Abstract
Anatomical evidence indicates that midcervical interneurons can be synaptically coupled with phrenic motoneurons. Accordingly, we hypothesized that interneurons in the C3–C4 spinal cord can display discharge patterns temporally linked with inspiratory phrenic motor output. Anesthetized adult rats were studied before, during, and after a 4-min bout of moderate hypoxia. Neuronal discharge in C3–C4 lamina I–IX was monitored using a multielectrode array while phrenic nerve activity was extracellularly recorded. For the majority of cells, spike-triggered averaging (STA) of ipsilateral inspiratory phrenic nerve activity based on neuronal discharge provided no evidence of discharge synchrony. However, a distinct STA phrenic peak with a 6.83 ± 1.1 ms lag was present for 5% of neurons, a result that indicates a monosynaptic connection with phrenic motoneurons. The majority (93%) of neurons changed discharge rate during hypoxia, and the diverse responses included both increased and decreased firing. Hypoxia did not change the incidence of STA peaks in the phrenic nerve signal. Following hypoxia, 40% of neurons continued to discharge at rates above prehypoxia values (i.e., short-term potentiation, STP), and cells with initially low discharge rates were more likely to show STP (P < 0.001). We conclude that a population of nonphrenic C3–C4 neurons in the rat spinal cord is synaptically coupled to the phrenic motoneuron pool, and these cells can modulate inspiratory phrenic output. In addition, the C3–C4 propriospinal network shows a robust and complex pattern of activation both during and following an acute bout of hypoxia.
Keywords: cervical interneurons, phrenic, hypoxia, plasticity, short-term potentiation
spinal interneurons which discharge in phase with the respiratory cycle have been well documented (Lane 2011). For example, the thoracic spinal cord contains a large number of such “respiratory interneurons” (Kirkwood et al. 1988), and these cells can relay inhibitory expiratory drive to intercostal motoneurons (Kirkwood 1995) and may also provide phasic (Davies et al. 1985) and tonic excitatory inputs (Kirkwood 1995). In the cervical spinal cord, respiratory interneurons are also prominent, and prior studies have operationally grouped these cells into upper- (C1–C3) and midcervical locations (C4–C6). Upper cervical cord interneurons appear to modulate both intercostal and phrenic motor output (Douse et al. 1992; Lipski et al. 1993; Lu et al. 2004; Qin et al. 2002) and also may have rhythmogenic properties (Okada et al. 2009; Oku et al. 2008). In the midcervical spinal cord (i.e., C3–C5) interneurons with both inspiratory- and expiratory-related discharge have been confirmed by independent laboratories (Bellingham and Lipski 1990; Iscoe and Duffin 1996; Palisses et al. 1989).
The functional role of midcervical respiratory interneurons, however, is not clear. Several groups have advanced the hypothesis that respiratory midcervical interneurons can modulate phrenic motoneuron excitability and help to “shape” the phrenic inspiratory burst (Bellingham and Lipski 1990; Douse and Duffin 1993; Lane et al. 2008a; Lane et al. 2008b; Palisses et al. 1989). However, Duffin and Iscoe found no evidence to support this hypothesis in a comprehensive study of cervical interneuron discharge in decerebrate cats (Duffin and Iscoe 1996). Robust inspiratory-related bursting was recorded from C5 interneurons, but cross-correlation with phrenic nerve activity produced no evidence for synaptic connectivity (Duffin and Iscoe 1996). On the other hand, anatomical studies indicate that midcervical interneurons are synaptically coupled to phrenic motoneurons in rat (Lane et al. 2008b) and cat (Lois et al. 2009). While it may be that anatomically identified “prephrenic interneurons” do not have a respiratory function, confirming this hypothesis presents a challenging experimental problem. In addition to the issue of sampling (i.e., recording sufficient cells), cervical interneurons also show state dependence in discharge properties (Grelot et al. 1993), and thus the specific conditions of the recording are likely to impact on the ability to detect interneuron-phrenic motoneuron relationships. In the present study, we used a multielectrode recording approach to explore the discharge patterns of midcervical interneurons in relation to phrenic motor activity. Specifically, we tested the hypothesis that the discharge of nonphrenic neurons in the C3–C4 spinal cord can be temporally linked to phrenic motor output in a manner consistent with a direct synaptic connection. Recordings were first conducted during a baseline normocapnic condition with stable inspiratory phrenic bursting, and then respiratory drive was increased by a moderate hypoxic stimulus. An anecdotal report suggests that hypoxia stimulates cervical interneuron discharge (Lane et al. 2009), and we reasoned that increasing cervical interneuron discharge could potentially increase the incidence of synaptic coupling between phrenic motoneurons and cervical interneurons.
Another important consideration is that hypoxia is a potent stimulus for inducing cervical motor plasticity (Lovett-Barr et al. 2012) and has intriguing potential as an adjunct to conventional neurorehabilitation therapies (Hayes et al. 2014; Tester et al. 2014; Trumbower et al. 2012). Previous studies of hypoxia-induced cervical spinal plasticity, however, have focused on phrenic motoneurons and phrenic motor output (Devinney et al. 2013). Here, we focused on a form of hypoxia-induced respiratory motor plasticity known as short-term potentiation or STP (Powell et al. 1998). This response is manifest as a slow increase in respiratory motor activity during hypoxia (i.e., after the acute response) and then a short-lasting (i.e., minutes) potentiation of output after oxygen levels are restored. Thus the secondary purpose of this work was to examine the time course of changes in C3–C4 neuronal discharge during and following an acute bout of hypoxia. We hypothesized that plasticity triggered by hypoxia would not be exclusive to phrenic motor output and that changes in cervical interneuron discharge rates would also persist beyond the period of hypoxia. To our knowledge, this is the first description of how hypoxia—a stimulus associated with increased respiratory motor output and plasticity—alters the pattern of cervical interneuron activity.
MATERIALS AND METHODS
Adult male Sprague-Dawley rats (N = 15, weight = 385 ± 11 g) were purchased from Harlan (Indianapolis, IN). All procedures were approved by the Institutional Animal Care and Use Committee at the University of Florida.
Neurophysiology.
Animals were initially anesthetized with isoflurane (5% in O2) in a closed chamber and then transferred to a nose cone (2–3% isoflurane, 50% O2, balance N2). The trachea was cannulated below the larynx with PE-240 tubing, and rats were mechanically ventilated (model 683; Harvard Apparatus, South Natick, MA; volume = 7 ml/kg; frequency = 60–65/min). Lungs were briefly hyperinflated (2–3 s) once per hour to minimize atelectasis. The tracheal pressure was monitored with a pressure transducer (Statham P-10EZ pressure transducer, CP122 AC/DC strain-gauge amplifier, Grass Instruments, West Warwick, RI) connected to the tracheal cannula.
The femoral artery and vein were catheterized (PE-50 tubing) for blood pressure measurement and drug administration, respectively. Rats were converted from isoflurane to urethane anesthesia (1.6 g/kg iv; 0.12 g/ml in distilled water), and anesthesia was initially monitored by observing the limb withdrawal response to toe pinch. During neurophysiological recordings, the anesthetic depth was monitored by observing the blood pressure and phrenic nerve responses to a noxious stimuli (toe pinch). Supplemental urethane was given if indicated (0.3 g/kg iv). A femoral arterial catheter (PE-50) was used to measure blood pressure (Statham P-10EZ pressure transducer, CP122 AC/DC strain-gauge amplifier, Grass Instruments, West Warwick, RI) and to withdraw samples for blood gas analysis (i-stat, Abbott Point of Care, Princeton, NJ). The rectal temperature was monitored by an electrical thermometer and maintained at 37.5 ± 1°C by a servo-controlled heating pad (model TC-1000, CWE, Ardmore, PA).
Rats were bilaterally vagotomized to prevent entrainment of the phrenic activity with the ventilator. A slow infusion of lactated Ringer's solution and sodium bicarbonate (3:1, 1.5 ml/h) was maintained to promote acid-base balance. Animals were then placed in a stereotaxic instrument (David Kopf Instruments, Tujunga, CA) and neuromuscularly paralyzed with pancuronium bromide (2.5 mg/kg iv, Hospira, Lake Forest, IL) to eliminate spontaneous breathing efforts. The partial pressure of end-tidal CO2 (PetCO2) was analyzed with a Capnogard neonatal CO2 monitor placed on the expiratory line of the ventilator circuit (Novametrix Medical Systems, Wallingford, CT) and maintained at 40–45 mmHg by adjusting inspired CO2.
The left phrenic nerve was isolated via a dorsal approach and sectioned distally. Efferent activity was recorded from the phrenic nerve by silver electrodes and covered with a mineral oil and vaseline mixture. The nerve signal was amplified (1000×, model 1700, A-M Systems, Carlsborg, WA), band-pass filtered (0.3–10 kHz), and integrated (time constant 100 ms; model MA-1000; CWE). The raw and integrated neural signals were digitized by the CED Power 1401 data acquisition interface and recorded on a PC (Dell Precision T1600) using Spike2 software (Cambridge Electronic Design, Cambridge, UK).
The C3–C4 vertebrate bone and dura were removed to expose the cervical spinal cord. A bilateral thoracotomy was performed to minimize spinal cord movement that could result from changes in the thoracic pressure during ventilation. Positive end-expiratory pressure of 1–3 cmH2O was applied by placing the expired ventilator line in water. Single unit activity from C3–C4 spinal segments was recorded using either a commercially available multiunit probe or a custom multielectrode array. The commercially available multiunit silicone probe (NeuroNexus Technologies, Ann Arbor, MI) consisted of 16 iridium recording sites (impedance = 0.7–1 MΩ, area = 703 μm2/site) 100 μm apart to allow for recording from separate neurons. The probe was placed 0.7 mm lateral to the midline and depth was adjusted using a micropositioner (model 640, Kopf Instruments, Tujunga, CA) until single units could be discriminated from background activity using an audio monitor. The single unit activity was amplified (5,000×), filtered (0.5–6 kHz), digitized at a 12-kHz sampling rate with an analog-to-digital converter (model RP2.1, Tucker Davis Technologies, Alachua, FL), and recorded on a PC using Tucker Davis Technologies software. The custom recording array has been described previously (Baekey et al. 2001; Kowalski et al. 2013). It consists of two independently controlled planar arrays of tungsten microelectrodes (impedance = 10–12 MΩ). Each array consisted of eight microelectrodes 150–200 μm apart. The depth of each electrode was adjusted using micromotor controllers, and the neural signals from single electrode were amplified, band-pass filtered (0.5–6 kHz), digitized (Power 1401, Cambridge Electronic Design, Cambridge, UK), and recorded on a PC (Spike 2 software, Cambridge Electronic Design). After stable recording of the cervical neurons during baseline (inspired gas = 50% O2 and 50% N2), animals were exposed to a 4-min hypoxic episode (inspired gas = 13–15% O2, balance N2).
Data analyses.
Phrenic nerve signals were analyzed using Spike 2 software (CED, UK). The integrated phrenic neurogram (∫Phr) was used to calculate respiratory frequency (bursts/min) and peak amplitude. Action potentials from the individual neurons were converted to waveforms using offline spike-sorting analyses with Spike2 software. Spikes were clustered together based on the criteria of at least 95% matching shapes, assessment of auto-correlograms, and the principle component analyses feature of the Spike2 software as previously described (Baekey et al. 2010). In addition, cycle-triggered histograms (Lindsey et al. 1992) were used to evaluate neural spike activity relative to the respiratory cycle. The cycle-triggered histograms were constructed by dividing the respiratory period (based on the phrenic neurogram) into 100 bins of equal size and measuring cumulative spike counts within each bin over 50 consecutive neural breaths. Phrenic nerve activity, averaged over 50 consecutive neural breaths, was also plotted over the spike counts, thereby providing a visualization of neural discharge in relation to the respiratory cycle. The relative degree of “respiratory modulation” for each recorded cell was calculated using the η2 statistic (Orem and Dick 1983). The η2 calculation evaluates the variance in neural activity during the respiratory cycle in relation to the total variance of activity. Values of η2 can range from 0 (no relationship of discharge patterns to the respiratory cycle) to 1.0 (all variance in discharge can be attributed to respiratory bursting). Orem and Dick concluded that η2 values greater than 0.3 indicate discharge that is highly correlated with the respiratory cycle (Orem and Dick 1983).
Spike-triggered averaging (STA) of the phrenic nerve activity in relation to spinal neuron discharge was used to examine the temporal relationship of neuronal spikes and phrenic motor output (Lipski et al. 1983). Unrectified and full-wave rectified digitized signals from the phrenic nerve were averaged, using the spikes of each recorded neuron as trigger events. In accordance with previous publication (Baekey et al. 2004), short-latency, short-duration offset peaks (e.g., 1- to 2-ms latency, ∼ 1-ms half-width) in the STA phrenic nerve output were taken as evidence that the cell was phrenic motoneuron. A clear peak in the STA of phrenic nerve activity occurring with a lag time greater than 5 ms taken as evidence that the recorded cell was synaptically antecedent to the phrenic motoneuron pool (Baekey et al. 2004). For cells considered to be phrenic motoneurons or prephrenic interneurons, STA was performed across the entire respiratory period over 50 cycles and also selectively during the inspiratory and expiratory periods as defined by the phrenic nerve recordings. The STA procedures enabled identification of phrenic motoneurons, but we cannot exclude the possibility that a portion of the recorded cells are nonphrenic motoneurons. We suggest, however, that these cells are likely to be interneurons because the spinal recording location used in our study is more medial than forelimb motoneurons and shoulder muscles would be expected in the adult rat (McKenna et al. 2000).
We are confident that the spike trains recorded in our study do not reflect recordings from “fibers of passage” (i.e., ascending or descending axons) because the recordings were held as the microelectrode was moved over a tracking distance of ∼100 μm with a steadily increasing or decreasing amplitude. Recordings from axons are more sensitive to movement of the electrode and usually disappear with a relatively smaller tracking distance (Bellingham and Lipski 1990; Kirkwood et al. 1988). In addition, the action potentials from the spike trains exhibited biphasic (i.e. negative/positive) spikes, which is typical of recordings from the neuronal somas located close to the recording electrode (Bellingham and Lipski 1990).
Neuronal activity was averaged over 1-min periods before, during, and following hypoxia. One-way repeated-measures analysis of variance (ANOVA) and the Student-Newman-Keuls post hoc test (SigmaPlot 12.5, Systat Software, San Jose, CA) was used to analyze changes in the blood pressure and heart rate. Neuronal firing behaviors were analyzed using two-way repeated-measures ANOVA and the Student-Newman-Keuls post hoc test. Regression analyses were used to examine the relationship between baseline discharge frequency (Hz) and short-term potentiation during following hypoxia. P < 0.05 was considered statistically significant for all analyses. All data are presented as means ± SE.
RESULTS
Blood gases, arterial pressure, and phrenic nerve activity.
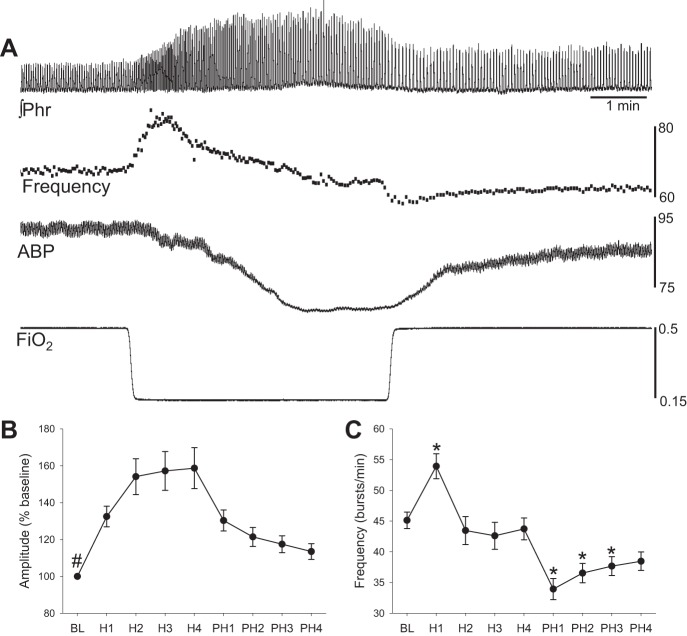
Prior to advancing the electrodes through the spinal cord, an arterial blood gas sample was taken that confirmed adequate oxygenation and normocapnia (PaO2 = 158 ± 5 mmHg, PaO2 = 41 ± 1 mmHg, pH = 7.3 ± 0.02). Mean arterial blood pressure averaged 94 ± 6 mmHg at baseline and decreased during the brief hypoxic episode. However, arterial pressure returned to baseline values within 4 min posthypoxia (Table 1). The drop in blood pressure was accompanied by an increase in heart rate that also rapidly returned to the baseline levels upon removal of the hypoxic stimulus (Table 1). The amplitude of the ∫Phr inspiratory burst increased progressively during the hypoxic exposure and then gradually declined during the posthypoxia period (Fig. 1). The mean inspiratory frequency (neural breaths/min) initially increased during hypoxia, but then showed a “roll off” over the course of hypoxia, and declined below baseline values during the posthypoxia period (i.e., posthypoxia frequency decline) (Powell et al. 1998).
Table 1.
Cardiovascular responses during hypoxia
| Baseline | Onset | End | 4 min | |
|---|---|---|---|---|
| MAP, mmHg | 94 ± 6 | 91 ± 5 | 80 ± 5* | 89 ± 6 |
| HR, beats/min | 407 ± 8 | 420 ± 9* | 424 ± 9* | 409 ± 8 |
Values are means ± SE. Mean arterial blood pressure (MAP) and heart rate (HR) during baseline, onset of hypoxia, end of hypoxia, and 4 min posthypoxia are displayed.
P < 0.05 compared with baseline.
Fig. 1.
Impact of hypoxia on phrenic nerve activity. A: moving averaged phrenic nerve activity (∫Phr), breath-by-breath inspiratory burst frequency (breaths/min), arterial blood pressure (ABP, mmHg) and the fraction of inspired oxygen (FiO2). B and C: the mean ∫Phr amplitude (% baseline) (B) and frequency (C) during and following hypoxia. BL indicates baseline, H1–H4 indicates hypoxia minutes 1–4, and PH1–PH4 indicates posthypoxia minutes 1–4. #P < 0.05 vs. all other time points; *P < 0.05 vs. baseline.
Neuronal discharge during baseline and hypoxia.
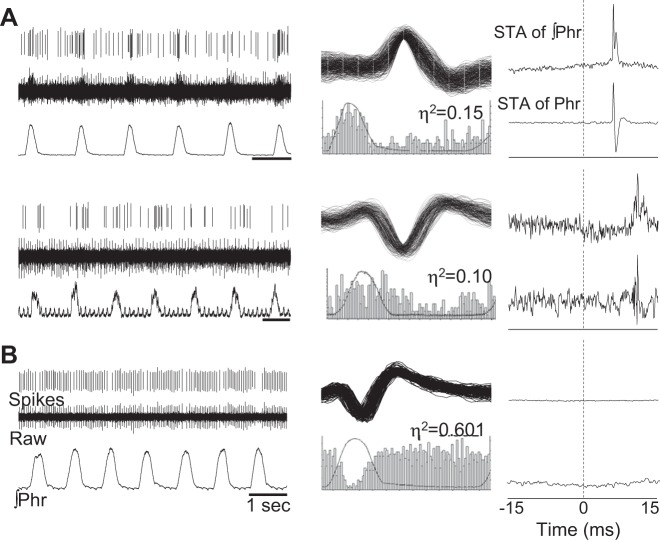
Approximately 5% of the total cells (i.e., 5/95), were classified as nonphrenic neurons that were synaptically antecedent to phrenic motor neurons (Fig. 2A). This conclusion was reached based on a clear peak in the STA of phrenic nerve activity occurring with a lag time of >5 ms [i.e., consistent with a synaptic delay (Baekey et al. 2004; Giraudin et al. 2008)]. Baseline firing rate was 14 ± 6 Hz, and on average, the STA peak (averaged across the entire respiratory cycle) for these five recordings was located 6.83 ± 1.1 ms from zero (see example in Fig. 2A). When the STA procedure was applied selectively to either the inspiratory or the expiratory period, 4/5 cells showed peaks only during inspiration, and the remaining cell showed peaks during both inspiration and expiration, as shown in Fig. 3. The average η2 value of 0.18 ± 0.04 (range 0.02–0.33) was indicative of a weak respiratory modulation of burst activity. This group of cells had a variable response to hypoxia (range 102–277% baseline), and on average, there was not a significant increase in discharge frequency (Fig. 4). We also noted a tendency for a greater η2 value during hypoxia, but this did not reach statistical significance (0.32 ± 0.10, P = 0.26 vs. baseline value). These cells were recorded across a wide anatomical range (500–2,000 μm below the dorsal spinal surface) (Fig. 5).
Fig. 2.
Representative examples of respiratory modulated interneurons. A: 2 separate examples of recordings from neurons that appear to be synaptically antecedent to the phrenic motor pool (i.e., prephrenic) based on the results of spike triggered averaging (STA). Sorted waveforms (i.e., spikes) and raw unit bursting, along with the phrenic neurograms (∫Phr), are presented in the left column. The middle column shows waveform overlay plots and cycle-triggered histograms for each cell. The η2 values (see materials and methods) are provided above the histogram. The panels on the right provide the output of STA of the integrated phrenic neurogram (∫Phr, top) and raw phrenic neurogram (Phr, bottom). For both the cells designated as “prephrenic,” a distinct peak can be seen in the STA result that is ∼7 ms from zero. The peak could be detected for STA performed on both the integrated and raw signal. B: an example of an expiratory modulated recording. Note the complete absence of STA peak for this cell.
Fig. 3.
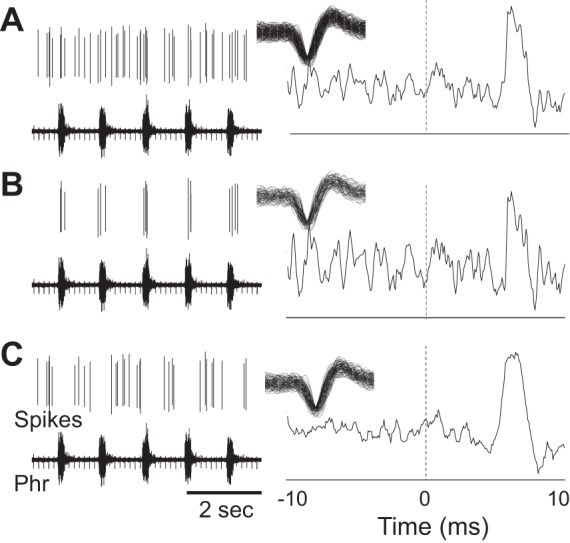
A representative example showing that the same neuron can modulate phrenic output during both inspiration and expiration. A–C show recordings of the same neuron. Depicted are the sorted neural spikes, raw phrenic nerve activity (Phr), and overlay plot of the spikes, and the result of spike triggered averaging of the phrenic nerve signal. In this example, the recorded neuron burst throughout the respiratory cycle as shown in A. To examine the potential impact of this cell on phrenic nerve activity during inspiration vs. expiration, spike triggered averaging of phrenic nerve activity was done across the entire respiratory cycle (A), only during the inspiratory period (B), and only during the expiratory period (C). A distinct peak in the spike triggered average result was evident in each case and occurred at ∼7 ms from zero. Therefore, the functional impact of this cell on phrenic nerve activity was similar across the inspiratory and expiratory phases.
Fig. 4.
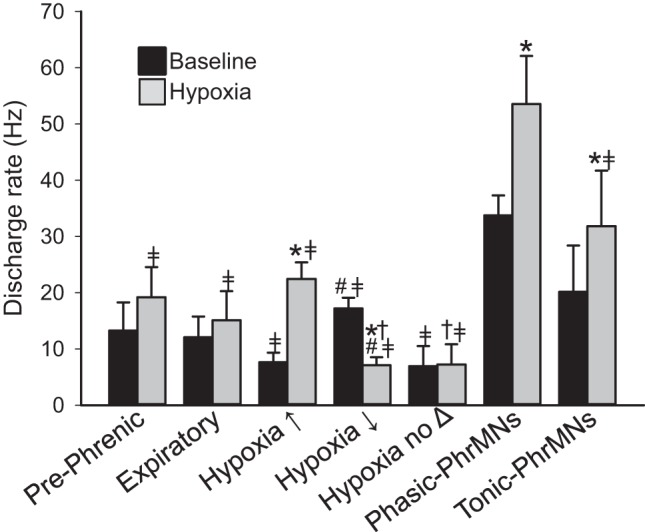
The mean discharge rate (Hz) during the baseline condition and subsequent exposure to hypoxia for each of the categories of recorded spinal neurons. The classification of neurons was based on the detailed analysis of spike activity in relation to the respiratory cycle as outlined in Fig. 1 and materials and methods. Prephrenic cells appeared to be synaptically antecedent to the phrenic motor pool; expiratory cells were active only during the expiratory period; cells with no apparent respiratory modulation were classified based on their response to hypoxia as excited (hypoxia↑), inhibited (hypoxia↓), or unresponsive (hypoxia no Δ). Phrenic motoneurons (PhrMNs) were classified as phasic (active only during the inspiratory phase) or tonic (active across the entire respiratory cycle). *P < 0.001 vs. baseline;  P < 0.03 vs. Phasic-PhrMNs; #P < 0.006 vs. hypoxia↑; †P < 0.04 vs. Tonic-PhrMNs.
P < 0.03 vs. Phasic-PhrMNs; #P < 0.006 vs. hypoxia↑; †P < 0.04 vs. Tonic-PhrMNs.
Fig. 5.
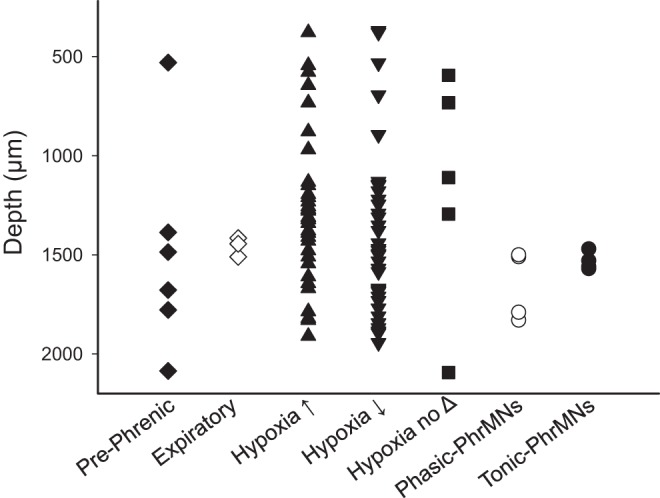
The distance (μm) from the dorsal surface of the spinal cord at which each cell was recorded. The microelectrode array was inserted into the spinal cord at 700 μm lateral to the midline at the C4 segment. The classification of cells into groups is discussed in the text as well as Fig. 4 legend. Phasic and tonic phrenic motoneurons (PhrMNs) were recorded at locations consistent with the anatomical location of the phrenic motor pool (i.e., 1,500–1,800 μm below the surface). Most cells classified as “prephrenic” were also recorded in this region, with one exception. Interneurons which responded to hypoxia with increased (hypoxia↑), or decreased (hypoxia↓) burst activity were recorded throughout the entire range of the recording tract.
A few cells (N = 3) were recorded that discharged more prominently during the expiratory period (e.g., Fig. 2B), and these neurons had no evidence of a peak in the STA of phrenic nerve activity. Two of these neurons were active during baseline and had η2 values of 0.06 and 0.18, and these changed to 0.6 and 0.21, respectively, during hypoxia. The third neuron was silent during baseline and was recruited during hypoxia (η2 = 0.75). These cells had a baseline firing rate of 0, 8, and 17 Hz, which increased to 9, 8, and 20 Hz during hypoxia (Fig. 4).
The majority of recorded cells (78/95 or 82%) displayed bursting patterns that did not relate in any discernable way to the respiratory cycle (e.g., see representative cycle-triggered histogram in Fig. 6). Nevertheless, most of these cells (73/78 or 94%) were “hypoxia sensitive” and responded to hypoxia with a change in firing frequency. As illustrated by the representative examples in Fig. 7, cells tended to respond to hypoxia with either an increase (37/78 or 47%) or decrease (36/78 or 46%) in firing frequency. Accordingly, we used this response as a criterion to classify non-respiratory-modulated interneurons as excited, inhibited, or insensitive based on the hypoxic response (Fig. 4). These cell groups did not cluster in a particular area within the gray matter, but rather were distributed through the gray matter at depths ranging from 398 to 2,094 μm from the dorsal surface (Fig. 5).
Fig. 6.
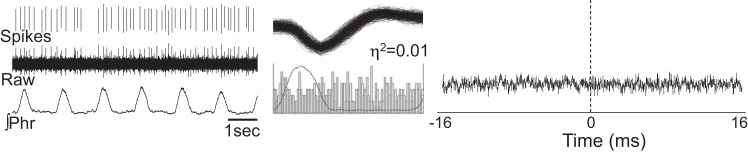
Representative example of a cell with no evidence for respiratory-related discharge patterns. The intraspinal recording is shown (raw) along with the sorted neural spikes, phrenic nerve activity (∫Phr), the associated cycle triggered histogram, an overlay plot of the spikes, and the result of spike triggered averaging of the phrenic nerve signal. The complete lack of respiratory modulation in this cell is evident by the cycle-triggered-histogram and η2 value close to zero. The complete absence of a peak in the phrenic nerve spike-triggered average was typical of neurons with similar discharge patterns.
Fig. 7.
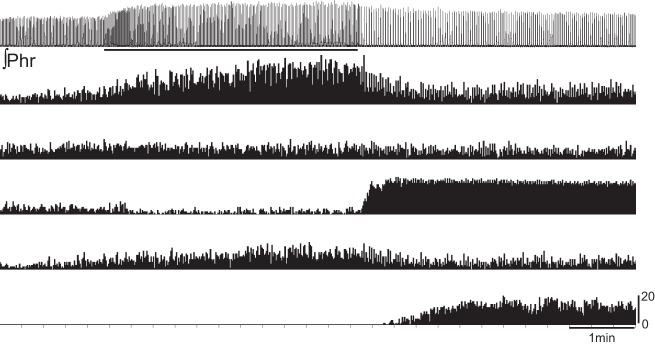
A representative example illustrating the diversity of neuronal responses to hypoxic exposure. The phrenic nerve signal (∫Phr) is presented at the top of the trace, with the horizontal bar indicating the period of hypoxic exposure. Below the phrenic nerve signal are plots of instantaneous burst frequency for five different neurons recorded simultaneously using the multielectrode array. This example illustrates that some cells responded robustly during the period of hypoxia, while others responded with a robust increase in bursting following the hypoxic exposure.
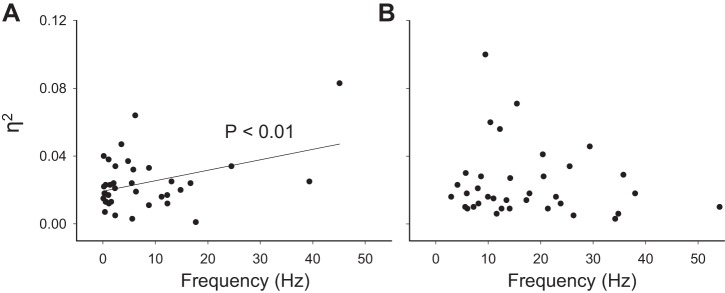
During baseline conditions, nonrespiratory interneurons that were subsequently inhibited by hypoxia fired at a higher rate (17 ± 2 Hz) than those that were subsequently excited by hypoxia (8 ± 2 Hz) (Fig. 4). The η2 analyses was consistent with the apparent lack of respiratory modulation in these cells (0.024 ± 0.003 for hypoxia-excited, 0.024 ± 0.004 for hypoxia-inhibited). There was a significant relationship between baseline discharge rate and the η2 value for hypoxia-excited cells, but no apparent relationship between these two variables for hypoxia-inhibited neurons (Fig. 8). During hypoxia, the hypoxia-excited cells had a 408 ± 130% increase in η2 values (P = 0.002 vs. baseline), but the overall degree of respiratory modulation nevertheless remained quite low (mean hypoxia η2 = 0.041 ± 0.004). Hypoxia-inhibited cells showed a range of η2 during hypoxia from 0.001 to 0.127 (mean = 0.026 ± 0.004, P = 0.59 vs. baseline). Approximately 6% of cells (5/78) were classified as “hypoxia-insensitive” with no discernable change in discharge frequency (100 ± 4 % baseline) or η2 (122 ± 41 % baseline) during the period of hypoxia.
Fig. 8.
The relationship between the relative degree of respiratory bursting (assessed via the η2 calculation) and burst frequency (Hz). These plots are based on data collected during the baseline condition and are subdivided into cells that responded to hypoxia with increased discharge (A) and cells that decreased discharge during hypoxia (B). The intent was to determine if the η2 values of cells that displayed little evidence for respiratory-related discharge could be predicted by the discharge rate. There was a significant positive correlation between η2 value and discharge rate for hypoxia-excited interneurons (A). However, no significant relationship was detected for hypoxia-inhibited interneurons (B).
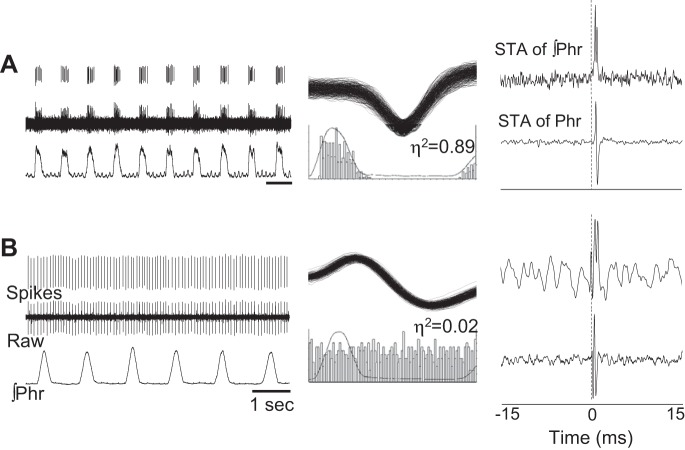
The focus of our intraspinal recordings was on nonphrenic cells; however, a small number of phrenic motoneurons were recorded (N = 8). Bursting patterns in cells considered to be phrenic motoneurons resulted in a distinct peak in the STA of phrenic nerve signal with a lag time that was below the possibility of synaptic delay [<1 ms (Christakos et al. 1994)] (e.g., Fig. 9). Thus STA based on the discharge in these 8 cells produced a peak at 0.68 ± 0.05 ms from zero. Two distinct bursting patterns were observed among cells classified as phrenic motoneurons. One group of neurons was active only during the inspiratory period (e.g., Fig. 9A) and thus had predictably high η2 values (0.57 ± 0.17). Classified as “rhythmic,” these cells fired at 34 ± 4 Hz during baseline, a value similar to prior reports (reviewed in Lee and Fuller 2011). Another group of cells were active throughout the respiratory cycle (i.e., tonic discharge), but nevertheless showed a very distinct peak in the phrenic nerve STA with a short lag time (e.g., Fig. 9B). In these recordings, a peak was present in the STA result regardless of whether the averaging was done during the inspiratory period, the expiratory period, or across the entire respiratory cycle (Fig. 10). Accordingly, these cells were classified as nonrhythmic or “tonic” and fired at frequencies ranging from 4 to 50 Hz (mean 15 ± 7, Fig. 4), but the burst patterns were not related in any discernable manner to the respiratory cycle as shown by cycle-triggered histograms (e.g., Fig. 9B) and η2 values (mean = 0.02 ± 0.01).
Fig. 9.
Representative examples of phrenic motoneurons that burst during inspiration (A) or tonically across the entire respiratory cycle (B). Sorted waveforms (i.e., spikes) and raw unit bursting, along with the phrenic neurograms (∫Phr), are presented in the left column. The middle column shows waveform overlay plots and cycle-triggered histograms for each cell. The η2 values (see materials and methods) are provided above the histogram. The panels on the right provide the output of STA of the integrated phrenic neurogram (∫Phr, top) and raw phrenic neurogram (Phr, bottom). The phasic cell that is shown in A displays strong inspiratory-related modulation (see cycle-triggered histogram) and has an accordingly high η2 value. In contrast, the tonic phrenic motoneuron shows no evidence for respiratory modulation and the η2 value is close to zero. Nevertheless, both of these cells display a distinct latency peak in the phrenic spike-triggered average that can be observed in both the raw and integrated phrenic signal.
Fig. 10.
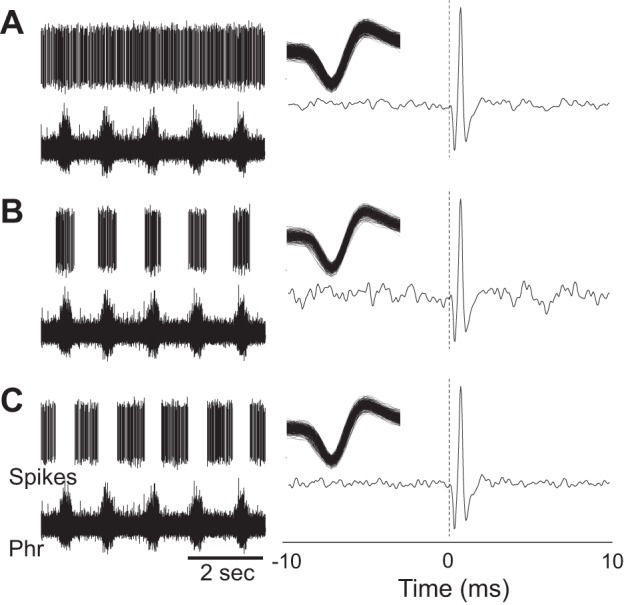
An example of a tonically discharging phrenic motor with spike triggered averaging performed across entire respiratory cycle (A), only during the inspiratory period (B), or selectively during the expiratory period (C). Sorted waveforms (i.e., spikes) and phrenic neurograms (Phr) are presented in the left column. The right column shows waveform overlay plots and the output of STA of the phrenic neurogram. A short-latency STA peak could be detected regardless of whether the averaging was done across the entire cycle (A) or selectively during the inspiratory (B) and expiratory phases (C).
Hypoxia triggered a robust increase in discharge rate of both rhythmic phrenic motoneuron (141 ± 8%) and tonic phrenic motoneurons (190 ± 52% baseline) (Fig. 4). During the hypoxic exposure, the rhythmically phrenic motoneurons maintained bursting only during the inspiratory period, while tonically active cells continued to burst throughout the respiratory cycle. There was no evidence for a change in the η2 values during hypoxia in either group (P > 0.23).
Neuronal discharge following hypoxia.
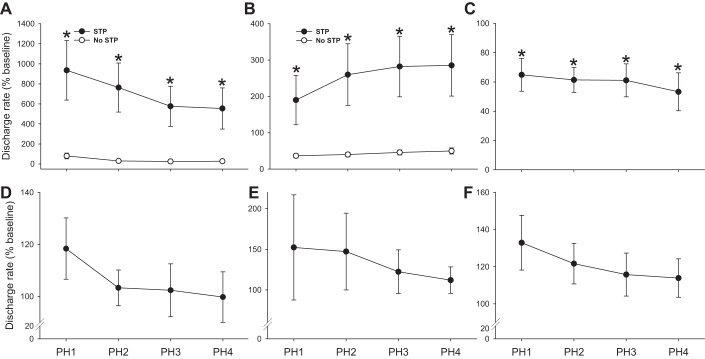
As illustrated by Fig. 7, a variety of discharge patterns were noted upon return to baseline conditions following hypoxia. For presentation of the posthypoxia data, the same general classifications, based on the acute hypoxic response, are maintained. For neurons with the largest sample size (i.e., hypoxia-excited and hypoxia-inhibited), a bimodal distribution in the posthypoxia discharge patterns was noted with some cells continuing to burst at values above baseline (i.e., STP of discharge rates) and others immediately returning to baseline discharge rates. Figure 11A demonstrates the posthypoxic response for cells that were excited by hypoxia. Of this group, 27% of cells immediately returned to prehypoxia baseline discharge rates, whereas the remaining 73% showed STP of burst discharge. For hypoxia-inhibited neurons, most (81%) did not show STP but a subset of cells (19%) robustly increased discharge rates when the hypoxic stimulus was removed (see example in Fig. 7). The mean posthypoxic response for hypoxia-inhibited neurons is shown in Fig. 11B.
Fig. 11.
Neuronal discharge during the posthypoxic period. For presentation of the posthypoxia data, the same general classifications used in prior figures are maintained. Thus data are presented from non-respiratory-modulated interneurons that increased bursting during hypoxia (A), decreased bursting during hypoxia (B), or did not change bursting during hypoxia (C). The mean posthypoxic response is also shown for cells classified as prephrenic (D), and both rhythmic (E) and tonic phrenic motoneurons (F). For neurons with the largest sample size (i.e., hypoxia-excited and hypoxia-inhibited), a bimodal distribution in the posthypoxia discharge patterns was noted with some cells continuing to burst at values above baseline (i.e., STP of burst discharge) and others immediately returning to baseline discharge rates. Therefore, in A and B the data are grouped into cells that did (●) or did not (○) show STP following hypoxia. PH1–PH4 indicates posthypoxia minutes 1–4. *P < 0.05 vs. baseline.
We observed that all neurons (N = 5) that did not change bursting during hypoxia (classified as hypoxia-insensitive) promptly reduced discharge rates following the brief hypoxic episode. Thus, despite the small sample size, there was a statistically significant posthypoxic depression of firing frequency in these cells (Fig. 11C). Cells classified as prephrenic interneurons (Fig. 11D) did not show a consistent pattern of posthypoxia STP, with discharge frequency ranging from 79 to 127% baseline at 4 min posthypoxia. Rhythmic and tonic phrenic motoneuron discharge was variable following hypoxia, and the tendency for posthypoxia STP in these neurons was not statistically significant (P > 0.05; Fig. 11, E and F).
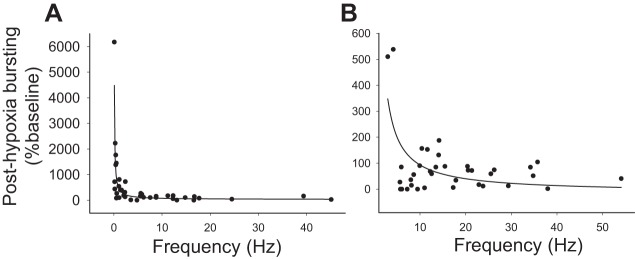
Finally, we observed that presence of posthypoxia STP in both hypoxia-excited (P < 0.001, Fig. 12A) and hypoxia-inhibited neurons (P < 0.001, Fig. 12B) showed a correlation with the initial baseline discharge rate. Thus cells with a low discharge rate at baseline were more likely to show persistent increases in discharge rate following the hypoxic stimulus. This relationship was most robust for the hypoxia-excited cells. In this group, posthypoxia STP was not evident in neurons that fired at greater than ∼5 Hz during the baseline period (Fig. 12A).
Fig. 12.
Relationship between baseline burst frequency and STP following hypoxia. The results of nonlinear regression analyses of the baseline discharge frequency (Hz) and posthypoxic bursting (%baseline) are presented for hypoxia-excited (A) and hypoxia-inhibited interneurons (B). For both groups, a statistically significant relationship was present (P < 0.001) indicating that cells with a low discharge rate at baseline were more likely to show persistent increases in discharge rate following the hypoxic stimulus.
DISCUSSION
Spinal interneurons play a significant role in shaping motoneuron output in many motor systems (Jankowska 2008; Krutki et al. 2011), but their role in the control of the diaphragm is less clear. While anatomical studies in both rat (Lane et al. 2008b) and cat (Lois et al. 2009) have revealed a midcervical interneuronal network that is synaptically coupled to the phrenic motor pool, previous studies have failed to find neurophysiological evidence that midcervical interneurons can modulate inspiratory phrenic activity (Duffin and Iscoe 1996). Here we demonstrated that ∼5% of interneurons recorded in the C3–C4 rat spinal cord burst with a pattern that is temporally linked to inspiratory phrenic motor output. We conclude that these cells are synaptically antecedent to the phrenic motor pool (i.e., prephrenic interneurons) and can modulate inspiratory phrenic motor output as suggested previously (Lane et al. 2008b; Palisses et al. 1989; Sandhu et al. 2009). We have also shown that C3–C4 propriospinal neurons have a diverse and complex response to hypoxia. The vast majority of cervical neurons recorded in this study responded to hypoxia with a change in burst patterns, and many cells displayed altered bursting following the hypoxic episode. These data suggest that interneurons in the C3–C4 spinal cord are participating in the autonomic and/or somatic motor response to hypoxia. Accordingly, we suggest that models of hypoxia-induced cervical spinal plasticity (Dale et al. 2014) should not be restricted to motoneurons but should also consider the potential impact of hypoxia on the propriospinal network.
Prephrenic interneurons in the cervical spinal cord.
The primary objective of this work was to look for neurophysiological evidence in support of the hypothesis that midcervical spinal interneurons are functionally connected to phrenic motoneurons. This hypothesis derived from prior work using both anatomical and neurophysiological methods. For example, Lane and colleagues used a transsynaptic viral neural tracer to show that the rat midcervical spinal cord contains a population of interneurons that are synaptically antecedent to phrenic motoneurons (i.e., prephrenic interneurons). A portion of these cells appear to receive synaptic inputs from the ventral medulla as shown via anterograde bulbospinal tracing (Lane et al. 2008b). Anatomical results from independent laboratories are also consistent with the presence of prephrenic interneurons in rat (Dobbins and Feldman 1994) and cat (Lois et al. 2009; Rice et al. 2009).
Neurophysiological studies from multiple species have shown that midcervical interneurons can display respiratory-related phasic bursting (Bellingham and Lipski 1990; Iscoe and Duffin 1996; Palisses et al. 1989; Sandhu et al. 2009), with one study confirming synaptic inputs from the ventral medulla (Duffin and Iscoe 1996). Palisses and colleagues provided one of the first reports on this topic (Palisses et al. 1989). These authors “tried to directly demonstrate relay respiratory interneurones at the C3–C6 spinal cord” and reported a wide range of respiratory-related discharge patterns in midcervical interneurons of decorticate rabbits. However, to our knowledge, previously there have been no clear demonstrations that cervical interneurons are functionally connected to phrenic motoneurons (Duffin and Iscoe 1996), although several groups have advanced this hypothesis (Bellingham and Lipski 1990; Douse and Duffin 1993; Lane et al. 2008a; Lane et al. 2008b; Palisses et al. 1989; Sandhu et al. 2009).
Here we demonstrated that ∼5% of the interneurons that we recorded in the C3–C4 rat spinal cord burst with a pattern that is temporally linked to inspiratory phrenic motor output. The conclusion that the recorded cells were synaptically antecedent (i.e., prephrenic) was reached based on a clear peak in the phrenic nerve STA occurring with a lag time of over 5 ms (i.e., consistent with a synaptic delay) (Baekey et al. 2004; Giraudin et al. 2008). Thus activation of these neurons was associated with an electrical potential in the phrenic nerve occurring within a timeframe that was consistent with synaptic delay. Of cells that were considered to be prephrenic interneurons, 4 of 5 showed evidence for synaptic connectivity (i.e., a clear STA peak in the phrenic neurogram) only during the inspiratory phase, and the remaining cell produced a STA peak during both the inspiratory and expiratory phase. While this represents a limited sample size, these data provide proof-of-concept that midcervical interneurons have the ability to modulate respiratory-related phrenic motor output. However, a considerable amount of evidence indicates that depolarization of phrenic motoneurons during inspiration occurs primarily due to excitatory monosynaptic bulbospinal projections (Duffin and van Alphen 1995; Ellenberger and Feldman 1988; Ellenberger et al. 1990). Thus, in the context of “normal” breathing, cervical interneurons are likely to play only a relatively minor role and may serve to modulate phrenic motoneuron excitability. These cells may become more functionally important when bulbospinal pathways are disrupted [e.g., after spinal cord injury (Lane et al. 2008a; Lane et al. 2008b; Sandhu et al. 2009)] or during postural and other nonrespiratory behaviors involving the diaphragm (Butler et al. 2014; Grelot et al. 1993). Another consideration is that cervical interneurons may serve to coordinate phrenic and intercostal motor outputs (Bellingham 1999; Decima et al. 1967). For example, reflex changes in phrenic activity can be induced by intercostal muscle activation (Bellingham 1999; Remmers 1973), and some cervical interneurons are synaptically coupled to both intercostal and phrenic motoneurons (Lane et al. 2008b).
Response to hypoxia.
A considerable literature exists on the phrenic motoneuron response to hypoxia (Lee and Fuller 2011), but to our knowledge this is the first report of the cervical interneuron response to diminished oxygen. In our experiments, hypoxia did not cause non-respiratory-modulated cells to adopt a respiratory pattern nor did it cause a substantial change in the η2 measure of relative degree of respiratory bursting. Nevertheless, there was a robust and complex neuronal response to the hypoxic stimulus. Establishing the functional significance of the diverse neuronal response to hypoxia is beyond the scope of this study, and indeed will represent a particularly challenging experimental question. However, the salient point is that the great majority of recorded neurons (i.e., >80%) altered their bursting patterns during hypoxia and thus could be considered as “hypoxia sensitive.” These neurons could be responding directly to reduced oxygen and/or blood flow, or may be receiving synaptic inputs through hypoxic chemoreception pathways. It should be pointed out, however, that cells were also recorded that displayed no alteration in bursting during the hypoxic stimulus. Thus the midcervical spinal cord contains interneurons with a wide range of sensitivity to alterations in oxygen, and the complex but seemingly orchestrated response is likely to be a part of the autonomic and somatic motor response to reduced oxygen. We also noted that alterations in neuronal discharge patterns persisted beyond the period of hypoxia and have termed this response as “STP of cervical interneuron discharge,” to be consistent with established definitions of hypoxia-induced plasticity (Powell et al. 1998). One straightforward conclusion that can be drawn from the current data set is that the cervical spinal neuroplasticity triggered by hypoxia may not be unique to phrenic motoneurons. As the field of hypoxia-induced spinal respiratory plasticity moves forward, we suggest that a potential role for cervical interneurons should be incorporated into discussions and physiological models of cervical spinal neural plasticity (Devinney et al. 2013) and rehabilitation (Hayes et al. 2014; Trumbower et al. 2012).
Tonic phrenic motoneurons.
Compared with rhythmic phrenic motoneuron discharge (reviewed in Lee and Fuller 2011), relatively little is known about tonic discharge patterns in phrenic motoneurons. At least two prior publications, however, have reported phrenic motoneurons that fire across the entire respiratory cycle in a tonic manner (Lee et al. 2013; Marchenko et al. 2012). In the present study, tonically active cells were recorded at a location consistent with anatomical descriptions of phrenic motoneurons [i.e., midcervical spinal cord; 1.53 ± 0.02 mm beneath the dorsal surface of the spinal cord (Goshgarian and Rafols 1981; Lane et al. 2008b)], but more importantly these recordings produced a distinct, short-latency STA peak in the phrenic nerve recording during both the inspiratory and expiratory phase (e.g., Fig. 10). In a prior study from our group, tonic phrenic motoneuron bursting transformed into a phasic pattern when respiratory drive was increased by gradually raising inspired CO2 levels (Lee et al. 2013). In contrast, in the present study tonic phrenic motoneurons increased overall firing rate during hypoxia, but also maintained bursting throughout the respiratory cycle. Thus the impact of inspiratory drives on tonic phrenic motoneuron discharge remains an open question. Whether phrenic motoneurons discharge tonically during spontaneous breathing without anesthesia is also not clear (Gransee et al. 2013), but it should be noted that Butler and Gandevia concluded that tonic discharge of motor units is “rare in the diaphragm” in humans (Butler and Gandevia 2008). The present results confirm that phrenic motoneurons can burst tonically under certain conditions and suggest that this discharge pattern can be maintained even during respiratory challenge.
Conclusion.
Our data indicate that at least a small percentage of midcervical interneurons can impact phrenic motor output during breathing, and we suggest that these cells are in fact “pre-phrenic interneurons” as hypothesized by previous authors (Bellingham and Lipski 1990; Douse and Duffin 1993; Lane et al. 2008a; Lane et al. 2008b; Palisses et al. 1989). Collectively, the existing literature suggests that spinal interneurons are more prominently involved in regulating respiratory intercostal motor activity (Kirkwood et al. 1988; Kirkwood et al. 1993; Saywell et al. 2011; Schmid et al. 1993) compared with phrenic motor output. However, the current data as well as several recent reports (Alilain et al. 2008; Lane 2011; Lane et al. 2008b) indicate that propriospinal cervical neurons are also likely to have a role in regulating phrenic output. Delineating the precise functional significance of spinal interneurons to breathing poses an experimentally challenging problem, and these cells are very likely to play a role in coordinating diaphragm and trunk muscle activity during postural tasks (Butler et al. 2014; Grelot et al. 1993). Butler and colleagues recently made a compelling argument that “spinal integration” of corticospinal and bulbospinal inputs is a fundamental and important part of ventilatory control in humans (Butler et al. 2014). Interneurons, both in the cervical (present study) and thoracic spinal cord (Saywell et al. 2011) are likely to play a fundamental role in this process.
GRANTS
Financial support for this work was provided by the following grants from the National Institutes of Health: 1-R01-NS-080180-01A1 (D. D. Fuller) and 1-R01-NS-054025-06 (P. J. Reier). Support was also provided by the State of Florida Brain and Spinal Cord Injury Research Program administered through the McKnight Brain Institute at the University of Florida. M. S. Sandhu was supported by a fellowship grant from the Craig H. Neilsen Foundation (Grant no. 220521).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.S.S., D.M.B., J.C.S., P.J.R., and D.D.F. conception and design of research; M.S.S. and D.M.B. performed experiments; M.S.S., D.M.B., and N.G.M. analyzed data; M.S.S., D.M.B., J.C.S., P.J.R., and D.D.F. interpreted results of experiments; M.S.S. and D.D.F. prepared figures; M.S.S. drafted manuscript; M.S.S., D.M.B., N.G.M., and D.D.F. edited and revised manuscript; M.S.S., D.M.B., J.C.S., P.J.R., and D.D.F. approved final version of manuscript.
ACKNOWLEDGMENTS
Present address for M. S. Sandhu: Rehabilitation Institute of Chicago, 345 E. Superior St., Rm. 1435, Chicago, IL 60611.
REFERENCES
- Alilain WJ, Li X, Horn KP, Dhingra R, Dick TE, Herlitze S, Silver J. Light-induced rescue of breathing after spinal cord injury. J Neurosci 28: 11862–11870, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baekey DM, Molkov YI, Paton JF, Rybak IA, Dick TE. Effect of baroreceptor stimulation on the respiratory pattern: insights into respiratory-sympathetic interactions. Respir Physiol Neurobiol 174: 135–145, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baekey DM, Morris KF, Gestreau C, Li Z, Lindsey BG, Shannon R. Medullary respiratory neurones and control of laryngeal motoneurones during fictive eupnoea and cough in the cat. J Physiol 534: 565–581, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baekey DM, Morris KF, Nuding SC, Segers LS, Lindsey BG, Shannon R. Ventrolateral medullary respiratory network participation in the expiration reflex in the cat. J Appl Physiol (1985) 96: 2057–2072, 2004. [DOI] [PubMed] [Google Scholar]
- Bellingham MC. Synaptic inhibition of cat phrenic motoneurons by internal intercostal nerve stimulation. J Neurophysiol 82: 1224–1232, 1999. [DOI] [PubMed] [Google Scholar]
- Bellingham MC, Lipski J. Respiratory interneurons in the C5 segment of the spinal cord of the cat. Brain Res 533: 141–146, 1990. [DOI] [PubMed] [Google Scholar]
- Butler JE, Gandevia SC. The output from human inspiratory motoneurone pools. J Physiol 586: 1257–1264, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JE, Hudson AL, Gandevia SC. The neural control of human inspiratory muscles. Prog Brain Res 209: 295–308, 2014. [DOI] [PubMed] [Google Scholar]
- Christakos CN, Cohen MI, Sica AL, Huang WX, See WR, Barnhardt R. Analysis of recurrent laryngeal inspiratory discharges in relation to fast rhythms. J Neurophysiol 72: 1304–1316, 1994. [DOI] [PubMed] [Google Scholar]
- Dale EA, Ben Mabrouk F, Mitchell GS. Unexpected benefits of intermittent hypoxia: enhanced respiratory and nonrespiratory motor function. Physiology 29: 39–48, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JG, Kirkwood PA, Sears TA. The detection of monosynaptic connexions from inspiratory bulbospinal neurones to inspiratory motoneurones in the cat. J Physiol 368: 33–62, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decima EE, von Euler C, Thoden U. Spinal intercostal-phrenic reflexes. Nature 214: 312–313, 1967. [DOI] [PubMed] [Google Scholar]
- Devinney MJ, Huxtable AG, Nichols NL, Mitchell GS. Hypoxia-induced phrenic long-term facilitation: emergent properties. Ann NY Acad Sci 1279: 143–153, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins EG, Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rat. J Comp Neurol 347: 64–86, 1994. [DOI] [PubMed] [Google Scholar]
- Douse MA, Duffin J. Axonal projections and synaptic connections of C5 segment expiratory interneurones in the cat. J Physiol 470: 431–444, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douse MA, Duffin J, Brooks D, Fedorko L. Role of upper cervical inspiratory neurons studied by cross-correlation in the cat. Exp Brain Res 90: 153–162, 1992. [DOI] [PubMed] [Google Scholar]
- Duffin J, Iscoe S. The possible role of C5 segment inspiratory interneurons investigated by cross-correlation with phrenic motoneurons in decerebrate cats. Exp Brain Res 112: 35–40, 1996. [DOI] [PubMed] [Google Scholar]
- Duffin J, van Alphen J. Bilateral connections from ventral group inspiratory neurons to phrenic motoneurons in the rat determined by cross-correlation. Brain Res 694: 55–60, 1995. [DOI] [PubMed] [Google Scholar]
- Ellenberger HH, Feldman JL. Monosynaptic transmission of respiratory drive to phrenic motoneurons from brainstem bulbospinal neurons in rats. J Comp Neurol 269: 47–57, 1988. [DOI] [PubMed] [Google Scholar]
- Ellenberger HH, Feldman JL, Goshgarian HG. Ventral respiratory group projections to phrenic motoneurons: electron microscopic evidence for monosynaptic connections. J Comp Neurol 302: 707–714, 1990. [DOI] [PubMed] [Google Scholar]
- Giraudin A, Cabirol-Pol MJ, Simmers J, Morin D. Intercostal and abdominal respiratory motoneurons in the neonatal rat spinal cord: spatiotemporal organization and responses to limb afferent stimulation. J Neurophysiol 99: 2626–2640, 2008. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG, Rafols JA. The phrenic nucleus of th albino rat: a correlative HRP and Golgi study. J Comp Neurol 201: 441–456, 1981. [DOI] [PubMed] [Google Scholar]
- Gransee HM, Zhan WZ, Sieck GC, Mantilla CB. Targeted delivery of TrkB receptor to phrenic motoneurons enhances functional recovery of rhythmic phrenic activity after cervical spinal hemisection. PloS one 8: e64755, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grelot L, Milano S, Portillo F, Miller AD. Respiratory interneurons of the lower cervical (C4–C5) cord: membrane potential changes during fictive coughing, vomiting, and swallowing in the decerebrate cat. Pflügers Arch 425: 313–320, 1993. [DOI] [PubMed] [Google Scholar]
- Hayes HB, Jayaraman A, Herrmann M, Mitchell GS, Rymer WZ, Trumbower RD. Daily intermittent hypoxia enhances walking after chronic spinal cord injury: a randomized trial. Neurology 82: 104–113, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iscoe S, Duffin J. Effects of stimulation of phrenic afferents on cervical respiratory interneurones and phrenic motoneurones in cats. J Physiol 497: 803–812, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E. Spinal interneuronal networks in the cat: elementary components. Brain Res Rev 57: 46–55, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood PA. Synaptic excitation in the thoracic spinal cord from expiratory bulbospinal neurones in the cat. J Physiol 484: 201–225, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood PA, Munson JB, Sears TA, Westgaard RH. Respiratory interneurones in the thoracic spinal cord of the cat. J Physiol 395: 161–192, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood PA, Schmid K, Sears TA. Functional identities of thoracic respiratory interneurones in the cat. J Physiol 461: 667–687, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski KE, Hsieh YH, Dick TE, DiMarco AF. Diaphragm activation via high frequency spinal cord stimulation in a rodent model of spinal cord injury. Exp Neurol 247: 689–693, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krutki P, Jelen S, Jankowska E. Do premotor interneurons act in parallel on spinal motoneurons and on dorsal horn spinocerebellar and spinocervical tract neurons in the cat? J Neurophysiol 105: 1581–1593, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA. Spinal respiratory motoneurons and interneurons. Respir Physiol Neurobiol 179: 3–13, 2011. [DOI] [PubMed] [Google Scholar]
- Lane MA, Fuller DD, White TE, Reier PJ. Respiratory neuroplasticity and cervical spinal cord injury: translational perspectives. Trends Neurosci 31: 538–547, 2008a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, Lee KZ, Fuller DD, Reier PJ. Spinal circuitry and respiratory recovery following spinal cord injury. Respir Physiol Neurobiol 169: 123–132, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, White TE, Coutts MA, Jones AL, Sandhu MS, Bloom DC, Bolser DC, Yates BJ, Fuller DD, Reier PJ. Cervical prephrenic interneurons in the normal and lesioned spinal cord of the adult rat. J Comp Neurol 511: 692–709, 2008b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KZ, Dougherty BJ, Sandhu MS, Lane MA, Reier PJ, Fuller DD. Phrenic motoneuron discharge patterns following chronic cervical spinal cord injury. Exp Neurol 249: 20–32, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KZ, Fuller DD. Neural control of phrenic motoneuron discharge. Respir Physiol Neurobiol 179: 71–79, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey BG, Hernandez YM, Morris KF, Shannon R. Functional connectivity between brain stem midline neurons with respiratory-modulated firing rates. J Neurophysiol 67: 890–904, 1992. [DOI] [PubMed] [Google Scholar]
- Lipski J, Duffin J, Kruszewska B, Zhang X. Upper cervical inspiratory neurons in the rat: an electrophysiological and morphological study. Exp Brain Res 95: 477–487, 1993. [DOI] [PubMed] [Google Scholar]
- Lipski J, Kubin L, Jodkowski J. Synaptic action of R beta neurons on phrenic motoneurons studied with spike-triggered averaging. Brain Res 288: 105–118, 1983. [DOI] [PubMed] [Google Scholar]
- Lois JH, Rice CD, Yates BJ. Neural circuits controlling diaphragm function in the cat revealed by transneuronal tracing. J Appl Physiol 106: 138–152, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett-Barr MR, Satriotomo I, Muir GD, Wilkerson JE, Hoffman MS, Vinit S, Mitchell GS. Repetitive intermittent hypoxia induces respiratory and somatic motor recovery after chronic cervical spinal injury. J Neurosci 32: 3591–3600, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu F, Qin C, Foreman RD, Farber JP. Chemical activation of C1–C2 spinal neurons modulates intercostal and phrenic nerve activity in rats. Am J Physiol Regul Integr Comp Physiol 286: R1069–R1076, 2004. [DOI] [PubMed] [Google Scholar]
- Marchenko V, Ghali MG, Rogers RF. Motoneuron firing patterns underlying fast oscillations in phrenic nerve discharge in the rat. J Neurophysiol 108: 2134–2143, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna JE, Prusky GT, Whishaw IQ. Cervical motoneuron topography reflects the proximodistal organization of muscles and movements of the rat forelimb: a retrograde carbocyanine dye analysis. J Comp Neurol 419: 286–296, 2000. [DOI] [PubMed] [Google Scholar]
- Okada Y, Yokota S, Shinozaki Y, Aoyama R, Yasui Y, Ishiguro M, Oku Y. Anatomical architecture and responses to acidosis of a novel respiratory neuron group in the high cervical spinal cord (HCRG) of the neonatal rat. Adv Exp Med Biol 648: 387–394, 2009. [DOI] [PubMed] [Google Scholar]
- Oku Y, Okabe A, Hayakawa T, Okada Y. Respiratory neuron group in the high cervical spinal cord discovered by optical imaging. Neuroreport 19: 1739–1743, 2008. [DOI] [PubMed] [Google Scholar]
- Orem J, Dick T. Consistency and signal strength of respiratory neuronal activity. J Neurophysiol 50: 1098–1107, 1983. [DOI] [PubMed] [Google Scholar]
- Palisses R, Persegol L, Viala D. Evidence for respiratory interneurones in the C3–C5 cervical spinal cord in the decorticate rabbit. Exp Brain Res 78: 624–632, 1989. [DOI] [PubMed] [Google Scholar]
- Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respir Physiol 112: 123–134, 1998. [DOI] [PubMed] [Google Scholar]
- Qin C, Farber JP, Chandler MJ, Foreman RD. Chemical activation of C1–C2 spinal neurons modulates activity of thoracic respiratory interneurons in rats. Am J Physiol Regul Integr Comp Physiol 283: R843–R852, 2002. [DOI] [PubMed] [Google Scholar]
- Remmers JE. Extra-segmental reflexes derived from intercostal afferents: phrenic and laryngeal responses. J Physiol 233: 45–62, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice CD, Lois JH, Kerman IA, Yates BJ. Localization of serotoninergic neurons that participate in regulating diaphragm activity in the cat. Brain Res 1279: 71–81, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu MS, Dougherty BJ, Lane MA, Bolser DC, Kirkwood PA, Reier PJ, Fuller DD. Respiratory recovery following high cervical hemisection. Respir Physiol Neurobiol 169: 94–101, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saywell SA, Ford TW, Meehan CF, Todd AJ, Kirkwood PA. Electrophysiological and morphological characterization of propriospinal interneurons in the thoracic spinal cord. J Neurophysiol 105: 806–826, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid K, Kirkwood PA, Munson JB, Shen E, Sears TA. Contralateral projections of thoracic respiratory interneurones in the cat. J Physiol 461: 647–665, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tester NJ, Fuller DD, Fromm JS, Spiess MR, Behrman AL, Mateika JH. Long-term facilitation of ventilation in humans with chronic spinal cord injury. Am J Respir Crit Care Med 189: 57–65, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumbower RD, Jayaraman A, Mitchell GS, Rymer WZ. Exposure to acute intermittent hypoxia augments somatic motor function in humans with incomplete spinal cord injury. Neurorehabil Neural Repair 26: 163–172, 2012. [DOI] [PubMed] [Google Scholar]