Abstract
Measuring the excitability of individual axons is complicated by the prohibitive difficulty in obtaining intracellular recordings. Here, we present an innovative methodology that enables local excitability to be measured anywhere in a channelrhodopsin (ChR2)-expressing neuron. The approach hinges on activating ChR2 in a spatially and temporally precise manner while recording the resulting spike train from a remote site. We validated this approach in primary afferent neurons (PANs). Initial encoding of somatosensory stimuli relies on transduction of the physical stimulus into a receptor potential and transformation of the receptor potential into a spike train; the transformation process depends on the excitability of the most distal PAN endings but, as explained above, is extraordinarily difficult to study in situ using traditional methods. Using ChR2-based photoactivation, we show 1) that excitability differs between the distal endings and more proximal portions of PAN axons, 2) that the transformation process differs between PANs, and 3) that the transformation process is directly affected by inflammation. Beyond presenting an innovative method by which to study axonal excitability, this study has validated its utility in helping to decipher the earliest stages of somatosensory encoding.
Keywords: axon, excitability, optogenetics, channelrhodopsin, somatosensory
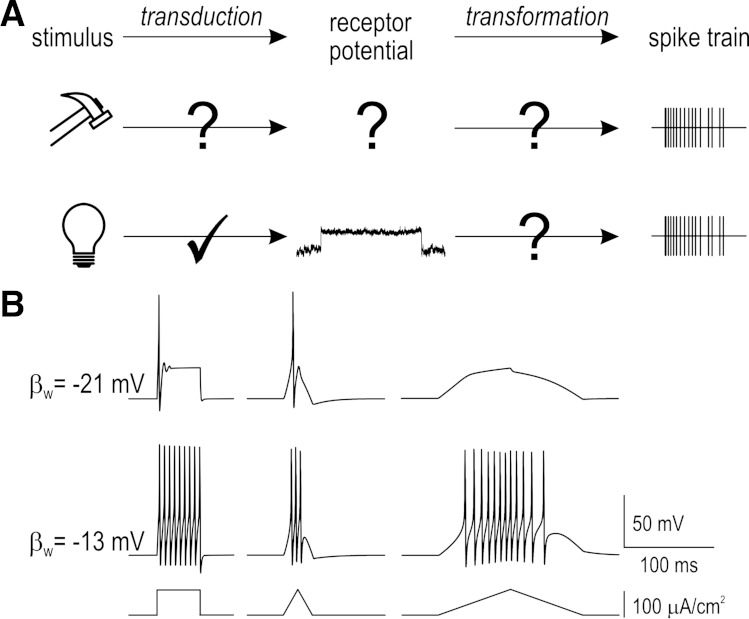
primary afferent neurons (PANs) must detect physical stimuli and transmit that information to the spinal cord or brain. Activation of mechano-, chemo-, or thermosensitive ion channels causes local depolarization known as a receptor potential (Gray and Sato 1953) or generator potential (Loewenstein and Rathkamp 1958) but that depolarization cannot travel appreciable distances down the axon and must, therefore, be converted into spike trains. We refer to generation of a receptor potential as the transduction step and to conversion of the receptor potential into a spike train as the transformation step (Fig. 1A). The same receptor potential may produce very different spike trains depending on the transformation step, as illustrated by computer simulations in Fig. 1B. Both steps must, therefore, be understood to provide a complete account of how neural representations of sensory input are formed.
Fig. 1.
Excitability and the transformation process. A: schematic showing transduction of physical stimuli into receptor potentials and transformation of receptor potentials into spike trains. If the receptor potential cannot be measured, neither transduction nor transformation can be inferred from the spike train. By replacing natural receptor potentials (with unknown kinetics) with photopotentials (with known kinetics), the transformation step can be isolated and studied. B: computer simulations showing generation of different spike trains in response to equivalent stimulation. Responses are from a 2-dimensional Morris-Lecar model with parameters set so that the neuron behaves as a coincidence detector (top) or integrator (bottom). Parameter βw represents the balance of voltage-dependent currents whose interaction controls spike initiation (for details, see Prescott et al. 2008; Rho and Prescott 2012). Spike initiation in coincidence detectors requires fast depolarization, which means large but slow-onset receptor potentials fail to elicit spiking in such neurons. These simulations illustrate the importance of characterizing spiking patterns through combined testing with fast- and slow-onset stimuli (e.g., steps and ramps).
Studying transduction and transformation is complicated because those steps occur together within exquisitely small axon terminals. The spike train can be recorded extracellularly but measuring the receptor potential requires intracellular access. Intracellular recordings, even if they were possible, risk damaging the axon and/or its surrounding tissue and disrupting the processes being studied. Rather than measuring the receptor potential, we sought to replace it with precisely controlled photopotentials (made possible through optogenetics) and thereby isolate the transformation step. We reasoned that applying spatially and temporally controlled photopotentials while remotely monitoring the emitted spike train would reveal the local transformation properties of the photostimulated region.
Driven by the specific goal of studying somatosensory encoding and further motivated by the prospect of enabling a multitude of other experiments on axonal excitability, we crossed Advillin-Cre mice with floxed channelrhodopsin (ChR2) mice to produce mice that express ChR2 in all PANs. Applying our optophysiological method for measuring excitability in these mice, we show that excitability differs 1) between different regions of the axon, 2) between the distal endings of different axons, and 3) before vs. after inflammation. These data thoroughly validate our novel excitability testing strategy and provide new insights into the earliest stages of somatosensory encoding.
MATERIALS AND METHODS
Transgenic mice.
All procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Male heterozygous Advillin-Cre mice (Zhou et al. 2010) (kindly provided by Fan Wang at Duke University) were crossed with Ai32 mice (C57BL/6 background) purchased from The Jackson Laboratory. Male Advillin-Cre mice must be used for breeding to ensure sensory neuron-specific expression of Cre recombinase (da Silva et al. 2011). Ai32 mice carry the ChR2(H134R)-EYFP gene in the Gt(ROSA)26Sor locus but that gene is separated from its CAG promoter by a STOP cassette flanked by loxP sites (Madisen et al. 2012) thus making its expression Cre dependent. Heterozygous male mice were used for all experiments.
Cell culture and patch-clamp recordings.
Acutely dissociated PANs were prepared from adult mice (Malin et al. 2007). Neurons were recorded in whole cell mode with >70% series resistance compensation using an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA). Intracellular recording solution contained the following (in mM): 125 KMeSO4, 5 KCl, 10 HEPES, 2 MgCl2, 4 Na2ATP, and 0.4 Tris-GTP (Sigma, Ronkonkoma, NY); pH was adjusted to 7.2 with KOH. Traces were low-pass filtered at 2 kHz and digitized at 20 kHz using a 1401 computer interface and Signal 5 software (CED, Cambridge, UK).
Colon-nerve preparation and teased fiber recordings.
The colon-nerve preparation was prepared as previously described (Feng and Gebhart 2011). In brief, the distal colorectum (∼3-cm long) was removed with a length of pelvic nerve still attached but with the dorsal root ganglion (where the PAN somata are located) removed. The colon was opened longitudinally along the antimesenteric border and pinned flat on a Sylgard-lined chamber with the mucosal side up. The nerve sheath surrounding the pelvic nerve was peeled back, and the nerve trunk was teased into fine bundles ∼10-μm thick that were placed individually onto a platinum-iridium electrode for recording. The signal was filtered (0.3–10 kHz) and amplified (×10,000) by a low-noise AC differential amplifier (DAM 80; World Precision Instruments, Sarasota, FL) and digitized at 20 kHz using a 1401 computer interface (CED) and Spike2 v5 software (CED).
Stimulation.
Controlled mechanical stretch was applied using a computer-controlled force actuator (series 300B; Aurora Scientific, Toronto, ON, Canada) whose headstage was modified to enable isotonic pulling. The antimesenteric edge of the colon was attached to the actuator by a custom-made set of claws so that stretch was applied homogeneously in the circumferential direction of the colon (Feng and Gebhart 2011). Photostimulation was applied using a 470-nm LED (OptoLED; Cairn Instruments, Kent, UK) focused to a point ∼1-mm wide on the colon mucosa or on the pelvic nerve using a fiber optic probe. Since blue light does not penetrate deep into tissue, photostimulation is likely to preferentially activate the most superficial axon terminals (which approach the mucosal surface from below). Previous work using electrical stimulation has established that PAN receptive fields are ∼1 mm2 (Feng and Gebhart 2011), meaning the photostimulus fills most, if not all, of the receptive field. For recordings from cultured neurons, the OptoLED was coupled directly to the epifluorescence port of the Zeiss AxioExaminer microscope. In both configurations, light intensity was measured using a PM100D power meter coupled to a S170C photodiode sensor (Thorlabs, Newton, NJ) that was positioned at the tissue stimulation site. Maximal light intensity was 5.4 mW/mm2 for stimuli delivered via the fiber optic and 12.6 mW/mm2 for stimuli delivered via the microscope. Signal or Spike2 software (CED) was used to control the timing of all photo and stretch stimuli.
RESULTS
ChR2 activation properties and conductance density.
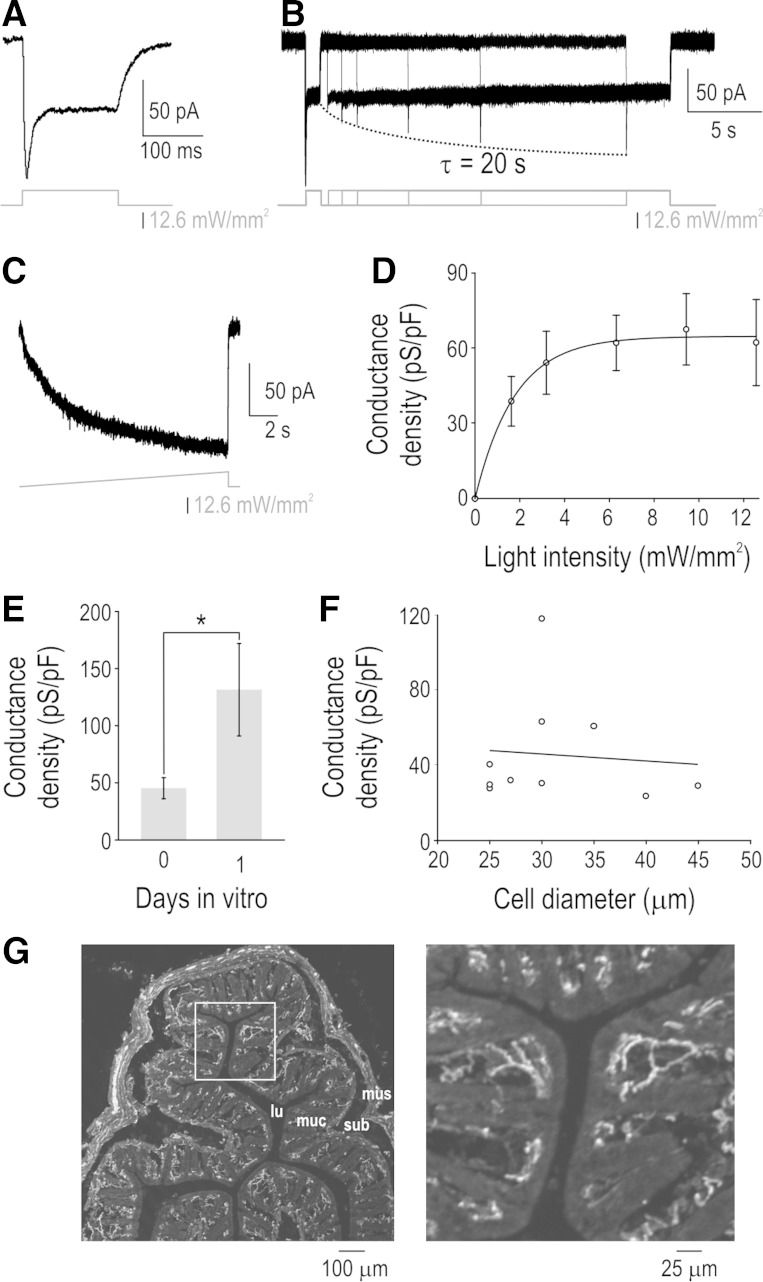
To begin, we measured the photoactivation properties and conductance density of ChR2 in acutely dissociated PAN somata using voltage clamp. Figure 2A shows a typical response to “step” photostimulation with 470 nm of light in a neuron clamped at −60 mV. Consistent with previous descriptions of the H134R form of ChR2 (Lin et al. 2009), we observed a short transient phase of photocurrent followed by a sustained phase. Applying a second photostimulus shortly after the first stimulus evoked an inward current comprising only the sustained phase (Fig. 2B) because inactivated ChR2 channels did not have time to deinactivate (e.g., Lin et al. 2009). Repeating the second photostimulus after different intervals revealed a slow deinactivation time constant of ∼20 s. This property of ChR2 allows square-shaped (i.e., abrupt onset but nonovershooting) photocurrents to be produced by closely spaced photostimuli. Furthermore, because ChR2 inactivation happens quickly, increasing the light intensity slowly (e.g., as a ramp) selectively reveals the sustained ChR2 current (Fig. 2C). Hence, Fig. 2, B and C, presents two stimulation protocols by which to mitigate effects of ChR2 inactivation so that the ChR2 response follows the photostimulus intensity more linearly. Distinguishing between different patterns of excitability is best achieved by testing with both fast- and slow-onset stimuli (i.e., steps and ramps). Consistent with ramped photostimulation, testing photostimulus steps with differing intensities revealed that ChR2 activation saturates at low light levels (Fig. 2D). Whereas most ChR2 experiments use short pulses of saturating-intensity light, these data show how differently shaped photopotentials can be created using deliberately chosen photostimulus waveforms.
Fig. 2.
Channelrhodopsin (ChR2) activation properties and conductance densities. A: sample voltage-clamp recording at −60 mV showing transient and sustained components of ChR2-mediated inward current. B: second photostimulus applied after a short interval failed to evoke transient component of ChR2 current because inactivated channels have not yet deinactivated. Varying the interstimulus interval revealed a ChR2 deinactivation rate of ∼20 s. C: ramping light intensity slower than the rate of ChR2 inactivation selectively reveals the sustained component of the ChR2 current. By inducing inactivation, the photostimulation protocols in B and C allow the photocurrent to follow changes in light intensity in a more linear fashion. D: conductance density was measured by dividing the sustained ChR2 current by driving force (=60 mV based on holding potential of −60 mV and ChR2 reversal potential of 0 mV) and membrane capacitance (C = τ/R). Means ± SE ChR2 conductance densities are plotted as a function of light intensity (n = 5 cells). E: responses to maximal light intensity from neurons tested on day 0 in vitro (n = 10) and day 1 in vitro (n = 5) show that ChR2 conductance density increased significantly with days in vitro (P < 0.05; unpaired t-test). F: responses to maximal light intensity plotted against soma diameter. Slope of the regression line (−0.4 pS·pF−1·μm−1) did not differ significantly from 0 (P = 0.81; t-test), indicating comparable expression across primary afferent neuron (PAN) types. Somata with a diameter <30 μm or >30 μm typically correspond to unmyelinated C fibers and myelinated fibers, respectively (Harper and Lawson 1985). G: immunostaining shows that enhanced yellow fluorescent protein (EYFP)-tagged ChR2 channels are present in the distal endings of PANs innervating the colon. Individual fibers can be seen at right, which is an enlarged view of the boxed region at left (lu, lumen; muc, mucosa; sub, submucosa; mus, muscularis).
Next, we measured ChR2 conductance density based on the sustained response to maximal photostimulation. ChR2 conductance density was 45 ± 9 pS/pF when measured on the same day as culturing (day 0 in vitro) but increased significantly to 132 ± 40 pS/pF by day 1 in vitro (P < 0.05; unpaired t-test; Fig. 2E). All other in vitro data are from day 0. Double immunolabeling of enhanced yellow fluorescent protein (EYFP) with isolectin B4 (IB4), calcitonin gene-related peptide (CGRP), or neurofilament-200 (NF-200) confirmed expression of the ChR2-EYFP transgene in nonpeptidergic C fibers, peptidergic C fibers, and myelinated fibers, respectively (data not shown). The flat regression line (P = 0.81, t-test) found by plotting the sustained responses to maximal light intensity against soma diameter (Fig. 2F) indicates that ChR2 is expressed at comparable levels regardless of cell size. Although there is no clear relationship between ChR2 expression levels and cell size, expression is evidently quite variable from one neuron to the next. It is unclear if the variability in somatic expression might arise as a consequence of culturing or if it correlates with expression elsewhere in the neuron. Immunolabeling confirmed that EYFP-tagged ChR2 channels are present in PAN axon terminals (Fig. 2G), but it is not possible to directly measure the ChR2 conductance density there. Not knowing the absolute amplitude of the ChR2 stimulus is an inherent limitation of the technique, but valuable insights can nonetheless be obtained by varying the shape of that stimulus, as demonstrated below.
Qualitative differences in excitability revealed by ChR2-based photoactivation.
Having characterized ChR2 activation properties and somatic expression levels in our transgenic mouse line, we began testing whether axonal excitability can be assessed using ChR2-based photoactivation. For this, we conducted teased fiber recordings while applying photostimuli focused to a point ∼1-mm wide to an ex vivo colon-nerve preparation (see materials and methods). Figure 3A shows a typical response from a colon afferent when its axon in the pelvic nerve was photostimulated (left) and when its terminals in the colon were photostimulated (right). Figure 3B shows a different fiber exhibiting transient spiking in response to photostimulation of its terminals; note that the fiber continued to spike transiently across a range of stimulus intensities (bottom trace). Photostimulation applied to the nerve elicited transient spiking in five of five-well isolated units, consistent with published examples of intra-axonal current injection (e.g., Birch et al. 1991; Kriz et al. 2000; Shu et al. 2007a,b) and with responses to somatic current injection in PAN (e.g., Liu et al. 2002; Ratté et al. 2014). Single units could not be isolated in the other nerves tested, but multiunit spiking was only ever observed at stimulus onset, which argues that all axons within the pelvic nerve respond to sustained focal depolarization with a single spike. By comparison, photostimulus steps applied to peripheral axon terminals in the colon elicited repetitive spiking (i.e., >3 spikes) in 10 of 25 neurons; the other 15 neurons responded with transient spiking (≤3 spikes, usually just 1). This represents a significantly lower proportion of transient spiking than observed with nerve photostimulation (χ2 = 6.0; P < 0.05). Notably, because we do not know whether ChR2 is distributed uniformly throughout the neuron, we cannot exclude that axons spike transiently because of lower ChR2 levels (and lower maximal photodepolarization); however, experiments described below argue that transient spiking is not attributable to insufficient depolarization.
Fig. 3.
Photostimulation-based measurement of excitability. A: sample traces from the same colon afferent activated by photostimulation applied to the pelvic nerve (left) or to its receptive field in the colon (right). Note that transient spiking was seen in response to closely spaced photostimuli; the transient ChR2 current is absent from all but the first stimulus (see Fig. 2B), meaning the spiking pattern is not attributable to the transient ChR2 current. B: example of fiber that responded with transient spiking to photostimulus trains applied to its terminals in the colon (top). This fiber continued to spike transiently across a broad range of stimulus intensities (bottom). C: sample responses from another transient spiking fiber tested with multistage steps, incrementing steps, and ramps. Note that the slowly ramped photostimulus did not elicit spiking despite photostimulus intensity exceeding threshold as inferred from photostimulus steps. Traces in A–C are filtered recordings but subsequent spiking responses are shown as rasters based on extracted spike times; all rasters are shown on a gray background so that responses without spikes are evident. D: comparison of responses in transient and repetitive spiking fibers to multistage photostimulus steps. The former responds with transient spiking at the start of each step whereas the latter spikes at a rate proportional to stimulus intensity. E: ramped stretching (left) elicited repetitive spiking (>3 spikes) in 10 of 25 units tested, designated type 1 (top) but did not elicit spiking in the other 15 units, designated type 2 (bottom). Ramped photostimulation (right) elicited repetitive spiking in 7 of 10 type 1 neurons but in only 1 of 15 type 2 neurons; all other type 2 neurons spiked transiently (≤3 spikes) or not at all. F: sample rasters from another fiber that did not respond to either type of stimulus ramp under control conditions (although it did respond with transient spiking to a photostimulus step; not shown). The fiber responded vigorously to both stimuli after blockade of voltage-gated potassium channels by 2.5 mM 4-aminopyridine (4-AP), therein demonstrating that the absence of the response to stretch under control conditions was due to the transformation step, not the transduction step. Three of 4 transient spiking neurons converted to repetitive spiking and 2 of 2 repetitive spiking neurons exhibited increased repetitive spiking after application of 4-AP.
Figure 3C illustrates a sequence of stimuli applied to a transient spiking neuron. Consistent with simulations in Fig. 1B, such fibers tend not to respond to slow photostimulation ramps even when photostimulus intensity exceeds the threshold intensity by severalfold. However, the threshold photostimulus intensity required to elicit spiking in most transient spiking fibers is near the maximum we can apply and so we could not routinely apply photostimuli double or triple the threshold intensity to rule out a switch to repetitive spiking (but see also summation experiments in Fig. 4). This is a limitation of the ChR2 rather than the photostimulus per se and could be overcome by the development of better (i.e., higher single-channel conductance) opsins or higher ChR2 expression. When fibers did respond to weak stimuli, it was possible to test their responses to photostimulus increments. Transient spiking neurons responded to abrupt increases in stimulus intensity (Fig. 3D, left), whereas repetitive spiking neurons can encode the stimulus intensity (Fig. 3D, right). The repetitive spiking shown in Fig. 3D is regular (i.e., with consistent interspike intervals), which makes the stimulus-dependent increase in the rate obvious, but spiking was quite irregular in other repetitively spiking neurons (see below).
Fig. 4.
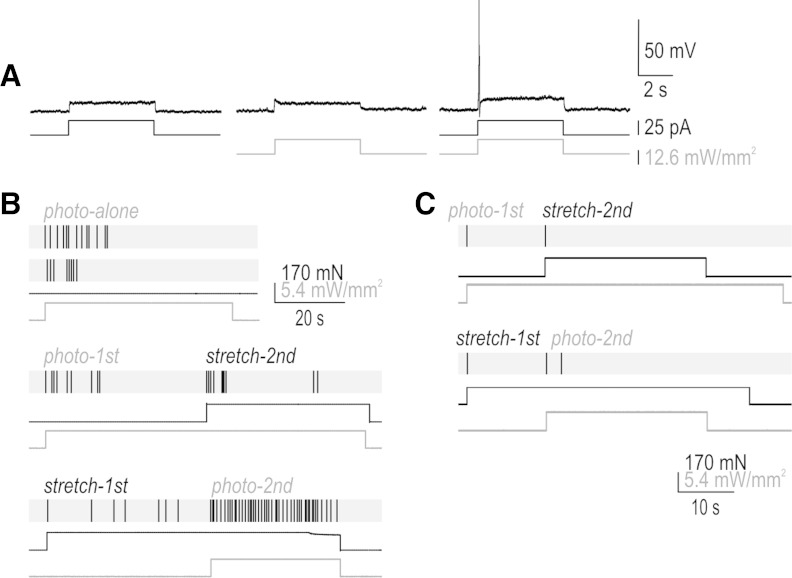
Summation of receptor potentials. A: somatic recording in current clamp showing that current injection and photostimulation causing subthreshold depolarization can, when combined, evoke a spike because the underlying depolarization summates. B: sample responses in a typical repetitive spiking fiber showing the effects of combining stretch and photostimulation. Stretch applied during photostimulation evoked more spiking than the same stretch applied before photostimulation (cf. responses labeled stretch-2nd and stretch-1st); this summation is expected since the photopotential is sustained (see Fig. 2A). Photostimulation applied during stretch evoked more spiking than the same photostimulus applied before stretching (cf. responses labeled photo-2nd and photo-1st); this summation shows that the stretch-induced receptor potential is sustained despite the eventual cessation of spiking. Similar results were observed in 4 of 4 repetitive spiking fibers tested. Multiple responses to the same photostimulus intensity are shown to highlight the irregular spike timing typical of repetitive spiking fibers. C: sample response of transient spiking fiber to equivalent combined stimulation. Most importantly, stretch applied during photostimulation elicited the same response as stretch applied before photostimulation despite the sustained photopotential, which argues that this fiber encodes changes in depolarization rather than absolute depolarization, consistent with Figs. 1B and 3D. This response pattern, which was observed in 3 of 3 transient spiking neurons tested, argues that transient spiking is not a consequence of insufficient net depolarization.
Next, we sought to compare excitability between cells and to relate those differences with differential responses to stretch stimuli. Of the 10 neurons that responded to slow stretching with repetitive spiking (designated type 1), 7 responded to slowly ramped photostimuli with repetitive spiking (Fig. 3E, top traces). Of the 15 neurons that that did not respond to slow stretching (designated type 2), only 1 exhibited repetitive spiking in response to ramped photostimuli (Fig. 3E, bottom traces). The proportion of neurons responding to photostimulation with repetitive spiking differed significantly between type 1 and 2 neurons (χ2 = 11.1; P < 0.001), demonstrating that differences in mechanoresponsiveness (which are typically ascribed to differences in transduction properties) correlate with, and may be caused by, differences in transformation properties.
Comparing photostimulus steps and ramps, we found that steps elicited repetitive spiking in 10 of 25 neurons whereas ramps elicited repetitive spiking in 8 of those 10 neurons. The remaining 15 and 17 neurons, respectively, responded with transient spiking or with no spiking at all; specifically, ramps tended to elicit no spikes whereas steps elicited transient spiking that typically comprised a single spike. The difference between ramp and step photostimulation is accounted for by 2 of the 10 neurons that responded with repetitive spiking to step stimulation responding with transient spiking to ramp stimulation. This difference in classification depending on the stimulus protocol is statistically insignificant (χ2 = 0.35; P = 0.56).
To test the role of transformation in the lack of mechanoresponsiveness in type 2 neurons, we applied 4-aminopyridine (4-AP) and retested both types of stimuli. 4-AP is known to increase excitability and encourage repetitive spiking according to somatic recording (Ratté et al. 2014) by blocking voltage-gated potassium channels, but it has no effects on ChR2 or mechanosensitive channels. Figure 3F shows a typical response in which a fiber that was unresponsive to ramped stretching and photostimulation under control conditions (although it responded with a single spike to a photostimulus step) responded vigorously to both types of stimulation after application of 4-AP. 4-AP reduced the photostimulus threshold in four of four type 2 neurons tested and caused three of them to develop repetitive spiking in response to stretch and photostimulation. 4-AP increased repetitive spiking in two of two type 1 neurons tested. These results demonstrate the importance of transformation properties for the initial encoding of somatosensory stimuli: if the neuron was mechanoinsensitive because of the transduction process (i.e., for lack of mechanosensitive ion channels), it should not start responding to mechanical stimulation after selective manipulation of the transformation process.
Inferring receptor potential kinetics through summation of responses to combined stimulation.
Repetitive spiking has two requirements: 1) sustained depolarization (receptor potential) and 2) the capacity to spike repetitively during sustained depolarization. Transient spiking will occur if either requirement is not met. The absence of repetitive spiking during sustained light-induced depolarization argues that requirement 2 is not met in transient spiking neurons, but it does not tell us about requirement 1. To investigate this, namely whether stretch-induced depolarization in transient spiking neurons is brief or sustained, we tested stimulus combinations. We reasoned that presence or absence of summation evidenced by spiking would reveal whether the unknown stretch-induced receptor potential was sustained or brief, respectively. Testing first in cultured neurons, Fig. 4A shows simply that receptor potentials induced by different stimulus modalities can summate to evoke spiking; equivalent results were obtained in four of four neurons tested. Repeating similar experiments on peripheral axon terminals, we found that stretch applied during a photostimulus evoked more spiking than the same stretch applied before photostimulation (Fig. 4B, cf. responses labeled stretch-2nd and stretch-1st). This temporal summation is expected since we know the ChR2-based photopotential is sustained (see Fig. 2). More importantly, photostimulation applied during a stretch stimulus evoked more spiking than the same photostimulus applied before stretching (Fig. 4B, cf. responses labeled photo-2nd and photo-1st). Temporal summation in the latter scenario shows that the stretch-induced receptor potential is sustained. Similar results were obtained in four of four repetitive spiking neurons tested.
Figure 4C shows the same experiments in a transient spiking neuron. The most notable observation here is the absence of summation effects. In particular, the observation that the stretch-2nd response still comprises only a single spike (despite summation of the underlying depolarization, since we know that ChR2 photoactivation causes sustained depolarization) argues that transient spiking is attributable to the transformation step rather than to an insufficient receptor potential. Of the three transient spiking neurons tested, none switched to repetitive spiking when photostimulation was superimposed on stretch stimulation. These data support the argument that transient spiking is a direct reflection of intrinsic excitability rather than because of insufficient ChR2 conductance density (see above).
Some additional observations about repetitive spiking are notable. Cessation of spiking during photostimulation is attributable to adaptation of the transformation process given that the photopotential is sustained. The same adaptation presumably shapes the spiking response to stretch; we cannot rule out diminution of the stretch-induced receptor potential (i.e., adaptation of the transduction process) but the similarity of spiking responses to photostimulation and stretch suggests that the underlying receptor potentials are similar. That said, spike timing in Fig. 4B was quite irregular although one should also bear in mind that the spike rate was low, which argues against bursting as one might think of it in the central nervous system. Irregular spiking has been observed previously in colon afferents (e.g., Feng and Gebhart 2011) and even more so in cutaneous afferents (e.g., Adrian and Zotterman 1926; Horch et al. 1974) during mechanical stimulation. Since photodepolarization is smooth, observation that photostimulation elicits irregular spiking implicates the transformation process as the main source of irregularity, consistent with recent work by Lesniak et al. (2014), whose simulations accounted for such irregularity on the basis of multiple independent spike initiation zones.
Measuring inflammation-induced changes in the excitability of peripheral axon terminals.
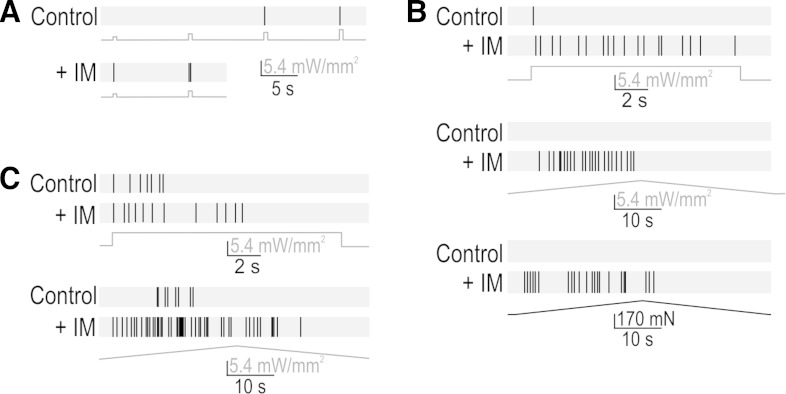
Inflammatory mediators (IM) enhance PAN excitability (e.g., Nicol and Cui 1994) and play an important role in chronic pain (Dawes et al. 2013). Therefore, we tested whether ChR2-based photoactivation could reveal inflammation-induced changes in transformation. Photostimulation of axon terminals revealed dramatically increased excitability following application of IM to the colon. Figure 5A shows the reduction in photostimulus threshold in a transient spiking neuron. Figure 5B shows other responses from the same neuron: the same photostimulus step that elicited transient spiking under normal conditions elicited repetitive spiking after IM, and ramped photostimulation and stretching both elicited repetitive spiking after IM whereas neither had elicited any spiking under control conditions. This pattern resembles the 4-AP effects in Fig. 3F and is consistent with a switch in spike initiation mechanism (Fig. 1B; see also Ratté et al. 2014; Rho and Prescott 2012). Figure 5C shows the IM-induced increase in responsiveness in a fiber that spiked repetitively under control conditions. In both fiber types, the IM-induced change in spiking evoked by ramped stretching was consistent with the change in spiking evoked by ramped photostimulation. IM-application experiments were completed for five fibers; all three transient spiking neurons switched to repetitive spiking after IM and both repetitive spiking neurons increased their spiking after IM. Whereas the altered responses to ramped stretching might be due to altered transduction or transformation, the altered photoresponses suggests that inflammation has a direct and functionally important impact on transformation. We cannot be certain that ChR2 function is not altered by IM, but whereas increased ChR2 conductance could in theory account for the increased responsiveness observed in repetitive spiking fibers, it could not account for the qualitative change in spiking pattern observed in transient spiking fibers based on evidence (see above) showing that insufficient depolarization is not the basis for transient spiking. Notably, the altered excitability observed among transient spiking neurons could explain inflammation-induced unsilencing of “silent” nociceptors (Dray 1995) insofar as such neurons could now respond to slow depolarization that would normally never excite those neurons (see Fig. 1B).
Fig. 5.
Effects of inflammation on transformation. A: inflammatory mediators (IM) reduced threshold in 5 of 5 fibers tested, as illustrated here for a transient spiking fiber. B: typical responses in a transient spiking fiber to photostimulus steps (top), photostimulus ramps (middle), and stretch ramps (bottom) before and after IM. Equivalent results were observed in 3 of 3 transient spiking fibers tested. Based on other evidence showing that increased depolarization does not cause transient neurons to start spiking repetitively, unaccounted for effects of IM on ChR2 function could not explain the observed switch in spiking pattern, which argues that IM has a direct effect on the transformation process. C: typical response in a repetitive spiking fibers to photostimulus steps (top) and ramps (bottom) before and after IM. The IM-induced increase in spiking was observed in 2 of 2 repetitive spiking fibers tested. IM soup comprised 5 μM histamine, 5 μM prostaglandin E2, 5 μM serotonin, and 5 μM bradykinin (all from Sigma).
DISCUSSION
This study describes a novel use of optogenetics to assess excitability in parts of the neuron that are inaccessible to conventional electrophysiological techniques. This methodological advance is especially important for studying PANs, which detect and encode stimuli in their peripheral axon terminals. If one hopes to provide a complete biophysical account of how somatosensory stimuli are initially encoded, one must understand the transformation process where it normally occurs, in the periphery. Using our approach, we have shown that transformation properties are indeed crucial for determining how different types of colon afferents encode stretch. We have also shown that transformation properties are modulated by inflammation and that this modulation can profoundly alter encoding.
The conceptual advance at the root of the photostimulation strategy we present here is to replace natural yet hard-to-measure receptor potentials with artificial yet controllable photopotentials. This capitalizes on breakthroughs in optogenetics that have enabled virtually any neuron to be made activatable by light. Light has several beneficial properties that include being easy to deliver in precise spatial and temporal patterns. Capitalizing on the spatial precision, one can photostimulate any part of a ChR2-expressing neuron and assess the ability of the photostimulated region to initiate spikes. Capitalizing on the temporal precision, one can apply different photostimulus waveforms to probe the dynamics of spike initiation within the photostimulated region. Because spikes are actively propagated along the axon, the resulting spike train can be recorded outside the photostimulated region.
Despite the popularity of optogenetics, this is the first study to use ChR2-based photoactivation to probe subcellular differences in excitability. Herman et al. (2014) recently showed that different types of neurons are differentially susceptible to photo-induced depolarization block, but regional differences within the same neuron were not tested. Computer simulations have suggested that photostimulation of different parts of a pyramidal neuron will evoke different spiking patterns (Grossman et al. 2013). Beyond the spatial considerations, almost all past studies have used short, intense photostimuli to reliably evoke spikes via maximal ChR2 activation; the use of rectified sine wave photostimulation by Wen et al. (2010) is a notable exception. In contrast, we have exploited differently shaped photostimulus waveforms whose intensities vary within the dynamic range of ChR2. Importantly, because ChR2 is a nonselective cation channel distributed in the membrane, ChR2 activation mimics the conductance-based excitation mediated by natural receptor molecules more accurately than current injection through an electrode (even if such an electrode could be used).
Unlike our focus on cellular processes (and subcellular differences therein), most ChR2-based photoactivation experiments have focused on activating large numbers of a certain type of neuron based on the selective expression of ChR2 in that neuron type. To that end, emphasis is placed on the cell-type specific expression of ChR2 or other light-sensitive molecules (Zhao et al. 2011). The same is true of recent efforts to use optogenetics to study peripheral mechanisms of somatosensation and pain. Wang and Zylka (2009) were the first to express ChR2 in Mrgprd-expressing nociceptors. More recently, Daou et al. (2013) crossed Ai32 mice with NaV1.8-Cre mice to express ChR2 in Nav1.8-expressing neurons, most of which are nociceptors. Draxler et al. (2014) used the same approach with VGluT3-Cre mice. Expression of ChR2 behind a Thy1.2 promoter resulted in preferential expression in large myelinated fibers (Ji et al. 2012) whereas viral delivery using adeno-associated virus serotype 6 (AAV-6) gave preferential expression amongst unmyelinated fibers (Iyer et al. 2014). The methodological advance presented here can obviously be applied to mice that express ChR2 in specific cell types. We used Advillin-Cre mice to ensure a heterogeneous ChR2-expressing cell population with which to validate our approach.
Like the photoactivation-based approach described here, advances in optical recording methods based on measurement of intracellular calcium or membrane potential (Akerboom et al. 2013; Hochbaum et al. 2014; Tian et al. 2009) will facilitate investigation of processes in parts of the neuron inaccessible to traditional electrophysiological techniques. Those optophysiological methods will be especially important for investigating transduction and, in that respect, will complement photoactivation-based investigation of transformation. The ChR2-based photoactivation strategy described here can also be used to probe for changes in axonal excitability following injury, demyelination, hypoxia, hyperexcitation, or any one of other countless manipulations that reproduce some aspect of neurological disease. Excitability testing by ChR2-based photoactivation has great potential.
GRANTS
This work was supported by a scholar award from the Edward Mallinckrodt Jr. Foundation (to S. A. Prescott) and by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-093525 (to G. F. Gebhart). S. A. Prescott is also a Canadian Institutes of Health Research New Investigator.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Y.Z., B.F., and E.S.S. performed experiments; Y.Z. and S.A.P. analyzed data; Y.Z., B.F., E.S.S., G.F.G., and S.A.P. interpreted results of experiments; Y.Z. and S.A.P. prepared figures; Y.Z., B.F., E.S.S., G.F.G., and S.A.P. edited and revised manuscript; Y.Z., B.F., E.S.S., G.F.G., and S.A.P. approved final version of manuscript; S.A.P. conception and design of research; S.A.P. drafted manuscript.
ACKNOWLEDGMENTS
We thank Fan Wang for providing Advillin-Cre mice, Stéphanie Ratté and Amber Shaffer for feedback on the manuscript, and Sona Joyce, Chris Sullivan, and Mike Burcham for expert technical assistance.
REFERENCES
- Adrian ED, Zotterman Y. The impulses produced by sensory nerve endings. Part II. The response of a single end organ. J Physiol 61: 151–171, 1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerboom J, Carreras Calderon N, Tian L, Wabnig S, Prigge M, Tolo J, Gordus A, Orger MB, Severi KE, Macklin JJ, Patel R, Pulver SR, Wardill TJ, Fischer E, Schuler C, Chen TW, Sarkisyan KS, Marvin JS, Bargmann CI, Kim DS, Kugler S, Lagnado L, Hegemann P, Gottschalk A, Schreiter ER, Looger LL. Genetically encoded calcium indicators for multi-color neural activity imaging and combination with optogenetics. Front Mol Neurosci 6: 2, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch BD, Kocsis JD, Di Gregorio F, Bhisitkul RB, Waxman SG. A voltage- and time-dependent rectification in rat dorsal spinal root axons. J Neurophysiol 66: 719–728, 1991. [DOI] [PubMed] [Google Scholar]
- da Silva S, Hasegawa H, Scott A, Zhou X, Wagner AK, Han BX, Wang F. Proper formation of whisker barrelettes requires periphery-derived Smad4-dependent TGF-beta signaling. Proc Natl Acad Sci USA 108: 3395–3400, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daou I, Tuttle AH, Longo G, Wieskopf JS, Bonin RP, Ase AR, Wood JN, De Koninck Y, Ribeiro-da-Silva A, Mogil JS, Seguela P. Remote optogenetic activation and sensitization of pain pathways in freely moving mice. J Neurosci 33: 18631–18640, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes JM, Andersson DA, Bennett DL, Bevan S, McMahon SB. Inflammatory mediators and modulators of pain. In: Wall and Melzack's Textbook of Pain (6 ed.), edited by McMahon SB, Koltzenburg M, Tracey I, Turk DC. New York: Elsevier, 2013, p. 48–67. [Google Scholar]
- Draxler P, Honsek SD, Forsthuber L, Hadschieff V, Sandkuhler JV. GluT3(+) primary afferents play distinct roles in mechanical and cold hypersensitivity depending on pain etiology. J Neurosci 34: 12015–12028, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dray A. Inflammatory mediators of pain. Br J Anaesth 75: 125–131, 1995. [DOI] [PubMed] [Google Scholar]
- Feng B, Gebhart GF. Characterization of silent afferents in the pelvic and splanchnic innervations of the mouse colorectum. Am J Physiol Gastrointest Liver Physiol 300: G170–G180, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA, Sato M. Properties of the receptor potential in Pacinian corpuscles. J Physiol 122: 610–636, 1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman N, Simiaki V, Martinet C, Toumazou C, Schultz SR, Nikolic K. The spatial pattern of light determines the kinetics and modulates backpropagation of optogenetic action potentials. J Comput Neurosci 34: 477–488, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper AA, Lawson SN. Conduction velocity is related to morphological cell type in rat dorsal root ganglion neurones. J Physiol 359: 31–46, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman AM, Huang L, Murphey DK, Garcia I, Arenkiel BR. Cell type-specific and time-dependent light exposure contribute to silencing in neurons expressing channelrhodopsin-2. Elife 3: e01481, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochbaum DR, Zhao Y, Farhi SL, Klapoetke N, Werley CA, Kapoor V, Zou P, Kralj JM, Maclaurin D, Smedemark-Margulies N, Saulnier JL, Boulting GL, Straub C, Cho YK, Melkonian M, Wong GK, Harrison DJ, Murthy VN, Sabatini BL, Boyden ES, Campbell RE, Cohen AE. All-optical electrophysiology in mammalian neurons using engineered microbial rhodopsins. Nat Methods 11: 825–833, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horch KW, Whitehorn D, Burgess PR. Impulse generation in type I cutaneous mechanoreceptors. J Neurophysiol 37: 267–281, 1974. [DOI] [PubMed] [Google Scholar]
- Iyer SM, Montgomery KL, Towne C, Lee SY, Ramakrishnan C, Deisseroth K, Delp SL. Virally mediated optogenetic excitation and inhibition of pain in freely moving nontransgenic mice. Nat Biotechnol 32: 274–278,2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji ZG, Ito S, Honjoh T, Ohta H, Ishizuka T, Fukazawa Y, Yawo H. Light-evoked somatosensory perception of transgenic rats that express channelrhodopsin-2 in dorsal root ganglion cells. PLoS One 7: e32699, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriz J, Meier J, Julien JP, Padjen AL. Altered ionic conductances in axons of transgenic mouse expressing the human neurofilament heavy gene: a mouse model of amyotrophic lateral sclerosis. Exp Neurol 163: 414–421, 2000. [DOI] [PubMed] [Google Scholar]
- Lesniak DR, Marshall KL, Wellnitz SA, Jenkins BA, Baba Y, Rasband MN, Gerling GJ, Lumpkin EA. Computation identifies structural features that govern neuronal firing properties in slowly adapting touch receptors. Elife 3: e01488, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY, Lin MZ, Steinbach P, Tsien RY. Characterization of engineered channelrhodopsin variants with improved properties and kinetics. Biophys J 96: 1803–1814, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CN, Devor M, Waxman SG, Kocsis JD. Subthreshold oscillations induced by spinal nerve injury in dissociated muscle and cutaneous afferents of mouse DRG. J Neurophysiol 87: 2009–2017, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein WR, Rathkamp R. Localization of generator structures of electric activity in a Pacinian corpuscle. Science 127: 341, 1958. [DOI] [PubMed] [Google Scholar]
- Madisen L, Mao T, Koch H, Zhuo JM, Berenyi A, Fujisawa S, Hsu YW, Garcia AJ 3rd, Gu X, Zanella S, Kidney J, Gu H, Mao Y, Hooks BM, Boyden ES, Buzsaki G, Ramirez JM, Jones AR, Svoboda K, Han X, Turner EE, Zeng H. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat Neurosci 15: 793–802, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin SA, Davis BM, Molliver DC. Production of dissociated sensory neuron cultures and considerations for their use in studying neuronal function and plasticity. Nat Protoc 2: 152–160, 2007. [DOI] [PubMed] [Google Scholar]
- Nicol GD, Cui M. Enhancement by prostaglandin E2 of bradykinin activation of embryonic rat sensory neurones. J Physiol 480: 485–492, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott SA, De Koninck Y, Sejnowski TJ. Biophysical basis for three distinct dynamical mechanisms of action potential initiation. PLoS Comput Biol 4: e1000198, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratté S, Zhu Y, Lee KY, Prescott SA. Criticality and degeneracy in injury-induced changes in primary afferent excitability and the implications for neuropathic pain. Elife 3: 02370, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rho YA, Prescott SA. Identification of molecular pathologies sufficient to cause neuropathic excitability in primary somatosensory afferents using dynamical systems theory. PLoS Comput Biol 8: e1002524, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y, Duque A, Yu Y, Haider B, McCormick DA. Properties of action-potential initiation in neocortical pyramidal cells: evidence from whole cell axon recordings. J Neurophysiol 97: 746–760, 2007a. [DOI] [PubMed] [Google Scholar]
- Shu Y, Yu Y, Yang J, McCormick DA. Selective control of cortical axonal spikes by a slowly inactivating K+ current. Proc Natl Acad Sci USA 104: 11453–11458, 2007b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, Bargmann CI, Jayaraman V, Svoboda K, Looger LL. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods 6: 875–881, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zylka MJ. Mrgprd-expressing polymodal nociceptive neurons innervate most known classes of substantia gelatinosa neurons. J Neurosci 29: 13202–13209, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, Wang H, Tanimoto S, Egawa R, Matsuzaka Y, Mushiake H, Ishizuka T, Yawo H. Opto-current-clamp actuation of cortical neurons using a strategically designed channelrhodopsin. PLoS One 5: e12893, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Ting JT, Atallah HE, Qiu L, Tan J, Gloss B, Augustine GJ, Deisseroth K, Luo M, Graybiel AM, Feng G. Cell type-specific channelrhodopsin-2 transgenic mice for optogenetic dissection of neural circuitry function. Nat Methods 8: 745–752, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Wang L, Hasegawa H, Amin P, Han BX, Kaneko S, He Y, Wang F. Deletion of PIK3C3/Vps34 in sensory neurons causes rapid neurodegeneration by disrupting the endosomal but not the autophagic pathway. Proc Natl Acad Sci USA 107: 9424–9429, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]