Abstract
Transection of a peripheral nerve results in withdrawal of synapses from motoneurons. Some of the withdrawn synapses are restored spontaneously, but those containing the vesicular glutamate transporter 1 (VGLUT1), and arising mainly from primary afferent neurons, are withdrawn permanently. If animals are exercised immediately after nerve injury, regeneration of the damaged axons is enhanced and no withdrawal of synapses from injured motoneurons can be detected. We investigated whether delaying the onset of exercise until after synapse withdrawal had occurred would yield similar results. In Lewis rats, the right sciatic nerve was cut and repaired. Reinnervation of the soleus muscle was monitored until a direct muscle (M) response was observed to stimulation of the tibial nerve. At that time, rats began 2 wk of daily treadmill exercise using an interval training protocol. Both M responses and electrically-evoked H reflexes were monitored weekly for an additional seven wk. Contacts made by structures containing VGLUT1 or glutamic acid decarboxylase (GAD67) with motoneurons were studied from confocal images of retrogradely labeled cells. Timing of full muscle reinnervation was similar in both delayed and immediately exercised rats. H reflex amplitude in delayed exercised rats was only half that found in immediately exercised animals. Unlike immediately exercised animals, motoneuron contacts containing VGLUT1 in delayed exercised rats were reduced significantly, relative to intact rats. The therapeutic window for application of exercise as a treatment to promote restoration of synaptic inputs onto motoneurons following peripheral nerve injury is different from that for promoting axon regeneration in the periphery.
Keywords: synaptic plasticity, exercise, nerve injury, regeneration
peripheral nerve injuries are common, with between 2.0–2.8% and 5% of the trauma population, depending upon the injuries inclusion criteria (Eser et al. 2009), resulting in hundreds of thousands of injuries per year (Taylor et al. 2008). Following a peripheral nerve transection, the conduit between muscle fibers in the periphery and neural circuits in the spinal cord is disrupted. Axons in cut nerves can regenerate, but functional recovery is poor. Based on a number of outcome measures, no or poor functional recovery has been reported for 83% of patients with full nerve transection injuries (Sunderland stage 5) (Noble et al. 1998). These poor functional outcomes are often attributed to the process of axon regeneration. Although axons in injured peripheral nerves do regenerate, the process is slow, and if long distances must be traveled, as is often found in humans, long recovery periods are required before any functional restoration can occur. Some axons in cut nerves do not regenerate immediately but are delayed for days or weeks, a phenomenon known as “staggered” regeneration (Al-Majed et al. 2000b). Once regeneration begins, some axons are misdirected, resulting in decreased fidelity in the way both sensory and motor targets are reinnervated (Sabatier et al. 2011). Enhancing the process of axon regeneration has thus emerged as a target for the development of novel treatments for peripheral nerve injury (Hoke and Brushart 2010).
In addition to the notable effects of nerve injury in the periphery, transection of peripheral nerves is known to produce marked changes in the circuitry in the central nervous system (CNS) (Brushart 2011). The most widely studied of these changes is the postinjury withdrawal of synaptic inputs from the somata and proximal-most dendrites of motoneurons, known as synaptic stripping (Alvarez et al. 2010; Alvarez et al. 2011). More than half of all of the synaptic terminals are withdrawn within 2 wk of injury (Blinzinger and Kreutzberg 1968; Sumner 1975; Svensson et al. 1991; Titmus and Faber 1990). Many of these inputs are later restored, but those containing vesicular glutamate transporter 1 (VGLUT1), and arising mainly from primary afferent neurons (Alvarez et al. 2004; Todd et al. 2003), continue to be withdrawn and remain so permanently (Alvarez et al. 2011). The small number of these somatodendritic inputs that remain also are reorganized. They are smaller and no longer clustered around the proximal-most dendrites of the motoneurons (Rotterman et al. 2014). This permanent stripping of the synaptic inputs arising from large, stretch-sensitive afferents and the reorganization of the small proportion that remain are major contributors to the permanent loss of the stretch reflex in self-reinnervated muscles (Bullinger et al. 2011; Cope and Clark 1993). This synaptic plasticity also may underlie the marked reduction in the amplitudes of electrically evoked composite excitatory postsynaptic potentials (EPSPs) found in motoneurons after peripheral nerve transection and repair (Mendell et al. 1995). Thus synaptic stripping could contribute substantially to poor functional recovery following peripheral nerve injury. Any treatments aimed at enhancing axon regeneration after peripheral nerve injury also should be evaluated for their longer term impact on the plasticity of synaptic inputs, especially inputs containing VGLUT1.
One effective approach to enhancing axon regeneration is to stimulate the production of growth-promoting neurotrophins using physical means, such as brief electrical stimulation (ES) (Al-Majed et al. 2000b). Application of as little as 1 h of ES to the proximal segment of the injured nerve results in a marked increase in the expression of BDNF and trkB in neurons whose axons are regenerating (Al-Majed et al. 2000a) and a striking enhancement of axon regeneration (Ahlborn et al. 2007; Alrashdan et al. 2010; Asensio-Pinilla et al. 2009; English et al. 2007b; Franz et al. 2013; Geremia et al. 2007; Hetzler et al. 2008; Huang et al. 2009; Lu et al. 2008; Nix and Hopf, 1983; Sharma et al. 2009; Sharma et al. 2010; Singh et al. 2012). Similarly, modest treadmill exercise begun on the third day after peripheral nerve injury results in enhancement of axon regeneration comparable to or even exceeding that found after ES (English et al. 2011). This exercise paradigm also resulted in a marked increase in the recovery of evoked direct muscle (M) responses to nerve stimulation, electrically evoked H reflexes, and a modest recovery of locomotor function (Boeltz et al. 2013). Both ES and treadmill exercise have been used to promote axon regeneration in a number of different animal models (reviewed in English et al. 2014; Udina et al. 2011). Treadmill exercise also has been shown to have a marked impact on synaptic stripping. If training is begun on the third day following nerve injury, no withdrawal of synaptic inputs is noted, including those containing VGLUT1 (Liu et al. 2014).
Although treadmill exercise as a potential therapy for treating peripheral nerve injury has shown promise, both in enhancing axon regeneration in the periphery and on synaptic inputs onto motoneurons, the timing of application of that training may be critical to its overall success in restoring function. Immediate training would be expected to enhance axon regeneration and effect synaptic withdrawal only at its earliest stages. Allowing synaptic stripping to begin before treadmill training is begun might be expected to affect the nature of postinjury plasticity in spinal circuits differently. The goal of this study was to compare the effects of treadmill exercise begun at the first signs of muscle reinnervation to the effects of training begun shortly after peripheral nerve injury (Boeltz et al. 2013). In this paper, we report the effect of this delayed training on restoration of nerve conduction and on the extent of contacts made by two types of synaptic inputs onto the somata and proximal-most dendrites of motoneurons.
METHODS
Animals and surgical methods.
All experimental procedures conformed to the Guidelines for the Use of Animals in Research of the Society for Neuroscience and were approved by the Institutional Animal Care and Use Committee of Emory University (Protocol No. 2002017).
Experiments were conducted on 32 Lewis rats, body wt ∼250 g. Females were chosen because they autotomize (chew toes) less following peripheral nerve injuries than male rats. These animals were assigned to treatment groups for electromyographic recording and anatomical analysis of synaptic inputs to motoneurons, as indicated in Table 1. Data from 12 of these rats have been reported in a previous publication (Boeltz et al. 2013), and they serve as controls for the experiments whose results are reported in this paper. Numbers of animals in each group were determined using an a priori power sample size estimate (Lenth 2006–9) based on the intergroup variability of our previously published study (Boeltz et al. 2013), and using a power of 0.8 and α = 0.05.
Table 1.
Experimental groups
| Group | N | No. Cells/Animal | |
|---|---|---|---|
| Electromyographic recording | |||
| Untreated* | 6 | ||
| Immediate* | 6 | ||
| Delayed | 4 | ||
| Anatomic analysis of synaptic inputs onto motoneurons | |||
| 4 wk | 10 wk | ||
| Intact | 4‡ | 15 | |
| Untreated | 4 | 4 | 20 |
| Immediate | 4 | 15 | |
| Delayed | 4 | 15 | |
Data from Boeltz et al. 2013.
Contralateral to Untreated 10 wk.
Recording hardware was implanted into four isoflurane anesthetized animals for chronic use. All implanted electrodes were constructed as described previously (Boeltz et al. 2013; Sabatier et al. 2008). Bipolar fine wire EMG electrodes (Cooner Wire No. AS631, Chatsworth, CA http://www.coonerwire.com/) were implanted into the right soleus (SOL) and tibialis anterior (TA) muscles to record electrical potentials produced by these muscles. The wires were secured in place with fine (6-0 nylon) sutures to minimize their movement while the muscle was contracting. A bipolar stimulating cuff electrode (Stein et al. 1977), made of Silastic tubing and the same fine wire, was implanted around the exposed right tibial nerve below its branching from the sciatic nerve. All EMG wires, and those attached to the stimulating cuff, were lead subcutaneously to a small connector (Plastics One, part no. 363/CP, http://www.plastics1.com/), ∼1 cm in diameter, mounted on the head of the animal with stainless steel bone screws implanted into the skull and secured with dental acrylic.
Recordings were made twice over a 2-wk period prior to sciatic nerve transection and repair. In each recording session, the tibial nerve was stimulated through the cuff and evoked EMG activity was recorded from SOL in awake animals (see below). Following the second recording session, the rats were anesthetized, the entire sciatic nerve was cut ∼10 mm proximal to the implanted cuff, using sharp scissors, and the proximal and distal stumps of the cut nerve were aligned and secured in place using fibrin glue. The glue was manufactured at the time of use from equal parts of thrombin and a 1:1 mixture of fibrinogen and fibronectin (MacGillivray 2003). Once the sciatic nerve was repaired and the fibrin glue was cured (1–2 min) to form a clot about the repaired nerve, the surgical site was closed in layers with appropriate sutures.
Electromyographic recordings.
We studied axon regeneration after peripheral nerve injury indirectly, using stimulus-evoked EMG potentials. The SOL muscle is innervated exclusively by axons coursing in the tibial branch of the sciatic nerve. By placing the stimulating cuff on the tibial branch distal to the transection site on the sciatic nerve, only sensory and motor axons that have regenerated and reinnervated the SOL muscle are stimulated. Prior to and at weekly intervals over a 10-wk period following sciatic nerve transection and repair, we evoked an M response, or compound muscle action potential, by stimulation of motor axons in the tibial nerve, via the implanted cuff electrode, and recording EMG activity in the SOL muscle. We also recorded a longer latency H reflex, evoked by stimulating axons of primary afferent neurons (dorsal root ganglion cells) that synapse directly onto motoneurons. In untreated animals, successful regeneration of motor axons and reinnervation of muscle fibers results in a restored M response beginning ∼3 wk after transection and repair. A restored H reflex is not found until ca. 1 wk later (English et al. 2007a).
Details of the recording methods in awake rats can be found in our earlier publications (Boeltz et al. 2013; English et al. 2007a). During each recording session, we monitored EMG activity in SOL continuously in awake animals and evaluated the average rectified activity over 10-ms intervals. When this average activity was maintained at a predetermined level for 50 ms, a constant-voltage monophasic stimulus pulse (0.1-ms duration) was applied to the tibial nerve via the implanted nerve cuff electrode. We used this approach in an effort to apply repeated stimuli to the animals under similar conditions. Activity was sampled at 10 kHz, beginning 20 ms prior to the stimulus and extending to 80 ms following the pulse, and stored on a laboratory computer system. Stimuli were applied no more frequently than once every 3 s to avoid muscle fatigue. Following sciatic nerve transection and repair, animals were tested daily until an M response was noted and then 5 days/wk during 2 wk of treadmill training. After treadmill training was completed, animals were tested one to two times per week for an additional 5 wk.
During each recording session, the evoked M responses and H reflexes were sampled using stimuli over a large range of voltages to determine the intensity required to evoke maximum amplitudes of each response. Once found, 100 trials were taken at each of these stimulus intensities. Average maximal M responses and maximal H reflexes for each muscle were extracted from these collected records. In intact rats, the M response and H reflex occur at predictable time intervals (0.5–4.0 ms and 4.5–11.0 ms, respectively) following tibial nerve stimulation (English et al. 2007a). Following recovery from sciatic nerve transection, the evoked EMG responses first reappear as a series of small potentials at much longer latencies than found in intact rats. Over time, these consolidate into two single, easily recognizable responses found in the same time windows as intact animals (English et al. 2007a). Thus during the reinnervation period, the time windows for the two responses were adjusted to accommodate these latency changes.
To investigate the time course of changes in the amplitude of the M response and H reflex, multiple linear regression methods were used (Boeltz et al. 2013). For each rat, the amplitude of the recorded M responses and H reflexes were expressed as a proportion of the M response and H reflex recorded from that animal prior to nerve transection and repair. Results from the three different treatment groups studied (Table 1) were compared. Changes in these relative M response or H reflex amplitudes over time were fit with linear least-squares trendlines, and the significance of each of the regression coefficients and of differences in slopes and intercepts of the lines for the three groups were compared using analysis of variance (ANOVA). Significance was set at P < 0.05 for all tests.
Treadmill exercise.
Tibial nerve stimulation was conducted daily following nerve injury. An evoked EMG response in SOL to tibial nerve stimulation with amplitude significantly greater than the prestimulus background activity was taken as evidence of a restored evoked M-response. Treadmill exercise began following the first noted reoccurrence of this motor response, usually around 3 wk after nerve transection and repair (mean 22.25 days, SD = 6.02 days, N = 4). Rats walked on a custom treadmill on a level surface at 20 m/min for 2 min with a 5-min rest for four trials, 5 days/wk for 2 wk. Animals were selected for inclusion into this study if they were able to run on the treadmill in presurgical screenings, at a speed of up to 22 m/min without the need for reinforcement. To avoid any complications of preinjury exercise, rats were not exposed to the treadmill for at least 2 wk prior to nerve repair surgery.
Anatomical analysis of synaptic inputs to motoneurons.
We studied the extent of contact of the somata and proximal-most dendrites of motoneurons by two different types of synaptic inputs in four treatment groups (Table 1). The extent of synaptic inputs onto motoneurons was studied in Untreated, Immediate, and Delayed groups 10 wk after unilateral sciatic nerve transection and repair. Because the left sciatic nerve remained intact in rats in the Untreated group, data from the left sides of the spinal cords harvested from theses rats were treated as an Intact group (Table 1). Data in these four groups were compared with similar observations made in a fifth group of rats 4 wk after sciatic nerve transection and repair without treatment.
Contacts made by structures immunoreactive (IR) for VGLUT1, which arise mainly from primary afferent neurons (Alvarez et al. 2004; Todd et al. 2003), and those immunoreactive to glutamic acid decarboxylase protein 67 (GAD67), the rate-limiting enzyme in the synthesis of the inhibitory neurotransmitter gamma amino butyric acid (GABA) (Hughes et al. 2005), were studied. These types of inputs are just two of several different types of inputs to motoneurons, but they serve as examples of synaptic inputs which are permanently (VGLUT1) or only transiently (GAD67) withdrawn following peripheral nerve transection. We assumed that because the proportions of inputs immunoreactive for VGLUT1 arising from sources other than primary afferent neurons is quite small, any contributions that they might make to the findings presented below were not considered.
Motoneurons reinnervating SOL muscles were marked by the intramuscular injection of 1 μl of a 1% solution of the beta subunit of cholera toxin conjugated to Alexafluor 555 (Molecular Probes-Life Technologies, Eugene, OR, http://www.lifetechnologies.com/) 3 days prior to tissue harvesting. Similar injections were made on the left sides of rats in the Untreated group. Following an overdose of pentobarbital sodium (150 mg/kg ip), all animals were perfused transcardially with periodate-lysate-paraformaldehyde (PLP) fixative (McLean and Nakane 1974). The L3–L5 segments of spinal cords were removed and stored overnight in 20% sucrose solution at 4°C for cryoprotection. Serial transverse sections were cut at 40 μm on a cryostat and mounted directly onto subbed slides. Tissue sections from most rats were reacted on slides with antibodies to the synapse-associated proteins GAD67 (Millipore no. MAB5406, Billerica, MA; http://www.merckmillipore.com/) and VGLUT1 (Synaptic Systems GmbH no. TO30326, Göttingen, Germany; www.sysy.com/). Sections were incubated in primary antibodies, diluted in 0.1 M phosphate buffer solution (PBS), 1:2,000 for rabbit anti-VGLUT1, 1:200 for mouse anti-GAD67, for 24 h at 4°C. After washing in 0.1 M PBS three times, sections were incubated with secondary antibodies (goat anti-rabbit IgG conjugated to Alexafluor 647 and goat anti-mouse IgG conjugated to Alexafluor 488) for 2 h at room temperature to detect immunofluorescence. All the incubations and reactions were separated by 3 × 10 min washes in 0.1 M PBS. Sections were cover slipped using Vectashield.
In rats whose sciatic nerves were cut and repaired but remained untreated for 4 wk, the retrograde labeling did not always fill the motoneurons completely enough to outline their borders clearly. Tissue sections from these rats were reacted with antibodies to the neuronal nuclear antigen (NeuN), followed by an Alexafluor 647-conjugated secondary antibody. As has been shown by others (Alvarez et al. 2010), NeuN immunoreactivity is very effective in outlining the boundaries of motoneurons. In these animals, alternate sections were reacted either with a monoclonal anti-NeuN antibody (Millipore, no. MAB377) and polyclonal anti-VGLUT1 or a polyclonal anti-NeuN (Millipore, no. ABN78) and monoclonal anti-GAD67. In either scenario, antibody binding to synapse-associated proteins was visualized after reaction with species-appropriate secondary antibodies conjugated to Alexafluor 488. Thus, in images of the cells selected for study in these cases, we could identify the retrograde label (marked by Alexafluor 555), immunoreactivity to NeuN (marked by Alexafluor 647), and immunoreactivity to either VGLUT1 or GAD67 (marked by Alexafluor 488). When NeuN immunoreactivity was used to recognize cell boundaries, cells were selected for study only if they also contained the retrograde label.
All analyses of tissue sections in this study were performed in such a way that the persons collecting images and taking measurements from the collected images were unaware, while performing these tasks, of the experimental conditions applied to the tissues. Histological sections of L3–L5 spinal cord were viewed with a laser scanning confocal microscope (Zeiss LSM510). Labeled motoneurons were selected for study only if they contained labeling that filled the somata and extended into the proximal dendrites, contained a clear nucleus that was not fluorescent, and their cell borders could be recognized (Fig. 1A). Twenty to 40 high-magnification (40×) RGB images of individual labeled motoneuron cell bodies were obtained, at a confocal Z-dimension thickness of 1 μm, from each side of each spinal cord studied (Table 1).
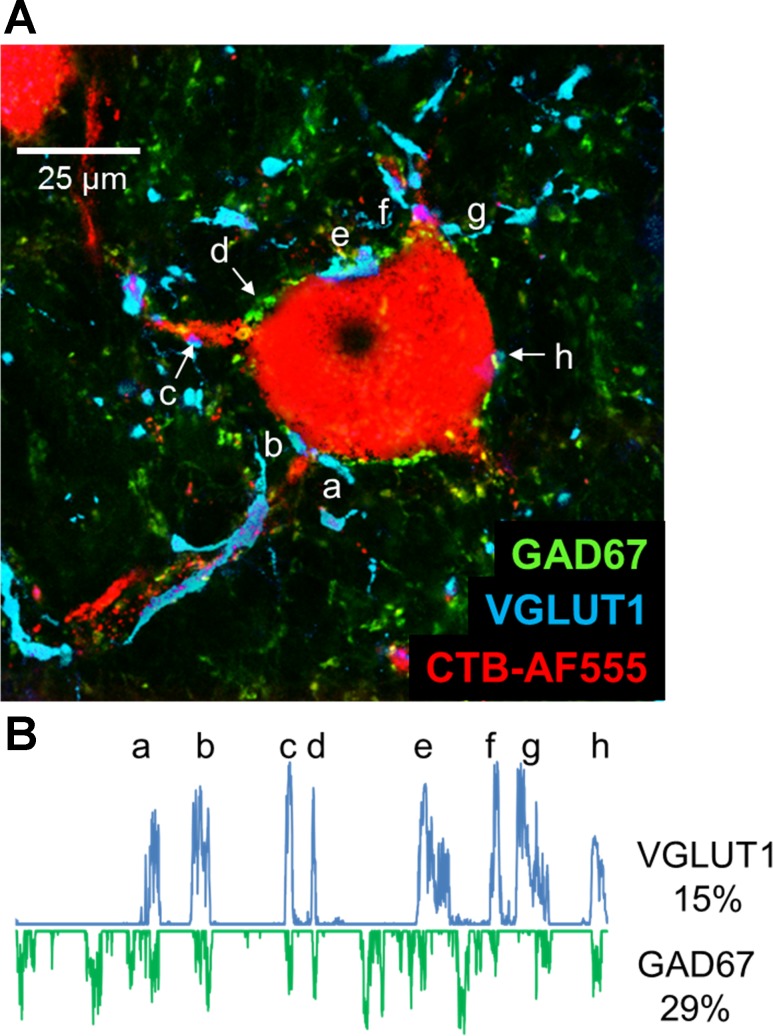
Fig. 1.
A: a single confocal image of a retrogradely labeled motoneuron (red), taken from a histological section of the spinal cord of an intact rat, is shown to demonstrate the methods used. The section was reacted with antibodies for the demonstration of the synapse-associated proteins, VGLUT1 (cyan) and GAD67 (green). A region of interest (ROI) was created around the perimeter of the cell, using the threshold function of ImageJ and only the red channel of the image. From this ROI, profile plots were generated to measure the fluorescence intensity along a 1-μm-wide line about the cell perimeter. B: profile plots for the cell in A are shown for immunoreactivity to VGLUT1 (positive values) and for the exact same locations for GAD67 (negative values). Lowercase letters a–h indicate places on the profile plot for VGLUT1 and their corresponding locations in the image in A. In this cell, 15% of the perimeter of the cell was in contact with VGLUT1-IR structures, and 29% was contacted by GAD67-IR structures.
All measurements from these images were made using ImageJ software, using a method analogous to that of Wang et al. (Wang et al. 2006). A region of interest (ROI) was created around the perimeter of each motoneuron studied (Fig. 1A) and used to study contacts made by structures immunoreactive to synapse-associated proteins. Because we considered that the identification of this ROI was critical to all subsequent analyses, we tried to make the determination of cell outline as objective as possible, viewing only the red channel of the RGB images, the one containing images of the retrograde label in the motoneuron, while creating the ROI, because the contrast between the border of the cells studied and the surrounding neuropil is so substantial. Cells in which such contrast in the red channel was not entirely clear were not selected for study. When using NeuN immunoreactivity to identify the cell outline, cells were selected for study only if they contained the retrograde label and were immunoreactive for NeuN. In establishing the ROI around the perimeter of the cell profile, only the RGB channel assigned to Alexafluor 647 was used, usually the blue channel. Care was taken not to extend the ROI further along motoneuron dendrites than 25 μm from the somata of the cells studied. Once the ROI was established, a plot profile (Fig. 1B) was created as a measure the fluorescence intensity, along the ROI, in both the green and blue channels of the RGB image in which immunoreactivity for GAD67 and VGLUT1 were displayed, respectively. When NeuN immunoreactivity was used to establish the ROI, profile plots of only the green channel were studied. Any fluorescence intensity values in the profile plot for each cell studied that were greater than one standard deviation more than the mean fluorescence intensity along the ROI were assumed to represent contact of the motoneuron by structures immunoreactive for the synapse-associated proteins studied. For each cell studied, the proportion of the entire ROI that exceeded this threshold was determined and was termed the percent synaptic coverage. Mean values of percent coverage by structures immunoreactive for VGLUT1 (VGLUT1-IR) and GAD67 (GAD67-IR) structures were determined for each rat studied.
We assume that these structures immunoreactive for synapse-associated proteins that are in very close proximity to the outline of the labeled motoneurons represent synaptic contacts made on the motoneurons. This assumption has been supported by others. Immunoreactive structures identified using the same antibodies as used here have been shown to contain synaptic active zones and, after extensive high-magnification reconstruction, to be in very close contact to the somata of intracellularly filled motoneurons (Rotterman et al. 2014). However, without higher resolution images available through electron microscopy, we acknowledge that we cannot rule out that at least some of the contacts we have studied might be separated from the motoneuron soma by ultrafine processes of glial cells.
Data from motoneurons studied on the intact left side of the spinal cords of the Untreated group of rats were treated as Intact controls (Table 1), as has been justified by others (Alvarez et al. 2010). Data from rats treated with delayed treadmill exercise were compared with findings from rats whose sciatic nerves had been cut and repaired and either not treated or treated with treadmill exercise begun immediately following nerve transection and repair. Comparisons of average median synaptic coverage by VGLUT1-IR and GAD67-IR structures was made using ANOVA, and post hoc paired comparisons (Tukey's HSD). Significance was set at P < 0.05 in all comparisons.
RESULTS
Effects of delayed exercise on restoration of muscle reinnervation.
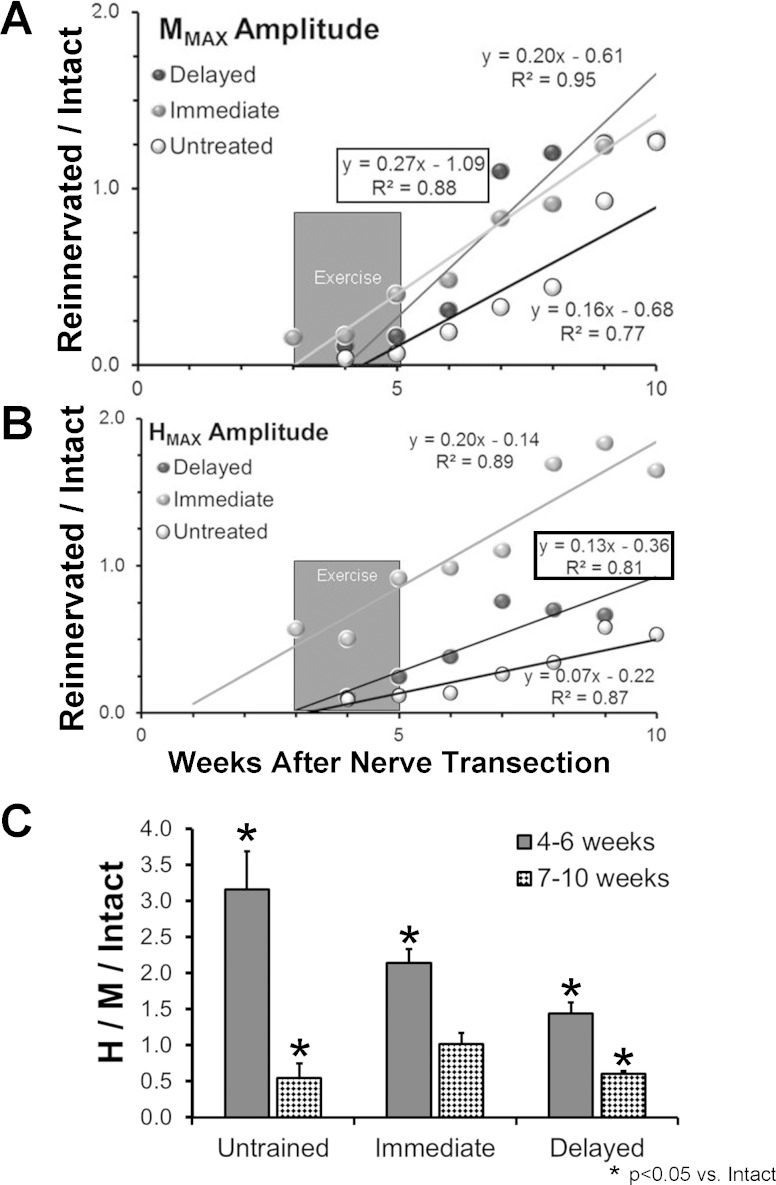
Following transection and surgical repair of the sciatic nerve, innervation of the SOL is lost, but then restored as axons in the cut nerve regenerate and reinnervate this muscle (English et al. 2007a). Over the course of 10 postinjury weeks, the amplitudes of the restored M responses increased in a linear manner with time (Fig. 2A). Such temporal changes in maximal M response amplitudes in each group of rats studied were scaled to the maximum M response amplitude measured prior to nerve transection and repair and then were fit with a linear regression model. The slopes of all of the regression lines and correlation coefficients were significant (P < 0.05). Significance of differences in the time course of these changes was evaluated between the different rat groups studied using multiple linear regression methods. As we have shown previously (Boeltz et al. 2013), the slope of the regression line for the Immediate exercised group is significantly greater than that found in Untreated animals. In the Delayed exercised animals, the slope of this line (Fig. 2A, boxed equation) was significantly (P < 0.01) greater than found in Untreated rats, but not significantly different from that found in Immediate exercised rats. Thus delaying the onset of exercise until the first signs of muscle reinnervation (ca. 3 wk) did not change the overall time course of functional recovery of muscle reinnervation.
Fig. 2.
EMG activity from the soleus muscle of the right hindlimb was evoked by tibial nerve stimulation before and at different times after transection and surgical repair of the sciatic nerve in 3 groups of rats: Untreated; rats exercised for 2 wk beginning 3 days after nerve transection (Immediate); and rats exercised for 2 wk beginning at the first sign of muscle reinnervation (mean ± SD: 22.25 ± 6.02 days) after nerve repair (Delayed). At each time studied, data were collected separately at stimulus intensities evoking the maximal direct muscle (M) response and maximal H reflex. A: changes in the amplitude of the M response, scaled to the M response amplitude encountered prior to nerve transection and repair, as a function of recovery time for the 3 groups of rats. Each symbol represents the average of 6 (Untreated or Immediate) or 4 (Delayed) rats. Data were fit with a least-squares linear regression line, and the formula for the line and the correlation coefficient are shown next to each regression line. Regression equations and correlation coefficients for the Delayed group are enclosed in boxes. The darkly shaded box marks the approximate time of exercise in the Delayed exercise group. B: data for the maximal H reflex are shown in a format similar to A. C: mean scaled Hmax/Mmax ratio (±95% confidence limits) over the periods of 4–6 wk and 7–10 wk post-nerve transection and repair are shown for each of the 3 groups studied.
Effects of delayed exercise on the H reflex.
A similar analysis of the recovery of the amplitude of the H reflex was conducted (Fig. 2B). The slopes of all of the regression lines and correlation coefficients were significant (P < 0.05). As previously reported (Boeltz et al. 2013), in Immediate exercised rats, the amplitude of the H reflex recovers at a significantly faster rate than found in Untreated animals. The slope of the regression line for Immediate exercised rats is significantly greater (P < 0.01) than found in Untreated rats. The amplitude of the H reflex recovered more rapidly in the Delayed exercised rats. The slope of the regression line (Fig. 2B, boxed equation) was significantly greater (P < 0.01) than that from data from Untreated rats, but also significantly smaller (P < 0.01) than that based on data from Immediate exercised rats. Thus delaying the onset of exercise markedly attenuated the restoration of the H reflex.
If the maximum amplitude of the H reflex is compared with the amplitude of the maximal M response in the same animal on the same day, a global measure of the proportion of the available motoneuron pool that is recruited into the reflex is obtained (Boeltz et al. 2013; Navarro et al. 2007). If that Hmax/Mmax ratio is then scaled to the same ratio in intact rats, and is studied during recovery from sciatic nerve transection and repair, a transient period of much enlarged reflex efficacy is noted, followed by a decline to a stable level beginning around 7 wk after injury (Boeltz et al. 2013; Valero-Cabre and Navarro 2001). To evaluate the significance of delayed exercise, we compared the mean, scaled Hmax/Mmax ratio in Untreated, Immediate, and Delayed groups of rats during the period of exaggerated reflex (4–6 wk after injury) and again at 7–10 wk after sciatic nerve transection and repair, when the reflex efficacy was more stabilized (Fig. 2C).
At the 4–6 wk survival time, the mean, scaled Hmax/Mmax ratios for the Untreated, Immediate, and Delayed groups all were significantly greater than unity, indicating a degree of exaggerated reflex activity. Significant differences were found among these three groups (ANOVA, F2,9 = 5.47, P < 0.03), and using post hoc paired testing (Tukey's honest significant differences, HSD), the scaled Hmax/Mmax ratio in the Delayed group was significantly smaller than both the Untreated (HSD, P < 0.04) and Immediate (HSD, P < 0.03) groups. No significant difference was found between the Untreated and Immediate groups. Thus delaying the onset of postinjury exercise attenuates but does not eliminate the period of exaggerated H reflex efficacy.
At the time interval 7–10 wk after nerve transection and repair, we also found a significant difference in scaled Hmax/Mmax ratios between the three groups studied (ANOVA, F2,9 = 8.24, P < 0.01) (Fig. 2C). Among rats exercised beginning on the third day following sciatic nerve transection and repair, no significant reduction in the scaled Hmax/Mmax ratio was found, suggesting a complete or nearly complete restoration of the reflex. In Untreated rats and rats in the Delayed group, this measure of efficacy of the H reflex pathway was reduced significantly (HSD, P < 0.01) from that found in Intact rats. No significant difference between rats in the Delayed group and Untreated rats was found. Thus the timing of onset of treadmill exercise after peripheral nerve injury has a significant effect on the ultimate restoration of the H reflex.
Effects of delayed exercise on synaptic inputs onto motoneurons.
We examined the extent of synaptic contacts made by structures immunoreactive for the synapse-associated proteins VGLUT1 and GAD67 onto the somata and proximal-most dendrites of axotomized motoneurons in two ways. First, we pooled the measurements made from the four animals in each of the five different treatment groups (Table 1) and examined the frequencies with which different values of percent synaptic coverage occurred. We then constructed cumulative distributions of these frequencies and evaluated the significance of differences between the groups using nonparametric statistical methods. These distributions are shown for the five groups studied for VGLUT1-IR contacts in Fig. 3A and for GAD67-IR contacts in Fig. 4A. Second, in each rat, we determined the median synaptic coverage and then evaluated the significance of differences between groups using ANOVA, and post hoc paired (Tukey's HSD) testing, where appropriate. Medians were chosen for study, rather than means, because the distribution of means was not normal. These data are shown in Fig. 3B for VGLUT1 and in Fig. 4B for GAD67.
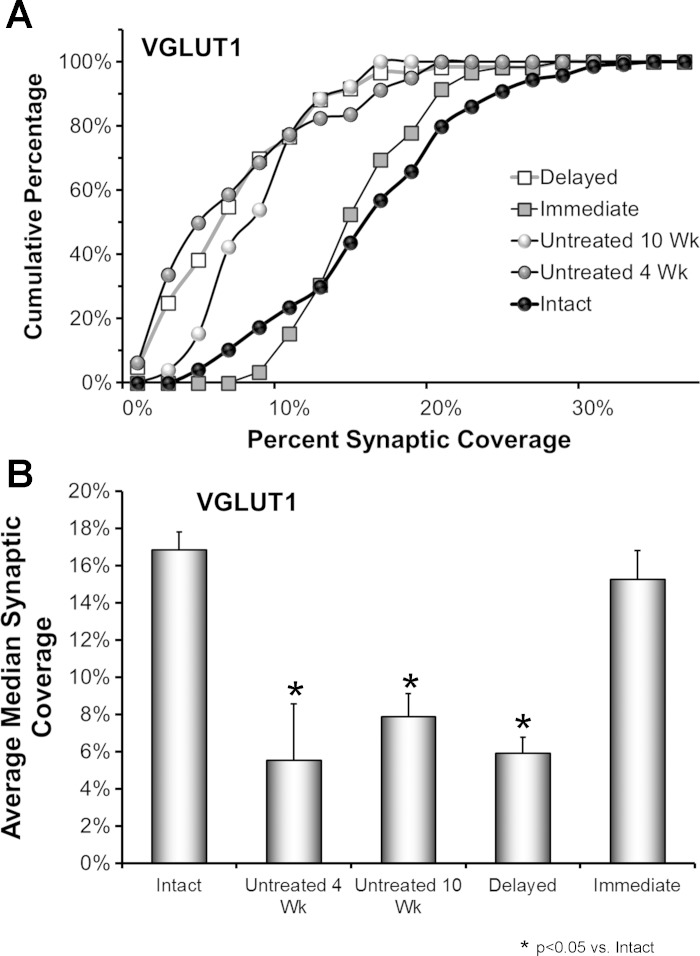
Fig. 3.
The effects of delayed treadmill exercise on synaptic coverage by VGLUT1-IR terminals on motoneurons are shown. A: data from 4 rats in each group were pooled and used to construct cumulative frequency distributions. Results are presented for Untreated animals at 2 different survival times (4 wk and 10 wk, N = 80 cells in each group), and for animals treated with 2 wk of daily treadmill exercise, begun either 3 days (Immediate) or ca. 3 wk (Delayed) after nerve injury (N = 60 cells per group) and examined 10 wk after the initial peripheral nerve transection and repair. Control data are presented from the Intact sides of the 4 rats in the Untreated 10 wk group (N = 80 cells). The values on the X-axis correspond to the percent of the cell perimeter in contact with VGLUT-IR structures in the different cells studied. Values on the Y-axis represent the cumulative frequencies with which the different values were observed. B: the average median (±SE) synaptic coverage by VGLUT1-IR structures is shown for the same groups of rats as in A. N = 4 rats in each group.
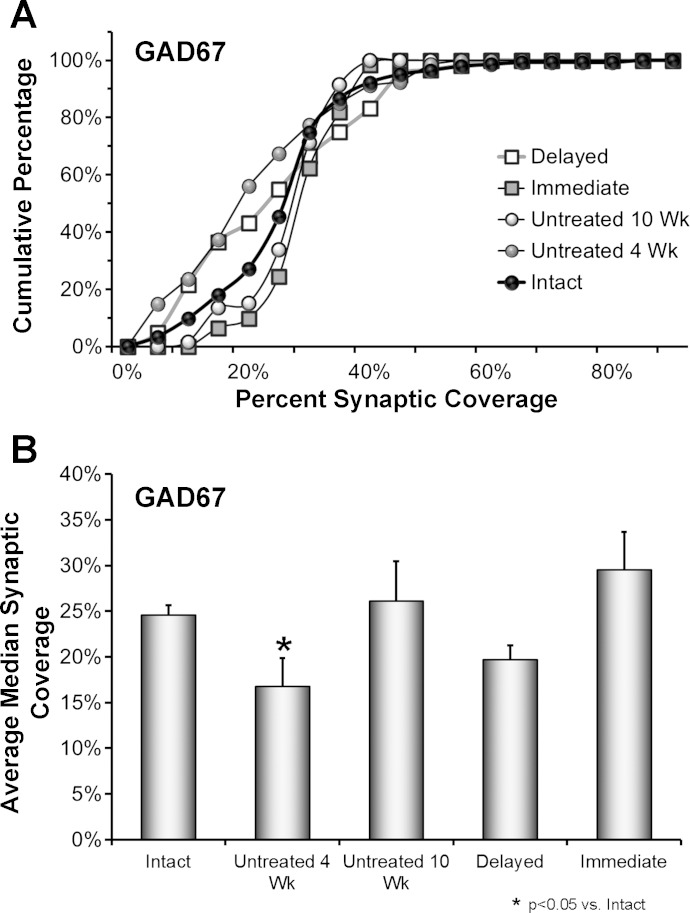
Fig. 4.
The effects of delayed treadmill exercise on synaptic coverage by GAD67-IR terminals on motoneurons are shown. A: data from 4 rats in each group were pooled and used to construct cumulative frequency distributions. Results are presented for Untreated animals at 2 different survival times (4 wk and 10 wk, N = 80 cells in each group), and for animals treated with 2 wk of daily treadmill exercise, begun either 3 days (Immediate) or ca. 3 wk (Delayed) after nerve injury (N = 60 cells per group) and examined 10 wk after the initial peripheral nerve transection and repair. Control data are presented from the Intact sides of the 4 rats in the Untreated 10 wk group (N = 80 cells). The values on the X-axis correspond to the percent of the cell perimeter in contact with GAD67-IR structures in the different cells studied. Values on the Y-axis represent the cumulative frequencies with which the different values were observed. B: the average median (±SE) synaptic coverage by GAD67-IR structures is shown for the same groups of rats as in A. N = 4 rats in each group.
In Untreated rats, a significant (Mann-Whitney U-test, P < 0.01) leftward shift in the distribution of synaptic coverages, relative to that of Intact rats, was found at both 4 and 10 wk after sciatic nerve transection and repair (Fig. 3A, open circles, gray circles), indicating a significant reduction in this synaptic coverage. Indeed, the results of the omnibus test of the ANOVA for average median percent VGLUT1-IR contacts were significant (F4,14 = 9.19, P < 0.01). At both 4 wk and 10 wk time points, significantly (HSD, P < 0.02) fewer VGLUT1-IR inputs were found on the motoneurons of Untreated rats than on motoneurons from Intact rats (Fig. 3B). If animals were treated with 2 wk of daily treadmill exercise, starting on the third day after sciatic nerve transection and repair, no such shift to the left in the distribution of synaptic coverages was noted 10 wk later (U-test, ns) (Fig. 3A, gray squares) and no significant reduction in average median coverage by VGLUT1-IR synaptic inputs was found (HSD, ns) (Fig. 3B). However, if the onset of exercise was delayed, the distribution of synaptic coverages studied 10 wk after sciatic nerve transection and repair was shifted to the left in a manner indistinguishable from that found in Untreated rats at that survival time (Fig. 3A, open squares), and average median synaptic coverage was reduced significantly (HSD, P < 0.02) (Fig. 3B).
By 4 wk after sciatic nerve transection, the distribution of GAD67-IR coverages on motoneurons in Untreated rats is shifted significantly to the left (toward smaller proportions of coverage) of that found in Intact rats (U-test, P < 0.01) (Fig. 4A, gray circles). By 10 wk after sciatic nerve transection and repair, this distribution is no longer significantly different from that observed in Intact rats, in both Untreated (Fig. 4A, open circles) and Immediately exercised rats (Fig. 4A, gray squares), suggesting that the withdrawn inhibitory inputs have reformed. In rats exercised beginning ca. 3 wk after sciatic nerve transection and repair and studied at 10 wk after injury, the distribution of coverages also was shifted significantly to the left of that of Intact rats (U-test, P < 0.02) (Fig. 4A, open squares) and not significantly different from that observed at the 4 wk postinjury time in Untreated rats. The outcome of the omnibus test from the ANOVA conducted on the average median GAD67-IR coverage data set was significant (F4,14 = 3.49, P < 0.03). In post hoc paired testing, average median coverage by GAD67-IR structures was found to be significantly (HSD, P < 0.03) different (smaller) than that found in Intact rats only in Untreated rats 4 wk after sciatic nerve transection and repair (Fig. 4B).
DISCUSSION
Following peripheral nerve transection, both sensory and motor axons are capable of regeneration and reinnervation of peripheral targets, but functional recovery is poor. In the CNS, peripheral axotomy is followed by significant changes in CNS circuitry at several levels (Brushart 2011). Among the best documented of these changes is the withdrawal of synaptic inputs from the somata and proximal-most dendrites of motoneurons (Alvarez et al. 2011). Many of these synaptic inputs are restored as axons regenerate and reinnervate their targets, but one population of inputs, those containing VGLUT1, is lost permanently following peripheral nerve transection, irrespective of whether the regeneration of motor axons is successful (Alvarez et al. 2011; Rotterman et al. 2014). Several cellular processes have been invoked to explain the transient withdrawal of most synaptic inputs (reviewed in Liu et al. 2014), but this permanent loss of VGLUT1-IR synapses has been attributed to injury to the peripheral process of the axons of these dorsal root ganglion neurons (Alvarez et al. 2011). The loss of these inputs is thought to be a major contributor to the permanent loss of the stretch reflex from self-reinnervated muscles (Bullinger et al. 2011; Cope and Clark 1993).
We have shown that if mice and rats are treated with moderate daily exercise for 2 wk following sciatic nerve transection and repair, an enhancement of axon regeneration (English et al. 2011) and a modest improvement in functional recovery (Boeltz et al. 2013) is found. In addition, the extent of synaptic inputs onto the axotomized motoneurons in immediately exercised mice is unchanged from intact mice, even those containing VGLUT1 (Liu et al. 2014). Based on these findings, we speculated that the process of synaptic withdrawal following peripheral nerve transection could represent an opportunity for therapeutic interventions that might affect the restoration of synaptic inputs to the motoneurons in the CNS to match the patterns of their reinnervation in the periphery.
In this study, we delayed the onset of exercise after sciatic nerve transection and repair until the first signs of successful axon regeneration and muscle reinnervation were noted (ca. 3 wk) and studied the effect of this delayed exercise on axon regeneration in the periphery, the restoration of a simple reflex circuit in the spinal cord (the H reflex), and on the extent of connections of two types of synaptic inputs onto the injured motoneurons. The main findings were 1) delayed exercise was as effective as immediate exercise in promoting motor axon regeneration and muscle fiber reinnervation; 2) delayed exercise was significantly less effective than immediate exercise in restoring the H reflex; and 3) delayed exercise had very different effects on VGLUT1-IR synaptic inputs onto motoneurons than immediate exercise.
The finding that the time course of restoration of the direct muscle (M) response to peripheral stimulation was influenced equally in rats in both of the exercised groups might have been anticipated. It is well known that motor axon regeneration after peripheral nerve transection and repair is not synchronous but temporally “staggered” (Al-Majed et al. 2000b), with some motor axons apparently not initiating the regeneration process for days or weeks following injury. By initiating the exercise at the first sign of muscle reinnervation, it is likely that only the earliest regenerating axons will have been successful so that mainly later regenerating axons would be affected by the treatment. Enhancing the outgrowth of that subset of the regenerating axons might be expected to result in a restored M response that was indistinguishable from that found in rats exercised immediately after injury, especially when evaluated several weeks later.
As we have reported previously for rats exercised beginning 3 days after sciatic nerve transection and repair (Boeltz et al. 2013), the magnitude of this scaled M response amplitude is greater than unity at the longest posttransection times studied in rats where the onset of the same exercise is delayed. The reasons for this bigger response are not entirely clear, as we have discussed previously (Boeltz et al. 2013), but we believe that they serve to emphasize the remarkable difference between the results from both groups of exercised animals and the slower and/or less complete reinnervation of the SOL muscle in unexercised rats.
The amplitude of the H reflex recorded 7–10 wk after sciatic nerve transection and repair without any treatment is only about 50% of that of the same rats prior to injury (Boeltz et al. 2013). The same reduction in size of the restored H reflex was found in the present study in exercised rats if the application of the treatment was delayed. This weakly restored H reflex correlates well with earlier observations made from motoneurons that had successfully reinnervated cat muscles. The amplitudes of electrically evoked composite EPSPs recorded from these motoneurons is smaller than controls (Mendell et al. 1995). Extensive withdrawal of the VGLUT1-IR inputs onto the somata and proximal-most dendrites of motoneurons of untreated rats has occurred by 10 wk after sciatic nerve injury, as reported here, and in the literature (Alvarez et al. 2011). A similar amount of withdrawal of these excitatory connections was found in exercised rats in our Delayed group. In a recent paper Rotterman and colleagues (Rotterman et al. 2014) provide a compelling explanation for this modest reflex (or EPSP) restoration despite a striking loss of the synaptic inputs responsible for them. Using an elegant analysis of all of the VGLUT1-IR inputs onto motoneurons marked by intracellular injection of neurobiotin, they found a marked reduction in contacts in the somatodendritic regions of the neurons but only a sparse reduction of contacts made on dendrites. These retained inputs are smaller, not clustered, and more heavily distributed in the pathway of any remaining arbors of afferent axons (Rotterman et al. 2014). They argued that the responses of motoneurons to synchronous electrical stimulation of sensory axons in the periphery might make them capable of producing EPSPs in motoneurons (Bullinger et al. 2011) through this reorganization of dendritic inputs, but that the temporally dispersed activation of different afferent axons anticipated during muscle stretch might not be sufficient. We speculate that in our Untreated rats and Delayed exercise rats this reorganization of VGLUT1-IR synaptic inputs onto motoneuron dendrites might be sufficient to produce a restored, albeit diminished, H reflex in awake animals.
These findings were in stark contrast to those in rats where exercise was begun 3 days after sciatic nerve transection and repair. The amplitude of the restored H reflex in these rats during the 7–10 wk interval after the sciatic nerve injury is not significantly different from that observed in the same rats prior to injury (Boeltz et al. 2013). We report here that the coverage of the somata and proximal-most dendrites by VGLUT1-IR structures 10 wk after injury and 8 wk after the cessation of exercise also was nearly identical to that found in Intact animals. We speculate that the fully restored size of the electrically evoked H reflex in these rats is a consequence of the fully restored VGLUT1-IR inputs onto the somatodendritic regions of the motoneurons.
In all of the rats in this study, the amplitude of the restored H reflex was transiently exaggerated, as has been reported elsewhere (Boeltz et al. 2013; Navarro et al. 2007). When scaled to the amplitude of the reflex recorded prior to injury, the amplitude of the restored H reflex was as much as three times larger than found in intact rats during the period of 4–6 wk after sciatic nerve transection and repair. In rats in which exercise was conducted for 2 wk beginning 1 wk prior to this period, an exaggerated H reflex was found but its magnitude was significantly smaller than found during the same period in the other groups. During this postinjury period, the coverage of motoneurons by inhibitory, GAD67-IR terminals was reduced significantly but restored to preinjury conditions, with or without exercise, by 10 wk posttransection. It is possible that this transient reduction in inhibitory inputs could account for the large exaggeration of the amplitude of the H reflex observed during this period, but the marked reduction of excitatory VGLUT1-IR synaptic contacts also found at this time complicates such an explanation. It is also possible that those motoneurons whose axons have regenerated successfully are simply more excitable during this period. Such transient hyperexcitability of the axotomized motoneurons could result from the well-documented changes in their intrinsic properties, specifically rheobase (Foehring et al. 1986; Nakanishi et al. 2005; Pinter and Vanden Noven 1989). It also is possible that during this recovery period the efficacy of inhibitory GABA-ergic synapses might be reduced or even made excitatory by changes in chloride homeostasis mediated by the neuron-specific K+-Cl− cotransporter (KCC2). Changes in chloride homeostasis and correlated changes in motoneuron excitability have been shown after spinal cord injury, and these could be blocked with 4 wk of daily exercise (Côté et al. 2014). Peripheral nerve injury results in a rapid and long-lasting reduction in phosphorylation of KCC2 in the spinal cord dorsal horn (Mòdol et al. 2014). Whatever the mechanism, we interpret our findings to mean that if exercise is conducted during the period of expected heightened H reflex efficacy, but not earlier, a dampening effect on the exaggerated H reflex found in early reinnervated muscles occurs.
The moderate exercise used in the present study may be considered a potential therapeutic approach to improving functional recovery following peripheral nerve injury. It is low-tech and could empower patients to take responsibility for their recovery. However, to translate these basic research findings to clinical use, it will be important to establish a therapeutic window for effective treatment. We have shown here that depending on whether such exercise is applied immediately (3 days) or 3 wk after nerve transection and repair, the regeneration of motor axons and reinnervation of muscle fibers will be enhanced significantly. The therapeutic window for motor axon regeneration thus extends at least until the first successful muscle fiber reinnervation is encountered. It is not yet clear whether the same is true for the regeneration of sensory axons in the periphery, and this remains to be a focus for future study.
The same broad therapeutic window is not the case for the effects of exercise on synaptic plasticity, at least as regards synaptic inputs to motoneurons. If rats were exercised immediately following sciatic nerve injury, a profound effect on VGLUT1-IR synaptic inputs was observed that was retained for at least 8 wk after the cessation of the exercise. By the time muscles have started to be reinnervated after sciatic nerve transection and repair, our moderate daily exercise paradigm no longer has an effect on the withdrawal of these inputs from motoneurons. If this therapy, or perhaps other activity-related therapies, were to be applied with the intent of influencing this synaptic plasticity in the spinal cord, then it needs to be applied within the first 3 wk following injury. We have argued that the immediate effect of exercise on synaptic withdrawal following peripheral nerve injury is dependent on motoneuron expression of brain-derived neurotrophic factor (BDNF) and androgens (Liu et al. 2014). However, the mechanisms by which this relatively short amount of exercise produces such a long-lasting effect on synaptic inputs to motoneurons is not clear at this time. Further study of the timing of onset and the duration of the exercise will be required to provide more clues.
GRANTS
This work was supported by National Institutes of Health Grant P01-HD-032571.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.B., J.T.E., T.M., A.M., A.K., S.J.R., and A.W.E. performed experiments; J.B., J.T.E., T.M., A.M., A.K., S.J.R., and A.W.E. analyzed data; J.B., J.T.E., T.M., A.M., A.K., S.J.R., and A.W.E. interpreted results of experiments; J.B., J.T.E., T.M., A.K., S.J.R., and A.W.E. drafted manuscript; J.B., J.T.E., T.M., A.M., A.K., S.J.R., and A.W.E. edited and revised manuscript; J.B., J.T.E., T.M., A.M., A.K., S.J.R., and A.W.E. approved final version of manuscript; A.W.E. conception and design of research; A.W.E. prepared figures.
ACKNOWLEDGMENTS
We thank members of our Program Project Group (T. R. Nichols, H. Maas, R. Gregor, B. Prilutsky, X. Y. Chen, J. Wolpaw, and R. Segal), and E. Field-Fote and T. Brushart, members of its Clinician Science Advisory Board, for comments on earlier presentations of this work.
REFERENCES
- Ahlborn P, Schachner M, Irintchev A. One hour electrical stimulation accelerates functional recovery after femoral nerve repair. Exp Neurol 208: 137–144, 2007. [DOI] [PubMed] [Google Scholar]
- Al-Majed AA, Brushart TM, Gordon T. Electrical stimulation accelerates and increases expression of BDNF and trkB mRNA in regenerating rat femoral motoneurons. Eur J Neurosci 12: 4381–4390, 2000a. [PubMed] [Google Scholar]
- Al-Majed AA, Neumann CM, Brushart TM, Gordon T. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J Neurosci 20: 2602–2608, 2000b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alrashdan MS, Park JC, Sung MA, Yoo SB, Jahng JW, Lee TH, Kim SJ, Lee JH. Thirty minutes of low intensity electrical stimulation promotes nerve regeneration after sciatic nerve crush injury in a rat model. Acta Neurol Belg 110: 168–179, 2010. [PubMed] [Google Scholar]
- Alvarez FJ, Villalba RM, Zerda R, Schneider SP. Vesicular glutamate transporters in the spinal cord, with special reference to sensory primary afferent synapses. J Comp Neurol 472: 257–280, 2004. [DOI] [PubMed] [Google Scholar]
- Alvarez FJ, Bullinger KL, Titus HE, Nardelli P, Cope TC. Permanent reorganization of Ia afferent synapses on motoneurons after peripheral nerve injury. Ann NY Acad Sci 1198: 231–241, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez FJ, Titus-Mitchell HE, Bullinger KL, Kraszpulski M, Nardelli P, Cope TC. Permanent central synaptic disconnection of proprioceptors after nerve injury and regeneration. I. Loss of VGLUT1/IA synapses on motoneurons. J Neurophysiol 106: 2450–2470, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asensio-Pinilla E, Udina E, Jaramillo J, Navarro X. Electrical stimulation combined with exercise increase axonal regeneration after peripheral nerve injury. Exp Neurol 219: 258–265, 2009. [DOI] [PubMed] [Google Scholar]
- Blinzinger K, Kreutzberg G. Displacement of synaptic terminals from regenerating motoneurons by microglial cells. Z Zellforsch 85: 145–157, 1968. [DOI] [PubMed] [Google Scholar]
- Boeltz T, Ireland M, Mathis K, Nicolini J, Poplavski K, Rose SJ, Wilson E, English AW. Effects of treadmill training on functional recovery following peripheral nerve injury in rats. J Neurophysiol 109: 2645–2657, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brushart TM. Nerve Repair. New York: Oxford University Press, 2011. [Google Scholar]
- Bullinger KL, Nardelli P, Pinter MJ, Alvarez FJ, Cope TC. Permanent central synaptic disconnection of proprioceptors after nerve injury and regeneration. II. Loss of functional connectivity with motoneurons. J Neurophysiol 106: 2471–2485, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope TC, Clark BD. Motor-unit recruitment in self-reinnervated muscle. J Neurophysiol 70: 1787–1796, 1993. [DOI] [PubMed] [Google Scholar]
- Côté MP, Gandhi S, Zambrotta M, Houle JD. Exercise modulates chloride homeostasis after spinal cord injury. J Neurosci 34: 8976–8987, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English AW, Chen Y, Carp JS, Wolpaw JR, Chen XY. Recovery of electromyographic activity after transection and surgical repair of the rat sciatic nerve. J Neurophysiol 97: 1127–1134, 2007a. [DOI] [PubMed] [Google Scholar]
- English AW, Mulligan A, Meador W, Sabatier MJ, Schwartz G. Electrical stimulation promotes peripheral axon regeneration by enhanced neuronal neurotrophin signaling. Dev Neurobiol 67: 158–172, 2007b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English AW, Wilhelm JC, Sabatier MJ. Enhancing recovery from peripheral nerve injury using treadmill training. Ann Anat 193: 354–361, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English AW, Wilhelm JC, Ward PJ. Exercise, neurotrophins, and axon regeneration in the PNS. Physiology 29: 437–445, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eser FAL, Bodur H, Atan C. Etiological factors of traumatic peripheral nerve injuries. Neurol India 57: 434–437, 2009. [DOI] [PubMed] [Google Scholar]
- Foehring RC, Sypert GW, Munson JB. Properties of self-reinnervated motor units of medial gastrocnemius of cat. II. Axotomized motoneurons and time course of recovery. J Neurophysiol 55: 947–965, 1986. [DOI] [PubMed] [Google Scholar]
- Franz CK, Singh B, Martinez JA, Zochodne DW, Midha R. Brief transvertebral electrical stimulation of the spinal cord improves the specificity of femoral nerve reinnervation. Neurorehabil Neural Repair 27: 260–268, 2013. [DOI] [PubMed] [Google Scholar]
- Geremia NM, Gordon T, Brushart TM, Al-Majed AA, Verge VM. Electrical stimulation promotes sensory neuron regeneration and growth-associated gene expression. Exp Neurol 205: 347–359, 2007. [DOI] [PubMed] [Google Scholar]
- Hetzler LE, Sharma N, Tanzer L, Wurster RD, Leonetti J, Marzo SJ, Jones KJ, Foecking EM. Accelerating functional recovery after rat facial nerve injury: Effects of gonadal steroids and electrical stimulation. Otolaryngol Head Neck Surg 139: 62–67, 2008. [DOI] [PubMed] [Google Scholar]
- Hoke A, Brushart T. Introduction to special issue: challenges and opportunities for regeneration in the peripheral nervous system. Exp Neurol 223: 1–4, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Hu X, Lu L, Ye Z, Wang Y, Luo Z. Electrical stimulation accelerates motor functional recovery in autograft-repaired 10 mm femoral nerve gap in rats. J Neurotrauma 26: 1805–1813, 2009. [DOI] [PubMed] [Google Scholar]
- Hughes DI, Mackie M, Nagy GG, Riddell JS, Maxwell DJ, Szabo G, Erdelyi F, Veress G, Szucs P, Antal M, Todd AJ. P boutons in lamina IX of the rodent spinal cord express high levels of glutamic acid decarboxylase-65 and originate from cells in deep medial dorsal horn. Proc Natl Acad Sci USA 102: 9038–9043, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenth RV. Java Applets for Power and Sample Size [Computer software] Retrieved 04/10/2010 from http://www.stat.uiowa.edu/∼rlenth/Power 2006–9. [Google Scholar]
- Liu C, Ward PJ, English AW. The effects of exercise on synaptic stripping require androgen receptor signaling. PLoS ONE 9: e98633, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu MC, Ho CY, Hsu SF, Lee HC, Lin JH, Yao CH, Chen YS. Effects of electrical stimulation at different frequencies on regeneration of transected peripheral nerve. Neurorehabil Neural Repair 22: 367–373, 2008. [DOI] [PubMed] [Google Scholar]
- MacGillivray TE. Fibrin sealants and glues. J Card Surg 18: 480–485, 2003. [DOI] [PubMed] [Google Scholar]
- McLean IW, Nakane PK. Periodate-lysate-paraformaldehyde fixative. A new fixative for immunoelectron microscopy. J Histochem Cytochem 22: 1077–1083, 1974. [DOI] [PubMed] [Google Scholar]
- Mendell LM, Taylor JS, Johnson RD, Munson JB. Rescue of motoneuron and muscle afferent function in cats by regeneration into skin. II. Ia-Motoneuron synapse. J Neurophysiol 73: 662–673, 1995. [DOI] [PubMed] [Google Scholar]
- Mòdol L, Cobianchi S, Navarro X. Prevention of NKCC1 phosphorylation avoids downregulation of KCC2 in central sensory pathways and reduces neuropathic pain after peripheral nerve injury. Pain 155: 1577–1590, 2014. [DOI] [PubMed] [Google Scholar]
- Nakanishi ST, Cope TC, Rich MM, Carrasco DI, Pinter MJ. Regulation of motoneuron excitability via motor endplate acetylcholine receptor activation. J Neurosci 25: 2226–2232, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro X, Vivo M, Valero-Cabre A. Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol 82: 163–201, 2007. [DOI] [PubMed] [Google Scholar]
- Nix WA, Hopf HC. Electrical stimulation of regenerating nerve and its effect on motor recovery. Brain Res 272: 21–25, 1983. [DOI] [PubMed] [Google Scholar]
- Noble J, Munro CA, Prasad VS, Midha R. Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. J Trauma 45: 116–122, 1998. [DOI] [PubMed] [Google Scholar]
- Pinter MJ, Vanden Noven S. Effects of preventing reinnervation on axotomized spinal motoneurons in the cat. I. Motoneuron electrical properties. J Neurophysiol 62: 311–324, 1989. [DOI] [PubMed] [Google Scholar]
- Rotterman TM, Nardelli P, Cope TC, Alvarez FJ. Normal distribution of VGLUT1 synapses on spinal motoneuron dendrites and their reorganization after nerve injury. J Neurosci 34: 3475–3492, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier M, Redmon N, Schwartz G, English A. Treadmill training promotes axon regeneration in injured peripheral nerves. Exp Neurol 211: 489–493, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier M, Ngoc To B, Nicolini J, English AW. Effect of axon misdirection on recovery of EMG activity and kinematics after peripheral nerve injury. Cells Tissues Organs 193: 298–309, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Coughlin L, Porter RG, Tanzer L, Wurster RD, Marzo SJ, Jones KJ, Foecking EM. Effects of electrical stimulation and gonadal steroids on rat facial nerve regenerative properties. Restor Neurol Neurosci 27: 633–644, 2009. [DOI] [PubMed] [Google Scholar]
- Sharma N, Marzo SJ, Jones KJ, Foecking EM. Electrical stimulation and testosterone differentially enhance expression of regeneration-associated genes. Exp Neurol 223: 183–191, 2010. [DOI] [PubMed] [Google Scholar]
- Singh B, Xu QG, Franz CK, Zhang R, Dalton C, Gordon T, Verge VM, Midha R, Zochodne DW. Accelerated axon outgrowth, guidance, and target reinnervation across nerve transection gaps following a brief electrical stimulation paradigm. J Neurosurg 116: 498–512, 2012. [DOI] [PubMed] [Google Scholar]
- Stein RB, Nichols TR, Jhamandas J, Davis L, Charles D. Stable long-term recordings from cat peripheral nerves. Brain Res 128: 21–38, 1977. [DOI] [PubMed] [Google Scholar]
- Sumner BE. A quantitative analysis of boutons with different types of synapse in normal and injured hypoglossal nuclei. Exp Neurol 49: 406–417, 1975. [DOI] [PubMed] [Google Scholar]
- Svensson M, Tornqvist E, Aldskogius H, Cova JL. Synaptic detachment from hypoglossal neurons after different types of nerve injury in the cat. J Hirnforsch 32: 547–552, 1991. [PubMed] [Google Scholar]
- Taylor CA, Braza D, Rice JB, Dillingham T. The incidence of peripheral nerve injury in extremity trauma. Am J Phys Med Rehabil 87: 381–385, 2008. [DOI] [PubMed] [Google Scholar]
- Titmus M, Faber D. Axotomy-induced alterations in the electrophysiological characteristics of neurons. Prog Neurobiol 35: 1–51, 1990. [DOI] [PubMed] [Google Scholar]
- Todd AJ, Hughes DI, Polgar E, Nagy GG, Mackie M, Ottersen OP, Maxwell DJ. The expression of vesicular glutamate transporters VGLUT1 and VGLUT2 in neurochemically defined axonal populations in the rat spinal cord with emphasis on the dorsal horn. Eur J Neurosci 17: 13–27, 2003. [DOI] [PubMed] [Google Scholar]
- Udina E, Cobianchi S, Allodi I, Navarro X. Effects of activity-dependent strategies on regeneration and plasticity after peripheral nerve injuries. Ann Anat 193: 347–353, 2011. [DOI] [PubMed] [Google Scholar]
- Valero-Cabre A, Navarro X. H reflex restitution and facilitation after different types of peripheral nerve injury and repair. Brain Res 919: 302–312, 2001. [DOI] [PubMed] [Google Scholar]
- Wang Y, Pillai S, Wolpaw JR, Chen XY. Motor learning changes GABAergic terminals on spinal motoneurons in normal rats. Eur J Neurosci 23: 141–150, 2006. [DOI] [PubMed] [Google Scholar]