Abstract
Careful, clean and controlled preparation of samples for mass spectrometry proteomics is crucial to obtain reproducible and reliable data. This is especially important when carrying out quantitative proteomics by chemical isobaric labeling (aka tandem mass tagging) since the differentially labeled samples are combined quite late during the sample processing. Addressing this need for robust and reliable sample processing for quantitative proteomics, we describe here iFASP, a simple protocol for combining isobaric mass tagging with the recently introduced Filter-Aided Sample Preparation (FASP) method. iFASP provides a quick, simple and effective method for obtaining clean samples, ensuring efficient digestion and providing excellent labeling yields for quantitative proteomics experiments. We have carried out our iFASP protocol using several highly complex Xenopus laevis egg and embryo lysates and compared the labeling yields and number of high-confidence peptide identifications to a standard in-solution digestion and labeling protocol. Although the labeling efficiency with both techniques is in the 99+% range, the number of peptides identified with a 1% false discovery rate and the corresponding number of quantified peptide spectral matches are as much as doubled with iFASP compared to the corresponding non-FASP-based method.
Keywords: FASP, TMT, iTRAQ, Xenopus laevis, Orbitrap, Q Exactive
INTRODUCTION
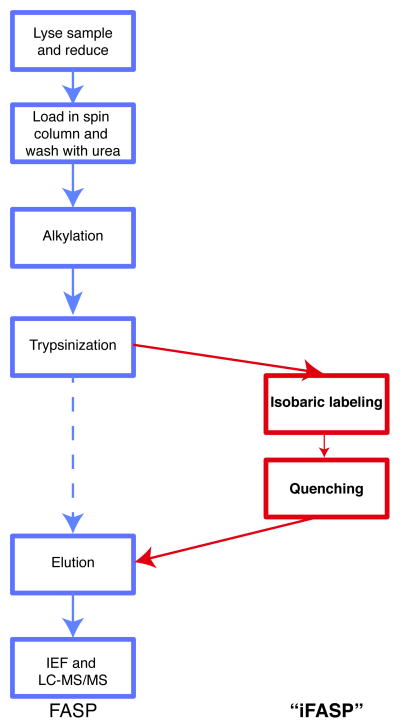
Reproducible and convenient ways of preparing samples for mass spectrometry-based proteomics are essential for ensuring good quality data. The technique of Filter-Aided Sample Preparation (FASP) was recently developed by the Mann lab1 by modifying and optimizing a method from the Liebler group2. Ultrafiltration spin columns are used to retain proteins whilst they are denatured with urea and subsequently digested. It has been widely employed for many proteomic studies and has been further developed for e.g. multi-enzyme digestion reactions3. However this technique has not yet been combined with isobaric labeling techniques, such as TMT and/or iTRAQ labeling4, which are widely used in quantitative proteomics experiments. Here we describe and assess a simple protocol which combines isobaric mass tagging with FASP (‘iFASP’, see Figure 1 for a comparison of workflows), thereby providing a very quick and easy sample preparation, digestion and isobaric mass tagging protocol to enable accelerated and streamlined multiplexed quantitative proteomics studies.
Figure 1.
FASP and iFASP workflow comparison. The regular FASP workflow with the steps for the iFASP workflow added and highlighted.
MATERIALS AND METHODS
Detailed experimental procedures can be found in the Supplementary Information.
Frog embryo lysis
Xenopus laevis embryos were snap-frozen in liquid nitrogen and lysed in Xenopus Lysis Buffer (XLB) and spun for 10 minutes at 14k rpm 4 °C. The supernatant was removed and protein concentrations in each sample were determined.
iFASP and TMT-/iTRAQ-labeling
100 μg protein was reduced with tris(2-carboxyethyl)phosphine (TCEP) and the FASP protocol was then followed using the manufacturer’s protocol for the FASP™ Protein Digestion Kit (#44250, Protein Discovery) with exceptions as described in the Supplementary Methods. Alkylation with 0.05 M iodoacetamide solution was performed in the dark at room temperature (23 °C) for 20 minutes. Trypsin digestion was carried out using 1:100 enzyme-to-protein at 37 °C for 16 hours.
TMT-labeling
TMTsixplex™ Isobaric Label Reagent Set (Thermo #90062) labels were dissolved in 41 μl acetonitrile, added to samples in the spin filters and the reactions were incubated at room temperature for one hour. To quench the reaction, 8 μl of 5% hydroxylamine was added to the samples in the spin filters and incubated at room temperature for a further 30 minutes. Peptides were eluted from the spin columns then combined and acidified.
iTRAQ labeling
iTRAQ® 8plex labels (AB Sciex) were supplemented with 50 μl isopropanol, added to the samples in the spin filters and the reactions were incubated at room temperature for one hour. Peptides were eluted from the columns then combined and acidified.
In-solution digest and TMT-labeling
100 μg protein was precipitated by methanol/chloroform precipitation and the pellet resuspended. Samples were reduced with TCEP and alkylated with iodoacetamide solution. Samples were digested with trypsin at 37 °C for 16 hours. Acidification with formic acid was carried out for 30 minutes at 37 °C. The sample was centrifuged at 21k × g for 15 minutes at 4 °C and the supernatant removed for cleanup.
Sample cleanup, electrophoresis and mass spectrometry (MS)
Peptides were desalted on an OASIS HLB column (Waters) and evaporated to dryness. Samples were separated into 24 fractions by isoelectric focusing (OFFGEL, Agilent) according to isoelectric point. Peptide fractions were cleaned using TARGA C18 Microspin Columns (The Nest Group) and dried before resuspension in MS loading buffer (5% acetonitrile, 0.1% formic acid in H2O).
Samples were analyzed by LC-MS/MS using a 60 minute gradient. Pulsed Q-Dissociation (PQD) in the LTQ-Orbitrap Classic or High-energy Collision Dissociation (HCD) in the Q Exactive (both Thermo Scientific) were used to fragment the peptide precursor ions.
Data analysis
Thermo RAW files were loaded into Proteome Discoverer 1.3 and searched against the Xenopus laevis protein database downloaded from Xenbase (www.xenbase.org) using a target-decoy database generated by Proteome Discoverer to calculate the false discovery rate (FDR) using Percolator. TMT 6-plex modifications were set as dynamic modifications to calculate labeling efficiency, and as static modifications for all other data analysis. Only peptides identified with a high rate of confidence (1% FDR) were used for analysis. Labeling efficiency was calculated as the percentage of the total number of unique peptides that were modified by TMT-labeling.
RESULTS AND DISCUSSION
This iFASP protocol was performed using multiple lysates from Xenopus laevis eggs and embryos. As a comparison, one of these lysates was also used for a conventional in-solution digestion and TMT-labeling protocol. Detailed protocols for iFASP and the conventional in solution protein digestion protocol including protein precipitation from the lysate, resuspension, digestion, and labeling are described in the Supplementary Methods. In particular, critical points along the workflow are highlighted in order to maximize the labeling yields (see Supplementary Methods and e.g. Table 2, Sample T7, where a cold centrifuge resulted in urea precipitation and thus poor labeling efficiency). The Xenopus samples were reduced with TCEP before loading 100 μg protein from each lysate into the FASP kit spin column supplied with the FASP Protein Digestion Kit. The manufacturer’s protocol was then followed for FASP including the denaturation in urea, reduction, alkylation and trypsinization in 50 mM ammonium bicarbonate (ABC) or triethylammonium bicarbonate (TEAB). Diverting from the normal FASP protocol, TMT 6plex reagents or iTRAQ 8plex reagents were resuspended in acetonitrile or isopropanol, respectively and a separate label was added to each sample in a spin column after the digestion, preceding the peptide elution steps. Samples were incubated with mixing before the elution steps of the FASP protocol were carried out as normal by washing with ABC or TEAB solution followed by NaCl solution.
Table 2.
Comparison of labeling efficiencies. Multiple Xenopus laevis egg and embryo lysates were prepared and labeled using iFASP with TMT labeling (T1–T15) and iTRAQ labeling (i1) and analyzed on the Q Exactive. Unique Peptides: total number of peptides with unique sequence identified at a confidence level of 1% peptide FDR. Unlabeled peptides: peptides with no TMT labels detected.
| Sample | Unique peptides | Unlabeled | Labeling efficiency |
|---|---|---|---|
| T1 | 21352 | 26 | 99.9 |
| T2 | 17376 | 162 | 99.1 |
| T3 | 16811 | 357 | 97.9 |
| T4 | 19605 | 19 | 99.9 |
| T5 | 13505 | 183 | 98.6 |
| T6 | 20174 | 15 | 99.9 |
| T7 | 16759 | 1796 | 89.3* |
| T8 | 18974 | 77 | 99.6 |
| T9 | 19000 | 75 | 99.6 |
| T10 | 14717 | 20 | 99.9 |
| T11 | 10992 | 19 | 99.8 |
| T12 | 12527 | 368 | 97.1 |
| T13 | 13541 | 394 | 97.1 |
| T14 | 7347 | 75 | 99.0 |
| T15 | 19191 | 223 | 98.8 |
| i1 | 10268 | 71 | 99.3 |
See main text for a discussion of this sample.
Peptides were acidified and desalted before separation into 24 fractions by isoelectric focusing. Each fraction was analyzed by LC-MS/MS using a 60 minute gradient on a LTQ-Orbitrap Classic, or a Q Exactive (both Thermo Scientific).
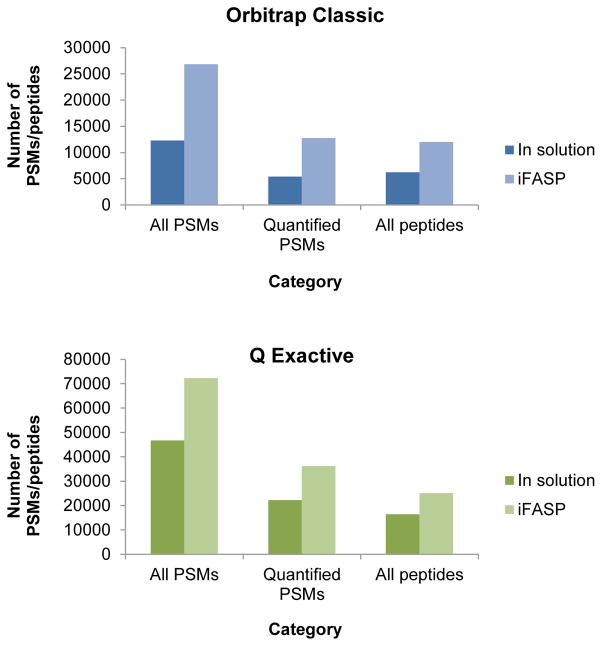
The qualitative and quantitative data analysis was carried out with the Proteome Discoverer (v1.3) analysis software as described in the Methods. The percentage labeling efficiency was calculated based on the total number of high confidence unique peptide sequence identifications (denominator, determined at a 1% false discovery rate (FDR) cutoff using Percolator) and the number of unique peptides carrying a TMT label (numerator) which was considered as variable during the database search5 (see Table 1). Irrespective of the instrument used and the resulting analytical depth, the labeling efficiencies of the two protocols, i.e. iFASP or conventional in-solution protocol, was greater than 99% (99.1% and 99.4% for the conventional protocol; 99.7% and 99.8% with iFASP). In addition to providing excellent labeling efficiencies, the iFASP protocol provides a much larger number of peptide spectrum matches and peptides for each machine than its respective conventional protocol sample, increasing the number of identified peptide spectral matches (PSMs) and/or peptides by at least 50 to 60% (e.g. 16400 peptides with the conventional protocol vs. 25097 with the iFASP protocol, see Figure 2). This agrees well with the increase in peptide identification reported by the Mann lab1a. Furthermore, the number of peptides recovered from the spin filters is not affected by the presence or absence of TMT labeling solution, as demonstrated by comparing the results of an iFASP sample preparation without TMT label added (TMT Mock iFASP, Table 1).
Table 1.
Comparison of data acquisition. Various factors are compared between the conventional in-solution digestion samples and isobaric filter-aided sample preparation (iFASP) samples. TMT-labeling was carried out for all samples except for the TMT Mock iFASP experiment. Peptides: number of peptides identified at a confidence level of 1% peptide FDR. PSMs: total number of peptide spectral matches (PSMs).
| Sample | All PSMs | All Peptides | Peptide Labeling Efficiency (%) |
|---|---|---|---|
| Conventional protocol/Orbitrap | 12304 | 6223 | 99.1 |
| iFASP/Orbitrap | 26846 | 12021 | 99.7 |
| Conventional protocol/Q Exactive | 46705 | 16400 | 99.4 |
| iFASP/Q Exactive | 72324 | 25097 | 99.9 |
| TMT Mock iFASP/Q Exactive | 63142 | 24644 | - |
Figure 2.
Comparison of PSM and peptide identifications. Graphs for the LTQ-Orbitrap Classic and the Q Exactive showing the numbers of all PSMs (total number of peptide spectral matches), all PSMs with associated quantification information and all peptides identified at a confidence level of 1 % peptide FDR for the sample comparing iFASP with a standard in solution digestion protocol.
Upon establishing a robust TMT-based iFASP protocol we further investigated three additional aspects: i) amount of starting material needed, ii) compatibility with the iTRAQ reagent and iii) use of primary and tertiary amine-containing buffer systems. For i) we applied our iFASP protocol also to 30 and 10 ug of starting material as well as the usual 100 ug of starting material. Apart from the lower number of identified samples as expected for smaller amounts of starting material, our iFASP protocol is fully compatible with smaller amounts of protein down to 10 ug (see Supplementary Table 1). To test the compatibility with iTRAQ we applied our iFASP protocol established for TMT with the only exception being that the labeling reagent was supplemented with 50 ul of isopropanol instead of 41 ul of acetonitrile prior to addition to the sample. The results clearly show that our iFASP protocol is compatible with iTRAQ as well as TMT (see Supplementary Table 2). To confirm the surprising finding that the iFASP protocol does indeed work with primary amine-containing buffers such as ammonium bicarbonate (ABC) and not only with tertiary amine-buffers such a triethylammonium bicarbonate (TEAB), we split samples and processed the aliquots simultaneously, once with ABC and once with TEAB (for TMT-labeling) or four times with ABC and four times with TEAB (for iTRAQ-labeling). These experiments confirmed our initial findings and clearly showed that ABC is compatible with the iFASP protocol giving similar labeling yields as TEAB (see Supplementary Tables 1 and 2).
Encouraged by this initial result, we applied our iFASP-based TMT labeling to further X. laevis egg and embryo lysates, to a) ensure general applicability and b) to compare labeling efficiencies (Table 2). The protocol was able to combine a more rapid sample preparation, to process the large number of samples more quickly, with a high labeling efficiency. In summary, we have demonstrated that isobaric mass tagging can be successfully combined with the FASP protocol. The iFASP protocol offers several advantages over the conventional protocols provided by the manufacturers. Firstly, peptide losses are minimized using the iFASP method. Secondly, there is no loss in labeling efficiency by performing TMT-labeling in the ultrafiltration columns compared to the regular in-solution protocol; in fact, the labeling efficiency is slightly higher. Thirdly, we noted that changes to the well-established FASP protocol are minimal as the labeling can be carried out in the spin columns even in the presence of the standard ammonium bicarbonate solution. No substitution of this primary amine-containing solution, with tertiary amine-based buffers such as triethylammonium bicarbonate (TEAB), was necessary (as compared for TMT in Supplementary Table 1 and for iTRAQ in Supplementary Table 2), but can easily be done if needed or desired. Although the major part of this work was carried out with the TMT label, we also demonstrated compatibility with the iTRAQ reagent (Supplementary Table 2); considering the significant similarity of the TMT and iTRAQ-based derivatization reactions, we are confident that the TMT-derived conclusions are all applicable to iTRAQ as well. Thus, our iFASP method is a quick and simple method for labeling peptide samples for multiplexing experiments.
Supplementary Material
Acknowledgments
Funding Sources
This work is funded in part by the US National Institute of Health grants R01GM094844, R01HD020991, and U01DK082316.
Thanks to Oliver Serang, Waltraud Mair and Michelle Brook for comments. Access to Xenopus laevis was kindly provided by Marc Kirschner.
ABBREVIATIONS
- MS
mass spectrometry
- MS/MS
tandem mass spectrometry
- LC-MS/MS
liquid chromatography tandem mass spectrometry
- FASP
Filter-Aided Sample Preparation
- FDR
false discovery rate
- iTRAQ
isobaric tag for relative and absolute quantitation
- TMT
tandem mass tag
- XLB
Xenopus lysis buffer
- HCl
hydrogen chloride
- NaCl
sodium chloride
- PQD
pulsed Q-dissociation
- HCD
high-energy Collision Dissociation
- PSM
peptide spectral match
- TEAB
triethylammonium bicarbonate
- TCEP
tris(2-carboxyethyl)phosphine
- ABC
ammonium bicarbonate
Footnotes
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Supporting Information Available: This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Wisniewski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation method for proteome analysis. Nature methods. 2009;6(5):359–62. doi: 10.1038/nmeth.1322. [DOI] [PubMed] [Google Scholar]; (b) Zeng HH, Thompson RB, Maliwal BP, Fones GR, Moffett JW, Fierke CA. Real-time determination of picomolar free Cu(II) in seawater using a fluorescence-based fiber optic biosensor. Analytical chemistry. 2003;75(24):6807–12. doi: 10.1021/ac0345401. [DOI] [PubMed] [Google Scholar]
- 2.(a) Manza LL, Stamer SL, Ham AJ, Codreanu SG, Liebler DC. Sample preparation and digestion for proteomic analyses using spin filters. Proteomics. 2005;5(7):1742–5. doi: 10.1002/pmic.200401063. [DOI] [PubMed] [Google Scholar]; (b) Liebler DC, Ham AJ. Spin filter-based sample preparation for shotgun proteomics. Nature methods. 2009;6(11):785. doi: 10.1038/nmeth1109-785a. author reply 785–6. [DOI] [PubMed] [Google Scholar]
- 3.(a) Sun X, Jiang X. Combination of FASP and fully automated 2D-LC-MS/MS allows in-depth proteomic characterization of mouse zymogen granules. Biomedical chromatography: BMC. 2012 doi: 10.1002/bmc.2805. [DOI] [PubMed] [Google Scholar]; (b) Wisniewski JR, Mann M. Consecutive proteolytic digestion in an enzyme reactor increases depth of proteomic and phosphoproteomic analysis. Analytical chemistry. 2012;84(6):2631–7. doi: 10.1021/ac300006b. [DOI] [PubMed] [Google Scholar]
- 4.(a) Thompson A, Schafer J, Kuhn K, Kienle S, Schwarz J, Schmidt G, Neumann T, Johnstone R, Mohammed AK, Hamon C. Tandem mass tags: a novel quantification strategy for comparative analysis of complex protein mixtures by MS/MS. Analytical chemistry. 2003;75(8):1895–904. doi: 10.1021/ac0262560. [DOI] [PubMed] [Google Scholar]; (b) Ross PL, Huang YN, Marchese JN, Williamson B, Parker K, Hattan S, Khainovski N, Pillai S, Dey S, Daniels S, Purkayastha S, Juhasz P, Martin S, Bartlet-Jones M, He F, Jacobson A, Pappin DJ. Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol Cell Proteomics. 2004;3(12):1154–69. doi: 10.1074/mcp.M400129-MCP200. [DOI] [PubMed] [Google Scholar]
- 5.Nogueira FC, Palmisano G, Schwammle V, Campos FA, Larsen MR, Domont GB, Roepstorff P. Performance of isobaric and isotopic labeling in quantitative plant proteomics. J Proteome Res. 2012;11(5):3046–52. doi: 10.1021/pr300192f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.