Abstract
The extent to which motor learning is generalized across the limbs is typically very limited. Here, we investigated how two motor learning hypotheses could be used to enhance the extent of interlimb transfer. According to one hypothesis, we predicted that reinforcement of successful actions by providing binary error feedback regarding task success or failure, in addition to terminal error feedback, during initial training would increase the extent of interlimb transfer following visuomotor adaptation (experiment 1). According to the other hypothesis, we predicted that performing a reaching task repeatedly with one arm without providing performance feedback (which prevented learning the task with this arm), while concurrently adapting to a visuomotor rotation with the other arm, would increase the extent of transfer (experiment 2). Results indicate that providing binary error feedback, compared with continuous visual feedback that provided movement direction and amplitude information, had no influence on the extent of transfer. In contrast, repeatedly performing (but not learning) a specific task with one arm while visuomotor adaptation occurred with the other arm led to nearly complete transfer. This suggests that the absence of motor instances associated with specific effectors and task conditions is the major reason for limited interlimb transfer and that reinforcement of successful actions during initial training is not beneficial for interlimb transfer. These findings indicate crucial contributions of effector- and task-specific motor instances, which are thought to underlie (a type of) model-free learning, to optimal motor learning and interlimb transfer.
Keywords: interlimb, intermanual, model-based, model-free, generalization
transfer of motor learning across the limbs has been studied widely in various disciplines including psychology, kinesiology, neuroscience, and engineering. A proper understanding of the mechanisms underlying interlimb transfer can not only provide substantial insights into how the two brain hemispheres communicate with each other but also have implications for rehabilitation of unilateral motor impairment. However, interlimb transfer has not been utilized in rehabilitation settings, probably because its extent is very limited. Whereas transfer of motor learning across different movement conditions within the same arm can occur completely (Lei et al. 2013; Morton et al. 2001), the extent of interlimb transfer typically ranges from 10 to 60% (Joiner et al. 2013; Morton et al. 2001; Sainburg and Wang 2002; Taylor et al. 2011; Wang et al. 2011). Various neural mechanisms underlying interlimb transfer have been suggested (Anguera et al. 2007; Block and Celnik 2013; Carroll et al. 2014; Perez et al. 2007; Taylor and Heilman 1980), although it remains unknown why its extent is so limited.
Motor adaptation tasks, such as adapting to a rotated visual display during reaching movements, are frequently employed to study how motor learning generalizes across different conditions. One motor learning hypothesis, which recently gained much attention, posits that motor adaptation involves two distinct yet complementary processes: model-based learning in which improvements in motor performance are guided by a forward model of the environment that is updated based on prediction errors, and model-free learning in which learning is driven by reinforcement of successful actions (Haith and Krakauer 2013; Huang et al. 2011). A similar but somewhat different hypothesis posits that motor adaptation involves algorithmic learning, in which one successively improves a rule-based method of control, and instance-reliant learning, in which effector-specific instances are accrued during repeated performances of a motor task and automatically retrieved later to allow fast and automatized performances of the task (Lei and Wang 2014; Wang and Sainburg 2003, 2004). While the ideas of model-based learning and algorithmic learning are in line with each other, the ideas of model-free learning and instance-reliant learning are somewhat different, in that reinforcement of successful actions is considered necessary only for the model-free learning idea, while effector-specific instances are emphasized only for the instance-reliant learning idea.
The two aforementioned models of motor learning can be used to explain the phenomenon of limited interlimb transfer. According to the hypothesis involving model-based vs. model-free learning, both types of learning occur during initial training with one arm, but only the model-based learning can transfer to the other arm (Xu et al. 2012), thus limiting the extent of interlimb transfer. The hypothesis involving algorithmic vs. instance-reliant learning provides a similar explanation, that is, algorithmic learning can transfer across the arms, although instance-reliant learning cannot. However, there is a difference between the two explanations: whereas the former explanation does not clarify why model-free learning cannot transfer across the arms, the latter explanation clarifies this point by arguing that the algorithm can transfer across the arms because it is effector independent, but the instances cannot because they are effector specific.
In the present study, we conducted two experiments in which we attempted to increase the extent of interlimb transfer following visuomotor adaptation based on the two motor learning hypotheses. In experiment 1, we examined whether strengthening the formation of model-free learning during initial training could lead to a greater extent of interlimb transfer. Model-free learning was strengthened by providing binary error feedback regarding task success or failure during initial training. Providing binary error feedback, compared with vector error feedback that offers spatial information such as movement direction and amplitude, has been suggested to reinforce successful actions to a greater extent, thus strengthening model-free learning (Shmuelof et al. 2012). In experiment 2, we examined whether providing movement instances associated with one arm during initial training with the other arm could lead to a greater extent of interlimb transfer. The instances were provided by having the participants perform a reaching task repeatedly with one arm without receiving performance feedback (which prevented adapting to a visuomotor rotation with this arm) while they concurrently adapted to the rotation with the other arm. Taylor et al. (2011) have employed a similar experimental paradigm and demonstrated increased interlimb transfer.
EXPERIMENT 1
Materials and Methods
Subjects.
Subjects were 16 healthy young adults (18–30 yr old, right-handed). Subjects were paid for their participation. Informed consent approved by the Institutional Review Board of the University of Wisconsin - Milwaukee was solicited prior to participation. Subjects were randomly assigned to one of two groups (8 subjects per group).
Apparatus.
A robotic exoskeleton called KINARM (BKIN Technologies, Kingston, ON, Canada) was used to collect data. Subjects were seated on a chair facing a table with both arms supported horizontally. The KINARM was incorporated with a virtual reality system that projected visual targets on a horizontal display to make them appear in the same plane as the arm. Direct vision of the arm was blocked, and a cursor representing the index fingertip was provided to guide the subject's reaching movement. The position of arm segments was sampled at 1,000 Hz, low-pass filtered at 15 Hz, and differentiated to yield resultant velocity values. Data were processed and analyzed using MATLAB.
Experimental design.
In general, subjects performed rapid reaching movements from a start circle to a target (2 cm in diameter, 10 cm away from the start circle) repeatedly with either arm (Fig. 1A). They were instructed to move their index finger to the target rapidly and as straight as possible in response to a “go” signal and stop without correcting their movement. The experiment consisted of four sessions: baseline with the left arm and with the right arm, visuomotor adaptation with the left arm (training) and with the right arm (transfer). Each session consisted of 20, 20, 100, and 80 trials, respectively. In the baseline sessions, the subjects were familiarized with the general reaching movement with each arm; in the training and transfer sessions, they adapted to a visual display that was rotated 30 degrees counterclockwise about the start circle (i.e., hand movement made in the “12 o'clock” direction resulted in cursor movement made in the “11 o'clock” direction) with the left and the right arm, respectively. During the left-arm training session, the subjects were divided into two groups. In one group, they only received continuous visual feedback, which is equivalent to vector error feedback that provides detailed spatial information such as movement direction and amplitude; in the other group, they received continuous feedback for the first 50 trials, then binary error feedback about task success or failure for the next 50 trials. [The latter is analogous to the condition that Shmuelof et al. (2012) used to strengthen model-free learning.] The continuous visual feedback was provided in the form of a cursor representing the fingertip location throughout the movement, and the binary error feedback was provided in such a way that the color of the target changed to red or blue upon completion of the movement depending on whether the subject hit the target successfully or not (Fig. 1B). During the right-arm transfer session, all subjects received the continuous visual feedback only.
Fig. 1.
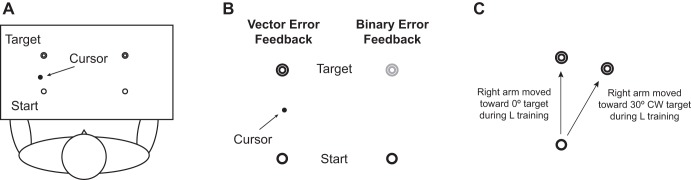
A: experimental setup. Target was displayed on 1 of 2 target locations, one for left and the other for right arm. B: experiment 1. In continuous visual feedback condition, a cursor representing fingertip location was shown throughout movement. In binary error (BE) feedback condition, no cursor was shown; instead, target color changed to red (success) or blue (failure) upon completion of reaching movement. C: experiment 2. One group of subjects reached toward 0-degree target (where target visually appeared), and another group toward 30-degree clockwise target (where they would reach toward following complete visuomotor adaptation) with right arm during left-arm training session.
Data analysis.
To examine performance accuracy, we calculated initial direction error (DE), which was the angular difference (in absolute terms) between a vector from the start circle to the target and another vector from the hand position at movement start to that at peak arm velocity. Using this measure, we also computed the percentage of interlimb transfer for each subject as follows: [(left arm DE at the first block of the training session − right arm DE at the first block of the transfer session)/(left arm DE at the first block of the training session − left arm DE at the last block of the training session)] × 100 (%). A block represents the mean of five consecutive trials.
Initial direction errors from the adaptation sessions were subjected to a repeated-measures ANOVA with group (vector error only, vector error + binary error) as a between-subject factor and block (the first and the last blocks of the training session, the first and the last blocks of the transfer session) as a within-subject factor (i.e., 2 × 4 ANOVA using the data from both arms together) to determine if there was any difference between the subject groups throughout the training and the transfer sessions.
Following this, two independent t-tests were conducted: one to determine if the extent of initial transfer from the left to the right arm was different between the subject groups, and the other to determine if the rate of visuomotor adaptation with the right arm following initial training with the left arm was different between the groups. The first t-test was conducted using the percentage of interlimb transfer. For the second t-test, a line of approximation was constructed for each subject in the two groups by fitting a logarithmic regression line to the right arm performance data, and the slope values were used to conduct the test. The alpha level was set at 0.017 (i.e., 0.05/3) for the three analyses after a Bonferroni correction was made, and at 0.05 for post hoc comparisons [Tukey's tests for between-group comparisons, Fisher's least significant difference (LSD) tests for within-group comparisons].
Results
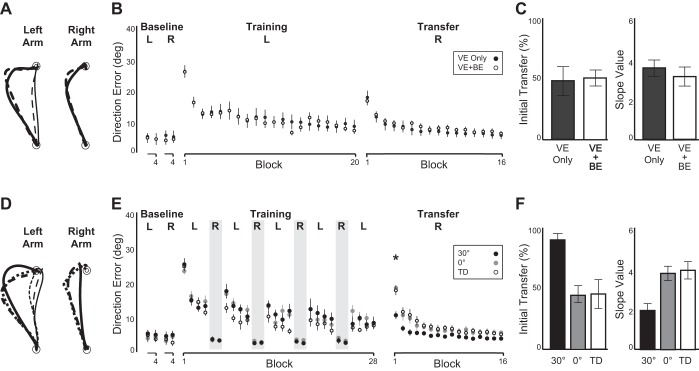
In experiment 1, all subjects adapted to the rotated visual display with the left arm first, then with the right arm. Table 1 shows the average movement duration and the average time to peak velocity of reaching movements in each condition separately. Figure 2A illustrates the hand-paths of a representative subject from the continuous visual feedback group and another from the binary error feedback group, both of whom demonstrated a largely curved hand-path at the beginning of the left-arm training session (left panel, thick lines), which became relatively straight by the end of the session (left panel, thin lines). The hand-paths at the beginning of the right-arm transfer session (right panel) were substantially straighter than those observed at the beginning of the left arm training session, although not as straight as those shown at the end of the left arm training session. These hand-paths suggest substantial, though incomplete, transfer of visuomotor adaptation from the left to the right arm in both subject groups.
Table 1.
Average movement duration (s) and time to peak velocity (s)
| Duration (±SE) | Time to Peak Velocity (±SE) | |
|---|---|---|
| Experiment 1 | ||
| VE only | 0.48 ± 0.21 | 0.25 ± 0.10 |
| VE + BE | 0.45 ± 0.32 | 0.23 ± 0.18 |
| Experiment 2 | ||
| 30 degrees | 0.46 ± 0.23 | 0.21 ± 0.14 |
| 0 degree | 0.45 ± 0.21 | 0.20 ± 0.11 |
| time delay | 0.46 ± 0.19 | 0.22 ± 0.09 |
VE, vector error; BE, binary error.
Fig. 2.
A–C, experiment 1. A: hand-paths from representative subjects observed on 1st (thick lines) and last (thin lines) trials with left arm during initial training session (left panel), and those observed on 1st trial with right arm during subsequent transfer session (right panel). Solid lines represent the group who received continuous visual feedback only [vector error (VE) only], broken lines the group who received continuous visual feedback for first 50 trials and binary error (VE) feedback for last 50 trials (VE + BE). B: mean performance measure. Every data point shown on x-axis represents the average of 5 consecutive trials (block) across all subjects within each group (means ± SE). C: percentages of initial transfer from left to right arm (left panel), and slope values during right-arm transfer session (right panel). Slope values obtained from nonlinear logarithmic regression equations were used to calculate the adaptation rate. D–F, experiment 2. D: hand-paths from representative subjects observed on first (thick lines) and last (thin lines) trials with left arm during initial training session (left panel), and those observed on first trial with right arm during subsequent transfer session (right panel). Solid lines represent the group who reached toward 30-degree target with right arm (30°), broken lines the group who reached toward 0-degree target with right arm (0°), dotted lines the group who did not move right arm during left-arm training session (Time Delay). E: mean performance measure, same as B (* < 0.05). F: percentages of initial transfer (left panel) and slope values (right panel), same as C. TD, time delay.
The repeated-measures ANOVA showed a significant main effect of block (P = 0.001). The post hoc analyses indicate that the direction errors at the first block of right arm performance were significantly smaller than those at the first block of left arm performance and significantly greater than those at the last block of either left or right arm performance (Fig. 2B). Neither the main effect of group nor the interaction effect between group and block was significant (P = 0.942 and 0.376, respectively). This indicates that the extent of interlimb transfer following visuomotor adaptation was not significantly different between the two subject groups. The lack of difference between the two subject groups was further confirmed by calculating the extent of initial transfer from the left-arm training to the right-arm transfer session (Fig. 2C, left panel), as well as the rate of right arm adaptation (i.e., slope value) (Fig. 2C, right panel), neither of which indicated a significant difference between the two groups (P = 0.83 and 0.607, respectively). Overall, these results suggest that the reinforcement of successful actions by providing binary error feedback has no beneficial effects over continuous visual feedback for increasing the extent of interlimb transfer.
EXPERIMENT 2
In this experiment, we investigated, according to the idea of instance-reliant learning, whether performing a reaching task repeatedly with one arm without providing performance feedback, while concurrently adapting to a visuomotor rotation with the other arm, would increase the extent of interlimb transfer.
Materials and Methods
Subjects.
Subjects were 24 healthy young adults (18–30 yr old, right-handed), who were randomly assigned to one of three groups (8 subjects per group). No subject tested in this experiment participated in experiment 1.
Apparatus.
The same apparatus used in experiment 1 was used in this experiment.
Experimental design.
As in experiment 1, experiment 2 also consisted of four sessions: baseline with the left arm and with the right arm, visuomotor adaptation with the left arm (training) and with the right arm (transfer). In the baseline sessions, the subjects were familiarized with the general reaching movement with each arm. In the training and transfer sessions, they adapted to a visual display that was rotated 30 degrees counterclockwise about the start circle with the left and the right arm, respectively. During the training session, however, some of the subjects also performed reaching movements with the right arm. Specifically, the subjects were divided into three groups. Two of the three groups performed reaching movements with the right arm in either the 0-degree direction (i.e., 12 o'clock direction, in which the hand would move prior to visuomotor adaptation) or the 30-degree clockwise direction (i.e., 1 o'clock direction, in which the hand would move following complete adaptation) for 10 trials after every 20 adaptation trials with the left arm (Fig. 1C). Visual feedback (continuous visual feedback) was provided for the left arm performances, but not for the right arm performances, during the training session. This allowed specific instances that were associated with the task to be performed later with the right arm (in the transfer session) to be accrued in advance, while preventing the right arm from adapting to the visuomotor rotation during the training session. The last group (named “time delay” group) took a short break (1 min) each time the other groups performed right arm movements for 10 trials. During the transfer session, all subjects received continuous visual feedback. Each of the four sessions (baseline with the left and the right arms, adaptation with the left and the right arms) consisted of 20, 20, 140 (100 for the left, 40 for the right arm), and 80 trials, respectively.
Data analysis.
As in experiment 1, we calculated initial direction error, on the basis of which we also computed the percentage of interlimb transfer for each subject. Initial direction errors from the adaptation sessions were subjected to a 3 × 4 repeated-measures ANOVA with group (0 degree, 30 degree, time-delay) as a between-subject factor and block (the first and the last blocks of the training session, the first and the last blocks of the transfer session) as a within-subject factor to determine if there was any difference among the subject groups throughout the training and the transfer sessions.
Following this, two simple ANOVAs with group as a between-subject factor were conducted: one, using the percentage of interlimb transfer, to determine if the extent of initial transfer from the left to the right arm was different among the subject groups; and the other, using the slope values from regression lines, to determine if the rate of visuomotor adaptation with the right arm following initial training with the left arm (i.e., slope value) was different among the groups.
Finally, we conducted an additional repeated-measures ANOVA with trial as a within-subject factor to determine how the right arm performance changed across the first few trials during the transfer session in the subject group who performed reaching movements toward the 30-degree target with the right arm during the initial left-arm training. For this analysis, we included the left arm data from the last five trials of the training session along with the right arm data from the first five trials of the transfer session. The alpha level was set at 0.0125 (i.e., 0.05/4) for the four ANOVAs after a Bonferroni correction was made and at 0.05 for post hoc comparisons (Tukey's tests for between-group comparisons, Fisher's LSD tests for within-group comparisons).
Results
Table 1 shows the average movement duration and the average time to peak velocity of reaching movements in each condition separately. Figure 2D illustrates the hand-paths of a representative subject from each of the three subject groups in experiment 2. The hand-paths during the left-arm training session were similar across the subjects, in that they were largely curved at the beginning (left panel, thick lines), but became relatively straight by the end of the session (left panel, thin lines). During the right arm transfer session, the hand-paths upon initial exposure to the visual rotation appeared different across the subjects, in that the subject who performed reaching movements toward the 30-degree target with the right arm during initial training showed relatively straight hand-paths from the beginning of the transfer session (right panel, solid line), whereas the other subjects' hand-paths were noticeably more curved. These hand-paths suggest that the extent of interlimb transfer following visuomotor adaptation may differ across the subject groups.
We quantified the difference by subjecting direction error measures to a repeated-measures ANOVA, which revealed a significant interaction effect between group and block (P = 0.004, Fig. 2E). Post hoc analyses indicate that the direction errors at the first block of the transfer session were significantly smaller than those at the first block of the training session in all subject groups. However, the errors at the last block of the training session and those at the first block of the transfer session were not significantly different in the group who performed reaching movements toward the 30-degree target with the right arm during initial training, whereas they were significantly different in the other two groups. Simple ANOVAs also revealed a significant effect of group for the percentage of initial transfer and the slope value (P = 0.002 and 0.002, respectively) (Fig. 2F). Post hoc comparisons indicate that the percentage of transfer observed in the group who performed reaching movements toward the 30-degree target with the right arm during initial training was significantly higher than that observed in the other two groups, and the mean slope value obtained from the former group was significantly lower than that of the other two groups. This indicates that the extent of interlimb transfer can increase substantially when movement instances directly associated with the task to be learned (i.e., 30-degree target direction) can be accrued during initial training.
With regard to the subjects who performed reaching movements toward the 30-degree target with the right arm during initial training, their right arm performance at the beginning of the transfer session was already superb, yet it still improved for the first couple of blocks. In fact, the data from the first 10 trials of the transfer session indicated that the direction errors decreased substantially within the first few trials (Fig. 3). Thus, we performed a trial-by-trial analysis using the left arm data from the last five trials of the training session and the right arm data from the first five trials of the transfer session of this subject group. The repeated-measures ANOVA indicated a significant effect of trial (P = 0.006). The post hoc comparisons showed that the direction error on the last trial of the training session was not significantly different from the error on any of the first five trials of the transfer session, and the error on the third trial from the last of the training session (trial 138), which was the lowest among the five trials, was significantly different only from the first two trials of the transfer session. This indicates that the direction errors in the transfer session approximated the lowest error observed in the training session within the first three trials.
Fig. 3.
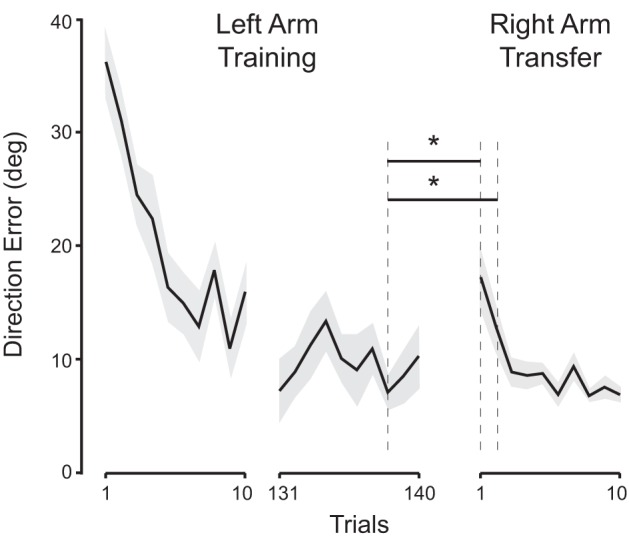
Trial-by-trial analysis. Thick black lines indicate mean direction errors (DE), shaded gray areas indicate SE. DE on last trial of left-arm training session was not significantly different from DE on any trial of right-arm transfer session; DE on trial 138, which was lowest in left-arm training session, was significantly different from DE on trials 1 and 2 of right-arm transfer session (* < 0.05).
DISCUSSION
In this study, we investigated how two motor learning hypotheses (one that involves model-free learning, another that involves instance-reliant learning) could explain the phenomenon of limited interlimb transfer. In experiment 1, we predicted based on the former hypothesis that reinforcement of successful actions would increase the extent of interlimb transfer following visuomotor adaptation. To reinforce successful actions, we provided continuous visual feedback for the first 50 trials and then binary error feedback for the next 50 trials. Shmuelof et al. (2012) suggest that providing binary error feedback once visuomotor adaptation reached asymptote would promote reinforcement learning. Our subjects were provided with the binary error feedback at asymptote, yet the extent of transfer was similar between the subject groups. This suggests that the reinforcement of successful actions using binary error feedback regarding performance success or failure provides no benefit for interlimb transfer. This is in line with the finding reported by Xu et al. (2012), who also compared two similar conditions (i.e., one in which subjects received both vector and binary errors, the other in which subjects only received binary errors later) and observed no difference between the two conditions in terms of interlimb transfer. The only difference between Xu et al.'s findings and our current findings is that we observed ∼50% transfer from the left to the right arm, but they observed minimal transfer from the right to the left arm. The experimental setup employed by Xu et al. might have been similar to that employed in our previous study, which demonstrated substantial transfer from the left to the right arm, but no transfer from the right to the left arm (Sainburg and Wang 2002).
In experiment 2, we predicted based on the instance-reliant learning hypothesis that providing effector-specific instances (i.e., by performing the reaching task repeatedly with the right arm without providing performance feedback during initial training with the left arm) would increase the extent of interlimb transfer. Here, we assumed that visuomotor adaptation would not occur with the right arm because visual feedback was not available and also that interlimb transfer would not occur during the training session because the trained direction was different between the two arms (i.e., 0-degree target for the left, 30-degree target for the right arm). Even within the same arm, the literature indicates, sensorimotor adaptation does not transfer across different directions very easily (e.g., Krakauer et al. 2000; Mattar and Ostry 2010). In fact, the right arm performance did not change much at all throughout the training session. This confirms that adaptation did not occur with the right arm and also indicates that the left arm adaptation that was occurring through the training session did not influence it either. If it did, the right arm performance in the training session would have demonstrated an increase in direction error. Following the training session, our data revealed substantially greater transfer in the subject group who reached toward the 30-degree target with the right arm during the training session compared with the other groups. The percentage of initial transfer in the former group was >90%, while that in the other groups was <50%. (We have also computed the percentage of initial transfer using the direction errors after they were subtracted by the mean direction errors from the last baseline block for each arm. The percentage of transfer in the former group was ∼80% and that in the other groups ∼40%.) This is consistent with the findings reported by Taylor et al. (2011), who demonstrated ∼50% of transfer from the right to the left arm following visuomotor adaptation when their subjects also performed reaching movements with the left arm, without visual feedback, during the right-arm training session. The reported extent of transfer is greater than the extent typically reported in interlimb transfer studies that employed similar visuomotor adaptation tasks (∼25%). The extent of transfer in our 30-degree target condition was even greater, probably because our subjects only experienced a single target (Taylor et al.'s subjects experienced 8 targets) and also because the target direction experienced with the right arm during the training session was associated with the target direction to be learned in the transfer session. In Taylor et al.'s study, the target direction experienced with the right arm during the training session was not associated with the direction to be learned but, rather, with the direction toward the actual target location (i.e., their paradigm was analogous to our 0-degree target condition).
Our data from the trial-by-trial analysis are also noteworthy. They indicate that the direction error on the first trial of the transfer session was not statistically different from that on the last trial of the training session. This indicates nearly complete transfer of visuomotor adaptation from the trained left to the untrained right arm. Interestingly, the right arm performance in the transfer session still showed additional improvements during the first few trials. The direction error on the first trial of the transfer session was significantly larger than the lowest direction error observed during the training session (trial 138), but the error on the third trial of the transfer session was no longer different from the lowest error in the training session. This is similar to our previous finding that visuomotor adaptation with one arm did not lead to a reduction of direction errors in the subsequent performance with the other arm until the second trial (Wang and Sainburg 2003). In that study, we demonstrated that interlimb transfer of visuomotor adaptation occurred when the direction of visuomotor rotations was extrinsically, but not intrinsically, consistent between the arms. However, the direction error on the first trial of the transfer session was very similar to that on the first trial of the training session regardless of the consistency of rotation directions between the arms. This finding, along with our current finding, suggests that the motor system may use the first few trials to probe whether the movement information obtained during a previous condition is useful or not for subsequent performance in another condition, when these conditions involve different motor effectors.
Our findings demonstrate, for the first time, that interlimb transfer of visuomotor adaptation can occur (nearly) completely, which can be explained by the idea of algorithmic and instance-reliant learning (Lei and Wang 2014; also see Logan 1988 for the original idea). When an individual adapts to a novel sensorimotor condition, not only an algorithm, which is equivalent to an internal forward model (Kawato 1999; Wolpert and Miall 1996), is developed, but also a set of instances are accrued. Here, the instances are thought to be effector specific, such that the adaptation acquired with the left arm results in the accrual of instances specifically associated with the left arm, and that acquired with the right arm in the accrual of instances associated with the right arm. The algorithm developed during initial training with one arm can be used by the motor system to facilitate subsequent performance with the other, because the algorithm is thought to be effector independent. However, the extent of interlimb transfer is typically limited because the instances accrued during initial training are associated with one particular arm, and they cannot be used to facilitate subsequent performance with the other arm, thus resulting in limited interlimb transfer. This interpretation is in agreement with the argument made by Morton et al. (2001), that interlimb generalization involves an internal representation that is effector independent, whereas intralimb generalization involves a separate effector-specific representation. This is also line with the fact that certain factors that can influence intralimb generalization following visuomotor adaptation, such as the number of target directions (e.g., 1 vs. 8 targets) and the length of initial training (e.g., 160 vs. 400 trials), do not have the same effects on interlimb generalization (Joiner et al. 2013; Lei and Wang 2014; Wang and Sainburg 2004).
An alternative explanation for limited interlimb transfer has been suggested by Carroll et al. (2014), who demonstrated that matching the coordinate frames of the visual display and the limb configuration between the arms appropriately could lead to a great extent of interlimb transfer following visuomotor adaptation (i.e., up to 86%). Based on this finding, the authors argued that the visual display should be extrinsically consistent, and the limb configuration intrinsically consistent, between the arms for the transfer to occur completely. In that study, however, the authors employed an isometric force control task. When they employed a reaching task in another study, the extent of transfer was much smaller (i.e., 51% or less) (Carroll et al. 2013). Given this, along with the fact that we did not manipulate the limb configurations in the present study, it seems unlikely that unmatched coordinate frames between the arms is the primary reason for limited interlimb transfer.
Our current findings provide additional insights into the neural processes underlying motor adaptation per se. Krakauer and colleagues (Haith and Krakauer 2013; Huang et al. 2011) argue that motor adaptation involves both model-based and model-free learning, which provide the motor system with robustness and redundancy. We agree with, and strengthen, that argument by demonstrating the importance of instance-reliant learning. In fact, instance-reliant learning is also a sort of “model-free” learning, because it does not involve any computational model that requires the updating of a control policy based on prediction errors. However, the idea of instance-reliant learning is somewhat different from that of model-free learning promoted by Krakauer and colleagues. The former idea emphasizes the assumption that this type of learning is effector and task dependent, while the latter does not. Also, the latter idea emphasizes the importance of reinforcing successful actions, while the former does not. Here, we compare between these two ideas not to argue that one idea is superior to the other but, rather, to suggest that the current notion of model-free learning may need to be modified. Our current findings suggest that model-free learning involves a neural representation that is effector specific (e.g., instances associated with a specific arm) and task specific (e.g., instances associated with a specific target direction) and that reinforcement of successful actions may not be as critical as it is thought to be for this learning mechanism.
Instance-reliant learning is also related to, and can explain, another type of model-free learning called use-dependency, which refers to a phenomenon that current movements are often biased to become similar to previously experienced movements (Classen et al. 1998; Diedrichsen et al. 2010; Verstynen and Sabes 2011). Instances from previous movements would already be available while those from current movements were being accrued, and a competition might occur between the two sets of instances in such a way that the instances associated with previous movements would be automatically retrieved (Logan 1988), thus causing current movements to be biased toward previous movements, until the instances associated with current movements were accrued sufficiently. This explanation is consistent with the neurophysiological findings reported by Billalta et al. (2013), which suggest that a brain region involved in model-free learning deals with competitions between different sets of motor memories. These authors demonstrate that depressing corticospinal excitability by applying repetitive transcranial magnetic stimulation (rTMS) over the primary motor cortex (M1) following initial motor adaptation, and prior to washout trials, increased the amount of savings during readaptation. They argue that rTMS over M1, a region thought to underlie both use-dependent and model-free learning (Diedrichsen et al. 2010; Haith and Krakauer 2013), prevented a competition between motor memories at recall, one associated with the motor adaptation and the other associated with the washout trials. Their study, along with other studies that suggest the coexistence of multiple memories (Krakauer 2009; Ogawa and Imamizu 2013; Pekny et al. 2011), thus supports the idea that multiple motor memories, or instances, stored in M1 can compete with each other for retrieval.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.W., Y.L., and J.R.B. conception and design of research; J.W. and Y.L. analyzed data; J.W. interpreted results of experiments; J.W. and Y.L. prepared figures; J.W. drafted manuscript; J.W., Y.L., and J.R.B. edited and revised manuscript; J.W., Y.L., and J.R.B. approved final version of manuscript; Y.L. performed experiments.
REFERENCES
- Anguera JA, Russell CA, Noll DC, Seidler RD. Neural correlates associated with intermanual transfer of sensorimotor adaptation. Exp Brain Res 1185: 136–151, 2007. [DOI] [PubMed] [Google Scholar]
- Billalta JI, Landi SM, Flo A, Della-Maggiore V. Extinction interferes with the retrieval of visuomotor memories through a mechanism involving the sensorimotor cortex. Cereb Cortex: bht346, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block H, Celnik P. Stimulating the cerebellum affects visuomotor adaptation but not intermanual transfer of learning. Cerebellum 12: 781–793, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll T, Poh E, Duarte Ferreira T, de Rugy A. Inter-limb generalization of visuomotor adaptation is similar for sagittal and horizontal plane reaching. Program No. 471.13. Society for Neuroscience 2013, Society for Neuroscience: San Diego. [Google Scholar]
- Carroll T, Poh E, de Rugy A. New visuomotor maps are immediately available to the opposite limb. J Neurophysiol 111: 2232–2243, 2014. [DOI] [PubMed] [Google Scholar]
- Classen J, Liepert J, Wise SP, Hallett M, Cohen LG. Rapid plasticity of human cortical movement representation induced by practice. J Neurophysiol 79: 1117–1123, 1998. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, White O, Newman D, Lally N. Use-dependent and error-based learning of motor behaviors. J Neurosci 30: 5159–5166, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haith AM, Krakauer JW. Model-based and model-free mechanisms of human motor learning. Adv Exp Med Biol 782: 1–21, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang VS, Haith A, Mazzoni P, Krakauer JW. Rethinking motor learning and savings in adaptation paradigms: model-free memory for successful actions combines with internal models. Neuron 70: 787–801, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner WM, Brayanov JB, Smith MA. The training schedule affects the stability, not the magnitude, of the interlimb transfer of learned dynamics. J Neurophysiol 110: 984–998, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawato M. Internal models for motor control and trajectory planning. Curr Opin Neurobiol 9: 718–727, 1999. [DOI] [PubMed] [Google Scholar]
- Krakauer JW, Pine ZM, Ghilardi MF, Ghez C. Learning of visuomotor transformations for vectorial planning of reaching trajectories. J Neurosci 20: 8916–8924, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW. Motor learning and consolidation: the case of visuomotor rotation. Adv Exp Med Biol 629: 405–421, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y, Wang J. Prolonged training does not result in a greater extent of interlimb transfer following visuomotor adaptation. Brain Cog 91: 95–99, 2014. [DOI] [PubMed] [Google Scholar]
- Lei Y, Johnson MJ, Wang J. Separation of visual and motor workspaces during targeted reaching results in limited generalization of visuomotor adaptation. Neurosci Lett 541: 243–247, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD. Toward an instance theory of automatization. Psychol Rev 95: 492, 1988. [Google Scholar]
- Mattar AA, Ostry DJ. Generalization of dynamics learning across changes in movement amplitude. J Neurophysiol 104: 426–438, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton SM, Lang CE, Bastian AJ. Inter-and intra-limb generalization of adaptation during catching. Exp Brain Res 141: 438–445, 2001. [DOI] [PubMed] [Google Scholar]
- Ogawa K, Imamizu H. Human sensorimotor cortex represents conflicting visuomotor mappings. J Neurosci 33: 6412–6422, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekny SE, Criscimagna-Hemminger SE, Shadmehr R. Protection and expression of human motor memories. J Neurosci 31: 13829–13839, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez MA, Tanaka S, Wise SP, Sadato N, Tanabe HC, Willingham DT, Cohen LG. Neural substrates of intermanual transfer of a newly acquired motor skill. Curr Biol 17: 1896–1902, 2007. [DOI] [PubMed] [Google Scholar]
- Sainburg RL, Wang J. Interlimb transfer of visuomotor rotations: independence of direction and final position information. Exp Brain Res 145: 437–447, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuelof L, Huang VS, Haith AM, Delnicki RJ, Mazzoni P, Krakauer JW. Overcoming motor “forgetting” through reinforcement of learned actions. J Neurosci 32: 14617–14621, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor HG, Heilman KM. Left-hemisphere motor dominance in righthanders. Cortex 16: 587–603, 1980. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Wojaczynski GJ, Ivry RB. Trial-by-trial analysis of intermanual transfer during visuomotor adaptation. J Neurophysiol 106: 3157–3172, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstynen T, Sabes PN. How each movement changes the next: an experimental and theoretical study of fast adaptive priors in reaching. J Neurosci 31: 10050–10059, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sainburg RL. Mechanisms underlying interlimb transfer of visuomotor rotations. Exp Brain Res 149: 520–526, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sainburg RL. Limitations in interlimb transfer of visuomotor rotations. Exp Brain Res 155: 1–8, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Joshi M, Lei Y. The extent of interlimb transfer following adaptation to a novel visuomotor condition does not depend on awareness of the condition. J Neurophysiol 106: 259–264, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert DM, Miall RC. Forward models for physiological motor control. Neural Netw 9: 1265–1279, 1996. [DOI] [PubMed] [Google Scholar]
- Xu J, Haith AH, Okolie J, Krakauer JW. Is inter-manual transfer of motor learning model-based or model free? Program No. 679.19. Society for Neuroscience 2012, Society for Neuroscience: New Orleans. [Google Scholar]