Abstract
The massive computational capacity of the cerebellar cortex is conveyed by Purkinje cells onto cerebellar and vestibular nuclei neurons through their GABAergic, inhibitory output. This implies that pauses in Purkinje cell simple spike activity are potentially instrumental in cerebellar information processing, but their occurrence and extent are still heavily debated. The cerebellar cortex, although often treated as such, is not homogeneous. Cerebellar modules with distinct anatomical connectivity and gene expression have been described, and Purkinje cells in these modules also differ in firing rate of simple and complex spikes. In this study we systematically correlate, in awake mice, the pausing in simple spike activity of Purkinje cells recorded throughout the entire cerebellum, with their location in terms of lobule, transverse zone, and zebrin-identified cerebellar module. A subset of Purkinje cells displayed long (>500-ms) pauses, but we found that their occurrence correlated with tissue damage and lower temperature. In contrast to long pauses, short pauses (<500 ms) and the shape of the interspike interval (ISI) distributions can differ between Purkinje cells of different lobules and cerebellar modules. In fact, the ISI distributions can differ both between and within populations of Purkinje cells with the same zebrin identity, and these differences are at least in part caused by differential synaptic inputs. Our results suggest that long pauses are rare but that there are differences related to shorter intersimple spike intervals between and within specific subsets of Purkinje cells, indicating a potential further segregation in the activity of cerebellar Purkinje cells.
Keywords: Purkinje cell, cerebellar modules, simple spikes, pausing, zebrin
as one of the largest integrators in the brain, the cerebellar Purkinje cell (PC) is contacted by tens of thousands of, predominantly excitatory, synaptic inputs that it, combined with intrinsic pacemaker activity, converges into the sole output of the cerebellar cortex. Hence, and in contrast to neurons in the cerebral cortex, PCs in the cerebellar cortex commonly operate in the upstate and have a high firing rate, even at rest (Crunelli and Hughes 2009; McCormick 2005; Thach 1968; Zhou et al. 2014). In addition to their alternative preferred state of membrane potential, PCs also have an alternative output, in that they inhibit their target neurons in the cerebellar nuclei. How PCs use their inhibitory control to convey their information onto the downstream targets remains enigmatic, even to date. Despite several lines of evidence identifying possible methods of information transfer, including those based on rate coding, spatiotemporal coding, or a combination of both (De Zeeuw et al. 2011; Jirenhed et al. 2007; Medina and Lisberger 2009; Person and Raman 2011; Shin et al. 2007), there is to date no consensus regarding the patterns of activity required for proper behavior or learning.
The occurrence of two different states of membrane potential was already observed in the first intracellular recordings of cerebellar PCs (Llinas and Sugimori 1980a and 1980b). Subsequent studies have identified several factors that modulate the switch between the two states, including molecular layer interneurons (Oldfield et al. 2010), Bergmann glia cells (Wang et al. 2012), climbing fibers (Libster and Yarom 2013), and the hyperpolarization-activated cation current (Williams et al. 2002). In vivo, in anesthetized mammals, the in vitro findings were confirmed, in that a significant portion of PCs switch between upstates with a high simple spike (SS) firing rate and downstates with seconds long silence of SS (Loewenstein et al. 2005), and later also replicated in awake cats and mice during chronic recordings (Cheron et al. 2014; Yartsev et al. 2009). Long pauses were suggested to be functionally relevant, since whisker stimulation-induced complex spikes can toggle transitions from up- to downstates and vice versa (Loewenstein et al. 2005). These conclusions were challenged by other studies, which showed that long SS pauses are rare in awake behaving mice (Cao et al. 2012; Schonewille et al. 2006) and that the probability is significantly increased by anesthesia (Schonewille et al. 2006). However, the cerebellum is not a homogeneous structure; differences in gene expression, anatomical connectivity, and physiological properties have been identified. Parasagittal stripes, or bands, of cerebellar cortex show alternating levels of gene expression for particular genes, most notable of which is aldolase C, typically referred to as zebrin II (Ahn et al. 1994; Brochu et al. 1990). PCs that have a similar zebrin “identity,” i.e., have a similar expression level of zebrin II (from here on: zebrin), typically receive inputs from the same part of the inferior olive, and project their axons to the same part of the cerebellar nuclei (Apps and Hawkes 2009; Brochu et al. 1990; Pijpers et al. 2006; Sugihara 2011; Sugihara and Quy 2007; Voogd and Ruigrok 2004), creating functional units, or modules. More recently physiological differences between modules have been described in terms of plasticity (Wadiche and Jahr 2005; Wang et al. 2011) and firing dynamics and rates (Kim et al. 2012; Xiao et al. 2014; Zhou et al. 2014). In addition, the cerebellar cortex can also be subdivided in zones on the basis of development and function by borders that run perpendicular to the sagittal bands, i.e., into transverse zones (Ozol et al. 1999; Reeber et al. 2013). Together these subdivisions raise a question that could answer previous discrepancies: How are PCs with longer or shorter SS pauses distributed over the cerebellum?
Here, we show that a fraction of cerebellar PCs, recorded in vivo in awake mice at rest, displays varying degrees of long (>500-ms) SS-pausing behavior. We identify nearby tissue damage and local temperature as two important factors affecting PC SS activity. Moreover, whereas longer pauses do not correlate with cerebellar location, there are difference in the occurrence of shorter pauses (<500 ms) and the shape of their interspike interval distributions between zebrin-identified cerebellar modules and transverse zones. These differences can be ablated by blocking input, suggesting that, whereas the firing rate is largely determined by intrinsic differences, the differential input shapes the dynamic range of PC activity, creating differences between and within the same functional units.
METHODS
All experiments involving animals were approved by the Dutch Ethical Committee for animal experiments. A large part of the dataset of PC recordings used for this study has also been used in a previous study (Zhou et al. 2014), and consequently the methods sections largely overlap.
In vivo extracellular recordings.
As recently described (Zhou et al. 2014), PCs were recorded in vivo in adult male C57Bl/6 mice (10–35 wk old; Charles River, Wilmington, MA). An immobilizing pedestal was constructed on the skull overlying the frontal and parietal bones to enable fixation of the mouse head, and a craniotomy was made in the interparietal or occipital bone to allow the entrance of the recording pipettes. The locations of the craniotomy are variable, as illustrated in Fig. 1B, allowing recordings in different cerebellar regions. After recovery of at least 24 h, mice were ready for in vivo recordings. The head of the mouse was fixed to a metal bar, and the body was supported by a plastic tube. Electrophysiological activity of PCs was recorded using double-barrel borosilicate glass pipettes (theta septum, 1.5 OD, 1.02 ID; WPI) (see Fig. 1A for details). To do so, one of the barrels was opened laterally to place the recording wire and sealed at the back with glass glue; the other barrel was filled with Alcian Blue to mark the recording sites (0.1–0.2% solution in saline). The recording signals were preamplified (custom-made), filtered (CyberAmp; Axon), digitized (Power1401; CED), and stored for offline analysis. Recording duration was on average 220 ± 9 s, and at least 120 s for all recordings (except when combined with 2-photon imaging, see below). To mark the recording location, brief air pulses were applied to the barrel filled with Alcian Blue after every recording. The temperature for different recording depths was measured by a BAT-12 Microprobe Thermometer (Physitemp Instruments); the tip size of the probe was 200 μm.
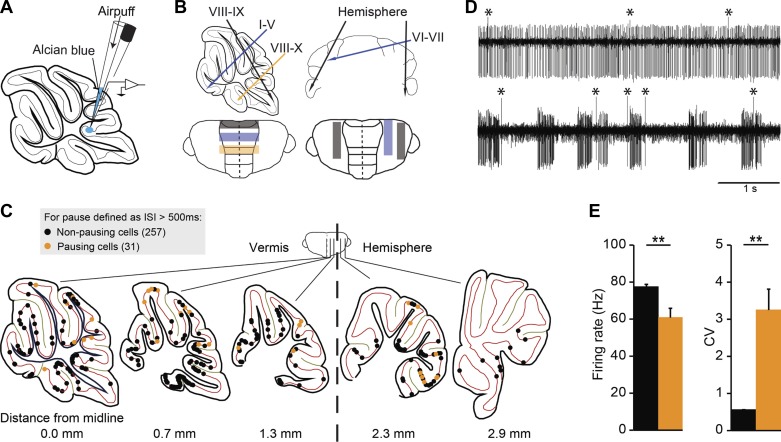
Fig. 1.
Distribution of all recorded Purkinje cells (PCs) in relation to the occurrence of long simple spike (SS) pauses. A: we performed extracellular recordings of PC activity from awake C57Bl/6 mice at rest. After each recording, the recording site was marked by a small injection of Alcian Blue. B: the location of the craniotomy was varied to be able to obtain PCs from different parts of the cerebellum at different recording depths. C: after histological processing or imaging, the location of 288 cells was successfully determined. The locations of all recording sites were projected on the mediolaterally nearest of five sagittal sections [schematic drawings were adapted from Franklin and Paxinos atlas (Paxinos and Franklin 2001) with permission]. PCs were subdivided based on the presence of a “long” pause in the recording, i.e., the occurrence of at least one interspike interval (ISI) with a duration of 500 ms (cells with long pause, orange, n = 31; without, black, n = 257). Note that the recordings obtained in combination with 2-photon imaging (n = 17) are located in the superficial layer of lobules IV-V, VI, and Crus I. D: example traces of PC recordings without (top) and with (bottom) long pauses; complex spikes are marked by asterisks (*). E: PCs with long pauses on average have a lower SS firing rate and higher CV. Error bars represent SE. **P < 0.01.
Analysis of in vivo recordings.
Analysis was performed using SpikeTrain (Neurasmus, www.neurasmus.com) for Matlab (Mathworks). The single-unit nature of the recording was confirmed by the occurrence of a pause in SS activity following each complex spike, termed the climbing fiber pause. First, the average firing rate, CV (standard deviation divided by the mean per cell), and mean interspike interval (ISI) were determined for SS. Next, we examined various features of SS activity that are independent of the mean ISI, or firing rate, as much as possible. To do so, we first determined the occurrence of pauses, for which there is currently no generally accepted definition (Cao et al. 2012; Schonewille et al. 2006; Shin et al. 2007; Yartsev et al. 2009). Shin and De Schutter (2006) developed formal criteria to identify pauses, based on the climbing fiber pause, to study the correlation between pairs of PCs. This elegant method is less suitable in our study, for instance, because the resulting definition of a pause (12 ms in their study) is between the mean ISI of zebrin-positive (Z+) and zebrin-negative (Z−) PCs (see Fig. 5D), and thus would automatically result in more pauses in Z+ PCs. In an attempt to avoid this bias, we considered ISIs pauses when they clearly deviated from the mean. We considered pauses “long” when the ISI exceeded 500 ms in duration, based on previous descriptions of pausing PCs (Loewenstein et al. 2005; Yartsev et al. 2009). Pauses were considered short if the interval between SS clearly deviated from the average or “normal” ISIs. To determine a standard value we calculated, based on the entire dataset, the mean ISI plus three standard deviations, which gave 14.8 + (3 × 11.9) = 50.8 ms, rounded to 50 ms. To evaluate if differences between groups are dependent on the pause length that was chosen, we tested for pauses longer than an ISI duration of 50, 100, 200, 500, 1,000, and 2,000 ms, termed “pause length” in Figs. 1–7. Cells were labeled “pausing cell” if at least one interval of the indicated pause length was present in the recorded period. “Pause time” refers to the cumulative time of all pauses longer than indicated pause length, divided by the total recording length.
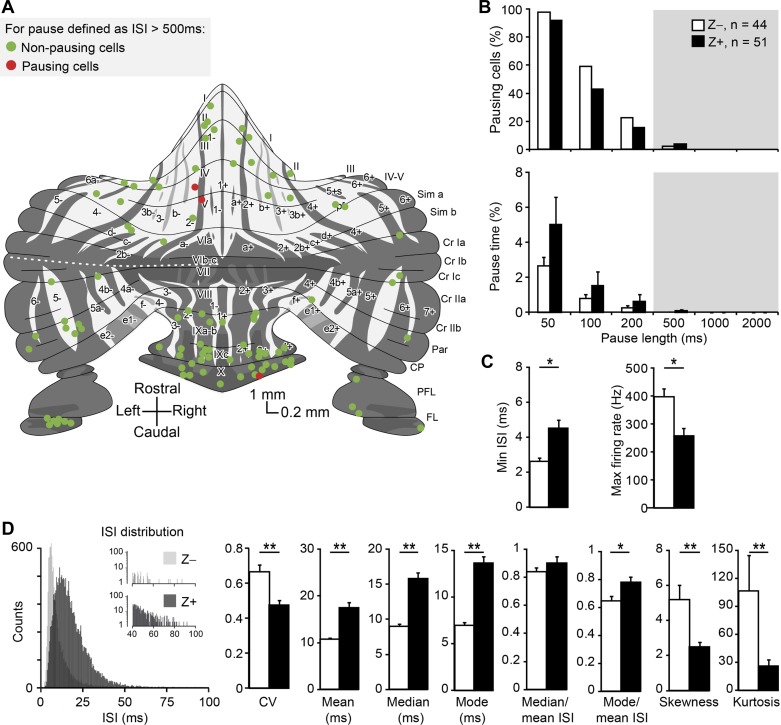
Fig. 5.
SS distribution shape and short pauses correlate with functional modules. A: distribution of PCs of which the zebrin identity and location were determined, throughout the unfolded cerebellum. Note that only 3 out of 95 cells have SS pauses longer than 500 ms. Schematic drawing adapted from Sugihara and Quy (2007) and Zhou et al. (2014). B: the percentages of pausing cells and pause time are not different between Z+ and Z− modules (Z−: n = 44, Z+: n = 51 cells; all P > 0.1, Mann-Whitney U-test). C: Z− cells have shorter minimum ISI and higher maximum firing rate than Z+ cells. D: left, examples of ISI distributions for a Z+ (dark gray) and Z− (light gray) PC. Right, detailed analysis of short SS pauses (<500 ms) based on these ISI distributions revealed differences in most ISI characteristics between Z− and Z+ modules. The differences in median and mode of ISIs are larger than that in mean, suggesting a difference in shape of the ISI distributions. This was confirmed as skewness and kurtosis; the 3rd and the 4th moment describing distributions and measures for asymmetry and “peakedness” that are independent of the mean, respectively, differed between modules. Both kurtosis and skewness are higher in Z− PCs, indicating their ISI distributions have a more preferred ISI combined a larger tail to the right, i.e., more short pauses (50–500 ms). No. of cells is indicated in parentheses. Note that the no. of ISIs in the example distributions is higher for the Z+ PC. Error bars represent SE. *P < 0.05 and **P < 0.01.
Fig. 7.
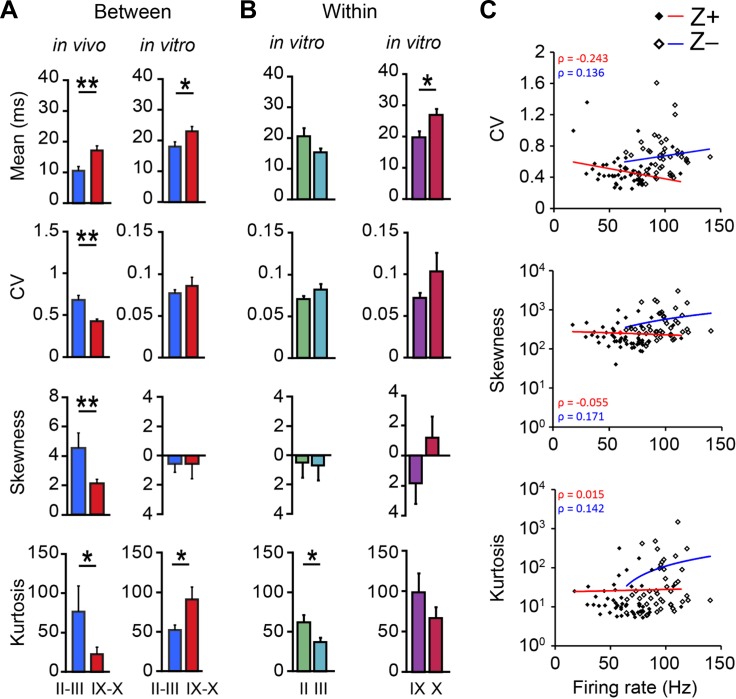
Differences in the shape of ISI distributions are absent in vitro. A: to test if the differences in the shape of the ISI distributions are intrinsic to PCs or depend on the input PCs receive, we compared all ISI features of the Z− PCs of lobules II and III with the Z+ PCs of lobules IX and X, recorded in vivo and in vitro (in vivo: n = 16 vs. 35 and in vitro: n = 27 vs. 33 for Z− and Z+ PCs, respectively). In vitro recordings were performed in the presence of blockers for AMPA, NMDA, and GABA, to block synaptic inputs to PCs. As a result, the SS activity only reflects intrinsic activity, which is characterized by a higher mean ISI and skewness and a lower CV and kurtosis. Whereas in vivo, in line with the results of Fig. 5D, the mean, CV, skewness, and kurtosis are all significantly different between Z− and Z+ PCs (P = 0.003, P < 0.001, P = 0.004, and P = 0.038, respectively), in vitro only the difference in the mean ISI persisted (P = 0.027). The other parameters are no longer different, or the difference is even reversed (kurtosis, P = 0.039). B: moreover, the differences we found in vivo between PCs with the same zebrin identity from different lobules, presented in Fig. 6D, are also no longer present in vitro (for II, III, IX, and X, n = 13, 14, 19, and 14, respectively). In the absence of synaptic inputs the differences between Z− PCs of lobules II and III are ablated (skewness, P = 0.87) or even inverted (kurtosis, P = 0.029). Note that for PCs recorded in vitro zebrin identity was not confirmed. C: whereas the difference between Z− and Z+ PCs was found to be largely dependent on differences in intrinsic activity, the differences in shape of the ISI distribution are absent in vitro. This implicates that firing rate and ISI distribution should be independent factors, and thus not correlate with each other. To evaluate this, we plotted these parameters against each other and determined the correlation coefficients, for in vivo recorded Z− and Z+ PCs. Indeed, we found no significant correlations in any of the plots (with and without log-linear transformations, all P > 0.05, Spearman's correlation). Error bars represent SE. *P < 0.05 and **P < 0.01.
To further analyze the SS activity, independently from the mean ISI, we calculated the median, mode, skewness, and kurtosis of ISIs. Skewness (s) was calculated as:
and kurtosis as:
where μ is the mean of x, σ is the standard deviation of x, and E is the expectation operator. To prevent bias by long ISIs (>500 ms) these values were excluded for this analysis.
Immunohistochemistry.
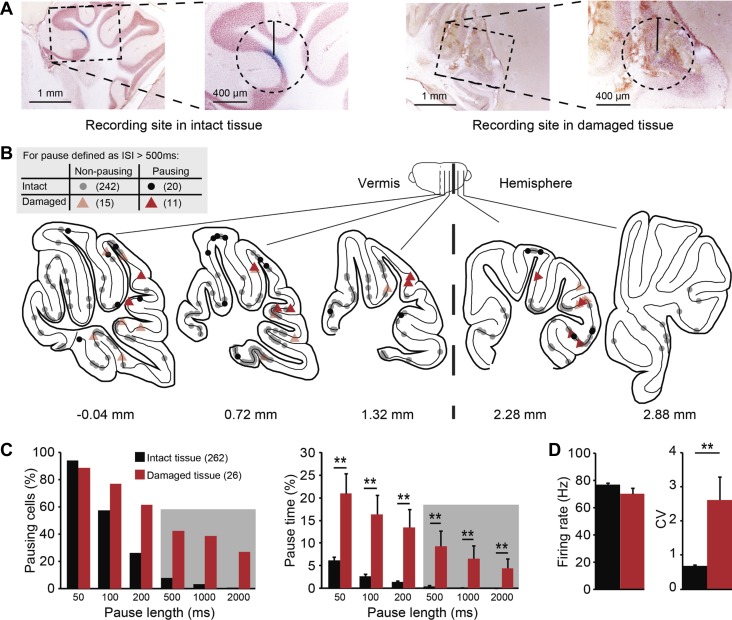
Mice were anesthetized and then perfused using 75 ml of 4% paraformaldehyde. The brains were postfixed and stored in 0.1 M phosphate buffer (PB) with 10% sucrose overnight. The brains were embedded in 10% gelatin with 10% sucrose, hardened in 10% formalin with 30% sucrose solution for 1–2 h, and stored overnight in 0.1 M PB with 30% sucrose (at 4°C). Sagittal sections with a thickness of 80 μm were processed by neutral red staining in experiments in which only the recording sites were determined. Coronal sections were cut at 40 μm followed by standard immunostaining to determine the zebrin identity. Zebrin bands were revealed using goat-derived zebrin II primary antibody (diluted at 1:1,000; Santa Cruz) and rabbit anti-goat secondary antibody (diluted at 1:200; Dako, Glostrup, Denmark). After staining, the recording sites were determined by light microscope and plotted on a 2D schematic drawing of the unfolded cerebellar cortex (adapted from Sugihara and Quy 2007). The injection site was assigned to the section with the highest Alcian Blue density, although the same injection could typically be found in several sections. For those cells that were recorded at the border of two cerebellar lobules, the cells were allocated to the rostral lobule. The locations of seven out of eight Flocc cells were estimated based on their responses to visual stimulation. Recorded PCs were determined to be from “damaged tissue” if we observed an obvious, i.e., visually identifiable, disruption in the tissue within a circle with a radius of 400 μm. Due to the highly organized, layered structure of the cerebellar cortex, tissue disruptions could be easily identified. Nevertheless, the person assessing the tissue quality was blind for the related PC activity, and the reproducibility of the assessment was confirmed by another, blind experimenter.
In vivo two-photon imaging of EAAT4-eGFP mice.
Two-photon imaging-based recordings of PC activity (included in Figs. 1–3) were obtained using EAAT4-eGFP mice (Gincel et al. 2007), which express enhanced green fluorescent protein (eGFP) under the EAAT4 promoter, in a pattern similar to that of zebrin. In five awake EAAT4-eGFP mice (3 females, 2 males, 10–26 wk old, Z−: n = 9 cells, Z+: n = 8 cells), PC activity was recorded after placing the recording pipette using two-photon imaging. PC recordings were aimed at lobule V–VI and Crus I. PCs were visualized with an excitation wavelength of 920 nm and recorded at a depth around 250 μm with a minimum recording duration of 30 s. The zebrin identity of each recorded cell was determined online. The recording pipettes were filled with 2 M NaCl and 10 μM Alexa-594, which can be visualized using an excitation wavelength of 800 nm.
Fig. 3.
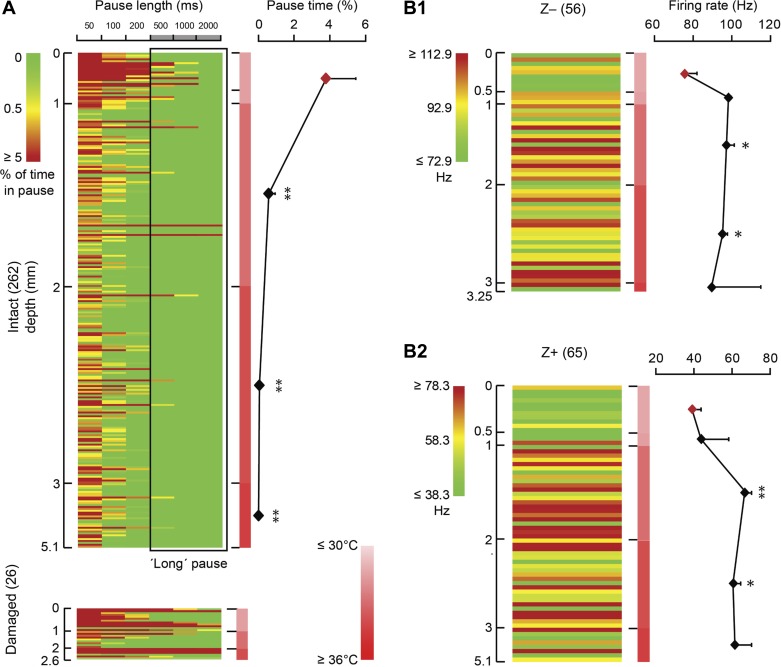
The potential influence of temperature on the occurrence of long SS pauses and firing rate. A: in total 262 cells recorded from intact tissue (see Fig. 2B) are presented in a matrix, in which rows represent individual cells ordered based on recording depth, columns represent different pause lengths, and color indicates the percentage of pause time per cell for each pause length. As indicated by the scale bar, the color represents the percentage of the total recording time each cell is pausing, with green indicating no pauses, and dark red indicates that the cell was pausing, at the indicated pause length for 5% of the total recording time or more. The scale bar in the middle indicates the temperature from low (light red) to high (dark red). Note that the maximum temperature (35°C) we detected is lower than the normal mouse body temperature (∼37–38°C), which is presumably an artifact induced by the relatively large temperature probe (diameter ∼200 μm). Recording depth correlates with pausing behavior in that superficial (depth ≤1 mm) PCs in lower temperature have a higher percentage of long pauses than the cells recorded in warmer tissue, at depths deeper than 1 mm (1-way ANOVA followed by Tukey's post hoc test). PCs from damaged tissue are added for comparison (bottom). B1 and B2: lower temperature also correlates with decreased SS firing rate. To eliminate a possible confounding effect, PCs were subdivided based on zebrin identity; both in zebrin-negative (Z−, B1) and zebrin-positive (Z+, B2) PCs the SS firing rate was significantly higher in most deeper regions compared with those at ≤0.5 mm depth (1-way ANOVA followed by Tukey's post hoc test). Row color is determined by the average SS firing rate for Z− and Z+ PCs ± 20 Hz, respectively. No. of cells is indicated in parentheses. Error bars represent SE. *P < 0.05 and **P < 0.01.
In vitro cell-attached recordings and data analysis.
Acute sagittal slices with the thickness of 250 μm were prepared from the cerebellar vermis (C57BL/6J mice) in ice-cold slicing medium that contains (in mM): 240 sucrose, 2.5 KCl, 1.25 Na2HPO4, 2 MgSO4, 1 CaCl2, 26 NaHCO3, and 10 d-glucose, and 95% O2 and 5% CO2 was bubbled into solution. Subsequently, slices were incubated at 34.0°C for 30 min and then at room temperature in ACSF solution, which contains: 124 mM NaCl, 2.5 mM KCl, 1.25 mM Na2HPO4, 2 mM MgSO4, 2 mM CaCl2, 26 mM NaHCO3, 10 mM d-glucose, 95% O2, and 5% CO2. α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), N-methyl-d-aspartic acid (NMDA), and γ-aminobutyric acid (GABA) subtype A receptors were blocked by 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (10 μM), DL-AP5 (50 μM), and picrotoxin (100 μM) in the bath, respectively. Slices were then incubated in the recording chamber for at least 10 min before recordings, and PCs were recorded in cell-attached mode with a temperature at 33.0 ± 1.0°C. Electrophysiological recordings, with a minimum recording duration of 120 s, were acquired, filtered, and digitized. Data were analyzed using Mini-analysis (Synaptosoft, Fort Lee, NJ) or Matlab. Cells were only included if: 1) CV <0.2 and 2) the changes in firing rate between the first and the last 30 s were <20%.
Statistics.
All values are shown as means ± SE; differences were tested for statistical significance by unpaired Student's t-test, unless stated otherwise, and were considered to be significant if P < 0.05.
RESULTS
A fraction of PCs show SS pauses longer than 500 ms.
To study the occurrence of long pauses in SS activity in relation to the location in the cerebellum, we analyzed extracellular recordings from PCs in awake C57Bl/6 mice at rest, and determined their location using immunohistochemistry or based on two-photon imaging (see methods). PCs were identified by the occurrence of the characteristic complex spike, and the consistent presence of the climbing fiber pause, a pause in SS following each complex spike, was taken as evidence that the recording was from a single unit. For immunohistochemical analysis, Alcian Blue was injected to identify the recording site after each recording in an attempt to correlate cerebellar subpopulations with the occurrence of pauses (Fig. 1A). The craniotomy was rectangular, strip-like shaped to allow access to the different parts of the cerebellar cortex without exposing a large surface (Fig. 1B). The location of in total 288 PCs was identified by histological retrieval or imaging (Fig. 1C). In a fraction of PCs, in both vermis and hemisphere, we observed at least one prolonged ISI, ranging in duration from hundreds of milliseconds to seconds (see example in Fig. 1D) during the recording period of at least 2 min. Notably, the location of most of these cells was close to the surface of lobules IV/V, VI/VII, Crus I/II, and paramedian lobe, i.e., typically near the craniotomy. When we consider ISIs with a duration longer than 500 ms a long pause, 31 out of 288 PCs are categorized as pausing cells. Not surprisingly, these PCs with long SS pauses show lower SS firing rate and higher CV compared with PCs without long pauses (Fig. 1E).
Percentages of pausing cells and pause time per cell correlate with local tissue damage.
The observation that most PCs with long pauses are located close to the surface of cerebellar cortex, under the craniotomy, suggests that some nonphysiological factors may induce the occurrence of SS pauses. To facilitate the entrance of the pipette and record from PCs in the cerebellum, bone and underlying dura were removed (Fig. 1A). After each successful recording the recording site was labeled by dye injection, which did not cause damage, and the pipette was moved at least 500 μm away from the recording site. Despite all precautions, tissue damage did occur, particularly in the less common chronic recordings over multiple sessions (Fig. 2A). To test the possible influence of tissue damage on the occurrence of pauses, we evaluated the integrity of the tissue surrounding every cell under light microscopy (Fig. 2A) in relation to pausing. There is, to our knowledge, no generally accepted ISI duration that constitutes a pause (see, e.g., Cao et al. 2012; Loewenstein et al. 2005; Schonewille et al. 2006; Shin and De Schutter 2006; Yartsev et al. 2009). Here, we aimed to identify the ISIs that are decidedly longer than normal ISIs to quantify pausing behavior. Hence, we evaluated pauses in PC SS activity based on different minimum ISI durations labeled as pause, ranging from 50 to 2,000 ms (see methods for details). Even though, due to the sectioning procedure, we could only identify damage in a single (cutting) plane, we found that cells near damaged tissue showed strikingly higher incidence of long pauses (>500 ms) compared with cells from intact tissue (11/26, or 42.3% vs. 20/262, or 7.6%, respectively, Fig. 2, B and C). In fact, this difference was present even when different ISI durations were considered a pause, both in the form of a higher percentage of pausing cells and a longer pause time. In contrast to pausing cells (Fig. 1E), cells near tissue damage on average do not fire SS at a lower rate (Fig. 2D), indicating that these cells are as a population less distinct from the norm and that tissue damage does not, by definition, affect all firing properties.
Fig. 2.
Correlating the quality of surrounding tissue with PC SS pauses. A: evaluation of the tissue quality under light microscope. Cells are classified as “intact” if there is no clear tissue damage in a circle with a radius of 400 μm around the injection site (left). If the structure of the surrounding tissue is disrupted, the cell was labeled as “damaged” (right). B: distribution of all cells (n = 288) over the cerebellar cortex, with color indicating the quality of surrounding tissue (black circles, intact; red triangle, damaged) and with the presence (light) or absence (dark) of long SS pauses (>500 ms) indicated by transparency [schematic drawings were adapted from Franklin and Paxinos atlas (Paxinos and Franklin 2001) with permission]. C: histograms comparing pausing cells, indicating the percentage of cells displaying at least one pause of the indicated length, and pause time, indicating the cumulative time of all pauses longer than indicated pause length divided by the total recording time per cell. Note that long pauses (>500 ms) are shaded. PCs recorded from damaged tissue show both higher percentages of pausing cells and pause time for different pause lengths than PCs recorded from the intact tissue (all P < 0.001, Mann-Whitney U-test). D: in line with the increased occurrence of pauses, tissue damage also correlates with higher SS CV, whereas SS firing rate is not affected. No. of cells is indicated in parentheses. Error bars represent SE. **P < 0.01.
Temperature differences correlate with the occurrence of long pauses and a lower firing rate.
In addition to possible damage, PCs in the vicinity of the craniotomy can potentially be affected by other nonphysiological changes, such as temperature changes. In the neocortex, radiative temperature loss under the craniotomy has been reported to negatively affect the temperature of brain surface, reducing it by several degrees centigrade and altering the up-down transitions and firing rate (Kalmbach and Waters 2012; Long and Fee 2008). Could the proportion of PCs that shows longer SS pauses, even though they were recorded from undisrupted tissue, be explained by a correlation with local dysregulation of temperature near the craniotomies? To answer this question, we measured the brain temperature at different recording depths. Repeated measurements show that the temperature difference between the cerebellar surface immediately below a saline-covered craniotomy and the deepest cerebellar parts at about 4 mm in awake mice can be up to 5°C. The absolute temperature is relatively low compared with the core temperature of mice (37–38.5°C), suggesting a small artifact of the recording method itself. Correlating the local temperature with the occurrence of SS pauses in PCs, excluding the cells near tissue damage, roughly identifies two populations, based on recording depth. PCs recorded <1 mm from the entrance point show more pauses in general, including long pauses (>500 ms), whereas the probability of long pauses is minimized in PCs recorded beyond 1 mm depth (Fig. 3A). This difference could, at least in part, be the result of a general decrease in SS firing rate, as described for the cortex (Kalmbach and Waters 2012; Long and Fee 2008). As the average firing rate differs substantially between Z+ and Z− PCs (Zhou et al. 2014), we subdivived PCs accordingly and analyzed the effect of temperature per group. Independent of their zebrin identity, PC firing rate correlates with the temperature, in that PCs in the first 0.5 mm of tissue in both subgroups have a significantly lower SS firing rate compared with those at larger depth (Fig. 3B1 and B2).
This is, to our knowledge, the first analysis of the effect of a craniotomy on cerebellar temperature and PC activity. Our results suggest that recording conditions can significantly reduce PC SS rate and increase the probability of longer pauses.
Distribution of short pauses over cerebellar lobules and hemispheral parts.
We have demonstrated how tissue damage and reduced temperature can affect PC activity. If long pauses represent a common property of PC firing, one would expect that, even after excluding cells obtained from damaged tissue or near the craniotomy (depth from entrance point <1.0 mm), long pauses can be readily identified in a significant portion of the recordings, as described previously (Cheron et al. 2014; Loewenstein et al. 2005; Yartsev et al. 2009). This was not the case; only 4.3% of PCs (10/234) have one or more pauses of >500 ms.
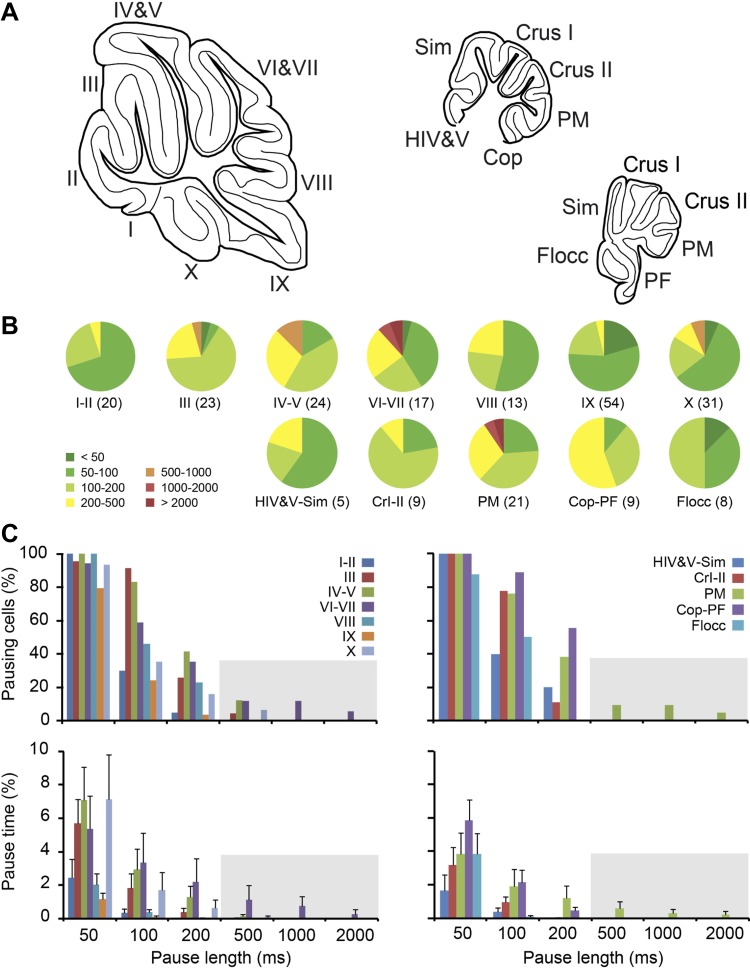
To test if there are any regional differences in the occurrence of pauses, we grouped cells based on their longest ISI, i.e., longest pause length, and determined the percentage of cells per lobule (Fig. 4, B and C, top) and the average percentage of total recording time that the cells were pausing at each given pause length (Fig. 4C, bottom). Both the percentage of pausing cells and pause time drop off when an ISI duration of longer than 500 ms is taken as the definition of a pause. We consider inter-SS intervals of >500 ms to be a long pause, but as the typical PC fires SS at 50 to 100 Hz, the typical inter-SS interval is ∼10 to 20 ms, and thus intervals much shorter than 500 ms could be considered a pause too. Whereas long pauses are rare and are not linked to specific lobules, shorter pauses, i.e., 50- to 500-ms intervals, are more omnipresent and vary more commonly in occurrence between lobules.
Fig. 4.
The occurrence of long pauses throughout the cerebellar cortex. Based on the evidence that firing properties can potentially be affected by nonphysiological conditions, we excluded PCs recorded from superficial layers (recording depth ≤1 mm) or damaged tissue. A: schematic drawing of sagittal sections of the vermis and hemisphere (at 2 levels), indicating the lobules and hemispheral regions [schematic drawings were adapted from Franklin and Paxinos atlas (Paxinos and Franklin 2001) with permission]. B: pie charts of the percentages of PCs displaying at least one ISI, or pause length, of the durations indicated by the colors. For example, in lobule III 1 out of 23 PCs (4%) showed at least one ISI of 500-1,000 ms, indicated in orange, and in 1 out of 23 PCs all ISIs were shorter than 50 ms, indicated in dark green. C: top, similar to B, but now displayed per pause length. Bottom, the average pause time compared between different lobules and regions of the vermis and hemisphere for different pause lengths. Whereas long pauses (>500 ms) are rare after imposing the criteria mentioned above, there is little indication of a preferred location of PCs showing shorter SS pauses. Lobules marked by Roman numbers I to X, hemispheral regions are: HIV&V, hemispheric parts of lobules IV and V; Sim, lobus simplex; PM, paramedian lobule; Cop, copula pyramidis; PF, paraflocculus; Flocc, flocculus. No. of cells per area is indicated between brackets.
These results indicate that long pauses are not a common characteristic of PC firing, and that shorter pauses are not only more common, but also appear to have a more differential distribution across cerebellar lobules.
Functional modules differ in distribution of short SS pauses.
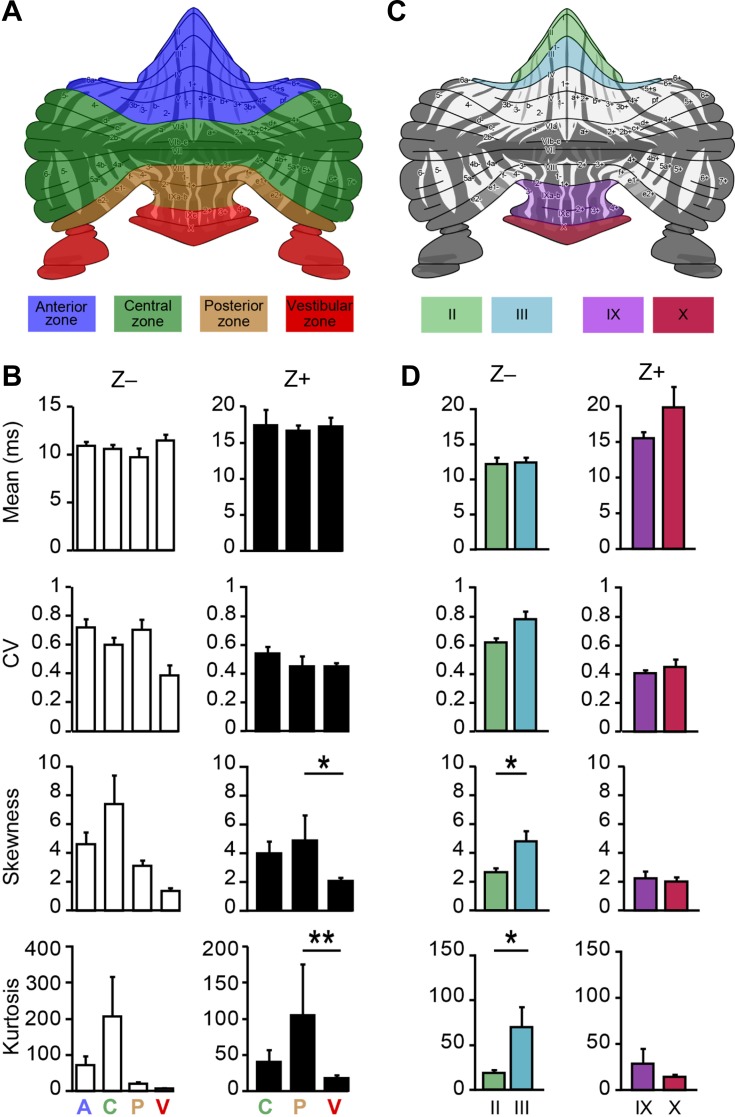
Functionally linked parts of the cerebellar cortex, organized in sagittal bands of cerebellar modules, can be identified based on their expression of zebrin (Apps and Hawkes 2009; Brochu et al. 1990; Sugihara 2011; Voogd 2011; Voogd and Ruigrok 2004). We have previously demonstrated that the firing rate of PCs in Z− bands is higher than that of those in Z+ bands (Zhou et al. 2014). To test if pausing behavior is also related to cerebellar modules, we analyzed SS activity in a subset of the PCs of which the zebrin identity was determined by immunohistochemistry (n = 95). When we grouped PCs based on their zebrin expression we find that, consistent with Fig. 4, only a small percentage of PCs show long pauses (Fig. 5A). Moreover, both the percentage of pause time and pausing cells are not different between cerebellar modules, independent of the chosen pause length (Fig. 5B). To evaluate whether Z+ and Z− PCs also differ in parameters other than firing rate, we determined several other parameters related to their ISI distributions. Anatomical evidence suggests that Z+ PCs have thinner axons than Z− cells (Haines and Manto 2011; Voogd 1964). This would be in line with the higher average firing frequency of Z− cells, since thicker axons are capable of sustaining higher firing rates, but also suggests that the maximum instantaneous frequency should be higher in Z− PCs. This prediction holds, as the minimum ISI, i.e., the maximum instantaneous firing rate, of Z− PCs is shorter than that of Z+ cells and correspondingly their maximum firing rate is higher (Fig. 5C). Moreover, the fact that the difference in mean ISI, compared with that in median and mode, is relatively small, i.e., that the mode over mean and median over mean ratios are larger in Z+ PCs (Fig. 5D), suggests that the proportion of longer ISIs is also relatively larger in Z− PCs. To test this, we calculated the third and fourth moment of distributions, skewness and kurtosis (the 1st and 2nd moment are mean and variance, respectively). Our prediction turned out to be correct; Z− cells have a larger, positive skewness, a signature feature of a larger “tail” to the right in the ISI distribution, caused by relatively less shorter, and more longer ISIs and independent of average firing rate. Kurtosis, or “peakedness,” is also larger in Z− PCs, which implicates that Z− PCs, more so than Z+ PCs, fire SS at a “preferred” ISI, or preferred firing rate.
Thus, subdividing PCs based on functional modules marked by zebrin effectively identifies differences in SS firing characteristics, including an anatomically correlated maximum firing rate and a relatively higher proportion of short pauses.
The shape of ISI distributions can differ between subpopulations of PCs with the same zebrin identity.
The zebrin expression pattern subdivides the cerebellar cortex in parasagittal stripes, generally considered to be functional modules based on their anatomical connectivity. In addition to the sagittally oriented subdivision related to zebrin, and also based on the discontinuity of these bands, the cerebellar cortex can also be subdivided horizontally into transverse zones (Ozol et al. 1999; Reeber et al. 2013). Interestingly, PCs with the same zebrin identity but located in different transverse zones appeared to differ in firing rate too (Zhou et al. 2014), suggesting that perhaps the occurrence of pauses or shape of the ISI distributions could differ within modules too. Indeed, specific characteristics of ISIs vary between transverse zones in that the skewness and kurtosis of Z+ PCs are different between posterior and vestibular zones. In all other comparisons we did not detect significant differences, suggesting that in general PCs with the same zebrin identity are rather similar between transverse zones.
Alternatively, PCs can be attributed to the lobule or hemispheral region in which they are located. To test the possibility that instead the differences occur within specific subpopulations of the same zebrin identity in the same transverse zone, we compared Z− PCs from lobules II and III and Z+ PCs from IX and X (Fig. 6D), lobules for which we had sufficient numbers of cells to compare. Although the PCs from lobule II and III are confirmed zebrin negative, ISI distribution analysis revealed that skewness and kurtosis are significantly lower in lobule II in vivo compared with lobule III, indicating that, even within groups with the same zebrin identity from the same transverse zone, there can be subpopulations differing in characteristics.
Fig. 6.
Differences in spiking activity between PCs with the same zebrin identity. A: based on cerebellar development and discontinuity of the zebrin bands, PCs can also be subdivided in four functionally distinct regions, called transverse zones: the anterior (A), central (C), posterior (P), and vestibular (V) zone. Schematic drawing adapted from Sugihara and Quy (2007) and Zhou et al. (2014). B: analysis of the four moments describing a distribution: mean, variance (here CV), skewness, and kurtosis of ISI distributions per transverse zone for Z− and Z+ PCs. Whereas Z− cells in A, C, P, and V (n = 24, 14, 4, and 2 cells, respectively) are not significantly different, Z+ cells (C, P, and V, n = 4, 4, and 42 cells, respectively) in the posterior zone have a higher skewness and kurtosis of ISI distributions (1-way ANOVA followed by Tukey's post hoc test) compared with the vestibular transverse zones. C: similarly, the ISI distribution shapes of PCs with the same zebrin identity, but from different lobules, were compared. D: analysis of recordings from the confirmed Z− PCs of lobules II (n = 6 cells) and III (n = 10) and of the Z+ PCs of lobules IX (n = 19) vs. X (n = 16) revealed that significant differences can occur within subpopulations of PCs with the same zebrin identity, since Z− PCs of lobule III have a higher skewness and kurtosis than those of lobule II. *P < 0.05 and **P < 0.01.
The presence of differences in shape of the ISI distributions between subpopulations of PCs with the same zebrin identity, but located in different transverse zones or lobules, suggests a further segregation in the activity of PCs in addition to that related to zebrin expression.
Differences between and within populations of zebrin-identified PCs are absent or reversed in vitro.
The difference in firing rate between Z− and Z+ PCs has been determined to be largely the result of intrinsic differences, as it was still present in the absence of synaptic inputs (Zhou et al. 2014). To verify if the differences in higher-order properties of ISI distributions, skewness and kurtosis, between and within groups of PCs with the same zebrin identity are also related to input or depend more on the presence of synaptic inputs, we recorded PC activity in vitro in the presence of blockers of synaptic inputs. These conditions have obvious, strong effects on all properties of the ISI distribution, resulting in a lower CV and skewness, and a higher kurtosis. We compared the in vivo activity of the confirmed Z− and Z+ PCs from lobules II-III and IX-X, respectively, with that of PCs from these lobules recorded in vitro to evaluate the contribution of synaptic inputs to the difference between zebrin-identified cerebellar modules (see Fig. 6C). As to be expected, the difference in mean ISI, which is strongly linked to firing rate, persisted in vitro (Fig. 7A, top). In contrast, the differences in skewness and kurtosis found in vivo are no longer present in vitro, or even reversed (Fig. 7A). As the differences in shape of the ISI distribution between Z+ and Z− modules are absent under conditions of blocked synaptic inputs, one could assume that the differences within subpopulations with the same zebrin identity are also no longer present in vitro. This was found to be true; when comparing the firing characteristics of lobules II vs. III and IX vs. X, the differences observed in vivo were no longer present, or even reversed (Figs. 6D vs. 7B). Together these results suggest that ISI distribution characteristics are the result of synaptic inputs, and not intrinsic to the PC. If indeed the firing rate is predominantly dependent on intrinsic properties and other ISI distribution features, such as CV, skewness, and kurtosis, are more dependent on the synaptic inputs to PCs, there should be no clear correlation between firing rate and CV, skewness, and the other parameters. This was confirmed, in that we found no significant correlations between firing rate and CV, skewness, or kurtosis for Z+ or Z− PCs (Fig. 7C).
Overall we conclude that, whereas the differences in firing rate between functional modules are largely intrinsic, the differences in the shape of ISI distributions between and within subpopulation appear mostly dependent on synaptic inputs.
DISCUSSION
Neurons commonly confer information by means of action potentials. Whereas in most brain regions the resting activity of neurons is relatively low and thus the information is transferred by action potentials, in the cerebellum there is an alternative configuration. The combination of a high level of activity, even at rest (Zhou et al. 2014), and the fact that their output is inhibitory make it tempting to assume that PCs communicate through the absence of action potentials, i.e., pauses (see, e.g., De Schutter and Steuber 2009; De Zeeuw et al. 2011). The occurrence and relevance of short (50–500 ms) and long (>500 ms) SS pauses under physiological conditions have been a topic under debate for nearly a decade (Cao et al. 2012; Cheron et al. 2014; Libster and Yarom 2013; Loewenstein et al. 2005; Oldfield et al. 2010; Schonewille et al. 2006; Shin et al. 2007; Wang et al. 2012; Yartsev et al. 2009). Understanding where and under what conditions pauses occur is crucial for evaluating their functional importance. By recording PCs throughout the whole cerebellum in awake mice and marking each recording site, we found that PCs that show long SS pauses were typically close to the brain surface, in the vicinity of the craniotomy. Evaluation of the tissue quality and measurements of the tissue temperature in relation to the recording depth suggest that local disruption of surrounding tissue and drop in temperature can contribute to an increase in the occurrence of long SS pauses. In contrast to long pauses, short pauses are more abundant and differentially distributed between modules and, in some cases, even differ between subpopulations of PCs with the same zebrin identity. Blocking all synaptic inputs to PCs in vitro ablated the differences between and within subpopulations of PCs with the same zebrin identity, suggesting that differences in the shape of ISI distributions are related to differential inputs to PCs.
Methodological considerations.
Although intensively studied, previous work has focused on the charaterization and possible role of SS pauses, with limited interest in the cerebellar cortical location of the studied PCs (Kim et al. 2012; Sugihara and Quy 2007; Wadiche and Jahr 2005; Wang et al. 2011; Xiao et al. 2014; Zhou et al. 2014). In the current study, we systemically correlated the locations of PCs with the occurrence of long pauses and found that these cells are often located close to the exposed brain surface, near the craniotomy. Craniotomies are widely used in brain research for probing the neural activity using electrophysiology or imaging. However, creating a cranial window to allow access for an objective or single or multiple pipette can profoundly compromise the physiological conditions especially in the superficial layer (Kalmbach and Waters 2012). Our data show that the brain tissue near a craniotomy, which is commonly disrupted, correlates with increased occurrence of long pauses. The tissue temperature in more superficial locations is also affected by the craniotomy, correlating with, and presumably causing, a decrease in the firing rate (Kalmbach and Waters 2012; Long and Fee 2008), but may also, more specifically for the cerebellum, induce long SS pauses. Our data are inconsistent with previous reports (Loewenstein et al. 2005; Yartsev et al. 2009), in that the percentage of PCs that fire SS with several hundred milliseconds or even seconds long pauses was significantly smaller. We have previously shown that anesthesia can increase the occurrence of long pauses (Schonewille et al. 2006). Yartsev and colleagues (Yartsev et al. 2009) performed 35 recording sessions in lobule VI of one cat and 17 in Crus II of another using one to four glass-coated tungsten microelectrodes. This method could be more prone to induce tissue damage, but alternative explanations cannot be excluded. It is, for instance, possible that longer pauses are rare and require either recordings of longer duration or specific sensory stimulation and/or motor activity to be revealed. This assumption, however, does not explain the eminent difference between the various obtained results. Although other factors, such as species differences, may be at play, the effects of tissue disruption and temperature could also explain the discrepancies between earlier reports on PC pausing behavior (Cao et al. 2012; Cheron et al. 2014; Loewenstein et al. 2005; Schonewille et al. 2006; Yartsev et al. 2009; Zhou et al. 2014). Particularly due to the recent developments in two-photon imaging techniques, recording in vivo has become increasingly popular. Relating our results to the typical recording depth for two-photon imaging of up to several hundreds of micrometers suggests these measurements could also be affected by tissue damage and lower temperatures. Future experiments will have to evaluate if keeping the dura in tact together with isolation or perfusion methods will prevent these unfavorable effects. In summary, our results demonstrate that, by opening the skin, bone, and/or dura, the local environment is disrupted, and thus the biophysical properties of the cell and circuits are affected.
PC SS activity.
There is currently no consensus on how cerebellar PCs convey their information onto cerebellar nuclear neurons. Elements of rate coding and spatiotemporal coding have been identified (for review, see De Zeeuw et al. 2011). Analysis of smooth pursuit learning has revealed an important role for rate coding (Medina and Lisberger 2009), whereas similar rate but disrupted regularity impaired cerebellar functioning (Hoebeek et al. 2005; Wulff et al. 2009). At the level of the major output targets of PCs, the cerebellar nuclei, particular temporal patterns are thought to evoke rebound potentiation (De Zeeuw et al. 2011; Hoebeek et al. 2010), with a role for the rate coding (De Schutter and Steuber 2009). Alternatively, or in addition, the level of synchrony of the inhibitory PC inputs is thought to control the cerebellar nuclei neuron output in a temporal manner (Person and Raman 2011; 2012). Independent of the proposed mechanisms, the SS pauses will be of relevance, and our data show that there are clear differences in the occurrence and distribution of these pauses between cerebellar modules. We observed a higher CV, i.e., a lower regularity, in Z− compared with Z+ PCs, whereas we did not detect a significant difference in CV2 in our previous study (Zhou et al. 2014). Although the datasets are also slightly different, this difference is predominantly related to the difference between the two measures. CV reflects the standard deviation over all ISIs, whereas CV2 is the mean of all CVs for adjacent ISIs. This suggests that the difference is in the range, rather than in the rapid fluctuations in ISI length. Notably, the higher CV is opposite to recently published results by Xiao et al. (2014) who found that Z− PCs are more regular. This discrepancy could be related to the difference in recording locations, but could also be caused by the use of anesthetics in that study, which have been previously shown to affect PC regularity (Schonewille et al. 2006).
The combination of a higher skewness and kurtosis in Z− PCs indicates that these cells commonly operate at a preferred particular interval (high kurtosis), but when deviating from this rate, do so typically with more, longer pauses (high skewness). Conversely, the lower skewness and kurtosis in Z+ PCs suggest that these cells have a less pronounced preferred interval, and fewer longer pauses. This would roughly match with the spiking activity observed in relation to eye blink conditioning and that during compensatory eye movements, which are thought to be predominantly dependent on Z− and Z+ PCs, respectively (Apps and Hawkes 2009; Mostofi et al. 2010; Sugihara and Quy 2007). Eye blink conditioning, i.e., acquiring a new, conditioned response to a previously unrelated stimulus, correlates with a short pause in an otherwise stable SS activity rate (Jirenhed et al. 2007). In contrast, compensatory eye movements and their adaptation, i.e., modulating existing reflexes, are typically linked to more gradually changing SS firing rates (see, e.g., Kimpo et al. 2014; Wulff et al. 2009), in line with a lower CV (Fig. 5D). Together, this suggests that differential information coding principles are used in different cerebellar modules that correlate not only with firing rate, but also result in distinct ISI distributions. Interestingly, even though the SS firing rates of PCs with the same zebrin identity are quite homogenous, the distributions of short ISIs can also show variations between lobules of the same zebrin identity, which are probably caused by differential synaptic inputs as they are ablated in conditions without input (Fig. 7B). These differences in pausing behavior between groups of PCs with the same zebrin identity suggest an additional layer of complexity in the physiology of the cerebellar cortex, potentially correlating with the functional requirements of particular subpopulations.
Functional importance of maintaining the physiological conditions.
Previous studies in song production showed that small changes at the brain surface can alter the functional interactions between superficial and deep structures (Long and Fee 2008). Similarly, in the cerebellum changes in biophysical dynamics of PCs near the craniotomy can potentially influence the synchronization of PCs and thereby their control on downstream target neurons in the cerebellar nuclei, ultimately affecting the cerebellar functional output. Unraveling the physiological features of cellular activity in the absence of nonphysiological perturbations is crucial for interpretation of experimental data, not only in cerebellar research, but in neuroscience in general. Our current results underline these potentially disruptive effects and identify the physiological characteristics of PC activity and how they differ throughout the cerebellar cortex.
GRANTS
This work was supported by the Dutch Organization for Life Sciences (M. Schonewille) and the Erasmus University Rotterdam Fellowship (M. Schonewille).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: H.Z. and M.S. conception and design of research; H.Z., Z.L., C.J., and M.S. performed experiments; H.Z., K.V., Z.L., and M.S. analyzed data; H.Z., K.V., and M.S. interpreted results of experiments; H.Z. and M.S. prepared figures; H.Z. and M.S. drafted manuscript; H.Z., K.V., Z.L., C.J., and M.S. edited and revised manuscript; M.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Mandy Rutteman, Erika Goedknegt, Elize Haasdijk, Laura Post. and Daphne Groeneveld for technical assistance. We thank Chris I. De Zeeuw for valuable discussion and Jeremy Rothstein for providing the EAAT4-eGFP mice.
REFERENCES
- Ahn AH, Dziennis S, Hawkes R, Herrup K. The cloning of zebrin II reveals its identity with aldolase C. Development 120: 2081–2090, 1994. [DOI] [PubMed] [Google Scholar]
- Apps R, Hawkes R. Cerebellar cortical organization: a one-map hypothesis. Nat Rev Neurosci 10: 670–681, 2009. [DOI] [PubMed] [Google Scholar]
- Brochu G, Maler L, Hawkes R. Zebrin II: a polypeptide antigen expressed selectively by Purkinje cells reveals compartments in rat and fish cerebellum. J Comp Neurol 291: 538–552, 1990. [DOI] [PubMed] [Google Scholar]
- Cao Y, Maran SK, Dhamala M, Jaeger D, Heck DH. Behavior-related pauses in simple-spike activity of mouse Purkinje cells are linked to spike rate modulation. J Neurosci 32: 8678–8685, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheron G, Prigogine C, Cheron J, Marquez-Ruiz J, Traub RD, Dan B. Emergence of a 600-Hz buzz UP state Purkinje cell firing in alert mice. Neuroscience 263: 15–26, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V, Hughes SW. The slow (<1 Hz) rhythm of non-REM sleep: a dialogue between three cardinal oscillators. Nat Neurosci 13: 9–17, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Schutter E, Steuber V. Patterns and pauses in Purkinje cell simple spike trains: experiments, modeling and theory. Neuroscience 162: 816–826, 2009. [DOI] [PubMed] [Google Scholar]
- De Zeeuw CI, Hoebeek FE, Bosman LW, Schonewille M, Witter L, Koekkoek SK. Spatiotemporal firing patterns in the cerebellum. Nat Rev Neurosci 12: 327–344, 2011. [DOI] [PubMed] [Google Scholar]
- Gincel D, Regan MR, Jin L, Watkins AM, Bergles DE, Rothstein JD. Analysis of cerebellar Purkinje cells using EAAT4 glutamate transporter promoter reporter in mice generated via bacterial artificial chromosome-mediated transgenesis. Exp Neurol 203: 205–212, 2007. [DOI] [PubMed] [Google Scholar]
- Haines DE, Manto MU. The saga of zones in the cerebellar cortex as reflected in the corticonuclear system: a different approach, a specific hypothesis, and the proof begins (Voogd, 1969). Cerebellum 10: 307–333, 2011. [DOI] [PubMed] [Google Scholar]
- Hoebeek FE, Stahl JS, van Alphen AM, Schonewille M, Luo C, Rutteman M, van den Maagdenberg AM, Molenaar PC, Goossens HH, Frens MA, De Zeeuw CI. Increased noise level of purkinje cell activities minimizes impact of their modulation during sensorimotor control. Neuron 45: 953–965, 2005. [DOI] [PubMed] [Google Scholar]
- Hoebeek FE, Witter L, Ruigrok TJ, De Zeeuw CI. Differential olivo-cerebellar cortical control of rebound activity in the cerebellar nuclei. Proc Natl Acad Sci USA 107: 8410–8415, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirenhed DA, Bengtsson F, Hesslow G. Acquisition, extinction, and reacquisition of a cerebellar cortical memory trace. J Neurosci 27: 2493–2502, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmbach AS, Waters J. Brain surface temperature under a craniotomy. J Neurophysiol 108: 3138–3146, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Oh SH, Lee JH, Chang SO, Kim J, Kim SJ. Lobule-specific membrane excitability of cerebellar Purkinje cells. J Physiol 590: 273–288, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimpo RR, Rinaldi JM, Kim CK, Payne HL, Raymond JL. Gating of neural error signals during motor learning. Elife 3: e02076, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libster AM, Yarom Y. In and out of the loop: external and internal modulation of the olivo-cerebellar loop (Abstract). Front Neural Circuits 7: 73, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R, Sugimori M. Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J Physiol 305: 197–213, 1980a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R, Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol 305: 171–195, 1980b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein Y, Mahon S, Chadderton P, Kitamura K, Sompolinsky H, Yarom Y, Hausser M. Bistability of cerebellar Purkinje cells modulated by sensory stimulation. Nat Neurosci 8: 202–211, 2005. [DOI] [PubMed] [Google Scholar]
- Long MA, Fee MS. Using temperature to analyse temporal dynamics in the songbird motor pathway. Nature 456: 189–194, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA. Neuronal networks: flip-flops in the brain. Curr Biol 15: R294–R296, 2005. [DOI] [PubMed] [Google Scholar]
- Medina JF, Lisberger SG. Encoding and decoding of learned smooth-pursuit eye movements in the floccular complex of the monkey cerebellum. J Neurophysiol 102: 2039–2054, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofi A, Holtzman T, Grout AS, Yeo CH, Edgley SA. Electrophysiological localization of eyeblink-related microzones in rabbit cerebellar cortex. J Neurosci 30: 8920–8934, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield CS, Marty A, Stell BM. Interneurons of the cerebellar cortex toggle Purkinje cells between up and down states. Proc Natl Acad Sci USA 107: 13153–13158, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozol K, Hayden JM, Oberdick J, Hawkes R. Transverse zones in the vermis of the mouse cerebellum. J Comp Neurol 412: 95–111, 1999. [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. San Diego, CA: Academic, 2001. [Google Scholar]
- Person AL, Raman IM. Purkinje neuron synchrony elicits time-locked spiking in the cerebellar nuclei. Nature 481: 502–505, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person AL, Raman IM. Synchrony and neural coding in cerebellar circuits (Abstract). Front Neural Circuits 6: 97, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijpers A, Apps R, Pardoe J, Voogd J, Ruigrok TJ. Precise spatial relationships between mossy fibers and climbing fibers in rat cerebellar cortical zones. J Neurosci 26: 12067–12080, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeber SL, White JJ, George-Jones NA, Sillitoe RV. Architecture and development of olivocerebellar circuit topography (Abstract). Front Neural Circuits 6: 115, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonewille M, Khosrovani S, Winkelman BH, Hoebeek FE, De Jeu MT, Larsen IM, Van der Burg J, Schmolesky MT, Frens MA, De Zeeuw CI. Purkinje cells in awake behaving animals operate at the upstate membrane potential. Nat Neurosci 9: 459–461, 2006. [DOI] [PubMed] [Google Scholar]
- Shin SL, De Schutter E. Dynamic synchronization of Purkinje cell simple spikes. J Neurophysiol 96: 3485–3491, 2006. [DOI] [PubMed] [Google Scholar]
- Shin SL, Hoebeek FE, Schonewille M, De Zeeuw CI, Aertsen A, De Schutter E. Regular patterns in cerebellar Purkinje cell simple spike trains. PLoS One 2: e485, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugihara I. Compartmentalization of the deep cerebellar nuclei based on afferent projections and aldolase C expression. Cerebellum 10: 449–463, 2011. [DOI] [PubMed] [Google Scholar]
- Sugihara I, Quy PN. Identification of aldolase C compartments in the mouse cerebellar cortex by olivocerebellar labeling. J Comp Neurol 500: 1076–1092, 2007. [DOI] [PubMed] [Google Scholar]
- Thach WT. Discharge of Purkinje and cerebellar nuclear neurons during rapidly alternating arm movements in the monkey. J Neurophysiol 31: 785–797, 1968. [DOI] [PubMed] [Google Scholar]
- Voogd J. Cerebellar zones: a personal history. Cerebellum 10: 334–350, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voogd J. The Cerebellum of the Cat: Structure and Fiber Connections (PhD thesis). Leiden: Univ. of Leiden, 1964. [Google Scholar]
- Voogd J, Ruigrok TJ. The organization of the corticonuclear and olivocerebellar climbing fiber projections to the rat cerebellar vermis: the congruence of projection zones and the zebrin pattern. J Neurocytol 33: 5–21, 2004. [DOI] [PubMed] [Google Scholar]
- Wadiche JI, Jahr CE. Patterned expression of Purkinje cell glutamate transporters controls synaptic plasticity. Nat Neurosci 8: 1329–1334, 2005. [DOI] [PubMed] [Google Scholar]
- Wang F, Xu Q, Wang W, Takano T, Nedergaard M. Bergmann glia modulate cerebellar Purkinje cell bistability via Ca2+-dependent K+ uptake. Proc Natl Acad Sci USA 109: 7911–7916, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Chen G, Gao W, Ebner TJ. Parasagittally aligned, mGluR1-dependent patches are evoked at long latencies by parallel fiber stimulation in the mouse cerebellar cortex in vivo. J Neurophysiol 105: 1732–1746, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SR, Christensen SR, Stuart GJ, Hausser M. Membrane potential bistability is controlled by the hyperpolarization-activated current I(H) in rat cerebellar Purkinje neurons in vitro. J Physiol 539: 469–483, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff P, Schonewille M, Renzi M, Viltono L, Sassoe-Pognetto M, Badura A, Gao Z, Hoebeek FE, van Dorp S, Wisden W, Farrant M, De Zeeuw CI. Synaptic inhibition of Purkinje cells mediates consolidation of vestibulo-cerebellar motor learning. Nat Neurosci 12: 1042–1049, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Cerminara NL, Kotsurovskyy Y, Aoki H, Burroughs A, Wise AK, Luo Y, Marshall SP, Sugihara I, Apps R, Lang EJ. Systematic regional variations in purkinje cell spiking patterns. PLoS One 9: e105633, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yartsev MM, Givon-Mayo R, Maller M, Donchin O. Pausing purkinje cells in the cerebellum of the awake cat (Abstract). Front Syst Neurosci 3: 2, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Lin Z, Voges K, Ju C, Gao Z, Bosman LW, Ruigrok TJ, Hoebeek FE, De Zeeuw CI, Schonewille M. Cerebellar modules operate at different frequencies. Elife 3: e02536, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]