Abstract
The caudal cingulate motor area (CMAc) and the supplementary motor area (SMA) play important roles in movement execution. The present study aimed to characterize the functional organization of these regions during movement by investigating laterality representations in the CMAc and SMA of monkeys via an examination of neuronal activity during a button press movement with either the right or left hand. Three types of movement-related neuronal activity were observed: 1) with only the contralateral hand, 2) with only the ipsilateral hand, and 3) with either hand. Neurons in the CMAc represented contralateral and ipsilateral hand movements to the same degree, whereas neuronal representations in the SMA were biased toward contralateral hand movement. Furthermore, recording neuronal activities using a linear-array multicontact electrode with 24 contacts spaced 150 μm apart allowed us to analyze the spatial distribution of neurons exhibiting particular hand preferences at the submillimeter scale. The CMAc and SMA displayed distinct microarchitectural organizations. The contralateral, ipsilateral, and bilateral CMAc neurons were distributed homogeneously, whereas SMA neurons exhibiting identical hand preferences tended to cluster. These findings indicate that the CMAc, which is functionally organized in a less structured manner than the SMA is, controls contralateral and ipsilateral hand movements in a counterbalanced fashion, whereas the SMA, which is more structured, preferentially controls contralateral hand movements.
Keywords: macaque, bimanual movement, linear-array multicontact electrode, microarchitecture
four motor areas have been identified in the medial aspect of the primate frontal cortex: the supplementary motor area (SMA), the presupplementary motor area (pre-SMA), the rostral cingulate motor area (CMAr), and the caudal cingulate motor area (CMAc) (Amiez and Petrides 2014; Dum and Strick 1991; Matelli et al. 1991; Picard and Strick 1996; Tanji 1996; Vogt 2009; Vogt et al. 1987). The pre-SMA and CMAr are only sparsely interconnected with the primary motor cortex (M1) and the spinal cord (Hatanaka et al. 2003; Lu et al. 1994; Shima et al. 1991; Wang et al. 2001, 2004), but the SMA and CMAc exhibit strong interconnections with M1 (Hatanaka et al. 2003; Lu et al. 1994; Morecraft and Van Hoesen 1992; Shima et al. 1991; Wang et al. 2001) and the spinal cord (Dum and Strick 1991; He et al. 1995; Hutchins et al. 1988; Maier et al. 2002). This suggests that the CMAc and SMA are located hierarchically closer to the output stage of motor control. In fact, studies of single-cell activity in monkeys have revealed that both the CMAc and SMA are involved in the generation of arm movements (Backus et al. 2001; Cadoret and Smith 1997; Crutcher et al. 2004; Nachev et al. 2008; Nakajima et al. 2013; Paus 2001; Picard and Strick 1996; Shima et al. 1991; Shima and Tanji 1998; Tanji 1994).
A number of studies have assessed the response properties of neurons in the CMAc and SMA. For example, Kermadi et al. (1998, 2000) demonstrated that neuronal activities in the CMAc and SMA are similarly modulated during unilateral and bilateral arm movements, and Russo et al. (2002) found that neurons in these regions exhibit vision-, set-, and movement-related activities. However, although it is evident that the directional selectivity of movement is greater in the SMA than in the CMAc (Richardson et al. 2008), the specific functional roles played by the CMAc and SMA remain largely elusive.
In human neuroimaging studies, the CMAc and SMA are active when subjects move either the ipsilateral or the contralateral hand (Babiloni et al. 2003; Baraldi et al. 1999; Diedrichsen et al. 2006; Immisch et al. 2001; Kollias et al. 2001; Naito et al. 2007; Roland et al. 1980). This suggests that the CMAc and SMA of a single hemisphere are involved with the movement of either hand. Similarly, single-cell studies in monkeys have shown that neurons in the SMA are active when subjects pull a lever with either arm (Brinkman and Porter 1979) and that the SMA is involved in the planning and execution of both single and bimanual hand movements in subjects trained to press a small button with their right hand, left hand, or both hands together (Tanji et al. 1987, 1988). However, to date, no studies have investigated the involvement of individual CMAc neurons in hand movements. Thus the present study examined neuronal activity in the CMAc and SMA of monkeys during the performance of a button press movement with either the right or left hand.
The present study had two major findings. First, an analysis of neuronal response selectivity revealed that the CMAc represents contralateral and ipsilateral hand movements in a counterbalanced manner to a greater extent than does the SMA. Second, an analysis of the spatial distribution of selective neurons within a small volume (spatial resolution: ∼150 μm) revealed that CMAc neurons selective for the movement of either hand or both hands in this region are intermingled homogeneously, whereas SMA neurons with same selectivity tend to form a cluster.
MATERIALS AND METHODS
Animals and apparatus.
Two male monkeys (Macaca fuscata) weighing 8.0 kg (monkey 1) and 10.0 kg (monkey 2) were used in the present study. They were cared for as prescribed by the National Institutes of Health guidelines and the guidelines of the Tokyo Metropolitan Institute of Medical Science. All animal care and experimental procedures were approved by the Animal Care and Use Committee of Tokyo Metropolitan Institute of Medical Science. During the experimental sessions, each monkey sat in a primate chair with its head restrained and with small push buttons (23 mm in diameter) beneath each hand. Both forelimbs were comfortably restrained with straps, and the monkey could easily press the buttons using only hand movement. A 19-in. LCD monitor was placed 22 cm in front of the monkey, and its eye positions were monitored at 240 Hz with an infrared eye-tracking system (resolution: 0.25° visual angle; RHS-M; Applied Science Laboratories, Bedford, MA). The TEMPONET system (Reflective Computing, Olympia, WA) was used to control the behavioral task and the opening and closing of a solenoid valve serially installed in the reward delivery system.
Surgery.
Before the initiation of physiological recording, anesthesia was induced in the monkeys with ketamine hydrochloride (10 mg/kg im) and atropine sulfate. The monkeys then underwent aseptic surgery while anesthetized with pentobarbital sodium (20–25 mg/kg iv), and antibiotics and analgesics were used to prevent infection and pain. During the surgery, polycarbonate and titanium screws were implanted in the skulls of the monkeys, and two plastic pipes were rigidly attached with acrylic resin. A section of the skull of each monkey over the left frontal lobe was removed, and a recording chamber was implanted to permit access to the CMAc and SMA.
Behavioral paradigm.
The monkeys were trained to press a small button using the right or left hand or to press both buttons using both hands according to visual go signals on the LCD display (Fig. 1A). A trial was initiated when the monkey gazed at a fixation point (white circle, 1.4° visual angle), and if the monkey continued to fixate on this point for 500 ms, it disappeared and a visual go signal (white square, 2.7°) was presented in conjunction with a 1,000-Hz tone. The visual go signal, which appeared to the right, to the left, or on both sides of the fixation point (4.1° from center), instructed the monkey to press the right button, left button, or both buttons, respectively. Furthermore, the go signals were presented either once (i.e., requiring a single movement) or twice (i.e., requiring two movements). In the trials requiring two movements, each of the first and second go signals was randomly selected from the three movement types, resulting in nine combinations of the first and second movements. Of these trial types, the present study focused on trials in which a single movement of either the left or right hand was required.
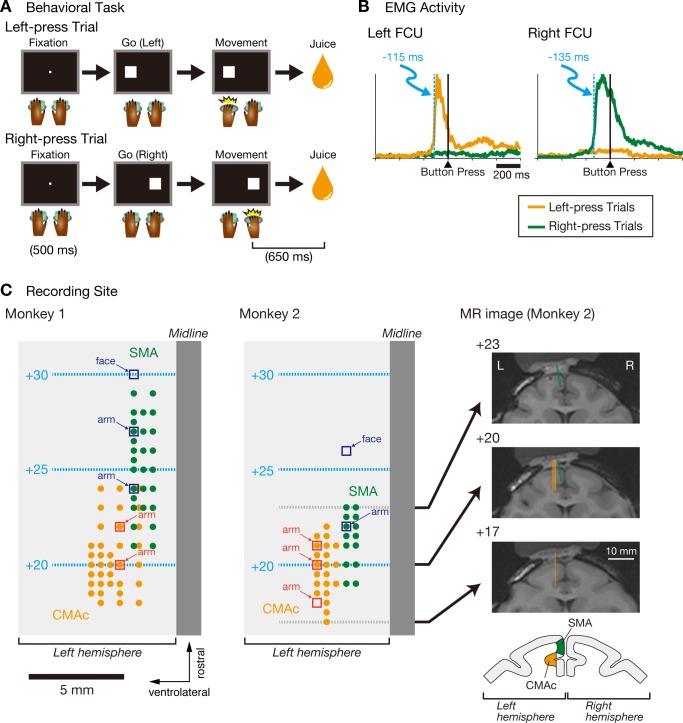
Fig. 1.
Behavioral task, muscle activity data, and recording sites. A: temporal sequence of the behavioral task in which monkeys were required to press a button with their left (top) or right hand (bottom). B: examples of electromyographic (EMG) activity shown in the flexor carpi ulnaris (FCU) muscles of the left and right arms, respectively. Activities in the left-press trials (orange) and right-press trials (green) are aligned with the performance of the button press. EMG activity was smoothed with a boxcar average of 11 time bins (i.e., 11 ms), and the light blue dashed lines indicate the first of 10 consecutive bins (10 ms) that exhibited a significant difference between the left-press and right-press trials (Welch's t-test, α = 0.01). The tick marks on the horizontal axis are placed at 200-ms intervals. C, left and middle: top views of the surfaces of the left frontal cortices of the 2 monkeys. The orange circles indicate the sites of penetration where the caudal cingulate motor area (CMAc) neurons were recorded; the electrode was advanced vertically in the dorsoventral direction. The green circles indicate the sites of penetration where the supplementary motor area (SMA) neurons were recorded; the electrode was inclined at a 10° angle lateral to the vertical axis. Open squares indicate sites where intracortical microstimulation (ICMS) was used to evoke movement (44 cathodal pulses, 200-μs width at 333 Hz, ≤80 μA) or where neurons responded to somatosensory stimuli in the CMAc (red) or SMA (blue); the corresponding body parts are labeled. The light blue dashed lines and numbers indicate the distance (mm) anterior to the Horsley-Clarke interaural plane. Right, coronal magnetic resonance images taken +17, +20, and +23 mm anterior to the Horsley-Clarke interaural plane in monkey 2. The thick lines indicate sites where electrodes penetrated the CMAc (orange) and SMA (green). Scale bars, 10 mm. L, left hemisphere; R, right hemisphere. Schematic at bottom right shows where neurons were recorded in the CMAc (orange) and SMA (green).
The monkey was required to respond promptly, i.e., within 3.0 s, after each visual go signal with the tone was presented. During the single-movement trials, a correct response resulted in immediate removal of the visual go signal and the tone, followed 650 ms later by delivery of a drop of apple juice as a reward; the reward was followed by an intertrial interval of ≥3 s. If there was an incorrect response (pressing the button opposite to the go signal) or the monkey simultaneously pressed the right and left buttons (within 150 ms of each other), the visual go signal immediately disappeared, and a low tone (153 Hz) was presented for 0.5 s to indicate that the monkey had made an error. In this case, an intertrial interval of ≥3 s occurred without any reward. Identical visual go signals were presented successively in a block.
In addition to the trials in which the visual go signals were presented with the tone (visible condition), 60% of trials in the latter part of each block (>10 successful trials) were presented with the 1,000-Hz tone (auditory go signal) in the absence of a visual go signal (invisible condition). Under the invisible condition, the monkeys were still able to perform the task because the identical movement had been previously executed in that particular block of trials. In total, in >90% of cases, each block consisted of 13–21 trials. When each block ended, 5 bursts of the 1,000-Hz tone were presented to indicate that a new block of trials would follow. The types of movement in the subsequent blocks were selected randomly.
Physiological recording.
All neuronal activity was recorded with a linear-array multicontact electrode that had 24 contacts with a spacing of 150 μm (Plexon, Dallas, TX). The electrode was inserted into the brain through a 25-gauge guide tube that penetrated the dura mater under the power of a hydraulic microdrive (MO-971; Narishige, Tokyo, Japan) that moved the electrode in micrometer steps. To record neural activity in the SMA, the guide tube and electrode were inclined at a 10° angle lateral to the vertical axis to avoid bleeding from the superior sagittal sinus. To record neural activity in the CMAc, the guide tube and electrode were set vertically in the dorsoventral direction. All neuronal activity from the 24 contacts was simultaneously recorded, amplified, filtered (band-pass filter: 500 Hz to 8 kHz), digitized (sampling rate: 48,828 Hz; PZ2 and RZ2; Tucker-Davis Technologies, Alachua, FL), and saved on a laboratory computer (Z210; Hewlett-Packard, Palo Alto, CA). Additionally, electromyographic (EMG) activity was recorded at 1,000 Hz with pairs of single-stranded Teflon-coated stainless steel wires (0.0762 mm in diameter; A-M Systems, Sequim, WA) that were inserted percutaneously. All EMG activity was amplified and digitized with an analog-to-digital converter, and the digital values were stored on the laboratory computer.
Neuronal activity was recorded in the left CMAc and SMA of the two monkeys. To reveal the somatotopic organizations of the CMAc and SMA, the present study assessed the neuronal responses evoked by somatosensory stimuli and the body movements produced by intracortical microstimulation (ICMS) applied through the tip of glass-coated tungsten electrodes inserted into these brain regions (44 cathodal pulses of 200 μs at 333 Hz, ≤80 μA; Luppino et al. 1993; Matsuzaka et al. 1992; Mitz and Wise 1987; Richardson et al. 2008; Shima et al. 1991; Takada et al. 2001). The recording sites were verified by examining magnetic resonance (MR) images (3.0T Magnetom Trio; Siemens, Erlangen, Germany; Fig. 1C).
Analysis of neuronal activity.
Single-unit potentials were sorted offline using a spike-sorting software package (OpenSorter; Tucker-Davis Technologies), and all analyses of neuronal activity were performed using custom-made programs on MATLAB R2012b (The MathWorks, Natick, MA) and R version 3.0.2 software (R Foundation for Statistical Computing, Vienna, Austria).
We first counted the number of spikes from each neuron in successive 200-ms bins around two task events: go signal onset (5 bins: 3 before and 2 after onset) and movement onset (3 bins: 1 before and 2 after onset). We defined a neuron as “task related” if the distribution of the discharge rate (in spikes/s) differed significantly in at least one of the two trial types (right press or left press) as revealed by one-way ANOVA using the eight 200-ms bins as independent variables (α = 0.005). The numbers of spikes from each task-related neuron were counted during the baseline period (−500 to −300 ms relative to the onset of the visual go signal) and the movement period (−200 to 0 ms relative to the button press). Under the visible condition, neuronal activity in the baseline period was compared with neuronal activity in the movement period for each of the right-press and left-press trials (paired t-test, α = 0.01, uncorrected). Neuronal activity was classified as movement related if there was a significant difference in the number of spikes between the baseline period and the movement period in either type of trial. Subsequently, movement-related neurons were classified into three categories: 1) contralateral neurons (activity changes observed only during right-press trials), 2) ipsilateral neurons (activity changes observed only during left-press trials), and 3) bilateral neurons (activity changes observed during both right- and left-press trials). All movement-related responses were further analyzed to determine whether they were associated with increased or decreased activity. To accomplish this, the preferred side for each movement-related neuron (right press or left press associated with a greater change in activity) was determined, and if the activity of the preferred side was greater (or smaller) in the movement period than in the baseline period, then the neuron was defined as exhibiting increased (or decreased) activity.
A laterality index was calculated to quantify the laterality representation of the right or left hand:
where FRcontra refers to the difference in firing rates between the baseline period and the movement period for the right-press trials (i.e., the hand contralateral to the left hemisphere from which the neurons were recorded), and FRipsi refers to this difference for the left-press trials (i.e., the hand ipsilateral to the left hemisphere from which the neurons were recorded). This index ranged from −1 to +1 and provided information regarding the extent of selectivity (with greater the absolute value indicating greater selectivity) and laterality (positive for contralateral preference and negative for ipsilateral preference).
RESULTS
Muscle activity.
EMG activity was recorded from the forelimbs, neck, and paravertebral muscles. The deltoid, trapezius, supraspinatus, infraspinatus, biceps, triceps, neck, and paravertebral muscles did not exhibit consistent changes in activity relative to movement execution. In contrast, the flexor carpi ulnaris (FCU), which is the primary muscle involved in the hand-only button press movement, did exhibit changes in activity that were associated with movement execution (Fig. 1B). More specifically, in the limb required to move, the FCU exhibited phasic bursts of activity during movement execution, whereas the FCU in the limb not required to move did not exhibit phasic changes in activity. These findings indicate that the monkeys executed the button press using hand movement on only the required side.
We calculated the onset time of the FCU activity in relation to the completion of the button press for each monkey by identifying the first of 10 consecutive 1-ms bins (10 ms) that exhibited a significant difference between the left-press and right-press trials (Welch's t-test, α = 0.01). The FCU onset times of ipsilateral hand movement were −115 ms for monkey 1 and −94 ms for monkey 2; those of contralateral hand movement were −135 ms for monkey 1 and −107 ms for monkey 2 (Fig. 1B).
Recording of neuronal activity in the CMAc and SMA.
During task performance, neuronal activities were recorded using a linear-array multicontact electrode inserted into the CMAc or SMA (Fig. 1C). Neurons in the SMA were recorded from medial wall of the frontal cortex, and those in the CMAc were recorded from the dorsal and ventral banks of the cingulate sulcus. The recording sites in the CMAc were confined to lateral aspects of the banks of the cingulate sulcus, and the most medial aspects were not explored. In separate sessions, the tungsten microelectrode (rather than the multicontact electrode) was employed to identify the somatotopic organizations of the CMAc and SMA, which were identified using movements evoked by ICMS or by recording neuronal responses to somatosensory stimuli applied by the experimenter. The present study observed neuronal spikes in and around the forelimb regions of the CMAc and SMA (Fig. 1C).
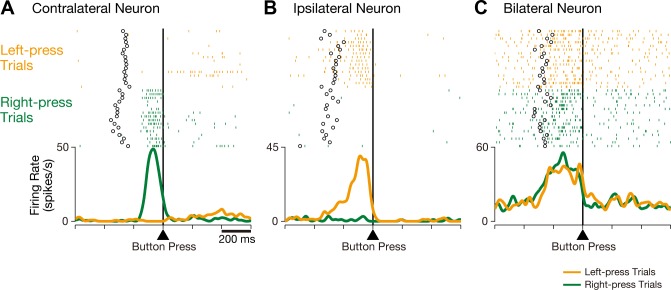
The present study primarily focused on neural activity recorded during successful trials because the success rate for both monkeys exceeded 94% (98.1% in monkey 1 and 94.5% in monkey 2). The CMAc and SMA neurons were sampled during at least two blocks of each of the right-press and left-press trials. We found three classes of neurons in these areas. The activity of contralateral neurons changed only in association with right-handed button presses, i.e., contralateral to the recorded hemisphere. Figure 2A shows an example of a contralateral neuron that exhibited increased activity during button pressing with the right (contralateral), but not the left (ipsilateral), hand. The activity of ipsilateral neurons changed only in association with left-handed button presses, i.e., ipsilateral to the recorded hemisphere. Figure 2B shows an example of an ipsilateral neuron that exhibited increased activity during button pressing with the ipsilateral, but not the contralateral, hand. The activity of bilateral neurons changed in association with both contralateral and ipsilateral button pressing. Figure 2C shows an example of a bilateral neuron that exhibited increased activity during button pressing with either the ipsilateral or contralateral hand. All three classes of neurons were found in both the CMAc and SMA, indicating that these areas are involved in movement of either hand.
Fig. 2.
Three representative examples of CMAc neurons. A: activity in this CMAc neuron increased when the monkey executed a button press with the contralateral (right) hand but not with the ipsilateral (left) hand. B: activity in this CMAc neuron increased when the monkey executed a button press with the ipsilateral (left) hand but not with the contralateral (right) hand. C: activity in this CMAc neuron increased when the monkey executed a button press with either the contralateral or ipsilateral hand. Rasters and mean spike density functions (smoothed using a Gaussian kernel; σ = 10 ms) indicate activity during right-press trials (green) and left-press trials (orange), and the ordinate represents the instantaneous firing rate (in spikes/s). Neuronal activity is aligned with the button press, open circles indicate the time of onset for the go signal, and the tick marks on the horizontal axis are placed at 200-ms intervals.
Population neuronal activity in the CMAc and SMA.
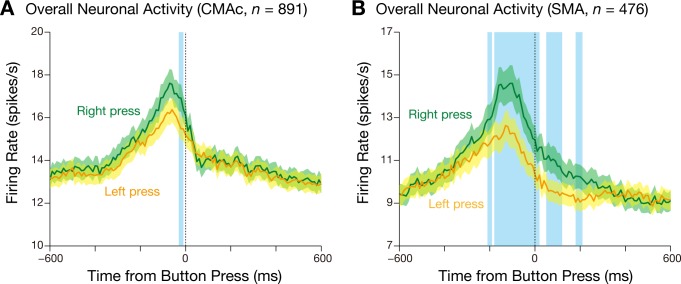
In total, we sampled the activity of 970 CMAc neurons (659 in monkey 1 and 311 in monkey 2) and 525 SMA neurons (356 in monkey 1 and 169 in monkey 2). Of these, 891 CMAc neurons and 476 SMA neurons exhibited task-related activity. To explore the overall hand representations of these task-related neurons, we calculated population activity for both the right-press and left-press trials (Fig. 3). In both areas, the population activity was greatest in relation to movement execution. The activity peaked just before completion of the button press. In the CMAc (Fig. 3A), the peaks occurred at −70 ms in the right-press trials and at −60 ms in the left-press trials relative to the button press. In the SMA (Fig. 3B), they occurred at −110 ms in the right-press trials and at −130 ms in the left-press trials. In the CMAc, the population activity did not differ between the right-press and left-press trials except for 6 of the 120 10-ms bins [−600 to 600 ms relative to the button press; a paired t-test (α = 0.01) was used to compare the activity of successive bins measured by the inverse-interspike interval method; Hoshi and Tanji 2006]. In contrast, in the SMA, the population activity for the right-press trials was greater than that for the left-press trials. In 34 of the 120 10-ms bins, the activity was greater in the right-press trials than the left-press trials.
Fig. 3.
Mean firing rate (±SE; bin width = 10 ms) of all task-related neurons in the CMAc (A) and SMA (B) for the right-press (green) and left-press trials (orange). The blue translucent areas indicate 10-ms bins in which the firing rate for the right-press trials differed from that for the left-press trials (paired t-test, α = 0.01) in two or more consecutive bins.
Laterality representation of hand movement by neurons in the CMAc and SMA.
To statistically verify the activity profile of each neuron, we analyzed all neurons in terms of movement-related activity (see materials and methods). This activity was observed in 612 CMAc neurons (431 in monkey 1 and 181 in monkey 2; 63% of 970 sampled neurons; 69% of 891 task-related neurons) and 345 SMA neurons (236 in monkey 1 and 109 in monkey 2; 66% of 525 sampled neurons; 72% of 476 task-related neurons). Welch's t-test (α = 0.01; 200-ms period preceding the button press) was used to compare the discharge rates of movement-related neurons under visible and invisible conditions in the context of the right-press or left-press trials. This analysis showed that the activity of 44 CMAc neurons and 20 SMA neurons differed significantly depending on conditions. These neurons were excluded from subsequent analyses to focus on neurons involved in movement execution per se. Thus, in the present study, 568 CMAc neurons and 325 SMA neurons were analyzed.
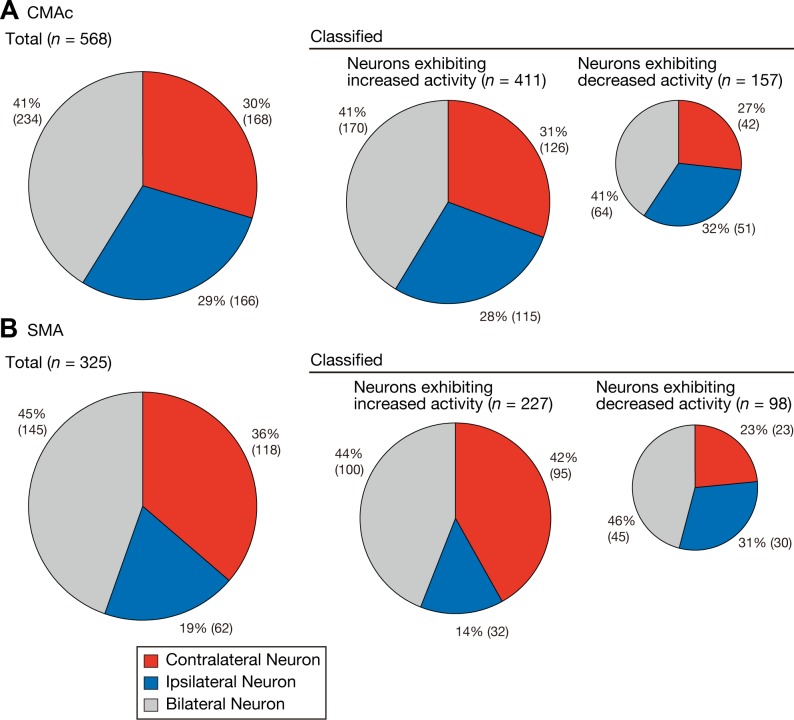
Movement-related neurons were classified into three categories: contralateral (right hand, Fig. 2A), ipsilateral (left hand, Fig. 2B), and bilateral (either hand, Fig. 2C) (see materials and methods). Of the 568 CMAc neurons, 168 (30%) were classified as contralateral selective, 166 (29%) were classified as ipsilateral selective, and 234 (41%) were classified as bilateral selective (Fig. 4A). The proportion of contralateral neurons in the CMAc did not significantly differ from that of ipsilateral neurons (binomial test for equal proportions, P = 0.9564). Of the 325 SMA neurons, 118 (36%) were classified as contralateral selective, 62 (19%) were classified as ipsilateral selective, and 145 (45%) were classified as bilateral selective (Fig. 4B). In contrast to what was noted in the CMAc, the proportion of contralateral neurons in the SMA was greater than that of ipsilateral neurons (binomial test for equal proportions, P < 0.0001).
Fig. 4.
Frequencies of neurons selective for contralateral, ipsilateral, or bilateral movements. The pie charts summarize the proportions of neurons in the CMAc (A) and SMA (B) that were classified into each of the three categories. Left, the classification of all movement-related neurons; middle and right, the classifications of the neurons associated with increased and decreased activity, respectively. The area of each pie chart is proportional to the total number of neurons in the CMAc and SMA; the percentage and actual numbers of neurons in each category are shown next to the corresponding portion of each pie chart. Each category is color coded: red, contralateral neurons; blue, ipsilateral neurons; and gray, bilateral neurons.
The laterality selectivity of the neurons was examined separately for neurons that displayed increased activity and those that exhibited decreased activity. In the CMAc, the frequencies of ipsilateral neurons exhibiting increased (decreased) activities did not differ from those of contralateral neurons (binomial test for equal proportions, P = 0.5196 for neurons with increased activity and P = 0.4069 for neurons with decreased activity; Fig. 4A). In contrast, analysis of SMA neurons exhibiting increased activity showed that the proportion of contralateral neurons was greater than that of ipsilateral neurons (P < 0.0001; Fig. 4B), but the proportions of neurons exhibiting decreased activity did not differ (P = 0.4101; Fig. 4B). These findings indicate that SMA neurons with increased activity preferentially represented contralateral hand movement, whereas CMAc neurons with increased activity represented contralateral and ipsilateral hand movements in a counterbalanced manner. Furthermore, neurons with decreased activity in both the CMAc and SMA represented the movements of either hand in a counterbalanced manner.
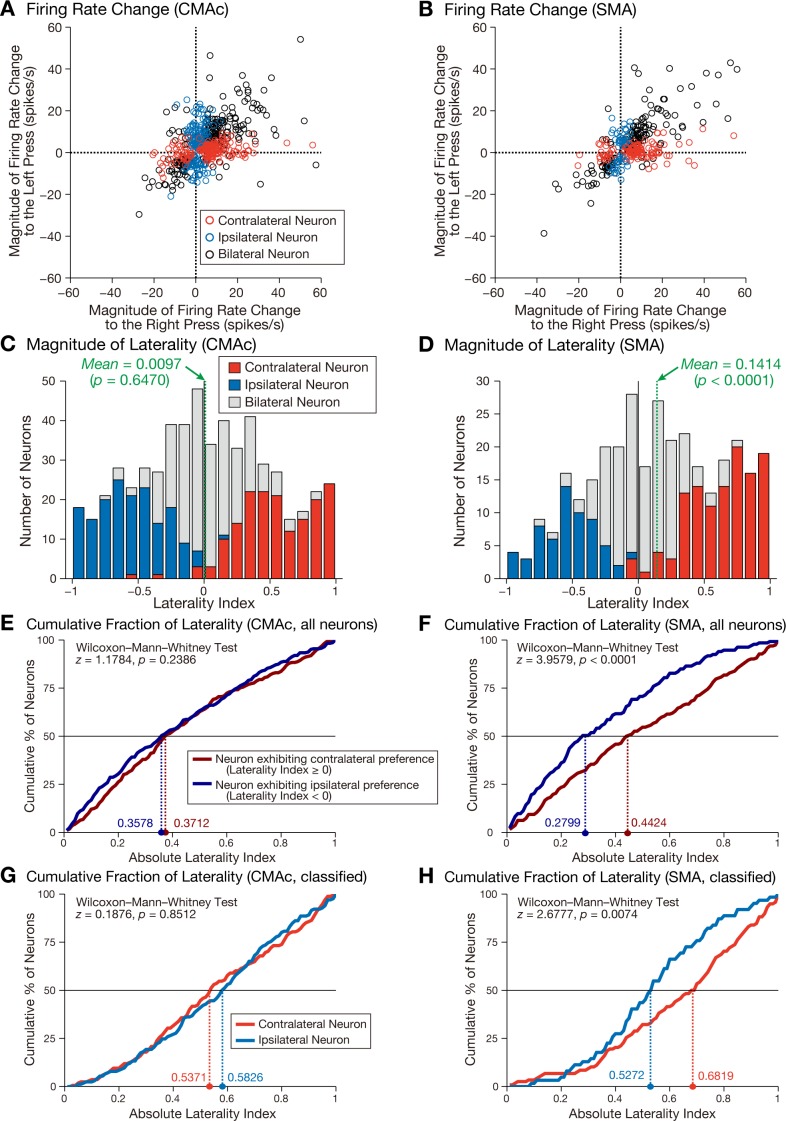
For each neuron, changes in activity during the movement period relative to the baseline period were calculated separately for the right-press and left-press trials. These data were used to plot the activity modulation for each neuron in the CMAc (Fig. 5A) and SMA (Fig. 5B), revealing that contralateral neurons were clustered around the x-axis and ipsilateral neurons were grouped around the y-axis, consistent with the notion that activity modulation in the contralateral (ipsilateral) neurons was greater during contralateral (ipsilateral) hand movement. In contrast, bilateral neurons were clustered around the line-of-unity slope, which indicates that the direction of activity modulation (increase/decrease) in these neurons was similar between the right-press and left-press trials.
Fig. 5.
Selectivity of neuronal activity for right-press and left-press movements. A and B: relationships of the changes in firing rates between the right-press and left-press trials in the CMAc (A) and SMA (B). The changes in movement-related firing rates from the baseline period for the right-press trials are plotted along the abscissa, and those for the left-press trials are plotted along the ordinate. The positive and negative values denote increased and decreased activity, respectively. Each open circle represents the data of an individual neuron: red, contralateral neuron; blue, ipsilateral neuron; and black, bilateral neuron. Five CMAc neurons (2 contralateral neurons and 3 bilateral neurons) and two SMA neurons (both bilateral neurons) are not shown because they displayed values that were greater than 60 spikes/s for the right-press trials. C and D: distributions of the laterality indexes in the CMAc (C) and SMA (D). The results are compiled into histograms: red, contralateral neurons; blue, ipsilateral neurons; and gray, bilateral neurons. Positive values indicate greater activity for contralateral movement, and negative values indicate greater activity for ipsilateral movement. The green dashed lines indicate the mean values of the laterality index. E and F: cumulative distributions of the absolute values of the laterality indexes for neurons exhibiting contralateral preference (neurons with positive values, dark red) and ipsilateral preference (neurons with negative values, dark blue) in the CMAc (E) and SMA (F). Colored dashed lines indicate the median absolute values for neurons with contralateral preference (dark red) and ipsilateral preference (dark blue). G and H: cumulative distributions of the absolute values of the laterality indexes for contralateral (red) and ipsilateral (blue) neurons, as determined by statistical analysis (paired t-test, P < 0.01) in the CMAc (G) and SMA (H). Colored dashed lines indicate the median absolute values for contralateral neurons (red) and ipsilateral neurons (blue).
For each neuron, the laterality index was calculated to quantify the representations for contralateral and ipsilateral hand movements (see materials and methods). The mean value of the laterality indexes in the CMAc (Fig. 5C) was not significantly different from zero (mean = 0.0097; one-sample t-test: t = 0.4582, P = 0.6470), whereas that of the laterality indexes in the SMA (Fig. 5D) was significantly greater than zero (mean = 0.1414; one-sample t-test: t = 5.1548, P < 0.0001). To further explore the laterality representation, we divided all neurons into those exhibiting contralateral and those exhibiting ipsilateral preferences on the basis of the signs of the laterality indexes. In the CMAc (Fig. 5E), the cumulative distributions of the absolute values of the laterality indexes did not differ between contralateral-preferring neurons (neurons with positive laterality index values, n = 282, median = 0.3712) and ipsilateral-preferring neurons (neurons with negative laterality index values, n = 286, median = 0.3578) (Wilcoxon-Mann-Whitney test: z = 1.1784, P = 0.2386). By contrast, in the SMA (Fig. 5F), the cumulative distribution of the absolute values of the laterality indexes of contralateral-preferring neurons (n = 192, median = 0.4424) was greater than that of ipsilateral-preferring neurons (n = 133, median = 0.2799; Wilcoxon-Mann-Whitney test: z = 3.9579, P < 0.0001). In a similar manner, we analyzed the contralateral and ipsilateral neurons using a paired t-test (Fig. 4). In the CMAc (Fig. 5G), the absolute values of the laterality indexes did not differ between the contralateral (n = 168, median = 0.5371) and ipsilateral neurons (n = 166, median = 0.5826) (Wilcoxon-Mann-Whitney test: z = 0.1876, P = 0.8512), whereas in the SMA (Fig. 5H), the absolute values of the laterality indexes of the contralateral neurons (n = 118, median = 0.6819) were significantly greater than those of the ipsilateral neurons (n = 62, median = 0.5272; Wilcoxon-Mann-Whitney test: z = 2.6777, P = 0.0074). These findings indicate that the overall activity of CMAc neurons reflected contralateral and ipsilateral hand movements in a counterbalanced manner, whereas the overall activity of neurons in the SMA preferentially reflected movement of the contralateral hand.
Temporal profiles of neuronal activities in the CMAc and SMA.
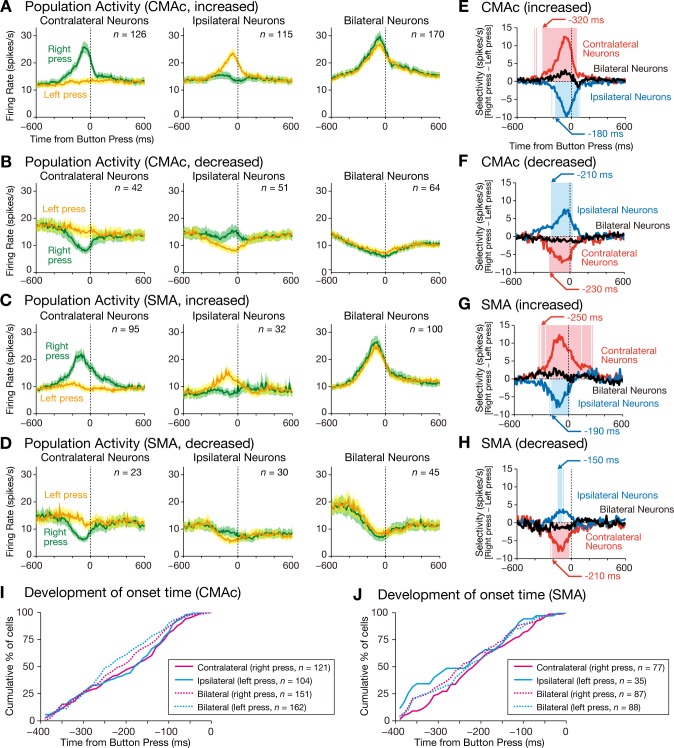
The population activities of the contralateral, ipsilateral, and bilateral neurons were calculated separately. The population activity of CMAc and SMA neurons exhibiting increased activity gradually increased and formed peaks during movement execution distributed from −130 to −60 ms relative to completion of the button press (Fig. 6, A and C, and Table 1). In contrast, the population activity of CMAc and SMA neurons with decreased activity gradually decreased and formed troughs during movement execution that were distributed from −90 to −10 ms relative to the completion of the button press (Fig. 6, B and D, and Table 1).
Fig. 6.
Time courses of the population selectivity. A–H: population activities are shown for CMAc neurons exhibiting increased activity (A) and decreased activity (B) and for SMA neurons exhibiting increased activity (C) and decreased activity (D). The mean firing rate (±SE; bin width = 10 ms) is aligned with the button press separately for right-press trials (green) and left-press trials (orange); left, contralateral neurons; middle, ipsilateral neurons; and right, bilateral neurons. Population selectivity is shown for CMAc neurons exhibiting increased activity (E) and decreased activity (F) and for SMA neurons exhibiting increased activity (G) and decreased activity (H). The population selectivity (bin width = 10 ms) was calculated by subtracting activity in the left-press trials from activity in the right-press trials. The selectivity is aligned with the button press; red, contralateral neurons, blue, ipsilateral neurons, and black, bilateral neurons. The red and blue translucent areas indicate 10-ms bins in which selectivity was significantly different from zero for the contralateral and ipsilateral neurons, respectively (1-sample t-test, α = 0.01). Arrows indicate the first of 5 consecutive bins that exhibited significant selectivity. I and J: cumulative distributions of onset time for individual neurons in the CMAc (I) and SMA (J). Magenta solid line, contralateral neurons for right-press trials; cyan solid line, ipsilateral neurons for left-press trials; magenta dashed line, bilateral neurons for right-press trials; cyan dashed line, bilateral neurons for left-press trials.
Table 1.
Peak and trough times of neurons
| Area |
||||
|---|---|---|---|---|
| Neuron Class | Trial | Modulation Type | CMAc | SMA |
| All neurons | Right press (peak time) | −70 | −110 | |
| Left press (peak time) | −60 | −130 | ||
| Contralateral neurons | Right press | Increased (peak time) | −80 | −100 |
| Decreased (trough time) | −60 | −80 | ||
| Ipsilateral neurons | Left press | Increased (peak time) | −60 | −130 |
| Decreased (trough time) | −20 | −90 | ||
| Bilateral neurons | Right press | Increased (peak time) | −60 | −90 |
| Decreased (trough time) | −30 | −70 | ||
| Left press | Increased (peak time) | −60 | −120 | |
| Decreased (trough time) | −10 | −40 | ||
Values are peak and trough times (ms) of neurons in the caudal cingulate motor area (CMAc) and supplementary motor area (SMA) of monkeys during left-press or right-press trials.
We next calculated the onset time of each movement-related neuron as determined by the times of the first of three consecutive 10-ms time bins where the discharge rates deviated by >3 SD of the mean value calculated during the baseline period (−500 ms to −300 ms relative to go signal onset). In the CMAc (Fig. 6I), the median values of onset time were −180 ms for contralateral neurons in the right-press trials (n = 121), −175 ms for ipsilateral neurons in the left-press trials (n = 104), −240 ms for bilateral neurons in the right-press trials (n = 151), and −210 ms for bilateral neurons in the left-press trials (n = 162). In the SMA (Fig. 6J), the values were −210 ms for contralateral neurons in the right-press trials (n = 77), −230 ms for ipsilateral neurons in the left-press trials (n = 35), −240 ms for bilateral neurons in the right-press trials (n = 87), and −225 ms for bilateral neurons in the left-press trials (n = 88). When CMAc and SMA data were compared, the onset times of the identical neuron types did not differ (P ≥ 0.01, Wilcoxon-Mann-Whitney test). As mentioned above, the onset times of muscle activity were distributed from −135 to −94 ms relative to the completion of the button press. By the time the earliest muscle activity was detected (−135 ms), modulation of neuronal activity had already commenced in 70% of neurons (376 of 538) in the CMAc and 76% of neurons (218 of 287) in the SMA, showing that modulation of neuronal activity preceded modulation of muscle activity in the great majority of cases.
To explore the duration of hand-movement modulation of movement-related neurons, we next calculated the activity duration of each movement-related neuron for which the onset time was calculated above. First, we determined offset times, i.e., the times of the first of three consecutive 10-ms time bins after onset time when the discharge rates deviated by ≤3 SD of the mean value calculated during the baseline period. Next, the duration was calculated on the basis of temporal difference between the onset time and the offset time. In the CMAc, the median duration values were 190 ms for contralateral neurons in the right-press trials, 170 ms for ipsilateral neurons in the left-press trials, 250 ms for bilateral neurons in the right-press trials, and 220 ms for bilateral neurons in the left-press trials. In the SMA, the values were 210 ms for contralateral neurons in the right-press trials, 140 ms for ipsilateral neurons in the left-press trials, 230 ms for bilateral neurons in the right-press trials, and 255 ms for bilateral neurons in the left-press trials. The durations for each neuron type did not differ between the CMAc and the SMA (P ≥ 0.01, Wilcoxon-Mann-Whitney test).
Finally, to examine the timing of the development of selectivity, population selectivity was calculated as the difference in activity between the right-press and left-press trials (i.e., mean activity in the right-press trials minus mean activity in the left-press trials). CMAc neurons with increased activity began to discriminate between right and left hand movements at −320 ms for contralateral neurons and at −180 ms for ipsilateral neurons relative to the button press (Fig. 6E), whereas SMA neurons did so at −230 ms for contralateral neurons and at −210 ms for ipsilateral neurons (Fig. 6G). CMAc neurons with decreased activity began to discriminate between right and left hand movements at −230 ms for contralateral neurons and at −210 ms for ipsilateral neurons relative to the button press (Fig. 6F), whereas SMA neurons did so at −210 ms for contralateral neurons and at −150 ms for ipsilateral neurons (Fig. 6H). These results showed that selectivity of hand use commenced before the onset of muscle activity.
Spatial distributions of neuronal selectivity within a small cortical volume.
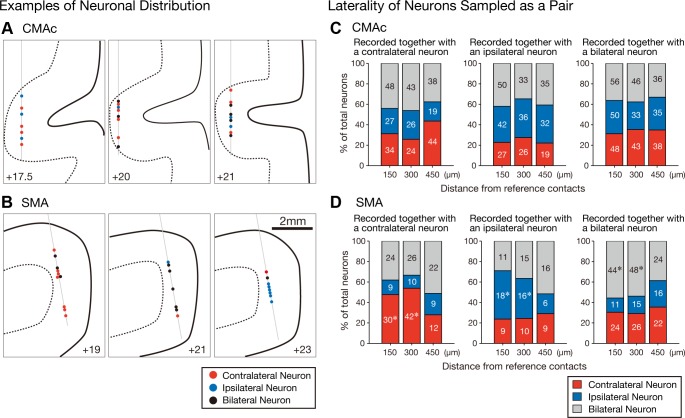
The use of multicontact electrodes allowed for the simultaneous recording of multiple neurons within a small volume of cortex by monitoring neuronal spikes using a pair of contact points. Six examples of selectivity distributions are shown in Fig. 7, A and B. In all cases, neurons exhibiting various forms of hand selectivity were simultaneously recorded. However, in the CMAc (Fig. 7A), neurons exhibiting distinct selectivity were intermingled, whereas in the SMA (Fig. 7B), neurons with identical selectivity were recorded from successive contacts (contralateral neurons, left; bilateral neurons, middle; and ipsilateral neurons, right).
Fig. 7.
A and B: 6 sample recordings from the CMAc (A) and SMA (B). Contralateral (red circles), ipsilateral (blue circles), and bilateral neurons (black circles) are plotted on the coronal sections reconstructed from the magnetic resonance images (solid line, cortical surface; broken line, border between gray matter and white matter). Neurons in each panel were simultaneously recorded with a linear-array multicontact electrode with 24 contacts spaced 150 μm apart. If 2 neurons were simultaneously recorded from 1 contact, 2 circles were plotted side by side. Thin gray thin indicate the electrodes. C and D: distributions of the laterality selectivity of CMAc (C) and SMA (D) neurons that were sampled as pairs of simultaneously recorded neurons (i.e., counterpart neurons) by 2 contacts with reference to a contact containing reference neurons. The stacked bar graphs summarize the distributions of selectivity among the counterpart neurons (150, 300, or 450 μm distant from reference neurons) recorded together with a contralateral-selective reference neuron (left), an ipsilateral-selective reference neuron (middle), or a bilateral-selective reference neuron (right). Each category is color coded as indicated: red, contralateral neurons; blue, ipsilateral neurons; and gray, bilateral neurons. The actual number of neurons in each category is shown within the corresponding portion of each bar graph. The asterisks indicate values that were greater than expected (post hoc residual analysis, P < 0.01).
The distribution of hand selectivity within a small cortical volume was assessed by comparing the selectivity of a pair of simultaneously recorded neurons. We first identified a reference neuron that 1) exhibited any hand selectivity and 2) had an adjacent contact with a cell that exhibited any hand selectivity. In this manner, 135 contralateral, 123 ipsilateral, and 167 bilateral CMAc neurons and 71 contralateral, 42 ipsilateral, and 101 bilateral SMA neurons were identified as reference neurons. Next, the distribution of selectivity in the counterpart neurons of the adjacent contacts (150 μm apart) was examined. Figure 7 shows the frequencies of neuronal selectivity observed in the counterpart neurons that were recorded simultaneously with the reference neurons for the contralateral (left), ipsilateral (middle), and bilateral (right) hand movements. The frequencies of the contralateral, ipsilateral, and bilateral counterpart neurons in the CMAc did not significantly differ by the selectivity of the reference neurons (chi-square test: χ2 = 5.2917, P = 0.2587; Fig. 7C). On the other hand, the frequencies of the contralateral, ipsilateral, and bilateral counterpart neurons in the SMA differed significantly depending on the selectivity of the reference neurons (chi-square test: χ2 = 25.3958, P < 0.0001; Fig. 7D). A post hoc residual analysis (Haberman 1973) applied to the SMA neurons revealed that for each of the contralateral, ipsilateral, and bilateral reference neurons, the proportions of counterpart neurons with the same selectivity as the reference neurons were greater than the expected values (contralateral neurons: 48%, P = 0.0091; ipsilateral neurons: 47%, P < 0.0001; and bilateral neurons: 56%, P = 0.0048; Fig. 7D). This finding indicates that neurons in the SMA with identical selectivity tended to form clusters, which is evident in the three SMA sample recordings (Fig. 7B).
In the same manner, the selectivity distributions of the counterpart neurons were analyzed at 300 and 450 μm. A reference neuron was identified where 1) a neuron exhibited any hand selectivity and 2) a contact located at 300 (for the 300-μm analysis) or 450 μm (for the 450-μm analysis) distant from the contact with the reference neuron involved a cell that exhibited any hand selectivity. In the CMAc, the selectivity distributions of counterpart neurons at 300 or 450 μm did not differ with respect to the selectivity of reference neurons (chi-square test: P ≥ 0.01; Fig. 7C). Next, the distributions of neurons in the SMA were assessed. Those of the counterpart SMA neurons at 300 μm significantly differed depending on the selectivity of the reference neurons (chi-square test: χ2 = 23.6539, P < 0.0001). Additionally, a post hoc residual analysis showed that neurons with an identical selectivity tended to form a cluster (contralateral neurons: 54%, n = 42, P = 0.0002; ipsilateral neurons: 39%, n = 16, P = 0.0005; and bilateral neurons: 54%, n = 48, P = 0.0050). In contrast, the selectivity distributions of the counterpart SMA neurons at 450 μm did not significantly differ depending on the selectivity of the reference neurons (chi-square test: χ2 = 2.2025, P = 0.6986). These findings indicate that the radii of the clusters of the contralateral, ipsilateral, and bilateral SMA neurons were <450 μm.
Although the above analyses revealed that the neuronal distributions of the SMA and CMAc were significantly different, this contrast may have been derived from differences in the electrode perpetration within the cortex. For example, the contacts in the SMA were arranged in a manner more parallel to the cortical surface of the brain than were the contacts in the CMAc because electrodes in the CMAc were more vertically oriented than those in the SMA. To assess the impact of electrode angle, the distributions of the CMAc neurons that were recorded from penetrations that were ≤0.5 mm from the most lateral level were analyzed. Because this site corresponds to the fundus of the cingulate sulcus, these contacts were arranged less vertically to the cortical surface of the brain than were the electrodes that were placed more medially. Even in the lateral recordings from the CMAc, the frequencies of the contralateral, ipsilateral, and bilateral counterpart neurons (150 μm apart) did not differ depending on the selectivity of the reference neurons (chi-square test: χ2 = 2.4806, P = 0.6481). This finding indicates that the distinct SMA and CMAc neuronal distributions did not result solely from the manner in which the contacts were arranged within the cortex but that the patterns of neuronal distributions within these regions were truly different.
DISCUSSION
In the present study, neuronal activity was recorded while the monkeys performed a button press task with either the right or left hand. Analyses of neuronal activity immediately before and during movement execution revealed that the CMAc represented contralateral, ipsilateral, and bilateral hand movements in a counterbalanced manner, whereas the SMA preferentially represented contralateral and bilateral hand movements with only minor representation of ipsilateral hand movement. Furthermore, an analysis of the spatial distributions of neuronal activity at a submillimeter scale revealed that the CMAc and SMA displayed distinct microarchitectural organizations. CMAc neurons representing contralateral and ipsilateral hand movements were homogeneously intermingled, whereas SMA neurons with identical hand preferences tended to aggregate.
Laterality representations of movement-related activity in the CMAc.
Human studies have demonstrated that the caudal cingulate zone (CCZ), which is analogous to the CMAc in monkeys, is active regardless of which arm is used during task performance (Dinstein et al. 2008; Immisch et al. 2001; Kollias et al. 2001). This suggests that the CMAc may be involved during the execution of bilateral arm movements by monkeys. Kermadi et al. (2000) examined neuronal activity in the CMAc of monkeys performing reach and grasp movements and found that a vast majority of neurons (77%) were active during the movement of either arm (bilateral neurons). In contrast, the present study found that a smaller proportion (41%) of CMAc neurons were active during bilateral movements. This disparity may result from differences in the types of movements required by the different tasks; Kermadi et al. (2000) measured whole arm movements, whereas the present study assessed hand-only movements. The remaining neurons of the present study were selective for either right or left hand movements. The activity modulation of ipsilateral neurons did not differ from that of contralateral neurons, and the neuronal activity began before the initiation of the muscle activity. Together, the data presented in this report provide compelling evidence that the CMAc is not only involved in the general control of hand movements irrespective of which hand is used but also is actively engaged during specific control of contralateral and ipsilateral hand movements.
Laterality representations of movement-related activity in the SMA.
Previous investigations of the laterality representations of movement execution in SMA neurons have measured whole arm movements (Brinkman and Porter 1979; Donchin et al. 1998, 2002; Kermadi et al. 1998). Similar to the laterality representations in CMAc neurons (Kermadi et al. 2000), a great majority of SMA neurons are classified as bilateral. Because whole arm movements are often accompanied by muscle activity in the nonperforming arm and inevitably include postural muscle activity, it is possible that bilateral neurons are overrepresented. To resolve this issue, Tanji et al. (1987, 1988) required monkeys to execute the button press action using hand-only movements. A reanalysis of the reported data from Tanji et al. (1987, 1988) revealed that 54%, 37%, and 9% of SMA neurons were classified as bilateral, contralateral, and ipsilateral neurons, respectively. The results of the present study are consistent with these findings in that more neurons were classified as contralateral than as ipsilateral. Additionally, the present study found that the activity modulation was biased toward the contralateral hand movement, indicating that the SMA preferentially represented the contralateral hand movement, although a substantial number of neurons also represented ipsilateral hand movement.
Temporal profiles of movement-related activity.
We found that the activity and hand use selectivity of CMAc and SMA neurons commenced earlier than did the onset of muscle activity. This indicates that both the CMAc and SMA are involved in the genesis of hand movement. We also found that the onset time of movement-related activity of the contralateral, ipsilateral, and bilateral neurons was similar in the CMAc (Fig. 6I) and SMA (Fig. 6J). Moreover, the duration of movement-related activity of these neurons did not differ between the two brain areas. These findings suggest that movement-related activity in the CMAc and SMA begin simultaneously and are of comparable duration. However, the timing of activity peaks differed between the CMAc and SMA. The activity of all task-related neurons in the SMA peaked earlier than that in the CMAc (Fig. 3 and Table 1). In the contralateral, ipsilateral, and bilateral neurons, the peak (trough) times associated with increased (decreased) activity of SMA neurons were earlier than those of CMAc neurons (Fig. 6, A–H, and Table 1). These results indicate that the SMA consistently reaches activity peaks earlier than does the CMAc, raising the possibility that the SMA may play a leading role in commitment to action execution, whereas the CMAc may make a greater contribution to the actual execution of unfolding action.
Distinct microarchitectural organizations of the CMAc and SMA.
We employed linear-array multicontact electrodes to simultaneously sample the activity of multiple neurons within a small cortical volume (several millimeters) (Bonini et al. 2014; Takeuchi et al. 2011). There are two major limitations to this method. First, the spatial resolution of neuronal recordings (∼100 μm) is 10 times coarser than that obtained via in vivo imaging (∼10 μm) (Komiyama et al. 2010; Sato et al. 2007). Second, neuronal sampling is linear or one dimensional, whereas two- or three-dimensional sampling is possible using in vivo imaging. However, our use of linear-array multicontact electrodes allowed us to explore neuronal organization in the brain of a nonhuman primate without introducing major changes to the recording system. Also, we were able to sample neurons from virtually any region of the brain, including mesial aspects and banks from which it is often difficult to obtain in vivo images; the monkey CMAc and SMA are typical examples of such tissues. Furthermore, direct recording of extracellular spikes allowed us to perform cross-correlation analysis between the spike trains of different neurons, yielding detailed data on information flow among neurons and computations performed within local circuits.
We could not monitor movement-related neurons from all successive contacts. Rather, movement-related neurons were sampled in clusters, in a rather sparse manner, as shown in Fig. 7, A and B. We offer two possible explanations for this sparseness. First, silent contacts may contain neurons encoding other movement types, such as wrist extension, reaching, or grasping, or neurons encoding movement of other body parts, such as the elbow or shoulder. Second, silent contacts may be too far away from actively discharging neurons to register electric activity.
However, our focus on the distributions of contacts adjacent to (within ≤450 μm) reference neurons suggests that the microarchitecture of the CMAc may differ from that of the SMA in several respects. In the SMA, neurons with the same selectivity tended to cluster in a manner that was suggestive of spatial grouping, and the radii of the clusters of the contralateral, ipsilateral, and bilateral neurons were estimated to be <450 μm. In the CMAc, the proportions of neurons selective for contralateral (30%) and ipsilateral (29%) hand movements were comparable (Fig. 4A), and the contralateral, ipsilateral, and bilateral neurons were distributed homogeneously without any clustering.
It has been suggested that there are three protogradations (directions of progressive cortical differentiation) in the frontal cortex of primates (Goldberg 1985; Sanides 1964, 1970; Sanides and Krishnamurti 1967): 1) the lateral protogradation, which originates in the insular cortex; 2) the most recent evolutional protogradation, which originates in the central sulcus; and 3) the medial protogradation, which originates in the cingulate gyrus. The CMAc is situated immediately next to the origin of the medial protogradation (the cingulate gyrus) and may be an evolutionally older aspect of the motor cortex. An intriguing possibility is that, much like the more homogenous cellular architecture found in evolutionarily older cortex (Barbas 1986; Barbas and Pandya 1987, 1989; Garcia-Cabezas and Barbas 2014; Shipp 2005), the evolutionally older CMAc may also employ a more homogenous functional architecture. If this is the case, evolutionarily newer motor areas may employ a more structured organization than that of the CMAc, as is observed in the SMA. Future studies that record neurons across all cortical layers with the use of higher spatial resolution (Hira et al. 2013) to investigate these possibilities may lead to a better understanding of the basic principles and mechanisms underlying the motor control that is realized by the neural circuitry in the motor areas of the frontal lobes (Cisek et al. 2003; Hoshi and Tanji 2007; Kalaska and Crammond 1992; Kurata 2010; Tanji 1994; Wise and Mauritz 1985).
GRANTS
This work was supported by Core Research for Evolutional Science and Technology (CREST) (to E. Hoshi) and by Japan Society for the Promotion of Science Grants-in-Aid for Young Scientists KAKENHI 30585906 (to Y. Nakayama) and for Research Activity Start-up KAKENHI 22830086 (to Y. Nakayama).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.N., O.Y., and E.H. conception and design of research; Y.N. performed experiments; Y.N., O.Y., and E.H. analyzed data; Y.N., O.Y., and E.H. interpreted results of experiments; Y.N. prepared figures; Y.N., O.Y., and E.H. drafted manuscript; Y.N., O.Y., and E.H. edited and revised manuscript; Y.N., O.Y., and E.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. J. Tanji for helpful comments on an earlier version of the manuscript and T. Ogata, S. Hoffman, Dr. A. Miyazaki, and Dr. M. Sakagami for technical assistance.
REFERENCES
- Amiez C, Petrides M. Neuroimaging evidence of the anatomo-functional organization of the human cingulate motor areas. Cereb Cortex 24: 563–578, 2014. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Carducci F, Del Gratta C, Demartin M, Romani GL, Babiloni F, Rossini PM. Hemispherical asymmetry in human SMA during voluntary simple unilateral movements. An fMRI study. Cortex 39: 293–305, 2003. [DOI] [PubMed] [Google Scholar]
- Backus DA, Ye S, Russo GS, Crutcher MD. Neural activity correlated with the preparation and execution of visually guided arm movements in the cingulate motor area of the monkey. Exp Brain Res 140: 182–189, 2001. [DOI] [PubMed] [Google Scholar]
- Baraldi P, Porro CA, Serafini M, Pagnoni G, Murari C, Corazza R, Nichelli P. Bilateral representation of sequential finger movements in human cortical areas. Neurosci Lett 269: 95–98, 1999. [DOI] [PubMed] [Google Scholar]
- Barbas H. Pattern in the laminar origin of corticocortical connections. J Comp Neurol 252: 415–422, 1986. [DOI] [PubMed] [Google Scholar]
- Barbas H, Pandya DN. Architecture and frontal cortical connections of the premotor cortex (area 6) in the rhesus monkey. J Comp Neurol 256: 211–228, 1987. [DOI] [PubMed] [Google Scholar]
- Barbas H, Pandya DN. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. J Comp Neurol 286: 353–375, 1989. [DOI] [PubMed] [Google Scholar]
- Bonini L, Maranesi M, Livi A, Fogassi L, Rizzolatti G. Space-dependent representation of objects and other's action in monkey ventral premotor grasping neurons. J Neurosci 34: 4108–4119, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkman C, Porter R. Supplementary motor area in the monkey: activity of neurons during performance of a learned motor task. J Neurophysiol 42: 681–709, 1979. [DOI] [PubMed] [Google Scholar]
- Cadoret G, Smith AM. Comparison of the neuronal activity in the SMA and the ventral cingulate cortex during prehension in the monkey. J Neurophysiol 77: 153–166, 1997. [DOI] [PubMed] [Google Scholar]
- Cisek P, Crammond DJ, Kalaska JF. Neural activity in primary motor and dorsal premotor cortex in reaching tasks with the contralateral versus ipsilateral arm. J Neurophysiol 89: 922–942, 2003. [DOI] [PubMed] [Google Scholar]
- Crutcher MD, Russo GS, Ye S, Backus DA. Target-, limb-, and context-dependent neural activity in the cingulate and supplementary motor areas of the monkey. Exp Brain Res 158: 278–288, 2004. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Grafton S, Albert N, Hazeltine E, Ivry RB. Goal-selection and movement-related conflict during bimanual reaching movements. Cereb Cortex 16: 1729–1738, 2006. [DOI] [PubMed] [Google Scholar]
- Dinstein I, Gardner JL, Jazayeri M, Heeger DJ. Executed and observed movements have different distributed representations in human aIPS. J Neurosci 28: 11231–11239, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donchin O, Gribova A, Steinberg O, Bergman H, Vaadia E. Primary motor cortex is involved in bimanual coordination. Nature 395: 274–278, 1998. [DOI] [PubMed] [Google Scholar]
- Donchin O, Gribova A, Steinberg O, Mitz AR, Bergman H, Vaadia E. Single-unit activity related to bimanual arm movements in the primary and supplementary motor cortices. J Neurophysiol 88: 3498–3517, 2002. [DOI] [PubMed] [Google Scholar]
- Dum RP, Strick PL. The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci 11: 667–689, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Cabezas MA, Barbas H. Area 4 has layer IV in adult primates. Eur J Neurosci 39: 1824–1834, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg G. Supplementary motor area structure and function: review and hypothesis. Behav Brain Sci 8: 567–616, 1985. [Google Scholar]
- Haberman SJ. The analysis of residuals in cross-classified tables. Biometrics 29: 205–220, 1973. [Google Scholar]
- Hatanaka N, Tokuno H, Hamada I, Inase M, Ito Y, Imanishi M, Hasegawa N, Akazawa T, Nambu A, Takada M. Thalamocortical and intracortical connections of monkey cingulate motor areas. J Comp Neurol 462: 121–138, 2003. [DOI] [PubMed] [Google Scholar]
- He SQ, Dum RP, Strick PL. Topographic organization of corticospinal projections from the frontal lobe: motor areas on the medial surface of the hemisphere. J Neurosci 15: 3284–3306, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hira R, Ohkubo F, Ozawa K, Isomura Y, Kitamura K, Kano M, Kasai H, Matsuzaki M. Spatiotemporal dynamics of functional clusters of neurons in the mouse motor cortex during a voluntary movement. J Neurosci 33: 1377–1390, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi E, Tanji J. Differential involvement of neurons in the dorsal and ventral premotor cortex during processing of visual signals for action planning. J Neurophysiol 95: 3596–3616, 2006. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Tanji J. Distinctions between dorsal and ventral premotor areas: anatomical connectivity and functional properties. Curr Opin Neurobiol 17: 234–242, 2007. [DOI] [PubMed] [Google Scholar]
- Hutchins KD, Martino AM, Strick PL. Corticospinal projections from the medial wall of the hemisphere. Exp Brain Res 71: 667–672, 1988. [DOI] [PubMed] [Google Scholar]
- Immisch I, Waldvogel D, van Gelderen P, Hallett M. The role of the medial wall and its anatomical variations for bimanual antiphase and in-phase movements. Neuroimage 14: 674–684, 2001. [DOI] [PubMed] [Google Scholar]
- Kalaska JF, Crammond DJ. Cerebral cortical mechanisms of reaching movements. Science 255: 1517–1523, 1992. [DOI] [PubMed] [Google Scholar]
- Kermadi I, Liu Y, Rouiller EM. Do bimanual motor actions involve the dorsal premotor (PMd), cingulate (CMA) and posterior parietal (PPC) cortices? Comparison with primary and supplementary motor cortical areas. Somatosens Mot Res 17: 255–271, 2000. [DOI] [PubMed] [Google Scholar]
- Kermadi I, Liu Y, Tempini A, Calciati E, Rouiller EM. Neuronal activity in the primate supplementary motor area and the primary motor cortex in relation to spatio-temporal bimanual coordination. Somatosens Mot Res 15: 287–308, 1998. [DOI] [PubMed] [Google Scholar]
- Kollias SS, Alkadhi H, Jaermann T, Crelier G, Hepp-Reymond MC. Identification of multiple nonprimary motor cortical areas with simple movements. Brain Res Brain Res Rev 36: 185–195, 2001. [DOI] [PubMed] [Google Scholar]
- Komiyama T, Sato TR, O'Connor DH, Zhang YX, Huber D, Hooks BM, Gabitto M, Svoboda K. Learning-related fine-scale specificity imaged in motor cortex circuits of behaving mice. Nature 464: 1182–1186, 2010. [DOI] [PubMed] [Google Scholar]
- Kurata K. Conditional selection of contra- and ipsilateral forelimb movements by the dorsal premotor cortex in monkeys. J Neurophysiol 103: 262–277, 2010. [DOI] [PubMed] [Google Scholar]
- Lu MT, Preston JB, Strick PL. Interconnections between the prefrontal cortex and the premotor areas in the frontal lobe. J Comp Neurol 341: 375–392, 1994. [DOI] [PubMed] [Google Scholar]
- Luppino G, Matelli M, Camarda R, Rizzolatti G. Corticocortical connections of area F3 (SMA-proper) and area F6 (pre-SMA) in the macaque monkey. J Comp Neurol 338: 114–140, 1993. [DOI] [PubMed] [Google Scholar]
- Maier MA, Armand J, Kirkwood PA, Yang HW, Davis JN, Lemon RN. Differences in the corticospinal projection from primary motor cortex and supplementary motor area to macaque upper limb motoneurons: an anatomical and electrophysiological study. Cereb Cortex 12: 281–296, 2002. [DOI] [PubMed] [Google Scholar]
- Matelli M, Luppino G, Rizzolatti G. Architecture of superior and mesial area 6 and the adjacent cingulate cortex in the macaque monkey. J Comp Neurol 311: 445–462, 1991. [DOI] [PubMed] [Google Scholar]
- Matsuzaka Y, Aizawa H, Tanji J. A motor area rostral to the supplementary motor area (presupplementary motor area) in the monkey: neuronal activity during a learned motor task. J Neurophysiol 68: 653–662, 1992. [DOI] [PubMed] [Google Scholar]
- Mitz AR, Wise SP. The somatotopic organization of the supplementary motor area: intracortical microstimulation mapping. J Neurosci 7: 1010–1021, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morecraft RJ, Van Hoesen GW. Cingulate input to the primary and supplementary motor cortices in the rhesus monkey: evidence for somatotopy in areas 24c and 23c. J Comp Neurol 322: 471–489, 1992. [DOI] [PubMed] [Google Scholar]
- Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci 9: 856–869, 2008. [DOI] [PubMed] [Google Scholar]
- Naito E, Nakashima T, Kito T, Aramaki Y, Okada T, Sadato N. Human limb-specific and non-limb-specific brain representations during kinesthetic illusory movements of the upper and lower extremities. Eur J Neurosci 25: 3476–3487, 2007. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Hosaka R, Tsuda I, Tanji J, Mushiake H. Two-dimensional representation of action and arm-use sequences in the presupplementary and supplementary motor areas. J Neurosci 33: 15533–15544, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci 2: 417–424, 2001. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL. Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex 6: 342–353, 1996. [DOI] [PubMed] [Google Scholar]
- Richardson AG, Lassi-Tucci G, Padoa-Schioppa C, Bizzi E. Neuronal activity in the cingulate motor areas during adaptation to a new dynamic environment. J Neurophysiol 99: 1253–1266, 2008. [DOI] [PubMed] [Google Scholar]
- Roland PE, Larsen B, Lassen NA, Skinhoj E. Supplementary motor area and other cortical areas in organization of voluntary movements in man. J Neurophysiol 43: 118–136, 1980. [DOI] [PubMed] [Google Scholar]
- Russo GS, Backus DA, Ye S, Crutcher MD. Neural activity in monkey dorsal and ventral cingulate motor areas: comparison with the supplementary motor area. J Neurophysiol 88: 2612–2629, 2002. [DOI] [PubMed] [Google Scholar]
- Sanides F. The cyto-myeloarchitecture of the human frontal lobe and its relation to phylogenetic differentiation of the cerebral cortex. J Hirnforsch 7: 269–282, 1964. [PubMed] [Google Scholar]
- Sanides F. Functional architecture of motor and sensory cortices in primates in the light of a new concept of neocortex evolution. In: The Primate Brain: Advances in Primatology, edited by Noback CR, Montagna W. New York: Appleton-Century-Crofts Educational Division/Meredith, 1970, p. 137–201. [Google Scholar]
- Sanides F, Krishnamurti A. Cytoarchitectonic subdivisions of sensorimotor and prefrontal regions and of bordering insular and limbic fields in slow loris (Nycticebus coucang coucang). J Hirnforsch 9: 225–252, 1967. [PubMed] [Google Scholar]
- Sato TR, Gray NW, Mainen ZF, Svoboda K. The functional microarchitecture of the mouse barrel cortex. PLoS Biol 5: e189, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima K, Aya K, Mushiake H, Inase M, Aizawa H, Tanji J. Two movement-related foci in the primate cingulate cortex observed in signal-triggered and self-paced forelimb movements. J Neurophysiol 65: 188–202, 1991. [DOI] [PubMed] [Google Scholar]
- Shima K, Tanji J. Role for cingulate motor area cells in voluntary movement selection based on reward. Science 282: 1335–1338, 1998. [DOI] [PubMed] [Google Scholar]
- Shipp S. The importance of being agranular: a comparative account of visual and motor cortex. Philos Trans R Soc Lond B Biol Sci 360: 797–814, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada M, Tokuno H, Hamada I, Inase M, Ito Y, Imanishi M, Hasegawa N, Akazawa T, Hatanaka N, Nambu A. Organization of inputs from cingulate motor areas to basal ganglia in macaque monkey. Eur J Neurosci 14: 1633–1650, 2001. [DOI] [PubMed] [Google Scholar]
- Takeuchi D, Hirabayashi T, Tamura K, Miyashita Y. Reversal of interlaminar signal between sensory and memory processing in monkey temporal cortex. Science 331: 1443–1447, 2011. [DOI] [PubMed] [Google Scholar]
- Tanji J. The supplementary motor area in the cerebral cortex. Neurosci Res 19: 251–268, 1994. [DOI] [PubMed] [Google Scholar]
- Tanji J. New concepts of the supplementary motor area. Curr Opin Neurobiol 6: 782–787, 1996. [DOI] [PubMed] [Google Scholar]
- Tanji J, Okano K, Sato KC. Relation of neurons in the nonprimary motor cortex to bilateral hand movement. Nature 327: 618–620, 1987. [DOI] [PubMed] [Google Scholar]
- Tanji J, Okano K, Sato KC. Neuronal activity in cortical motor areas related to ipsilateral, contralateral, and bilateral digit movements of the monkey. J Neurophysiol 60: 325–343, 1988. [DOI] [PubMed] [Google Scholar]
- Vogt BA. Cingulate Neurobiology and Disease. Oxford: Oxford University Press, 2009. [Google Scholar]
- Vogt BA, Pandya DN, Rosene DL. Cingulate cortex of the rhesus monkey: I. Cytoarchitecture and thalamic afferents. J Comp Neurol 262: 256–270, 1987. [DOI] [PubMed] [Google Scholar]
- Wang Y, Matsuzaka Y, Shima K, Tanji J. Cingulate cortical cells projecting to monkey frontal eye field and primary motor cortex. Neuroreport 15: 1559–1563, 2004. [DOI] [PubMed] [Google Scholar]
- Wang Y, Shima K, Sawamura H, Tanji J. Spatial distribution of cingulate cells projecting to the primary, supplementary, and pre-supplementary motor areas: a retrograde multiple labeling study in the macaque monkey. Neurosci Res 39: 39–49, 2001. [DOI] [PubMed] [Google Scholar]
- Wise SP, Mauritz KH. Set-related neuronal activity in the premotor cortex of rhesus monkeys: effects of changes in motor set. Proc R Soc Lond B Biol Sci 223: 331–354, 1985. [DOI] [PubMed] [Google Scholar]