Abstract
Pathologic reorganization of spinal networks and activity-dependent plasticity are common neuronal adaptations after spinal cord injury (SCI) in humans. In this work, we examined changes of reciprocal Ia and nonreciprocal Ib inhibition after locomotor training in 16 people with chronic SCI. The soleus H-reflex depression following common peroneal nerve (CPN) and medial gastrocnemius (MG) nerve stimulation at short conditioning-test (C-T) intervals was assessed before and after training in the seated position and during stepping. The conditioned H reflexes were normalized to the unconditioned H reflex recorded during seated. During stepping, both H reflexes were normalized to the maximal M wave evoked at each bin of the step cycle. In the seated position, locomotor training replaced reciprocal facilitation with reciprocal inhibition in all subjects, and Ib facilitation was replaced by Ib inhibition in 13 out of 14 subjects. During stepping, reciprocal inhibition was decreased at early stance and increased at midswing in American Spinal Injury Association Impairment Scale C (AIS C) and was decreased at midstance and midswing phases in AIS D after training. Ib inhibition was decreased at early swing and increased at late swing in AIS C and was decreased at early stance phase in AIS D after training. The results of this study support that locomotor training alters postsynaptic actions of Ia and Ib inhibitory interneurons on soleus motoneurons at rest and during stepping and that such changes occur in cases with limited or absent supraspinal inputs.
Keywords: locomotor training, neuromodulation, neuroplasticity, reciprocal inhibition, Ib inhibition, spinal cord injury
during walking, a multitude of neuronal interactions at multiple spinal segments occurs simultaneously, with sensory afferent feedback and descending inputs to adjusting amplitude, rate, and periodicity of motoneuron discharges based on the demands of the motor task (Rossignol 2006; Knikou 2010a). Animal studies have provided clear evidence on the contribution of the spinal nonreciprocal Ib and reciprocal Ia inhibition to the reflex regulation of locomotion and the spinal central pattern generator. Stimulation of group I afferents mediating load information from ankle extensors potentiates activity of extensor motoneurons at the stance phase, initiates extension, and terminates or delays flexor bursts in the ipsilateral hind limb (Duysens and Pearson 1980; Conway et al. 1987; Gossard et al. 1994; Guertin et al. 1995; Whelan et al. 1995). Similarly, recordings from Ia inhibitory interneurons during fictive locomotion in complete spinally transected cats showed that hyperpolarization of extensor alpha motoneurons during the swing phase is directly related to their activity (Pratt and Jordan 1987; Degtyarenko et al. 1996; Geertsen et al. 2011), which is determined largely by intraspinal rhythmic processes (Feldman and Orlovsky 1975). Given the important contribution of reciprocal and Ib inhibitory interneuronal circuits to spinal locomotor centers, in this study we sought to determine the function of these circuits in people with spinal cord injury (SCI) after locomotor training.
In uninjured humans, Golgi tendon group Ib afferents evoke short-latency soleus H-reflex depression, which is reduced when the triceps surae is voluntarily activated and the limb is loaded and reverses to excitation during walking (Pierrot-Deseillingny et al. 1982; Stephens and Yang 1996; Faist et al. 2006). Both reciprocal and Ib inhibition are modulated in a phase- and task-dependent manner in uninjured human subjects (Capaday et al. 1990, 1995; Lavoie et al. 1997; Duysens et al. 2000; Ethier et al. 2003; Mummidisetty et al. 2013). In addition to their online modulation to meet motor task demands, these particular spinal circuits can be altered with operant conditioning (Chen et al. 2006), sensory stimulation (Perez et al. 2003), and strength training (Geertsen et al. 2008). Reciprocal and nonreciprocal Ib inhibition are heavily influenced by sensory inputs (Lundberg et al. 1977; Rossi et al. 1988; Knikou 2006; Mummidisetty et al. 2013) and are under descending control (Fournier et al. 1983; Crone 1993; Crone and Nielsen 1994). SCI in humans is associated with pathologic changes of these spinal inhibitory circuits, with alterations in strength, timing, and modulation at rest, during contraction, and during walking being reported (Crone et al. 1994; Boorman et al. 1996; Morita et al. 2001; Knikou and Mummidisetty 2011).
We have recently shown that locomotor training induces plastic changes of flexor and extensor reflexes, presynaptic inhibition of soleus Ia afferents, and soleus H-reflex habituation at rest and during stepping in individuals with SCI (Knikou 2013; Knikou and Mummidisetty 2014; Smith et al. 2014, 2015). Most notable is the reemergence of the soleus H-reflex phase-dependent modulation (Knikou 2013), which can be viewed as a net plasticity of multiple spinal neuronal circuits. Accordingly, the objective of this study was to assess the plastic changes of reciprocal and nonreciprocal Ib inhibition in response to locomotor training in people with SCI. We hypothesized that the pathologic behavior of these two spinal circuits observed in motor incomplete SCI (Knikou and Mummidisetty 2011; Knikou 2012) will be changed following locomotor training. We will show that locomotor training changes actions of Ia and Ib inhibitory interneurons on soleus motoneurons at rest resembling that seen in neurological intact humans and that their modulatory reflex actions are adjusted in a phase-dependent pattern during assisted stepping in both the motor complete and incomplete SCI conditions. These neurophysiologic changes suggest that task-dependent-mediated spinal neuronal reorganization occurs in humans with SCI.
METHODS
Subjects
Sixteen people with chronic SCI participated in the study. Study participation varied depending on the number of locomotor training sessions attended (Table 1) and ranged from 1.5 to 3.5 mo for each subject. Data collection for this study lasted 3 yr. One participant had neurological deficit grade A (no sensory or motor function preserved below the lesion) on the American Spinal Injury Association Impairment Scale (AIS), one had AIS B (sensory but not motor function preserved below the lesion), six had AIS C (more than half muscles below the lesion level had muscle grade <3), and nine had AIS D (at least half key muscles below the lesion level had muscle grade ≥3) (Table 1). The level of SCI ranged from Cervical 3 to Thoracic 10. All participants signed an informed consent form before participation to the study for neurophysiological tests, clinical evaluation, and locomotor training, which was approved by the Northwestern University Institutional Review Board. Subjects' consent was obtained according to the Declaration of Helsinki. Subjects also participated in previous studies (Knikou 2013; Knikou and Mummidisetty 2014; Smith et al. 2014, 2015) and are identified here with the same code. Subjects R09 and R20 did not participate in the Ib inhibition experiment. Data from the reciprocal inhibition experiment from subject R10 were rejected because the unconditioned M waves and H reflexes before and after training had different amplitudes (%Mmax).
Table 1.
Characteristics of participants
| ASIA (motor) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Gender | Age, yr | Postinjury, yr | Level | Cause of SCI | AIS Scale | Clonus | ASIA (light touch) | ASIA (pin prick) | LL | RL | Medication | #Training Sessions |
| R04 | F | 35 | 12 | C3-C4 | MVA | C | 1LL, 1RL | 72 | 72 | 4 | 4 | Not known | 57 |
| R06 | F | 46 | 1.5 | C5-C7 | MVA | B | 3LL, 3RL | 77 | 77 | 0 | 0 | Baclofen: 10 mg not frequent | 53 |
| R07 | M | 31 | 8 | C5-C7 | MVA | A | 3LL, 3RL | 76 | 40 | 0 | 0 | None | 53 |
| R08 | F | 49 | 4 | T5-T7 | Fall | D | 1LL, 1RL | 75 | 75 | 22 | 14 | None during the study | 60 |
| R09 | M | 44 | 3 | C5-C6 | Fall | D | 0LL, 0RL | 112 | 112 | 19 | 24 | Gabapentin: 3.6 g; Diazepam: 15 mg | 30 |
| R10 | F | 52 | 11 | T7 | Fall | D | 1LL, 0RL | 78 | 78 | 16 | 24 | Neurontin: 27 mg; Baclofen: 60 mg | 65 |
| R11 | M | 39 | 6 | C4 | GSW | D | 3LL, 3RL | 106 | 106 | 24 | 22 | Coumadin | 64 |
| R12 | M | 41 | 1.5 | C5-C6 | MVA | D | 3LL, 3RL | 54 | 54 | 19 | 16 | None during the study | 55 |
| R13 | F | 39 | 7 | T4 | Transverse myelitis | C | 3LL, 3RL | 112 | 74 | 9 | 2 | Gabapentin: 0.3 g; Baclofen: 20 mg | 53 |
| R14 | M | 25 | 0.5 | C5-C6 | Diving | D | 1LL, 1RL | 112 | 110 | 25 | 13 | None during the study | 44 |
| R15 | M | 37 | 1.0 | C1 | Spinal Tumor | C | 3LL, 2RL | 64 | 64 | 12 | 5 | Not known | 36 |
| R16 | M | 49 | 2.5 | C5 | MVA | C | 0LL, 0RL | 64 | 34 | 17 | 12 | Baclofen: 15 mg | 41 |
| R17 | M | 21 | 3 | T10 | GSW | D | 3LL, 3RL | 105 | 105 | 13 | 15 | Baclofen: 60 mg; Gabapentin: 50.9 g | 48 |
| R18 | M | 29 | 2 | C7 | MVA | D | 1LL, 1RL | 86 | 86 | 25 | 21 | None | 26 |
| R19 | M | 26 | 1 | C6 | Diving | C | 3LL, 3RL | 112 | 97 | 21 | 8 | Dexapam: 10 mg | 20 |
| R20 | M | 55 | 3 | T6-T7 | Blood clot during spinal surgery | C | 2LL, 3RL | 82 | 82 | 33 | 34 | None | 21 |
Level of spinal cord injury (SCI) corresponds to the neurological injury level. For each subject, the American Spinal Injury Association (ASIA) standard neurological classification of SCI for sensation (sensory light touch and pin prick; out of 112 maximal points) is shown and evaluated as 0 = absent, 1 = impaired, 2 = normal. ASIA motor score (out of 50 maximal points for each leg) is indicated for the left leg (LL) and right leg (RL) based on the manual muscle test of key muscles and evaluated as 0 = no contraction; 1 = flicker or trace of contraction; 2 = active movement, with gravity eliminated; 3 = active movement against gravity; 4 = active movement against gravity and resistance; 5 = normal power. The ankle clonus for both legs leg is also indicated. Ankle clonus was clinically assessed as follows: 0 = no clonus present; 1 = mild, clonus was maintained <3 s; 2 = moderate, clonus persisted between 3 and 10 s; 3 = severe, clonus persisted for >10 s. Medication for each subject is indicated as total milligrams taken per day. C, cervical; T, thoracic; MVA, motor vehicle accident; GSW, gunshot wound; M, male; F, female.
Locomotor Training
Subjects received body weight support (BWS) assisted locomotor training with a robotic exoskeleton system (Lokomat Pro, Hocoma, Switzerland) and were trained 1 h/day, 5 days/wk. The protocol employed to train individuals with SCI and progression of training has been previously published in detail (see Fig. 1 and Pierrot-Deseilligny sec-type="methods"Table 2 in Knikou 2013). Briefly, in AIS A-B subjects, training on the first day started with 60% BWS at 1.58 km/h treadmill speed. At each subsequent session, the treadmill speed was targeted to be adjusted by 0.07 km/h and BWS to decrease by 5%. In AIS C-D subjects, when quadriceps manual muscle test score was ≥3/5, training started with 40% BWS at 1.98 km/h treadmill speed. At each subsequent session, the treadmill speed was targeted to be adjusted by 0.07 km/h and BWS to decrease by 5%. When quadriceps and triceps surae strength was increased by a full grade, then the BWS was decreased by 10%. Treadmill speed and BWS were adjusted differently for each individual over the course of training based on the presence or absence of knee buckling and toe dragging during the stance and swing phases, respectively. In all subjects, the position of the ankle strap was determined based on the tibialis anterior (TA) muscle strength, which was assessed every five training sessions. The ultimate training goal in AIS C-D subjects was to reach a treadmill speed of 2.98 km/h at the lowest BWS possible without the ankle straps.
Fig. 1.
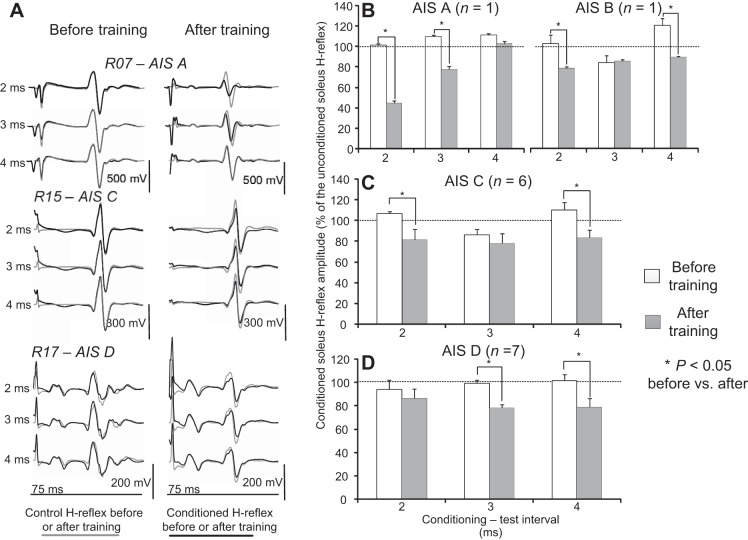
Reciprocal inhibition before and after locomotor training during seated. A: nonrectified waveform averages (n = 20) of conditioned soleus H reflexes (black lines) by common peroneal nerve (CPN) stimulation at 2-, 3-, and 4-ms conditioning-test (C-T) intervals are shown superimposed on the associated unconditioned (grey lines) soleus H reflexes recorded before and after locomotor training. Reflexes are indicated for motor complete [American Spinal Injury Association Impairment Scale A (AIS A) R07] and motor incomplete (AIS C, R15; AIS D, R17) spinal cord injuries (SCIs). B–D: overall amplitude of the conditioned soleus H reflex as a percentage of the unconditioned H reflex recorded in AIS A, B, C, and D subjects at 2-, 3-, and 4-ms C-T intervals before and after training. *Statistically significant differences between the conditioned H reflexes recorded before and after training. Error bars denote the SE.
Peripheral Nerve Stimulation
Posterior tibial nerve.
With subject seated, a stainless steel plate electrode of 4 cm in diameter was placed and secured proximal to the patella (anode electrode). Rectangular single pulse stimuli of 1-ms duration were delivered by a custom-built constant current stimulator to the posterior tibial nerve at the popliteal fossa. A hand-held monopolar stainless steel head electrode (cathode electrode) was used as a probe (Knikou 2008) to locate the optimal stimulation site, at which the M wave had a similar shape to that of the H reflex and the H reflex could be evoked without an M wave at low stimulation intensity levels (Knikou 2008). After the optimal stimulation site was identified, the monopolar electrode was replaced by a pregelled disposable electrode (SureTrace, Conmed, Utica, NY) that was maintained under constant pressure throughout the experiment with an athletic wrap.
Common peroneal nerve.
The common peroneal nerve (CPN) was stimulated by a bipolar stainless steel electrode placed distal to the head of the fibula. The optimal stimulation site corresponded to the one that at increased levels of intensities the peroneus longus muscle was silent, the TA motor threshold (MT) was always lower than that of the peroneus longus muscle, and at increased stimulation intensities selective ankle dorsiflexion without ankle eversion was induced (Knikou 2005, 2008). The CPN was stimulated with a single shock of 1 ms in duration, generated by a constant current stimulator (DS7A, Digitimer, UK). The stimulus to the CPN was delivered at 0.9 to 1.2 TA M-wave threshold across subjects. The TA M wave was monitored throughout the experiment to ensure consistency of the conditioning stimulation. For each subject, the conditioning stimulus after training was delivered at similar multiples of TA M-wave threshold utilized before training.
Medialis gastrocnemius nerve.
The medialis gastrocnemius (MG) nerve was stimulated with a bipolar electrode placed 7–10 cm distal and medial to the cathode electrode for posterior tibial nerve stimulation where a clear contraction of the MG muscle could be evoked (Knikou et al. 2006). The position of the bipolar stimulating electrode was based on the criterion that an M wave or an H reflex in the soleus muscle was not evoked at stimulation intensities above MG motor threshold. The stimulus to the MG nerve was expressed in multiples of MG MT and was delivered at 0.95 × MT to ensure that the conditioning effects were not contaminated by recurrent inhibition (Rossi et al. 1994).
Changes in Postsynaptic Inhibition of Soleus Motoneurons
The neurophysiological tests described below were conducted before training and 2 days after training (both in the morning on separate days). Recordings posttraining were conducted at similar BWS levels, treadmill speeds, and M-wave amplitudes to those utilized before training.
Experiment 1-reciprocal inhibition.
Having established the most optimal stimulation sites, the soleus maximal M wave (Mmax) was evoked with subjects seated and saved for offline analysis. The stimulation intensity was adjusted to evoke control H reflexes that ranged from 20 to 40% of the Mmax (Crone et al. 1990). Twenty reflexes elicited at 0.2 Hz were recorded with subjects seated. Then, the effects of CPN stimulation on the soleus H reflex at the conditioning-test (C-T) intervals of 2, 3, and 4 ms were established. The soleus H-reflex depression at these C-T intervals in uninjured humans is exerted from flexor group Ia afferents on soleus α-motoneurons involving the well described pathway of reciprocal inhibition because the inhibition is strictly between antagonists, is evoked by pure Ia volleys, and is reduced by recurrent inhibition (Pierrot-Desseilligny and Burke 2012). The C-T interval during which the soleus H reflex was depressed, remained unaltered, or was less facilitated with subjects seated was utilized during assisted stepping. The optimal stimulation site was rechecked with subjects during BWS standing, and the TA MT was reestablished. During stepping, CPN stimulation was delivered at 1.1–1.2 × TA MT, because the amount of reciprocal inhibition depends on the conditioning stimulation strength (Petersen et al. 1998, 1999).
Experiment 2-Ib inhibition.
The preparation proceeded in the same fashion as the reciprocal inhibition experiment. The effects of MG nerve stimulation on the soleus H reflex at the C-T intervals of 4, 5, and 6 ms were established with subjects seated (Knikou and Rymer 2002; Knikou 2005). In uninjured human subjects, the soleus H-reflex depression at these C-T intervals is mediated by nonreciprocal Ib inhibition (Bouaziz et al. 1975; Pierrot-Deseilligny et al. 1979). The C-T interval during which the soleus H reflex was most depressed, remained unaltered, or was less facilitated with subjects seated, was utilized during assisted stepping.
Reciprocal and Ib Inhibition During Assisted Stepping
Each subject was transferred to the treadmill and wore an upper body harness that was connected to overhead pulleys. Thigh and shank segments of the exoskeleton were adjusted based on each subject's leg length and diameter, and both feet were secured into the foot lifters. With the subject standing at a BWS similar to that utilized during stepping, the stimulation sites were reevaluated, and the MG and/or TA M-wave MT was reestablished. Then, 80 to 130 stimuli were delivered at 0.2 Hz to assemble the soleus M-wave and H-reflex recruitment curves (Knikou et al. 2009, 2011; Smith et al. 2015).
The orientation of the recording EMG electrode with respect to the underlying muscle fibers changes during walking. The knee joint during the swing phase moves the stimulating electrode away from the tibial nerve, while knee extension during the stance phase has the opposite effect. To counteract these confounding factors, a supramaximal stimulus to the tibial nerve was delivered at 60 ms after the unconditioned or the conditioned H reflex at each bin of the step cycle (Knikou et al. 2009, 2011; Knikou and Mummidisetty 2011, 2014). The customized LabVIEW software measured the peak-to-peak amplitude of the M wave and Mmax recorded during stepping and used a self-teaching algorithm to adjust the stimulus intensity at each bin of the step cycle. Adjustment of stimulation intensity was based on two criteria: the amplitude of the M wave as a percentage of the Mmax that was set to range from 2 to 12% of the Mmax, and the stimulation intensities that evoked H reflexes only on the ascending limb of the recruitment curve (Knikou 2008, 2013; Knikou et al. 2009, 2011). These criteria applied to both conditioned and unconditioned H reflexes, which were randomly recorded during stepping. The experiment was concluded when at least five accepted conditioned and unconditioned H reflexes were recorded at each bin of the step cycle.
During stepping, stimulation was triggered based on the signal from the ipsilateral foot switch (MA153; Motion Lab Systems, Baton Rouge, LA). In all subjects, the step cycle was divided into 16 equal bins. Bin 1 corresponds to heel contact. Bins 8, 9, and 16 correspond approximately to stance-to-swing transition, swing phase initiation, and swing-to-stance transition, respectively. EMG and foot switch signals (Motion Lab Systems) were filtered with a cut-off frequency of 10–1,000 Hz and sampled at 2,000 Hz using a data acquisition card (NI PCI-6225; National Instruments, Austin, TX).
Data Analysis
Soleus M waves, H reflexes, and maximal M waves recorded during seated and stepping were measured as peak-to-peak amplitudes of the nonrectified waveforms. For each subject, the soleus H reflexes recorded following CPN or MG nerve stimulation during seated were expressed as a percentage of the mean amplitude of the unconditioned (or control) H reflex. The mean normalized conditioned soleus H reflex from each subject was grouped based on the C-T interval and time of testing, and two factor repeated-measures ANOVA was used to determine the effects of training across the C-T intervals tested for each AIS group.
The conditioned and unconditioned soleus H reflexes during stepping were expressed as a percentage of the associated maximal M wave evoked at each corresponding bin of the step cycle. This was done separately for all reflexes recorded before and after training. The mean amplitude of the conditioned and unconditioned soleus H reflexes was estimated for each bin of the step cycle and grouped based on time of testing and AIS scale. Three-way repeated-measures ANOVAs (pre- and posttraining, conditioned/unconditioned H reflexes, 16 bins) were performed to determine the effects of training on the amplitude of the H reflexes for each bin of the step cycle and differences between conditioned and unconditioned H reflexes. Post hoc Bonferroni tests were used to test for significant comparisons. This analysis was also performed separately for the M waves of the conditioned and unconditioned H reflexes in AIS C and AIS D subjects before and after training. The percentage of change of the conditioned H reflex after training from the conditioned reflex before training following CPN and/or MG nerve stimulation was estimated, and grouped separately based on the number of locomotor training sessions attended (20–50 and 51–65) and time postinjury (0.5–1 yr, 1.5–3.0 yr, and more than 4.0 yr) for all subjects. A one-way ANOVA was performed to determine whether the percentage of change changed with varying sessions of training or time postinjury.
To determine the net modulation of reciprocal and/or Ib inhibition before and after training during stepping, the unconditioned H reflex at each bin of the step cycle was subtracted from the associated conditioned H reflex (M waves for both reflexes ranged from 2 to 8% of the Mmax), both normalized to the Mmax evoked at each bin (Mummidisetty et al. 2013). This was done to counteract the soleus H-reflex phase-dependent modulation (pathological or reorganized after training) during walking. The mean amplitude of the subtracted conditioned H reflex was grouped across subjects based on the bin number and time of testing, and the overall amplitude was estimated. A resultant positive value indicates a condition associated with decreased inhibition, while a negative value indicates increased inhibition (Mummidisetty et al. 2013). Two factor repeated-measures ANOVA was used to determine changes in the net modulation of reciprocal and Ib inhibition after training during stepping.
The background activity of the ipsilateral soleus, MG, and TA muscles for each bin of the step was calculated from the mean value of the filtered and rectified EMG (band-pass filtered 20–400 Hz) for 50 ms beginning 100 ms before tibial nerve stimulation. The soleus, MG, and/or TA background activity was then normalized to the associated maximal locomotor EMG background activity for each bin of the step cycle and was grouped based on bin number and time of testing. Two factor repeated-measures ANOVA was applied to the data. The mean amplitude of the conditioned soleus H reflexes (normalized to the Mmax evoked at each bin) was plotted on the y-axis (dependent variable) vs. the associated normalized soleus, MG, or TA background activity (independent variable) on the x-axis, respectively. The slope and the y-intercept from the linear least-square regression were estimated for each subject, and changes before and after training were established with a paired t-test. In all statistical tests, significant differences were tested at 95% of confidence level. Results are presented as means ± SE.
RESULTS
Reciprocal Inhibition at Rest and During Stepping After Locomotor Training in SCI
In both motor complete and incomplete SCI, the reciprocal inhibition exerted from ankle flexor Ia afferents on soleus motoneurons was reestablished with locomotor training (Fig. 1A). In all types of spinal injuries, reciprocal facilitation or absent reciprocal inhibition before training was replaced by reciprocal inhibition after training with subjects seated (Fig. 1A). Note that the control soleus H reflexes and M waves have similar amplitude before and after training (Fig. 1A), supporting that the observed changes after training could not be due to conditioning of different types of soleus motoneurons and that stimulation and recording procedures were not different.
In Fig. 1, B–D, the overall amplitude of the conditioned H reflexes for AIS A, B, C, and D subjects recorded in seated before and after training is indicated, respectively. In AIS A subject, the conditioned soleus H reflex was significantly different before and after training at the C-T intervals of 2 and 3 ms (F1,2 = 10.87, P = 0.01; Fig. 1B). In the AIS B subject, a significant change for the conditioned soleus H reflex was found for the C-T intervals of 2 and 4 ms (F1,2 = 11.49, P = 0.001). Similarly, in AIS C subjects, the conditioned soleus H reflex varied significantly with respect to the time of testing (F1 = 6.94, P = 0.015). Reciprocal inhibition increased at the C-T interval of 2 and 4 ms after training compared with that recorded before training (P < 0.05). In AIS D subjects, the conditioned soleus H reflex varied significantly with respect to the time of testing (F1 = 6.93, P = 0.01). Reciprocal inhibition increased at the C-T intervals of 3 and 4 ms after training compared with that recorded before training (P < 0.05). An overall change of −15.29 ± 10.2% and −18.13 ± 6.1% (P = 0.45) on the conditioned H reflex was observed for AIS C and D subjects after training, respectively.
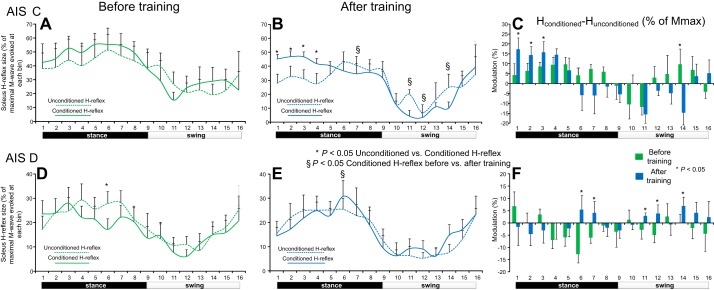
The mean normalized amplitudes of the conditioned and unconditioned soleus H reflexes during assisted stepping before and after locomotor training for AIS C subjects are indicated in Fig. 2, A and B, respectively. Before training, the conditioned soleus H reflex was not significantly different from the unconditioned soleus H reflex across all phases of the step cycle (P > 0.05; Fig. 2A). After training, the conditioned soleus H reflex was larger in amplitude from the unconditioned H reflex at early stance phase (bins 1–4; P < 0.05; Fig. 2B). The conditioned H reflex recorded after training was significantly different from the conditioned soleus H reflex recorded before training at midstance phase (bin 7) and at mid- and late-swing phases (bins 11, 12, and 14; P < 0.05). The net modulation of reciprocal inhibition before and after training in AIS C subjects during assisted stepping is indicated in Fig. 2C. Reciprocal inhibition was decreased at heel contact (bin 1; P < 0.05) and at early stance (bins 2 and 3; P < 0.05) and was increased at late swing (bins 14; P < 0.05) after training compared with that observed before training.
Fig. 2.
Reciprocal inhibition before and after locomotor training during stepping. Mean amplitudes of the conditioned and unconditioned soleus H reflexes recorded before and after locomotor training from AIS C (A and B) and AIS D (D and E) subjects during stepping as a percentage of the associated maximal M wave. *Decreased conditioned H reflexes compared with the unconditioned H reflexes. §Statistically significant differences between the conditioned H reflexes recorded before and after training. C and F: net modulation of reciprocal inhibition with positive values suggesting decreased reciprocal inhibition and negative values suggesting increased reciprocal inhibition. Bin 1 corresponds to heel contact. Bins 8, 9, and 16 correspond approximately to stance-to-swing transition, swing phase initiation, and swing-to-stance transition, respectively. Error bars in all graphs denote the SE.
The mean normalized amplitudes of the conditioned and unconditioned soleus H reflexes during assisted stepping for AIS D subjects before and after training are indicated in Fig. 2, D and E, respectively. Before training, the conditioned soleus H reflex was smaller at midstance (bin 6; P < 0.05; Fig. 2D) compared with the unconditioned soleus H reflex. After training, the conditioned soleus H reflex was not significantly different from the unconditioned soleus H reflex across all phases of the step cycle (P > 0.05; Fig. 2E). The conditioned H reflex recorded after training was significantly different from the conditioned soleus H reflex recorded before training at midstance phase (bin 6; P < 0.05). The net modulation of reciprocal inhibition in AIS D subjects before and after training during assisted stepping is indicated in Fig. 2F. Reciprocal inhibition was decreased at late stance phase (bins 6 and 7; P < 0.05) and during the swing phase (bins 11, 12, and 14; P < 0.05) after training compared with that observed before training (Fig. 2F), suggesting for a different reorganization pattern of reciprocal inhibition during walking in individuals with AIS C and AIS D (compare Fig. 2, C and 2F).
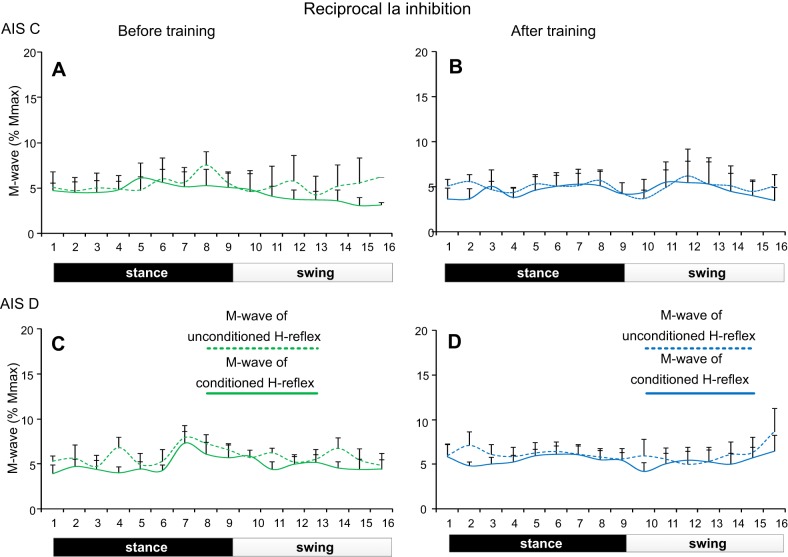
Changes in reciprocal Ia inhibition during stepping were observed at similar amplitudes of M waves before and after training in all subjects (Fig. 3). For AIS C subjects, the M-wave amplitudes of the conditioned and unconditioned soleus H reflexes did not vary across bins (F15 = 0.29, P = 0.99) or time of testing (F1 = 0.1, P = 0.74). A significant interaction for the normalized M waves among bins, time of testing, and type of H reflexes was not found (F1,15 = 0.13, P = 1.0; Fig. 3, A and B). Similar results were also found for the soleus M wave in AIS D subjects during stepping (across bins: F15 = 0.55, P = 0.91; time of testing: F1 = 0.52, P = 0.47; Fig. 3, C and D).
Fig. 3.
Soleus M waves during stepping before and after training in SCI. Normalized M-wave amplitudes (as a percentage of the maximal M wave) recorded during stepping before and after locomotor training for the unconditioned soleus H reflex and following common peroneal nerve stimulation (reciprocal Ia inhibition) in AIS C (A and B) and in AIS D (C and D) subjects. For all cases, the M waves did not change across bins or time of testing.
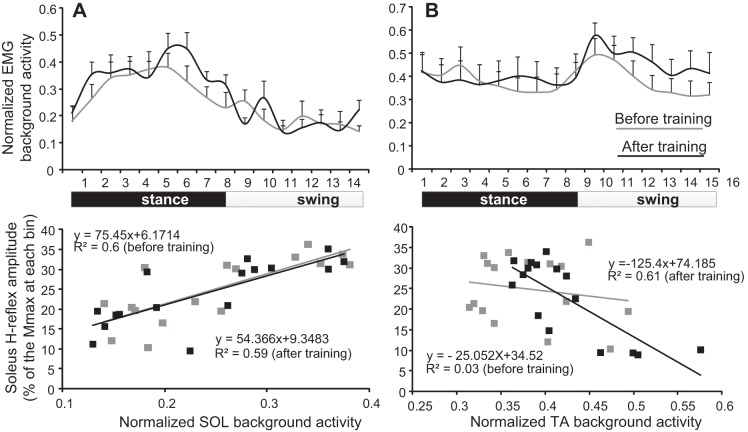
The normalized soleus background activity along with the linear regression between the soleus and TA background activity and the associated conditioned H reflexes from all AIS C and D subjects before and after training is indicated in Fig. 4. The normalized soleus EMG background activity was similar before and after training (F1 = 2.48, P = 0.11, two-factor ANOVA; Fig. 4A, top). Linear regression analysis between the soleus background activity and the conditioned H reflex from each AIS C and D subject showed that the y-intercept reached overall amplitudes of 10.21 ± 3.17 and −3.3 ± 10.42 (P = 0.11), and the slope reached overall amplitudes of 51.93 ± 12.07 and 55.99 ± 17.37 (P = 0.42) before and after training, respectively. The normalized TA EMG background activity from all AIS C and D subjects was also similar before and after training (two-factor ANOVA, F1 = 2.02, P = 0.15; Fig. 4B, top). Linear regression analysis between the TA background activity and the conditioned H reflex from each AIS C and D subject showed that the y-intercept reached overall amplitudes of 12.18 ± 8.18 and 17.17 ± 13.29 (P = 0.37), while the slope reached overall amplitudes of 28.15 ± 27.32 and −8.54 ± 22.03 (P = 0.15) before and after training, respectively.
Fig. 4.
Relationship between conditioned soleus H reflex and background EMG activity. A: normalized soleus background EMG activity (top) along with the mean normalized soleus EMG background activity plotted against the conditioned soleus (SOL) H reflex recorded at each bin of the step cycle (bottom) before and after locomotor training. B: normalized tibialis anterior (TA) background EMG activity (top) along with the mean normalized TA EMG background activity plotted against the conditioned soleus H reflex recorded at each bin of the step cycle (bottom) before and after locomotor training. For both graphs at the bottom, the 16 points correspond to the 16 bins of the step cycle.
Ib Inhibition at Rest and During Stepping After Locomotor Training in SCI
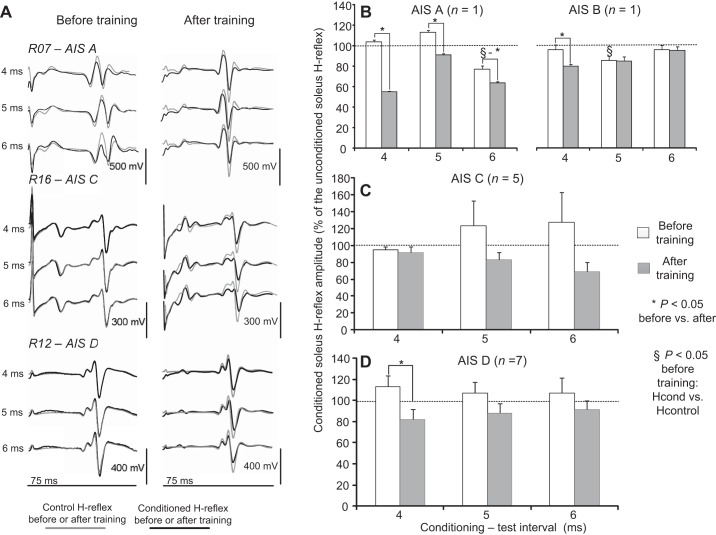
Locomotor training reestablished or potentiated the MG-mediated short-latency soleus H-reflex depression during seated in motor complete and incomplete SCI (Fig. 5A). Ib inhibition was evident in R07 (AIS A) but was absent in R16 (AIS C) and R12 (AIS D) before training (Fig. 5A) and was potentiated in all of them after training while seated (Fig. 5A).
Fig. 5.
Ib inhibition before and after locomotor training during seated. A: nonrectified waveform averages (n = 20) of conditioned soleus H reflexes (black lines) by medialis gastrocnemius (MG) nerve stimulation at 4-, 5-, and 6-ms C-T intervals are shown superimposed on the associated unconditioned (grey lines) soleus H reflexes recorded before and after locomotor training. Reflexes are indicated for motor complete (AIS A, R07) and motor incomplete (AIS C, R16; AIS D, R12) SCIs. B–D: overall amplitude of the conditioned soleus H reflex as a percentage of the unconditioned H reflex recorded in AIS A, B, C, and D subjects at 4-, 5-, and 6-ms C-T intervals before and after training. *Statistically significant differences between the conditioned H reflexes recorded before and after training. §Statistically significant differences between the conditioned and the unconditioned H reflexes recorded before training. Error bars denote the SE.
In Fig. 5, B–D, the overall amplitude of the conditioned H reflexes for AIS A, B, C, and D subjects recorded in seated before and after training is indicated, respectively. In the AIS A subject, the conditioned H reflex was significantly different before and after training at all C-T intervals tested (F1 = 487.06, P < 0.001), while the conditioned H reflex before training was reduced from control H-reflex values at the C-T interval of 6 ms (F3,71 = 33.29, P < 0.001; Fig. 5B). In the AIS B subject, the conditioned H reflex was significantly different before and after training at the C-T interval of 4 ms (F1 = 5.8, P = 0.018; Fig. 5B), while again at the C-T interval of 5 ms the conditioned H reflex before training was reduced from control H-reflex values (F2 = 7.12, P = 0.02; Fig. 5B), suggesting the presence of Ib inhibition in motor complete SCI.
In motor incomplete AIS C subjects, although there was a trend for Ib excitation to be replaced by Ib inhibition after training (Fig. 5C), a significant difference for the conditioned soleus H reflexes as a function of time of testing (F1,2 = 3.58, P = 0.07) or C-T interval tested (F1,2 = 0.09, P = 0.9) was not found. Ib inhibition was not present only in one AIS C subject (R15) after training, while in the remaining AIS C subjects an overall change of −26.72 ± 20.8% on the conditioned H reflex was observed. Ib facilitation before training was replaced by Ib inhibition after training at the C-T interval of 4 ms in AIS D subjects (F1 = 6.23, P = 0.018; Fig. 5D), and an overall change of −14.9 ± 7.4% on the conditioned H reflex was observed. When data from both AIS C and D subjects were taken into consideration, the conditioned soleus H reflex was decreased after training compared with that observed before training at all C-T intervals tested (F1 = 10.28, P = 0.002).
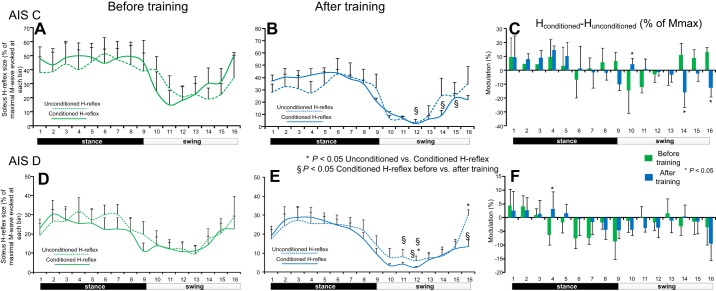
The mean normalized conditioned and unconditioned H reflexes during assisted stepping before and after training for AIS C subjects are indicated in Fig. 6, A and B, respectively. The conditioned soleus H reflex was not significantly different from the unconditioned soleus H reflex across all phases of the step cycle before and after training (P > 0.05; Fig. 6, A and B). The conditioned soleus H reflex after training was reduced compared with the conditioned soleus H reflex before training at mid- and late-swing phases (bins 12, 14, and 16; P < 0.05; Fig. 6B). The net modulation of Ib inhibition in AIS C subjects during stepping before and after training is indicated in Fig. 6C. Ib inhibition was decreased at early swing phase (bin 10) and increased at late swing phase (bin 14) and during the swing to-stance transition phase (bin 16) after training compared with that observed before training (Fig. 6C).
Fig. 6.
Ib inhibition before and after locomotor training during stepping. The mean amplitudes of the conditioned and unconditioned soleus H reflexes recorded before and after locomotor training from the right leg of AIS C (A and B) and AIS D (D and E) subjects during walking are shown as a percentage of the associated maximal M wave. *Decreased conditioned H reflexes compared with the unconditioned H reflexes. §Statistically significant differences between the conditioned H reflexes recorded before and after training. C and F: net modulation of Ib inhibition with positive values suggesting decreased Ib inhibition and negative values suggesting increased Ib inhibition. Bins 8, 9, and 16 correspond approximately to stance-to-swing transition, swing phase initiation, and swing-to-stance transition, respectively. Error bars in all graphs denote the SE.
The mean normalized conditioned and unconditioned soleus H reflexes in AIS D subjects during assisted stepping before and after training are indicated in Fig. 6, D and E, respectively. Before training, the conditioned soleus H reflex was not different from the unconditioned soleus H reflex across all phases of the step cycle (P > 0.05; Fig. 6D). After training, the conditioned soleus H reflex was decreased at midswing and at swing-to-stance transition (bins 12 and 16) compared with the unconditioned soleus H reflex (P < 0.05; Fig. 6E). Furthermore, the conditioned H reflex after training was decreased compared with the conditioned soleus H reflex before training at mid- and late-swing phases (bins 10, 11, 12, and 16; P < 0.05). The net modulation of Ib inhibition in AIS D subjects during stepping before and after training is indicated in Fig. 6F. Ib inhibition was increased at the early stance phase (bin 4; P < 0.05) after training compared with that observed before training and remained unaltered during the swing phase (Fig. 6F).
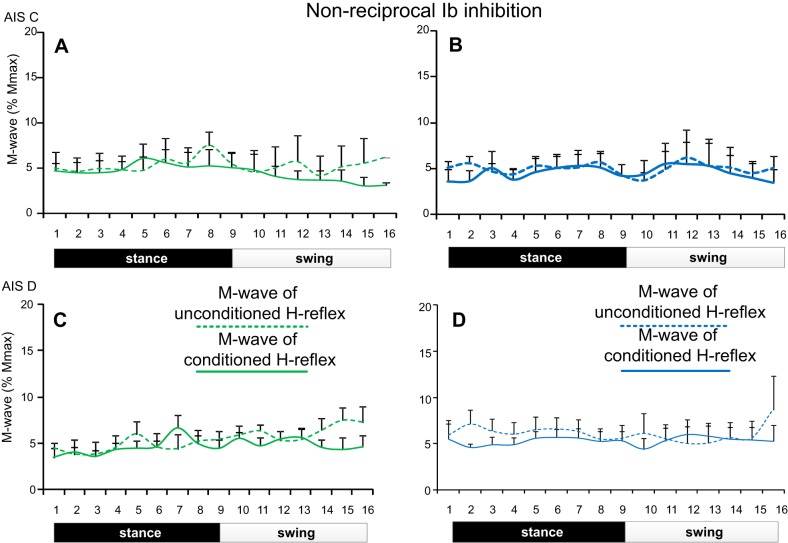
Changes in nonreciprocal Ib inhibition during stepping were observed at similar amplitudes of M waves before and after training in all subjects (Fig. 7). For AIS C subjects, the M waves of the conditioned and unconditioned soleus H reflexes were not significantly different across the step cycle (F15 = 0.29, P = 0.99) and time of testing (F1 = 0.09, P = 0.76). A significant interaction for the M waves among bins, time of testing, and type of H reflexes was not found (F1,15 = 0.13, P = 1.0). Similar results were also found for the M waves in AIS D subjects (across bins: F15 = 0.36, P = 0.98; time of testing: F1 = 0.31, P = 0.57; Fig. 7C, D). A significant interaction for the M-wave values among bins, time of testing, and type of H reflexes was not found (F1,15 = 0.31, P = 0.99).
Fig. 7.
Soleus M waves during stepping before and after training in SCI. Normalized M-wave amplitudes (as a percentage of the maximal M wave) recorded during stepping before and after locomotor training for the unconditioned soleus H reflex and following medial gastrocnemius nerve stimulation (nonreciprocal Ib inhibition) in AIS C (A and B) and in AIS D (C and D) subjects. For all cases, the M waves did not change across bins or time of testing.
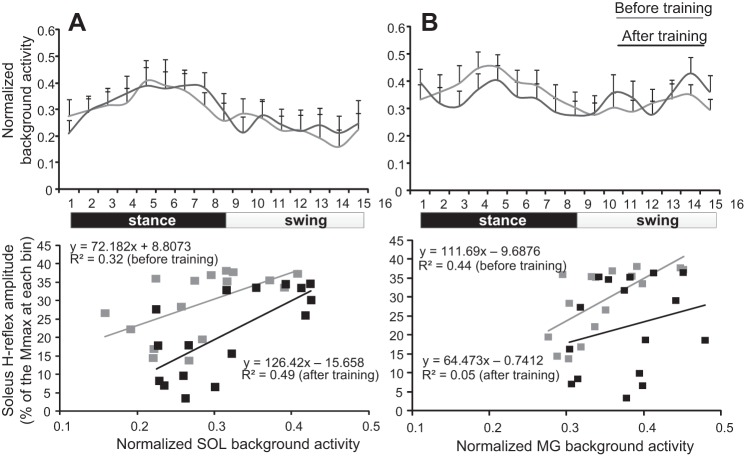
The normalized soleus EMG background activity along with the linear regression between the SOL and MG background activity and the associated conditioned H reflex from all AIS C and D subjects before and after training is indicated in Fig. 8. The normalized soleus EMG background activity was not significantly different before and after training (F1 = 0.15, P = 0.69; Fig. 8A, top). The y-intercept reached overall amplitudes of 27.55 ± 7.26 and 9.74 ± 3.65 (paired t-test, P = 0.02), and the slope reached overall amplitudes of 20.88 ± 25.19 and 31.56 ± 18.6 (paired t-test, P = 0.36) before and after training, respectively. The normalized MG background activity during walking from all AIS C and D subjects was also not significantly different before and after training (F1 = 0.14, P = 0.7; Fig. 8B, top). Linear regression analysis between the overall amplitudes of MG background activity and the conditioned H reflex from each AIS C and D subject showed that the y-intercept reached overall amplitudes of 19.92 ± 12.38 and 15.13 ± 8.19 (P = 0.37), while the slope reached overall amplitudes of 21.63 ± 21.45 and 20.19 ± 17.6 (P = 0.47) before and after training, respectively.
Fig. 8.
Relationship between conditioned soleus H reflex and background EMG activity. A: normalized soleus background EMG activity (top) along with the mean normalized soleus background EMG activity plotted against the conditioned soleus H reflex for each bin of the step cycle (bottom) before and after locomotor training. B: normalized MG background EMG activity (top) along with the mean normalized MG background EMG activity plotted against the conditioned soleus H reflex for each bin of the step cycle (bottom) before and after locomotor training. For both graphs at the bottom, the 16 points correspond to the 16 bins of the step cycle.
Changes in Magnitude of Spinal Inhibition as a Function of Locomotor Sessions and Time Postinjury
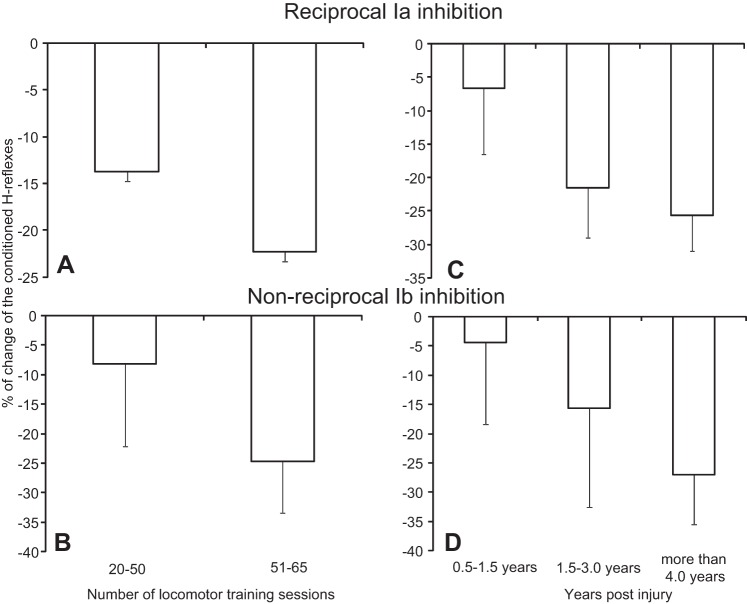
The magnitude of reciprocal Ia inhibition (Fig. 9A) and nonreciprocal Ib inhibition (Fig. 9B) during seated did not alter when locomotor training sessions attended ranged from 20 to 50 or from 51 to 65 (reciprocal inhibition: F = 2.24, P = 0.14; Ib inhibition: F = 0.43, P = 0.51). A similar result was also found for the magnitude of spinal inhibition based on time postinjury (Fig. 9, C and D; reciprocal inhibition: F = 3.03, P = 0.06; Ib inhibition: F = 1.05, P = 0.36).
Fig. 9.
Magnitude of spinal postsynaptic inhibition. A and B: percentage of change of the conditioned H reflex during seated from all subjects after training, reflecting the magnitude of reciprocal and nonreciprocal inhibition, is plotted against the number of locomotor training sessions attended. C and D: percentage of change of the conditioned H reflex during seated from all subjects after training, reflecting the magnitude of reciprocal and nonreciprocal inhibition, is plotted against the years postinjury.
DISCUSSION
This is the first report on plastic changes of reciprocal Ia and nonreciprocal Ib inhibition after locomotor training in people with motor complete or incomplete SCI. One of the most important findings was that locomotor training reversed reciprocal facilitation to reciprocal inhibition during seated regardless of the sensorimotor capacity before training (Fig. 1). Similarly, Ib inhibition returned in 13 out of 14 subjects during seated (Fig. 5) and increased at similar rates to that of reciprocal Ia inhibition. It should be noted that Ib inhibition was present in motor complete SCI before training during seated (Fig. 5B), a finding consistent with the physiologic behavior of force-sensitive Ib afferents in untrained complete spinal cord transected animal preparations (Conway et al. 1987; Pearson and Collins 1993).
During stepping, reorganization of postsynaptic control of soleus motoneurons occurred in a phase-dependent manner. Reciprocal inhibition was decreased in AIS C at early stance (bins 1–3) and in AIS D at late stance (bins 6 and 7) after locomotor training (Fig. 2, C and D). In uninjured subjects, cocontraction, which decreases reciprocal inhibition (Nielsen and Kagamihara 1992), of ankle flexors and extensors at early stance is needed to stabilize the ankle and ensure a physiological step progression (Misiaszek et al. 2000). The reduced reciprocal inhibition at early stance in AIS C is thus functional and consistent with observations during walking in uninjured humans (Petersen et al. 1999; Mummidisetty et al. 2013). Furthermore, reciprocal inhibition was increased at midswing in AIS C (Fig. 2C) but was decreased after training at midswing in AIS D (Fig. 2F). These findings suggest that reciprocal inhibition during stepping recovers at a greater level in AIS C than in AIS D with training, and that for both subject groups, the modulation pattern was not the same compared with that we reported under similar experimental procedures in healthy control subjects (i.e., reciprocal Ia inhibition was increased at stance-to-swing transition and throughout the swing phase) (Mummidisetty et al. 2013). Decreased levels of reciprocal inhibition in AIS D subjects are depicted by the absent full soleus H-reflex depression after training (Fig. 2C in Knikou 2013) and absent modulation of presynaptic inhibition of Ia afferents (Fig. 2F in Knikou and Mummidisetty 2014) during the swing phase in these patients.
Based on the amplitude of the unconditioned and conditioned soleus H reflexes before and after training by MG nerve stimulation, Ib inhibition was absent during the stance phase in both AIS C and D (Fig. 6, B and E) and was decreased at early stance after training in AIS D (Fig. 6F). These findings are consistent with the reduced short-latency group I inhibition of synergists at the stance phase of walking in healthy humans and during fictive locomotion in spinal animals (McCrea et al. 1995; Stephens and Yang 1996) and the reduced Ib inhibition during a rhythmic loading-unloading motor task in healthy humans (Faist et al. 2006). Furthermore, Ib inhibition in AIS C was decreased at early swing and increased at late swing (Fig. 6C), suggesting that synergistic group I afferents may contribute to the soleus H-reflex depression during the swing phase, supported by previous findings reported for untrained SCI subjects (Knikou 2012). Last, cyclic disinhibition of group Ib excitatory spinal interneurons was weak compared with that observed in animals (Angel et al. 1996), since locomotor training did not induce an extra facilitation of soleus motoneuron responses by group Ib afferents during the stance phase (Fig. 6, B and E).
The decreased reciprocal inhibition during the swing phase in AIS D (Fig. 2F) and the weak Ib excitation during the stance phase in AIS C and D (Fig. 6, C and F) may depend on the number of training sessions, the number of steps per session (de Leon et al. 2011), or even the BWS since loading can affect the net EMG output (Dietz et al. 2002). The BWS during training was adjusted separately for each participant based on his/her ability to step without knee buckling or toe dragging, and thus quantification of BWS effects is not possible. However, it should be noted that reciprocal inhibition at 27% BWS is still modulated in a physiologic pattern in uninjured subjects (Mummidisetty et al. 2013). Furthermore, the magnitude of reciprocal Ia and nonreciprocal Ib inhibition was not affected by the number of training sessions (Fig. 9, A and B) with subjects seated. However, the number of training sessions may affect differently the strength of postsynaptic inhibition during stepping.
The neuroplastic changes described above occurred at similar levels of soleus and MG motoneuron gain before and after training under control conditions and reflex conditioning during stepping (Figs. 4 and 8). The linear relationship between the conditioned soleus H-reflex and soleus background activity suggests that reflex actions of MG group I afferents on soleus alpha motoneurons follow the soleus background excitability pattern. However, because Ib inhibition is not affected by triceps surae contraction (Pierrot-Deseilligny et al. 1982), and reciprocal inhibition is not abolished following soleus muscle voluntary contraction (Yang and Whelan 1993), although it is modulated during ankle movement (Shindo et al. 1984), net changes of soleus motoneuron excitability and duration of hyperpolarization cannot account solely for the observed changes.
Possible Mechanisms for Plasticity of Postsynaptic Inhibition Posttraining
SCI in humans is characterized by extensive pathologic reorganization of spinal neuronal circuits that gate sensory afferent feedback and regulate amplitude and periodicity of motoneuron discharges (Knikou 2010a; Tansey et al. 2012). Reciprocal inhibition is replaced by facilitation (Crone et al. 1994; Morita et al. 2001; Okuma et al. 2002), attributed mostly to altered cortical control of Ia inhibitory interneurons (Nielsen et al. 1995). Findings on Ib inhibition are conflicting, with both physiologic and pathologic behaviors being reported (Downes et al. 1995; Morita et al. 2006; Knikou 2012).
Because both reciprocal and Ib inhibition were potentiated after training in complete SCI, direct descending inputs on spinal inhibitory interneurons may not be a key source for neuroplasticity but may be required for long-term support of inhibitory synaptic transmission and regulation of the depth of spinal inhibition. This is supported by the ability of the spinal cord after complete spinalization to undergo functional reorganization with locomotor training (Rossignol 2006) and that the plasticity of the glycinergic system, which mediates inhibitory neurotransmission, can occur independent of supraspinal influences (Sadlaoud et al. 2010). Locomotor training normalizes the proportion of inhibitory and excitatory synaptic inputs to spinal motoneurons (Ichiyama et al. 2011), improves synaptic inputs from Ia afferents (Petruska et al. 2007), alters the concentration levels of Na+-K+-ATPase (Ilha et al. 2011), and reverses disynaptic inhibition to polysynaptic excitation (Côté et al. 2003) in animals. We have recently shown that locomotor training changes the behavior of short- and long-latency flexion reflexes, reestablishes a physiologic soleus H-reflex phase-dependent modulation, increases presynaptic inhibitory control of soleus motoneurons, modifies excitability properties of soleus motoneurons, changes cocontraction levels between knee and ankle antagonistic muscles, and promotes interlimb and intralimb EMG coordination in the same people with SCI tested here (Knikou 2013; Knikou and Mummidisetty 2014; Smith et al. 2014, 2015). Because the modulation of soleus H-reflex inhibition by presynaptic inhibition after training (Knikou and Mummidisetty 2014) was completely different from the soleus H-reflex postsynaptic inhibition in the same patients tested in this study, the changes observed after locomotor training can be attributed to changes in the intrinsic properties, function, and synaptic inputs of Ia and Ib inhibitory interneurons. This is supported by the altered dorsal column-mediated synaptic activity after exercise training (Rank et al. 2015). Additional sources for changes in modulation of reciprocal Ia and nonreciprocal Ib inhibition after training include changes in the tonic presynaptic regulation of synaptic transmission from soleus group Ia and Ib afferents to motoneurons and Ia/Ib interneurons (Gossard 1996; Gosgnach et al. 2000) and altered tonic presynaptic regulation of Ia and Ib interneurons terminals (Enriquez-Denton et al. 2000). The central pattern generator may have changed the strength of inhibitory interneurons synaptic effect, driven in part by sensory afferent feedback from load and muscle stretch receptors (Knikou 2006, 2010b). Last, because Ia/Ib interneurons are susceptible to inputs from several descending motor pathways, we cannot ignore the possibility that in motor incomplete SCIs changes in corticospinal descending synaptic drive including the propriospinal system might have contributed to the observed spinal neuronal and network changes.
Limitations
A limitation of this study is that data were acquired from one person with AIS A and one person with AIS B, while the sample sizes for AIS C and D subjects were small. Neurophysiological research studies in a larger number of patients receiving locomotor training are needed to further delineate the neurophysiological mechanisms of locomotor training mediated plasticity in people with SCI. Patients who did not receive locomotor training were not tested in this study. However, it is unlikely that the observed changes were due to spontaneous neuronal plasticity. Spontaneous plasticity is mostly observed within the first 9 mo postinjury (Fawcett et al. 2007), and time postinjury was less than 1 yr only in 3 of 16 patients tested here. Furthermore, the BWS needed at the end of training was decreased by as much as 280 ± 66.6% and TA EMG activity for example increased by as much as 243% in the left leg and by 142% in the right leg (Knikou 2013; Knikou and Mummidisetty 2014), suggesting that stepping capabilities and full body-weight bearing changed beyond that expected by spontaneous plasticity (de Leon et al. 1998).
Conclusion
We describe here, for the first time reported in the literature, changes in the postsynaptic control of soleus motoneurons from antagonistic Ia and synergistic Ib afferents in people with chronic SCI after locomotor training. Our current and previous findings from the same patients (Knikou 2013; Knikou and Mummidisetty 2014; Smith et al. 2014, 2015) provide a comprehensive investigation on neurophysiological mechanisms underlying locomotor training in people with SCI. Task-dependent adaptation of neuronal and network excitability demonstrates that neuromodulation can express functional neuroplasticity and may be used as a biomarker of rehabilitation efforts and recovery of motor dysfunction. However, more studies are needed to establish to what extent neurophysiologic measures of activity-dependent neuroplasticity can predict recovery of motor function.
GRANTS
This work was supported by the New York State Department of Health, Wadsworth Center, Spinal Cord Injury Research Board Grant C023690 and The Craig Neilsen Foundation Grant 83607 (to M. Knikou). A. C. Smith is supported by National Institute of Child Health and Human Development Grant T32-HD-057845 and by the Foundation for Physical Therapy Promotion of Doctoral Studies.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.K. conception and design of research; M.K., A.C.S., and C.K.M. performed experiments; M.K. analyzed data; M.K. interpreted results of experiments; M.K. and C.K.M. prepared figures; M.K. drafted manuscript; M.K. and A.C.S. edited and revised manuscript; M.K., A.C.S., and C.K.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the research participants for participating in numerous experiments and training sessions and William Zev Rymer for support.
REFERENCES
- Angel MJ, Guertin P, Jiménez I, McCrea DA. Group I extensor afferents evoke disynaptic EPSPs in cat hindlimb extensor motorneurones during fictive locomotion. J Physiol 494: 851–861, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorman G, Lee R, Becker W, Windhorst U. Impaired natural reciprocal inhibition in patients with spasticity due to incomplete spinal cord injury. Electroencephalogr Clin Neurophysiol 101: 84–92, 1996. [DOI] [PubMed] [Google Scholar]
- Bouaziz Z, Bouaziz M, Hugon M. Modulation of soleus electromyogram during electrical stimulation of medial gastrocnemius nerve in man. Electroencephalogr Clin Neurophysiol 15: 31–42, 1975. [PubMed] [Google Scholar]
- Capaday C, Cody F, Stein R. Reciprocal inhibition of soleus motor output in humans during walking and voluntary tonic activity. J Neurophysiol 64: 607–616, 1990. [DOI] [PubMed] [Google Scholar]
- Capaday C, Lavoie BA, Cormeau F. Differential effects of a flexor nerve input on the soleus H-reflex during standing and walking. Can J Physiol Pharmacol 73: 436–449, 1995. [DOI] [PubMed] [Google Scholar]
- Chen XY, Chen L, Chen Y, Wolpaw JR. Operant conditioning of reciprocal inhibition in rat soleus muscle. J Neurophysiol 96: 2144–2150, 2006. [DOI] [PubMed] [Google Scholar]
- Conway BA, Hultborn H, Kiehn O. Proprioceptive input resets central locomotor rhythm in the spinal cat. Exp Brain Res 68: 643–656, 1987. [DOI] [PubMed] [Google Scholar]
- Côté MP, Ménard A, Gossard JP. Spinal cats on the treadmill: changes in load pathways. J Neurosci 23: 2789–2796, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C. Reciprocal inhibition in man. Dan Med Bull 40: 571–581, 1993. [PubMed] [Google Scholar]
- Crone C, Nielsen J. Central control of disynaptic reciprocal inhibition in humans. Acta Physiol Scand 152: 351–363, 1994. [DOI] [PubMed] [Google Scholar]
- Crone C, Nielsen J, Petersen N, Ballegaard M, Hultborn H. Disynaptic reciprocal inhibition of ankle extensors in spastic patients. Brain 117: 1161–1168, 1994. [DOI] [PubMed] [Google Scholar]
- Crone C, Hultborn H, Mazières L, Morin C, Nielsen J, Pierrot-Deseilligny E. Sensitivity of monosynaptic test reflexes to facilitation and inhibition as a function of the test reflex size: a study in man and the cat. Exp Brain Res 81: 35–45, 1990. [DOI] [PubMed] [Google Scholar]
- de Leon RD, Hodgson JA, Roy RR, Edgerton VR. Locomotor capacity attributable to step training versus spontaneous recovery after spinalization in adult cats. J Neurophysiol 79: 1329–1340, 1998. [DOI] [PubMed] [Google Scholar]
- de Leon RD, See PA, Chow CH. Differential effects of low versus high amounts of weight supported treadmill training in spinally transected rats. J Neurotrauma 28: 1021–1033, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degtyarenko AM, Simon ES, Burke RE. Differential modulation of disynaptic cutaneous inhibition and excitation in ankle flexor motoneurons during fictive locomotion. J Neurophysiol 76: 2972–2985, 1996. [DOI] [PubMed] [Google Scholar]
- Dietz V, Müller R, Colombo G. Locomotor activity in spinal man: significance of afferent input from joint and load receptors. Brain 125: 2626–2634, 2002. [DOI] [PubMed] [Google Scholar]
- Downes L, Ashby P, Bugaresti J. Reflex effects from Golgi tendon organ (Ib) afferents are unchanged after spinal cord lesions in humans. Neurology 45: 1720–1724, 1995. [DOI] [PubMed] [Google Scholar]
- Duysens J, Clarac F, Cruse H. Load-regulating mechanisms in gait and posture: comparative aspects. Physiol Rev 80: 83–133, 2000. [DOI] [PubMed] [Google Scholar]
- Duysens J, Pearson KG. Inhibition of flexor burst generation by loading ankle extensor muscles in walking cats. Brain Res 187: 321–332, 1980. [DOI] [PubMed] [Google Scholar]
- Enríquez-Denton M, Nielsen J, Perreault MC, Morita H, Petersen N, Hultborn H. Presynaptic control of transmission along the pathway mediating disynaptic reciprocal inhibition in the cat. J Physiol 526: 623–637, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethier C, Imbeualt MA, Ung V, Capaday C. On the soleus H-reflex modulation pattern during walking. Exp Brain Res 151: 420–425, 2003. [DOI] [PubMed] [Google Scholar]
- Faist M, Hoefer C, Hodapp M, Dietz V, Berger W, Duysens J. In humans Ib facilitation depends on locomotion while suppression of Ib inhibition requires loading. Brain Res 1076: 87–92, 2006. [DOI] [PubMed] [Google Scholar]
- Fawcett JW, Curt A, Steeves JD, Coleman WP, Tuszynski MH, Lammertse D, Bartlett PF, Blight AR, Dietz V, Ditunno J, Dobkin BH, Havton LA, Ellaway PH, Fehlings MG, Privat A, Grossman R, Guest JD, Kleitman N, Nakamura M, Gaviria M, Short D. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord 45: 190–205, 2007. [DOI] [PubMed] [Google Scholar]
- Feldman AG, Orlovsky GN. Activity of interneurons mediating reciprocal Ia inhibition during locomotion. Brain Res 84: 181–194, 1975. [DOI] [PubMed] [Google Scholar]
- Fournier E, Katz R, Pierrot-Deseilligny E. Descending control of reflex pathways in the production of voluntary isolated movements in man. Brain Res 288: 375–377, 1983. [DOI] [PubMed] [Google Scholar]
- Geertsen SS, Lundbye-Jensen J, Nielsen JB. Increased central facilitation of antagonist reciprocal inhibition at the onset of dorsiflexion following explosive strength training. J Appl Physiol 105: 915–922, 2008. [DOI] [PubMed] [Google Scholar]
- Geertsen SS, Stecina K, Meehan CF, Nielsen JB, Hultborn H. Reciprocal Ia inhibition contributes to motoneuronal hyperpolarisation during the inactive phase of locomotion and scratching in the cat. J Physiol 589: 119–134, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosgnach S, Quevedo J, Fedirchuk B, McCrea DA. Depression of group Ia monosynaptic EPSPs in cat hindlimb motoneurones during fictive locomotion. J Physiol 526: 639–652, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossard JP. Control of transmission in muscle group IA afferents during fictive locomotion in the cat. J Neurophysiol 76: 4104–4112, 1996. [DOI] [PubMed] [Google Scholar]
- Gossard JP, Brownstone RM, Barajon I, Hultborn H. Transmission in a locomotor related group Ib pathway from hindlimb extensor muscles in the cat. Exp Brain Res 98: 213–228, 1994. [DOI] [PubMed] [Google Scholar]
- Guertin P, Angel MJ, Perreault MC, McCrea DA. Ankle extensor group I afferents excite extensors throughout the hindlimb during fictive locomotion in the cat. J Physiol 487: 197–209, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiyama RM, Broman J, Roy RR, Zhong H, Edgerton VR, Havton LA. Locomotor training maintains normal inhibitory influence on both alpha- and gamma-motoneurons after neonatal spinal cord transection. J Neurosci 31: 26–33, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilha J, Centenaro L, Broetto Cunha N, de Souza DF, Jaeger M, do Nascimento PS, Kolling J, Ben J, Marcuzzo S, Wyse AT, Gottfried C, Achaval M. The beneficial effects of treadmill step training on activity-dependent synaptic and cellular plasticity markers after complete spinal cord injury. Neurochem Res 36: 1046–1055, 2011. [DOI] [PubMed] [Google Scholar]
- Knikou M. Effects of hip joint angle changes on intersegmental spinal coupling in human spinal cord injury. Exp Brain Res 167: 381–393, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knikou M. Effects of changes in hip position on actions of spinal inhibitory interneurons in humans. Int J Neurosci 116: 945–961, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knikou M. The H-reflex as a probe: pathways and pitfalls. J Neurosci Methods 171: 1–12, 2008. [DOI] [PubMed] [Google Scholar]
- Knikou M. Neural control of locomotion and training-induced plasticity after spinal and cerebral lesions. Clin Neurophysiol 121: 1655–1668, 2010a. [DOI] [PubMed] [Google Scholar]
- Knikou M. Plantar cutaneous afferents normalize the reflex modulation patterns during stepping in chronic human spinal cord injury. J Neurophysiol 103: 1304–1314, 2010b. [DOI] [PubMed] [Google Scholar]
- Knikou M. Function of group Ib inhibition during assisted stepping in human spinal cord injury. J Clin Neurophysiol 29: 271–277, 2012. [DOI] [PubMed] [Google Scholar]
- Knikou M. Functional reorganization of soleus H-reflex modulation during stepping after robotic-assisted step training in people with complete and incomplete spinal cord injury. Exp Brain Res 228: 279–296, 2013. [DOI] [PubMed] [Google Scholar]
- Knikou M, Angeli CA, Ferreira CK, Harkema SJ. Soleus H-reflex modulation during body weight support treadmill walking in spinal cord intact and injured subjects. Exp Brain Res 193: 397–407, 2009. [DOI] [PubMed] [Google Scholar]
- Knikou M, Chaudhuri D, Kay E, Schmit BD. Pre- and post-alpha motoneuronal control of the soleus H-reflex during sinusoidal hip movements in human spinal cord injury. Brain Res 1103: 123–139, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knikou M, Hajela N, Mummidisetty CK, Xiao M, Smith AC. Soleus H-reflex phase-dependent modulation is preserved during stepping within a robotic exoskeleton. Clin Neurophysiol 122: 1396–1404, 2011. [DOI] [PubMed] [Google Scholar]
- Knikou M, Mummidisetty CK. Reduced reciprocal inhibition during assisted stepping in human spinal cord injury. Exp Neurol 231: 104–112, 2011. [DOI] [PubMed] [Google Scholar]
- Knikou M, Mummidisetty CK. Locomotor training improves premotoneuronal control after chronic spinal cord injury. J Neurophysiol 111: 2264–2275, 2014. [DOI] [PubMed] [Google Scholar]
- Knikou M, Rymer WZ. Effects of changes in hip joint angle on H-reflex excitability in humans. [Erratum in Exp Brain Res 144: 558, 2002]. Exp Brain Res 143: 149–159, 2002. [DOI] [PubMed] [Google Scholar]
- Lavoie BA, Devanne H, Capaday C. Differential control of reciprocal inhibition during walking versus postural and voluntary motor tasks in humans. J Neurophysiol 78: 429–438, 1997. [DOI] [PubMed] [Google Scholar]
- Lundberg A, Malmgren K, Schomburg ED. Cutaneous facilitation of transmission in reflex pathways from Ib afferents to motoneurones. J Physiol 265: 763–780, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea DA, Shefchyk SJ, Stephens MJ, Pearson KG. Disynaptic group I excitation of synergist ankle extensor motoneurones during fictive locomotion in the cat. J Physiol 487: 527–539, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misiaszek JE, Stephens MJ, Yang JF, Pearson KG. Early corrective reactions of the leg to perturbations at the torso during walking in humans. Exp Brain Res 131: 511–523, 2000. [DOI] [PubMed] [Google Scholar]
- Morita H, Crone C, Christenhuis D. Modulation of presynaptic inhibition and disynaptic reciprocal Ia inhibition during voluntary movement in spasticity. Brain 124: 826–837, 2001. [DOI] [PubMed] [Google Scholar]
- Morita H, Shindo M, Momoi H, Yanagawa S, Ikeda S, Yanagisawa N. Lack of modulation of Ib inhibition during antagonist contraction in spasticity. Neurology 67: 52–56, 2006. [DOI] [PubMed] [Google Scholar]
- Mummidisetty CK, Smith AC, Knikou M. Modulation of reciprocal and presynaptic inhibition during robotic-assisted stepping in humans. Clin Neurophysiol 124: 557–564, 2013. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Kagamihara Y. The regulation of disynaptic reciprocal Ia inhibition during co-contraction of antagonistic muscles in man. J Physiol 456: 373–391, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J, Crone C, Sinkjaer T, Toft E, Hultborn H. Central control of reciprocal inhibition during fictive dorsiflexion in man. Exp Brain Res 104: 99–106, 1995. [DOI] [PubMed] [Google Scholar]
- Okuma Y, Mizuno Y, Lee RG. Reciprocal Ia inhibition in patients with asymmetric spinal spasticity. Clin Neurophysiol 113: 292- 297, 2002. [DOI] [PubMed] [Google Scholar]
- Pearson K, Collins D. Reversal of the influence of group Ib afferents from plantaris on activity in medial gastrocnemius muscle during locomotor activity. J Neurophysiol 70: 1009–1017, 1993. [DOI] [PubMed] [Google Scholar]
- Perez M, Field-Fote E, Floeter M. Patterned sensory stimulation induces plasticity in reciprocal Ia inhibition in humans. J Neurosci 23: 2014–2018, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen N, Morita H, Nielsen J. Evaluation of reciprocal inhibition of the soleus H-reflex during tonic plantar flexion in man. J Neurosci Methods 84: 1–8, 1998. [DOI] [PubMed] [Google Scholar]
- Petersen N, Morita H, Nielsen J. Modulation of reciprocal inhibition between ankle extensors and flexors during walking in man. J Physiol 520: 605–619, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruska JC, Ichiyama RM, Jindrich DL, Crown ED, Tansey KE, Roy RR, Edgerton VR, Mendell LM. Changes in motoneuron properties and synaptic inputs related to step training after spinal cord transection in rats. J Neurosci 27: 4460–4471, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Bergego C, Katz R. Reversal in cutaneous control of Ib pathways during human voluntary contraction. Brain Res 233: 400–403, 1982. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Burke D. Spinal and Corticospinal Mechanisms of Movement. New York: Cambridge Univ. Press, 2012. [Google Scholar]
- Pierrot-Deseilligny E, Katz R, Morin C. Evidence for Ib inhibition in human subjects. Brain Res 166: 176–179, 1979. [DOI] [PubMed] [Google Scholar]
- Pratt CA, Jordan LM. Ia inhibitory interneurons and Renshaw cells as contributors to the spinal mechanisms of fictive locomotion. J Neurophysiol 57: 56–71, 1987. [DOI] [PubMed] [Google Scholar]
- Rank MM, Flynn JR, Battistuzzo CR, Galea MP, Callister R, Callister RJ. Functional changes in deep dorsal horn interneurons following spinal cord injury are enhanced with different durations of exercise training. J Physiol 593: 331–345, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi A, Mazzochio R, Scarpini C. Changes in Ia reciprocal inhibition from the peroneal nerve to the soleus alpha-motoneurons with different static body positions in man. Neurosci Lett 84: 283–286, 1988. [DOI] [PubMed] [Google Scholar]
- Rossi A, Zalaffi A, Decchi B. Heteronymous recurrent inhibition from gastrocnemius muscle to soleus motoneurons in humans. Neurosci Lett 169: 141–144, 1994. [DOI] [PubMed] [Google Scholar]
- Rossignol S. Plasticity of connections underlying locomotor recovery after central and/or peripheral lesions in the adult mammals. Philos Trans R Soc Lond B Biol Sci 361: 1647–1671, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadlaoud K, Tazerart S, Brocard C, Jean-Xavier C, Portalier P, Brocard F, Vinay L, Bras H. Differential plasticity of the GABAergic and glycinergic synaptic transmission to rat lumbar motoneurons after spinal cord injury. J Neurosci 30: 3358–3369, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindo M, Harayama H, Kondo K, Yanagisawa N, Tanaka R. Changes in reciprocal Ia inhibition during voluntary contraction in man. Exp Brain Res 53: 400–408, 1984. [DOI] [PubMed] [Google Scholar]
- Smith AC, Mummidisetty CK, Rymer WZ, Knikou M. Locomotor training alters the behavior of flexor reflexes during walking in human spinal cord injury. J Neurophysiol 112: 2164–2175, 2014. [DOI] [PubMed] [Google Scholar]
- Smith AC, Rymer WZ, Knikou M. Locomotor training modifies soleus monosynaptic motoneuron responses in human spinal cord injury. Exp Brain Res 233: 89–103, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens M, Yang J. Short latency, non-reciprocal group I inhibition is reduced during the stance phase of walking in humans. Brain Res 743: 24–31, 1996. [DOI] [PubMed] [Google Scholar]
- Tansey KE, McKay WB, Kakulas BA. Restorative neurology: consideration of the new anatomy and physiology of the injured nervous system. Clin Neurol Neurosurg 114: 436–440, 2012. [DOI] [PubMed] [Google Scholar]
- Whelan PJ, Hiebert GW, Pearson KG. Stimulation of the group I extensor afferents prolongs the stance phase in walking cats. Exp Brain Res 103: 20–30, 1995. [DOI] [PubMed] [Google Scholar]
- Yang JF, Whelan PJ. Neural mechanisms that contribute to cyclical modulation of the soleus H-reflex in walking in humans. Exp Brain Res 95: 547–556, 1993. [DOI] [PubMed] [Google Scholar]