Abstract
The aim of the present work was to investigate the effects of the radiofrequency (RF) electromagnetic fields (EMFs) on human resting EEG with a control of some parameters that are known to affect alpha band, such as electrode impedance, salivary cortisol, and caffeine. Eyes-open and eyes-closed resting EEG data were recorded in 26 healthy young subjects under two conditions: sham exposure and real exposure in double-blind, counterbalanced, crossover design. Spectral power of EEG rhythms was calculated for the alpha band (8–12 Hz). Saliva samples were collected before and after the study. Salivary cortisol and caffeine were assessed by ELISA and HPLC, respectively. The electrode impedance was recorded at the beginning of each run. Compared with the sham session, the exposure session showed a statistically significant (P < 0.0001) decrease of the alpha band spectral power during closed-eyes condition. This effect persisted in the postexposure session (P < 0.0001). No significant changes were detected in electrode impedance, salivary cortisol, and caffeine in the sham session compared with the exposure one. These results suggest that GSM-EMFs of a mobile phone affect the alpha band within spectral power of resting human EEG.
Keywords: electroencephalogram, radio frequency, mobile phone, alpha band
emerging technologies in mobile telecommunications, such as radio frequency fields (RF) and microwave radiation, are widely used in our modern society. Prominent examples are the wireless Internet network and mobile phone communications, which are particularly widespread. The extensive use of mobile phones (MP) increases the exposure of human beings to radiofrequency electromagnetic fields. During a phone call, given the close proximity of the MP to the user's head, a part of the electromagnetic field (EMF) can be absorbed by the head and the brain (Schönborn et al. 1998). This exposure to EMF has raised questions about possible effects of the EMF of mobile phones on brain activity.
Some earlier studies have investigated the effects of EMFs on resting cerebral activity with somewhat mixed results, but more recently, there has been consistent data, indicating the existence of exposure effects on the alpha bands of the resting EEG.
Indeed, data reported by some authors showed an increase in EEG power in the alpha frequency band (Cook et al. 2004; Croft et al. 2002, 2008, 2010; Curcio et al. 2005; Hinrikus et al. 2008; Huber et al. 2002; Kramarenko and Tan 2003; Regel et al. 2007; Reiser et al. 1995), whereas other studies reported a decrease in EEG power or coherence in the alpha band (Maby et al. 2006; Perentos et al. 2013; Vecchio et al. 2007, 2010, 2012). Finally, other studies failed to show an effect on EEG power in the alpha bands (D'Costa 2003; Hietanen et al. 2000; Röschke and Mann, 1997; Perentos et al. 2007).
As the literature cited demonstrates, the most consistent effect observed is a change in alpha band power. However, these changes sometimes correspond to an increase in alpha power and sometimes to a decrease. The reason why alpha band power reacts differently to RF exposure remains unclear. The main problem lies in the use of different methods, different experimental protocols, and/or different intensities or frequencies (van Rongen et al. 2009), thus making the comparison of data more difficult. As also reported by Loughran et al. (2012), individual variability is also one of the important factors that may explain the discrepancies between the results.
Moreover, several other parameters could impact the EEG results as confounding factors. Among these parameters are electrode impedance changes. The battery and electronics of the phone causes it to heat up, which, in turn, causes heating of the skin and underlying tissue (Anderson and Rowley 2007; Ghosn et al. 2012, Straume et al. 2005). As exposure to heat causes the dilation of blood vessels, this phenomenon may result in a change in the skin impedance (Luck 2005), which, in turn, could explain some observed changes in the recorded EEG power.
In addition, changes in the alpha band power are related to changes in parameters, such as cortisol or caffeine, which, to our knowledge, have never been concretely measured in relation to EMF exposure. Changes in cortisol and ECG could result from stress linked to the experimental environment and protocol, and therefore, these parameters need to be controlled.
The aim of the present study was to examine the potential impact of GSM (global system for mobile) RF (radiofrequency) exposure to the alpha band of the resting EEG under controlled parameters and to, thus, bring additional information to fill certain gaps in our current knowledge of the effects of GSM RF exposure. This study was conducted on awake volunteers in two different conditions: open eyes and closed eyes. In addition, some parameters that are known to affect alpha band, such as electrode impedance, cortisol levels and caffeine concentrations were also investigated to ensure that if any effect was observed, that it was not attributable to one of the aforementioned parameters. Hence, electrode impedance was checked after each block of EEG recordings, and caffeine and cortisol were concurrently evaluated in the saliva.
METHODS
Participants.
Twenty-six healthy volunteers participated in the experiment (13 females and 13 males; mean age = 23.5 ± 3.1 yr). All women reported having regular menstrual cycles (25–32 days) during the year preceding the study, no vasomotor complaints (i.e., hot flashes, night sweats). These women were studied in the laboratory during the follicular phase of their menstrual cycle to avoid any interference with EEG rhythms and hemispheric activity. All participants provided informed written consent and were compensated for their participation. All procedures were approved by the local ethics committee (ID no. = RCB: 2011, A01455-36). The volunteers were selected following a routine clinical examination. The mean body mass index of the subjects was 22.3 ± 1.8. Systolic and diastolic blood pressures were 113.3 ± 9.2 and 74 ± 7.7 mmHg (mean ± SD), respectively. Inclusion criteria included regular sleep habits, no medication, no chronic disease or disability, no recent acute illness, no smoking, and no neurological or psychiatric illness. All participants were right-handed and had normal or corrected-to-normal vision. Those selected were instructed to abstain from consuming alcohol and coffee for 24 h before and during each experimental session. They were instructed to abstain from using a mobile phone on the day of the experiment. Participants declared that they did not use the mobile phone at all on the day of the experiment. Moreover, we are quite sure that they did not use their phones 2 to 3 h before the start of the experiment, since they were admitted into the facility of the hospital to fill some documents related to the experiment 2 to 3 h prior to the exposure.
Experimental design.
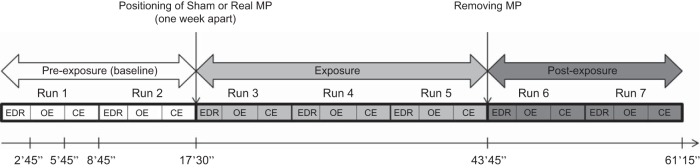
Participants attended two EEG recording sessions in a crossover, randomized, double-blind, and counterbalanced design experiment. During each session, the subject was exposed to 26 min and 15 s of sham or real GSM RF exposure (Fig. 1). In the case of sham exposure, the mobile phone was switched “on” but without RF radiation, while for real exposure, the mobile phone was switched “on” with RF radiation. For the same subject, the two sessions were at a 1-wk interval. Both the subjects and experimenters were unaware of the exposure condition. The experiment was conducted in a dimly lit, electrically shielded room. Subjects were seated in a comfortable chair, and a screen was placed 1 m in front of the volunteer to keep their eyes in a well-defined direction. In addition to the EEG recordings, ECG and galvanic skin responses (GSR) [also called electrodermal response (EDR)] were simultaneously recorded (EDR data will not be reported in the present paper). During the recordings, volunteers were asked to fix their eyes on a center point on the screen represented by a white square in the center of a black background. Each recording session was composed of seven experimental blocks distributed across the 3 experimental conditions: preexposure, exposure, and postexposure. Each block consisted of three recordings: EDR assay (2 min and 45 s), resting EEG with open eyes (3 min), and resting EEG with closed eyes (3 min) (Fig. 1). Vocal instructions were previously recorded by the experimenter. Loudspeakers placed on either side of the screen in front of the volunteer connected to a computer in the acquisition room allowed instructions to be sent to the volunteers. Auditory instructions to inform the volunteers when recording starts, when to open or to close their eyes, and the fixation point were given with Omnistim (stimulus presentation software developed at the MEG-EEG Center). Transistor-transistor logic (TTL) pulses were used to synchronize stimulus presentation and the EEG/BIOPAC systems. Instructions were at the beginning of the recording block, the open eyes and the closed-eye periods, and at the end of the block.
Fig. 1.
The experimental protocol included three periods: preexposure, exposure, and postexposure. Each volunteer participated in two recording sessions (sham and active exposure) in a crossover randomized double-blind design. Electrodermal response (EDR), open eyes (OE), and closed eyes (CE) were performed during resting EEG recordings.
The timeline of the two experimental sessions is presented in Fig. 1. The preexposure period consisted of two blocks of recordings (run 1 and run 2) with no mobile phone (baseline). Three blocks (run 3, run 4, and run 5) were recorded during the exposure period in which the actual mobile phone (genuine) was positioned and activated for the exposure session, and the sham phone was used in the sham session. The mobile phone was then removed, and two blocks were recorded in the postexposure period (runs 6 and 7).
Exposure system and dosimetry.
Subjects were exposed to RF EMF by a commercial dual-band GSM mobile phone (Nokia 6650). The mobile phone was positioned against the left ear. To set the standard exposure parameters, the phone was connected to a personal computer to control the required frequency and RF power by service software (Phoenix, Nokia, Finland). The sham or genuine exposure was carried out using a “load” or a “dummy load”, respectively. For this purpose an external power load was connected to the external antenna connector of the phone. A 50-Ω resistive load and an open-circuit dummy load were developed for sham and exposed conditions with the same shape and structure to allow for the double-blind protocol of the study. This implied that, when the telephone was on, the internal circuitry was regularly active, but no radiofrequency power was delivered in space by the antenna. The participants received GSM-modulated exposure with the full power of the mobile phone (2 W peak, 250 mW average, pulse modulated with 1/8 duty cycle) at 900 MHz for 26 min. The maximum specific absorption rates (SARs) were averaged on 10 g tissue, 1 g tissue, and the peak value was measured at 0.49 W/kg, 0.70 W/kg, and 0.93 W/kg, respectively. The SAR of the sham phone was below the detection level of the system (0.001 W/kg) at any position of the phantom, and no electric field was detected on the surface of the sham phone (for more details, see Ghosn et al. 2012).
EEG recording and data acquisition.
Electroencephalography data were recorded using BrainCap (EASYCAP Products, Herrsching, Germany) with 29 passive electrodes (Fp1, Fp2, F7, F3, Fz, F4, F8, FC5, FC1, FC2, FC6, T7, C3, Cz, C4, T8, CP5, CP1, CP2, CP6, P7, P3, Pz, P4, P8, PO3, PO4, O1, and O2) placed according to the international extended 10/10 system. The reference electrode was the AFz, and the ground electrodes were placed on the right shoulder of each participant. Repeated EEG blocks were recorded with respect to the AFz reference at a sampling rate of 1,000 Hz. The signal was amplified and band-pass filtered online between 0.016 Hz and 250 Hz. We used three bipolar derivations to monitor eye movements: one electrode was placed below the right eye for vertical eye movements, and two electrodes were placed at the outer canthi of the eyes for horizontal movements. Data acquisition was performed using BRAINAMP MR plus Amplifiers (Brain Products, Munich, Germany).
EEG interference with RF-EMF.
To test possible interference between radio frequencies emitted by the mobile phone and EEG signals recorded during exposure, a polystyrene phantom head was constructed to simulate a complete EEG chain. Time-frequency analysis was performed on the three recordings blocks (without a phone, with the sham phone, and the real phone) to detect any interference signals. Results showed no disturbance in the recording in the absence or presence of the two phones for the frequencies between 1 and 20 Hz. The two sham and real phones used in our experiment seem not to have disturbed the EEG recordings assessed during exposure.
Measurement of electrode impedance.
Electrode impedance was checked to be below 5 kΩ and was recorded throughout the experiment at the beginning of each run.
Heart rate data acquisition.
Heart rate was recorded by BIOPAC MP150 (GSR100C and ECG100C modules) at a sampling rate of 1,000 Hz by using two electrodes. One was placed at the base of the neck (above the right clavicle), while the other was placed on the left forearm.
ELISA for salivary cortisol.
Saliva was collected using a Salivette device (Sarstedt) and then centrifuged and immediately frozen. Each volunteer provided two saliva samples, the first before starting the experiment (T0) and the second after the recordings were complete (Tf). The cortisol was quantified in two samples collected at T0 and Tf using commercialized sandwich ELISA kits (human cortisol), according to the manufacturer's instructions.
Samples were centrifuged (1,000 g/20 min/4°C), and the supernatant was collected. Raw data were presented for sham and exposed groups.
Salivary caffeine concentration using HPLC.
Salivary caffeine concentration was assessed in T0 samples. A rapid HPLC method was used for the salivary caffeine analysis. The HPLC system consisted of a Spectra SYSTEM Pump and a Spectra SYSTEM UV detector (Ultimate 3000 Photodiode Array detector). An Envirodur C18 (3 μm) column (250 × 4.6 mm; Macherey Nagel) was used for the separation. The mobile phase was made of 85% of a 0.012 M KH2PO4 and 15% acetonitrile. The flow rate was set at 1 ml/min, and the injection volume was set at 20 μl. The detection wave length was set at 280 nm. The caffeine solution concentrations used for the standard curves were 1, 1.5, 2, 5, 8, 15, 25, 50, and 100 μg/ml. Standard curves were constructed by plotting concentration vs. area under the curve. Caffeine retention time was 5.2 min.
EEG data analysis.
Resting EEG data were analyzed for the periods “open eyes” and “closed eyes,” which lasted 3 min each for each run. In total, 7 runs were performed: the first two runs (runs 1 and 2) consisted of the preexposure period, the three following runs (runs 3, 4, and 5) constituted the exposure period, and the last two runs (runs 6 and 7) represented the postexposure period. Markers were placed in the data at 4-s intervals, and then we performed the time-frequency wavelet transform on individual EEG epochs comprising data from −2.5 to 2.5 ms around each marker. We used a family of complex Morlet wavelets, with an m parameter of 10 and a Blackman window of 100 ms, resulting in an estimate of signal power at each time sample and at each frequency between 1 and 20 Hz, with a frequency step of 1 Hz. The time-frequency transformed data were then averaged across epochs for each experimental eye condition, each run and for each subject, separately for the baseline trials and the exposure and postexposure trials to obtain spectral power, which were then subsequently averaged in the alpha (8–12 Hz) bands. The alpha band was divided into two subbands: the upper (10–12 Hz) and the lower (8–10 Hz) and were then analyzed. The log-transformation of the data was used to approach a normal distribution. Finally, the data were averaged over the three conditions of interest: preexposure (baseline), during exposure, and postexposure period, for each subject and for the grand mean of the 26 volunteers.
Statistical analysis.
A four-way repeated-measures ANOVA was run to determine the effect of exposure (sham or exposed), frequency bands (delta, theta, or alpha), period (before, during, or after), and eye conditions (closed or open) across subjects. Then, we restricted the analysis to alpha band (8–12 Hz) in closed-eye condition as follows: for each period (before, during, and after), we performed a paired t-test for each electrode across subjects in the two conditions (real exposure vs. sham exposure). Then, we averaged frequency power values for each portion of the alpha band (8–12 Hz, 8–10 Hz, and 10–12 Hz) on each electrode across subjects and performed a paired t-test in the two conditions (sham or real exposure). The family-wise error rate was controlled via permutations tests as showed by Groppe et al. (2011), which is, at most, as conservative as Bonferroni.
Heart rate, impedance, and cortisol data analyses were performed using two-way repeated-measures ANOVA. Statistical significance was set for P < 0.05.
RESULTS
EEG interference with RF-EMF.
No disturbance was seen in any recording in the absence or presence of the phones (actual or genuine) for the analyzed frequencies between 1 and 20 Hz. The two sham and real phones used in our experiment did not disturb EEG recordings assessed during exposure (data not shown).
Alpha spectral power.
There were significant differences between frequency bands and eye conditions for all of the electrode measurements. Period levels (before, during, and after) were statistically significantly different on all electrodes except in the frontal region (Fp1, Fp2, F7, F3, Fz, F4, FC1, FC2).
In the closed-eyes condition, a significant difference between sham and real exposure was found in alpha band power (8–12 Hz) for all electrodes during the exposure (except FP2, FC5, and P8) and postexposure period (except Cz, CP2, P7). Indeed, a paired permutation t-test analysis detected a significant and important decrease in alpha band power (8–12 Hz) (P < 0.0001) during the exposure and postexposure period (P < 0.001) (Table 1).
Table 1.
Statistical analyses of alpha band spectral power
| Exposure to GSM 900 Signal | Alpha Band |
||
|---|---|---|---|
| Closed Eyes | 8–12 | 8–10 | 10–12 |
| Before | P = 0.1116 → | P = 0.9412 → | P = 0.210 → |
| t = 1.6606 | t = −0.0758 | t = 1.2868 | |
| During | P < 0.0001 ⇊ | P < 0.001 ⇊ | P < 0.0001 ⇊ |
| t = 4.8816 | t = 5.1514 | t = 4.1656 | |
| After | P < 0.001 ⇊ | P < 0.0001 ⇊ | P < 0.0001 ⇊ |
| t = 5.2655 | t = 5.3638 | t = 4.4889 | |
For each period (before, during, and after), we performed a paired t-test for each electrode across all subjects. Then, the frequency power values were averaged across all subjects, and a paired t-test was performed on the averaged electrode values for the two conditions (sham/exposure). Comparisons were made between sham vs. real exposure (i.e., t >0 corresponds to a decrease, and t <0 corresponds to a power increase in the truly exposed condition).
Furthermore, the alpha band (8–12 Hz) was divided into two subbands—the upper (10–12 Hz) and lower (8–10 Hz) alpha bands—which were analyzed separately. Results showed that in the 8–10 Hz frequency band, alpha spectral power significantly decreased during the exposure and postexposure period (P < 0.001 and P < 0.0001, respectively). Likewise, data within the upper alpha band (10–12 Hz) showed a decrease in the spectral power during and also after exposure (P = 0.0001 and P < 0.0001, respectively) (Table 1).
Electrode impedance.
Figure 2 represents electrode impedance recorded at the beginning of each run. No significant differences have been detected when comparing sham and real exposure between runs. Repeated-measures two-way ANOVA and Bonferroni post hoc tests were applied. P and F values are given in Table 2. Impedance was not affected by the factor session (sham and real exposure) recorded 1 wk apart in all runs. Moreover, no significant differences were found in all electrode impedances when comparing the seven runs separately in the sham sessions and in the exposure sessions.
Fig. 2.
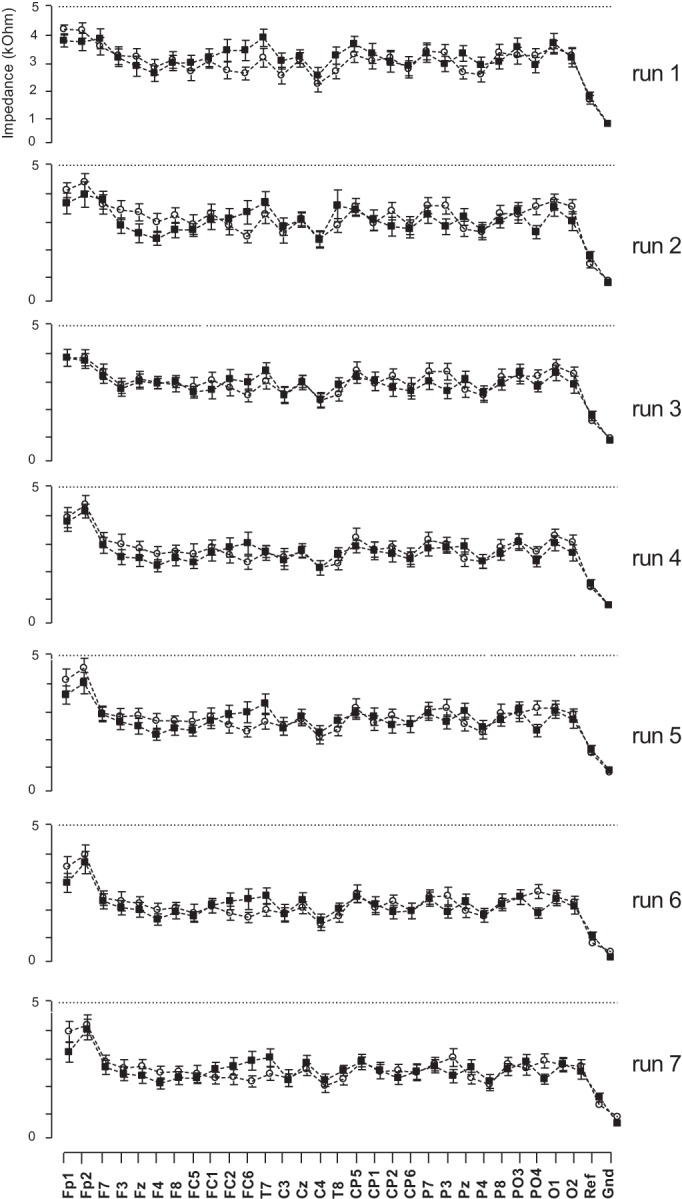
Changes in the electrical impedance of EEG electrodes during sham (○) and exposed (■) sessions. The impedances were maintained below 5 kΩ. No significant differences were detected comparing sham and real exposure in all runs.
Table 2.
Statistical findings: electrode impedance with two factors: session (sham and exposed), electrodes (29 electrodes), and interaction between the two factors
| Source of Variation | P Value | F |
|---|---|---|
| Run 1 | ||
| Interaction | 0.7391 | 0.8313 |
| Session | 0.0696 | 3.296 |
| Electrodes | <0.0001 | 18.89 |
| Run 2 | ||
| Interaction | 0.5268 | 0.9639 |
| Session | 0.0734 | 3.208 |
| Electrodes | <0.0001 | 18.47 |
| Run 3 | ||
| Interaction | 0.9862 | 0.5348 |
| Session | 0.2490 | 1.330 |
| Electrodes | <0.0001 | 19.66 |
| Run 4 | ||
| Interaction | 0.9944 | 0.4832 |
| Session | 0.0912 | 2.857 |
| Electrodes | <0.0001 | 20.09 |
| Run 5 | ||
| Interaction | 0.6613 | 0.8816 |
| Session | 0.1883 | 1.732 |
| Electrodes | <0.0001 | 18.43 |
| Run 6 | ||
| Interaction | 0.8481 | 0.7492 |
| Session | 0.3986 | 0.7129 |
| Electrodes | <0.0001 | 20.22 |
| Run 7 | ||
| Interaction | 0.5234 | 0.9659 |
| Session | 0.4799 | 0.4992 |
| Electrodes | <0.0001 | 18.74 |
Heart rate.
There were no significant variations in heart rate (Fig. 3), whether it be between the two sessions (sham and real exposure), eye condition (open eyes and closed eyes) within and between sessions [two-way ANOVA: exposure (F = 0.1, P = 0.75), and eye condition (F = 0.58, P = 0.71)].
Fig. 3.
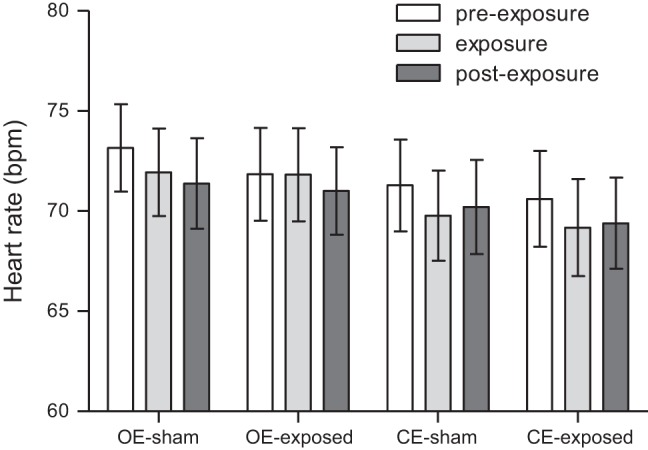
Heart rate during open eyes (OE) and closed eyes (CE) periods in sham and exposed sessions. Results are expressed as means ± SE.
Salivary cortisol.
Figure 4 shows the salivary cortisol concentration in sham and exposed sessions separately for participants recorded in the morning or in the afternoon. ANOVA analyses showed no significant differences in cortisol concentrations when comparing sham to exposed sessions, no differences between volunteers, and no significant interaction between exposure × subjects in the morning, respectively, in T0 and Tf (F = 2.72, P = 0.12; F = 0.08, P = 2.27; interaction: F = 0.42, P = 0.87). In the afternoon, no significant difference was observed between T0 and Tf when comparing sham to exposed sessions (F = 0.67, P = 0.78), but a significant difference was noted between subjects (F = 2.08, P = 0.04), and no exposure × subjects interaction (F = 0.55, P = 0.89).
Fig. 4.
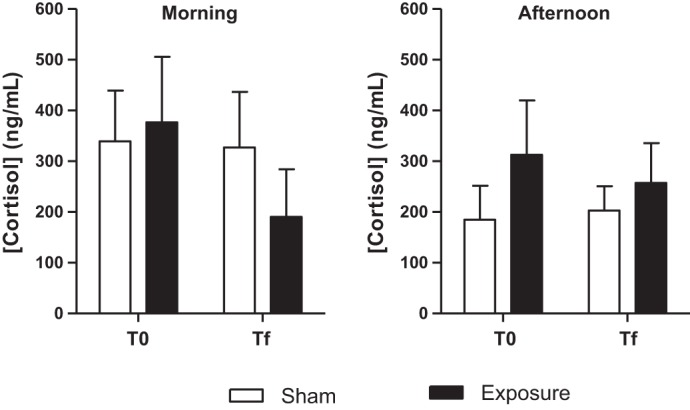
Salivary cortisol concentration (ng/ml) before starting the study protocol (T0) and after the end of the protocol (Tf) in sham and exposed sessions for the volunteers who attended the experiment in the morning or in the afternoon.
Salivary caffeine.
Results showed that caffeine concentrations in all samples were negligible and below the detection limit of 2 μg/ml.
DISCUSSION
The present study evaluated the effect of GSM (global system for mobile) signals of a mobile phone on the electrical activity of the human brain, especially in the alpha band of the resting EEG in young adults. In this study, healthy adults underwent two sessions of EEG recordings 1 wk apart as a washout period. Results showed that alpha spectral power decreased during exposure period to GSM signals. These results concur with previous findings on the effects of GSM signals on alpha power of resting EEG in humans (Croft et al. 2002; Curcio et al. 2005; Kramarenko and Tan 2003). When analyzing lower (8–10 Hz) and upper (10–12 Hz) alpha bands separately, results showed a similar significant decrease. This effect persisted in the postexposure period (Table 1), suggesting that the effect is sustained with lasting physiological changes and not solely during immediate interaction between exposure and the target tissue. This is in line with the results obtained in other studies that have exposed participants prior to the EEG recording (Curcio et al. 2005; Huber et al. 2002; Reiser et al. 1995), and where an effect of RF-EMF has been observed on brain activity. The persisted effect of RF-EMF on brain activity was also observed on the EEG during sleep, during which time some authors have reported a modification following the active period of exposure (Loughran et al. 2005, 2012; Regel et al. 2007).
As we know, interpreting alpha wave activity from the amplitude/power measurement is dependent on several factors, mainly the experimental conditions under which the amplitude is measured, such as open or closed eyes (Bazanova and Vernon 2013). Indeed, it was reported that an increase in the amplitude seen with closed eyes indicates less activation, whereas when eyes are open, there is a decrease in amplitude, indicating an increase in activation (Barry et al. 2007). It was assumed that neuronal activity generating the alpha rhythm is associated with areas of the cortex that are not processing information at rest. This is the usual explanation of why the rhythm may disappear when the eyes are open, while processing the visual information. Similarly, when a subject concentrates on a particular modality, the EEG activity in the alpha band specifically decreases in the corresponding brain region. Also, reduction in the power of alpha rhythms has been related to the speed of information processing, the subject's global attention, and cognitive performance (Klimesch 1997, 1998, 1999, 2003; Krause et al. 2000a, b; Neubauer and Freudenthaler 1995; Vogt et al. 1998).
The possible reasons why an effect was found only for eyes closed but not for eyes open may reside in the fact that amplitudes of alpha waves diminish when subjects open their eyes and, thereby, the effect of radiofrequency could not be significantly detected. However, when the subject is awake and relaxed with eyes closed, the alpha rhythm is prominent, thereby facilitating the observation of any effect.
According to these data, it seems that the effects observed in our study mimic, to some extent, the global reductions in alpha-band power observed in eyes-opened vs. eyes-closed conditions. One would suggest that the power decrease in alpha-band frequency resulting from the GSM signal exposure could be beneficial for memory process, global attention, and cognitive performance. The potential clinical significance of this effect, in this area, could be assessed in further studies.
The mechanisms behind these exposure-induced changes still remain unclear. However, on the basis of earlier reported data, it has been shown that intracortical excitability of the motor cortex was modified by acute exposure to GSM 900 (Ferreri et al. 2006). Intracortical inhibition/facilitation (ICI/ICF) curves were investigated, and results showed that ICI is reduced and ICF is enhanced after exposure to GSM signal (Ferreri et al. 2006). It has been suggested that ICI is mediated by GABA-A receptors (Hanajima et al. 1998), ICF is mediated by glutamatergic N-methyl-d-aspartate (NMDA) (Ziemann et al. 1998), and an imbalance between ICI and ICF may lead to changes in the intracortical excitability (Sanger et al. 2001). It has also been suggested that oxidative stress may play a role in this phenomenon, since it reduces the release of GABA and the activity of GABA-A receptors at presynaptic and postsynaptic sites (Sah et al. 1999, 2002), which correlates with the observed decrease in EEG amplitude.
The data reported in the present study were obtained while controlling certain parameters considered as confounding factors. Indeed, alpha rhythm is known to be sensitive to several factors, including caffeine and cortisol. To our knowledge, previous studies on RF's effect on EEG did not concretely and concurrently measure such factors that may modify alpha power. Therefore, our study was designed to include and assess salivary cortisol and caffeine.
As alpha rhythm has long been known to be sensitive to overall attentional states (i.e., intensity aspects such as arousal) (Adrian and Matthews 1934) and is also involved in the biasing of selective attention (Foxe et al. 1998; Kelly et al. 2006), we instructed subjects to refrain from any caffeinated drinks (e.g., coffee, tea, caffeinated soft drinks) 24 h before the study. It has been reported that caffeine increases alertness and speeds reaction time, dominant factors in relation to alpha power (Fredholm et al. 1999; Smith 2002). In addition, previous studies reported a drop in absolute alpha power during rest with eyes open when caffeine was ingested at high doses (Deslandes et al. 2005; Siepmann and Kirch 2002). In our study, caffeine assessed in the saliva did not show detectable values (above the device's quantification limit = 2 μg/ml), suggesting that caffeinated drinks did not bias the observed results.
Moreover, salivary cortisol was assessed because it has been shown that concentrations of cortisol within the blood or saliva can vary spontaneously with EEG power across a range of 6.5–14.0 Hz, which includes the alpha rhythm (Sannita et al. 1999). Our results showed no significant variations in salivary cortisol between sham and real exposure.
In regard to electrical impedance, no differences were detected in all runs when comparing sham to real exposure sessions. Thus, the reported effects could not be related to differences in electrode impedance throughout the experiment, caffeine consumption before the experiment, or cortisol differences between groups.
Conclusions.
Exposure to GSM-EMFs of a mobile phone can influence human dominant alpha rhythms in a resting state. Our results showed a power decrease of alpha band during and after exposure to GSM-EMFs compared with sham exposure in an eyes-closed condition. These findings were not correlated with impedance electrodes, cortisol, or caffeine, factors that can influence alpha power. However, extended postexposure duration should be tested since the observed effect persisted until the end of the postexposure period. Furthermore, it is also important to stress the potential clinical significance of this effect.
GRANTS
This study has been fully funded by INERIS and the program 190 “post Grenelle” for the “pôle applicatif en Toxicologie et Ecotoxicologie” (French Ministry of Ecology and Sustainable Development).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: R.G., L.H., A.D., and J.-D.L. performed experiments; R.G., L.Y.-C., L.H., and A.D. analyzed data; R.G., L.Y.-C., L.H., A.D., G.T., R.d.S., and B.S. interpreted results of experiments; R.G. prepared figures; R.G. and B.S. drafted manuscript; R.G., J.-D.L., G.T., R.d.S., and B.S. edited and revised manuscript; R.G., L.Y.-C., L.H., A.D., J.-D.L., G.T., R.d.S., and B.S. approved final version of manuscript; G.T., R.d.S., and B.S. conception and design of research.
ACKNOWLEDGMENTS
Sponsors have not been involved in the collection, analysis, and interpretation of data and in the writing of this article. The authors thank the staff of the MEG-EEG center CENIR at Paris Salpêtrière Hospital (where the study was conducted) for technical support. We also thank Dr. Alexandre Vallée for clinical examination and Jean-Pierre Gomez for the fabrication of the phantom head at the INERIS workshop.
REFERENCES
- Adrian ED, Matthews BHC. The Berger rhythm: Potential changes in the occipital lobes in man. Brain 57: 355–385, 1934. [Google Scholar]
- Anderson V, Rowley J. Measurements of skin surface temperature during mobile phone use. Bioelectromagnetics 28: 159–162, 2007. [DOI] [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, Johnstone SJ, Brown CR. EEG differences between eyes-closed and eyes-open resting conditions. Clin Neurophysiol 118: 2765–2773, 2007. [DOI] [PubMed] [Google Scholar]
- Bazanova OM, Vernon D. Interpreting EEG alpha activity. Neurosci Biobehav Rev 44: 94–110, 2014. [DOI] [PubMed] [Google Scholar]
- Cook CM, Thomas AW, Prato FS. Resting EEG is affected by exposure to a pulsed ELF magnetic field. Bioelectromagnetics 25: 196–203, 2004. [DOI] [PubMed] [Google Scholar]
- Croft RJ, Chandler JS, Burgess AP, Barry RJ, Williams JD, Clarke AR. Acute mobile phone operation affects neural function in humans. Clin Neurophysiol 113: 1623–1632, 2002. [DOI] [PubMed] [Google Scholar]
- Croft RJ, Hamblin DL, Spong J, Wood AW, McKenzie RJ, Stough C. The effect of mobile phone electromagnetic fields on the alpha rhythm of human electroencephalogram. Bioelectromagnetics 29: 1–10, 2008. [DOI] [PubMed] [Google Scholar]
- Croft RJ, Leung S, McKenzie RJ, Loughran SP, Iskra S, Hamblin DL, Cooper NR. Effects of 2G and 3G mobile phones on human alpha rhythms: resting EEG in adolescents, young adults, and the elderly. Bioelectromagnetics 31: 434–444, 2010. [DOI] [PubMed] [Google Scholar]
- Curcio G, Ferrara M, Moroni F, D'Inzeo G, Bertini M, De Gennaro L. Is the brain influenced by a phone call? An EEG study of resting wakefulness. Neurosci Res 53: 265–270, 2005. [DOI] [PubMed] [Google Scholar]
- D'Costa H, Trueman G, Tang L, Abdel-rahman U, Abdel-rahman W, Ong K, Cosic I. Human brain wave activity during exposure to radiofrequency field emissions from mobile phones. Australas Phys Eng Sci Med 26: 162–167, 2003. [DOI] [PubMed] [Google Scholar]
- Deslandes AC, Veiga H, Cagy M, Piedade R, Pompeu F, Ribeiro P. Effects of caffeine on the electrophysiological, cognitive and motor responses of the central nervous system. Braz J Med Biol Res 38: 1077–1086, 2005. [DOI] [PubMed] [Google Scholar]
- Ferreri F, Curcio G, Pasqualetti P, De Gennaro L, Fini R, Rossini PM. Mobile phone emissions and human brain excitability. Ann Neurol 60: 188–196, 2006. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Simpson GV, Ahlfors SP. Parieto-occipital ∼10-Hz activity reflects anticipatory state of visual attention mechanisms. Neuroreport 9: 3929–3933, 1998. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Bättig K, Holmén J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev 51: 83–133, 1999. [PubMed] [Google Scholar]
- Ghosn R, Thuróczy G, Loos N, Brenet-Dufour V, Liabeuf S, de Seze R, Selmaoui B. Effects of GSM 900 MHz on middle cerebral artery blood flow assessed by transcranial Doppler sonography. Radiat Res 178: 543–550, 2012. [DOI] [PubMed] [Google Scholar]
- Groppe DM, Urbach TP, and Kutas M. Mass univariate analysis of event-related brain potentials/fields I: A critical tutorial review. Psychophysiology 48: 1711–1725, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Sakai K, Furubayashi T, Machii K, Kanazawa I. Paired-pulse magnetic stimulation of the human motor cortex: differences among I waves. J Physiol 509: 607–618, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hietanen M, Kovala T, Hämäläinen AM. Human brain activity during exposure to radiofrequency fields emitted by cellular phones. Scand J Work Environ Health 26: 87–92, 2000. [DOI] [PubMed] [Google Scholar]
- Hinrikus H, Bachmann M, Lass J, Tomson R, Tuulik V. Effect of 7, 14 and 21 Hz modulated 450 MHz microwave radiation on human electroencephalographic rhythms. Int J Radiat Biol 84: 69–79, 2008. [DOI] [PubMed] [Google Scholar]
- Huber R, Treyer V, Borbély AA, Schuderer J, Gottselig JM, Landolt HP, Werth E, Berthold T, Kuster N, Buck A, Achermann P. Electromagnetic fields, such as those from mobile phones, alter regional cerebral blood flow and sleep and waking EEG. J Sleep Res 11: 289–295, 2002. [DOI] [PubMed] [Google Scholar]
- Kelly SP, Lalor EC, Reilly RB, Foxe JJ. Increases in alpha oscillatory power reflect an active retinotopic mechanism for distracter suppression during sustained visuospatial attention. J Neurophysiol 95: 3844–3851, 2006. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Russegger H, Doppelmayr M, Pachinger T. A method for the calculation of induced band power: implications for the significance of brain oscillations. Electroencephalogr Clin Neurophysiol 108: 123–130, 1998. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Gerloff C. Enhancing cognitive performance with repetitive transcranial magnetic stimulation at human individual alpha frequency. Eur J Neurosci 17: 1129–1133, 2003. [DOI] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Brain Res Rev 29: 169–195, 1999. [DOI] [PubMed] [Google Scholar]
- Klimesch W. EEG-alpha rhythms and memory processes. Int J Psychophysiol 26: 319–340, 1997. [DOI] [PubMed] [Google Scholar]
- Kramarenko AV, Tan U. Effects of high-frequency electromagnetic fields on human EEG: A brain mapping study. Int J Neurosci 113: 1007–1019, 2003. [DOI] [PubMed] [Google Scholar]
- Krause CM, Sillanmäki L, Koivisto M, Häggqvist A, Saarela C, Revonsuo A, Laine M, Hämäläinen H. Effects of electromagnetic field emitted by cellular phones on the EEG during a memory task. Neuroreport 11: 761–764, 2000a. [DOI] [PubMed] [Google Scholar]
- Krause CM, Sillanmäki L, Koivisto M, Häggqvist A, Saarela C, Revonsuo A, Laine M, Hämäläinen H. Effects of electromagnetic fields emitted by cellular phones on the electroencephalogram during a visual working memory task. Int J Radiat Biol 76: 1659–1667, 2000b. [DOI] [PubMed] [Google Scholar]
- Loughran SP, McKenzie RJ, Jackson ML, Howard ME, Croft RJ. Individual differences in the effects of mobile phone exposure on human sleep: rethinking the problem. Bioelectromagnetics 33: 86–93, 2012. [DOI] [PubMed] [Google Scholar]
- Loughran SP, Wood AW, Barton JM, Croft RJ, Thompson B, Stough C. The effect of electromagnetic fields emitted by mobile phones on human sleep. Neuroreport 16: 1973–1976, 2005. [DOI] [PubMed] [Google Scholar]
- Luck SJ. An introduction to the event-related potential technique. In: Averaging, Artifact Rejection and Artifact Correction. Cambridge, MA: MIT Press, 2005, chapt. 4, p. 166. [Google Scholar]
- Maby E, Le Bouquin Jeannes R, Faucon G, Le Bouquin Jeannes R, Faucon G. Short-term effects of GSM mobiles phones on spectral components of the human electroencephalogram. Conf Proc IEEE Eng Med Biol Soc 1: 3751–3754, 2006. [DOI] [PubMed] [Google Scholar]
- Neubauer AC, Freudenthaler HH. Ultradian rhythms in cognitive performance: no evidence for a 15-h rhythm. Biol Psychol 40: 281–298, 1995. [DOI] [PubMed] [Google Scholar]
- Perentos N, Croft RJ, McKenzie RJ, Cvetkovic D, Cosic I. Comparison of the effects of continuous and pulsed mobile phone like RF exposure on the human EEG. Australas Phys Eng Sci Med 30: 274–280, 2007. [DOI] [PubMed] [Google Scholar]
- Perentos N, Croft RJ, McKenzie RJ, Cosic I. The alpha band of the resting electroencephalogram under pulsed and continuous radio frequency exposures. IEEE Trans Biomed Eng 60: 1702–1710, 2013. [DOI] [PubMed] [Google Scholar]
- Regel SJ, Gottselig JM, Schuderer J, Tinguely G, Rétey JV, Kuster N, Landolt HP, Achermann P. Pulsed radio frequency radiation affects cognitive performance and the waking electroencephalogram. Neuroreport 18: 803–807, 2007. [DOI] [PubMed] [Google Scholar]
- Reiser H, Dimpfel W, Schober F. The influence of electromagnetic fields on human brain activity. Eur J Med Res 1: 27–32, 1995. [PubMed] [Google Scholar]
- Röschke J, Mann K. No short-term effects of digital mobile radio telephone on the awake human electroencephalogram. Bioelectromagnetics 18: 172–176, 1997. [PubMed] [Google Scholar]
- Sah R, Galeffi F, Ahrens R, Jordan G, Schwartz-Bloom RD. Modulation of the GABAA-gated chloride channel by reactive oxygen species. J Neurochem 80: 383–391, 2002. [DOI] [PubMed] [Google Scholar]
- Sah R, Schwartz-Bloom RD. Optical imaging reveals elevated intracellular chloride in hippocampal pyramidal neurons after oxidative stress. J Neurosci 19: 9209–9217, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger TD, Garg RR, Chen R. Interactions between two different inhibitory systems in the human motor cortex. J Physiol 530: 307–317, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sannita WG, Loizzo A, Garbarino S, Gesino D, Massimilla S, Ogliastro C. Adrenocorticotropin-related modulation of the human EEG and individual variability. Neurosci Lett 262: 147–150, 1999. [DOI] [PubMed] [Google Scholar]
- Schönborn F, Burkhardt M, Kuster N. Differences in energy absorption between heads of adults and children in the near field of sources. Health Phys 74: 160–168, 1998. [DOI] [PubMed] [Google Scholar]
- Siepmann M, Kirch W. Effects of caffeine on topographic quantitative EEG. Neuropsychobiology 45: 161–166, 2002. [DOI] [PubMed] [Google Scholar]
- Smith A. Effects of caffeine on human behavior. Food Chem Toxicol 40: 1243–1255, 2002. [DOI] [PubMed] [Google Scholar]
- Straume A, Oftedal G, Johnsson A. Skin temperature increase caused by a mobile phone: a methodological infrared camera study. Bioelectromagnetics 26: 510–519, 2005. [DOI] [PubMed] [Google Scholar]
- Van Rongen E, Croft R, Juutilainen J, Lagroye I, Miyakoshi J, Saunders R, de Seze R, Tenforde T, Verschaeve L, Veyret B, Xu Z. Effects of radiofrequency electromagnetic fields on the human nervous system. J Toxicol Environ Health B Crit Rev 12: 572–597, 2009. [DOI] [PubMed] [Google Scholar]
- Vecchio F, Babiloni C, Ferreri F, Curcio G, Fini R, Del Percio C, Rossini PM. Mobile phone emission modulates interhemispheric functional coupling of EEG alpha rhythms. Eur J Neurosci 25: 1908–1913, 2007. [DOI] [PubMed] [Google Scholar]
- Vecchio F, Babiloni C, Ferreri F, Buffo P, Cibelli G, Curcio G, van Dijkman S, Melgari JM, Giambattistelli F, Rossini PM. Mobile phone emission modulates inter-hemispheric functional coupling of EEG alpha rhythms in elderly compared to young subjects. Clin Neurophysiol 121: 163–171, 2010. [DOI] [PubMed] [Google Scholar]
- Vecchio F, Buffo P, Sergio S, Iacoviello D, Rossini PM, Babiloni C. Mobile phone emission modulates event-related desynchronization of alpha rhythms and cognitive-motor performance in healthy humans. Clin Neurophysiol 123: 121–128, 2012. [DOI] [PubMed] [Google Scholar]
- Vogt F, Klimesch W, Doppelmayr M. High-frequency components in the alpha band and memory performance. J Clin Neurophysiol 15: 167–172, 1998. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Hallett M, Cohen LG. Mechanisms of deafferentation-induced plasticity in human motor cortex. J Neurosci 18: 7000–7007, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]