Abstract
Vertical fusional vergence (VFV) normally compensates for slight vertical heterophorias. We employed magnetic resonance imaging to clarify extraocular muscle contributions to VFV induced by monocular two-prism diopter (1.15°) base-up prism in 14 normal adults. Fusion during prism viewing requires monocular infraduction. Scans were repeated without prism, and with prism shifted contralaterally. Contractility indicated by morphometric indexes was separately analyzed in medial and lateral vertical rectus and superior oblique (SO) putative compartments, and superior and inferior horizontal rectus extraocular muscle putative compartments, but in the whole inferior oblique (IO). Images confirmed appropriate VFV that was implemented by the inferior rectus (IR) medial compartment contracting ipsilateral and relaxing contralateral to prism. There was no significant contractility in the IR lateral compartment. The superior but not inferior lateral rectus (LR) compartment contracted significantly in the prism viewing eye, but not contralateral to prism. The IO contracted ipsilateral but not contralateral to the prism. In the infraducting eye, the SO medial compartment relaxed significantly, while the lateral compartment was unchanged; contralateral to prism, the SO lateral compartment contracted, while the medial compartment was unchanged. There was no contractility in the superior or medial rectus muscles in either eye. There was no globe retraction. We conclude that the vertical component of VFV is primarily implemented by IR medial compartment contraction. Since appropriate vertical rotation is not directly implemented, or is opposed, by associated differential LR and SO compartmental activity, and IO contraction, these actions probably implement a torsional component of VFV.
Keywords: eye movement, extraocular muscle, magnetic resonance imaging, vertical vergence
vertical binocular misalignment occurs under both normal and pathological conditions and induces diplopia, unless compensated by motor or sensory processes. Geometric considerations produce physiological vertical image disparity during near viewing in tertiary gazes (Schor et al. 1994). Some vertical image disparity can be fused into a single percept by sensory and motor mechanisms. Sensory fusion, which depends upon the vertical extent of Panum's fusional area (Schor and Tyler 1981), is typically limited to only about 0.25° amplitude (Hara et al. 1998). Most fusion of vertical disparity is therefore attributed to a dysconjugate motor mechanism, vertical fusional vergence (VFV).
Maximal amplitudes of VFV are typically much smaller than horizontal fusional vergence amplitudes (Sharma and Abdul-Rahim 1992), being typically 1–1.5° (Mottier and Mets 1990; von Noorden 1990). The amplitude of normal VFV is also greater at near than at distance (Bharadwaj et al. 2007; Hara et al. 1998), although viewing distance does not influence the sensory component of vertical fusion (Hara et al. 1998).
The extraocular muscle (EOM) mechanisms implementing VFV have been historically, yet controversially deduced from observed eye movements, as well as conventional notions of EOM anatomy and actions. Based upon video observations of cyclotorsion and globe translation during VFV of up to 1° in central gaze, Enright (1992) supposed that VFV may be implemented exclusively by the superior oblique (SO) muscles, acting against fixed tone in the inferior oblique (IO) muscles. Enright's view was supported by an afterimage study of VFV in response to a three- or four-prism diopter (PD; roughly 1.5 or 2°) prism that was suggestive of an oblique EOM contribution (Cheeseman and Guyton 1999). However, Enright's postulated SO mechanism was disputed by observations that the relative contributions of the two eyes were independent of the horizontal gaze angle, and therefore inconsistent with expected variation associated with SO actions (Van Rijn and Collewijn 1994). The foregoing discrepancy may have been due to small numbers of subjects in each study, and to what has since emerged to be idiosyncratic properties of VFV. Irsch et al. used scleral magnetic search coils to demonstrate cycloversion (similarly-directed torsion in the two eyes) during VFV, and argued that Enright was correct in attributing VFV to the oblique EOMs (Irsch et al. 2013). The VFV of standing subjects in response to a two PD base-down prism was found to be greater when the nondominant eye viewed through the prism than when the dominant eye viewed through the prism; both VFV responses exceeded the geometric demand of the prism (Matheron et al. 2008). In eight subjects viewing a virtual reality display, neither cyclovergence nor cycloversion was correlated with VFV (Hara et al. 1998). Therefore, even the direction of torsion associated with VFV has been considered individually idiosyncratic (Steffen et al. 2002).
Additional studies of prism-induced VFV reported the orientation of Listing's plane (LP), but again have been inconsistent. Mikhael et al. (1995) reported that monocular vertical prisms of 0.75–3.5° rotated the vertical primary position of either eye in proportion to the amount of VFV, but variably rotated horizontal primary position. Straumann and Muller (1994) found that fusion with 0.75° vertical prism induced nasal rotation of LP without change in vertical primary position. Steffen et al. (2002) found no consistent changes in horizontal primary position induced by 3.9–6.2° VFV, but a significant change in relative binocular orientation of the primary positions.
Analysis of the relationship between torsional and vertical eye movements has been used to infer EOM mechanisms of VFV in phoria-adapted normal subjects (Irsch et al. 2013) and pathological vertical heterophorias attributed to SO weakness (Mudgil et al. 2002). In 10 subjects with SO weakness, the kinematic behavior was highly diverse (Mudgil et al. 2002). The ratio of cyclovergence to VFV ranged from 0.2 to 1.5, and three different EOM actuators were inferred in different individuals: 1) predominantly the oblique EOMs; 2) predominantly the vertical rectus EOMs; and 3) the paretic SO in the hypertropic eye plus the contralateral superior rectus (SR) (Cheeseman and Guyton 1999). This study offered the dubious postulate that a weak or paralyzed SO implements VFV in some individuals.
Compartmentalization has recently emerged as a novel feature of EOM function that might alter the kinematic assumptions foundational to prior studies of VFV. Because human rectus EOM tendons are wide (Apt 1980; Apt and Call 1980; Demer 2009), their intrinsic parallel fibers individually insert at substantially differing scleral sites that have correspondingly different oculorotary actions. The motor nerve to each horizontal rectus EOM divides into superior and inferior branches supplying non-overlapping distributions, forming segregated neuromuscular compartments maintained throughout their entire lengths (da Silva Costa et al. 2011; Peng et al. 2010). These neuromuscular compartments contain roughly parallel EOM fibers having only sparse lateral interconnections (Demer et al. 2010; Lim et al. 2007) and thus minimal transverse mechanical coupling. Consequently, only about 5% of passive tensile loading of one transverse half of an isolated rectus EOM tendon is reflected in force observed in the opposite half-tendon (Shin et al. 2012). Calcium-induced depolarization in one bovine rectus compartment generates contractile force mostly confined to that compartment (Shin et al. 2014). The inferior rectus (IR) muscle is innervated diffusely by one intramuscular motor nerve branch, but its lateral third is innervated additionally but selectively by a separate nerve branch, providing the possibility of some differential compartmental function (da Silva Costa et al. 2011). While the intramuscular motor nerve arborization of the SR does not exhibit topographic segregation compatible with selective compartmental control, torsional and vertical actions could be implemented by differential compartmental contraction in the lateral rectus (LR) and medial rectus (MR) where motor innervation is highly segregated (da Silva Costa et al. 2011). For example, selective compartmental contraction of the inferior compartments of the LR (LRi), MR (MRi), or both might infraduct the eye, as required for VFV.
The potential for differential compartmental control of the SO has recently been recognized based upon three-dimensional reconstructions of the intramuscular trochlear nerve in monkeys and humans (Le et al. 2014b). The trochlear nerve bifurcates external to the SO into medial and lateral branches that innervate non-overlapping compartments of EOM fibers demarcated by a border slightly oblique to the long axis of the EOM cross section. Tracing of EOM and tendon fibers in the human SO indicates that medial compartment fibers insert near the globe equator and thus have predominantly torsional action in central gaze, while the lateral compartment fibers insert posteriorly and have predominantly vertical action in central gaze (Le et al. 2014b). Taken together, these findings imply that the torsional and vertical actions of the SO need not be linked, but might be controlled independently if inputs to their motor pools differ. If so, differential actions of the SO compartments could explain inconsistencies in prior studies of VFV. Moreover, the motor nerves to the IO muscle of rabbit, cow, monkey, and human also bifurcate prior to entry into the EOM belly, and each of the divisions innervates non-overlapping groups of EOM fibers (Le et al. 2014a). Thus differential compartmental control appears anatomically possible for both horizontal and both oblique EOMs; a total of 11 distinct oculorotary EOM compartments could contribute to eye movements.
It is clear that the three externally observable degrees of rotational freedom of each eye, horizontal, vertical, and torsional, are highly overdetermined by the number of EOM compartments available to effect ocular rotation. The contributions of particular EOMs or EOM compartments therefore cannot be ascertained from observations of eye movement behavior alone. It is necessary to directly observe the contractile behavior of EOMs and their compartments. Several direct measures of muscle behavior can indicate “contractility” (Clausen 2003). Magnetic resonance imaging (MRI) allows direct observation of function in individual EOMs. Unlike force measurement, MRI indicates contractility by morphological changes associated with EOM shortening. Miller (1989) introduced the approach for rectus EOMs by analyzing changes in cross-sectional area distribution, and that method has been extended by others (Tian et al. 2000). Analysis of EOM cross sections by MRI shows atrophy and reduced thickening in the fields of activation of denervated rectus EOMs (Demer 2003; Demer and Miller 1999), and the normal and paretic SO (Clark and Demer 2011; Clark et al. 1998; Demer and Miller 1995; Jiang and Demer 2008; Kono and Demer 2003; Kono et al. 2009) and IO (Demer et al. 2003b; Ela-Dalman et al. 2008; Kono and Demer 2003). After trochlear neurectomy in monkey, MRI and histology demonstrate SO atrophy comparable to MRI measurements in living humans with SO palsy (Demer et al. 2010).
Although isometric studies of function record developed tension at fixed muscle length (Lennerstrand et al. 2006), muscle contraction in a literal sense can be studied by measuring isotonic length changes during constant loading (Wilkie 1956). The concept of contractility includes shortening and thickening, features conveniently approached by imaging. Moreover, there is evidence that EOM volume increases during contraction (Yoo et al. 2014). For horizontal rectus EOMs, there are high correlations with conjugate horizontal duction angle for MRI measures of both maximum cross-sectional area and posterior partial volume (PPV), so that both measures can represent contractility (Clark and Demer 2012c). Differential compartmental contractility in human EOMs is demonstrable by MRI. In humans, the superior MR (MRs) compartment exhibits greater contractility that the MRi compartment (Demer and Clark 2014). Differential horizontal rectus contraction has been demonstrated in the LRi compartment during ocular counterrolling (Clark and Demer 2012a), in the MRs compartment during vertical duction (Demer and Clark 2013), and threefold greater in MRs during conjugate adduction than in horizontal convergence (Demer and Clark 2014).
During horizontal convergence to a target aligned to one eye, MRI has demonstrated extorsion of the rectus pulley array, absence of retraction of the aligned eye inconsistent with MR and LR cocontraction (Demer et al. 2003a), and corelaxation of all compartments of the aligned MR and LR (Demer and Clark 2014). Based upon the transverse widths of the horizontal rectus EOM tendons, it has been estimated that differential compartmental activity in the horizontal rectus EOMs alone could suffice to implement the amplitude of normal VFV (da Silva Costa et al. 2011). This study aimed to employ MRI to evaluate the contributions of individual EOM actuators, including the horizontal rectus and SO compartments, to human VFV induced by monocular prism viewing. Such mechanistic observations are essential to understanding the central neural control strategies for control of vergence.
MATERIALS AND METHODS
Subjects.
Fourteen healthy adult, paid volunteers of mean age 21.6 ± 2.1 yr (SD, range 19–27 yr) were recruited by advertisement and gave written, informed consent according to a protocol conforming to the Declaration of Helsinki and approved by the Institutional Review Board at the University of California, Los Angeles. Subjects underwent examinations by an ophthalmologist-author verifying normal corrected visual acuity, normal ocular versions, orthotropia at near and distance, 40 arcsec contour stereopsis by crossed polarization testing at near (Titmus), and ability to fuse at least two PD (1.14°) vertical prism before either eye.
MRI.
Each subject participated in a scanning session lasting 60–75 min. High-resolution, T2 fast spin echo (Demer and Dusyanth 2011) weighted MRI was performed using a 1.5 T General Electric Signa (Milwaukee, WI) scanner using a surface coil array (Medical Advances, Milwaukee, WI), and techniques are described in detail elsewhere (Clark and Demer 2012a; Demer and Clark 2014; Demer and Dusyanth 2011; Demer et al. 2003a). For hearing protection, subjects wore earplugs during imaging. Initially, a low-resolution triplanar scan was used to localize subsequent imaging. For each orbit under each viewing condition, sets of 18 contiguous, 2-mm-thick quasi-coronal image planes were collected perpendicular to the long axis of each orbit employing a 256 × 256 matrix over an 8-cm field of view, so that in-plane resolution was 312 μm (Fig. 1A). Sets of quasi-sagittal images were also obtained parallel to the long axis of each orbit (Fig. 1B). The duration of each of the foregoing “scans” was about 2.5 min for quasi-coronal views and 2.0 min for quasi-sagittal views. Rest periods were assigned between scans.
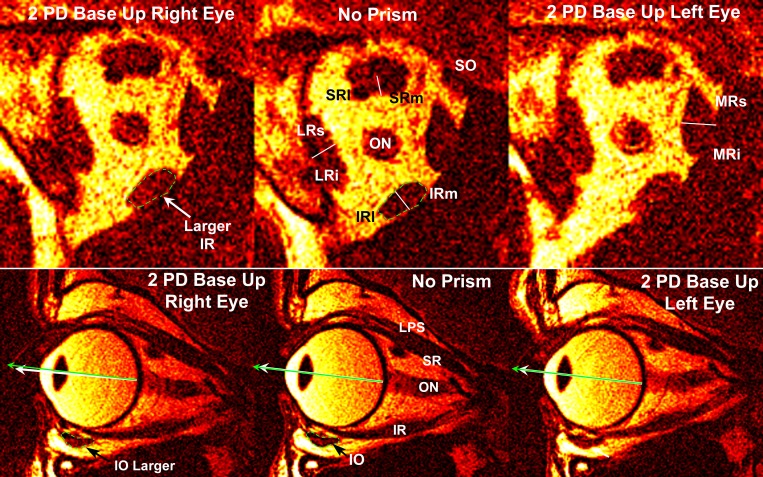
Fig. 1.
Quasi-coronal (top row) and quasi-sagittal (bottom row) magnetic resonance imaging of right orbit of subject viewing binocularly without prism (center column) and during vertical fusional vergence (VFV) with two prism diopter (PD) prism base up (BU) over the right eye (left column) and separately over the left eye (right column). Green arrows indicate visual direction to the target. White arrow at bottom left indicates infraduction of the eye viewing through BU prism. LR, lateral rectus muscle. Rectus cross sections have been divided into presumptive compartments as indicated by the fine white lines. Dotted green outlines of the inferior oblique (IO) and inferior rectus (IR) for the “No Prism” condition have been superimposed upon the corresponding muscles for the “2 PD Base Up Right Eye” condition to illustrate the corresponding small increases in muscle size for this viewing condition. SO, superior oblique; SR, superior rectus; SRl, lateral SR; SRm, medial SR; LRs, superior LR; LRi, inferior LR; IRm, medial IR; IRl, lateral IR; MR, medial rectus; MRs, superior MR; MRi, inferior MR; LPS, levator palpebrae superioris; ON, optic nerve.
Viewing conditions.
The surface coil array had a transparent faceplate through which the subject viewed targets, illuminated indirectly by white light inside the scanner bore. Subjects binocularly fixated an accommodative target affixed 25 cm above the nose on the inside of the scanner bore, consisting of a 5 × 5 mm, black on white cross of 1-mm stroke width surrounded by five finely ruled, concentric squares of progressively larger dimensions to a maximum of 20 × 20 mm.
Most normal subjects have sufficient VFV reserve to maintain fusion, despite a two PD vertical disparity during distance viewing, and slightly more during near viewing (von Noorden 1990). This condition requires 1.14° monocular infraduction by the viewing eye. Prior to MRI, each subject was evaluated for the ability to maintain VFV for the prism viewing condition. Initial subjects were also tested with three PD base up, but since many could not maintain VFV and reported vertical diplopia with the stronger prism, prism power was limited to two PD. Alternatively, the prism base could have been set down to evoke a monocular supraduction, or one PD split base up in one eye with one PD base down in the contraprism eye to evoke antisymmetric vertical ductions, but these alternatives were not chosen to permit simplification of analysis. Consequently, for scans involving VFV, a two PD acrylic prism was affixed base up to the transparent faceplate of the surface coil mask over one eye, and the subject's ability to binocularly fuse the target in the scanner without diplopia was verified subjectively. Quasi-sagittal and quasi-coronal images of both orbits were each obtained three times during the same scanning session: first, during viewing without prism; second, during right eye viewing through prism; and third, during left eye viewing through prism. Before each scan, subjects were verbally coached to fuse the target binocularly, and their success was confirmed after each scan.
Analysis.
Digital MRI images were quantified using ImageJ64 and custom analysis programs written in MatLab (MathWorks 2011, Boston, MA). Potential subjective bias was minimized by quasi-automated analysis subsequent to structure outlining in ImageJ64, and automated analysis of subsequent steps in MatLab. Moreover, the authors did not have strong a priori expectations of quantitative results for individual EOMs, and, as will be seen below, the results were often surprising. Anteroposterior globe position was determined in quasi-sagittal, midglobe images from the difference between horizontal coordinates of the anterior border of the inferior orbital rim and the globe centroid as manually outlined. Contractility of the IO was inferred from changes in its manually outlined cross section in quasi-sagittal images at the midpoint of the IR (Demer et al. 2003b). In quasi-coronal images, each rectus EOM and the SO was manually outlined and cropped to include only its belly (Clark and Demer 2012b, 2012c), avoiding inclusion of adjacent nonmuscular structures. Subsequent analytic steps were automated. For each rectus EOM, the angle of a linear best-fit through the maximum transverse dimension was computed (Clark and Demer 2012a), and the image rotated to align that best-fit line to vertical for horizontal EOMs, and horizontal for vertical EOMs. Superior and inferior horizontal rectus compartmental areas were calculated as cross-sectional areas above and below the perpendicular bisector of that best-fit line, omitting a band 20% of the length of the best fit line to account for anatomical variation in the compartmental border; corresponding medial and lateral vertical rectus compartmental areas were calculated relative to that perpendicular bisector (Clark and Demer 2012a), but again omitting the central 20% to account for anatomical variation in the border. For the SO, a bootstrap analysis was performed as described in the results section to determine the angular orientation of a line, oblique to the long axis of the SO cross section that optimally discriminated compartmental function, again omitting the central 20% of the cross section to account for possible curvature or other irregularities in the compartmental boundary.
Longitudinal cross-sectional area distributions of rectus EOMs and the SO were plotted to evaluate data trends. For this purpose, image plane sets were referenced to the globe-optic nerve junction, permitting averaging over multiple subjects. However, because intersubject variation in overall EOM size can obscure the effects of small changes in contractility in each subject, a normalized analysis was then performed for statistical purposes.
Cross sections in four contiguous image planes −4, −5, −6, and −7 (8 to 14 mm posterior to the globe-optic nerve junction) were summed and multiplied by the 2-mm slice thickness to form PPVs. Change in PPV in horizontal rectus EOMs is a robust correlate of duction angle, accounting for >85% of variance in duction of groups of subjects, and >97% of variance within individuals (Clark and Demer 2012c). We have also demonstrated that, in 13 normal subjects, change in PPV of vertical rectus EOMs is also highly correlated with vertical duction angle in normal subjects (unpublished data), accounting for >84% of variance in duction in groups of subjects, and >90% of variance within individuals (unpublished data). The change in PPV of the normal SO also correlates well with vertical duction, accounting for >50% of variance in duction in groups of subjects, and >70% of variance within individuals (unpublished data).
As conventionally done in MRI studies of EOM function (Demer and Clark 2014), contractility was defined as the percentage change from that measured without prism viewing. Because of the highly curved path of the IO, the only image plane analyzed for the IO was that closest to the center of the IR muscle; whole-muscle cross-sectional area changes in this plane are considered to best reflect IO contractility (Demer and Clark 2005; Demer et al. 2003a, 2003b).
While every EOM contains an oculorotary global layer (GL) that inserts upon the sclera, and an orbital layer (OL) that inserts upon connective tissues, the borders between these layers are seldom discriminable by MRI. The automated parsing of EOMs into compartments therefore included both GL and OL contributions. Cross-sectional data were analyzed using ANOVA; two-way comparisons of means were made using the two-tailed Student's t-test, both by GraphPad Prism (GraphPad Software, La Jolla, CA).
Although data were analyzed separately for right eyes alone and left eyes alone, trends were similar for both. Right and left eyes were, therefore, pooled for analysis reported here.
RESULTS
Angular eye positions.
Differences in horizontal and vertical eye positions were determined from the measured globe radius, and changes in positions of the globe-optic nerve junction relative to the centroid of the bony orbit. As described elsewhere (Demer et al. 2003a), these values can be determined at subpixel resolution, providing a precise measure of rotational ocular orientation. Horizontal and vertical eye positions were computed as prism viewing minus contraprism positions, including each eye in each role for 28 total observations. Eyes during prism viewing averaged 0.07 ± 0.26° (standard error of the mean, SEM) abduction relative to that during contraprism viewing, a difference not significantly different from zero. Eyes during prism viewing averaged 0.85 ± 0.30° infraduction relative to during contraprism viewing, significantly different from zero (P < 0.01), but not significantly different from the ideal VFV of 1.15° (P > 0.1). This means that, on average, subjects performed the motor task accurately, achieving the desired amount of VFV without confounding horizontal vergence.
Globe position.
Anteroposterior globe position was analyzed in quasi-sagittal images to seek evidence of EOM cocontraction or corelaxation during VFV. The mean horizontal globe centroid was located 5.98 ± 0.57 (SE) mm posterior to the anterior border of the inferior orbital rim during viewing without prism, and during VFV with two PD monocular base up, prism was 6.15 ± 0.56 mm posterior in the orbit contralateral to prism, and 6.07 ± 0.59 mm posterior in the orbit ipsilateral to prism. While ANOVA demonstrated highly significant variation among individual orbits (P < 0.0001), there was no significant effect of prism viewing condition (P = 0.346), nor a significant effect by paired t-testing of the change in anteroposterior globe position within individual orbits (P > 0.5). This means that VFV was not associated with globe retraction or proptosis exceeding measurement variability.
EOM cross sections.
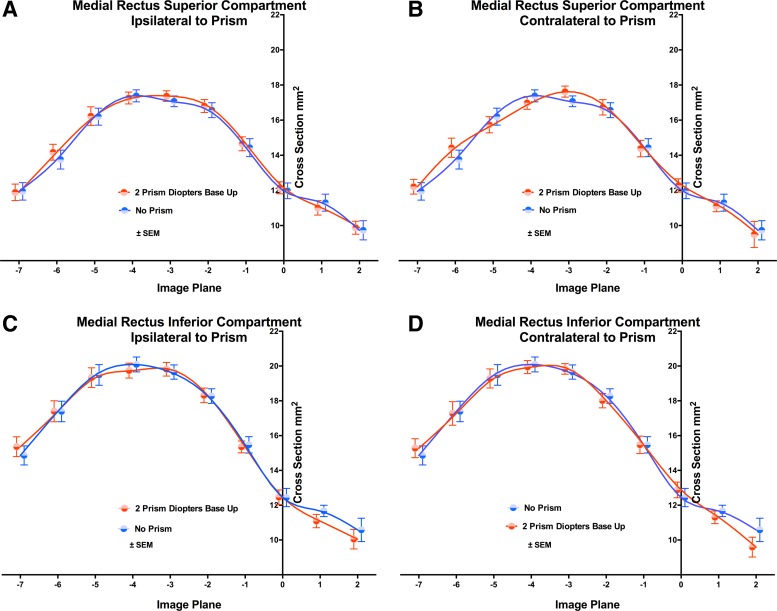
The primary data reflecting EOM function was the distribution of cross-sectional area along the length of EOMs; these data include both physiological effects of VFV, but also a large contribution from intersubject anatomical variability. There was a suggestion in some MRI images that the IR contracted in the infraducting eye that viewed through base-up prism, but quantitative analysis of pooled subject data demonstrated that the increase in cross-sectional area was small in any single image plane (Fig. 1, left). There was no significant effect of prism viewing on IR cross sections, although ANOVA demonstrated significant variation among individual orbits and image planes (P < 0.0001). Figure 2 plots mean (±SE) IR cross section for the eye ipsilateral vs. contralateral to the prism, and in the IR's lateral (IRl) and medial (IRm) regions that are tentatively considered “compartments” for purposes of analysis, irrespective of neuromuscular anatomy. The central 20% of EOM width was omitted from analysis to account for potential variability in the intercompartmental border. In every instance, IR cross-sectional area was maximal three image planes (6 mm) posterior to the reference plane at the globe-optic nerve junction, which was defined as plane zero. In the eye contralateral to the prism, cross-sectional distributions of both IRl and IRm along the anteroposterior extent of the orbit were similar with or without prism viewing (Fig. 2, right).
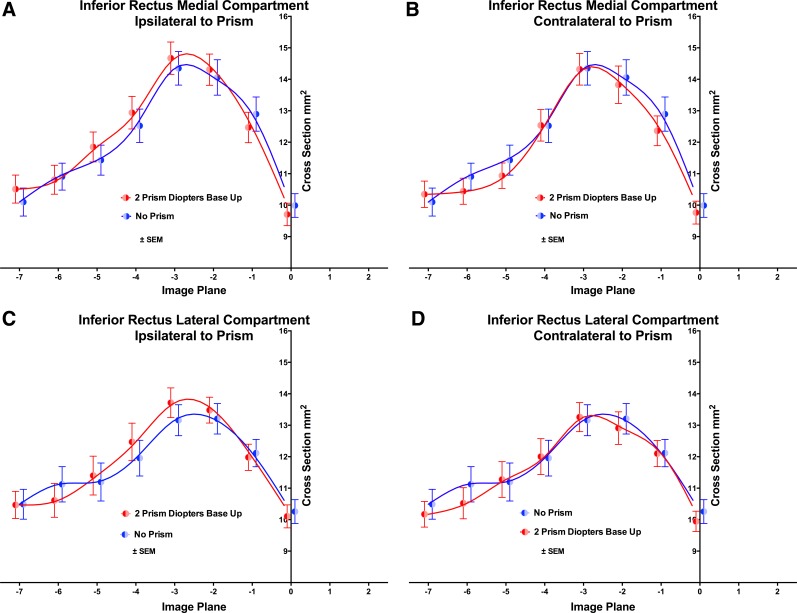
Fig. 2.
Cross-sectional area distributions of the IR muscle in 2-mm-thick image planes numbered negatively going posteriorly from zero at the globe-ON junction, in medial (A and B) and lateral (C and D) 40% compartments, omitting the central 20% of muscle dimension in 27 orbits. A and C: ipsilateral to prism. B and D: contralateral to prism. Prism viewing had no significant effect by ANOVA. Symbols and spline fits have been offset slightly on the abscissa to avoid overlap.
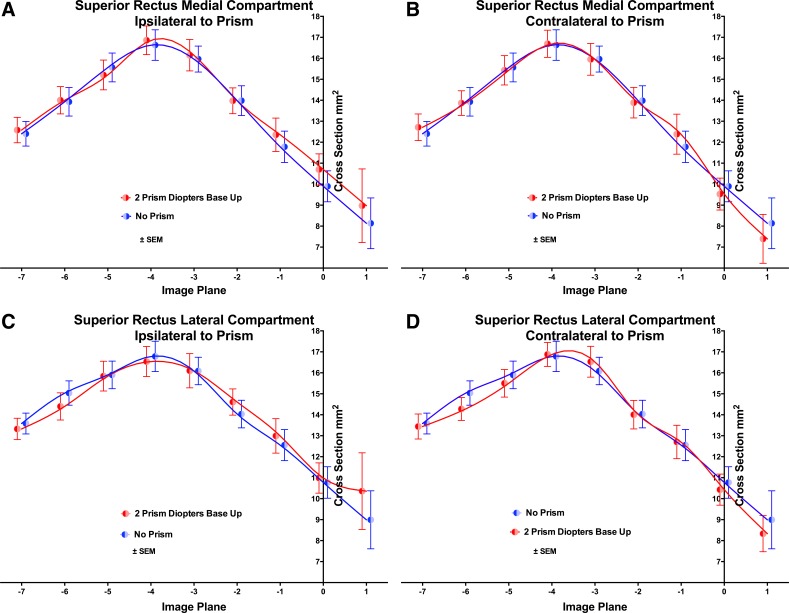
The SR muscle exhibited maximum cross-sectional area four image planes (8 mm) posterior to the glob-optic nerve junction (Fig. 3). The SR's lateral (SRl) and medial (SRm) 40% regions were tentatively considered “compartments” for purposes of analysis, irrespective of neuromuscular anatomy that makes differential function improbable. There was no significant effect of prism viewing in either the eye ipsilateral or contralateral to the prism, in either SRl or SRm, although ANOVA demonstrated a highly significant (P < 0.0001) effect of individual orbit and of image plane.
Fig. 3.
Cross-sectional area distributions of the SR muscle in 2-mm-thick image planes numbered as in Fig. 2, in medial (A and B) and lateral (C and D) 40% compartments, omitting the central 20% of muscle dimension in 27 orbits. A and C: ipsilateral to prism. B and D: contralateral to prism. Prism viewing had no significant effect by ANOVA. Symbols and spline fits have been offset slightly on the abscissa to avoid overlap.
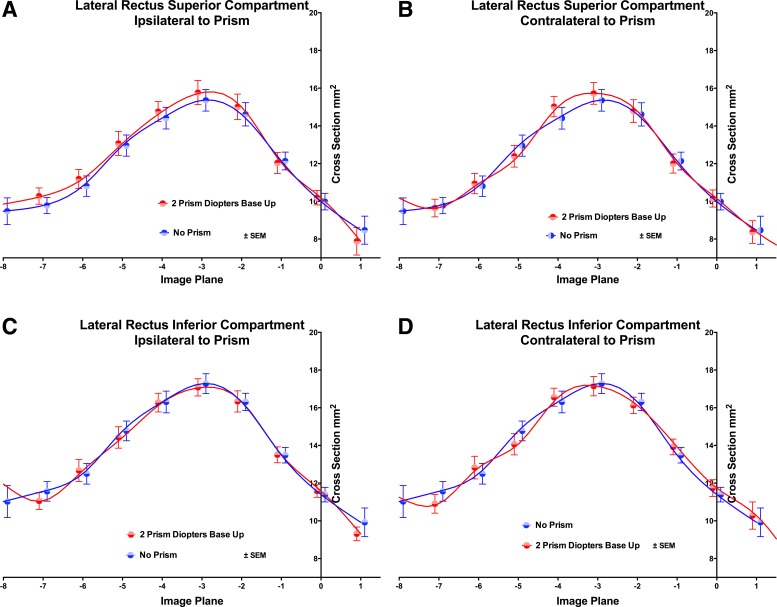
The LR has not been classically recognized to have any infraducting function, but differential activation of its LRi and superior LR (LRs) compartments could generate vertical duction. The LR exhibited maximum cross-sectional area three to four image planes (6–8 mm) posterior to the globe-optic nerve junction (Fig. 4). The cross-sectional distribution of LRs ipsilateral to the base-up prism was significantly different than during nonprism viewing (P = 0.0056), while of course varying significantly by image plane (P < 0.0001). Prism viewing had no significant effect on LRi cross-sectional distribution ipsilateral to prism, nor on distributions of either LRs or LRi contralateral to prism, although ANOVA demonstrated significant effect of image plane and individual orbit (P < 0.0001).
Fig. 4.
Cross-sectional area distributions of the LR muscle in 2-mm-thick image planes numbered as in Fig. 2. A and B: superior compartment. C and D: inferior compartment. A and C: ipsilateral to prism. B and D: contralateral to prism. Viewing through two PD BU prism had a significant effect on the distribution of the LR superior compartment ipsilateral (P = 0.0056; A) but not contralateral to the prism, and no significant effect in the inferior compartment. Symbols and spline fits have been offset slightly on the abscissa to avoid overlap.
The MR also has not been classically recognized to have any infraducting function, but differential activation of its MRi and MRs compartments might potentially generate vertical duction. The MR exhibited maximum cross-sectional area two to four image planes (4–8 mm) posterior to the globe-optic nerve junction (Fig. 5). Prism viewing had no significant effect on MRi or MRs cross-sectional distributions, despite significant effect of image plane (P < 0.0001).
Fig. 5.
Cross-sectional area distributions of the MR muscle in 2-mm-thick image planes numbered as in Fig. 2. A and B: superior compartment. C and D: inferior compartment. A and C: ipsilateral to prism. B and D: contralateral to prism. Viewing through two PD BU prism had no effect on the cross-sectional distribution of either compartment ipsilateral or contralateral to the prism. Symbols and spline fits have been offset slightly on the abscissa to avoid overlap.
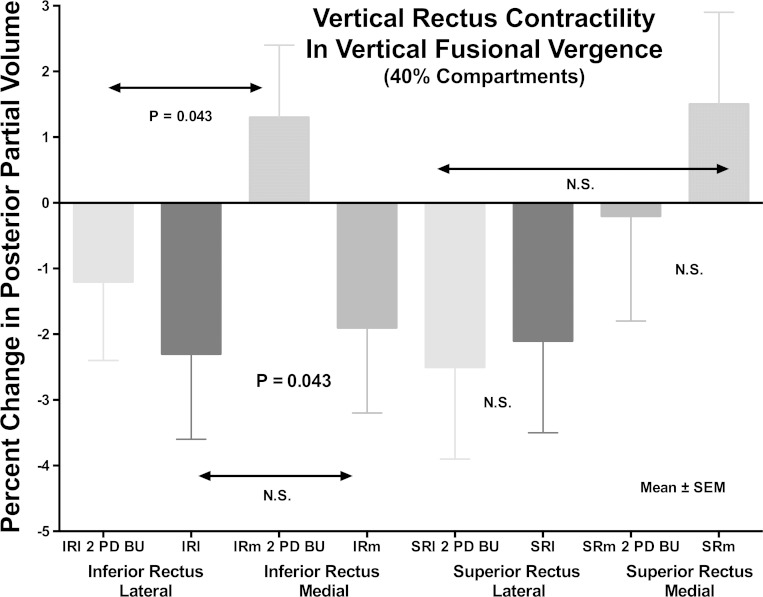
The foregoing plots consist of raw data substantially influenced by interindividual anatomical variability that was analytically reduced in the following manner. Contractility of EOMs was evaluated quantitatively primarily by computation of changes in PPV (Fig. 6). Considering the entire IR homogeneously, PPV increased by 1.3 ± 0.9% in the orbit viewing through prism, and decreased by 1.4 ± 09% in the orbit contralateral to prism, for a significant difference between orbits (P = 0.013). This is consistent with optically appropriate, relative infraduction by the eye viewing through prism. However, compartmental analysis revealed that this effect was limited to the medial compartment: ipsilateral to prism, IRm contracted by 1.3 ± 1.1% as significantly different from 1.9 ± 1.3% relaxation in IRm contralateral to prism (P = 0.016). By contrast, in IRl there was 1.2 ± 1.2% relaxation ipsilateral to prism, and 2.3 ± 1.3% relaxation contralateral to prism (P > 0.36). The difference in PPV between IRl and IRm ipsilateral to prism was significant (P = 0.043), indicating differential compartmental function in the ipsilateral IR.
Fig. 6.
Percent change in posterior partial volume (PPV) from control condition for vertical rectus muscle compartments during VFV for prism viewing (2 PD BU) and contraprism orbits. Note significant differential contractile increase in PPV in the IRm compartment for the prism-viewing orbit, but similar decrease in the contraprism orbit. Note absence of significant change for the SRl or SRm compartment of the SR. NS, nonsignificant.
Considering the SR as a monolithic EOM, there was no significant change in PPV for either eye. There was also no significant change in PPV in response to prism viewing for the lateral (SRl) or medial (SRm) halves, nor differential changes in PPV between SRl and SRm in either eye (Fig. 6).
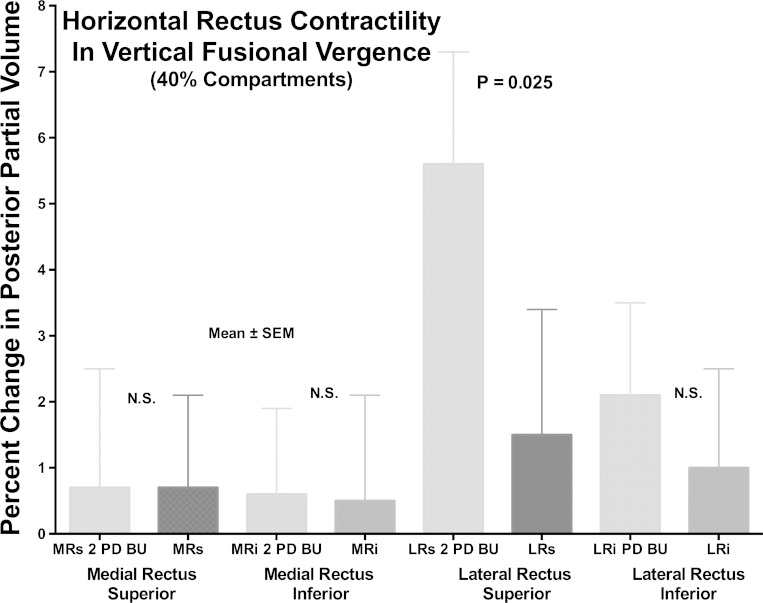
While the horizontal rectus EOMs are traditionally considered to lack a role in vertical eye movement, even when considered as a monolithic EOM, the LR of the prism-viewing eye contracted significantly during VFV. For the monolithic LR, PPV in the prism viewing eye was 2.6 ± 1.4% greater than without prism (P < 0.05, one-tail t-test), but there was no comparable significant change for the contraprism eye (0.4 ± 1.4%). As illustrated in Fig. 7, the bulk of the contractile change was observed in the LRs compartment, where the PPV increased significantly by 5.6 ± 1.7% in the prism-viewing eye (P < 0.01), but did not change significantly in the contraprism eye at 1.5 ± 1.9%; the difference between eyes was significant (P = 0.012). There was no significant change in PPV for LRi compartment of the prism and contraprism eyes during VFV, nor a significant difference between eyes during VFV (Fig. 7). The difference in change in PPV between LRs and LRi in the prism-viewing eye was significant (P = 0.025).
Fig. 7.
Percent change in PPV from control condition for horizontal rectus muscle 40% compartments during VFV for the prism viewing (2 PD BU) and contraprism orbits. Note absence of significant contractility for either MR compartment, but significant contractile increase in PPV in the LRs but not LRi compartments for the prism-viewing orbit.
Analysis of individual subjects was performed to ascertain if differential compartmental contractility of LRi might idiosyncratically contribute to VFV in some subjects. However, contraction in LRi exceeded that of LRs in only 4 of 28 ipsiprism orbits, and relaxation in LRi exceeded that of LRs in 8 of 28 contraprism orbits. None of the foregoing were the same orbits, and all of the remaining orbits exhibited contractile behavior opposite of that consistent with an LR contribution to the expected vertical direction of VFV.
Unlike for the LR, VFV was not associated with any significant changes in PPV in either MR compartment in either the prism viewing or contraprism eye (Fig. 7). This means that LR contraction ipsilateral to base-up prism during VFV was not reciprocated by MR relaxation.
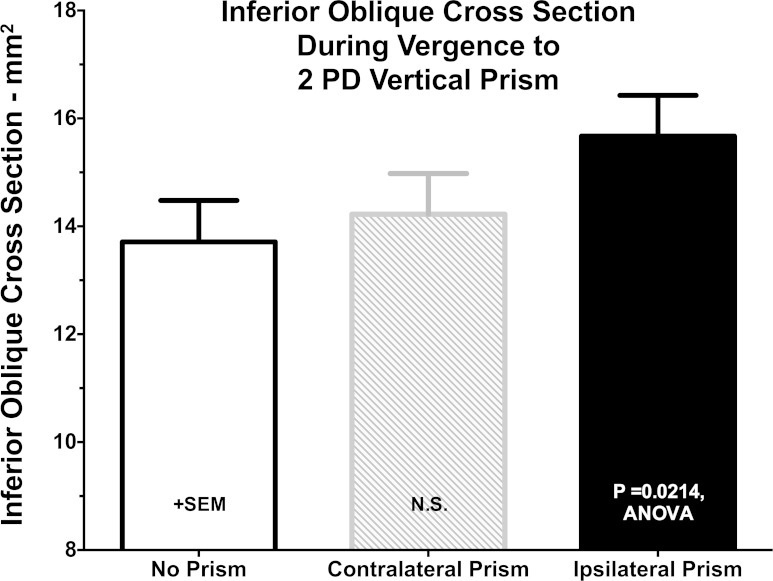
It was possible to evaluate IO cross section reliably only in the one image plane that contains the IR center in quasi-sagittal images, so change in this cross section was the only available measure of contractility. As illustrated in Fig. 8, IO cross section increased significantly by 18.6 ± 5.6% in the prism-viewing eye during VFV (ANOVA, P = 0.0214), but in the contraprism eye the comparable measure was not significantly different from zero at 5.8 ± 4.0%. This implies that the IO, normally assumed to have a supraducting action, was significantly but paradoxically contracting in the infraducting eye during VFV.
Fig. 8.
Mean changes in IO muscle cross section at point of IR muscle crossing during VFV.
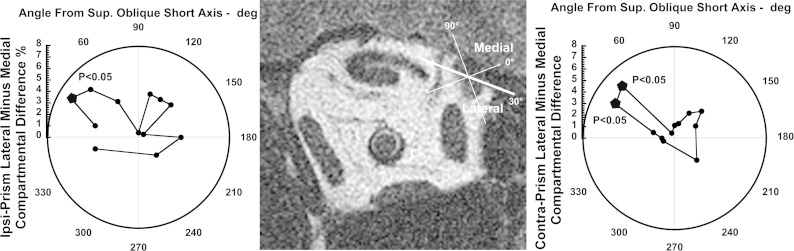
The SO has well-established infraducting function, so it is interesting that, when the whole SO is considered as a monolithic EOM, VFV was associated with a significant 4.2 ± 1.2% reduction in PPV ipsilateral to the prism (P < 0.01), and no significant change contralateral to prism. Whole muscle analysis of the SO confounds possible differential contributions in its compartments that can be appreciated only after determination of the orientation of the compartmental border. This was accomplished by a bootstrap method that systematically varied the orientation of the angle of an assumed border in 15° increments, seeking the angle that maximized intercompartmental differences during VFV after the central 20% of SO width was excluded to account for curvature or other irregularities in the border. The reference angle was the short axis of a best-fit ellipse to the SO cross section. The approach is illustrated in Fig. 9, which shows significant (P < 0.05) intercompartmental differences for the orbit ipsilateral to prism for 30° orientation, and for the orbit contralateral to prism for 30° and 45° angles. Since in each instance the same orbits contributed data under varying viewing conditions, the 30° orientation was chosen, since it was associated with statistically significant differential compartmental function in both ipsiprism and contraprism roles, and therefore most likely reflects the actual anatomy. This approach was confirmed by determination of the border angle in each individual orbit that gave the maximum differential compartmental contractility. For each orbit, the angle was the same whether viewing through prism or contraprism. Maximum differential compartmental contractility was observed for 15° orientation in 2 orbits, for 30° orientation in 19 orbits, and 45° orientation for 6 orbits. The average of these maximum differential angles was 32°.
Fig. 9.
Bootstrap analysis for identification of the intercompartmental SO boundary orientation optimizing differential function during VFV. Center: quasi-coronal MR image illustrating that reference angle 0° was taken as the short axis of the ellipse that best fit the SO cross section. Flanking polar plots represent the difference in PPV change between the medial and lateral peripheral 40% compartments resulting from VFV compared with nonprism viewing. Positive angles are clockwise. Points significantly different from zero are indicated by bold pentagons, and included 30° for the SO ipsilateral to the prism, and both 30 and 45° for the SO contralateral to prism. Consistent significance at 30° both ipsi- and contralateral to prism justifies this angle as the best provisional intercompartmental border.
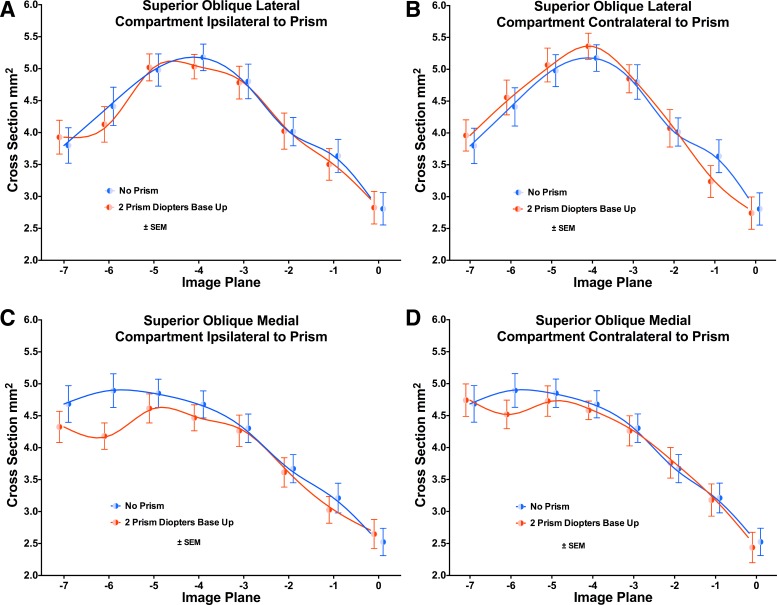
The SO exhibited maximum cross-sectional area four image planes (8 mm) posterior to the globe-optic nerve junction (Fig. 10). A highly significant anatomic effect of image plane was demonstrated by ANOVA for both SO compartments under all conditions (P < 0.0001). Prism viewing had a significant functional effect on the cross-sectional distribution for the medial compartment (SOm) ipsilateral to the prism (P = 0.0075), but the change was a reduction in posterior cross-sectional area indicative of paradoxical relaxation in the infraducting eye. Prism viewing had no significant effect on the cross-sectional area distribution of lateral compartment (SOl), either ipsi- or contralateral to the prism (Fig. 10). More sensitive statistical evaluation requires construction of PPVs, as shown below.
Fig. 10.
Cross-sectional area distributions of the SO muscle during VFV, in 2-mm-thick image planes numbered as in Fig. 2. A and B: SOl. C and D: SOm. A and C: ipsilateral to prism. B and D: contralateral to prism. Viewing through two PD BU prism significantly influenced the cross-sectional distribution of the SOm 40% compartment ipsi- but not contralateral to the prism, and not in the SOl compartment either ipsi- or contralateral to the prism. The effect of ipsilateral prism was a paradoxical reduction in posterior SOm cross section reflective of relaxation during infraduction of the eyes (C). Symbols and spline fits have been offset slightly on the abscissa to avoid overlap.
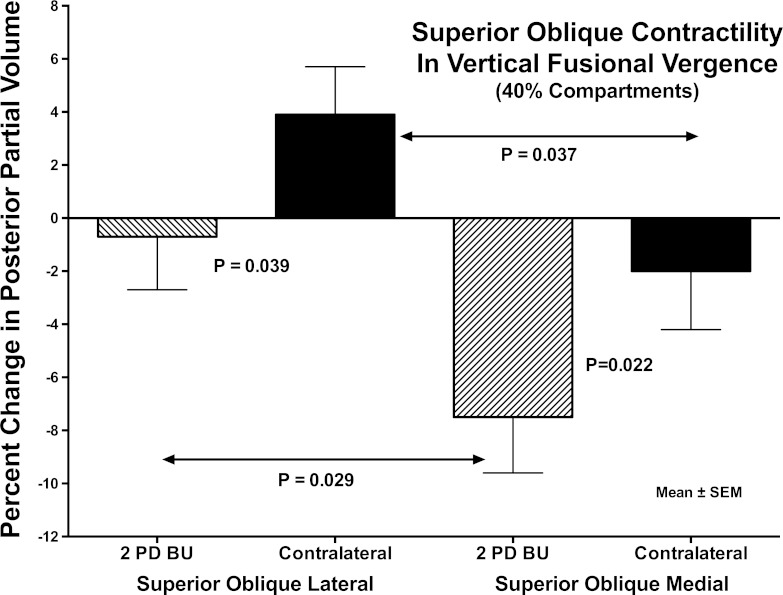
For the whole SO ipsilateral to prism during VFV, there was a 4.2 ± 1.2% reduction in PPV (P < 0.02). However, this paradoxical SO relaxation in the infraducting eye was accomplished almost entirely in the SOm compartment, which exhibited significant 7.5 ± 2.1% (P < 0.01) reduction in PPV (Fig. 11). The SOl compartment ipsilateral to prism exhibited no significant change in PPV (−0.7 ± 2.0%, P > 0.5, Fig. 11). The difference in PPV change between SOm and SOl during VFV was significant (P = 0.029).
Fig. 11.
Percent change in PPV from control condition for SO muscle compartments. Note significant differences in contractile change in PPV between SOl and SOm compartments in both the prism viewing and contraprism orbits. Also note paradoxical relaxation of SOm during prism viewing, corresponding to image planes −4 through −7 in Fig. 10.
During VFV, PPV in the SOl contralateral to prism was significantly greater by 3.9 ± 1.8% than during nonprism viewing (P < 0.05). Contralateral to prism, SOm was 2.2 ± 2.2% less than during nonprism viewing, not significantly different from zero, but significantly less than for SOl contralateral to prism (P = 0.037). Similar analysis was performed based upon demarcation angles between SOl and SOm of 15°, 30°, or 45° as optimized to maximize the contractility difference for each individual orbit; this confirmed all of the foregoing compartmental differences at a higher level of statistical significance (P < 0.001).
Table 1 summarizes contractile behavior during VFV to monocular base-up prism of all of the oculorotary EOMs and their selective compartments, where anatomically justified. The primary appropriate vertical action was implemented by IRm in the infraducting eye ipsilateral to the prism. This was associated with relaxation of IRm contralateral to the prism, which would have paradoxically produced supraduction by that eye, albeit appropriately augmenting vertical vergence. The most striking contractility observed during VFV was contraction in LRs in the infraducting eye ipsilateral to prism; this robust yet unexpected behavior would be expected to produce supraduction, and possibly cycloduction, yet was not reciprocated by MR contractility. The IO of the infraducting eye ipsilateral to the prism contracted, action expected to cause excycloduction but paradoxical supraduction. During VFV, SOm relaxed in the infraducting eye ipsilateral to prism, also expected to produce excycloduction; in the contraprism eye, there was contraction of SOl, which is expected from geometric considerations to produce mainly paradoxical infraduction.
Table 1.
Extraocular muscle contractility during vertical fusional vergence
| Prism Viewing Eye |
Contraprism Viewing Eye |
|||
|---|---|---|---|---|
| Muscle Compartment | Contractility | Expected Effect | Contractility | Expected Effect |
| Inferior oblique (whole muscle) | Contraction | Excycloduction, supraduction | — | — |
| Inferior rectus lateral | — | — | — | — |
| Inferior rectus medial | Contraction | Infraduction | Relaxation | Supraduction |
| Lateral rectus inferior | — | — | — | — |
| Lateral rectus superior | Contraction | Abduction, supraduction | — | — |
| Medial rectus inferior | — | — | — | — |
| Medial rectus superior | — | — | — | — |
| Superior oblique lateral | — | — | Contraction | Infraduction |
| Superior oblique medial | Relaxation | Excycloduction | — | — |
| Superior rectus (whole muscle) | — | — | — | — |
—, No change.
DISCUSSION
This study employed high-resolution MRI in humans to demonstrate disconjugate vertical eye rotation appropriate in direction and magnitude to binocular motor fusion during monocular viewing through a two PD (1.15°) prism. Despite the small magnitude of the physiologically required eye movement during VFV, associated contractile changes in EOMs were indicated by significant changes in PPV, similar to those demonstrated for larger eye movements associated with conjugate horizontal duction (Demer and Clark 2014), convergence (Demer and Clark 2014), and ocular counterrolling (Demer and Clark 2005). Unlike Enright's (1992) video study of similar magnitude VFV, the MRI technique detected no significant retraction or proptosis of either eye that might have suggested EOM cocontraction or corelaxation. Nonetheless, significant contractile changes were demonstrated in multiple EOMs. This investigation included novel analysis of EOM regions corresponding to known histological patterns of selective compartmental intramuscular innervation. Functional compartmental behavior demonstrated here during VFV include extension of previously known differential compartmental activity in LR (Clark and Demer 2012a), as well as novel demonstration of differential compartmental activity in IR and SO.
Direct actuator of VFV.
Earlier investigators used eye movement patterns to infer the EOMs responsible for VFV, thus variously implicating the SO (Cheeseman and Guyton 1999; Enright 1992), or idiosyncratic combinations of the oblique and vertical EOMs (Mudgil et al. 2002). The present study exploits MRI capabilities to detect changes in PPV associated with VFV, allowing novel direct observation of the contractile changes in EOM compartments, as summarized in Table 1. The ideal motor response to maintain binocular fusion during monocular viewing through a base-up prism would consist of ipsilateral eye infraduction by an angle equal to the prism's power. Indeed, MRI demonstrated that the prism viewing eye's vertical position was significantly lower than that of the contraprism eye, by an amount not significantly different from prism power. An appropriate motor fusional response was therefore achieved during this experiment. However, the only EOM compartment whose contraction was appropriate to this vergence eye movement was the IRm, which contracted in the infraducting, prism viewing eye, and relaxed in the contraprism eye. Differential compartmental activation of the horizontal rectus EOMs did not implement the vertical component of VFV. Ipsiprism IRm contraction is appropriate to infraduction of that eye, and contraprism IRm relaxation, while inappropriate to foveal alignment, is at least appropriate to the disconjugacy of the vergence. Surprisingly, this occurred without significant contractile change in either SR. These results therefore suggest the novel phenomenon of vertical rectus cocontraction during VFV, especially remarkable since both EOM force measurements and MRI indicate horizontal rectus corelaxation during horizontal fusional convergence (Demer and Clark 2014; Miller et al. 2002, 2011). The conclusion of vertical rectus cocontraction must be regarded as questionable, however, since there was no significant change in anteroposterior globe position.
While ipsiprism contraction of IRm was the only striated EOM mechanism demonstrating contractile behavior consistent with implementing the optically appropriate infraduction during VFV, not enough is known about ocular motor mechanics to conclude that IRm contractility is sufficient for the behavior. Some perspective is provided by our unpublished data for conjugate infraduction averaging 8.2° in 14 normal orbits indicates 1.7% PPV increase in both IRl and IRm per degree, which is roughly proportionate to the 1.3 ± 1.1% contractility in IRm observed here during 0.85 ± 0.30° infraduction associated with VFV. Also comparable is the roughly 1.5%/° change in PPV for each horizontal rectus EOM over a wide range of conjugate duction (Clark and Demer 2012c). A possible role for smooth muscle (SM) around the MR pulley has been postulated augmenting horizontal convergence (Demer and Clark 2014). The inframedial SM extending between the IR and MR pulleys (Miller et al. 2003) might similarly play a role in augmenting VFV, particularly in resolving the paradoxical absence of SR relaxation reciprocal to IR contraction.
Role of SO.
As might have been anticipated, the present MRI measurements confirmed that the SO participates in VFV, but not as predicted by conventional notions. In the infraducting, prism-viewing eye, the SOl compartment, expected in central gaze to have primarily infraducting action based upon its posterior insertion, instead exhibited no contractility. The SOm compartment relaxed significantly, which would be expected in central gaze to produce excycloduction because of the primarily equatorial scleral insertion fibers of SOm tendon fibers. In the contraprism eye, there was no contractile change in SOm during VFV, but there was significant contraction in SOl that would be expected to produce infraduction. While infraduction of the contraprism eye is optically counterproductive, this behavior has nonetheless been observed in behavioral studies of prism-induced VFV (Matheron et al. 2008), and infraduction produced by SOl contraction would tend to offset supraduction resulting from IRm relaxation in the contraprism eye. The present findings therefore contradict Enright's (1992) claim that VFV is implemented solely by the SO acting against fixed IO tone, yet concur with the general proposition of Enright, and Cheeseman and Guyton (1999) and that the oblique EOMs are involved in VFV.
Role of IO.
The present study also demonstrated IO contraction in the prism-viewing eye during VFV, without change in the contralateral IO. Such IO contractility would be expected to produce counterproductive supraduction by the infraducting, prism-viewing eye. This finding of IO contraction during VFV contradicts Enright's (1992) supposition of fixed IO tone. Excycloduction by the prism-viewing eye during VFV might have a sensory objective, similar to the manner in which the shift from extorsion in converged downward gaze to intorsion in converged upward gaze (Minken and Van Gisbergen 1994) improves binocular correspondence of retinal meridia and hence stereo perception (Schreiber et al. 2001). Irrespective of purpose, ipsiprism IO contraction and bilateral but compartmentally differential SO contractility during VFV are likely to be among the mechanisms implementing the change in the LP orientation reported by multiple investigators (Mikhael et al. 1995; Steffen et al. 2002; Straumann and Muller 1994).
Compartmental LR function.
The present study demonstrated selective compartmental contraction of LRs ipsilateral to base-up prism during VFV, without change in ipsilateral LRi, in either contralateral LR compartment, or in the MR. Contraction of LRs would be expected to produce abduction and supraduction and might also have a torsional effect, depending upon the location of the LRs force centroid at the LR pulley. This selective compartmental contractility in the LR extends the finding of LR differential compartmental contractility first reported for ocular counterrolling, in which selective contractility was demonstrated in LRi (Clark and Demer 2012a). No contractile change in LRi was observed in the present study during VFV, nor in either MRi or MRs. Absence of contribution of the MR to VFV stands in contrast to MRI evidence for significantly less MRs, but not MRi, contractility during horizontal convergence than during adduction (Demer and Clark 2014). Selective compartmental control of LR is possible because the abducens nerve bifurcates into inferior and superior divisions prior to entry into the LR belly (da Silva Costa et al. 2011; Peng et al. 2010) and then arborizes within non-overlapping compartments of fibers that have only weak transverse mechanical coupling among generally parallel muscle (Shin et al. 2012, 2014) and tendon fibers (Shin et al. 2013). There is preliminary evidence that topographically distinct abducens motoneuron pools may selectively innervate the two LR compartments (Demer et al. 2013), consistent with evidence that neuoropathic LR atrophy in human LR abducens paresis is often limited to LRs (Clark and Demer 2014). The frequent occurrence of small-angle vertical strabismus in the setting of human abducens palsy (Pihlblad and Demer 2014) also supports a differential compartmental role for the LRs in VFV.
Paradoxical horizontal rectus behavior.
Robust contractility of LRs of the prism-viewing eye during VFV was not reciprocated by contractile change in either MR compartment, nor reflected in horizontal eye movement. During static VFV, force balance between MR and LR would always be expected, unless other EOMs or passive elastic forces contribute. The present finding of absence of reciprocal behavior in the horizontal rectus agonist-antagonist pair is reminiscent of the recent, perplexing finding in humans that LR contractility did not significantly differ between convergence and conjugate adduction, yet contractility in MRs during convergence was only about one-third of that in conjugate adduction (Demer and Clark 2014). However, during VFV, contraction in LRs may be required to offset the secondary adducting effect of contraction in IRm during infraduction. Another possible contributor to force balance is SM, which is abundant in a band over 1 mm thick from the IR to MR pulleys (Demer et al. 1997; Kono et al. 2002b; Miller et al. 2003). Most of this SM is composed of bundles arranged in the anteroposterior direction (Kono et al. 2002b) that could load or unload the IR pulley suspension, and so indirectly alter IR tension. While the SM in the pulley suspensions receives autonomic innervation (Demer et al. 1997) whose presumed slow dynamic properties could be compatible with VFV, nothing is presently known about this SM's function.
Compartmental SO function.
The present finding during VFV of robust differential compartmental SO contractility is the first demonstration of this phenomenon and may be understood in light of recent anatomical findings. Dissection of human and bovine orbits demonstrates that the structure of the SO and its tendon does not differ significantly from the rectus EOMs (Le et al. 2014a, 2014b). The SO tendon readily unrolls into a configuration of parallel muscle fibers in continuity with corresponding parallel tendon fibers that can be traced to its broad scleral insertion (Le et al. 2014a; Le et al. 2014b). External to the SO belly, the trochlear nerve bifurcates into medial and lateral divisions arborizing within non-overlapping medial and lateral compartments of muscle fibers that in turn correspond with predominantly equatorial and retroequatorial scleral insertions, respectively (Le et al. 2014a, 2014b). From a starting position in central gaze as employed here, geometry dictates that the equatorial insertion of the SOm fibers must generate predominantly cycloduction, while the retroequatorial insertion of SOl fibers must generate predominantly infraduction. Thus the observed relaxation of SOm in the prism-viewing eye during VFV contributes to the excycloduction implemented by the IO and IR. The observed SOl contraction in the non-prism-viewing eye during VFV would contribute to a counterproductive infraduction of the eye that was already aligned on the target without prism viewing.
Compartmental vertical rectus function.
Anatomical studies of intramuscular SR innervation suggested absence of compartmentally segregated peripheral innervation, although a selective lateral motor nerve division was demonstrated in the IR supplying partially overlapping innervation of diffuse, whole-muscle innervation provided by the main division (da Silva Costa et al. 2011). Since this study detected no significant SR contractility during VFV, the data provide no further insight into possible SR compartmentalization. However, the IR contraction observed during VFV was present only in IRm, constituting the first demonstration of differential compartmental function in this EOM. Given the pattern of overlapping intramuscular innervation in IR, this behavior might be explained by profound reduction in selective partial innervation to IRl in the eye infraducting during prism viewing, while the same region of EOM fibers remains partly innervated by moderately increased firing by the nerve branch that diffusely projects to the entire IR. No data are available concerning possible selective innervation to specific fiber types in either IR compartment. While the present MRI method was not designed to detect compartmental function in the IO, there is anatomical evidence for compartmental innervation of that EOM as well (Le et al. 2014a).
Because experimental time constraints did not permit comparative study of conjugate infraduction similar to the 1.15° angle of VFV, is it impossible to know if differential compartmental function in the IR is unique to vergence as opposed to conjugate vertical gaze. We have obtained unpublished data using identical MRI and analytic technique in normal human subjects during conjugate infraduction, showing no significant difference between IRl and IRm contractility for conjugate infraduction averaging 8° (13 orbits of 8 subjects), or for maximal infraduction averaging 21° (25 orbits of 13 subjects). Systematic studies of small-angle conjugate infraduction, conjugate supraduction, and VFV in response to base-down prism are warranted in further investigations.
Analytic considerations for compartmentalization.
The foregoing compartmental analysis should be considered capable of demonstrating differential compartmental function in EOMs, but incapable of excluding it for several reasons. The present analysis parsed rectus EOMs into two compartments having equal transverse dimensions corresponding to the average location of anatomical demarcations occasionally observable by MRI in the LR (Demer and Clark 2014), and to average proportions consistently demonstrated by histological reconstruction in a small number of serially sectioned orbits for LR (da Silva Costa et al. 2011; Peng et al. 2010) and MR (da Silva Costa et al. 2011). The present analysis parsed the SO into equal medial and lateral regions, again based upon histological observations consistently made in a small number of serially sectioned orbits (Le et al. 2014b). While these assumptions seem reasonable for average relative compartmental proportions for the horizontal rectus EOMs and the SO, the relative proportions are expected to vary among individual subjects. This implies that there was probably some misattribution of function in one EOM compartment to that of the fellow compartment, which would have the statistical effect of diminishing differences between compartments, and increasing variance, so that small but real differential compartmental effects could appear statistically insignificant. Moreover, for the SO, the mediolateral intercompartmental border might be variably inclined from the vertical as a result of curvature of the adjacent superomedial orbital wall, along which the SO belly courses. An incorrect assumption about the anatomical orientation of the intercompartmental SO border would also reduce observed differences in compartmental behavior and might increase variance. In every case, the analytic assumptions here would all tend to diminish the chances of detecting differential compartmental behavior, but never create an illusion of it. Moreover, the significant differential compartmental behavior that was detected here in LR and SO during VFV probably underestimates the actual magnitude of differential effect and might have missed more subtle differential behavior altogether.
GL and OL.
The analytic method employed here also pools the GL and OL in each EOM compartment, although the latter do not directly generate ocular rotation. The GL and OL would probably have contributed equally to each assumed compartmental parsing. The two layers probably contract simultaneously during static fixations (Kono et al. 2002a), and no anatomical evidence has yet emerged of separate motor nerve trunks to these two layers. Elucidation of physiological relationships among the transverse EOM compartments, and the OL/GL, will require higher resolution methods of observing EOM function that have yet to be developed and should ideally be correlated with companion studies of motoneuron behavior.
GRANTS
This study was supported by National Eye Institute Grants EY-08313 and core grant EY-00331. J. L. Demer was supported by an unrestricted award from Research to Prevent Blindness and holds the Leonard Apt Chair of Pediatric Ophthalmology.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.L.D. conception and design of research; J.L.D. performed experiments; J.L.D. and R.A.C. analyzed data; J.L.D. and R.A.C. interpreted results of experiments; J.L.D. prepared figures; J.L.D. drafted manuscript; J.L.D. and R.A.C. edited and revised manuscript; J.L.D. approved final version of manuscript.
ACKNOWLEDGMENTS
Nicolasa de Salles provided technical assistance.
REFERENCES
- Apt L. An anatomical reevaluation of rectus muscle insertions. Trans Am Ophthalmol Soc 78: 365–375, 1980. [PMC free article] [PubMed] [Google Scholar]
- Apt L, Call NB. An anatomical reevaluation of rectus muscle insertions. Ophthalmic Surg 12: 108–112, 1980. [PubMed] [Google Scholar]
- Bharadwaj SR, Hoenig MP, Sivaramakrishnan VC, Karthikeyan B, Simonian D, Mau K, Rastani S, Schor CM. Variation of binocular-vertical fusion amplitude with convergence. Invest Ophthalmol Vis Sci 48: 1592–1600, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman EW, Guyton DL. Vertical fusional vergence: the key to dissociated vertical deviation. Arch Ophthalmol 117: 1188–1191, 1999. [DOI] [PubMed] [Google Scholar]
- Clark RA, Demer JL. Differential lateral rectus compartmental contraction during ocular counter-rolling. Invest Ophthalmol Vis Sci 53: 2887–2896, 2012a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RA, Demer JL. Enhanced vertical rectus contractility by magnetic resonance imaging in superior oblique palsy. Arch Ophthalmol 129: 904–908, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RA, Demer JL. Functional magnetic resonance imaging (MRI) with selective zonal analysis suggests that differential compartmental medial rectus (MR) contractility augments vertical duction. Program No. 371.01. In: 2012 Neuroscience Meeting Planner. New Orleans, LA: Society for Neuroscience, 2012b. [Google Scholar]
- Clark RA, Demer JL. Functional morphometry of horizontal rectus extraocular muscles during ocular duction. Invest Ophthalmol Vis Sci 53: 7375–7379, 2012c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RA, Demer JL. Lateral rectus superior compartment palsy. Am J Ophthalmol 15: 479–487, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RA, Miller JM, Demer JL. Displacement of the medial rectus pulley in superior oblique palsy. Invest Ophthalmol Vis Sci 39: 207–212, 1998. [PubMed] [Google Scholar]
- Clausen T. Na+-K+ pump regulation and skeletal muscle contractility. Physiol Rev 83: 1269–1324, 2003. [DOI] [PubMed] [Google Scholar]
- da Silva Costa RM, Kung J, Poukens V, Yoo L, Tychsen L, and Demer JL. Intramuscular innervation of primate extraocular muscles: unique compartmentalization in horizontal recti. Invest Ophthalmol Vis Sci 52: 2830–2836, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demer JL. A 12-year, prospective study of extraocular muscle imaging in complex strabismus. J AAPOS 6: 337–347, 2003. [DOI] [PubMed] [Google Scholar]
- Demer JL. Extraocular muscles. In: Duane's Clinical Ophthalmology, edited by Tasman W and Jaeger EA. Hagerstown, MD: Lipincott, 2009, p. 1–30. [Google Scholar]
- Demer JL, Clark RA. Differential compartmental function of medial rectus muscle during converged and conjugate ocular adduction. J Neurophysiol 112: 845–855, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demer JL, Clark RA. Magnetic resonance imaging (MRI) demonstrates differential compartmental contractility of medial rectus muscle during vertical duction. In: ARVO 2013 Annual Meeting Abstracts. Rockville: MD, ARVO, 2013, p. 1302. [Google Scholar]
- Demer JL, Clark RA. Magnetic resonance imaging of human extraocular muscles during static ocular counter-rolling. J Neurophysiol 94: 3292–3302, 2005. [DOI] [PubMed] [Google Scholar]
- Demer JL, Dusyanth A. T2 fast spin echo magnetic resonance imaging of extraocular muscles. J AAPOS 15: 17–23, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demer JL, Kono R, Wright W. Magnetic resonance imaging of human extraocular muscles in convergence. J Neurophysiol 89: 2072–2085, 2003a. [DOI] [PubMed] [Google Scholar]
- Demer JL, Miller JM. Magnetic resonance imaging of the functional anatomy of the superior oblique muscle. Invest Ophthalmol Vis Sci 36: 906–913, 1995. [PubMed] [Google Scholar]
- Demer JL, Miller JM. Orbital imaging in strabismus surgery. In: Clinical Strabismus Management: Principles and Techniques, edited by Rosenbaum AL and Santiago AP. Philadelphia, PA: Saunders, 1999, p. 84–98. [Google Scholar]
- Demer JL, Mittelman-Smith M, Micevyh P, Poukens V, Foeller P, Tychsen LT. Do topographically distinct abducens motor neuron pools innervate the superior and inferior compartments of the lateral rectus muscle? Program No. 363.09. In: 2013 Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience, 2013. [Google Scholar]
- Demer JL, Oh SY, Clark RA, Poukens V. Evidence for a pulley of the inferior oblique muscle. Invest Ophthalmol Vis Sci 44: 3856–3865, 2003b. [DOI] [PubMed] [Google Scholar]
- Demer JL, Poukens V, Miller JM, Micevych P. Innervation of extraocular pulley smooth muscle in monkeys and humans. Invest Ophthalmol Vis Sci 38: 1774–1785, 1997. [PubMed] [Google Scholar]
- Demer JL, Poukens V, Ying H, Shan X, Tian J, Zee DS. Effects of intracranial trochlear neurectomy on the structure of the primate superior oblique muscle. Invest Ophthalmol Vis Sci 51: 3485–3493, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ela-Dalman N, Velez FG, Demer JL, Rosenbaum AL. High resolution magnetic resonance imaging demonstrates reduced inferior oblique muscle size in isolated inferior oblique palsy. J AAPOS 12: 602–607, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enright JT. Unexpected role of the oblique muscles in the human vertical fusional reflex. J Physiol 451: 279–293, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara N, Steffen H, Roberts DC, Zee DS. Effects of horizontal vergence on the motor and sensory components of vertical fusion. Invest Ophthalmol Vis Sci 39: 2268–2276, 1998. [PubMed] [Google Scholar]
- Irsch K, Guyton DL, Ramey NA, Adyanthaya RS, Ying HS. Vertical vergence adaptation produces objective vertical deviation that changes with head tilt. Invest Ophthalmol Vis Sci 54: 3108–3114, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Demer JL. Magnetic resonance imaging of the functional anatomy of the inferior rectus muscle in superior oblique muscle palsy. Ophthalmology 115: 2079–2086, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono R, Clark RA, Demer JL. Active pulleys: magnetic resonance imaging of rectus muscle paths in tertiary gazes. Invest Ophthalmol Vis Sci 43: 2179–2188, 2002a. [PubMed] [Google Scholar]
- Kono R, Demer JL. Magnetic resonance imaging of the functional anatomy of the inferior oblique muscle in superior oblique palsy. Ophthalmology 110: 1219–1229, 2003. [DOI] [PubMed] [Google Scholar]
- Kono R, Okanobu H, Ohtsuki H, Demer JL. Absence of relationship between oblique muscle size and Bielschowsky head tilt phenomenon in clinically diagnosed superior oblique palsy. Invest Ophthalmol Vis Sci 50: 175–179, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono R, Poukens V, Demer JL. Quantitative analysis of the structure of the human extraocular muscle pulley system. Invest Ophthalmol Vis Sci 43: 2923–2932, 2002b. [PubMed] [Google Scholar]
- Le A, Poukens V, Demer JL. Compartmental innervation scheme for the mammalian superior oblique (SO) and inferior oblique (IO) muscles. Program No. 62.22. In: 2014 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience, 2014a. [Google Scholar]
- Le A, Poukens V, Demer JL. Evidence for compartmental innervation of the mammalian superior oblique (SO) muscle by the trochlear nerve. In: ARVO 2014 Annual Meeting Abstracts. Rockville, MD: ARVO, 2014b, p. 2559. [Google Scholar]
- Lennerstrand GL, Schiavi C, Tian S, Benassi M, Camppos EC. Isometric force measured in human horizontal eye muscles attached or detached from the globe. Graefes Arch Clin Exp Ophthalmol 244: 539–544, 2006. [DOI] [PubMed] [Google Scholar]
- Lim KH, Poukens V, Demer JL. Fascicular specialization in human and monkey rectus muscles: evidence for anatomic independence of global and orbital layers. Invest Ophthalmol Vis Sci 48: 3089–3097, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheron E, Yang Q, Le TT, Kapoula Z. Effects of ocular dominance on the vertical vergence induced by a 2-diopter vertical prism during standing. Neurosci Lett 333: 176–180, 2008. [DOI] [PubMed] [Google Scholar]
- Mikhael S, Nicolle D, Vilis T. Rotation of Listing's plane by horizontal, vertical and oblique prism-induced vergence. Vision Res 35: 3243–3254, 1995. [DOI] [PubMed] [Google Scholar]
- Miller JM. Functional anatomy of normal human rectus muscles. Vision Res 29: 223–240, 1989. [DOI] [PubMed] [Google Scholar]
- Miller JM, Bockisch CJ, Pavlovski DS. Missing lateral rectus force and absence of medial rectus co-contraction in ocular convergence. J Neurophysiol 87: 2421–2433, 2002. [DOI] [PubMed] [Google Scholar]
- Miller JM, Davison RC, Gamlin PD. Motor nucleus activity fails to predict extraocular muscle forces in ocular convergence. J Neurophysiol 105: 2863–2873, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JM, Demer JL, Poukens V, Pavlowski DS, Nguyen HN, Rossi EA. Extraocular connective tissue architecture. J Vis 3: 240–251, 2003. [DOI] [PubMed] [Google Scholar]
- Minken AWH, Van Gisbergen JAM. A three-dimensional analysis of vergence movements at various levels of elevation. Exp Brain Res 101: 331–345, 1994. [DOI] [PubMed] [Google Scholar]
- Mottier ME, Mets MB. Vertical fusional vergences in patients with superior oblique muscle palsies. Am Orthopt J 40: 88–93, 1990. [Google Scholar]
- Mudgil AV, Walker M, Steffen H, Guyton DL, Zee DS. Motor mechanisms of vertical fusion in individuals with superior oblique paresis. J AAPOS 6: 145–153, 2002. [DOI] [PubMed] [Google Scholar]
- Peng M, Poukens V, da Silva Costa RM, Yoo L, Tychsen L, Demer JL. Compartmentalized innervation of primate lateral rectus muscle. Invest Ophthalmol Vis Sci 51: 4612–4617, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlblad M, Demer JL. Hypertropia in unilateral, isolated abducens palsy. J AAPOS 18: 235–240, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schor CM, Maxwell JS, Stevenson SB. Isovergence surfaces: the conjugacy of vertical eye movements in tertiary positions of gaze. Ophthalmic Physiol Opt 14: 279–286, 1994. [DOI] [PubMed] [Google Scholar]
- Schor CM, Tyler CW. Spatio-temporal properties of Panum's fusional area. Vision Res 21: 683–692, 1981. [DOI] [PubMed] [Google Scholar]
- Schreiber K, Crawford JD, Fetter M, Tweed D. The motor side of depth vision. Nature 410: 819–822, 2001. [DOI] [PubMed] [Google Scholar]
- Sharma K, Abdul-Rahim AS. Vertical fusional aplitude in normal adults. Am J Ophthalmol 114: 636–637, 1992. [DOI] [PubMed] [Google Scholar]
- Shin A, Yoo L, Chaudhuri Z, Demer JL. Independent passive mechanical behavior of bovine extraocular muscle compartments. Invest Ophthalmol Vis Sci 53: 8414–8423, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin A, Yoo L, Demer JL. Biomechanics of superior oblique Z-tenotomy. J AAPOS 17: 612–617, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin A, Yoo L, Demer JL. Independent active contraction of bovine extraocular muscle (EOM) compartments. Program No. 62.13. In: 2014 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience, 2014. [Google Scholar]
- Steffen H, Walker MD, Zee DS. Changes in Listing's plane after sustained vertical fusion. Invest Ophthalmol Vis Sci 43: 668–672, 2002. [PubMed] [Google Scholar]
- Straumann D, Muller E. Is Listing's law preserved in the vertical fusional reflex? In: Contemporary Ocular Motor and Vertibular Research: A Tribute to David A. Robinson, edited by Fuchs AF, Brandt T, Buttner U, and Zee DS. Stuttgart, Germany: Georg Thieme Verlag, 1994, p. 336–338. [Google Scholar]
- Tian S, Nishida Y, Isberg B, Lennerstrand G. MRI measurements of normal extraocular muscles and other orbital structures. Graefes Arch Clin Exp Ophthalmol 238: 393–404, 2000. [DOI] [PubMed] [Google Scholar]
- Van Rijn LJ, Collewijn H. Eye torsion associated with disparity-induced vertical vergence in humans. Vision Res 34: 2307–2316, 1994. [DOI] [PubMed] [Google Scholar]
- von Noorden GK. Binocular Vision and Ocular Motility: Theory and Management of Strabismus (4th Ed.). St. Louis, MO: Mosby, 1990, p. 193. [Google Scholar]
- Wilkie DR. The mechanical properties of muscle. Br Med Bull 12: 177–182, 1956. [DOI] [PubMed] [Google Scholar]
- Yoo L, Clark RA, Shin A, Demer JL. High Poisson ratio (PR) of contracting human superior rectus (SR) muscle indicates reverse compressibility. Program No.62.15. In: 2014 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience, 2014. [Google Scholar]