the Ca2+ flux through a single open Ca2+ channel. Further, we show that Ca2+ flux through a single open Ca2+ channel triggers fusion of a closely associated vesicle with only low probability (5–6%). Based on this insight, we propose that in the frog NMJ the observed low probability of release from individual single vesicle release sites within active zones is governed both by a low probability of Ca2+ channel opening and a low probability that flux through an open channel will trigger vesicle fusion.
MATERIALS AND METHODS
Tissue preparation.
Adult frogs (Rana pipiens) were decapitated and pithed following anesthesia in 0.6% tricaine methane sulfonate solution as approved by the University of Pittsburgh Institutional Animal Care and Use Committee. The cutaneous pectoris nerve-muscle preparation was dissected bilaterally and bathed in normal frog Ringer (NFR; in mM: 116 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl2, 5 HEPES, pH 7.4) as previously described (Luo et al. 2011; Wachman et al. 2004).
Dye loading.
The Ca2+-sensitive dye Calcium Green-1 (3,000 MW dextran conjugate; Molecular Probes) was loaded through the cut end of the nerve as previously described (Luo et al. 2011; Wachman et al. 2004). Briefly, the nerve was immersed in a drop of 30 mM dye dissolved in distilled water for 6–8 h at room temperature. After rinsing in NFR, the preparation was stored at 4°C for 2–3 h.
Ca2+ imaging and processing.
These procedures were similar to those described by Wachman et al. (2004) and Luo et al. (2011). The muscle was pinned over an elevated Sylgard (Dow Corning) platform in a 35-mm dish mounted on a microscope stage. The postsynaptic acetylcholine receptors were labeled by exposure to 2 μg/ml Alexa 594-conjugated α-bungarotoxin (α-BTX) for 10 min. α-BTX staining was used to locate and focus the postsynaptic receptor bands, which are positioned directly opposite from the presynaptic active zones, and evaluate the z-axis drift over the course of data collection. Superficially positioned nerve terminals, the majority of which were in a single focal plane as judged by α-BTX staining, were chosen for study. All Ca2+ imaging was performed in NFR (except as noted) with 10 μM curare added to prevent nerve-evoked muscle contractions that were not completely blocked by the α-BTX.
An acousto-optic tunable filter (AOTF; ChromoDynamics) was employed to select wavelengths and gate the laser illumination with sub-millisecond time resolution (Krypton-Argon laser; Innova 70 Spectrum, Coherent). The laser was fiber-coupled into the epi-illumination port of an upright fluorescence microscope equipped with a ×100 water-immersion long working-distance objective with 1.0 NA (Lumplan/FL IR, Olympus). Calcium Green-1 was excited at 488 nm and emission light was collected through a 530 ± 20 nm filter. Alexa 594-α-BTX was excited at 567 nm and emission light was collected through a 620 ± 30 nm filter. The timing of laser illumination was triggered with nerve stimulation following a 1.5-ms delay (for nerve conduction), and dye was illuminated for only 1 ms during action potential invasion of the nerve terminal to limit spatial diffusion of Ca2+ entering through voltage-gated Ca2+ channels. Images were recorded on a liquid nitrogen-cooled, back-thinned CCD camera (LN1300B, Roper Scientific), which provided the high-sensitivity low-noise detection necessary for the measurement of fluorescence changes sampled during brief 1-ms time windows.
Raw resting photoelectron counts ranged between 1,200 and 4,000 in different dye-loaded nerve terminals, and fluorescence changes during nerve stimulation (using a suction electrode at a current intensity of 5× threshold) were significantly above resting fluctuations (dominated by shot noise). Images were collected at 0.5 Hz in sets of 20: the first 10 images with stimulator off (background) and the second 10 images with stimulator on (nerve-evoked signals). Images were processed on a Pentium-based computer using MATLAB (MathWorks). Prior to analysis, images were coregistered to correct for slight fluctuation in the lateral position of the preparation during data collection using custom software written by Greg Hood (Pittsburgh Supercomputer Center). Differences in fluorescence above rest were determined for individual images by subtracting mean resting fluorescence (generated by averaging the 10 background images). The resulting “difference images” represented the relative fluorescence changes, computed as ΔF/F0 = (F − F0)/F0, and were displayed in pseudo-color. In multiple trial experiments, 5–10 sets of Ca2+ images (each set consisting of 10 Ca2+ images with stimulation off and 10 Ca2+ images with stimulation on as described above) were collected for each well-focused nerve terminal and coregistered to the first image of the first dataset. Between each set, we confirmed/adjusted focus onto the active zone in reference to α-BTX staining and discarded image sets from analysis if they showed noticeable z-axis drift.
Imaging analysis.
We restricted our quantitative analysis to the nerve terminal regions as defined by a mask based on the resting fluorescence intensity. The blocking effect of titrating ω-conotoxin GVIA (CgTX) on action potential-evoked Ca2+ influx was measured by calculating the ratio of total Ca2+ entry into active zones before and after CgTX treatment from the same terminal regions. The total Ca2+ entry was estimated by the sum of pixel fluorescence intensities above background (ΔF/F0, see above). Only pixels that reported significant action potential-evoked fluorescence change under control condition (i.e., before drug treatment, t-test, P < 0.01) were included.
Exposure to relatively high concentrations of CgTX greatly reduced action potential-evoked Ca2+ entry and therefore fluorescence change (e.g., by 92% in 600 nM CgTX). We have previously shown that the calcium signal reported using this approach is linearly related to calcium entry (Luo et al. 2011). In order to reliably identify Ca2+ entry under this condition, we used a per-pixel selection criterion that required that stimulated fluorescence change above rest (ΔF) should exceed 3 times the standard deviation (3 × SD) of the resting fluorescence. This criterion was chosen based on the following reasons. First, the use of 2 × SD as a criterion was too sensitive and yielded the selection of a large number of pixels between active zones, and a significant number of false positives selected even under unstimulated conditions. On the other hand, the use of 4 × SD was too stringent and eliminated so many pixels from the selection that there were almost none detected within active zone regions. Second, in testing a variety of criteria (2, 3, or 4 SD), we compared the number of pixels that were chosen to the expected number of channels remaining based on the use of such a high concentration of CgTX (600 nM). This CgTX treatment decreased total average Ca2+ entry (as measured by the decrease in Ca2+-sensitive fluorescent signal) into the nerve terminal by 92% (see Table 1). In a previous study of untreated control synapses, we estimated that there are 20–40 Ca2+ channels in each active zone, and that each open during an action potential with a probability (po) of ∼0.2 (see Luo at al. 2011). Therefore, a 92% reduction in total Ca2+ entry would correspond to ∼2 Ca2+ channels remaining in each active zone. Calculating the probability of at least one of these two channels A and B opening during an action potential yields a probability of po(A or B) = po(A) + po(B) - po(AB) = 0.36. Using the 3 × SD criterion led to a detection of a number of Ca2+ entry sites that was roughly consistent with this expectation for po (see Figs. 4 and 5), providing further support for this measure.
Table 1.
The effect of CgTX titration on single action potential-evoked Ca2+ entry and neurotransmitter release (as measured by changes in quantal content)
| [CgTX], nM: | 25 | 50 | 75 | 100 | 400 | 600 |
|---|---|---|---|---|---|---|
| %Ca2+ entry blocked | 22 ± 1.0 | 41 ± 2.6 | 59 ± 4.6 | 70 ± 5.9 | 85 ± 8.0 | 92 ± 1.7 |
| %Vesicle release blocked | 34 ± 2.4 | 64 ± 2.9 | 79 ± 5.0 | 84 ± 1.8 | 96 ± 2.5 | 98 ± 1.4 |
Values are means ± SD.
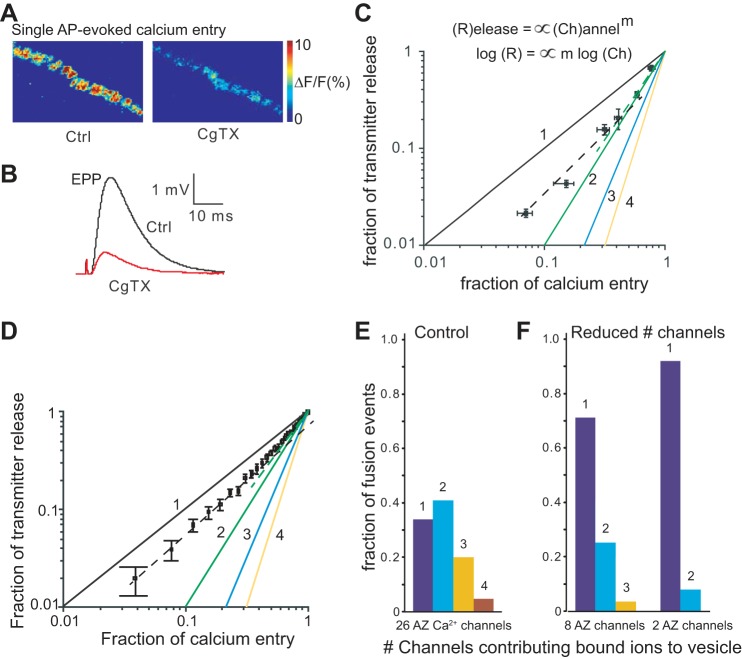
Fig. 4.
Imaging Ca2+ entry through single Ca2+ channel openings in the frog NMJ active zone. A: postsynaptic labeling of acetylcholine receptors [Alexa 594-conjugated α-bungarotoxin (BTX)] is used for estimating the number of active zones within the imaged regions of the nerve terminal. For visual clarity, offset yellow ovals show schematic outlines of the 31 AZs identified in this fluorescence image. B: average Ca2+ signal for a representative control nerve terminal (100 stimuli at 0.5 Hz). C: average Ca2+ signal remaining after block of presynaptic Ca2+ channels using a 60-min exposure to 600 nM CgTX (100 stimuli at 0.5 Hz). D: histogram distribution of single pixel fluorescence intensity under resting (unstimulated), stimulated control, and stimulated CgTX treatment conditions. Exposure to 400 or 600 nM CgTX resulted in a distribution that took a lognormal form suggesting that individual pixels sampled single Ca2+ channel openings. This was distinct from the Gaussian-like distribution of resting intensities, and the distribution of intensities following stimulation under control conditions (no toxin block). E: representative recordings of miniature endplate potentials (mEPPs) from a muscle fiber (top), and averaged EPP response evoked by nerve stimulation (bottom), after exposure to 600 nM CgTX. Using these recordings, quantal content (QC) was calculated (QC = average EPP amplitude/average mEPP amplitude) as listed in the text. Scale bars = 2 μm.
Fig. 5.
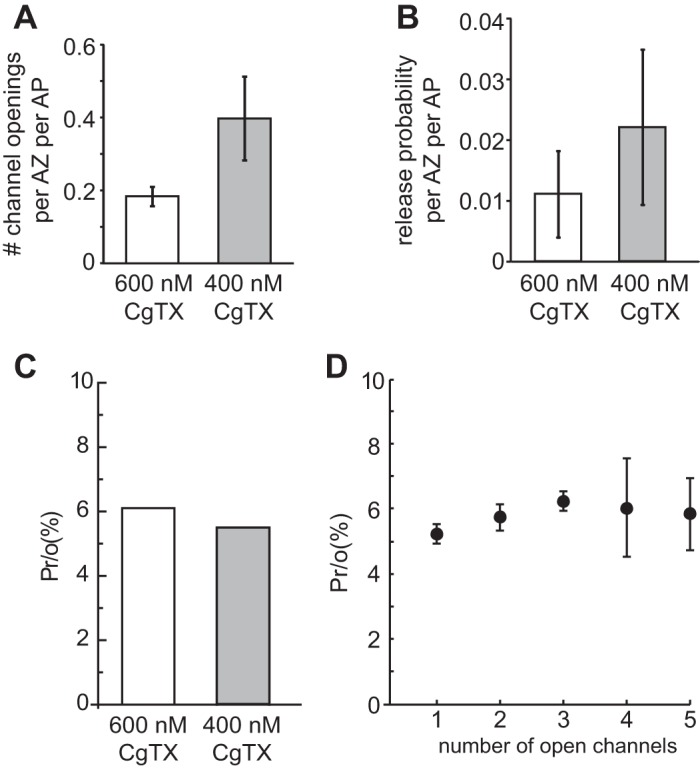
The coupling of Ca2+ influx to vesicle fusion events after exposure to a high concentration of CgTX. A: shown is the number of detected Ca2+ channel openings per active zone and action potential after exposure to either 400 nM or 600 nM CgTX. These data are obtained by dividing the number of imaged Ca2+ entry sites per action potential by the number of active zones within an imaged area (see Fig. 3). B: normalized release probability per active zone and action potential after exposure to 400 nM or 600 nM CgTX determined by dividing the average QC by the average number of active zones in the nerve terminal of adult frog cutaneous pectoris NMJs (700). C: experimental estimate of release probability per Ca2+ channel opening [Pr/o(%)] is similar (5–6%) when calculated following exposure to 400 nM or 600 nM CgTX (plot generated using the data in A and B). D: MCell simulations predict that release probability following a single Ca2+ channel opening is small (5–6%) and comparable to our experimental estimate shown in C. Further, the release probability per Ca2+ channel opening remains relatively constant even as the number of open channels within an active zone is increased in our simulation.
For statistical analysis, the number of detected openings was normalized (as openings per active zone per action potential) for each terminal by dividing the total number of openings by the number of active zones and the number of stimulation trials.
Electrophysiology.
Intracellular recordings from the adult frog cutaneous pectoris nerve-muscle preparation were performed as described previously (Douthitt et al. 2011; Meriney and Grinnell 1991). In brief, the nerve of the cutaneous pectoris muscle was stimulated via a suction electrode at 5× the threshold intensity required to elicit muscle twitch. Intracellular micropipettes were made from glass pipettes (Warner Instruments, filament glass catalog no. 64-0787; O.D. 1 mm; I.D. 0.58 mm) and had a resistance of 25–50 MΩ after filling with 3 M potassium acetate. Surface muscle fibers were penetrated close to nerve endings under visual control with a long working distance water-immersion objective (×40, 3 mm working distance). Only muscles with a resting potential more hyperpolarized than −70 mV were recorded for analysis. Spontaneous miniature endplate potentials (mEPPs) and action potential-evoked endplate potentials (EPPs) were recorded. To calculate the number of synaptic vesicles released following each action potential, quantal content was calculated using the direct method (EPP amplitude/mEPP amplitude). Data were amplified using a Dagan BBC 700 amplifier and acquired using Clampex 9 software (Axon Instruments, Foster City, CA). Clampfit 9.2 was used for data analysis.
Drug treatment.
CgTX is known to block N-type Ca2+ channels in a manner that is essentially irreversible (Stocker et al. 1997). For titrating channel block, the frog nerve-muscle preparation was incubated with various doses of CgTX for 45–60 min and then washed in NFR. Superficially positioned nerve terminals and muscle fibers were chosen for study to avoid variability in toxin access to nerve terminals positioned at different depths of the preparation. Blocking effects on transmitter release and Ca2+ influx were determined in separate experiments to optimize the health of the terminal for imaging experiments and to avoid potential complications due to the buffering effects of Ca2+-sensitive dye during recordings of transmitter release.
Monte Carlo simulations.
All computational modeling was carried out with version 3.1 of the MCell software developed in our lab (Kerr et al. 2008; www.mcell.org). The active zone model used in this paper was identical to our recently developed spatially realistic 3-D model of the frog NMJ active zone (Dittrich et al. 2013) and used here without modification. This model is heavily constrained by a wealth of anatomical, physiological, and biochemical data that have been collected over decades of study on the frog NMJ and other model synapses, as well as more recent work in our laboratory. The geometry of our active zone model was based on published ultrastructural data from the adult frog NMJ (Harlow et al., 2001; Heuser et al. 1974; Pawson et al. 1998) and contained 26 synaptic vesicles arranged in two double rows (Fig. 1B). In addition, 26 N-type voltage-gated Ca2+ channels were positioned in a one-to-one topographic relationship with each docked synaptic vesicle at an average distance from the vesicle of 35 nm (Fig. 1B, see also Dittrich et al. 2013). The total number of Ca2+ channels and their opening probability following single action potentials were constrained by our recent Ca2+ imaging experiments (Luo et al. 2011). During each simulation, an invading action potential led to the stochastic opening of a varying number of voltage gated Ca2+ channels based on their open probability (p ∼ 0.2). Once open, Ca2+ channels would permit Ca2+ ions to enter the presynaptic space according to the instantaneous driving force and the experimentally known channel conductance. Presynaptic, freely diffusing Ca2+ ions could then either bind to Ca2+ sensor sites on synaptic vesicles (synaptotagmin) or 2 mM fixed buffer molecules with kinetic rates as reported in Dittrich et al. (2013). Synaptic vesicle release was determined via our excess-calcium-binding-site model (Dittrich et al. 2013), which unifies recent biochemical evidence regarding the number, arrangement, and properties of available synaptotagmin molecules on synaptic vesicles (our model has 8 synaptotagmin molecules near the base of the vesicle with 5 low-affinity Ca2+ binding sites each, see Fig. 1A) and classical physiology such as the 4th-order calcium-release relationship. The number and positioning of synaptotagmin molecules in the model was chosen to be consistent with previously published proteomic studies (Mutch et al. 2011; Takamori et al. 2006), recently hypothesized orientations of these proteins (Kummel et al. 2011; Wang et al. 2014), and the best fit of the physiology data with these model parameters (Dittrich et al. 2013). In particular, the detailed arrangement of synaptotagmin sites on the bottom of synaptic vesicles does not impact the qualitative features of our model as we have recently shown for second sensor sites within an extension of the model presented in this paper (Ma et al. 2015). Our model captures the well-known narrow distribution of release latencies at the frog NMJ (Katz and Miledi 1965). In contrast to earlier computational studies (Shahrezaei et al. 2006) our model is significantly more detailed and includes the previously determined estimated number of presynaptic voltage-gated Ca2+ channels and their opening probability (Luo et al. 2011), proper timing of stochastic channel opening driven by an action potential waveform, and the resulting narrow latency distribution of synaptic vesicle release. To simulate the experimental titration with increasing concentrations of ω-CgTX GVIA we randomly removed increasing numbers of voltage-gated Ca2+ channels from the model and then measured the resulting synaptic vesicle release.
Fig. 1.

Diagram of the MCell model representation of synaptic vesicles and their arrangement into an active zone. A: schematic view of the bottom of a docked synaptic vesicle used in our MCell model. In this view, the 40 Ca2+ binding sites in groups of 5 are depicted in various shades of gray and each represent a synaptotagmin molecule. B: view of our MCell frog neuromuscular junction (NMJ) active zone (AZ) model including 26 docked synaptic vesicles and their closely associated presynaptic Ca2+ channels (VGCCs). During an action potential, only a small subset of these Ca2+ channels are predicted to open. Depicted is a sample single model run in which open channels are represented as white pentagons and closed channels as black pentagons.
For each model condition we performed 10,000 statistically independent runs on a computer cluster at the Pittsburgh Supercomputing Center (codon.psc.edu, a cluster of 23 dual processor machines, each with two 1.6-GHz AMD Opteron processors and 8 gigabytes of memory). Data analysis was performed with custom-written scripts.
RESULTS
The relationship between presynaptic Ca2+ entry domains and triggering of transmitter release.
In order to explore the quantitative relationship between Ca2+ channel openings and transmitter release at the frog NMJ, which predominantly expresses N-type Ca2+ channels, we titrated the concentration of CgTX to block different fractions of Ca2+ channels, and thus presynaptic Ca2+ entry. Although similar experiments have been performed previously (Shahrezaei et al. 2006), our study improves on these in two significant ways. First, we determined changes in Ca2+ influx and transmitter release in separate experiments to avoid potential buffering effects of Ca2+-sensitive dye on our measures of transmitter release. Second, we imaged Ca2+ entry with fast spatial and temporal resolution to detect restricted sites of Ca2+ entry as opposed to volume averaged signals within the whole nerve terminal. Furthermore, we compare these data to our computer simulations presented below.
When the nerve was stimulated at low frequency (0.5 Hz), strong Ca2+-dependent fluorescence signals were detected within presynaptic active zones (Luo et al. 2011; Wachman et al. 2004). An example of averaged action potential-evoked fluorescence change (normalized as ΔF/F0) over 10 stimulus trials is shown in Fig. 2A. When compared with control, exposure to 100 nM CgTX caused a dramatic decrease in action potential-evoked fluorescence signals at the nerve terminal. The ratio of action potential-evoked-Ca2+ influx before and after drug treatment was used to represent the blocking percentage of Ca2+ channels by various concentrations of CgTX (see Table 1).
Fig. 2.
Titrating ω-conotoxin (CgTX) GVIA blockade and comparing block of Ca2+ entry with block of vesicle fusion reveals a low Ca2+ channel cooperativity in transmitter release. A: CgTX (100 nM) significantly reduced single action potential-evoked Ca2+ entry into the nerve terminals. B: EPP amplitude decreased after exposure to 100 nM CgTX. C: a log-log plot of fractional Ca2+ entry and neurotransmitter release after treatment with various concentrations of CgTX (25, 50, 75, 100, 400, and 600 nM) reveals a relationship with a slope between 1 and 2 (lines with indicated slopes are represented in black, green, blue, and yellow). The dashed green line is a fit to the first few data points during only a small fractional block of Ca2+ channels. This dashed green line has a slope close to 2 (similar to the solid green line). However, after a large fraction block of Ca2+ channels the data are fit using a dashed black line and show a relationship with a slope close to 1 (similar to the solid black line). D: MCell computer simulations reproduce experimental data and predict that each vesicle fusion event is triggered by the Ca2+ flux through very few Ca2+ channels. A log-log plot compares changes in Ca2+ entry and simulated neurotransmitter release after randomly removing increasing numbers of Ca2+ channels from the modeled active zone (which mimics the CgTX titration shown in C). The simulated data fall on a slope between 1 and 2, similar to experimental data shown in C. As in C, the data collected after the removal of only a few calcium channels are fit using a dashed green line, which has a slope close to 2, while the data collected after the removal of many calcium channels are fit using a dashed black line, which has a slope close to 1. E: histogram of the fraction of released synaptic vesicles that bound Ca2+ ions contributed by various numbers of Ca2+ channels under the control condition (26 Ca2+ channels in the AZ). F: histograms of the fraction of released synaptic vesicles that bound Ca2+ ions that originated from various numbers of Ca2+ channels when most of the AZ Ca2+ channels were removed from the simulation (8 channels present; 70% block, and 2 channels present; 92% block). With increasing removal of AZ Ca2+ channels, vesicle fusion increasingly is triggered by the Ca2+ flux through a single Ca2+ channel (dark blue bar).
We next examined the effects of the same concentrations of CgTX on the magnitude of neurotransmitter release using intracellular recording of endplate potentials (EPPs). EPPs were recorded from the same muscle fiber before and after incubation with CgTX. For these recordings, 3–7 μM curare (for partial block of acetylcholine receptors) and 2 μM μ-conotoxin PIIIA (a muscle-specific sodium channel blocker) were added to the Ringer bathing the preparation to block muscle contraction. The concentration of curare was titrated to reduce EPP amplitude to less than 10 mV to avoid significant effects of nonlinear summation of quantal events underlying the EPP, while still permitting the EPP to be measured both before and after exposure to various concentrations of CgTX. Figure 2B shows an example of averaged EPP traces before and after exposure to 100 nM CgTX, and the summary data are reported in Table 1.
We estimated the stoichiometric relationship between Ca2+ entry and transmitter release by plotting the blocking effects of titrating CgTX on both Ca2+ entry and transmitter release on a log-log scale. We have previously demonstrated that our imaged Ca2+ signals are linearly related to Ca2+ influx under these experimental conditions (Luo et al. 2011). As shown in Fig. 2C, the resulting relationship is not completely linear over the range of CgTX doses examined. At low doses of CgTX (25–50 nM) the relationship appeared to fall on a line with a slope of ∼2, but as the concentration of CgTX was increased, this relationship approached a slope closer to 1. These results suggest that under control conditions, only a small number of Ca2+ channels contribute Ca2+ to individual vesicle release events, consistent with previous observations at this synapse (Shahrezaei et al. 2006; Yoshikami et al. 1989). However, as presynaptic Ca2+ channels are blocked, release events depend increasingly on single open channels.
We then tested whether our spatially realistic excess-Ca2+-binding-site model of the frog NMJ active zone (Dittrich et al. 2013) was able to reproduce the effect of titrating CgTX to block presynaptic Ca2+ entry on transmitter release. This model has been shown to reproduce many properties of neuromuscular transmission faithfully including the normal release rate, the 4th power relationship between vesicle release and extracellular Ca2+ concentration, and synaptic delay (Dittrich et al. 2013). To model CgTX titration, an increasing number of Ca2+ channels were randomly removed from the active zone, and the resulting amount of vesicle release following single action potential stimulation was simulated. As shown in Fig. 2D and Table 2, the model reproduced accurately the experimental observations (compare with Fig. 2C and Table 1), and thus could be used to aid in interpreting these data.
Table 2.
The effect of titrating blockade of Ca2+ channels on action potential-evoked neurotransmitter release using Monte Carlo simulation
| Active Zone Channel Number: | 26 | 20 | 15 | 8 | 4 | 2 |
|---|---|---|---|---|---|---|
| %Channels removed | Control | 23 | 42 | 69 | 85 | 92 |
| %Vesicle release blocked | Control | 32 | 57 | 79 | 91 | 96 |
Since our experimental data indicated that very few, but likely more than one Ca2+ channel contributed Ca2+ ions to triggering single vesicle release from control terminals, we used our MCell results to estimate the percentage of released vesicles that bound Ca2+ ions from one, two, or more Ca2+ channels (Fig. 2E). The simulations showed that 5.2 ± 0.3 Ca2+ channels opened in each active zone following a single action potential stimulation. Interestingly, we found that about 34% of released synaptic vesicles bound only Ca2+ ions that entered the nerve terminal through a single open Ca2+ channel, and 41% of vesicle fusion events were triggered by Ca2+ ions derived from only two open Ca2+ channels (see also Dittrich et al. 2013). Vesicle fusion triggered by Ca2+ ions from more than two channel openings was less likely. On average, synaptic vesicle fusion was triggered by Ca2+ ions derived from 1.9 Ca2+ channels, consistent with our experimental CgTX titration data. The spatial distribution of the average contribution of specific Ca2+ channels to a given vesicle fusion event is graphically depicted in Fig. 3B. As expected, this relationship changed when we decreased the number of available Ca2+ channels in the model active zone (Fig. 2F). When 80% of Ca2+ channels were blocked, the average number of Ca2+ channels that open was reduced to 1.0 ± 0.2 and the average number of vesicles released per single active zone was reduced to 0.06 ± 0.007. Under these conditions, using large simulation runs (10,000) to increase the reliability of our estimates, we determined that 460 of 550 (84%) released vesicles were bound by Ca2+ ions from a single Ca2+ channel. Therefore, the model predicts that vesicle fusion will still occur with only a small number of active Ca2+ channels in the active zone and that these events will be triggered almost exclusively by Ca2+ ions derived from a single open Ca2+ channel.
Fig. 3.

Ca2+ flux through a single open Ca2+ channel primarily provides the trigger for vesicle fusion in our simulated active zone model, even when other channels open nearby. A: given a particular vesicle fusion event in the AZ (red circle), the average probability that individual Ca2+ channels have opened is represented by the red area in the pie chart and the corresponding percentage associated with each channel icon. B, left: for the majority of vesicle fusion cases in which the closely-associated Ca2+ channel has opened (94% as represented in A above), the number in each Ca2+ channel circle icon indicates the percentage of Ca2+ ions contributed by each Ca2+ channel to the total number of ions bound by the vesicle at the time of fusion (red circle). B, right: representative model schematic depicting the 40 Ca2+ binding sites (gray areas) at the bottom of synaptic vesicles (SV). The colored dots indicate Ca2+ ions (color coded by their channel of origin) bound to different synaptotagmin binding sites at the time of vesicle fusion for a representative single model run under the condition that the closely associated Ca2+ channel has opened (94% of fusion events). C, left: for the minority of vesicle fusion cases in which the closely associated Ca2+ channel has not opened (6% as represented in A above), the number in each Ca2+ channel circle icon indicates the percentage of Ca2+ ions contributed by each Ca2+ channel to total ions bound by the vesicle at the time of fusion (red circle). C, right: representative model schematic of the bound Ca2+ ions on vesicular synaptotagmin binding sites at the time of fusion for a representative single model run under the conditions that the closely associated Ca2+ channel has not opened (6% of fusion events). Schematic is organized as described above for B.
Single Ca2+ channel openings predominate in contributing Ca2+ ions to vesicle fusion even when more than one channel opens in the active zone.
To further understand the quantitative contribution of Ca2+ channels to triggering vesicle release, we tracked the pattern of Ca2+ channel opening and Ca2+ ion binding for each given fused vesicle using our recently published excess-calcium-binding-site model of the frog NMJ active zone (Dittrich et al. 2013). Using this approach, Dittrich et al. (2013) showed that known properties of action potential-evoked transmitter release at the frog NMJ could be best fit if synaptic vesicle fusion was triggered by at least two Ca2+ ions binding to at least three of the modeled synaptotagmin molecules on a given synaptic vesicle. In fact, on average, 7–8 Ca2+ ions bound to a synaptic vesicle (among the 40 total Ca2+ binding sites available, see materials and methods; Dittrich et al. 2013) at the time of vesicle fusion. Here, we extended our use of this model to evaluate the Ca2+ channel of origin for these bound Ca2+ ions. Interestingly, we found that when a particular vesicle fused, 94% of the time the nearest, closely-associated Ca2+ channel opened (Fig. 3A). For these cases, the Ca2+ ions that bound to the fused vesicle were found 82% of the time to originate from the single nearest Ca2+ channel (Fig. 3B, left panel). Therefore, our model suggests that of the average 7–8 Ca2+ ions that bind to trigger vesicle fusion, 6–7 were derived from the closest Ca2+ channel and only 1–2 Ca2+ ions originate from other open Ca2+ channels (Fig. 3B, right panel). On the other hand, when the nearest, closely-associated Ca2+ channel did not open, a synaptic vesicle rarely fused (only 6% of total fusion events). Under these rare conditions, the Ca2+ ions that bound to the fused synaptic vesicle derived predominately from a nearby cluster of 3–5 Ca2+ channels, with each of these channels contributing 1–2 Ca2+ ions to the synaptic vesicle Ca2+ binding sites (Fig. 3C). In conclusion, our simulations predict that action potential-evoked transmitter release at the frog NMJ is controlled by the Ca2+ ions that derive predominantly from the opening of one closely associated Ca2+ channel, even when several Ca2+ channels open in the active zone (Fig. 3, A and B).
Experimental evidence that single channel openings can trigger synaptic vesicle release.
Our previous Ca2+ imaging data (Luo et al. 2011; Wachman et al. 2004) have shown that each active zone in the adult frog NMJ has relatively few Ca2+ channels (20–40), and that each Ca2+ channel has a relatively low opening probability during an action potential (∼0.2). Therefore, only a few of these Ca2+ channels would be predicted to open (4–8) within each active zone during a single action potential. Thus we attempted to reduce the number of functional Ca2+ channels so that a single action potential would open at most one Ca2+ channel within each active zone. To block most Ca2+ channels, we exposed the preparation to 600 nM CgTX for 60 min. We imaged action potential-evoked Ca2+ influx at active zones and found that the total Ca2+ entry was reduced by 92 ± 1.7% (Fig. 4, A–C; n = 3). Following a reduction of this magnitude, the number of available Ca2+ channels within individual active zones is expected to be very small (1∼2), and given the low probability of opening (∼0.2), the likelihood that more than one channel would open in the same active zone during a single action potential stimulus would be very small (∼0.04). In agreement with this expectation, the Ca2+-sensitive fluorescent signals detected by each imaging pixel in our imaging system had a large coefficient of variation (CV = 1.6 ± 0.5) after 600 nM CgTX treatment, compared with control conditions (CV = 0.5 ± 0.3), consistent with a large reduction in the number of channels. To increase the total number of Ca2+ signals detected for these experiments, we collected 70–100 trials of stimulus-evoked Ca2+ images after CgTx treatment. After exposure to 600 nM CgTX, we identified discrete, sparse, and spatially distributed signals evoked by single action potentials within well-focused regions of the nerve terminal (Fig. 4C). As shown in Fig. 4D, under 600 nM CgTX block the histogram distribution of the fluorescence intensity detected by individual pixels underlying Ca2+ channel openings takes a log-normal form, similar to the distribution of the single channel current integral (see figure 2, C and D, in Dittrich et al. 2013). This distribution is generated as single channels open with variable mean open time under conditions of rapidly changing driving force at different times during the action potential waveform. The similarity of the fluorescence and single channel current histogram distributions is consistent with our prediction that at most one Ca2+ channel opens in each active zone under these conditions. A similar lognormal distribution was observed after exposure to 400 nM CgTX, which reduced total action potential-evoked Ca2+ influx evoked by 84.7 ± 3.3%. In contrast, for control terminals, the histogram distribution of signal intensities from individual pixels was significantly shifted to higher magnitude and took a more Gaussian shape (Fig. 4D). We hypothesize that exposure to either 400 or 600 nM CgTX blocked sufficient numbers of Ca2+ channels in each active zone that imaged Ca2+ signals were derived predominantly from a single open channel. Under these conditions, we predict that transmitter release would be rare, and when it occurred, it would be triggered by single channel openings.
In order to test this hypothesis, we carried out postsynaptic recording of EPPs from muscle fibers after exposure to either 600 nM or 400 nM CgTX. As shown in Fig. 4E, we still detected significant transmitter release from nerve terminals after exposure to either 400 or 600 nM CgTX, despite the rare Ca2+ channel openings in each active zone as predicted under these conditions. After exposure to 400 nM CgTX quantal content measured 15.2 ± 8.8, while after exposure to 600 nM CgTX quantal content was only 7.6 ± 4.9. These values are obviously much lower than what has been reported in untreated control nerve terminals (quantal content ∼350; Dittrich et al. 2013; Katz and Miledi 1979), but support the hypothesis that isolated single Ca2+ channel openings can trigger transmitter release from the frog NMJ.
Estimating the probability of transmitter release triggered by the Ca2+ flux through a single Ca2+ channel opening.
How reliably can single Ca2+ channel openings trigger synaptic vesicle fusion? In order to evaluate the vesicle fusion probability due to single Ca2+ channel openings, we measured the frequency of observing Ca2+ channel openings at the single active zone level after exposure to different concentrations of CgTX. We then compared the channel opening frequency with the release probability of a single active zone under each treatment condition. To calculate Ca2+ channel openings per active zone, we limited our analysis to those nerve terminal regions where we could clearly identify individual active zones (see Fig. 4A). For example, 31 active zones can be distinguished within the region of interest identified in the representative nerve terminal shown in Fig. 4A. After exposure to 600 nM CgTX, we collected imaging data from 100 stimulus trials and calculated that each action potential evoked an average of 0.23 Ca2+ channel openings per active zone. Similar results were obtained from 8 nerve terminals and the mean number of openings evoked by a single action potential was estimated to be 0.18 ± 0.07 per active zone (Fig. 5A). This value is very close to our estimate of the average opening probability of individual Ca2+ channels (Luo et al. 2011), which provides further support for our hypothesis that the number of functional Ca2+ channels within individual active zones is reduced to approximately one after exposure to 600 nM CgTX. We also calculated the number of detected Ca2+ entry sites for nerve terminals treated with 400 nM CgTX. In these experiments, we found that the number of Ca2+ channel openings per active zone was 0.40 ± 0.11. This value is roughly double the value observed after treatment with 600 nM CgTX and consistent with a doubling in the total Ca2+ entry that remained after block (15.3% vs. 7% of control for 400 and 600 nM, respectively).
Next we calculated the average number of released vesicles from individual active zones during an action potential. Because the quantal content indicates the total number of synaptic vesicles released from the entire nerve terminal, which on average has about 700 active zones at the frog NMJ (Dittrich et al. 2013), the number of released vesicles per active zone can be calculated by dividing the quantal content by the total number of active zones. As shown above, our measured quantal content was 7.6 ± 4.9 (n = 42) and 15.1 ± 8.8 (n = 42) after exposure to 600 nM and 400 nM CgTX, respectively. Therefore, the release probability per active zone during an action potential is estimated to be ∼0.01 (7.6/700) and ∼0.02 (15.1/700), respectively, after 600 nM and 400 nM CgTX treatment (Fig. 5B). This is much lower than the release probability of control terminals (∼0.5, Dittrich et al. 2013; Katz and Miledi 1979).
Finally, the probability that a single Ca2+ channel opening triggers synaptic vesicle fusion can be estimated by dividing the average number of release events per active zone by the average number of single Ca2+ channel openings in each active zone. Using this approach, the probability that Ca2+ flux through a single open Ca2+ channel can trigger vesicle fusion was calculated to be ∼6% (Fig. 5C).
We then used our Monte Carlo simulation approach to validate these results by examining the release probability of synaptic vesicles in the model when the number of Ca2+ channels in the active zone was greatly reduced. Under the extreme condition when very few Ca2+ channels were available, the probability that only a single Ca2+ channel opened clearly followed the binomial distribution. For example, if the total number of Ca2+ channels within the active zone was reduced to 3, the probability that only one channel opened during an action potential was predicted as [(3!)/(1! × 2!)]P1(1 − P)2 = 0.37 (for P equal to 0.19; see Luo et al. 2011). Indeed, under these conditions, we observed 3,823 single channel opening events out of 10,000 modeling runs (generated by distinct random number seeds) and 221 of these released a synaptic vesicle. Therefore, our model predicted that the release probability per opening was equal to 5.8% (221/3,823; Fig. 5C), very close to our experimental measurement (∼6%). We then varied the number of available Ca2+ channels in the active zone from 1 to 10 and determined that the release probability was 5.2 ± 0.3% (mean ± SD) for release events in which only one Ca2+ channel opened during an action potential (see Table 3).
Table 3.
Release probability from a single active zone. The numbers present averages over active zone models that had 1 to 10 available Ca2+ channels
| Number of Ca2+ Channel Openings: | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Total release probability | 5.2 ± 0.3 | 11.4 ± 0.8 | 18.6 ± 0.9 | 24.0 ± 6.0 | 29.0 ± 5.5 |
| Release probability per channel opening | 5.2 ± 0.3 | 5.7 ± 0.4 | 6.2 ± 0.3 | 6.0 ± 1.5 | 5.8 ± 1.1 |
Values are means ± SD. The numbers present averages over active zone models that had 1 to 10 available Ca2+ channels.
We then used our MCell model to ask whether additional open Ca2+ channels in the active zone would enhance the release probability per opening. If each vesicle release event is mainly triggered by a single open channel, the release probability per opening should not change significantly regardless how many channels open in the active zone. On the other hand, if several open channels cooperate to trigger fusion of a given vesicle, the release probability per opening should increase with more open channels, perhaps even in a supralinear fashion. With the full complement of Ca2+ channels available in our active zone model (26), the average number of channels that opened with each action potential was ∼5, but even under this condition, the probability of vesicle release per opening was only 6–7%. As shown in Fig. 5D and Table 3, our model predicted that the release probability per Ca2+ channel opening was little affected by the total number of open Ca2+ channels in the active zone. These data underscore the predominance of vesicle fusion events triggered by Ca2+ flux through a single open channel, even when several Ca2+ channels open in the active zone.
DISCUSSION
We have provided experimental and computational evidence that at the adult frog NMJ vesicle release is triggered by a spatially localized nanodomain of Ca2+ ions overwhelmingly derived from the single Ca2+ channel most tightly associated with the fused vesicle, with contributions from a small number of additional channels nearby. In fact, we show that a single open Ca2+ channel is able to trigger synaptic vesicle release from presynaptic active zones at the adult frog NMJ. Beyond what was previously reported in the literature (Shahrezaei et al. 2006), using realistic estimates of the channel-vesicle stoichiometry and channel open probability (Luo et al. 2011), we provide an estimate for the release probability of a synaptic vesicle triggered by the Ca2+ flux through a single open Ca2+ channel, which is only about 6%. Thus, despite a tight 1:1 coupling of Ca2+ channels to synaptic vesicles, open Ca2+ channels trigger release unreliably. These data advance our understanding of presynaptic active zone function at a model synapse (the frog NMJ).
The role of Ca2+ channel cooperativity in triggering transmitter release.
An ongoing debate centers on whether release of individual synaptic vesicles is triggered by Ca2+ flux through a single open Ca2+ channel or through multiple open Ca2+ channels in the active zone (Tarr et al. 2013). Since the spatial relationship between docked synaptic vesicles and presynaptic Ca2+ channels is not known at most synapses, previous studies typically relied on measuring the power relationship between transmitter release and Ca2+ entry by altering the number of available Ca2+ channels (Augustine 1990; Mintz et al. 1995; Scimemi and Diamond 2012; Shahrezaei et al. 2006; Wu et al. 1999; Yoshikami et al. 1989). Conceptually, the effectiveness of gradual Ca2+ channel blockade on transmitter release is determined by the spatial relationship between Ca2+ channels and synaptic vesicles, and therefore reflects the cooperative coupling (denoted by m) of Ca2+ channels in controlling vesicle secretion. The value of m has been shown to vary for different synapses and different subtypes of Ca2+ channels at a single synapse. For example, at cerebellar parallel fiber synapses, Mintz et al. (1995) determined that transmitter release triggered by N-type Ca2+ channels had an m value of 2.5, whereas transmitter release triggered by P/Q type Ca2+ channels had an m value of 4.0. Similarly, at the young calyx of Held synapse, transmitter release triggered by P/Q type Ca2+ channels has a different m value (m = 3.7) than release triggered by N-type Ca2+ channels (m = 1.3; Wu et al. 1999). As the calyces mature, the m value for the P/Q type Ca2+ channels decreases (Fedchyshyn and Wang 2005), suggesting that the spatial coupling between Ca2+ channels and docked vesicles is tightened during development. At the chick ciliary ganglion calyx, where N-type channels trigger release, m has been reported to be 1.3 (Gentile and Stanley 2005). Such a large variability in the cooperative coupling of Ca2+ channels with neurotransmitter release suggests a wide variety of spatial organization of Ca2+ channels within individual release sites at different synapses. Lower values of m (1–2) suggest that a small number of open Ca2+ channels are sufficient for triggering the fusion of a synaptic vesicle and which may thus be tightly associated with those open channels. In contrast, higher values of m (4–5) indicate that many Ca2+ channels need to open simultaneously to contribute the necessary Ca2+ ions to trigger the release of a single synaptic vesicle. Under conditions where m values are high, the Ca2+ channel cooperativity measurement is governed by the molecular cooperativity of the Ca2+ sensor (Dodge and Rahamimoff 1967) as titrating channel block is essentially similar to changing extracellular Ca2+ concentration at these synapses (Meinrenken et al. 2002).
In agreement with previous studies (Shahrezaei et al. 2006; Yoshikami et al. 1989), we have shown at the adult frog NMJ that Ca2+ channels have a low cooperativity in triggering transmitter release (m = 1–2). In contrast to previous reports, our studies are built upon our recent high-resolution Ca2+ imaging data that support the hypothesis that there are relatively few functional Ca2+ channels in each frog NMJ active zone (Luo et al. 2011). These data predict a 1:1 relationship between presynaptic Ca2+ channels and docked synaptic vesicles and allow our current study to make detailed predictions regarding the probability with which individual channel openings trigger vesicle fusion (see below). The small m value we report here further supports the close association between individual docked synaptic vesicles and a single Ca2+ channel. As evidenced by our Monte Carlo simulations, Ca2+ ions that bound to released synaptic vesicles were primarily derived from the nearest open Ca2+ channel. This dominant control of vesicle fusion by a single Ca2+ channel (nanodomain coupling) becomes more pronounced when a majority of Ca2+ channels are blocked. On the other hand, a significant number of vesicle release events involved small contributions from neighboring Ca2+ channels, and it will be interesting to investigate possible functional implications of this finding in future work.
Functional organization of single vesicle release sites within active zones of the frog NMJ.
At individual release sites in a variety of synapses the measured Ca2+ channel-release site cooperativity (the m value) for triggering transmitter release has been used to infer the stoichiometric relationship between Ca2+ channels and docked synaptic vesicles. However, there has been no direct evidence on how reliably the Ca2+ flux through a single open Ca2+ channel can trigger synaptic vesicle fusion. Past studies have at most determined the approximate number of Ca2+ channels contributing ions to vesicle release (Shahrezaei et al. 2006). By greatly reducing the number of Ca2+ channels in each active zone using drug treatments combined with high-resolution Ca2+ imaging techniques, we were able to capture sparsely distributed Ca2+ influx from single Ca2+ channel openings. In addition we conducted Monte Carlo simulations of a realistic active zone model with small numbers of active Ca2+ channels and estimated the coupling between single Ca2+ channel openings and vesicle fusion. Analysis of our data supports the conclusion that after block of 85 and 92% of Ca2+ influx within active zones (using 400 or 600 nM CgTX, respectively), we were able to image the Ca2+ flux through single open Ca2+ channels in individual active zones during single action potential stimulation. Further, our results show that Ca2+ flux through single open voltage-gated Ca2+ channels triggered synaptic vesicle fusion with a probability of only ∼6%. Importantly, even under control conditions with an average of five Ca2+ channel openings per action potential stimulus, the vesicle release probability per open channel remained approximately constant at ∼6% (Table 3). This finding provides a mechanistic explanation for the observation that action potential stimulation only unreliably triggers vesicle fusion at each single vesicle release site in the frog NMJ. Importantly, however, these highly unreliable single vesicle release sites (composed of one synaptic vesicle and its closely associated Ca2+ channel) can be assembled to build a strong and reliable synapse using thousands of such units. Such assembly of unreliable single vesicle release sites into active zones and nerve terminals is important for conserving presynaptic resources and allows both strength and reliability of synaptic transmission at the entire NMJ during repetitive nerve activity (Tarr et al. 2013).
Even though vesicle fusion is predominately triggered by the closest Ca2+ channel during single activation events, the long, linear arrangement of Ca2+ channels at frog active zones might lead to significant contributions of adjacent channels during trains of action potentials. Indeed, an evaluation of short-term synaptic plasticity mechanisms at the frog NMJ combining physiological data and MCell computer simulations showed that the contribution of adjacent Ca2+ channels to vesicle release increased during subsequent stimuli (Ma et al. 2015). Clearly, the precise relationship between active zone structural organization (especially with respect to Ca2+ channels and synaptic vesicles) and physiological function requires further examination.
GRANTS
This work was supported by National Institutes of Health Grants R01-NS-043396 (to S. D. Meriney), R01-NS090644 (to S. D. Meriney and M. Dittrich), R01-GM-068630, P41-RR-06009, and P41-GM-103712 (to J. R. Stiles and M. Dittrich) and by National Science Foundation Grants 0844174 (to M. Dittrich), 0844604 (to S. D. Meriney), and 1249546 (to M. Dittrich and S. D. Meriney).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: F.L., M.D., S.C., J.R.S., and S.D.M. conception and design of research; F.L., M.D., S.C., and S.D.M. performed experiments; F.L., M.D., S.C., and S.D.M. analyzed data; F.L., M.D., and S.D.M. interpreted results of experiments; F.L., M.D., and S.D.M. prepared figures; F.L., M.D., and S.D.M. drafted manuscript; F.L., M.D., and S.D.M. edited and revised manuscript; F.L., M.D., S.C., and S.D.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank G. Hood for writing the image alignment routine and T. Tarr for feedback during the preparation of this manuscript.
REFERENCES
- Augustine GJ. Regulation of transmitter release at the squid giant synapse by presynaptic delayed rectifier potassium current. J Physiol 431: 343–364, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine GJ, Neher E. Neuronal Ca2+ signaling takes the local route. Curr Opin Neurobiol 2: 302–307, 1992. [DOI] [PubMed] [Google Scholar]
- Bennett MR. Neuromuscular transmission at an active zone: the secretosome hypothesis. J Neurocytol 25: 869–891, 1996. [DOI] [PubMed] [Google Scholar]
- Borst JG, Sakmann B. Calcium influx and transmitter release in a fast CNS synapse. Nature 383: 431–434, 1996. [DOI] [PubMed] [Google Scholar]
- Brandt A, Khimich D, Moser T. Few CaV1.3 channels regulate the exocytosis of a synaptic vesicle at the hair cell ribbon synapse. J Neurosci 25: 11577–11585, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucurenciu I, Bischofberger J, Jonas P. A small number of open Ca2+ channels trigger transmitter release at a central GABAergic synapse. Nat Neurosci 13: 19–21, 2010. [DOI] [PubMed] [Google Scholar]
- Dittrich M, Pattillo JM, King JD, Cho S, Stiles JR, Meriney SD. An excess-calcium-binding-site model predicts neurotransmitter release at the neuromuscular junction. Biophys J 104: 2751–2763, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge FA, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol 193: 419–432, 1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douthitt HL, Luo F, McCann SD, Meriney SD. Dynasore, an inhibitor of dynamin, increases the probability of transmitter release. Neuroscience 172: 187–195, 2011. [DOI] [PubMed] [Google Scholar]
- Eggermann E, Bucurenciu I, Goswami SP, Jonas P. Nanodomain coupling between Ca2+ channels and sensors of exocytosis at fast mammalian synapses. Nat Rev Neurosci 13: 7–21, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedchyshyn MJ, Wang LY. Developmental transformation of the release modality at the calyx of Held synapse. J Neurosci 25: 4131–4140, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile L, Stanley EF. A unified model of presynaptic release site gating by calcium channel domains. Eur J Neurosci 21: 278–282, 2005. [DOI] [PubMed] [Google Scholar]
- Grinnell AD. Dynamics of nerve-muscle interaction in developing and mature neuromuscular junctions. Physiol Rev 75: 789–834, 1995. [DOI] [PubMed] [Google Scholar]
- Harlow ML, Ress D, Stoschek A, Marshall RM, McMahan UJ. The architecture of active zone material at the frog's neuromuscular junction. Nature 409: 479–484, 2001. [DOI] [PubMed] [Google Scholar]
- Heuser JE, Reese TS, Landis DM. Functional changes in frog neuromuscular junctions studied with freeze-fracture. J Neurocytol 3: 109–131, 1974. [DOI] [PubMed] [Google Scholar]
- Jarsky T, Tian M, Singer JH. Nanodomain control of exocytosis is responsible for the signaling capability of a retinal ribbon synapse. J Neurosci 30: 11885–11895, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B, Miledi R. The effects of calcium on acetylcholine release from motor nerve terminals. Proc R Soc L B Biol Sci 161: 496–503, 1965. [DOI] [PubMed] [Google Scholar]
- Katz B, Miledi R. Estimates of quantal content during “chemical potentiation” of transmitter release. Proc R Soc Lond B Biol Sci 205: 369–378, 1979. [DOI] [PubMed] [Google Scholar]
- Kerr R, Bartol TM, Kaminsky B, Dittrich M, Chang JCJ, Baden S, Sejnowski TJ, Stiles JR. Fast Monte Carlo simulation methods for biological reaction-diffusion systems in solution and on surfaces. SIAM J Sci Comput 30: 3126–3149, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kümmel D, Krishnakumar SS, Radoff DT, Li F, Giraudo CG, Pincet F, Rothman JE, Reinisch KM. Complexin cross-links prefusion SNAREs into a zigzag array. Nat Struct Mol Biol 18: 927–933, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo F, Dittrich M, Stiles JR, Meriney SD. Single-pixel optical fluctuation analysis of calcium channel function in active zones of motor nerve terminals. J Neurosci 31: 11268–11281, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Kelly L, Ingram J, Price TJ, Meriney SD, Dittrich M. New insights into short-term synaptic facilitation at the frog neuromuscular junction. J Neurophysiol 113: 71–87, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinrenken CJ, Borst JG, Sakmann B. Calcium secretion coupling at calyx of held governed by nonuniform channel-vesicle topography. J Neurosci 22: 1648–1667, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meriney SD, Grinnell AD. Endogenous adenosine modulates stimulation-induced depression at the frog neuromuscular junction. J Physiol 443: 441–455, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meriney SD, Dittrich M. Organization and function of transmitter release sites at the neuromuscular junction. J Physiol 591: 3159–3165, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz IM, Sabatini BL, Regehr WG. Calcium control of transmitter release at a cerebellar synapse. Neuron 15: 675–688, 1995. [DOI] [PubMed] [Google Scholar]
- Mutch SA, Kensel-Hammes P, Chiu DT. Protein quantification at the single vesicle level reveals that a subset of synaptic vesicle proteins are trafficked with high precision. J Neurosci 31: 1461–1470, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson PA, Grinnell AD, Wolowske B. Quantitative freeze-fracture analysis of the frog neuromuscular junction synapse-I. Naturally occurring variability in active zone structure. J Neurocytol 27: 361–377, 1998. [DOI] [PubMed] [Google Scholar]
- Schmidt H, Brachtendorf S, Arendt O, Hallermann S, Ishiyama S, Bornschein G, Gall D, Schiffmann SN, Heckmann M, Eilers J. Nanodomain coupling at an excitatory cortical synapse. Curr Biol 23: 244–249, 2013. [DOI] [PubMed] [Google Scholar]
- Scimemi A, Diamond JS. The number and organization of Ca2+ channels in the active zone shapes neurotransmitter release from Schaffer collateral synapses. J Neurosci 32: 18157–18176, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahrezaei V, Cao A, Delaney KR. Ca2+ from one or two channels controls fusion of a single vesicle at the frog neuromuscular junction. J Neurosci 26: 13240–13249, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley EF. Single calcium channels and acetylcholine release at a presynaptic nerve terminal. Neuron 11: 1007–1011, 1993. [DOI] [PubMed] [Google Scholar]
- Stocker JW, Nadasdi L, Aldrich RW, Tsien RW. Preferential interaction of omega-conotoxins with inactivated N-type Ca2+ channels. J Neurosci 17: 3002–3013, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamori S, Holt M, Jahn R. Molecular anatomy of a trafficking organelle. Cell 127: 831–846, 2006. [DOI] [PubMed] [Google Scholar]
- Tarr TB, Dittrich M, Meriney SD. Are unreliable release mechanisms conserved from NMJ to CNS? Trends Neurosci 36: 14–22, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachman ES, Poage RE, Stiles JR, Farkas DL, Meriney SD. Spatial distribution of calcium entry evoked by single action potentials within the presynaptic active zone. J Neurosci 24: 2877–2885, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Bello O, Auclair SM, Wang J, Coleman J, Pincet F, Krishnakumar SS, Sindelar SV, Rothman JE. Calcium sensitive ring-like oligomers formed by synaptotagmin. Proc Natl Acad Sci USA 111: 13966–13971, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LG, Westenbroek RE, Borst JG, Catterall WA, Sakmann B. Calcium channel types with distinct presynaptic localization couple differentially to transmitter release in single calyx-type synapses. J Neurosci 19: 726–736, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikami D, Bagabaldo Z, Olivera BM. The inhibitory effects of omega-conotoxins on Ca2+ channels and synapses. Ann NY Acad Sci 560: 230–248, 1989. [DOI] [PubMed] [Google Scholar]