Abstract
Mirror visual feedback (MVF) during motor training has been shown to improve motor performance of the untrained hand. Here we thought to determine if MVF-induced performance improvements of the left hand can be augmented by upregulating plasticity in right primary motor cortex (M1) by means of anodal transcranial direct current stimulation (a-tDCS) while subjects trained with the right hand. Participants performed a ball-rotation task with either their left (untrained) or right (trained) hand on two consecutive days (days 1 and 2). During training with the right hand, MVF was provided concurrent with two tDCS conditions: group 1 received a-tDCS over right M1 (n = 10), whereas group 2 received sham tDCS (s-tDCS, n = 10). On day 2, performance was reevaluated under the same experimental conditions compared with day 1 but without tDCS. While baseline performance of the left hand (day 1) was not different between groups, a-tDCS exhibited stronger MVF-induced performance improvements compared with s-tDCS. Similar results were observed for day 2 (without tDCS application). A control experiment (n = 8) with a-tDCS over right M1 as outlined above but without MVF revealed that left hand improvement was significantly less pronounced than that induced by combined a-tDCS and MVF. Based on these results, we provide novel evidence that upregulating activity in the untrained M1 by means of a-tDCS is capable of augmenting MVF-induced performance improvements in young normal volunteers. Our findings suggest that concurrent MVF and tDCS might have synergistic and additive effects on motor performance of the untrained hand, a result of relevance for clinical approaches in neurorehabilitation and/or exercise science.
Keywords: transcranial direct current stimulation (tDCS), motor learning, mirror visual feedback, primary motor cortex
mirror visual feedback (MVF) during practice of a novel motor skill has been shown to improve performance not only of the trained but also of the untrained hand (Nojima et al. 2012). The fact that MVF leads to behavioral gains in the untrained body part suggests that it might be an interesting adjuvant approach for neurorehabilitation. Indeed, the concept of MVF was originally used to reduce phantom-limb pain after upper limb amputation (Ramachandran and Rogers-Ramachandran 1996). Since then, the technique has been successfully applied to improve upper limb function in specific neurological diseases such as in patients suffering from stroke (Hamzei et al. 2012) or complex regional pain syndrome (Moseley 2004). Similarly, specific noninvasive brain stimulation (NIBS) protocols have also been shown to improve training outcomes (Reis et al. 2008), an effect that could be used to complement MVF.
Although the underlying neural mechanisms of MVF-induced behavioral gains still remain elusive, there is ample evidence that plasticity within primary motor cortex (M1) ipsilateral to the trained hand might play an important role in mediating performance improvements of the stationary (untrained) hand (Garry et al. 2005; Giraux and Sirigu 2003; Nojima et al. 2012; Waters-Metenier et al. 2014). For example, Nojima and colleagues found that MVF is associated with an increase in corticospinal excitability within M1 representing the untrained hand and that such M1 plasticity is directly correlated with behavioral improvements in a ball-rotation task. Furthermore, disrupting activity within ipsilateral M1 by means of continuous theta burst stimulation (cTBS), a specific form of NIBS, blocked MVF-induced performance improvements of the untrained hand (Nojima et al. 2012). Apart from local alterations in M1, a recent neuroimaging study in stroke patients revealed MVF-induced functional alterations in other motor-related brain areas such as dorsal and ventral premotor cortex (Hamzei et al. 2012).
Based on the aforementioned findings, the present study was designed to further investigate the role of M1 in MVF-induced performance improvements in the untrained hand by assessing the interaction with NIBS. We hypothesized that upregulating excitability within M1 by means of a single session of anodal transcranial direct current stimulation (a-tDCS) will augment MVF-induced behavioral gains in a ball-rotation task compared with sham stimulation (s-tDCS). Because unilateral tDCS might have the potential to modulate performance of both hands (Vines et al. 2008; Vines et al. 2006), we also tested the effect of a-tDCS without MVF. We hypothesized here that a-tDCS without MVF will also improve performance in the untrained hand but to a smaller degree compared with a-tDCS with MVF. To investigate the stability of the potential tDCS-mediated MVF effects, performance of the trained and untrained hand was reevaluated 24 h after initial training of the groups that received combined a-tDCS or s-tDCS and MVF.
MATERIALS AND METHODS
A total number of 29 right-handed healthy young participants (mean age: 26.64 ± 3.58 yr; range: 20–37 yr; 11 female) were enrolled in the study and gave written informed consent. Twenty one participants (mean age: 25.40 ± 2.80 yr; range: 20–30 yr; 9 female) participated in a randomized double-blind, sham controlled study design (main experiment). Eight additional participants were tested in a post hoc control experiment (mean age: 29.75 ± 3.57 yr; range: 25–37 yr; 2 female, see below).
The study was performed in accordance with the Declaration of Helsinki and was approved by the local ethics committee of the University of Leipzig. All participants were right handed, as assessed by the Edinburgh Handedness Questionnaire (mean score 90.93; range 63–100) (Oldfield 1971), and underwent a detailed neurological examination to exclude any evidence for neurological disease and/or contraindications relevant for the study procedures outlined below. None of the participants was taking any central-acting drugs during the time of the experiment. All participants were task naïve. We did not include highly skilled musicians, typists, or sportsmen even though some of the participants were experienced in playing a musical instrument or were currently doing sports as a regular leisure activity. One participant was excluded from the final analysis (main experiment) since performance in the ball-rotation task (see below) could not be analyzed because of technical problems while videotaping. Hence, a total of 28 participants (17 male, 11 female) were included in the final analyses.
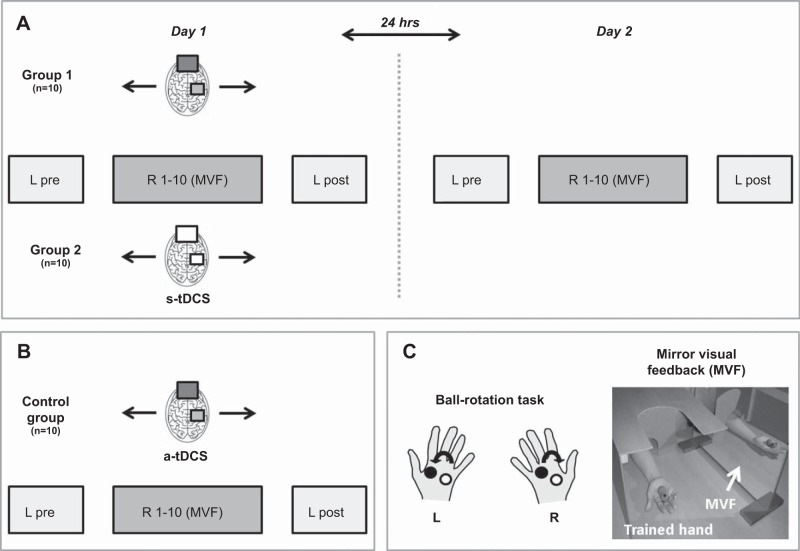
For the main experiment (n = 20), participants were invited to take part in the study over two consecutive days (day 1 and day 2). Detailed study procedures have been described previously (Nojima et al. 2012). In brief, participants performed a complex ball-rotation task where they were asked to rotate two cork balls with their left and right hand in separate sessions and specific rotations. On the first day (day 1), participants first performed the task with their left (untrained) hand (L pre) and had to rotate the cork balls in counterclockwise orientation for a single trial as quickly as possible (1 min trial length). This trial served as baseline performance. Subsequently, the ball-rotation task was performed with the right (trained) hand in clockwise orientation for 20 min (10 trials with a trial length of 1 min with 1 min rest periods in between) while participants received MVF. Here, subjects were instructed to observe the movement of the hand in a mirror; the performing hand was covered by a wooden box. During the right hand training period, participants were instructed to relax the left (untrained) hand as much as possible. The experimenter monitored the left hand by visual inspection to ensure that the left hand was not moving throughout the training period. After this training period, performance of the left (untrained) hand was retested (L post). During MVF, 20 min of anodal tDCS (a-tDCS + MVF group, n = 10, 4 female) or sham stimulation (s-tDCS + MVF group, n = 10, 5 female) were applied over the right (untrained) M1. To investigate the stability and/or reversibility of the potential tDCS-induced behavioral effects, the ball-rotation task was performed again on the second day (day 2, 24 h later) under the same experimental conditions as described above but without tDCS application (Fig. 1).
Fig. 1.
Experimental setup and design. A: participants performed a complex ball-rotation task on two consecutive days (day 1 and day 2). On day 1, participants performed the task first with the left (untrained) hand (L pre) in counterclockwise orientation. Subsequently, the right (trained) hand performed the task for 10 consecutive trials (R1–10) in clockwise orientation while watching the movements of the performing hand in a mirror [mirror visual feedback (MVF), see C]. Total duration of MVF training was 20 min. During MVF, participants either received anodal transcranial direct current stimulation (a-tDCS) or sham stimulation (s-tDCS) over right primary motor cortex (M1). At the end of the training period, the left (untrained) hand was reevaluated in counterclockwise orientation (L post). On day 2 the ball-rotation task was performed again under the exact experimental conditions as on day 1 but without tDCS. B: control experiment. The experimental setup and design was comparable to A. However, participants received no MVF (without MVF) during 20 min of a-tDCS while the right hand was trained for 10 consecutive trials (R1–10). Here, participants were instructed to watch the left stationary hand while the right hand was covered with a box. C: illustration of the ball-rotation task. The left hand (L) was supposed to rotate the balls in counterclockwise orientation. Training of the right hand (R) was performed in clockwise orientation. During training, the right hand was always covered with a box. Groups 1 and 2 received MVF and a-tDCS or s-tDCS during training of the right hand, whereas the control group had to perform the task without MVF but with a-tDCS. See text for details.
In a post hoc control experiment, a total number of eight participants performed the ball-rotation task (day 1 only) as outlined above but without MVF during 20 min of a-tDCS over right M1 (a-tDCS without MVF group, n = 8). During training of the right hand, participants were instructed to watch the stationary left hand; the right hand was covered with a box (see also Fig. 1 for experimental setup and design). This control experiment was performed to investigate the sole effect of a-tDCS on performance of the left (untrained) hand.
In summary, the common feature of all experimental groups was the training of the ball-rotation task with the right hand and the pre- and postinvestigation of motor performance of the left (untrained) hand. The difference between groups was either the type of tDCS stimulation (a-tDCS vs. s-tDCS) and/or the feedback provided during training of the right hand (with or without MVF; see also Fig. 1). Motor performance in the ball-rotation task was videotaped throughout the experiment and analyzed (number of ball rotations/min) offline by an experimenter who was blinded to the study procedures.
tDCS was delivered via saline-soaked sponge electrodes using a weak direct current of 1 mA generated from a battery-driven stimulator (NeurConn, Ilmenau, Germany). a-tDCS or s-tDCS stimulation was applied over the right (untrained) M1, during training of the right hand concurrent with MVF. The target electrode (anode; 35 cm2) was placed over the following Montreal Neurological Institute (MNI) coordinates: 40, −20, 54 (x, y, z), which correspond to the right M1 (Mayka et al. 2006). To minimize stimulation effects of the “reference” electrode (cathode), a 100-cm2 electrode was placed over the frontal orbit. Flexible elastic straps were used to fixate the electrodes on the head. Electrode positioning was guided by a three-dimensional (3D) neuronavigation device (Brainsight Version 2; Rogue Research, Montreal, Canada). In brief, for localization of right M1 in MNI coordinates, participants were first registered to an individual magnetic resonance scan using predefined landmarks (nasion, left and right tragus). Subsequently, anatomical images were transformed into 3D-MNI normalized space. Target coordinates were then individually localized on the head of the participant with a 3D motion-tracked pointer stick to guide electrode placement.
Impedance during tDCS was always kept below 10 kΩ. During a-tDCS, the current was increased at the start and decreased at the end of tDCS for 30 s in a ramp-like fashion. Current density under the anode (right M1) was 0.028 mA/cm2 (total charge 0.033 C/cm2) and 0.01 mA/cm2 (total charge 0.012 C/cm2) under the cathode (frontal orbit). During s-tDCS, the current was increased, maintained, and then decreased for 30 s each.
Statistical analyses.
Statistical analyses were performed using the Statistical Software Package for Social Sciences (IBM SPSS Version 22). Initially, baseline performance of the left (untrained) hand was compared between groups (a-tDCS + MVF vs. s-tDCS + MVF, main experiment) using an independent-samples t-test. Differences in performance of the untrained hand after MVF were evaluated by repeated-measures ANOVA (ANOVA-RM) with factor TRIAL (L pre vs. L post) and GROUP (a-tDCS + MVF vs. s-tDCS + MVF). To investigate the stability of tDCS-induced behavioral effects, performance of L post (day 1) was compared with L pre (day 2) for both groups separately using paired t-tests. In this and all subsequent analyses, post hoc t-tests were Bonferroni corrected for multiple comparisons.
Right (trained) hand performance was evaluated using another ANOVA-RM with factor TRIAL (R1–10) and GROUP (a-tDCS + MVF vs. s-tDCS + MVF). The same comparisons were performed for day 1 and day 2 for both hands. Performance differences between the trained (R10) and untrained (L post) hand were investigated using paired t-tests to evaluate if concurrent MVF and a-tDCS improved performance of the untrained hand to a similar amount as the trained hand.
Performance of the left (untrained) hand in the control experiment (a-tDCS without MVF) was evaluated using an ANOVA-RM with factor TRIAL (L pre vs. L post). Subsequently, absolute performance changes (L post − L pre) of the untrained hand across groups (a-tDCS + MVF, s-tDCS + MVF, a-tDCS without MVF) were compared using an univariate ANOVA with factor GROUP.
Levene's tests were performed to check for differences in variance, and (if necessary) P values were corrected accordingly. Behavioral data are presented as mean ± SE values.
RESULTS
Performance of the left (untrained) hand.
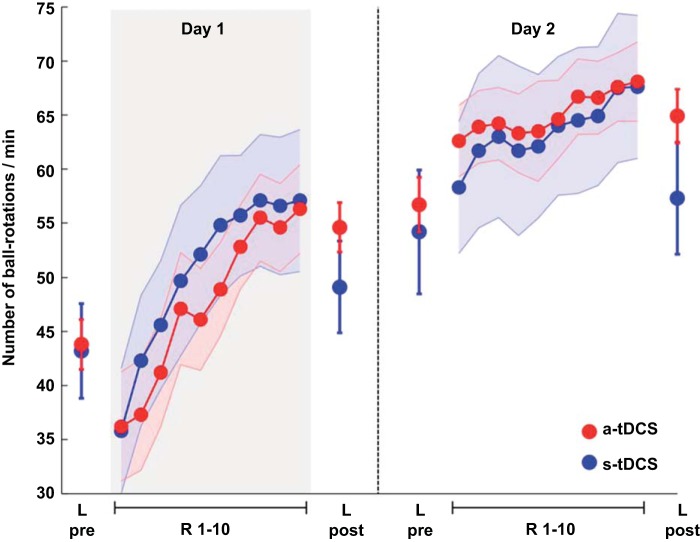
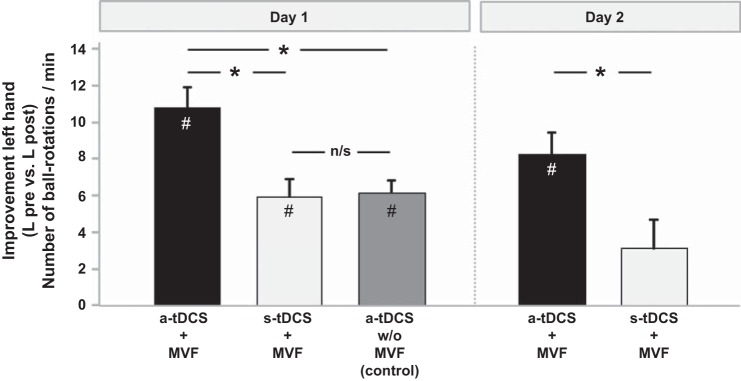
At the beginning of the experiment (day 1), baseline performance of the untrained hand (L pre) did not differ between groups [a-tDCS + MVF: 43.80 ± 2.30; s-tDCS + MVF: 43.20 ± 4.38 ball rotations/min, t(18) = 0.121; P = 0.905; Fig. 2 and Table 1]. However, 20 min of concurrent a-tDCS and MVF resulted in superior performance gains of the untrained hand compared with s-tDCS [ANOVA-RM with factor TRIAL (L pre vs. L post) × GROUP (a-tDCS + MVF vs. s-tDCS + MVF): F(1,18) = 10.778; P = 0.004]. Manual dexterity improved significantly in both groups by 10.80 ± 1.11 ball rotations/min in the a-tDCS + MVF group [t(9) = −9,699; P < 0.0001] and by 5.90 ± 0.99 after sham stimulation [t(9) = −5,936; P = 0.002; Fig. 3].
Fig. 2.
Behavioral data for the ball-rotation task. Performance of the left (untrained) hand before (L pre) and after (L post) MVF-training with the right hand (R1–10). Results are shown for day 1 and day 2, separated by 24 h (broken line). The gray bar (day 1) indicates the period where either 20 min of a-tDCS + MVF or sham (s-tDCS + MVF) was applied. Please note that tDCS was applied on day 1 only. Application of a-tDCS over right M1 during MVF resulted in superior performance gains for the left (untrained) hand (L pre vs. L post) compared with s-tDCS. The induced effects were specific for the left hand since no such changes were observed for the trained hand. The plot of mean performance and performance range shows mean ± SE values.
Table 1.
Group data of the left (L pre, L post) and right hand (R1–R10) in the ball-rotation task for day 1
| L Pre | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | R9 | R10 | L Post | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a-tDCS + MVF (n = 10) | ||||||||||||
| Mean | 43.80 | 36.20 | 37.30 | 41.20 | 47.10 | 46.10 | 48.90 | 52.80 | 55.50 | 54.60 | 56.30 | 54.60 |
| SD | 7.28 | 15.99 | 16.26 | 15.77 | 16.33 | 14.87 | 13.62 | 12.25 | 12.71 | 12.83 | 12.98 | 7.20 |
| SE | 2.30 | 5.06 | 5.14 | 4.99 | 5.16 | 4.70 | 4.31 | 3.88 | 4.02 | 4.06 | 4.10 | 2.28 |
| s-tDCS + MVF (n = 10) | ||||||||||||
| Mean | 43.20 | 35.80 | 42.30 | 45.60 | 49.70 | 52.10 | 54.80 | 55.70 | 57.10 | 56.60 | 57.10 | 49.10 |
| SD | 13.85 | 18.27 | 19.12 | 18.75 | 21.96 | 20.14 | 20.33 | 17.57 | 19.27 | 20.07 | 20.67 | 13.38 |
| SE | 4.38 | 5.78 | 6.05 | 5.93 | 6.95 | 6.37 | 6.43 | 5.56 | 6.09 | 6.35 | 6.54 | 4.23 |
| a-tDCS w/o MVF (n = 8) | ||||||||||||
| Mean | 51.88 | 46.38 | 46.50 | 46.63 | 49.38 | 48.88 | 50.63 | 51.88 | 53.63 | 56.00 | 56.00 | 58.00 |
| SD | 10.82 | 14.75 | 15.42 | 16.71 | 16.09 | 16.11 | 16.12 | 16.11 | 15.75 | 16.04 | 16.71 | 11.55 |
| SE | 3.82 | 5.22 | 5.45 | 5.91 | 5.69 | 5.70 | 5.70 | 5.70 | 5.57 | 5.67 | 5.91 | 4.08 |
n, No. of subjects; L, left; R, right; a-tDCS, anodal transcranial direct current stimulation; MVF, mirror visual feedback; s-tDCS, sham transcranial direct current stimulation. Please note that a-tDCS without (w/o) MVF did not receive MVF during training of the right hand.
Fig. 3.
Absolute improvements in the ball-rotation task for the left (untrained) hand. Please note that a-tDCS + MVF augmented behavioral gains compared with s-tDCS + MVF not only on day 1 but also on day 2. These results indicate that superior performance gains were still present on day 2, 24 h after termination of a-tDCS. Participants that received a-tDCS without MVF showed similar behavioral gains compared with s-tDCS + MVF. n/s, Not significant. Bars represent mean values, and whiskers represent SE values. *Significant between-group differences; #significant within-group comparisons (L pre vs. L post). See text for details.
On day 2 (without tDCS), baseline performance of the left hand (L pre) did not differ between groups [t(12.410) = 0.400; P = 0.696]. However, left hand performance improved from day 1 (L post) to day 2 (L pre) in the s-tDCS + MVF group [day 1 L post: 49.10 ± 4.23 vs. 54.20 ± 4.14 ball rotations/min (day 2 L pre), t(9) = −2.735; P = 0.023, significant P threshold = 0.025 to account for multiple comparisons], whereas no such changes could be observed for the a-tDCS + MVF group [t(9) = −1.561; P = 0.153]. After MVF on day 2, however, the a-tDCS + MVF group again showed superior performance improvements of the left hand (L pre vs. L post) compared with sham [8.25 ± 1.24 (a-tDCS group) vs. 2.67 ± 1.58 ball rotations/min (s-tDCS + MVF group); ANOVA-RM with factor TRIAL (L pre vs. L post on day 2) × GROUP (a-tDCS + MVF vs. s-tDCS + MVF): F(1,18) = 6.422; P = 0.021; Fig. 2].
Within-trial performance of the left (untrained) hand.
We performed an additional analysis to investigate if performing the ball-rotation task for 1 min with the left hand is already capable of inducing performance gains within a single trial. After separating performance of the left hand in four time bins (15 s each; Table 2), we found no significant performance changes within each trial (L pre or L post) in participants that received a-tDCS + MVF [day 1 L pre: ANOVA-RM with factor BIN: F(3,27) = 0.866, P = 0.471; day 1 L post: F(3,27) = 0.599, P = 0.621] or s-tDCS+MVF [day 1 L pre: ANOVA-RM with factor BIN: F(3,27) = 1.570, P = 0.219; day 1 L post: F(3,27) = 1.502, P = 0.219]. Similar results were obtained when comparing within-trial performance on day 2 (P > 0.05 for all comparisons). These results indicate that performing the ball-rotation task for 1 min does not lead to performance changes within each trial.
Table 2.
Performance of the left hand within each trial (L pre and L post for day 1 and day 2)
|
Day 1 |
Day 2 |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L Pre |
L Post |
L Pre |
L Pos t |
|||||||||||||||||
| 0–15 s | 15–30 s | 30–45 s | 45–60 s | Sum | 0–15 s | 15–30 s | 30–45 s | 45–60 s | Sum | 0–15 s | 15–30 s | 30–45 s | 45–60 s | Sum | 0–15 s | 15–30 s | 30–45 s | 45–60 s | Sum | |
| a-tDCS + MVF | ||||||||||||||||||||
| P1 | 10 | 9 | 11 | 11 | 41 | 13 | 14 | 13 | 12 | 52 | 17 | 14 | 13 | 13 | 57 | 18 | 14 | 14 | 16 | 62 |
| P2 | 10 | 13 | 15 | 13 | 51 | 15 | 16 | 14 | 15 | 60 | 17 | 15 | 13 | 16 | 61 | 14 | 17 | 15 | 20 | 64 |
| P3 | 13 | 12 | 14 | 14 | 53 | 15 | 18 | 15 | 14 | 62 | 16 | 14 | 15 | 16 | 61 | 15 | 17 | 17 | 20 | 69 |
| P4 | 11 | 10 | 12 | 8 | 41 | 17 | 14 | 13 | 12 | 56 | 14 | 13 | 12 | 14 | 53 | 17 | 17 | 15 | 14 | 63 |
| P5 | 8 | 9 | 5 | 7 | 29 | 9 | 10 | 9 | 10 | 38 | 9 | 9 | 9 | 9 | 36 | 11 | 13 | 10 | 11 | 45 |
| P6 | 12 | 10 | 14 | 12 | 48 | 13 | 14 | 13 | 14 | 54 | 13 | 14 | 17 | 13 | 57 | 17 | 16 | 18 | 16 | 67 |
| P7 | 10 | 10 | 11 | 13 | 44 | 17 | 16 | 15 | 14 | 62 | 16 | 15 | 17 | 17 | 65 | 18 | 14 | 19 | 18 | 69 |
| P8 | 14 | 13 | 12 | 12 | 51 | 15 | 14 | 15 | 15 | 59 | 15 | 15 | 13 | 14 | 57 | 18 | 18 | 18 | 20 | 74 |
| P9 | 9 | 10 | 9 | 12 | 38 | 12 | 12 | 13 | 13 | 50 | 17 | 14 | 15 | 15 | 58 | 19 | 17 | 15 | 18 | 66 |
| P10 | 10 | 10 | 12 | 10 | 42 | 12 | 12 | 13 | 16 | 53 | 17 | 14 | 15 | 16 | 62 | 19 | 17 | 13 | 21 | 70 |
| Mean | 10.70 | 10.60 | 11.50 | 11.20 | 43.80 | 13.80 | 14.00 | 13.30 | 13.50 | 54.60 | 15.10 | 13.70 | 13.90 | 14.30 | 56.70 | 16.60 | 16.00 | 15.40 | 17.40 | 64.90 |
| SD | 1.83 | 1.51 | 2.88 | 2.25 | 7.28 | 2.49 | 2.31 | 1.77 | 1.78 | 7.20 | 2.56 | 1.77 | 2.42 | 2.31 | 8.01 | 2.55 | 1.70 | 2.72 | 3.17 | 7.87 |
| s-tDCS + MVF | ||||||||||||||||||||
| P1 | 11 | 9 | 11 | 10 | 41 | 12 | 10 | 9 | 11 | 42 | 10 | 12 | 10 | 11 | 43 | 11 | 11 | 10 | 11 | 43 |
| P2 | 5 | 4 | 5 | 6 | 20 | 8 | 8 | 8 | 8 | 32 | 9 | 10 | 7 | 10 | 36 | 11 | 11 | 11 | 11 | 44 |
| P3 | 14 | 13 | 15 | 11 | 53 | 15 | 16 | 17 | 12 | 60 | 17 | 14 | 15 | 16 | 62 | 18 | 16 | 15 | 15 | 64 |
| P4 | 6 | 7 | 8 | 7 | 28 | 8 | 9 | 8 | 8 | 33 | 8 | 9 | 8 | 8 | 33 | 10 | 8 | 9 | 12 | 39 |
| P5 | 14 | 16 | 16 | 18 | 64 | 18 | 19 | 15 | 16 | 68 | 19 | 22 | 20 | 22 | 83 | 21 | 26 | 24 | 17 | 88 |
| P6 | 12 | 12 | 13 | 12 | 49 | 13 | 13 | 15 | 11 | 52 | 13 | 14 | 14 | 14 | 55 | 14 | 14 | 11 | 14 | 53 |
| P7 | 7 | 7 | 7 | 7 | 28 | 9 | 8 | 8 | 7 | 32 | 8 | 8 | 6 | 8 | 30 | 11 | 10 | 9 | 9 | 39 |
| P8 | 12 | 11 | 12 | 10 | 45 | 13 | 15 | 13 | 12 | 53 | 16 | 11 | 16 | 18 | 61 | 16 | 16 | 16 | 16 | 64 |
| P9 | 13 | 12 | 14 | 13 | 52 | 14 | 15 | 13 | 17 | 59 | 18 | 19 | 19 | 18 | 74 | 17 | 15 | 16 | 19 | 67 |
| P10 | 13 | 12 | 12 | 15 | 52 | 17 | 14 | 15 | 14 | 60 | 17 | 15 | 16 | 17 | 65 | 17 | 19 | 17 | 19 | 72 |
| Mean | 10.70 | 10.30 | 11.30 | 10.90 | 43.20 | 12.70 | 12.70 | 12.10 | 11.60 | 49.10 | 13.50 | 13.40 | 13.10 | 14.20 | 54.20 | 14.60 | 14.60 | 13.80 | 14.30 | 57.30 |
| SD | 3.40 | 3.53 | 3.59 | 3.78 | 13.85 | 3.53 | 3.77 | 3.51 | 3.37 | 13.38 | 4.40 | 4.43 | 5.02 | 4.78 | 18.07 | 3.75 | 5.21 | 4.73 | 3.50 | 16.37 |
P, participant no.
Performance of the right (trained) hand.
Performing the ball-rotation task on day 1 during MVF resulted in significant performance improvements of the trained hand in both groups [ANOVA-RM with factor TRIAL (R1–10): F(9,161) = 38.373; P < 0.0001] while there was no difference in the learning rate between groups [ANOVA-RM with factor TRIAL (R1–10) × GROUP (a-tDCS + MVF vs. s-tDCS + MVF): F(9,161) = 0.861; P = 0.490; Fig. 2]. These findings suggest that a-tDCS over right M1 might selectively affect performance gains of the untrained hand only and does not seem to affect performance of the trained hand. On day 2, further performance gains [ANOVA-RM with factor TRIAL (R1–10): F(9,161) = 6.920; P < 0.0001] were observed, whereas there was no difference in the learning rate between groups [ANOVA-RM with factor TRIAL (R1–10) × GROUP (a-tDCS + MVF vs. s-tDCS + MVF): F(9,161) = 0.439; P = 0.820].
Control experiment (a-tDCS without MVF) and comparison between groups.
Baseline performance (L pre) in a group of participants that received a-tDCS during training of the right hand but without MVF (a-tDCS without MVF) did not differ from the main experimental groups [a-tDCs + MVF and s-tDCS + MVF, univariate ANOVA with factor TRIAL (L pre): F(2,25) = 1.665; P = 0.209; see Table 1 for details]. However, application of a-tDCS during training of the right hand but without MVF (a-tDCS without MVF) resulted in significant performance improvements of the untrained (left) hand [ANOVA-RM with factor TRIAL (L pre vs. L post), F(1,7) = 72.758; P = 0.0001]. Here, manual dexterity of the untrained hand improved by 6.12 ± 0.71 ball rotations/min. However, comparing performance improvements of the untrained (left) hand on day 1 revealed significant differences between groups [univariate ANOVA with factor GROUP (a-tDCS + MVF, s-tDCS + MVF, a-tDCS without MVF): F(2,25) = 8.084; P = 0.020] such that the a-tDCS + MVF group showed significantly stronger behavioral gains compared with s-tDCS + MVF [t(18) = 3.283; P = 0.004] and a-tDCS without MVF [t(16)=3.326; P = 0.004], whereas no significant difference could be observed between s-tDCS + MVF and a-tDCS without MVF [t(16) = −0.175; P = 0.863, significant P threshold = 0.016 to account for multiple comparisons; Fig. 3].
DISCUSSION
The aim of the present study was to further investigate the role of M1 (ipsilateral to a trained hand) in mediating MVF-induced behavioral gains of the untrained (left) hand. We provide novel evidence that upregulating excitability in M1 by means of a-tDCS in conjunction with MVF resulted in superior performance changes of the left (untrained) hand compared with sham stimulation. Performance of the right (trained) hand was not affected by tDCS over right M1, indicating the local specificity of a-tDCS-induced behavioral gains. In summary, these findings suggest that combining NIBS with MVF could be a novel strategy to facilitate motor performance of untrained limbs. Our findings might have important implications in the context of neurorehabilitation to restore motor function without extensive training of the affected limb. Furthermore, the concept of MVF and concurrent NIBS could also be used to optimize performance gains of both limbs after unilateral skill training in the context of exercise and sport science.
Unilateral skill training typically improves performance on both the trained and untrained limb, a phenomenon called intermanual transfer of learning or cross-training effect (Kwon et al. 2013; Obayashi 2004; Perez et al. 2007). This phenomenon has been observed for a number of motor paradigms such as serial reaction time tasks (Perez et al. 2007), sequential pinch force tasks (Camus et al. 2009), and prism adaptation (Taub and Goldberg 1973). The amount of intermanual transfer seems to depend on several factors such as task complexity, structural integrity of the corpus callosum (Bonzano et al. 2011), and degree of hand dominance (Chase and Seidler 2008). On the other hand, neural mechanisms mediating intermanual transfer seem to be divergent from those underlying MVF-induced performance changes: previous experiments that used the same complex ball-rotation task as in the present study revealed performance improvements of the untrained hand only when MVF was provided (Nojima et al. 2012). Hence, the observed behavioral changes in the present study cannot solely be explained by a pure intermanual transfer effect. In fact, a subsequent study by Nojima and colleagues indicated that MVF can induce motor learning of the untrained hand even in patients with callosal disconnection (Nojima et al. 2013).
Unilateral short- and long-term motor skill training typically results in functional brain alterations in a variety of motor-related brain regions, including the M1 representing the trained limb (for review, see Dayan and Cohen 2011). Whereas the underlying mechanisms of intermanual transfer still remain obscure, there is preliminary evidence that, apart from intracortical modulations within M1 representing the trained limb, interhemispheric alterations between homologous M1s might be potential candidate mechanisms mediating the observed behavioral effect (Camus et al. 2009; Perez et al. 2007). Evidence for the underlying mechanisms of MVF-induced performance changes comes from several noninvasive brain imaging and brain stimulation studies. For example, it has been shown that right hand mirror training in stroke patients is associated with learning-related functional brain alterations in the left primary sensorimotor cortex (SMC) as well as in the mirror neuron system including the right dorsal (dPMC) and left ventral (vPMC) premotor cortex (Hamzei et al. 2012). In healthy participants, MVF training resulted in increased corticospinal excitability, within M1 representing the untrained hand while no such changes could be observed for intracortical or interhemispheric inhibitory circuits. However, changes in corticospinal excitability within untrained M1 were directly correlated with behavioral improvements in a ball-rotation task (Nojima et al. 2012). The results indicate that M1 seems to be one of the important drivers in mediating MVF-induced effects of the untrained hand.
Recently, specific NIBS techniques have been used to modulate training-related neuroplasticity within M1 to augment skill learning. For example, it has been shown that application of repetitive transcranial magnetic stimulation (rTMS) and tDCS (for review, see Reis et al. 2008) is capable of evoking superior learning rates of the trained hand compared with sham stimulation (Nitsche et al. 2003; Teo et al. 2011). A recent study provided compelling evidence that repeated application of NIBS over multiple days might even prolong such NIBS-induced behavioral effects (Reis et al. 2009). However, NIBS-induced behavioral and brain changes are highly variable across subjects and seem to depend on a huge variety of factors such as age, handedness, brain morphology, internal brain state, attention, genetic predisposition, etc. (Conde et al. 2012; Ridding and Ziemann 2010; Vallence et al. 2013). Although the effects of NIBS on unilateral skill learning are well documented, little is known about the efficacy of NIBS to augment MVF-induced behavioral gains. In the present study, all but one participant that received a-tDCS during MVF on day 1 showed superior behavioral gains of the untrained hand (range 6–18 ball rotations/min) compared with the mean ± SE improvement in the sham group (5.90 ± 0.99 ball rotations/min). Interestingly, a similar effect could be observed on day 2. Here, participants that received a-tDCS + MVF on day 1 also showed superior performance improvements of the left hand (L pre vs. L post) on day 2 compared with s-tDCS + MVF, even though no tDCS was applied. Together, these results point to a consistent and robust tDCS effect on a behavioral level and provide further evidence for the role of M1 ipsilateral to the trained hand in mediating MVF-induced behavioral gains.
Even though we cannot make direct inferences about the underlying neural mechanisms of a-tDCS-induced behavioral gains during MVF, it is reasonable to assume that MVF alone results in excitability changes within M1 of the untrained hand (Nojima et al. 2012) that are potentiated by concurrent a-tDCS, which by itself transiently upregulates excitability (Nitsche et al. 2008). Such a potentiation of excitation within M1 by a-tDCS in combination with MVF might enable superior performance changes of the untrained hand compared with s-tDCS. However, the underlying mechanisms of the observed behavioral effects still need to be determined in future studies.
In summary, we provide further support for the important role of M1 ipsilateral to the trained hand in mediating MVF-induced performance changes in the untrained hand. Crucially, we demonstrate that a combined application of NIBS and MVF is capable of optimizing the outcome of MVF or NIBS (a-tDCS) alone, a strategy that could be beneficial for treating individuals with unilaterally reduced function such as sufferers of unilateral stroke. Furthermore, our findings could also be important in the context of exercise and sport science where unilateral motor training in conjunction with MVF and NIBS might enhance the training outcome of both, the trained and untrained hand.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
B.S., C.J.S., A.V., and P.R. conception and design of research; E.v.R., M.H., and E.K. performed experiments; E.v.R. and P.R. analyzed data; E.v.R., M.H., E.K., B.S., C.J.S., A.V., and P.R. interpreted results of experiments; C.J.S. and P.R. prepared figures; P.R. drafted manuscript; M.H., E.K., B.S., C.J.S., and P.R. edited and revised manuscript; E.v.R., M.H., E.K., B.S., C.J.S., A.V., and P.R. approved final version of manuscript.
REFERENCES
- Bonzano L, Tacchino A, Roccatagliata L, Mancardi GL, Abbruzzese G, Bove M. Structural integrity of callosal midbody influences intermanual transfer in a motor reaction-time task. Hum Brain Mapp 32: 218–228, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camus M, Ragert P, Vandermeeren Y, Cohen LG. Mechanisms controlling motor output to a transfer hand after learning a sequential pinch force skill with the opposite hand. Clin Neurophysiol 120: 1859–1865, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase C, Seidler R. Degree of handedness affects intermanual transfer of skill learning. Exp Brain Res 190: 317–328, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde V, Vollmann H, Sehm B, Taubert M, Villringer A, Ragert P. Cortical thickness in primary sensorimotor cortex influences the effectiveness of paired associative stimulation. Neuroimage 60: 864–870, 2012. [DOI] [PubMed] [Google Scholar]
- Dayan E, Cohen LG. Neuroplasticity subserving motor skill learning. Neuron 72: 443–454, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garry MI, Loftus A, Summers JJ. Mirror, mirror on the wall: viewing a mirror reflection of unilateral hand movements facilitates ipsilateral M1 excitability. Exp Brain Res 163: 118–122, 2005. [DOI] [PubMed] [Google Scholar]
- Giraux P, Sirigu A. Illusory movements of the paralyzed limb restore motor cortex activity. Neuroimage 20, Suppl 1: S107–S111, 2003. [DOI] [PubMed] [Google Scholar]
- Hamzei F, Lappchen CH, Glauche V, Mader I, Rijntjes M, Weiller C. Functional plasticity induced by mirror training: the mirror as the element connecting both hands to one hemisphere. Neurorehabil Neural Repair 26: 484–496, 2012. [DOI] [PubMed] [Google Scholar]
- Kwon YH, Kwon JW, Park JW. Changes in brain activation patterns according to cross-training effect in serial reaction time task: an functional MRI study. Neural Reg Res 8: 639–646, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayka MA, Corcos DM, Leurgans SE, Vaillancourt DE. Three-dimensional locations and boundaries of motor and premotor cortices as defined by functional brain imaging: a meta-analysis. Neuroimage 31: 1453–1474, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley GL. Graded motor imagery is effective for long-standing complex regional pain syndrome: a randomised controlled trial. Pain 108: 192–198, 2004. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, Paulus W, Hummel F, Boggio PS, Fregni F, Pascual-Leone A. Transcranial direct current stimulation: State of the art 2008. Brain Stimul 1: 206–223, 2008. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Schauenburg A, Lang N, Liebetanz D, Exner C, Paulus W, Tergau F. Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J Cogn Neurosci 15: 619–626, 2003. [DOI] [PubMed] [Google Scholar]
- Nojima I, Mima T, Koganemaru S, Thabit MN, Fukuyama H, Kawamata T. Human motor plasticity induced by mirror visual feedback. J Neurosci 32: 1293–1300, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nojima I, Oga T, Fukuyama H, Kawamata T, Mima T. Mirror visual feedback can induce motor learning in patients with callosal disconnection. Exp Brain Res 227: 79–83, 2013. [DOI] [PubMed] [Google Scholar]
- Obayashi S. Possible mechanism for transfer of motor skill learning: implication of the cerebellum. Cerebellum 3: 204–211, 2004. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113, 1971. [DOI] [PubMed] [Google Scholar]
- Perez MA, Wise SP, Willingham DT, Cohen LG. Neurophysiological mechanisms involved in transfer of procedural knowledge. J Neurosci 27: 1045–1053, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran VS, Rogers-Ramachandran D. Synaesthesia in phantom limbs induced with mirrors. Proc Biol Sci 263: 377–386, 1996. [DOI] [PubMed] [Google Scholar]
- Reis J, Robertson E, Krakauer JW, Rothwell J, Marshall L, Gerloff C, Wassermann E, Pascual-Leone A, Hummel F, Celnik PA, Classen J, Floel A, Ziemann U, Paulus W, Siebner HR, Born J, Cohen LG. Consensus: “Can tDCS and TMS enhance motor learning and memory formation?” Brain Stimul 1: 363–369, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, Celnik PA, Krakauer JW. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci USA 106: 1590–1595, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridding MC, Ziemann U. Determinants of the induction of cortical plasticity by non-invasive brain stimulation in healthy subjects. J Physiol 588: 2291–2304, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub E, Goldberg LA. Prism adaptation: control of intermanual transfer by distribution of practice. Science 180: 755–757, 1973. [DOI] [PubMed] [Google Scholar]
- Teo JT, Swayne OB, Cheeran B, Greenwood RJ, Rothwell JC. Human theta burst stimulation enhances subsequent motor learning and increases performance variability. Cereb Cortex 21: 1627–1638, 2011. [DOI] [PubMed] [Google Scholar]
- Vallence AM, Kurylowicz L, Ridding MC. A comparison of neuroplastic responses to non-invasive brain stimulation protocols and motor learning in healthy adults. Neurosci Lett 549: 151–156, 2013. [DOI] [PubMed] [Google Scholar]
- Vines BW, Nair D, Schlaug G. Modulating activity in the motor cortex affects performance for the two hands differently depending upon which hemisphere is stimulated. Eur J Neurosci 28: 1667–1673, 2008. [DOI] [PubMed] [Google Scholar]
- Vines BW, Nair DG, Schlaug G. Contralateral and ipsilateral motor effects after transcranial direct current stimulation. Neuroreport 17: 671–674, 2006. [DOI] [PubMed] [Google Scholar]
- Waters-Metenier S, Husain M, Wiestler T, Diedrichsen J. Bihemispheric transcranial direct current stimulation enhances effector-independent representations of motor synergy and sequence learning. J Neurosci 34: 1037–1050, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]