Abstract
The relationship between mono- and polysynaptic strength and action potential synchronization was explored using a reduced external Mg2+ model. Single and dual whole cell patch-clamp recordings were performed in hippocampal cultures in three concentrations of external Mg2+. In decreased Mg2+ medium, the individual cells transitioned to spontaneous bursting behavior. In lowered Mg2+ media the larger excitatory synaptic events were observed more frequently and fewer transmission failures occurred, suggesting strengthened synaptic transmission. The event synchronization was calculated for the neural action potentials of the cell pairs, and it increased in media where Mg2+ concentration was lowered. Analysis of surrogate data where bursting was present, but no direct or indirect connections existed between the neurons, showed minimal action potential synchronization. This suggests the synchronization of action potentials is a product of the strengthening synaptic connections within neuronal networks.
Keywords: dual whole cell patch-clamp, hippocampal culture, synchronization, action potential
seizures are defined as hypersynchronous discharge of neurons (Browne and Holmes 2000). It is important to understand the mechanisms of synchronization of action potentials to improve treatment of seizures (Golomb et al. 2006; Kopell and Ermentrout 2004; Netoff et al. 2005a; Netoff and Schiff 2002). Synchronization of neuronal bursting activity is a characteristic of many in vitro seizure models (Fujiwara-Tsukamoto et al. 2004; Golomb et al. 2006; Haas and Jefferys 1984; Miles et al. 1984; Robinson et al. 1993; Scharfman 1994; Traub et al. 1987; Traub et al. 1994; Ziburkus et al. 2006) even though the underlying mechanisms causing synchronized bursting may be different (Gulyás-Kovács et al. 2002). Often the synchronization of the bursting activity is examined using cross-correlation (Netoff and Schiff 2002; Traub et al. 2004; Ziburkus et al. 2006), field potentials (Bianchi and Wong 1994; Haas and Jefferys 1984; Niespodziany et al. 2003) or by visual inspection (Fujiwara-Tsukamoto et al. 2004; Miles and Wong 1983; Miles et al. 1984; Scharfman 1994; Traub et al. 1994). Few experimental studies quantify the synchronization of action potentials to make comparisons between various in vitro conditions.
An underlying assumption of in vitro bursting activity is that the activity is more synchronous than under more “natural” external conditions where no bursting is present. However, it is possible for action potential synchrony to exist in neurons with synaptic connections (Borgers and Kopell 2003; Mancilla et al. 2007; Netoff et al. 2005b). Under bursting conditions, action potentials in connected cells do not always exhibit exact synchronization (Golomb et al. 2006), but they may have varying time delays (Scharfman 1994) for which action potentials could be considered synchronous.
Low and zero Mg2+ in the extracellular solution can be used as a seizure-like condition (Gulyás-Kovács et al. 2002; Mody et al. 1987; Traub et al. 1994). Decreasing Mg2+ creates bursting behavior predominantly by unblocking NMDA receptors (Mangan and Kapur 2004; Mody et al. 1987; Traub et al. 1994; Velisek et al. 1994). Bursts of action potentials in decreased Mg2+ conditions were shown to be synchronized using extracellular measurements (Robinson et al. 1993). Additionally, decreasing Mg2+ has been shown to increase the amplitude and frequency of spontaneous excitatory postsynaptic currents (sEPSCs) (Mangan and Kapur 2004). This suggests that the strength of the synaptic connections has increased. Mangan and Kapur (Mangan and Kapur 2004) implied that the increase in synchronized activity is related to the increase in synaptic strength, but the relationship between synaptic strength and synchronization has not been studied directly.
In this study we examine the relationship between synchronization and network synaptic strength by measuring the synchronization of action potentials using dual whole cell recordings in various Mg2+ external conditions. We also examine the change in synaptic strength between two cells in the network by counting the number of failures, measuring spontaneous EPSC amplitudes.
MATERIALS AND METHODS
Ethical Approval
Animals were treated according to a protocol approved by the University of Virginia Animal Care and Use Committee (ACUC), and efforts were made to minimize animal stress and discomfort.
Mixed Culture Preparation
Cultures were prepared from postnatal day 0 to postnatal day 1 (P0–P1) Sprague-Dawley newborn rats using methods similar to those described elsewhere (Banker and Goslin 1998; Goodkin et al. 2008; Nunez 2008). The newborn rats were decapitated, and their brains removed and placed in cold HEPES-buffered Hanks' balanced salt solution (HEPES-HBSS). The hippocampi were removed under a dissecting microscope and collected in a small petri dish containing HEPES-HBSS. Tissues were incubated in 0.125% trypsin for 15 min at 37°C. Trypsin solution was replaced with 5 ml HEPES-HBSS, and the cells were rinsed twice more with HEPES-HBSS at 5-min intervals. Hippocampi were triturated until no fragments of tissue remained. Neurons were collected by centrifugation and resuspended in 5 ml of Dulbecco's modified Eagles medium (DMEM) and F-12 supplement (1:1) (Invitrogen) with 10% fetal bovine serum (heat-inactivated, Invitrogen), 2 mM l-glutamine (Invitrogen), and penicillin (100 U/ml)-streptomycin (100 U/ml). Culture dishes were coated with poly-lysine and filled with 2 ml of culture medium. Cells were plated at a minimum density of 50,000 per 35 mm2 dish and kept at 37°C in a 5% CO2 incubator. After 24 h, the culture medium was changed to serum-free medium containing 2% B27 and 2 mmol/l glutamine. The medium was replaced with fresh medium every 2–3 days.
Electrophysiology
Neurons were 14 days in vitro (DIV) at time of study because recurrent bursting is best observed after synapse maturation (Mangan and Kapur 2004). Three external media were used containing various concentrations of Mg2+: control medium containing 3 mM Mg2+, low magnesium containing 0.5 mM Mg2+, and zero magnesium, with 0 mM added Mg2+. The external media consisted of either (in mM) 147 NaCl, 2.6 KCl, 2 CaCl2, 3 MgCl2, 10 glucose, and 10 HEPES, pH 7.4, osmolarity 310–316 mOsm (control medium); 153 NaCl, 2.6 KCl, 2.1 CaCl2, 0.5 MgCl2, 10 glucose, and 10 HEPES, pH 7.4, osmolarity 314–316 mOsm (low magnesium); or 153 NaCl, 2.6 KCl, 2.2 CaCl2, 10 glucose, and 10 HEPES, pH 7.4, osmolarity 314–316 mOsm (zero magnesium). All chemicals were from Sigma-Aldrich (St. Louis, MO) unless otherwise stated. The external medium was sterile filtered before use. In solution-switch experiments a perfusion system was used to exchange the medium while maintaining the dual patch.
Micropipettes were pulled on a P-97 Flaming/Brown Micropipette puller (Sutter Instruments, Novato, CA) from borosilicate glass (O.D. 1.5 mm, I.D. 0.86 mm, World Precision Instruments, Sarasota, FL) using a 1-stage pull protocol. Micropipettes with resistances of 5–10 MΩ were filled with a solution of (in mM) 155 K-gluconate, 0.6 EGTA, 12 HEPES, 8 KCl, 3 NaCl and 4 MgATP, pH 7.3, osmolarity 297–301 mOsm. The internal solution was sterile filtered before use. In the voltage-clamp recordings, lidocaine N-ethyl bromide (6 mM) was added to the internal solution to block action potentials. NaCl, CaCl2, and NaOH were from Fischer Scientific (Pittsburgh, PA).
Experiments were done at room temperature, and cultured neurons were viewed on a Scientifica stage (Uckfield, East Sussex, UK) using an inverted Nikon (Melville, NY) microscope. Distance between cells was measured using LinLab (Uckfield, East Sussex, UK). All dual patched cells were within 450 μm of one another. Micropipettes were mounted on SD Instruments micromanipulators (Grants Pass, OR). A separate silver chloride reference/counter electrode was placed in the dish for each cell. Data were recorded at 25 kHz using an FPGA card and user defined LabVIEW software (National Instruments, Austin, TX) with an Axopatch 200A and an Axopatch 200B amplifiers (Molecular Devices, Sunnyvale, CA), filtered using a four-pole Bessel filter at 5 kHz. Only the amplifiers were used to apply current or potential.
Dual current-clamp recordings.
Connections were explored in control medium. Dual patch-clamped neurons were recorded in current-clamp mode. One cell was given a suprathreshold current (presynaptic cell) and the other cell was hyperpolarized to −65 mV (postsynaptic cell). Then the process was repeated by switching the presynaptic and postsynaptic cell.
In control medium, both cells were depolarized with a suprathreshold current to generate action potentials in both cells. In zero magnesium and low magnesium, cells were either depolarized with a supratheshold current, or if bursting was present the cells were injected with the appropriate current to set the minimum membrane potential to −65 mV.
In grouped experiments, cells were measured in either control medium or zero-magnesium conditions only. In solution-switched experiments, cells were initially patch-clamped in control medium and then the solution was exchanged using a whole bath perfusion system to either low-magnesium or zero-magnesium conditions.
Simultaneous current-clamp and voltage-clamp recordings.
Whole cell recordings of two neurons were made in control medium, then the solution was switched to low magnesium and then to zero magnesium. The membrane potential of the presynaptic neuron was measured in current-clamp mode, and the currents were recorded from the postsynaptic neuron in voltage-clamp mode. The external solution was exchanged using a bath perfusion system. A suprathreshold current was injected into the presynaptic neuron, and the postsynaptic neuron was held at a potential of −65 mV. Postsynaptic neurons that required less than −150 pA to hold the potential in control medium were discarded.
Data Analysis
Event synchronization.
The event synchronization described by Quiroga et al. (Quiroga et al. 2002) was used to quantify the degree of action potential synchronization. Event synchronization quantifies the number of times an event occurs in both time series within a small window. This measure can tell whether the two time series have events that are consistently together. A brief description of the event synchronization is below, but more details are available elsewhere (Quiroga et al. 2002).
For two neurons, action potential times in neuron x and y can be defined as txi and tyj. Action potentials that occur in both neurons within a time interval ±τ are considered to be synchronous. For each neuron, a count of these events is defined as
| (1) |
with
| (2) |
where nx is the number of action potentials in neuron x, and ny is the number of action potentials for neuron y, with i = 1, . . .,nx and j = 1, . . .,ny. The values of cτ(x|y) and cτ(y|x) are then combined symmetrically:
| (3) |
where Qτ is the strength of the event synchronization. Qτ varies from 0 to 1 with 0 being no synchronized events and 1 being completely synchronized events.
To avoid double counting, a local definition of τ for each event pair ij is defined as:
| (4) |
where τc is a constant representing the maximum allowable time interval between action potentials.
Analysis of strength of connection.
Synaptic strength analysis was done using two methods, the method of failures and amplitude histogram analysis (Redman 1990). Failures were identified in windows of 30 ms following an action potential in the presynaptic neuron. First, the EPSC onsets were determined by five consecutive negative derivatives in the current of the postsynaptic neuron (Bykhovskaia 2008). Then, the falling phase of the EPSC was determined by the sum of the derivative being over 0.0156 pA/ms over a moving window of 1 ms (Bykhovskaia 2008). If the minimum value between the onset and falling phase was greater than 3 SDs of the root mean square (RMS) noise it was considered a “success.” If there was no onset or if the minimum was not greater than 3 SDs of the noise, the response was considered a “failure.” The number of failures, N0, and number of action potentials, N, were combined to create the mean quantal content, mf = −log(N0/N). For cells where no failures were measured, a value of N0 = 1 was used to obtain an mf for comparison purposes.
Amplitude histogram analysis was performed on all EPSC amplitudes in the first 2 min of the recording of the postsynaptic neuron. EPSC amplitudes were analyzed using MiniAnalysis (Synaptosoft, Decatur, GA). The threshold was set to three times the RMS of baseline noise. Amplitudes were discarded if they met either of the following sets of criteria: 1) the decay time was less than the rise time, the amplitude was less than 40 pA, and the peak area was less than 150 pA·ms; or 2) the decay time was greater than 10 ms, the rise time was greater than 5 ms, and the amplitude was less than 40 pA. Amplitude histograms were fit with Gaussian curves using MATLAB (MathWorks, Natick, MA).
Classification of synaptic connections.
Responses in the postsynaptic cell as a result of action potentials in the presynaptic cell were analyzed using a user-defined program in MATLAB. First, action potentials >100 ms apart were identified in the presynaptic cell. The 100-ms limit avoids convolution of responses from multiple action potentials. Then the mean (overall baseline) and RMS noise of the membrane potential in postsynaptic cell was measured. For each action potential in the presynaptic cell, a minimum and maximum of the membrane potential of the postsynaptic cell were found within a window of 30 ms from the peak of the action potential. Maximum and minimum values at the edge of the 30-ms window were rejected, and then the program moved to the next action potential. The mean and SD of a 3-ms window just prior to the peak of the action potential created the local baseline and local noise, respectively. If the local baseline is greater than the overall baseline ±2 mV, or the local noise was greater than 1.25 times the RMS noise or a maximum of 1 mV, the response was rejected and the program moved to the next action potential. The membrane potential was averaged for 3 ms at the minimum and maximum values, and the local baseline was subtracted to get the maximum amplitude and minimum amplitude within the 30-ms window. If the minimum amplitude was more hyperpolarized than three times the RMS noise, the response was identified as inhibitory. If the maximum amplitude was more depolarized than three times the RMS noise, the response was identified as excitatory. Otherwise the response was considered a failure. For excitatory responses, the time from the peak of the presynaptic action potential to the peak of the excitatory postsynaptic potential was recorded.
Burst analysis.
Identification of bursts was done using a user-defined program in MATLAB similar to the methods by Pasquale et al. (Pasquale et al. 2010) and Selinger et al. (Selinger et al. 2007). First, the action potentials were identified, and the interspike intervals (time between subsequent spikes) were calculated. A log interspike interval histogram was created using equally spaced bins of 0.1 in log10(interspike interval) units (Pasquale et al. 2010; Selinger et al. 2007). Next, the histogram was smoothed using weighted linear least squares and a 1st-degree polynomial model in MATLAB (smooth with “lowess” method). Peaks of the smoothed histogram were identified in MATLAB using “findpeaks” with a minimum difference of 2 between peaks. The minimum between the two peaks was identified as the burst cutoff. If only one peak was found, the data were identified as having no bursts. If there were more than two peaks, then peaks greater than 10 s were ignored. If two peaks were less than 10 s, the minimum between those two peaks was identified as the burst cutoff. If there was less than 1 peak below 10 s, then the minimum between the two smallest [log(interspike intervals)] was the burst threshold. Finally, if more than two peaks were below 10 s, then the minimum between the two largest [log(interspike intervals)] peaks under 10 s was defined as the burst cutoff. Interspike intervals longer than the burst cutoff were used to identify the beginning and end of bursts and each burst had to contain ≥3 action potentials (Cocatre-Zilgien and Delcomyn 1992).
Generally action potential bursts have two characteristic time scales: the fast time scale, between action potentials in the burst, and the slow time scale between the last action potential of one burst and the first action potential of the next burst. However, in our data there was often more than these two time scales. To define the interspike interval used for the burst cutoff (i.e., interspike intervals between two action potentials for which smaller values are considered part of the burst and larger values define the ending/beginning of a burst) we needed to define other contingencies rather than relying on only the minimum between two peaks. We defined the burst cutoff on the log(inter-spike interval) scale as the minimum closest to 10 s. This way the burst interval was not too small or too large so that the bursts were more closely defined as one would do visually. The results of this analysis were averaged and reported.
Statistical Tests
Significance was determined by α < 0.05 for all statistical tests performed. Data were reported as means ± SD unless noted otherwise. Statistical tests used were Student's t-test (MATLAB), ANOVA (MATLAB), repeated-measures ANOVA (rmANOVA, Minitab16, State Collage, PA), Fisher's Exact Test (Fishers, MATLAB, program created by Giuseppe Cardillo), and Kolmogorov-Smirnov test (K-S test, MATLAB) as noted in the text and figures. Post hoc multicomparison tests were done using the Tukey method (MATLAB, Minitab). Numbers of pairs are denoted as np, and number of individual cells are denoted as nc.
RESULTS
Grouped Current-Clamp Experiments
A total of 20 pairs of neurons were examined for the grouped experiments. Of the 20 pairs, 13 were recorded in control medium only. The other 7 pairs were recorded in zero added Mg2+ (zero magnesium). In solution-switched experiments, 17 pairs were measured in control medium and switched to zero magnesium. Another 9 pairs were measured in control medium and switched to low Mg2+ (low magnesium) solutions. Behaviors of the individual cells in control medium, low magnesium, and zero magnesium are described below. Results for the pairs of cells can be found in their respective sections.
Control medium.
Control medium contained 3 mM Mg2+ in the external solution. Cell pairs where one or both cells did not fire action potentials when subjected to a suprathreshold current were discarded (np = 5). Rarely, neurons in control medium fired spontaneous action potentials when held near a membrane potential of −65 mV (np = 2/72). Occasionally, bursting activity was seen in control medium (np = 4) at potentials of −65 mV. The bursting activity was not continuous, and these pairs were either discarded (np = 3) or sections of data without bursting activity were used for further analysis (np = 1).
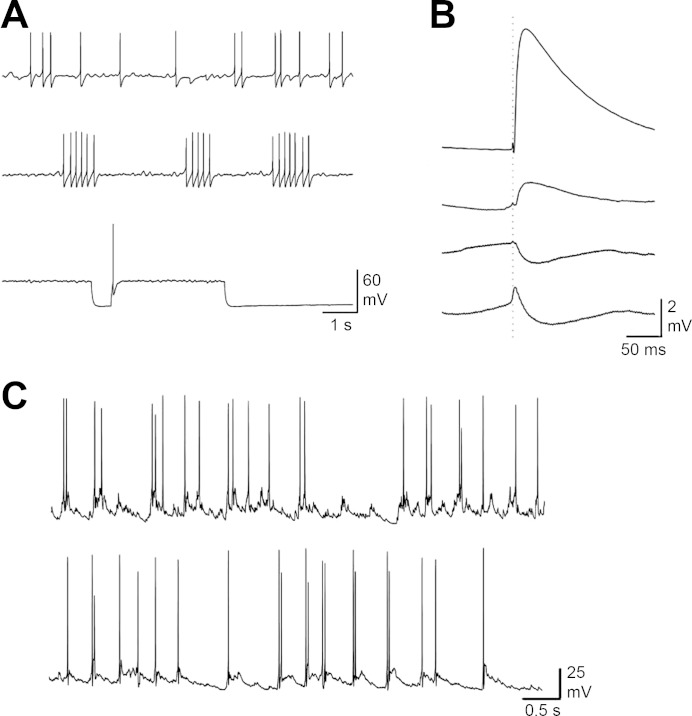
When a suprathreshold current was injected to cells in control medium, three types of activity were commonly seen: recurrent action potentials (nc = 73), bursts of action potentials (nc = 17), or action potentials only at the beginning of the depolarization (nc = 11, Fig. 1A).
Fig. 1.
A: whole cell recordings in current-clamp mode for cells in control medium. Top: trace of a cell with recurrent action potential. Cells could also have more periodic action potentials as well. Middle: trace for a cell with bursts of action potentials. Bottom: trace for a cell that only fired action potentials with application of a depolarizing current. Application of suprathreshold current in the top and middle panel was constant and lasted the duration of the recording. In the bottom panel, the suprathreshold current was applied and released. B: examples of connection types seen in the dual current-clamp experiments. Top: strong excitatory connections had an average depolarizing response with amplitude greater than 3 mV and less than 10% failures. Middle top: all other excitatory connections were considered weak. Middle bottom: inhibitory connections had hyperpolarizing membrane potential shift greater than 3 times the RMS noise. Shown is the averaged inhibitory postsynaptic potential (IPSP) response for one cell. Bottom: electrical connections were identified visually. The program identifying the connections could classify the electrical response as either excitatory, inhibitory, or failure because of their shape and relatively small amplitudes (generally <1 mV). Shown are the average for all responses (excitatory, inhibitory, and failures) for a cell with an electrical connection. The action potential peak in presynaptic cell occurred at the dotted line. Potentials were pinned to 0 mV at the dotted line before averaging. C: dual whole cell recordings in current-clamp mode for two cells recorded on different days. These two traces were used together to create a surrogate pair for synchronization analysis. Q for this surrogate pair was 0.12.
Low magnesium and zero magnesium.
Decreased extracellular Mg2+ causes recurrent bursting activity (Golomb et al. 2006; Robinson et al. 1993; Traub et al. 1994; Ziburkus et al. 2006). Two decreased Mg2+ models were used, containing 0.5 mM Mg2+ (low magnesium) or 0 mM Mg2+ (zero magnesium). In both conditions, spontaneous bursting activity was observed. Eleven pairs of neurons in low magnesium or zero magnesium were depolarized to generate action potentials when bursting was not present (mean minimum potential, −52.1 ± 0.5 mV, nc = 22). They were not included in the bursting analysis. The burst duration, number of action potentials per burst, and burst cutoff were identified using a user-defined program. The burst threshold varied depending on the log10(interspike interval) histogram for each cell. The log(interspike interval) histograms had either one peak (no bursts), two peaks, or three peaks.
The burst cutoff was identified as the local minimum between the two peaks of the log(interspike interval) histograms as described in materials and methods. Burst cutoffs for low-magnesium cells were 0.99 ± 1.63 s (nc = 23). Burst cutoffs for zero-magnesium cells were 0.58 ± 0.49 s (nc = 24). The burst cutoffs were not significantly different (P = 0.24, t-test). The number of action potentials per burst for low magnesium was 15.2 ± 30.1 (nc = 23) and zero magnesium was 11.3 ± 18.2 (nc = 24), which was significantly different (P = 0.005, t-test). Burst durations were 2.08 ± 4.37 s in low magnesium (nc = 23) and 1.64 ± 2.86 s in zero magnesium (nc = 24), which were significantly different (P = 0.03, t-test). From these data, it can be concluded that bursts in low magnesium were longer and included more action potentials than bursts in zero magnesium.
Classification of Synaptic Connections
The types of connections between the cell pairs were recorded to test whether the type of connection affected the amount of synchronization significantly. Type of connections between the cell pairs studied under current-clamp mode varied. The connection between the neurons was studied in control (control medium) external conditions. One neuron was depolarized to induce action potentials, while the other cell was held at −65 mV. Then the process was repeated for the other member of the pair. The connections could either be strong excitatory (s, Fig. 1B), weak excitatory (w, Fig. 1B), inhibitory (i), or electrical (e). Connections were classified as strong excitatory when in response to a presynaptic action potential, postsynaptic neuron membrane depolarization had amplitudes greater than 3 mV and there were less than 10% failures (nc = 11). Connections were classified as weak excitatory when peak depolarization was less than 3 mV and failure occurred more often than 10% (nc = 62). Connections were inhibitory when hyperpolarization was greater than 3 times the RMS of the noise. The number of inhibitory connections is similar to those expected in hippocampal cultures, about 7% (nc = 10/102) (Benson et al. 1994). Electrical connections had small amplitude peaks close to the time of the action potential in the presynaptic cell, followed by a small hyperpolarizing response (nc = 19). The response could be classified as either excitatory, inhibitory, or a failure by the detection program, and therefore had to be identified by visual inspection. Each cell in the pair was the presynaptic and postsynaptic cell. Table 1 shows the frequency of each connection for all the cell pairs in grouped and solution-switched experiments. The distribution of connections measured in control medium was not statistically significant between the experiments (P = 0.83, Fisher's). The average time from the peak of action potential in the presynaptic cell to the peak of excitatory postsynaptic potential (EPSP) was 17.0 ± 7.8 ms (nc = 73).
Table 1.
Type of connections obtained in the control medium for grouped and solution-switched experiments
| Connection |
||||
|---|---|---|---|---|
| Experiments | w | s | i | e |
| Grouped | 13 | 3 | 3 | 7 |
| Solution-switched | 31 | 5 | 5 | 11 |
w, weak excitatory; s, strong excitatory; i, inhibitory; e, electrical.
Event synchronization.
The synchronization of the action potentials was measured by using the event synchronization. The maximum window, τc, was set to 25 ms based on the average latency from action potential peak to EPSP peak.
To examine whether the type of connection affected the synchronization results, the connections of the solution-switched experiments were grouped into weak-weak (np = 13), strong-other (np = 6), weak-electrical or -inhibitory (np = 4), and electrical-electrical or -inhibitory (np = 3). For each of the three Mg2+ concentrations studied, no significant difference in synchronization was found between the groups of connections (ANOVA, P > 0.05). This is in contrast to modeling and experimental studies where the synchronization is altered based on the type of connections between the cells (Borgers and Kopell 2003; Mancilla et al. 2007; Netoff et al. 2005b).
Event synchronization is just one possible measure of synchronization, and it was chosen because it does not show a bias of synchronization for periods of bursts or single spikes (Lyttle and Fellous 2011), which is important because bursting was not present in all of the data. To ensure that the increased frequency of action potentials during bursting activity did not artificially increase Q, surrogate pairs of neurons were created from all of the zero-magnesium and low-magnesium current-clamp recordings. These surrogate pairs mimic dual current-clamp recordings where the cells are not connected either directly or indirectly through other cells. Cell “pairs” were made by randomly assigning cells using two nonrepeating permutations. The first minute of each recording was used to create the synchronization values for the pair. Their synchronization is expected to be very low because they are not connected (recorded in different cultures on different days). An example of surrogate data is shown in Fig. 1C. The top cell was recorded in July 2010 and the second cell was recorded in November 2010. Their synchronization was 0.12. The average surrogate data synchronization was 0.09 ± 0.05 (np = 84, see Table 2).
Table 2.
Synchronization and distances for dual current-clamp experiments
| Control | Low | Zero | Surrogate | |
|---|---|---|---|---|
| Grouped experiments | ||||
| Q (np) | 0.13 ± 0.19 (13) | 0.26 ± 0.20 (7) | ||
| Distance, μm (np) | 131.6 ± 73.8 (8) | 230.3 ± 61.7 (4) | ||
| Solution-switched experiments | ||||
| Q (np) | 0.14 ± 0.23 (17) | 0.43 ± 0.16* (17) | ||
| Q (np) | 0.27 ± 0.30 (9) | 0.55 ± 0.22* (9) | ||
| Distance, μm (np) | 164.0 ± 84.8 (23) | |||
| Total | ||||
| Q (np) | 0.17 ± 0.24 (39) | 0.55 ± 0.22* (9) | 0.37 ± 0.19* (28) | 0.09 ± 0.05 (84) |
| Distance, μm (np) | 164.2 ± 82.9† (35) | |||
Values are means ± SD.
Significantly different from control, P < 0.05.
Value is for all experiments.
Effect of Decreased Magnesium on Synchronization
Grouped experiments in control medium and zero magnesium.
Modeling of synaptically connected neurons with recurrent action potentials (not bursting) has suggested that biologically connected neurons could exhibit synchronized action potentials (Borgers and Kopell 2003; Netoff et al. 2005b). To examine whether synchronization of synaptically connected neurons was enhanced by decreasing extracellular Mg2+, two cells in whole cell current-clamp mode were recorded under either control medium or zero-magnesium external conditions.
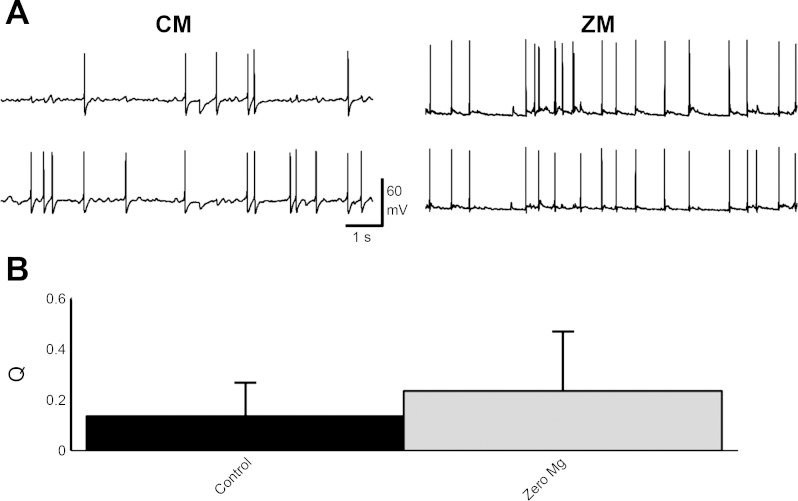
In control medium, both cells were given a suprathreshold current to generate recurrent action potentials (Fig. 2A, control medium). Cells in zero-magnesium conditions had current injected to adjust the membrane potential to −65 mV (−64 ± 8 mV, nc = 16, Fig. 2A, zero magnesium). Synchronizations for the data from two pairs of neurons shown in Fig. 2A were 0.38 and 0.53 for control medium and zero magnesium, respectively. For all grouped experiments, Q for control medium was 0.13 ± 0.19 (np = 13) and 0.26 ± 0.20 (np = 7) for zero magnesium (see Table 2). The synchronizations between the control medium and zero-magnesium pairs were similar (P = 0.16, t-test).
Fig. 2.
A: dual whole cell recordings in current-clamp mode. Two traces are shown for control medium conditions where both cells were depolarized to induce recurrent action potentials. Two traces of two different cells are shown for zero-magnesium conditions. Q was 0.38 for the pair in control medium and 0.53 for the pair in zero magnesium. B: the difference in Q for cells in control medium and zero magnesium was not significant (P = 0.16, t-test). In control medium 13 cell pairs were recorded and in zero magnesium 7 pairs were recorded.
In the zero-magnesium experiments, it was difficult to measure the strength or type of connection between the cells due to the high amount of activity at −65 mV. No connection data were analyzed for the zero-magnesium pairs. The lack of significant change in synchronization could be attributed to any number of differences between the cells measured in control medium and the cells measured in zero magnesium. Some factors that may have contributed to the control medium and zero magnesium not being significantly different are that the cells were different distances apart, part of different cultures with a different number of connections to a variable number of other cells, and the type and strength of the connections in zero magnesium could not be determined accurately. To keep these variables the same in control medium and zero magnesium, solution-switch experiments were performed where the solution was exchanged from control medium to zero magnesium while maintaining the dual patch-clamp so cell pairs could be measured in both conditions. Q tended toward (nonsignificant) increase from control medium to zero magnesium. It would be possible that the increase in Q would not be significantly different in the solution-switch experiments. Solution-switch experiments were also performed by exchanging control medium to low-magnesium conditions. The addition of low magnesium can demonstrate a trend in synchronization even if the change in synchronization is not significant.
Solution-switch experiments.
In the grouped experiments, the type of connection could not be determined for the neuron pairs in zero magnesium. This may have been the reason the change in synchronization was not significant. To keep the type of connection the same in control medium and zero magnesium, the same cell pairs were measured in control medium and low magnesium or zero magnesium. The solution was switched from control medium to zero magnesium or low magnesium using a bath perfusion system. Low-magnesium conditions were studied to further examine the relationship between action potential synchronization and network synaptic strength.
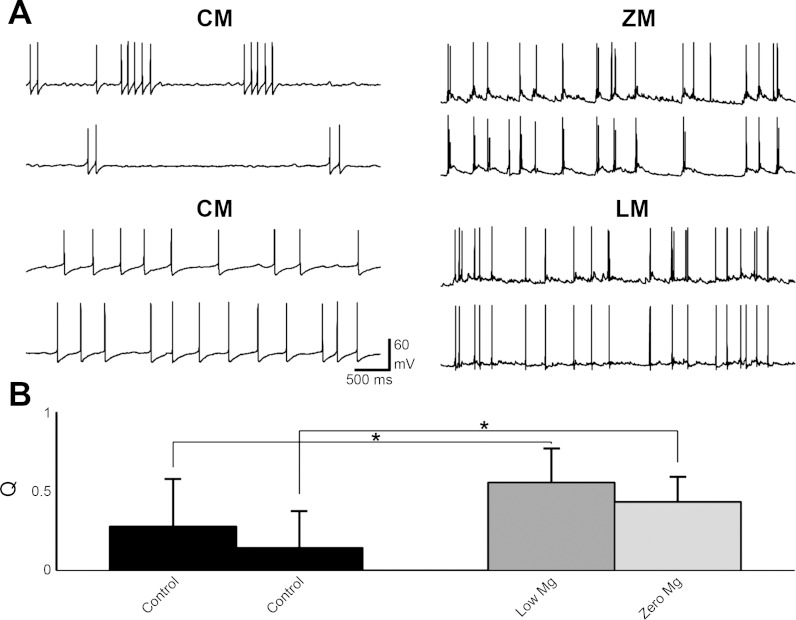
Recordings were obtained from two cells in current-clamp mode in control medium. The types of connections between the cells were measured as previously described. Both cells were depolarized with a suprathreshold current to generate action potentials. The external medium was exchanged to either zero magnesium or low magnesium. Recordings for two cells in control medium and the same two cells in zero magnesium are shown in Fig. 3A, top. Synchronization for the pair in control medium was 0.02 and in zero magnesium was 0.62. Figure 3B shows that the synchronization was significantly changed from control to zero magnesium (np = 17, P = 0.00007, paired t-test; see Table 1). Recordings for two cells in control medium and the same two cells in low magnesium are shown in Fig. 3A, bottom. Synchronization for the pair in control medium was 0.17 and it increased in low magnesium to 0.83. Synchronization was significantly changed from control to low magnesium (np = 9, P = 0.00007, paired t-test; see Fig. 3B, Table 1).
Fig. 3.
A: dual whole cell recordings in current-clamp mode under control medium conditions (top left) and zero-magnesium conditions (top right). Both cells were depolarized in control medium to induce recurrent action potentials. B: synchronization for the pair under control medium was 0.02 and under zero-magnesium the synchronization was increased to 0.62. For pairs solution-switched from control medium to zero magnesium, synchronization increased significantly from 0.14 ± 0.23 to 0.43 ± 0.16, respectively (np = 17, P = 0.00007, paired t-test). A: dual whole cell recordings in current-clamp mode under control medium conditions (bottom left) and low magnesium conditions (bottom right). Both cells were depolarized in control medium to induce recurrent action potentials. B: synchronization for the pair under control medium was 0.17 and under low magnesium the synchronization was increased to 0.83. For pairs solution-switched from control medium to low magnesium, synchronization increased significantly from 0.27 ± 0.30 to 0.55 ± 0.22, respectively (np = 9, P = 0.00007, paired t-test). *Significant, P < 0.05.
Ideally, to examine the relationship between synchronization and synaptic strength, the synchronization and change in synaptic strength would be measured in the same cell pair under control medium and zero magnesium (or low magnesium). However, the same problems that made it difficult to measure the type of connection in zero magnesium for the grouped experiments also affect the ability to detect change in synaptic strength in the solution-switch experiments. Action potentials were present in both the presynaptic and postsynaptic cells and they often occurred nearly simultaneously, obscuring the EPSP responses to those action potentials. Firing of multiple other cells in the culture created many EPSPs in the postsynaptic cell. The EPSPs in response to the presynaptic (recorded) cell could not be distinguished from other EPSPs. The membrane potential was highly variable because of the temporary depolarizations underlying the zero-magnesium bursting. The amplitudes of EPSPs depend on the initial membrane potential. The use of control medium to low magnesium solution-switched experiments did not alleviate any of these issues.
To measure the synaptic strength, dual whole cell patch-clamp experiments were performed with one cell in current-clamp mode (presynaptic cell) and the other cell in voltage-clamp mode (postsynaptic cell). Action potentials in the postsynaptic cell were blocked by addition of lidocaine to the internal solution so that only EPSCs were measured. In voltage-clamp mode, the membrane potential is held relatively constant making the measured amplitudes during bursting slightly more accurate. Because the specific response due to activity in the presynaptic neuron cannot be distinguished from activity due to network activity, two measures of synaptic strength were used: the method of failures, which depends on responses relative to the presynaptic cell, and the increase of EPSC amplitudes, which depends on all EPSCs recorded in the postsynaptic cell.
Effect of Decreased Magnesium on Synaptic Strength
Action potentials of the presynaptic cell were identified, and the cell pairs were separated by the strength of their excitatory connections in control medium, either strong or weak. The reversal potential for Cl− was −68 mV for these conditions, meaning inhibitory connections would cause responses in the opposite direction of the excitatory connections. Therefore, even though no GABAA receptor blockers were used in the experiments, the responses analyzed below are all considered excitatory.
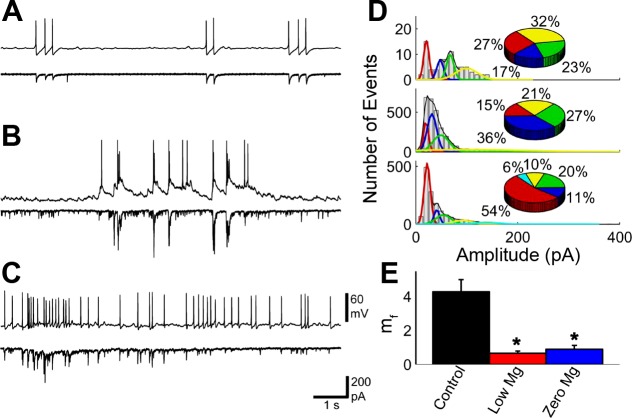
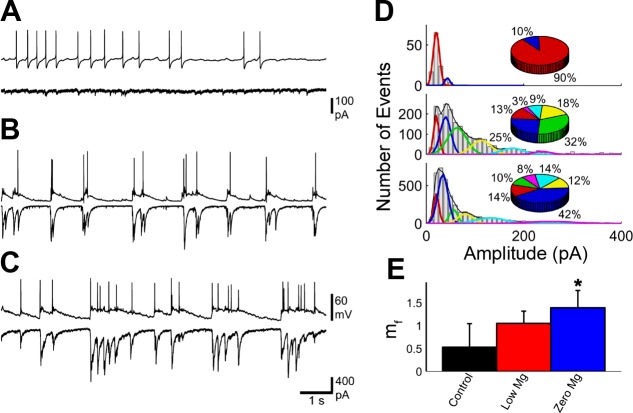
An example of two cells with a strong excitatory connection is shown in Fig. 4. All EPSC amplitudes in the first 2 min of the recordings were used to create amplitude histograms. The histograms were fit with multiple Gaussian curves. Peaks were at 20.9, 47.8, 67.8, and 99.6 pA in control; 20.0, 31.6, 49.3, and 100.4 pA in low magnesium; and 22.4, 41.3, 54.0, 92.0, and 139.5 pA in zero magnesium. As the concentration of Mg2+ was decreased to zero magnesium, a new population of high-amplitude EPSCs emerged with amplitudes around 140 pA, demonstrating that the mean quantal content increased. In the pairs with a strong connection, mf decreased from control to low magnesium (Fig. 4E). Both low magnesium and zero magnesium were significantly different from control medium (P < 0.0001 for both, np = 4, rmANOVA, post hoc Tukey method).
Fig. 4.
Connectivity was decreased from control medium to low magnesium and increased from low magnesium to zero magnesium in a neuron pair with a strong connection under control medium conditions. One cell in current-clamp mode (A, B, C, top) and one cell in voltage-clamp mode (A, B, C, bottom) were measured in control medium (A), low-magnesium (B), and zero-magnesium (C) external conditions. D: amplitude distributions for all EPSCs for the cell in voltage-clamp mode under control medium (top), low-magnesium (middle), and zero-magnesium (bottom) conditions. Amplitudes increased slightly from control medium to low magnesium and zero magnesium. Fits of the amplitudes reveal new populations of amplitudes as magnesium was decreased. Corresponding pie charts demonstrate the relative amount of amplitudes at each peak value. E: mf was calculated for responses based on the action potentials of the cell in current-clamp mode. The number of failures increased from control to low magnesium and then decreased slightly to zero magnesium. mf for control medium was significantly higher than low magnesium and zero magnesium (P < 0.0001 for both, np = 4, repeated-measures ANOVA, post hoc Tukey method). *Significant, P < 0.05.
An example of two cells with a weak excitatory connection is shown in Fig. 5. Again, amplitude histograms were made from the first 2 min of the recordings and fit with multiple Gaussian curves. For the weakly connected neurons, peaks were at 20.7 and 43.5 pA in control medium; 20.8, 39.7, 63.1, 113.2, 176.3, and 238.6 pA in low magnesium; and 20.7, 34.2, 55.9, 80.1, 132.7, and 256.0 pA in zero magnesium. As the concentration of Mg2+ was decreased to zero magnesium, a new population of high-amplitude EPSCs emerged with amplitudes around 140 pA, demonstrating that the mean quantal content increased. For pairs with a weak connection, mf was increased as Mg2+ was decreased (Fig. 5E). However, only the zero magnesium was significantly different from control (P = 0.026, np = 4, rmANOVA, post hoc Tukey method). Four new populations emerged in the low-magnesium conditions and were also present in the zero-magnesium conditions. This suggests that for the network activity for the strong excitatory connected neurons, the synaptic strength increased, even though the direct connection in the strong pairs decreased.
Fig. 5.
Connectivity was increased from control medium to low magnesium and from low magnesium to zero magnesium in a pair with a weak connection under control medium conditions. One cell in current-clamp mode (A, B, C, top) and one cell in voltage-clamp mode (A, B, C, bottom) were measured in control (A), low-magnesium (B), and zero-magnesium (C) external conditions. D: amplitude distributions for all EPSCs for the cell in voltage-clamp mode under control medium (top), low-magnesium (middle), and zero-magnesium (bottom) conditions. Amplitudes increased from control medium to low magnesium and zero magnesium. Fits of the amplitudes reveal new populations of amplitudes as magnesium was decreased. Corresponding pie charts demonstrate the relative amount of amplitudes at each peak value. E: mf was calculated for responses based on the action potentials of the cell in current-clamp mode. The number of failures decreased from control medium to low magnesium and then decreased slightly from low magnesium to zero magnesium. mf increased from control medium to low magnesium to zero magnesium, with control medium and zero magnesium being significantly different (P = 0.026, np = 4, repeated-measures ANOVA, post hoc Tukey method). *Significant, P < 0.05.
DISCUSSION
Normal brain functions like memory, attention, and motor control rely on synchronization of brain activity (Uhlhaas and Singer 2010). Deviation of the normal synchronization, either decreased or increased, can be linked to several diseases, such as schizophrenia, epilepsy, Alzheimer's disease, and autism (Uhlhaas and Singer 2006). In some diseases, such as epilepsy, synchronization is expected to increase across the brain (Uhlhaas and Singer 2006) even at the neuron level (Traub et al. 1984; Wong et al. 1986). It is important to understand the relationship of synchronization of neural activity and the factors that contribute to the presence (or absence) of synchronization to advance therapeutic treatments and disease models.
This paper examines the relationship between synchronization of action potentials and synaptic strength between pairs of neurons within network of cultured neurons. It has been suggested that synchronized behavior is increased as the synaptic strength is increased (Mangan and Kapur 2004; Traub et al. 1984). The strength of connections between neurons was examined as external Mg2+ was decreased. Decreasing Mg2+ strengthens synaptic connections by increasing release probability (more vesicles released per action potential) and unblocking NMDA receptors (Mangan and Kapur 2004; Traub et al. 1994). Decreasing Mg2+ also enhances the excitability of the cells by reducing the surface charge screening, increasing the threshold of voltage-gated channels (Isaev et al. 2011) and preventing the hyperpolarization effect of BK channels that are Mg2+ activated (Chen et al. 2011).
As Mg2+ was decreased from control to low magnesium, the action potential behavior transitioned from single action potentials to bursts of action potentials. The bursting behavior is a combined effect of the increase in synaptic connectivity strength and enhanced excitability of the cells. It has been suggested that stronger synaptic connections can also lead to bursting behavior in hippocampal neurons (McCormick and Contreras 2001; Yue and Yaari 2004). While both synaptic strength and excitability lead to bursting behavior, we believe that it is primarily the increase in synaptic strength that ultimately leads to action potential synchronization and that the increase of synchronization is not an effect of the bursting behavior.
Synchronization of neurons in culture may happen in a variety of ways, either direct connections through synapses, or indirect connections through common inputs. Analysis of surrogate data where bursting was present, but no direct synaptic connections or indirect connections through the culture existed between the neurons, showed minimal action potential synchronization, suggesting that the presence of bursts is not enough to create synchronized activity. The role of bursting behavior in synchronization was not tested further and the effect of decreasing Mg2+ on synaptic strength was the primary focus of the study.
Synchronization of the bursting activity has been examined using cross-correlation (Netoff and Schiff 2002; Traub et al. 2004; Ziburkus et al. 2006), field potentials (Bianchi and Wong 1994; Haas and Jefferys 1984; Niespodziany et al. 2003) and by visual inspection (Fujiwara-Tsukamoto et al. 2004; Miles and Wong 1983; Miles et al. 1984; Scharfman 1994; Traub et al. 1994). In this study, event synchronization was used to quantify the amount of action potential synchronization between two cells in various external concentrations of Mg2+. Event synchronization is just one possible measure of synchronization. Compared with other synchronization measures, event synchronization is robust in the presence of noise (Kreuz et al. 2007). Event synchronization was not altered by the increase of frequency during bursting activity, which was verified using surrogate data. Additionally, action potentials may not exhibit exact synchronization (Golomb et al. 2006; Scharfman 1994). By using event synchronization, slight variations in delay and order would be considered synchronous within a small time window.
Synchronization of cells with biological synaptic connections has been studied using neuronal models, and the type of connection often affects the ability of the cells to in-phase synchronize (Borgers and Kopell 2003; Netoff et al. 2005b). However, in these experiments the type of connection did not alter the synchronization of cells in control medium. However, high values of synchronization did appear occasionally in control medium, suggesting that it is possible for biologically connected cells to be highly synchronized, but it was not common under the conditions studied. Modeling of bursting activity suggests that synchronization occurs on the level of bursts but not at the level of action potentials (Golomb et al. 2006; Shi and Lu 2009) unless the synaptic strength is sufficiently strong (Buric et al. 2009). Results in solution-switch experiments confirm that the action potential synchronization was increased for decreased Mg2+ concentrations.
Performing synaptic analysis under decreased Mg2+ was difficult due to the increase of activity and superposition of multiple quantal releases. Because of these issues, synaptic strength was measured in two ways: method of failures and deconvolution of amplitude histograms. Both methods suggest that more vesicles were released per EPSC, indicating a strengthening of connections. The first peaks from amplitude distributions were all around 20 pA, suggesting that the quantal content of one vesicle was unaltered by decreasing Mg2+ as has been described before (Mangan and Kapur 2004). Also, decreasing Mg2+ increased the number and sizes of sEPSCs. New populations emerged at low-magnesium and zero-magnesium conditions, suggesting that the strength of the synapses increased.
One possible mechanism for increased excitability and strengthening of synaptic transmission was first proposed by Frankenhauser and Hodgkin (Frankenhauser and Hodgkin 1957). It proposed that reducing divalent cations would increase neuronal excitabilty by decreasing charge screening at the membrane surface. This would cause a smaller portion of the transmembrane potential to fall across the lipid bilayer (McLaughlin et al. 1971), thus decreasing the electric field sensed by voltage-dependent conductances such as calcium channels in the synaptic terminal. At the neuromuscular junction, lowering extracellular Mg2+ increases stimulus-evoked neurotransmitter release by lowering the surface membrane potential of the presynaptic terminal (Muller and Finkelstein 1974). Increasing Mg2+ concentration displaces calcium in the surface charge layer adjacent to the negatively charged membrane, thus decreasing the amount of calcium available for influx into synaptic terminals. Reducing or eliminating Mg2+ would have the opposite effect. Thus one presynaptic effect of reducing Mg2+ would be to increase the probability of release as proposed in the Katz model of neurotransmission. Previous studies in the central synapses have proposed a similar mechanism (Mangan and Kapur 2004), and the present study further supports this hypothesis.
The strength of synaptic transmission was increased as Mg2+ was decreased. Although the measurements were made between neuron pairs, they likely represent and reflect increasing synaptic strength within the whole network. Measurement of EPSCs with a long latency as carried out in the present study would detect both monosynaptic and polysynaptic events. Furthermore each pyramidal neuron in culture receives synaptic inputs from multiple surrounding neurons, only one of which was a part of the recorded pair.
These results have some limitations. Synaptic connections in cells are dynamic and can display short-term effects such as depression, facilitation, and plasticity (Kaplan et al. 2003). These properties were not accounted for in the analysis. Only three concentrations of Mg2+ were examined in this paper. In grouped experiments, neuronal synchrony did not increase significantly under low-magnesium conditions; this was either due to insufficient sample size or differences in the cell culture batches.
These findings suggest that the increase in synaptic strength also caused an increase in action potential synchronization for pairs of neurons in culture.
GRANTS
This study was funded by the National Institutes of Health under Grant Nos. RO1-NS-040337, RO1-NS-044370, UO1-NS-58204 and the National Institute of General Medical Sciences of the National Institutes of Health under Award No. T32-GM-008715 (Biotechnology Training Program), and by the Biomedical Innovation Fund of the Univ. of Virginia.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.E.J., J.L.H., and J.K. conception and design of research; S.E.J. performed experiments; S.E.J. analyzed data; S.E.J., J.L.H., and J.K. interpreted results of experiments; S.E.J. prepared figures; S.E.J. drafted manuscript; S.E.J., J.L.H., and J.K. edited and revised manuscript; S.E.J. and J.K. approved final version of manuscript.
REFERENCES
- Banker G, Goslin K (editors). Culturing Nerve Cells. Cambridge, MA: MIT Press, 1998. [Google Scholar]
- Benson DL, Watkins FH, Steward O, Banker G. Characterization of gabaergic neurons in hippocampal cell-cultures. J Neurocytol 23: 279–295, 1994. [DOI] [PubMed] [Google Scholar]
- Bianchi R, Wong RKS. Carbachol-induced synchronized rhythmic bursts in CA3 neurons of guinea-pig hippocampus in-vitro. J Neurophysiol 72: 131–138, 1994. [DOI] [PubMed] [Google Scholar]
- Borgers C, Kopell N. Synchronization in networks of excitatory and inhibitory neurons with sparse, random connectivity. Neural Comput 15: 509–538, 2003. [DOI] [PubMed] [Google Scholar]
- Browne TR, Holmes GL. Handbook of Epilepsy. Philadelphia, PA: Lippincott, Williams, and Wilkins, 2000. [Google Scholar]
- Buric N, Todorovic K, Vasovic N. Exact synchronization of noisy bursting neurons with coupling delays. Chaos Solitons Fractals 40: 1127–1135, 2009. [Google Scholar]
- Bykhovskaia M. Making quantal analysis more convenient, fast and accurate: user-friendly software quanta. J Neurosci Methods 168: 500–513, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RS, Geng Y, Magleby KL. Mg2+ binding to open and closed states can activate BK channels provided that the voltage sensors are elevated. J Gen Physiol 138: 593–607, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocatre-Zilgien JH, Delcomyn F. Identification of bursts in spike trains. J Neurosci Methods 41: 19–30, 1992. [DOI] [PubMed] [Google Scholar]
- Frankenhaeuser B, Hodgkin AL. The action of calcium on the electrical properties of squid axon. J Physiol 137: 218–244, 1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara-Tsukamoto Y, Isomura Y, Kaneda K, Takada M. Synaptic interactions between pyramidal cells and interneurone subtypes during seizure-like activity in the rat hippocampus. J Physiol 557: 961–979, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golomb D, Shedmi A, Curtu R, Ermentrout GB. Persistent synchronized bursting activity in cortical tissues with low magnesium concentration: a modeling study. J Neurophysiol 95: 1049–1067, 2006. [DOI] [PubMed] [Google Scholar]
- Goodkin HP, Joshi S, Mtchedlishvili Z, Brar J, Kapur J. Subunit-specific trafficking of GABA(A) receptors during status epilepticus. J Neurosci 28: 2527–2538, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyás-Kovács A, Dóczi J, Tarnawa I, Détári L, Banczerowski-Pelyhe I, Világi I. Comparison of spontaneous and evoked epileptiform activity in three in vitro epilepsy models. Brain Res 945: 174–180, 2002. [DOI] [PubMed] [Google Scholar]
- Haas HL, Jefferys JGR. Low-calcium field burst discharges of CA1 pyramidal neurons in rat hippocampal slices. J Physiol 354: 185–201, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaev D, Ivanchick G, Khmyz V, Isaeva E, Savrasova A, Krishtal O, Holmes GL, Maximyuk O. Surface charge impact in low-magnesium model of seizure in rat hippocampus. J Neurophysiol 107: 417–423, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan MP, Wilcox KS, Dichter MA. Differences in multiple forms of short-term plasticity between excitatory and inhibitory hippocampal neurons in culture. Synapse 50: 41–52, 2003. [DOI] [PubMed] [Google Scholar]
- Kopell N, Ermentrout B. Chemical and electrical synapses perform complementary roles in the synchronization of interneuronal networks. Proc Natl Acad Sci USA 101: 15482–15487, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuz T, Mormann F, Andrzejak RG, Kraskov A, Lehnertz K, Grassberger P. Measuring synchronization in coupled model systems: a comparison of different approaches. Physica D-Nonlinear Phenomena 225: 29–42, 2007. [Google Scholar]
- Lyttle D, Fellous JM. A new similarity measure for spike trains: Sensitivity to bursts and periods of inhibition. J Neurosci Methods 199: 296–309, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancilla JG, Lewis TJ, Pinto DJ, Rinzel J, Connors BW. Synchronization of electrically coupled pairs of inhibitory interneurons in neocortex. J Neurosci 27: 2058–2073, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan PS, Kapur J. Factors underlying bursting behavior in a network of cultured hippocampal neurons exposed to zero magnesium. J Neurophysiol 91: 946–957, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Contreras D. On the cellular and network bases of epileptic seizures. Annu Rev Physiol 63: 815–846, 2001. [DOI] [PubMed] [Google Scholar]
- McLaughlin SG, Szabo G, Eisenman G. Divalent ions and the surface potential of charged phospholipid membranes. J Gen Physiol 58: 667–687, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R, Wong RKS. Single neurons can initiate synchronized population discharge in the hippocampus. Nature 306: 371–373, 1983. [DOI] [PubMed] [Google Scholar]
- Miles R, Wong RKS, Traub RD. Synchronized afterdischarges in the hippocampus—contribution of local synaptic-interactions. Neuroscience 12: 1179–1189, 1984. [DOI] [PubMed] [Google Scholar]
- Mody I, Lambert JDC, Heinemann U. Low extracellular magnesium induces epileptiform activity and spreading depression in rat hippocampal slices. J Neurophysiol 57: 869–888, 1987. [DOI] [PubMed] [Google Scholar]
- Muller RU, Finkelstein A. The electrostatic basis of Mg2+ inhibition of transmitter release. Proc Natl Acad Sci USA 71: 923–926, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netoff TI, Acker CD, Bettencourt JC, White JA. Beyond two-cell networks: Experimental measurement of neuronal responses to multiple synaptic inputs. J Comput Neurosci 18: 287–295, 2005a. [DOI] [PubMed] [Google Scholar]
- Netoff TI, Banks MI, Dorval AD, Acker CD, Haas JS, Kopell N, White JA. Synchronization in hybrid neuronal networks of the hippocampal formation. J Neurophysiol 93: 1197–1208, 2005b. [DOI] [PubMed] [Google Scholar]
- Netoff TI, Schiff SJ. Decreased neuronal synchronization during experimental seizures. J Neurosci 22: 7297–7307, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niespodziany I, Klitgaard H, Margineanu DG. Desynchronizing effect of levetiracetam on epileptiform responses in rat hippocampal slices. Neuroreport 14: 1273–1276, 2003. [DOI] [PubMed] [Google Scholar]
- Nunez J. Primary culture of hippocampal neurons from P0 newborn rats. J Vis Exp 19: e895, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquale V, Martinoia S, Chiappalone M. A self-adapting approach for the detection of bursts and network bursts in neuronal cultures. J Comput Neurosci 29: 213–229, 2010. [DOI] [PubMed] [Google Scholar]
- Quiroga RQ, Kreuz T, Grassberger P. Event synchronization: A simple and fast method to measure synchronicity and time delay patterns. Physical Rev E 66: 2002. [DOI] [PubMed] [Google Scholar]
- Redman S. Quantal analysis of synaptic potentials in neurons of the central nervous system. Physiol Rev 70: 165–198, 1990. [DOI] [PubMed] [Google Scholar]
- Robinson HPC, Kawahara M, Jimbo Y, Torimitsu K, Kuroda Y, Kawana A. Periodic synchronized bursting and intracellular calcium transients elicited by low magnesium in cultured cortical-neurons. J Neurophysiol 70: 1606–1616, 1993. [DOI] [PubMed] [Google Scholar]
- Scharfman HE. Synchronization of area CA3 hippocampal pyramidal cells and non-granule cells of the dentate gyrus in bicuculline-treated rat hippocampal slices. Neuroscience 59: 245–257, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selinger JV, Kulagina NV, O'Shaughnessy TJ, Ma W, Pancrazio JJ. Methods for characterizing interspike intervals and identifying bursts in neuronal activity. J Neurosci Methods 162: 64–71, 2007. [DOI] [PubMed] [Google Scholar]
- Shi X, Lu QS. Burst synchronization of electrically and chemically coupled map-based neurons. Physica A Stat Mechanics Applications 388: 2410–2419, 2009. [Google Scholar]
- Traub RD, Bibbig A, LeBeau FEN, Buhl EH, Whittington MA. Cellular mechanisms of neuronal population oscillations in the hippocampus in vitro. Annu Rev Neurosci 27: 247–278, 2004. [DOI] [PubMed] [Google Scholar]
- Traub RD, Jefferys JGR, Whittington MA. Enhanced NMDA conductance can account for epileptiform activity-induced by low Mg2+ in the rat hippocampal slice. J Physiol 478: 379–393, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Knowles WD, Miles R, Wong RKS. Synchronized afterdischarges in the hippocampus—simulation studies of the cellular mechanism. Neuroscience 12: 1191–1200, 1984. [DOI] [PubMed] [Google Scholar]
- Traub RD, Miles R, Wong RKS. Models of synchronized hippocampal bursts in the presence of inhibition. 1. Single population events. J Neurophysiol 58: 739–751, 1987. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci 11: 100–113, 2010. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron 52: 155–168, 2006. [DOI] [PubMed] [Google Scholar]
- Velisek L, Dreier J, Stanton P, Heinemann U, Moshe S. Lowering of extracellular pH suppresses low-Mg2+ induced seizures in combined entorhinal cortex hippocampal slices. Exp Brain Res 101: 44–52, 1994. [DOI] [PubMed] [Google Scholar]
- Wong RKS, Traub RD, Miles R. Cellular basis of neuronal synchrony in epilepsy. Adv Neurol 44: 583–592, 1986. [PubMed] [Google Scholar]
- Yue CY, Yaari Y. KCNQ/M channels control spike afterdepolarization and burst generation in hippocampal neurons. J Neurosci 24: 4614–4624, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziburkus J, Cressman JR, Barreto E, Schiff SJ. Interneuron and pyramidal cell interplay during in vitro seizure-like events. J Neurophysiol 95: 3948–3954, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]