Abstract
Much is known on how select sensory feedback contributes to the activation of different motoneuron pools in the locomotor control system of stick insects. However, even though activation of the stance phase muscles depressor trochanteris, retractor unguis, flexor tibiae and retractor coxae is correlated with the touchdown of the leg, the potential sensory basis of this correlation or its connection to burst intensity remains unknown. In our experiments, we are using a trap door setup to investigate how ground contact contributes to stance phase muscle activation and burst intensity in different stick insect species, and which afferent input is involved in the respective changes. While the magnitude of activation is changed in all of the above stance phase muscles, only the timing of the flexor tibiae muscle is changed if the animal unexpectedly steps into a hole. Individual and combined ablation of different force sensors on the leg demonstrated influence from femoral campaniform sensilla on flexor muscle timing, causing a significant increase in the latencies during control and air steps. Our results show that specific load feedback signals determine the timing of flexor tibiae activation at the swing-to-stance transition in stepping stick insects, but that additional feedback may also be involved in flexor muscle activation during stick insect locomotion. With respect to timing, all other investigated stance phase muscles appear to be under sensory control other than that elicited through touchdown.
Keywords: sensory control, campaniform sensilla, load sensing, locomotion, species comparison
leg movement during steps in vertebrates and invertebrates alike is the joint result of central rhythmic activity combined with sensory feedback, which are ultimately also responsible for the coordinated movement of the limbs and body (e.g., insects: Büschges 2005; Büschges et al. 1995; turtle: Robertson et al. 1985; mammals: Brown 1911; for review see Kiehn and Kjaerulff 1998; Pearson 2008). The relative importance of central output vs. sensory feedback for the timing of the stance and swing muscles has been discussed since the early days of neuroscience (Brown 1911; Prochazka et al. 2000; Sherrington 1910). Since then, proprioceptive sensory feedback from leg sense organs has been known to be integrated with central pattern generator activity of the individual leg joints to control the magnitude of activation and the timing of phase transitions between muscle antagonists (for detailed reviews, see Büschges 2005; Büschges and Gruhn 2008; Duysens et al. 2000; Ekeberg et al. 2004; Orlovsky et al. 1999). The transition between the stance and swing phases of a step cycle has been investigated particularly well and is known to be affected both by position and load signals from the same as well as from neighboring legs (Bässler 1967, 1993; Conway et al. 1987; Cruse 1985, 1990; Duysens and Pearson 1980; Gorassini et al. 1994; Hiebert and Pearson 1999; Wendler 1964; Zill et al. 2009). In vertebrates, the relative contributions of central vs. peripheral influences during step cycle transitions were clarified through trap door experiments with cats. These experiments, in which a cat stepped into a suddenly appearing hole in the ground, have shown that the first phase of leg extensor activity is centrally driven up until at least 30 ms after touchdown (TD) (Gorassini et al. 1994; Hiebert et al. 1994) and sensory feedback appears to take effect only thereafter. In this case, loading of the leg only influences the magnitude of muscle activation. For invertebrates, namely the stick insect, several sensory inputs are known to provide a sensory input that facilitates transitions between antagonist muscle pairs (for review, see Büschges 2005). Position and load feedback, as well as coordinating influences from the neighboring legs, for example, are known to affect the stance-to-swing transition (Bässler 1967, 1977; Cruse 1985, 1990; Wendler 1964). Similarly, in the swing-to-stance transition, flexor muscle activation has been described to be tightly coupled with the TD of the leg (Bässler et al. 1991; Berendes et al. 2013; Cruse 1985; Gruhn et al. 2006; Rosenbaum et al. 2010). Yet, in this latter case, it is unresolved to what extent the coupling is dependent on sensory feedback or on central drive. Recently, a laser-assisted trap door setup has been developed to help the studying of the role of local sensory input for the activation of select stick insect leg muscles (Berendes et al. 2013). With this approach, similar to that used in the cat (Gorassini et al. 1994), it was shown that flexor activation was drastically delayed or even absent when ground contact was unexpectedly lowered during swing, and it was suggested that missing load feedback might be the cause of this delayed flexor activation (Berendes et al. 2013). Load sensing, which in vertebrates can be provided by tendon organs (Prochazka et al. 1997), in insects is provided by campaniform sensilla (CS) (Büschges and Gruhn 2008; Zill et al. 2004). In the stick insect and the cockroach, femoral (feCS) and trochanteral CS (trCS), are capable of signaling tarsal ground contact and have been reported to take part in the initiation and maintenance of stance (Akay et al. 2001, 2004; Rosenbaum et al. 2010; Zill et al. 2004, 2009). Their feedback can influence the magnitude and timing of motoneuron (MN) activity. A load increase has been shown to initiate and increase depressor activation (Borgmann et al. 2011; Cruse et al. 1993; Rosenbaum et al. 2010; Watson et al. 2002; Zill et al. 2004, 2009), as well as its duration (Pearson and Bradley 1972; Rosenbaum et al. 2010; Watson and Ritzmann 1998a, 1998b; Zill et al. 1999), and ablation of feCS was shown to reduce flexor tibiae (FlxTi) muscle electromyogram (EMG) magnitude (Akay et al. 2001). However, so far no direct demonstration exists for the sensory control of stance muscle activation through the ground contact signal in general, and the influence of CS or other sense organs in this context in particular (for review see Büschges 2005).
We have used the trap door approach to allow the systematic investigation of the influence that lack of ground support has on the timing of activation of the major stance phase muscles of the stick insect. The muscles investigated were the retractor coxae (RetCx) in forward and protractor coxae (ProCx) in backward walking, and the FlxTi, the depressor trochanteris (DepTr) and the retractor unguis (RetUng). Furthermore, we have analyzed the sensory influences from the leg onto flexor muscle activation. For this purpose, we selectively ablated either single or multiple sense organs, amputated parts of the leg, and compared latencies of the initial activation of the flexor muscle between normal steps on ground (SG) and steps into the hole (SIH). Finally, we compared the response in the stick insect Carausius morosus to that of the two other phasmid species Cuniculina impigra and Aretaon asperrimus, which are often used interchangeably in motor control research to determine if the findings are transferrable between species.
MATERIALS AND METHODS
Experimental animals.
The experiments were carried out on adult female stick insects of the species Carausius morosus, Cuniculina impigra and Aretaon asperrimus. The animals were taken from a colony at the Biocenter of the University of Cologne, kept at 20–22°C under a 12:12-h light-dark cycle. The stick insects were fed blackberry leaves (Rubus fructiosus) ad libitum.
Experimental setup.
The setup used has been described in detail in Berendes et al. (2013). In brief, animals were tethered (see below) above a slippery surface which had a separate platform for one leg (49 mm × 34 mm, stainless steel surface) integrated into it at the same height. This platform could be lowered pneumatically (SLS-6-25-P-A; FESTO mini slide, Esslingen, Germany) to levels in 2-mm intervals below the original walking surface level. Ground contact of the leg was detected electrically (Gruhn et al. 2006) by means of a lock-in-amplifier (electronics workshop, Zoological Institute, Cologne, Germany) that amplifies the voltage at the animal during application of a specific small current to the slippery surface at any platform level. In addition, a sheet of laser light (LG series, 1 mW, 660 nm, Lasertechs, Aschaffenburg, Germany) was used to detect virtual ground contact after lowering the platform (photo detector SLCD-61N4, Silonex, Montreal, Canada). Directly following manual initialization, the drop of the lowerable platform occurred after the next tarsal liftoff, which in turn was detected by the ground contact detection circuit. The activation of the laser light sheet, together with the drop of the platform, allowed signaling the next passing of the tarsus through the former ground level. The surface was brought back to its original position pneumatically at any time thereafter chosen by the experimenter. Tarsal ground and virtual ground detection signals were fed into an analog-to-digital converter (Micro1401, CED, Cambridge, UK), and recorded using Spike2 software (version 7, CED, Cambridge, UK). The period of maximum amplitude (Amax) in the tarsal contact trace marked the swing phase (tarsus lifted off), while that of minimum amplitude marked stance (tarsus in contact with surface). The onset of the digital pulse produced by the laser signal marked the time when the tarsus crossed the virtual ground level for the first time. Any additional deflection in the laser contact trace could be caused by either a passing of the leg through the laser signal, or the misreading of the laser signal by the detector, caused by positional changes of the leg in the light path. High-speed video (AOS S-PRI, AOS Technology AG, Baden Daettwil, Switzerland, resolution: 400 × 1,024 pixel, frame rate: 500 fps, shutter speed: 2000 μs) during some of the experiments was used to ensure the accuracy of the electrical and the laser-sheet measurements.
Preparation.
The animal was glued ventral side down (two component glue, ProTemp II, ESPE, Seefeld, Germany) onto a balsa stick as described in Berendes et al. (2013). The animal was induced to autotomize the hind and front legs as described in Schmidt and Grund (2003). If autotomy could not be induced, legs were removed by cutting them off with a pair of scissors at the level of the coxa. Forward walking was elicited by brush strokes to the abdomen, backward walking by gently pulling on the antennae. EMGs of the stance phase muscles RetCx in forward and ProCx in backward walking, the FlxTi, the DepTr, and the tibial branch of the RetUng were performed using two twisted copper wires (51 μm outer Ø) (Rosenbaum et al. 2010). The location of the EMG recording sites was according to described locations (Radnikow and Bässler 1991; Rosenbaum et al. 2010). The freshly cut-off tips of the wires were inserted through small holes in the cuticle into these muscles and held in place by ProTemp II glue. The signal was preamplified 100-fold with an isolated preamplifier (MA101, electronics workshop, Zoological Institute, Cologne, Germany), and further amplified 10-fold with a main amplifier/signal conditioner (MA102, electronics workshop, Zoological Institute, Cologne, Germany). For all experiments, the signal was band-pass filtered 100 Hz to 1,000 Hz. Cross talk from the extensor tibiae muscle was present in most of the flexor EMGs recorded. Flexor activity, however, was easily distinguished from extensor potentials, based on its larger amplitude and lower frequency. The FlxTi muscle is multiply innervated and shows different innervation patterns over the length of the muscle (Debrodt and Bässler 1989; Goldammer et al. 2012). As it has been previously reported that the latencies of activation in this muscle can depend on the site of the recording proximally or distally within the femur (Berendes et al. 2013), care was taken to place the EMG wires always at the end of the proximal third of the femur. A threshold line was placed above the level of the extensor potentials to determine the time of the first large flexor potential unit of every stance phase.
All experiments involving ablation and amputation were carried out 24 h after surgery on animals which had not been used for control experiments previously, because of the deterioration of the EMG signal over this prolonged period. However, care was taken to have equal numbers of recordings from control animals without ablation. CS were ablated as described previously (Zill et al. 2011) by destroying the caps and subsequent insertion of a fine minuten pin into the destroyed cap. Ablated CS included tibial CS (tiCS; groups 6A and B; Zill et al. 2011) and two additional groups anterior and posterior from these; feCS (Akay et al. 2001) and trCS (groups 1–4, Bässler 1977; Zill et al. 2012). In addition to the CS, the RetUng tendon was cut. For this purpose, a window was cut into the cuticle of the tibia. The tendon was lifted with an insect pin, while care was taken not to damage nerves or muscles in the leg, and then cut with a pair of scissors. Afterwards, the cuticle was closed. For tarsus ablation, the tibia was cut just above the tibia-tarsus joint. Different sense organs or the tendon were either destroyed alone or in combinations thereof, as described in the results section.
Evaluation of muscle latencies and magnitude of muscle activity.
Stance phase muscle activation latencies were calculated by using the first EMG spike of the stance phase muscle in question that was visible above noise level, with respect to TD, as measured by the tarsal contact signal or the virtual TD (vTD) signal at the pass through the laser light sheet. For comparison of EMG activities between SG and SIH, original recordings were rectified and smoothed (time constant 20 ms). The integral of muscle activity was compared between the first 200 ms after TD and 200 ms after the vTD, unless the antagonist was activated earlier, and a second step was initiated within the time window. In that case, the steps were not used for analysis. The time window of 200 ms was chosen after a comparison of the stance phase lengths to represent an average length before the start of swing phase muscle activity. In addition to the total value of the integral, the maximum value of the integrals in the 200 ms interval after TD and vTD, respectively, was also measured, and all values exported to Origin (version 8.5, Origin Lab, Northhampton, MA).
The number of animals used for each experiment were as follows: three each for the RetCx, ProCx, and DepTr; nine for the FlxTi; and four for the RetUng for the stance phase muscle activity analysis in C. morosus. For the comparative analysis of flexor muscle activity, we used data from nine animals of C. morosus, five of Cuniculina impigra and four of Aretaon asperrimus. For ablation experiments, the feCS were ablated in three, and the tiCS or trCS were ablated in five animals. In three animals, the feCS and trCS were ablated together. In five animals, the tarsus was cut off, and the tiCS, trCS, and feCS were ablated. Finally, the RetUng tendon was cut in seven animals. The number of animals used for a specific condition (N) and the number of steps evaluated (n) for a specific experiment are also given in the figures.
Statistics.
All statistics were performed using Origin (version 8.5, Origin Lab, Northhampton, MA). The data sets acquired in this study were tested for normal distribution using the Shapiro-Wilk test. According to this test, none of the data presented in this study were distributed normally. For this reason, all tests for statistical significance were performed using nonparametric statistical tests. For data sets dependent on each other, the Wilcoxon signed-rank test was used. For this purpose, latencies, the integral or the amplitude measured during three control steps in one walking sequence were pooled. The Mann-Whitney-U-test was used to compare independent data sets. Significance was marked in Figs. 2–5 with the following symbols: * for a significance level of P < 0.05; ** for a significance of P < 0.01 and *** for P < 0.001. N denotes the number of animals, while n represents the number of steps.
Fig. 2.
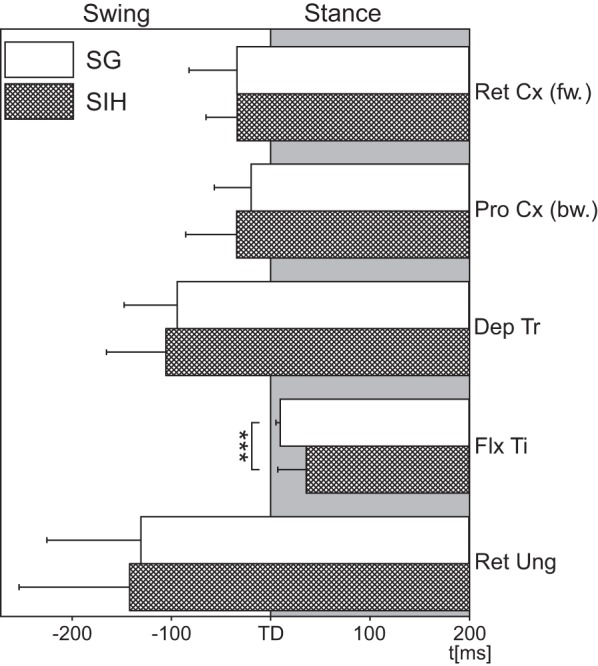
Schematic comparing activation latencies for the different stance muscles during SG (open bars) and SIH (filled bars). fw, Forward; bw, backward. RetCx: N = 3, nSG = 27, nSIH = 9; ProCx: N = 3, nSG = 27, nSIH = 9; DepTr: N = 3, nSG = 27, nSIH = 9; FlxTi: N = 9, nSG = 171, nSIH = 57; RetUng: N = 4, nSG = 36, nSIH = 12. N = number of animals; n = number of steps; the gray shaded area marks stance. Values are means ± SD. ***Significant difference, P < 0.001.
Fig. 5.
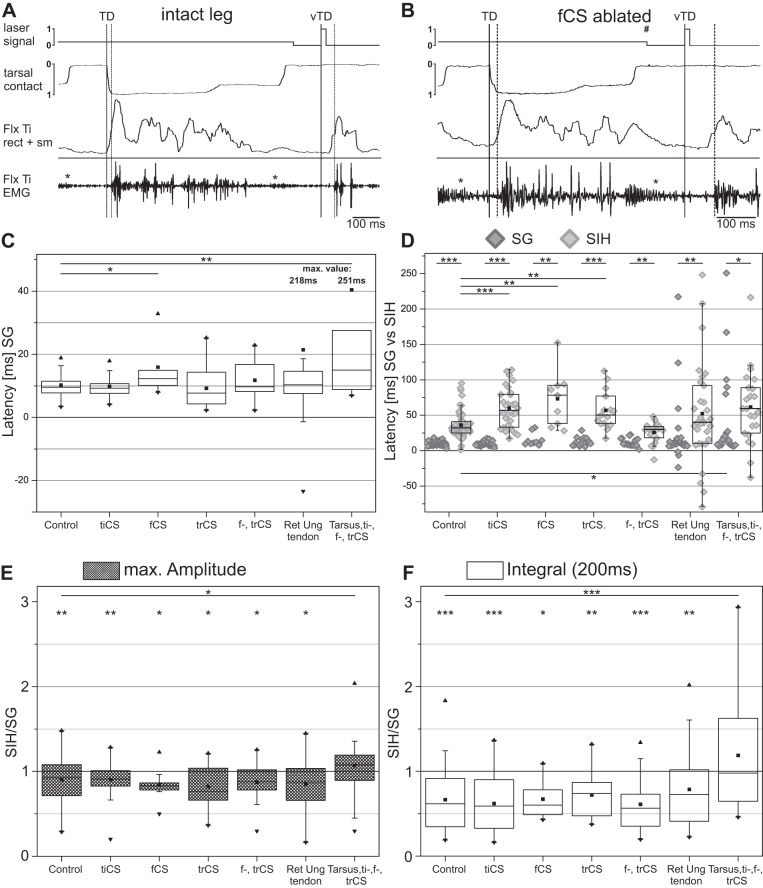
Effects of ablation experiments. A: example of FlxTi activation during SG and SIH in an intact animal. B: example of the FlxTi activation during SG and SIH. Solid vertical lines mark TD or vTD, dashed vertical lines mark beginning of muscle activity in EMG trace, asterisks mark crosstalk from the extensor tibiae muscle, the number sign marks the time of the platform drop. C: latencies of FlxTi activation after ablation of different sense organs during SG. D: scatter plot with the values of FlxTi activation latencies for comparison between SG and SIH after ablation of different sense organs; the median and interquartile range of the SG values are shown in C and therefore omitted for the sake of clarity. E and F: ratio of the maximal FlxTi burst amplitude (E) and total integral of rectified and smoothed FlxTi burst activity normalized to SG burst activity (F) during the 200-ms period after TD between SIH and SG after ablation of different sense organs. Control: N = 9, nSG = 171, nSIH = 57; tibial campaniform sensilla (tiCS) ablated: N = 5, nSG = 90, nSIH = 30; femoral campaniform sensilla (feCS) ablated: N = 3, nSG = 27, nSIH = 9; femoral (feCS) and trochanteral campaniform sensilla (trCS) ablated: N = 3, nSG = 54, nSIH = 18; trCS ablated: N = 5, nSG = 45, nSIH = 15; RetUng tendon cut: N = 7, nSG = 93, nSIH = 31; tarsus CS, tiCS, feCS, and trCS ablated: N = 5, nSG = 69, nSIH = 23. N = number of animals; n = number of steps. Values are means ± SD. Significant difference: *P < 0.05; **P < 0.01, and ***P < 0.001.
RESULTS
During walking, the different stance phase muscles are activated in a specific sequence that is influenced by sensory input (Büschges 2005; Rosenbaum et al. 2010). In this context, ground contact has been demonstrated to be of great importance for the activation of FlxTi muscle (Berendes et al. 2013). However, it remains unclear to what extent the activity of other stance phase muscles (ProCx and RetCx, DepTr, RetUng) may also be modified by sensory feedback elicited by ground contact of the tarsus. In addition, it also remains unclear what sensory signals are mediating the coupling between ground contact and stance muscle activation. Here we use a trap door setup described by Berendes et al. (2013) to investigate the influence of ground contact on all stance phase muscles and pin down potential sensory signals that could mediate this relationship.
Influence of ground contact on stance muscle activation latency.
In the first set of experiments we implanted EMG wires into the stance phase muscles RetCx (during forward walking), its antagonist the ProCx (during backward walking), and DepTr, FlxTi and RetUng, which act as stance muscles in both walking directions. Figure 1 shows example recordings from each of these muscles with EMG, rectified and smoothed EMG, and with the respective tarsal contact trace for one SG and one subsequent SIH (Fig. 1, A and C–G) and the recording sites (Fig. 1B).
Fig. 1.
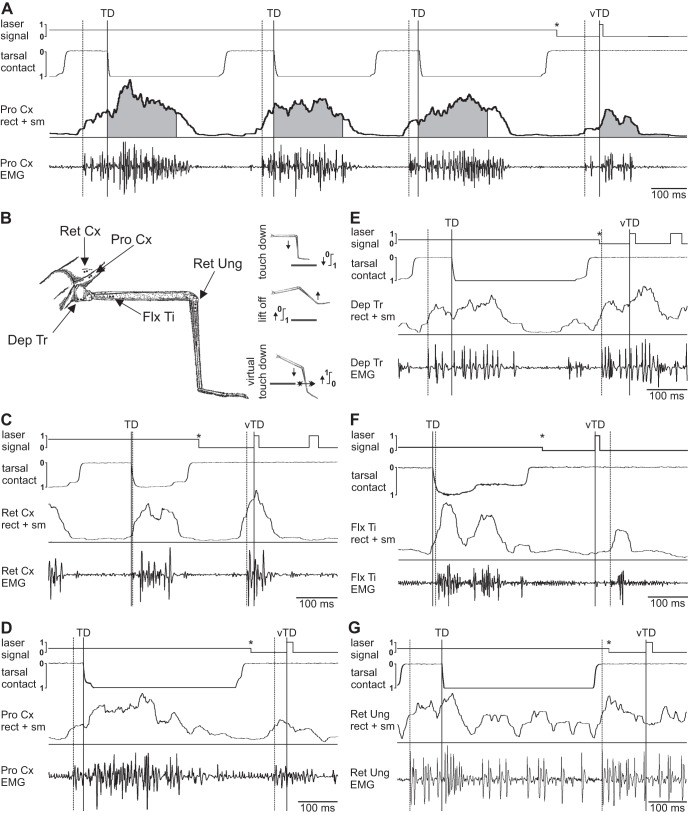
A: example trace for a recording of three consecutive steps of the protractor coxae (ProCx) muscle during backward walking on the ground [after touchdown (TD)] and one step into the hole (SIH) [after virtual touchdown (vTD)]. Top trace marks laser signal, 2nd trace from top is the electrical tarsal contact trace, followed by the rectified (rect) and smoothed (sm) EMG and the original EMG recording. Gray shaded area under the rectified trace marks 200-ms time window for analysis. B: schematic drawing of the right middle leg, with marked EMG recording sites and scheme for current flow during TD, lift off and the vTD. C–G: example EMGs of all stance phase muscles during one step on the ground (SG) (after TD) and one SIH (after vTD); traces are the same as in A. C: retractor coxae (RetCx) during forward walking. D: ProCx during backward walking. E: depressor trochanteris (DepTr). F: flexor tibiae (FlxTi). G: retractor unguis (RetUng). Solid vertical lines mark TD or vTD, dashed vertical lines mark beginning of muscle activity in EMG trace, asterisks mark platform drop. The additional deflections in the laser signal in C and E are movement artifacts caused by misreading of the detector.
The RetCx moves the coxa posteriorly during stance in forward steps. It was activated around the time of TD, and no difference in muscle activation was discernible between SG and SIH (Fig. 1C). Retractor activation latency in three animals during SG started on average −34.0 ms before TD in control steps (SD = 32.1; N = 3, n = 27), and not significantly different from the −33.5 ms for the SIH (SD = 21.0; N = 3, n = 9). During backward walking, the ProCx takes over the role of the retractor, but moves the leg forward during the stance phase. An example of the activity is shown in Fig. 1A and, at larger time resolution, in Fig. 1D. The average latency of ProCx activation for the control steps of three animals was −19.4 ms (SD = 24.7; N = 3, n = 27), not significantly different from the −34.1 ms for the SIH (SD = 34.3; N = 3, n = 9). The third stance phase muscle investigated, the DepTr, is located in the coxa and is responsible for lowering the leg. Its activation both, during SG and SIH occurred well before TD (Fig. 1E). In the animals tested, depressor activity began on average −93.5 ms before TD in control steps (SD = 35.6; N = 3, n = 27), which was not significantly different from the latency of −105.5 ms before SIH (SD = 39.9; N = 3, n = 9). The correlation of FlxTi activation to the time TD has been shown previously (Berendes et al. 2013; Gruhn et al. 2006; Rosenbaum et al. 2010). Figure 1F shows an example of flexor activity during a ground supported step and after stepping into the hole. Similar to earlier results by Berendes et al. (2013), upon SIH, the latency of activation for the flexor was shifted significantly (P < 0.001) to a later point in time (mean = 36.3 ms, SD = 19.1; N = 9, n = 57) compared with regular SG (mean = 10.2 ms, SD = 3.0; N = 9, n = 171). The last leg muscle tested was the second part of the RetUng, located in the proximal tibia, which is responsible for lowering and flexing the claw (Fig. 1G). The RetUng was activated around the time of lift off, on average −130.3 ms before TD for the control steps (SD = 63.3; N = 4, n = 36), and −142.1 ms, for the SIH (SD = 74.5; N = 4, n = 12), which was again not significantly different from one another. The results are summarized in Fig. 2. Of all stance muscles investigated, stepping into a hole only affected the latency of activation of the FlxTi significantly, confirming earlier results on this particular muscle (Berendes et al. 2013). All other muscles are activated before TD and do not show a change in latency. So, with respect to timing, this suggests that ground contact affects only in the flexor muscle.
Influence of ground contact on stance muscle activation strength.
Despite the lack of influence of ground contact signaling on the activation latency of the stance phase muscles other than the flexor, there was still the possibility that ground contact alters the activity in an ongoing stance phase after TD, as has been described for the flexor and depressor muscles (Gabriel and Büschges 2007; Rosenbaum et al. 2010). To explore this, we compared the strength of EMG activity within a time window of 200 ms either after real TD or after the passing through the laser sheet. As a measure, we took the ratio of either the maximal amplitudes during steps with and without ground contact, or that of the total area of the rectified and smoothed integrals of the EMG activity of the different stance phase muscles during steps with and without ground contact. The Amax gives an idea about the maximum intensity of muscle activation, whereas the total area can be an additional measure of the activity within the predefined time window.
We first compared the maximum of the integral amplitudes during SIH to those during SG. If the ratio was smaller than 1, then the maximum activity of the muscle during SIH was smaller, and if it was greater than 1, then activity during SIH had increased. No significant differences between the Amax under the two situations were seen in the RetCx (Amax-ratioRetCx = 1.04, SD = 0.23). The maximum depressor activity was significantly greater after SIH (Amax-ratioDepTr = 1.51, SD = 0.5, P < 0.01), whereas ProCx, FlxTi and RetUng maximum activity were significantly greater during SG (Amax-ratioProCx = 0.68, SD = 0.22, P < 0.5; Amax-ratioFlxTi = 0.9, SD = 0.25, P < 0.01; Amax-ratioRetUng = 0.86, SD = 0.16, P < 0.05) (Fig. 3A).
Fig. 3.
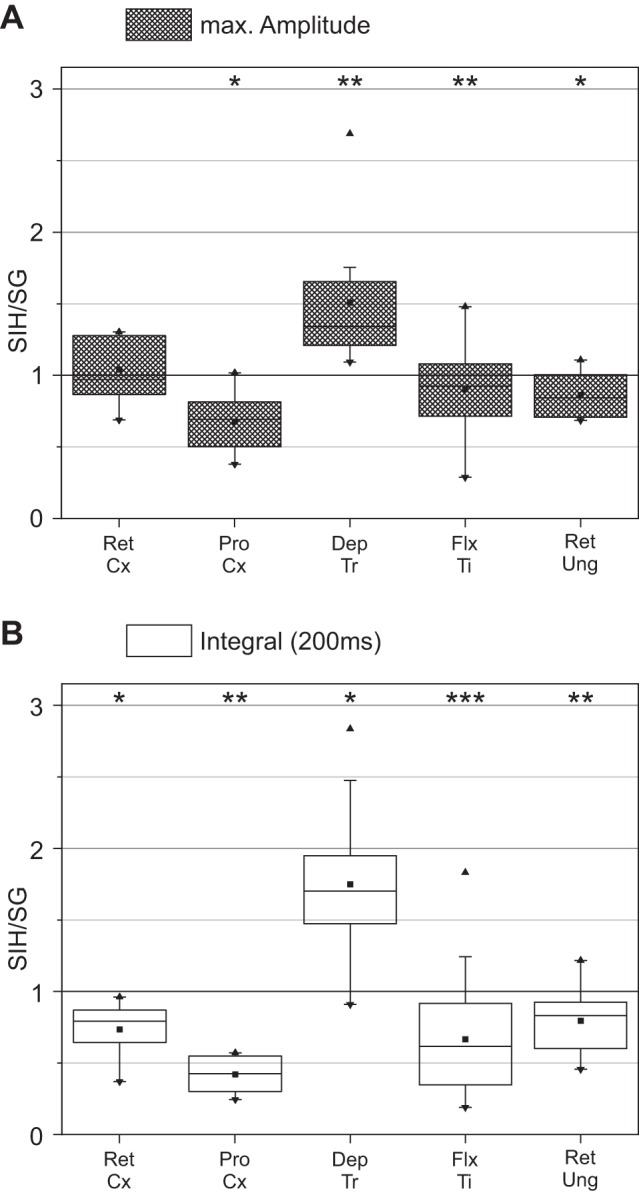
Ratio of the maximal burst amplitude (A) and total integral of rectified and smoothed burst activity normalized to SG burst activity (B) of the stance phase muscle during the 200-ms period after TD between SIH and SG. RetCx: N = 3, nSG = 21, nSIH = 7; ProCx: N = 3, nSG = 24, nSIH = 8; DepTr: N = 3, nSG = 27, nSIH = 9; FlxTi: N = 9, nSG = 171, nSIH = 57; RetUng: N = 4, nSG = 36, nSIH = 12. N = number of animals; n = number of steps. Values are means ± SD. Significant difference: *P < 0.05; **P < 0.01, and ***P < 0.001.
The analysis of the total area under the integral of the rectified EMG activity within a 200-ms time window after either the real or the fictive TD showed that the SIH-to-SG activity ratios in the depressor were clearly increased after stepping into the hole (mean ratioDepTr = 1.75, SD = 0.63, P < 0.05), whereas RetCx and ProCx, FlxTi and RetUng activity was clearly decreased (mean ratioRetCx = 0.73, SD = 0.19, P < 0.05; mean ratioProCx = 0.42, SD = 0.13, P < 0.01; mean ratioFlxTi = 0.67, SD = 0.38, P < 0.001; mean ratioRetUng = 0.8, SD = 0.22, P < 0.01) (Fig. 3B). All values including median and interquartile range (IQR) are summarized in Table 1.
Table 1.
Ratios of the burst integrals during SIH vs. SG for the stick insect stance muscles
| Mean | SD | Median | IQR | |
|---|---|---|---|---|
| FlxTi total area | 0.67 | 0.38 | 0.62 | 0.57 |
| FlxTi maximum burst amplitude | 0.9 | 0.25 | 0.93 | 0.37 |
| RetCx total area | 0.73 | 0.19 | 0.79 | 0.23 |
| RetCx maximum burst amplitude | 1.04 | 0.23 | 0.97 | 0.41 |
| ProCx total area | 0.42 | 0.13 | 0.42 | 0.25 |
| ProCx maximum burst amplitude | 0.68 | 0.22 | 0.69 | 0.31 |
| DepTr total area | 1.75 | 0.63 | 1.7 | 0.48 |
| DepTr maximum burst amplitude | 1.51 | 0.5 | 1.34 | 0.45 |
| RetUng total area | 0.8 | 0.22 | 0.83 | 0.32 |
| RetUng maximum burst amplitude | 0.86 | 0.16 | 0.84 | 0.3 |
SIH, steps into the hole; SG, steps on the ground; IQR, interquartile range; FlxTi, flexor tibiae; RetCx, retractor coxae; ProCx, protractor coxae; DepTr, depressor trochanteris; RetUng, retractor unguis.
This demonstrates that, despite the fact that the timing of muscle activation is only affected in the flexor, ground contact, nevertheless, does influence the magnitude of activity in all other stance muscles. This influence, however, only affects already ongoing muscle activity, and the sign of the influence can either be positive or negative.
Influence of ground feedback on FlxTi in different phasmid species.
Two additional phasmid species are often used in locomotor research in addition to the well known species Carausius morosus, which have been used above. These are Cuniculina impigra, which is similar in its body proportions to Carausius morosus but of bigger size, and Aretaon asperrimus, which is similar in size as Carausius morosus, but has shorter legs and shows more frequent walking activity. Since each preparation offers particular advantages to the experimenter (Berg et al. 2013; Jeck and Cruse 2007), and especially Cuniculina and Carausius are often used interchangeably, we wanted to verify that the observed effect is a general phenomenon in phasmids.
We focused the subsequent comparative analysis on the activity of the FlxTi muscle in the three species, since it is affected by ground contact in both the timing and the magnitude of the response. During normal SG, the latency of the first EMG spike in the flexor muscle is shortest in Carausius (mean = 10.2 ms, SD = 3.0; N = 9, n = 171), followed by that in Aretaon (mean = 14.9 ms, SD = 6.4; N = 4, n = 63), and that in Cuniculina (mean = 21.4 ms, SD = 17.9; N = 5, n = 75). Between Carausius and the other two species, this difference was significant (P < 0.01 and P < 0.001, respectively), while the difference was not significant between Aretaon and Cuniculina. Activation of the flexor muscle after SIH occurred in all three species significantly later than after their respective SG (Carausius morosus: mean = 36.3 ms, SD = 19.1; N = 9, n = 57; Aretaon asperrimus: mean = 190.5 ms, SD = 90.3; N = 4, n = 21; Cuniculina impigra: mean = 50.0 ms, SD = 28.0; N = 5, n = 25; for all species P < 0.001) and was also significantly different from one another in all species (Fig. 4A). Similar to C. morosus, the Amax of the rectified flexor EMG was also lower in Aretaon (93.3%, SD = 71.1) and Cuniculina (86.4%, SD = 29.1), but these changes were not significant over the values from the SG (Fig. 4B). However, as for C. morosus (see above), the overall FlxTi activity for a time window of 200 ms after TD was also significantly lower for the other two species during SIH compared with steps with ground contact (P < 0.001). While flexor activity in C. morosus dropped to 67% of its original value (SD = 38.6), A. asperrimus flexor activity dropped to 50.5% (SD = 38.5), and C. impigra flexor activity dropped to 62.8% (SD = 28.4) (Fig. 4C).
Fig. 4.
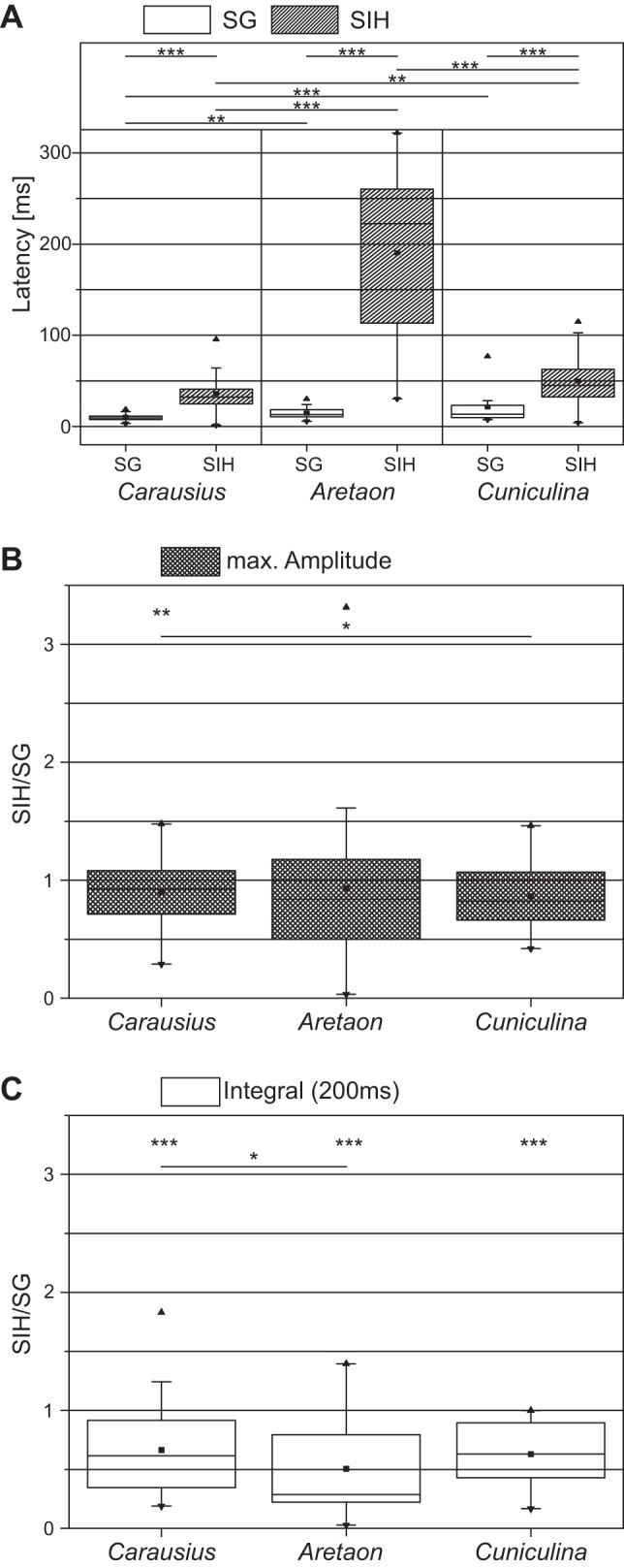
Comparison of latency of FlxTi activation and burst properties for the three species, Carausius morosus, Cuniculina impigra and Aretaon asperrimus, during SG and SIH. A: latency of FlxTi activation. Carausius morosus: N = 9, nSG = 171, nSIH = 57; Aretaon asperrimus: N = 4, nSG = 63, nSIH = 21; Cuniculina impigra: N = 5, nSG = 75, nSIH = 25. B and C: ratio of the maximal FlxTi burst amplitude (B) and total integral of rectified and smoothed FlxTi burst activity normalized to SG burst activity (C) during the 200-ms period after TD between SIH and SG. Carausius morosus: N = 9, nSG = 171, nSIH = 57; Aretaon asperrimus: N = 4, nSG = 63, nSIH = 21; Cuniculina impigra: N = 3, nSG = 39, nSIH = 13. N = number of animals; n = number of steps. Values are means ± SD. Significant difference: *P < 0.05; **P < 0.01, and ***P < 0.001.
In summary, while there are differences between the three phasmid species, all of them show the same dependency of both timing and magnitude of flexor activation on the presence of a ground contact signal. Aretaon shows a particularly dramatic dependence of flexor activation on the TD signal. Thus all three species lend themselves to investigating the relationship between sensory feedback caused by ground contact and the activation of the FlxTi muscle.
Potential sources of TD feedback for FlxTi activation.
We next investigated potential sources for the sensory feedback that influences flexor latency in Carausius morosus. Candidate sense organs that could influence either the timing or magnitude of the FlxTi activity by measuring the moment of ground contact are the trCS (groups 1–4; Bässler 1977; Zill et al. 2012) and feCS (Akay et al. 2001), as well as tiCS (groups 6A and B; Zill et al. 2011), including two additional groups anterior and posterior of tiCS 6A and 6B. In addition, in a subset of experiments, we cut the RetUng tendon to remove all potential input associated with activity in this muscle. A multipolar receptor connected to the RetUng tendon similar to ones found for more proximal muscles (Bässler 1977; Bräunig et al. 1981; Matheson and Field 1995) would be another potential candidate for the detection of the ground contact. Removal of sense organs or of the tarsus were performed either individually or in combinations thereof, and recordings were made 24 h following the procedure. Examples of a FlxTi recording during SG and SIH in a control animal and in a different animal 24 h after ablation of the feCS are shown in Fig. 5, A and B, respectively.
Ablation of either all trCS or tiCS did not have an influence on the latency of FlxTi activation during regular SG (trCSabl: SGmean = 12.9 ms, SD = 6.2, N = 5, n = 15; tiCSabl: SGmean = 9.9 ms, SD = 3.2, N = 5, n = 90) (Fig. 5C). However, the flexor latency was significantly prolonged (P < 0.05) if the feCS were ablated (feCSabl SGmean = 15.9 ms, SD = 9.2 ms, N = 3, n = 27). Interestingly, if the feCS were ablated together with the trCS, the latency of FlxTi activation after TD was not significantly prolonged compared with control animals (feCStrCSabl SGmean = 11.8 ms, SD = 5.6, N = 3, n = 54). Cutting the RetUng tendon at the level of the mid-tibia prevented the flexion of the tarsus before TD and a passive stretch of the tendon at TD, effectively removing proper feedback from such a hypothesized stretch receptor. This procedure produced a large increase in the variability of flexor latencies after TD (Vartendon|int: 15.5 ms; Vartendon|cut: 240.9 ms), but the mean values were not significantly different from controls (SGmean = 21.4 ms, SD = 43.5, N = 7, n = 93) (Fig. 5C).
For all these experiments, we also measured the latencies for the SIH with respect to the vTD. In all cases, the SIH latencies of flexor muscle activation increased significantly (Fig. 5D). In all three cases where single CS groups were ablated did this procedure also increase the latency of the flexor response to SG significantly over control SIH (trCSabl: SIHmean = 57.0 ms, SD = 27.0; N = 5, n = 15, P < 0.01; tiCSabl SIHmean = 60.1 ms, SD = 28.3, N = 5, n = 30, P < 0.001; feCSabl: SIHmean = 73.2 ms, SD = 39.4, N = 3, n = 9, P < 0.01). Interestingly, when feCS and trCS were ablated together (feCStrCSabl SIHmean = 25.7 ms, SD = 14.9, N = 3, n = 18), no significant difference in SIH latency over controls existed. Cutting the RetUng tendon had the same effect on SIH latencies as on latencies during SG, i.e., the variability was greatly increased (Vartendon|int: SIH = 94.1 ms; Vartendon|cut: SIH = 326.8 ms), but the mean latency was unchanged (tendon cut SIHmean = 52.2 ms, SD = 70.3, N = 7; n = 31). To test if redundancy from a potential interplay of different sense organs was responsible for the absence of effects during SG in most ablation experiments, we then ablated all the above CS groups and additionally removed the tarsus. Animals without these CS and without tarsus showed a great reluctance to perform continuous stepping with that leg, and the procedure greatly increased the variability of latencies recorded (VarSG = 243.9 ms; VarSIH = 254.2). In addition, mean latency for flexor activation during SG and SIH was delayed significantly (SGmean = 40.4 ms, SD = 61.0, P < 0.05; SIHmean = 61.7 ms, SD = 52.8, P < 0.05, N = 5, nSG = 69, nSIH = 23; Fig. 5, C and D).
Finally, we wanted to know if ablation of any of the sense organs or the tendon, on top of the effect on the latency of flexor activation, also affected the magnitude of activation of the flexor muscle. In intact animals, flexor activity during SIH was significantly reduced, both in Amax and total activity, as measured through the integral of the rectified and smoothed EMG (see above and Fig. 5, E and F). In all ablation situations but one, the maximum integral amplitude and total flexor activity were significantly reduced during SIH over SG (for a summary of all values, including the median and IQR range, see Table 2), but never significantly different from the reduction in the intact animal (Fig. 5, E and F). The Amax were reduced to between 82–90% of the SG value, while the total integral area was reduced to between 61–78% of the SG value. The observed changes, however, are not a result of qualitative changes in the flexor EMG compared with the control animals, such as through changes in the occurrence of large or small units or delayed recruitment of single units, in any of our single or double ablation protocols (compare Figs. 1F and 5A to Fig. 5B). The only case with a result significantly different from control animals was the experiment where all of the above CS fields and the tarsus were ablated. These animals showed a great reluctance to perform steps with the leg that had undergone the ablations. Here again, the variability was greatly increased, but neither the mean Amax nor the mean total activity were significantly different between SG and SIH. Instead, these animals often lacked large flexor units (data not shown), and both values were significantly different from the control animals without any surgery.
Table 2.
Burst integral percentage and maximum burst percentage of FlxTi muscle during SIH compared with SG for the intact leg and for different ablation scenarios
| Mean | SD | Median | IQR | |
|---|---|---|---|---|
| Burst integral, %FlxTi activity in SIH compared with SG | ||||
| Control | 66.513 | 38.359 | 61.582 | 57.03 |
| trCS ablation | 72.163 | 27.531 | 73.731 | 39.347 |
| RU tendon cut | 78.739 | 41.844 | 72.729 | 60.608 |
| fCS ablation | 67.147 | 22.96 | 60.186 | 29.023 |
| fCS, trCS ablation | 60.967 | 32.096 | 56.497 | 37.74 |
| tiCS ablation | 61.981 | 33.512 | 59.022 | 57.247 |
| Tarsus, tiCS, fCS, trCS ablation | 118.767 | 65.525 | 97.796 | 97.754 |
| Maximum burst amplitude, %FlxTi SIH compared with SG | ||||
| Control | 90.209 | 25.275 | 92.557 | 36.607 |
| trCS ablation | 82.17 | 24.943 | 76.424 | 37.807 |
| RU tendon cut | 85.144 | 27.115 | 87.457 | 37.787 |
| fCS ablation | 84.246 | 19.082 | 82.846 | 8.113 |
| fCS, trCS ablation | 87.246 | 22.483 | 87.721 | 23.658 |
| tiCS ablation | 90.072 | 22.778 | 90.918 | 18.317 |
| Tarsus, tiCS, fCS, trCS ablation | 106.828 | 37.488 | 107.608 | 29.781 |
trCS, trochanteral campaniform sensilla; RU tendon, retractor unguis tendon; fCS, femoral campaniform sensilla; tiCS, tibial campaniform sensilla.
In summary, the only single CS group with a clear effect on flexor activation latency during regular ground steps is that of the feCS. However, feCS ablation and all other single CS ablations did also affect the flexor activation latency during SIH. Cutting the RetUng tendon only affected the variability of the response, while ablation of all of the above CS groups and the tarsus (including the tendon) together affected the latencies during SG and SIH in addition to increasing the variability. Only this latter procedure affected the change in magnitude of the flexor response seen between SG and SIH, which was absent after this particular procedure only.
DISCUSSION
We have used a recently developed foot-in-the-hole setup to study systematically which leg muscles depend on the TD of the leg they are moving for their activation during walking, and to what extent. We have compared data from three different phasmid species and singled out the FlxTi muscle as the only muscle that is dependent on TD for its activation. We performed a series of ablation experiments to determine the source of the sensory feedback that contributes to this dependence. Only ablation of the local feCS directly affected flexor activation. However, a greatly increased variability in the latency of activation after lesioning the tendon of the tarsal flexor muscle RetUng suggests the involvement of additional sensory signals in flexor muscle activation at TD.
Dependence of stance muscle latency on TD.
The influences of sensory feedback about TD on the timing of the stance muscles in the stick insect are only partly known (Berendes et al. 2013). We therefore first analyzed the latencies of these muscles with respect to the electrically determined TD in a two-middle-leg preparation during regular steps on a slippery surface. We found that the flexor and the depressor latencies matched values reported in earlier studies for the intact, six-legged animal (Gruhn et al. 2006; Rosenbaum et al. 2010). Similarly, the RetUng muscle was activated during swing as reported earlier (Fischer et al. 2001). Only the latencies for the activation of RetCx (forward) and ProCx (backward) were shifted forward from the mean latencies which had been reported for the six-legged stick insect by Rosenbaum et al. (2010). However, Rosenbaum and colleagues (2010) also reported a significant forward shift for RetCx activation in the two-leg preparation, and the data reported show that activity very often began well before TD for stance in the respective directions for both muscles. Part of the discrepancy to the data from the six-legged animal could be the result of a generally large variability in the latencies of this muscle. The latency shift observed by Rosenbaum and colleagues, and our latencies, however, also suggest a timing influence caused by the activity of adjacent legs for this particular muscle. Previous studies have already shown that front leg stepping influences activation of the middle leg RetCx MNs during forward walking (Borgmann et al. 2007, 2009), but the source or sources for such an interleg sensory influence are as yet not clear. One of them could be the front leg femoral chordotonal organ (fCO) (Ludwar et al. 2005).
Effects of lack of ground support on stance muscles.
SIH should reveal if ground contact affects the activation of a given muscle through a shift in its activation latency. A similar approach in cat, in which the animal stepped into a suddenly appearing hole in the ground, showed that ground contact influences the leg stance muscle lateral gastrocnemius only in the magnitude of its activation, and not until 30 ms after TD (Gorassini et al. 1994; Hiebert et al. 1994). The SIH scenario in the stick insect affected activation strength and latency of the different stance muscles to a different extent, and only in select muscles. The latency of activation was only affected in the FlxTi muscle, where it was significantly greater for SIH than for SG. This matches and confirms earlier reports by Berendes and colleagues (2013) and shows that the TD signal appears to be only necessary for the timing of this muscle. In contrast, the intensity, or magnitude of muscle activity was influenced in all muscles, albeit in different directions. RetCx, ProCx, FlxTi and RetUng showed a significant decrease, and the DepTr a significant increase in activity. This corroborates the importance of local feedback for the magnitude control.
For all of these muscles, at least single local sources for sensory influences are known. Activation of the RetCx during forward walking is facilitated by activation of the fCO through extension of the femur-tibia joint (Bässler 1986). In addition, the ventral coxal hair plate is known to report the position of the coxa, and its stimulation initiates and sustains RetCx activation (Bässler 1977; Cruse et al. 1984). ProCx activation, on the other hand, is influenced by stimulation of coxal hair rows (Cruse et al. 1984), and it does not seem unlikely that their stimulation also helps in initiating protraction during stance in backward walking. Local load signals are also known to influence both RetCx and ProCx activation and activity. Feedback from the trCS supports termination of ProCx and initiation of RetCx (Akay et al. 2004, 2007), as well as an increase in RetCx activity (Schmitz 1993) during forward and vice versa for ProCx activity during backward walking (Akay et al. 2007). In addition, stimulation of the trCS is able to sustain stance (Bässler 1977). It is therefore interesting that the latency in the RetCx in forward, or ProCx in backward walking, is unchanged during SIH. This suggests that the local TD signal is of relatively little importance for their timing when they are used as stance muscles in the two-leg preparation. The lack of change in DepTr activation latency upon SIH supports earlier studies (Bässler 1967; Bucher et al. 2003; Graham and Bässler 1981; Hess and Büschges 1999), in which it was shown that the extension of the tibia, as measured by the fCO, is highly influential for depressor activation. It is also known that sustained load, such as that caused by ground support and signaled by trCS increases depressor activity, but only during ongoing stance (Rosenbaum et al. 2010; Zill et al. 2012, 2013). Interestingly, we found that also lack of ground support leads to an increase in DepTr activity. This is likely caused by a second mechanism at work in this case, providing the positive feedback. Its source is probably the trochanteral hair plate that is located at the base of the trochanter and signals the position of the joint (Schmitz 1986). The ablation of this hair plate causes a strong reduction in downward directed force, in turn suggesting that its activation during the lowering of the leg in SIH increases DepTr activation (Schmitz 1986). The RetUng displayed no change in activation latency during SIH but showed a marked decrease in activity following the passing through the fictive ground level. Up until recently, very little information was available on sources of sensory feedback for this muscle in the stick insect. Several sense organs on the tarsi of the locust have been reported to feed back onto RetUng MNs (Laurent and Hustert 1988), and recent studies in stick insects have shown that RetUng activity and the forces generated by the resulting tarsal flexion are reported back and further enhance RetUng activity with an average delay of 35 ms (Zill et al. 2010, 2014). This finding explains the reduction of mean activity when the animal steps into a hole.
Finally, for the FlxTi muscle, we found both a drastic increase in latency and a reduction in overall activity, confirming earlier results by Berendes et al. (2013). Clearly, the initial ground contact has a strong influence on both timing and magnitude of this muscle. This effect was seen in all three phasmid species investigated, albeit to different degrees. While the morphologically more similar species Carausius morosus and Cuniculina impigra showed similar changes in FlxTi latencies during SIH, Aretaon asperrimus expressed a particularly strong dependence of FlxTi activation on TD. In any case, however, all three species lend themselves to the further investigation of this phenomenon.
All magnitude changes most likely represent underestimations of the effect that loading in an intact freely walking animal would have (Schmitz and Dallmann 2014), owing to the fact that the animals were tethered above a slippery surface and did not have to carry their own weight. Furthermore, the effect of the SIH on the magnitude in all but the DepTr muscle parallels the reduction in EMG amplitude in cat lateral gastrocnemius and vastus lateralis muscles, the ankle and knee extensors (Gorassini et al. 1994). The increase in DepTr activity could be part of a corrective response elicited by the above-mentioned stimulation of the trochanteral HP (Schmitz 1986).
Interestingly, the studies on the EMG response to missing ground support in cat have demonstrated that the magnitude control also strongly depended on the posture and state of the contralateral leg, which we did not monitor (Gorassini et al. 1994). In the cat, extensor muscle activity was increased compared with control during SIH when the contralateral leg was in swing (Gorassini et al. 1994). In the stick insect, we never observed a reduction in the response to stepping into the hole, suggesting that the response is quite stereotypical and the state of the contralateral leg is of relatively little importance in this context. Nevertheless, it cannot be excluded that the state of the ipsilateral front or hind legs might have been able to influence the response had they not been removed for the experiment.
Sensory influences on FlxTi activation.
The tight correlation of TD and FlxTi activation seen in our and in previous studies suggests that this particular leg muscle is strongly influenced by the transient loading of the leg at TD signaled through CS (Berendes et al. 2013; Gruhn et al. 2006). Since the swing-to-stance transition in a step cycle provides an excellent opportunity to investigate the relative importance of central and peripheral influences during such a phase transition, we directed our attention to ablating potential candidate sources for this feedback. This excluded ablating the tarsus which had previously been shown not to be directly involved in FlxTi timing (Berendes et al. 2013). The FlxTi is known to receive sensory input from movement and load sensors. Movement signals from the fCO are known to support and enhance FlxTi activity at the beginning of and during stance (for review, see Büschges et al. 2008). However, this feedback is most likely not instrumental for the initial activation of the muscle. Furthermore, load feedback from various leg CS has been shown to influence FlxTi MN activity (Akay et al. 2001, 2004; Zill et al. 2014). Yet no direct demonstration exists that stimulation of these or other CS is causal for FlxTi timing at the beginning of stance (for review, see Büschges 2005). When ablating single groups of sense organs, we found that only ablation of the feCS caused a significant increase in FlxTi latency during normal ground steps, the response one would expect for sensory feedback that is a major contributor to the activation of the muscle. The only other single ablation to also cause an increase in latency was that of the trCS. This change, however, was not significant.
This suggests that the main timing information for the activation of the FlxTi through TD in the two-leg preparation is provided by the local feCS. These particular CS had previously only been shown to provide important feedback for the magnitude control of FlxTi activity (Akay et al. 2001, 2004). No previous evidence existed that trCS affected flexor activity at all (Büschges and Gruhn 2008), and future intracellular recordings will have to verify this influence. Cutting the RetUng tendon in the tibia only affected the variability of the response, suggesting that any multipolar receptor associated with the tendon is not directly involved in FlxTi activation.
FlxTi activation during SIH.
Despite a further increase in activation latency and a marked decrease in FlxTi activity, stepping into the hole nevertheless results in FlxTi activation in 81% of the cases (Berendes et al. 2013). This could suggest a weak centrally controlled activation of the muscle, not completely unlike that in the cat (Gorassini et al. 1994). The presence of networks able to generate such rhythmicity in the stick insects is known from pharmacological experiments which have used the muscarinic agonist pilocarpine in deafferented preparations (Büschges 1995; Büschges et al. 1995). The fact, however, that the FlxTi fails to become active in 19% of the SIH shows that this central influence is not very strong in the non-deafferented animal. In addition to feedback on TD through the feCS, it is therefore likely that additional sensory feedback adds to eliciting flexor activation during SIH in the two-leg preparation we used.
One possible sensory mechanism compensating for the lack of input from the feCS could be passive flexion in the femur tibia joint through passive forces at the end of extensor activity. This could elicit enough flexion movement (Hooper et al. 2009) to assist in activating FlxTi MNs during SIH through stimulating the fCO which has long been known to be involved in reinforcement of flexion movement (Bässler 1973; Bässler 1977). During normal steps, the time course of this passive flexion is too slow, as extensor tibiae MN activity often lasts just up to the beginning of FlxTi MN activity (Hooper et al. 2009; Rosenbaum et al. 2010). However, during SIH this movement could help in the belated FlxTi activation. Compared with the intact leg, the latency of FlxTi activation during SIH was increased even further in all single CS ablation experiments, while no single ablation changed the SIH effect on the magnitude of FlxTi activity. This implies that not only the trCS or the feCS, together with the tarsal CS (Zill et al. 2014), but also the tiCS has an influence on the flexor MNs. Such an influence from the latter has not been shown yet. Two reasons for these effects of CS ablation on FlxTi timing during SIH are conceivable. For one, ablation of any given CS could reduce inherent tonic activity (Zill et al. 2012, 2013, 2014) to zero and thereby decrease the likelihood of flexor activation. On the other hand, it is known that CS do not only report transient or continuous load that is externally applied, but also load that is self-generated by cuticular strain due to muscle contractions (Zill et al. 2012, 2014). This means that strain developed by muscles such as the DepTr and the RetUng, which are activated independently of TD, theoretically could in turn stimulate trCS and tarsal CS and thereby activate the flexor muscle. So far, however, such an indirect effect has not been shown, and CS activation through muscle contractions has been shown in resisted movements only (Zill et al. 2011, 2012, 2014). Both mechanisms could serve as explanations for the finding that all single CS ablations further increased the FlxTi activation latency during SIH compared with the increase observed in the intact leg. Finally, it cannot be excluded that additional interleg sensory influences that we eliminated through tethering a reduced preparation may play a role in the freely walking animal, especially on a nonslippery ground. Such sensory feedback might add up to help activating the FlxTi in combination with local feedback in the intact animal.
The effect of the ablation of all CS in conjunction with removing the tarsus on the flexor muscle activation and why this procedure prevented the reduction in Amax that is seen in the intact animal, while still increasing the FlxTi activation latency, remains unclear.
In summary, we have shown that, while ground contact influences all stance phase muscles of the stick insect middle leg, only the timing of the FlxTi is largely controlled by loading feedback upon TD. This is different from the current assumption that also the timing of RetCx MNs is dependent on the onset of load signaling (Ekeberg et al. 2004). The source for the activation signal for this latter muscle remains unknown. The sensory feedback controlling flexor muscle timing, on the other hand, originates mostly from the local feCS, which had previously only been implied in FlxTi magnitude control. In the absence of ground support, lack of phasic CS activity contributes to a belated FlxTi activation. This could assist in the following searching movements for renewed ground support.
GRANTS
This work was supported by Deutsche Forschungsgemeinschaft Grant Bu857/11.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.S., M.G., and A.B. conception and design of research; J.S. performed experiments; J.S. analyzed data; J.S., M.G., and A.B. interpreted results of experiments; J.S. prepared figures; J.S. and M.G. drafted manuscript; J.S., M.G., and A.B. approved final version of manuscript; M.G. and A.B. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Drs. Joachim Schmidt and Till Bockemühl, as well as Volker Berendes for valuable comments and discussions. We also thank Jan Sydow and Michael Dübbert for help with the technical realization.
REFERENCES
- Akay T, Bässler U, Gerharz P, Büschges A. The role of sensory signals from the insect coxa-trochanteral joint in controlling motor activity of the femur-tibia joint. J Neurophysiol 85: 594–604, 2001. [DOI] [PubMed] [Google Scholar]
- Akay T, Haehn S, Schmitz J, Büschges A. Signals from load sensors underlie interjoint coordination during stepping movements of the stick insect leg. J Neurophysiol 92: 42–51, 2004. [DOI] [PubMed] [Google Scholar]
- Akay T, Ludwar BC, Göritz ML, Schmitz J, Büschges A. Segment specificity of load signal processing depends on walking direction in the stick insect leg muscle control system. J Neurosci 27: 3285–3294, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bässler U. [On the regulation of the position of the femur-tibial joint of the walking-stick insect Carausius morosus at rest and in motion]. Kybernetik 4: 18–26, 1967. [DOI] [PubMed] [Google Scholar]
- Bässler U. [Control of active movements of the femur-tibia-joint of the stick-insect Carausius morosus]. Kybernetik 13: 38–53, 1973. [DOI] [PubMed] [Google Scholar]
- Bässler U. Sensory control of leg movement in the stick insect Carausius morosus. Biol Cybern 25: 61–72, 1977. [DOI] [PubMed] [Google Scholar]
- Bässler U. Afferent control of walking movements in the stick insect Cuniculina impigra II. J Comp Physiol 158: 351–362, 1986. [Google Scholar]
- Bässler U. The femur-tibia control system of stick insects–a model system for the study of the neural basis of joint control. Brain Res Brain Res Rev 18: 207–226, 1993. [DOI] [PubMed] [Google Scholar]
- Bässler U, Rohrbacher J, Karg G, Breutel G. Interruption of searching movements of partly restrained front legs of stick insects, a model situation for the start of a stance phase? Biol Cybern 65: 507–514, 1991. [Google Scholar]
- Berendes V, Dubbert M, Bockemuhl T, Schmitz J, Buschges A, Gruhn M. A laser-supported lowerable surface setup to study the role of ground contact during stepping. J Neurosci Methods 215: 224–233, 2013. [DOI] [PubMed] [Google Scholar]
- Berg E, Büschges A, Schmidt J. Single perturbations cause sustained changes in searching behavior in stick insects. J Exp Biol 216: 1064–1074, 2013. [DOI] [PubMed] [Google Scholar]
- Borgmann A, Hooper SL, Büschges A. Sensory feedback induced by front-leg stepping entrains the activity of central pattern generators in caudal segments of the stick insect walking system. J Neurosci 29: 2972–2983, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgmann A, Scharstein H, Büschges A. Intersegmental coordination: influence of a single walking leg on the neighboring segments in the stick insect walking system. J Neurophysiol 98: 1685–1696, 2007. [DOI] [PubMed] [Google Scholar]
- Borgmann A, Toth TI, Gruhn M, Daun-Gruhn S, Buschges A. Dominance of local sensory signals over inter-segmental effects in a motor system: experiments. Biol Cybern 105: 399–411, 2011. [DOI] [PubMed] [Google Scholar]
- Bräunig P, Hustert R, Pfluger HJ. Distribution and specific central projections of mechanoreceptors in the thorax and proximal leg joints of locusts. I. Morphology, location and innervation of internal proprioceptors of pro- and metathorax and their central projections. Cell Tissue Res 216: 57–77, 1981. [DOI] [PubMed] [Google Scholar]
- Brown TG. The intrinsic factors in the act of progression in the mammal. Proc R Soc Lond B Biol Sci 84: 308–319, 1911. [Google Scholar]
- Bucher D, Akay T, DiCaprio RA, Büschges A. Interjoint coordination in the stick insect leg-control system: the role of positional signaling. J Neurophysiol 89: 1245–1255, 2003. [DOI] [PubMed] [Google Scholar]
- Büschges A. Role of local nonspiking interneurons in the generation of rhythmic motor activity in the stick insect. J Neurobiol 27: 488–512, 1995. [DOI] [PubMed] [Google Scholar]
- Büschges A. Sensory control and organization of neural networks mediating coordination of multisegmental organs for locomotion. J Neurophysiol 93: 1127–1135, 2005. [DOI] [PubMed] [Google Scholar]
- Büschges A, Akay T, Gabriel JP, Schmidt J. Organizing network action for locomotion: insights from studying insect walking. Brain Res Rev 57: 162–171, 2008. [DOI] [PubMed] [Google Scholar]
- Büschges A, Gruhn M. Mechanosensory feedback in walking: From joint control to locomotor patterns. Adv In Insect Phys 34: 193–230, 2008. [Google Scholar]
- Büschges A, Schmitz J, Bässler U. Rhythmic patterns in the thoracic nerve cord of the stick insect induced by pilocarpine. J Exp Biol 198: 435–456, 1995. [DOI] [PubMed] [Google Scholar]
- Conway BA, Hultborn H, Kiehn O. Proprioceptive input resets central locomotor rhythm in the spinal cat. Exp Brain Res 68: 643–656, 1987. [DOI] [PubMed] [Google Scholar]
- Cruse H. Coactivating influences between neighboring legs in walking insects. J Exp Biol 114: 513–519, 1985. [Google Scholar]
- Cruse H. What mechanisms coordinate leg movement in walking arthropods? Trends Neurosci 13: 15–21, 1990. [DOI] [PubMed] [Google Scholar]
- Cruse H, Dean J, Suilmann M. The contribution of diverse sense organs to the control of leg movement by a walking insect. J Comp Physiol A 154: 695–705, 1984. [Google Scholar]
- Cruse H, Schmitz J, Braun U, Schweins A. Control of body height in a stick insect walking on a treadwheel. J Exp Biol 181: 141–155, 1993. [Google Scholar]
- Debrodt B, Bässler U. Motor neurones of the flexor tibiae muscle in phasmids. Zool Jb Physiol 93: 481–494, 1989. [Google Scholar]
- Duysens J, Clarac F, Cruse H. Load-regulating mechanisms in gait and posture: comparative aspects. Physiol Rev 80: 83–133, 2000. [DOI] [PubMed] [Google Scholar]
- Duysens J, Pearson KG. Inhibition of flexor burst generation by loading ankle extensor muscles in walking cats. Brain Res 187: 321–332, 1980. [DOI] [PubMed] [Google Scholar]
- Ekeberg Ö, Blümel M, Büschges A. Dynamic simulation of insect walking. Arthropod Struct Dev 33: 287–300, 2004. [DOI] [PubMed] [Google Scholar]
- Fischer H, Schmidt J, Haas R, Büschges A. Pattern generation for walking and searching movements of a stick insect leg. I. Coordination of motor activity. J Neurophysiol 85: 341–353, 2001. [DOI] [PubMed] [Google Scholar]
- Gabriel JP, Büschges A. Control of stepping velocity in a single insect leg during walking. Philos Trans A Math Phys Eng Sci 365: 251–271, 2007. [DOI] [PubMed] [Google Scholar]
- Goldammer J, Büschges A, Schmidt J. Motoneurons, DUM cells, and sensory neurons in an insect thoracic ganglion: a tracing study in the stick insect Carausius morosus. J Comp Neurol 520: 230–257, 2012. [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Prochazka A, Hiebert GW, Gauthier MJ. Corrective responses to loss of ground support during walking. I. Intact cats. J Neurophysiol 71: 603–610, 1994. [DOI] [PubMed] [Google Scholar]
- Graham D, Bässler U. Effects of afference sign reversal on motor activity in walking stick insects (Carausius morosus). J Exp Biol 91: 179–193, 1981. [Google Scholar]
- Gruhn M, Hoffmann O, Dübbert M, Scharstein H, Büschges A. Tethered stick insect walking: a modified slippery surface setup with optomotor stimulation and electrical monitoring of tarsal contact. J Neurosci Methods 158: 195–206, 2006. [DOI] [PubMed] [Google Scholar]
- Hess D, Büschges A. Role of proprioceptive signals from an insect femur-tibia joint in patterning motoneuronal activity of an adjacent leg joint. J Neurophysiol 81: 1856–1865, 1999. [DOI] [PubMed] [Google Scholar]
- Hiebert GW, Gorassini MA, Jiang W, Prochazka A, Pearson KG. Corrective responses to loss of ground support during walking. II. Comparison of intact and chronic spinal cats. J Neurophysiol 71: 611–622, 1994. [DOI] [PubMed] [Google Scholar]
- Hiebert GWP, Pearson KG. Contribution of sensory feedback to the generation of extensor activity during walking in the decerebrate cat. J Neurophysiol 81: 758–770, 1999. [DOI] [PubMed] [Google Scholar]
- Hooper SL, Guschlbauer C, Blümel M, Rosenbaum P, Gruhn M, Akay T, Büschges A. Neural control of unloaded leg posture and of leg swing in stick insect, cockroach, and mouse differs from that in larger animals. J Neurosci 29: 4109–4119, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeck T, Cruse H. Walking in Aretaon asperrimus. J Insect Physiol 53: 724–733, 2007. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Kjaerulff O. Distribution of central pattern generators for rhythmic motor outputs in the spinal cord of limbed vertebrates. Ann N Y Acad Sci 860: 110–129, 1998. [DOI] [PubMed] [Google Scholar]
- Laurent G, Hustert R. Motor neuronal receptive fields delimit patterns of motor activity during locomotion of the locust. J Neurosci 8: 4349–4366, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwar BC, Göritz ML, Schmidt J. Intersegmental coordination of walking movements in stick insects. J Neurophysiol 93: 1255–1265, 2005. [DOI] [PubMed] [Google Scholar]
- Matheson T, Field L. An elaborate tension receptor system highlights sensory complexity in the hind leg of the locust. J Exp Biol 198: 1673–1689, 1995. [DOI] [PubMed] [Google Scholar]
- Orlovsky GN, Deliagina TG, Grillner S. Neuronal Control of Locomotion. New York: Oxford University Press, 1999. [Google Scholar]
- Pearson KG. Role of sensory feedback in the control of stance duration in walking cats. Brain Res Rev 57: 222–227, 2008. [DOI] [PubMed] [Google Scholar]
- Pearson KG, Bradley AB. Specific regeneration of excitatory motoneurons to leg muscles in the cockroach. Brain Res 47: 492–496, 1972. [DOI] [PubMed] [Google Scholar]
- Prochazka A, Clarac F, Loeb GE, Rothwell JC, Wolpaw JR. What do reflex and voluntary mean? Modern views on an ancient debate. Exp Brain Res 130: 417–432, 2000. [DOI] [PubMed] [Google Scholar]
- Prochazka A, Gillard D, Bennett D. Positive force feedback control of muscles. J Neurophysiol 77: 3226–3236, 1997. [DOI] [PubMed] [Google Scholar]
- Radnikow, Bässler U. Function of a muscle whose apodeme travels through a joint moved by other muscles: why the retractor unguis muscle in stick insects is tripartite and has no antagonist. J Exp Biol 157: 87–99, 1991. [Google Scholar]
- Robertson GA, Mortin LI, Keifer J, Stein PS. Three forms of the scratch reflex in the spinal turtle: central generation of motor patterns. J Neurophysiol 53: 1517–1534, 1985. [DOI] [PubMed] [Google Scholar]
- Rosenbaum P, Wosnitza A, Büschges A, Gruhn M. Activity patterns and timing of muscle activity in the forward walking and backward walking stick insect Carausius morosus. J Neurophysiol 104: 1681–1695, 2010. [DOI] [PubMed] [Google Scholar]
- Schmidt J, Grund M. Rhythmic activity in a motor axon induced by axotomy. Neuroreport 14: 1268–1271, 2003. [DOI] [PubMed] [Google Scholar]
- Schmitz J. Properties of the feedback system controlling the coxa-trochanter joint in the stick insect Carausius morosus. Biol Cybern 55: 35–42, 1986. [Google Scholar]
- Schmitz J. Load-compensating reactions in the proximal leg joints of stick insects during standing and walking. J Exp Biol 183: 15–33, 1993. [Google Scholar]
- Schmitz J, Dallmann CJ. Force and torque profiles of stick insects walking on compliant surfaces. Program No. 830.16 In: 2014 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience, 2014. [Google Scholar]
- Sherrington CS. Flexion-reflex of the limb, crossed extension-reflex, and reflex stepping and standing. J Physiol 40: 28–121, 1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson JT, Ritzmann RE. Leg kinematics and muscle activity during treadmill running in the cockroach, Blaberus discoidalis. I. Slow running. J Comp Physiol A 182: 11–22, 1998a. [DOI] [PubMed] [Google Scholar]
- Watson JT, Ritzmann RE. Leg kinematics and muscle activity during treadmill running in the cockroach, Blaberus discoidalis. II. Fast running. J Comp Physiol A 182: 23–33, 1998b. [DOI] [PubMed] [Google Scholar]
- Watson JT, Ritzmann RE, Pollack AJ. Control of climbing behavior in the cockroach, Blaberus discoidalis. II. Motor activities associated with joint movement. J Comp Physiol A 188: 55–69, 2002. [DOI] [PubMed] [Google Scholar]
- Wendler G. Laufen und Stehen der Stabheuschrecke Carausius morosus: Sinnesborstenfelder in den Beingelenken als Glieder von Regelkreisen. Zeitschr f vergl Physiologie 48: 198–250, 1964. [Google Scholar]
- Zill SN, Büschges A, Schmitz J. Encoding of force increases and decreases by tibial campaniform sensilla in the stick insect, Carausius morosus. J Comp Physiol A 197: 851–867, 2011. [DOI] [PubMed] [Google Scholar]
- Zill SN, Chaudhry S, Büschges A, Schmitz J. Directional specificity and encoding of muscle forces and loads by stick insect tibial campaniform sensilla, including receptors with round cuticular caps. Arthropod Struct Dev 42: 455–467, 2013. [DOI] [PubMed] [Google Scholar]
- Zill SN, Chaudhry S, Exter A, Buschges A, Schmitz J. Positive force feedback in development of substrate grip in the stick insect tarsus. Arthropod Struct Dev 43: 441–455. 2014. [DOI] [PubMed] [Google Scholar]
- Zill SN, Keller BR, Chaudhry S, Duke ER, Neff D, Quinn R, Flannigan C. Detecting substrate engagement: responses of tarsal campaniform sensilla in cockroaches. J Comp Physiol A 196: 407–420, 2010. [DOI] [PubMed] [Google Scholar]
- Zill SN, Keller BR, Duke ER. Sensory signals of unloading in one leg follow stance onset in another leg: transfer of load and emergent coordination in cockroach walking. J Neurophysiol 101: 2297–2304, 2009. [DOI] [PubMed] [Google Scholar]
- Zill SN, Ridgel AL, DiCaprio RA, Frazier SF. Load signalling by cockroach trochanteral campaniform sensilla. Brain Res 822: 271–275, 1999. [DOI] [PubMed] [Google Scholar]
- Zill SN, Schmitz J, Büschges A. Load sensing and control of posture and locomotion. Arthropod Struct Dev 33: 273–286, 2004. [DOI] [PubMed] [Google Scholar]
- Zill SN, Schmitz J, Chaudhry S, Büschges A. Force encoding in stick insect legs delineates a reference frame for motor control. J Neurophysiol 108: 1453–1472, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]