Abstract
Lack of visual input early in life results in occipital cortical responses to auditory and tactile stimuli. However, it remains unclear whether cross-modal plasticity also occurs in subcortical pathways. With the use of functional magnetic resonance imaging, auditory responses were compared across individuals with congenital anophthalmia (absence of eyes), those with early onset (in the first few years of life) blindness, and normally sighted individuals. We find that the superior colliculus, a “visual” subcortical structure, is recruited by the auditory system in congenital and early onset blindness. Additionally, auditory subcortical responses to monaural stimuli were altered as a result of blindness. Specifically, responses in the auditory thalamus were equally strong to contralateral and ipsilateral stimulation in both groups of blind subjects, whereas sighted controls showed stronger responses to contralateral stimulation. These findings suggest that early blindness results in substantial reorganization of subcortical auditory responses.
Keywords: blindness, cross-modal plasticity, medial geniculate nucleus, subcortical pathways, superior colliculus
in blind individuals, the occipital cortex responds during basic auditory tasks such as sound localization (Lessard et al. 1998; Weeks et al. 2000; Gougoux et al. 2005), auditory motion (Poirier et al. 2006; Bedny et al. 2010; Lewis et al. 2010; Jiang et al. 2014), and detection of changes in sounds (Kujala et al. 2005), as well as during more complex linguistic-based tasks requiring semantic processing and verbal memory (Burton et al. 2002a,b; Röder et al. 2002; Amedi et al. 2003).
In contrast, although substantial rewiring of subcortical connections can occur in response to developmental disruption of sensory pathways (Frost 1981; Sur et al. 1988), few studies have addressed whether blindness leads to changes in the functional properties of subcortical pathways. Dynamic causal modeling of data obtained in congenitally blind humans during an auditory discrimination task did not indicate increased thalamo-cortical connectivity between the medial geniculate nucleus (MGN) and primary visual cortex (Klinge et al. 2010). In the (ZRDCT-an) anophthalmic mouse, in which eyes never develop, auditory stimulation elicited activity in the dorsal lateral geniculate nucleus (LGN) and occipital cortex (Piché et al. 2004; Chabot et al. 2008) that was not observed in neonatally enucleated or sighted mice (Tucker et al. 2001).
One possible reason that differences in subcortical responses between blind and sighted people have not yet been observed is because it is relatively difficult to measure activity in these small structures using functional MRI. A second possibility is that subcortical reorganization of function may be limited to anophthalmic individuals, whereas previous studies have focused on mixed populations of blind subjects that have primarily consisted of individuals who became blind postnatally (i.e., early blind). In human bilateral anophthalmia, both eyes fail to develop or development is arrested at a very early stage. Therefore, unlike other causes of congenital blindness, the anophthalmic visual pathway does not experience prenatal retinal activity. As described above, early “visual” areas such as the LGN and V1 have auditory c-Fos activity in anophthalmic but not neonatally enucleated mice (Chabot et al. 2007). Previous imaging work in the human brain suggests that reorganization at the cortical level may differ between anophthalmic and early blind individuals; for example, in anophthalmia, the pericalcarine region (V1 in sighted subjects) appears to be a “low level” auditory structure that does not distinguish between language and backwards speech (Watkins et al. 2012), while it does for early blind subjects (Bedny et al. 2011).
Here, we measured subcortical auditory responses to both binaural and monaural stimulation in individuals with anophthalmia as well as early blind subjects and sighted controls using an auditory stimulus specifically designed to elicit robust auditory responses in the auditory thalamus (Jiang et al. 2013). We found evidence of subcortical reorganization in both blind groups, suggesting that the lack of visual input early in life does indeed alter functional response properties of both auditory and the visual subcortical pathways.
MATERIALS AND METHODS
Participants
Due to subject availability, the data used in this article were obtained using the same paradigm but at two separate sites. To control for site differences, each group of blind people was compared with a group of sighted controls scanned at the same site.
University of Oxford.
Five bilateral anophthalmic participants were recruited (mean age 29 yr, range 23–35 yr, 2 females). All had participated in previous imaging studies (Bridge et al. 2009; Watkins et al. 2012, 2013) and are referred to as cases 1, 2, 4, 5, and 6. The data presented here were acquired 4–5 yr after the first study. Case 3 from that study was not scanned again. All anophthalmic participants except one read Braille and had no neurological history beyond the cause of their blindness (Table 1). Ten age-matched control participants with normal or corrected-to-normal vision were also recruited (mean age 26 yr, range 23–37 yr, 7 females). This study was granted ethical approval by the Oxford University Central Ethical Committee, and all subjects gave informed written consent before participation.
Table 1.
Brief case descriptions of anophthalmic and early blind participants
| Subject | Gender | Age | Clinical Description |
|---|---|---|---|
| Ano 1 | M | 33 | Bilateral anophthalmia associated with OTX2 mutation, mother carrier, delayed speech and motor development. |
| Ano 2 | F | 35 | Isolated bilateral anophthalmia. No family history. |
| Ano 4 | F | 23 | Isolated bilateral anophthalmia, right orbital cyst. No family history. |
| Ano 5 | M | 27 | Isolated bilateral anophthalmia. No family history. |
| Ano 6 | M | 28 | Isolated bilateral anophthalmia. No family history. |
| EB 1 | F | 63 | Ruptured right eye at 2 mo, detached retina at 5 yr. No residual light perception. |
| EB 2 | F | 59 | Optic nerve virus infection at 1.5 yr, low residual light perception. |
| EB 3 | M | 46 | Congenital blindness, caused by congenital glaucoma. Low residual light perception. |
| EB 4 | M | 60 | Congenital blindness, caused by retinopathy of prematurity. No residual light perception. |
| EB 5 | F | 61 | Congenital blindness, caused by cataracts. Low residual light perception and color in right eye. |
| EB 6 | M | 31 | Congenital blindness, caused by Leber's congenital amaurosis. No residual light perception. |
| EB 7 | M | 39 | Congenital blindness, caused by congenital glaucoma. Low residual light perception in right eye. |
Age is at time of scan. All participants except Ano 1 are Braille readers. Anophthalmic participants were scanned at the University of Oxford site and early blind participants were scanned at the University of Washington site. M, male; F, female.
University of Washington.
Seven blind participants were recruited (mean age 51 yr, range 31–63 yr, 3 females). All subjects were blind at birth or within the first 5 yr of life due to mixed etiologies and were left with no or very low light perception (Table 1). This group is referred to as “early blind” consistent with previous literature (Burton et al. 2002b; Gougoux et al. 2005; Collignon et al. 2007). Eight control participants with normal or corrected-to-normal vision were also scanned at the same site (mean age 30 yr, range 23–39 yr, 5 females). All subjects gave written and informed consent before the experiment, following procedures approved by the University of Washington (UW).
MR Imaging Acquisition
University of Oxford.
Images were acquired using a Siemens Verio 3-Tesla whole body MRI scanner and a 32-channel coil at the Functional Magnetic Resonance Imaging of the Brain Centre (University of Oxford). Structural images were acquired at 1-mm isotropic resolution using a T1-weighted MPRAGE sequence [repetition time (TR) = 2,040 ms, echo time (TE) = 4.7 ms, flip angle = 8°, 192 transverse slices, and 1-mm isotropic voxels]. A total of six functional runs were acquired using a sparse echo-planar imaging pulse sequence [TR = 10 s, acquisition time (TA) = 2 s, TE = 30 ms, flip angle = 90°, 36 transverse slices, and 3-mm isotropic voxels]. Transverse slices were positioned to cover most of the brain (cerebellum, temporal, and occipital lobes) although depending on head size, superior portions of the parietal lobe were sometimes omitted.
University of Washington.
Images were acquired using a Philips 3-Tesla whole body MRI scanner and an eight-channel coil at the UW Diagnostic Imaging Sciences Center (DISC). Structural images were acquired at 1-mm isotropic resolution using a T1-weighted MPRAGE sequence (TR = 2200 ms, TE = 3.55 ms, flip angle = 8°, 160 sagittal slices, and 1-mm isotropic voxels). The six functional runs were acquired using a sparse echo-planer imaging pulse sequence (TR = 10 s, TA = 1.4 s, TE = 16.5 ms, flip angle = 76°, 32 transverse slices, and 2.75 × 2.75 × 3 mm voxels). Again, slices covered as much of the brain as possible, but in some subjects superior portions of the parietal lobe were not scanned.
Auditory Stimuli
Auditory stimuli were presented to participants via MRI-compatible earphones (model S14; Sensimetrics), and sound levels were adjusted to a comfortable listening level. Sighted control participants were scanned in darkness with eyes closed. Stimuli consisted of scrambled musical segments (1 s each), extracted using MATLAB (Mathworks) from three songs: God Shuffled His Feet (Crash Test Dummies), Will O' the Wisp (Miles Davis), and Saeta (Miles Davis). The first song contained lyrics, and the second two were music only. These scrambled segments were presented binaurally, monaurally to the right ear, or monaurally to the left ear. The intensity in the binaural condition was scaled by −6 dB in each ear relative to the monaural conditions to equate total sound amplitude across monaural and binaural conditions. Equating the amplitude in this way reduces differences in loudness across conditions.
Each 10-s trial consisted of either scrambled segments (binaural, monaural left, or monaural right) or silence for 8 s, followed by 2 s when no stimuli were presented and an MR volume was acquired (Fig. 1). One advantage of this “sparse sampling” technique is that it minimizes masking of the auditory stimulus by acoustic scanner noise. A second advantage is that, because of the hemodynamic delay in the blood oxygen-level dependent (BOLD) signal (which is ∼4–5 s to peak response in A1), each volume acquisition measures the BOLD response to auditory stimuli with minimal contribution of scanner acoustic noise to the BOLD response. Furthermore, as a result of reduced magnetic saturation, the signal-to-noise for each individual volume is higher than during continuous acquisition (Hall et al. 1999).
Fig. 1.
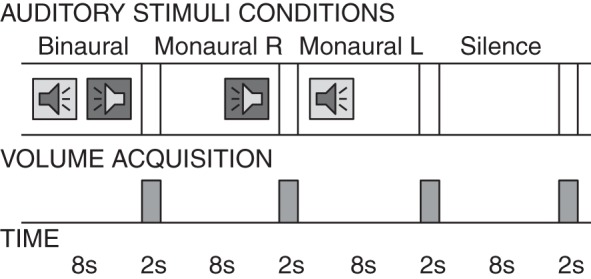
Auditory stimulation using sparse sampling to minimize scanner acoustical noise: 8-s auditory stimulus (binaural, monaural right, monaural left, and silence) followed by 2 s for volume acquisitions.
The presentation order of conditions was fixed for all subjects (binaural, monaural right, monaural left, and silence, repeated 8 times in a run). A total of 32 trials were presented in each of the 6 scans (each scan lasted ∼6 min). Each scan contained scrambled segments from only one of the three songs. The order of songs was fixed for all participants: scans one and four sampled segments from God Shuffled His Feet (Crash Test Dummies), scans two and five sampled from Will O' the Wisp (Miles Davis), and scans three and six sampled from Saeta (Miles Davis).
Attentional Task
During all music conditions, a 1-s musical segment was sometimes repeated consecutively. Participants were required to respond whenever this occurred (one back task), and responses were recorded on a MRI-compatible response box. The task was only used to maintain participant attention.
Data Analysis
MRI analysis.
Functional MRI data processing was performed using FEAT (FMRI Expert Analysis Tool) Version 6.00, part of FSL (FMRIB's Software Library: www.fmrib.ox.ac.uk/fsl). Preprocessing of functional images included motion correction using MCFLIRT (Jenkinson et al. 2002), nonbrain removal using BET (Smith 2002), and high-pass temporal filtering (50s). No spatial smoothing was applied to prevent distortion in the small thalamic regions of interest. A general linear model was applied, with explanatory variables for binaural, right-ear and left-ear stimulation. Functional images were initially registered to each participant's T1-weighted structural image (BBR) for subject-specific analysis and then registered to T1-weighted MNI-152 (Montreal Neurological Institute) 2-mm standard space using nonlinear registration (FNIRT) for group analyses. Higher level analyses were performed using fixed-effects analysis for an average of each participant's six runs and mixed-effects for group comparison. For whole brain inspection, each subject's statistical maps showing BOLD activity during any of the three music conditions (binaural, monaural right, and monaural left) relative to baseline were thresholded at z ≥ 2.5 (Gaussianized T/F) and projected onto native T1-weighted 1-mm space. For group whole brain comparisons, statistical maps were thresholded at z ≥ 2.8 (Gaussianized T/F, cluster corrected, P < 0.05) and projected onto MNI 2-mm standard space.
Region of interest analysis.
The two cortical regions of interest, primary visual cortex (V1) and primary auditory cortex (A1), were derived from the Juelich histological atlas as implemented in FSL with fslview (version 3.2.0). A1 was the combination of TE1.0, TE1.1, and TE1.2 (Morosan et al. 2001). Cortical probabilistic definitions were thresholded at 30%. Anatomical definitions of the lateral and medial geniculate nuclei were also derived from Juelich histological atlas, with the probabilistic maps thresholded at 15% (because of its more variable location across subjects in the atlas, a lower threshold was used to ensure we captured activation in our participants in this region).
Due to the known variability in the location of the auditory thalamus between participants and hemispheres (Rademacher et al. 2002), it was defined for each participant in their native space based on BOLD activation to any of the three auditory conditions (binaural, monaural right, and monaural left). The activation overlapped with the expected location of the MGN (based on the Juelich Histological Atlas) and was defined separately for the left and right sides by increasing statistical thresholds until reaching a volume of ∼100 mm3, which is the approximate size of the MGN (Winer 1984).
The inferior colliculus was not reliably activated in each subject, and therefore, individual functional masks could not be made. Instead, the inferior colliculus was defined anatomically based on each subject's T1-weighted 1-mm structural scan and divided at the midline into right and left regions of interest. The superior colliculus was defined using the same method.
To determine the magnitude of activation evoked by the auditory stimulus, percent BOLD signal change was extracted from these cortical and subcortical regions of interest. Percentage BOLD signal change during each of the three auditory conditions relative to the silent baseline was calculated using Featquery, and statistical analyses were performed using SPSS software (IBM, SPSS Version 20 for Mac). Repeated-measures ANOVAs and two-tailed Student t-tests (independent and paired samples) were performed to investigate differences in percent BOLD signal change during the three auditory conditions (binaural, monaural ipsilateral, and monaural contralateral) relative to baseline. Results were considered statistically significant at P < 0.05. Homogeneity of variance was assessed using Mauchly's test of sphericity; if significant, the Greenhouse-Geisser correction and adjusted degrees of freedom were reported.
RESULTS
We begin by showing whole brain responses in blind and sighted subjects, followed by a discussion of findings of the reorganization evident within visual subcortical (superior colliculus and LGN) and auditory subcortical (inferior colliculus and the auditory thalamus) pathways. Finally, we report cortical responses within A1 and V1.
Whole Brain Responses to Sound in Blind and Sighted Subjects
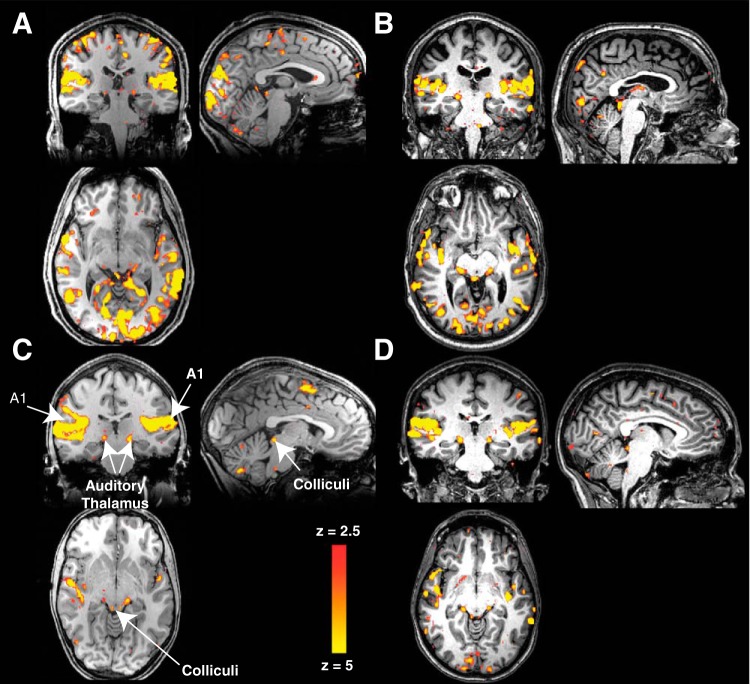
Figure 2 shows examples of the responses in single participants to all auditory stimulation conditions combined (binaural, monaural right, and monaural left). As expected, all major auditory structures responded to our stimuli in both sighted and blind groups. The example subjects (Fig. 2A, anophthalmic; Fig. 2B, early blind; Fig. 2C, Oxford control; Fig. 2D, UW control) showed extensive, highly significant activation in the auditory cortex, auditory thalamus, and inferior colliculus. However, in the example anophthalmic (Fig. 2A) and early blind (Fig. 2B) participants, there is additional, significant activation in the occipital cortex, including both pericalcarine and extrastriate regions.
Fig. 2.
Example activation across all auditory stimulus conditions (binaural, monaural ipsilateral, and monaural contralateral) for an anophthalmic (A), early blind (B), and sighted control participants [C: Oxford site; D: University of Washington (UW) site]. Statistical maps are thresholded voxelwise at z ≥ 2.5 for visualization purposes.
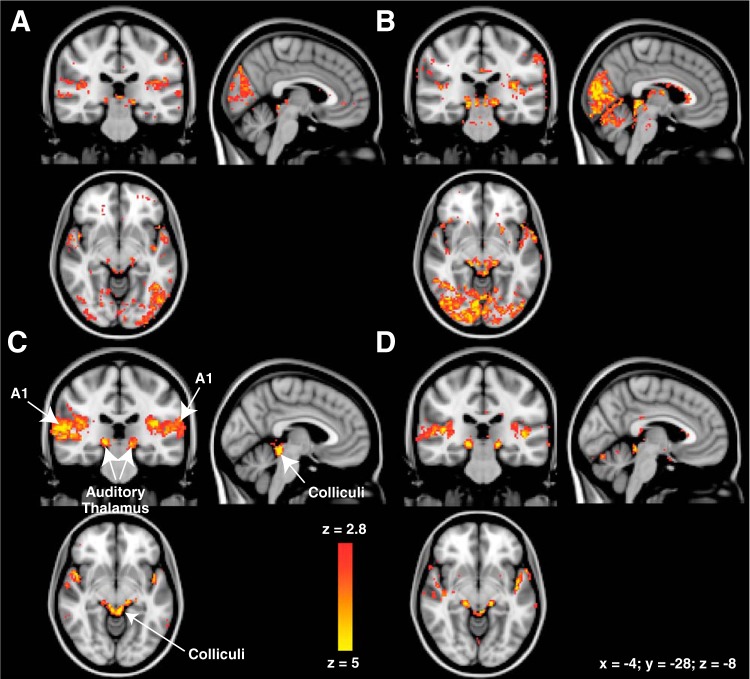
Group activity maps revealed a pattern very similar to the example subjects described above. Figure 3 shows the activation from a mixed-effects analysis performed separately for each group (Fig. 3A, anophthalmic; Fig. 3B, early blind; Fig. 3C, Oxford controls; Fig. 3D, UW controls). Group statistical maps show clear activation in the auditory cortex, thalamus, and colliculi for all subject groups. As predicted from previous studies, anophthalmic and early blind groups show additional occipital activation.
Fig. 3.
Group activation [in Montreal Neurological Institute (MNI) 2-mm standard space] combined across all auditory stimulus conditions for anophthalmic (A), early blind (B), and sighted control subjects (C: Oxford site; D: UW site). Group statistical maps (mixed effects) are thresholded at z ≥ 2.8 (P < 0.003).
The Superior Colliculus Shows Responses to Auditory Stimuli in Blind Individuals
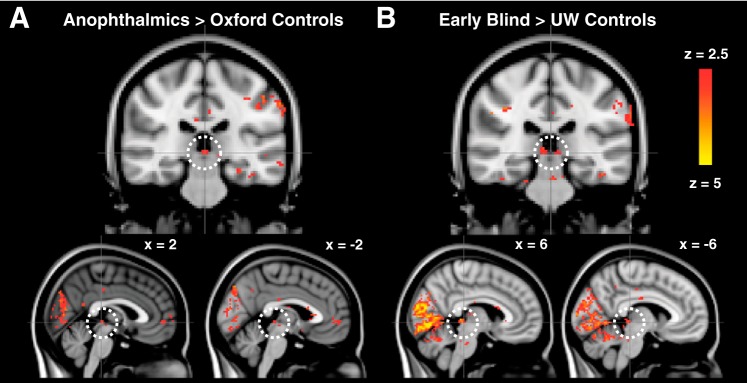
Group data comparing responses within all four subject groups to all three auditory conditions within the region of the superior colliculus are shown in Fig. 4. Figure 4A shows responses that are greater in the anophthalmic group compared with Oxford controls, and Fig. 4B shows responses that are greater in the early blind group compared with UW controls. Both blind groups show greater auditory activity than their respective sighted controls in the expected location of the superior colliculus.
Fig. 4.
Contrasts of anophthalmia > Oxford sighted group (A) and early blind > UW sighted group (B) in all 3 auditory conditions show auditory activity in the expected location of the superior colliculus in blind subjects. Group statistical maps (mixed effects) are thresholded at z ≥ 2.5.
The superior colliculus was then defined anatomically for individual subjects using the T1-weighted 1-mm structural scan. Since there was no significant difference between the left and right superior colliculus activation in any condition, data were collapsed across the left and right hemispheres. Percentage BOLD signal change within this region during the three auditory stimulation conditions is shown in Fig. 5 for anophthalmic subjects and their sighted controls (Fig. 5A) and early blind subjects and their sighted controls (Fig. 5B). Both blind groups showed significantly higher activity in the superior colliculus relative to their sighted controls [main effect of group: F(1,24) = 5.08, P = 0.034]. The enhancement of auditory responses was greatest in the binaural condition, but differences between the three conditions did not reach significance (main effect of condition: P = 0.053). There were no significant differences in BOLD responses between the left and right superior colliculi or between scan sites (Oxford and UW) nor was there a significant interaction between group and condition.
Fig. 5.
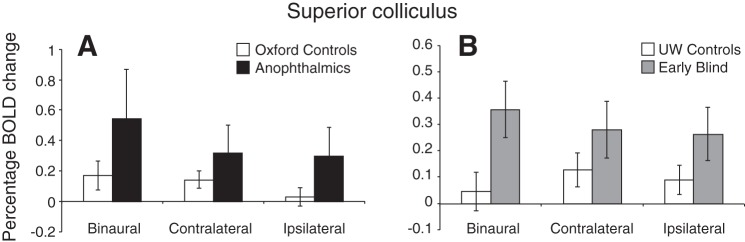
Mean %blood oxygenation-level dependent (BOLD) signal change within the superior colliculus during each of the auditory stimulation conditions (binaural, contralateral, and ipsilateral). Results are separated by scan site (A: Oxford site; B: UW site). Error bars represent means ± SE. Note the y-axis scale difference between the 2 sites reflecting the overall reduced response in the UW data.
The LGN Shows No Evidence for Cross-Modal Plasticity
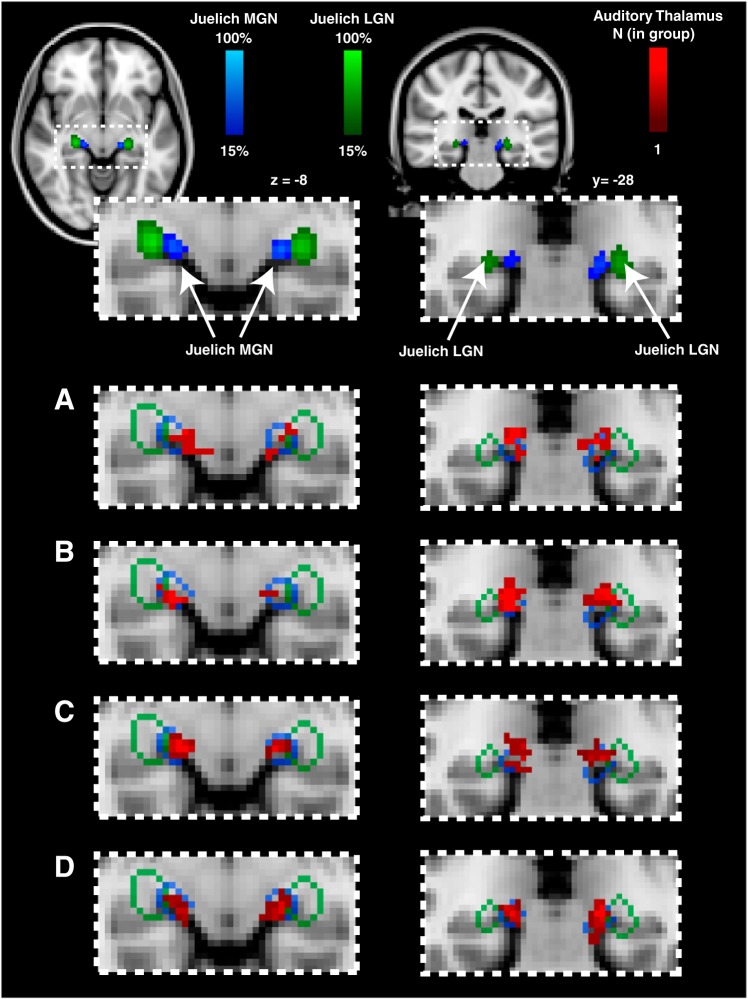
The anatomical definition of the LGN from the Juelich histological atlas was also used to examine whether there were any novel responses in this structure in blind participants. We found no response in the LGN for any subject group for our paradigm (see Fig. 6). Thus, for the auditory stimuli we used, we found no evidence of cross-modal plasticity in the LGN for either anophthalmic or early blind participants.
Fig. 6.
Functional definitions for all subjects (red) are superimposed on the location of anatomically defined medial geniculate nucleus (MGN; blue) and lateral geniculate nucleus (LGN; green). A: anophthalmic group. B: early blind group. C: Oxford control group. D: UW control group.
The Auditory Thalamus Shows Increased Ipsilateral Activity as a Result of Blindness
With the use of methods previously validated by Jiang et al. (2013), the auditory thalamus was defined functionally in each subject's native space based on activation across all auditory stimulation conditions. When converted into MNI standard space, the mean coordinates (and SD) for the central voxel within the auditory thalamus across all subjects were X = −15 (1.64), Y = −27 (1.71), Z = −5 (2.31) in the left hemisphere and X = +16 (1.59), Y = −27 (1.82), Z = −5 (2.29) in the right hemisphere. These match previous reports of MGN coordinates located with structural imaging (Rademacher et al. 2002; Devlin et al. 2006), with functional imaging (Griffiths et al. 2001), and from post mortem brains (Morel et al. 1997; Niemann et al. 2000). No differences in these central coordinates were found among participant groups.
To further confirm our localization of the MGN, functional definitions were overlaid with probabilistic anatomical definitions of the MGN and LGN from the Juelich histological atlas implemented in FSL (thresholded at 15%; Fig. 6). Almost all of the functionally defined auditory thalamus (red) fell within the anatomically expected location of MGN (blue border) and very little fell within the anatomically expected location of neighboring LGN (green border). This provided further confirmation that our functionally defined auditory thalamus activity was centered on the anatomical definition of MGN for all subject groups.
No difference between left and right auditory thalamus activation was found for any condition or group, so data were collapsed across left and right monaural presentations to give ipsilateral and contralateral monaural stimulation. Responses within the functionally defined auditory thalamus to the three auditory stimulation conditions are shown in Fig. 7. Data from participants scanned at Oxford and UW are presented in Fig. 7, A and B, respectively. A four-way ANOVA [hemisphere × auditory condition × group (sighted or blind) × site] indicated significantly higher BOLD responses at the Oxford site compared with UW [F(1,24) = 12.46, P = 0.002]. There were no significant differences between binaural and contralateral auditory conditions in any group; sound amplitude was normalized to match loudness across binaural and monaural conditions so no differences related to loudness were expected. In sighted subjects, the largest responses were to binaural and contralateral stimulation with significantly lower activation levels to ipsilateral stimulation [main effect of condition: F(1.58,37.9) = 16.52, P < 0.001]. This pattern of responses is predicted from the known anatomical pathways, which are partially crossed at this stage in the auditory system. In contrast, neither blind group showed an attenuated response to the ipsilateral stimulation [group × condition interaction: F(1.58,37.9) = 3.53, P = 0.037]. Within-group paired t-tests revealed that contralateral activation was significantly higher than ipsilateral activation in the sighted groups [Oxford: t(8) = 7.9, P < 0.001; UW: t(7) = 5.79, P < 0.001], but responses to ipsilateral and contralateral stimulation were not significantly different in the blind subject groups. Independent t-tests between conditions confirmed that the blind groups showed significantly higher activation than the sighted groups during ipsilateral stimulation only [t(26) = 2.35, P = 0.027].
Fig. 7.
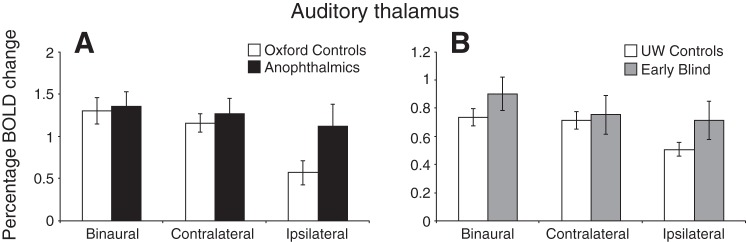
Mean %BOLD change extracted from the auditory thalamus (A: Oxford site; B: UW site) during each of the auditory stimulation conditions (binaural, contralateral and ipsilateral). Error bars represent means ± SE. In the auditory thalamus, both control groups show an attenuated response to ipsilateral auditory stimulation, a pattern not found in either blind group. Note the y-axis scale difference between the 2 sites reflecting the overall reduced response in the UW data.
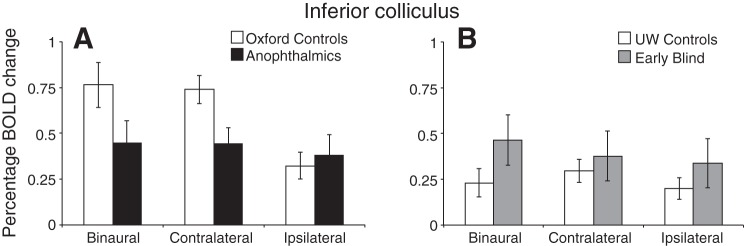
The Inferior Colliculus Shows a Mixed Pattern of Results
The inferior colliculus was defined anatomically for individual subjects using the T1-weighted 1-mm structural scan. Mean percent BOLD signal changes are shown in Fig. 8.
Fig. 8.
Mean %BOLD change extracted from the inferior colliculus (A: Oxford site; B: UW site) during each of the auditory stimulation conditions (binaural, contralateral, and ipsilateral). Error bars represent means ± SE.
The results reported here for the inferior colliculus should be interpreted with caution given that eliciting robust inferior colliculus activity was difficult. The stimulus we used was not specifically designed for this area, and in some individual subjects we could not identify activity in the inferior colliculus. The overall magnitude of BOLD responses was lower for anophthalmic subjects compared with Oxford controls, although this did not reach significance [main effect of group at the Oxford site only: F(1,12) = 3.31, P = 0.094]. This difference might possibly be due to a greater distribution of processing across the inferior and superior colliculi in the anophthalmic individuals, although for UW subjects the opposite pattern of results was observed. However, three sighted subjects from the UW site demonstrated very little activity in the inferior colliculus, thus reducing the control means for the UW site.
The general pattern of contralateral bias in the inferior colliculus in anophthalmic subjects (see Fig. 8) is similar to that seen in the auditory thalamus, in that the anophthalmic subjects show the same magnitude of responses for contralateral and ipsilateral ear stimulation rather than the attenuated ipsilateral responses seen in sighted controls.
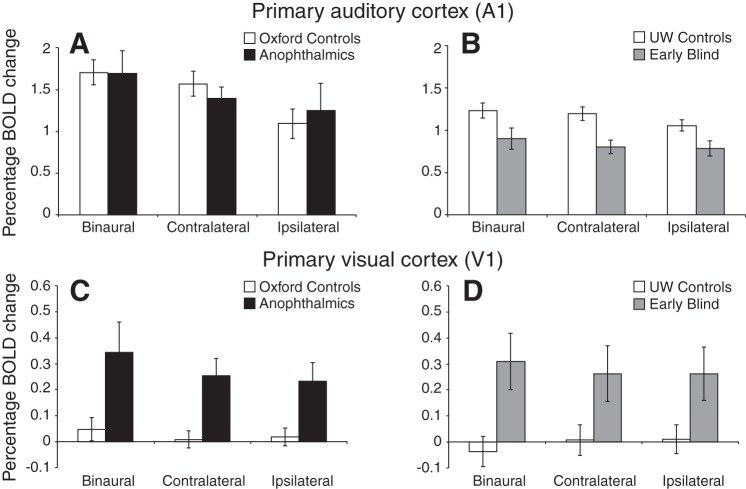
A1 Shows Reduced Contralateral Bias and V1 Shows Cross-Modal Plasticity as a Result of Blindness
Primary auditory (A1) and visual (V1) cortices were defined in MNI 2-mm standard space using the Juelich histological atlas as implemented in FSL. The responses of A1 and V1 to the auditory stimulation are shown in Fig. 9. For A1, the BOLD responses from the UW site were once again significantly lower than those from the Oxford site [main effect of site: F(1,24) = 10.02, P = 0.004].
Fig. 9.
Mean %BOLD change within A1 (A: Oxford site; B: UW site) and V1 (C: Oxford site; D: UW site) during each of the auditory stimulation conditions (binaural, contralateral, and ipsilateral). Error bars represent means ± SE.
Across all groups, in A1 there was a significant effect of stimulation condition [F(2,28) = 28.83, P < 0.001]. The blind groups again failed to show the control pattern of attenuated ipsilateral responses, but this effect was not as strong as for the auditory thalamus and did not quite reach significance (group × condition interaction: P = 0.056). Within-group paired-samples t-tests showed that the contralateral activation was significantly higher than ipsilateral activation in the sighted groups [Oxford: t(8) = 6.04, P < 0.001; UW: t(7) = 4.63, P = 0.002], but the difference between ipsilateral and contralateral conditions was not significant in either of the blind groups [anophthalmia: t(4) = 0.6, P = 0.58; early blind: t(5) = 1.11, P = 0.32]. There was no difference between hemispheres for any condition or group at either scan site (Oxford and UW).
As expected from previous studies, in the expected location of V1, sighted participants did not show significant activation for any of the three auditory conditions, whereas the blind groups showed robust activation across all three conditions. Both blind groups had significantly greater BOLD signal change than sighted subjects [main effect of group: F(1,24) = 15.74, P < 0.001]. Interestingly, although there were no main effects of hemisphere or scan site on mean BOLD signal in V1, the activation in anophthalmic subjects was slightly left lateralized whereas in the early blind subjects it was slightly right lateralized resulting in a significant hemisphere by site interaction [F(1,24) = 5.79, P = 0.024]. However, within each of these two blind groups the levels of activity in the right and left hemispheres were not significantly different. Additionally, there was no significant effect of stimulation condition in the responses of the blind groups.
DISCUSSION
We examined whether visual deprivation in early life (pre- and postnatal) alters subcortical responses to auditory stimulation in addition to the well-established cortical changes. Using a stimulus designed to elicit robust auditory thalamus activity, we found that the absence of visual input early in human development leads to changes in subcortical response properties within both visual and auditory pathways. Specifically, our data suggest that the superior colliculus is recruited for auditory processing in both anophthalmic and early blind individuals. Furthermore, auditory pathways show a reduced contralateral bias; auditory-evoked responses in subcortical and possibly cortical structures ipsilateral to the stimulated ear were higher in blind subjects compared with sighted controls.
Eliciting Subcortical Activity Using a Complex Auditory Stimulus
A variety of methods have been used to localize and measure responses within the MGN (Guimaraes et al. 1998; Giraud et al. 2000; Griffiths et al. 2001; Harms and Melcher 2002; Krumbholz et al. 2005). The complex sound stimulus (scrambled music) we used was previously shown to reliably elicit responses within the auditory thalamus (Jiang et al. 2013). We chose this particular stimulus because it appears to elicit much stronger responses in the auditory thalamus at the individual level compared with dynamic rippled noise (Jiang et al. 2013) and contains both speech and nonspeech components. Neurons within the three subdivisions of the MGN (ventral, dorsal, and medial) respond to a range of natural and artificial sounds with varying spectral and temporal features in nonhuman primates (Symmes et al. 1980; Allon and Yeshurun 1985; Bartlett and Wang 2011). In particular, the MGN appears to respond well to complex and speech-like stimuli with low predictability (von Kriegstein et al. 2008; Diaz et al. 2012). Our auditory stimuli consisted of speech and nonspeech music scrambled into short segments; these segments ensure low predictability while sampling various spectrotemporally complex features found in speech. In addition, as an ecologically valid stimulus it is perhaps better suited to maintaining participant attention. Finally, the sound vs. the silent rest contrast was chosen because we were primarily interested in eliciting robust subcortical responses to auditory stimulation, rather than characterizing more subtle functionally specific responses between different types of auditory stimuli. Using this stimulus, we saw significant bilateral auditory thalamus activation in 26/28 individual participants.
A series of studies have suggested that auditory noise (Devlin et al. 2003) and “speech-like” stimuli containing fast temporal modulations may be preferentially processed in left auditory structures (Zatorre and Belin 2001; Schonwiesner et al. 2007), while musical stimuli or spatial or motion information may be preferentially processed in the right hemisphere (Zatorre et al. 2002; Krumbholz et al. 2005), although this lateralization may depend on context (Brechmann and Scheich 2005; Shtyrov et al. 2005; Schonwiesner et al. 2007). In this study, similar to Jiang et al. (2013), we consistently saw larger responses to contralateral compared with ipsilateral stimuli in sighted subjects, but we saw no evidence of any hemispheric specialization. This may be because the stimuli that we used contained a combination of auditory-noise, music-like, and speech-like features.
The Superior Colliculus Shows Responses to Auditory Stimuli in Blind Individuals
The superior colliculus is known to have multimodal input even in sighted animals (Meredith and Stein 1986; Covey et al. 1987; Jiang et al. 1997; King et al. 1998), and these nonvisual responses may be larger in the absence of visual input. Electrophysiology studies with dark reared rats (Vidyasagar 1978) and visually deprived cats (Rauschecker and Harris 1983) found significant increases in the mean number of somatic and auditory responsive cells in the superior colliculus. This increase was found in intermediate and deep layers of the superior colliculus in visually deprived rats (Vidyasagar 1978) and across all layers in visually deprived cats (Rauschecker and Harris 1983). Consistent with these animal studies, our data suggest recruitment of the superior colliculus for auditory processing in anophthalmic and early blind subjects; both blind groups showed significantly greater BOLD responses in the superior colliculus, defined anatomically, compared with the sighted groups.
One possibility, given the animal data, is that the reorganization observed here occurs within the colliculus itself. Several groups have reported low level auditory responses within the occipital cortex, including auditory motion (Poirier et al. 2006; Saenz et al. 2008; Bedny et al. 2010; Jiang et al. 2014) and, more recently, a tonotopic map of auditory frequency in visual motion area hMT+ (Watkins et al. 2013). It is possible that these responses might be mediated by connections with the superior colliculus, either directly or via the pulvinar, as discussed extensively with relation to blindsight (Cowey 2010). A second possibility is that the responses we see in the superior colliculus are the result of descending feedback from cortex, as described further below.
The LGN Shows No Evidence for Cross-Modal Plasticity
Based on the mouse model of anophthalmia (Piché et al. 2004; Laemle et al. 2006; Chabot et al. 2007, 2008), one might expect to see a recruitment of the LGN for auditory processing. However, we found no significant modulation of neural activity within the anatomical location of LGN as a result of early blindness. This could be due to a number of factors: humans may differ from the mouse model, the LGN might not respond to the particular auditory stimulus that we used, or atrophy of the LGN [as has been demonstrated in congenital blindness (Cecchetti et al. 2015) and anophthalmia (Bridge et al. 2009)] might have precluded detection of an evoked auditory response. Scanning this region at higher field strength using other auditory stimuli (e.g., stimuli containing information about spatial position) may yet reveal cross-modal plasticity in this area.
In sighted individuals, retinal input projects separately to the superior colliculus and LGN, and both pathways then project to striate cortex. The superior colliculus receives feedback projections from layers 5 and 6 of V1 (Nhan and Callaway 2012), while striate feedback to LGN is from layers 4–6 (Ichida and Casagrande 2002). One possibility is that cross-modal plasticity is driven by subcortical reorganization that is limited to the superior colliculus. Alternatively, if cross-modal responses are due to feedback projections from V1, then the restriction to the superior colliculus suggests that distinct cell types within the layers must used, since feedback comes from the same layers. A further possibility is that feedback is from extrastriate areas such as hMT+, which projects to LGN via V1 (Jones et al. 2012), while there are direct projections from hMT+ to the superior colliculus (Fries 1984).
The Auditory Thalamus Shows Increased Ipsilateral Activity as a Result of Blindness
In the auditory pathway, inputs from the ear cross to the contralateral brainstem at the level of the superior olivary nucleus (Langers et al. 2005). However, some information stays in the ipsilateral brainstem pathways while some information reaching the contralateral side crosses back at the level of the inferior colliculus. As a consequence, subcortical and cortical auditory responses are stronger in the hemisphere contralateral to the stimulated ear in both animal models (Langers et al. 2005; Schnupp et al. 2011) and humans (subcortex: Langers et al. 2005; cortex; Woldorff et al. 1999; Suzuki et al. 2002). This contralateral bias is clearly replicated in our sighted data, within both the MGN and A1. In contrast to our sighted subjects, both anophthalmic and early blind subjects showed a similar magnitude of auditory thalamus BOLD responses to ipsilateral stimulation as for contralateral or binaural stimulation. This effect was significant in the auditory thalamus and was marginally significant within A1.
This reduction in the contralateral bias in the blind subcortical auditory pathway suggests that the visual system may have a role in shaping the contralateral bias. In the ferret, this contralateral bias develops at the cortical level ∼1 mo after the onset of hearing, suggesting that it requires exogenous input and consists of a boosting of the contralateral response rather than a suppression of the ipsilateral one (Mrsic-Flogel et al. 2006). One possibility is that this contralateral bias is driven by the development of cross-modal correspondences between visual (which are exclusive to the contralateral visual field) and auditory maps.
It is again not clear whether the reduction in the contralateral bias that we observe within the auditory thalamus occurs within subcortical structures and is passed forward to cortex, or whether this reorganization is observable as a consequence of descending feedback from cortex. In the case of descending feedback, the lack of a contralateral bias in the auditory thalamus might simply be a reflection of the absence of a contralateral bias in cortex. Although we found that the enhancement of ipsilateral responses in blind compared with sighted subjects seemed to be weaker in A1 than in the MGN, feedback projections are presumably selective and thus might result in a selective boosting of MGN ipsilateral responses. A third potential source of this reduced contralateral bias is feedback from pericalcarine cortex to the auditory thalamus in blind individuals. Projections from the MGN to the primary visual cortex have been found in bilaterally enucleated opposums (Karlen et al. 2006), and it is possible that these projections are reciprocal. Differentiating between these (and other) possibilities is an interesting avenue for future work.
The Inferior Colliculus Shows a Mixed Pattern of Results
Across most subcortical and cortical structures we found a very similar pattern of results for blind and anophthalmic subjects. The exception was the inferior colliculus, where anophthalmic subjects appear to show reduced inferior colliculus activation relative to the Oxford controls as well as the early blind and sighted controls scanned at UW. This result should be treated with caution, given the difficulty of eliciting robust activity in this small subcortical structure. However, this potential difference may reflect differences in processing between anophthalmic and early blind individuals. Higher resolution scanning and an auditory stimulus (perhaps containing spatial information) specifically designed to elicit strong activity in this structure could be used to further investigate this potential group difference.
Study Limitations
The anophthalmic and early blind participants in the current study differed in age and were scanned at different sites with different scanners and head coils. As a result we were not able to directly compare activation patterns across anophthalmic and early blind subjects, so each group was compared with sighted control subjects scanned at the same site. Furthermore, both groups were relatively small for the types of group comparisons presented here.
Further limitations include the variety of causes of blindness in the early blind group (common in such studies of blindness) and age differences between the early blind and controls at the UW site. In particular, sighted controls at UW were significantly younger than the early blind subjects. This is a concern, given that previous studies have shown that increased age reduces BOLD responses in auditory cortex (Cliff et al. 2013). We found a similar result in auditory cortex, where responses in the older early blind group show an overall reduction in magnitude compared with the younger UW control group. However, the main findings described in this article are unlikely to be due to age differences since they were observed in anophthalmic (who were age-matched to their sighted controls) as well as early blind subjects.
Summary
In conclusion, we describe for the first time subcortical reorganization of function in the human brain as a result of absent visual input occurring early in development. Changes included an apparent recruitment of the superior colliculus for auditory processing and increased ipsilateral BOLD activity in the auditory thalamus. Future work is needed to determine the full extent of functional reorganization within subcortical structures and how these changes contribute to enhanced processing in cortex.
GRANTS
This work is supported by the National Institutes of Health (to H. Bridge and I. Fine) and the Royal Society (to H. Bridge). G. S. L. Coullon is funded by St. John's College Oxford.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: G.S.L.C., F.J., I.F., K.E.W., and H.B. performed experiments; G.S.L.C. analyzed data; G.S.L.C., F.J., I.F., K.E.W., and H.B. interpreted results of experiments; G.S.L.C. prepared figures; G.S.L.C. drafted manuscript; G.S.L.C., F.J., I.F., K.E.W., and H.B. edited and revised manuscript; G.S.L.C., F.J., I.F., K.E.W., and H.B. approved final version of manuscript; F.J. and I.F. conception and design of research.
ACKNOWLEDGMENTS
We thank our participants for their contributions to this research.
REFERENCES
- Allon N, Yeshurun Y. Functional organization of the medial geniculate body's subdivisions of the awake squirrel monkey. Brain Res 360: 75–82, 1985. [DOI] [PubMed] [Google Scholar]
- Amedi A, Raz N, Pianka P, Malach R, Zohary E. Early “visual” cortex activation correlates with superior verbal memory performance in the blind. Nat Neurosci 6: 758–766, 2003. [DOI] [PubMed] [Google Scholar]
- Bartlett EL, Wang X. Correlation of neural response properties with auditory thalamus subdivisions in the awake marmoset. J Neurophysiol 105: 2647–2667, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedny M, Konkle T, Pelphrey K, Saxe R, Pascual-Leone A. Sensitive period for a multimodal response in human visual motion area MT/MST. Curr Biol 20: 1900–1906, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedny M, Pascual-Leone A, Dodell-Feder D, Fedorenko E, Saxe R. Language processing in the occipital cortex of congenitally blind adults. Proc Natl Acad Sci USA 108: 4429–4434, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brechmann A, Scheich H. Hemispheric shifts of sound representation in auditory cortex with conceptual listening. Cereb Cortex 15: 578–587, 2005. [DOI] [PubMed] [Google Scholar]
- Bridge H, Cowey A, Ragge N, Watkins K. Imaging studies in congenital anophthalmia reveal preservation of brain architecture in “visual” cortex. Brain 132: 3467–3480, 2009. [DOI] [PubMed] [Google Scholar]
- Burton H, Snyder AZ, Conturo TE, Akbudak E, Ollinger JM, Raichle ME. Adaptive changes in early and late blind: a fMRI study of Braille reading. J Neurophysiol 87: 589–607, 2002a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton H, Snyder AZ, Diamond JB, Raichle ME. Adaptive changes in early and late blind: a FMRI study of verb generation to heard nouns. J Neurophysiol 88: 3359–3371, 2002b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchetti L, Ricciardi E, Handjaras G, Kupers R, Ptito M, Pietrini P. Congenital blindness affects diencephalic but not mesencephalic structures in the human brain. Brain Struct Funct 2015. January 6 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Chabot N, Charbonneau V, Laramée ME, Tremblay R, Boire D, Bronchti G. Subcortical auditory input to the primary visual cortex in anophthalmic mice. Neurosci Lett 433: 129–134, 2008. [DOI] [PubMed] [Google Scholar]
- Chabot N, Robert S, Tremblay R, Miceli D, Boire D, Bronchti G. Audition differently activates the visual system in neonatally enucleated mice compared with anophthalmic mutants. Eur J Neurosci 26: 2334–2348, 2007. [DOI] [PubMed] [Google Scholar]
- Cliff M, Joyce DW, Lamar M, Dannhauser T, Tracy DK, Shergill SS. Aging effects on functional auditory and visual processing using fMRI with variable sensory loading. Cortex 49: 1304–1313, 2013. [DOI] [PubMed] [Google Scholar]
- Collignon O, Lassonde M, Lepore F, Bastien D, Veraart C. Functional cerebral reorganization for auditory spatial processing and auditory substitution of vision in early blind subjects. Cereb Cortex 17: 457–465, 2007. [DOI] [PubMed] [Google Scholar]
- Covey E, Hall WC, Kobler JB. Subcortical connections of the superior colliculus in the mustache bat, Pteronotus parnellii. J Comp Neurol 263: 179–197, 1987. [DOI] [PubMed] [Google Scholar]
- Cowey A. The blindsight saga. Exp Brain Res 200: 3–24, 2010. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Raley J, Tunbridge E, Lanary K, Floyer-Lea A, Narain C, Cohen I, Behrens T, Jezzard P, Matthews PM, Moore DR. Functional asymmetry for auditory processing in human primary auditory cortex. J Neurosci 23: 11516–11522, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin JT, Sillery EL, Hall DA, Hobden P, Behrens TE, Nunes RG, Clare S, Matthews PM, Moore DR, Johansen-Berg H. Reliable identification of the auditory thalamus using multi-modal structural analyses. Neuroimage 30: 1112–1120, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz B, Hintz F, Kiebel SJ, von Kriegstein K. Dysfunction of the auditory thalamus in developmental dyslexia. Proc Natl Acad Sci USA 109: 13841–13846, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries W. Cortical projections to the superior colliculus in the macaque monkey: a retrograde study using horseradish peroxidase. J Comp Neurol 230: 55–76, 1984. [DOI] [PubMed] [Google Scholar]
- Frost DO. Orderly anomalous retinal projections to the medial geniculate, ventrobasal, and lateral posterior nuclei of the hamster. J Comp Neurol 203: 227–256, 1981. [DOI] [PubMed] [Google Scholar]
- Giraud AL, Lorenzi C, Ashburner J, Wable J, Johnsrude I, Frackowiak R, Kleinschmidt A. Representation of the temporal envelope of sounds in the human brain. J Neurophysiol 84: 1588–1598, 2000. [DOI] [PubMed] [Google Scholar]
- Gougoux F, Zatorre RJ, Lassonde M, Voss P, Lepore F. A functional neuroimaging study of sound localization: visual cortex activity predicts performance in early-blind individuals. PLoS Biol 3: e27, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths TD, Uppenkamp S, Johnsrude I, Josephs O, Patterson RD. Encoding of the temporal regularity of sound in the human brainstem. Nat Neurosci 4: 633–637, 2001. [DOI] [PubMed] [Google Scholar]
- Guimaraes AR, Melcher JR, Talavage TM, Baker JR, Ledden P, Rosen BR, Kiang NY, Fullerton BC, Weisskoff RM. Imaging subcortical auditory activity in humans. Hum Brain Mapp 6: 33–41, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DA, Haggard MP, Akeroyd MA, Palmer AR, Summerfield AQ, Elliott MR, Gurney EM, Bowtell RW. “Sparse” temporal sampling in auditory fMRI. Hum Brain Mapp 7: 213–223, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms MP, Melcher JR. Sound repetition rate in the human auditory pathway: representations in the waveshape and amplitude of fMRI activation. J Neurophysiol 88: 1433–1450, 2002. [DOI] [PubMed] [Google Scholar]
- Ichida JM, Casagrande VA. Organization of the feedback pathway from striate cortex (V1) to the lateral geniculate nucleus (LGN) in the owl monkey (aotus trivirgatus). J Comp Neurol 454: 272–283, 2002. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17: 825–841, 2002. [DOI] [PubMed] [Google Scholar]
- Jiang F, Stecker GC, Fine I. Functional localization of the auditory thalamus in individual human subjects. Neuroimage 78: 295–304, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F, Stecker GC, Fine I. Auditory motion processing after early blindness. J Vis 14: 1–18, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang ZD, Moore DR, King AJ. Sources of subcortical projections to the superior colliculus in the ferret. Brain Res 755: 279–292, 1997. [DOI] [PubMed] [Google Scholar]
- Jones HE, Andolina IM, Ahmed B, Shipp SD, Clements JT, Grieve KL, Cudeiro J, Salt TE, Sillito AM. Differential feedback modulation of center and surround mechanisms in parvocellular cells in the visual thalamus. J Neurosci 32: 15946–15951, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlen SJ, Kahn DM, Krubitzer L. Early blindness results in abnormal corticocortical and thalamocortical connections. Neuroscience 142: 843–858, 2006. [DOI] [PubMed] [Google Scholar]
- King AJ, Jiang ZD, Moore DR. Auditory brainstem projections to the ferret superior colliculus: anatomical contribution to the neural coding of sound azimuth. J Comp Neurol 390: 342–365, 1998. [PubMed] [Google Scholar]
- Klinge C, Eippert F, Röder B, Büchel C. Corticocortical connections mediate primary visual cortex responses to auditory stimulation in the blind. J Neurosci 30: 12798–12805, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Kriegstein K, Patterson RD, Griffiths TD. Task-dependent modulation of medial geniculate body is behaviorally relevant for speech recognition. Curr Biol 18: 1855–1859, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumbholz K, Schönwiesner M, Rübsamen R, Zilles K, Fink GR, von Cramon DY. Hierarchical processing of sound location and motion in the human brainstem and planum temporale. Eur J Neurosci 21: 230–238, 2005. [DOI] [PubMed] [Google Scholar]
- Kujala T, Palva MJ, Salonen O, Alku P, Huotilainen M, Järvinen A, Näätänen R. The role of blind humans' visual cortex in auditory change detection. Neurosci Lett 379: 127–131, 2005. [DOI] [PubMed] [Google Scholar]
- Laemle LK, Strominger NL, Carpenter DO. Cross-modal innervation of primary visual cortex by auditory fibers in congenitally anophthalmic mice. Neurosci Lett 396: 108–112, 2006. [DOI] [PubMed] [Google Scholar]
- Langers DR, van Dijk P, Backes WH. Lateralization, connectivity and plasticity in the human central auditory system. Neuroimage 28: 490–499, 2005. [DOI] [PubMed] [Google Scholar]
- Lessard N, Paré M, Lepore F, Lassonde M. Early-blind human subjects localize sound sources better than sighted subjects. Nature 395: 278–280, 1998. [DOI] [PubMed] [Google Scholar]
- Lewis LB, Saenz M, Fine I. Mechanisms of cross-modal plasticity in early-blind subjects. J Neurophysiol 104: 2995–3008, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith MA, Stein BE. Visual, auditory, and somatosensory convergence on cells in superior colliculus results in multisensory integration. J Neurophysiol 56: 640–662, 1986. [DOI] [PubMed] [Google Scholar]
- Morel A, Magnin M, Jeanmonod D. Multiarchitectonic and stereotactic atlas of the human thalamus. J Comp Neurol 387: 588–630, 1997. [DOI] [PubMed] [Google Scholar]
- Morosan P, Rademacher J, Schleicher A, Amunts K, Schormann T, Zilles K. Human primary auditory cortex: cytoarchitectonic subdivisions and mapping into a spatial reference system. Neuroimage 13: 684–701, 2001. [DOI] [PubMed] [Google Scholar]
- Mrsic-Flogel TD, Versnel H, King AJ. Development of contralateral and ipsilateral frequency representations in ferret primary auditory cortex. Eur J Neurosci 23: 780–792, 2006. [DOI] [PubMed] [Google Scholar]
- Nhan HL, Callaway EM. Morphology of superior colliculus- and middle temporal area-projecting neurons in primate primary visual cortex. J Comp Neurol 520: 52–80, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann K, Mennicken VR, Jeanmonod D, Morel A. The Morel stereotactic atlas of the human thalamus: atlas-to-MR registration of internally consistent canonical model. Neuroimage 12: 601–616, 2000. [DOI] [PubMed] [Google Scholar]
- Piché M, Robert S, Miceli D, Bronchti G. Environmental enrichment enhances auditory takeover of the occipital cortex in anophthalmic mice. Eur J Neurosci 20: 3463–3472, 2004. [DOI] [PubMed] [Google Scholar]
- Poirier C, Collignon O, Scheiber C, Renier L, Vanlierde A, Tranduy D, Veraart C, De Volder AG. Auditory motion perception activates visual motion areas in early blind subjects. Neuroimage 31: 279–285, 2006. [DOI] [PubMed] [Google Scholar]
- Rademacher J, Bürgel U, Zilles K. Stereotaxic localization, intersubject variability, and interhemispheric differences of the human auditory thalamocortical system. Neuroimage 17: 142–160, 2002. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Harris LR. Auditory compensation of the effects of visual deprivation in the cat's superior colliculus. Exp Brain Res 50: 69–83, 1983. [DOI] [PubMed] [Google Scholar]
- Röder B, Stock O, Bien S, Neville H, Rösler F. Speech processing activates visual cortex in congenitally blind humans. Eur J Neurosci 16: 930–936, 2002. [DOI] [PubMed] [Google Scholar]
- Saenz M, Lewis LB, Huth AG, Fine I, Koch C. Visual motion area MT+/V5 responds to auditory motion in human sight-recovery subjects. J Neurosci 28: 5141–5148, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnupp J, Nelken I, King AJ. Auditory Neuroscience–Making Sense of Sound. Cambridge, MA: MIT, 2011. [Google Scholar]
- Schonwiesner M, Krumbholz K, Rubsamen R, Fink GR, von Cramon DY. Hemispheric asymmetry for auditory processing in the human auditory brain stem, thalamus, and cortex. Cereb Cortex 17: 492–499, 2007. [DOI] [PubMed] [Google Scholar]
- Shtyrov Y, Pihko E, Pulvermüller F. Determinants of dominance: Is language laterality explained by physical or linguistic features of speech? Neuroimage 27: 37–47, 2005. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp 17: 143–155, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur M, Garraghty PE, Roe AW. Experimentally induced visual projections into auditory thalamus and cortex. Science 242: 1437–1441, 1988. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Kitano H, Kitanishi T, Itou R, Shiino A, Nishida Y, Yazawa Y, Ogawa F, Kitajima K. Cortical and subcortical activation with monaural monosyllabic stimulation by functional MRI. Hear Res 163: 37–45, 2002. [DOI] [PubMed] [Google Scholar]
- Symmes D, Alexander GE, Newman JD. Neural processing of vocalizations and artificial stimuli in the medial geniculate body of squirrel monkey. Hear Res 3: 133–146, 1980. [DOI] [PubMed] [Google Scholar]
- Tucker P, Laemle L, Munson A, Kanekar S, Oliver ER, Brown N, Schlecht H, Vetter M, Glaser T. The eyeless mouse mutation (ey1) removes an alternative start codon from the Rx/rax homeobox gene. Genesis 31: 43–53, 2001. [DOI] [PubMed] [Google Scholar]
- Vidyasagar TR. Possible plasticity in the rat superior colliculus. Nature 275: 140–141, 1978. [DOI] [PubMed] [Google Scholar]
- Watkins KE, Cowey A, Alexander I, Filippini N, Kennedy JM, Smith SM, Ragge N, Bridge H. Language networks in anophthalmia: maintained hierarchy of processing in “visual” cortex. Brain 135: 1566–1577, 2012. [DOI] [PubMed] [Google Scholar]
- Watkins KE, Shakespeare TJ, O'Donoghue MC, Alexander I, Ragge N, Cowey A, Bridge H. Early auditory processing in area V5/MT+ of the congenitally blind brain. J Neurosci 33: 18242–18246, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks R, Horwitz B, Aziz-Sultan A, Tian B, Wessinger CM, Cohen LG, Hallett M, Rauschecker JP. A positron emission tomographic study of auditory localization in the congenitally blind. J Neurosci 20: 2664–2672, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer JA. The human medial geniculate body. Hear Res 15: 225–247, 1984. [DOI] [PubMed] [Google Scholar]
- Woldorff MG, Tempelmann C, Fell J, Tegeler C, Gaschler-Markefski B, Hinrichs H, Heinz HJ, Scheich H. Lateralized auditory spatial perception and the contralaterality of cortical processing as studied with functional magnetic resonance imaging and magnetoencephalography. Hum Brain Mapp 7: 49–66, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ, Belin P, Penhune VB. Structure and function of auditory cortex: music and speech. Trends Cogn Sci 6: 37–46, 2002. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Belin P. Spectral and temporal processing in human auditory cortex. Cereb Cortex 11: 946–953, 2001. [DOI] [PubMed] [Google Scholar]