Abstract
Low-voltage fast (LVF)- and hypersynchronous (HYP)-seizure onset patterns can be recognized in the EEG of epileptic animals and patients with temporal lobe epilepsy. Ripples (80–200 Hz) and fast ripples (250–500 Hz) have been linked to each pattern, with ripples predominating during LVF seizures and fast ripples predominating during HYP seizures in the rat pilocarpine model. This evidence led us to hypothesize that these two seizure-onset patterns reflect the contribution of neural networks with distinct transmitter signaling characteristics. Here, we tested this hypothesis by analyzing the seizure activity induced with the K+ channel blocker 4-aminopyridine (4AP, 4–5 mg/kg ip), which enhances both glutamatergic and GABAergic transmission, or the GABAA receptor antagonist picrotoxin (3–5 mg/kg ip); rats were implanted with electrodes in the hippocampus, the entorhinal cortex, and the subiculum. We found that LVF onset occurred in 82% of 4AP-induced seizures whereas seizures after picrotoxin were always HYP. In addition, high-frequency oscillation analysis revealed that 4AP-induced LVF seizures were associated with higher ripple rates compared with fast ripples (P < 0.05), whereas picrotoxin-induced seizures contained higher rates of fast ripples compared with ripples (P < 0.05). These results support the hypothesis that two distinct patterns of seizure onset result from different pathophysiological mechanisms.
Keywords: seizure onset, high-frequency oscillation, 4-aminopyridine, picrotoxin
recent studies on ictogenesis in animals and humans have revealed the existence of two main patterns of seizure onset that were termed low-voltage fast (LVF) and hypersynchronous (HYP) onset patterns (Bragin et al. 2005; Lévesque et al. 2012; Perucca et al. 2014; Velasco et al. 2000). LVF seizures start with a positive or negative going spike followed by low-amplitude, high-frequency activity, whereas HYP seizures are characterized at onset by the occurrence of focal periodic spiking. Imaging studies have suggested that these two seizure onset patterns are linked to distinct underlying pathologies; patients with HYP seizures are indeed more likely to show focal onset and greater neuronal loss in the hippocampus than those showing LVF seizures that are often associated with more diffuse lesions (Ogren et al. 2009; Velasco et al. 2000).
We recently discovered in the pilocarpine model of temporal lobe epilepsy (TLE) that these two patterns of seizure onset are associated with distinct patterns of occurrence of high-frequency oscillations (HFOs) (Lévesque et al. 2012). Specifically, LVF seizures show high rates of ripples (80–200 Hz) compared with fast ripples (250–500 Hz), whereas HYP seizures are associated with high rates of fast ripples compared with ripples (Lévesque et al. 2012). This evidence led us to speculate that HFOs may mirror different functional properties of neural networks during ictogenesis. Ripples are thought to reflect summated inhibitory postsynaptic potentials generated by pyramidal cells in response to inhibitory interneuron firing, whereas fast ripples should mirror the hypersynchronous bursting of principal (glutamatergic) cells (Bragin et al. 1999; Dzhala and Staley 2004; Foffani et al. 2007; Jefferys et al. 2012). Because 4-aminopyridine (4AP) is known to enhance interneuron excitability and to induce GABAergic synchronization in vitro (Avoli and de Curtis 2011), we used in this study systemic injections of 4AP (Lévesque et al. 2013) or of the GABAA receptor antagonist picrotoxin to test the hypothesis that LVF seizures are mainly caused by GABAergic-mediated synchronization, whereas glutamatergic interactions, enhanced by the blockade of GABAA receptor signaling, predominate during HYP seizures. We also postulated that 4AP-induced LVF seizures would be associated with higher rates of ripples compared with fast ripples in temporal lobe regions shortly before and after the onset of ictal activity, whereas picrotoxin would induce HYP seizures associated with the occurrence of higher rates of fast ripples compared with ripples.
MATERIALS AND METHODS
Animal housing.
Male Sprague-Dawley rats (250–300 g) were obtained from Charles River (St-Constant, QC, Canada) and let habituate for 72 h after delivery before the implantation of electrodes. They were housed in controlled conditions at 22 (±2)°C and under a 12:12-h light/dark cycle (lights on from 7:00 a.m. to 7:00 p.m.) with food and water ad libitum. All procedures were approved by the Canadian Council of Animal Care, and all efforts were made to minimize suffering and the number of animals used.
Surgery for the implantation of electrodes.
A total of nine animals underwent surgery for the implantation of electrodes before 4AP or picrotoxin treatment. Before surgery, rats received topical Lidocain (5%; Odan). An incision was then made in the skin to expose the skull plate from bregma to lambda. Four stainless steel screws (2.4 mm length) were fixed to the skull as anchor screws, and in addition four small holes were drilled to allow the implantation of bipolar electrodes (20–30 kΩ; 5–10 mm length; distance between exposed tips: 500 μm; MS303/2-B/spc, Plastics One). Electrodes were implanted in the CA3 region of the right hippocampus [anterioposterior (AP): −4.3, mediolateral (ML): ±4, dorsoventral (DV): −7.8], the right entorhinal cortex (EC) (AP: −8.6, ML: ± 5.2, DV: −6.8), and the left subiculum (AP: −6.0, ML: ± 4.3, DV: −8.3) (Paxinos and Watson 1998). A fourth bipolar electrode was placed under the frontal bone, after the removal of insulating material, and used as reference. During surgery, animals received a preventive antibiotherapy (Enrofloxacine, 10 mg/kg sc). After surgery, rats were injected with Ketoprofen (5 mg/kg sc; Merail), Buprenorphine (0.01–0.05 mg/kg sc, repeated every 12 h; CDMV) and 2 ml of 0.9% sterile saline (subcutaneously) repeated every 12 h if necessary.
EEG recording.
Two days after surgery, rats were placed in custom-made Plexiglas boxes (30×20×40 cm) and provided with food and water ad libitum. Electrodes were then connected to multichannel cables and swivels. EEGs were amplified using an interface kit (Mobile 36 chlTMPro Amp, Stellate) and low-pass filtered at 500 Hz and sampled at 2 kHz per channel. On the day of injection, animals were given either 4AP (4–5 mg/kg ip) or picrotoxin (3–5 mg/kg ip) to induce acute seizures (Pitkänen et al. 2005). EEG video monitoring was performed using the Stellate system for 4 h after the injection (Lévesque et al. 2013).
Seizure detection and classification.
Seizures were detected visually, and only seizures with good quality recordings were selected. They were then exported to Matlab 7.11.0 (R2010b) (Mathworks, Natick, MA) and analyzed offline using custom-built routines. In the 4AP-treated group, 11 seizures were selected for analysis, whereas 12 seizures were selected in the picrotoxin-treated group. All seizures were visually analyzed by four reviewers, and the seizure-onset time was marked. The reviewers were blind to the treatment that was used to induce seizures. At least three of the four reviewers agreed on the onset time for all seizures. Selected seizures in the 4AP- and picrotoxin-treated group were then classified based on their onset pattern, i.e., LVF and HYP seizures (Lévesque et al. 2012; Velasco et al. 2000; see Fig. 1 for examples). The seizures that could not classify in any of the groups were excluded from the study.
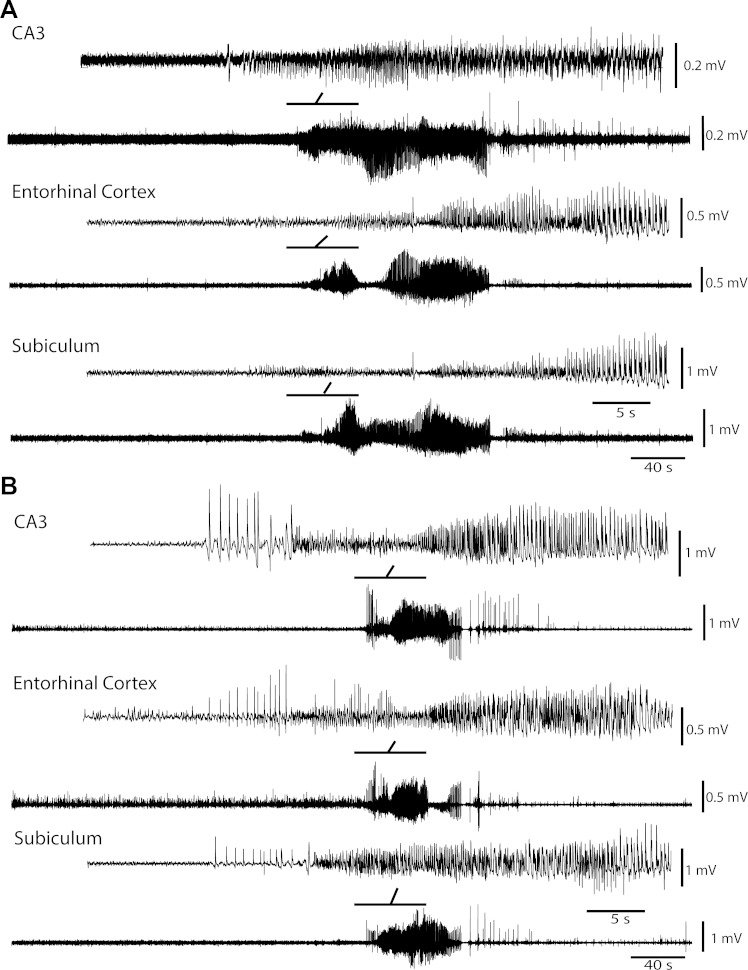
Fig. 1.
Representative seizures induced by 4-aminopyridine (4AP) or picrotoxin. A: low-voltage fast (LVF) seizure recorded simultaneously from the CA3 region of hippocampus, the entorhinal cortex, and the subiculum in a rat 12 min after 4AP treatment. Insets show the onset of the seizure that is characterized by the occurrence of a spike followed by a low-voltage fast activity. B: hypersynchronous (HYP) seizure recorded simultaneously from the CA3 region of hippocampus, the entorhinal cortex, and the subiculum 30 min after picrotoxin treatment. Insets indicate the onset of the seizure and the expanded time scale of the periodic focal spikes at its onset. Bold lines on top of the traces represent the part of the seizure shown above with an expanded time scale.
High-frequency oscillation analysis.
HFOs were analyzed from 60 s before the onset of the seizure up to 60 s after. Raw EEG recordings were first band-pass filtered in the 80-to 200-Hz and in the 250- to 500-Hz frequency range using a finite impulse response filter; zero-phase digital filtering was used to avoid phase distortion. Filtered EEGs from each region were then normalized using a 10-s reference period selected from 70 to 60 s before the onset of the seizure. To be considered as an HFO candidate, oscillatory events in each frequency band had to show at least four consecutive cycles having amplitude of 3 SD above the mean of the reference period. The time lag between two consecutive cycles had to be between 5 and 12.5 ms for ripples and between 2 and 4 ms for fast ripples. Ripples and fast ripples occurring at the same time (overlapping events) were removed from analysis to avoid the effect of sharp events (Bénar et al. 2010; Lévesque et al. 2012; Salami et al. 2012).
Statistical analysis.
To compare the rates of occurrence of ripples and fast ripples in each region during pre-ictal and ictal periods, we divided each period in three equal parts of 20 s. Wilcoxon signed-rank tests were used for multiple comparisons followed by Bonferroni-Holm corrections. Kruskall-Wallis tests were used to compare the duration of ripples and fast ripples in between regions and during pre-ictal and ictal periods. The level of significance was set to P < 0.05.
RESULTS
Seizure-onset patterns.
Seizures were recorded from five rats treated with 4AP. Eleven seizures were recorded in these experiments, from which 82% (9/11) were categorized as LVF seizures and 18% (2/11) were categorized as unclassified. LVF seizures had an average duration of 134 (±16) s, whereas unclassified seizures lasted 131 (±42) s. Figure 1A shows an example of an LVF seizure recorded from a 4AP-treated animal. In the picrotoxin-treated group, twelve seizures were recorded from four animals. All seizures in the picrotoxin-treated group were classified as HYP (Fig. 1B). The average duration of HYP seizures was 77 (±9) s. The number of injected animals in each group was balanced to have a comparable number of seizures in the two groups.
Rates of occurrence over time of high-frequency oscillations during seizures.
During both 4AP- and picrotoxin-induced seizures, HFOs could be detected in all recorded regions. Representative examples of HFOs detected during an HYP seizure after picrotoxin injection recorded in subiculum are shown in Fig. 2A. The average duration of ripples during 4AP-induced seizures was 44.8 (±3.4) ms, and the average duration of ripples during picrotoxin-induced seizures was 37.1 (±1.6) ms. The average duration of fast ripples during 4AP-induced seizures was 17.4 (±1.0) ms, and the average duration of fast ripples during picrotoxin-induced seizures was 15.8 (±1.1) ms. These values were not significantly different under the two pharmacological procedures (Kruskal-Wallis test).
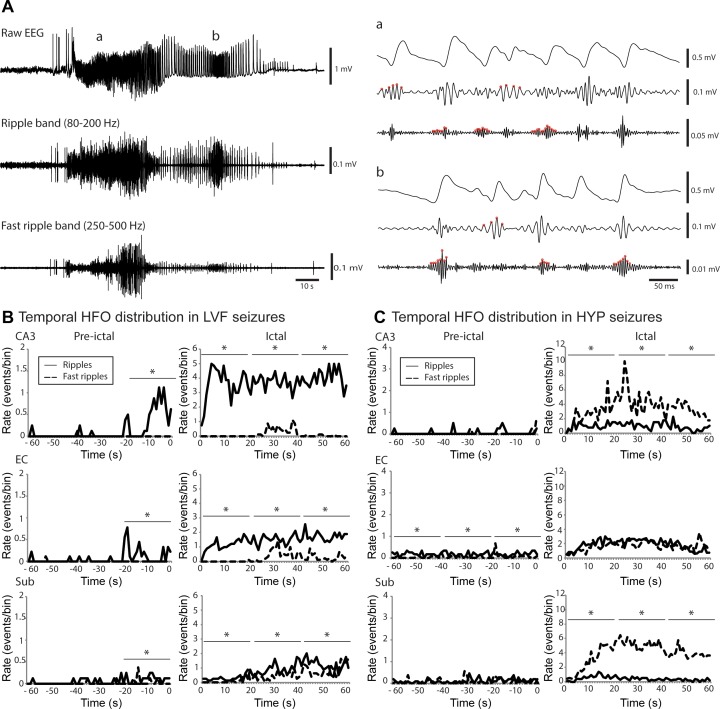
Fig. 2.
High-frequency oscillations (HFOs) during seizures after the injection of 4AP and picrotoxin. A: raw EEG recordings of a HYP seizure recorded from the subiculum in a picrotoxin-treated rat as well as filtered traces in the ripple frequency band (80–200 Hz) and fast ripple frequency band (250–500 Hz). Expanded time scale of the selected part (a and b) of raw EEG recording, ripple frequency band, and the fast ripple frequency band of the seizure shown in A. B: temporal distribution of HFOs in all LVF recorded seizures from 4AP-treated animals in the hippocampal CA3 region, the entorhinal cortex, and the subiculum. Left, temporal evolution of HFOs 60 s before the seizure onset; right, distribution from the onset to 60 s after. Note that all regions show higher rates of ripples compared with fast ripples in the 20 s time period that preceded the onset of the seizure and during the seizure (*P < 0.05). C: temporal distribution of HFOs in all HYP recorded seizures from picrotoxin-treated rats in the CA3, the entorhinal cortex, and the subiculum. Note that the CA3 region and subiculum show higher rates of fast ripples compared with ripples during the 60 s time-period that followed seizure onset (*P < 0.05) as well as that there was no significant difference during the ictal period between the rate of occurrence of ripples and fast ripples in the entorhinal cortex. Note the difference in the scale of pre-ictal and ictal period.
To study the temporal evolution of HFOs over time, the number of HFOs per second was calculated for each seizure and then averaged. The average rate of HFO occurrence was calculated from 60 s before the seizure onset up to 60 s after the start of the seizure. This analysis revealed that 20 s before the onset of LVF seizures induced with 4AP, ripples occurred at higher rates compared with fast ripples in all recorded regions (P < 0.05) (Fig. 2B). This difference was maintained over time during the ictal period, because ripples occurred at higher rates compared with fast ripples from the onset of the seizure up to 60 s later (P < 0.05) (Fig. 2B).
Figure 2C shows the temporal changes of HFOs during picrotoxin-induced HYP seizures. Ripples and fast ripples occurred at similar rates during the pre-ictal period in the CA3 region and in the subiculum. However, a significantly higher rate of fast ripples compared with ripples was observed in both of these regions during seizures (P < 0.05) (Fig. 2C). No significant differences were observed between the rate of ripples and fast ripples in the EC during the ictal period; however, in this limbic structure, ripple rate was higher than that of fast ripples during the pre-ictal period in HYP recorded seizures after the injection of picrotoxin (Fig. 2C).
DISCUSSION
The main findings of our study can be summarized as follows. First, 4AP mostly induced LVF seizures, whereas picrotoxin always induced HYP seizures; second, LVF seizures induced with 4AP were mostly associated with ripples, and their rate of occurrence increased 20 s before the onset of the seizure; third, seizures induced with picrotoxin were mostly associated with fast ripples.
Seizure-onset patterns and GABA receptor-mediated activity.
With the systemic administration of drugs that either block or enhance GABA receptor-mediated transmission, we were able to reproduce the two main patterns of seizure onset observed in clinical studies and in animal models of TLE (Bragin et al. 2005; Lévesque et al. 2012; Velasco et al. 2000). Seizures after the administration of the K+ channel blocker 4AP were mostly characterized by an LVF onset, suggesting that GABAA receptor signaling may be involved in the initiation of this pattern. On the other hand, all seizures after the administration of the GABAA antagonist picrotoxin had the characteristics of HYP onset patterns, indicating that excitatory signaling could result in the initiation of this seizure-onset pattern.
Our results thus support the hypothesis that the activity of distinct neuronal networks may underlie different seizure onset types (Bragin et al. 2009). Indeed, in vitro studies have suggested that LVF ictal-like activity induced by 4AP in brain slices depends on synchronous GABA receptor-mediated potentials, because it is abolished by GABAA receptor antagonists (Avoli and de Curtis 2011). In addition, we recently reported that LVF seizures can be triggered by optogenetic activation of parvalbumin-positive interneurons (Shiri et al. 2015). Contrary to what was observed with 4AP, picrotoxin only induced HYP seizures. These results are in line with studies performed in human tissue maintained in vitro, in which it was shown that these periodic spikes at the onset of ictal activity reflect pyramidal cell firing and depend on glutamatergic mechanisms (Huberfeld et al. 2011).
High-frequency oscillations associated with seizures induced with 4AP and picrotoxin.
We also found that the two seizure-onset patterns were characterized by two distinct patterns of HFO occurrence. After the injection of 4AP, LVF seizures were associated with higher rates of ripples compared with fast ripples shortly before seizure onset as well as during the ictal discharge. On the contrary, the rate of occurrence of fast ripples was higher than that of ripples during HYP seizures recorded in the CA3 region and subiculum after the administration of picrotoxin. Because HFOs are believed to reflect neuronal network activity in epileptic regions, these findings indicate that different mechanisms should underlie the two seizure-onset patterns. Surprisingly, in the EC of picrotoxin-treated rats we observed a higher rate of ripple occurrence during the pre-ictal period and we did not observe any significant difference between ripple and fast ripple rates of occurrence during the ictal period. This finding is unexpected because high rates of fast ripples compared with ripples occur in the EC during HYP spontaneous seizures in pilocarpine-treated animals (Lévesque et al. 2012). This difference may result from the fact that picrotoxin-induced seizures were acutely induced in control animals, whereas spontaneous seizures in pilocarpine-treated epileptic rats occur after a latent period that is associated with cellular and molecular changes. Despite this difference, our findings support the view that ripples should reflect population inhibitory postsynaptic potentials generated by principal cells entrained by synchronously active interneuron networks, whereas fast ripples are thought to reflect the hypersynchronous firing of principal (glutamatergic) neurons (Jefferys et al. 2012; Staba et al. 2014); in fact, higher rates of ripples, compared with fast ripples, were associated with LVF seizures induced by 4AP (which enhance both inhibitory and excitatory currents), whereas higher rates of fast ripples compared with ripples occurred during HYP seizures induced by picrotoxin (which is known to antagonize GABAA receptor-mediated inhibition). More studies using single cell recordings could, however, be helpful to identify the mechanisms underlying ripples or fast ripples in both seizure-onset patterns. For instance, a recent study by Alvarado-Rojas et al. (2015) suggests that the mechanisms underlying ripples occurring during interictal spikes differ from those responsible for ripples occurring during the pre-ictal period. It would thus be interesting to establish whether HFOs recorded during LVF and HYP seizures share the same mechanisms.
Distinct patterns of HFOs occur during the two seizure-onset patterns in the pilocarpine model of TLE (Lévesque et al. 2012). In line with what was previously reported by us, we propose here a possible diagnostic role for both types of HFOs depending on the pattern of seizure onset. More specifically, in patients with LVF-onset pattern seizures, ripples could be more significant compared with fast ripples, whereas in patients with HYP-onset pattern seizures, the occurrence of fast ripples could be more useful in identifying the seizure onset zone. Therefore, the two types of HFOs could be used differently to help localize the seizure onset zone. However, the validity of this hypothesis remains to be proved in humans by identifying the exact mechanisms underlying ripples and fast ripples and by demonstrating similar relationships between seizure onset pattern and ripples/fast ripples distributions.
This study could improve our understanding of the basic mechanisms underlying ictogenesis, and as a result our findings can have an important role in identifying therapeutic targets to treat epilepsy, because the two seizure-onset patterns reported here were almost specific to the pharmacological treatment. It has been previously shown that both of these seizure-onset patterns can be recorded in humans and in animal models of TLE (Bragin et al. 2005; Lévesque et al. 2012; Perucca et al. 2014; Velasco et al. 2000). Our findings thus suggest that patients with apparently the same pathology (mesial temporal sclerosis) but with different seizure-onset patterns could benefit from different pharmacological treatments.
GRANTS
This study was supported by the Canadian Institutes of Health Research (8109 and 74609). P. Salami received a student scholarship from the Savoy Foundation for Epilepsy.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: P.S. conception and design of research; P.S. performed experiments; P.S. analyzed data; P.S., M.L., J.G., and M.A. interpreted results of experiments; P.S. prepared figures; P.S., M.L., J.G., and M.A. drafted manuscript; P.S., M.L., J.G., and M.A. edited and revised manuscript.
REFERENCES
- Alvarado-Rojas C, Huberfeld G, Baulac M, Clemenceau S, Charpier S, Miles R, de la Prida LM, Le Van Quyen M. Different mechanisms of ripple-like oscillations in the human epileptic subiculum. Ann Neurol 77: 281–290, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avoli M, de Curtis M. GABAergic synchronization in the limbic system and its role in the generation of epileptiform activity. Prog Neurobiol 95: 104–132, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bénar CG, Chauvière L, Bartolomei F, Wendling F. Pitfalls of high-pass filtering for detecting epileptic oscillations: a technical note on “false” ripples. Clin Neurophysiol 121: 301–310, 2010. [DOI] [PubMed] [Google Scholar]
- Bragin A, Azizyan A, Almajano J, Engel J. The cause of the imbalance in the neuronal network leading to seizure activity can be predicted by the electrographic pattern of the seizure onset. J Neurosci 29: 3660–3671, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Azizyan A, Almajano J, Wilson CL, Engel J. Analysis of chronic seizure onsets after intrahippocampal kainic acid injection in freely moving rats. Epilepsia 46: 1592–1598, 2005. [DOI] [PubMed] [Google Scholar]
- Bragin A, Engel J Jr, Wilson CL, Fried I, Buzsáki G. High-frequency oscillations in human brain. Hippocampus 9: 137–142, 1999. [DOI] [PubMed] [Google Scholar]
- Dzhala VI, Staley KJ. Mechanisms of fast ripples in the hippocampus. J Neurosci 24: 8896–8906, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foffani G, Uzcategui YG, Gal B, Menendez de la Prida L. Reduced spike-timing reliability correlates with the emergence of fast ripples in the rat epileptic hippocampus. Neuron 55: 930–941, 2007. [DOI] [PubMed] [Google Scholar]
- Huberfeld G, Menendez de la Prida L, Pallud J, Cohen I, Le Van Quyen M, Adam C, Clemenceau S, Baulac M, Miles R. Glutamatergic pre-ictal discharges emerge at the transition to seizure in human epilepsy. Nat Neurosci 14: 627–634, 2011. [DOI] [PubMed] [Google Scholar]
- Jefferys JGR, Menendez de la Prida L, Wendling F, Bragin A, Avoli M, Timofeev I, Lopes da Silva FH. Mechanisms of physiological and epileptic HFO generation. Prog Neurobiol 98: 250–264, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévesque M, Salami P, Behr C, Avoli M. Temporal lobe epileptiform activity following systemic administration of 4-aminopyridine in rats. Epilepsia 54: 596–604, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévesque M, Salami P, Gotman J, Avoli M. Two seizure-onset types reveal specific patterns of high-frequency oscillations in a model of temporal lobe epilepsy. J Neurosci 32: 13264–13272, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogren JA, Bragin A, Wilson CL, Hoftman GD, Lin JJ, Dutton RA, Fields TA, Toga AW, Thompson PM, Engel J, Staba RJ. Three-dimensional hippocampal atrophy maps distinguish two common temporal lobe seizure-onset patterns. Epilepsia 50: 1361–1370, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates (4th ed). San Diego: Academic, 1998. [Google Scholar]
- Perucca P, Dubeau F, Gotman J. Intracranial electroencephalographic seizure-onset patterns: effect of underlying pathology. Brain J Neurol 137: 183–196, 2014. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Schwartzkroin PA, Moshé SL. Models of Seizures and Epilepsy (1st ed). San Diego: Academic, 2005. [Google Scholar]
- Salami P, Lévesque M, Gotman J, Avoli M. A comparison between automated detection methods of high-frequency oscillations (80–500 Hz) during seizures. J Neurosci Methods 211: 265–271, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiri Z, Manseau F, Lévesque M, Williams S, Avoli M. Interneuron activity leads to initiation of low-voltage fast-onset seizures. Ann Neurol 77: 541–546, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staba RJ, Stead M, Worrell GA. Electrophysiological biomarkers of epilepsy. Neurotherapeutics 11: 334–346, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco AL, Wilson CL, Babb TL, Engel J. Functional and anatomic correlates of two frequently observed temporal lobe seizure-onset patterns. Neural Plast 7: 49–63, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]