Abstract
Serotonin (5-hydroxytryptamine, 5-HT) neurons from the mouse and rat rostral medulla are stimulated by increased CO2 when studied in culture or brain slices. However, the response of 5-HT neurons has been variable when animals are exposed to hypercapnia in vivo. Here we examined whether halogenated inhalational anesthetics, which activate TWIK-related acid-sensitive K+ (TASK) channels, could mask an effect of CO2 on 5-HT neurons. During in vivo plethysmography in mice, isoflurane (1%) markedly reduced the hypercapnic ventilatory response (HCVR) by 78–96% depending upon mouse strain and ambient temperature. In a perfused rat brain stem preparation, isoflurane (1%) reduced or silenced spontaneous firing of medullary 5-HT neurons in situ and abolished their responses to elevated perfusate Pco2. In dissociated cell cultures, isoflurane (1%) hyperpolarized 5-HT neurons by 6.52 ± 3.94 mV and inhibited spontaneous firing. A subsequent decrease in pH from 7.4 to 7.2 depolarized neurons by 4.07 ± 2.10 mV, but that was insufficient to reach threshold for firing. Depolarizing current restored baseline firing and the firing frequency response to acidosis, indicating that isoflurane did not block the underlying mechanisms mediating chemosensitivity. These results demonstrate that isoflurane masks 5-HT neuron chemosensitivity in vitro and in situ and markedly decreases the HCVR in vivo. The use of this class of anesthetic has a particularly potent inhibitory effect on chemosensitivity of 5-HT neurons.
Keywords: chemoreceptor, breathing, anesthesia, TASK, raphe
breathing is controlled by neurons within the brain stem that generate rhythmic, patterned output to respiratory muscles. One of the most important sources of feedback comes from central respiratory chemoreceptors (CRCs) that monitor arterial Pco2, probably indirectly via tissue pH (Feldman et al. 2003; Richerson 2004; Wang et al. 2002). There are certain properties that cells must possess to be CRCs (Richerson et al. 2005). These include intrinsic chemosensitivity to physiologically relevant changes in CO2 and appropriate effects on other cells so that any changes induced by hypercapnia ultimately lead to stimulation of respiratory output. There are many different pH/CO2-sensitive neurons, as well as some glia, that have been identified as candidates for CRCs (Feldman et al. 2003; Fukuda et al. 1978; Gourine et al. 2010; Mulkey et al. 2004; Nichols et al. 2008; Pineda and Aghajanian 1997; Richerson 2004; Richerson et al. 1995, 2005; Trapp et al. 2008). It is possible that all or most of these various cells contribute to the whole-animal response to hypercapnia in vivo. If they actually are all chemoreceptors, it remains unknown whether they play an equal, widely distributed role under all conditions or if they each make unique contributions (e.g., depending on pathological states, developmental age, level of CO2, etc.) (Nattie and Li 2006).
Some serotonin (5-hydroxytryptamine, 5-HT) neurons in the medulla are putative CRCs. 5-HT neurons are located in regions of high blood flow, similar to peripheral chemoreceptors. A subset increase their firing rate in vitro in response to hypercapnia due to intrinsic chemosensitivity, project to respiratory nuclei, and stimulate output of the respiratory control network (Bernard et al. 1996; Bradley et al. 2002; Corcoran et al. 2009; Depuy et al. 2011; Feldman et al. 2003; Hodges et al. 2004; Ptak et al. 2009; Richerson 1995, 2004; Richerson et al. 2005; Taylor et al. 2005; Wang et al. 1998, 2001). 5-HT neurons in the medulla respond to hypercapnia in vivo with an increase in c-fos staining (Corcoran et al. 2009; Larnicol et al. 1994; Richerson 2004; Sato et al. 1992). Recently, 5-HT neurons in the medulla of a decerebrate in situ perfused brain stem preparation have also been shown to increase their firing rate in response to acidosis of the perfusate (Iceman and Harris 2014). However, single-unit electrophysiological recordings from an in vivo preparation are considered by some to be the gold standard for defining normal neuronal activity, and there have been contradictory experimental findings reported with this approach. One laboratory has reported that 5-HT neurons in the raphe obscurus (Veasey et al. 1995) and dorsal raphe (Veasey et al. 1997) of unanesthetized, behaving cats increase their firing frequency in response to as little as 3% inhaled CO2. In contrast, a different laboratory has reported that 5-HT neurons in the raphe obscurus of anesthetized mice and ventrolateral medulla (VLM) of anesthetized rats in vivo do not increase their firing frequency in response to inhalation of 10% CO2 (Depuy et al. 2011; Mulkey et al. 2004). It is important to understand why these different results have been obtained. A lack of consistent chemosensitivity in vivo has led some to conclude that 5-HT neurons are not CRCs (Depuy et al. 2011; Guyenet et al. 2005; Richerson et al. 2005). These contradictory data constitute the major remaining argument against the hypothesis that 5-HT neurons in the medulla are CRCs (Teran et al. 2014). One potential confounding factor is that in the two studies that failed to show 5-HT neuron chemosensitivity animals were anesthetized with halothane or isoflurane (Depuy et al. 2011; Mulkey et al. 2004).
The halogenated anesthetics family are commonly used in humans and in laboratory animals (Eger 1981). The mechanisms of halogenated anesthetic action are not precisely understood. Importantly, however, halogenated anesthetics activate TWIK-related acid-sensitive K+ (TASK) channels (Patel et al. 1999; Sirois et al. 2000). These channels are expressed widely in the central nervous system and, when activated, hyperpolarize cells that express them (Duprat et al. 1997; Talley et al. 2001). Halothane and isoflurane have been commonly used as research anesthetics, although halothane is currently less popular because of hepatotoxicity. Isoflurane is considered to be advantageous over many other inhalational anesthetics, because it has low blood solubility and does not induce cardiovascular depression (Eger 1981).
While it is widely acknowledged that halogenated anesthetics can depress breathing, there is not uniform agreement on their impact on ventilatory responses to hypercapnia. The literature is inconsistent, and differences have been reported with different anesthetic agents, concentrations, and species investigated (Groeben et al. 2003; Hirshman et al. 1977; Knill et al. 1983; Martin-Body and Sinclair 1985; Pandit 2014). Recognizing the potential for halogenated anesthetics to alter breathing, ventilatory responsiveness, and mechanisms of chemosensitivity is critical when interpreting the results of studies conducted under anesthesia.
Here we used experimental preparations at increasing levels of complexity ranging from cultured neurons to whole animals to test the hypothesis that anesthetics might prevent detection of an effect of hypercapnia on 5-HT neurons. As previously reported, 5-HT neurons were chemosensitive to acidosis in culture (Wang et al. 1998, 2001, 2002; Wang and Richerson 1999), and this property was retained in a perfused brain preparation (Iceman et al. 2013). In culture and in the perfused brain stem, 1% isoflurane abolished the firing of 5-HT neurons under control conditions and prevented the increase in firing frequency normally induced by hypercapnic acidosis. These results demonstrate that the use of halogenated anesthetics masks 5-HT neuron chemosensitivity and suggest why 5-HT neurons were unresponsive in vivo to inhalation of CO2 in previous studies (Depuy et al. 2011; Mulkey et al. 2004). Isoflurane (1%) also caused a severe reduction in the hypercapnic ventilatory response (HCVR) in vivo. Inhibition of the HCVR in vivo could be due to inhibition of any combination of the putative CRCs (Corcoran et al. 2009; Mulkey et al. 2004; Nichols et al. 2008; Pineda and Aghajanian 1997; Richerson 2004), including peripheral chemoreceptors (Lahiri and DeLaney 1975; O'Regan and Majcherczyk 1982). However, abolition of the CO2 response of 5-HT neurons would be predicted to contribute to blunting of the HCVR by isoflurane.
MATERIALS AND METHODS
Ethical approval.
All procedures and experiments involving mice were carried out under the approval of The University of Iowa Institutional Animal Care and Use Committee. All procedures and experiments involving rats were carried out under the approval of The University of Alaska Fairbanks Institutional Animal Care and Use Committee. All animal procedures were carried out in accordance with the recommendations of the American Veterinary Medical Association Guidelines for the Euthanasia of Animals (2013 edition). A minimal number of animals was used, and care was taken to reduce the possibility of any discomfort.
Plethysmography.
Minute ventilation (V̇e) was measured with standard open-flow (700 ml/min), whole-body plethysmography as previously used in our laboratory (Hodges et al. 2008). The chamber was a commercially available model (Buxco, Wilmington, NC), but the remainder of the plethysmography equipment was custom-designed and built. The protocol consisted of >20 min of baseline recording in 0% CO2, 50% O2, balance N2 followed by ∼7-min exposures to 3%, 5%, or 7% CO2, 50% O2, balance N2. The same sequence of increased CO2 from 0% to 7% was then performed with 1% isoflurane. Lmx1bf/f mice (n = 16) and ePet-EYFP mice (n = 10), both of which have a wild-type phenotype, were used for the whole-animal experiments (Hodges et al. 2008; Scott et al. 2005; Zhao et al. 2006). Mice were exposed to 1% isoflurane mixed with 50% O2 for at least 15 min prior to CO2 exposure. Anesthesia was fully induced with 1% isoflurane, which was the only concentration used. The plethysmograph chamber was maintained at 30°C with a heat lamp and a feedback controller (TCAT-2AC; Physitemp Instruments, Clifton, NJ). All data were acquired with custom-written MATLAB software. Body temperature measurements were recorded by telemetry probes (IPTT-300; BMDS, Seaford, DE) inserted into the abdominal cavity at least 5 days before recordings. We did not collect O2 consumption data because engineers from AEI Technologies (Pittsburgh, PA) advised us that isoflurane would damage the O2 analyzer. Isoflurane levels were maintained at 1% with a precision vaporizer (Summit Anesthesia Solutions, Bend, OR).
Since body temperature regulation may be compromised by isoflurane, and 30°C is below the thermoneutral range of mice, an additional set of plethysmography studies were performed with body temperature maintained constant at the normal level. In these experiments, the protocol was altered to expose wild-type mice (n = 4) to two different gas mixtures (0% CO2, 50% O2, balance N2; 7% CO2, 50% O2, balance N2) with and without isoflurane, while body temperature was maintained at 36°C with a heat lamp to control for confounding influences of an isoflurane-induced reduction in body temperature.
Cell culture.
ePet-EYFP mice were used to prepare cultures to allow identification of 5-HT neurons prior to patch-clamp recordings (Scott et al. 2005). In these mice, the enhancer region of the Pet-1 ETS gene drives expression of enhanced yellow fluorescent protein (YFP). Neonatal ePet-EYFP pups (n = 20) were killed on postnatal days 0–2 (P0–P2), and a wedge of tissue from the ventromedial portion of the rostral half of the medulla (including the raphe pallidus, r. magnus, and r. obscurus) was removed. The tissue was digested, triturated, and plated on poly-l-ornithine- and laminin-coated coverslips. Cultures were fed and maintained as previously described (Wang et al. 1998). Recordings were performed on YFP-positive cells after P21 (19–21 days after culturing) to allow maturation of chemosensitivity (Wang and Richerson 1999).
Patch-clamp recordings.
The gramicidin perforated-patch technique was used for recordings. Electrodes (6–14 MΩ; borosilicate glass) were pulled on a micropipette puller (model P-97; Sutter Instrument, Novato, CA) and filled with intracellular solution containing (in mM) 135 KOH, 135 methanesulfonic acid, 10 KCl, 5 HEPES, and 1 EGTA (pH 7.2; osmolarity 275 ± 5 mosM). Coverslips were transferred to a recording chamber on the stage of an Axiovert 200 inverted microscope (Carl Zeiss USA, Thornwood, NY). Recordings were performed with a Multiclamp 700B microelectrode amplifier (Molecular Devices, Sunnyvale, CA), and data were collected with pCLAMP software and a Digidata 1440A acquisition system (Molecular Devices).
Bath solutions.
aCSF (pH 7.4) contained (in mM) 124 NaCl, 3 KCl, 2 MgCl2, 2 CaCl2, 1.3 NaH2PO4, 26 NaHCO3, and 10 dextrose. Acidic aCSF (pH 7.15) contained (in mM) 136 NaCl, 3 KCl, 2 MgCl2, 2 CaCl2, 1.3 NaH2PO4, 13 NaHCO3, and 10 dextrose. Both solutions had an osmolarity of 305 ± 5 mosM and were maintained isocapnic by equilibration with 5% CO2-95% O2. Fast glutamatergic, glycinergic, and GABAergic ionotropic synaptic transmission was blocked by 100 μM picrotoxin (PTX) (Sigma-Aldrich, St. Louis, MO), 50 μM (±)-2-amino-5-phosphonopentanoic acid (AP-5) (Tocris, Ellisville, MO), and 10 μM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) (Tocris). Solutions were equilibrated with isoflurane (1%) with a precision vaporizer (Summit Anesthesia Solutions).
In situ brain stem recordings.
Juvenile (n = 22; 60–120 g) male Sprague-Dawley rats (Simonsen Laboratories, Gilroy, CA) were used for a perfused brain stem preparation, whose advantages have been described previously (Harris and St-John 2003; Richerson and Getting 1990; St-John and Paton 2000; Toppin et al. 2007). Rats were heparinized (0.3 ml of 1,000 IU/ml ip) and briefly anesthetized with isoflurane until spontaneous respirations ceased. The portion of the body caudal to the diaphragm was removed, and the temperature of the rostral portion was reduced by placing it in chilled perfusate containing (in mM) 125 NaCl, 3.75 KCl, 2.5 CaCl2, 1.25 MgSO4, 1.25 KH2PO4, 25 NaHCO3, 10 glucose, and 0.18 Ficoll-70. Preparations were decerebrated at a precollicular level, and the forebrain was removed by aspiration. A catheter with a double lumen was inserted retrogradely into the descending aorta, and perfusate was pumped into the aorta. Perfusion pressure was increased gradually to 50–75 mmHg and then held constant. Gallamine triethiodide (60 mg/l) was added to the perfusate. Perfusate passed through a heat exchanger, filter (25 μm), and “bubble trap.” The perfusate was maintained at 30–31°C. Venous outflow of perfusate was collected and recirculated.
In situ protocol.
The levels of O2 and CO2 in the perfusate were maintained by equilibrating a perfusate reservoir with gas mixtures produced with a precision GSM-2 gas mixer (CWE, Ardmore, PA) and verified with a CD-3A CO2 analyzer (AEI Technologies). Control conditions approximated normocapnic plasma in vivo: perfusate equilibrated with 95% O2-5% CO2 entered the aorta with Pco2 and pH of 33 mmHg and 7.4, respectively. Neuronal recordings were always initiated under control gas conditions and followed by a hypercapnic challenge (91% O2-9% CO2; Pco2 60 mmHg, pH 7.2) for 5 min. Preparations were then perfused with isoflurane (1%) added to the perfusate with an anesthetic vaporizer (Draeger Medical, Telford, PA).
Extracellular recordings were made with pulled glass electrodes fabricated to produce a tip resistance of 15–20 MΩ and filled with biotinamide hydrobromide (Life Technologies, Grand Island, NY) dissolved at 5% in 0.5 M sodium acetate. With a dorsal approach, electrodes were placed in the rostral medullary raphe (r. magnus and r. pallidus) under stereotactic guidance using surface landmarks. Electrodes were placed with a stepping motor (Burleigh Inchworm) held in a stereotaxic five-axis micropositioner integrated with a Benchmark Angle Two digital brain atlas (MyNeuroLab, St. Louis, MO). Recordings were made with a Multiclamp 700B intracellular amplifier (Molecular Devices) with high-pass filter at 300 Hz and low-pass filter at 1 kHz Bessel via an Axon CV7B high-impedance headstage (Molecular Devices). Signals were digitized with Spike 2 (CED, Cambridge, UK), sampled (>10 kHz), and stored as computer data files.
Spike sorting and analysis.
Stable 1- to 3-min periods of single-unit firing frequency were analyzed with Spike 2 software (CED). Mean single-unit firing frequencies were analyzed before (baseline), during the last minute of the 5-min hypercapnic challenge, and after a 5-min return to control conditions. Control recordings and hypercapnic challenges were repeated after at least a 10-min exposure to 1% isoflurane. If a neuron responded to hypercapnic perfusate with an increase in firing frequency >20% relative to baseline the neuron was considered chemosensitive.
Identification of 5-HT neurons.
Putative serotonergic neurons were identified with electrophysiological criteria similar to those used by Veasey et al. (1995, 1997). Mason (1997) and Mulkey et al. (2004) used intracellular or juxtacellular labeling of recorded neurons and demonstrated that electrophysiological criteria identify 5-HT neurons with ∼90% accuracy.
5-HT raphe neurons typically display a stereotypical slow tonic firing pattern (0.5–2.5 Hz; Mason 1997). Stable 1- to 3-min periods of single-unit firing were analyzed to provide a mean value for unit firing frequency, mean interspike interval, and SD and SE of mean interspike interval. Cell firing frequency and regularity were used to classify neurons as putative 5-HT neurons (Mason 1997). Briefly, the mean interspike interval (x̄, in ms) and the SD of the interspike interval (s, in ms) were derived as independent variables and used to solve the discriminant function
A value resulting from this function <0 indicates that the cell is likely to be serotonergic (putative 5-HT cell), while a value >0 indicates the cell is not likely to be serotonergic. We and others have characterized recordings as putative 5-HT neurons by firing pattern analysis and confirmed that it is accurate at least 90% of the time compared with juxtacellular labeling and immunohistochemical neurotransmitter phenotyping (Iceman et al. 2013; Mulkey et al. 2004).
Recorded neurons were filled with biotinamide by applying positive-current pulses (400-ms duration, 50% duty cycle) of gradually increasing intensity (0–10 nA max in 0.2-nA steps) until entrainment of cell discharge to the current pulse was achieved (Iceman et al. 2013). Cell entrainment was maintained for at least 30 s. Current pulses trigger the iontophoretic ejection of biotinamide, and entrainment facilitates uptake of this marker by the entrained cell. Entrainment was never initiated when multiple units were visible, and double neuron or ectopic labeling was not observed. Spike height, width, and shape were monitored before, during, and after juxtacellular entrainment to ensure that only one cell was recorded and labeled.
Immunohistochemistry.
Preparations were perfused with 4% paraformaldehyde in PBS. Brain stems were removed and submerged in fixative overnight prior to sectioning (Vibratome; 60-μm coronal sections). Biotinamide was revealed with a streptavidin-Alexa Fluor 546 conjugate (2 μg/ml; Life Technologies). Sections were then blocked with 1% bovine serum albumin, 3% normal goat serum, and 0.3% Triton X-100 and then incubated at 4°C overnight in a mouse anti-tryptophan hydroxylase (TpOH) monoclonal antibody diluted 1:1,000 in blocking buffer (catalog no. T0678; Sigma-Aldrich), followed by a goat anti-mouse secondary antibody conjugated to Alexa Fluor 488 (1:500; Life Technologies). Local biotinamide label and TpOH immunoreactivity were visualized with a Zeiss LSM510 confocal microscope.
Statistical analysis.
All statistical differences were calculated with a two-way repeated-measures ANOVA and Holm-Sidak pairwise multiple-comparison procedures with an overall significance level set to P < 0.05, unless otherwise indicated (GraphPad Prism v6.01 and SigmaPlot v12). F statistic values reported are for the interaction between isoflurane and pH/CO2. When data are presented as X ± Y, X is the group mean and Y is the SD. For whole-animal experiments with mice, each animal was exposed to increased CO2 levels both in the absence and in the presence of isoflurane to act as in-subject controls.
RESULTS
Isoflurane severely impaired hypercapnic ventilatory response in vivo.
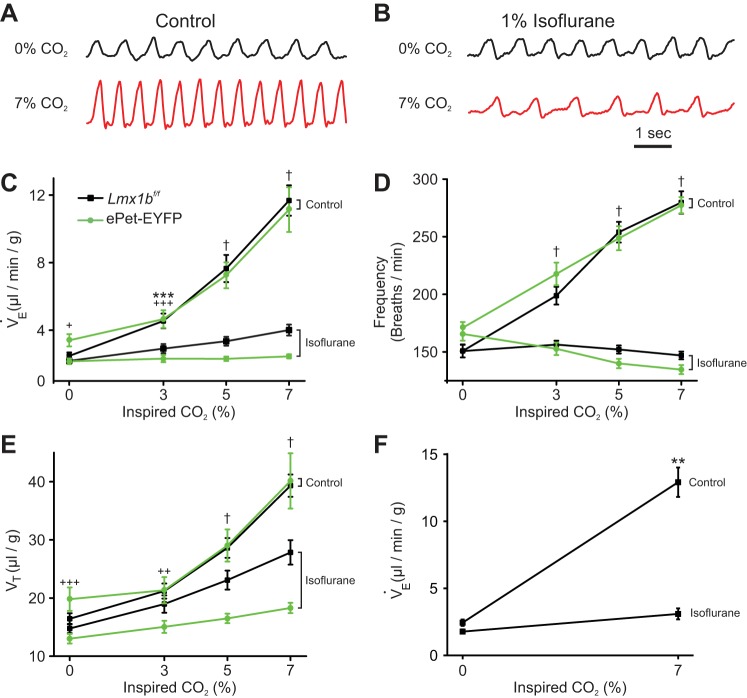
We measured the HCVR in awake (unanesthetized) Lmx1bf/f mice (Fig. 1A), which are on a mixed background of C57BL/6 and 129 strains (Zhao et al. 2006) and are functionally wild type, and again after at least 15 min of exposure to 1% isoflurane (Fig. 1B), the approximate mean alveolar concentration required for surgical anesthesia (1 MAC) (Eger 1981). Increases in inspired CO2 from 0% to 3%, 5%, and 7% were performed with O2 maintained at 50% (increased from the normal 21%; balance N2) to reduce the contribution from peripheral chemoreceptors as shown by others previously (Lahiri and DeLaney 1975). In response to 7% CO2, V̇e increased by 338% in unanesthetized Lmx1bf/f mice (from 2.52 ± 0.86 μl·g−1·min−1 to 11.03 ± 2.95 μl·g−1·min−1; P < 0.0001, n = 16; Fig. 1C) compared with only 83% in 1% isoflurane (from 2.23 ± 0.61 μl·g−1·min−1 to 4.08 ± 1.31 μl·g−1·min−1; P = 0.0004, n = 16; Fig. 1C), a reduction in the HCVR to 22% of control (F3,90 = 50.03, P < 0.0001, n = 16). In contrast, isoflurane had no effect on baseline breathing (from 2.52 ± 0.86 μl·g−1·min−1 in awake Lmx1bf/f mice to 2.23 ± 0.61 μl·g−1·min−1 in isoflurane; P = 0.4880, n = 16). V̇e in 7% CO2 with isoflurane (4.08 ± 1.31 μl·g−1·min−1) was significantly reduced compared with V̇e in 7% CO2 in the absence of isoflurane (11.03 ± 2.95 μl·g−1·min−1; P < 0.0001). The slope of the HCVR curve between 3% and 7% CO2 was decreased in anesthetized Lmx1bf/f mice to 16% of that in unanesthetized mice (Fig. 1C).
Fig. 1.
Isoflurane severely impaired the hypercapnic ventilatory response (HCVR) in vivo. A: whole-animal plethysmography recordings from an Lmx1bf/f mouse in 0% CO2 (top) and 7% CO2 (bottom), both in 50% O2. B: identical recordings from the mouse in A except for the addition of 1% isoflurane to the inspired gas. C: isoflurane caused a large reduction in the slope of minute ventilation (V̇e) vs. inspired CO2 in both Lmx1bf/f (F3,45 = 50.03, P < 0.001, n = 16) and ePet-EYFP (F3,27 = 39.37, P < 0.001, n = 10) mice. D: isoflurane ablated the increase in breathing frequency (FR) as inspired CO2 increased in both Lmx1bf/f (F3,45 = 69.08, P < 0.001, n = 16) and ePet-EYFP (F3,27 = 35.64, P < 0.001, n = 10) mice. E: increases in tidal volume (Vt) were reduced in isoflurane as inspired CO2 increased to 5% and above in both Lmx1bf/f (F3,45 = 14.42, P < 0.001, n = 16) and ePet-EYFP (F3,27 = 20.85, P < 0.001, n = 10) mice. F: isoflurane greatly reduced the HCVR in response to 7% CO2 when body temperature during anesthesia was held at 36°C (F1,3 = 73.04, P = 0.0034, n = 4). All error bars represent SE. **P < 0.01, ***P < 0.001 for Lmx1bf/f mice; +P < 0.05, ++P < 0.01, +++P < 0.001 for ePet-EYFP mice; †P < 0.0001 for both Lmx1bf/f and ePet-EYFP mice.
We also tested a separate strain of mice, ePet-EYFP, because we used these mice for in vitro experimentation (see below). ePet-EYFP mice are on a mixed background of C57BL/6 and 129 strains (Scott et al. 2005) and are functionally wild type. The effects of isoflurane on V̇e were more robust in ePet-EYFP mice. V̇e increased by 227% in response to 7% CO2 in unanesthetized ePet-EYFP mice (from 3.41 ± 1.15 μl·g−1·min−1 to 11.14 ± 4.18 μl·g−1·min−1; P < 0.0001, n = 10; Fig. 1C). In isoflurane, ePet-EYFP mice no longer had a HCVR, as there was no difference in V̇e at 0% CO2 compared with 7% CO2 (2.16 ± 0.53 vs. 2.45 ± 0.38 μl·g−1·min−1, which was 4% of control; P = 0.9947, n = 10). In contrast to Lmx1bf/f mice, isoflurane did decrease baseline breathing (from 3.41 ± 1.15 μl·g−1·min−1 in awake ePet-EYFP mice to 2.16 ± 0.53 μl·g−1·min−1 in isoflurane; P = 0.0257, n = 10). It is not known why isoflurane had greater effects in ePet-EYFP mice than in Lmx1bf/f mice. In ePet-EYFP mice, V̇e in 7% CO2 with isoflurane was significantly reduced compared with V̇e in 7% CO2 in the absence of isoflurane (2.45 ± 0.38 vs. 11.14 ± 4.18 μl·g−1·min−1; P < 0.0001, n = 10).
Isoflurane had a particularly profound effect on the breathing frequency (FR) component of the HCVR in both Lmx1bf/f and ePet-EYFP mice (Fig. 1D). In unanesthetized Lmx1bf/f mice, when CO2 was increased to 7%, FR increased by 85% (from 150.85 ± 22.67 breaths/min to 279.71 ± 38.95 breaths/min; P < 0.0001, n = 16). In 1% isoflurane, there was no longer any change in FR with 7% CO2 in Lmx1bf/f mice (from 150.69 ± 21.86 breaths/min to 146.94 ± 13.57 breaths/min; P = 0.9413, n = 16). In unanesthetized ePet-EYFP mice, when CO2 was increased to 7%, FR increased by 62% (from 170.94 ± 15.93 breaths/min to 277.29 ± 22.30 breaths/min; P < 0.0001, n = 10). However, in the presence of 1% isoflurane, there was no increase with 7% CO2 in ePet-EYFP mice (from 164.92 ± 18.39 breaths/min to 148.03 ± 44.50 breaths/min; P = 0.3506, n = 10).
Isoflurane also blunted the effect of CO2 on tidal volume (Vt), albeit less than on FR (Fig. 1E). In unanesthetized Lmx1bf/f mice Vt increased by 139% (from 16.44 ± 3.80 μl/g to 39.32 ± 7.67 μl/g; n = 16), while in 1% isoflurane Vt only increased 89% (from 14.76 ± 3.18 μl/g to 27.85 ± 8.40 μl/g; n = 16). The increase in Vt induced by 7% CO2 in Lmx1bf/f mice was reduced by 43% in the presence of isoflurane (P < 0.0001). In contrast, Vt in ePet-EYFP mice was more severely reduced. In unanesthetized ePet-EYFP mice, Vt increased by 103% (from 19.81 ± 6.36 μl/g to 40.13 ± 15.02 μl/g; P < 0.0001, n = 10); however, in isoflurane, Vt only increased 41% (from 13.01 ± 2.60 μl/g to 18.29 ± 2.81 μl/g; P = 0.0148, n = 10). The increase in Vt induced by 7% CO2 in ePet-EYFP mice was reduced by 74% in the presence of isoflurane (P < 0.0001).
Isoflurane treatment induced mild hypothermia (from 35.9 ± 1.6°C to 34.7 ± 1.0°C in 0% CO2; F3,45 = 5.190, P = 0.0036, n = 16). As such, it was important to determine whether changes in body temperature had a confounding effect on the results, perhaps indirectly through changes in metabolism. In a separate set of experiments body temperature was held constant at 36°C during isoflurane administration with a heat lamp controlled by a feedback loop. In the absence of isoflurane, V̇e increased by 430% (from 2.44 ± 0.47 μl·g−1·min−1 to 12.92 ± 2.18 μl·g−1·min−1; P = 0.0016, n = 4; Fig. 1F) in response to 7% CO2. In isoflurane, V̇e did not increase in response to 7% CO2 (from 1.78 ± 0.32 μl·g−1·min−1 to 3.09 ± 0.82 μl·g−1·min−1, which was 13% of control; P = 0.1806, n = 4; Fig. 1F). V̇e in 7% CO2 (3.09 ± 0.82 μl·g−1·min−1) was significantly reduced in the presence of isoflurane compared with V̇e in 7% CO2 in the absence of isoflurane (12.92 ± 1.09 μl·g−1·min−1; P = 0.002). Therefore, the effect of isoflurane on the HCVR was independent of body temperature.
Isoflurane abolished response of 5-HT neurons to CO2 in perfused brain stem.
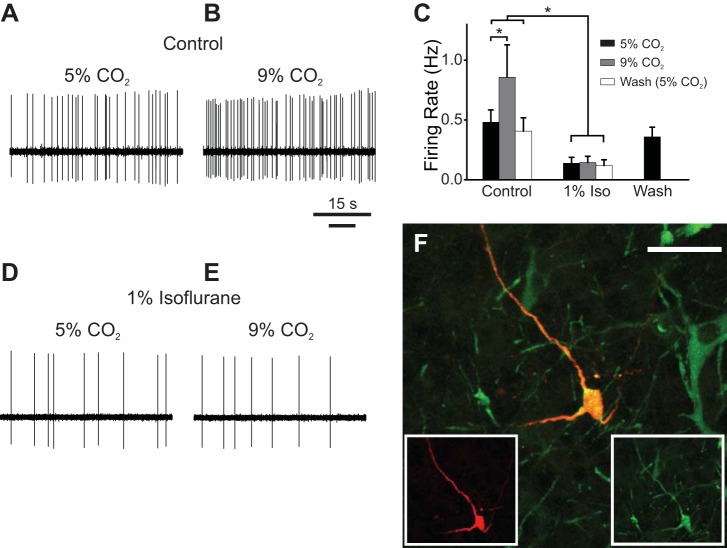
The severe depression of the HCVR by isoflurane without a significant effect on baseline ventilation in Lmx1bf/f mice was similar to previous observations that genetic deletion (Hodges et al. 2008) or selective inhibition (Ray et al. 2011) of 5-HT neurons also led to a decrease in the HCVR without changing baseline ventilation in adult mice. We hypothesized that isoflurane induced its effects on breathing in part by inhibition of 5-HT neurons, a possibility made more likely because 5-HT neurons express TASK channels at a high level (Talley et al. 2001). To test this possibility, we conducted experiments in an unanesthetized perfused in situ rat brain stem preparation. This preparation maintains an intact and functional respiratory control network and cardiorespiratory reflexes similar to those in vivo (Harris and St-John 2003; Richerson and Getting 1990; St-John and Paton 2000; Toppin et al. 2007). We first examined whether isoflurane affected baseline firing frequency of 5-HT neurons. Adding isoflurane (1%) to control perfusate (pH 7.4) reduced (Fig. 2A) or completely eliminated (Fig. 2, B and C) spontaneous firing in 64% (n = 14/22) and 36% (n = 8/22) of putative 5-HT neurons, respectively. This effect was reversible (Fig. 2, A and D). A subset of these neurons was verified to be serotonergic with juxtacellular labeling followed by TpOH immunohistochemistry (Fig. 2, E and F).
Fig. 2.
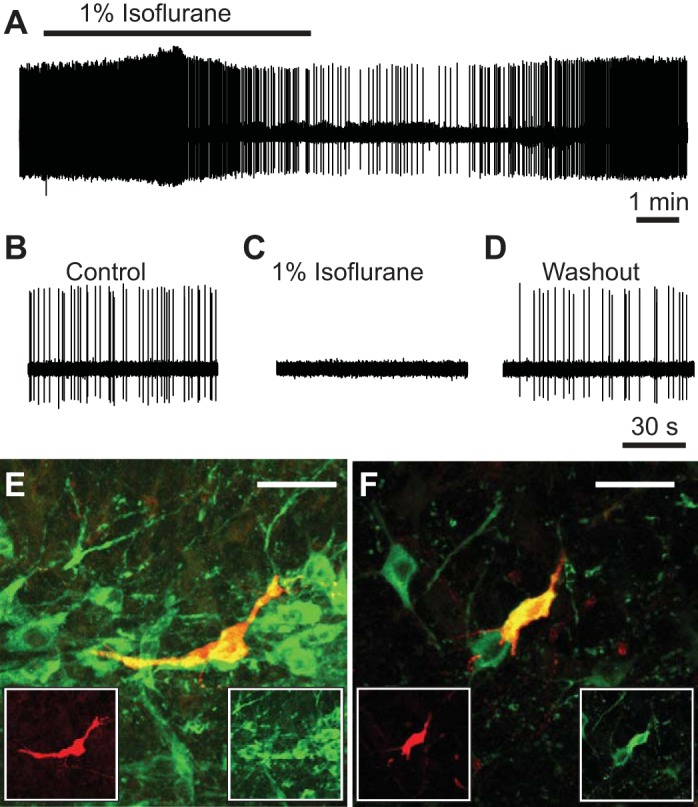
Isoflurane inhibited baseline firing of serotonin (5-hydroxytryptamine, 5-HT) neurons in the perfused brain stem preparation. A: a putative 5-HT neuron in the perfused brain stem preparation decreased its firing frequency in response to 1% isoflurane, with recovery upon washout. B: spontaneous firing of a putative 5-HT neuron (different from A) exposed to control perfusate. C: isoflurane (1%) abolished firing of neuron in B. D: spontaneous firing of neuron in B returned after washout of isoflurane. E and F: photomicrographs of the 2 juxtacellularly labeled cells in A (E) and B–D (F) confirmed that they were 5-HT neurons. Red, biotinamide; green, tryptophan hydroxylase (TpOH) immunohistochemistry; yellow, colocalization. Scale bars, 50 μm.
Extracellular recordings from CO2-stimulated neurons (n = 9) meeting the electrophysiological criteria for 5-HT neurons (see materials and methods) in the rostral portion of the medullary raphe (r. magnus and r. pallidus) revealed that these cells increased their firing frequency when in hypercapnic perfusate (Fig. 3, A–C), confirming that 5-HT neurons are chemosensitive in an intact brain stem (Iceman et al. 2013). The size of the response (71 ± 56% increase from control) was consistent with the sensitivity of 5-HT neurons documented in cats in vivo (Veasey et al. 1995, 1997) and the response of the respiratory system as a whole in this perfused brain preparation (St-John and Paton 2000; Toppin et al. 2007). These results verify that 5-HT neurons are chemosensitive even when the glial microenvironment is intact, in agreement with Veasey et al. (1995, 1997), who demonstrated that a subset of 5-HT neurons increase their firing rate in response to inspired CO2 in unanesthetized cats in vivo. These data also confirm our recent results with this perfused preparation (Iceman et al. 2013) and add to what has been considered to be the key, missing evidence needed to support the 5-HT neuron chemoreceptor hypothesis: chemosensitivity, in an intact brain stem, of neurons verified to be serotonergic by anatomical methods (Depuy et al. 2011).
Fig. 3.
Isoflurane abolished the change in firing rate of a 5-HT neuron in situ in response to acidosis. A: spontaneous firing of a putative 5-HT neuron exposed to control perfusate (pH 7.4) in situ. B: firing frequency increased in response to hypercapnic perfusate (pH 7.2). C: summary of recordings testing 5-HT neuron chemosensitivity in situ (n = 9). 5-HT neurons were chemosensitive under control conditions (P = 0.03). Isoflurane significantly reduced firing frequency (P = 0.02) and caused loss of the response to hypercapnic perfusate. Firing frequency returned to baseline levels after isoflurane was washed out. Error bars represent SE. D: isoflurane (1%) caused a decrease in firing frequency of the neuron in A. E: in isoflurane, the neuron no longer responded to hypercapnic perfusate with an increase in firing frequency. F: juxtacellular labeling confirmed that this was a 5-HT neuron. Red, biotinamide; green, TpOH immunostaining; yellow, colocalization. Scale bar, 50 μm. *P < 0.05.
Isoflurane (1%) eliminated the acidosis-induced increased firing frequency of 5-HT neurons in the in situ perfused brain stem preparation. As was found with the larger set of putative 5-HT neurons noted above, adding 1% isoflurane to the perfusate eliminated (n = 3/9) or markedly decreased (n = 6/9) firing in the subset of CO2-stimulated neurons. Subsequent hypercapnia in this isoflurane perfusate resulted in no increase in firing frequency (an increase of 15 ± 72%; n = 9); thus isoflurane completely eliminated the response to hypercapnia (Fig. 3, C–E). This effect of isoflurane was reversible (n = 8/9; Fig. 3C). A subset of these neurons were verified to be serotonergic with juxtacellular labeling followed by TpOH immunohistochemistry (Fig. 3F).
Isoflurane abolished firing of 5-HT neurons in culture and prevented increased firing in response to acidosis.
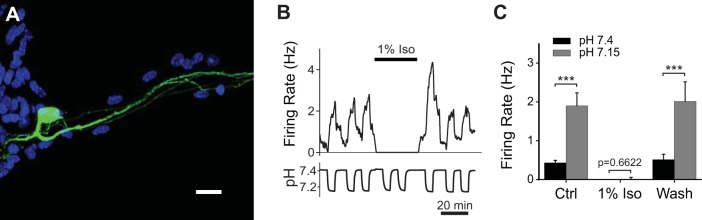
5-HT neurons can be studied in cell culture, where they maintain properties that closely resemble those exhibited in more intact preparations (Wang et al. 2001). We prepared medullary raphe cultures from ePet-EYFP mice (Scott et al. 2005) (Fig. 4A) to understand the cellular mechanisms of the effects of isoflurane on respiratory chemoreception. For the 5-HT neuron shown in Fig. 4B, acidic aCSF (pH = 7.15) induced increased firing that quickly reversed on return to aCSF (pH = 7.4). Acidosis consistently increased firing frequency in all 5-HT neurons tested (from 0.43 ± 0.25 Hz in aCSF to 1.90 ± 1.30 Hz in acidic aCSF, an increase of 342%; P < 0.0001, n = 15; Fig. 4C), consistent with the degree of chemosensitivity previously reported for 5-HT neurons in culture (Wang et al. 1998, 2001, 2002; Wang and Richerson 1999).
Fig. 4.
Isoflurane inhibited firing of 5-HT neurons in vitro and abolished their response to CO2. A: confocal microscopy image of a cultured medullary ePet-EYFP neuron. green, EYFP; blue, DAPI nuclear stain. Scale bar, 25 μm. B: firing frequency and pH of a recording from a cultured medullary 5-HT neuron. Firing increased during acidosis. Isoflurane (1%) eliminated firing in aCSF, and firing did not return in acidosis. C: summary of current-clamp recordings. 5-HT neurons had a robust increase in firing frequency in response to acidosis (Ctrl, n = 15). Isoflurane eliminated firing and prevented any change in response to acidosis (1% Iso, n = 15). These effects were reversible (Wash, n = 12). A 2-way repeated-measures ANOVA revealed a significant effect of pH (F1,39 = 41.77, P < 0.0001) and isoflurane (F2,39 = 12.25, P < 0.0001). Furthermore, there was an interaction between pH and isoflurane (F2,39 = 8.568, P = 0.0008). Error bars represent SE. ***P < 0.001.
In aCSF isoflurane (1%) completely eliminated 5-HT neuron firing, and firing did not return in acidic aCSF (Fig. 4B). Firing was restored in aCSF upon washout of isoflurane, as was the acidosis-induced increase in firing frequency. This effect of isoflurane was highly consistent across all recorded neurons (n = 15; Fig. 4C). When neurons were exposed to 1% isoflurane in aCSF, the firing frequency decreased from 0.43 ± 0.25 Hz to 0.00 ± 0.00 Hz (n = 15). Furthermore, in isoflurane there was no change in firing frequency in response to acidosis (from 0.00 ± 0.00 Hz to 0.12 ± 0.22 Hz; P = 0.6635, n = 15; Fig. 4C). The inhibition of 5-HT neurons was reversible upon washout of isoflurane (n = 12), as was the acidosis-induced increase in firing frequency (n = 12).
Isoflurane did not prevent underlying mechanisms of chemosensitivity in 5-HT neurons.
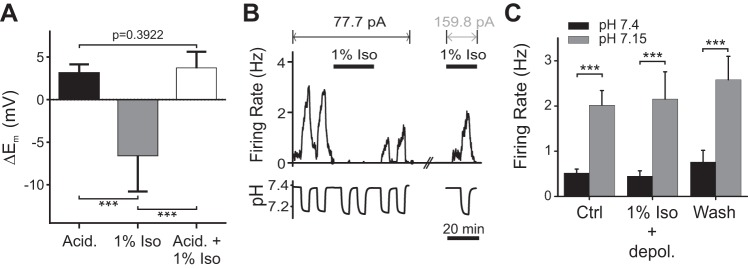
The protocol used above (Fig. 4B) did not distinguish between whether isoflurane blocked the underlying chemosensory mechanisms or, alternatively, simply hyperpolarized 5-HT neurons and prevented them from reaching threshold for action potential generation during acidosis. To differentiate between these possibilities, we examined changes in membrane potential (Em) in response to acidosis both in the absence and in the presence of isoflurane. In aCSF without isoflurane, acidosis induced a 3.10 ± 1.05-mV depolarization in Em (n = 13; Fig. 5A). In aCSF, isoflurane (1%) hyperpolarized 5-HT neurons by −6.53 ± 4.26 mV relative to aCSF without isoflurane (n = 13; Fig. 5A). When acidic aCSF with isoflurane was superfused into the chamber, the Em in these neurons depolarized by 3.68 ± 1.95 mV compared with aCSF with isoflurane (n = 13; Fig. 5A). However, this was not enough to reverse the hyperpolarization of Em caused by isoflurane. There was no difference in the depolarization induced by acidosis in control conditions compared with acidosis in isoflurane (P = 0.3922, Wilcoxon matched-pairs signed-rank test; n = 13; Fig. 5A). Thus 5-HT neurons retained chemosensitivity, but isoflurane caused sufficient hyperpolarization so that acidosis could not depolarize them enough to reach action potential threshold.
Fig. 5.
Isoflurane did not abolish underlying chemosensitive mechanisms in 5-HT neurons in culture. A: summary of changes in membrane potential (Em) of cultured 5-HT neurons induced by acidic aCSF (Acid.), isoflurane (1% Iso), and acidic aCSF in isoflurane (Acid. + 1% Iso) (F = 56.12, P < 0.001, n = 13). B: recording from a 5-HT neuron in culture whose firing was abolished by isoflurane. Current injection was increased from 77.7 pA to 159.8 pA to reverse the hyperpolarization induced by isoflurane. The response to acidosis was then as large as control despite the continued presence of isoflurane. C: summary of the effect of acidosis while giving current injection to reverse the hyperpolarization induced by isoflurane. Shown are firing frequencies in aCSF (pH 7.4) and acidic aCSF (pH 7.15) under control conditions (Ctrl, n = 12), in isoflurane while giving extra depolarizing current (1% Iso + depol., n = 12), and during washout of isoflurane (Wash, n = 7). Chemosensitivity remained intact in isoflurane (F1,28 = 40.01, P < 0.0001), and there was no effect of isoflurane after an increase in current injection (F2,28 = 0.4537, P = 0.6399) or interaction between pH and isoflurane (F2,28 = 0.1227; P = 0.8850). All error bars represent SE. ***P < 0.001.
We next used depolarizing current injection to reverse the hyperpolarization of 5-HT neurons that was induced by isoflurane and determined whether acidosis would then alter their firing rate. 5-HT neurons were first exposed to acidic aCSF without isoflurane to quantify their chemosensitivity (Fig. 5B). Bath solutions were then switched to aCSF with 1% isoflurane, which hyperpolarized neurons and caused them to stop firing. A sufficient amount of current was then injected to return firing frequency back to the baseline level, and chemosensitivity was reassessed. Prior to isoflurane exposure, tested cells increased action potential frequency in response to acidosis from 0.52 ± 0.31 Hz to 2.02 ± 1.12 Hz, an increase of 288% (P = 0.0022, n = 12; Fig. 5C). Despite continued exposure to isoflurane, neurons with compensatory current injection still increased their firing frequency when exposed to acidic aCSF from 0.45 ± 0.40 Hz to 2.15 ± 2.09 Hz, an increase of 378% (P = 0.0009, n = 12). Thus, when hyperpolarization induced by isoflurane was reversed with current injection, there was no difference in firing frequency between control and isoflurane conditions in aCSF (P = 0.9285) and acidic aCSF (P = 0.7842), indicating that isoflurane did not abolish underlying chemosensory mechanisms in 5-HT neurons.
DISCUSSION
Here we studied the effects of isoflurane on pH/CO2 chemosensitivity of 5-HT neurons in culture and correlated our findings with that of 5-HT neurons in situ and with respiratory motor output in vivo. Using this approach, we found that isoflurane hyperpolarized 5-HT neurons in culture and eliminated their firing, consistent with activation of TASK channels. This hyperpolarization prevented expression of chemosensitivity, as normally assessed by a change in firing rate in response to acidosis. However, hyperpolarized neurons still depolarized with acidosis, and the firing response to acidosis returned when hyperpolarization was reversed by current injection. Recordings from a perfused brain preparation demonstrated that similar cellular responsiveness existed when 5-HT neurons were embedded within an intact nervous system. Spontaneous firing of 5-HT neurons in situ was reduced or abolished, and firing frequency responses of CO2-stimulated neurons were absent in the presence of isoflurane.
Our collection of data from three different preparations and from different species and strains provides confidence that the results are of general relevance. ePet-EYFP mice were used for both in vivo and in vitro experiments, allowing the data to be directly compared. Data from whole-animal plethysmography were obtained from two different mouse strains, and in both cases there was a similar strong reduction in the HCVR. The inhibition of 5-HT neuron chemosensitivity in cultures from ePet-EYFP mice was recapitulated in the perfused rat brain stem preparation. These findings at the single-cell level can explain, in part, why isoflurane (1%) greatly attenuated the ventilatory response to hypercapnia in vivo. These data demonstrate that isoflurane introduces a significant decrease in the ability to detect chemoreception of 5-HT neurons and other neurons that express significant levels of TASK channels (Talley et al. 2001).
5-HT neurons and central CO2 chemoreception.
5-HT neurons are chemosensitive across many different preparations (see above). However, conflicting interpretations of data (Depuy et al. 2011; Mulkey et al. 2004) have led to the suggestion that chemosensitivity of 5-HT neurons may be an unnatural property induced by culture conditions. Despite evidence of chemosensitivity in vitro, the absence of 5-HT neuron response to CO2 in anesthetized animals in vivo has been taken to indicate that 5-HT neurons do not express chemosensitivity within the intact nervous system and, thus, are not central chemoreceptors (Depuy et al. 2011; Mulkey et al. 2004). However, our results demonstrate that 5-HT neurons are chemosensitive in an intact nervous system. The two studies that failed to document 5-HT neuron chemosensitivity in vivo used either halothane (Mulkey et al. 2004) or isoflurane (Depuy et al. 2011) anesthesia. Our present results suggest that halogenated anesthetics may have prevented detection of 5-HT neuron chemosensitivity in these two previous in vivo studies. This possibility is further supported when the ventilatory sensitivities reported in the various studies are considered. Studies in unanesthetized mice (Hodges et al. 2008) or rats (Davis et al. 2006; Taylor et al. 2005) typically report at least 250% increases in V̇e with elevation of inspired CO2 from 0% to 7%. In contrast, during experiments in isoflurane-anesthetized rats in which 5-HT neurons were reported to be insensitive to CO2, V̇e increased by only 35% when inspired CO2 was elevated from 0% to 10% (Depuy et al. 2011). Thus in the studies that failed to detect 5-HT neuron chemosensitivity it is likely that halogenated anesthetics hyperpolarized 5-HT neurons, obscuring their chemosensitivity and blunting the HCVR.
Previous recordings from 5-HT neurons in the parapyramidal (ppy) region showed that these neurons are not responsive to hypercapnia/hyperoxia in urethane-anesthetized animals after carotid body denervation (Takakura and Moreira 2013). This is consistent with the likelihood that 5-HT neurons in different nuclei and regions serve different physiological roles. For example, 5-HT neurons from rhombomere 5 (primarily in raphe magnus) are more chemosensitive than 5-HT neurons originating from rhombomeres 6 and 7, and the former project to integrative respiratory nuclei (Brust et al. 2014).
Role of TASK channels in 5-HT neurons.
Isoflurane is well known to potentiate TASK channel currents (Sirois et al. 2000). TASK channels mediate a leak K+ current, and potentiation of this current causes hyperpolarization. TASK channels are present throughout the nervous system, and 5-HT neurons express them at very high levels (Talley et al. 2001). Thus isoflurane will hyperpolarize and inhibit many neurons, including 5-HT neurons. The effects seen when 5-HT neurons were exposed to isoflurane in cell culture and in the in situ perfused brain preparation were consistent with what would be expected with activation of TASK channels. Additionally, it is possible that depression of arousal is due in part to isoflurane-induced inhibition of 5-HT neurons in the midbrain, which have previously been shown to be involved with CO2-induced arousal (Buchanan and Richerson 2010).
TASK channels are inhibited by acidosis (Duprat et al. 1997) and have been proposed to mediate the response of neonatal mouse 5-HT neurons in brain slices to changes in pH from 7.5 to 6.9 (Mulkey et al. 2007). As rat and mouse 5-HT neurons mature, they develop a much larger response to acidosis and are responsive over a narrower pH range [such as between pH 7.4 and 7.2 (Brust et al. 2014; Wang and Richerson 1999)]. Preliminary evidence indicates that a calcium-activated nonselective cation current is the major contributor to chemosensitivity in adult rats and mice (Wu et al. 2009). It is possible that TASK channels also contribute to chemosensitivity of mature 5-HT neurons, but this has not yet been examined. If so, it is unlikely that TASK channels are the sole mediator, as we show here that chemosensitivity of 5-HT neurons is seemingly unchanged by TASK channel potentiation from isoflurane, when the hyperpolarization expected by the influence of isoflurane on TASK channels is compensated by experimental injection of depolarizing current. Whole-animal plethysmography of two different transgenic mouse lines demonstrated that TASK channels were not required for the HCVR in vivo (Mulkey et al. 2007) but were necessary for peripheral chemoreception (Trapp et al. 2008). Previous work from our laboratory has shown that inhibition or deletion of 5-HT neurons decreases central respiratory chemoreception in vivo (Brust et al. 2014; Hodges et al. 2008; Ray et al. 2011). Therefore, if TASK channels mediate chemosensitivity of 5-HT neurons then there should also be a decrease in the HCVR after genetic deletion of TASK channels, but that is not the case (Mulkey et al. 2007).
In 5-HT neurons, the change in conductance of TASK channels is small over the pH range studied here (7.4-7.15) (Teran et al. 2014; Washburn et al. 2002), so it would not be expected to cause the large change in firing frequency seen in 5-HT neurons over this pH range. It is also unlikely that such a small shift in TASK conductance caused by acidosis would be enough to cause the firing frequency of these neurons to increase to 300% of baseline, when motor neurons express higher levels of TASK channels but are not strongly depolarized by acidosis (Talley et al. 2001; Washburn et al. 2002). In fact, the effect of pH on motor neurons is actually the opposite, with the common, reproducible clinical observation that respiratory alkalosis causes tetany (Brown 1953). The firing frequency responses of 5-HT neurons to acidosis were neither exaggerated nor blunted in isoflurane, when the hyperpolarizing influences were compensated. For these and other reasons, it is unlikely that TASK channels are the mediators of 5-HT neuron chemosensitivity (Corcoran et al. 2009; Teran et al. 2014).
Effect of isoflurane on respiratory chemoreception.
Here we have shown that cell culture can be used to study 5-HT neuron chemosensitivity and how it is influenced by the anesthetic isoflurane. We then recorded from 5-HT neurons in a perfused brain stem preparation and determined how isoflurane affected those neurons and their chemosensitivity within an intact respiratory network and a normal glial-vascular microenvironment. Finally, we studied the effect of isoflurane on the response of the whole animal to inhalation of CO2 and illustrated that the influence of isoflurane on chemoreception in vivo is consistent with its influence on chemosensitive 5-HT neurons in vitro and in situ. Our ability to correlate the effect of a perturbation on a single neuron with the effect of that perturbation on the motor behavior of the intact animal allowed us to define a cellular mechanism that may contribute to the effect of isoflurane on respiratory chemoreception in vivo.
Isoflurane, halothane, and other halogenated anesthetics have been frequently used in both research and clinical settings. These agents can alter the outcome of experiments on chemoreception and control of breathing more than is appreciated by some investigators. In the present experiments, isoflurane markedly depressed the HCVR but had no effect on baseline breathing in Lmx1bf/f mice. This is reminiscent of what is seen when 5-HT neurons are genetically deleted in adult Lmx1bf/f/p mice (Hodges et al. 2008) or selectively silenced in adult mice expressing DREADD receptors on 5-HT neurons (Brust et al. 2014; Ray et al. 2011). The reason for the dissociation of the effects on baseline breathing and the HCVR in Lmx1bf/f is unknown. TASK channels are widely expressed in the brain stem, including on 5-HT neurons and motor neurons and in respiratory nuclei, and when activated would cause inhibition at multiple sites in the respiratory network. Therefore, isoflurane should cause a blunted HCVR and depressed breathing at baseline, as seen in ePet-EYFP mice. However, neurons in the retrotrapezoid nucleus (RTN) are one of the exceptions, because they express TWIK-related halothane-inhibited K+ (THIK-1) channels, which are K+ channels that are inhibited, rather than activated, by halogenated anesthetics (Lazarenko et al. 2010; Rajan et al. 2001). As a result, RTN neurons would be activated by isoflurane and halothane and should stimulate the respiratory network, counteracting inhibition at other sites, potentially explaining why ventilation is maintained constant at baseline in isoflurane in Lmx1bf/f mice.
TASK channels are also expressed in peripheral chemoreceptors. Our use of 50% O2 during plethysmography recordings was designed to minimize contributions of peripheral chemoreceptors as previous work has demonstrated (Hodges et al. 2008; Lahiri and DeLaney 1975). The effect of halogenated anesthetics on the HCVR in our experiments could potentially be explained largely via inhibition of central chemoreceptors, including 5-HT neurons. Our data do not, however, exclude an effect on other isoflurane-sensitive chemoreceptors, including peripheral chemoreceptors. They also do not exclude an effect on other elements of the respiratory network.
The gold standard for defining normal neuronal activity is widely considered to be extracellular recording from neurons in vivo. However, this approach typically requires the use of anesthesia, and two of the most common and convenient agents used are halothane and isoflurane. These halogenated anesthetics alter respiratory physiology so severely that in vivo preparations using these agents should not be assumed to reflect normal physiology in studies of breathing. In particular, the use of these anesthetics may greatly underestimate the relative contribution of chemoreceptors that express TASK channels, such as 5-HT neurons.
GRANTS
Research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke (NINDS) under Grant P20 NS-076916 (G. B. Richerson), the National Institute of Child Health and Human Development under Grants P01 HD-36379 and R01 HD-052772 (G. B. Richerson), NINDS through a Diversity Research Development Program award [Specialized Neuroscience Research Program (SNRP)] under Grant 2U54 NS-041069 (M. B. Harris), and the National Institute of General Medical Sciences through an Institutional Development Award (IDeA) under Grant P20 GM-103395 (M. B. Harris).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.A.M., K.E.I., S.L.J., M.B.H., and G.B.R. conception and design of research; C.A.M., K.E.I., S.L.J., and Y.W. performed experiments; C.A.M., K.E.I., S.L.J., M.B.H., and G.B.R. analyzed data; C.A.M., K.E.I., S.L.J., M.B.H., and G.B.R. interpreted results of experiments; C.A.M., K.E.I., and S.L.J. prepared figures; C.A.M., M.B.H., and G.B.R. drafted manuscript; C.A.M., K.E.I., S.L.J., Y.W., M.B.H., and G.B.R. edited and revised manuscript; C.A.M., K.E.I., S.L.J., Y.W., M.B.H., and G.B.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Xiuqiong Zhou for mouse husbandry and genotyping. We thank Lori Smith for her technical contributions.
REFERENCES
- Bernard DG, Li A, Nattie EE. Evidence for central chemoreception in the midline raphe. J Appl Physiol 80: 108–115, 1996. [DOI] [PubMed] [Google Scholar]
- Bradley SR, Pieribone VA, Wang W, Severson CA, Jacobs RA, Richerson GB. Chemosensitive serotonergic neurons are closely associated with large medullary arteries. Nat Neurosci 5: 401–402, 2002. [DOI] [PubMed] [Google Scholar]
- Brown EB., Jr Physiological effects of hyperventilation. Physiol Rev 33: 445–471, 1953. [DOI] [PubMed] [Google Scholar]
- Brust RD, Corcoran AE, Richerson GB, Nattie E, Dymecki SM. Functional and developmental identification of a molecular subtype of brain serotonergic neuron specialized to regulate breathing dynamics. Cell Rep 9: 2152–2165, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan GF, Richerson GB. Central serotonin neurons are required for arousal to CO2. Proc Natl Acad Sci USA 107: 16354–16359, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran AE, Hodges MR, Wu Y, Wang W, Wylie CJ, Deneris ES, Richerson GB. Medullary serotonin neurons and central CO2 chemoreception. Respir Physiol Neurobiol 168: 49–58, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SE, Solhied G, Castillo M, Dwinell M, Brozoski D, Forster HV. Postnatal developmental changes in CO2 sensitivity in rats. J Appl Physiol 101: 1097–1103, 2006. [DOI] [PubMed] [Google Scholar]
- Depuy SD, Kanbar R, Coates MB, Stornetta RL, Guyenet PG. Control of breathing by raphe obscurus serotonergic neurons in mice. J Neurosci 31: 1981–1990, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprat F, Lesage F, Fink M, Reyes R, Heurteaux C, Lazdunski M. TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO J 16: 5464–5471, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eger EI., 2nd Isoflurane: a review. Anesthesiology 55: 559–576, 1981. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci 26: 239–266, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda Y, Honda Y, Schlafke ME, Loeschcke HH. Effect of H+ on the membrane potential of silent cells in the ventral and dorsal surface layers of the rat medulla in vitro. Pflügers Arch 376: 229–235, 1978. [DOI] [PubMed] [Google Scholar]
- Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science 329: 571–575, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeben H, Meier S, Tankersley CG, Mitzner W, Brown RH. Heritable differences in respiratory drive and breathing pattern in mice during anaesthesia and emergence. Br J Anaesth 91: 541–545, 2003. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bayliss DA, Mulkey DK. Retrotrapezoid nucleus: a litmus test for the identification of central chemoreceptors. Exp Physiol 90: 247–253, 2005. [DOI] [PubMed] [Google Scholar]
- Harris MB, St-John WM. Tonic pulmonary stretch receptor feedback modulates both eupnea and gasping in an in situ rat preparation. Am J Physiol Regul Integr Comp Physiol 285: R215–R221, 2003. [DOI] [PubMed] [Google Scholar]
- Hirshman CA, McCullough RE, Cohen PJ, Weil JV. Depression of hypoxic ventilatory response by halothane, enflurane and isoflurane in dogs. Br J Anaesth 49: 957–963, 1977. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Martino P, Davis S, Opansky C, Pan LG, Forster HV. Effects on breathing of focal acidosis at multiple medullary raphe sites in awake goats. J Appl Physiol 97: 2303–2309, 2004. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Tattersall GJ, Harris MB, McEvoy SD, Richerson DN, Deneris ES, Johnson RL, Chen ZF, Richerson GB. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci 28: 2495–2505, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iceman KE, Harris MB. A group of non-serotonergic cells is CO2-stimulated in the medullary raphe. Neuroscience 259: 203–213, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iceman KE, Richerson GB, Harris MB. Medullary serotonin neurons are CO2 sensitive in situ. J Neurophysiol 110: 2536–2544, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knill RL, Kieraszewicz HT, Dodgson BG, Clement JL. Chemical regulation of ventilation during isoflurane sedation and anaesthesia in humans. Can Anaesth Soc J 30: 607–614, 1983. [DOI] [PubMed] [Google Scholar]
- Lahiri S, DeLaney RG. Stimulus interaction in the responses of carotid body chemoreceptor single afferent fibers. Respir Physiol 24: 249–266, 1975. [DOI] [PubMed] [Google Scholar]
- Larnicol N, Wallois F, Berquin P, Gros F, Rose D. c-fos-like immunoreactivity in the cat's neuraxis following moderate hypoxia or hypercapnia. J Physiol (Paris) 88: 81–88, 1994. [DOI] [PubMed] [Google Scholar]
- Lazarenko RM, Fortuna MG, Shi Y, Mulkey DK, Takakura AC, Moreira TS, Guyenet PG, Bayliss DA. Anesthetic activation of central respiratory chemoreceptor neurons involves inhibition of a THIK-1-like background K+ current. J Neurosci 30: 9324–9334, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Body RL, Sinclair JD. Analysis of respiratory patterns in the awake and in the halothane anaesthetised rat. Respir Physiol 61: 105–113, 1985. [DOI] [PubMed] [Google Scholar]
- Mason P. Physiological identification of pontomedullary serotonergic neurons in the rat. J Neurophysiol 77: 1087–1098, 1997. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci 7: 1360–1369, 2004. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Talley EM, Stornetta RL, Siegel AR, West GH, Chen X, Sen N, Mistry AM, Guyenet PG, Bayliss DA. TASK channels determine pH sensitivity in select respiratory neurons but do not contribute to central respiratory chemosensitivity. J Neurosci 27: 14049–14058, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie E, Li A. Central chemoreception 2005: a brief review. Auton Neurosci 126–127: 332–338, 2006. [DOI] [PubMed] [Google Scholar]
- Nichols NL, Hartzler LK, Conrad SC, Dean JB, Putnam RW. Intrinsic chemosensitivity of individual nucleus tractus solitarius (NTS) and locus coeruleus (LC) neurons from neonatal rats. Adv Exp Med Biol 605: 348–352, 2008. [DOI] [PubMed] [Google Scholar]
- O'Regan RG, Majcherczyk S. Role of peripheral chemoreceptors and central chemosensitivity in the regulation of respiration and circulation. J Exp Biol 100: 23–40, 1982. [DOI] [PubMed] [Google Scholar]
- Pandit JJ. Volatile anaesthetic depression of the carotid body chemoreflex-mediated ventilatory response to hypoxia: directions for future research. Scientifica (Cairo) 2014: 394270, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AJ, Honore E, Lesage F, Fink M, Romey G, Lazdunski M. Inhalational anesthetics activate two-pore-domain background K+ channels. Nat Neurosci 2: 422–426, 1999. [DOI] [PubMed] [Google Scholar]
- Pineda J, Aghajanian GK. Carbon dioxide regulates the tonic activity of locus coeruleus neurons by modulating a proton- and polyamine-sensitive inward rectifier potassium current. Neuroscience 77: 723–743, 1997. [DOI] [PubMed] [Google Scholar]
- Ptak K, Yamanishi T, Aungst J, Milescu LS, Zhang R, Richerson GB, Smith JC. Raphe neurons stimulate respiratory circuit activity by multiple mechanisms via endogenously released serotonin and substance P. J Neurosci 29: 3720–3737, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan S, Wischmeyer E, Karschin C, Preisig-Muller R, Grzeschik KH, Daut J, Karschin A, Derst C. THIK-1 and THIK-2, a novel subfamily of tandem pore domain K+ channels. J Biol Chem 276: 7302–7311, 2001. [DOI] [PubMed] [Google Scholar]
- Ray RS, Corcoran AE, Brust RD, Kim JC, Richerson GB, Nattie E, Dymecki SM. Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition. Science 333: 637–642, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richerson GB. Response to CO2 of neurons in the rostral ventral medulla in vitro. J Neurophysiol 73: 933–944, 1995. [DOI] [PubMed] [Google Scholar]
- Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci 5: 449–461, 2004. [DOI] [PubMed] [Google Scholar]
- Richerson GB, Getting PA. Preservation of integrative function in a perfused guinea pig brain. Brain Res 517: 7–18, 1990. [DOI] [PubMed] [Google Scholar]
- Richerson GB, Wang W, Hodges MR, Dohle CI, Diez-Sampedro A. Homing in on the specific phenotype(s) of central respiratory chemoreceptors. Exp Physiol 90: 259–266, 2005. [DOI] [PubMed] [Google Scholar]
- Richerson HB, Coon JD, Lubaroff D. Selective early increases of bronchoalveolar CD8+ lymphocytes in a LEW rat model of hypersensitivity pneumonitis. J Allergy Clin Immunol 96: 113–121, 1995. [DOI] [PubMed] [Google Scholar]
- Sato M, Severinghaus JW, Basbaum AI. Medullary CO2 chemoreceptor neuron identification by c-fos immunocytochemistry. J Appl Physiol 73: 96–100, 1992. [DOI] [PubMed] [Google Scholar]
- Scott MM, Wylie CJ, Lerch JK, Murphy R, Lobur K, Herlitze S, Jiang W, Conlon RA, Strowbridge BW, Deneris ES. A genetic approach to access serotonin neurons for in vivo and in vitro studies. Proc Natl Acad Sci USA 102: 16472–16477, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirois JE, Lei Q, Talley EM, Lynch C 3rd, Bayliss DA. The TASK-1 two-pore domain K+ channel is a molecular substrate for neuronal effects of inhalation anesthetics. J Neurosci 20: 6347–6354, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-John WM, Paton JF. Characterizations of eupnea, apneusis and gasping in a perfused rat preparation. Respir Physiol 123: 201–213, 2000. [DOI] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS. Arterial chemoreceptor activation reduces the activity of parapyramidal serotonergic neurons in rats. Neuroscience 237: 199–207, 2013. [DOI] [PubMed] [Google Scholar]
- Talley EM, Solorzano G, Lei Q, Kim D, Bayliss DA. CNS distribution of members of the two-pore-domain (KCNK) potassium channel family. J Neurosci 21: 7491–7505, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor NC, Li A, Nattie EE. Medullary serotonergic neurones modulate the ventilatory response to hypercapnia, but not hypoxia in conscious rats. J Physiol 566: 543–557, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teran FA, Massey CA, Richerson GB. Serotonin neurons and central respiratory chemoreception: where are we now? Prog Brain Res 209: 207–233, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toppin VA, Harris MB, Kober AM, Leiter JC, St-John WM. Persistence of eupnea and gasping following blockade of both serotonin type 1 and 2 receptors in the in situ juvenile rat preparation. J Appl Physiol 103: 220–227, 2007. [DOI] [PubMed] [Google Scholar]
- Trapp S, Aller MI, Wisden W, Gourine AV. A role for TASK-1 (KCNK3) channels in the chemosensory control of breathing. J Neurosci 28: 8844–8850, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Response of serotonergic caudal raphe neurons in relation to specific motor activities in freely moving cats. J Neurosci 15: 5346–5359, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Single-unit responses of serotonergic dorsal raphe neurons to specific motor challenges in freely moving cats. Neuroscience 79: 161–169, 1997. [DOI] [PubMed] [Google Scholar]
- Wang W, Bradley SR, Richerson GB. Quantification of the response of rat medullary raphe neurones to independent changes in pHo and PCO2. J Physiol 540: 951–970, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Pizzonia JH, Richerson GB. Chemosensitivity of rat medullary raphe neurones in primary tissue culture. J Physiol 511: 433–450, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Richerson GB. Development of chemosensitivity of rat medullary raphe neurons. Neuroscience 90: 1001–1011, 1999. [DOI] [PubMed] [Google Scholar]
- Wang W, Tiwari JK, Bradley SR, Zaykin RV, Richerson GB. Acidosis-stimulated neurons of the medullary raphe are serotonergic. J Neurophysiol 85: 2224–2235, 2001. [DOI] [PubMed] [Google Scholar]
- Washburn CP, Sirois JE, Talley EM, Guyenet PG, Bayliss DA. Serotonergic raphe neurons express TASK channel transcripts and a TASK-like pH- and halothane-sensitive K+ conductance. J Neurosci 22: 1256–1265, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Hodges MR, Wang W, Zaykin A, Wylie CJ, Deneris ES, Richerson GB. Hypercapnic acidosis inhibits a calcium-activated non-selective cation current in mature serotonergic neurons (Abstract). Soc Neurosci Abstr 2009: 89 17, 2009. [Google Scholar]
- Zhao ZQ, Scott M, Chiechio S, Wang JS, Renner KJ, Gereau RWt Johnson RL, Deneris ES, Chen ZF. Lmx1b is required for maintenance of central serotonergic neurons and mice lacking central serotonergic system exhibit normal locomotor activity. J Neurosci 26: 12781–12788, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]