Abstract
Recent advances in recording and computing hardware have enabled laboratories to record the electrical activity of multiple brain regions simultaneously. Lagging behind these technical advances, however, are the methods needed to rapidly produce microdrives and head-caps that can flexibly accommodate different recording configurations. Indeed, most available designs target single or adjacent brain regions, and, if multiple sites are targeted, specially constructed head-caps are used. Here, we present a novel design style, for both microdrives and head-caps, which takes advantage of three-dimensional printing technology. This design facilitates targeting of multiple brain regions in various configurations. Moreover, the parts are easily fabricated in large quantities, with only minor hand-tooling and finishing required.
Keywords: microdrive, method, extracellular recording, tetrode, silicon probe
while there is a long-standing appreciation that neuronal interactions between brain regions underlie behavior (Sechenov 1965), investigating those interactions still constitutes a significant challenge. In the last 2 decades, some of the main technological hurdles have been surmounted, as evidenced by the proliferation of amplifiers and digitizers that allow simultaneous recording from hundreds of sites and at frequencies high enough to readily detect and sort spikes from multiple single units. Moreover, parallel advances in computing power and storage capacity have enabled the analysis of such data.
However, lagging behind these advances in recording and computing technologies are the methods used to target and drive electrodes to multiple brain regions. Many investigators aiming to record from numerous areas implant several individual drives (e.g., Igarashi et al. 2014), which extends surgery time, impairs animal recovery, and complicates experiments. An alternative strategy relies on custom-machined drive assemblies that are specifically built for a particular set of regions (Paz et al. 2007), limiting their general use. Other recently introduced designs allow targeting of multiple regions, but recording sites must be within a few millimeters (generally ≤5 mm) of each other (Lansink et al. 2007; Voigts et al. 2013). While these designs are suitable for mice, they do not allow access to the majority of the rat brain, which spans ∼15 × 20 mm. A further complication with most designs is the extensive time investment and skill involved in their fabrication.
Fortunately, the above issues can be resolved easily, by taking advantage of the recent availability of high-performance consumer-grade three-dimensional (3D) printing technology. Indeed, 3D printers enable the rapid fabrication of complex (difficult to machine) designs created with standard 3D modeling software. These designs can then be shared through online exchanges, some of which also provide fabrication services. Conveniently, 3D printers can produce numerous objects simultaneously, in a semiautomated manner. Here, we present novel head-cap and microdrive designs that can be fabricated at little cost using consumer-grade 3D printers. Importantly, our design allows targeting of multiple combinations of distant recording sites distributed throughout the entire rostrocaudal extent of the rat brain.
MATERIALS AND METHODS
The materials and methods section is organized as follows. We first explain the operation of 3D printers and outline the design process for 3D printable parts. Next, we describe how to build different types of microdrives and how to prepare a head-cap that can accommodate multiple microdrives in various spatial configurations. Last, we summarize our surgical and recording procedures. Detailed step-by-step descriptions of the printing, assembly, and implantation methods are provided in appendices a–f.
Basic principles of 3D printer operation.
There are numerous types of 3D printers available on the market, but two technologies in particular are widely available: stereolithography (SL) and fused deposition modeling (FDM). Both techniques build objects in layers from files generated by the designer. Both types of printers are used in our laboratory (SL, Asiga Freeform “Plus 33” Pico 3D, Anaheim Hills, CA; FDM, MakerBot Replicator 2X, Brooklyn, NY).
For SL printers, a liquid resin is cured in layers onto a metal plate using ultraviolet light. In the FDM process, a filament of Acrylonitrile butadiene styrene (ABS) or polylactic acid (PLA) plastic is fed into a heated nozzle that extrudes the molten plastic onto a stage. Each layer is extruded one at a time, with the nozzle guided so as to outline the edges of the object's surface. Due to the physical properties of the extruded plastic (viscosity, shrinkage upon cooling), the accuracy of the FDM device is inferior to that of our SL printer. Thus we use it only to print coarse components, such as the head-cap body and electrode interface board (EIB) holder. To our knowledge, PLA and ABS are not biocompatible. However, this problem is mitigated because dental acrylic is placed between the 3D printed components and the skull. Of note, ASIGA recently introduced a resin for 3D-printing parts that will have long-term contact with a patient's skin (“BioMOLD”). There are also specialized biocompatible forms of ABS (e.g., ABS-M30i), but they are not widely available outside of industrial settings.
To facilitate the adoption of our design by other laboratories, we have constrained all fabrication to a single model of 3D printer, the Asiga printer, which uses the SL method. Of note, the ultraviolet curable resin used by the Asiga printer adheres well to dental acrylic and is strong enough to resist the wear and tear incurred during chronic recordings lasting months. In the experiments described below, we did not observe a single case of 3D-printed component detaching from the dental acrylic, even though all rats were recorded for ≥3 wk and, in one case, for 2 mo. However, other printers or equipment may be used to fabricate our head-caps, and this will be indicated where appropriate (see appendix a for details).
Designing 3D printable parts.
Design and production of a 3D printed part typically involves four stages. First, the designers have an idea for the part they want to construct. This is usually sketched out on paper, with detailed dimensions noted so that any conflicting size issues become apparent.
For the second stage, the design is instantiated in 3D modeling software. Numerous software packages are available (e.g., AutoCAD, SolidWorks, Blender), and it is up to the designers to settle on one that best fits their needs in terms of geometric transformations and tools supported for easily translating the paper designs onto the computer. At the very least, the program should be able to export a Standard Tessellation Language file, which is accepted by many of the software controllers for 3D printers, including ours. For this paper, we used Blender, a free and open source (GNU General Public License) 3D modeling software package.
At the third stage, the digital version of the design has reached sufficient completion that it can be printed with most, if not all, features present. This step usually determines whether the design is feasible on the 3D printer, as it will expose the limitations of the material, along with the printer's resolution, printable area, and time to completion. Indeed, designs that theoretically conform to the printer's reported specifications may be impossible to print because the 3D geometry of the object interferes with normal printing operation. See appendix b for a list of problems often encountered during this design phase. Confronting these problems may require substantial revisions of the design, or its abandonment.
After a design is printed that is close enough to the desired tolerances for assembly, a fourth stage of iterative minor changes occurs. At this point, the various parts of the design are brought together and constructed into the finished product. Often hand-tooling, such as filing edges, enlarging holes, adding thin strips of material, will be necessary for construction of the part. These modifications, usually subtle, should be noted and eliminated by changing the original 3D model. Another aspect of the refinement stage is incorporating feedback from problems that occur after implantation, such as the sensitivity of parts to any cage debris or the forces exerted by the animal. However, once the design has been modified to accommodate these refinements, hand-tooling is nearly eliminated.
Overview of head-cap assembly and microdrives.
Now that the reader is familiar with basic principles of 3D design and printing, we introduce our approach to multisite recording and then explain how to construct the required components. The Standard Tessellation Language files for these various components can be downloaded from the National Institutes of Health (NIH) 3D Print Exchange (http://3dprint.nih.gov/discover; type “Microdrive” in the search box on upper left). Note that some online exchanges provide printing services (http://www.shapeways.com/). Computer-generated animations showing the construction process are available on our laboratory's web site (http://parelab.rutgers.edu).
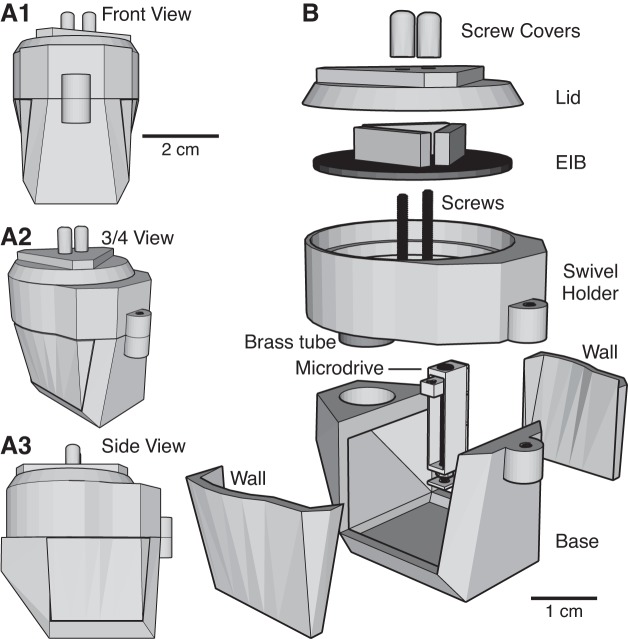
The two components are the head-cap (Fig. 1) and the microdrives, of which we will describe three types (Fig. 2). The head-cap is composed of five main components: the base, the two walls, the EIB holder, and the lid (Fig. 1B). The base of the cap is where the microdrives will be mounted. A bregma hole is made in the base to ensure stereotaxic accuracy (see Surgical procedures). For additional control over placement, a hole at lambda can be added as well. The left and right walls act as protective barriers after implantation, but they can be removed during construction to facilitate installation of the microdrives and electrodes onto the base. Resting atop the base is the EIB holder. This part can rotate in and out of position, exposing the microdrives below, and allowing adjustment of electrode positions during and after implantation. The final part is a lid that protects the EIB. An assembled head-cap weighs 16.9 g. For a breakdown of weights, see Table 1.
Fig. 1.
Head-cap design. A: head-cap viewed from various angles (A1: front view; A2: ¾ view; A3: side view). B: exploded view of the head-cap, allowing inspection of its various components. EIB, electrode interface board.
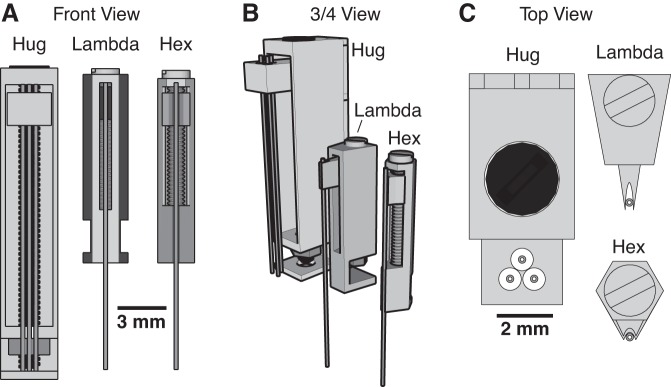
Fig. 2.
Three types of microdrives. The three types of microdrives (Hug, Lambda, Hex) are shown from various angles (A: front view; B: ¾ view; C: top view). Within each panel, the drives are represented with the same scale so their relative sizes can be appreciated.
Table 1.
Weight of 3D-printed and commercially available components
| Components | Weight, g | |
|---|---|---|
| 3D-printed | NIH Exchange Web Address | |
| Headstage base | 5.40 | http://3dprint.nih.gov/discover/3dpx-000883 |
| EIB holder (with brass tube) | 7.08 | http://3dprint.nih.gov/discover/3dpx-000883 |
| EIB lid | 2.59 | http://3dprint.nih.gov/discover/3dpx-000883 |
| Screw covers (pair) | 0.15 | http://3dprint.nih.gov/discover/3dpx-000883 |
| Walls (pair) | 1.71 | http://3dprint.nih.gov/discover/3dpx-000883 |
| “Hug” microdrive | 0.54 | http://3dprint.nih.gov/discover/3dpx-000880 |
| “Lambda” microdrive | 0.10 | http://3dprint.nih.gov/discover/3dpx-000881 |
| “Lambda” microdrive cluster | 0.34 | http://3dprint.nih.gov/discover/3dpx-000881 |
| “Hex” microdrive | 0.09 | http://3dprint.nih.gov/discover/3dpx-000897 |
| “Hex” microdrive cluster | 0.26 | http://3dprint.nih.gov/discover/3dpx-000897 |
| Commercially available | Vendor and Catalog No. | |
| EIB | 1.95 | Plexon no. ADP/24TET-32V |
| 0-80 screw (∼18 mm) | 0.22 | McMaster-Carr no. 91792A071 |
3D, three-dimensional; NIH, National Institutes of Health; EIB, electrode interface board.
While the basic structure of the head-cap is fixed, it accommodates several types of microdrives in various combinations, numbers, and relative positions, depending on the objectives of the study. Thus the base will be printed with holes that match these requirements. appendices c and d provide step-by-step instructions for the printing and assembly of the microdrives and head-cap, respectively. For advice on how to modify our designs, see appendix e.
Microdrive designs.
3D printing allowed us to create different types of microdrives, each tailored to a different task (Fig. 2). The first design is the “Hug” drive (0.5 g), so-called because its walls hug the electrode carrier. Given its relatively large size, it is the easiest to assemble of all the designs. Its size also makes it ideal for advancing arrays of electrodes that cover a large area (∼2 × 4 mm), and which must travel long distances in the dorsoventral plane to reach their targets (∼12 mm). This extended driving depth also makes it ideal for approaching structures at an angle, such as those near the midline. However, it performs poorly when targeting structures with thin layers, such as the hippocampus, where it is unlikely the entire array of electrodes will simultaneously be in the same layer.
To overcome this limitation, we designed another drive that is smaller. Called the “Lambda” drive (0.1 g; Fig. 2), because of its outline when viewed from above, it features a wedge-shaped electrode carrier nestled in a triangular groove, with the electrode bearing tip protruding. Due to its thinner walls, which allows multiple drives to be tightly packed, it must be shorter than the Hug drive to prevent flexing during advancing. This means it can only target superficial structures (less than ∼5 mm from skull surface). However, deeper structures can be reached if the electrodes already protrude from the head-cap upon implantation. To facilitate tight packing of multiple drives, we also created a version with three independent drives that can be printed as a cluster (Fig. 3A).
Fig. 3.
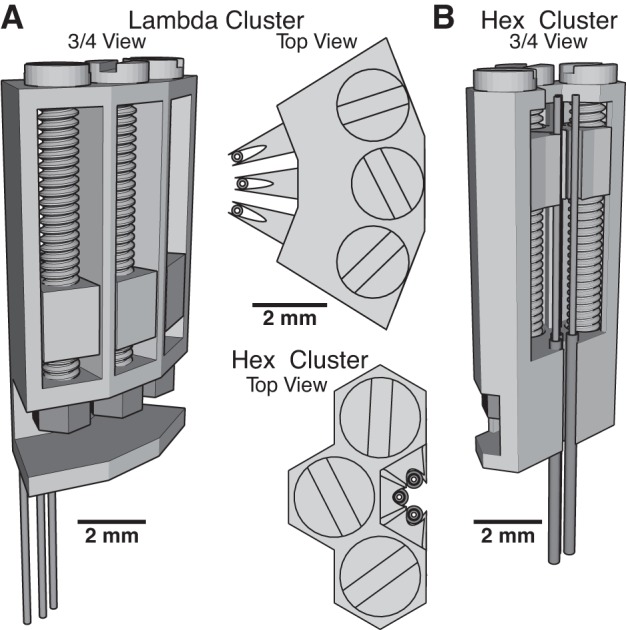
Microdrive clusters. For situations where tight packing of electrodes is required, we have designed microdrive clusters of the Lambda (A) and Hex (B) types. The sharing of inner walls between microdrives allows for closer spacing of tetrodes. Top views of the two cluster types are shown in between A and B.
If many drives need to be placed in a small area, then our third microdrive design would be ideal. It uses the same screw and nut as the Lambda drive, but the drive body barely extends outside of the borders of the screw head. Referred to as the “Hex” drive (Fig. 2), it has the finest tolerances of the three during fabrication and may require adjustments to the printer settings. While it has comparable travel to the Lambda drive, more of them can be placed closer together, allowing for multiple independent control of up to six electrodes within a circular region less than 1 mm in diameter. We also provide a version with three Hex drives attached together, as a cluster (Fig. 3B). Additionally, its low weight (0.09 g) makes it the best drive of the three for use in mice, whose small body size precludes head-caps greater than ∼2.5 g in weight. As illustrated in Fig. 4, and detailed in appendix c, the assembly of the three types of microdrives is simple and rapid.
Fig. 4.
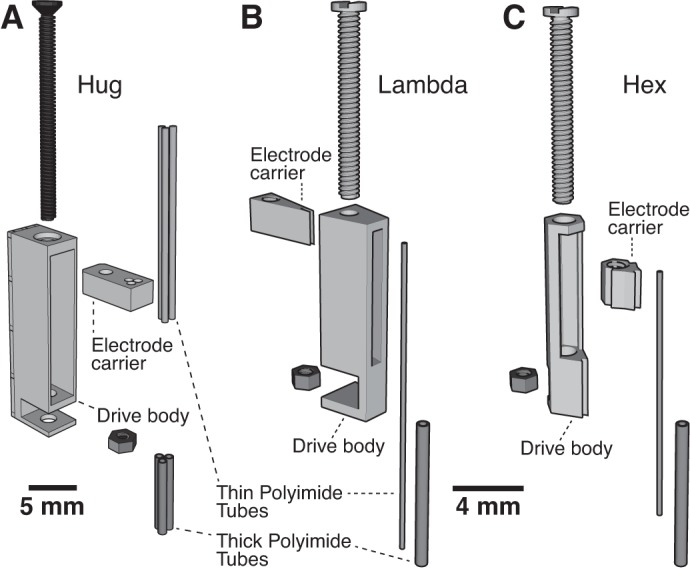
Microdrive components. Exploded ¾ view of the three types of microdrives is shown: Hug (A), Lambda (B), Hex (C).
Surgical procedures.
All surgical and recording procedures were approved by the Institutional Animal Care and Use Committee at Rutgers University. Male Long Evans rats (300–600 g; n = 6), anesthetized with isoflurane, were mounted in a stereotaxic apparatus and administered atropine sulfate (0.05 mg/kg im) to aid breathing. Once shaven, the scalp was washed with betadine and isopropyl alcohol, and a local anesthetic (bupivacaine, sc) was injected in the scalp. Fifteen minutes later, the scalp was incised, the skull exposed, and the periosteum removed. Segments of muscle overlying the parietal bones were resected bilaterally, and cranial muscles were separated from the posterior sutures so that skull screws could be placed outside the footprint of the head-cap. A ground screw was placed over the cerebellum. Craniotomies were then performed above the regions of interest, the dura mater opened, and silicone gel applied over the exposed brain surfaces. Next, a thin (<0.5 mm) layer of dental acrylic was applied across intact bone surfaces and around the skull screws.
After the microdrives were secured to the head-cap, which is usually done a day or more prior to the surgery, the head-cap was mounted onto a stereotaxic arm using a 3D printed holder, and its antero-posterior axis was aligned with that of the skull. To guide the placement of the head-cap, a polyimide tube (length ∼1 cm) was cemented vertically at bregma. A hole in the head-cap's base at bregma was lined up with this polyimide tube, and the head-cap was lowered until flush with the skull, ensuring its stereotaxic placement. For additional accuracy in placement, an additional guide can be placed at lamda, ensuring that the cap is aligned with the antero-posterior axis. The head-cap was then cemented to the skull. appendix f provides a step-by-step description of the implantation procedure.
Recording and data processing.
Field potentials and unit activity were recorded with either a Plexon (Dallas, TX) or Intan system (Los Angeles, CA). The data were sampled at 30 kHz/channel and saved as binary files that were subsequently preprocessed using the NDManager suite of applications (Hazan et al. 2006). Filtering of unit activity, sorting of spike waveforms (KlustaKwik), and manual clustering were carried out using this software package. For clustering we used the first four principal components from each electrode (4 per tetrode, 16 principal components total). Well-isolated single units exhibited a refractory period lasting at least several milliseconds, and their distribution of waveforms had less than 5% overlap with any other unit recorded at that site. Common noise in the unit traces (e.g., EMG) was removed by subtracting from each channel the median signal recorded from all others. Local field potentials (LFPs) were extracted from the wideband signals using a second-order Butterworth low-pass filter with a cutoff of 300 Hz, and filtered both forwards and backwards to remove phase distortion (MATLAB “filtfilt” command).
To measure the quality of single-unit isolation across a training session, we measured the isolation distance for each single unit from all other single units recorded at the same site (Harris et al. 2001; Schmitzer-Torbert et al. 2005). To calculate the isolation distance, all single-unit waveforms are projected into principal component space. The Mahalanobis distance was measured for each detected spike with respect to the single unit of interest. Mahalanobis distance measures the distance between a point and a reference distribution's mean center, with each dimension rescaled by the reference's covariance matrix. For our spike data, the reference distribution was the selected single unit, and the Mahalanobis distance of all other units from the selected single unit was measured. These distances were sorted in ascending order, and the distance of the point at the location equal to the number of spikes from the selected single unit is defined as the isolation distance. Within this distance half the spikes are not from the selected single unit. A larger isolation distance corresponds to better single-unit isolation. Isolation distance was calculated for each 30-min period of a recording session.
RESULTS
To demonstrate the utility of our approach to the design of head-caps and microdrives, we will show examples of unit and LFP recordings obtained throughout the cortico-hippocampal loop using 3D printed components. Although the examples illustrated below are restricted to cortical recordings, the same components can be used to monitor neuronal activity in subcortical regions or in a mixture of cortical and subcortical targets.
Examples of recording configurations.
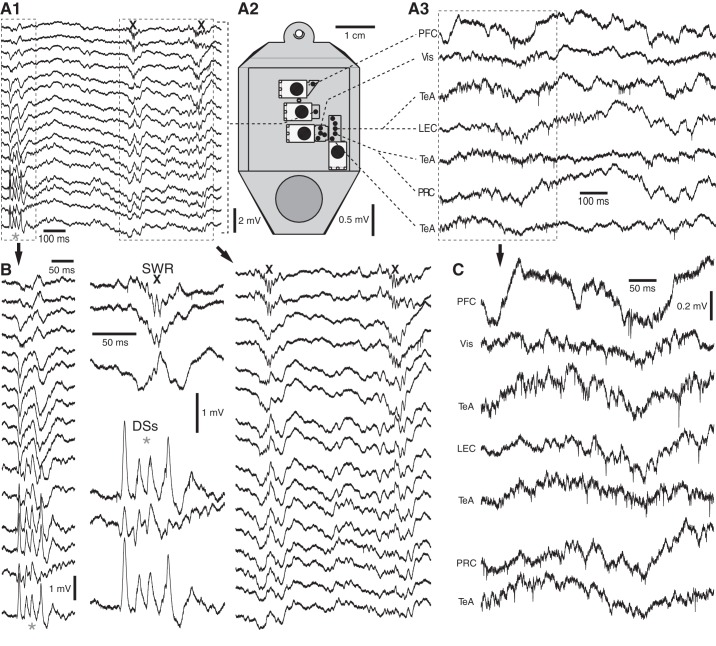
Figure 5 shows a case where we combined hippocampal recordings using a linear 16-channel silicon probe (Fig. 5, A1 and B) with neocortical and rhinal recordings using tetrodes (Fig. 5, A3 and C), of which we only show a subset. The spatial arrangement of the microdrives in the head-cap is illustrated in Fig. 5A2. Together, the various recording sites ranged from the rostral (prefrontal cortex) to the caudal poles of the brain (visual and perirhinal cortices). The position of the silicon probe in the dorsal hippocampus was adjusted so that the recording leads spanned from stratum oriens in CA1 to the hilus of the dentate gyrus (top and bottom traces in Fig. 5, A1 and B, respectively). Note that, by adjusting the relative lengths of the tetrodes controlled by a single microdrive, simultaneous recordings were obtained from several regions. For instance, the most caudal microdrive in Fig. 5A2 controlled tetrodes that reached the perirhinal and lateral entorhinal cortices, as well as the temporal association area. During the epoch depicted in Fig. 5, the rat was in slow-wave sleep. This is evidenced by the presence of dentate spikes and sharp wave ripples (asterisks and Xs, respectively), as well as the prevalence of large-amplitude slow waves in the LFPs recorded at neocortical and rhinal sites (Fig. 5, A3 and C).
Fig. 5.
Multisite extracellular recordings in the cortico-hippocampal loop. A: examples of signals and relative position of microdrives. A1: recordings obtained with a linear 16-channel silicon probe in the dorsal hippocampus. The linear array spanned multiple layers of the dorsal hippocampus, from stratum oriens (top trace) to the hilus of the dentate gyrus (DG; bottom trace). In this and following panels, asterisks and Xs mark dentate spikes (DSs) and sharp wave ripples (SWRs), respectively. A2: top view of head-cap with the various microdrives. A3: neocortical and rhinal recording sites. The signals of A1 and A3 are expanded in B and C, respectively. The middle part of B shows select hippocampal signals during SWRs (top) and DSs (bottom), respectively. LEC, lateral entorhinal cortex; PFC, prefrontal cortex; PRC, perirhinal cortex; TeA, temporal association cortex; Vis, visual cortex.
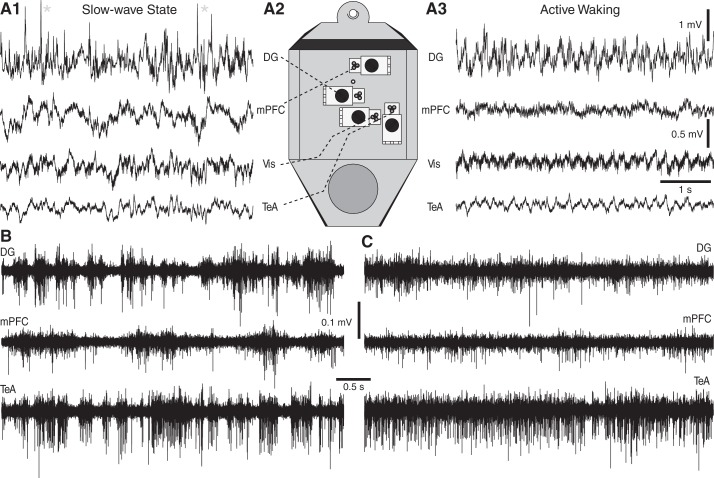
Figure 6 shows the results obtained with another recording configuration (Fig. 6A2) where, in contrast with the above, tetrodes were used to monitor neuronal activity at all recording sites, including the hippocampus. In this case, the animal transitioned between slow-wave sleep (Fig. 6A1) and active waking with prominent theta activity (Fig. 6A3). High-pass filtered versions of the signals depicted in Fig. 6, A1 and A3, are shown in Fig. 6, B and C, respectively. Note synchronized burst-pause firing of many units in slow-wave sleep (Fig. 6B) and their tonic firing pattern during wakefulness (Fig. 6C).
Fig. 6.
Multisite recordings of state-related shifts in hippocampal and neocortical activity. A: examples of signals and relative position of microdrives. Recordings obtained with tetrodes in the DG, medial PFC (mPFC), Vis, and the TeA during slow-wave sleep (A1) and active waking (A3) are shown. Asterisks mark DSs. A2: top view of head-cap with the various microdrives. B and C: high-pass filtered (0.3–3 kHz) versions of the signals depicted in A1 and A3, respectively.
Incidence and quality of unit recordings.
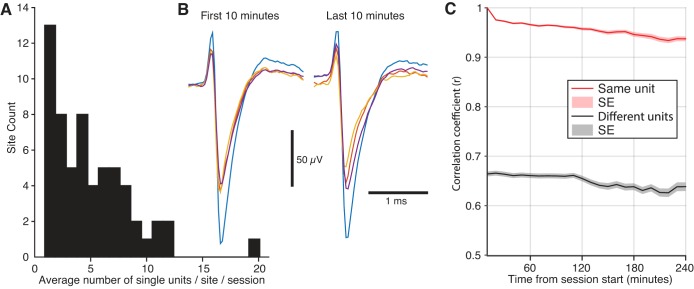
To assess the incidence and stability of unit recordings with our head-cap and microdrive designs, six rats were implanted with multiple tetrodes in the configurations shown in Figs. 5 and 6. After recovery, rats were recorded for 1–5 h (187 ± 62 min; 102.8 total hours recorded) daily for a combined total of 33 recording sessions. At the end of each session, tetrodes were moved ventrally <0.1 mm, and the animals returned to their home-cage overnight. This approach ensured signal stability the next day. Of 62 recording sites, two sites yielded only multiunit activity. At all other recording sites, single units could be detected regularly, for a combined total of 1,161 single units. The number of single units observed daily per site ranged between 1 and 20 (average 4.9 ± 3.6 single units·site−1·session−1; Fig. 7A). With respect to recording stability, the vast majority (>95%) of single units were held throughout the recording session (>96% of session duration). Further underscoring the stability at the recording site, for those units that were held, the spike shapes remained relatively unchanged across the session (Fig. 7B).
Fig. 7.
Stable single-unit activity was readily obtained with our recording electrodes. A: we calculated a histogram of the average number of single units at each recording site across all sessions. Most sites had less than 5 single units on average per session, but some had over 10. Site count (y-axis) refers to the number of recording sites that had a particular average yield of single units across sessions. B: example waveform of a single unit recorded at the beginning (left) and end (right) of a 4-h recording session. Each trace illustrates the same spike, but recorded from the different leads of the same tetrode. C: correlation between spike waveforms averaged every 10 min with those occurring in the first 10 min of the session. On average, when a unit that was held for the entire session was compared with itself (red line, n = 1038), the correlation remained above 0.9 throughout a typical session duration of 4 h. This average never dipped below the mean correlation between two different units recorded at the same site (black line, n = 3,344), which remained below 0.7. Lines and shaded regions are means ± SE.
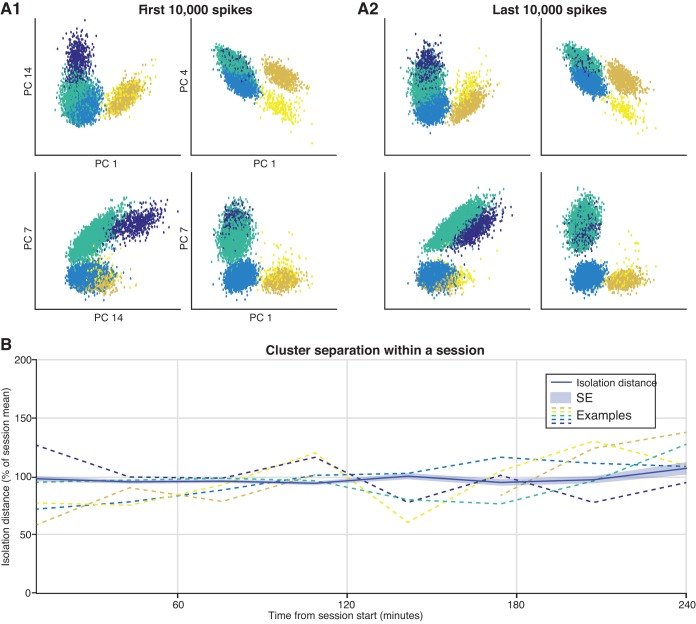
To further characterize waveform stability across a recording session, two approaches were used. In the first (Fig. 7C), we calculated the Pearson correlation coefficient (r) between the mean spike waveform (77 samples, 2.6 ms) during the first 10 min of the session with that for each additional 10-min period. On average, when a unit was compared with itself, the correlation coefficient remained above 0.9 throughout a typical session of 4 h. On the other hand, the correlation between two different units recorded at the same site remained below 0.7 on average. In the second approach (Fig. 8), we calculated the isolation distance, a measure of cluster quality, for each single unit at 0.5-h intervals (see materials and methods). Then, to facilitate comparisons across single units, we divided these values by their session mean. There was no apparent change in single-unit isolation across a recording session.
Fig. 8.
Single-unit isolation quality remained unchanged across a recording session. A: example of single-unit clusters in principal component (PC) space. Five distinct single units were recorded from this tetrode, with each color corresponding to a different user-defined cluster. The clusters are plotted for a subset of PC dimensions. There is little change in cluster separation between the first (A1) and last (A2) 10,000 spikes recorded during this session. B: to determine if cluster isolation changed across the recording session, we calculated the isolation distance for each single unit at 0.5-h intervals and then divided these values by their session mean. There was no apparent change in single-unit isolation across a recording session. The dark blue line is the mean across all units, whereas the shaded region corresponds to the SE (n = 1,161). The colored dashed lines correspond to the clusters shown above.
Impact of swiveling EIB holder on tetrode connections to EIB.
One area of concern with our head-cap design is the possibility that tetrode wires are damaged over time when the EIB holder is repeatedly swiveled between the open and shut positions to access the microdrive screws. One might worry that this repeated movement could kink some wires, damage their insulation as they rub against each other or head-cap walls, or even cause enough tension to break them. However, we found that, provided the wires are long enough to easily accommodate half a rotation of the board holder, and by making sure it is not rotated more than 180°, such problems occur rarely, if at all. In six rats, it did not occur once.
Short circuits caused by access of cerebrospinal fluid into the head-cap.
During chronic extracellular recordings in behaving animals, electrical short circuits between electrodes are a common occurrence. This problem typically results from seepage of cerebrospinal fluid or blood to the EIB. We found that the containment of the EIB in the EIB holder actually protects the EIB from such leakage. In six rats, this problem did not occur.
In addition, seepage of cerebrospinal fluid can interfere with movement of electrodes. With tetrodes, we found that this problem could be eliminated with a simple precaution: by printing holes in the head-cap base that are just wide enough for the thick polyimide tubes. However, with silicon probes, this problem sometimes arises.
DISCUSSION
This paper presents a novel approach to the construction of head-caps and microdrives for chronic multisite recordings in rats. The novelty of our approach resides in the way the parts are produced, the flexible recording configurations it allows, the drastically reduced dependence on hand-tooling and skill, the consequent reduction in construction time, and shortened surgical duration. Below, we compare our method to current practices and consider the likely impacts of 3D printer technology on neurophysiology.
Evolution of multisite implants.
The importance of recording many sites to understand brain function has been appreciated for a long time (e.g., Gerstein and Clark 1964). Shortly after World War II, investigators routinely performed multisite recordings, in some cases monitoring as many as 610 cortical and subcortical sites simultaneously (Lilly 1958). However, these experiments typically relied on fixed electrodes and were performed in cats and monkeys, whose larger skulls can accommodate more electrodes (e.g., Yoshii et al. 1957).
In rats, multisite recordings typically targeted single brain regions (e.g., McGinty and Harper 1976; O'Keefe and Dostrovsky 1971) and relied on microwires mounted onto microdrives. Most microdrive designs can be traced back to the Harper-McGinty style drive, which requires skilled labor for its construction and modification of off-the-shelf parts (Harper and McGinty 1973; Keating and Gerstein 2002). The same basic principles pioneered then are still in use today. However, they have been adapted to accommodate other electrode types, such as silicon probes (Bragin et al. 2000). As to head-caps, for much of the history of chronic recordings, they were custom designed to the particular task. This still happens, for instance with the cap being constructed at the time of surgery out of conductive mesh and dental acrylic (Vandecasteele et al. 2012).
Incorporating 3D printing technology in the design of multisite implants.
Since the species of choice for chronic recordings is currently the rat (with the mouse becoming a close second), it is necessary to construct multisite implants that are light-weight, compact, multifunctional, and relatively easy to assemble. All of these requirements can be met by incorporating 3D printing technology in the preparation of implants, as we did here.
An important feature of our head-cap design is that it can be reconfigured to record from practically any combination of brain areas. Thus, to put our design in perspective, we will focus on prefabricated (notably, often with 3D printers) head-caps that were designed for general use. In this realm, several differences immediately stand out. In the past, most head-caps had preconstructed areas for placing microdrives, something our design has moved away from. Instead, we have a stage for inserting guide tubes and a space large enough to incorporate several microdrives, in various configurations.
Our head-cap also uses the EIB holder to provide protection for the delicate microdrives, something which was typically done by a separate shield or cone (Kloosterman et al. 2009). Perhaps the most significant departure is the use of a rotational linkage between the EIB holder and base of the head-cap containing the microdrives. Such a design eliminates the need for a cone and allows easy access to the entire area of the microdrive stage. Since most previous designs assumed a particular placement of the microdrives on the head-cap, the EIB was attached such that those drives could be readily accessed. However, this approach limits the orientation and positioning of microdrives, precluding access to certain combinations of brain regions. An alternative approach would be to have the EIB holder completely offset from the microdrive area, but that would introduce extra torque on the cap when the animal has a preamplifier and cable attached. By using a swivel joint between the EIB and base of the head-cap, our design allows easy access to the microdrive area and protection of the microdrives when they are not being advanced.
Importantly, our microdrive designs can be constructed in just a few minutes. In the past, when we used a milling machine, it took approximately 8 h to build the components required for four microdrives. In contrast, with the 3D printer, the components of 8 to 15 drives can be produced in 1 h (depending on the drive type). Moreover, even when performed by a novice, drive assembly requires less than 5 min, because there are only two construction steps, both of which are easy to perform. However, one disadvantage of our design over others (see Yamamoto and Wilson 2008) is a slight amount of lag and slip when advancing the drive, due to subtle variations in the printing of the threads on the carrier. This problem is mitigated, however, by the surfeit of drives that can be quickly made, allowing the experimenter to discard drives that are substandard.
For our demonstration, we chose to monitor multiple brain regions accessed with several independent microdrives. Typically, implanting so many microdrives individually requires extended surgical times. In contrast, by positioning the microdrives in the head-cap relative to bregma prior to the surgery, its duration can be reduced by 50–75%, increasing postsurgical survival rates and accelerating recovery.
Potential impacts of recent advances in 3D printing.
The rapid rise of affordable desktop 3D printers enables any laboratory to begin fabricating customized chronic implants. But beyond increased access to in-house fabrication of specialized parts, what else does 3D printing offer? Several developments may fundamentally change how chronic implants will be created and used.
The first is the inclusion of 3D scanning into the design of printed parts. This is best illustrated in dentistry, where custom-fitted prosthetic teeth can be created for patients while they wait in the office (Bidra et al. 2013). The dentist uses a 3D hand scanner to image the area where a tooth replacement will be placed. Then, a computer controlled milling machine produces the false tooth, incorporating the contours of the surfaces that were just scanned. One can envision such a technique applied to the implantation of chronic head-caps, where the skull of the animal is scanned after craniotomies are drilled, and a base for the head-cap is created that exactly fits all the grooves and ridges of the skull.
Another trend is the advance of 3D printing for metal components. Several techniques are available (Cheah et al. 2005; Stübinger et al. 2013), but the most common is laser sintering of fine metal powder. As the resolution and finish quality of this technique improves, it may become possible to print custom microdrive screws. Such screws are already common for numerous microdrive designs (Brunetti et al. 2014), but must be ordered specially. Besides this, screws could be designed that minimize slippage during advancing, or that have the desired thread density to make estimation of the distance traveled simplified (e.g., 100 μm per full rotation). Metal printing may also allow for miniature metal drives, since even conventional machinable plastics are lacking in rigidity. Finally, further advances in printable materials may result in a drastic divergence from traditional principles of head-cap design. For instance, it may become possible to integrate biocompatible scaffolds into the base of the chronic implant, allowing it to fuse with the skull (Hutmacher et al. 2004; Vail et al. 1999).
Conclusions.
We presented head-cap and microdrive designs that can be created with consumer grade 3D printing technology. Besides the novel aspects of their design, the ability to create them with 3D printers lowers the time and effort required for their construction. Parts can be created in bulk runs and stored for later use. The designs can be adapted for a wide variety of experimental preparations. Hopefully, with the increased adoption of desktop 3D printing, our design will become one of many that are freely available, lowering the barrier to entry for laboratories interested in pursuing chronic recording.
GRANTS
This work was supported by National Institute of Mental Health R01 Grants MH-098738, MH-083710, and MH-073610 to D. Paré.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.B.H., D.H., and D.P. conception and design of research; D.B.H., M.V.D., and D.H. performed experiments; D.B.H. analyzed data; D.B.H., D.H., and D.P. interpreted results of experiments; D.B.H., M.V.D., and D.P. prepared figures; D.B.H. and D.P. drafted manuscript; D.B.H., M.V.D., D.H., and D.P. edited and revised manuscript; D.B.H., M.V.D., D.H., and D.P. approved final version of manuscript.
Appendix A: OPTIMIZING PRINTING
Printing Speed vs. Resolution
Printing can be accelerated by increasing the thickness of each printed layer. However, there is a trade-off between speed and resolution, as the thickness of the printed layer sets the maximal resolution of the printed part. If a printed part lacks fine features, then printing in thick layers speeds up the process with little or no negative impact. By contrast, when printing screw holes with threading, the minimum layer thickness available should be used (∼0.025 mm for our device) to achieve maximal spatial resolution.
Weight of the Printed Components
The density of the materials used by SL printers is higher than that of FDM printers. However, FDM printers have a lower resolution. Thus optimal results can be obtained by printing larger and coarser components (e.g., head-cap walls) with FDM printers and using SL printers for small components with details requiring high-resolution printing (e.g., drive components). However, designs that have been calibrated for printing by SL printers may need to be slightly resized to get the same dimensions on FDM printers. Moreover, because FDM printers cannot produce the precise holes needed for the guide tubes in the base of the head-cap, those will need to be added separately with a drill press, milling machine, or stereotaxically mounted microdrill.
Choice of Printer
Some designs will not be printable due to limitations of the printer's resolution. In our laboratory, the FDM type printer, the MakerBot Replicator 2X, has a layer thickness of 100 μm and extrudes a filament with at least a diameter of 400 μm. While this precludes creating walls and features smaller than that resolution, the shape of objects can be extremely precise since the reported accuracy of the nozzle positioner is 11 μm. In contrast, our SL printer is capable of printing objects with much finer features. The Asiga Freeform “Plus 33” Pico 3D has a minimum layer thickness of 10 μm and a minimum effective feature size of ∼100 μm. In general, it is recommended that large objects lacking fine features should be printed with an FDM printer, while those with requiring precise interlocking of small parts (e.g., microdrives) should be made with an SL printer.
Appendix B: COMMONLY ENCOUNTERED PROBLEMS WHEN PRINTING A DESIGN
Designs that theoretically conform to the printer's reported specifications may be impossible to print because the geometry of the object interferes with normal printing operation. For example, in liquid resin SL printers, walls can block the efflux of uncured material from crevices in the printed part. Repeated exposure of this excess resin to the curing light can cause partial curing, leading to bulky edges that obstruct the insertion of other parts that require fine tolerances for proper mating. Sometimes this problem can be solved by changing the orientation of the object being printed, facilitating drainage of uncured material. But this solution will not solve a related problem: when printing small holes in a block of material, uncured resin may not drain from the hole between printing steps and will inadvertently be cured in place. Another common problem is that a wall on a part is too thin to maintain the desired rigidity. Additionally, there are variations between print runs, typically when a new batch of printing material is used. Whenever possible, designs should be robust to slight changes in the type of material used. Doing so may take several iterations of refinement.
Appendix C: PRINTING AND ASSEMBLING THE MICRODRIVES
We now describe the construction of three types of microdrives. While they differ in their dimensions, the way they are assembled is similar. Thus we will describe construction of the first microdrive type (Hug drive) in detail and only highlight differences in the construction of the other two. See Fig. 4 for the components of the various drives.
Hug Microdrives
Each Hug microdrive is built from two printed components: the drive body and electrode carrier. Additional parts are a 0-80 screw (length 3/4 in., ∼19 mm), and a 0-80 nut (McMaster-Carr, Cleveland, OH). It is important to print the drive body with its long axis oriented parallel to the baseplate, its back facing the baseplate, and its open side facing down into the tray so that excess uncured material drips off the component, instead of being cured inadvertently (see appendix b). Printing the drive horizontally thus ensures that the rear inside face of the drive is smooth, allowing the electrode carrier to move easily. The electrode carrier should be printed with its top face parallel to the baseplate. Every part, including the drive body, should be elevated off the platform by at least 2 mm and supported by an ample number of sprues. Raising the carrier from the baseplate with sprues ensures proper printing of electrode holes.
Once both parts have been printed and cleaned, place the electrode carrier into the base of the drive body. Make sure the screw hole in the carrier lines up with the screw holes in the body. Insert a 0-80 screw into the drive body, down to the electrode carrier. Thread the screw through the electrode carrier until it starts to protrude from the carrier and out the drive body. Insert the 0-80 nut into the slot on the base of the drive body and proceed to thread the screw. It should pass entirely through the nut, but not extend farther than ∼0.5 mm. If it protrudes farther, than the screw was over-tightened, which causes buckling of the drive body walls.
After the screw is fully inserted, check that there is no extra space between the screw head and the top of the drive body, nor should there be space between the bottom of the drive body and the nut. Next, permanently secure the nut to the screw by applying an acid flux for soldering stainless steel where the screw threads through the nut, then solder. To prevent damage to the bottom of the drive body, the soldering iron should only contact the nut for a few seconds. Once this is done, the screw can now be turned to retract the electrode carrier up to the top of the drive body. This movement can be smoothed by adding petroleum jelly to the screw. Be sure not to add so much that it gets on the part of the carrier where the electrodes are to be affixed. Petroleum jelly should also surround the nut at the base, so that any dental acrylic or epoxy used to secure the drives to the head-cap do not occlude its motion.
Once these steps are completed, check that that electrode carrier advances smoothly. Turning the screw, the carrier should freely move between the upper and lower 0.5 mm of the microdrive base. It should extend straight from the microdrive, with less than ∼5° of angling, and this should not change when the direction of motion is reversed. Also, the carrier should not wobble when advanced. When any of these issues arise, the drive should be discarded. If the same problems persist, then the thickness of the drive body or carrier may need to be adjusted.
Lambda and Hex Microdrives
The Lambda and Hex drives are assembled in much the same way as the Hug drive, except for the use of smaller screws (M1, McMaster-Carr) and nuts (1.0 mm brass hex nut, Scale Hardware, Ft. Lauderdale, FL). For Lambda and Hex drives, once the electrode carrier is inserted into the drive body and the screw is threaded into the carrier, and, prior to fastening the nut, check that there is little resistance when turning the screw to raise and lower the electrode carrier over its extent of travel. High rotational torque can cause angling of the electrode carrier, which interferes with the independent travel of closely spaced microdrives (for example, in the Hex and Lambda clusters). Once the motion of either drive has been verified, and the nut secured with solder, the last step is to attach the thin polyimide guide tube to the electrode carrier with cyanoacrylate.
Appendix D: PRINTING AND ASSEMBLING THE HEAD-CAP
To assemble the head-cap, the microdrives must be installed in the base, the EIB holder attached to the head-cap base, and the electrodes threaded into the microdrive guide tubes, making sure the wires are long enough to reach the EIB and accommodate swiveling of the EIB holder. Also, the base should have been printed with holes in the desired recording configuration. To align the Hug drive with these guide holes, thick polyimide tubes (356 μm outer diameter, 198 μm inner diameter, 3 cm long, Polymicro Technologies, Phoenix, AZ) are inserted through the holes in the electrode carriers and into the guide holes in the base of the head-cap. The microdrive is then pressed against the base of the cap and the process repeated for each drive. Potential misalignment of the drives is easily detected by examination of the configuration from above. These can be corrected by gently nudging the drives.
To fix the Hug drives to the floor, dental acrylic is applied around the sides and back of the drives, but not over the electrode guide holes. Once the dental acrylic has dried, the polyimide tubes are removed, and shorter ones (8 mm) are inserted into the guide holes in the head-cap base. They should extend ∼2 mm from the underside of the base and approach the minimum level of the electrode carrier when it is fully lowered. A drop of epoxy is applied where the guide tubes enter the floor to fix them in place. Care should be taken not to block the tubes.
When securing the Lambda and Hex drives to the base of the head-cap, the thick polyimide tubes should already be fixed to the head-cap base. The thin polyimide tubes attached to the microdrives are then threaded into these guide tubes, and the drive is secured to the based as you would the Hug drive.
Microwire or tetrode electrodes are nested in polyimide tubes to prevent them from buckling upon penetration of the brain. A thin polyimide tube (169.2 μm outer diameter, 102.6 μm inner diameter, 4 cm long, Polymicro Technologies) is run from the electrode carrier into its corresponding guide tube. Leave an extra 1 cm protruding from the top of the electrode carrier. Where the thin polyimide tubes enter the large guide tubes, liberally apply petroleum jelly to facilitate transit of the thin tubes and prevent the back flow of cerebrospinal fluid and blood that can occur when the drives are lowered into the brain. A drop of cyanoacrylate is applied to the top of the electrode carrier to secure the thin polyimide tubes. It should fill any remaining space in the carrier holes not taken up by the polyimide tubes. Once the glue has dried, trim the top of the tubes so they extend <1 mm from the electrode carrier. Then cut the electrode guide tubes to their desired depths upon initial implantation during surgery.
The EIB holder is assembled next using an ∼11-mm segment of brass tubing and two 0-80 screws at least 2 cm long. Each screw is threaded into the holes in the holder's floor, with the head resting in the recessed portion on the underside. The brass tube is inserted into its hole, with its top flush with the holder's floor, and then glued in place with cyanoacrylate followed by epoxy. In addition, a thin layer of epoxy is placed on the bottom interior end of the tube. The exterior of the tube should remain free of adhesive. Once the glue has dried, petroleum jelly is applied around the tube, and it is inserted into the back of the head-cap base.
Prior to insertion of the EIB in its holder, connect all electrodes using EIB pins (Neuralynx). The electrode wires are connected so that they protrude from the bottom of the EIB, the side opposite the connectors. Make sure the EIB holder is swiveled away from the side of the base with the microdrives. Before the EIB is inserted into the holder, each electrode is threaded through the tube and out of the head-cap base (where the wall will eventually be), labeling each wire with its corresponding position on the board as you go. Once all electrodes have been threaded, lower the EIB onto the 0-80 screws protruding from the holder, taking care not to crimp or damage the wires. Nuts are then lowered on top of the EIB to lock it in place in the holder. If not done already, the electrodes are now inserted into the guide tubes, and a drop of cyanoacrylate is applied where they enter the top of the guide, preventing them from inadvertently slipping out. Cut the tips of the electrodes fresh on the day of the surgery with tungsten carbide scissors (no. 14568-09, Fine Science Tools, Foster City, CA).
The remaining parts of the head-cap, namely the EIB holder lid and the head-cap walls, are attached during surgery. The walls are cemented in place on the head-cap, while the lid is held down with 3D-printed screw covers.
Appendix E: MODIFYING OUR DESIGNS
Investigators should modify our designs to better suit their needs. There are three principal parts that will likely need to be changed. For this purpose, we provide “blank” versions of those parts (see the NIH 3D Print Exchange). The first is the electrode carrier for the Hug drive. While our design has three guide holes, others may wish more or fewer. They may also want to reduce their profile or how far they protrude from the drive body, especially when attempting to pack many drives into a small area.
Investigators may also want to change the base of the head-cap to accommodate guide tubes for their target regions. This process is relatively straightforward: add holes at the appropriate coordinates relative to the bregma hole. If the target is near bregma, then introduce another hole to facilitate stereotaxic placement of the cap. Care should be taken to space guide holes far enough apart so that the microdrives do not block one another. This will not always be possible, especially for close structures, in which case a single drive with appropriately staggered electrodes may be needed.
Finally, the top of the head-cap holding the EIB should be modified to fit the desired board or connectors. In general, make sure the board holder top has a large enough footprint to entirely cover the base of the head-cap, protecting the microdrives and wire contained therein from debris and damage. Holes can be added to the base to allow screws for fixing the EIB in place. The entry point of these holes should be large enough to accommodate the head of the screw, since its protrusion would disturb the microdrives below.
Appendix F: DETAILED DESCRIPTION OF IMPLANTATION PROCEDURES
Exposing and Cleaning the Skull
Perform a single scalpel incision starting at the frontal sinus and ending at the caudal-most edge of the skull, without cutting the neck muscles. This extended incision is necessary so that the entire surface of the skull, including the bones overlying temporal cortex, can be accessed easily. Segments of muscle overlying the parietal bones should also be resected bilaterally so that skull screws can be placed (if temporal or rhinal structures must be recorded from, then more muscle is removed to allow access). Muscles are then separated from the posterior sutures, since these too serve as anchor points for skull screws. If bleeding ensues, either from the muscle or the skull, then manage it until it has stopped. Once the skull is exposed, the periosteum can be removed with a cotton swab and forceps. Once the skull has been thoroughly cleaned and all muscles resected, apply hydrogen peroxide to the skull surface to further degrade any residual connective tissue. After the hydrogen peroxide has been cleared away, allow the skull to dry thoroughly.
Positioning Anchoring Screws
For most surgeries, anchoring screws are placed outside the footprint of the head-cap. This reduces the distance between the base of the cap and the skull, allowing for the maximal travel of the microdrives into the brain. But, if only dorsal structures (e.g., somatosensory cortex, dorsal hippocampus) are to be recorded from, then the screws may be placed underneath the cap. We have used both self-tapping 0-80 stainless steel screws (1/8 in., Plastics One) and 000-120 titanium screws (1/8 in.; Fasteners and Metal Product, Waltham, MA) to anchor the head-cap to the skull. Holes are drilled with an appropriately sized dental bur. One of the screws serves as a ground and is placed over the right cerebellum. The ground screw should be shorter than the others (1/16 in., Plastics One) so that its head is closer to the skull without the threaded portion protruding into the brain.
Performing the Craniotomies
After all the screws have been placed, the craniotomies can now be drilled. Use stereotaxic coordinates to outline the edges of the craniotomies. Because the cap necessitates blind placement of the microdrives over the craniotomies, include an additional 0.5 mm of space between each recording site and the craniotomy edge. Craniotomies are performed by thinning the skull with the same bur used to drill the screw holes. As the thinning progresses, saline should be washed over the drilling site to reduce heating caused by the friction of the bur on the bone. Once the bone has been thinned enough, it can be lifted away from the dural surface without resistance. We have found that this procedure produces less subdural bleeding than just drilling the outline of the craniotomy and lifting the remaining flap of bone. Immediately following the craniotomy, a durotomy is performed. Care is taken to minimize damage to the pial surface, since bleeding in the craniotomy lowers the chance of a successful implant. Upon completion of the durotomy, the entire craniotomy is covered in silicone gel (3-4680, Dow Corning, Midland, MI), protecting it from bone fragments released during any additional drilling. A skilled surgeon can routinely perform multiple craniotomies/durotomies in the same subject with little or no bleeding.
Preparation of Skull Surface and Stereotaxic Positioning of Head-cap
Once all the craniotomies have been completed, the skull is prepared for lowering the head-cap. A thin layer of dental acrylic is applied across all intact bone surfaces and around each of the skull screws (for implants lasting less than 1 mo use Teets Cold Cure; for longer lasting implants use C&B Metabond). More dental acrylic is applied around the anchor screws, which are either too rostral to be under the head-cap, or mounted to the side of the skull. Care is taken to avoid building up the dental acrylic more than 0.5 mm from the dorsal-most skull surface, since it would prevent the head-cap from being flush with the skull. To guide the placement of the head-cap, which encompasses most of the skull surface, a polyimide tube ∼1 cm in length is cemented vertically at bregma. This assumes that no craniotomy has been performed at bregma. If this is not the case, then another location can be used, but the head-cap must be modified to account for this. Prior to lowering the head-cap, the silicone gel overlying the various craniotomies is replaced, since bone fragments tend to cover them by this point. The silicone gel that is put onto the craniotomies should be partially cured so that it is solid enough to be built up ∼5 mm above the surface of the skull, but liquid enough to fill all the cracks in the edges of the craniotomy. The importance of this step will become clear below. The head-cap is mounted onto a stereotaxic arm using a 3D printed holder. Care should be taken to align the antero-posterior axis of the cap with that of the skull. A hole in the base of the cap at bregma serves as a guide for placing the cap. The tube that was vertically affixed to bregma should pass through this hole without any deviation once the cap is aligned with bregma. Lower the cap until it is flush with the skull. Doing so, the guide tubes that protruded from the head-cap will immerse in the silicone gel overlying the craniotomies. If not enough gel has been placed over the craniotomy to allow this, then retract the head-cap and apply more gel.
Dental acrylic (Teets Cold Cure, AM Systems, Sequin, WA), can now be liberally applied to the base of the skull cap. It must be liquid enough to flow underneath the cap and fill the space between the skull and cap. The silicone gel prevents the dental acrylic from entering the craniotomy or flowing up the guide tubes. Dental acrylic is also applied to the front and back walls of the cap, ensuring that it is rigidly attached to the skull. Once the dental acrylic cures, the cap can be removed from the stereotaxic holder. At this point, any wires such as ground or electromyographic electrodes are connected to their termination points in the cap. The walls of the head-cap are then attached using dental acrylic to hold them in place. When doing this, ensure that no dental acrylic leaks onto the microdrives or guide tubes, potentially damaging them.
Postsurgical Procedures, Maintenance, and Handling
Once all the dental acrylic has dried, the animal is removed from the stereotaxic apparatus. To reduce pain and infection during recuperation, topical lidocaine and Neosporin are applied to the wound surrounding the head-cap, and an injection of the anti-inflammatory drug ketoprofen (2 mg/kg sc) is administered daily for at least 3 days postsurgery. For the 3 days following the surgery, the subject is closely monitored for any signs of discomfort. Most often, subjects exhibit no discomfort and resume their normal eating, grooming, and sleeping activities within a day of the surgery.
Start advancing the microdrives the day after the surgery (up until this point, they will be fully retracted). Penetration of the brain is best indicated by the detection of unit activity in the superficial layers of cortex. This serves as a zero point, by which one can judge the dorsal-ventral distance from the target region of interest. For best results, the microdrive should only be advanced at most one or two full rotations per day (317–634 μm). Once the electrodes are within 1 mm of the target of interest, the rate of advance should be slowed to at most 1 rotation a day, but a quarter rotation (∼80 μm) is recommended. The ideal step size will vary with the target. For instance, the thin pyramidal cell layer of the hippocampus should be approached much more slowly.
Occasionally, cerebrospinal fluid or blood may leak out of the guide tubes and into the head-cap. This can happen when the guide tubes are too large for the electrodes passing through, or not sufficiently sealed with petroleum jelly. If the fluid is still moist, then it can be sopped up with a cotton swab. It should not be allowed to dry over the guide tube, since it may prevent the smooth transit of the electrode.
REFERENCES
- Bidra AS, Taylor TD, Agar JR. Computer-aided technology for fabricating complete dentures: systematic review of historical background, current status, and future perspectives. J Prosthet Dent 109: 361–366, 2013. [DOI] [PubMed] [Google Scholar]
- Bragin A, Hetke J, Wilson CL, Anderson DJ, Engel J Jr, Buzsáki G. Multiple site silicon-based probes for chronic recordings in freely moving rats: implantation, recording and histological verification. J Neurosci Methods 98: 77–82, 2000. [DOI] [PubMed] [Google Scholar]
- Brunetti PM, Wimmer RD, Liang L, Siegle JH, Voigts J, Wilson M, Halassa MM. Design and fabrication of ultralight weight, adjustable multi-electrode probes for electrophysiological recordings in mice. J Vis Exp 91: e51675, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheah CM, Chua CK, Lee CW, Feng C, Totong K. Rapid prototyping and tooling techniques: a review of applications for rapid investment casting. Int J Adv Manuf Technol 25: 308–320, 2005. [Google Scholar]
- Gerstein GL, Clark WA. Simultaneous studies of firing patterns in several neurons. Science 143: 1325–1327, 1964. [PubMed] [Google Scholar]
- Harper RM, McGinty DJ. A technique for recording single neurons from unrestrained animal. In: Brain Unit Activity During Behavior, edited by Phillips MI. Springfield, IL: Thomas, 1973. [Google Scholar]
- Harris KD, Hirase H, Leinekugel X, Henze DA, Buzsaki G. Temporal interaction between single spikes and complex spike bursts in hippocampal pyramidal cells. Neuron 32: 141–149, 2001. [DOI] [PubMed] [Google Scholar]
- Hazan L, Zugaro M, Buzsáki G. Klusters, NeuroScope, NDManager: a free software suite for neurophysiological data processing and visualization. J Neurosci Methods 155: 207–16, 2006. [DOI] [PubMed] [Google Scholar]
- Hutmacher DW, Sittinger M, Risbud MV. Scaffold-based tissue engineering: rationale for computer-aided design and solid free-form fabrication systems. Trends Biotechnol 22: 354–62, 2004. [DOI] [PubMed] [Google Scholar]
- Igarashi KM, Lu L, Colgin LL, Moser MB, Moser EI. Coordination of entorhinal-hippocampal ensemble activity during associative learning. Nature 510: 143–147, 2014. [DOI] [PubMed] [Google Scholar]
- Keating JG, Gerstein GL. A chronic multi-electrode microdrive for small animals. J Neurosci Methods 117: 201–206, 2002. [DOI] [PubMed] [Google Scholar]
- Kloosterman F, Davidson TJ, Gomperts SN, Layton SP, Hale G, Nguyen DP, Wilson MA. Micro-drive array for chronic in vivo recording: drive fabrication. J Vis Exp 26: 1094, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansink CS, Bakker M, Buster W, Lankelma J, van der Blom R, Westdorp R, Joosten RN, McNaughton BL, Pennartz CM. A split microdrive for simultaneous multi-electrode recordings from two brain areas in awake small animals. J Neurosci Methods 162: 129–138, 2007. [DOI] [PubMed] [Google Scholar]
- Lilly JC. Correlations between neurophysiological activity in the cortex and short-term behavior in the monkey. In: Biological and Biochemical Basis of Behavior, edited by Harlow HF and Woolsey CN. Madison, WI: University of Wisconsin Press, 1958, p. 83–100. [Google Scholar]
- McGinty DJ, Harper RM. Dorsal raphe neurons: depression of firing during sleep in cats. Brain Res 101: 569–575, 1976. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res 34: 171–175, 1971. [DOI] [PubMed] [Google Scholar]
- Paz R, Bauer EP, Paré D. Learning-related facilitation of rhinal interactions by medial prefrontal inputs. J Neurosci 27: 6542–6551, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sechenov IM. Reflexes of the Brain. Cambridge, MA: MIT, 1965. [Google Scholar]
- Schmitzer-Torbert N, Jackson J, Henze D, Harris K, Redish AD. Quantitative measures of cluster quality for use in extracellular recordings. Neuroscience 131: 1–11, 2005. [DOI] [PubMed] [Google Scholar]
- Stübinger S, Mosch I, Robotti P, Sidler M, Klein K, Ferguson SJ, von Rechenberg B. Histological and biomechanical analysis of porous additive manufactured implants made by direct metal laser sintering: a pilot study in sheep. J Biomed Mater Res B Appl Biomater 101: 1154–1163, 2013. [DOI] [PubMed] [Google Scholar]
- Vail NK, Swain LD, Fox WC, Aufdlemorte TB, Lee G, Barlow JW. Materials for biomedical applications. Materials & Design 20: 123–132, 1999. [Google Scholar]
- Vandecasteele M, MS, Royer S, Belluscio M, Berényi A, Diba K, Fujisawa S, Grosmark A, Mao D, Mizuseki K, Patel J, Stark E, Sullivan D, Watson B, Buzsáki G. Large-scale recording of neurons by movable silicon probes in behaving rodents. J Vis Exp 61: e3568, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigts J, Siegle JH, Pritchett DL, Moore CI. The flexDrive: an ultra-light implant for optical control and highly parallel chronic recording of neuronal ensembles in freely moving mice. Front Syst Neurosci 7: 8, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto J, Wilson MA. Large-scale chronically implantable precision motorized microdrive array for freely behaving animals. J Neurophysiol 100: 2430–2440, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii N, Pruvot P, Gastaut H. Electrographic activity of the mesencephalic reticular formation during conditioning in the cat. Electroencephalogr Clin Neurophysiol 9: 595–608, 1957. [DOI] [PubMed] [Google Scholar]