Abstract
Often, the brain receives more sensory input than it can process simultaneously. Spatial attention helps overcome this limitation by preferentially processing input from a behaviorally-relevant location. Recent neuropsychological and psychophysical studies suggest that attention is deployed to near-hand space much like how the oculomotor system can deploy attention to an upcoming gaze position. Here we provide the first neuronal evidence that the presence of a nearby hand enhances orientation selectivity in early visual processing area V2. When the hand was placed outside the receptive field, responses to the preferred orientation were significantly enhanced without a corresponding significant increase at the orthogonal orientation. Consequently, there was also a significant sharpening of orientation tuning. In addition, the presence of the hand reduced neuronal response variability. These results indicate that attention is automatically deployed to the space around a hand, improving orientation selectivity. Importantly, this appears to be optimal for motor control of the hand, as opposed to oculomotor mechanisms which enhance responses without sharpening orientation selectivity. Effector-based mechanisms for visual enhancement thus support not only the spatiotemporal dissociation of gaze and reach, but also the optimization of vision for their separate requirements for guiding movements.
Keywords: attention, peripersonal space, reaching, vision
a growing body of human psychophysical evidence shows that visual processing is altered near the hand. In blindsight, simply placing the hand in the blind field near to visual stimuli improves detection and size perception (Brown et al. 2008; Schendel and Robertson 2004). In extinction, patients fail to attend to a second stimulus presented in the contralesional hemifield, but, when the hand is placed within the affected field, detection of the second stimulus is improved (di Pellegrino and Frassinetti 2000). An improvement in detection near the hand, especially in cases involving extinction, would suggest that attention is deployed to near-hand space much like how the oculomotor system deploys spatial attention (Moore et al. 2003). Studies using classic spatial attention paradigms have shown this to be true. In a spatial cueing paradigm, reaction times to targets near the hand were facilitated, regardless of cue location (Reed et al. 2006). In another study involving visual search, inhibition of return and attentional blink paradigms, the presence of the hand slowed the shifting of attention between visual items (Abrams et al. 2008). These studies suggest that improved visual processing near the hand is linked to attentional prioritization of the space near the hand.
These behavioral studies suggest that attentional prioritization occurs in “near-hand space,” when movements are sustained. However, there is currently no neurophysiological evidence to support these findings, and the neuronal mechanisms underlying this enhancement are as yet unknown. To determine if and how a nearby hand affects early visual processing, we recorded from neurons in macaque area V2, an early visual area shown to be modulated by attention (Luck et al. 1997; Motter 1993), selective for orientation (Motter 1993), a feature necessary for accurate reaching (Fattori et al. 2009; Murata et al. 2000; Raos et al. 2004) and is directly connected with fronto-parietal reaching and grasping networks to guide the hand (Gattass et al. 1997; Passarelli et al. 2011). We measured the responses of V2 neurons to oriented rectangles when the animals maintained their grasp on a touch bar, placing their hand near to but outside the neuron's receptive field (RF) (Fig. 1, Hand-Near). As we wanted to be able to dissociate the effects of oculomotor driven spatial attention from those of near-hand attention, we separated the grasp target (touch bar) from the visual stimulus in the RF. Eye movements precede arm movements toward a reach target (Ballard et al. 1992; Biguer et al. 1982; Fisk and Goodale 1985; Neggers and Bekkering 2000, 2001, 2002; Prablanc et al. 1979) and the oculomotor system deploys spatial attention (Moore and Armstrong 2003; Moore and Fallah 2001, 2004; Müller et al. 2005). Thus, if the visual stimulus was also the reach target, oculomotor-driven spatial attention would be deployed to the reach target and would at the least confound and at the most completely mask modulation due to the nearby hand. To avoid this, we did not make the visual stimulus the reach target, but placed the hand nearby to take advantage of the spatial extent of attention afforded by the nearby hand.
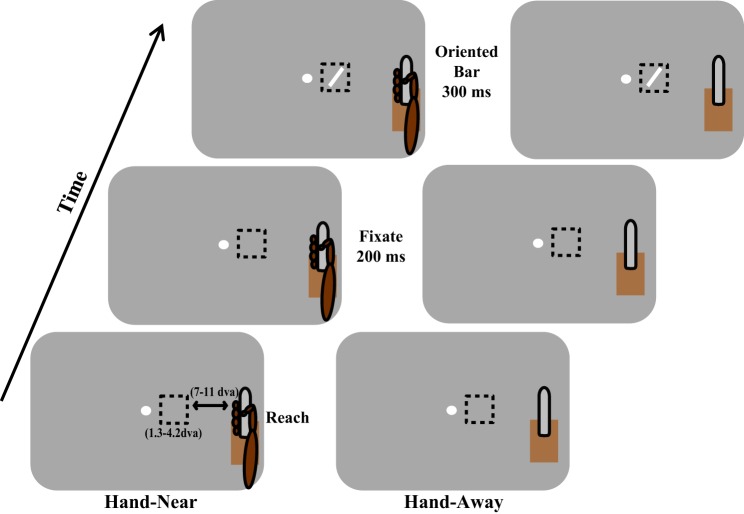
Fig. 1.
Experimental paradigm. In a Hand-Near block, the animal grasps a vertically orientated touch bar placed outside the receptive field (RF, dashed box) at which time a fixation point appears. Two hundred milliseconds later, an oriented bar is displayed within the RF for 300 ms. In a Hand-Away block, the touch bar apparatus remains visible, but no reach is made by the animal. Reward is given for maintaining fixation and grasp (Hand-Near) or simply maintaining fixation (Hand-Away). The bottom left panel shows the variation in RF diameter [1.3–4.2 degrees of visual angle (dva)] and also the distance between the right edge of the RF and the edge of the fingers (7–10.7 dva). This figure represents a depiction and is not drawn to scale or matched for the color and contrast of the experimental apparatus or the animal.
Prior studies of spatial attention (McAdams and Maunsell 1999; Moran and Desimone 1985; Motter 1993; Treue and Martinez-Trujillo 1999) have used “Attend-In” and “Attend-Away” paradigms to compare the neuronal modulation when a spatial location is attended vs. when attention is located elsewhere. In Attend-In conditions, a cue, presented prior to the visual target, is used to allocate attention to a certain spatial location. In Attend-Away conditions, the cue allocates attention to a location away from where the target is presented. Under these circumstances, neuronal responses undergo a gain modulation when the spatial location is attended. We modified this paradigm so that the presence of the hand acted in a similar manner as the spatial cue in those studies. We hypothesized that, if the hand is the center of an attentional field, as suggested by prior research (Abrams et al. 2008; Brown et al. 2008; di Pellegrino and Frassinetti 2000; Reed et al. 2006; Schendel and Robertson 2004), neuronal responses in “Hand-Near” and “Hand-Away” conditions should be similar to the neuronal responses seen in Attend-In and Attend-Away conditions, respectively. As the relationship between oculomotor-driven spatial attention and the effect of hand position on early visual responses is unknown, the stimuli measuring V2 neurons' orientation selectivity were task irrelevant. If the task had instead required attending to and making judgments of the stimuli within the RFs, the effects of spatial attention would have confounded neuronal responses associated with hand attention. This task design is similar to real-life situations where you are reading a paper and reach, without looking, to pick up your cup of coffee: would orientation processing improve when the hand is near the cup? Across the population, we found that, in the Hand-Near condition, orientation tuning sharpened. This suggests that the mechanisms of near-hand attention are different than gain modulation seen with oculomotor-driven spatial attention. In addition, we found that the presence of a nearby hand reduced the variability of neuronal responses. Together, these results show that orientation selectivity is improved near the hand, which could increase the accuracy of subsequent reaches and grasps in the peripersonal workspace.
MATERIALS AND METHODS
Electrophysiology
Two adult female rhesus monkeys were each implanted with a head-holding device and a recording chamber positioned over left V2 using stereotaxic coordinates. Placement was confirmed by assessing RF size and eccentricity, topographic organization and feature selectivity (Gattass et al. 1981; Hubel and Livingstone 1987; Levitt et al. 1994; Roe and Ts'o 1995). A microdrive (3-NRMD-A2, Crist Instruments) was used to advance a tungsten electrode (FHC). Neuronal data were acquired and stored using a Multichannel Acquisition Processor (Plexon). Single neurons were isolated online using Rasputin software (Plexon). RFs were mapped with a manually controlled flashing oriented bar that could be varied in orientation, size, and position. The diameter of the RF varied across neurons but ranged between 1.3 and 4.2 degrees of visual angle (“dva”; mean = 1.8, SD = 0.5). Note that the experiment was carried out if a RF was plotted; orientation selectivity was not tested at this point. This allowed for including neurons that only developed orientation selectivity in the presence of the hand. Neurons were isolated offline using Offline Sorter (Plexon) for subsequent analyses. All experimental and surgical procedures complied with animal care guidelines, as defined by the CACC (Canadian Animal Care Committee) and York University's Animal Care Committee. The study and all associated protocols were approved by York University's Animal Care Committee.
Stimuli and Task
Experimental control was maintained using Cortex software (http://dally.nimh.nih.gov/). Eye gaze was tracked using an infrared eye tracker (ISCAN model ETL-200, 240 Hz). Stimuli were presented on a computer monitor (Viewsonic G225f, 1,024 × 768 resolution, 60 Hz) that was placed 36 cm from the monkey. This distance allowed the animal to comfortably reach with its right hand to a vertically oriented touch bar immediately adjacent to the front of the monitor (Fig. 1) which was present throughout the experiment and positioned outside of the visual RF. The distance from the right edge of the RF to the touch bar ranged between 5.6 cm (8.8 dva) and 7.9 cm (12.5 dva). As the monkeys would grasp the touch bar by wrapping their fingers around it, the distance to the fingers (1.8 dva wide) ranged between 7 and 10.7 dva (see Fig. 1). This minimum distance of 7 dva reduced the possibility of the hand encroaching upon the RF and modulating baseline firing rates, even if hand-mapping underestimated the size of the RF center. With this spacing, visual stimulation within the RF was identical across both conditions (Hand-Away and Hand-Near). The experiments were conducted in a darkened room illuminated by the ambient light from the computer monitor. The hand and touch bar were low contrast but visible to the animals.
In a Hand-Near block, once the animal had grasped the touch bar, each trial began with the appearance of a fixation point (Fig. 1, left). When the animal maintained fixation within a 2 dva window for 200 ms, a task-irrelevant oriented rectangle was presented for 300 ms in the center of the RF. The rectangle varied in orientation (0, 22.5, 45, 67.5, 90, 112.5, 135, and 157.5°) and size (based on the size of the RF). If fixation and grasp of the touch bar were maintained throughout this period, the animal received a reward (Monkey A: juice, Monkey B: fruit). In a Hand-Away block (Fig. 1, right), the touch bar apparatus remained in place, but the animal did not reach and grasp the touch bar. Trials again commenced with the appearance of the fixation point. Each orientation was tested 10–20 times in each hand condition.
We used this paradigm as it replicates the hand position of studies in which a sustained reach placed the hand near visual stimuli and showed improved visual processing and attentional prioritization of near-hand space (Abrams et al. 2008; Brown et al. 2008; Reed et al. 2006). This links the current research to previous neurophysiological work on spatial attention, with “Hand-Near” and “Hand-Away” substituting for “Attend-In” and “Attend-Away” (McAdams and Maunsell 1999; Moran and Desimone 1985; Motter 1993; Treue and Martinez-Trujillo 1999).
Data Analysis
We computed baseline rates from −175 to 0 ms prior to the onset of the oriented rectangle and stimulus response rates from 0 to 300 ms after stimulus onset. From these we computed the following measures.
Orientation tuning index.
To quantify possible changes in tuning between Hand-Near and Hand-Away conditions, we computed an orientation tuning index (OTI): Rpref/Rorth in each condition, where R is the response rate of the neuron for preferred or orthogonal orientation. The preferred orientation was the orientation that produced a maximal response, and the orthogonal orientation was 90° to the preferred orientation. In contrast to curve-fitting, this index, based on response rates, avoids the use of interpolated data when determining changes in tuning.
Response modulation.
We quantified the effect of hand position by computing a number of modulation indexes. First, we computed the percent change of firing rate based on whether a reach had occurred or not: [(HN − HA)/HA] × 100, where HN represents the average response in the Hand-Near condition, and HA represents the average response rate in the Hand-Away condition. We similarly computed the percent change in the response rate to the preferred direction only: [(HNPref − HAPref)/HAPref] × 100. Finally, we computed the modulation of the tuning indexes to determine whether changes in tuning occurred between the Hand-Near and Hand-Away conditions: [(OTIHN − OTIHA)/OTIHA] × 100. Significant shifts were tested using the Wilcoxon signed-rank test.
Curve fitting.
We fit the orientation tuning data for unimodally oriented neurons with a von Mises (vM) function, a circular form of the Gaussian function, used for orientation selectivity (Kohn and Movshon 2004). The function takes the form:
where a is the multiplicative scaling factor, κ is the concentration or bandwidth of tuning, θ is the orientation at that point in the tuning curve, p is the preferred orientation, and m is the baseline rate. Fits were performed in Matlab with the nlinfit function (based upon least squares estimation). For each neuron, fits were computed for Hand-Near and Hand-Away conditions separately. Two neurons in the main population and two neurons in the baseline shifted population were removed from further analysis due to poor fits. For these neurons, nlinfit did not converge to a solution (ill-conditioned Jacobians), and they were rejected from further analysis (similar to Kohn and Movshon 2004). Significant shifts in the fit parameters were tested using the Wilcoxon signed-rank test.
Fano factors.
To quantify response variability we computed Fano factors (FF = spike count variance/mean spike count; Chang et al. 2012; Cohen and Maunsell 2009; McAdams and Maunsell 1999; Mitchell et al. 2007) in the HNpref and HApref conditions. To eliminate the possibility that changes in the FF were influenced by neuronal firing rates, we mean-matched response rates in the HNpref and HApref conditions and then compared the FFs in each using a Wilcoxon signed-rank test.
RESULTS
Inclusion and Exclusion Criteria
Neurons were only included for further analysis if they had a significant visual response over baseline (t-test). As we wanted to test the effect of the hand on orientation tuning, we then limited our analysis to neurons exhibiting significant orientation tuning (one-way ANOVA, e.g., Jansen-Amorim et al. 2011; Motter 1993) in either the Hand-Near or Hand-Away condition. The only difference between the conditions was the presence or absence of the hand on the touch bar. To eliminate the possibility that the hand or arm visually encroached on the classic RF, neurons were excluded if they showed a significant modulation in the baseline firing rate between Hand-Near and Hand-Away conditions (t-test). Eliminating cells from analysis that had a significant shift in their baseline firing rate also removed the possibility that responses were altered due to other variables, such as arousal. Of 93 neurons from which data were obtained, 41 were removed as they were not orientation tuned in either the Hand-Near or Hand-Away conditions (26) or did not have a significant visual response above baseline to the oriented bars (15 neurons). Fifty-two neurons were orientation selective. Although studies have shown that spatial attention can increase baseline responses in area V2 (Luck et al. 1997), 14 cells (baseline-shifted neurons) were analyzed separately as they had a significant baseline modulation between the Hand-Near and Hand-Away conditions, which could also reflect the animal's arm impinging on the RF center. The remaining 38 neurons became the main population for analysis. Note that, of the 52 orientation-selective neurons, 15 were only orientation selective in the Hand-Near condition. These cells would have been missed if we only tested neurons that exhibited orientation selectivity during the mapping of the RF.
Gaze Position
To ensure that gaze position did not shift dependent on hand placement, we calculated the difference between the average horizontal eye position shift between the baseline period and the presentation of the stimulus for each included neuron's experimental session and computed any potential shifts between the Hand-Near and Hand-Away conditions. There was no significant shift [F(1,27) = 0.34, P = 0.568] of the eye position toward the hand [Hand-Near − Hand-Away = −0.002 ± 0.001 (SE) dva]. This indicates that the presence or absence of the hand did not influence gaze position. There was, however, a significant shift of gaze toward the RF during stimulus presentation [F(1,27) = 7.73, P = 0.010]. This suggests that the onset of the stimulus was salient enough to slightly (0.046 dva) draw the eyes toward the RF, regardless of the hand position. This indicates that the hand was not the target of a saccade and an oculomotor-driven shift in attention.
Neuronal Analysis
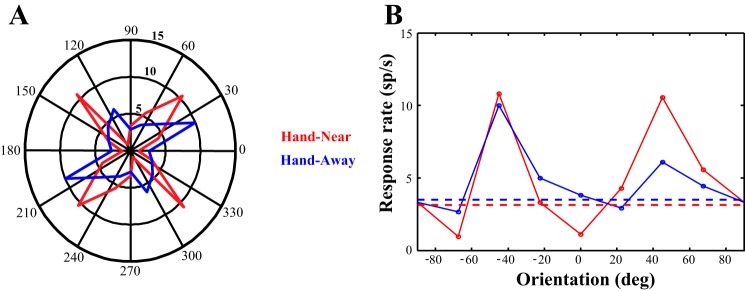
Figure 2 shows the tuning curves of two example neurons. Neuron A depicts a neuron whose responses increased slightly at the preferred orientation but sharpened during the Hand-Near condition (Fig. 2A) due to a reduction in response to the orientations on the flank of the tuning curve. Neuron B instead showed an increase in response to the preferred orientation, with no corresponding change in response at the orthogonal orientation (Fig. 2B). While spatial attention classically results in a proportional increase to responses across the tuning curve, neither neuron A nor B show this pattern of response.
Fig. 2.
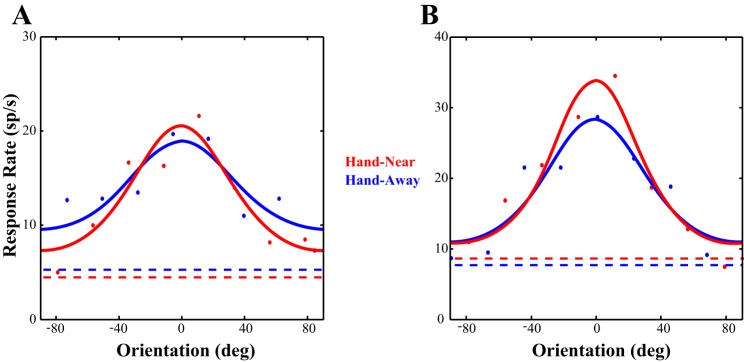
Example cells. The data from two cells fitted with von Mises functions are shown. A: this neuron shows responses that are increased at the preferred orientation and reduced at the orthogonal orientation, resulting in a sharpening of tuning. B: while showing a larger increase response at the preferred orientation, this neuron instead had no modulation at the orthogonal orientation. sp/s, Spikes per second. Dashed lines represent baseline firing rates.
Effect of Hand Position on Preferred and Orthogonal Responses
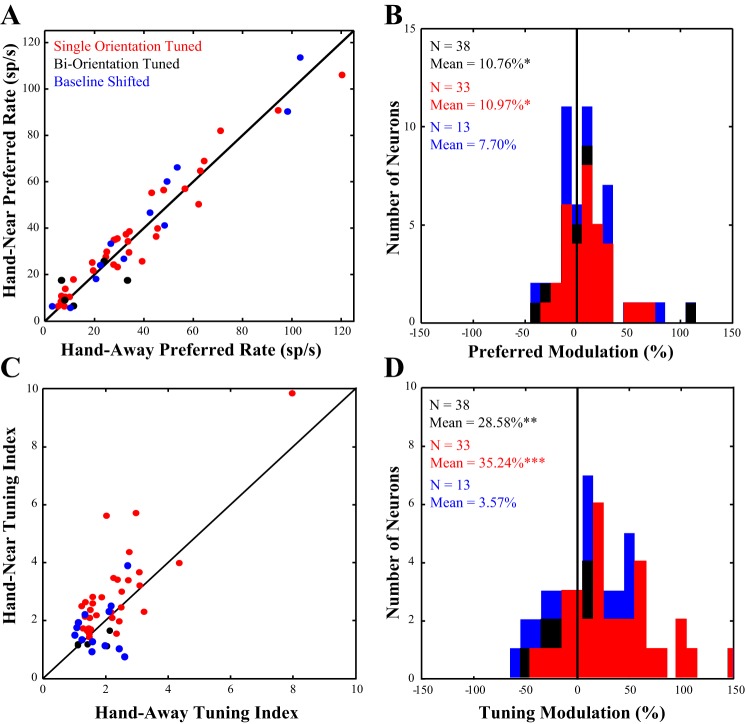
Figure 3A plots the distribution of neuronal responses to the preferred orientation in the Hand-Near vs. the Hand-Away condition. Points that lie above the line of unity indicate cells in which the response rate to the preferred orientation in the Hand-Near condition was greater than in the Hand-Away condition. More of the neurons lie above the line of unity than below (red and black dots). Across the population (n = 38), the response to the preferred orientation significantly increased (Z = 2.12, P = 0.034) by 10.76% (±4.69 SE) in the Hand-Near vs Hand-Away condition (Fig. 3B). In contrast, the population showed no significant increase in the response rate at the orthogonal orientation with the Hand-Near [Z = 1.50, P = 0.133, mean: −3.69 ± 6.69% (SE)]. It is important to note that previous studies of classic spatial attention have shown little to no effect on neuronal responses to irrelevant stimuli when attention is directed outside of the RF (e.g., Moran and Desimone 1985). Finding enhanced responses when grasping a touch bar outside of the RF is not only surprising but also provides neurophysiological evidence that attention is deployed to near-hand space. Based on the distance between the touch bar and the stimulus in the RF, near-hand-related visual enhancement appears to operate with a larger spatial focus than oculomotor-driven spatial attention. In addition, hand position preferentially enhances responses at the preferred orientation and not at the orthogonal orientation.
Fig. 3.
Modulation of preferred rate and tuning. A: the response of each neuron to the preferred orientation in the Hand-Away (x-axis) condition is plotted against the response of each neuron in the Hand-Near (y-axis) condition. The diagonal line on the plot represents the line of unity; the majority of points fall above this line, indicating an increased response to the preferred orientation when the hand was nearby. B: we quantified this change in response by computing a modulation index and found that the presence of the hand significantly increased neuronal response to the preferred orientation (seen as a shift of the population to the right of zero). C: the tuning index of each neuron in the Hand-Away condition (x-axis) is plotted against the tuning index in the Hand-Near condition (y-axis). Again more units fall above the line of unity. D: we used the same modulation index to quantify tuning modulation and found that the presence of the hand significantly sharpened tuning. Data in red represent all neurons in the dataset that were tuned for a single orientation. Data in black represent neurons that were tuned for two orientations, and data in blue are neurons that were excluded from the main analysis, as they showed a significant baseline shift between the Hand-Near and Hand-Away conditions.
Effect of the Hand on Orientation Tuning
Multiplicative gain modulation, proportional increases across stimulus selectivity (McAdams and Maunsell 1999), is a mechanism commonly used to describe how spatial attention affects the responses of visual neurons. That is, multiplicative gain modulation increases responses across the tuning curve without changing the shape of the tuning curve. If, similar to spatial attention, the presence of the hand enhances early visual processing through gain modulation, there should be no change in the OTI (OTI = RPref/ROrth). Plotting the tuning index in the Hand-Near vs the Hand-Away condition (Fig. 3C, red and black dots) shows that the majority of the neurons fall above the line of unity. Tuning is significantly sharpened by 28.58% [±7.71 SE, Z = 3.30, P = 0.001; Fig. 3D] across the population (n = 38). Thus, unlike classic spatial attention which does not affect tuning, hand-related attention sharpened orientation selectivity by almost 30%.
Effect without Biorientation Tuned Cells
Previous work (Anzai et al. 2007) has found that up to 20% of V2 neurons show enhanced responses at two orthogonal orientations (i.e., are biorientation tuned). These types of cells have been shown to be used to determine contours and occlusion (Rubin 2001). Figure 4 shows the responses of an example biorientation tuned neuron. While the polar plot (panel A) doubles up the orientation information, it clearly shows the crossed axes of the two preferred orientations. Panel B shows the same data plotted as an 180° tuning curve. When the hand is present (Hand-Near), the major axis of orientation (e.g., the longer one in the Hand-Away condition) is little changed. However, the response to the minor axis increases. The responses to the orientations in between these two axes are suppressed and drop below baseline. Changes in tuning, then, are hard to determine in biorientation cells as any increase in response in the lesser of the two preferred orientations would reduce the tuning index because the OTI reflects tuning to a single orientation. As tuning indices do not accurately reflect biorientation cells, we reexamined our population and found five biorientation cells (∼13%). We then removed them from our cell population and performed the analyses again. Due to the small sample size (n = 5), we did not analyze the biorientation tuned cells on their own; however, they are depicted separately (black dots and bars in Figs. 3 and Fig. 6).
Fig. 4.
Example biorientation tuned cell. Polar (A) and 180° (B) plots for the same example neuron are shown. The firing rate in the polar plot is depicted as the distance away from the center, and the responses to each orientation are mirrored 180° to depict a circular tuning plot. In the Hand-Near condition, there is an increased response along the minor axis (orthogonal orientation) with no change in the major axis (preferred orientation), compared with the Hand-Away condition. B: also, with the hand present, responses to orientations between the two axes are suppressed below baseline.
Fig. 6.
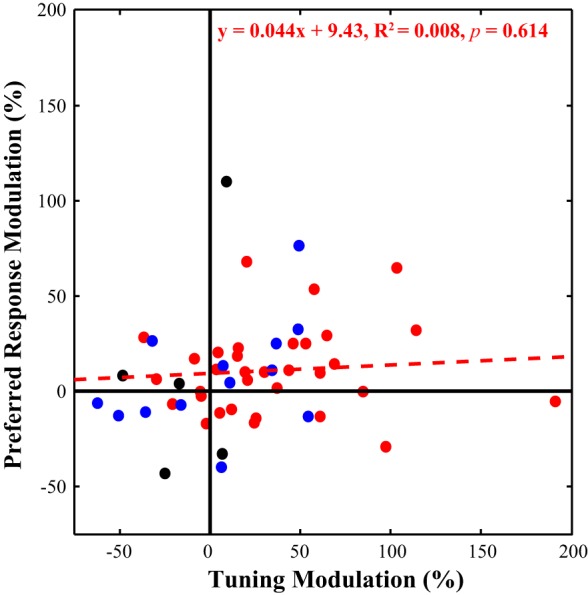
Response modulation vs. sharpened tuning. We plotted the tuning modulation (x-axis) against the preferred modulation (y-axis) for each unit. While the majority of the neurons fall within the top right quadrant, the population of single orientation tuned neurons (in red) did not show a significant relationship between modulation in the preferred response and changes in the tuning index. Biorientation neurons are shown in black, and baseline shifted neurons in blue for comparison.
With the biorientation cells removed, the single orientation population (n = 33) still produced an increase in response when the hand was present (Fig. 3B, red bars). Responses at the preferred orientation were significantly (Z = 2.53, P = 0.011) increased by 10.97% (±3.86 SE) in the Hand-Near vs Hand-Away condition (Fig. 5B). Responses to the orthogonal orientation were now significantly decreased in the presence of the hand [−9.41 ± 5.76% (SE), Z = 2.17, P = 0.030]. The presence of the hand not only improves responses to the preferred orientation, but also decreases responses to the orthogonal orientation. This was masked by the biorientation cells in the whole population because the biorientation cells also preferred the orthogonal orientation. Consistent with these results, the tuning index showed a greater decrease with the hand present when the biorientation cells are removed (Fig. 3D, red bars). Tuning was sharpened by 35.24% (±8.03 SE, Z = 3.76, P < 0.001) in Hand-Near vs Hand-Away, an increase from that seen across the full population (28.58%).
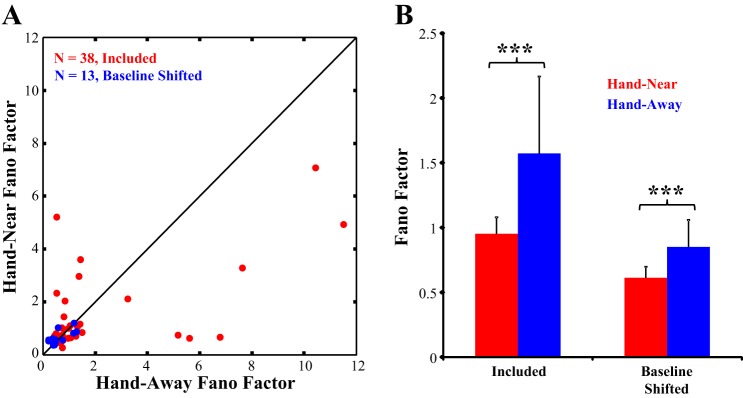
Fig. 5.
Changes in response variability. A: the Fano factor in the Hand-Near and Hand-Away conditions are plotted for the population of neurons included in the dataset (n = 38, in red) and baseline shifted neurons (n = 13, in blue). B: in both populations, response variability was significantly reduced when the hand was present. ***Significant difference, Hand-Near vs. Hand-Away.
Effect of Hand Position on Baseline Shifted Cells
Although studies have shown that spatial attention can increase baseline responses in area V2 (Luck et al. 1997), 14 neurons that had a significant baseline shift between the Hand-Near and the Hand-Away condition were not included in the main analysis. This was done to ensure that the effect of hand position was not being driven by the arm encroaching on the visual RF. We now analyzed the baseline-shifted neurons to determine whether their responses were similar to the rest of the population. None of the cells were biorientation tuned. It should be noted, however, that one cell within this population was removed as an extreme outlier (preferred modulation = 387%, orthogonal modulation = 742%). In the presence of the hand, the remaining baseline shifted cells (n = 13) were not significantly modulated by hand position in their responses to the preferred [mean: 7.70 ± 7.71% (SE), P = 0.267] or the orthogonal orientations [mean: 20.16 ± 14.87% (SE), P = 0.414]. Nor was there a significant modulation of the tuning index [mean: 3.57 ± 11.11% (SE), P = 0.787]. Figure 3 shows the distribution of the baseline shifted cells in blue with the rest of the population (red) and biorientation cells (black). The baseline shifted cells are also depicted in blue in Fig. 6, which shows the cells' distribution across the range of preferred response and tuning modulations. We cannot distinguish whether the lack of an effect of hand position in the baseline shifted cells is due to the arm impinging on the RF, the small sample size, some other factor, or a combination of these possibilities.
Effect of the Hand on Response Variability
Previous studies have shown reductions in response variability during reaching in premotor cortex (Churchland et al. 2010) and oculomotor preparation in frontal eye field (FEF) (Purcell et al. 2012). The reduction in oculomotor response variability has been shown to also propagate back to visual neurons in area V4, which show a similar reduction prior to a saccade (Steinmetz and Moore 2010). If near-hand attention is mediated by feedback from fronto-parietal reaching and grasping networks (Culham et al. 2003), we would expect to find a similar reduction in response variability in V2 neurons when a sustained reach places the hand nearby. To control for changes in firing rate, we first mean-matched response rates in the Hand-Near and Hand-Away conditions (as per Churchland et al. 2010) and then computed their FF (spike count variance/mean spike count). Figure 5A shows the FF distribution in the Hand-Near compared with the Hand-Away condition across the population of 38 neurons included in the dataset (in red). The FF of the preferred orientation response (Fig. 5B) significantly declined (Z = −8.68, P < 0.001) in the Hand-Near condition (mean: 0.96 ± 0.11 SE), compared with the Hand-Away condition (mean: 1.52 ± 0.31 SE). Response variability also significantly declined in the baseline shifted cells (Z = −8.76, P < 0.001, Fig. 5 in blue) in the Hand-Near condition (mean: 0.61 ± 0.086) compared with the Hand-Away condition (mean: 0.85 ± 0.13). This reduction in response variability within near-hand space is consistent with FF reductions seen due to spatial attention and/or motor feedback (Churchland et al. 2010; Purcell et al. 2012; Steinmetz and Moore 2010).
Relationship Between Changes in Response Rates and Orientation Tuning
We investigated the relationship between changes in preferred response and orientation tuning and found different patterns of activity (Fig. 6). While the top right quadrant contains the majority of cells, which exhibited both increased response and sharpened tuning when the hand was near, there was no significant relationship between preferred response modulation and tuning index modulation across the population of cells (F = 0.26, P = 0.614). The biorientation cells, as discussed previously, produced negative tuning index modulations in the Hand-Near condition due to increasing responsivity to the secondary preferred orientation. Thus they are predominantly found on the left-hand side of the distribution (black dots). Finally, the baseline shifted cells are plotted in blue, depicting where they fall among the rest of the population.
Effect of Hand Position on Fitted Tuning Curves
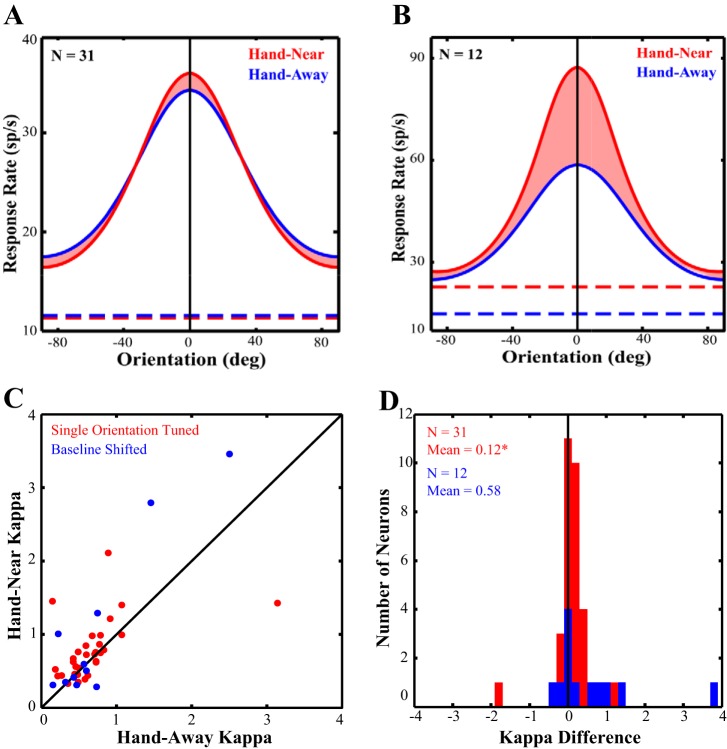
Of the 38 neurons used in the previous analysis, we removed the 5 biorientation tuned cells as they would not be fit by a unimodal von Mises function. We then used the von Mises function to fit the remaining 33 neurons. Two additional neurons were poorly fit (as per the nlinfit function due to ill-conditioned Jacobians) and thus were removed from the population. Figure 7A depicts the population tuning curves in both the Hand-Near and Hand-Away. The shaded area between the two curves highlights how tuning sharpens when the hand is present, with increased responsivity around the preferred orientation and decreased responsivity at orthogonal orientations, consistent with the previous results. κ is the concentration parameter from the fit that describes the tuning bandwidth: the larger the κ, the sharper the tuning. From each cell's individual curve fits, we have plotted the κ in the Hand-Near vs. Hand-Away conditions in Fig. 7C. Consistent with the population tuning curve and the raw data analyses, the majority of cells (Fig. 7C, red dots) fall above the line of unity. κ significantly increased by 17% (+0.114 ± 0.085 SE, Z = 2.49, P = 0.013, Fig. 7D) in the Hand-Near (mean: 0.79 ± 0.07 SE) compared with the Hand-Away (mean: 0.67 ± 0.09 SE) condition. The population amplitude (a), a multiplicative scaling factor that represents the scaling of the response above baseline, was not significantly different in the two hand conditions (Hand-Near: 11.2 ± 1.3 SE; Hand-Away: 11.6 ± 1.4 SE, Z = −0.53, P = 0.60). Thus there was no evidence in support of gain modulation. In addition, the preferred orientation across the population did not significantly differ between Hand-Away and Hand-Near conditions [mean difference: −7.09 ± 10.6° (SE), Z = −0.20, P = 0.85].
Fig. 7.
Curve fit data. The population averages of the tuning curve fits are depicted with the preferred directions aligned to vertical (0°). Baseline firing rates are indicated by the dashed lines. The shaded area between the curves for the Hand-Near (red) and Hand-Away (blue) conditions represents the change in κ (bandwidth) between the conditions. A: neurons tuned for one orientation (n = 31). B: neurons that had a significant baseline shift between the Hand-Near and Hand-Away conditions (n = 12). The majority of the neurons included in the dataset fall above the line of unity (C), producing a significant sharpening in tuning bandwidth (D). The baseline shifted neurons did not show a significant change in κ.
Of the 14 baseline shifted cells, 12 were fit with von Mises functions (Fig. 7B) and 2 were removed as they were poorly fit. In this population, there was a trend (P = 0.09) for an increase in κ in the Hand-Near (mean: 1.32 ± 0.42 SE) over the Hand-Away (mean: 0.738 ± 0.12 SE) condition (Fig. 7, C and D, in blue). Amplitudes were not significantly different (P = 0.233) between Hand-Near (mean: 17.2 ± 2.9 SE) and Hand-Away (mean: 20.9 ± 3.9 SE) conditions. Furthermore, there was no significant shift in preferred orientation between conditions [mean difference: 6.0 ± 3.6° (SE), P = 0.17]. Similar to the results with the raw data, there were no significant differences in this population of baseline shifted cell, although the trend for sharpened tuning may be due to a lack of statistical power due to the small sample size.
Relationship Between Orientation Selectivity and the Orientation of the Hand
While classic spatial attention does not differentially modulate preferred and nonpreferred stimulus response, such an effect has previously been seen with feature-based attention (Treue and Martinez-Trujillo 1999). The feature-similarity gain model (Treue and Martinez-Trujillo 1999) states that the strongest enhancement occurs when the attended feature is also the neuron's preferred stimulus, decreasing as the difference between the two gets larger. We would expect that, if the feature similarity gain model was responsible for the sharpened tuning seen in the current study, then because the task-relevant touch bar was vertical, neurons preferring vertical orientations should have the greatest enhancement, while neurons preferring horizontal orientations should have the least enhancement.
A similar effect would occur with far surround suppression. More recently, there has been a description of “far surrounds” distinct from “near surrounds” for visual neurons in areas V1 and V2 (e.g., Okamoto et al. 2009; Shushruth et al. 2009). The near surround is based on feedforward and horizontal connections, whereas the far surround is based on feedback from extrastriate areas (Shushruth et al. 2009). While the distance between the touch bar and the RF is large enough that the hand was not within the classical near surround of the V2 neurons, the hand may have fallen within the far surround. The effect of far surround stimulation has been shown to enhance orientation selectivity in area V1 in the cat (Okamoto et al. 2009), when large gratings covered from the center to the far surround. The hand and/or touch bar in our paradigm would be a much weaker stimulus, as it only covers a portion of the far surround, but if a similar effect occurred in V2 in the monkey, then we would once again expect that the magnitude of the sharpened tuning would be strongest when the cell's preferred direction was near vertical.
So the potential effects of far surround suppression and feature-based attention would be the same in the current paradigm: the hand/touch bar are vertically oriented in the surround and would have the greatest effect on cells that preferred that orientation and the least effect on cells that preferred horizontal. Instead we found no significant relationship between the neurons' vertical offsets [abs(90°-preferred orientation)] and κ (tuning bandwidth) for the main population (regression analysis, n = 31; F = 0.06, P = 0.81) or the baseline shifted population (n = 12; F = 0.51, P = 0.49). Therefore, near-hand modulation of visual processing was not dependent on the orientation of the touch bar, either through feature-based attention or far surround suppression.
Qualitative Analysis of Full Population
To determine if the presence of the hand had any effect on all of the neurons, regardless of responsivity and selectivity, we performed the following analysis. As a proportion of the neurons were not significantly visually responsive or orientation selective, we had to first estimate a preferred orientation. For each neuron, all trials (Hand-Near and Hand-Away) were averaged, and the peak response was selected as that neuron's preferred orientation. We then aligned the preferred orientations to produce population averages for Hand-Near and Hand-Away conditions. These population averages were then fit using the von Mises function. These results are depicted in Fig. 8. Across the population of all V2 neurons (n = 93), including cells that were not visually responsive or tuned for orientation, there was no evidence of gain modulation as the amplitude did not differ appreciably between the Hand-Near (9.84) and Hand-Away (9.66) conditions. However, there was still a qualitative sharpening of orientation tuning, as κ, the concentration parameter, showed an almost 20% increase in the Hand-Near condition (Hand-Near = 0.429, Hand-Away = 0.359). This pattern of results is similar to that seen in the previous analyses and may likely be driven by the visually responsive, orientation selective neurons.
Fig. 8.
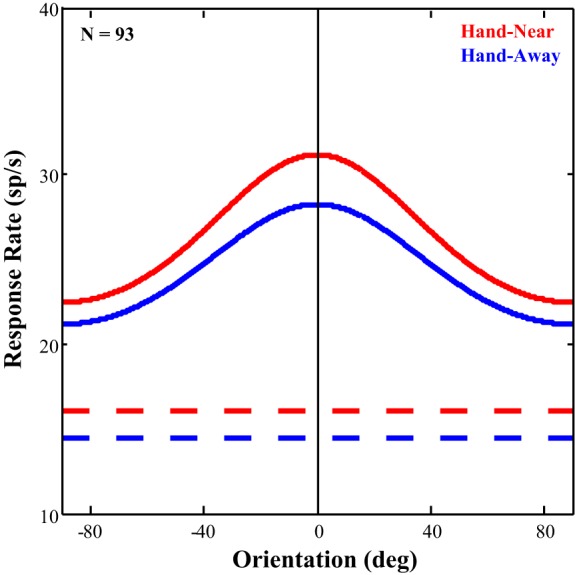
Effect of hand position across the full population. All neurons, regardless of responsivity, were aligned to the orientation of their maximum response, averaged with each hand condition and then fit with von Mises functions. The curve fits and baseline rates are depicted for qualitative comparison. Curve amplitude did not differ appreciably between conditions (Hand-Near = 9.84, Hand-Away = 9.66). There was, however, a qualitative sharpening of tuning which showed an almost 20% increase in the Near-Hand condition (0.429) over that in the Hand-Away (0.359) condition.
DISCUSSION
Previous studies on hand-related attention have focused on behavioral benefits only (Abrams et al. 2008; Bekkering and Neggers 2002; Brown et al. 2008; Craighero et al. 1999; Deubel et al. 1998; di Pellegrino and Frassinetti 2000; Fagioli et al. 2007; Reed et al. 2006; Schendel and Robertson 2004); this study provides the first neurophysiological evidence that a nearby hand affects neuronal responses in an early visual processing area. Our results show that hand position, like gaze position, alters visual processing, but they also show that the mechanisms for these two phenomena are somewhat different. The responses of area V2 neurons were preferentially enhanced to the preferred orientation over the orthogonal orientation (Fig. 7A) and produced sharpened orientation tuning. These results are not completely consistent with current models of spatial attention or feature-similarity gain. Instead, the results suggest a novel effector-based mechanism which improves sensitivity in early visual processing areas of a feature relevant for that effector, i.e., orientation for reaching and grasping with the hand. Furthermore, we showed that a maintained reach and grasp reduced the variability of V2 neuronal responses to nearby task-irrelevant visual stimuli, a result consistent with attentional deployment near the hand. We hypothesize that this reduction in response variability indicates feedback from parietal areas involved in the fronto-parietal motor network, proprioception and/or encoding of peripersonal space. These hand-specific tuning properties may be functionally advantageous because sharpened orientation tuning would allow for more accurate grasping of nearby objects.
Proposed Neural Mechanism
Prior studies of spatial attention (McAdams and Maunsell 1999; McAdams and Reid 2005; Seidemann and Newsome 1999) have shown that visual neurons undergo gain modulation when attended. However, the results of the present study on hand attention do not show gain modulation. In the main population (without biorientation cells), while the preferred response significantly increased in the Hand-Near condition, the orthogonal response significantly decreased. This was also evident in the population tuning curves (Fig. 7A). Thus hand attention sharpened orientation selectivity instead of increasing the gain of the responses across all orientations. Similar effects on direction selectivity have been found in area middle temporal neurons with feature-based attention (Treue and Martinez-Trujillo 1999). But feature-based attention is dependent on congruency between the attended feature, in this case the vertically oriented touch bar and hand, and the preferred orientation of the cell. Instead, we found no relationship between the orientation of the touch bar and the orientation of the visual stimulus within the RF. Therefore, the effects of the hand on visual processing were not driven by feature-based attention either.
A third potential mechanism is based on suppressive surrounds. When a preferred stimulus is presented in the RF and a matching stimulus is presented in the surround, that neuronal response is suppressed by the stimulus in the surround (Akasaki et al. 2002; DeAngelis et al. 1994; Li and Li 1994; Walker et al. 1999, 2000). This surround suppression is thought to be driven by feedforward and horizontal connections. Additionally, a far surround dependent on feedback from extrastriate areas has been described in areas V1 and V2 (e.g., Shushruth et al. 2009). In V1, orientation tuning can be enhanced when a large oriented stimulus covers a cell's classical RF and its far surround (Chen et al. 2005; Okamoto et al. 2009; Orban et al. 1979; Xing et al. 2005). While the hand and touch bar were placed outside the near surround, it is possible they fell within the far surround. However, it is unlikely that the sharpened orientation tuning seen in the Hand-Near condition in the current experiment is due to surround suppression. Surround effects are dependent on the similarity between the stimulus in the surround and the preferred orientation of the cell, but the results of our regression analysis showed no such relationship.
Orientation tuning improved in the Hand-Near over the Hand-Away condition, but this effect was not subserved by spatial attention (gain modulation), feature-based attention, or surround suppression. Therefore, near-hand attention is dependent on a novel mechanism wherein general orientation selectivity is enhanced in the space near the hand. The mechanism operates by enhancing the preferred responses while inhibiting the nonpreferred, which results in sharper tuning.
Proposed Neural Circuitry
As hand-related visual enhancement differs in effect from current models of spatial and feature-based attention, it would need to be served by separate neural circuitry. It has been proposed (Rizzolatti et al. 1987) and demonstrated (Moore and Fallah 2001, 2004; Moore et al. 2003) that recurrent feedback from the oculomotor system modulates visual attention and early visual responses. Reductions in neuronal variability due to movement preparation have been shown to occur in areas ventral premotor (Churchland et al. 2010) and FEF (Purcell et al. 2012). The reduction in response variability found in FEF coincides with reductions in response variability in area V4 prior to a saccade (Steinmetz and Moore 2010). Based on these studies, and given that behavioral studies have shown visual enhancement with both sustained and active arm movements (Abrams et al. 2008; Bekkering and Neggers 2002; Brown et al. 2008; Craighero et al. 1999; Deubel et al. 1998; di Pellegrino and Frassinetti 2000; Fagioli et al. 2007; Festman et al. 2013; Hannus et al. 2005; Langerak et al. 2013; Reed et al. 2006; Schendel and Robertson 2004; Symes et al. 2008), we hypothesize that reductions in V2 neuronal variability in the current study could also reflect feedback from fronto-parietal reaching and grasping networks that would influence subsequent feedforward orientation processing in a recurrent network. In fact, a recent study (Gutteling et al. 2013) showed that temporary inactivation of the anterior intraparietal sulcus (a parietal region associated with grasping movements) using transcranial magnetic stimulation eliminated an increased sensitivity to orientation seen when a grasping vs. a pointing movement was planned.
Areas in posterior parietal cortex both receive visual input to guide actions (sensorimotor integration) as well as providing feedback to extrastriate visual areas (Borra et al. 2008; Passarelli et al. 2011; Prime et al. 2008; Rizzolatti and Matelli 2003). For example, neurons in the anterior intraparietal area are associated with grasping movements and exhibit selectivity for the type, shape, size and orientation of objects that are to be grasped (Monaco et al. 2014; Murata et al. 2000). The inferior parietal lobule, which includes the anterior intraparietal area, also has feedback connections with extrastriate visual areas (Rizzolatti and Matelli 2003) that are thought to be crucial for tactile object recognition. Additionally, area V6A neurons are selective for the orientation of the hand (Fattori et al. 2009), are involved in grasping (Fattori et al. 2010), and have direct connections with area V2 (Passarelli et al. 2011). Therefore, feedback from parietal areas involved with control of the hand should be able to provide the signal that improves orientation selectivity in early visual processing.
These parietal areas receive visual, proprioceptive and motor efference information that could be used to guide (Kalaska 1988; Vesia et al. 2010) the spatial focus for reach-related visual enhancement. First of all, motor efference signals from active motor circuitry, such as motor and premotor cortexes, encode the end point of a reach. Second, proprioceptive processing in somatosensory cortex uses information from the joints, tendons and muscles to determine the location of the arm in space. Third, the visual system encodes the position of a visible arm. For example, vision of a fake arm affects neurons in area 5 that encode the position of the arm (Graziano et al. 2000). Thus area 5 encodes arm position by both vision and proprioception. It is possible that any of these sources could provide the spatial information necessary to guide hand-related attention as the brain regions involved in each are all integrated into the parietal portion of the reaching and grasping network (Buneo and Andersen 2006, 2012; Grea et al. 2002; Pisella et al. 2000). It would be through this integration that reciprocal connections from the parietal areas in the reaching and grasping network may drive hand-related attention. The broader spatial resolution of the motor system would be ideal to improve visual processing of the workspace near the hands, including nearby task-irrelevant stimuli and potential reach targets. In the present study, the arm is visible and the reach is sustained, meaning that visual, proprioceptive and motor efference information is all available. To determine the relative strength of each of these factors in deploying near-hand attention, future studies will need to be conducted with an occluded arm to isolate proprioception from vision of the hand, a fake arm to isolate the contribution of visual information, and with passive arm placement vs. active reaching to disambiguate motor efference feedback.
Other investigations also show that planned hand movements improve visual processing (Craighero et al. 1999; Fagioli et al. 2007; Symes et al. 2008). Specifically, orientation selection was improved when participants were to grasp the visual target (oriented bars) as opposed to when they were to point to the target (Bekkering and Neggers 2002; Gutteling et al. 2011; Hannus et al. 2005). These studies suggest a link between maintaining the plan for hand movement and altered visual processing near the endpoint of the movement. Such a mechanism parallels motor plans in the oculomotor system, deploying attention to the endpoint of the planned saccade (Moore and Fallah 2001, 2004) and enhancing visual responses in area V4 (Moore and Armstrong 2003). Having separate parallel effector-based mechanisms for deploying spatial attention has the advantage that the effectors can more easily be decoupled for movement. That is, we can grab an object while looking elsewhere. In fact, the parietal occipital junction has been implicated in reaching to a peripheral target (Prado et al. 2005), and damage to the posterior parietal cortex results in optic ataxia, an inability to accurately reach to peripheral targets (Carey et al. 1997; Jackson et al. 2005; Milner and Goodale 1995). Thus posterior parietal cortex has separate representations for the spatial locations of gaze and reach targets (Jackson et al. 2009). These parallel effector-based systems could not only maintain separate target locations, but may also provide the signals to improve visual processing of each target.
Note that the full range of the near-hand effect has not yet been determined. The spatial extent of these parietal feedback connections would likely be similar to the spatial extent of the far surround in area V2 (Shushruth et al. 2009), which is also dependent on feedback from extrastriate areas. The spatial extent of the hand effect on orientation selectivity found in this study varied between 8.3 and 14.9 dva, based on the spacing between the hand and the RF borders and the size of the RFs themselves. Alternatively, feedback from parietal cortex may not be spatially limited, but instead extend throughout the ipsilateral visual field. Determining the spatial extent of the near-hand effect may provide further insight into the underlying circuitry.
Oculomotor-Driven Spatial Attention
Prior research and the present results suggest that improved visual processing in near-hand space is due to attentional prioritization of the space near the hand and propose a neural mechanism based upon feedback from parietal areas involved in visual guidance of hand movements. However, similar results may have been found as a result of oculomotor-driven spatial attention. That is not likely due to the following factors. First of all, the stimulus in the RF is task-irrelevant: there is no need for the animals to attend to the oriented rectangle as they make no responses or judgments based upon it. Spatial attention may have been allocated to the touch bar for the animals to make accurate reaches in their visual periphery. However, the orientation stimuli only appear after the touch bar has been grasped. As there was no other location or stimulus requiring attention, oculomotor-driven attention may have been allocated to one of these locations during the task. If the animals had learned to attend to the oriented rectangle to judge the timing of the reward, this attentional allocation would have occurred whether the bar was grasped or not and there would be no modulation between the Hand-Near and Hand-Away conditions. If instead, oculomotor-driven spatial attention was allocated to the touch bar when the hand grasped it, attention would have been allocated away from the oriented stimulus and the recorded neuronal RFs, which would result in lower response rates and poorer orientation selectivity in the Hand-Near vs. Hand-Away conditions, as seen in biased competition (e.g., Desimone and Duncan 1995). Instead, we see increased orientation selectivity when the hand was present, a result opposite to any likely allocation of oculomotor-driven spatial attention.
Attentional Control, Task Design, and Future Studies
Thus this experimental paradigm does not specifically control for the locus of attention beyond requiring gaze fixation. An alternative would have been to use an attentional paradigm such as spatial cueing (e.g., Posner 1980) that controls for spatial attention by allocating attention toward and away from a RF, independent of hand position. While this type of manipulation is useful for determining whether the hand modifies behavior above and beyond that of spatial attention, it would also confound the effects of spatial and hand attention. In an effort to avoid this, the current paradigm was developed to specifically eliminate cues that would allocate spatial attention to the RF stimuli. This allowed for investigating hand attention without the confusion of spatial attention modulations also being involved. So it must be noted that the results of hand attention in this study cannot be directly related to spatial attention. While previous studies in humans have suggested that spatial cueing operates independently of hand attention in speeded reaction time studies (Abrams et al. 2008; Reed et al. 2006), future studies will be needed to determine how they interact on neurons in visual processing areas.
The experimental paradigm used in the present study also sought to dissociate the eyes and the hand and thus did not have the animal reach to the visual stimulus that was presented in the neuronal RF. It is known that, when reaching, the eyes move to the reach target prior to the hand arriving (Ballard et al. 1992; Biguer et al. 1982; Fisk and Goodale 1985; Neggers and Bekkering 2000, 2001, 2002; Prablanc et al. 1979). Since spatial attention is allocated to the target region of an oculomotor plan (Moore and Armstrong 2003; Moore and Fallah 2001, 2004; Müller et al. 2005), even though the eye movement output is inhibited (i.e., with continued fixation), the plan to move the eyes, and thus the shifting of spatial attention, would still occur if a reach was made to the stimulus in the RF. This would again mean that, in the Hand-Near condition, the results would be confounded with those of spatial attention. By placing the touch bar outside of the RF, it ensures that, when a reach occurs, spatial attention is not allocated to the experimental visual stimulus (the oriented bar within the RF). A limitation of this design is that only the effects of a maintained reach have been determined. Since we suggest that improved orientation selectivity near the hand would be useful in guiding the hand for more accurate grasps, it would be important to also determine the temporal aspects of near-hand attention that unfold before and during an active reach to a target in the RF. With the results of this study as a template, future studies could investigate hand attention during a dynamic reach.
Summary and Conclusion
In conclusion, we find that, when a hand is nearby, neurons in area V2 exhibit sharpened orientation selectivity and reduced response variability. It was not dependent on the relationship between the orientation of the touch bar and the oriented rectangle, suggesting it was a general improvement in orientation selectivity instead of feature-based attention or far surrounds suppression. These factors are advantageous for guiding subsequent or on-going hand movements. We propose that parietal areas involved in grasping and encoding peripersonal space are likely involved in deploying near-hand attention, although future work is necessary to support this hypothesis.
GRANTS
This study was supported by a National Sciences and Engineering Research Council Canadian Graduate Scholarship to C. J. Perry; CFI Leaders Opportunity Fund to M. Fallah; Ontario Research Fund to M. Fallah; J.P. Bickell Foundation Medical Research Grant to M. Fallah; and a Canadian Institute of Health Research Operating Grant to J. D. Crawford.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.J.P., L.E.S., J.D.C., and M.F. conception and design of research; C.J.P. performed experiments; C.J.P. and M.F. analyzed data; C.J.P. and M.F. interpreted results of experiments; C.J.P. prepared figures; C.J.P. and M.F. drafted manuscript; C.J.P., L.E.S., J.D.C., and M.F. edited and revised manuscript; C.J.P., L.E.S., J.D.C., and M.F. approved final version of manuscript.
REFERENCES
- Abrams RA, Davoli CC, Du F, Knapp WH, Paull D. Altered vision near the hands. Cognition 107: 1035–1047, 2008. [DOI] [PubMed] [Google Scholar]
- Akasaki T, Sato H, Yoshimura Y, Ozeki H, Shimegi S. Suppressive effects of receptive field surround on neuronal activity in the cat primary visual cortex. Neurosci Res 43: 207–220, 2002. [DOI] [PubMed] [Google Scholar]
- Anzai A, Peng X, Van Essen DC. Neurons in monkey visual area V2 encode combinations of orientations. Nat Neurosci 10: 1313–1321, 2007. [DOI] [PubMed] [Google Scholar]
- Ballard DH, Hayhoe MM, Li F, Whitehead SD. Hand-eye coordination during sequential tasks. Philos Trans R Soc Lond B Biol Sci 337: 331–338, 1992. [DOI] [PubMed] [Google Scholar]
- Bekkering H, Neggers SF. Visual search is modulated by action intentions. Psychol Sci 13: 370–374, 2002. [DOI] [PubMed] [Google Scholar]
- Biguer B, Jeannerod M, Prablanc. The coordination of eye, head, and arm movements during reaching at a single visual target. Exp Brain Res 46: 301–304, 1982. [DOI] [PubMed] [Google Scholar]
- Borra E, Belmalih A, Calzavara R, Gerbella M, Murata A, Rozzi S, Luppino G. Cortical connections of the macaque anterior intraparietal (AIP) area. Cereb Cortex 18: 1094–1111, 2008. [DOI] [PubMed] [Google Scholar]
- Brown LE, Kroliczak G, Demonet JF, Goodale MA. A hand in blindsight: hand placement near target improves size perception in the blind visual field. Neuropsychologia 46: 786–802, 2008. [DOI] [PubMed] [Google Scholar]
- Buneo CA, Andersen RA. The posterior parietal cortex: sensorimotor interface for the planning and online control of visually guided movements. Neuropsychologia 44: 2594–2606, 2006. [DOI] [PubMed] [Google Scholar]
- Buneo CA, Andersen RA. Integration of target and hand position signals in the posterior parietal cortex: effects of workspace and hand vision. J Neurophysiol 108: 187–199, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey DP, Coleman RJ, Della Sala S. Magnetic misreaching. Cortex 33: 639–652, 1997. [DOI] [PubMed] [Google Scholar]
- Chang MH, Armstrong KM, Moore T. Dissociation of response variability from firing rate effects in frontal eye field neurons during visual stimulation, working memory, and attention. J Neurosci 32: 2204–2216, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Dan Y, Li CY. Stimulation of non-classical receptive field enhances orientation selectivity in the cat. J Physiol 564: 233–243, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland MM, Yu BM, Cunningham JP, Sugrue LP, Cohen MR, Corrado GS, Newsome WT, Clark AM, Hosseini P, Scott BB, Bradley DC, Smith MA, Kohn A, Movshon JA, Armstrong KM, Moore T, Chang SW, Snyder LH, Lisberger SG, Priebe NJ, Finn IM, Ferster D, Ryu SI, Santhanam G, Sahani M, Shenoy KV. Stimulus onset quenches neural variability: a widespread cortical phenomenon. Nat Neurosci 13: 369–378, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MR, Maunsell HR. Attention improved performance primarily by reducing interneuronal correlations. Nat Neurosci 12: 1594–1601, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craighero L, Fadiga L, Rizzolatti G, Umiltà C. Action for perception: a motor-visual attentional effect. J Exp Psychol Hum Percept Perform 25: 1673–1692, 1999. [DOI] [PubMed] [Google Scholar]
- Culham JC, Danckert SL, DeSouza JF, Gati JS, Menon RS, Goodale MA. Visually guided grasping produces fMRI activation in dorsal but not ventral stream brain areas. Exp Brain Res 153: 180–189, 2003. [DOI] [PubMed] [Google Scholar]
- DeAngelis GC, Freeman RD, Ohzawa I. Length and width tuning of neurons in the cat's primary visual cortex. J Neurophysiol 71: 347–374, 1994. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective attention. Annu Rev Neurosci 18: 193–222, 1995. [DOI] [PubMed] [Google Scholar]
- Deubel H, Schneider WX, Paprotta I. Selective dorsal and ventral processing: evidence for a common attentional mechanism in reaching and perception. Vis Cogn 5: 81–107, 1998. [Google Scholar]
- di Pellegrino G, Frassinetti F. Direct evidence from parietal extinction of enhancement of visual attention near a visible hand. Curr Biol 10: 1475–1477, 2000. [DOI] [PubMed] [Google Scholar]
- Fagioli S, Ferlazzo F, Hommel B. Contolling attention through action: observing actions primes action-related stimulus dimensions. Neuropsychologia 45: 3351–3355, 2007. [DOI] [PubMed] [Google Scholar]
- Fattori P, Breveglieri R, Marzocchi N, Filippini D, Bosco A, Galletti C. Hand orientation during reach-to-grasp movements modulates neuronal activity in the medial posterior parietal area V6A. J Neurosci 29: 1928–1936, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattori P, Raos V, Breveglieri R, Bosco A, Marzocchi N, Galletti C. The dorsomedial pathway is not just for reaching: grasping neurons in the medial parieto-occipital cortex of the macaque monkey. J Neurosci 30: 342–349, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festman Y, Adam JJ, Pratt J, Fischer MH. Both hand position and movement direction modulate visual attention. Front Psychol 4: 657, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisk JD, Goodale MA. The organization of eye and limb movements during unrestricted reaching to targets in the contralateral and ipsilateral visual space. Exp Brain Res 60: 159–178, 1985. [DOI] [PubMed] [Google Scholar]
- Gattass R, Gross CG, Sandell JH. Visual topography of V2 in the macaque. J Comp Neurol 20: 519–539, 1981. [DOI] [PubMed] [Google Scholar]
- Gattass R, Sousa AP, Mishkin M, Ungerleider LG. Cortical projections of area V2 in the macaque. Cereb Cortex 7: 110–129, 1997. [DOI] [PubMed] [Google Scholar]
- Graziano MSA, Cooke DF, Taylor CSR. Coding the location of the arm by sight. Science 290: 1782–1786, 2000. [DOI] [PubMed] [Google Scholar]
- Grea H, Pisella L, Rossetti Y, Desmurget M, Tilikete C, Grafton S, Prablanc C, Vighetto A. A lesion of the posterior parietal cortex disrupts on-line adjustments during aiming movements. Neuropsychologia 40: 2471–2480, 2002. [DOI] [PubMed] [Google Scholar]
- Gutteling TP, Kenemans JL, Neggers SF. Grasping preparation enhances orientation change detection. PLos One 6: e17675, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteling TP, Park SY, Kenemans JL, Neggers SFW. TMS of the anterior intraparietal area selectively modulates orientation change detection during action preparation. J Neurophysiol 110: 33–41, 2013. [DOI] [PubMed] [Google Scholar]
- Hannus A, Cornelissen FW, Lindemann O, Bekkering H. Selection-for-action in visual search. Acta Psychol (Amst) 118: 171–191, 2005. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Livingstone MS. Segregation of form, color, and stereopsis in primate area 18. J Neurosci 7: 3378–3415, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SR, Newport R, Husain M, Fowlie JE, O'Donoghue M, Bajaj N. There may be more to reaching than meets the eye: re-thinking optic ataxia. Neuropsychologia 47: 1397–1408, 2009. [DOI] [PubMed] [Google Scholar]
- Jackson SR, Newport R, Mort D, Husain M. Where the eye looks, the hand follows; limb-dependent magnetic misreaching in optic ataxia. Curr Biol 15: 42–46, 2005. [DOI] [PubMed] [Google Scholar]
- Jansen-Amorim AK, Lima B, Fiorani M, Gattass R. GABA inactivation of visual area MT modifies the responsiveness and direction selectivity of V2 neurons in Cebus monkey. Vis Neurosci 28: 513–527, 2011. [DOI] [PubMed] [Google Scholar]
- Kalaska JF. The representation of arm movements in postcentral and parietal cortex. Can J Physiol Pharmacol 66: 455–463, 1988. [DOI] [PubMed] [Google Scholar]
- Kohn A, Movshon JA. Adaptation change the direction of tuning of macaque MT neurons. Nat Neurosci 7: 764–772, 2004. [DOI] [PubMed] [Google Scholar]
- Langerak RM, La Mantia CL, Brown LE. Global and local processing near the left and right hands. Front Psychol 4: 793, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CY, Li W. Extensive integration field beyond the classical receptive field of cat's striate cortical neurons-classification and tuning properties. Vision Res 34: 2337–2355, 1994. [DOI] [PubMed] [Google Scholar]
- Levitt JB, Kiper DC, Movshon JA. Receptive fields and functional architecture of macaque V2. J Neurophysiol 71: 2517–2542, 1994. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Chelazzi L, Hillyard SA, Desimone R. Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. J Neurophysiol 77: 24–42, 1997. [DOI] [PubMed] [Google Scholar]
- McAdams CJ, Maunsell JH. Effects of attention on orientation-tuning functions of single neurons in macaque cortical area V4. J Neurosci 19: 431–441, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdams CJ, Reid RC. Attention modulates the responses of simple cells in monkey primary visual cortex. J Neurosci 25: 11023–11033, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner AD, Goodale MA. The Visual Brain in Action. New York: Oxford, 1995. [Google Scholar]
- Mitchell JF, Sundberg KA, Reynolds JH. Differential attention-dependent response modulation across cell classes in macaque visual area V4. Neuron 55: 131–141, 2007. [DOI] [PubMed] [Google Scholar]
- Monaco S, Chen Y, Medendorp WP, Crawford JD, Fiehler K, Henriques DY. Functional magnetic resonance imaging adaptation reveals the cortical networks for processing grasp-relevant object properties. Cereb Cortex 24: 1540–1554, 2014. [DOI] [PubMed] [Google Scholar]
- Moore T, Armstrong KM. Selective gating of visual signals by microstimulation of frontal cortex. Nature 421: 370–373, 2003. [DOI] [PubMed] [Google Scholar]
- Moore T, Armstrong KM, Fallah M. Visuomotor origins of covert spatial attention. Neuron 40: 671–683, 2003. [DOI] [PubMed] [Google Scholar]
- Moore T, Fallah M. Control of eye movements and spatial attention. Proc Nat Acad Sci U S A 98: 1273–1276, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T, Fallah M. Microstimulation of the frontal eye field and its effects on covert spatial attention. J Neurophysiol 91: 152–162, 2004. [DOI] [PubMed] [Google Scholar]
- Moran J, Desimone R. Selective attention gates visual processing in the extrastriate cortex. Science 229: 782–784, 1985. [DOI] [PubMed] [Google Scholar]
- Motter BC. Focal attention produces spatially selective processing in visual cortical areas V1, V2, and V4 in the presence of competing stimuli. J Neurophysiol 70: 909–919, 1993. [DOI] [PubMed] [Google Scholar]
- Müller JR, Philiastides MG, Newsome WT. Microstimulation of the superior colliculus focuses attention without moving the eyes. Proc Natl Acad Sci U S A 102: 524–529, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata A, Gallese V, Luppino G, Kaseda M, Sakata H. Selectivity for the shape, size, and orientation of objects for grasping in neurons of monkey parietal area AIP. J Neurophysiol 83: 2580–2601, 2000. [DOI] [PubMed] [Google Scholar]
- Neggers SF, Bekkering H. Ocular gaze is anchored to the target of an ongoing pointing movement. J Neurophysiol 83: 639–651, 2000. [DOI] [PubMed] [Google Scholar]
- Neggers SF, Bekkering H. Gaze anchoring to a pointing target is present during the entire pointing movement and is driven by a non-visual signal. J Neurophysiol 86: 961–970, 2001. [DOI] [PubMed] [Google Scholar]
- Neggers SF, Bekkering H. Coordinated control of eye and hand movement in dynamic reaching. Hum Mov Sci 21: 349–376, 2002. [DOI] [PubMed] [Google Scholar]
- Okamoto M, Naito T, Sadakane O, Osaki H, Sato H. Surround suppression sharpens orientation tuning in the cat primary visual cortex. Eur J Neurosci 29: 1035–1046, 2009. [DOI] [PubMed] [Google Scholar]
- Orban GA, Kato H, Bishop PO. End-zone region in receptive fields of hypercomplex and other striate neurons in the cat. J Neurophysiol 42: 818–832, 1979. [DOI] [PubMed] [Google Scholar]
- Passarelli L, Rosa MG, Gamberini M, Bakola S, Burman KJ, Fattori P, Galletti C. Cortical connections of area V6Av in the macaque: a visual-input node to the eye/hand coordination system. J Neurosci 31: 1790–1801, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisella L, Grea H, Tilikete C, Vighetto A, Desmurget M, Rode G, Boisson D, Rossetti Y. An “automatic pilot” for the hand in human posterior parietal cortex: toward reinterpreting optic ataxia. Nat Neurosci 3: 729–736, 2000. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Q J Exp Psychol 32: 3–25, 1980. [DOI] [PubMed] [Google Scholar]
- Prablanc C, Echallier JF, Komilis E, Jeannerod M. Optimal response of eye and hand motor systems in point at a virtual target. I. Spatio-temporal characteristics of eye and hand movements and their relationships when varying the amount of visual information. Biol Cybern 35: 113–124, 1979. [DOI] [PubMed] [Google Scholar]
- Prado J, Clavagnier S, Otzenberger H, Scheiber C, Kennedy H, Perenin MT. Two cortical systems for reaching in central and peripheral vision. Neuron 48: 849–858, 2005. [DOI] [PubMed] [Google Scholar]
- Prime SL, Vesia M, Crawford JD. Transcranial magnetic stimulation over posterior parietal cortex disrupts transsaccadic memory of multiple objects. J Neurosci 28: 6938–6949, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell BA, Heitz RP, Cohen JY, Schall JD. Response variability of frontal eye field neurons modulates with sensory input and saccade preparation but not visual search salience. J Neurophysiol 108: 2737–2750, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raos V, Umilta MA, Gallese V, Fogassi L. Functional properties of grasping-related neurons in the dorsal premotor area F2 of the macaque monkey. J Neurophysiol 92: 1990–2002, 2004. [DOI] [PubMed] [Google Scholar]
- Reed CL, Grubb JD, Steele C. Hands up: attentional prioritization of space near the hand. J Exp Psychol Hum Percept Perform 32: 166–177, 2006. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Matelli M. Two different streams form the dorsal visual stream: anatomy and functions. Exp Brain Res 153: 146–157, 2003. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Riggio L, Dascola I, Umilta C. Reorienting attention across the horizontal and vertical meridians: Evidence in favor of a premotor theory of attention. Neuropsychologia 25: 31–40, 1987. [DOI] [PubMed] [Google Scholar]
- Roe AW, Ts'o DY. Visual topography in primate V2: multiple representation across functional stripes. J Neurosci 15: 3689–3715, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin N. The role of junctions in surface completion and contour matching. Perception 30: 339–366, 2001. [DOI] [PubMed] [Google Scholar]
- Schendel K, Robertson LC. Reaching out to see: arm position can attenuate human visual loss. J Cogn Neurosci 16: 935–943, 2004. [DOI] [PubMed] [Google Scholar]
- Seidemann E, Newsome WT. Effect of spatial attention on the responses of area MT neurons. J Neurophysiol 81: 1783–1794, 1999. [DOI] [PubMed] [Google Scholar]
- Shushruth S, Ichida JM, Levitt JB, Angelucci A. Comparison of spatial summation properties of neurons in macaque V1 and V2. J Neurophysiol 102: 2069–2083, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz NA, Moore T. Changes in the response rate and response variability of area V4 neurons during the preparation of saccadic eye movements. J Neurophysiol 103: 1171–1178, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symes E, Tucker M, Ellis R, Vainio L, Ottoboni G. Grasp preparation improves change detection for congruent objects. J Exp Psychol Hum Percept Perform 34: 854–871, 2008. [DOI] [PubMed] [Google Scholar]
- Treue S, Martinez-Trujillo JC. Feature-based attention influences motion processing gain in macaque visual cortex. Nature 399: 575–579, 1999. [DOI] [PubMed] [Google Scholar]
- Vesia M, Yan X, Sergio LE, Crawford JD. Specificity of human parietal saccade and reach regions during transcranial magnetic stimulation. J Neurosci 30: 13053–13065, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker GA, Ohzawa I, Freeman RD. Asymemtric suppression outside the classical receptive receptive field of the visual cortex. J Neurosci 19: 10536–10553, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker GA, Ohzawa I, Freeman RD. Suppression outside the classical cortical receptive field. Vis Neurosci 17: 369–379, 2000. [DOI] [PubMed] [Google Scholar]
- Xing D, Shapley RM, Hawken MJ, Ringbach DL. Effect of stimulus size on the dynamics of orientation selectivity in Macaque V1. J Neurophysiol 94: 799–812, 2005. [DOI] [PubMed] [Google Scholar]