Abstract
Progressive loss of plasticity during development prevents refined circuits from regressing to an immature state and is thought to depend on maturation of GABAergic inhibition. For example, a gradual reduction in size of visual receptive fields (RFs) occurs in the superior colliculus (SC) during development. Maintenance of the refined state throughout adulthood requires early light exposure. Here we investigate the potential role of changes in long- or short-term plasticity in experience-dependent maintenance of refined RFs. Using an acute SC slice preparation, we found that long-term plasticity was not affected by visual deprivation, indicating that it does not underlie deprivation-induced RF enlargement. In contrast, visual deprivation altered short-term plasticity in an unexpected way. Specifically, GABAB receptor (GABABR)-mediated paired pulse depression was increased in slices from dark-reared animals. This increase was mimicked by GABAAR blockade in slices from normally reared animals, suggesting that experience-dependent maintenance of GABAAR function prevents an increase in probability of neurotransmitter release. GABABR-mediated short-term depression in response to strong stimulation (such as occurs during vision) was reduced in slices from dark-reared animals. This change was mimicked in slices from normal animals by reducing GABA release. These results are consistent with the hypothesis that early visual experience maintains GABAergic inhibition and prevents later deprivation-induced alterations of short-term depression in SC. Identifying how plasticity is restricted in mature circuits could guide therapies to enhance recovery of function in adults.
Keywords: visual deprivation, critical period, dark rearing, rodent, inhibitory plasticity, synaptic plasticity
development of sensory pathways requires robust plasticity during critical periods, but when neural circuits have matured, this plasticity is no longer necessary and is restricted. This restriction stabilizes circuits and prevents regression from the mature state, but also limits recovery from injury and disease. Understanding the mechanisms that underlie the transition from flexible to stable circuits is of great importance for understanding how plasticity is regulated across life stages.
Visual receptive fields (RFs) refine to a smaller diameter during development, increasing perceptual acuity. This refinement is independent of visual experience in both the superior colliculus (SC) (Carrasco et al. 2005; Wang et al. 2010) and primary visual cortex (V1) (Balmer and Pallas 2013; Sarnaik et al. 2014). Thus spontaneous activity alone is sufficient to refine RFs. Late juvenile visual experience is necessary to maintain the refined RFs into adulthood, however (Balmer and Pallas 2013; Carrasco and Pallas 2006). Failure to maintain refined RFs in SC of dark-reared (DR) hamsters occurs at least in part through a deprivation-induced loss of lateral inhibition (Carrasco et al. 2011). Here we investigate the synaptic mechanism through which visual experience preserves inhibition and thus restricts SC RF plasticity in adulthood. We hypothesized that deprivation-induced changes in either long- or short-term plasticity could lead to a loss of RF refinement in adult DR SC. In visual cortex, reducing inhibition facilitates induction of long-term synaptic plasticity (Artola et al. 1990), which could increase RF size by strengthening synapses along the outskirts of the RF. DR reduces inhibition in V1 (Morales et al. 2002) and prolongs the early period during which long-term plasticity can be induced (Kirkwood et al. 1995). In addition, dysregulation of inhibition may alter presynaptic inhibition and/or neurotransmitter release probability, which in turn could affect short-term synaptic plasticity and enlarge RFs by increasing excitation or reducing inhibition.
We present evidence here from recordings of acute SC slices that early visual experience prevents RF plasticity in adult SC by preserving GABAergic inhibition and thus maintaining GABA-mediated short-term depression (STD) of excitatory synapses. We found that the reductions in GABAA receptor (GABAAR)-mediated inhibition and GABA content in DR SC shown in a previous study (Carrasco et al. 2011) cause dysregulation of GABABR-mediated inhibition, which disrupts short-term but not long-term plasticity. Deprivation-induced disruption of STD likely contributes to RF plasticity in adulthood, which is normally restricted by experience-dependent maintenance of inhibition.
METHODS
Animals and Rearing Conditions
Syrian hamsters (Mesocricetus auratus) of both sexes were used in this and the previous related studies due to their short gestation time, robust visual responses, and the availability of data on their developmental plasticity (Carrasco et al. 2005; Chalupa and Rhoades 1977; Razak et al. 2010). All of the procedures involving animals met or exceeded standards of humane care developed by the National Institutes of Health and the Society for Neuroscience and were approved by the Georgia State University Institutional Animal Care and Use Committee. Hamsters were obtained from Charles River Laboratories or Harlan Laboratories and bred in-house. Normally reared hamsters were kept on a 14:10-h light-dark cycle. DR hamsters were maintained in a light-tight dark room and exposed only briefly to a dim red light for husbandry purposes, at a wavelength not visible to Syrian hamsters (Huhman et al. 1999). Pregnant dams of DR pups were moved into the darkroom prior to parturition.
Slice Preparation
Hamsters were deeply anesthetized with isoflurane, and the brain was rapidly extracted into ice-cold 95% O2/5% CO2 saturated artificial cerebral spinal fluid containing kynurenic acid (KA-ACSF) (in mM): 123 NaCl, 2.5 KCl, 1 NaH2PO4, 1.3 MgSO4, 26.2, NaHCO3, 11 glucose, and 2.5 CaCl2, 0.8 thiourea, 2 sodium pyruvate, 0.4 ascorbic acid, and 1.2 kynurenic acid. The nonspecific ionotropic glutamate receptor antagonist kynurenic acid was included in the KA-ACSF to suppress any excitotoxic effects of glutamate release that may occur due to tissue slicing. Parasagittal (SC) or coronal (V1) slices were cut at 350 μm using a vibratome and incubated in oxygenated, 32°C KA-ACSF for 1 h followed by the same solution with kynurenic acid omitted for >1 h at room temperature. For recording, individual slices were transferred to a submerged type-recording chamber and superfused with the above solution heated to 28°C, minus the kynurenic acid, thiourea, sodium pyruvate and ascorbic acid, at a rate of 1–2 ml/min. In some long-term plasticity experiments, the bath temperature was raised to 32°C or 35°C, but the results were not different from the 28°C temperature results, and thus the data were combined.
Recording Procedures
Recording pipettes were pulled from borosilicate glass (1.5/0.86 mm outer diameter/inner diameter, Narashige PP-830) to resistances from 2–6 MΩ when filled with ACSF. Signals were amplified and low-pass filtered (2–3 kHz, Multiclamp 700B) and digitized using Signal software and CED hardware (50 kHz, Micro 1401; Cambridge Electronic Design). The amplitude of the field excitatory postsynaptic potential (fEPSP) response was measured at its negative peak. The short latency between the presynaptic response when observed and the fEPSP peak (∼2 ms), and the narrowness of the peak, suggest a largely monosynaptic response (Fig. 1A). Any polysynaptic response would arise through a feedforward interneuron and would be delayed at least 1 ms due to synaptic delay. Such longer latency responses would be unlikely to overlap with the initial fEPSP peak, although we do not rule out the possibility of minor polysynaptic components. The duration and slope of the fEPSP are influenced by polysynaptic components and thus were not analyzed.
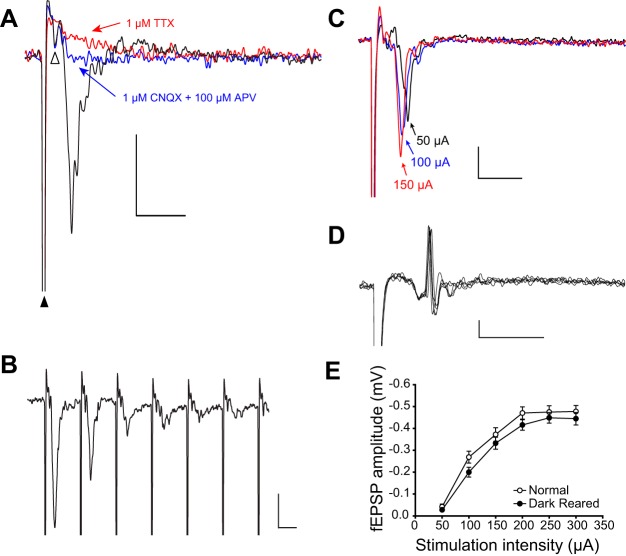
Fig. 1.
The evoked field potential in superior colliculus (SC) is an excitatory postsynaptic potential (fEPSP). A: the postsynaptic signal is excitatory, because blocking α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-D-aspartate (NMDA)-type glutamate receptors with 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and dl-2-amino-5-phosphonovaleric (APV) acid completely blocked the field potential (blue trace; stimulus artifact marked with black triangle). Under glutamate receptor blockade, a small presynaptic potential remained (open triangle), which was blocked by tetrodotoxin (TTX) (red trace). B: decreased responses during rapid (100 Hz) stimulation suggest a postsynaptic signal. C: monotonically decreasing latency at increasing stimulation intensities also suggests a postsynaptic signal. D: action potentials are occasionally observed that coincide with the peak of the fEPSP, indicating that it is likely a combination of current sources, including postsynaptic dendrites and somata. Several overlain traces show that the spike occurs at a consistent latency. Reducing stimulus intensity caused the spike to disappear. E: current evoked fEPSP responses were not significantly affected by visual experience. Scale bars in A–D: 0.2 mV, 5 ms.
Data Analysis
Analysis of long-term plasticity.
To evoke field potential responses, electrical stimuli with 50- to 100-μs pulse durations were delivered to the stratum opticum of the SC through a concentric bipolar stimulating electrode (Platinum/iridium, FHC, cat. no. CBBPC75). Stimulation intensity was adjusted to 50–75% of the maximum evoked response. Test stimuli were delivered at 20-s intervals over at least 10 min of stable recording before and 45 min after the inducing stimulation. Induction protocols that have been shown to produce long-term plasticity in SC in other rodents were used, including 50 Hz 20 s, 20 Hz 20 s and 1 Hz 300 s (Lo and Mize 2002; Okada and Miyamoto 1989; Zhao et al. 2006). To determine whether there was a long-term change in synaptic strength after application of the inducing stimulus, the mean peak response during baseline recordings was compared with the mean peak response during an interval 30–40 min after induction. The example fEPSPs shown in Figs. 1–6 are averages of 5–10 trials. To induce long-term potentiation (LTP) in V1, the stimulation electrode was placed between the white matter and layer 6, and the recording electrode was placed in layer 2/3. Theta burst stimulation consisted of 3 episodes delivered at 10-s intervals, consisting of 10 trains delivered at 5 Hz each, consisting of 4 pulses at 100 Hz, as described previously (Kirkwood et al. 1995).
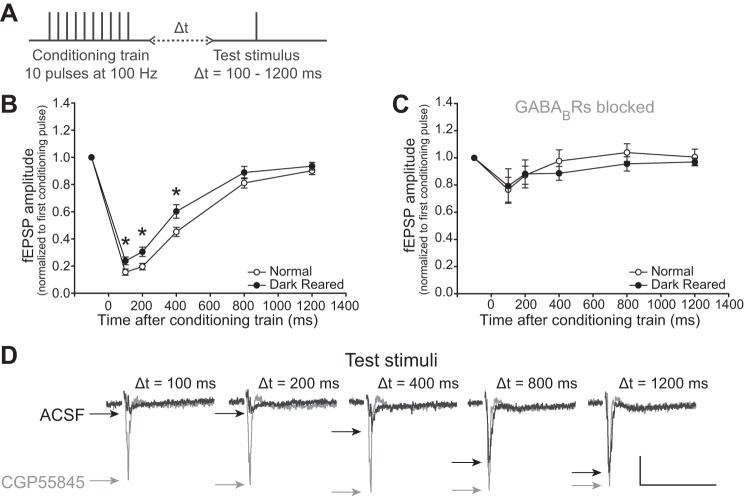
Fig. 6.
Train-induced short-term depression (STD) is maintained by visual experience, is reduced by DR, and is mediated by GABABR inhibition. A: a 10-pulse conditioning stimulus train delivered at 100 Hz was followed by test stimuli presented at either 100, 200, 400, 800 or 1,200 ms to measure the extent and recovery of STD. Δt, change in time. B: slices from normally reared animals were significantly more depressed by the conditioning stimulus than slices from DR animals. Thus, although DR increased STD after a single stimulus (PPD; cf., Fig 3), DR decreased STD after the train stimulus. *P < 0.05. C: 3 μM CGP55845 blocked STD from occurring in slices from both normal and DR animals, but there were no significant differences in the extent of the effect between groups. This shows that recovery from the train-induced STD is strongly inhibited by GABABR activity. D: example of a single experiment, using a slice from a normal animal, showing that at short intervals after the conditioning stimulus the fEPSP is quite depressed. This depression is relieved when GABABRs are blocked with CGP55845 (gray traces). Stimulus artifacts are omitted for clarity. Scale bar: 0.2 mV, 50 ms.
PPR analysis.
Paired pulse ratio (PPR) was calculated as the mean of the second response divided by the mean of the first response over a series of five trials (Kim and Alger 2001). In experiments in which the signal did not return to zero before the second pulse (PP2), the amplitude of PP2 was measured relative to the signal during 1 ms before the stimulus artifact. When multiple interpulse intervals were used, the stimulus intensity was set to 25% of the maximum to avoid overstimulation. To reduce the likelihood of a ceiling effect caused by complete depression of PP2, we increased the stimulus intensity to 75% of the maximum in experiments in which pharmacological agents were applied. Under these conditions, we did not always observe higher paired pulse depression (PPD) in slices from DR animals, which was not unexpected, given interactions between stimulation intensity and PPD (Kim and Alger 2001; Platt and Withington 1997).
Recovery from STD analysis.
A conditioning stimulus train consisting of ten 100-μs pulses was delivered at 100 Hz to cause strong STD. Stimulation intensity was set to 75% of maximal. A test pulse was delivered 100, 200, 400, 800 or 1,200 ms after the conditioning train to measure recovery from STD. Trials were repeated at 1-min intervals. Test stimuli were normalized relative to the first conditioning pulse under the same bath conditions. The time constant of recovery from STD was calculated as the time to reach 1 − e−1 = 63.2% recovery from an exponential that was fit to the relative fEPSP amplitudes from 100 to 1,200 ms. In experiments in which a drug was bath applied, stimuli were paused for 5–20 min for the drug to take maximal effect, based on the time course of drug action in preliminary experiments.
Statistical analysis.
Student's t-tests or one-way or two-way analyses of variance (ANOVA), followed by Tukey's post hoc tests were used for normally distributed data with equal variance between groups. For data that did not meet these requirements, Mann-Whitney rank sum tests were used. The “n” values in results refer to the number of slices. Data are presented as means ± SE of the mean.
RESULTS
The retinocollicular midbrain visual circuit is important for spatial orientation to visual objects (Schneider 1969). Visual experience is necessary to maintain refined RFs and high visual acuity (Balmer and Pallas 2013; Carrasco et al. 2005), but it is unclear what mechanisms underlie visual experience-dependent restriction of RF plasticity in the retinocollicular circuit. Here we examine the role of visual experience in long- and short-term changes of synaptic strength in acute brain slices from Syrian hamsters that were either normally reared or DR from birth until early or late adulthood [postnatal days 60–70 (P60-70) or >P90].
Characteristics of Evoked fEPSPs Recorded from Superficial SC
To test the role of visual experience in mechanisms of synaptic plasticity, we utilized an acute SC slice preparation. Retinocollicular axons are easily visualized in parasagittal brain slices as they travel within the stratum opticum (optic layer) below the superficial gray layer of the SC. The majority of the axons in the optic layer are retinal ganglion cell (RGC) axons, although some are visual cortical projections (Rhoades et al. 1991). Both projections are almost entirely glutamatergic (Lund and Lund 1971; Mize and Butler 1996), although a small proportion of GABAergic RGCs have been reported (Caruso et al. 1989; da Costa et al. 1997).
Figure 1 shows how extracellular fEPSPs were characterized for subsequent measurements. Electrically stimulating the glutamatergic afferents in the optic layer elicited a fEPSP in the superficial gray layer. These fEPSPs were blocked by bath-applied ionotropic glutamate receptor antagonists 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 1 μM) and DL-2-amino-5-phosphonovaleric acid (APV; 100 μM) (Fig. 1A), depressed during high-frequency stimulation (Fig. 1B), and decreased in latency with increased stimulation intensity (Fig. 1C), reflecting their excitatory, postsynaptic origin. The fEPSPs are likely caused by a combination of synaptic and somatic current sources, because single-unit spikes were occasionally observed near their peak (Fig. 1D). In these cases, the stimulus was reduced to below threshold for that spike, or the electrode was repositioned. Although feedforward inhibition would also be prevented by blocking glutamatergic transmission, GABAAR antagonists had little effect on the fEPSP peak. The short latency (∼3 ms) and short duration (∼5 ms) of the fEPSP are consistent with a monosynaptic population response, although we do not rule out the possibility of minor polysynaptic components. By analyzing the amplitude of the initial peak of the fEPSP, which was always the largest peak in the signal, we exclude most, if not all, polysynaptic components. Note that, although the fEPSP is a postsynaptic response caused by glutamate release, both excitatory and inhibitory postsynaptic neurons in SC are innervated by retinocollicular afferents (Mize 1988, 1992).
To test whether reduced inhibition in DR SC (Carrasco et al. 2011) could cause increased excitability that could contribute to enlarged RFs, we measured the amplitude of fEPSPs elicited by stimulating the optic layer with increasing stimulus intensity. The fEPSP amplitude was not different between rearing conditions at any stimulus intensity, supporting the conclusion that DR does not affect current-evoked excitability in SC [two-way repeated-measures ANOVA; F(1,233) = 1.985, N = 40, P = 0.167, Fig. 1E]. This result was not unexpected, given the preponderance of homeostatic regulatory mechanisms in the central nervous system that could prevent reduced inhibition from increasing overall excitability (Maffei et al. 2012; Turrigiano 2011; Wenner 2011) and given the lack of increased excitability in DR SC in vivo (Carrasco et al. 2005). Although we consider it unlikely, we cannot definitively rule out that DR caused precisely complementary changes in inhibition and excitation that resulted in no net change in the fEPSP response.
Long-term Plasticity in SC Is Not Affected by Visual Experience
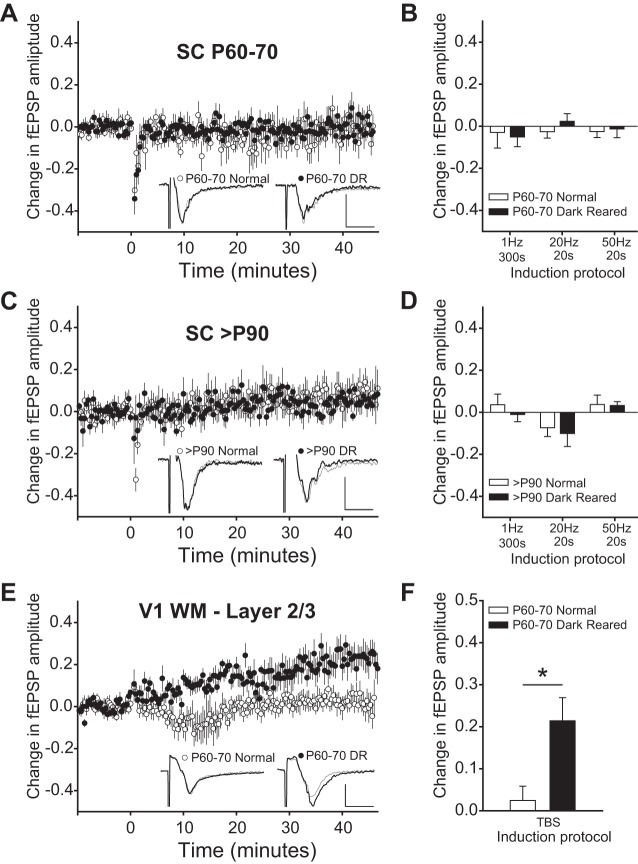
In V1, long-term plasticity is maximal during the critical period for ocular dominance plasticity and declines thereafter, but is maintained at the maximal level in V1 of DR animals (Kirkwood et al. 1995). In this series of experiments, we tested whether long-term plasticity varies in strength during the critical period for RF plasticity in the SC. SC RFs are large at birth and undergo a period of refinement between birth and adulthood at P60 (Carrasco et al. 2005). DR animals follow the same pattern of refinement, but between P60 and P70, the RFs enlarge. It is possible that LTP could have led to this RF enlargement in adulthood. For example, weak excitatory synaptic inputs on the outskirts of a neuron's RF, which would have remained weak with normal visual experience, could have been potentiated during DR, and the potentiation could have increased the RF size. If so, SC slices from DR but not normally reared animals would exhibit juvenile levels of long-term plasticity between P60 and P70.
As expected, long-term plasticity could not be induced in slices from normally reared adults (P60-70). However, the stimulation paradigm also failed to induce long-term plasticity in slices from age-matched DR animals. The summary of experiments using a 50-Hz, 20-s induction protocol is shown in Fig. 2A. Induction protocols of 1 Hz for 300 s and 20 Hz for 20 s were also used, with similar negative results. Although afferent stimulation patterns such as this are unlikely to occur in vivo, these protocols have been used to induce long-term plasticity in SC in vitro (Lo and Mize 2002; Okada and Miyamoto 1989; Zhao et al. 2006). The mean fEPSP amplitude from 10 min before the induction stimulus compared with 30–40 min postinduction was not significantly different between rearing conditions for any induction protocol (Student's t-tests: 1 Hz, 300 s normal: −0.030 ± 0.0739, n = 5 vs. DR: −0.053 ± 0.0288, n = 6, P = 0.758; 20 Hz 20 s normal: −0.025 ± 0.0307, n = 14 vs. DR: 0.029 ± 0.0399, n = 11, P = 0.282; 50 Hz 20 s normal: −0.025 ± 0.0287, n = 8 vs. DR: −0.013 ± 0.0415, n = 7, P = 0.826, Fig. 2B). Thus it is unlikely that RF enlargement in DR animals is the result of deprivation-induced maintenance of long-term plasticity beyond the critical period and into adulthood.
Fig. 2.
Long-term plasticity in SC is not affected by visual experience. A: fEPSPs were evoked at 20-s intervals. After the fEPSP amplitude was stable for >10 min, a 50-Hz, 20-s stimulus was applied. There was no significant change in slices from normal or from dark-reared (DR) animals between postnatal days 60–70 (P60-70). Example traces are averages during a 10-min baseline (thin gray) and 30–40 min after the induction protocol (thick black). Scale bar: 0.2 mV, 5 ms. B: other induction protocols at this age also did not cause long-term plasticity. Data plotted are mean change from 30–40 min after the induction stimulus normalized to a 10-min baseline. C: there was also no significant change after a 50-Hz, 20-s stimulus in slices from normal or DR animals >P90. Example traces and scale bar are as in A. D: there were also no differences between slices from normal or DR animals using 3 other induction protocols. E: in contrast, in primary visual cortex (V1) white matter (WM)-layer 2/3 long-term potentiation (LTP) was induced in slices from DR animals, but not in slices from normal animals. Example traces and scale bar are as in A. F: theta burst stimulation (TBS) caused significantly more LTP in V1 slices from DR than from normally reared animals. *P < 0.05.
Because the largest differences in synaptic plasticity may be in the age group with the largest differences in RF size, we also investigated long-term plasticity in >P90 animals that had either unrefined (DR) or refined (normal) RFs. By P90 the RFs of DR animals have reached their maximum size (Carrasco et al. 2005). We found no significant differences in mean levels of long-term plasticity between slices from >P90 normal and DR adults. The mean fEPSP amplitude from 10 min before the induction stimulus to 30–40 min postinduction was not significantly different between rearing conditions (Student's t-tests: 1 Hz 300 s normal: 0.036 ± 0.0507, n = 10 vs. DR: −0.011 ± 0.0344, n = 8, P = 0.489; 20 Hz 20 s normal: −0.073 ± 0.0417, n = 7 vs. DR: −0.101 ± 0.0617, n = 8, P = 0.726; 50 Hz 20 s normal: 0.037 ± 0.0445, n = 8 vs. DR: 0.032 ± 0.0189, n = 7, P = 0.920, Fig. 2, C and D). These results suggest that the deprivation-induced increases in adult RF size cannot be explained by increased susceptibility to LTP, and that neither enlargement of RFs nor the maintenance of enlarged RFs during DR require juvenile levels of long-term plasticity.
As a positive control to show that the brain slices were indeed healthy enough to exhibit long-term plasticity, we demonstrated our ability to induce LTP at a synapse known to produce it reliably: the white matter to layer 2/3 synapse in V1. As previously shown in rats (Kirkwood et al. 1995), theta burst stimulation (see methods) caused a significant potentiation of the fEPSP in V1 slices from DR hamsters but not in V1 slices from normally reared hamsters (normal: 0.025 ± 0.0337, n = 8 vs. DR: 0.214 ± 0.0550, n = 7, Student's t-test, P < 0.05, Fig. 2, E and F). Thus we argue that the inability to induce long-term plasticity in SC was not an artifact of slice preparation methods.
Short-term Plasticity in SC Is Affected by Visual Experience
Short-term plasticity shapes the ongoing activity of neural circuits, and, if affected by visual experience, could affect sensory processing and contribute to RF plasticity. The effect of visual experience on short-term plasticity in SC has not been investigated. Here we show that visual experience is necessary to maintain short-term plasticity.
Visual experience prevents a DR-induced increase in PPD.
Our first approach to studying short-term plasticity was to measure PPR. This experiment was designed to compare the level of short-term plasticity evoked by paired pulse stimulation between slices from normal and from DR animals as an assay for possible DR-induced changes in probability of neurotransmitter release. Decreased amplitude of PP2 relative to the first pulse (PP1) indicates PPD [PPR (PP2/PP1) < 1], whereas a relative increase in PP2 indicates facilitation (PPR > 1). Because inhibition is reduced by DR (Carrasco et al. 2011), we predicted an increase in the probability of neurotransmitter release that could increase PPD. Slices from normal and DR animals were stimulated by paired pulses with intervals ranging from 20 to 200 ms. The stimulus intensity was set to 25% of the maximal response that could be elicited to test depression in response to mild stimulation, as opposed to the stronger stimulation tested in later experiments.
As predicted, we found that PPD was increased in slices from DR animals compared with PPD in slices from normally reared animals [two-way repeated-measures ANOVA, F(1,202) = 5.160, N = 50, P = 0.028, Student's t-tests, P < 0.05 at 20-, 100- and 150-ms intervals, Fig. 3, A and B]. PP1 was not affected by rearing condition, indicating that the change in PPD was due to a weaker response to PP2 in slices from DR animals, rather than a stronger response to PP1 [two-way repeated-measures ANOVA, F(1,222) = 0.0261, N = 50, P = 0.872]. There was also a significant increase in PPD as interpulse interval increased [two-way repeated-measures ANOVA, F(4,202) = 6.958, N = 50, P < 0.001, Fig. 3A].
Fig. 3.
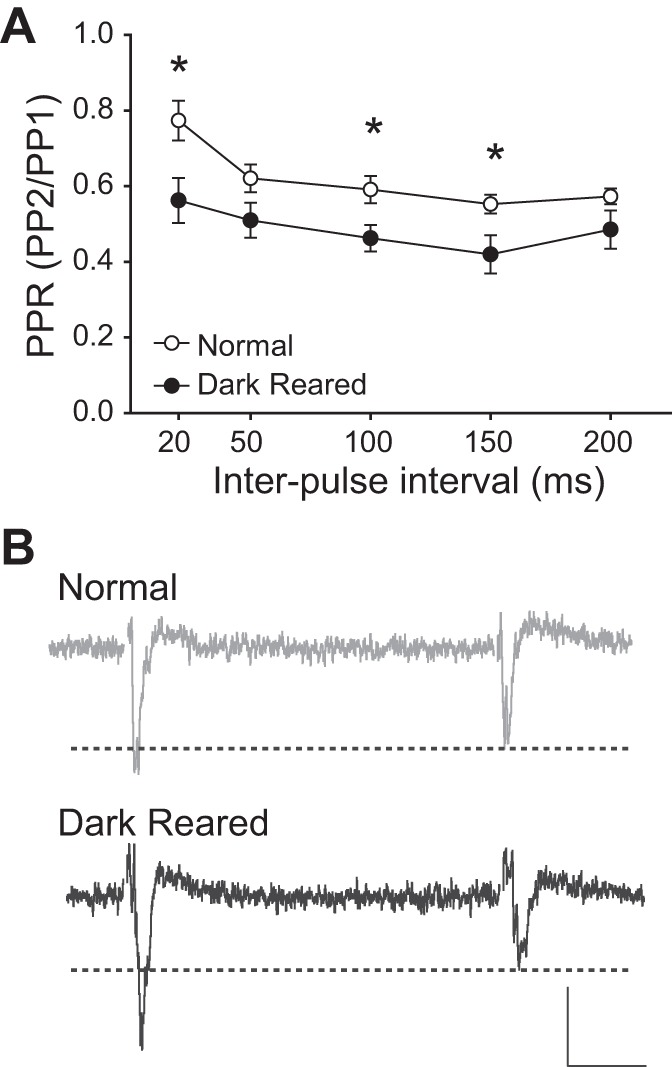
Lack of visual experience causes increases in paired pulse depression (PPD). A: DR animals have higher PPD [lower paired pulse ratio (PPR)], compared with normal animals. *P < 0.05. B: examples of PPD in a slice from a normal (gray) and a DR (black) animal. Note that the response to the second pulse (PP2) of the DR trace is much smaller than the response to the first pulse (PP1), whereas, in the normal trace, PP2 is only slightly smaller than PP1. Stimulus artifacts are omitted for clarity. Scale bar: 0.1 mV, 10 ms.
GABABR function underlies PPD, but is not directly affected by visual experience.
GABABRs are metabotropic receptors that hyperpolarize neurons with a slow onset and long duration (Benardo 1994) by increasing K+ channel conductance and/or decreasing Ca2+ channel conductance (Alger and Nicoll 1982; Benardo 1994; Chalifoux and Carter 2011). GABABRs could underlie PPD through either presynaptic inhibition of excitatory neurotransmitter release or postsynaptic inhibition that is activated by the first stimulus pulse and remains during the second stimulus pulse. We hypothesized that a deprivation-induced increase in GABABR function could underlie the increased PPD observed in slices from DR animals. If slices from DR animals are more sensitive to GABABR agonists and antagonists than slices from normal animals, the hypothesis would be supported.
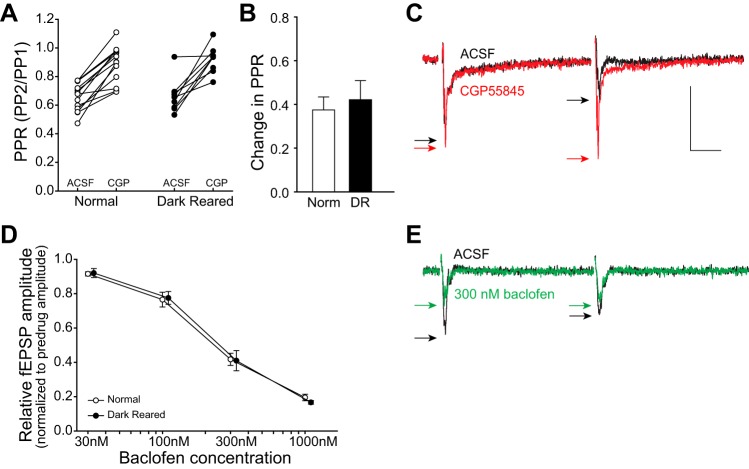
To test the role of GABABR-mediated inhibition on PPD, we used the GABABR antagonist CGP55845. For this and all subsequent experiments, the interpulse interval was 100 ms, and the stimulation intensity was set to 75% of the maximum response that could be elicited from each slice, to avoid a floor effect (saturated PPD). At this stimulation intensity, the difference in PPD between rearing conditions was not always apparent, which was not unexpected, given the known stimulus intensity-dependent dynamics of PPD (Kim and Alger 2001; Platt and Withington 1997). Application of 3 μM CGP55845 significantly decreased PPD (increased PPR) in slices from both rearing conditions (paired t-tests, normal PPR before CGP55845: 0.652 ± 0.0269 to normal PPR during 3 μM CGP55845: 0.887 ± 0.0354, n = 14, DR PPR before CGP55845: 0.662 ± 0.0390 to DR PPR during 3 μM CGP55845: 0.920 ± 0.0313, n = 10, P < 0.001, Fig. 4A). Contrary to our prediction, the ability of CGP55845 to reduce PPD was not significantly different between slices from normal and DR animals (Student's t-test, normal: 37.5 ± 5.96% increase in PPR, n = 14 vs. DR: 42.2 ± 8.71% increase in PPR, n = 10, P = 0.649, Fig. 4, B and C). In addition, the effect of CGP55845 on the amplitude of PP1 was not significantly different between rearing conditions (normal: −0.353 ± 0.0237 mV, normal with CGP 55845: −0.435 ± 0.0382 mV, n = 16; DR: −0.370 ± 0.0199 mV, n = 16, DR with CGP55845: −0.475 ± 0.0402 mV, n = 13, Student's t-test comparing effect between treatment conditions, P = 0.515). Decreasing GABABR-mediated inhibition with CGP55845 relieves the robust inhibition of PP2, but also increases the amplitude of PP1. This indicates that PP1 is slightly inhibited by GABABRs under normal conditions (without drug).
Fig. 4.
GABAB receptor (GABABR) function is required for PPD, but is not directly affected by visual experience. A: application of the GABABR antagonist CGP55845 (CGP; 3 μM) decreased PPD in slices from both normal and DR animals. This panel shows that GABABR-mediated inhibition underlies PPD in SC. B: the effect of CGP55845 on PPD [(gabazine − ACSF)/ACSF] was not significantly different between rearing conditions. ACSF, artificial cerebrospinal fluid. C: example trace showing the effect of CGP55845. The largest effect is the increase in PP2, which decreases PPD (increase in PPR). Black trace is before drug, and black arrows point to the peaks. Red trace is during bath application of CGP55845, and red arrows point to the peaks. Scale bar: 0.2 mV, 20 ms. D: application of the GABABR agonist baclofen reduced the fEPSP amplitude of the response to PP1, but the effect was not significantly different between rearing conditions. Data points are horizontally offset for clarity. E: baclofen decreased PPD (green trace/arrows, increased PPR). In contrast to the effect of CGP55845, baclofen affected PP1 more than PP2, suggesting that PP2 is nearly maximally affected by GABABR-mediated inhibition under normal (no drug) conditions. Stimulus artifacts are omitted for clarity. Scale bar is as in C.
As a second assay for deprivation-induced changes in GABABR function, we tested the effect of the GABABR agonist (RS)-baclofen on PPD. Increasing concentrations of baclofen reduced the fEPSP amplitude of PP1 monotonically, but, contrary to our prediction, there was no difference in response amplitude between slices from normal and DR animals at any concentration [two-way repeated-measures ANOVA, F(1,55) = 0.0323, N = 16, P = 0.860, Fig. 4, D and E], nor was there a difference in the effect of baclofen on PPR between treatment groups [normal: predrug PPR: 0.597 ± 0.0453, 30 nM PPR: 0.611 ± 0.0631, 100 nM PPR: 0.691 ± 0.0472, 300 nM PPR: 0.971 ± 0.0490, n = 9; vs. DR: predrug PPR: 0.550 ± 0.0332, 30 nM PPR: 0.598 ± 0.0270, 100 nM PPR: 0.677 ± 0.0311, 300 nM PPR: 0.896 ± 0.0764, n = 7; two-way repeated-measures ANOVA, F(1,57) = 0.221, P = 0.645, N = 16]. These experiments show that, although GABABR-mediated inhibition underlies PPD in SC, there is no apparent effect of visual experience on GABABR sensitivity or on the degree of tonic GABABR-mediated inhibition in SC.
Reduced GABAAR function underlies increased PPD in slices from DR animals.
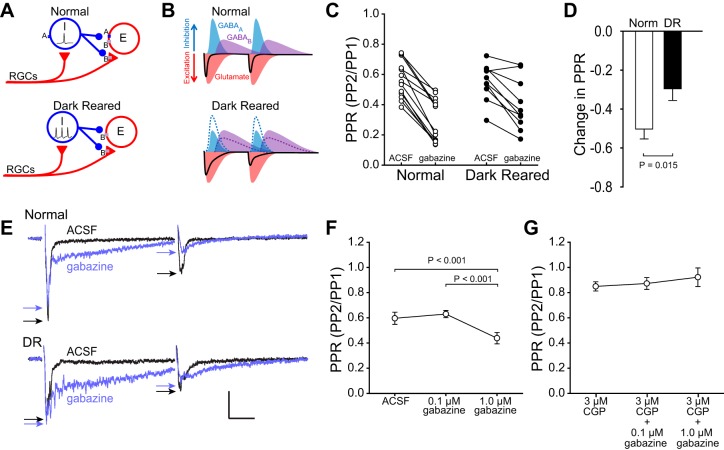
Although GABABR function appears to be equivalent in slices from normal and DR animals (cf., Fig. 4), it is possible that DR-induced decreases in GABAAR efficacy could lead to changes in GABA release. GABAARs are the largest contributor to fast synaptic inhibition in the SC (Bowery et al. 1987). We previously showed that GABAAR agonists and antagonists affect visual response strength and RF size less in DR than in normally reared animals, suggesting that visual experience is necessary to maintain GABAAR function (Carrasco et al. 2011). We hypothesized that reduced GABAAR function in DR SC could disinhibit GABAergic neurons, which would cause increased GABA release (Fig. 5A). This increased GABA release would enhance the GABABR mediated inhibition of PP2 and could underlie the increased PPD in slices from DR animals (Fig. 5B).
Fig. 5.
Reduced GABAAR function underlies increased PPD in slices from DR animals. A: hypothetical circuit model showing that, when GABAARs are reduced by DR (or blocked), the inhibitory neurons (I) are disinhibited, and GABA release increases, resulting in increased GABABR-mediated inhibition of excitatory neurons (E). Triangles are excitatory, circles are inhibitory. RGCs, retinal ganglion cells. B: model showing how reduced GABAAR function, increased GABA release, and increased GABABR-mediated inhibition could increase PPD in slices from DR animals. Increased GABA release enhances GABABR-mediated inhibition of excitatory neurons (E), which increases the fEPSP duration, and increases inhibition of PP2 (dotted lines are normal condition for comparison). C and D: the GABAAR antagonist gabazine (10 μM) reduced the PPR significantly more in slices from normal animals (50.3 ± 5.08% decrease in PPR) than in slices from DR animals (29.6 ± 6.04% decrease in PPR). E: gabazine caused a large increase in the duration of the fEPSP in slices from both normal and DR animals, which is likely caused by disinhibition of both excitatory and inhibitory neurons and increased duration of evoked responses. Stimulus artifacts are omitted for clarity. Scale bar: 0.2 mV, 20 ms. Arrows indicate peaks of fEPSPs. F: 1 μM gabazine was sufficient to increase PPD (decrease PPR) in slices from normal animals. Thus reducing GABAAR function in slices from normal animals mimicked the increased PPD seen in slices from DR animals (cf., Fig 3). G: blockade of GABABRs prevented the increase in PPD caused by 1 μM gabazine, supporting the model in which reduced GABAAR function increases PPD by increasing inhibitory neuron output and, in turn, increasing GABABR-mediated inhibition.
To test whether a DR-induced reduction in GABAAR function underlies increased PPD, the GABAAR antagonist SR-95531 (gabazine) was bath-applied on slices from normal and DR animals. GABAAR blockade with 10 μM gabazine caused a significant increase in PPD (decrease in PPR) in slices from both normal and DR animals (paired t-tests, normal prior to gabazine PPR: 0.568 ± 0.0340 compared with normal PPR during 10 μM gabazine: 0.293 ± 0.0398, n = 14, P < 0.001; DR PPR prior to gabazine: 0.550 ± 0.0383 compared with DR PPR during 10 μM gabazine: 0.398 ± 0.0538, n = 11, P = 0.001, Fig. 5C). However, this effect was significantly greater in slices from normal animals than in slices from DR animals (Student's t-test, normal: 50.3 ± 5.08% decrease in PPR, n = 14 vs. DR: 29.6 ± 6.04% decrease in PPR, n = 11, P = 0.015, Fig. 5, D and E), consistent with reduced GABAAR efficacy in DR SC. The change in PPR was due to changes in the amplitude of PP2, in that the amplitude of PP1 was affected only slightly (normal: 4.2% decrease, DR: 6.1% increase). Reducing GABAAR function in slices from normal animals mimicked the effect of DR on PPD (cf., Fig. 3), arguing that reduced GABAAR function such as occurs in DR SC (Carrasco et al. 2011) is likely to underlie increased PPD.
DR-induced reduction in GABAAR function increases PPD via GABABR-mediated inhibition.
Our observation that GABAAR blockade increased fEPSP duration (Fig. 5E) is consistent with reports that gabazine increases evoked activity of both excitatory and inhibitory neurons in SC (Kaneda et al. 2008). We hypothesized that reduced GABAAR efficacy, as occurs in DR SC (Carrasco et al. 2011), allows disinhibition of GABAergic neurons, leading to increased GABABR-mediated inhibition. This hypothesis predicts that blockade of GABABRs would prevent reduced GABAAR function (caused by DR or gabazine) from increasing PPD. Bath application of 1 μM gabazine was sufficient to cause an increase in PPD in slices from normal animals [one-way repeated-measures ANOVA, F(2,14) = 40.012, n = 5, P < 0.001; Tukey's tests, predrug (ACSF) PPR vs. 1 μM gabazine PPR, P < 0.001, Fig. 5F]. As predicted, blocking GABABRs with 3 μM CGP55845 prior to applying gabazine prevented the increase in PPD (Tukey's tests, 3 μM CGP55845 PPR vs. 3 μM CGP55845 and 0.1 μM gabazine PPR, P > 0.05; 3 μM CGP55845 PPR vs. 3 μM CGP55845 and 1.0 μM gabazine PPR, P > 0.05, Fig. 5G). These experiments support the interpretation that the reduced GABAAR function seen in DR animals could lead to an increase in the activity of GABAergic interneurons, which in turn could increase PPD by enhancing GABABR-mediated inhibition (cf., Fig. 5B), although intracellular recordings would be necessary to verify this directly. We hypothesize that this increased GABABR-mediated inhibition increases depression of PP2 through pre- and/or postsynaptic inhibition (cf., Fig. 5A).
Visual experience is necessary for GABABR-mediated recovery from train-induced STD.
The PPD experiments revealed a major contribution of GABABRs to STD, but it is unlikely that natural vision would produce such a short-duration response in the SC. During visual stimulation with moving spots of light, SC neurons are strongly activated with trains of high-frequency impulses (Cirone and Salt 2001; Razak and Pallas 2005). Thus, in a second set of experiments, we presented trains of current pulses rather than single pulses and tested the strength and duration of the resulting STD. A conditioning stimulus consisting of a train of 10 pulses at 100 Hz induces profound STD in SC that recovers after about 1 s (Kaneda et al. 2008).
To measure the strength and degree of recovery of the train-induced STD, test pulses were delivered at 100- to 1,200-ms intervals after the end of the conditioning stimulus train (Fig. 6A). The stimulation intensity of the train and test pulses was set to evoke 75% of the maximal response of each slice to elicit the strong response that is caused by visual activity in vivo. The fEPSP amplitudes evoked by the test pulses were normalized to the first conditioning pulse. Slices from DR animals were less depressed after the conditioning stimulus than the slices from normally reared animals 100, 200 and 400 ms after the conditioning stimulus [two-way repeated-measures ANOVA, F(1,149) = 5.064, N = 30, P = 0.032, Fig. 6B].
Informed by our finding that GABABR inhibition underlies PPD in SC, we tested whether or not GABABR-mediated inhibition also underlies the STD caused by train stimulation. In support of this idea, train-induced STD is strongest at ∼100 ms after the conditioning stimulus and lasts for about 1 s, following the time course of action of GABABR-mediated inhibition (Benardo 1994). If GABABRs underlie train-induced STD, then reducing GABABR-mediated inhibition will reduce the STD.
Indeed, in both slices from normal and DR animals, GABABR antagonist CGP55845 (3 μM) almost completely blocked STD from occurring after the 100-Hz train stimulus [two-way repeated-measures ANOVA, normal: F(1,124) = 209.970, n = 7, P < 0.001; DR: F(1,84) = 46.710, n = 5, P < 0.001, Fig. 6, C and D]. The remaining STD observed during GABABR blockade is likely caused by GABABR-independent mechanism of STD (Lambert and Wilson 1994).
Taken together, these studies indicate that recovery from train-induced STD in SC is mediated by GABABRs and is altered by visual experience. Thus, although DR increases PPD (cf., Fig. 3), it decreases the strength and duration of STD caused by a train of pulses. Indeed, a higher probability of neurotransmitter release in the disinhibited slices from DR animals could cause GABA to be depleted more quickly, which could contribute to reduced maximal STD duration.
Reduced synaptically released GABA mimics the effects of DR on reduced train-induced STD.
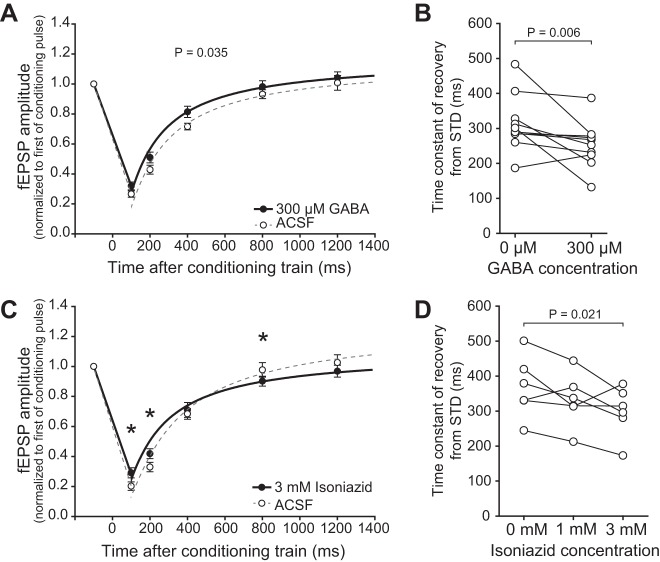
Although there was no effect of visual experience on GABABR function (cf., Fig. 4), reduced GABA content in SC cells could reduce stimulus-evoked GABABR-mediated inhibition. DR reduces GABA content in SC neurons and visual experience maintains GABA content (Carrasco et al. 2011). Here we tested whether reduced GABA in SC could mimic the reduced STD that we observed in slices from DR animals. We recorded the time course of recovery from STD in slices from normal animals as above and then reduced GABA in two different ways.
In the first approach, we utilized the pattern of GABACR expression in SC. GABACRs are ionotropic chloride channels similar to GABAARs. They are preferentially expressed by inhibitory neurons and have a higher affinity for GABA than do GABAARs or GABABRs (Pasternack et al. 1999; Platt and Withington 1998; Schmidt et al. 2001). Thus, when low concentrations of GABA are bath applied, inhibitory neurons are inhibited, reducing the amount of synaptically released GABA, increasing fEPSP amplitude, and potentially contributing to enlarged RFs. We applied 300 μM GABA, a concentration that binds to the high-affinity GABACRs and reduces GABA release, which we inferred by observing an increase in the fEPSP amplitude (124.0 ± 7.57%). Reducing synaptically released GABA in this indirect fashion caused the predicted reduction in STD [two-way repeated-measures ANOVA, F(1,119) = 5.803, P = 0.035, N = 12, Fig. 7A]. The time constant of recovery from STD was also significantly reduced by 300 μM GABA (paired t-test, P = 0.006, n = 10, Fig. 7B). This experiment suggests that reduced GABA, as occurs in DR SC (Carrasco et al. 2011), may underlie the reduced STD caused by stimulus trains.
Fig. 7.
Reducing GABA release reduced the duration of train-induced STD. A: 300 μM GABA significantly reduced STD induced by the 10-pulse, 100-Hz conditioning train (cf., Fig 6A) [one-way repeated-measures ANOVA (P = 0.035)]. Exponential fits are shown from 100 to 1,400 ms after the conditioning train for ACSF (dashed gray line) and 300 μM GABA (black line). B: exponentials were fit to the recovery from STD of each slice before and after GABA application. 300 μM GABA caused a significant decrease in the time constant of recovery. C: 3 mM isoniazid reduced GABA synthesis and significantly reduced the train-induced STD at the 100- and 200-ms time points. *P < 0.05. Exponential fits are as in A. D: the time constant of recovery from STD reached significance (P = 0.021) at 3 mM isoniazid.
Although unlikely, it is possible that constant application of even this low concentration of GABA could cause GABABR desensitization that could contribute to the effect seen. To control for this alternative hypothesis, we reduced GABA by applying a GABA depleting agent, isonicotinic acid hydrazide (isoniazid). Isoniazid inhibits pyridoxal phosphate, the cofactor of the GABA synthesizing enzyme glutamic acid decarboxylase, and has been used to reduce GABA in acute brain slices (Carta et al. 2008; De Koninck and Mody 1997). Bath application of 3 mM isoniazid caused an increase in the fEPSP amplitude (109.3 ± 5.94%) and reduced the duration of STD [two-way repeated-measures ANOVA, significant interaction between time after train and isoniazid concentration, F(8,104) = 4.370, Tukey's t-test, significant effect (P < 0.05) of 3 mM isoniazid at 100, 200 and 800 ms latencies, N = 6, Fig. 7C]. The time constant of recovery from STD was also significantly reduced by 3 mM isoniazid (one-way repeated-measures ANOVA, P = 0.026, Tukey's test, ACSF vs. 3 mM isoniazid, n = 6, P = 0.021, Fig. 7D), which is probably due to reduced depression 100 ms after the conditioning train in the slices from DR animals. Taken together, these experiments support the hypothesis that reduced GABA in DR SC is responsible for the faster than normal recovery from STD, and that maintenance of STD through visual experience depends on maintenance of presynaptic GABA.
How Does STD Affect RF Size?
The classical definition of a RF is the area of the visual field within which a stimulus causes a neuron to fire action potentials. STD affects excitability and action potential firing and thus necessarily affects measurements of RF size. V1 RFs are known to change size in reaction to visual stimulation (Pettet and Gilbert 1992) and contrast levels (Kapadia et al. 1999; Sceniak et al. 1999). Here we use a RF model to investigate how STD, which is maintained by visual experience, could contribute to the maintenance of refined RFs, and how DR, which reduces the magnitude and duration of train-induced STD, could contribute to enlarged RFs.
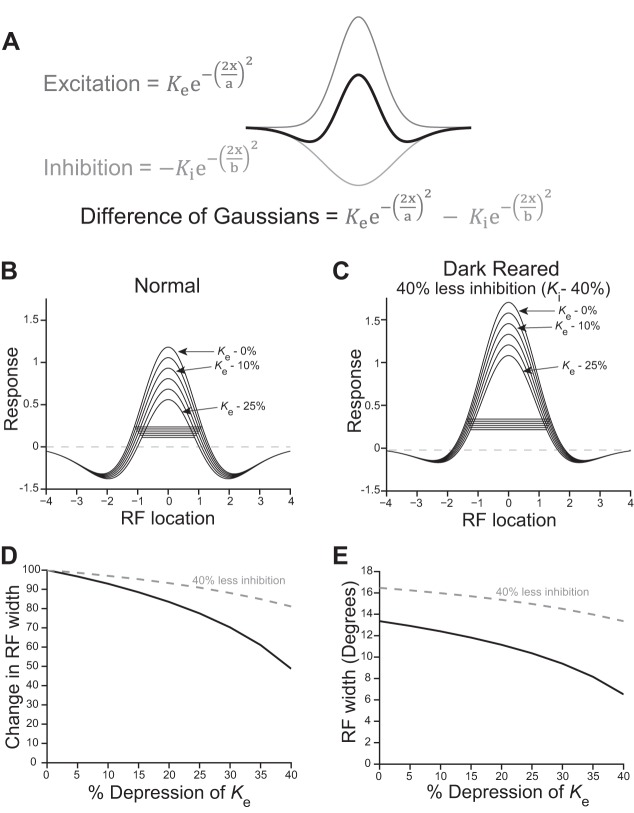
We showed increased PPD and decreased train-induced STD in slices from DR animals. The train stimulus more closely resembles the barrage of action potentials generated by natural vision than does a single stimulus pulse, thus we modeled the effect of reductions in train-induced STD rather than PPD on RF size. To investigate how STD affects RF size, we constructed a difference of Gaussians (DOG) model of RFs (Rodieck 1965). The DOG model results in a “Mexican hat” response function “R” by subtracting the inhibitory response Gaussian from the excitatory response Gaussian.
Ke and Ki are the peaks and a and b are the space constants of the excitatory and inhibitory Gaussians, respectively. The SC responds best to visual stimuli that are smaller than the excitatory RF due to strong within-RF inhibition. We based the normal RF Gaussians in our model on size-tuning data from Syrian hamster SC (Razak and Pallas 2006), optimizing Ke, Ki, a and b for best fit using the following equation, as previously described (Chen et al. 2013; DeAngelis et al. 1994; Sceniak et al. 1999).
As expected, inhibition is strong, more than half as strong as the peak of excitation (Ki = 0.52 × Ke), and the width of the inhibitory region of the RF is more than one and a half times that of the excitatory region (b = 1.66 × a). The DOG function using these values is plotted in Fig. 8A.
Fig. 8.
Reduced STD could contribute to enlarged receptive fields (RFs) in DR animals. A: difference of Gaussians (DOG) model of SC RFs. See text for definition of equation terms. B: different levels of depression were modeled as reductions in Ke of 0–25%, in 5% intervals. The horizontal lines are the RF widths at 20% of the peak, corresponding to the method used for measuring RF widths in vivo. C: lower levels of inhibition, as is seen in slices from DR animals, cause RFs to be larger. Ki was reduced by 40% to model the reduced inhibition caused by DR. The model supports the interpretation that reduced inhibition increases RF size. In addition, the RF size in DR cases was less affected by depression than in normal cases, as can be seen in D. D: change in RF width resulting from different levels of depression (RF widths from B, black line) and resulting from both depression and 40% reduced inhibition (data from C, gray dashed line). As the level of depression is increased, RF sizes shrink more. Reducing inhibition by 40% caused depression to have less of an effect on RF size (gray dashed line). E: plotting absolute RF widths shows that the reduced inhibition increases RF size at all levels of depression (gray dashed line is shifted upward). Both increased depression and reduced inhibition caused by DR may contribute to RF enlargement.
To model the effect of STD on RF size, we reduced the amplitude of the excitatory Gaussian Ke at intervals of 5% and plotted these DOGs (Fig. 8B). RF size was calculated as the width of these DOG at 20% of the peak (indicated by a horizontal line), ignoring responses below zero. The 20% level reflects how SC RFs were measured in vivo (Balmer and Pallas 2013; Carrasco et al. 2005). At the point when depression reduced the peak of the excitatory response Gaussian Ke by a total of 25%, the RF width was reduced to 78% of its initial, predepression size. When depression is reduced, as is the case in DR SC, RF width does not shrink to the same extent. For example, when depression was only 10%, the RF width remained at 93% of its initial size (Fig. 8B). This shows that, when depression is reduced, the RF shrinks less than it does at higher levels of depression.
It is clear that DR reduces inhibition in the SC, thus we modeled the reduced inhibition to test how it changes the effect of STD on RF size. We made a model of a DR RF by reducing the peak inhibitory response Ki by 40% before calculating the effect of different levels of depression of the excitatory Gaussian as above. Irrespective of depression, this reduction in the peak of the inhibitory Gaussian caused a large increase in initial RF size, because the excitatory response was less opposed by inhibition (Fig. 8C). This reduced inhibition in DR SC is likely to be a major contributor to the RF enlargement seen after DR (Carrasco et al. 2011). In the DR RF model, depression of the excitatory Gaussian caused the RF to shrink less than in the normal model: 25% depression reduces the DR RF width to 91% of its predepressed value (compared with 78% under conditions of more inhibition, as in Fig. 8B). Figure 8D plots the RF widths with different levels of depression. The RF width shrinks more when depression is higher. The black line represents RF widths taken from Fig. 8B. In addition, when Ki is reduced, as would be the case in DR SC (Fig. 8C), depression has less of an effect on the RF width (gray dashed line, RF widths taken from Fig. 8C). Figure 8E plots absolute RF size. Although reduced STD contributes to enlarged RFs, the decrease in inhibition (plotted as the gray dashed line) also has a marked effect on initial RF size (indicated by an upward shift in the gray dashed line) (Fig. 8E).
In summary, this analysis supports the idea that the reduced STD observed in slices from DR animals could underlie increased RF size measured in vivo. The combination of reduced inhibition and reduced STD in SC that occurs after DR combine to prevent RF size from shrinking during the initial moments of visual stimulation.
DISCUSSION
The goal of this study was to investigate the mechanisms of critical period closure in SC. Importantly, we showed that the mechanisms regulating plasticity might differ across different sensory pathways. We showed that in hamster SC, visual experience protects against deprivation-induced changes in short-term plasticity, but, unlike in V1, deprivation does not maintain long-term plasticity. Our data support a mechanism through which visual experience-dependent maintenance of GABAAR function prevents plasticity of GABABR-mediated STD. DR increased PPD, likely by releasing inhibitory neurons from inhibition due to reduced GABAAR function, thus increasing evoked GABA release onto GABABRs of local SC neurons (Fig. 5, A and B). In addition, stimulation with a high-frequency train of current pulses, more closely approximating natural vision than stimulation with single pulses, showed that the strength and duration of STD is reduced in slices from DR animals (Fig. 6). Thus visual experience-dependent maintenance of GABA content is essential to maintain maximal levels of visual stimulus-evoked STD in normally-reared animals. Our computational RF model shows that reduced STD during visual stimulation, which would maintain excitatory responses at a higher level, could explain the increased RF size in SC after DR.
DR Does Not Extend Long-Term Plasticity in SC
In V1, DR reduces inhibition, prolonging the age at which long-term synaptic plasticity occurs (Kirkwood and Bear 1995; Kirkwood et al. 1995). DR causes reduced inhibition in SC as well and could allow long-term plasticity and contribute to RF enlargement by strengthening synapses on the outskirts of the excitatory RF. We were unable to induce long-term plasticity in SC slices from DR animals at the time of RF enlargement (P60-70) or once RFs were at their maximally enlarged state >P90, however. Thus, unlike in V1, in SC DR did not maintain the ability to induce long-term plasticity in acute slices, indicating that changes in the ability to induce LTP are unlikely to underlie DR-induced RF enlargement in SC.
This experiment provides evidence that visual experience affects the retinocollicular and retinogeniculocortical pathways in different ways. This difference provides an opportunity to study differing mechanisms of development and plasticity in pathways that receive input from the same cells. Studies comparing plasticity in SC and V1 could elucidate differences in how experience affects midbrain and cortical visual circuits and identify the developmental processes underlying their distinct mechanisms.
Visual Experience-Dependent Maintenance of GABAergic Inhibition Maintains GABABR Activation
Reduced GABAAR function that occurs in DR SC may lead to disinhibition of inhibitory interneurons that would enhance GABA release during a single stimulus. The disinhibited GABA release could cause more GABABR-mediated inhibition and thus increased PPD in DR animals. Intense stimulation, such as occurs during vision, leads to GABA depletion. This depletion likely occurs more rapidly in DR SC because of the reduced GABA content in these interneurons (Carrasco et al. 2011). This loss of inhibition and STD could contribute to RF enlargement in DR SC. Increased GABA release and GABA depletion could affect extrasynaptic receptors and other synapses in the circuit that are important for visual processing, although this possibility was not examined in this study.
GABABRs Underlie STD in SC and Are Not Affected by Visual Experience
SC expresses GABABRs at an extremely high level relative to other areas of the brain (Bowery et al. 1984) at both pre- and postsynaptic sites (Endo and Isa 2002). We report here that GABABRs almost completely underlie STD in SC, under both normal and deprived conditions. We showed that PPD increases as interpulse interval increases. This indicates that depression of PP2 depends on a delayed mechanism that lasts over 200 ms, such as metabotropic receptor signaling, rather than vesicle depletion. GABABRs fit this pattern (Chalifoux and Carter 2011), whether operating through pre- or postsynaptic inhibition (Endo and Isa 2002).
GABA levels and effectiveness of GABAARs in SC are reduced by DR (Carrasco et al. 2011), but little is known regarding the activity-dependent regulation of GABABR expression in SC. We did not find differences in sensitivity to GABABR agents between slices from normal and DR animals. GABABR expression is reduced by TTX blockade of retinal signaling in monkey lateral geniculate nucleus (LGN) (Muñoz et al. 1998) and V1 (Muñoz et al. 2001), but TTX treatment is not directly comparable to DR, because it blocks both spontaneous and light-evoked activity. If DR causes reduced GABABR function in V1 but not in SC, it could explain differences in mechanisms of experience-dependent plasticity between cortical and midbrain visual areas. Comparing visual experience-dependent regulation of GABABRs between SC and V1 is necessary to test this prediction.
Reduced STD Caused by High-Frequency Stimulation Could Contribute to RF Enlargement in DR SC
The finding that high-frequency train stimulation evokes less STD in slices from DR animals than in slices from normal animals led us to test how STD could affect RF size in a DOG RF model. In short, reduced STD in DR SC will yield higher than normal excitability during visual stimulation. Higher excitability that is not offset by higher inhibition would increase RF size. The DOG model was first applied to retina (Enroth-Cugell and Robson 1966; Rodieck 1965), but has also been used for modeling RFs in V1 (DeAngelis et al. 1994; Sceniak et al. 1999), frontal eye fields (Cavanaugh et al. 2012), and LGN (Cai et al. 1997; Dawis et al. 1984) and is a good first approximation to understanding center-surround RFs in general, with the important caveat that inhibition is stronger within the RF in SC than in V1, LGN, or RGCs. Our RF model predicts how RF size could be affected by visual stimulus properties and suggests that high STD contributes to small RFs (normal), and low STD contributes to larger RFs (DR). Experiments measuring the effects of DR on depression of excitatory and inhibitory currents are necessary to add detail to this model.
In an in vivo study in rat SC, iontophoresis of GABABR agents did not affect the size of RFs measured by varying stimulus sizes (Binns and Salt 1997), which initially appears to be at odds with our hypothesis. Iontophoresis likely affects receptors on the soma of the recorded cell and to a lesser extent its processes and nearby cells, whereas our bath-applied drugs may have affected somata and processes more uniformly. It is possible that for the drugs to affect STD, they need to affect presynaptic receptors on the retinocollicular afferents that make contact with the dendrites of the SC neurons, which may be too distal to be affected by iontophoresis.
Experience-Dependent Changes in STD Vary Between Synapses and Sensory Areas
Sensory experience is necessary to decrease the STD of inhibition (iSTD) that is present during development at the layer 4 to 2/3 synapse in V1 (Jiang et al. 2005, 2010) and auditory cortex (Takesian et al. 2010). Thus sensory experience causes inhibition to maintain its strength during stimulus trains. Sensory deprivation causes inhibition to depress more (higher iSTD) than it would under normal rearing conditions during repeated stimulation. Increased iSTD in DR V1 may be due to the DR-induced reduction of GABAergic inhibition (Bakkum et al. 1991; Benevento et al. 1995; Gabbott and Stewart 1987; Morales et al. 2002). As in DR V1, iSTD is increased in GAD65 knockout mice (Choi et al. 2002), indicating that the strength of inhibition weakens during stimulus trains under conditions of reduced GABA. This weakening of inhibition during stimulus trains may cause the reduced STD of fEPSPs that occurs in these mice (Fagiolini and Hensch 2000). If, as reported in V1 and in auditory cortex, inhibition is maintained during train stimulation (low iSTD) in normal SC and inhibition weakens during train stimulation (higher iSTD) in DR SC, then this change in iSTD could mediate the reduced STD of fEPSPs in DR SC. Investigation of iSTD at a single neuron level in SC is necessary to test these predictions.
Dysregulation of Inhibition and Short-Term Plasticity in Psychiatric Disorders
Dysregulation of inhibition can lead to altered short-term plasticity, which, depending on the synapse, can adversely affect sensory and cognitive processing (Abbott and Regehr 2004). Short-term plasticity is affected by environmental factors, such as brain injury (Li et al. 2005), early life seizures (Hernan et al. 2013), and hearing loss (Takesian et al. 2010). Preventing dysregulation of inhibition from occurring, or intervening with therapies that affect inhibitory plasticity, may ameliorate disrupted short-term plasticity and provide a therapeutic approach to some aspects of pervasive psychiatric disorders (Arguello and Gogos 2012).
GRANTS
This work was supported by grants from the National Science Foundation, the National Institutes of Health, and the Georgia State University Research Foundation to S. L. Pallas; and the Brains and Behavior Fellowship, the Honeycutt Fellowship, and two Sigma Xi Grants-in-Aid-of-Research to T. S. Balmer.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.S.B. and S.L.P. conception and design of research; T.S.B. performed experiments; T.S.B. analyzed data; T.S.B. and S.L.P. interpreted results of experiments; T.S.B. prepared figures; T.S.B. drafted manuscript; T.S.B. and S.L.P. edited and revised manuscript; T.S.B. and S.L.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Profs. Peter Wenner, Paul Katz, and Vincent Rehder for providing comments on the manuscript and advice on experimental design, members of the Donald Rainnie Laboratory for technical assistance, members of the Pallas Laboratory for technical assistance and comments on the manuscript, the Department of Animal Resources staff at Georgia State University for animal care, and Bryce Chung for assistance with the computational model.
Present address of T. S. Balmer: Vollum Institute, Oregon Health & Science Univ., Portland, OR.
REFERENCES
- Abbott LF, Regehr W. Synaptic computation. Nature 431: 796–803, 2004. [DOI] [PubMed] [Google Scholar]
- Alger BE, Nicoll RA. Pharmacological evidence for two kinds of GABA receptor on rat hippocampal pyramidal cells studied in vitro. J Physiol 328: 125–141, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arguello PA, Gogos J. Genetic and cognitive windows into circuit mechanisms of psychiatric disease. Trends Neurosci 35: 3–13, 2012. [DOI] [PubMed] [Google Scholar]
- Artola A, Bröcher S, Singer W. Different voltage-dependent thresholds for inducing long-term depression and long-term potentiation in slices of rat visual cortex. Nature 347: 69–72, 1990. [DOI] [PubMed] [Google Scholar]
- Bakkum BW, Benevento LA, Cohen RS. Effects of light/dark- and dark-rearing on synaptic morphology in the superior colliculus and visual cortex of the postnatal and adult rat. J Neurosci Res 28: 65–80, 1991. [DOI] [PubMed] [Google Scholar]
- Balmer TS, Pallas SL. Refinement but not maintenance of visual receptive fields is independent of visual experience. Cereb Cortex doi: 10.1093/cercor/bht281, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benardo LS. Separate activation of fast and slow inhibitory postsynaptic potentials in rat neocortex in vitro. J Physiol 476: 203–215, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benevento LA, Bakkum BW, Cohen RS. Gamma-aminobutyric acid and somatostatin immunoreactivity in the visual cortex of normal and dark-reared rats. Brain Res 689: 172–182, 1995. [DOI] [PubMed] [Google Scholar]
- Binns KE, Salt TE. Different roles for GABAA and GABAB receptors in visual processing in the rat superior colliculus. J Physiol 504: 629–639, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery NG, Hudson AL, Price GW. GABAA and GABAB receptor site distribution in the rat central nervous system. Neuroscience 20: 365–383, 1987. [DOI] [PubMed] [Google Scholar]
- Bowery NG, Price GW, Hudson AL, Hill DR, Wilkin GP, Turnbull MJ. GABA receptor multiplicity. Visualization of different receptor types in the mammalian CNS. Neuropharmacology 23: 219–231, 1984. [DOI] [PubMed] [Google Scholar]
- Cai D, DeAngelis GC, Freeman RD. Spatiotemporal receptive field organization in the lateral geniculate nucleus of cats and kittens. J Neurophysiol 78: 1045–1061, 1997. [DOI] [PubMed] [Google Scholar]
- Carrasco MM, Mao YT, Balmer TS, Pallas SL. Inhibitory plasticity underlies visual deprivation-induced loss of receptive field refinement in the adult superior colliculus. Eur J Neurosci 33: 58–68, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco MM, Pallas SL. Early visual experience prevents but cannot reverse deprivation-induced loss of refinement in adult superior colliculus. Vis Neurosci 23: 845–852, 2006. [DOI] [PubMed] [Google Scholar]
- Carrasco MM, Razak KA, Pallas SL. Visual experience is necessary for maintenance but not development of receptive fields in superior colliculus. J Neurophysiol 94: 1962–1970, 2005. [DOI] [PubMed] [Google Scholar]
- Carta M, Murru L, Barabino E, Talani G, Sanna E, Biggio G. Isoniazid-induced reduction in GABAergic neurotransmission alters the function of the cerebellar cortical circuit. Neuroscience 154: 710–719, 2008. [DOI] [PubMed] [Google Scholar]
- Caruso DM, Owczarzak MT, Goebel DJ, Hazlett JC, Pourcho RG. GABA-immunoreactivity in ganglion cells of the rat retina. Brain Res 476: 129–134, 1989. [DOI] [PubMed] [Google Scholar]
- Cavanaugh J, Joiner WM, Wurtz RH. Suppressive surrounds of receptive fields in monkey frontal eye field. J Neurosci 32: 12284–12293, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalifoux JR, Carter AG. GABAB receptor modulation of synaptic function. Curr Opin Neurobiol 21: 339–344, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalupa LM, Rhoades RW. Responses of visual, somatosensory, and auditory neurones in the golden hamster's superior colliculus. J Physiol 270: 595–626, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Song XM, Li CY. Contrast-dependent variations in the excitatory classical receptive field and suppressive nonclassical receptive field of cat primary visual cortex. Cereb Cortex 23: 283–292, 2013. [DOI] [PubMed] [Google Scholar]
- Choi SY, Morales B, Lee HK, Kirkwood A. Absence of long-term depression in the visual cortex of glutamic acid decarboxylase-65 knock-out mice. J Neurosci 22: 5271–5276, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirone J, Salt TE. Group II and III metabotropic glutamate receptors contribute to different aspects of visual response processing in the rat superior colliculus. J Physiol 534: 169–178, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa BL, Hokoc JN, Pinaud RR, Gattass R. GABAergic retinocollicular projection in the New World monkey Cebus apella. Neuroreport 8: 1797–1802, 1997. [DOI] [PubMed] [Google Scholar]
- Dawis S, Shapley R, Kaplan E, Tranchina D. The receptive field organization of X-cells in the cat: spatiotemporal coupling and asymmetry. Vision Res 24: 549–564, 1984. [DOI] [PubMed] [Google Scholar]
- De Koninck Y, Mody I. Endogenous GABA activates small-conductance K+ channels underlying slow IPSCs in rat hippocampal neurons. J Neurophysiol 77: 2202–2208, 1997. [DOI] [PubMed] [Google Scholar]
- DeAngelis GC, Freeman RD, Ohzawa I. Length and width tuning of neurons in the cat's primary visual cortex. J Neurophysiol 71: 347–374, 1994. [DOI] [PubMed] [Google Scholar]
- Endo T, Isa T. Postsynaptic and presynaptic GABA(B) receptor-mediated inhibition in rat superficial superior colliculus neurons. Neurosci Lett 322: 126–130, 2002. [DOI] [PubMed] [Google Scholar]
- Enroth-Cugell C, Robson JG. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol 187: 517–552, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagiolini M, Hensch TK. Inhibitory threshold for critical-period activation in primary visual cortex. Nature 404: 183–186, 2000. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Stewart MG. Quantitative morphological effects of dark-rearing and light exposure on the synaptic connectivity of layer 4 in the rat visual cortex (area 17). Exp Brain Res 68: 103–114, 1987. [DOI] [PubMed] [Google Scholar]
- Hernan AE, Holmes GL, Isaev D, Scott RC, Isaeva E. Altered short-term plasticity in the prefrontal cortex after early life seizures. Neurobiol Dis 50: 120–126, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhman KL, Jasnow AM, Sisitsky AK, Albers HE. Glutamic acid decarboxylase mRNA in the suprachiasmatic nucleus of rats housed in constant darkness. Brain Res 851: 266–269, 1999. [DOI] [PubMed] [Google Scholar]
- Jiang B, Huang S, de Pasquale R, Millman D, Song L, Lee HK, Tsumoto T, Kirkwood A. The maturation of GABAergic transmission in visual cortex requires endocannabinoid-mediated LTD of inhibitory inputs during a critical period. Neuron 66: 248–259, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Huang ZJ, Morales B, Kirkwood A. Maturation of GABAergic transmission and the timing of plasticity in visual cortex. Brain Res Rev 50: 126–133, 2005. [DOI] [PubMed] [Google Scholar]
- Kaneda K, Phongphanphanee P, Katoh T, Isa K, Yanagawa Y, Obata K, Isa T. Regulation of burst activity through presynaptic and postsynaptic GABA(B) receptors in mouse superior colliculus. J Neurosci 28: 816–827, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapadia MK, Westheimer G, Gilbert CD. Dynamics of spatial summation in primary visual cortex of alert monkeys. Proc Natl Acad Sci U S A 96: 12073–12078, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Alger BE. Random response fluctuations lead to spurious paired-pulse facilitation. J Neurosci 21: 9608–9618, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood A, Bear MF. Elementary forms of synaptic plasticity in the visual cortex. Biol Res 28: 73–80, 1995. [PubMed] [Google Scholar]
- Kirkwood A, Lee HK, Bear MF. Co-regulation of long-term potentiation and experience-dependent synaptic plasticity in visual cortex by age and experience. Nature 375: 328–331, 1995. [DOI] [PubMed] [Google Scholar]
- Lambert NA, Wilson WA. Temporally distinct mechanisms of use-dependent depression at inhibitory synapses in the rat hippocampus in vitro. J Neurophysiol 72: 121–130, 1994. [DOI] [PubMed] [Google Scholar]
- Li H, Bandrowski AE, Prince DA. Cortical injury affects short-term plasticity of evoked excitatory synaptic currents. J Neurophysiol 93: 146–156, 2005. [DOI] [PubMed] [Google Scholar]
- Lo FS, Mize RR. Properties of LTD and LTP of retinocollicular synaptic transmission in the developing rat superior colliculus. Eur J Neurosci 15: 1421–1432, 2002. [DOI] [PubMed] [Google Scholar]
- Lund RD, Lund JS. Modifications of synaptic patterns in the superior colliculus of the rat during development and following deafferentation. Vision Res Suppl 3: 281–298, 1971. [DOI] [PubMed] [Google Scholar]
- Maffei A, Bucher D, Fontanini A. Homeostatic plasticity in the nervous system. Neural Plast 2012: 913472, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mize RR. Immunocytochemical localization of gamma-aminobutyric acid (GABA) in the cat superior colliculus. J Comp Neurol 276: 169–187, 1988. [DOI] [PubMed] [Google Scholar]
- Mize RR. The organization of GABAergic neurons in the mammalian superior colliculus. Prog Brain Res 90: 219–248, 1992. [DOI] [PubMed] [Google Scholar]
- Mize RR, Butler GD. Postembedding immunocytochemistry demonstrates directly that both retinal and cortical terminals in the cat superior colliculus are glutamate immunoreactive. J Comp Neurol 371: 633–648, 1996. [DOI] [PubMed] [Google Scholar]
- Morales B, Choi SY, Kirkwood A. Dark rearing alters the development of GABAergic transmission in visual cortex. J Neurosci 22: 8084–8090, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz A, DeFelipe J, Jones EG. Patterns of GABABR1a,b receptor gene expression in monkey and human visual cortex. Cereb Cortex 11: 104–113, 2001. [DOI] [PubMed] [Google Scholar]
- Muñoz A, Huntsman MM, Jones EG. GABA(B) receptor gene expression in monkey thalamus. J Comp Neurol 394: 118–126, 1998. [DOI] [PubMed] [Google Scholar]
- Okada Y, Miyamoto T. Formation of long-term potentiation in superior colliculus slices from the guinea pig. Neurosci Lett 96: 108–113, 1989. [DOI] [PubMed] [Google Scholar]
- Pasternack M, Boller M, Pau B, Schmidt M. GABA(A) and GABA(C) receptors have contrasting effects on excitability in superior colliculus. J Neurophysiol 82: 2020–2023, 1999. [DOI] [PubMed] [Google Scholar]
- Pettet MW, Gilbert CD. Dynamic changes in receptive-field size in cat primary visual cortex. Proc Natl Acad Sci U S A 89: 8366–8370, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt B, Withington DJ. GABA-induced long-term potentiation in the guinea-pig superior colliculus. Neuropharmacology 37: 1111–1122, 1998. [DOI] [PubMed] [Google Scholar]
- Platt B, Withington DJ. Paired-pulse depression in the superficial layers of the guinea-pig superior colliculus. Brain Res 777: 131–139, 1997. [DOI] [PubMed] [Google Scholar]
- Razak KA, Fuzessery ZM, Pallas SL. Developmental plasticity of inhibitory receptive field properties in the auditory and visual systems. In: Developmental Plasticity of Inhibitory Circuitry, edited by Pallas SL. New York: Springer, 2010, p. 71–89. [Google Scholar]
- Razak KA, Pallas SL. Dark rearing reveals the mechanism underlying stimulus size tuning of superior colliculus neurons. Vis Neurosci 23: 741–748, 2006. [DOI] [PubMed] [Google Scholar]
- Razak KA, Pallas SL. Neural mechanisms of stimulus velocity tuning in the superior colliculus. J Neurophysiol 94: 3573–3589, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades RW, Figley B, Mooney RD, Fish SE. Development of the occipital corticotectal projection in the hamster. Exp Brain Res 86: 373–383, 1991. [DOI] [PubMed] [Google Scholar]
- Rodieck RW. Quantitative analysis of cat retinal ganglion cell response to visual stimuli. Vision Res 5: 583–601, 1965. [DOI] [PubMed] [Google Scholar]
- Sarnaik R, Wang BS, Cang J. Experience-dependent and independent binocular correspondence of receptive field subregions in mouse visual cortex. Cereb Cortex 24: 1658–1670, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sceniak MP, Ringach DL, Hawken MJ, Shapley R. Contrast's effect on spatial summation by macaque V1 neurons. Nat Neurosci 2: 733–739, 1999. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Boller M, Ozen G, Hall WC. Disinhibition in rat superior colliculus mediated by GABAC receptors. J Neurosci 21: 691–699, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider GE. Two visual systems. Science 163: 895–902, 1969. [DOI] [PubMed] [Google Scholar]
- Takesian AE, Kotak VC, Sanes DH. Presynaptic GABA(B) receptors regulate experience-dependent development of inhibitory short-term plasticity. J Neurosci 30: 2716–2727, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano G. Too many cooks? Intrinsic and synaptic homeostatic mechanisms in cortical circuit refinement. Annu Rev Neurosci 34: 89–103, 2011. [DOI] [PubMed] [Google Scholar]
- Wang L, Sarnaik R, Rangarajan K, Liu X, Cang J. Visual receptive field properties of neurons in the superficial superior colliculus of the mouse. J Neurosci 30: 16573–16584, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenner P. Mechanisms of GABAergic homeostatic plasticity. Neural Plast 2011: 489470, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JP, Phillips MA, Constantine-Paton M. Long-term potentiation in the juvenile superior colliculus requires simultaneous activation of NMDA receptors and L-type Ca2+ channels and reflects addition of newly functional synapses. J Neurosci 26: 12647–12655, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]