Abstract
A hallmark of tactile texture exploration is that it involves movement between skin and surface. When we scan a surface, small texture-specific vibrations are produced in the skin, and specialized cutaneous mechanoreceptors convert these vibrations into highly repeatable, precise, and informative temporal spiking patterns in tactile afferents. Both texture-elicited vibrations and afferent responses are highly dependent on exploratory kinematics, however; indeed, these dilate or contract systematically with decreases or increases in scanning speed, respectively. These profound changes in the peripheral response that accompany changes in scanning speed and other parameters of texture scanning raise the question as to whether exploratory behaviors change depending on what surface is explored or what information is sought about that surface. To address this question, we measure and analyze the kinematics as subjects explore textured surfaces to evaluate different types of texture information, namely the textures' roughness, hardness, and slipperiness. We find that the exploratory movements are dependent both on the perceptual task, as has been previously shown, but also on the texture that is scanned. We discuss the implications of our findings regarding the neural coding and perception of texture.
Keywords: texture perception, exploratory procedures, scanning speed, touch, haptics
we are exquisitely sensitive to surface texture and able to distinguish surfaces whose features vary on a submicron scale (Skedung et al. 2013). A hallmark of texture exploration is lateral movement between skin and surface (Lederman and Klatzky 1993), without which our ability to discern fine surface texture is severely compromised (Hollins and Risner 2000). Scanning a texture's surface produces vibrations in the skin (Bensmaia and Hollins 2003, 2005; Manfredi et al. 2014) that in turn produce temporal spiking patterns in two types of cutaneous mechanoreceptive afferents that innervate the skin: PC afferents, which innervate Pacinian corpuscles, and rapidly adapting (RA) afferents, which innervate Meissner corpuscles (Weber et al. 2013). The frequency composition of the vibrations reflects the spatial structure of the texture and that of the fingerprint (Manfredi et al. 2014).
Of course, the frequency composition of texture-elicited vibrations also depends heavily on scanning speed (Bensmaia and Hollins 2003; Manfredi et al. 2014). Indeed, to a first approximation, the frequency composition of the vibrations translate left and right along the frequency axis with decreases and increases in scanning speed, respectively (Manfredi et al. 2014). As might be expected, then, afferent responses are also highly dependent on surface texture: Temporal spiking patterns contract or dilate systematically with increases or decreases in scanning speed, respectively (Weber et al. 2013). While this temporal mechanism is highly sensitive to changes in scanning speed, it is complemented by another mechanism, responsible for the coding of coarse textures, that is less so. Indeed, coarse textural features are reflected in the spatial pattern of activation in another class of peripheral afferents, namely slowly adapting type 1 (SA1) afferents (Connor et al. 1990; Connor and Johnson 1992; Weber et al. 2013). While the spatial layout of this SA1-mediated texture image is relatively invariant with respect to scanning speed, SA1 firing rates depend on scanning speed (Johnson and Lamb 1981). The relative movement dependence of the two mechanisms is reflected in the fact that perception of coarse textural features is possible even in the absence of movement, while that of fine textural features is abolished without movement. Note, however, that even the perception of coarse features depends on scanning speed, as evidenced by the fact that the roughness of coarse gratings changes slightly but systematically with changes in scanning speed (Cascio and Sathian 2001).
Taken together, these findings suggest that the peripheral neural representation of texture depends at least in some part on the exploratory kinematics. As such, to understand the coding of texture, one needs to know how texture is explored under natural conditions. In fact, the kinematics of texture exploration can provide clues as to the neural basis of texture perception: To the extent that different perceptual textural dimensions, such as roughness or hardness, rely on different neural codes, different exploratory actions might be used to optimally extract information about each dimension. Similarly, exploratory movements might be optimized to extract information on a texture by texture basis. Finally, the biophysical properties of the textures themselves will affect contact mechanics and thereby shape exploratory actions, for example by exerting different amounts of frictional force.
In the present study, then, we sought to objectively and quantitatively characterize the kinematics of texture exploration and do so across a wide range of tangible textures. Specifically, we wished to 1) measure exploratory movements produced by surfaces that vary widely in textural properties, 2) determine whether exploratory kinematics are dependent on the type of textural information that is being sought, and 3) determine whether exploratory kinematics are dependent on the texture itself. Unlike many previous studies, the texture swaths were large, so that the movements were not constrained by the geometry of the workspace but rather reflected each subject's natural exploratory strategies.
MATERIALS AND METHODS
Participants.
All testing procedures were approved by the Institutional Review Board for Human Use of the University of Chicago. All subjects were paid for their participation. Six subjects (four men and two women, mean age = 19.8 ± 2.6 yr) participated in the study. All subjects were University of Chicago undergraduate or graduate students and reported normal tactile sensitivity and no history of neurological disease.
Stimuli.
Stimuli consisted of 14 different textures that spanned a wide range of perceptual qualities (see Table 1). Textures were mounted on 30.5 × 30.5 × 0.3 cm Plexiglas panels with double-sided adhesive tape (3M). The large stimulus surface was chosen to provide subjects with unconstrained freedom of movement in all directions during texture exploration.
Table 1.
Texture stimuli used in the study
| Texture Name |
|---|
| Taffeta |
| Medium corduroy |
| Sandpaper (240 grit) |
| Wool gabardine |
| Silk jacquard |
| Satin |
| Nylon (200 denier) |
| Careerwear flannel |
| Sandpaper (800 grit) |
| Snowflake fleece fuzz |
| Crinkled silk |
| Microsuede |
| Stretch velvet |
| Denim |
These textures comprise a subset of stimuli described in detail in a previous study (Manfredi et al. 2014).
Psychophysical procedure.
Subjects were seated at the experimental table behind a curtain and passed their arm under the curtain to explore each texture, so they could see neither their hand nor the texture. The subjects lifted their hands between trials while the previous texture was removed and the next texture was positioned, so they did not have to reposition their hand between trials. Instructed to explore each texture with a single finger, all six subjects used the index finger of their right hand. When the texture was in place, the experimenter told the subject they could begin exploring. Subjects could explore the texture for as long as they wanted, and the trial ended when the subject had provided a perceptual rating (see below).
After exploring each surface, subjects produced a free magnitude estimate (rating) on one of three textural continua, which have been previously identified as the most perceptually salient (Bensmaia and Hollins 2005; Hollins et al. 2000): softness/hardness, roughness/smoothness, and stickiness/slipperiness. Subjects were instructed to report a number proportional to the perceived position of the surface along that continuum. Subjects were told that surfaces were to be judged on a ratio scale and encouraged to use any range of numbers they chose. For example, if the second texture was twice as hard as the first texture, they were to assign to it a number that was twice as large. The 14 texture stimuli were each presented to each subject five times, in quasi-random order, for a total of 70 stimulus presentations for each of three tasks. On each experimental block, subjects rated surfaces along one of the three dimensions. Magnitude estimates were normalized by dividing by the mean of all the ratings obtained in each experimental block and then averaged within subjects and then across subjects. Five subjects completed the three tasks in one experimental session, and one subject completed them in two experimental sessions.
Tracking of exploratory kinematics.
A colored circle (diameter = 0.5 cm) was fixed to the nail of the exploratory finger to serve as color marker for the tracking algorithm. A high-speed digital camera (Lumenera Lt225) was fixed 90 cm above the workspace and aimed directly downward. A tape measure ran along the table to indicate the spatial scale of the filmed workspace and was used to convert pixels to mm. The workspace was lit with four high-intensity fluorescent bulbs (55 W each) providing even illumination. The camera captured exploratory movements at a rate of 120 frames per second with a resolution of 512 × 512 pixels, resulting in a spatial resolution of ∼0.5 mm. Raw data were collected with the Streampix 5 (Norpix) software and converted to AVI files with no compression, which were then used to track the kinematics of the fingertip using custom Matlab code. Specifically, the tracker converted each video frame to hue-saturation-value (HSV) color space and then calculated the spatial centroid of all pixels that fell within a manually set range of HSV values. The color marker on the finger was sufficiently different in color from the rest of the frame to allow for reliable tracking. Ultimately, the tracker provided a timestamp and the x and y position of the color marker fixed to the exploratory finger. High-frequency jitter in marker position was eliminated by convolving the position traces with a narrow Gaussian-like Hann window (total width = 125 ms), which resulted in smoother and more accurate trajectories.
As described above, the tracking was automated. Nonetheless, the resulting position traces were verified manually for each trial to ensure the color marker was tracked properly. Occasionally, a subject would lift their scanning finger midtrial and reposition it elsewhere before reestablishing contact with the texture and continuing their exploratory movements. In these cases, the frames corresponding to the repositioning movement were recorded and the data from those frames were excluded from all analyses. This ensured that we only considered movements while the finger was in contact with the textured surface. On some trials (particularly on the hardness rating task), subjects only pressed their fingers onto the stimuli and made no exploratory movements in the x-y plane, yielding scanning speeds of zero.
Analysis.
Time-varying speed was obtained by dividing the change in tracked x–y coordinates by the difference in timestamps between adjacent frames. The speed signal tended to oscillate, peaking in the middle of each sweeping motion and dropping off to zero during reversals of scanning direction. Several metrics were considered to characterize scanning speed: the mean speed on each trial, the maximum speed on each trial, and mean of peak speed (across sweeps) on each trial. These metrics were found to behave similarly: the correlation between trial mean speeds and trial maximum speeds and trial mean peak speed were 0.92 and 0.95, respectively, so mean speed was used for further analysis. The mean speed in each trial was first calculated, and then these means were averaged across repetitions to yield one value for each combination of texture, subject, and task.
To understand how much time subjects spent using different exploratory strategies, we grouped each individual trial into one of three categories of increasing complexity. First, if the subject moved within an area of <1 cm2, the trial was categorized as stationary. Second, if the subject moved mostly in linear sweeps, the trial was categorized as linear. We assessed this condition by performing a principal component analysis on the raw trajectories. If at least 90% of the movement variance was captured by a single principal component (i.e., movement along a straight line), we counted this trial as linear. Third, all other cases denoted complex movement, such as circles or meandering trajectories over the workspace.
To assess the temporal evolution of scanning movements during individual trials, we divided the movement traces into 25 ms wide bins, starting at the onset of movement. To ensure that each time bin comprised data from the same number of trials, we restricted our analysis to the first 2.1 s of movement and only included trials that were at least that long; using this criterion, ∼85% of all trials were included.
Finally, movements were characterized by frequent stops, for example when subjects reversed direction during lateral movements. We identified movement epochs where the speed was near-zero and used these stops to split each trial into individual sweeps.
RESULTS
Types of exploratory movements.
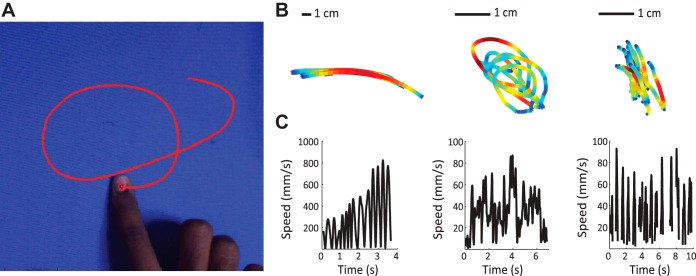
The main objective of the study was to characterize the kinematics of unconstrained texture exploration. To this end, we tracked exploratory finger movements with a high-speed camera, while subjects explored each of 14 textures and then rated each texture along one of three textural dimensions, namely roughness, slipperiness, and hardness (Fig. 1A). Subjects were free to move in any direction in the x-y plane, could lift and reposition their fingers, and could do so for however long they chose.
Fig. 1.
A: example tracked trajectory. B: 3 trajectories, measured on different trials, color-coded for speed. C: speed vs. time for the trajectories shown in B. Discontinuities in the traces denote periods when the finger was not in contact with the surface.
When afforded the freedom to explore, subjects exhibited highly stereotyped and repetitive exploratory movements (Fig. 1, B and C): The most commonly observed strategy was lateral scanning while pivoting around the elbow, wrist, or metacarpophalangeal joint, with the finger itself kept relatively straight and rigid. The more proximal the pivot point, the faster and more strictly lateral the motion was. We also observed circular motion involving wrist rotation and bending and extending of the finger. Subjects sometimes scanned their finger in the distal-to-proximal direction, often with a finger lift and reset rather than reversing the scanning direction from proximal to distal. Similarly, subjects sometimes scanned their finger laterally in only one direction, with a finger lift and reset. In some cases, particularly in the hardness task, exploration was characterized by pressing into and tapping on the surface with little lateral movement.
To quantify the degree to which different exploratory strategies were used, we assigned each trial to one of three categories based on the complexity of the movement trajectory (see materials and methods): stationary (barely any movement such as when pressing the finger down), linear (such as when rapidly sweeping laterally or proximal-distally), and complex (such as for circular movements). In rare cases, different exploratory behaviors were used within a single trial, either sequentially or simultaneously. Across all tasks, subjects spent most trials (70%) doing simple linear sweeps, followed by complex movements (22%), and stationary trials (8%). While the number of trials using each strategy differed somewhat between subjects, the breakdown of strategies was highly correlated between subjects (r = 0.87 on average).
The average time of exploration was consistent across tasks (6.37, 6.24, and 6.08 s per trial for hardness, roughness, and slipperiness, respectively) but individual trial lengths varied widely from <1 to 23.4 s for the hardness task, 1.1 to 38 s for the roughness task, and <1 to 23.9 s for the slipperiness task.
Subjects used a wide range of speeds, with mean trial speeds ranging from 6 to 407 mm/s and max speeds ranging from 15 to 1,416 mm/s (excluding trials with no x-y plane motion) (Fig. 2A). The aforementioned differences in exploratory strategies were reflected in differences in scanning speeds as well: overall mean scanning speed varied from 52 to 120 mm/s and max scanning speed varied from 133 to 307 mm/s between subjects (Fig. 2B). Across all tasks and textures, correlations of the mean trial speeds between pairs of subjects were on average 0.66 (with correlation coefficients for individual pairs ranging from 0.31 to 0.85), indicating that subjects' scanning speed varied across tasks and textures in broadly similar ways.
Fig. 2.
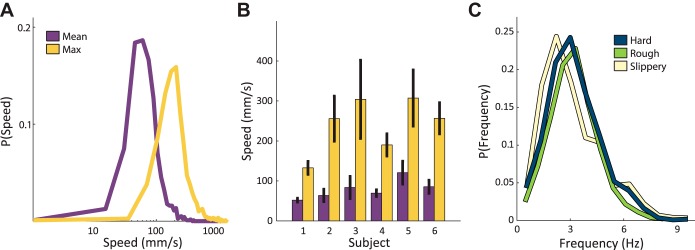
A: probability distribution of trial mean speeds (purple) and max speeds (orange) for all subjects, textures, and tasks; B: average trial mean speed (purple) and maximum speed (orange) for each subject over all tasks and textures. Error bars denote the standard error of the mean across all trials; C: probability distribution (over 12 equally sized bins) of scanning frequencies for the hardness (blue), roughness (green), and slipperiness (yellow), tasks calculated using all extracted sweeps during all trials over all subjects for each task.
As can be seen in the speed traces (Fig. 1C), kinematics tended to be oscillatory, especially during lateral sweeps. Using the time interval between speed reversals as an estimate of the cycle period (and its inverse as an estimate of frequency), we found that the frequencies of exploratory movements were consistent across tasks (Fig. 2C) and textures (data not shown). The mean scanning frequencies were 3.2, 3.2, and 3.1 Hz in the roughness, hardness, and slipperiness tasks, respectively. Thus, while speed was modulated by task (see below), frequency was not; indeed, longer distances were traversed on the faster tasks (Fig. 3A).
Fig. 3.
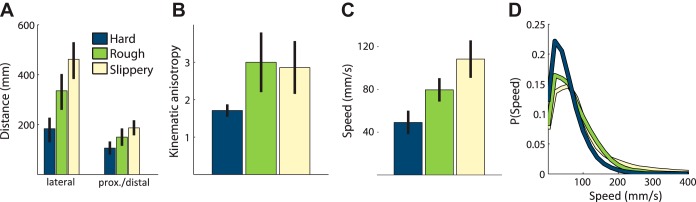
A: mean distance traveled in the lateral (left) and proximal-distal (right) directions for the hardness (blue), roughness (green), and slipperiness (yellow) tasks calculated for each trial and averaged over textures and subjects. B: kinematic anisotropy (ratio of distance traveled along the lateral axis to that along the distal-proximal axis) in the three tasks. C: mean speeds in the three tasks averaged across all subjects. D: distributions of instantaneous speeds for each task using a bin size of 20 mm/s. In A–C, error bars denote the standard error of the mean across subjects.
Task and texture dependence of exploratory kinematics.
Exploratory strategies differed across the three tasks: The roughness and slipperiness tasks yielded an especially high proportion of linear movements (77 and 85%, respectively), while the hardness task elicited a high proportion of stationary and complex movements (20 and 33% respectively). Lateral motion (as opposed to distal-proximal motion) tended to dominate for all three tasks, but the slipperiness and roughness tasks tended to involve more lateral motion, whereas the hardness task exhibited a more pronounced distal-proximal component (Fig. 3B).
Scanning speeds also differed across the three tasks (Fig. 3, C and D). Indeed, while the distributions of speed overlapped considerably across tasks, speed was significantly modulated by task [two-way ANOVA with task as main effect, F(2, 210) = 51.6, P < 0.01]. Speeds were lowest when subjects judged hardness and highest when they judged slipperiness, with the mean speed changing more than twofold (from 49 to 108 mm/s) between both conditions.
Subjects also tended to use different speeds when scanning different textures (see Fig. 4A for an illustration of the range of speeds used across textures for the slipperiness task), and this texture-related speed modulation was consistent across tasks and subjects [two-way ANOVA with texture as main effect, F(13, 210) = 2.89, P < 0.01]. Indeed, the scanning speeds used for different textures were correlated across tasks, with correlation values ranging from 0.75 to 0.84. In other words, a texture that was scanned rapidly in one task tended to be scanned rapidly in the other two as well. Consistent with this result, the interaction term of the two-way ANOVA was not significant [F(26,210) = 0.62, P = 0.93], and thus the effects of task and texture identity on the scanning speeds were independent. Mean scanning speeds ranged from 31 to 58, 43 to 95, and 51 to 150 mm/s for the hardness, roughness, and slipperiness tasks, respectively. Finally, another way in which the textures themselves influence subjects' movements was that harder surfaces tended to elicit more proximal-distal motion than did soft ones (Fig. 4B).
Fig. 4.
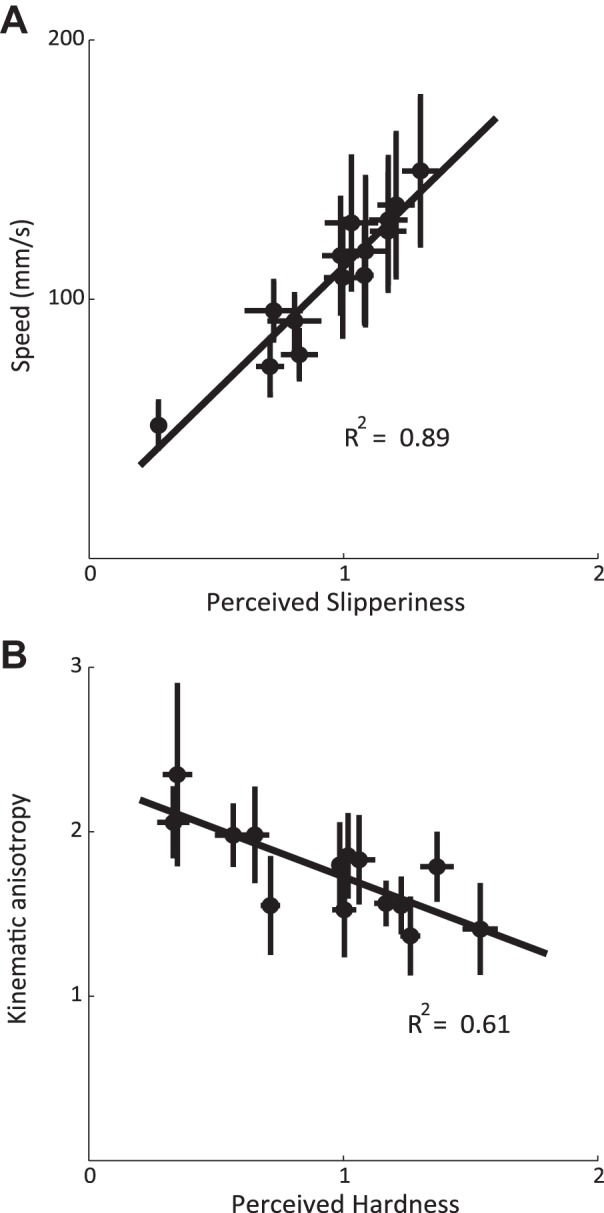
A: scanning speed measured in the slipperiness task vs. slipperiness ratings averaged over subjects. B: kinematic anisotropy vs. perceived hardness averaged over all subjects. Error bars denote the standard error of the mean across subjects.
In summary, we found that subjects' movements were determined both by the task and by the identity of the texture they were exploring. Depending on the task and the texture, mean exploration speeds could vary up to fivefold.
Relationship between scanning speed and perceptual ratings.
Having established that both task and texture identity affected the kinematics of texture exploration, we asked whether there is also a relationship between kinematic parameters and the perceptual ratings obtained in the three different tasks. We found that subjects tended to scan surfaces that were judged to be slippery faster than surfaces that were judged to be sticky, regardless of the task (see Fig. 4A for the relationship between slipperiness ratings and speed). The correlation between slipperiness rating and scanning speed was 0.85, 0.88, and 0.94 when subjects were rating roughness, hardness, and slipperiness, respectively. In other words, subjects tended to scan slippery textures faster even if they were not judging slipperiness, though the effect was more pronounced if they were.
Dynamic adaptation of scanning speed.
Next, we wished to determine whether subjects adapted their exploratory strategy as they obtained more information about the texture during exploration. To this end, we examined how scanning speed evolved over time (see materials and methods). We found that scanning speed increased significantly as the trial progressed [two-way repeated-measures ANOVA, F(80, 800) = 9.68, P < 0.01, Fig. 5A], with mean speed increasing from 28 mm/s at trial onset to 65 mm/s more than 2 s into the trial. This effect is not simply due to the speed starting out at zero at trial onset because mean speed continues to increase over multiple scans of the texture. The within-trial increase in speed was observed for all subjects individually and therefore appears to be a robust effect. The interaction between time and task was highly significant [two-way repeated-measures ANOVA interaction term of time and task: F(160, 800) = 1.83, P < 0.01]. Specifically, speed was much more similar across tasks at the beginning of the trial than it was at the end. Note, however, that even the initial speeds tended to differ, with slipperiness speeds highest, and hardness speeds lowest. Thus, task-related differences in kinematics are observed as early as 25 ms into a given trial. Splitting trials into individual “sweeps” by detecting when subjects made short stops (see materials and methods), for example to reverse direction, also showed that the speed of the very first sweep in each trial differed by task and that subsequent sweep speeds increased differentially depending on the task. These speed increases by sweep were associated with increases in the traversed distance (data not shown), but not in frequency, which remained constant within trials (see above).
Fig. 5.
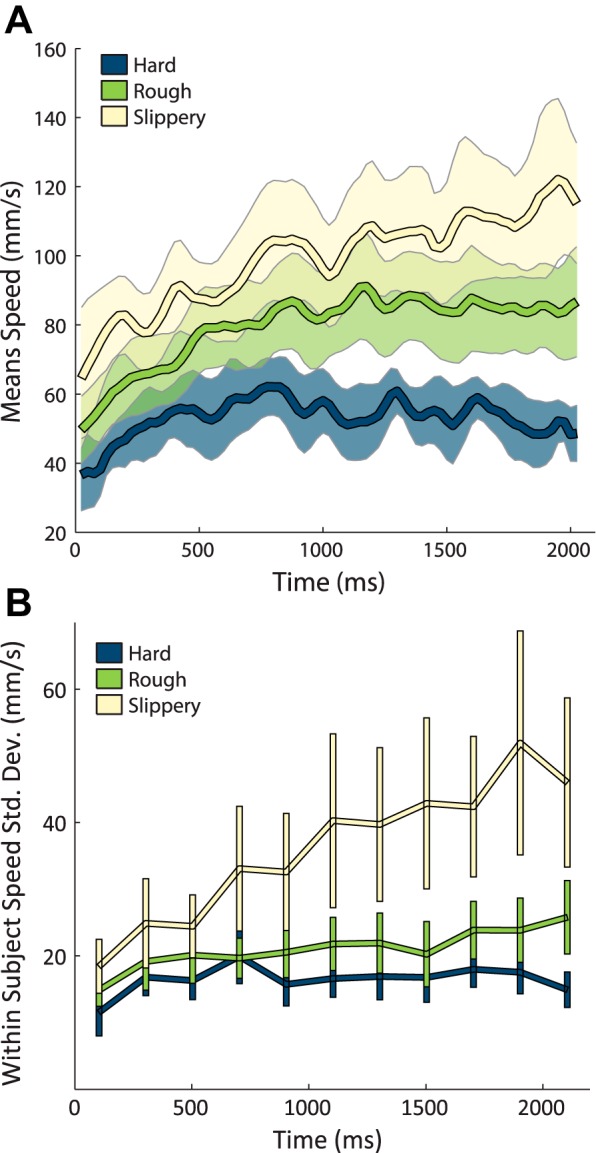
A: temporal evolution of scanning speed within trials for the hardness (blue), roughness (green), and slipperiness (yellow) tasks (bin size 25 ms) across all trials for all subjects. Only trials lasting at least 2.1 s are included. Error bars denote the standard error of the mean across all subjects. B: standard deviation of scanning speed across textures averaged across subjects for the 3 tasks as a function of time (bin size 200 ms) for the same trials included in A. Scanning speeds become increasingly texture dependent as the trial progresses. Error bars denote the standard error across subjects.
We also examined how scanning speed changed within a single trial for each texture individually. Because subjects did not know in advance which texture would be presented on any given trial, any texture-specific differences in scanning speed should arise gradually over the course of a single trial. We found that speeds became increasingly divergent as the trial progressed, especially on the slipperiness and roughness tasks (Fig. 5B).
Finally, we examined whether exploratory strategy changed across repeated presentations of the same texture as subjects performed the same task. We found that the speed of exploratory movements did not change as subjects became more familiar with the textures and the task (data not shown). Specifically, surfaces judged to be slippery were scanned faster than surfaces judged to be sticky even in the first experimental block.
In summary, both task and stimulus identity affect the kinematics of texture exploration. While differences in scanning speed across textures and tasks are present right from the start, the differences become more pronounced during the task.
DISCUSSION
Tactile experience is typically the consequence of active movements and is shaped in large part by those movements. The movements themselves are in turn shaped by the type of sensory information that is sought or relevant to the ongoing motor behavior. Here, our goal was to quantitatively characterize the movements used to explore textured surfaces across a range of textural properties with stimulus swaths that were large enough to minimize geometrical constraints on the exploratory behaviors. Moreover, we wished to determine whether task instructions - the specific type of sensory information that was sought - and the textures themselves affected exploratory kinematics. As has previously been shown, we observed that subjects tended to use repetitive, stereotyped movements such as lateral scanning, distal-proximal scanning, or tapping and pressing onto the textures. Movements were mostly oscillatory and thus involved large fluctuations in speed. Importantly, we found that exploratory movements depended both on the task and the explored surface, and these factors interacted in shaping exploratory kinematics.
Parameters of texture exploration.
Previous studies on the kinematic parameters used in texture exploration restricted subjects' movements by explicitly instructing them to only perform lateral sweeps or by using very small patches of textures that could only be explored using such movements (Gamzu and Ahissar 2001; Libouton et al. 2010; Morley et al. 1983; Smith et al. 2002a; Tanaka et al. 2014). While these restrictions made it easier to measure subjects' exploratory behaviors (to track scanning speed, e.g.), these constrained movements might differ from unconstrained ones. Using large patches of textures that subjects could explore freely, we found that lateral sweeps were indeed quite commonly used, but subjects also employed proximal-distal sweeps, followed circular trajectories, or tapped on the textures, indicating that more complex behaviors were often preferred to simple lateral sweeps.
Scanning speed has been measured in several previous studies on texture exploration. A pair of studies reported extreme values for scanning speeds, ranging from 50 mm/s (Libouton et al. 2010) to 160 mm/s (Morley et al. 1983). Scanning speed has also been found to vary widely across subjects, ranging from 10 to 160 mm/s (Smith et al. 2002a). In general, however, scanning speeds commonly falls in the range from 80 to 100 mm/s (Gamzu and Ahissar 2001; Smith et al. 2002a,b; Tanaka et al. 2014), matching reasonably well the average speeds averaged across tasks, textures, and subjects in the present study. Determining the range of scanning speeds during natural texture exploration has implications for studies on the neural basis of texture, which often impose very slow scanning speeds that would only rarely be used under natural conditions (e.g., Oddo et al. 2011).
Kinematic parameters other than scanning speed and contact force remain largely unexplored. Morley et al. (1983) examined the frequency with which lateral sweeps occurred and reported a value ∼4 Hz. This is slightly higher than our estimate (3.2 Hz), which was relatively robust across textures and tasks, a difference that might reflect the restricted workspace of the former study.
Task dependence of exploratory movements.
We corroborate previous findings that surface texture and hardness exploration involve different exploratory movements, namely lateral movement and pressure application, respectively (Lederman and Klatzky 1987, 1990). In the present study, these differences were reflected in trial-averaged kinematic parameters, such as tendency for lateral vs. proximal-distal motion (Fig. 3B), mean distance traveled (Fig. 3A), and mean speed (Fig. 3, C and D). While we did not measure force per se, it was evident from the recorded videos that subjects also used higher normal forces in the hardness task than in the roughness or slipperiness ones, especially in trials when the finger appeared stationary. The task-dependent differences in kinematics appeared not to be entirely driven by sensory feedback. For example, scanning speed differed significantly across tasks even when measured in the first 25 ms of movement, indicating that the specific task shaped in part the exploratory plan before the arrival of any sensory feedback.
In addition to replicating the result that texture and hardness elicit different exploratory movements, we show that even two closely related properties of surface texture, namely slipperiness and roughness, elicit different exploratory movements. Indeed, movements tended to differ in distance traveled and mean speed depending on whether subjects performed the roughness or the slipperiness tasks (Fig. 3). The difference in the exploratory movements associated with roughness and slipperiness is to be expected when one considers that these two dimensions are perceptually distinct (Hollins et al. 2000) and probably involve different neural mechanisms. The perception of roughness relies on two mechanisms: one spatial, the other temporal (Weber et al. 2013). The temporal mechanism is highly dependent on scanning speed and involves the transduction of texture-elicited vibrations by rapidly adapting mechanoreceptors and the processing of precisely timed spiking sequences upstream. In contrast, slipperiness perception is probably mediated by slowly adapting afferents, which encode both the amount of skin-stretch produced during scanning, (Westling and Johansson 1987) and the normal force exerted on the surface; from these signals, the kinetic friction of the surface can be estimated (see below). The task dependence of exploratory strategy has also been previously shown in a study demonstrating that identifying sandpapers elicited different exploratory movements than did discriminating them (Tanaka et al. 2014).
Texture dependence of exploratory movements.
Interestingly, not only were exploratory movements dependent on the task, they were also dependent on the explored surface. Specifically, while the speed at which all surfaces were scanned was approximately equivalent at the beginning of a trial for a given task, speeds progressively diverged as the trial progressed (Fig. 5B). This texture dependence was modulated by task, in that the progressive divergence in scanning speed was very strong for slipperiness trials, weak for hardness trials, and intermediate for roughness trials.
Previous studies have either reported no effect of texture on exploratory parameters (Libouton et al. 2010; Morley et al. 1983) or found differences only under certain circumstances, such as during free exploration in the absence of a task (Tanaka et al. 2014) or when tactile cues were obscured by a glove (Gamzu and Ahissar 2001). However, these studies generally used similar textures of a common class (such as only sandpapers or gratings), which might not have imparted enough variety to bring to light any texture-dependent effects.
We found that speed scaled most closely with the perceived slipperiness of the textures regardless of the task. Perceived slipperiness is closely associated with the frictional properties of the texture (Smith and Scott 1996; Yoshioka et al. 2007). A parsimonious explanation of the texture effect, then, is that it reflects the frictional properties of the surfaces. Given that normal force is generally relatively consistent across textures within subjects (Bensmaia and Hollins 2003; Smith et al. 2002a,b), the progressive increases in speed are likely due to progressive increases in lateral force exerted as each trial proceeds. This increase in lateral force will produce a larger increase in speed for low-friction (and thus perceptually slippery surfaces) than for high-friction (sticky) ones. It is important to note, however, that this relationship between slipperiness and scanning speed is stronger for slippery trials than for roughness or hardness ones. This suggests that the modulation of lateral force is more stereotyped and repeatable across textures in the slipperiness tasks than it is in the two other tasks, an interpretation that is broadly compatible with previous ones (Smith and Scott 1996). According to this interpretation, however, the texture dependence of the exploratory movements does not reflect online changes in motor behavior that depend on sensory input but rather reflects an exploratory plan that is dependent on the task but relatively independent of the texture. In this view, texture-dependent finger kinematics arise purely from the biophysical interaction with surfaces themselves rather than any changes in motor behavior.
Implications for texture perception and its neural basis.
The speed at which a texture is scanned across the skin exerts a major influence on the vibrations produced in the skin: As the speed increases (or decreases), the frequency composition of these vibrations translates to the right (or left) along the frequency axis (Delhaye et al. 2012; Manfredi et al. 2014). In other words, texture-elicited vibrations dilate or contract systematically with decreases or increases in scanning speed, respectively. This systematic effect of speed on vibrations is then reflected as a systematic effect of speed on afferent responses: the texture-specific patterns of spiking in the nerve also dilate or contract systematically with decreases or increases in scanning speed (Weber et al. 2013). Exploratory movements that involve large, continuous changes in scanning speed, such as those that are naturally used, will therefore result in a continuously changing peripheral texture representation that reflects not just the texture but the instantaneous speed as well. Given this rapidly changing neural representation, one might expect that the perception of texture might also depend on speed. Remarkably, it does not. All previous experiments investigating the effects of speed on texture perception have shown that tactile texture perception is almost completely invariant with respect to changes in scanning speed (Bensmaia and Hollins 2003; Lederman 1983; Meftah el et al. 2000). In other words, even when textures are passively scanned across the finger, the way they feel is independent of how fast they are scanned across the skin. Thus, the nervous system is able to extract invariant information about texture based on a neural signal stemming from the hand that is highly speed dependent. There is some evidence that an estimate of scanning speed is perceptually available even during passive scanning of textures (Dépeault et al. 2008), and this estimate could be used to compensate for the speed-dependent scaling of the neural responses. Another possibility is that textural information is extracted in a similar way as timbre is in the auditory system (Yau et al. 2009), which might not require an explicit correction for scanning speed. Regardless, sampling a variety of scanning speeds when exploring a texture might be beneficial for acquiring and maintaining these invariant representations.
GRANTS
This work was supported by National Science Foundation Grant IOS-1150209.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.C., H.P.S., E.C.D.-B., and S.J.B. conception and design of research; T.C. performed experiments; T.C. and H.P.S. analyzed data; T.C., H.P.S., E.C.D.-B., and S.J.B. interpreted results of experiments; T.C. and H.P.S. prepared figures; T.C., H.P.S., and S.J.B. drafted manuscript; T.C., H.P.S., E.C.D.-B., and S.J.B. edited and revised manuscript; T.C., H.P.S., E.C.D.-B., and S.J.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Stephanie Palmer for lending us her high-speed camera and Zoe Boundy-Singer for help with data collection and analysis.
REFERENCES
- Bensmaia S, Hollins M. Pacinian representations of fine surface texture. Percept Psychophys 67: 842–854, 2005. [DOI] [PubMed] [Google Scholar]
- Bensmaia SJ, Hollins M. The vibrations of texture. Somatosens Motor Res 20: 33–43, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio CJ, Sathian K. Temporal cues contribute to tactile perception of roughness. J Neurosci 21: 5289–5296, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor CE, Hsiao SS, Phillips JR, Johnson KO. Tactile roughness: neural codes that account for psychophysical magnitude estimates. J Neurosci 10: 3823–3836, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor CE, Johnson KO. Neural coding of tactile texture: comparison of spatial and temporal mechanisms for roughness perception. J Neurosci 12: 3414–3426, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaye B, Hayward V, Lefevre P, Thonnard JL. Texture-induced vibrations in the forearm during tactile exploration. Front Behav Neurosci 6: 37, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dépeault A, Meftah el-M, Chapman CE. Tactile speed scaling: contributions of time and space. J Neurophysiol 99: 1422–1434, 2008. [DOI] [PubMed] [Google Scholar]
- Gamzu E, Ahissar E. Importance of temporal cues for tactile spatial- frequency discrimination. J Neurosci 21: 7416–7427, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollins M, Bensmaia S, Karlof K, Young F. Individual differences in perceptual space for tactile textures: evidence from multidimensional scaling. Percept Psychophys 62: 1534–1544, 2000. [DOI] [PubMed] [Google Scholar]
- Hollins M, Risner SR. Evidence for the duplex theory of tactile texture perception. Percept Psychophys 62: 695–705, 2000. [DOI] [PubMed] [Google Scholar]
- Johnson KO, Lamb GD. Neural mechanisms of spatial tactile discrimination: neural patterns evoked by braille-like dot patterns in the monkey. J Physiol 310: 117–144, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederman SJ. Tactual roughness perception - spatial and temporal determinants. Can J Psychol 37: 498–511, 1983. [Google Scholar]
- Lederman SJ, Klatzky RL. Extracting object properties through haptic exploration. Acta Psychol (Amst) 84: 29–40, 1993. [DOI] [PubMed] [Google Scholar]
- Lederman SJ, Klatzky RL. Hand movements: a window into haptic object recognition. Cogn Psychol 19: 342–368, 1987. [DOI] [PubMed] [Google Scholar]
- Lederman SJ, Klatzky RL. Haptic classification of common objects: knowledge-driven exploration. Cogn Psychol 22: 421–459, 1990. [DOI] [PubMed] [Google Scholar]
- Libouton X, Barbier O, Plaghki L, Thonnard JL. Tactile roughness discrimination threshold is unrelated to tactile spatial acuity. Behav Brain Res 208: 473–478, 2010. [DOI] [PubMed] [Google Scholar]
- Manfredi LR, Saal HP, Brown KJ, Zielinski MC, Dammann JF 3rd, Polashock VS, Bensmaia SJ. Natural scenes in tactile texture. J Neurophysiol 111: 1792–1802, 2014. [DOI] [PubMed] [Google Scholar]
- Meftah el-M, Belingard L, Chapman CE. Relative effects of the spatial and temporal characteristics of scanned surfaces on human perception of tactile roughness using passive touch. Exp Brain Res 132: 351–361, 2000. [DOI] [PubMed] [Google Scholar]
- Morley JW, Goodwin AW, Darian-Smith I. Tactile discrimination of gratings. Exp Brain Res 49: 291–299, 1983. [DOI] [PubMed] [Google Scholar]
- Oddo CM, Beccai L, Wessberg J, Wasling HB, Mattioli F, Carrozza MC. Roughness encoding in human and biomimetic artificial touch: spatiotemporal frequency modulation and structural anisotropy of fingerprints. Sensors (Basel) 11: 5596–5615, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skedung L, Arvidsson M, Chung JY, Stafford CM, Berglund B, Rutland MW. Feeling small: exploring the tactile perception limits. Scientific reports 3: 2617, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM, Chapman CE, Deslandes M, Langlais JS, Thibodeau MP. Role of friction and tangential force variation in the subjective scaling of tactile roughness. Exp Brain Res 144: 211–223, 2002a. [DOI] [PubMed] [Google Scholar]
- Smith AM, Gosselin G, Houde B. Deployment of fingertip forces in tactile exploration. Exp Brain Res 147: 209–218, 2002b. [DOI] [PubMed] [Google Scholar]
- Smith AM, Scott SH. Subjective scaling of smooth surface friction. J Neurophysiol 75: 1957–1962, 1996. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Bergmann Tiest WM, Kappers AM, Sano A. Contact force and scanning velocity during active roughness perception. PLoS One 9: e93363, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber AI, Saal HP, Lieber JD, Cheng JW, Manfredi LR, Dammann JF 3rd, Bensmaia SJ. Spatial and temporal codes mediate the tactile perception of natural textures. Proc Natl Acad Sci USA 110: 17107–17112, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westling G, Johansson RS. Responses in glabrous skin mechanoreceptors during precision grip in humans. Exp Brain Res 66: 128–140, 1987. [DOI] [PubMed] [Google Scholar]
- Yau JM, Hollins M, Bensmaia SJ. Textural timbre: the perception of surface microtexture depends in part on multimodal spectral cues. Commun Integr Biol 2: 344–346, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka T, Bensmaia SJ, Craig JC, Hsiao SS. Texture perception through direct and indirect touch: an analysis of perceptual space for tactile textures in two modes of exploration. Somatosens Motor Res 24: 53–70, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]