Abstract
Broca (Broca P. Bull Soc Anat Paris 36: 330–357, 1861) influentially argued that posterior left inferior frontal gyrus supports speech articulation. According to an alternative proposal (e.g., Dronkers NF. Nature 384: 159–161, 1996; Wise RJ, Greene J, Buchel C, Scott SK. Lancet 353: 1057–1061, 1999; Baldo JV, Wilkins DP, Ogar J, Willock S, Dronkers NF. Cortex 47: 800–807, 2011), a region in the anterior insula [specifically, the superior precentral gyrus of the insula (SPGI)] is the seat of articulatory abilities. Moreover, Dronkers and colleagues have argued that the SPGI is functionally specialized for (complex) speech articulation. Here, we evaluate this claim using individual-subject functional MRI (fMRI) analyses (e.g., Fedorenko E, Hsieh PJ, Nieto-Castanon A, Whitfield-Gabrieli S, Kanwisher N. J Neurophysiol 104: 1177–1194, 2010). We find that the SPGI responds weakly, if at all, during articulation (parts of Broca's area respond 3–4 times more strongly) and does not show a stronger response to higher articulatory demands. This holds regardless of whether the SPGI is defined functionally (by selecting the most articulation-responsive voxels in the vicinity of the SPGI in each subject individually) or anatomically (by using masks drawn on each individual subject's anatomy). Critically, nonspeech oral movements activate the SPGI more strongly than articulation, especially under the anatomical definition of the SPGI. In line with Hillis et al. (Hillis AE, Work M, Barker PB, Jacobs MA, Breese EL, Maurer K. Brain 127: 1479–1487, 2004; also Trupe L, Varma DD, Gomez Y, Race D, Leigh R, Hillis AE, Gottesman RF. Stroke 44: 740–744, 2013), we argue that previous links between the SPGI, and perhaps anterior insula more generally, and articulation may be due to its high base rate of ischemic damage (and activation in fMRI; Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Nat Methods 8: 665–670, 2011), combined with its proximity to regions that more directly support speech articulation, such as the precentral gyrus or the posterior aspects of the inferior frontal gyrus (Richardson JD, Fillmore P, Rorden C, Lapointe LL, Fridriksson J. Brain Lang 123: 125–130, 2012), and thus susceptibility to joint damage.
Keywords: articulation, Broca's area, fMRI, insula, SPGI
broca (1861, 1865) hypothesized that the posterior aspects of the left inferior frontal gyrus (LIFG) support speech production. Broca's claim has been frequently challenged, and some studies suggest instead that the anterior insula, or some part thereof, is critically involved in speech articulation (e.g., Shuren 1993; Dronkers 1996; Donnan et al. 1997; Nagao et al. 1999; Wise et al. 1999; Nestor et al. 2003). For example, Dronkers (1996) reported a lesion overlap study where all patients with chronic apraxia of speech, a motor planning disorder characterized by articulatory difficulties (Ogar et al. 2005), showed damage to the superior precentral gyrus of the left insula (SPGI; cf. Mohr et al. 1978; Hillis et al. 2004; Davis et al. 2008; Richardson et al. 2012). Dronkers thus argued that the SPGI is critically engaged in, and functionally specialized for, articulatory coordination. Later, Dronkers and colleagues additionally suggested that the SPGI is especially important for complex articulatory movements (Ogar et al. 2006; Baldo et al. 2011).
Several subsequent neuroimaging studies have reported activation within the insula for articulation tasks (e.g., Wise et al. 1999; Riecker et al. 2000a, 2005, 2008; Bohland and Guenther 2006; Moser et al. 2009), with at least one reporting activation specifically in the SPGI (Bohland and Guenther 2006), thus corroborating the role of parts of the insula in articulatory processes. One thing that these and other functional MRI (fMRI) investigations of articulation (e.g., Brown et al. 2009; Peeva et al. 2010; Grabski et al. 2012) have made clear is that articulation is not spatially restricted to the SPGI, or any other single brain region, but rather engages a number of cortical, subcortical, and cerebellar regions (see Kent et al. 2000, for a review of cortical and subcortical dysfunctions that may give rise to speech motor deficits). In fact, several studies did not find activation in the anterior insula while reporting activation in other brain regions (e.g., Riecker et al. 2000b; Price et al. 2003; Bonilha et al. 2006), although a failure of a region to emerge in a group analysis does not necessarily imply lack of its engagement (e.g., Nieuwenhuis et al. 2011; Nieto-Castañon and Fedorenko 2012).
The fact that the SPGI is not the only brain region engaged in articulation (e.g., Duffy 2005; Bohland and Guenther 2006) does not diminish this region's potential importance for articulatory processing. On the other hand, a discovery that the SPGI is not functionally specialized for (complex) articulation, contra Dronkers (1996) and Baldo et al. (2011), would fundamentally alter the hypotheses we entertain about the possible computations conducted in this region. This question is especially important given that the anterior insula is among the most common foci of activation across a wide range of fMRI studies (Yarkoni et al. 2011); thus any claim about some part of the anterior insular cortex being functionally specialized for some mental function requires serious scrutiny.
In the current study, we used a functional localization fMRI approach, which has been shown to be more sensitive than traditional group analyses (e.g., Nieto-Castañon and Fedorenko 2012), to ask three questions: 1) is the SPGI engaged in complex articulatory movements; 2) does the SPGI respond reliably more strongly to complex than simple articulatory movements; and critically, and 3) is the SPGI functionally specialized for articulation?
To address our research questions, we designed several overt motor imitation tasks. The first directly targeted articulation and thus served as our “localizer” task. It included the production of easy-to-articulate and harder-to-articulate pseudowords, as described below. The other tasks shared some features with articulation and were designed to probe the degree of functional specificity of the SPGI. They included vowel production, nonspeech oral movements, and respiration. Vowel production is a motor speech task but has only minimal articulatory requirements (e.g., Shankweiler et al. 1968). Nonspeech oral movements share many features with speech articulation but have been shown to rely on distinct patterns of muscle control both in healthy adults (e.g., Ostry and Flanagan 1989; Smith 1992; Ostry and Munhall 1994; Moore and Ruark 1996) and individuals with neurological impairments (e.g., LaPointe and Wertz 1974; Dworkin and Aronson 1986; McHenry et al. 1994; Theodoros et al. 1995; Thompson et al. 1995a,b; Ziegler 2003a,b,c; cf. Ballard et al. 2003; see Bunton 2008, for a review). Although several prior studies have compared neural responses to articulation and nonspeech oral movements (e.g., Wildgruber et al. 1996; Riecker et al. 2000b; Bonilha et al. 2006; Sörös et al. 2006; Dhanjal et al. 2008), none have focused on the SPGI, the only region that has been explicitly argued to be functionally specialized for articulatory control. Finally, speech production requires complex coordination of respiratory processes, and it has been suggested that the insula may play a role in this coordination (Ackermann and Riecker 2010).
We additionally included a spatial working memory task to test whether responses to articulation in the anterior insula may be due to responses to any demanding task. In particular, the anterior insula has been previously reported to be active across a wide range of manipulations, plausibly due to this region being part of the domain-general fronto-parietal cognitive control, or “multiple-demand,” network (e.g., Duncan and Owen 2000; Duncan 2010; Fedorenko et al. 2013). The regions of this network participate in many goal-directed behaviors and are robustly activated by working memory manipulations (e.g., Fedorenko et al. 2013). The inclusion of this task thus allows us to distinguish between the alleged articulation-responsive portions of the anterior insula and its domain-general portions.
MATERIALS AND METHODS
Participants.
Twenty right-handed native speakers of English (19 females; mean age = 26) from the University of South Carolina and surrounding community volunteered for the study. When applicable, they received course credit for participating. All participants gave informed consent in accordance with protocols reviewed and approved by the Institutional Review Board at University of South Carolina and were naïve to the purposes of the study.
Design, materials, and procedure.
For all the tasks except for spatial working memory, participants viewed 2,500-ms-long video clips of a female actor (native English speaker) and were asked to imitate each action (during the subsequent 2,500 ms) in a blocked design. The clips were created so that the duration was as close to the 2,500 ms as possible, and some behavioral piloting of the tasks suggested that participants generally follow the timing closely in their imitations. Each block included four trials and lasted 20 s. The sound was delivered via Resonance Technology's MRI-compatible Serene Sound headphones.
For the articulation task, 72 bisyllabic pseudowords were used: the second syllable was always a simple consonant-vowel (CV) syllable (e.g., fʌ, si, gɒ), and the first syllable varied between the hard and the easy condition. The hard condition included phonotactically legal consonant clusters, including many that require fast transitions between articulator positions, and took the form of CCVCC syllables (e.g., kru:rd, dreilf, sfi:lt), and the easy condition included no clusters and took the form of CV syllables (e.g., mɔ:, koi, rei). For example, sample items in the hard condition sounded like snoirb-gʌ or kweips-ki, and sample items in the easy condition sounded like lɔ:-kʌ or gu:mɒ (a complete list of the pseudowords is available from the authors upon request).1 Participants completed two 460-s-long runs, each containing nine blocks per condition and five fixation blocks. Comparison tasks used 10 tokens each of vowels (e.g., i: as in feet), nonspeech oral movements (e.g., pucker of the lips), or respiration sequences (e.g., inhale slowly then exhale quickly; see appendix for a complete list of the materials). Each trial (2,500 ms) included a single vowel, oral movement or respiration sequence. The oral movements and respiration conditions did not involve phonation. Participants completed three 480-s-long runs, each containing six blocks per condition and six fixation blocks.
For the spatial working memory task (Fedorenko et al. 2013), participants saw a 3 × 4 grid and kept track of four or eight locations in the easy and hard condition, respectively, in a blocked design. At the end of each trial, participants had to choose the grid with the correct locations in a two-alternative forced-choice question. Each block included four trials and lasted 34 s. Participants completed two 404-s-long runs, each containing five blocks per condition and five 16-s-long fixation blocks.
Condition order was counterbalanced across runs and participants for all tasks. The entire scanning session lasted ∼1.5 h.
fMRI data acquisition.
Structural and functional data were collected on a whole-body 3 Tesla Siemens Trio scanner with a 12-channel head coil at the McCausland Center for Brain Imaging. T1-weighted structural images were collected in 192 axial slices with 1-mm isotropic voxels [repetition time (TR) = 2,250 ms, echo time (TE) = 4.2 ms]. Functional, blood oxygenation level-dependent (BOLD) data were acquired using an EPI sequence (with a 76 degree flip angle), with the following acquisition parameters: 31 3.75-mm-thick near-axial slices acquired in sequential order (with 25% distance factor), 2 × 2 mm in-plane resolution, field of view (FoV) = 208 × 20 8 mm, A > P phase encoding, TR = 2,000 ms, and TE = 30 ms. The first 8 s of each run were excluded to allow for steady state magnetization.
fMRI data analyses.
MRI data were analyzed using SPM5 and custom MATLAB scripts (available from the first author). Each subject's data were motion corrected. Then the data were preprocessed in two different ways: for most analyses, the data were normalized into a common brain space (the Montreal Neurological Institute, MNI template), but for the analysis that used individually defined anatomical ROI masks the data were analyzed in native space. Finally, the data were resampled into 2-mm isotropic voxels and smoothed using a 4-mm Gaussian filter, high-pass filtered (at 200 s).
ROIs were defined anatomically or using a combination of anatomy and functional data in each individual participant. For functional definitions, we used the hard articulation > fixation contrast in the localizer task to pick out voxels that are most sensitive to articulatory demands. To ensure that we captured the region in question, we defined the SPGI in three different ways, as described next.
First, we used the mask of the SPGI hand-drawn in the MNI space by Dronkers and colleagues (size: 79 voxels; Fig. 1A). This mask was intersected with each individual subject's activation map for the hard articulation > fixation contrast: voxels within each mask were sorted based on their t-values, and the top 10% of voxels were chosen as that subject's functional region of interest (fROI). Given the small size of this mask, individual fROIs are only 7 voxels in size. However, the observed response pattern (see results) was similar even when no subject-specific functional masking was used, i.e., when the same set of 79 voxels were used in each participant.
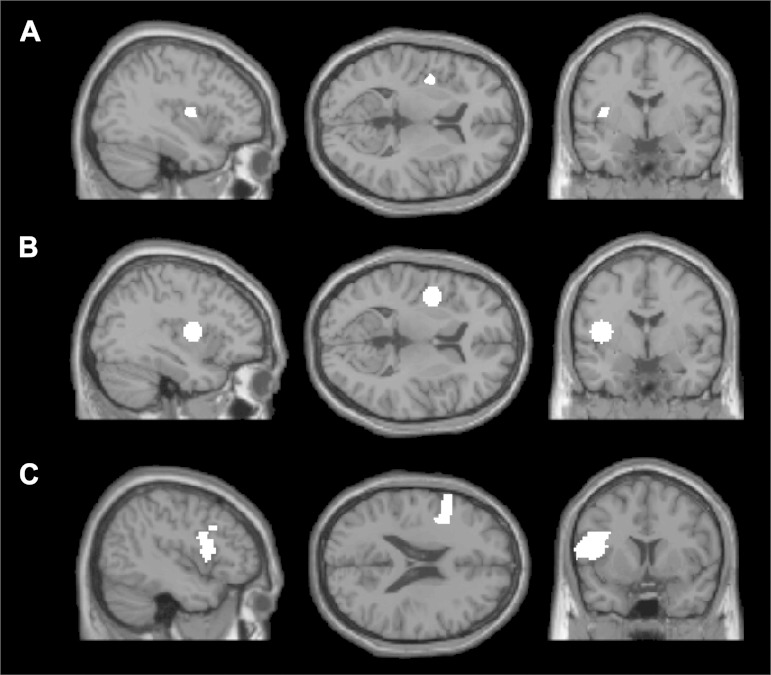
Fig. 1.
The anatomical region of interest (ROI) masks, shown on 3 sample slices on the Montreal Neurological Institute (MNI)152 brain template: A: superior precentral gyrus of the insula (SPGI) mask hand-created by Dronkers and colleagues. B: SPGI mask created by drawing a sphere (radius = 10 mm) around the peak coordinate reported in Dronkers (1996). C: opercular portion of the left inferior frontal gyrus (LIFG). Each mask was intersected with each subject's activation map for the hard articulation > fixation contrast, and the top 10% of voxels in each mask were taken as that subject's functional ROI.
Second, we created a spherical mask of the SPGI by taking the peak coordinate from Dronkers (1996), {−41, −2, 10} and drawing a sphere around it with a radius of 10 mm (size: 515 voxels; Fig. 1B). This mask was intersected with each individual subject's activation map for the hard articulation > fixation contrast, as described above. Thus, individual fROIs are 51 voxels in size under this definition.
Third, the SPGI was defined manually on each individual subject's anatomy (e.g., Nieto-Castañon et al. 2003; Fig. 2). This manual delineation was performed by one of the authors (L. Bonilha), who is a neurologist with special training in quantitative neuroanatomy (e.g., Bonilha et al. 2004). The posterior short gyrus of the insula was identified on a sagittal slice, and its cortex from the middle point upwards was included in the ROI. This definition was based on the descriptions in the literature (Baldo et al. 2011) and the senior author's (J. Fridriksson) prior discussions with Dronkers and colleagues. These individual anatomical ROIs are 31 voxels on average (SE = 2.9), varying between 6 and 55 voxels. No functional masking was used for this analysis, due to the small size of some of these ROIs.
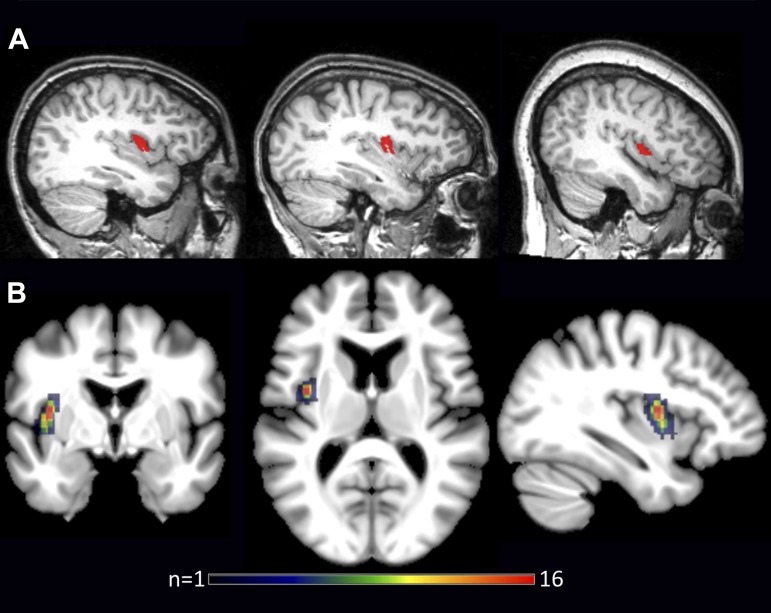
Fig. 2.
Subject-specific anatomical SPGI masks shown in sagittal view (native space) for 3 individual participants (A) and as a binary overlay map (MNI space; B), illustrating the variability across participants [the maximum overlap, n = 16, occurred at MNI coordinates {−37, −2, 10}, which is remarkably close to the peak coordinate reported in Dronkers (1996), i.e., {−41, −2, 10}].
To demonstrate that our localizer task produces robust responses somewhere in the brain, we examined the brain region originally hypothesized to support speech articulation (Broca 1861, 1865; also Hillis et al. 2004), i.e., the opercular portion of the LIFG (Tzourio-Mazoyer et al. 2002; Fig. 1C; size: 1,038 voxels), in addition to our three definitions of the SPGI. This mask was intersected with each individual subject's activation map for the hard articulation > fixation contrast, as in the first two definitions of the SPGI. Thus individual fROIs are 103 voxels in size under this definition.
For the analyses where functional masking was used, to estimate the responses to vowel production, nonspeech oral movements, and respiration, all the data from the localizer experiment were used for defining the fROIs. To estimate the responses to the conditions of the localizer task, we used an across-runs cross-validation procedure. In particular, each subject's activation map was computed for the hard articulation > fixation contrast using the first run, and the 10% of voxels with the highest t-value within a given ROI mask were selected as that subject's fROI. The response was then estimated using the second run. This procedure was repeated, using the second run for defining the fROI and the first run for estimating the response. Finally, the responses were averaged across runs to derive a single response magnitude for each condition in a given ROI/subject. This n-fold cross-validation procedure (where n is the number of runs) allows one to use all of the data for defining the ROIs and for estimating the responses (see Nieto-Castañon and Fedorenko 2012, for discussion), while ensuring the independence of the data used for fROI definition vs. response estimation (Kriegeskorte et al. 2009). Statistical tests across subjects were performed on the percent signal change values extracted from the fROIs as defined above.
RESULTS
Before asking our critical question of whether the SPGI is functionally specialized for articulation, we need to establish that this region responds during articulation. A further question is whether the SPGI responds more to complex than simple articulation, as has been argued by Baldo et al. (2011). The results are shown in Table 1 and Fig. 3 (grey bars). The fROIs defined using either of the two SPGI masks based on the definition of Dronkers [“SPGI” and “SPGIsph” (sph = spherical) in Table 1 and Fig. 3] show significant, albeit small, responses to both the hard and easy articulation conditions relative to the low-level fixation baseline but no reliable difference between the two conditions (ts < 1.34). When using subject-specific anatomical SPGI masks (“SPGI subj-spec anat” in Table 1 and Fig. 3), responses to the two articulation conditions are not significantly different from the low-level baseline (t < 1), with no difference between the hard and easy articulation conditions (t < 1). Finally, in the LIFGop, a region originally implicated in articulation (Broca 1861, 1865; Hillis et al. 2004), we find large and robust responses to both articulation conditions and a highly reliable hard > easy articulation effect (P < 0.001).
Table 1.
Effects for 3 contrasts
| fROI | HardArtic > Fix | EasyArtic > Fix | Hard > EasyArtic |
|---|---|---|---|
| SPGI | t = 1.77; P < 0.05 | t = 1.89; P < 0.05 | t < 1; n.s. |
| SPGIsph | t = 3.75; P < 0.001 | t = 3.68; P < 0.001 | t = 1.33; n.s. |
| SPGI subj-spec anat | t < 1; n.s. | t < 1; n.s. | t < 1; n.s. |
| LIFGop | t = 8.00; P < 0.0001 | t = 7.25; P < 0.0001 | t = 3.78; P < 0.001 |
Effect for 3 contrasts (hard articulation > fixation, easy articulation > fixation, and hard > easy articulation) estimated in data not used for defining the functional regions of interest (ROIs) using across-runs cross-validation and using one-tailed paired-samples t-tests. Degrees of freedom = 19. SPGI, superior precentral gyrus of the insula; sph, spherical; subj-spec anat, subject-specific anatomical; LIFGop, left inferior frontal gyrus region.
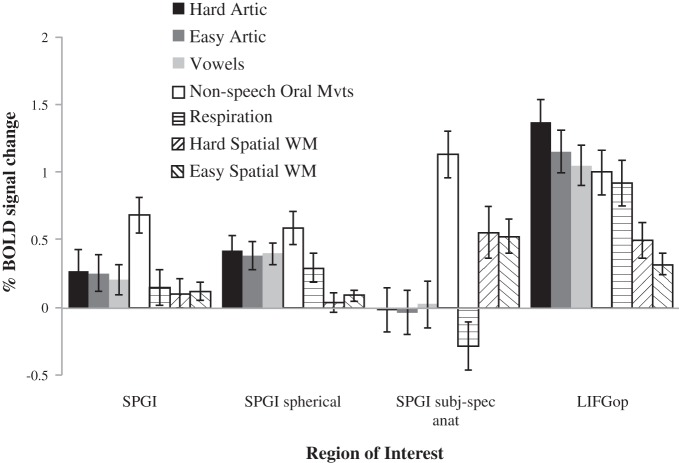
Fig. 3.
Responses [in %blood oxygenation level-dependent (BOLD) signal change] in the SPGI defined using 3 different masks (as described above) to the hard and easy articulation conditions of the localizer experiment, the 3 conditions designed to evaluate the functional specificity of the SPGI for articulation, and the spatial working memory (WM) task designed to evaluate potential overlap with the cognitive control network. Error bars denote means ± SE across participants.
Given that the SPGI shows some response to articulation, at least under the functional definitions (even though this response is about a third to a quarter of the size of that observed in the LIFGop fROI), we can ask whether the SPGI responds to articulatory processing in a functionally specific manner. If so, then it should be engaged only (or at least much more strongly) during articulation than during the other, nonspeech, conditions. The results are shown in Fig. 3. All three conditions (vowel production, nonspeech oral movements, and respiration) produce some response in the SPGI, defined functionally (Ps < 0.05). In the subject-specific anatomical ROI analysis, responses to vowel production and respiration are not different from the low-level baseline (t < 1), but the nonspeech oral movements condition produces a large and significantly robust response (P < 0.0001). Thus, across the three ways of defining the SPGI, the nonspeech oral movement condition produces a response that is stronger than the two articulation conditions. This pattern provides evidence against a functionally selective engagement of the SPGI in articulation.
The response in the LIFGop fROI also does not look strongly selective for articulation, but the numerical pattern of responses is at least more consistent with a selective role in articulatory processing: the response is numerically the strongest to the two articulation conditions (with the harder condition producing a reliably stronger response than the easier condition, as discussed above); furthermore, the hard articulation condition produces a response that is reliably stronger than that produced by the vowels condition (P < 0.05, by a one-tailed paired-samples t-test), the nonspeech oral movements condition (P < 0.05), and the respiration condition (P < 0.01).
Finally, with respect to the question of overlap between regions sensitive to articulatory demands and regions sensitive to cognitive effort in general (e.g., Duncan 2010; Fedorenko et al. 2013), the SPGI defined using the definition of Dronkers (SPGI and SPGIsph) does not respond much to spatial working memory (WM). When using subject-specific anatomical SPGI masks, we see some response to spatial WM, although the hard condition does not produce a reliably stronger response than the easier condition (t < 1). These results suggest that our localizer contrast (hard articulation > fixation) does not overlap much with the domain-general cognitive control or “multiple demand” network (Duncan 2010) in the vicinity of the SPGI, in spite of the fact that this network has a prominent component in the anterior insula/frontal operculum. We also see an above-baseline response to the two spatial WM conditions in the LIFGop fROI, but the response to these conditions is substantially and reliably (P < 0.001) lower than that elicited by the articulation conditions.
DISCUSSION
To summarize the key findings: in contrast to prior claims (e.g., Shuren 1993; Dronkers 1996; Donnan et al. 1997; Nagao et al. 1999; Wise et al. 1999; Nestor et al. 2003; Ogar et al. 2006; Baldo et al. 2011), the SPGI, responds weakly, if at all, during articulation of even difficult-to-pronounce words and is not modulated by articulatory complexity. This is in contrast to the opercular portion of the left IFG, which shows a large and robust response during articulation and responds reliably more strongly during the more demanding articulation condition. Critically, even when using the most sensitive methods available, functionally defining articulation-responsive regions in each participant individually (e.g., Nieto-Castañon and Fedorenko 2012) or manually defining the SPGI's anatomical boundaries in each individual brain (e.g., Nieto-Castañon et al. 2003), the SPGI does not exhibit a functionally selective response for speech articulation (cf. Dronkers 1996). Instead, under all definitions, it responds more strongly to the production of nonspeech oral movements, consistent with some prior fMRI studies that failed to observe speech selectivity in the anterior insula using traditional group-based analyses (e.g., Riecker et al. 2000b; Bonilha et al. 2006).
How do we reconcile the lack of a strong and selective response to articulation in the SPGI in the current study with neuropsychological data linking the left anterior insula damage to apraxia of speech and with fMRI studies that have observed responses to articulation in the anterior insula? We argue that these earlier results are due to high base rates of ischemic damage (in cases of stroke) and activation (in fMRI studies) in the anterior insula, combined with a high probability of joint damage between the anterior insula and nearby brain structures that may be critical for speech production.
Hillis et al. (2004) made this point clearly in their critique of Dronkers' (1996) original lesion overlap study. They argued that although it may be the case that all patients with apraxia of speech have SPGI damage, this does not imply that the SPGI supports speech articulation. In particular, given the anterior insula's vulnerability to ischemic damage (Caviness et al. 2002; Finley et al. 2003), there may be just as many, or more, patients with SPGI damage but no apraxia of speech. Hillis et al. (2004) examined the probability of apraxia of speech given damage to the SPGI and failed to observe a relationship between the SPGI damage (or insular damage more generally) and apraxia of speech, although they did observe a relationship between apraxia of speech and left posterior inferior frontal damage.
With respect to fMRI, Yarkoni et al. (2011) made a similar argument: a failure to control for base rates of activation in a brain region may lead to flawed inferences about this region's contribution to some mental process. In particular, although some parts of the anterior insula may quite consistently emerge in studies of articulation (e.g., Wise et al. 1999; Riecker et al. 2000a; Bohland and Guenther 2006; Moser et al. 2009), the link between the anterior insula and articulation may not be warranted, because this region is reported across many fMRI studies that do not target articulation (e.g., Duncan and Owen 2000; Duncan 2010; Fedorenko et al. 2013).
To conclude, although we do not rule out that the SPGI, or other parts of the insula, may play some role in articulation, it does not appear likely that the SPGI is a core speech articulation region given its lack of a selective response and, under some definitions, any response, to articulatory demands (cf. Dronkers 1996; Wise et al. 1999; Ogar et al. 2006; Baldo et al. 2011). In contrast, LIFGop, a region originally implicated in speech articulation (Broca 1861, 1865), showed a large and robust response to articulation, with a reliably greater response to the hard compared with the easy articulation condition. Furthermore, the response to the hard articulation condition in this region was slightly but reliably higher than the response to the vowels, nonspeech oral movements, and respiration conditions and much higher than the response to the spatial working memory conditions, suggesting at least some degree of articulatory selectivity. Thus LIFGop, but probably not the SPGI, should be probed further in future work on articulation, along with other regions that are engaged by articulatory demands (e.g., Duffy 2005; Bohland and Guenther 2006; Brown et al. 2009; Moser et al. 2009; Peeva et al. 2010; Grabski et al. 2012).
GRANTS
This work was supported by National Institute of Deafness and Other Communications Disorders Grant DC-009571 (to J. Fridriksson), National Institute of Child Health and Human Development Grant HD-057522 (to E. Fedorenko), and the Simons Center for the Social Brain at MIT Grant (to E. Fedorenko).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: E.F., P.F., K.S., and J.F. conception and design of research; E.F. and L.B. analyzed data; E.F., P.F., K.S., L.B., and J.F. interpreted results of experiments; E.F. prepared figures; E.F. drafted manuscript; E.F., P.F., K.S., and J.F. edited and revised manuscript; E.F., P.F., K.S., L.B., and J.F. approved final version of manuscript; P.F. and K.S. performed experiments.
ACKNOWLEDGMENTS
We thank Peter Graff and Morris Alper for help in the construction of pseudowords, Taylor Hanayik for help with recording the materials, Nina Dronkers for providing the SPGI mask, and Chris Rorden, Alfonso Nieto-Castañon, and Sheeba Anteraper for help with some preprocessing steps. For comments on this work, we thank the audience at the Neurobiology of Language Conference (2013, San Diego) and Nancy Kanwisher.
Appendix
Table A1 shows the materials used in the nonspeech conditions.
Table A1.
Materials used in the nonspeech conditions
| Vowels | Oral Movements | Respiration Sequences |
|---|---|---|
| /i/ as in feet | Stick tongue out straight | Regular breathing |
| /I/ as in it | Puff out your cheeks | Long inhale, short exhale |
| /ɛ/ as in bed | Bite your lower lip | Short inhale, long exhale |
| /æ/ as in apple | Smile/Show your teeth | Breath hold for a second, then release |
| /ʌ/ as in up | Lick your lips | Alternate inhale/exhale quickly |
| /u/ as in too | Pucker your lips | Inhale short, short, exhale long |
| /ʊ/ as in book | Stick tongue out, then move up then down (touch chin/nose) | Inhale long, exhale short, short |
| /o/ as in go | Push inside of cheek with tongue (left then right) | Exaggerated slow breathing |
| /ɔ/ as in dog | Press lips tightly together | Inhale and exhale quickly, then long inhale/exhale |
| /e/ as in eight | Pucker then smile | Inhale and exhale long, then inhale and exhale quickly |
Footnotes
All the consonant clusters were legal (i.e., attested in English). Sometimes, illegal clusters resulted across the syllable boundaries (e.g., ps-k or rb-g). However, these kinds of clusters often occur in natural speech (e.g., “maps can,” or “crepes cooked,” or “Barb gave,” etc.).
REFERENCES
- Ackermann H, Riecker A. The contribution(s) of the insula to speech production: a review of the clinical and functional imaging literature. Brain Struct Funct 214: 419–433, 2010. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Wilkins DP, Ogar J, Willock S, Dronkers NF. Role of the precentral gyrus of the insula in complex articulation. Cortex 47: 800–807, 2011. [DOI] [PubMed] [Google Scholar]
- Ballard KJ, Robin DA, Folkins JW. An integrative model of speech motor control: a response to Ziegler. Aphasiology 17: 37–48, 2003. [Google Scholar]
- Bohland JW, Guenther FH. An fMRI investigation of syllable sequence production. Neuroimage 32: 821–841, 2006. [DOI] [PubMed] [Google Scholar]
- Bonilha L, Moser D, Rorden C, Baylis G, Fridriksson J. Speech apraxia without oral apraxia: can normal brain function explain the physiopathology? Neuroreport 17: 1027–1031, 2006. [DOI] [PubMed] [Google Scholar]
- Bonilha L, Kobayashi E, Cendes F, Min Li L. Protocol for volumetric segmentation of medial temporal structures using high-resolution 3-D magnetic resonance imaging. Hum Brain Mapp 22: 145–154, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broca P. Remarques sur le siège de la faculté du langage articulé, suivies d'une observation d'aphéme (perte de la parole). Bull Soc Anat Paris 36: 330–357, 1861. [Google Scholar]
- Broca P. Localisation of speech in the third left frontal convolution. Bull Soc Anthropol Paris 6: 377–393, 1865. [Google Scholar]
- Brown S, Laird AR, Pfordresher PQ, Thelen SM, Turkeltaub P, Liotti M. The somatotopy of speech: phonation and articulation in the human motor cortex. Brain Cogn 70: 31–41, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunton K. Speech versus nonspeech: different tasks, different neural organization. Semin Speech Lang 29: 267, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviness V, Makris N, Montinaro E, Sahin N, Bates J, Schwamm L, Caplan D, Kennedy DN. Anatomy of stroke, part I: an MRI-based topographic and volumetric system of analysis. Stroke 33: 2549–2556, 2002. [DOI] [PubMed] [Google Scholar]
- Davis C, Kleinman JT, Newhart M, Gingis L, Pawlak M, Hillis AE. Speech and language functions that require a functioning Broca's area. Brain Lang 105: 50–58, 2008. [DOI] [PubMed] [Google Scholar]
- Dhanjal NS, Handunnetthi L, Patel MC, Wise RJ. Perceptual systems controlling speech production. J Neurosci 28: 9969–9975, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnan GA, Darby DG, Saling MM. Identification of brain region for coordinating speech articulation. Lancet 349: 221, 1997. [DOI] [PubMed] [Google Scholar]
- Dronkers NF. A new brain region for coordinating speech articulation. Nature 384: 159–161, 1996. [DOI] [PubMed] [Google Scholar]
- Duffy JR. Motor Speech Disorders: Substrates, Differential Diagnosis, and Management (2nd ed.). St. Louis, MO: Elsevier Mosby, 2005. [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci 23: 475–483, 2000. [DOI] [PubMed] [Google Scholar]
- Duncan J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn Sci 14: 172–179, 2010. [DOI] [PubMed] [Google Scholar]
- Dworkin JP, Aronson AE. Tongue strength and alternate motion rates in normal and dysarthric subjects. J Commun Disord 19: 115–132, 1986. [DOI] [PubMed] [Google Scholar]
- Fedorenko E, Hsieh PJ, Nieto-Castanon A, Whitfield-Gabrieli S, Kanwisher N. A new method for fMRI investigations of language: defining ROIs functionally in individual subjects. J Neurophysiol 104: 1177–1194, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Duncan J, Kanwisher N. Broad domain generality in focal regions of frontal and parietal cortex. Proc Natl Acad Sci USA 110: 16616–16621, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley A, Saver J, Alger J, Pregenzer M, Leary M, Ovbiagele B. Diffusion weighted imaging assessment of insular vulnerability in acute middle cerebral artery infarctions (Abstract). Stroke 34: 259, 2003. [Google Scholar]
- Grabski K, Lamalle L, Vilain C, Schwartz JL, Vallée N, Tropres I, Baciu M, Le Bas JF, Sato M. Functional MRI assessment of orofacial articulators: neural correlates of lip, jaw, larynx, and tongue movements. Hum Brain Mapp 33: 2306–2321, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis AE, Work M, Barker PB, Jacobs MA, Breese EL, Maurer K. Re-examining the brain regions crucial for orchestrating speech articulation. Brain 127: 1479–1487, 2004. [DOI] [PubMed] [Google Scholar]
- Kent RD. Research on speech motor control and its disorders: a review and prospective. J Commun Disord 33: 391–428, 2000. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci 12: 535–540, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPointe LL, Wertz RT. Oral-movement abilities and articulatory characteristics of brain-injured adults. Percept Mot Skills 39: 39–46, 1974. [DOI] [PubMed] [Google Scholar]
- McHenry MA, Minton JT, Wilson RL, Post YV. Intelligibility and nonspeech orofacial strength and force control following traumatic brain injury. J Speech Lang Hear Res 37: 1271–1283, 1994. [DOI] [PubMed] [Google Scholar]
- Mohr JP, Pessin MS, Finkelstein S, Funkenstein HH, Duncan GW, Davis KR. Broca aphasia: pathologic and clinical. Neurology 28: 311–324, 1978. [DOI] [PubMed] [Google Scholar]
- Moore CA, Ruark JL. Does speech emerge from earlier appearing oral motor behaviors? J Speech Lang Hear Res 39: 1034–1047, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser D, Fridriksson J, Bonilha L, Healy EW, Baylis G, Baker JM, Rorden C. Neural recruitment for the production of native and novel speech sounds. Neuroimage 46: 549–557, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao M, Takeda K, Komori T, Isozaki E, Hirai S. Apraxia of speech associated with an infarct in the precentral gyrus of the insula. Neuroradiology 41: 356–357, 1999. [DOI] [PubMed] [Google Scholar]
- Nestor PJ, Graham NL, Fryer TD, Williams GB, Patterson K, Hodges JR. Progressive non-fluent aphasia is associated with hypometabolism centred on the left anterior insula. Brain 126: 2406–2418, 2003. [DOI] [PubMed] [Google Scholar]
- Nieto-Castanon A, Ghosh SS, Tourville JA, Guenther FH. Region of interest based analysis of functional imaging data. Neuroimage 19: 1303–1316, 2003. [DOI] [PubMed] [Google Scholar]
- Nieto-Castañón A, Fedorenko E. Subject-specific functional localizers increase sensitivity and functional resolution of multi-subject analyses. Neuroimage 63: 1646–1669, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S, Forstmann BU, Wagenmakers E. Erroneous analyses of interactions in neuroscience: a problem of significance. Nat Neurosci 14: 1105–1107, 2011. [DOI] [PubMed] [Google Scholar]
- Ogar J, Slama H, Dronkers NF, Amici S, Gorno-Tempini M. Apraxia of speech: an overview. Neurocase 11: 427–432, 2005. [DOI] [PubMed] [Google Scholar]
- Ogar J, Willock S, Baldo JV, Wilkins D, Ludy C, Dronkers NF. Clinical and anatomical correlates of apraxia of speech. Brain Lang 97: 343–350, 2006. [DOI] [PubMed] [Google Scholar]
- Ostry DJ, Flanagan JR. Human jaw movement in mastication and speech. Arch Oral Biol 34: 685–693, 1989. [DOI] [PubMed] [Google Scholar]
- Ostry DJ, Munhall KG. Control of jaw orientation and position in mastication and speech. J Neurophysiol 71: 1528–1545, 1994. [DOI] [PubMed] [Google Scholar]
- Peeva MG, Guenther FH, Tourville JA, Nieto-Castanon A, Anton JL, Nazarian B, Alario FX. Distinct representations of phonemes, syllables, and supra-syllabic sequences in the speech production network. Neuroimage 50: 626–638, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Gorno-Tempini ML, Graham KS, Biggio N, Mechelli A, Patterson K, Noppeney U. Normal and pathological reading: converging data from lesion and imaging studies. Neuroimage 20: S30–S41, 2003. [DOI] [PubMed] [Google Scholar]
- Richardson JD, Fillmore P, Rorden C, Lapointe LL, Fridriksson J. Reestablishing Broca's initial findings. Brain Lang 123: 125–130, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riecker A, Ackermann H, Wildgruber D, Dogil G, Grodd W. Opposite hemispheric lateralization effects during speaking and singing at motor cortex, insula and cerebellum. Neuroreport 11: 1997–2000, 2000a. [DOI] [PubMed] [Google Scholar]
- Riecker A, Ackermann H, Wildgruber D, Mayer J, Dogil G, Haider H, Grodd W. Articulatory/phonetic sequencing at the level of the anterior perisylvian cortex: a functional magnetic resonance imaging (fMRI) study. Brain Lang 75: 259–276, 2000b. [DOI] [PubMed] [Google Scholar]
- Riecker A, Mathiak K, Wildgruber D, Erb M, Hertrich I, Grodd W, Ackermann H. fMRI reveals two distinct cerebral networks subserving speech motor control. Neurology 64: 700–706, 2005. [DOI] [PubMed] [Google Scholar]
- Riecker A, Brendel B, Ziegler W, Erb M, Ackermann H. The influence of syllable onset complexity and syllable frequency on speech motor control. Brain Lang 107: 102–113, 2008. [DOI] [PubMed] [Google Scholar]
- Shankweiler D, Harris K, Taylor M. Electromyographic studies of articulation in aphasia. Arch Phys Med Rehabil 49: 1–8, 1968. [PubMed] [Google Scholar]
- Shuren J. Insula and aphasia. J Neurol 240: 216–218, 1993. [DOI] [PubMed] [Google Scholar]
- Smith A. The control of orofacial movements in speech. Crit Rev Oral Biol Med 3: 233–267, 1992. [DOI] [PubMed] [Google Scholar]
- Sörös P, Sokoloff LG, Bose A, McIntosh AR, Graham SJ, Stuss DT. Clustered functional MRI of overt speech production. Neuroimage 32: 376–387, 2006. [DOI] [PubMed] [Google Scholar]
- Thompson EC, Murdoch BE, Stokes PD. Lip function in subjects with upper motor neuron type dysarthria following cerebrovascular accidents. Eur J Disord Commun 30: 451–466, 1995a. [DOI] [PubMed] [Google Scholar]
- Thompson EC, Murdoch BE, Stokes PD. Tongue function in subjects with upper motor neuron type dysarthria following cerebrovascular accident. J Med Speech Lang Pathol 3: 27–40, 1995b. [DOI] [PubMed] [Google Scholar]
- Trupe L, Varma DD, Gomez Y, Race D, Leigh R, Hillis AE, Gottesman RF. Chronic apraxia of speech and Broca's area. Stroke 44: 740–744, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15: 273–289, 2002. [DOI] [PubMed] [Google Scholar]
- Wildgruber D, Ackermann H, Klose U, Kardatzki B, Grodd W. Functional lateralization of speech production at primary motor cortex: a fMRI study. Neuroreport 7: 2791–2796, 1996. [DOI] [PubMed] [Google Scholar]
- Wise RJ, Greene J, Buchel C, Scott SK. Brain regions involved in articulation. Lancet 353: 1057–1061, 1999. [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods 8: 665–670, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler W. Speech motor control is task-specific: evidence from dysarthria and apraxia of speech. Aphasiology 17: 3–36, 2003a. [Google Scholar]
- Ziegler W. To speak or not to speak: distinctions between speech and nonspeech motor control. Aphasiology 17: 99–105, 2003b. [Google Scholar]
- Ziegler W. Review. Aphasiology 17: 3–36, 2003c. [Google Scholar]