Abstract
The Q175 knockin mouse model of Huntington's disease (HD) carries a CAG trinucleotide expansion of the human mutant huntingtin allele in its native mouse genomic context and recapitulates the genotype more closely than transgenic models. In this study we examined the progression of changes in intrinsic membrane properties and excitatory and inhibitory synaptic transmission, using whole cell patch-clamp recordings of medium-sized spiny neurons (MSNs) in the dorsolateral striatum and cortical pyramidal neurons (CPNs) in layers 2/3 of the primary motor cortex in brain slices from heterozygous (Q175+/−) and homozygous (Q175+/+) mice. Input resistance in MSNs from Q175+/+ and Q175+/− mice was significantly increased compared with wild-type (WT) littermates beginning at 2 mo. Furthermore, the frequency of spontaneous and miniature excitatory postsynaptic currents (EPSCs) was significantly reduced in MSNs from Q175+/+ and Q175+/− mice compared with WTs beginning at 7 mo. In contrast, the frequency of spontaneous inhibitory postsynaptic currents (sIPSCs) and IPSC-to-EPSC ratios were increased in MSNs from Q175+/+ mice beginning at 2 mo. Morphologically, significant decreases in spine density of MSNs from Q175+/− and Q175+/+ mice occurred at 7 and 12 mo. In CPNs, sIPSC frequencies and IPSC-to-EPSC ratios were significantly increased in Q175+/− mice compared with WTs at 12 mo. There were no changes in intrinsic membrane properties or morphology. In summary, we show a number of alterations in electrophysiological and morphological properties of MSNs in Q175 mice that are similar to other HD mouse models. However, unlike other models, CPN inhibitory activity is increased in Q175+/− mice, indicating reduced cortical excitability.
Keywords: cerebral cortex, electrophysiology, Huntington's disease, Q175, striatum
huntington's disease (HD), a progressive autosomal dominant neurodegenerative disorder, is characterized by motor, cognitive, and psychiatric symptoms (Haddad and Cummings 1997). It is caused by an abnormal expansion of CAG repeats in the coding region of the huntingtin (Htt) gene (Huntington's Disease Collaborative Research Group 1993). In HD, the most striking neuropathology is a massive loss of medium-sized spiny neurons (MSNs) in the striatum (Vonsattel and DiFiglia 1998) as well as laminar thinning and white matter and neuronal loss in the cerebral cortex (Rosas et al. 2006).
The discovery of the gene responsible for HD enabled the study of mechanisms and disease progression through development of genetic animal models. The most currently used models include fragment, full-length, and knockin (KI) models. These models differ in CAG repeat length, copy numbers, and transgene expression levels. One of the first transgenic mouse models of HD, the R6/2 fragment model, carries exon 1 of the mutant gene and produces an aggressive phenotype more akin to the juvenile form of HD (Mangiarini et al. 1996). The most widely studied full-length mouse models express human Htt in yeast or bacterial artificial chromosomes (YAC and BAC, respectively) (Gray et al. 2008; Hodgson et al. 1999; Slow et al. 2003). These models tend to display a more protracted and less severe phenotype.
KI mouse models usually express full-length mutant (m)Htt, with varying CAG repeat lengths, in its native genomic context and therefore recapitulate the human disease more faithfully than transgenic mouse models (Heng et al. 2007, 2010; Lin et al. 2001; Menalled 2005; Menalled et al. 2003, 2012; Shelbourne et al. 1999; Yu et al. 2003). In such models early overt behavioral changes are often subtle, but careful testing demonstrates abnormalities as early as 1–2 mo of age (Menalled et al. 2002) and a more severe phenotype develops as the mice age (Hickey et al. 2008). Electrophysiological analyses have demonstrated alterations of synaptic activity in striatum and cerebral cortex in all HD models examined (Cepeda et al. 2007; Cummings et al. 2009; Raymond et al. 2011). In striatum, transient and progressive changes in spontaneous excitatory synaptic activity and biphasic changes in receptor sensitivity have been shown (Cepeda et al. 2003; Graham et al. 2009; Joshi et al. 2009). In contrast, inhibitory GABAergic synaptic activity is increased in a subset of MSNs (Centonze et al. 2005; Cepeda et al. 2004, 2013; Cummings et al. 2010; Dvorzhak et al. 2013).
Less is known about alterations in cortical electrophysiology, but significant changes have been described in several HD models (Cummings et al. 2007, 2009; Spampanato et al. 2008). This is important, as MSNs receive major excitatory glutamatergic inputs from the cortex and, to a lesser degree, from the thalamus. We previously demonstrated a disruption of corticostriatal communication that increases with disease progression in the R6/2 mouse model of HD (Cepeda et al. 2003). How, when, and why this occurs remains unknown, but a progressive corticostriatal disconnection supports the notion that cell-cell interactions are essential to understanding of HD pathophysiology (Gu et al. 2007). Indeed, removing cortical mHtt ameliorates the motor and psychiatric disturbances in HD mice (Wang et al. 2014). It is thus important to examine the chronology and progression of striatal and cortical synaptic alterations to determine whether changes in one region precede changes in the other.
In the present study, we examined the onset and progression of electrophysiological alterations in the cortex and striatum of a recently described KI model, the Q175. This KI mouse line was derived from a spontaneous germline CAG expansion from the CAG140 line (Menalled et al. 2012) and carries the human mHtt gene in the mouse genomic context (Heng et al. 2007; Menalled 2005). Recent studies have characterized some of the behavioral, morphological, and electrophysiological alterations in these mice. Behavioral changes include motor, cognitive, and circadian deficits (Loh et al. 2013; Menalled et al. 2012). Morphological alterations consist of decreases in the number of MSNs and striatal volume loss (Heikkinen et al. 2012). Electrophysiological studies have shown that MSNs of 6- to 9-mo-old Q175 mice exhibit intrinsic hyperexcitability, but there is a significant decrease in corticostriatal excitatory synaptic input (Heikkinen et al. 2012). However, a systematic evaluation of correlated excitatory and inhibitory synaptic inputs to individual MSNs, combined with single-cell morphology, has not yet been performed. In addition, alterations in cortical pyramidal neurons (CPNs) have not been examined in this model. Thus the present study provides a comprehensive characterization of the electrophysiological and morphological phenotype of the Q175 mouse model that includes evaluation of both excitatory and inhibitory synaptic inputs to MSNs and CPNs, as well as more detailed morphological examination of somatic size, dendritic complexity, and spine density of recorded neurons.
MATERIALS AND METHODS
Animals.
All experimental procedures were performed in accordance with the US Public Health Service Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of California, Los Angeles (UCLA). Male and female Q175 KI mice and wild-type (WT) littermates were obtained from our colony at UCLA. The Q175 KI mouse line was derived from a spontaneous expansion of the CAG copy number in CAG140 KI mice, in which a fragment extending upstream of Htt exon 1 has been replaced with the human HTT sequence containing 140 repeats of the CAG tract (Menalled et al. 2003, 2012). These mice were microinjected with the targeting vector into embryonic stem cells and backcrossed with C57BL/6J mice. The Q175 mice used in this study had a range of 166–196 CAG repeats (mean = 183 ± 1.8, n = 40). All mice were genotyped twice, once at weaning and again after electrophysiological recordings. For experiments in striatum, homozygous (Q175+/+) and heterozygous (175+/−) animals were examined at three ages: 2 mo (behaviorally presymptomatic), 7 mo (overt behavioral phenotype), and 12 mo (full behavioral phenotype). Our experiments in the cortex of Q175 mice were limited to two genotypes (WT and Q175+/−) and two time points (2 and 12 mo). This limitation was caused by breeding difficulties. Every effort was made to minimize pain, discomfort, and the number of mice used.
Cell visualization and electrophysiology.
Detailed procedures for slice preparation have been published previously (Andre et al. 2011; Cepeda et al. 2008, 2013). Briefly, mice were deeply anesthetized with isoflurane and killed. The brain was rapidly removed and placed in ice-cold low-Ca2+ artificial cerebrospinal fluid (ACSF) containing the following (in mM): 130 NaCl, 3 KCl, 1.25 NaH2PO4, 26 NaHCO3, 5 MgCl2, 1 CaCl2, and 10 glucose. Coronal slices were cut (350 μm) with a microslicer (Leica VT1000S; Leica Microsystems) and transferred to an incubating chamber containing ACSF (with 2 mM CaCl2 and 2 mM MgCl2) oxygenated with 95% O2-5% CO2 (pH 7.2–7.4, 290–310 mosM). Recordings began after at least 1-h incubation at room temperature. The microscope (Olympus BX51WI) was equipped with differential interference contrast optics and infrared imaging. Whole cell patch-clamp recordings in voltage-clamp mode were obtained with a MultiClamp 700B Amplifier (Molecular Devices) and pCLAMP 10.2. Patch pipettes (3–5 MΩ) contained a Cs-methanesulfonate-based internal solution with 0.2% biocytin and the following salt concentrations (in mM): 130 Cs-methanesulfonate, 10 CsCl, 4 NaCl, 1 MgCl2, 5 MgATP, 5 EGTA, 10 HEPES, 5 GTP, 10 phosphocreatine, and 0.1 leupeptin (pH 7.2 with CsOH, 270 mosM). Electrode access resistances were <25 MΩ.
Basic membrane properties were determined with a depolarizing step voltage command (5 mV) using the membrane test function integrated in the pCLAMP software. MSNs in the dorsolateral striatum and CPNs in layers 2/3 of the primary motor cortex (M1) were recorded. They were initially voltage-clamped at −70 mV, and spontaneous currents were recorded. These currents were mixtures of spontaneous excitatory postsynaptic currents (sEPSCs) and spontaneous inhibitory postsynaptic currents (sIPSCs), and the data are not reported here. The holding potential (VHold) was stepped to +10 mV to record sIPSCs. The same cells were then held at −70 mV in the presence of bicuculline (BIC, 10 μM) to block γ-aminobutyric acid type A (GABAA) receptor-mediated currents and to record sEPSCs in isolation. Tetrodotoxin (TTX, 1 μM) was added to record miniature (m)EPSCs. Recorded postsynaptic currents were filtered at 1 kHz with Clampex 10.2 in gap-free mode. sEPSCs and sIPSCs were analyzed off-line with the automated detection protocol within the Mini Analysis program (Justin Lee, Synaptosoft, version 6.0) and subsequently checked manually for accuracy. Event analyses were performed blind to genotype. The threshold amplitude for the detection of an event (5 pA for sEPSCs; 10 pA for sIPSCs) was set above the root mean square noise (<2 pA at VHold = −70 mV and <4 pA at VHold = +10 mV). sEPSCs and IPSCs with peak amplitudes between 10 and 50 pA and between 10 and 100 pA, respectively, were grouped, aligned by half-rise time, and normalized by peak amplitude to calculate event kinetics. For each cell, grouped events were averaged to calculate average amplitudes, rise times, and decay times.
Histochemistry.
After recordings, slices containing biocytin-filled MSNs were fixed in formalin and stored in PBS with 0.05% sodium azide. The slices were washed with 1× TBS, permeabilized with 0.7% Triton, incubated overnight with the fluorophore Alexa Fluor 488-streptavidin (1:1,000), mounted on glass slides, and examined on an ApoTome confocal microscope (Zeiss, Thornwood, NY). Neurons were imaged at ×20 and ×100 magnification. Somatic areas were calculated and spine densities were counted with ImageJ (National Institutes of Health). Dendritic arborization was determined by Sholl analysis. To assess the complexity of the dendritic arborization, the number of intersections was counted using 14 concentric circles with increasing diameters in steps of 10 μm. Circles had a distance between 20 and 150 μm from the soma.
Statistical analyses.
Values reported are means ± SE. Differences between group means were assessed with t-tests (for 2 groups) or one-way ANOVAs (multiple groups) followed by Bonferroni post hoc t-tests. Amplitude-frequency and cumulative interevent interval or amplitude distributions were compared with two-way repeated-measures ANOVAs followed by Bonferroni post hoc t-tests. Differences in the proportions of sIPSC-to-sEPSC ratios were assessed with the nonparametric χ2 statistic. Differences were considered statistically significant when P < 0.05. All statistical analyses were performed with Microsoft Excel, Sigma Stat 12.0, or SPSS 21.
RESULTS
Basic membrane properties of MSNs.
Basic membrane properties of MSNs were recorded at a VHold of −70 mV (Table 1). There was a significant increase in mean cell capacitance for Q175+/+ at 2 mo compared with WTs and Q175+/− (P = 0.017 and 0.005, respectively) but not at later ages. Mean input resistance was significantly increased at 2 mo for Q175+/+ compared with WTs (P = 0.036). Although there also was an increase in mean input resistance for the Q175+/− group, it was not statistically significant. At 7 mo, input resistances of MSNs from both Q175+/+ and Q175+/− mice were increased compared with WTs. Again, only the difference between Q175+/+ and WTs was statistically significant (P = 0.003). At 12 mo, mean input resistances of MSNs from both Q175+/+ and Q175+/− were statistically significantly increased compared with those of WTs (P < 0.001 and P = 0.003, respectively). There also was a statistically significant decrease in the mean decay time constant (τ) at both 2 (P = 0.037) and 7 (P = 0.002) mo in Q175+/− compared with Q175+/+, but not compared with WTs.
Table 1.
Basic membrane properties of Q175 mice
| Cm, pF | Rm, MΩ | τ, ms | Somatic Area, μm2 | |
|---|---|---|---|---|
| MSNs | ||||
| 2 mo | ||||
| WT (21) | 62.64 ± 4.80 | 65.23 ± 5.30 | 2.00 ± 0.14 | 107.31 ± 7.53 (19) |
| Q175+/− (19) | 59.74 ± 4.86†† | 90.89 ± 11.64 | 1.85 ± 0.12† | 122.29 ± 14.52 (8) |
| Q175+/+ (24) | 81.21 ± 4.00** | 96.25 ± 8.50* | 2.33 ± 0.13 | 157.45 ± 14.47 (11)*** |
| 7 mo | ||||
| WT (21) | 57.01 ± 4.08 | 83.48 ± 12.72 | 1.96 ± 0.11 | 137.98 ± 19.84 (14) |
| Q175+/− (19) | 55.00 ± 3.85 | 107.79 ± 12.88 | 1.74 ± 0.10†† | 119.00 ± 9.99 (14) |
| Q175+/+ (23) | 63.87 ± 2.67 | 142.22 ± 11.11** | 2.28 ± 0.11 | 130.86 ± 8.96 (17) |
| 12 mo | ||||
| WT (20) | 64.05 ± 2.74 | 65.85 ± 3.45 | 2.18 ± 0.09 | 134.78 ± 7.90 (15) |
| Q175+/− (21) | 65.05 ± 2.91 | 107.48 ± 9.75** | 2.24 ± 0.11 | 151.69 ± 11.09 (10) |
| Q175+/+ (22) | 66.50 ± 2.45 | 130.91 ± 9.50*** | 2.37 ± 0.11 | 151.09 ± 10.52 (11) |
| CPNs | ||||
| 2 mo | ||||
| WT (15) | 89.80 ± 4.66 | 62.80 ± 4.50 | 2.57 ± 0.12 | |
| Q175+/− (17) | 89.53 ± 5.38 | 54.47 ± 5.53 | 2.56 ± 0.10 | |
| 12 mo | ||||
| WT (16) | 84.56 ± 5.67 | 101.93 ± 10.48 | 2.17 ± 0.13 | 163.87 ± 9.81 (12) |
| Q175+/− (15) | 81.53 ± 4.22 | 103.33 ± 8.02 | 2.41 ± 0.12 | 185.24 ± 17.34 (8) |
Values are means ± SE for no. of cells in parentheses. Cm, cell membrane capacitance; Rm, input resistance; τ, time constant; MSN, medium-sized spiny neuron; CPN, cortical pyramidal neuron. Different from wild type (WT):
P < 0.05,
P < 0.01,
P < 0.001. Different from Q175+/+:
P < 0.05,
P < 0.01.
sEPSCs and mEPSCs decrease in frequency in MSNs.
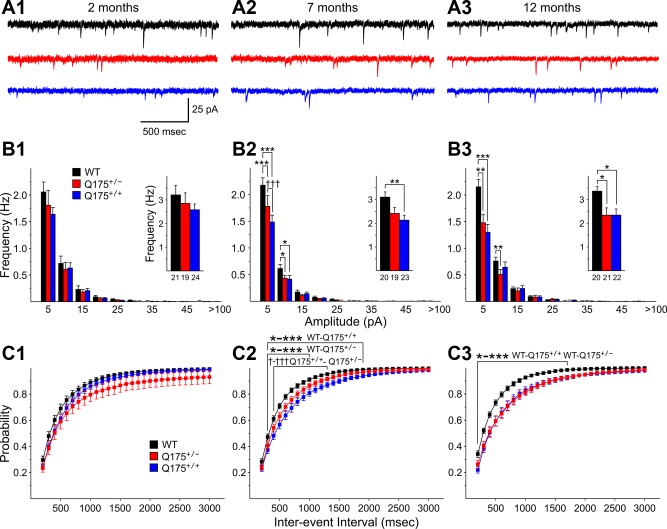
Overall, sEPSC frequency of MSNs in Q175 mice decreased with phenotype progression and the decreases were generally greater in Q175+/+ than in Q175+/− mice (Fig. 1). At 2 mo, mean sEPSC frequencies of MSNs from Q175+/− and Q175+/+ mice were similar to values from WTs (Fig. 1B1, inset). Similarly, amplitude-frequency histograms and cumulative interevent interval distributions were not significantly different among the groups (Fig. 1, B1 and C1). At 7 mo, mean sEPSC frequency was decreased in MSNs from both Q175+/+ and Q175+/− mice compared with WTs (Fig. 1B2, inset). However, this decrease was statistically significant only for MSNs from Q175+/+ mice compared with those of WTs (P = 0.009). Amplitude-frequency histograms displayed bins with statistically significant decreases in frequency for both Q175+/+ and Q175+/− mice compared with WT values (P < 0.001–0.021 for 5–10 and 10–15 pA bins) and in the 5–10 pA bin for comparisons between values from Q175+/+ and Q175+/− mice (P < 0.001) (Fig. 1B2). Cumulative interevent interval distributions were shifted to the right in MSNs from both Q175+/+ and Q175+/− mice compared with those of WTs, indicating that there was a greater proportion of long interevent intervals (Fig. 1C2). The interevent interval distribution of Q175+/+ mice was statistically significantly different from that of WTs for intervals from 100 to 1,900 ms (P < 0.001–0.038). The distribution from Q175+/− mice was significantly shifted from that of WTs for intervals from 100 to 1,000 ms (P < 0.001–0.049). In addition, the distribution from Q175+/+ mice was significantly different from that of Q175+/− mice for intervals from 200 to 1,300 ms (P < 0.05-0.001). At 12 mo, the mean frequencies of sEPSCs from MSNs of both Q175+/+ and Q175+/− were significantly reduced compared with those of WTs (P = 0.021 and 0.024, respectively) (Fig. 1B3, inset). Similar to findings at 7 mo, amplitude-frequency histograms at 12 mo displayed bins with significant decreases in frequency for both Q175+/+ and Q175+/− mice (P < 0.001–0.004 for 5–10 and 10–15 pA bins for WT and Q175+/+ comparisons; P < 0.001 for 5–10 pA bin for WT and Q175+/− comparison) (Fig. 1B3). Cumulative interevent interval distributions at 12 mo remained shifted to the right in MSNs from both Q175+/+ and Q175+/− mice compared with those of WTs mice (P < 0.001–0.05 for 100- to 1,700-ms intervals for comparisons between both WTs and Q175+/+ and WTs and Q175+/−) (Fig. 1C3). At 12 mo the cumulative interevent interval distributions for MSNs of Q175+/+ and Q175+/− mice were similar.
Fig. 1.
A: representative traces of spontaneous excitatory postsynaptic currents (sEPSCs) from striatal medium-sized spiny neurons (MSNs) at 2 (A1), 7 (A2), and 12 (A3) mo for wild-type (WT), Q175+/−, and Q175+/+ mice at a holding potential (VHold) of −70 mV in the presence of 10 μM bicuculline (BIC). B: amplitude-frequency histograms for WT, Q175+/−, and Q175+/+ mice at 2 (B1), 7 (B2), and 12 (B3) mo. Significant decreases in frequency occurred in 5–10 and 10–15 pA bins at both 7 and 12 mo for both Q175+/− and Q175+/+ mice. The average frequency of sEPSCs (insets) was reduced significantly for Q175+/+ compared with WTs at both 7 and 12 mo and for Q175+/− compared with WTs at 12 mo, although there was a strong trend for a reduction at 7 mo as well. C: cumulative interevent interval histograms for WT, Q175+/−, and Q175+/+ mice at 2 (C1), 7 (C2), and 12 (C3) mo. Histograms were significantly shifted to the right for both Q175+/+ and Q175+/− compared with WTs at both 7 and 12 mo, indicating a higher proportion of occurrence of longer interevent intervals. In this and subsequent figures numbers below bar graphs are group ns of recorded neurons. All comparisons of Q175+/− or Q175+/+ with WT mice are indicated by asterisks, while comparisons of Q175+/− with Q175+/+ mice are indicated by daggers. For all comparisons * or † indicates P < 0.05, ** or †† indicates P < 0.01, and *** or ††† indicates P < 0.001. Single asterisks or daggers followed by a hyphen and multiple asterisks or daggers indicate multiple levels of significance for the bracketed comparisons. Values in all figures are means ± SE.
No significant changes were observed in mean sEPSC amplitudes for MSNs from Q175 mice at each age examined (Table 2). sEPSC kinetics, including rise time, decay time, and half-amplitude duration, of MSNs from Q175 mice also were unchanged compared with kinetics of sEPSCs from WTs (data not shown).
Table 2.
Amplitude of postsynaptic currents
| sEPSCs, pA | mEPSCs, pA | sIPSCs, pA | |
|---|---|---|---|
| MSNs | |||
| 2 mo | |||
| WT (21) | 12.18 ± 0.65 | 11.27 ± 0.61 | 17.62 ± 0.57 |
| Q175+/− (19) | 11.93 ± 0.91 | 11.54 ± 0.35 | 21.14 ± 1.33 |
| Q175+/+ (24) | 12.44 ± 0.34 | 10.64 ± 1.26 | 18.01 ± 1.12 |
| 7 mo | |||
| WT (20) | 11.11 ± 0.73 | 10.63 ± 0.66 | 16.42 ± 0.79 |
| Q175+/− (19) | 12.27 ± 0.41 | 11.25 ± 0.31 | 19.51 ± 1.34 |
| Q175+/+ (23) | 12.58 ± 0.43 | 11.90 ± 0.48 | 20.15 ± 0.89 |
| 12 mo | |||
| WT (20) | 13.32 ± 0.37 | 12.94 ± 0.22 | 18.95 ± 0.91 |
| Q175+/− (21) | 13.63 ± 0.42 | 12.59 ± 0.30 | 20.05 ± 1.35 |
| Q175+/+ (22) | 12.40 ± 0.52 | 11.47 ± 0.69 | 22.04 ± 1.16 |
| CPNs | |||
| 2 mo | |||
| WT (15) | 12.84 ± 0.34 | 10.79 ± 0.20 | 18.80 ± 0.78 |
| Q175+/− (17) | 12.98 ± 0.33 | 10.94 ± 0.22 | 18.65 ± 1.01 |
| 12 mo | |||
| WT (16) | 13.50 ± 0.45 | 11.72 ± 0.34 | 27.62 ± 2.16 |
| Q175+/− (15) | 13.52 ± 0.70 | 11.95 ± 0.48 | 20.85 ± 1.57* |
Values are means ± SE for no. of cells in parentheses. sEPSC, spontaneous excitatory postsynaptic current; mEPSC, miniature excitatory postsynaptic current; sIPSC, spontaneous inhibitory postsynaptic current. Different from WT:
P < 0.05.
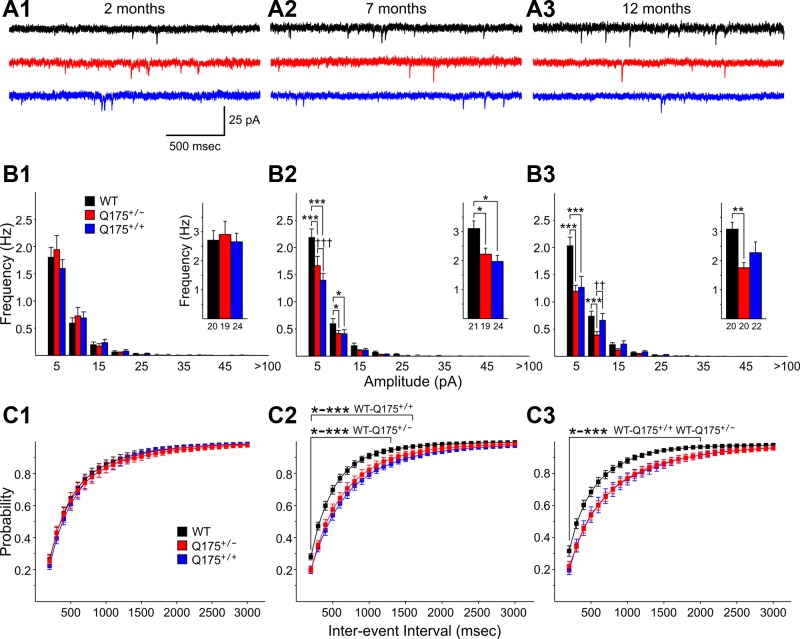
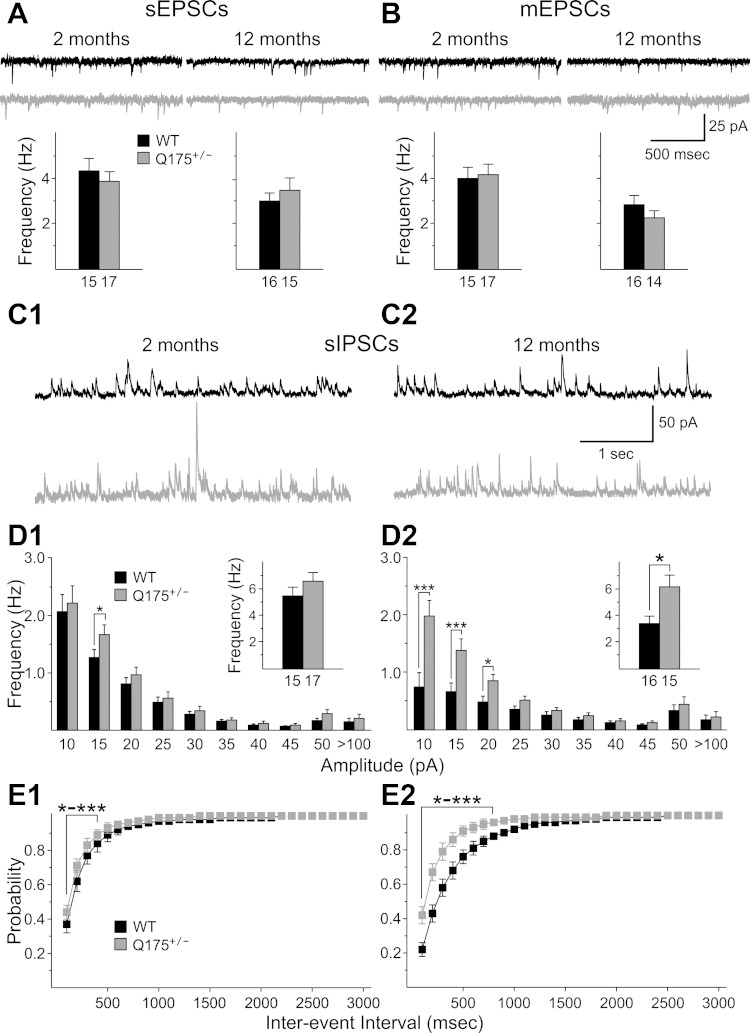
After completion of the recordings of sEPSCs, mEPSCs were recorded from the same neurons by perfusion of slices with 1 μM TTX (Fig. 2A). At 2 mo, there were no differences in mEPSCs among the groups (Fig. 2, B1 and C1). At 7 mo, mEPSCs were significantly reduced in MSNs from both Q175+/+ and Q175+/− mice compared with WTs (P = 0.029 and 0.022, respectively) (Fig. 2B2, inset). Amplitude-frequency histograms displayed bins with significant decreases in frequency for both Q175+/+ and Q175+/− mice compared with those of WTs (P < 0.001–0.032 for 5–10 and 10–15 pA bins for both WT vs. Q175+/+ and WT vs. Q175+/−, respectively) (Fig. 2B2). Cumulative interevent interval distributions were shifted to the right in MSNs from Q175+/+ and Q175+/− mice compared with those of WTs, indicating that there was a higher proportion of longer interevent intervals (Fig. 2C2). The interevent interval distributions of Q175+/+ were statistically significantly different from those of WTs for intervals from 100 to 1,600 ms (P < 0.001–0.045) and for WT vs. Q175+/− for intervals from 100 to 1,300 ms (P < 0.001 to P = 0.045) (Fig. 2C2). At 12 mo the mean frequencies of mEPSCs from MSNs of both Q175+/+ and Q175+/− were reduced compared with that of WTs, but only the difference between Q175+/− and WTs was statistically significant (P = 0.005) (Fig. 2B3, inset). Similar to findings at 7 mo, amplitude-frequency histograms at 12 mo displayed bins with significant decreases in frequency for both Q175+/+ and Q175+/− mice (P < 0.001 for 5–10 pA bin for WT vs. either Q175+/+ or Q175+/−; P < 0.001 for WT vs. Q175+/− for the 10–15 pA bin; P = 0.004 for WT vs. Q175+/− for the 10–15 pA bin) (Fig. 2B3). Cumulative interevent interval distributions at 12 mo were significantly shifted to the right in MSNs from Q175+/+ and Q175+/− mice compared with that of WT mice (P < 0.001–0.05 for 100- to 2,000-ms intervals) (Fig. 2C3).
Fig. 2.
A: representative traces of miniature excitatory postsynaptic currents (mEPSCs) from striatal MSNs at 2 (A1), 7 (A2), and 12 (A3) mo for WT, Q175+/−, and Q175+/+ mice at a VHold of −70 mV in the presence of 10 μM BIC and 1 μM tetrodotoxin (TTX). B: amplitude-frequency histograms for WT, Q175+/−, and Q175+/+ mice at 2 (B1), 7 (B2), and 12 (B3) mo. Significant decreases in frequency occurred in 5–10 and 10–15 pA bins at both 7 and 12 mo for both Q175+/− and Q175+/+ mice. The average frequency of mEPSCs (insets) was reduced significantly for both Q175+/− and Q175+/+ mice compared with WTs at 7 mo and for Q175+/− compared with WTs at 12 mo. C: cumulative interevent interval histograms for WT, Q175+/−, and Q175+/+ mice at 2 (C1), 7 (C2), and 12 (C3) mo. Histograms were significantly shifted to the right for both Q175+/+ and Q175+/− compared with WTs at both 7 and 12 mo, indicating a higher proportion of occurrence of longer interevent intervals.
Similar to sEPSCs, there were no statistically significant differences in mEPSC amplitudes among the groups at each age (Table 2) and there were no differences in mEPSC kinetics (data not shown).
sIPSCs increase in frequency in MSNs.
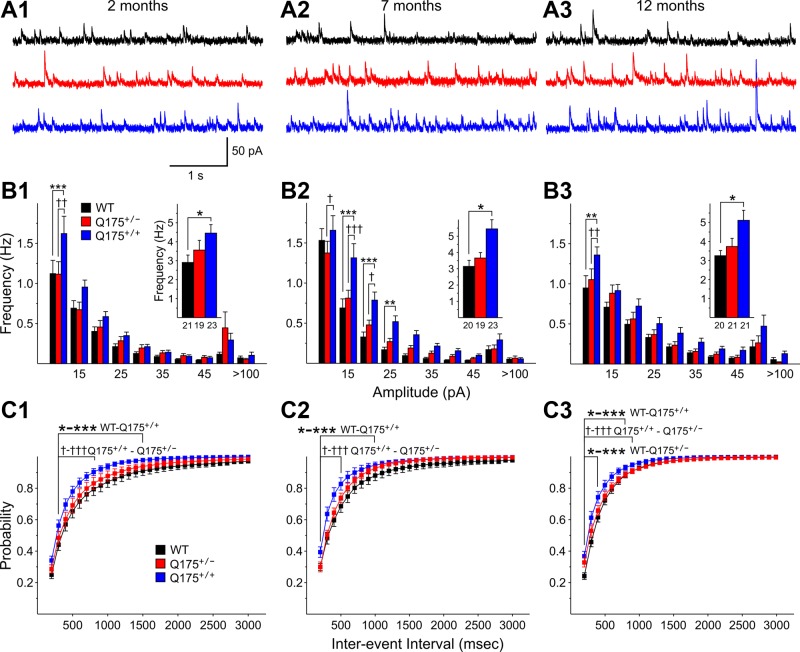
sIPSCs were recorded at a VHold of +10 mV (Fig. 3A). At 2 mo, there was a statistically significant increase in the mean frequencies of sIPSCs from Q175+/+ mice compared with those of WTs (P = 0.013) (Fig. 3B1, inset). The amplitude-frequency histograms for sIPSCs of Q175+/+ displayed one bin (10–15 pA) with a statistically significant increase compared with values from both WT and Q175+/− mice (P = 0.005 and 0.006, respectively) (Fig. 3B1). Cumulative interevent interval distributions were shifted to the left for sIPSCs of MSNs from both Q175+/+ and Q175+/− mice compared with those of WTs, indicating that proportionately more interevent intervals were shorter (Fig. 3C1). The interevent interval distribution of Q175+/+ mice was statistically significantly different from that of WTs for intervals of 100-1,500 ms (P < 0.001–0.039) as well as significantly different from that of Q175+/− mice at interevent intervals of 200–800 ms (P = 0.010–0.033). At 7 mo, Q175+/+ mice displayed a significantly increased sIPSC frequency (P = 0.002). The amplitude-frequency histogram for sIPSCs of Q175+/+ mice displayed several bins with significantly increased frequencies compared with WTs (P < 0.001–0.078, 15–20, 20–25, and 25–30 pA) and Q175+/− mice (P < 0.001–0.036, 10–15, 15–20 pA) (Fig. 3B2). Cumulative interevent interval distributions were shifted to the left for MSNs from both Q175+/+ and Q175+/− mice compared with those of WTs (Fig. 3C2). The interevent interval distribution of Q175+/+ was statistically significantly different from that of WTs for intervals of 100-1,000 ms (P < 0.001–0.038) as well as significantly different from that of Q175+/− mice at interevent intervals of 100–500 ms (P < 0.001–0.012). At 12 mo, the mean sIPSC frequency of MSNs from Q175+/+ mice was significantly increased compared with WTs (P = 0.009) (Fig. 3B3). The amplitude-frequency histogram for sIPSCs of Q175+/+ mice displayed one bin (10–15 pA) with a significantly increased frequency compared with both WTs and Q175+/− mice (P < 0.001 and P = 0.007, respectively) (Fig. 3B3). The cumulative interevent interval distributions for sIPSCs of MSNs from Q175+/+ and Q175+/− mice were shifted to the left compared with those of WTs (Fig. 3C3). The interevent interval distribution of Q175+/+ was statistically significantly different from that of WTs for intervals of 100–800 ms (P < 0.001–0.035) as well as significantly different from that of Q175+/− mice at interevent intervals of 100–900 ms (P < 0.001–0.05). In addition, the interevent interval distribution of Q175+/− mice was statistically significantly different from that of WTs for intervals of 100–300 ms (P < 0.001–0.018).
Fig. 3.
A: representative traces of spontaneous inhibitory postsynaptic currents (sIPSCs) from striatal MSNs at 2 (A1), 7 (A2), and 12 (A3) mo for WT, Q175+/−, and Q175+/+ mice at a VHold of −70 mV. B: amplitude-frequency histograms for WT, Q175+/−, and Q175+/+ mice at 2 (B1), 7 (B2), and 12 (B3) mo. Significant increases in frequency occurred for the 5–10 pA bin for Q175+/+ mice compared with Q175+/− and WTs at 2 mo. At 7 and 12 mo most of the significant increases occurred for comparisons between Q175+/+ and WT or Q175+/− mice. The average frequency of sIPSCs (insets) was increased significantly for Q175+/+ mice at all ages, for both Q175+/− and Q175+/+ mice compared with WTs at 7 mo, and for Q175+/− at 12 mo. C: cumulative interevent interval histograms for WT, Q175+/−, and Q175+/+ mice at 2 (C1), 7 (C2), and 12 (C3) mo. Histograms were significantly shifted to the left for Q175+/+ compared with either WT or Q175+/− mice at each age, indicating a higher proportion of occurrence of shorter interevent intervals. At 12 mo there also was a significant left shift in the distribution of cumulative interevent intervals for Q175+/− compared with that of WT mice.
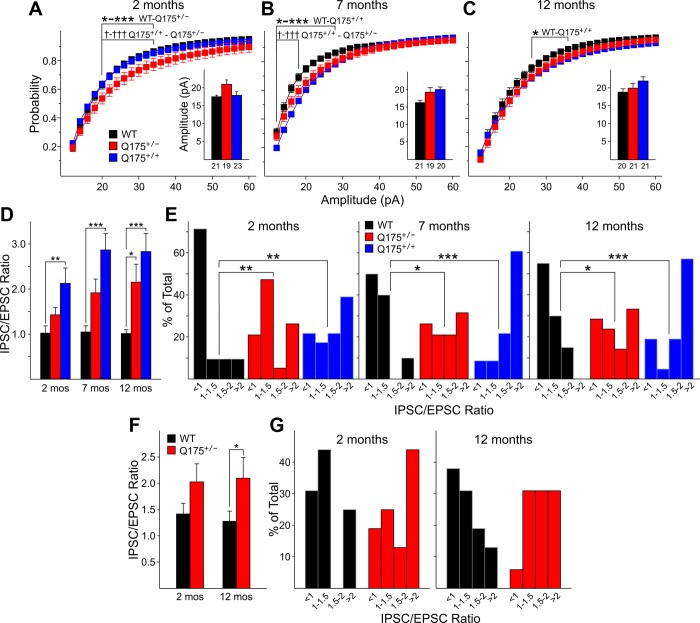
sIPSC amplitudes also were significantly increased. Although mean amplitudes were not significantly changed, there were shifts in the distributions of amplitudes. At 2 mo, cumulative amplitude histograms were shifted significantly to the right for Q175+/− mice compared with distributions from WTs or Q175+/+ mice, indicating proportionately more events with higher amplitudes from 20 to 34 pA (P = 0.045-0.021) (Fig. 4A). At 7 mo, again, cumulative amplitude histograms were shifted significantly to the right, indicating proportionately more events with higher amplitudes from 12 to 28 pA for Q175+/+ compared with values for WTs (P = 0.012 to P < 0.001) and for Q175+/+ compared with Q175+/− for amplitudes of 12–18 pA (P = 0.048-0.003) (Fig. 4B). At 12 mo, cumulative amplitude histograms were shifted significantly to the right, indicating proportionately more events with higher amplitudes from 26 to 36 pA for Q175+/+ compared with values for WTs (P = 0.05) (Fig. 4C). There were no consistent differences in the kinetics of sIPSCs at each age (data not shown).
Fig. 4.
A–C: cumulative amplitude histograms for sIPSCs from WT, Q175+/−, and Q175+/+ mice at 2 (A), 7 (B), and 12 (C) mo. The proportionate distribution of larger amplitudes was significantly greater at 2 mo in Q175+/− compared with either WTs or Q175+/− mice, as shown by the distributional shift to the right. Similarly at 7 and 12 mo, distributions of events for either Q175+/− or Q175+/+ mice also were shifted to the right. The average amplitudes of sIPSCs (insets) from Q175+/− or Q175+/+ mice also were increased compared with WTs, but these increases were not statistically significant. D: average sIPSC-to-sEPSC ratios for MSNs were significantly increased for Q175+/+ compared with those from WT mice at all ages. The average ratio was significantly increased for Q175+/− compared with those from WT at 12 mo. E: normalized distributions of sIPSC-to-sEPSC frequency ratios for all MSNs in each age group [divided into 4 categorical bins: <1 (fewer sIPSCs than sEPSCs), 1–1.5 (more sIPSCs than sEPSCs), 1.5–2.0, and >2.0]. For all 3 ages these distributions were significantly shifted to the right for Q175+/− or Q175+/+ compared with WT mice, indicating that proportionately more neurons had higher ratios of sIPSCs to sEPSCs. F: average sIPSC-to-sEPSC ratios for CPN were increased for Q175+/− compared with those from WT mice at both 2 and 12 mo, but the difference only was significant at 12 mo. G: normalized distributions of sIPSC-to-sEPSC frequency ratios for all MSNs in each age group (similar to E). For both ages the distribution shifted proportionately to the greater ratios.
Ratios of frequencies of sIPSCs to sEPSCs are increased.
To further examine changes in inputs to MSNs, the ratio of sIPSCs to sEPSCs was calculated for each neuron. This ratio provides a normalized index of the inhibitory and excitatory inputs to each cell independent of the absolute values of the frequencies. If the inputs are equal the ratio would be ∼1. If the frequency of sIPSCs exceeds that of sEPSCs the ratio is >1, and if sIPSCs are reduced in frequency compared with sEPSCs the ratio is <1. At 2 mo, both Q175+/+ and Q175+/− mice displayed increases of the ratio, but only the difference between Q175+/+ and WTs was statistically significant (P = 0.002) (Fig. 4D). Examination of distributions of ratios, however, demonstrated significant differences between the distribution from WTs and those from Q175+/+ (P < 0.01) and Q175+/− (P = 0.006) mice (Fig. 4E, left). In both Q175+/+ and Q175+/− mice there was a significant shift in the proportion of neurons displaying higher sIPSC-to-sEPSC ratios. At 7 mo the results were similar. Both Q175+/+ and Q175+/− mice displayed increases of the mean ratio, with the difference in means being statistically significant for the comparison between Q175+/+ and WT mice (P < 0.001) (Fig. 4D) while the distributions of ratios were significantly different between WTs and both Q175+/+ (P < 0.001) and Q175+/− (P = 0.03) mice (Fig. 4E, center). At 12 mo the increase in mean sIPSC-to-sEPSC ratios for both Q175+/+ and Q175+/− mice was statistically significantly different from that of WTs (P < 0.001 and P = 0.04, respectively) (Fig. 4D). Similarly, the distributions were shifted significantly to higher values for both Q175+/+ and Q175+/− mice compared with those of WTs (P < 0.001 and P = 0.039, respectively).
Altered morphology of MSNs.
To determine changes in MSN morphology, biocytin-filled recorded MSNs were examined for changes in somatic cross-sectional area (Table 1), dendritic arborizations (Fig. 5), and spine density (Fig. 6). Mean somatic cross-sectional area was significantly increased for MSNs from Q175+/+ mice compared with that of WTs at 2 mo (P < 0.001). There were no other significant differences between Q175 and WT mice at any of the other ages. The increase in somatic cross-sectional area at 2 mo in Q175+/+ mice is consistent with the increased MSN capacitance.
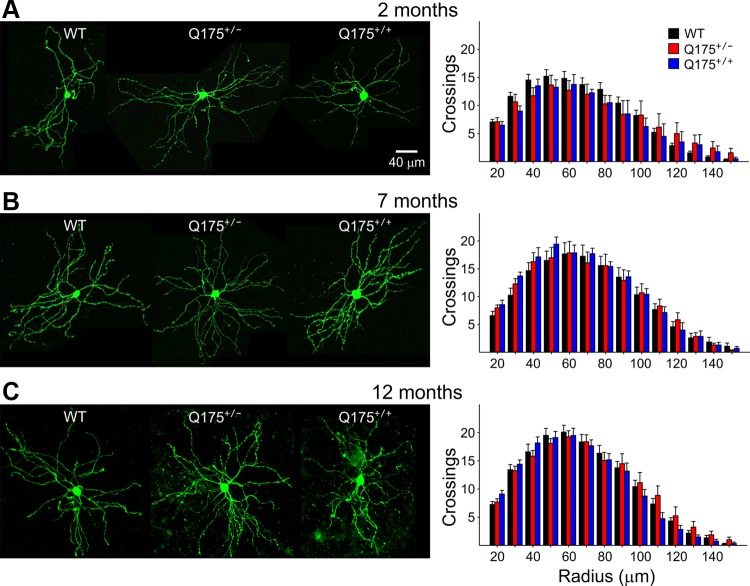
Fig. 5.
Representative confocal images of biocytin-labeled MSNs at ×20 magnification for WT, Q175+/−, and Q175+/+ mice at 2 (A; n = 21, 11, 4, respectively), 7 (B; n = 12, 14, 7, respectively), and 12 (C; n = 12, 16, 18, respectively) mo. Histograms on right of images show Sholl analyses performed with increments of 10-μm diameter. Although dendritic complexity increased with age, there were no significant differences in dendritic complexity for MSNs from Q175+/− or Q175+/+ mice compared with WTs at any age.
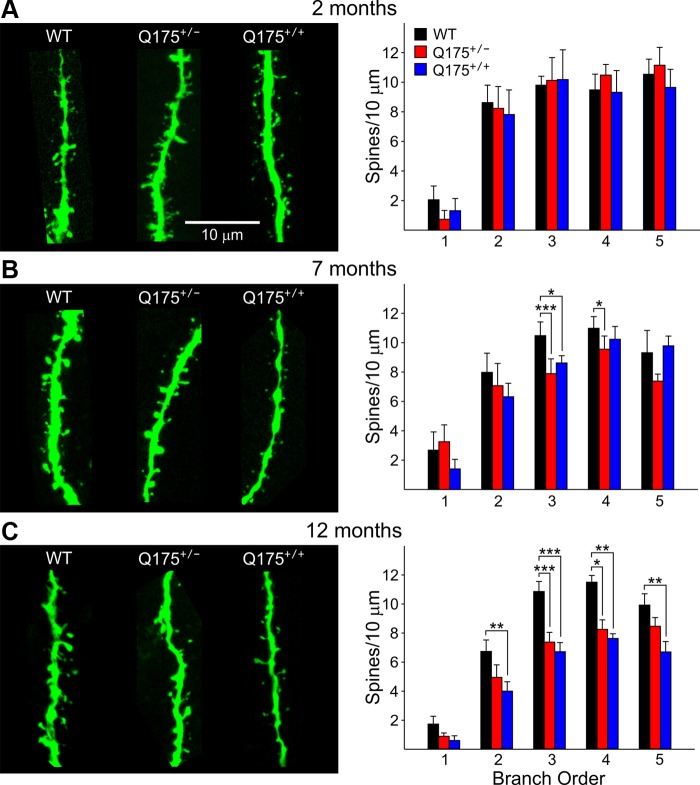
Fig. 6.
Representative confocal images of biocytin-labeled dendritic shafts of MSNs at ×100 magnification for WT, Q175+/−, and Q175+/+ mice at 2 (A; n = 14, 8, 6, respectively), 7 (B; n = 10, 11, 12, respectively), and 12 (C; n = 15, 19, 15, respectively) mo to examine spine density. Spine densities were determined by counting the number of spines on 10-μm segments. Histograms on right show spine density at each branch order. At 2 mo there were no significant differences among the groups. Spine density was significantly decreased in both Q175+/− and Q175+/+ mice at several branch orders at 7 and 12 mo compared with WTs.
Dendritic complexity determined by Sholl analysis revealed that dendritic arborization as measured by the number of crossings at each radius maximizes at ∼50 μm from the center of the soma and is more complex at 7 and 12 mo compared with 2 mo of age (Fig. 5). At 2 mo the maximum number of crossings is ∼15, while at 7 and 12 mo it is close to 20. However, the distributions of dendritic crossings were similar in Q175+/+, Q175+/−, and WT mice at each age (Fig. 5).
Dendritic spine density of MSNs of Q175 and WT mice was examined by counting the number of spines on 10-μm segments of dendrites of increasing branch order by an observer blind to genotype (Fig. 6). As has been shown previously for MSNs, spine density increased with distance from the soma (Klapstein et al. 2001). No significant differences in spine density were apparent between Q175 and WT mice at 2 mo. However, there were significant decreases in spine density at 7 and 12 mo of age for both Q175+/+ and Q175+/− mice compared with WTs. Spine densities were significantly reduced in Q175+/− mice at 7 mo for branch orders 3 and 4 compared with WTs (P < 0.001 and P < 0.023, respectively) and in Q175+/+ mice compared with WTs for branch order 3 (P = 0.04). At 12 mo, spine densities were reduced for Q175+/− mice at branch orders 3 and 4 compared with WTs (P < 0.001 and P = 0.017) and reduced for Q175+/+ mice at branch orders 2–5 compared with WTs (P = 0.007, P < 0.001, P = 0.009, P = 0.005, respectively).
sEPSCs are similar while sIPSCs are increased in frequency in CPNs.
For these experiments recordings were only obtained from Q175+/− and WT mice at two ages, 2 and 12 mo, because of the availability of mice. These were not the same mice used to evaluate alterations in MSNs. sEPSCs and mEPSCs were recorded at a VHold of −70 mV with a Cs-methanesulfonate internal solution. Neuron capacitance, input resistance, and time constants were similar for CPNs from Q175+/− mice and WTs at both ages, although there was an increase in input resistance for all recorded CPNs between 2 and 12 mo (Table 1). sEPSC and mEPSC frequencies were not significantly different between CPNs from Q175+/− and WT mice at either age (Fig. 7, A and B). There also were no significant differences in amplitude-frequency and cumulative interevent interval distributions among the groups at either age (data not shown).
Fig. 7.
A and B: representative traces of sEPSCs (A) and mEPSCs (B) from CPNs at 2 and 12 mo for WT and Q175+/− mice at a VHold of −70 mV. Bar graphs below each set of traces show the average frequencies of sEPSCs and mEPSCs at each age for each group. There were no significant changes between groups at each age. C: representative traces of sIPSCs recorded from CPNs at a VHold of +10 mV at 2 (C1) and 12 (C2) mo. D: amplitude-frequency histograms for WT and Q175+/− and Q175+/+ mice at 2 (D1) and 12 (D2) mo. Significant increases in frequency occurred for the 10–15 pA bin for Q175+/− mice compared with WTs at 2 mo. At 12 mo significant increases occurred for comparisons between Q175+/− and WT mice for 10–20 pA bins. The average frequency of sIPSCs (insets) was increased significantly for Q175+/− mice at 12 mo. E: cumulative interevent interval histograms for Q175+/− and WT mice at 2 (E1) and 12 (E2) mo. Histograms were significantly shifted to the left for Q175+/− compared with WT at both ages.
At 2 mo, mean sIPSC frequency was similar in Q175+/− mice compared with WT values (Fig. 7, C1 and D1, inset). The amplitude-frequency histogram for sIPSCs of Q175+/− mice displayed one bin (10–15 pA) with a significantly increased frequency (P = 0.024) (Fig. 7D1). The interevent interval distributions for Q175+/− mice were statistically significantly different from those of WTs for intervals of 100–400 ms (P < 0.001 to P = 0.03) (Fig. 7E1). At 12 mo, the increase in sIPSC frequency in Q175+/− mice was statistically significantly different from that of WTs (P = 0.009) (Fig. 7D2, inset). The increase also was reflected by a significant difference in three amplitude-frequency bins (10, 15, and 20 pA) (P < 0.001, P < 0.001, and P = 0.036, respectively). Similarly, cumulative interevent interval distributions were significantly shifted to the left from 100 to 800 ms, indicating a proportionately greater number of short interevent intervals (P < 0.001 to P = 0.036). There also were significant differences between Q175+/− and WT mice in sIPSC amplitude and kinetics at 12 mo. sIPSC amplitude was significantly lower in Q175+/− compared with WTs (P = 0.018) (Table 2). Q175+/− mice also displayed significantly longer sIPSC rise times (2.48 ± 0.19 vs. 3.12 ± 0.25 for WTs and Q175+/−, respectively; P = 0.048), and half-amplitude durations (15.6 ± 0.65 vs. 19.2 ± 0.93 for WTs and Q175+/−, respectively; P = 0.004) compared with values from WTs.
To further examine changes in inputs to CPNs, the ratio of sIPSCs to sEPSCs was calculated for each neuron as for MSNs (Fig. 4F). There was an increase in the mean ratio for CPNs of Q175+/− mice compared with values from WTs at both ages, but the increase was statistically significant only for the 12-mo groups (P = 0.027). Examination of distributions of ratios demonstrated a shift in the proportion of neurons displaying higher sIPSC-to-sEPSC ratios at both ages. However, the differences between distributions of values from Q175+/− and WTs were not statistically significant.
We also examined morphological alterations, but only for CPNs at 12 mo, as there were no changes in sEPSCs or mEPSCs and few changes in sIPSCs at 2 mo. There were no significant differences in CPN morphology between Q175+/− and WT mice. Mean somatic cross-sectional areas (Table 1), dendritic arborizations, and spine densities were similar (data not shown for arborizations and spine densities).
DISCUSSION
The present study examined the progression of electrophysiological and morphological alterations that occur in striatal MSNs and CPNs of Q175+/− and Q175+/+ mice. The findings indicate that, in the striatum, there are significant decreases in excitatory inputs to MSNs at both 7 and 12 mo combined with a correlated increase in inhibitory inputs that was more pronounced in neurons from Q175+/+ than Q175+/− mice. These alterations indicate that in MSNs there is an imbalance in the ratio of inhibition to excitation as the phenotype develops. This imbalance occurs in parallel with changes in intrinsic membrane properties and loss of spines on dendritic shafts of higher-branch order dendrites, without the loss of dendritic segments or an alteration in dendritic fields. In CPNs excitatory inputs in Q175+/− and WT mice are similar at the ages studied, whereas inhibitory inputs are increased at 12 mo in the Q175+/−. Thus the findings demonstrate that, depending on gene dosage and disease progression, Q175 mice exhibit a number of phenotypes that are similar to other mouse models of HD, but other alterations are at variance. It is important to note the similarities and differences among the various mouse models, as the Q175+/− mice, in contrast to some of the other models, closely resemble the typical human heterozygotic HD condition (Menalled and Brunner 2014).
Increased input resistance is robust and occurs consistently across almost all animal models of HD. Significant increases are observed as early as 2 mo for MSNs from Q175+/+ mice and at 12 mo for cells from Q175+/− mice, although a very strong trend is present at both 2 and 7 mo for Q175+/− mice. Increased input resistance in MSNs from Q175 mice also has been previously reported (Dvorzhak et al. 2013; Heikkinen et al. 2012). This increased input resistance could result from abnormal K+ conductances as reported in the R6/2 and TgCAG100 mouse models of HD (Ariano et al. 2005; Cepeda et al. 2003). Similar to R6/2 (Klapstein et al. 2001), there was a decrease in cell membrane decay time constant but only in MSNs from 2 and 7 mo Q175+/− mice. Unexpectedly, cell capacitance was increased in MSNs from 2-mo-old Q175+/+. We do not have an explanation for this observation, but the increase in cell capacitance in these mice is consistent with observations of increased somatic cross-sectional areas in MSNs of Q175+/+ mice. Although changes in biophysical membrane properties are expected to influence synaptic integration, those changes did not lead to consistent effects on amplitude or kinetics of synaptic inputs, suggesting that other factors, e.g., receptor subunit composition and neurotransmitter transporters, could be involved.
Changes in intrinsic membrane properties occurred in parallel with morphological changes in MSNs as determined by biocytin labeling. There was a gradual loss of spines as early as 7 mo in Q175+/− and Q175+/+ mice. In CAG140 mice, there is reduced spine density and loss of dendritic complexity in 20- to 26-mo-old homozygous animals (Lerner et al. 2012). Therefore, the onset of spine density loss in Q175 mice occurs much earlier than in CAG140 mice, further demonstrating a more severe phenotype. However, we did not observe a reduction in dendritic complexity in 12-mo-old Q175 mice. This indicates that spine loss precedes pathological reduction of dendritic fields. It is thus plausible that Q175 mice will have a reduced dendritic complexity at a later age. In other mouse models of HD decreases in MSN spine density, as well as alterations in dendritic field complexity, occur as the phenotype progresses (Klapstein et al. 2001; Lerner et al. 2012).
The frequency of sEPSCs and mEPSCs was significantly decreased in MSNs from Q175 mice. The reduction in EPSC frequency was more pronounced and occurred earlier in MSNs from Q175+/+ than Q175+/− mice. These findings are similar to the decrease in 7- to 9-mo-old Q175 mice found previously (Heikkinen et al. 2012). However, unlike the findings reported by these authors, we did not observe changes in sEPSC amplitude for MSNs from Q175+/− or Q175+/+ mice at any age. sEPSC kinetics (rise time, decay time, or half-amplitude duration) of MSNs from Q175+/− and Q175+/+ mice were unchanged compared with WTs at each age tested. It is therefore unlikely that glutamate receptor subunit composition or transporters are altered in MSNs from Q175 mice. Changes in mEPSC amplitude and frequency were similar to changes found in sEPSCs and indicated that the effects were independent of action potential frequency. Similar alterations in the frequency of sEPSCs and mEPSCs with phenotype progression have been observed in multiple mouse models of HD including the R6/2, R6/1, YAC, and BAC models and have been associated more mechanistically with both pre- and postsynaptic alterations in the striatum, including reductions in synaptic proteins, loss of dendritic spines, and loss of synapses (Andre et al. 2011; Cepeda et al. 2003; Gray et al. 2008; Milnerwood and Raymond 2007; Raymond et al. 2011; Wang et al. 2014). The overall agreement of these effects in multiple mouse models provides considerable validity to this change and indicates further that a major change in MSNs is loss of excitatory synaptic communication as the phenotype progresses.
In contrast, sIPSC frequency in MSNs from Q175+/+ mice was markedly increased as early as 2 mo of age. Furthermore, sIPSC amplitude was increased in MSNs from Q175+/+ mice compared with WTs. An increase in sIPSC frequency in MSNs from Q175+/+ mice also has been reported (Dvorzhak et al. 2013). The changes in frequency of sIPSCs are congruent with alterations in inhibitory inputs to MSNs observed in R6/2, YAC128, BACHD, and other mouse models of HD (Centonze et al. 2005; Cepeda et al. 2004; Cummings et al. 2010). The increase in the frequency of sIPSCs combined with the decrease in frequency of sEPSCs and mEPSCs generates a marked imbalance in the ratio of inhibition to excitation, which is relevant for understanding phenotype progression. In support, there is recent evidence for a decrease in the glutamate-to-GABA ratio measured by HPLC in 6-mo-old Q175 mice (Smith et al. 2014). Inhibitory inputs to MSNs are derived from two sources, feedback inhibition from other MSNs and feedforward inhibition from several populations of striatal interneurons. We have shown previously that in the R6/2 and BACHD models a major source of the increase in inhibition observed in MSNs is generated by striatal interneurons, both fast-firing parvalbumin-expressing interneurons and persistent low-threshold-spiking somatostatin-expressing interneurons (Cepeda et al. 2013). However, this issue remains somewhat unclear. Since MSNs are more depolarized in some of the HD models, especially the R6/2 (Klapstein et al. 2001), they may produce more spontaneous action potentials not only in vivo (Rebec et al. 2006) but also in vitro, thus providing another potential source of increased GABA inputs in HD. Furthermore, a recent study suggested that increased GABA activity could be the result of disinhibition of spontaneous action potential generation by MSNs (Dvorzhak et al. 2013).
Our experiments in the cortex of Q175 mice were limited to two genotypes (WT and Q175+/−) and two time points (2 and 12 mo). This limitation was caused by breeding difficulties. We chose to record CPNs only from 2- and 12-mo-old animals, since we found no significant pathological progression in MSNs at 12 mo compared with 7 mo. There were no significant differences in input resistance, capacitance, and time constant in CPNs from Q175+/− mice at 12 mo. However, all recordings in the present study used a Cs-methanesulfonate-based internal solution. We previously reported increased input resistance in CPNs from symptomatic YAC128 and CAG140 mice (Cummings et al. 2009). Similar increases were observed in 80-day-old R6/2 mice but only when K-gluconate was used as the internal pipette solution. It is therefore possible that differences in CPN membrane properties in Q175 mice would become apparent when K-gluconate is used as the internal solution. In agreement, progressive alterations in passive and active membrane properties were observed in CPNs from perirhinal cortex of R6/1 mice with a K+-based recording solution (Cummings et al. 2006). Unlike MSNs, CPNs from Q175+/− mice did not display altered morphology, indicating that CPNs are less vulnerable to morphological alterations than MSNs. In the R6/2 and CAG100 mouse models significant dendritic abnormalities and spine loss were reported (Klapstein et al. 2001; Laforet et al. 2001).
In symptomatic R6/2, YAC128, and CAG140 homozygotic KI mice, sEPSC frequency of CPNs is significantly increased (Cummings et al. 2009). This change could correlate with the observation that CPNs from behaving, symptomatic R6/2 animals display faster spontaneous firing rates compared with WT littermates (Walker et al. 2008). In the present study, however, we did not observe alterations in sEPSCs or mEPSCs in CPNs from Q175+/− mice at 12 mo. It is possible that in Q175+/+ mice or in older Q175+/− mice increased EPSC frequency may occur. In contrast, CPNs from Q175+/− mice had significantly increased sIPSC frequencies. Some increases were already apparent at 2 mo and became more significant at 12 mo. In CPNs from R6/2 mice we reported biphasic changes in IPSC frequency. At 21 days of age before overt behavioral symptoms develop IPSC frequencies were increased, whereas at 80 days, in fully symptomatic mice, they were reduced (Cummings et al. 2009). In the same study we found that CPNs from YAC128 and CAG140 mice at 12 mo displayed increased IPSC frequencies. As the R6/2 fragment model presents with a very aggressive phenotype, it is possible that reduced inhibition to CPNs represents a very late stage in disease progression that has not yet occurred in YAC128, CAG140, or Q175 mice at 12 mo.
The increase in sIPSC frequency that we observed in cortical layer 2/3 of Q175 mice beginning at 2 mo could represent a compensatory mechanism that prevents excitotoxic activation of MSNs by CPNs. The source of increased inhibition remains unanswered, but we can postulate that it originates from local interneurons. Preliminary studies in our laboratory indicate increased GABA responses evoked in CPNs by optogenetic stimulation of somatostatin-expressing interneurons (Holley et al. 2014). In contrast to our previous study in R6/2 mice, we did not observe large-amplitude events in MSNs from Q175 mice, nor did we find robust changes in excitatory inputs into CPNs in layer 2/3. Thus we postulate that increased inhibition in CPNs keeps cortical excitability in check and prevents excessive excitation of striatal neurons. However, in a full-length mouse model of HD, the BACHD, reduced inhibition onto CPNs, along with decreased excitation onto CPNs and parvalbumin-expressing interneurons at 6 mo, has been reported (Spampanato et al. 2008), further emphasizing the importance of the HD model used and the stage of phenotype progression.
In conclusion, while the Q175 is a relatively new model, electrophysiologically it displays many properties similar to other KI and transgenic models including the early increases in MSN membrane input resistance, loss of excitatory inputs to MSNs, which appear to be associated with loss of dendritic spines, increase in inhibitory inputs to MSNs, and increases in inhibitory inputs to CPNs. With several exceptions, the degree of these alterations in Q175 mice was greater or occurred earlier in homozygotic compared with heterozygotic mice and increased with progression of the phenotype over age. Similar gene dosage and age effects have been observed in Q175 mice with regard to disruption of circadian rhythms (Loh et al. 2013). Changes in intrinsic membrane and synaptic properties provide potential therapeutic targets. Particularly intriguing is the early increase in frequency of sIPSCs in CPNs from Q175 mice at a presymptomatic stage, when no changes in striatal EPSC synaptic activity are yet apparent. If this increase truly represents a compensatory mechanism to reduce cortical hyperexcitability and prevent striatal damage, reinforcing cortical inhibition could be a clinical target.
The present findings, taken in conjunction with the electrophysiological findings from other mouse models, emphasize both the similarities and differences among models. Conclusions based on findings from just one model, while very useful for beginning to understand outcomes in HD, need to be validated with multiple models at different stages of disease progression. The primary advantage of the Q175 model is more rapid progression of the phenotype compared with the CAG140 model and the early appearance of alterations in heterozygotic mice, which more faithfully recapitulate the human condition and are more adequate for preclinical studies.
GRANTS
This work was supported by a contract from CHDI, Inc. and National Institute of Neurological Disorders and Stroke Grant NS-41574.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.I. performed experiments; T.I. and C.H.T. analyzed data; T.I., C.C., and M.S.L. interpreted results of experiments; T.I., C.H.T., C.C., and M.S.L. prepared figures; T.I. and M.S.L. drafted manuscript; T.I., C.H.T., C.C., and M.S.L. approved final version of manuscript; C.C. and M.S.L. conception and design of research; C.C. and M.S.L. edited and revised manuscript.
ACKNOWLEDGMENTS
The authors thank My N. Huynh for helping with biocytin processing. Donna Crandall helped with illustrations. Michael Haim participated in data analysis.
REFERENCES
- Andre VM, Cepeda C, Fisher YE, Huynh M, Bardakjian N, Singh S, Yang XW, Levine MS. Differential electrophysiological changes in striatal output neurons in Huntington's disease. J Neurosci 31: 1170–1182, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariano MA, Cepeda C, Calvert CR, Flores-Hernandez J, Hernandez-Echeagaray E, Klapstein GJ, Chandler SH, Aronin N, DiFiglia M, Levine MS. Striatal potassium channel dysfunction in Huntington's disease transgenic mice. J Neurophysiol 93: 2565–2574, 2005. [DOI] [PubMed] [Google Scholar]
- Centonze D, Rossi S, Prosperetti C, Tscherter A, Bernardi G, Maccarrone M, Calabresi P. Abnormal sensitivity to cannabinoid receptor stimulation might contribute to altered gamma-aminobutyric acid transmission in the striatum of R6/2 Huntington's disease mice. Biol Psychiatry 57: 1583–1589, 2005. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Andre VM, Yamazaki I, Wu N, Kleiman-Weiner M, Levine MS. Differential electrophysiological properties of dopamine D1 and D2 receptor-containing striatal medium-sized spiny neurons. Eur J Neurosci 27: 671–682, 2008. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Galvan L, Holley SM, Rao SP, Andre VM, Botelho EP, Chen JY, Watson JB, Deisseroth K, Levine MS. Multiple sources of striatal inhibition are differentially affected in Huntington's disease mouse models. J Neurosci 33: 7393–7406, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Hurst RS, Calvert CR, Hernandez-Echeagaray E, Nguyen OK, Jocoy E, Christian LJ, Ariano MA, Levine MS. Transient and progressive electrophysiological alterations in the corticostriatal pathway in a mouse model of Huntington's disease. J Neurosci 23: 961–969, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Starling AJ, Wu N, Nguyen OK, Uzgil B, Soda T, Andre VM, Ariano MA, Levine MS. Increased GABAergic function in mouse models of Huntington's disease: reversal by BDNF. J Neurosci Res 78: 855–867, 2004. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Wu N, Andre VM, Cummings DM, Levine MS. The corticostriatal pathway in Huntington's disease. Prog Neurobiol 81: 253–271, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings DM, Andre VM, Uzgil BO, Gee SM, Fisher YE, Cepeda C, Levine MS. Alterations in cortical excitation and inhibition in genetic mouse models of Huntington's disease. J Neurosci 29: 10371–10386, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings DM, Cepeda C, Levine MS. Alterations in striatal synaptic transmission are consistent across genetic mouse models of Huntington's disease. ASN Neuro 2: e00036, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings DM, Milnerwood AJ, Dallérac GM, Vatsavayai SC, Hirst MC, Murphy KP. Abnormal cortical synaptic plasticity in mice transgenic for exon 1 of the human Huntington's disease mutation. Brain Res Bull 72: 103–107, 2007. [DOI] [PubMed] [Google Scholar]
- Cummings DM, Milnerwood AJ, Dallerac GM, Waights V, Brown JY, Vatsavayai SC, Hirst MC, Murphy KP. Aberrant cortical synaptic plasticity and dopaminergic dysfunction in a mouse model of Huntington's disease. Hum Mol Genet 15: 2856–2868, 2006. [DOI] [PubMed] [Google Scholar]
- Dvorzhak A, Semtner M, Faber DS, Grantyn R. Tonic mGluR5/CB1-dependent suppression of inhibition as a pathophysiological hallmark in the striatum of mice carrying a mutant form of huntingtin. J Physiol 591: 1145–1166, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham RK, Pouladi MA, Joshi P, Lu G, Deng Y, Wu NP, Figueroa BE, Metzler M, Andre VM, Slow EJ, Raymond L, Friedlander R, Levine MS, Leavitt BR, Hayden MR. Differential susceptibility to excitotoxic stress in YAC128 mouse models of Huntington disease between initiation and progression of disease. J Neurosci 29: 2193–2204, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M, Shirasaki DI, Cepeda C, Andre VM, Wilburn B, Lu XH, Tao J, Yamazaki I, Li SH, Sun YE, Li XJ, Levine MS, Yang XW. Full-length human mutant huntingtin with a stable polyglutamine repeat can elicit progressive and selective neuropathogenesis in BACHD mice. J Neurosci 28: 6182–6195, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Andre VM, Cepeda C, Li SH, Li XJ, Levine MS, Yang XW. Pathological cell-cell interactions are necessary for striatal pathogenesis in a conditional mouse model of Huntington's disease. Mol Neurodegener 2: 8, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad MS, Cummings JL. Huntington's disease. Psychiatr Clin North Am 20: 791–807, 1997. [DOI] [PubMed] [Google Scholar]
- Heikkinen T, Lehtimaki K, Vartiainen N, Puolivali J, Hendricks SJ, Glaser JR, Bradaia A, Wadel K, Touller C, Kontkanen O, Yrjanheikki JM, Buisson B, Howland D, Beaumont V, Munoz-Sanjuan I, Park LC. Characterization of neurophysiological and behavioral changes, MRI brain volumetry and 1H MRS in zQ175 knock-in mouse model of Huntington's disease. PLoS One 7: e50717, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng MY, Duong DK, Albin RL, Tallaksen-Greene SJ, Hunter JM, Lesort MJ, Osmand A, Paulson HL, Detloff PJ. Early autophagic response in a novel knock-in model of Huntington disease. Hum Mol Genet 19: 3702–3720, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng MY, Tallaksen-Greene SJ, Detloff PJ, Albin RL. Longitudinal evaluation of the Hdh(CAG)150 knock-in murine model of Huntington's disease. J Neurosci 27: 8989–8998, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey MA, Kosmalska A, Enayati J, Cohen R, Zeitlin S, Levine MS, Chesselet MF. Extensive early motor and non-motor behavioral deficits are followed by striatal neuronal loss in knock-in Huntington's disease mice. Neuroscience 157: 280–295, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson JG, Agopyan N, Gutekunst CA, Leavitt BR, LePiane F, Singaraja R, Smith DJ, Bissada N, McCutcheon K, Nasir J, Jamot L, Li XJ, Stevens ME, Rosemond E, Roder JC, Phillips AG, Rubin EM, Hersch SM, Hayden MR. A YAC mouse model for Huntington's disease with full-length mutant huntingtin, cytoplasmic toxicity, and selective striatal neurodegeneration. Neuron 23: 181–192, 1999. [DOI] [PubMed] [Google Scholar]
- Holley SM, Rudberg KN, Cepeda C, Levine MS. Differential and region-specific contributions of GABAergic interneurons in mouse models of Huntington's disease (Abstract). Soc Neurosci Abstr 2014: 46.21, 2014. [Google Scholar]
- Huntington's Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell 72: 971–983, 1993. [DOI] [PubMed] [Google Scholar]
- Joshi PR, Wu NP, Andre VM, Cummings DM, Cepeda C, Joyce JA, Carroll JB, Leavitt BR, Hayden MR, Levine MS, Bamford NS. Age-dependent alterations of corticostriatal activity in the YAC128 mouse model of Huntington disease. J Neurosci 29: 2414–2427, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapstein GJ, Fisher RS, Zanjani H, Cepeda C, Jokel ES, Chesselet MF, Levine MS. Electrophysiological and morphological changes in striatal spiny neurons in R6/2 Huntington's disease transgenic mice. J Neurophysiol 86: 2667–2677, 2001. [DOI] [PubMed] [Google Scholar]
- Laforet GA, Sapp E, Chase K, McIntyre C, Boyce FM, Campbell M, Cadigan BA, Warzecki L, Tagle DA, Reddy PH, Cepeda C, Calvert CR, Jokel ES, Klapstein GJ, Ariano MA, Levine MS, DiFiglia M, Aronin N. Changes in cortical and striatal neurons predict behavioral and electrophysiological abnormalities in a transgenic murine model of Huntington's disease. J Neurosci 21: 9112–9123, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner RP, Trejo Martinez Ldel C, Zhu C, Chesselet MF, Hickey MA. Striatal atrophy and dendritic alterations in a knock-in mouse model of Huntington's disease. Brain Res Bull 87: 571–578, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Tallaksen-Greene S, Chien WM, Cearley JA, Jackson WS, Crouse AB, Ren S, Li XJ, Albin RL, Detloff PJ. Neurological abnormalities in a knock-in mouse model of Huntington's disease. Hum Mol Genet 10: 137–144, 2001. [DOI] [PubMed] [Google Scholar]
- Loh DH, Kudo T, Truong D, Wu Y, Colwell CS. The Q175 mouse model of Huntington's disease shows gene dosage- and age-related decline in circadian rhythms of activity and sleep. PLoS One 8: e69993, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, Lawton M, Trottier Y, Lehrach H, Davies SW, Bates GP. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell 87: 493–506, 1996. [DOI] [PubMed] [Google Scholar]
- Menalled L, Brunner D. Animal models of Huntington's disease for translation to the clinic: best practices. Mov Disord 29: 1375–1390, 2014. [DOI] [PubMed] [Google Scholar]
- Menalled LB. Knock-in mouse models of Huntington's disease. NeuroRx 2: 465–470, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menalled LB, Kudwa AE, Miller S, Fitzpatrick J, Watson-Johnson J, Keating N, Ruiz M, Mushlin R, Alosio W, McConnell K, Connor D, Murphy C, Oakeshott S, Kwan M, Beltran J, Ghavami A, Brunner D, Park LC, Ramboz S, Howland D. Comprehensive behavioral and molecular characterization of a new knock-in mouse model of Huntington's disease: zQ175. PLoS One 7: e49838, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menalled LB, Sison JD, Dragatsis I, Zeitlin S, Chesselet MF. Time course of early motor and neuropathological anomalies in a knock-in mouse model of Huntington's disease with 140 CAG repeats. J Comp Neurol 465: 11–26, 2003. [DOI] [PubMed] [Google Scholar]
- Menalled LB, Sison JD, Wu Y, Olivieri M, Li XJ, Li H, Zeitlin S, Chesselet MF. Early motor dysfunction and striosomal distribution of huntingtin microaggregates in Huntington's disease knock-in mice. J Neurosci 22: 8266–8276, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milnerwood AJ, Raymond LA. Corticostriatal synaptic function in mouse models of Huntington's disease: early effects of huntingtin repeat length and protein load. J Physiol 585: 817–831, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond LA, Andre VM, Cepeda C, Gladding CM, Milnerwood AJ, Levine MS. Pathophysiology of Huntington's disease: time-dependent alterations in synaptic and receptor function. Neuroscience 198: 252–273, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebec GV, Conroy SK, Barton SJ. Hyperactive striatal neurons in symptomatic Huntington R6/2 mice: variations with behavioral state and repeated ascorbate treatment. Neuroscience 137: 327–336, 2006. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Tuch DS, Hevelone ND, Zaleta AK, Vangel M, Hersch SM, Salat DH. Diffusion tensor imaging in presymptomatic and early Huntington's disease: selective white matter pathology and its relationship to clinical measures. Mov Disord 21: 1317–1325, 2006. [DOI] [PubMed] [Google Scholar]
- Shelbourne PF, Killeen N, Hevner RF, Johnston HM, Tecott L, Lewandoski M, Ennis M, Ramirez L, Li Z, Iannicola C, Littman DR, Myers RM. A Huntington's disease CAG expansion at the murine Hdh locus is unstable and associated with behavioural abnormalities in mice. Hum Mol Genet 8: 763–774, 1999. [DOI] [PubMed] [Google Scholar]
- Slow EJ, van Raamsdonk J, Rogers D, Coleman SH, Graham RK, Deng Y, Oh R, Bissada N, Hossain SM, Yang YZ, Li XJ, Simpson EM, Gutekunst CA, Leavitt BR, Hayden MR. Selective striatal neuronal loss in a YAC128 mouse model of Huntington disease. Hum Mol Genet 12: 1555–1567, 2003. [DOI] [PubMed] [Google Scholar]
- Smith GA, Rocha EM, McLean JR, Hayes MA, Izen SC, Isacson O, Hallett PJ. Progressive axonal transport and synaptic protein changes correlate with behavioral and neuropathological abnormalities in the heterozygous Q175 KI mouse model of Huntington's disease. Hum Mol Genet 23: 4510–4527, 2014. [DOI] [PubMed] [Google Scholar]
- Spampanato J, Gu X, Yang XW, Mody I. Progressive synaptic pathology of motor cortical neurons in a BAC transgenic mouse model of Huntington's disease. Neuroscience 157: 606–620, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonsattel JP, DiFiglia M. Huntington disease. J Neuropathol Exp Neurol 57: 369–384, 1998. [DOI] [PubMed] [Google Scholar]
- Walker AG, Miller BR, Fritsch JN, Barton SJ, Rebec GV. Altered information processing in the prefrontal cortex of Huntington's disease mouse models. J Neurosci 28: 8973–8982, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Gray M, Lu XH, Cantle JP, Holley SM, Greiner E, Gu X, Shirasaki D, Cepeda C, Li Y, Dong H, Levine MS, Yang XW. Neuronal targets for reducing mutant huntingtin expression to ameliorate disease in a mouse model of Huntington's disease. Nat Med 20: 536–541, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu ZX, Li SH, Evans J, Pillarisetti A, Li H, Li XJ. Mutant huntingtin causes context-dependent neurodegeneration in mice with Huntington's disease. J Neurosci 23: 2193–2202, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]