Abstract
Cortical spreading depression (CSD), a putative migraine trigger, has been shown recently to promote multiple activation patterns of meningeal nociceptors. In the current study we used a modified experimental approach in a rat model to: 1) reassess the responses of meningeal nociceptors following a single CSD episode, 2) examine factors that may influence the propensity of meningeal nociceptors to develop a prolonged activation following a CSD, and 3) test the responses of meningeal nociceptors following multiple CSDs. A single CSD episode promoted persistent activation in about 50% of the nociceptors tested, similar to our previous report. Only two patterns of prolonged nociceptor activation were observed: biphasic activation and one with a delayed onset. Aδ units had shorter mean onset latency for the prolonged activation than C units. The prolonged activation onset latency was inversely correlated with the number of the nociceptors' receptive fields. The propensity to develop the prolonged activation following CSD was related to the presence of basal ongoing activity, but neither to the emergence of brief activation during the CSD phase nor to the nociceptors' responsiveness to inflammatory mediators or ATP. Finally, multiple CSDs did not promote a heightened nociceptive response compared with a single CSD. The present study confirms the ability of a single CSD to elicit persistent activation of meningeal nociceptors. CSD-evoked prolonged nociceptive responses may not be related to the inflammatory and ATP chemosensitivity of the neurons but rather to other neuronal properties, such as basal ongoing activity and number of receptive fields.
Keywords: CSD, meningeal nociceptors, in vivo electrophysiology, migraine headache
migraine headaches affect about 15% of the adult population worldwide (Stovner et al. 2007). The head pain of migraine is most likely initiated as a result of the activation of primary afferent nociceptive neurons that innervate the cranial meninges and their related large blood vessels. The mechanisms underlying the initial activation of these meningeal nociceptors during a migraine attack are not completely understood but may be related to abnormal cortical metabolic changes that occur during the attack. In one type of migraine, migraine with aura, the attack is associated with abnormal visual symptoms such as blind spots and flashing lights (i.e., the visual aura) as well other sensory disturbances. It is now well accepted that these neural disturbances are the result of cortical spreading depression (CSD), a self-propagating slow wave of neuronal and glial depolarizations coupled to cerebral hemodynamic and metabolic changes including hyperemia followed by a prolonged hypoperfusion, or oligemia (Pietrobon and Moskowitz 2014). The finding that the aura symptoms precede or coincide with the early stages of the migraineous headache (Hansen et al. 2012) points to CSD, its associated cortical metabolic changes and the ensuing release of nociceptive molecules as potential endogenous events that play a role in mediating the activation of meningeal nociceptive neurons and the resultant headache (Bolay et al. 2002). Indeed, in a recent electrophysiological study we have shown that a single episode of CSD, initiated experimentally in the visual cortex of rats, can lead to increased ongoing activity in a sizable population of meningeal nociceptors (Zhang et al. 2010).
In that earlier study, CSD was associated with a variety of nociceptor activation patterns, including a brief increase in activity as well as a prolonged activation with either an immediate or delayed onset. These different neural responses could have stemmed from distinct response properties of meningeal nociceptors (Strassman and Levy 2006). Alternatively, these responses could have been influenced by the methods employed in that experimental preparation. For example, in that previous study (Zhang et al. 2010), CSD was evoked by stimulating the visual cortex in the vicinity of the transverse sinus, a meningeal region in which all the units tested had receptive fields (RF). The CSD-evoking stimulus thus could have led to direct stimulation of the nociceptors themselves, promoting the immediate and short-lasting activation pattern noted, which potentially could have contributed, at least in part to the emergence of the delayed and prolonged pattern of activation. In that previous work, similar to all previous recordings of meningeal nociceptors, the activity of the meningeal nociceptors was recorded using a sharp microelectrode advanced into the trigeminal ganglion through a track made in the ipsilateral cortex. During the search for a meningeal nociceptor, the microelectrode was advanced many times through the cortex and these multiple pinprick events were very likely to promote one or more uncontrolled CSDs before the experimentally evoked ones. Furthermore, in that earlier study the insertion of another sharp electrode into the ipsilateral cortex to record the CSD event was also very likely to induce another earlier episode of CSD. Whether these earlier uncontrolled CSD episodes played a role in mediating the diverse responses of the nociceptors noted following the experimental controlled CSD was never tested.
In the present study we reassessed the responses of meningeal nociceptors following CSD by employing a novel approach that minimized these technical issues and their potential influence on the responses of the neurons. First, we recorded the activity of meningeal nociceptors in the trigeminal ganglion using an electrode that was inserted into the ganglion through the contralateral cortex (Zhao and Levy 2014), thus preventing a resultant CSD in the ipsilateral cortex. We further minimized the possibility of direct stimulation of meningeal nociceptors with RF over the transverse sinus by inducing CSD experimentally in the frontal cortex, a caudal cortical region covered by dural tissue that is innervated by only a small percentage of neurons that also have RF on the transverse sinus. Finally, by recording the occurrence of the CSD episode using a laser Doppler flowmetry (LDF) probe, which does not penetrate the cortex, we further limited the occurrence of uncontrolled CSD.
In addition, we examined in this work whether baseline ongoing discharge, location of RFs, and chemosensitivity constitute factors that influence the propensity of meningeal nociceptors to become activated following CSD. Finally, we tested changes in the activity of meningeal nociceptors following a controlled session of multiple CSDs, an experimental paradigm that is often used in preclinical studies to investigate anti-migraine drugs.
MATERIALS AND METHODS
Animals and anesthesia.
Similar to our previous CSD study that used male rats (Zhang et al. 2010), the current study employed male rats. A total of 36 Sprague-Dawley animals (250–350 g) were used. Animals were handled in compliance with the experimental protocol approved by the Institutional Animal Care and Use Committee of the Beth Israel Deaconess Medical Center and adhered to the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain (Zimmermann 1986). Animals were deeply anesthetized with urethane (1.5–1.8 g/kg, ip) and mounted on a stereotaxic frame. Core temperature was kept at 37°C using a homoeothermic control system. Animals were ventilated with room air supplemented with O2 to achieve oxygen saturation values >95%. Heart rate and end-tidal CO2 were monitored and kept within a physiological range of 350–450 beats/min and 3.5–4.5%, respectively.
Surgery and electrophysiological recordings.
A craniotomy was made to expose the left transverse sinus as well as the adjacent dura extending ∼2 mm rostral to the sinus. The exposed dura was bathed with a modified synthetic interstitial fluid (SIF) consisting of (in mM) 135 NaCl, 5 KCl, 1 MgCl2, 5 CaCl2, 10 glucose, and 10 HEPES at pH 7.2. Single-unit activity of meningeal nociceptors (1 U/rat) was recorded in the ipsilateral (left) trigeminal ganglion using a contralateral approach, as described recently (Zhao and Levy 2014) and see also Fig. 1. Briefly, a small craniotomy (2 × 2 mm) was made in the calvaria over the right hemisphere, 2 mm caudal and 2 mm lateral to bregma. A platinum-coated tungsten microelectrode (impedance 100 kΩ, FHC) was advanced through the right hemisphere into the left trigeminal ganglion using an angled (22.5°) trajectory. In preliminary studies, we verified that inserting the recording electrode using this approach did not produce CSD in the left cortical hemisphere. To identify meningeal nociceptors, single-shock electrical search stimuli (0.5 ms, 5 mA, 0.5 Hz) were delivered through bipolar stimulating electrodes to the outer dural surface of the left transverse sinus. Units were classified based on their conduction velocity (CV), either as C units (CV ≤ 1.5 m/s) or slow Aδ (1.5 < CV ≤ 5 m/s) (Levy and Strassman 2002b). Faster conducting A units (CV > 5 m/s) were not included in this study because in our earlier preliminary studies they exhibited very poor responsiveness to CSD (Zhao and Levy, unpublished data). All units had at least 1 RF on the left transverse sinus or its near vicinity (<0.5 mm). A real-time waveform discriminator (spike 2 software, CED, Cambridge, UK) was used to create and store a template for the action potential evoked in the neurons, which was employed later to acquire and analyze the activity of the neurons.
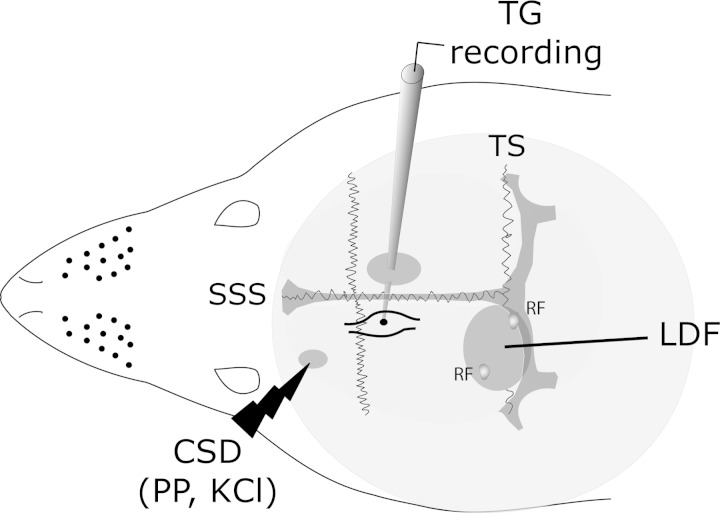
Fig. 1.
Representation of the experimental setup. Three skull openings (shaded ovals) were made. A small craniotomy was made over the left frontal cortex to elicit cortical spreading depression (CSD) events using a pinprick (PP) or KCl injections. Meningeal nociceptor activity was recorded in the left trigeminal ganglion (TG) using a tungsten microelectrode inserted through a craniotomy made over the contralateral hemisphere. An ipsilateral craniotomy was made to expose the left transverse sinus (TS) and its vicinity to search for meningeal nociceptors and identify receptive fields (RFs). Laser Doppler flowmetry (LDF) probe was placed over the cortex near the RF to record CSD-related changes in cerebral blood flow. SSS, superior sagittal sinus.
Induction and recording of CSD.
Single CSD episodes were induced by pinpricking the cortex with a fine glass micropipette (diameter 10 μm) at ∼2 mm depth for 2 s. To study the effect of a series of CSD events, 30 nl of 1 M KCl were microinjected into the cortex every 5 min. Injections were made at a depth of ∼150 μm using a Hamilton syringe (2–4 μm tip) driven by an electronic-geared syringe pump (Ultra micro pump UMPIII; WPI, Sarasota, FL). The initial insertion of the micropipette did not trigger CSD. All CSD-evoking stimuli were made in the left frontal cortex through a small burr hole (∼0.5 mm). The occurrence of a CSD episode was recorded using LDF with the probe positioned, within the craniotomy, just above the exposed dura near the RF of the unit. A successful induction of CSD was considered when the typical hemodynamic signature characterized by a brief (∼1–2 min) cortical cerebral hyperemia followed a persistent (>1 h) post-CSD oligemia (Fordsmann et al. 2013) was observed.
Experimental paradigm.
Before the induction of CSD, baseline ongoing activity of meningeal nociceptors was recorded for at least 45 min. Only units that displayed a stable baseline were tested further. Recording of ongoing activity was then continued for at least 60 min following a single episode of CSD. In units in which multiple CSDs were evoked, ongoing activity was recorded for at least 90 min (30 min during the CSDs events and then for an additional 60 min). For testing chemosensitivity, acute changes in ongoing activity were examined in response to local application of a mix of inflammatory mediators (IM) or ATP. For IM stimulation, we used a topical application of a 50-μl mixture of histamine, serotonin, and bradykinin (at 100 μM), and prostaglandin E2 (at 10 μM) in pH 7.2 SIF. For ATP stimulation, we applied freshly made ATP (1 mM in pH 7.2 SIF). Chemical stimuli were applied for 180 s and the concentrations used were based on previous finding (Hockley et al. 2014; Jankowski et al. 2013; Levy and Strassman 2002a). Units were stimulated with IM, ATP, or both agents. Testing for chemosensitivity begun at least 60 min after the end of a single CSD experiment. In units tested with both ATP and IM, the second agent was tested 45–60 min after the application of the first agent, when the activity of the unit returned to baseline. To determine the number of RFs and their dural localization, at the end of the study, the calvarial bone covering the area between the CSD elicitation site and the transverse sinus was removed by using careful drilling, and RFs were mapped using von Frey monofilament stimulation.
Data analysis.
Data are means ± SE. Statistical analyses were conducted using parametric statistics, with two-tailed level of significance set at α = 0.05. Differences between means were calculated using t-tests. A prolonged activation following CSD was considered when a nociceptor displayed an increase in firing rate above the upper end point of the 95% confidence interval calculated for the baseline mean and which lasted >10 min. For calculating the onset latency for such activation, time 0 was considered as the time when the first sign of the CSD (i.e., the initial increase in cerebral blood flow) appeared. Activation by IM or ATP was considered when the discharge rate increased above the upper end point of the 95% confidence interval for the baseline mean for duration of at least 60 s for ATP and 300 s for IM (Hockley et al. 2014; Jankowski et al. 2013; Levy and Strassman 2002a). Group differences were analyzed using two-tailed, Fisher's exact test. Linear regression was used to determine relationships between the meningeal nociceptors' response following CSD and the number of their RF.
RESULTS
Activation of meningeal nociceptors following a single CSD.
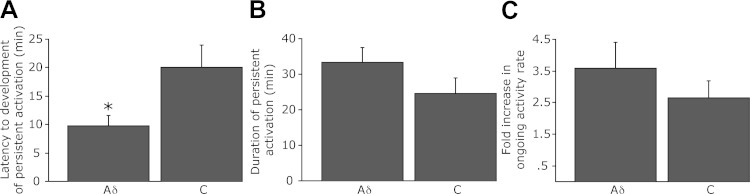
Changes in the activity of meningeal nociceptors following a single CSD were evaluated in 17 Aδ (mean CV 2.5 ± 0.20, range 1.58–4.69 m/s) and 19 C units (mean CV 0.64 ± 0.07, range 0.31–1.42 m/s). At baseline, ongoing activity was present in 6/17 of the Aδ units (mean 0.28 ± 0.17, range 0–2.44 Hz) and 18/19 of the C-units (mean 0.72 ± 0.19, range 0–2.7 Hz, P < 0.05 between the populations). After the induction of CSD, a prolonged nociceptor activation (>10 min) was noted in 9/17 (53%) of the Aδ units and 10/19 (53%) of the C units. Two major patterns of activation were observed. One pattern consisted of biphasic activation, involving a brief activation that occurred around the time of the CSD-related cortical hyperemia followed by a delayed and more persistent activation later on (Fig. 2A). This pattern of activation was observed primarily in the Aδ population (6/9 Aδ units and 2/10 C units). A second pattern, which was observed primarily in the C unit population (3/9 Aδ units and 8/10 C units), consisted only of persistent activation with a delayed onset (Fig. 2B). The differences in the activation pattern between the Aδ and C unit populations showed, however, only a trend toward significance (P = 0.069, Fisher's exact test). Nonetheless, the Aδ population exhibited a statistically significant shorter mean onset latency for the prolonged activation phase (9.8 ± 1.8, range 3–17 min in Aδ vs. 20 ± 3.9, range 3–30 min in C units, P < 0.05, Fig. 3A). The average duration and magnitude of this activation did not differ between the two nociceptor populations: the duration of the activation in the Aδ population was 33.3 ± 4.1 min (range 20–55 min) compared with 24.5 ± 4.5 min (range 15–50 min) in the C units (Fig. 3B) while ongoing activity rate in the Aδ units rose by 358 ± 82.9% (range 124–630%) compared with 264 ± 54% (range 136–638%) in the C unit population (Fig. 3C).
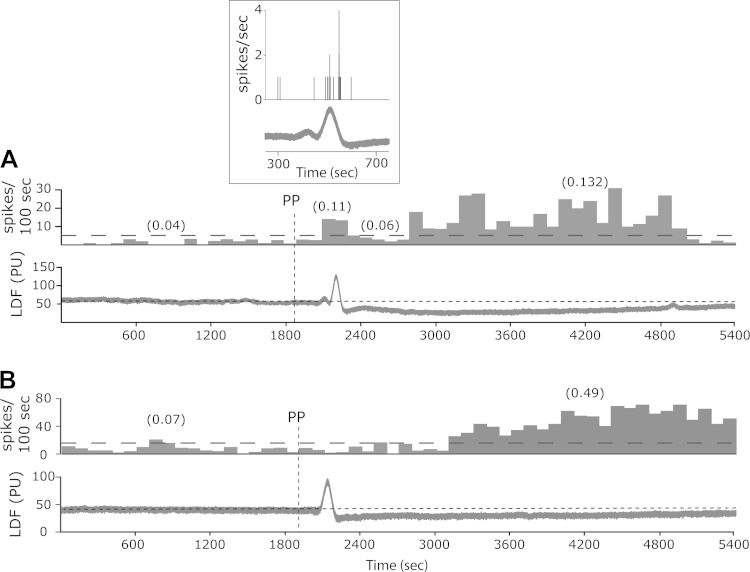
Fig. 2.
Selected 90-min peristimulus time histograms (PSTH, bin size 100 s) demonstrating the changes in neuronal activity of two meningeal nociceptors after the induction of a single CSD in the frontal cortex using PP stimulation. Average discharge rates (in Hz) are shown in parentheses. The horizontal dotted line indicates the level above which the unit was considered to be activated. The bottom traces in each example illustrate LDF recordings of the CSD-related changes in cerebral blood flow (in arbitrary perfusion units, PU). Note the brief hyperemia (during the CSD) and an ensuing post-CSD persistent oligemia. A: example of a meningeal nociceptor displaying a biphasic type of response including a brief neuronal activation around the time of the hyperemia followed by a delayed and prolonged activation during the oligemic phase. The inset box provides an expended time scale view illustrating the neural activation during the hyperemic phase. B: example of a meningeal nociceptor displaying only a prolonged activation with delayed onset that emerged during the oligemic phase.
Fig. 3.
Characterizations of the delayed and prolonged activation of Aδ and C meningeal nociceptors following the induction of a single CSD. A: latency to the onset of delayed activation. B: duration of the prolonged increases in activity. C: fold increases in ongoing discharge rate during the prolonged activation phase compared with baseline. *P < 0.05, t-test.
During the induction of a single CSD using pinprick a short burst of increased activity (∼1 s) was noted in 3 Aδ and 1 C units (4/36, 11.1%, of the total population tested). In only one of those units (an Aδ) a prolong increase in activity developed later (1/19, 5.2% of the activated unit population). In all of the 4 units that displayed this burst of activity in response to the pinprick stimulation, a dural RF was found at the pinprick site or very close by (<0.5 mm).
Relationship between CSD-evoked prolonged nociceptor activation and nociceptor activation during the CSD phase.
Nociceptor activation during the arrival of the CSD wave (i.e., the hyperemic stage) near the RF of meningeal nociceptors has been suggested to promote the ensuing delayed prolonged activation (Bolay et al. 2002). A short-term increase in ongoing activity during the time of the CSD-evoked hyperemic phase was noted in 8/19 (42%) of the units that demonstrated a delayed and prolonged activation subsequently. This response rate nonetheless was not different from that observed in the units that failed to develop this pattern of activation (4/17, 23.5%, Table 1). In the units demonstrating a prolonged activation, the average duration of the short-term activation during the hyperemic stage of the CSD was 78.5 ± 7.9 s (range 60–120 s), which was also not different from that observed in units that did not develop a prolonged activation later on (84.0 ± 14.7, range 60–120 s).
Table 1.
Numbers of units/total tested displaying basal ongoing activity, short-term activation during the CSD, and responsiveness to local chemical stimulation in CSD-affected and nonaffected meningeal nociceptors
| Persistent Activation After CSD |
||
|---|---|---|
| Yes | No | |
| Basal ongoing activity | 18/19 (94.7%)* | 10/17 (58.9%) |
| Brief activation during CSD | 8/19 (42.1%) | 4/17 (23.5%) |
| Activated by IM | 5/12 (41.7%) | 6/13 (46.2%) |
| Activated by ATP | 10/16 (62.5%) | 6/14 (42.9%) |
CSD, cortical spreading depression; IM, inflammatory mediators.
P < 0.05, Fisher's exact test.
Relationship between CSD-evoked prolonged nociceptor activation, baseline ongoing activity, and RFs.
The propensity of meningeal nociceptors to develop a delayed and prolonged type of activation following CSD was related to the presence of ongoing activity during the baseline-recording period. Among the 19 units that developed a prolonged activation, 18 units (95%) displayed varying levels of baseline ongoing activity, a significantly higher percentage than in the group of neurons that did not develop such a response (10/17, 59%, P < 0.05, Table 1), suggesting that neurons with no ongoing activity are less likely to develop persistent activation following CSD. Nonetheless, there was no difference in the mean baseline ongoing activity rate between the persistently activated neurons (mean 0.38 ± 0.14, range 0–2.4 Hz) and those that did not develop such a response (mean 0.68 ± 0.23, range 0–2.7 Hz).
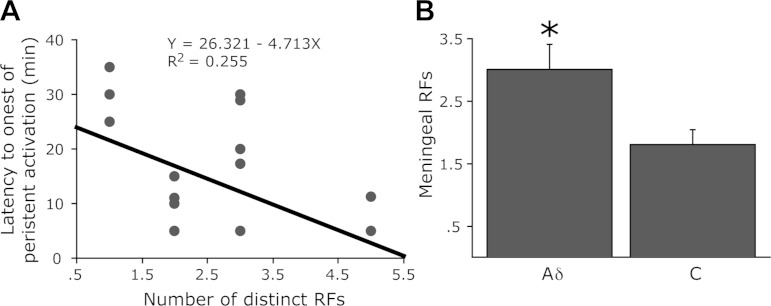
The propensity to develop a prolonged activation following a single CSD event was not related to the number of dural RFs located for each unit. In units that developed a prolonged activation there were on average 2.1 ± 0.28 RFs (range 1–5). A similar number of RFs (mean 2.25 ± 0.27, range 1–5) were obtained for units that did not develop such a response following CSD. In neurons showing a persistent activation there was, however, an inverse correlation between the number of RFs and the latency to develop prolonged activations (R2 = 0.255, P < 0.05, Fig. 4A). A further analysis between the activated Aδ and C units revealed that Aδ units, which had shorter onset for such activation, also had significantly more dural RFs (3.0 ± 0.4 vs. 1.6 ± 0.3, P < 0.01, Fig. 4B).
Fig. 4.
Relationship between dural RFs and the prolonged activation of meningeal nociceptors after a single CSD. A: linear regression analysis of the onset latency to the delayed and persistent activation of meningeal nociceptors vs. the number of their dural RFs. Note the inverse correlation. B: mean number of dural RFs found for the Aδ and C units recorded in this study. *P < 0.05, t-test.
Relationship between CSD-evoked prolonged nociceptor activation and meningeal nociceptors' chemosensitivity.
We next tested whether neurons that became persistently activated after a single CSD episode displayed different chemosensitivity than units that did not develop such a response. Chemosensitivity was assessed by testing responses to a mix of mediators that are released during inflammation and to ATP, which is released during CSD (Karatas et al. 2013; Mies and Paschen 1984). Most of the units tested (23/37) were stimulated with both IM and ATP, 2 only with IM, and 7 only with ATP. In units tested with both agents, ATP was tested as the first agent in 15/23 cases and IM was introduced first in the remainder of the cases. In this experimental paradigm, the response rates to ATP and to IM were not affected by the order of their administration. IM activated 3/8 units when it was administered first and 8/15 units were when it was administered as the second agent (P = 0.667, Fisher's exact test). Similarly, ATP activated 10/15 units when it was administered first and 4/8 units when it was administered as the second agent (P = 0.657, Fisher's exact test). Because of the similarities in the response rates obtained in these two administration protocols, we analyzed the responses to the mediators in CSD responders and nonresponders regardless of the protocol used to administer them.
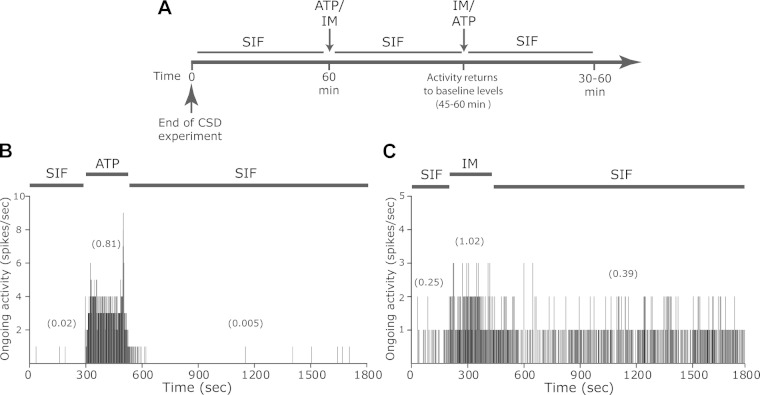
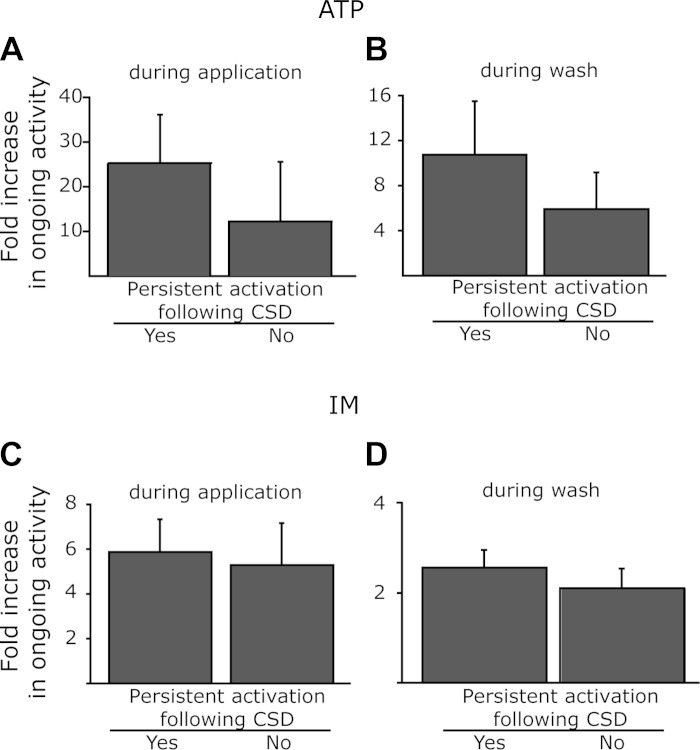
Topical application of ATP activated similar proportions of Aδ (8/14, 57.1%) and C units (9/16, 56.2%). Topical application of IM also activated similar proportions of Aδ (6/11, 54.5%) and C units (6/14, 42.9%). Overall, the propensity to develop a prolonged activation following CSD was not related to the chemosensitivity of the neurons tested, neither to their responsiveness to ATP nor to IM (Table 1). The response rate after local administration of ATP among the units that developed a prolonged activation after CSD was 62.5% (10/16, 6Aδ, 4C) units, which was not statistically different from that noted for the units that did not develop such CSD-evoked response (42.8%, 6/14, 2Aδ, 5C). In units that developed a prolonged activation following a single CSD, the ATP-evoked neural activation lasted on average 72.9 ± 40.2 s (see an example in Fig. 5B), which was not different from the response observed in the units that did not display this pattern of activation after CSD (81 ± 43 s). The magnitudes of responses during the application of ATP as well as during the wash period were also similar between the two groups (Fig. 6).
Fig. 5.
Responses of CSD-responsive meningeal nociceptors to ATP and to inflammatory mediators (IM). A: illustration of the experimental timeline where the same unit was exposed to ATP and to IM. B: PSTH (bin size 1 s) demonstrating the brief activation of a CSD-responsive meningeal nociceptor after topical application of 1 mM ATP. Average discharge rates (in Hz) are given in parentheses. Note the increase in activity during the exposure to ATP and its return to baseline immediately during the wash [synthetic interstitial fluid (SIF)] period. C: PSTH (bin size 1 s) demonstrating the activation of the same unit following topical application of IM.
Fig. 6.
Relationship between the responses of meningeal nociceptors to CSD and their chemosensitivity. A: responses during the application of ATP. B: residual responses to ATP during the initial (1 min) wash period. C: responses during the application of IM. D: residual responses to IM during the initial (10 min) wash period.
The response rate after stimulation with IM was 41.7% (5/12, 3 Aδ, 2C) among the units that developed a prolonged activation, which was not different from that observed among the units that did not demonstrate such a persistent response (46.2%, 6/13, 3Aδ, 3C). The magnitude of responses observed during the application of IM as well as during the wash period was also not statistically different between units that displayed persistent response following CSD and those that did not (Fig. 6).
Responses of meningeal nociceptors after multiple CSDs.
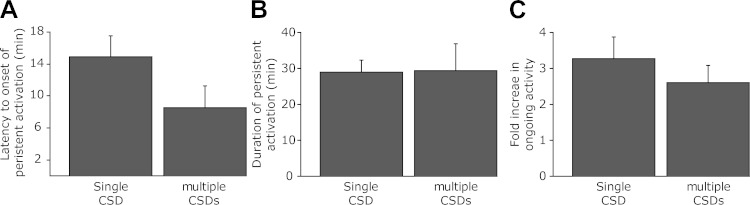
Finally, we tested the responses of meningeal nociceptors to a series of 5 subsequent CSD, induced during a 30-min period. A total of 18 units that were not exposed previously to a single CSD paradigm were tested. Ten units were Aδ (mean CV 2.64 ± 0.27, range 1.76–4.31 m/s) and had a mean baseline ongoing activity rate of 0.43 ± 0.19 Hz (range 0–1.74 Hz) and 8 were C units (mean CV 0.71 ± 0.15, range 0.34–1.25 m/s) and had a mean baseline ongoing activity rate of 0.76 ± 0.41 Hz (range 0–2.97 Hz). In response to a series of CSD episodes, a prolonged increase in ongoing activity rate was noted in 7/18 (38.9%) units, which included 3 Aδ and 4 C units. Overall, this incidence of persistence activation was not statistically different from that observed after a single CSD (7/18 vs. 19/36, respectively). A prolonged increase in ongoing activity was observed with an overall delay of 8.6 ± 1.4 min (range 4–13 min, following the first CSD). Although there was a trend toward a shorter delay compared with that observed in the single CSD episode group (i.e., 14.9 ± 2.6 min), this difference did not reach a statistical significance (P = 0.06, Fig. 7A). As Fig. 7B depicts, the duration of the prolonged nociceptor activation phase in the multiple CSD group was 29.3 ± 7.6 min (range 10–55), which was also not statistically different from that observed in the single CSD group (28.68 ± 2.9 min). The magnitude of the nociceptors' activation following multiple CSD was also not different from that observed following a single CSD episode (260 ± 48% vs. 327 ± 60%, respectively, Fig. 7C).
Fig. 7.
Characterization of the delayed onset activation of meningeal nociceptors after single and multiple episodes of CSDs. A: latency to onset of delayed activation. B: duration of prolonged increases in activity. C: fold increase in ongoing discharge rate during the prolonged activation compared with baseline.
DISCUSSION
In the current study we have used a novel experimental setup (Fig. 1) to study the effect of CSD on meningeal nociceptors. This setup employed a contralateral approach for inserting the recording electrode into the left trigeminal through the right forebrain together with a laser blood flowmetry to record CSD-related changes in cerebral blood flow, both of which eliminated the induction of uncontrolled CSD episodes in the ipsilateral (left) cortex. We also induced CSD in the frontal cortex remotely from most of the neurons' RFs on the transverse sinus and its vicinity to minimize the possibility of direct neuronal activation by the CSD-evoking stimulus. Using these modified approaches, we obtained data confirming our previous work (Zhang et al. 2010), which demonstrated the development of a prolonged activation of meningeal nociceptors following a single CSD episode induced in the visual cortex. In the current study, the incidence rate of a prolonged nociceptor activation that exceeded 10 min after the induction of a single CSD in the frontal cortex, using a pinprick stimulus, was comparable to that reported earlier after a similar elicitation of CSD in the visual cortex (53% vs. 59%). In the current study we also noted a similar (although slightly shorter) mean duration of this activation: ∼29 min vs. ∼37 min in the previous study. A similar mean onset latency for the prolonged activation phase was also observed: in this study ∼15 min vs. ∼12 min in the previous work. These similarities suggest that the elicitation of uncontrolled CSD episodes before the experimental one (as likely occurred in the previous study) is unlikely to affect the propensity of the nociceptors to develop a prolonged activation following CSD, the latency of such response or its overall duration. Our current finding further suggests that a CSD event can promote a prolonged activation of meningeal nociceptors regardless of the cortical region it arises from. This notion adds support to the view that even in migraine attacks that occur without a visual aura, the headache may be the result of a CSD that involves areas of the cortex that would not generate a perceived aura (i.e., silent CSD) (Pietrobon and Moskowitz 2012).
Our finding indicate that multiple CSD episodes promote persistent activation of meningeal nociceptors that is no different from that evoked by a single CSD episode. A paradigm using the elicitation of a train of CSD events is often used in preclinical studies to explore the effects of anti-migraine agents. Recent studies, employing this paradigm, have found that agents used in migraine prophylaxis can suppress the frequency of CSD events within a train (Ayata et al. 2006; Eikermann-Haerter et al. 2015). Given that multiple CSDs do not exert an additive nociceptive effect compared with a single CSD event, we propose that a reduction in the number of CSD episodes within a train may not serve as a good predictor of a drug's anti-migraine effect.
While some of the findings in the current study were similar to those reported previously (Zhang et al. 2010), there were also some notable differences. In the current study we observed a higher mean of increased ongoing discharge rate, ∼3.5-fold in our study versus ∼2-fold in the previous one. Also different were the major patterns of responses noted: in the previous study, three major patterns of activation were observed while in the current study we observed only two patterns. Here, we noted in 42% of the cases a biphasic response, which included a short-term activation during the arrival of the CSD wave near the meningeal nociceptors' RF that was followed by a delayed phase of prolonged activation. In the previous study, this particular biphasic pattern of activation was noted in only about 10% of the cases. Also different in the current study was the lack of one type of specific response that was reported earlier in ∼30% of the cases: a prolonged activation with an immediate onset. Finally, in the current study the incidence rate of an immediate, short-lived burst of nociceptor firing during the pinprick event (i.e., before the emergence of the CSD) was much smaller and occurred in only ∼5% of the units that displayed a prolonged activation compared with 28% of the units in the previous study. The factors contributing to the differences in the neuronal responsiveness between these two studies are unclear at present. It is possible that injury to the nociceptors' nerve endings evoked mechanically by the pinprick or chemically by the high dose of KCl used to elicit the CSD in the previous study could have played a role. Regardless of the mechanism underlying these differences, we propose that the modified experimental preparation used in the current study affords significant improvements over previously used techniques to examine the responses of meningeal nociceptors after CSD and explore pharmacological agents that could modulate these responses.
The current study is the first to investigate potential factors that affect the propensity of meningeal nociceptors to develop a prolonged increase in ongoing activity rate after CSD. Our data suggest that one key factor that influences such susceptibility is the presence of basal ongoing activity. The exact mechanism underlying such ongoing activity in meningeal nociceptors is not completely understood but is likely related to the craniotomy that is required to expose the dura and the ensuing local immune response (Levy et al. 2007). The higher rate of activation after CSD in meningeal nociceptors with a basal level of ongoing activity could suggest that these neurons were already in a sensitized (or a semisensitized) state and thus were more readily available to become activated by the nociceptive factors released as a result of the CSD. Another possibility is that meningeal nociceptors that do not display basal ongoing activity in this preparation do not possess the cellular machinery to allow the development of ongoing discharge in response to the milieu found at baseline conditions as well as after CSD. It is noteworthy that this sensitivity is unlikely to be related to the propensity of the neurons to become activated in response to a chemical stimulus evoked by ATP or the mixture of inflammatory mediators tested given that we did not find any difference in the responsiveness to these nociceptive agents between neurons that were affected by CSD and those that did not. Finally, the short duration of the nociceptors' activation after stimulation with ATP, similar to its action on nociceptors innervating other tissues (Hockley et al. 2014; Jankowski et al. 2013), suggest that local action of ATP may not play a significant role, at least not by itself, in mediating the prolonged nociceptor response after CSD.
The short-term increase in ongoing activity during the arrival of the CSD wave near the meningeal nociceptors' RF has been suggested to promote the ensuing prolonged activation through a mechanism that involves the release of sensory neuropeptides such as CGRP and the resultant degranulation of meningeal mast cells, a process known as neurogenic inflammation (Bolay et al. 2002). While testing this hypothesis specifically will require further experiments, we propose that in this preparation neurogenic inflammation is unlikely to mediate the delayed and prolonged activation of meningeal nociceptors following CSD. This argument is supported by the lack of differences in the incidence rate of short-term activation between units that developed a prolonged activation and those, which did not. We also argue against the role of neurogenic inflammation in mediating the persistent activation after CSD given that in this, as well as in other studies that involve a craniotomy, the cranial dura is already in a state of inflammation, a process mediated at least in part by the degranulation of dural mast cells (Levy et al. 2007). Although the contribution of the initial activation of meningeal nociceptors during the CSD phase to the emergence of the delayed and prolonged activation phase remains to be investigated, factors other than meningeal neurogenic inflammation, such as cortical release of vasoactive mediators that mediate the changes in cerebral blood flow may play a role. A recent study implicated the opening of cortical neuronal pannaxin-1 megachannels and its associated neuroimmune response in mediating the meningeal vasodilatation and pain following CSD (Karatas et al. 2013). Whether this mechanisms can promote the prolonged activation of meningeal nociceptors will require further studies.
Finally, the current study points to an inverse correlation between the number of dural RFs of meningeal nociceptors and the latency to develop a prolonged activation after CSD. Our data showing that meningeal Aδ nociceptors, which overall had a shorter mean latency for such activation than C units, also had more dural RFs than C units further suggests a possible role for the number of dural RFs in controlling this latency. That units with more RFs had shorter latencies suggests a mechanism that involves the spread of noxious mediators after the CSD event and spatial summation in mediating the onset of the persistent nociceptors' activation.
GRANTS
The study was supported by National Institutes of Health Grants AA020305, NS078263, NS086830 and grants from the National Headache Foundation to D. Levy.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.Z. and D.L. conception and design of research; J.Z. performed experiments; J.Z. and D.L. analyzed data; J.Z. and D.L. interpreted results of experiments; J.Z. and D.L. prepared figures; J.Z. and D.L. edited and revised manuscript; J.Z. and D.L. approved final version of manuscript; D.L. drafted manuscript.
REFERENCES
- Ayata C, Jin H, Kudo C, Dalkara T, Moskowitz MA. Suppression of cortical spreading depression in migraine prophylaxis. Annals Neurol 59: 652–661, 2006. [DOI] [PubMed] [Google Scholar]
- Bolay H, Reuter U, Dunn AK, Huang Z, Boas DA, Moskowitz MA. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat Med 8: 136–142, 2002. [DOI] [PubMed] [Google Scholar]
- Eikermann-Haerter K, Lee JH, Yalcin N, Yu ES, Daneshmand A, Wei Y, Zheng Y, Can A, Sengul B, Ferrari MD, van den Maagdenberg AM, Ayata C. Migraine prophylaxis, ischemic depolarizations, and stroke outcomes in mice. Stroke 46: 229–236, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordsmann JC, Ko RW, Choi HB, Thomsen K, Witgen BM, Mathiesen C, Lonstrup M, Piilgaard H, MacVicar BA, Lauritzen M. Increased 20-HETE synthesis explains reduced cerebral blood flow but not impaired neurovascular coupling after cortical spreading depression in rat cerebral cortex. J Neurosci 33: 2562–2570, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen JM, Lipton RB, Dodick DW, Silberstein SD, Saper JR, Aurora SK, Goadsby PJ, Charles A. Migraine headache is present in the aura phase: A prospective study. Neurology 79: 2044–2049, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockley JR, Boundouki G, Cibert-Goton V, McGuire C, Yip PK, Chan C, Tranter M, Wood JN, Nassar MA, Blackshaw LA, Aziz Q, Michael GJ, Baker MD, Winchester WJ, Knowles CH, Bulmer DC. Multiple roles for NaV1.9 in the activation of visceral afferents by noxious inflammatory, mechanical, and human disease-derived stimuli. Pain 155: 1962–1975, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski MP, Rau KK, Ekmann KM, Anderson CE, Koerber HR. Comprehensive phenotyping of group III and IV muscle afferents in mouse. J Neurophysiol 109: 2374–2381, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatas H, Erdener SE, Gursoy-Ozdemir Y, Lule S, Eren-Kocak E, Sen ZD, Dalkara T. Spreading depression triggers headache by activating neuronal Panx1 channels. Science 339: 1092–1095, 2013. [DOI] [PubMed] [Google Scholar]
- Levy D, Burstein R, Kainz V, Jakubowski M, Strassman AM. Mast cell degranulation activates a pain pathway underlying migraine headache. Pain 130: 166–176, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D, Strassman AM. Distinct sensitizing effects of the cAMP-PKA second messenger cascade on rat dural mechanonociceptors. J Physiol (Lond) 538: 483–493, 2002a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D, Strassman AM. Mechanical response properties of A and C primary afferent neurons innervating the rat intracranial dura. J Neurophysiol 88: 3021–3031, 2002b. [DOI] [PubMed] [Google Scholar]
- Mies G, Paschen W. Regional changes of blood flow, glucose, and ATP content determined on brain sections during a single passage of spreading depression in rat brain cortex. Experimental Neurol 84: 249–258, 1984. [DOI] [PubMed] [Google Scholar]
- Pietrobon D, Moskowitz MA. Chaos and commotion in the wake of cortical spreading depression and spreading depolarizations. Nat Rev Neurosci 15: 379–393, 2014. [DOI] [PubMed] [Google Scholar]
- Pietrobon D, Moskowitz MA. Pathophysiology of migraine. Annu Rev Physiol 75: 365–91, 2013. [DOI] [PubMed] [Google Scholar]
- Stovner L, Hagen K, Jensen R, Katsarava Z, Lipton R, Scher A, Steiner T, Zwart JA. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia 27: 193–210, 2007. [DOI] [PubMed] [Google Scholar]
- Strassman AM, Levy D. Response properties of dural nociceptors in relation to headache. J Neurophysiol 95: 1298–1306, 2006. [DOI] [PubMed] [Google Scholar]
- Zhang X, Levy D, Noseda R, Kainz V, Jakubowski M, Burstein R. Activation of meningeal nociceptors by cortical spreading depression: implications for migraine with aura. J Neurosci 30: 8807–8814, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Levy D. The sensory innervation of the calvarial periosteum is nociceptive and contributes to headache-like behavior. Pain 155: 1392–1400, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M. Ethical considerations in relation to pain in animal experimentation. Acta Physiol Scand 128: 221–233, 1986. [PubMed] [Google Scholar]