Abstract
Neurons in the pontomedullary reticular formation (PMRF) give rise to the reticulospinal tract. The motor output of the PMRF was investigated using stimulus-triggered averaging of electromyography (EMG) and force recordings in two monkeys (M. fascicularis). EMG was recorded from 12 pairs of upper limb muscles, and forces were detected using two isometric force-sensitive handles. Of 150 stimulation sites, 105 (70.0%) produced significant force responses, and 139 (92.5%) produced significant EMG responses. Based on the average flexor EMG onset latency of 8.3 ms and average force onset latency of 15.9 ms poststimulation, an electromechanical delay of ∼7.6 ms was calculated. The magnitude of force responses (∼10 mN) was correlated with the average change in EMG activity (P < 0.001). A multivariate linear regression analysis was used to estimate the contribution of each muscle to force generation, with flexors and extensors exhibiting antagonistic effects. A predominant force output pattern of ipsilateral flexion and contralateral extension was observed in response to PMRF stimulation, with 65.3% of significant ipsilateral force responses directed medially and posteriorly (P < 0.001) and 78.6% of contralateral responses directed laterally and anteriorly (P < 0.001). This novel approach permits direct measurement of force outputs evoked by central nervous system microstimulation. Despite the small magnitude of poststimulus EMG effects, low-intensity single-pulse microstimulation of the PMRF evoked detectable forces. The forces, showing the combined effect of all muscle activity in the arms, are consistent with reciprocal pattern of force outputs from the PMRF detectable with stimulus-triggered averaging of EMG.
Keywords: reticulospinal, electrophysiology, stimulus-triggered averaging, macaque
the reticulospinal tract, originating in the pontomedullary reticular formation (PMRF), is a major motor pathway involved in bilateral coordination (Kuypers 1958, 1981; Peterson et al. 1974, 1975, 1979). The main targets of reticulospinal axons are interneurons in lamina VII and VIII and motoneurons in lamina IX that innervate axial and proximal limb muscles (Grillner and Lund 1966, 1968; Jankowska et al. 2003; Matsuyama et al. 1997; Peterson 1979; Peterson et al. 1979). Although they primarily descend ipsilaterally, reticulospinal axons produce contralateral effects through commissural interneurons and decussating axon collaterals (Bannatyne et al. 2003; Jankowska et al. 2003; Sakai et al. 2009). Because of these bilateral outputs to proximal limb muscles, the reticulospinal system is uniquely suited to coordinate bilateral movement of the limbs.
Microstimulation studies in the cat using short stimulus trains have demonstrated reticulospinal recruitment of axial and proximal limb muscles bilaterally (Drew and Rossignol 1990a, 1990b; Schepens and Drew 2006; Sprague and Chambers 1954). A reciprocal motor output pattern was observed following stimulation, characterized by ipsilateral limb flexion, contralateral limb extension, and head rotation toward the ipsilateral side (Sprague and Chambers 1954). Stimulus trains in the monkey PMRF have reproduced the same motor pattern in the upper limb (Herbert et al. 2010). Motor outputs of stimulus trains have exhibited high agreement with those of stimulus-triggered averaging (StimulusTA) of single-pulse microstimulation (Herbert et al. 2010). StimulusTA studies in the monkey have revealed a double reciprocal pattern of electromyography (EMG) activity from upper limb muscles, consisting of facilitation of ipsilateral flexors and contralateral extensors and suppression of ipsilateral extensors and contralateral flexors (Davidson and Buford 2004, 2006).
Spike-triggered averaging (SpikeTA) is considered to be the most direct method of investigating motor outputs in electrophysiological studies. The major motor effects revealed by SpikeTA are thought to be primarily mediated through monosynaptic and disynaptic connections to motoneurons (Baker and Lemon 1998; Cheney and Fetz 1985; Davidson et al. 2007). StimulusTA is also thought to activate monosynaptic and disynaptic pathways, but with a larger number of neurons engaged (Cheney and Fetz 1985). StimulusTA and SpikeTA from the same recording sites in the motor cortex and PMRF exhibit similar EMG activity (Cheney and Fetz 1985; Davidson et al. 2007). These findings support the view that StimulusTA reveals the output of reticulospinal neurons through relatively direct pathways which more accurately depict the physiological effects of PMRF neural activity than trains of stimuli.
To our knowledge, no SpikeTA or StimulusTA studies have investigated the movements and force outputs resulting from motor activity within the central nervous system (CNS). There have been multiple studies, however, that have investigated the relationship between neural activity and force. Evarts (1968) devised one of the first single-unit recording studies to determine whether primary motor cortex neuron activity related to force or displacement. Over time, investigators have devised analytic approaches to extract correlates of force, velocity, and position signals from neural activity in cortical motor areas (Ashe 1997; Georgopolous et al. 1992; Moran and Schwartz 1999). However, in the reticulospinal system, no comparable studies exist. In cats standing on force platforms, reticulospinal motor outputs were associated with stereotyped force output patterns for postural adjustments (Gahery et al. 1980), but there have been no purely upper limb studies relevant to reaching where forces were measured.
The purpose of the present study was to determine the force effects of PMRF motor output evoked with single-pulse stimuli by using the StimulusTA approach to measure forces in addition to EMG activity. Despite the small magnitude of previously detected poststimulus EMG effects (PStEs), we expected measureable forces to be exerted by the upper limbs in response to PMRF stimulation. Furthermore, we expected the pattern of forces to correspond to the double reciprocal pattern of EMG activity associated with PMRF output. Demonstrating the effectiveness of this novel technique for measuring motor outputs could have broad applications for motor systems neurophysiology by permitting direct measurement of force outputs evoked by CNS microstimulation. This would allow for identification of physiologically relevant force effects of CNS neural activity. A portion of these results have previously been presented in abstracts (Buford and Montgomery 2011; Hirschauer and Buford 2014).
MATERIAL AND METHODS
Subjects, task, and surgery.
Two male monkeys (Macaca fascicularis) were trained to perform a bilateral isometric force control task administered by Tempo software (Reflective Computing, Olympia, WA). Subjects sat in a primate chair with their head restrained and simultaneously controlled two cursors on a computer screen via two stationary force-sensitive joysticks. The joysticks were located at waist level, so during the task both of the subject's arms were positioned much like a person's would be to hold a gearshift in a car. The subjects tended to grip the joysticks with their palms down and their forearms pronated. Each hand controlled one cursor via a single joystick, as shown in Fig. 1. Cursors were primarily controlled by pushing and pulling along the anteroposterior y-axis, also shown in Fig. 1. A trial began once the subject was holding both joysticks with the cursors within the range of two central targets corresponding to zero y-direction force. Once this starting position was held for 1.0 s, targets appeared indicating the necessary forces that must be applied to complete the task. The subject had to push or pull on each joystick to move the cursors to the targets. Once the cursors were on targets, that effort had to be maintained for 0.5 s. This was implemented as a simple reaction time task, with the subject free to begin the effort to move the cursors as soon as the targets appeared. Pushing or pulling forces of ∼30 N were required to hold the cursors on the targets. The next trial could be initiated after a 1.0-s reward period; the subject self-paced the start of the next trial. Within each session, the trial types were presented in a pseudorandomized order. The start positions were in the center left and center right of the screen for the left and right joysticks. For each arm, the target positions were either directly above the start position (for a trial requiring pushing) or directly below it (for a trial requiring pulling). Two targets appeared for each trial type (one for each arm), in one of four possible combinations.
Fig. 1.
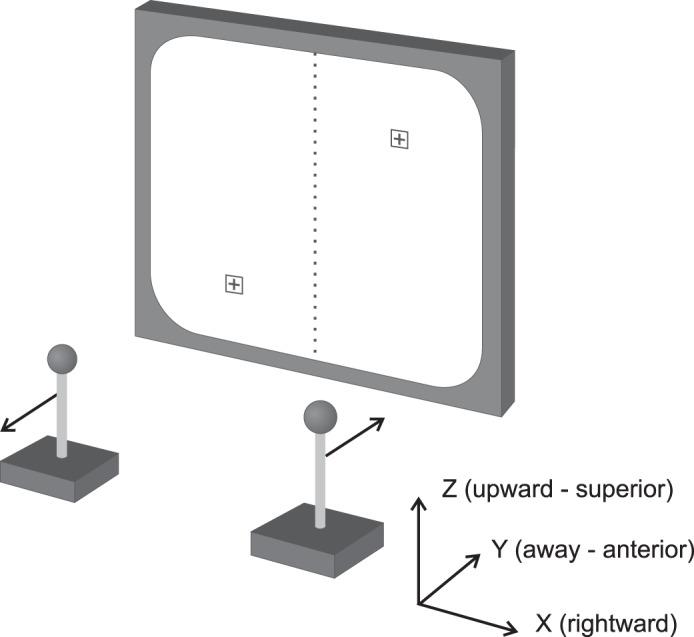
Schematic of the apparatus for the isometric force control task. The force-sensitive joysticks control the cursors on the display screen. The target locations correspond to the positions for a task requiring left-sided pulling and right-sided pushing. The coordinate system demonstrates the orientations of the x- (mediolateral), y- (anteroposterior), and z-axes (superoinferior).
Surgical procedures for implanting the recording chamber and EMG electrodes were similar to those provided in previous reports (Davidson and Buford 2004, 2006). A stainless steel recording chamber was mounted to the skull over a craniotomy of the left parietal bone and secured with bone screws and dental acrylic. The center of the recording chamber was directed toward Horsley-Clark stereotaxic coordinates anteroposterior 0, mediolateral 0, dorsoventral −12, allowing bilateral access to the PMRF (Szabo and Cowan 1984). The chamber was tilted ∼10° laterally to avoid penetration of midline vascular structures. EMG electrodes consisted of pairs of multistranded, Teflon-coated stainless steel wires that were led subcutaneously from the cranial implant to the electrode implant sites. Insulation was removed from the last 2 mm of the tips of the wires, which were inserted intramuscularly with a hypodermic needle (Betts et al. 1976; Park et al. 2000). Twelve bilateral arm and shoulder muscle pairs were implanted for a total of 24 muscles. The implanted muscles with their functions and abbreviations are presented in Table 1.
Table 1.
List of EMG implantation sites
| No. | Muscle | Function(s) | Acronym |
|---|---|---|---|
| 1 | Flexor carpi ulnaris | Wrist flexion | FCU |
| 2 | Extensor carpi radialis | Wrist extension | ECR |
| 3 | Brachioradialis | Elbow flexion | BRAC |
| 4 | Biceps brachii | Elbow flexion | BIC |
| 5 | Triceps brachii, lateral | Elbow extension | TRIC |
| 6 | Anterior deltoid | Humerus flexion & internal rotation | ADLT |
| 7 | Posterior deltoid | Humerus extension & external rotation | PDLT |
| 8 | Subscapularis | Humerus adduction & internal rotation | SUB |
| 9 | Supraspinatus | Humerus abduction & external rotation | SUP |
| 10 | Pectoralis major | Humerus flexion & adduction | PMJ |
| 11 | Latissimus dorsi | Humerus extension & adduction | LAT |
| 12 | Upper trapezius | Scapula elevation | UTR |
Electromyography (EMG) implants were located in left and right muscles, with 12 muscles per side, for a total of 24 muscles.
Subject care complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the institutionally approved animal care protocol for our laboratory. Surgeries were performed under veterinary supervision in aseptic conditions. Animals were pretreated with antibiotics and anesthetized with ketamine HCl (13 mg/kg im) followed by isoflurane gas (1–2%) through endotracheal intubation. While under anesthesia, the subject's respiration, heart rate, and body temperature were monitored. Antibiotics and nonsteroidal anti-inflammatory analgesics (e.g., buprenorphine, ibuprofen) were administered postoperatively to prevent infection and discomfort.
Stimulation procedures.
Microstimulation was delivered by polyimide- and epoxy-insulated tungsten microelectrodes (Frederick Haer, Bowdoin, ME). Electrodes were inserted through a 23-gauge thin-walled stainless steel guide cannula, which was aligned with stimulation sites by placement through a two-dimensional grid in the recording chamber. Guide tubes were advanced into the brain and stopped a few millimeters into the cerebellum. The electrode was lowered through the guide tube and into the brain stem with a manual hydraulic microdrive.
A total of 311 stimulation sites from 83 penetrations were located throughout the PMRF, which is bounded by the abducens nucleus dorsally, the inferior olive and pyramidal tract inferiorly, and the facial nucleus laterally. The abducens nucleus was identified during electrode insertion by recordings from cells with firing rates proportional to ipsilateral eye abduction (Fuchs and Luschei 1970). Prior to stimulation, electrophysiological activity was recorded as the electrode was inserted to its maximal depth. As the electrode was retracted, stimulation was applied at 0.5-mm intervals to regions in which task-related neural activity had been detected.
At each StimulusTA site, 2,000 biphasic pulses were delivered at a rate of 5 Hz by a digital stimulus controller (Master-8, AMPI) connected to an analog stimulus isolator (model 2200, AM-Systems, Carlsborg, WA). A stimulus current of 30 μA was used, unless this current level produced observable muscle twitches or movement, in which case the current was reduced in 5-μA increments until the response was no longer visible. A default current of 30 μA was chosen because previous studies in PMRF output indicate this is an effective stimulus intensity for StimulusTA in the cat (Drew and Rossignol 1990a, 1990b) and monkey (Cowie and Robinson 1994; Davidson and Buford 2004, 2006; Herbert et al. 2010). While some stimulus currents were as low as 10 μA, the vast majority of currents were 30 μA (>95%). For each session, stimulation was delivered continuously for 400 s throughout all phases of the task and regardless of subject behavior. Both subjects appeared to be unaware of this background stimulation.
EMG recording.
EMG data were collected from 12 pairs of chronically-implanted intramuscular electrodes. The electrode wires were led subcutaneously to three 17-pin plugs (WPI no. 223-161) mounted in the dental acrylic of the cranial implant. The integrity of the EMG implants was verified by periodic testing of electrode impedances. For both subjects, electrodes were located in both the left and right of 12 pairs of upper limb muscles, listed in Table 1. EMG activity was recorded at a sampling rate of 4 kHz with 20- to 2,000-Hz band-pass filtering from all implanted muscles during stimulation using Spike2 software and a Power 1401 data acquisition unit (CED, Cambridge, UK). A template subtraction algorithm removed ECG artifact prior to processing.
Force recording.
Bilateral forces were recorded using two stationary, force-sensitive joysticks, which the subjects were trained to grasp. To maintain an isometric condition, the joysticks measured the magnitude and direction of forces applied by the subject, but did not move. The joysticks consisted of a ball handle on top of a shaft screwed into the mounting site of a 6 degree-of-freedom load cell (Gamma Model, ATI Industrial Automation, Apex, NC). The single-trial resolution of the load cells was 3.5 mN, which was subsequently improved by signal averaging. Each load cell output controlled the movement of its respective cursor on the display screen. Force measurements were sampled at 4 kHz along the x-, y-, and z-axes from each joystick, shown in Fig. 1. Force response vectors were calculated using simple vector addition of the axial components. Force measurements were recalibrated for each recording session by defining the average force of periods in which the subject was not contacting the joysticks to be 0 N. The firmware in the controller for the load cell was supplied parameters about the length of the joystick so that output readings were calibrated for the point of force application.
EMG averaging and analysis.
Procedures for compiling StimulusTAs of EMG data have been previously described in detail (Davidson and Buford 2006; Herbert et al. 2010). Briefly, for each muscle, EMG records were adjusted to remove DC offsets, rectified, and averaged off-line using custom scripts for Spike2 and MATLAB. Averages were compiled over an 80-ms window with a 20-ms prestimulus period and a 60-ms poststimulus period. Triggers were selected for averaging only if the mean EMG of the 80-ms window was at least 10% greater than the peak baseline noise (McKiernan et al. 1998). Periods during which the EMG level exceeded the maximum amplifier range (4.5 V) were also excluded. As a result, the number of stimulus triggers varied for the StimulusTA of each muscle, with a typical analysis, including more than 1,900 triggers. A minimum of 500 triggers were required for a StimulusTA to be included in the analysis (McKiernan et al. 1998).
To test for PStEs, multiple fragment statistical analysis (MFSA) (Poliakov and Schieber 1998) was performed by dividing the stimulation period into N non-overlapping fragments, where N is the square root of the number of triggers in the trial. For each fragment, the average poststimulus EMG response was determined by subtracting the average EMG during a target interval from the average EMG during preceding and following control intervals. The target interval was a 10-ms period centered at 11-ms poststimulus (Poliakov and Schieber 1998), and control intervals were 10-ms periods centered at 9 ms prestimulus and 31 ms poststimulus, chosen to avoid the stimulus artifact observed immediately following stimulation. The distribution of the average poststimulus EMG response for each fragment was used to determine a P value for the significance of poststimulus effects in each muscle using Student's t-test with significance level α = 0.05. Onset and duration were calculated for significant responses, with onset and offset latencies defined as the times at which the EMG amplitude crossed the 2 SD threshold, shown in Fig. 3A.
Fig. 3.
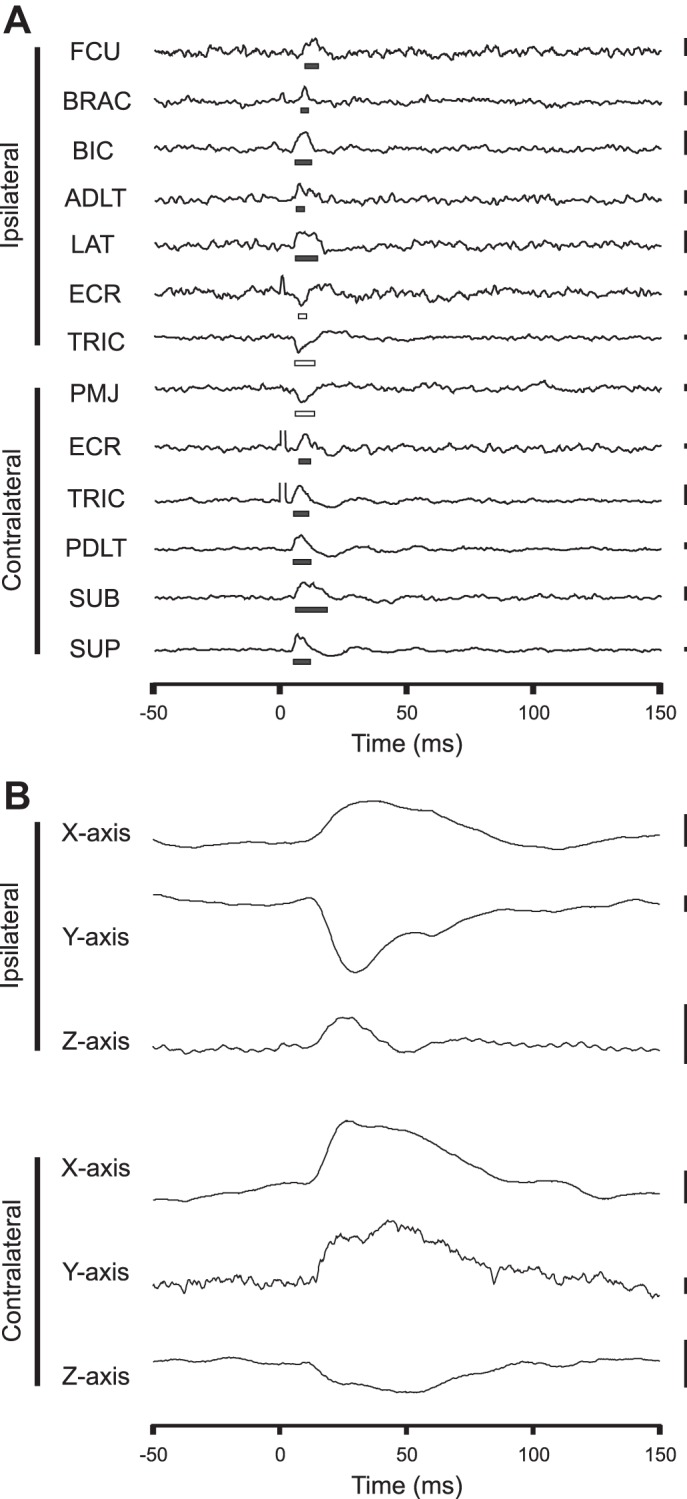
Selected example of EMG and force recordings of ipsilateral flexion and contralateral extension in response to left-sided pontomedullary reticular formation (PMRF) stimulation. A: significant poststimulus EMG effects. Significant periods of poststimulus facilitation (PStF) are indicated by solid bars, and poststimulus suppression (PStS) by open bars. Scale bars on right represent 0.01 mV, except those for extensor carpi radialis (ECR) and right TRIC, which represent 0.001 mV. FCU, flexor carpi ulnaris; BRAC, brachioradialis; ADLT, anterior deltoid; LAT, latissimus dorsi; PMJ, pectoralis major; PDLT, posterior deltoid; SUP, supraspinatus; SUB, subscapularis. B: corresponding poststimulus force responses showing posteromedial forces toward the body from the left (ipsilateral) arm and anterolateral forces away from the body from the right (contralateral) arm. Scale bars represent 10 mN. For this site, all averages included at least 1,355 triggers. The 30-μA stimulation was applied at time 0 s.
Force averaging and analysis.
Because force measurements could only be collected while the subjects made contact with the joysticks, only data from stimulation sites for which the subject contacted each joystick for cumulatively greater than 100 s (25.0%) of the 400-s stimulation period were included in the StimulusTA force analysis, with 162/311 (52.1%) of stimulation sites meeting these criteria. Subjects were considered to not be making contact with the joysticks if the net force on the joystick was less than 50 mN for greater than 15 ms. Force data were averaged over a 200-ms peristimulus window, consisting of a 50-ms pretrigger and 150-ms posttrigger period. Only triggers during which contact was maintained throughout the entire 200-ms time window were included in the study. Since sites with less than 500 triggers were excluded from the analysis, the number of triggers for which contact was maintained ranged from 500 to 2,000 for each stimulation site. To remove slow drift from force recordings, a baseline ramp subtraction procedure was used to level the baseline and set it to zero (Cheney and Fetz 1985).
MFSA was again used to test for poststimulus force effects (Poliakov and Schieber 1998). For the force response average, the target interval was a 20-ms period centered at 40-ms poststimulus, and control intervals were 20-ms periods centered at 0-ms and 80-ms poststimulus. These periods were chosen based on force onset and time-to-peak estimates from trials in which the change in force deviated from baseline by >4 SD (Soto and Cros 2011). The N averages of the difference between target period and control for all three components of force were used to determine a P value for the significance of poststimulus force effects using Hotelling's T-squared statistic. The distributions of the direction of force responses for each arm were compared using Pearson's χ2 test. The statistical significance of bilateral force responses was determined using Fisher's method to combine unilateral significance values. Bilateral force patterns were quantified in Fig. 10 by defining “pure flexion” (directed equally medially and posteriorly) as 1 and “pure extension” (directed equally laterally and anteriorly) as −1 for the ipsilateral side, and pure flexion as −1 and pure extension as 1 for the contralateral side, and then finding the average of both sides.
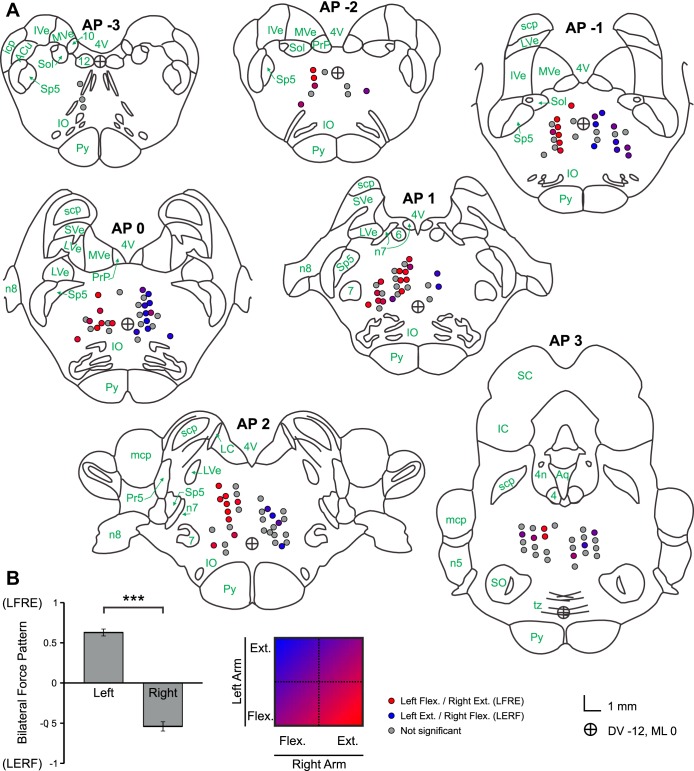
Fig. 10.
A: anatomical locations of stimulation sites with associated force response patterns. Brainstem sections were adapted from Szabo and Cowan (1984), with nomenclature from Martin and Bowden (1996). Colored circles represent a significant force response, with red representing a pattern of left-sided flexion and right-sided extension (LFRE), blue representing left-sided extension and right-sided flexion (LERF), and intermediate values defined by the color scale at the bottom. Gray circles indicate nonsignificant bilateral force responses. n4, Fourth cranial nerve; n5, fifth cranial nerve; 6, sixth cranial nerve nucleus; n7, seventh cranial nerve; 7, seventh cranial nerve nucleus; n8, eighth cranial nerve; 10, 10th cranial nerve nucleus; 12, 12th cranial nerve nucleus; icp, inferior cerebellar peduncle; mcp, middle cerebellar peduncle; scp, superior cerebellar peduncle; Py, pyramidal tract; tz, trapezoid body; IO, inferior olivary nucleus; SO, superior olivary nucleus; IVe, inferior vestibular nucleus; SVe, superior vestibular nucleus; LVe, lateral vestibular nucleus; MVe, medial vestibular nucleus; PrP, nucleus prepositus; ACu, anterior cuneate nucleus; Sol, nucleus solitarius; Pr5, principal nucleus of fifth cranial nerve; Sp5, spinal nucleus of fifth cranial nerve; LC, locus coeruleus; IC, inferior colliculus; SC, superior colliculus; 4V, fourth ventricle; Aq, cerebral aqueduct. The circle with crosshairs represents dorsoventral (DV) −12, mediolateral (ML) 0 in the stereotaxic coordinates. B: bilateral force output patterns by laterality of stimulation site (left vs. right). Output patterns corresponding to LFRE are closer to 1, and those corresponding to RFLE are closer to −1. ***P < 0.001. Error bars, SEM. [Fig. 10A adapted from Szabo and Cowan (1984). Reprinted with permission from Wiley. Copyright 1984.]
Multiple multivariate linear regression.
To test for agreement between EMG and force measurements, a multiple multivariate linear regression was performed with the poststimulus changes in EMG activity of all 12 muscles as explanatory variables and the components of the force responses along all 3 axes as dependent variables. For each muscle, regression coefficients were used to determine if the EMG activity significantly predicted the components (along the x-, y-, or z-axis) of the force response. The change in EMG activity, defined as the average activity during the control intervals subtracted from the average activity during the target interval (Poliakov and Schieber 1998), was calculated for each muscle and used to predict forces recorded from the joystick on the corresponding side only. Because of the bilateral symmetry of the subject's body and posture, the data from both sides were combined into one set. There were no significant differences between the quality or behavior of EMG and force recordings from the left and right arms.
Anatomy and histology.
Electrolytic lesions were made with 20-μA stimulation applied for 20 s (DC anodal) in the final tracks at multiple points of interest. After being placed under deep anesthesia with sodium pentobarbital, the subjects were transcardially perfused with phosphate-buffered saline and phosphate-buffered formalin. The brain was then removed and submerged in phosphate-buffered formalin with 30% sucrose for cryoprotection. A freezing microtome was used to cut 50-μm frontal sections, and every fourth section was mounted and stained with cresyl violet. The locations of brain stem structures were identified using a stereotaxic atlas (Szabo and Cowan 1984). The locations of EMG recording electrodes were verified by postmortem dissection. Following histological reconstruction, 12 additional stimulation sites that were outside of the PMRF or within 0.5 mm of midline were excluded from the analysis. As a result, 150 sites met all of the criteria for inclusion in the analysis.
RESULTS
Task-related EMG activity and force outputs.
EMG activity was present in all muscles at various times during performance of the task. The EMG activity of every muscle was significantly modulated between the hold phase and the target phase of the task (P < 0.001). The average force output during the target phase of the task was ∼30 N along the y-axis, with smaller magnitude forces (<10 N) measured along the x- and z-axes during the same time period. A representative example of task-related changes in y-axis force output and biceps brachii (BIC) and lateral triceps brachii (TRIC) EMG activity is shown in Fig. 2. No PStEs or force effects were evident in the raw data from single pulses before StimulusTA was applied.
Fig. 2.
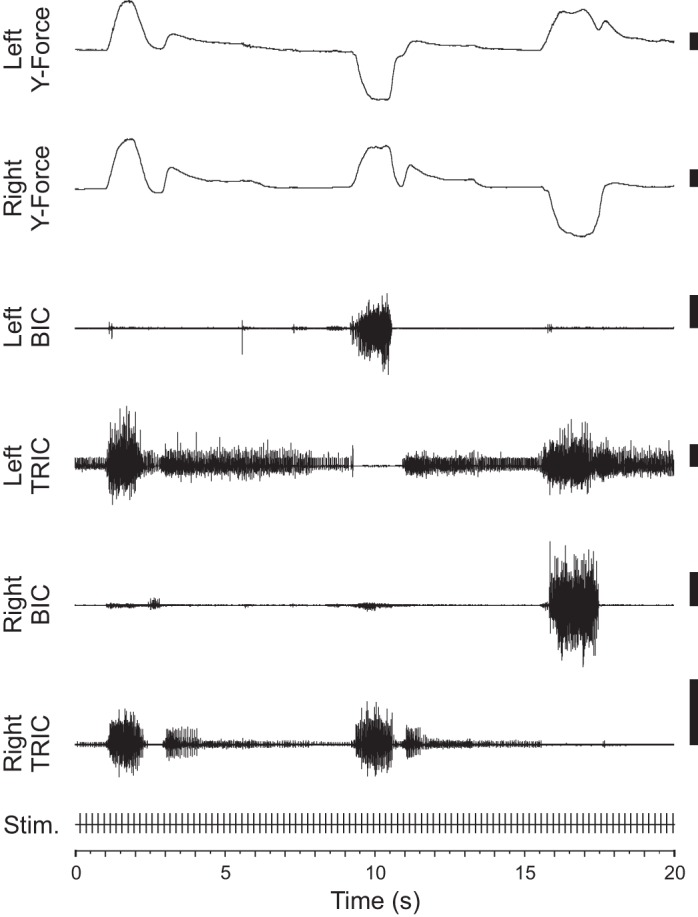
Representative example of task-related changes in y-axis force and biceps brachii (BIC) and lateral triceps brachii (TRIC) electromyography (EMG) activity. Three trials are shown over a 20-s period. The first trial required bilateral pushing, the second left-sided pulling and right-sided pushing, and the last left-sided pushing and right-sided pulling. EMG recordings show increased BIC activity during trials requiring pulling for that arm and increased TRIC activity during trials requiring pushing. Scale bars represent 10 N for force recordings and 1 mV for EMG recordings. Throughout the session, 30-μA stimulation was applied at 200-ms intervals (5 Hz).
There was typically a low-to-moderate level of EMG activity during pretrial hold periods when the subject waited with its hands on the force sensors but before it was instructed to apply force. This resting level of EMG activity was usually sufficient for intertrial triggers to meet the inclusion criteria for the analysis. The force output was greatest during the target phase of the task. All muscles showed a significant difference in the EMG activity for the pulling vs. pushing aspects of the task. The greatest differences in EMG for pulling vs. pushing were found in the flexors BIC, pectoralis major (PMJ), and anterior deltoid (ADLT) and the extensors TRIC and posterior deltoid (PDLT). The flexors were preferentially facilitated during pulling trials (negative y-direction), whereas the extensors were facilitated during pushing (positive y-direction).
General poststimulus effect characteristics.
In total, 733 PStEs were evoked from 139 of the 150 sites included in the analysis, accounting for 92.7% of all PMRF sites tested. On average, 22.0 ± 13.9% of the muscles analyzed responded per effective stimulus site, corresponding to five or six muscles. The overall range of EMG responses was 4.2% (one muscle) to 70.8% (17 muscles) of the 24 muscles tested. A selected example of PStEs and force effects is shown in Fig. 3.
Of the 733 PStEs, 423 (57.7%) were poststimulus suppression (PStS) and 310 (42.3%) were poststimulus facilitation (PStF). Consistent with its overall prevalence, PStS was evoked from more sites (132/150, 88.0%) than PStF (103/150, 68.7%). Exclusive PStS was observed from 36 sites (24.0%), while exclusive PStF was observed from only 7 sites (4.7%). For each effective stimulus site, 12.7 ± 8.2% of the muscles analyzed were suppressed, and 9.3 ± 10.1% were facilitated. The average onset for PStF of 8.3 ± 2.9 ms was significantly earlier than the average onset for PStS of 9.7 ± 2.9 ms, t(731) = 6.46, P < 0.001, as shown in Table 2.
Table 2.
Characteristics of poststimulus effects
| Timing |
Amplitude |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| EMG PStF, ms |
EMG PStS, ms |
Force, ms |
EMG PStF, % | EMG PStS, % | Force, mN | ||||
| Onset | Duration | Onset | Duration | Onset | TTP | ||||
| Mean | 8.3 | 7.3 | 9.7 | 6.6 | 15.9 | 45.2 | 11.0 | 9.2 | 12.0 |
| SD | 2.9 | 5.0 | 2.9 | 3.7 | 2.6 | 18.3 | 4.0 | 3.4 | 8.5 |
Timing and amplitude characteristics of poststimulus force responses and EMG facilitation and suppression. Onset latency is defined as the earliest time at which the EMG or force recording deviated from baseline activity by >2 ± SD. PStF, poststimulus facilitation; PStS, poststimulus suppression; TTP, time to peak.
PStEs by muscle.
Figure 4 shows the effectiveness of stimulation for each muscle, calculated as the number of stimulus-evoked responses in a muscle divided by the number of sites for which EMG was recorded from that muscle. Stimulation was most effective at limb girdle muscles compared with limb flexors and extensors, χ2 (1, N = 733) = 29.9, P < 0.001. EMG effects were most commonly observed in axial muscles like upper trapezius (UTR) (58.0%) and latissimus dorsi (LAT) (51.3%) and least commonly in distal muscles like flexor carpi ulnaris (FCU) (27.3%) and extensor carpi radialis (ECR) (14.0%).
Fig. 4.
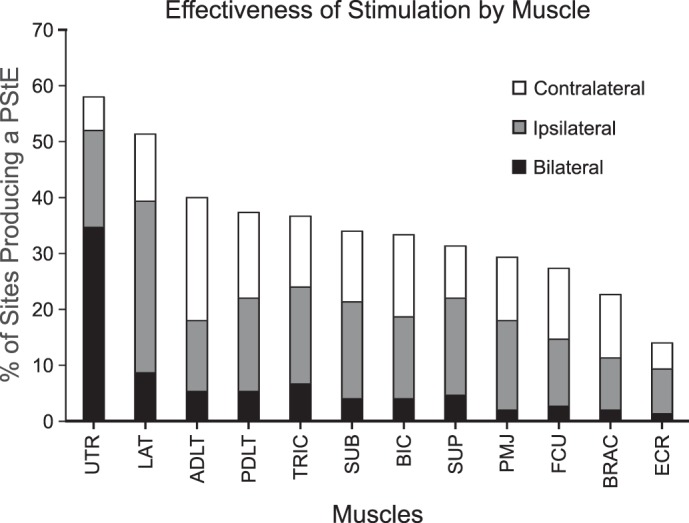
Effectiveness of stimulation at producing poststimulus EMG effects, determined for each muscle by calculating the percentage of sites from which a poststimulus EMG effect (PStE) was observed for each muscle. Percentages are subdivided based on the laterality of the responses evoked from each muscle. The height of the black bar represents the proportion of PStEs that produced a bilateral response, the gray bar represents ipsilateral responses, and the white bar represents contralateral responses. UTR, upper trapezius.
Poststimulus muscle activation patterns.
An average-linkage clustering algorithm was used to classify each of the 12 muscles into two clusters based on their poststimulus changes in EMG activity from all 150 stimulation sites, as calculated by MFSA (Poliakov and Schieber 1998). Muscles that had similar changes in EMG activity across many stimulation sites were clustered nearer each other. The clustering dendrogram is shown in Fig. 5A. The clustering assignments segregated muscles into a “flexor-like” muscle group, which contains the primary elbow and shoulder flexors (BIC and ADLT), and an “extensor-like” muscle group, which contains the primary elbow and shoulder extensors (TRIC and PDLT). The wrist muscles, FCU and ECR, were the muscles whose activity was least correlated with either group. While FCU and ECR are most correlated with each other, this may simply be due to the lower effectiveness of stimulation on distal muscles.
Fig. 5.
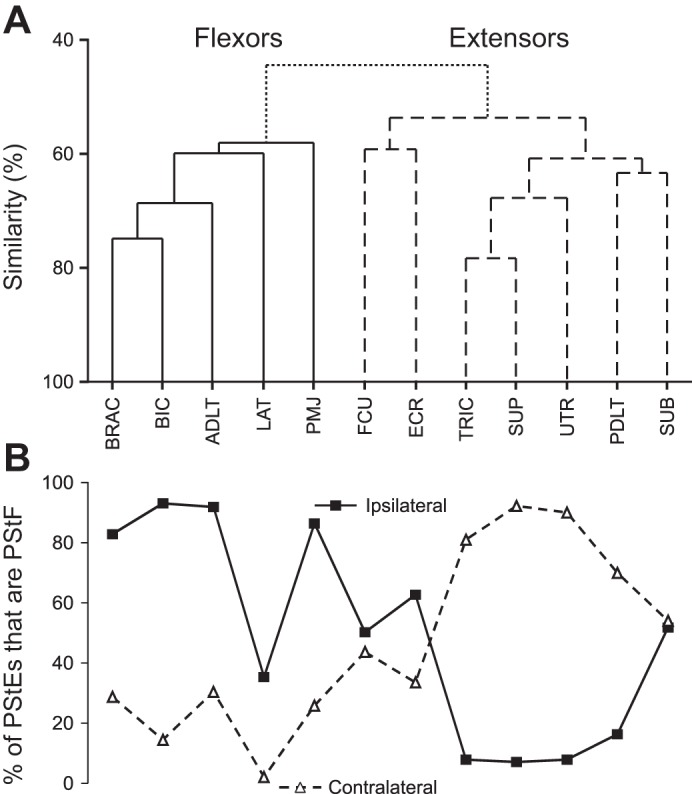
Muscle activation patterns and distribution of PStF and PStS by muscle. A: dendrogram showing muscle activation groupings based on average-linkage clustering algorithm of relative change in EMG activity following stimulation. The solid line indicates the flexor-like cluster; the dashed line indicates the extensor-like cluster. Similarity in activity between muscles is denoted by the vertical location of the branch point. B: each point represents the percentage of PStEs that were PStF for that muscle, organized along the horizontal axis to match clustering results. The solid line represents ipsilateral muscles; the dashed line represents contralateral muscles.
Facilitation was the most common poststimulus effect seen in ipsilateral flexors [brachioradialis (BRAC), BIC, ADLT, PMJ] and contralateral extensors (TRIC, PDLT, UTR), while suppression was the most common effect seen in ipsilateral extensors and contralateral flexors, as shown in Fig. 5B. LAT exhibited generalized suppression, but more so for the contralateral side of the body, consistent with its classification as a flexor. In general, this agrees with previously published reports (Davidson and Buford 2004), with the new finding that supraspinatus (SUP) and subscapularis (SUB) both group with the extensors.
General characteristics of transient force responses.
Significant force responses were detected from 105 (70.0%) of the 150 stimulation sites. Of those sites, 64 sites (61.0%) produced significant force responses bilaterally. Unilateral force responses were detected from the ipsilateral arm for 19 sites (18.1%) and from the contralateral arm for 22 sites (21.0%). In total, 169 significant force responses were detected from either arm. The average magnitude of significant force responses was 12.0 ± 8.5 mN with a maximum of 40.9 mN. For perspective, this mean is about 1.2 g and the maximum is about 5.0 g by weight. The forces required for task performance were ∼1,000 times greater than the observed poststimulus force responses. The magnitudes were not significantly different between ipsilateral and contralateral responses.
Force onset latencies were determined for poststimulus effects in which the instantaneous change in force deviated from baseline by >4 SD. This threshold was reached in 65 (38.5%) of the 169 significant force responses from either arm. For these force responses, the force onset latency was defined as the time at which the change in force crossed the 2 SD threshold relative to the baseline mean. The average onset latency was 15.9 ± 2.6 ms, as shown in Table 2. No significant difference in onset latency was detected between ipsilateral and contralateral force responses. The average time-to-peak, defined as the length of time between stimulus delivery and the maximum force deviation from baseline, was 45.2 ± 18.3 ms (median = 38.8 ms). There was no significant difference in time-to-peak between ipsilateral and contralateral force responses.
Bilateral force output patterns.
For the 105 stimulation sites in the PMRF that produced a significant force response in at least one arm, the majority of forces were directed along one of two directions, either medially and posteriorly or laterally and anteriorly. The direction of these force responses was significantly dependent on the laterality of the response relative to the location of the stimulation site (i.e., ipsilateral vs. contralateral), χ2 (3, N = 193) = 114.9, P < 0.001. The direction of force responses in the x–y plane can be divided into four groups, determined by the signs of the x- and y-components of force, with each group consisting of one 180° quadrant. 65.3% of significant force responses from the ipsilateral arm were directed medially and posteriorly, χ2 (3, N = 95) = 86.5, P < 0.001, shown in Fig. 6, while 78.6% of responses from the contralateral arm were directed laterally and anteriorly, χ2 (3, N = 98) = 151.6, P < 0.001, also shown in Fig. 6. For simplicity, results are presented as if the left side were always ipsilateral. Because of the bilateral symmetry of the subject and task design, this was done by mirroring results from right-sided stimulation across midline. In this frame of reference, the average ipsilateral force response was directed at an angle of 317° (medially and posteriorly), and the average contralateral force response was directed at an angle of 47° (laterally and anteriorly). This output pattern is consistent with the facilitation of ipsilateral flexors and contralateral extensors and the reciprocal suppression of contralateral flexors and ipsilateral extensors observed in EMG recordings.
Fig. 6.

Polar histograms of the direction of poststimulus force responses in the horizontal (x–y) plane. Left: distribution of force responses from side ipsilateral to the stimulus site, with 0° medial and 270° posterior, shown as if the ipsilateral side were always on the left. Ipsilateral force responses were primarily directed medially and posteriorly, P < 0.001. Right: distribution of force responses contralateral to stimulus site, with 0° lateral and 90° anterior, shown as if the contralateral side were always on the right. Contralateral force responses were primarily directed laterally and anteriorly, P < 0.001.
Relationships between force responses and PStEs.
At all 105 sites with a significant force effect in the StimulusTA, there was also at least one muscle with a significant PStE. As noted above, there were 139 sites of the 150 that resulted in a significant PStE, leaving 34 sites from which at least one PStE was evoked, but the force response was not significant. These 34 sites evoked, on average, fewer PStEs than their counterparts, t(126) = 7.36, P < 0.001.
For those sites from which significant PStEs were found in EMG records and in the forces, the average magnitude of the force responses was significantly correlated with the average poststimulus change in EMG activity elicited for the same site, r2 = 0.425, F(1,148) = 109.4, P < 0.001, as shown in Fig. 7. Similarly, the average force magnitude was significantly correlated with the number of muscles exhibiting a PStE, r2 = 0.373, F(1,148) = 85.3, P < 0.001. The magnitude of the force and EMG responses was determined by subtracting the average value during the control intervals from the average value during the target interval. For Fig. 7, the average EMG response was calculated by finding the average percent change from baseline during the target interval for all 24 EMG recordings. Similarly, the average force response was found for recordings from both force sensors. As noted above, the magnitude of the stimulus triggered force responses was about 1/1,000th of the force required to perform the task. Likewise, the sizes of the PStEs ranged from 1/100th to 1/1,000th of the task-related modulation of the EMG levels. The average onset of the PStEs at 8.3 ± 2.9 ms was significantly earlier than the average onset of the force responses at 15.9 ± 2.6 ms, t(373) = 19.5, P < 0.001, allowing sufficient time between events for the electromechanical delay (EMD).
Fig. 7.
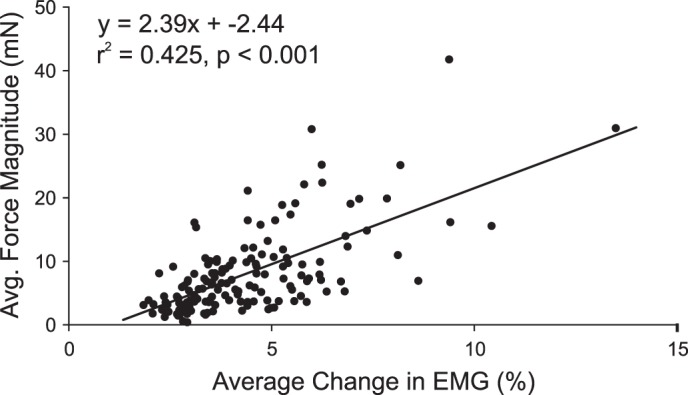
Correlation between the magnitude of poststimulus EMG effects with the magnitude of poststimulus force responses. A significant correlation was observed between the average change in EMG activity during the target interval as a percentage of the baseline activity and the average force magnitude, r2 = 0.425, P < 0.001, n = 150.
Multiple multivariate linear regression.
To test how well poststimulus changes in EMG activity account for the observed force responses, a multiple multivariate linear regression model was developed. The explanatory variables were the changes in average EMG activity between control and target intervals for all 12 muscles on one side, measured in multiples of the standard deviation of the EMG levels recorded during the baseline period. The response variables were the components of the force responses along each axis, recorded from the same side as the EMG recordings. For each stimulation site, the force responses were calculated by determining the change in force between the average force onset time and the average time-to-peak. To determine the force contributions of all the muscles from which EMG was recorded, a stepwise regression was not used.
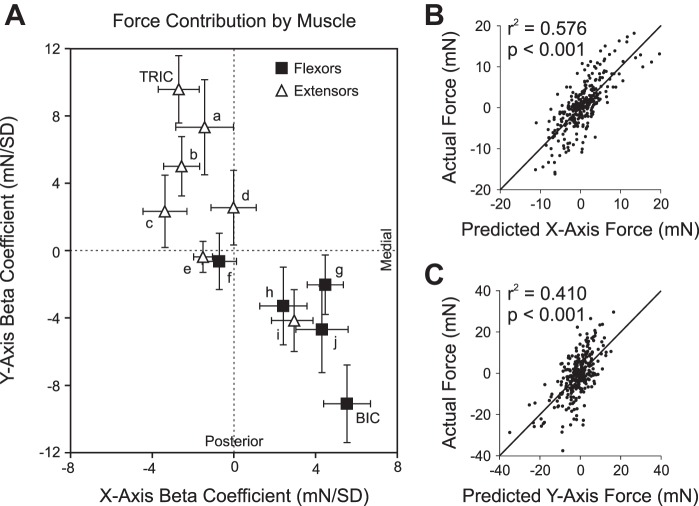
The β-coefficients of the regression analysis represent the contributions of each muscle to the generation of force in the ipsilateral arm, as shown in Fig. 8A. Facilitation of flexors and suppression of extensors were significantly associated with a resultant medial and posterior deflection in the force response of the corresponding arm, χ2 (3, N = 7) = 8.43, P = 0.038, while facilitation of extensors and suppression of flexors were associated with a resultant lateral and anterior deflection, χ2 (3, N = 5) = 8.60, P = 0.035. Additionally, the relative change in the EMG activity significantly correlated with the component of the force responses along the x-axis, r2 = 0.576, F(12,287) = 32.54, P < 0.001; y-axis, r2 = 0.410, F(12,287) = 16.63, P < 0.001; and z-axis, r2 = 0.294, F(12,287) = 9.98, P < 0.001. Every muscle except the FCU made a significant contribution to the regression model, shown in Fig. 8A. Variance inflation factors for all muscles were <5, indicating tolerable multicollinearity of PStEs. The goodness of fit for the x- and y-components of the linear regression model is shown in Fig. 8, B and C, respectively.
Fig. 8.
A: contributions of each muscle to force responses as determined by the linear regression model. Solid squares represent flexor-like muscles, and open triangles represent extensor-like muscles. BIC and TRIC are directly labeled, and superscripts specify the other muscles: a ECR, b PDLT, c SUP, d FCU, e UTR, f LAT, g ADLT, h PMJ, i SUB, j BRAC. The positive x-axis is medial, and the positive y-axis is anterior. Error bars correspond to the standard error. B: linear regression model predictions compared with actual x-axis (mediolateral) components of force responses and best-fit line, r2 = 0.576, P < 0.001, n = 300. C: linear regression model predictions compared with actual y-axis (anteroposterior) components of force responses and best-fit line, r2 = 0.410, P < 0.001, n = 300.
Force contributions of antagonistic muscle pairs.
According to the regression model, BIC and TRIC made the largest and most significant contributions to the force response (β = 10.7, P < 0.001 and β = 10.0, P < 0.001, respectively). To determine the ability of BIC and TRIC EMG activity alone to explain forces in the x–y plane, a directional tuning curve for the BIC and TRIC was generated, shown in Fig. 9. For each muscle, the correlation coefficients were found between the EMG activity and the projection of the force response vectors in 12 directions at 30° intervals. The correlation coefficient curves were fit well by sinusoidal functions, r2 = 0.962 for both BIC and TRIC. The EMG activity of BIC was best correlated with the components of force directed along an angle of 295° (medially and posteriorly). The TRIC EMG activity was best correlated with the components of force directed along an angle of 118° (laterally and anteriorly). As expected of antagonistic muscles, the BIC and TRIC correlated with components of force in opposite directions (177° difference).
Fig. 9.
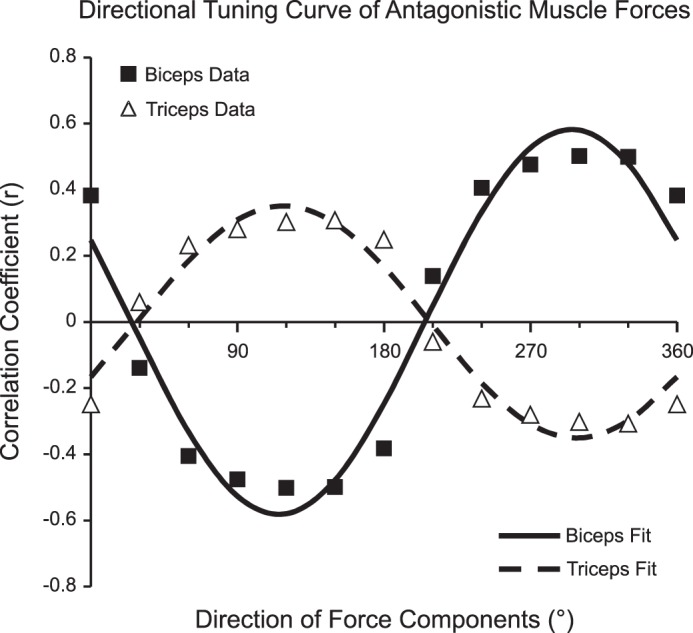
Directional tuning curve showing the correlation of EMG activity with the components of force responses in each direction. For BIC and TRIC, the correlation coefficients were found between the EMG activity and the components of force in 12 directions at 30° intervals. Standard cosine functions were used to fit data for BIC (solid line, solid squares) and TRIC (dashed line, open triangles). BIC activity was best correlated with the force components for 295°, and TRIC activity was best correlated with the force components for 118°. For both BIC and TRIC, r2 = 0.962.
Force patterns by location of stimulation.
Stimulus sites were distributed throughout the PMRF with no significant preference for laterality. For bilaterally significant responses, the average force output pattern was significantly different between left- and right-sided stimulation, t(71) = 16.4, P < 0.001, shown in Fig. 10B. The degree of flexion in the response from one arm was significantly correlated with the degree of extension in the other, r2 = 0.379, F(1,71) = 43.3, P < 0.001. In Fig. 10A, stimulation sites are categorized by their corresponding force output pattern, with different colors representing the directions of the poststimulus forces effects. Sites that produced a force pattern of left-sided flexion and right-sided extension are shown in red, and sites that produced a pattern of right-sided flexion and left-sided extension are shown in blue. Sites for which no significant bilateral force responses were detected are shown in gray.
DISCUSSION
Detection of transient force responses.
Single-pulse microstimulation produces small EMG responses detectable with StimulusTA, but does not produce obvious muscle twitches, and is thought to be imperceptible to the subject (Cheney and Fetz 1985). Measuring this poststimulus EMG activity has been a valuable method for studying motor outputs of cortical and brain stem neurons (Cheney and Fetz 1985; Davidson and Buford 2004, 2006). While individual movements evoked by a single stimulus may be too small to observe, the present study shows that force responses associated with muscle recruitment can be detected using StimulusTA. The overall effect of StimulusTA on arm movement is difficult to predict using only the complex pattern of facilitation and suppression of the muscles in that limb. But direct recording of these small force changes can reveal general patterns of arm movement resulting from muscle recruitment. To our knowledge, this is the first demonstration of such a measurement. This approach could also have considerable applications in other brain regions where questions about the coordinate representation encoded remain under study (Kakei et al. 1999, 2001).
While many studies have produced limb and head movements with short stimulus trains in the motor cortex (Lemon 2008) and a few have done so in the brain stem (Cowie and Robinson 1994; Drew and Rossignol 1990a; Herbert et al. 2010; Riddle et al. 2009), this type of stimulation produces spatial and temporal summation, leading to activation of polysynaptic pathways. In contrast, the low frequency (5 Hz) and low-current intensity (30 μA) of single-pulse microstimulation minimizes temporal and spatial summation and primarily activates monosynaptic and disynaptic pathways, producing a more physiologically relevant response (Cheney and Fetz 1985). StimulusTA with single-pulse microstimulation produces motor outputs similar to those from SpikeTA (Davidson et al. 2007), indicating that results from StimulusTA offer a better approximation of the motor effects of spontaneous neural activity than techniques that use higher frequency stimulation. Hence, it seems likely the force responses measured here are from relatively direct reticulospinal pathways.
EMD.
Muscle fiber contraction and force generation trails the motor unit action potential onset by a time period known as the EMD. Muscle contraction following excitation in the frog sartorius muscle has been shown to occur with a latency as short as 2.5 ms (Sandow 1952). In human finger muscles, forces associated with contraction of single motor units have been measured based on the triggered averaging approach (Kilbreath et al. 2002, Yu et al. 2007). In those studies, forces of 20–50 mN began in single fingers within 2–3 ms of the beginning of the motor unit action potential. In the present study, StimulusTA produced force responses with an average onset time of 15.9 ms poststimulation, which, considering an average flexor EMG onset latency of around 8.3 ms, corresponds to an EMD of ∼7.6 ms. An EMD of 8.0 ms has been observed in humans in response to a maximal electrical stimulation of the posterior tibial nerve (Grosset et al. 2008). Winter and Brookes (1991) measured the time interval from the change in electrical activity to the registration of force from a human soleus muscle to be 10.2 ms, and Muro and Nagata (1985) reported similar results of 11.7 ms in unstretched soleus muscle and 7.0 ms in heavily stretched muscle. Hence, the timing of the present results is consistent with the hypothesis that the detected force responses resulted directly from the poststimulus EMG changes measured by StimulusTA and not through an indirect mechanism. Studies relying on movement onset have reported longer estimates of EMD (Hoffmann and Strick 1999; Norman and Komi 1979; Philipp and Hoffmann 2014), but measurements of force onset in a pretensed, isometric condition would be expected to reveal relatively short EMDs.
Linear regression analysis.
Multivariate linear regression is used to determine the effects of multiple explanatory variables on their associated dependent variables. Linear regression is appropriate for these data because the mean rectified EMG varies approximately linearly with the isometric force generated by a muscle over the midrange recruitment levels studied (Lippold 1952; Milner-Brown and Stein 1975). Furthermore, because of the stationary position of the subject's arms, activity from a specific muscle will generate forces in a consistent direction. If the force responses are caused by the change in EMG activity following stimulation, a multivariate linear regression model should be able to describe this relationship. Our model uses the change in EMG activity from 12 muscles on one side to predict the direction and magnitude of the force response generated from the corresponding arm.
Linear regression analysis determined the contributions of each muscle to the forces generated at the joysticks, based on muscle EMG activity. The force responses of the studied muscles matched their expected functions, with elbow and shoulder flexors producing forces toward the body and extensors producing forces away from the body. BIC and TRIC made the largest contributions to force generation. Activation of wrist muscles was generally weak, and hand position on the joysticks was free to vary with the subject's style across trials, as long as the overall force was properly directed. As a result, the wrist muscles did not strongly contribute to the regression model. The limited contribution of more proximal muscles to the regression model may be dependent on the arm position required by the task and could vary with another recording setup.
Muscle groupings.
Davidson and Buford (2006) identified functional groups of upper limb muscles based on reciprocal patterns of EMG activation following PMRF stimulation. Of the muscles analyzed in this study, BIC, BRAC, ADLT, and PMJ have previously been shown to be facilitated during ipsilateral PMRF stimulation and suppressed during contralateral stimulation. Similarly, TRIC, PDLT, and UTR have been shown to be suppressed during ipsilateral PMRF stimulation and facilitated during contralateral stimulation. These groupings were preserved in this study by the clustering algorithm with grouped muscles based only on similarity of poststimulus EMG activity. The cluster encompassing the BIC, BRAC, ADLT, and PMJ was designated as the “flexor-like” muscle group, while the cluster containing TRIC, PDLT, and UTR was designated as the “extensor-like” muscle group.
Possibly owing to their less consistent responses to stimulation, the distal FCU and ECR were the last muscles to be clustered into either group. Although stimulation has proven less effective at activating more distal muscles, the reticulospinal tract has been shown to make monosynaptic and disynaptic projections to both forearm and intrinsic hand muscles (Riddle et al. 2009). While our results indicate a relatively low level of consistency between the flexor and extensor synergies in the proximal limb muscles and the activation patterns in wrist muscles, hemiparetic stroke patients have been shown to exhibit flexion synergy between proximal arm muscles and those of the wrist and fingers (Miller and Dewald 2012). One possible factor in this discrepancy is that our subjects were free to adopt individual styles and vary their grip on the joysticks across trials, which may have introduced some variability and reduced the consistency of muscle activation patterns. In other words, this might be due to experimental conditions. It is also possible that this is a species difference between humans and macaques.
Rotator cuff muscles, such as SUB and SUP, act as stabilizers of the shoulder joint in addition to other actions on the humeroscapular joint. The primary functions of SUP are external rotation and elevation of the glenohumeral joint. The primary functions of SUB are internal rotation and adduction of the glenohumeral joint. Despite their seemingly opposite functions, both of these muscles strongly clustered with the extensors. This is consistent with their role as stabilizers, both acting during extension particularly to stabilize the glenohumeral joint. However, it is difficult to determine if this pattern of synergy is contingent on the subject being in the position required by the task.
Reciprocal force output pattern.
Stimulation of the PMRF has been known to produce bilateral motor output patterns in the cat (Drew and Rossignol 1990a, 1990b; Sprague and Chambers 1954) and monkey (Davidson and Buford 2004, 2006). These movement patterns include flexion of the spine, ipsilateral head turning, ipsilateral arm flexion, and contralateral arm extension. The StimulusTA results agree that PMRF stimulation produces a bilateral pattern of EMG activity. Consistent with previous studies, ipsilateral facilitation and contralateral suppression of flexor-like muscles and reciprocal effects on extensor-like muscles were observed. Generalized suppression of LAT was compatible with flexion of the spine, and ipsilateral suppression and contralateral facilitation of UTR was compatible with ipsilateral head turning. Poststimulus force responses indicate a strong pattern of flexion of the ipsilateral arm and extension of the contralateral arm, as shown in Fig. 10. This pattern was also detected in force responses corresponding to stimulation sites in which only unilateral EMG activity was identified.
This bilateral action of the PMRF is supported anatomically by evidence of bilaterally distributed monosynaptic and disynaptic pathways. While the majority of reticulospinal axons terminate ipsilaterally, they have also been shown to project bilaterally (Matsuyama et al. 1997). Additionally, ipsilaterally projecting neurons can evoke bilateral motor outputs via commissural interneurons (Bannatyne et al. 2003; Jankowska et al. 2003; Jankowska and Edgley 2006). The pattern of flexion of one limb and the extension of the other is closely tied to locomotion (Drew and Rossignol 1984). Supraspinal locomotor command signals to central pattern generators in the spinal cord are primarily conveyed via reticulospinal pathways (Eidelberg et al. 1981; Matsuyama et al. 2004). However, PMRF neurons have also been shown to be modulated during voluntary reaching (Buford and Davidson 2004; Schepens et al. 2008; Schepens and Drew 2004, 2006) and finger movements (Soteropoulos et al. 2012). These results suggest that reticulospinal pathways involved in locomotion are also activated during reaching (Drew et al. 2004).
Because of its bilateral pattern of projection to the upper limbs, the reticulospinal system has been considered a candidate pathway for alternative motor control of the impaired limb after stroke (Ellis et al. 2012; Jankowska and Edgley 2006). One line of evidence supporting this concept is related to the asymmetric tonic neck reflex (ATNR), a primitive reflex thought to be mediated by the PMRF (Brink et al. 1985; Srivastava et al. 1984). The ATNR is normally suppressed in the adult nervous system, but can reemerge following cortical injury, such as from trauma or stroke. It is characterized by a classic “fencing” posture, with ipsilateral arm flexion and contralateral arm extension (Ellis et al. 2012). This reemergence of the ATNR is presumably due to an increased reliance on reticulospinal pathways, which also results in a loss of independent joint control and increased flexion synergy during voluntary reaching (Dewald et al. 1995; Ellis et al. 2012). The strong agreement of the ATNR motor pattern with the pattern of force responses evoked by PMRF provides further evidence that reticulospinal motor patterns are the basis of this response.
Task-dependent facilitation of poststimulus effects.
It is possible that, because of the requirements of the task, the behavior of the subjects preferentially facilitated force responses along the y-axis. If the behavioral task required exertion of forces in the x-direction, that may have affected the relative magnitudes of the components of force in the x- and y-directions. However, because the flexion/extension pattern of the force responses was produced with bilaterally symmetric task requirements, we would expect the force responses to display the observed reciprocal force output pattern, regardless of the exact nature of the task. While it would be interesting to subdivide the averages into periods of different patterns of exertion to construct an average from data collected only while both arms were pushing, another with one arm pulling and the other pushing, etc., the design of the study did not permit that. We would have needed to apply at least four times as many stimuli to support that analysis, perhaps more, and this would have made data collection four times as slow. As a practical matter in the design of this study, there was simply not enough time to perform so much stimulation. Perhaps a future study could be designed to allow dividing the stimuli for averaging in this manner.
Conclusions.
This novel technique for measuring force responses from StimulusTA provides strong confirmation of a bilateral double reciprocal pattern of PMRF output. The high internal consistency of our results, especially the EMD determined by the force onset latency and the strong correlation between EMG activity and force direction and magnitude, supports the validity of these findings. To our knowledge, this is the first report of low-intensity, single-pulse microstimulation in the CNS producing measureable force outputs. Our study provides a new approach to measuring very small forces on the order of a few grams by weight generated by single-pulse microstimulation in the CNS. Potentially, this approach could also reveal force responses associated with spontaneous neural activity in the motor system. This technique could be applied to other brain regions, such as the motor cortex, to detect physiological motor outputs.
GRANTS
This work was supported in part by National Institute of Neurological Disorders and Stroke Grant R01 NS-037822 to J. A. Buford.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.J.H. and J.A.B. performed experiments; T.J.H. and J.A.B. analyzed data; T.J.H. and J.A.B. interpreted results of experiments; T.J.H. and J.A.B. prepared figures; T.J.H. and J.A.B. drafted manuscript; T.J.H. and J.A.B. edited and revised manuscript; T.J.H. and J.A.B. approved final version of manuscript; J.A.B. conception and design of research.
ACKNOWLEDGMENTS
The authors thank Jacob Banks, Stephanie Moran, Rebecca Slattery, Chelsea Ellis, and Amanda Feddersen for technical assistance with this project. Wendy Herbert, Lynnette Montgomery, and Alexis Ortiz-Rosario assisted with data collection.
REFERENCES
- Ashe J. Force and the motor cortex. Behav Brain Res 87: 255–269, 1997. [DOI] [PubMed] [Google Scholar]
- Baker SN, Lemon RN. Computer simulation of post-spike facilitation in spike-triggered averages of rectified EMG. J Neurophysiol 80: 1391–1406, 1998. [DOI] [PubMed] [Google Scholar]
- Bannatyne BA, Edgley SA, Hammar I, Jankowska E, Maxwell DJ. Networks of inhibitory and excitatory commissural interneurons mediating crossed reticulospinal actions. Eur J Neurosci 18: 2273–2284, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts B, Smith JL, Edgerton VR, Collatos TC. Telemetered EMG of fast and slow muscles in cats. Brain Res 117: 529–533, 1976. [DOI] [PubMed] [Google Scholar]
- Brink EE, Suzuki I, Timerick SJ, Wilson VJ. Tonic neck reflex of the decerebrate cat: a role for propriospinal neurons. J Neurophysiol 54: 978–987, 1985. [DOI] [PubMed] [Google Scholar]
- Buford JA, Davidson AG. Movement-related and preparatory activity in the reticulospinal system of the monkey. Exp Brain Res 159: 284–300, 2004. [DOI] [PubMed] [Google Scholar]
- Buford JA, Montgomery L. Small force transients in the upper limbs recorded in association with stimulus triggered averaging in the reticulospinal system of the monkey. Soc Neurosci Abstr 591–02, 2011. [Google Scholar]
- Cheney PD, Fetz EE. Comparable patterns of muscle facilitation evoked by individual corticomotoneuronal (CM) cells and by single intracortical microstimuli in primates: evidence for functional groups of CM cells. J Neurophysiol 53: 786–804, 1985. [DOI] [PubMed] [Google Scholar]
- Cowie RJ, Robinson DL. Subcortical contributions to head movements in macaques. I. Contrasting effects of electrical stimulation of a medial pontomedullary region and the superior colliculus. J Neurophysiol 72: 2648–2664, 1994. [DOI] [PubMed] [Google Scholar]
- Davidson AG, Buford JA. Motor outputs from the primate reticular formation to shoulder muscles as revealed by stimulus triggered averaging. J Neurophysiol 92: 83–95, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AG, Buford JA. Bilateral actions of the reticulospinal tract on arm and shoulder muscles in the monkey: stimulus triggered averaging. Exp Brain Res 173: 25–39, 2006. [DOI] [PubMed] [Google Scholar]
- Davidson AG, Schieber MH, Buford JA. Bilateral spike-triggered average effects in arm and shoulder muscles from monkey pontomedullary reticular formation. J Neurosci 27: 8053–8058, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewald JP, Pope PS, Given JD, Buchanan TS, Rymer WZ. Abnormal muscle coactiviation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain 118: 495–510, 1995. [DOI] [PubMed] [Google Scholar]
- Drew T, Prentice S, Schepens B. Cortical and brainstem control of locomotion. Prog Brain Res 143: 251–261, 2004. [DOI] [PubMed] [Google Scholar]
- Drew T, Rossignol S. Phase-dependent responses evoked in limb muscles by stimulation of medullary reticular formation during locomotion in thalamic cats. J Neurophysiol 52: 653–675, 1984. [DOI] [PubMed] [Google Scholar]
- Drew T, Rossignol S. Functional organization within the medullary reticular formation of intact unanesthetized cat. I. Movements evoked by microstimulation. J Neurophysiol 64: 767–781, 1990a. [DOI] [PubMed] [Google Scholar]
- Drew T, Rossignol S. Functional organization within the medullary reticular formation of intact unanesthetized cat. II. Electromyographic activity evoked by microstimulation. J Neurophysiol 64: 782–795, 1990b. [DOI] [PubMed] [Google Scholar]
- Eidelberg E, Story JL, Walden JG, Meyer BL. Anatomical correlates of return of locomotor function after partial spinal cord lesions in cats. Exp Brain Res 42: 81–88, 1981. [DOI] [PubMed] [Google Scholar]
- Ellis MD, Drogos J, Carmona C, Keller T, Dewald JP. Neck rotation modulates flexion synergy torques, indicating an ipsilateral reticulospinal source for impairment in stroke. J Neurophysiol 108: 3096–3104, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evarts EV. Relation of pyramidal tract activity to force exerted during voluntary movement. J Neurophysiol 31: 14–27, 1968. [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Luschei ES. Firing patterns of abducens neurons of alert monkeys in relationship to horizontal eye movement. J Neurophysiol 33: 382–392, 1970. [DOI] [PubMed] [Google Scholar]
- Gahery Y, Ioffe ME, Massion J, Polit A. The postural support of movement in cat and dog. Acta Neurobiol Exp (Warsz) 40: 741–756, 1980. [PubMed] [Google Scholar]
- Georgopolous AP, Ashe J, Smyrnis N, Taira M. The motor cortex and the coding of force. Science 256: 1692–1695, 1992. [DOI] [PubMed] [Google Scholar]
- Grillner S, Lund S. A descending pathway with monosynaptic action on flexor motoneurones. Experientia 22: 390–390, 1966. [DOI] [PubMed] [Google Scholar]
- Grillner S, Lund S. The origin of a descending pathway with monosynaptic action on flexor motoneurones. Acta Physiol Scand 74: 274–284, 1968. [DOI] [PubMed] [Google Scholar]
- Grosset JF, Piscione J, Lambertz D, Perot C. Paired changes in electromechanical delay and musculo-tendinous stiffness after endurance or plyometric training. Eur J Appl Physiol 105: 131–139, 2008. [DOI] [PubMed] [Google Scholar]
- Herbert WJ, Davidson AG, Buford JA. Measuring the motor output of the pontomedullary reticular formation in the monkey: do stimulus-triggered averaging and stimulus trains produce comparable results in the upper limbs? Exp Brain Res 203: 271–283, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschauer TJ, Buford JA. Bilateral force transients in the upper limbs following single-pulse microstimulation in the ponto-medullary reticular formation. Soc Neurosci Abstr 71–16, 2014. [Google Scholar]
- Hoffmann DS, Strick PL. Step-tracking movements of the wrist. IV. Muscle activity associated with movements in different directions. J Neurophysiol 81: 319–333, 1999. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Edgley SA. How can corticospinal tract neurons contribute to ipsilateral movements? A question with implications for recovery of motor functions. Neuroscientist 12: 67–79, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Slawinska U, Maleszak K, Edgley SA. Neuronal basis of crossed actions from the reticular formation on feline hindlimb motoneurons. J Neurosci 23: 1867–1878, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakei S, Hoffman DS, Strick PL. Muscle and movement representations in the primary motor cortex. Science 285: 2136–2139, 1999. [DOI] [PubMed] [Google Scholar]
- Kakei S, Hoffman DS, Strick PL. Direction of action is represented in the ventral premotor cortex. Nat Neurosci 4: 1020–1025, 2001. [DOI] [PubMed] [Google Scholar]
- Kilbreath SL, Gorman RB, Raymond J, Gandevia SC. Distribution of the forces produced by motor unit activity in the human flexor digitorum profundus. J Physiol 543: 289–296, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuypers HG. Anatomical analysis of cortico-bulbar connexions to the pons and the lower brain stem in the cat. J Anat 92: 198–218, 1958. [PMC free article] [PubMed] [Google Scholar]
- Kuypers HG. Anatomy of descending pathways. In: Handbook of Physiology. The Nervous System. Motor Control. Bethesda, MD: Am. Physiol. Soc, 1981, sect. 1, vol. II, pt. 1, chapt. 13, p. 597–666. [Google Scholar]
- Lemon RN. Descending pathways in motor control. Annu Rev Neurosci 31: 195–218, 2008. [DOI] [PubMed] [Google Scholar]
- Lippold OCJ. The relation between integrated action potentials in a human muscle and its isometric tension. J Physiol 117: 492–499, 1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RF, Bowden DM. A stereotaxic template atlas of the macaque brain for digital imaging and quantitative neuroanatomy. Neuroimage 4: 119–150, 1996. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Mori F, Nakajima K, Drew T, Aoki M, Mori S. Locomotor role of the corticoreticular-reticulospinal-spinal interneuronal system. Prog Brain Res 143: 239–249, 2004. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Takakusaki K, Nakajima K, Mori S. Multi-segmental innervation of single pontine reticulospinal axons in the cervico-thoracic region of the cat: anterograde PHA-L tracing study. J Comp Neurol 377: 234–250, 1997. [PubMed] [Google Scholar]
- McKiernan BJ, Marcario JK, Karrer JH, Cheney PD. Corticomotoneuronal postspike effects in shoulder, elbow, wrist, digit, and intrinsic hand muscles during a reach and prehension task. J Neurophysiol 80: 1961–1980, 1998. [DOI] [PubMed] [Google Scholar]
- Miller LC, Dewald JPA. Involuntary paretic wrist/finger flexion forces and EMG increase with shoulder abduction load in individuals with chronic stroke. Clin Neurophysiol 123: 1216–1225, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner-Brown HS, Stein RB. The relation between the surface electromyogram and muscular force. J Physiol 246: 549–569, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran DW, Schwartz AB. Motor cortical representation of speed and direction during reaching. J Neurophysiol 82: 2676–2692, 1999. [DOI] [PubMed] [Google Scholar]
- Muro A, Nagata A. The effects on electromechanical delay of muscle stretch of the human triceps surae. In: Biomechanics IX-A, edited by Winter DA, Norman RW, Wells RP, Hayes KC, Patla AE. Champaign, IL: Human Kinetics, 1985, p. 86–90. [Google Scholar]
- Norman RW, Komi PV. Electromechanical delay in skeletal muscle under normal movement conditions. Acta Physiol Scand 106: 241–248, 1979. [DOI] [PubMed] [Google Scholar]
- Park MC, Belhaj-Saif A, Cheney PD. Chronic recording of EMG activity from large numbers of forelimb muscles in awake macaque monkeys. J Neurosci Methods 96: 153–160, 2000. [DOI] [PubMed] [Google Scholar]
- Peterson BW. Reticulospinal projections to spinal motor nuclei. Annu Rev Physiol 41: 127–140, 1979. [DOI] [PubMed] [Google Scholar]
- Peterson BW, Anderson ME, Filion M. Response of pontomedullary reticular neurons to cortical, tectal and cutaneous stimuli. Exp Brain Res 21: 19–44, 1974. [DOI] [PubMed] [Google Scholar]
- Peterson BW, Maunz RA, Pitts NG, Mackel RG. Patterns of projection and braching of reticulospinal neurons. Exp Brain Res 23: 333–351, 1975. [DOI] [PubMed] [Google Scholar]
- Peterson BW, Pitts NG, Fukushima K. Reticulospinal connections with limb and axial motoneurons. Exp Brain Res 36: 1–20, 1979. [DOI] [PubMed] [Google Scholar]
- Philipp R, Hoffmann KP. Arm movements induced by electrical microstimulation in the superior colliculus of the macaque monkey. J Neurosci 34: 3350–3363, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliakov AV, Schieber MH. Multiple fragment statistical analysis of post-spike effects in spike-triggered averages of rectified EMG. J Neurosci Methods 94: 3325–3341, 1998. [DOI] [PubMed] [Google Scholar]
- Riddle CN, Edgley SA, Baker SN. Direct and indirect connections with upper limb motoneurons from the primate reticulospinal tract. J Neurosci 29: 4993–4999, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai ST, Davidson AG, Buford JA. Reticulospinal neurons in the pontomedullary reticular formation of the monkey (Macaca fascicularis). Neuroscience 163: 1158–1170, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandow A. Excitation-contraction coupling in muscular response. Yale J Biol Med 25: 176–201, 1952. [PMC free article] [PubMed] [Google Scholar]
- Schepens B, Drew T. Independent and convergent signals from the pontomedullary reticular formation contribute to the control of posture and movement during reaching in the cat. J Neurophysiol 92: 2217–2238, 2004. [DOI] [PubMed] [Google Scholar]
- Schepens B, Drew T. Descending signals from the pontomedullary reticular formation are bilateral, asymmetric, and gated during reaching movements in the cat. J Neurophysiol 96: 2229–2252, 2006. [DOI] [PubMed] [Google Scholar]
- Schepens B, Stapley P, Drew T. Neurons in the pontomedullary reticular formation signal posture and movement both as an integrated behavior and independently. J Neurophysiol 100: 2235–2253, 2008. [DOI] [PubMed] [Google Scholar]
- Soteropoulos DS, Williams ER, Baker SN. Cells in the monkey ponto-medullary reticular formation modulate their activity with slow finger movements. J Physiol 590: 4011–4027, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto O, Cros D. Direct corticospinal control of force derivative. J Neurosci 31: 1944–1948, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague JM, Chambers WW. Control of posture by reticular formation and cerebellum in the intract, anesthetized and unanesthetized and in the decerebrated cat. Am J Physiol 176: 52–64, 1954. [DOI] [PubMed] [Google Scholar]
- Srivastava UC, Manzoni D, Pompeiano O, Stampacchia G. Responses of medullary reticulospinal neurons to sinusoidal rotation of neck in decerebrate cat. J Neurosci 11: 473–486, 1984. [DOI] [PubMed] [Google Scholar]
- Szabo J, Cowan WM. A stereotaxic atlas of the brain of the cynomolgus monkey (Macaca fascicularis). J Comp Neurol 222: 265–300, 1984. [DOI] [PubMed] [Google Scholar]
- Winter EM, Brookes FBC. Electromechanical response times and muscle elasticity in men and women. Eur J Appl Physiol 63: 124–128, 1991. [DOI] [PubMed] [Google Scholar]
- Yu WS, Kilbreath SL, Fitzpatrick RC, Gandevia SC. Thumb and finger forces produced by motor units in the long flexor of the human thumb. J Physiol 583: 1145–1154, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]