Abstract
Social defeat (SD) induced stress causes physiological and behavioral deficits in rodents, including depression and anxiety-like behaviors, as well as memory impairment. Anxiolytic and mood elevating effects of physical exercise are also known. However, rescue effect of physical exercise in social defeat-induced anxiety, depression or memory impairment has not been addressed. Role of epigenetic mechanisms that potentially contribute to these rescue or protective effects are also not known. Present study investigated the effect of moderate treadmill exercise on anxiety-like behavior and memory function in rats subjected to SD using a modified version of the resident-intruder model for social stress (defeat). Changes in histone acetylation and histone-modifying enzymes were examined in hippocampus, amygdala and frontal cortex which are considered critical for anxiety, depression and cognition. Sprague Dawley rats were randomly assigned in four groups; control, exercised, social defeat, social defeat and exercise. At the end of the SD or control exposure lasting 30 min daily for 7 days, one group of SD rats was subjected to treadmill exercise for 2 weeks, whereas the other SD group was handled without exercise. Anxiety-like behavior tests and radial arm water maze test suggested that moderate treadmill exercise rescued social defeat induced anxiety-like behavior and memory impairment. Moreover, exercise normalized SD-induced increase in oxidative stress, most likely by adjusting antioxidant response. Our data suggests involvement of epigenetic mechanisms including histone acetylation of H3 and modulation of methyl-CpG-binding in the hippocampus that might contribute to the rescue effects of exercise in SD-induced behavioral deficits in rats.
Keywords: Social defeat, stress, treadmill, exercise, anxiety and depression, Physical exercise, Anxiety, Cognition, Oxidative stress
1. Introduction
Negative impact of chronic psychological stress on an individual’s physical and social performance as well as overall quality of life is well recognized (Cohen S and Wills TA 1985). While some negative effects of stress cause acute psychological reactions such as nervous breakdown, poor concentration, irritability and sleeplessness [1], others result into a chronic prolonged state of compromised mental health often leading to serious psychiatric disorders [2]. Actually, chronic stress is believed to contribute to anxiety disorders, depression [3, 4], and cognitive impairment [5–14]. Physical exercise is considered beneficial against stress, anxiety and depression, and also known to improve executive functioning and working memory [15, 16]. In fact, regular physical exercise is proposed as a neuroprotective strategy [17–19]. While antidepressant, anxiolytic and pro-cognitive effects of physical exercise are commonly accepted [20–22], the molecular basis for beneficial effects of exercise on stress-induced anxiety, depression and learning-memory impairment and mechanisms by which physical exercise alters brain function to enable neuroprotective properties are unclear.
Two major questions were addressed in this study. First, whether physical exercise rescues adverse behavioral consequences of stress in an animal model of social stress (defeat)? Second, reveal molecular pathways including oxidative stress and epigenetic mechanisms which potentially enable rescue of socially defeated phenotype. Social conflicts in humans are known to cause severe stress leading to serious psychological problems [23, 24]. Thus researchers have often utilized an ethologically relevant animal model of social stress (resident-intruder paradigm) to understand the etiology of stressor-related illnesses [25–28]. In this model, social stress induces long-lasting, adverse physiological, behavioral and neuronal deficits, which seem to resemble certain human psychopathologies of depression and anxiety [29]. Socially defeated animals also exhibit cognitive impairment [30]. This model involves aggressive encounters by a large, aggressive male rat (resident) toward a smaller male rat (intruder) [28]. Effect of physical exercise to rescue social defeat-induced deficits, have not been examined, therefore, using the social defeat model of stress, we have investigated exercise mediated behavioral and biochemical effects in rats.
Relevant to this, oxidative stress has been implicated in the response to stress [31] and in the pathogenesis of psychiatric diseases [32]. Earlier, we have published causal role of oxidative stress in anxiety-like behavior and cognitive impairment in rats and preventive effect of moderate treadmill exercise on oxidative stress–induced anxiety-like behavior [33, 34]. Moreover, social defeat has been shown to alter brain-derived neurotrophic factor (BDNF) [25] in the hippocampus [35] and exercise is known to exert a strong influence on brain plasticity and cognition, through mechanisms centered on the action of BDNF involving epigenetic mechanisms. Indeed, recent studies have found changes in modifications at specific gene promoter regions in association with social defeat [36, 37] and exercise has been related to chromatin remodeling, specifically the induction of histone acetylation through modulation of histone deacetylases (HDAC) and histone acetyltransferases (HAT) activities. Furthermore, increased acetylation at specific promoter regions of brain-derived neurotrophic factor has been associated with a reversal of depressive-like behavior following electroconvulsive shock therapy, while overall increased acetylation in the nucleus accumbens has been associated with depressive-like symptoms in mice [37, 38]. Finally, oxidative stress is known to regulate histone acetylation/deacetylation [39, 40] and also reported to lead to the release of pro-inflammatory cytokine interleukin-8 (IL-6) in human alveolar epithelial cells via modulation of histone acetylation/deacetylation processes [41]. With these previous studies in mind, using the social defeat model of social stress, we investigated the association between oxidative stress, inflammation, histone H3 acetylation/deacteylation, methyl-CpG-binding protein (MeCP)-2 levels and BDNF levels, in the hippocampus, the amygdala, and the frontal cortex, brain areas implicated in the symptomatology of anxiety, depression and cognition both in rodent models and humans [42–47].
2. Materials and Methods
2.1. Animals
Male Sprague Dawley rats (275–300 g) were used as controls or intruders, and male Long-Evans (LE), retired breeders (400–500 g) served as residents (Charles River, Wilmington, MA). Rats were singly housed with a 12-h light, 12-h dark cycle (lights on at 0600 h) in a climate-controlled room with food and water provided ad libitum. All experiments were conducted in accordance with the NIH guidelines using approved protocols from the University of Houston Animal Care Committee.
2.2. Social Defeat Model
2.2.1. Experimental design
The social defeat model used in the present study was modified from the resident-intruder model originally developed by Miczek [48]. Rats were randomly assigned to either a social defeat or control group [28, 49, 50]. This paradigm consisted of 7 encounters, carried out for 7 consecutive days, with an aggressive male Long Evans (LE) rat. Each intruder (Sprague Dawley) was defeated by six different resident LE rats. [50, 51]. A typical social defeat was observed by intruder defeat, indicated by the intruder surrendering or acquiring a supine position for approximately 3 sec. After defeat, a perforated Plexiglas partition was placed in the cage to avoid direct physical contact between the LE and intruder. The Plexiglas partition with holes allowed intense visual, auditory, and olfactory interactions for the remainder of the 30-min session. If a resident struggled to defeat the intruder for 10 min, rats were separated with the Plexiglas partition for the remainder of the 30-min session. Controls were placed behind a Plexiglas partition in a fresh cage for 30 min daily. Rats were returned to their home cage after each social defeat session, and body weight was recorded on days 1 and 8. All Sprague Dawley rats were used for behavioral assessment before sacrifice.
2.3. Moderate treadmill exercise
The rats were subjected to treadmill exercise on a motorized rodent treadmill (Columbus Instruments, Columbus, OH). The treadmills are equipped with bars that deliver a mild electric shock (0–0.5 mA with a 1–2 s inter-pulse interval) when the rat stops running. This is a very mild shock which causes no stress and minimal discomfort to the animals but serves as a cue for the rat to continue running [33]. The rats were subjected to the following treadmill exercise protocol for a total of 2 weeks: 30 min daily for 2 weeks. For the first week the animals ran at 10meters/min for 30 min, followed by 15meters/min for the second week [33]. The rats were given a rest period of 5 min after 15 min of exercise in each setting.
2.4. Tests for Anxiety-Like Behavior
2.4.1. Open Field (OF) activity
Rats were placed in the center of the OF (60×40 cm) and left free to explore the arena for 15 min and movement quantified using Opto-Varimex Micro Activity Meter v2.00 system (Optomax, Columbus Instruments; OH) as previously published by us [33, 34]. Total activity, ambulatory activity, distance covered and fecal boli were examined.
2.4.2. Light-Dark (LD) exploration
Time spent in light is considered as a measure of anxiety-like behavior. The light-dark box consisted of a light and a dark compartment separated with a single opening for passage from one compartment to the other and total time spent in the lit area was recorded [33, 34].
2.4.3. Elevated plus-maze
A standard rat elevated plus-maze with 43 cm arms extending from a 10 cm central area was obtained from Med Associates Inc., (St. Albans, VT). The arms of the maze were approximately 90 cm above the floor. The rat’s movements were tracked manually. The observer was blinded to the group classification to avoid bias. Each session was started by placing the rat in the central area facing the open arms of the maze and lasted 5 min. In between rats, the maze was wiped down with alcohol. The amount of time the rat spent in the open arms was noted [52].
2.5. Memory Function
2.5.1. Radial Arm Water Maze (RAWM)
The RAWM procedures were done as previously published by us [53, 54]. Basically, the apparatus consisted of a black circular pool filled with water containing six swim paths in a dimly lit room. Each rat was randomly assigned a goal arm which contains a hidden black platform near the end of the arm. The rats were randomly released at an arm different from the goal arm, allowed to swim and locate the platform which is submerged 1 cm under water. The rats were allowed 1 minute for each learning trial or memory test. An error was counted when the rat entered more than halfway into an arm other than the goal arm or if the rat entered more than half of the goal arm but failed to approach the platform. Number of errors ranged from 1–7, as the rat can only swim into 7 arms within 1 minute. If the rat failed to locate the platform within 1 minute, the rat was manually guided to the platform and was scored with 7 errors. Upon reaching the platform, the rat was allowed 15 seconds rest before the next trial began.
2.5.2. Short-term and long-term memory tests
The rats were subjected to the first set of six learning trials (trials # 1–6) followed by a five min rest period and then another set of six learning trials (trials # 7–12) and tested for short-term memory 30 min after the end of 12th trial. The rats were subjected to learning trials (trials #1–12) as above. At the end of the 12th trial, the rats were returned to their home cages and 24 h later subjected to long-term memory test.
Brain Dissections and Preparation of Homogenates
Experimental and control rats were anesthetized using mild anesthesia (Isoflurane, #57319-479-06, Phoenix Pharmaceuticals) immediately after anxiety behavior tests. The brains were quickly removed and hippocampus, frontal cortex and amygdala were identified according to the atlas of Paxinos and Watson [55] and isolated and quickly frozen in liquid nitrogen and stored at −80°C until analysis as previously published by us [33, 56, 57].
2.6.1. Western Blotting
Equal amounts of brain tissue homogenate proteins diluted with 4X laemmli sample buffer were subjected to SDS-polyacrylamide gel electrophoresis (PAGE) and western blotting as previously published by us [34]. Primary antibody dilutions used were as follows; GLO-1 (1:200 dilution; Abcam, Cambridge, MA – Cat No: ab81461), GSR-1 (1:100 dilution), Cu/Zn SOD (1:1000 dilution; Cat No: 07-403), Mn SOD (1:1000 dilution; Cat No: 06-984), Acetyl-H3 (1:500 dilution; Cat No: 07-013), total-H3 (1:1000; Cat No: 05-499), HDAC5 (1:1000 dilution; Cat No:07-045) and MeCP-2 (1:1000 dilution; Cat No: 07-013), were from Millipore, Temecula, CA. CAMKIV (1:1000 dilution; Cat No: 4032S), p-p44/42 MAPK (1:200 dilution; Cat No: 9106S) and p44/42 MAPK (1:1000 dilution; 9107S) were from Cell Signaling technology, Danvers, MA. IL-6 (1:1000 dilution, Cat No: ARC0062) was from Invitrogen, Grand Island, NY. BDNF (1:1000 dilution; Cat No: sc-546), p-CREB (1:200 dilution; Cat No: sc-7978), t-CREB (1:1000 dilution; Cat No: sc-58) and β-actin (1:1000 dilution; Cat No: sc-47778) were from Santacruz biotechnology, Santacruz, CA. The membranes were incubated with respective antibody for 1 h, followed by incubation with an anti-rabbit horseradish peroxidase (HRP)-conjugated secondary antibody (1:1000), anti-rat horseradish peroxidase (HRP)-conjugated secondary antibody (1:1000) or anti-mouse HRP-linked secondary antibody (1:1000) at room temperature for 1 h. The images of immunoblots were captured by a Fluorchem 8900 imaging system with intensity of each immunoreactive band determined using Alpha Ease FC 4.0 (Alpha Innotech Corp., San Leandro, CA) that were normalized to β-actin protein loading control.
2.6. Indices of oxidative stress
8-isoprostane levels in serum and urine were measured using EIA kit (Cayman, Ann Arbor, MI). Isoprostanes are a family of eicosanoids of non-enzymatic origin produced by the random oxidation of tissue phospholipids by oxygen radicals [33]. The OxyBlot™ Protein Oxidation Detection Kit (EMD Millipore Corp. #S7150) was used for immunoblot detection of carbonyl groups introduced into proteins by oxidative reactions. Equal amount (20 μg) of protein homogenate from different brain regions (prepared as indicated above) were subjected to this kit based reaction following manufacturer’s instructions, which allows detection of carbonylation of proteins in the homogenates using western blotting method.
2.7. Corticosterone measurement
Serum corticosterone levels, released in response to stress and anxiety [58] were measured using an EIA based kit (cat#500651, Cayman Chem. Co., Ann Arbor, MI) per manufacturer’s instructions.
2.8. Statistical Analysis
Data are expressed as mean ± SEM. Significance was determined by one-way ANOVA and Tukey’s post-hoc test (GraphPad Software, Inc. San Diego, CA). A value of p< 0.05 was considered significant.
3. Results
3.1. General parameters
Control groups including sedentary (CON) and exercise alone (EX) gained similar amount of weight while the SD and SD+EX animals gained less weight during the 7-day social defeat protocol [Control, EX, SD and SD+EX (gain in body weight in g/7days): 20 ± 5.5, 21.2 ± 6.5, 5.5 ± 2.5 and 8.5 ± 3.5, F(3,36) = 9.33, p<0.05] (Fig 2A). Food intake during 7-day social defeat protocol was not different between control groups including, sedentary and EX alone when compared to SD and SD+EX rats [Control, SD and SD+EX (g/rat/day): 25.3 ± 2.1, 25.4 ± 1.9, 23.3 ± 3.3 and 27 ± 1.6, F(3,36) = 2.91, p<0.05] (Fig. 2B). However, daily water intake increased in SD and SD+EX [Control, EX, SD and SD+EX (ml/rat/day): 31.4 ± 4.1, 37.4 ± 3.8, 51.8 ± 18.4 and 55.2 ± 15.4, F(3,36) = 2.45, p<0.05] rats when compared to controls (Fig. 2C).
Fig. 2. General body parameters.
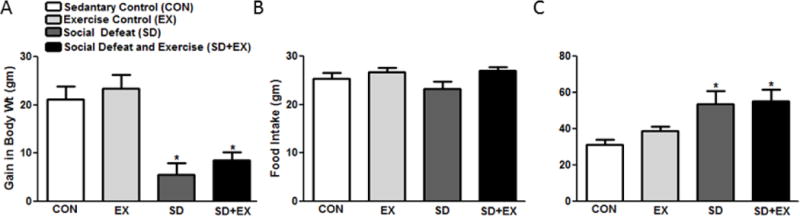
Examination of general parameters including gain in body weight (A), food (B) and water (C) intake was measured. Four groups of male Sprague-Dawley rats were utilized in this study. Group 1: control (sedentary), group 2: exercised, group 3: social defeat; group 4: social defeat and exercise. Bars are means ± SEM, n = 10 rats/group. *significantly different from control (sedentary and exercise alone) rats, #significantly different from SD (social defeat) rats, p<0.05.
3.2. Anxiety-like behavior tests
In light-dark test, a rat is exposed to a novel environment with protected (dark) and unprotected (light) areas. Unwillingness to explore the lit area and willingness to spend more time in the dark during a 5-min test session is indicative of high anxiety-like behaviors. Control rats spent more time (sec) in the light compartment (CON: 78.6.3 ± 16.9, EX: 97.2 ± 22.7), when compared to SD rats (41.2 ± 8.66, F(3,36) = 3.83, p<0.05). SD+EX rats spent significantly more time in the lit area (73.8 ± 17.1) as compared to SD rats (41.2 ± 8.66) (Fig. 3A). Elevated-plus maze model is based on rat’s dislike for open spaces. This aversion leads to the behavior termed as thigmotaxis, which means avoidance to open areas by restricting movements to enclosed spaces or to the edges of a confined space. Increased amount of time spent in the closed arms during a 5-min session is indicative of high anxiety-like behavior. Amount of time (sec) the control rats spent in the open arms (CON: 68.6 ± 11.1, EX: 89.0 ± 9.2, F(3,36) = 3.89, p<0.05) and SD+EX (104.6 ± 23.5) was significantly higher than the SD (46.3 ± 11.2) rats (Fig 3B).
Fig. 3. Examination of anxiety-like behavior using light-dark, elevated-plus maze and open-field tests.
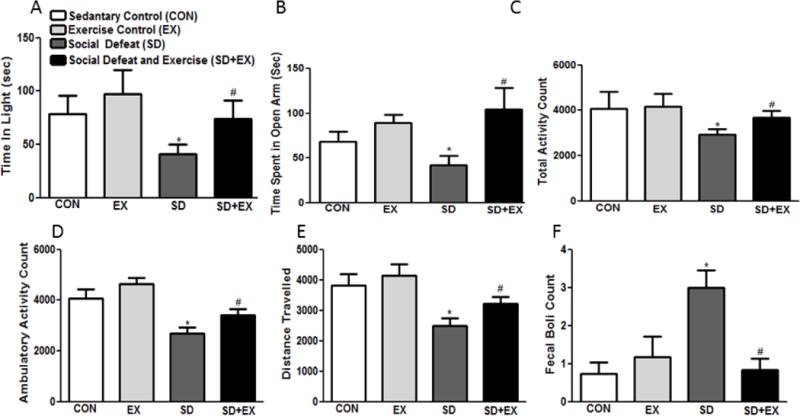
Light-dark test determined time spent in light (A), elevated-plus maze test determined time spent in open arms (B) while the open-field test determined total activity (C), ambulatory activity (D), distance traveled (E) and fecal boli (D). Bars are means ± SEM, n = 10 rats/group. *significantly different from control (sedentary and exercise alone) rats (p<0.05), #significantly different from SD rats (p<0.05) using one way ANOVA analysis.
Furthermore, socially defeated rats had lower total (CON: 4064 ± 758.6, EX: 4161 ± 579.0, SD: 2927 ± 242.5 and SD+EX: 3680 ± 298.9, F(3,36) = 6.13, p<0.05) (Fig. 3C) and ambulatory activity (CON: 4072 ± 371.1, EX: 4639 ± 247.4, SD: 2705 ± 212.5 and SD+EX: 3409 ± 233.0, F(3,36) = 5.13, p<0.05) (Fig. 3D) and covered lesser distance (CON: 3824 ± 369.0, EX: 4133 ± 395.5, SD: 2503 ± 249.4 and SD+EX: 3217 ± 234.0, F(3,36) = 6.55, p<0.05) than the control and SD+EX rats (Fig. 3E). Moreover, number of fecal boli of SD rats was significantly higher than controls and SD+EX rats (CON: 0.72 ± 0.30, EX: 1.1 ± 0.536, SD: 3.0 ± 0.462 and SD+EX: 0.83 ± 0.307, F(3,36) = 3.53, p<0.05) (Fig. 3F).
3.3. Memory function
Controls, SD and SD+EX rats on an average made comparable errors in the STM test, with each group making 0.5 ± 0.24, 0.6 ± 0.14, 0.5 ± 0.10 and 0.72 ± 0.33 errors, F(3,36) = 0.37, p<0.05 respectively. On the other hand, in the LTM, social defeat significantly increased the number of errors as compared to the control rats with control rats (CON and EX) making 0.25 ± 0.2 and EX: 0.6 ± 0.5 errors while SD rats made 2.6 ± 0.7 errors. SD+EX groups made 1.1 ± 0.3 error, F(3,36) = 3.43, p<0.05. Thus, social defeat did not significantly affect STM but the long-term memory consolidation was affected in these rats (Fig. 4 A, B).
Fig. 4. Examination of short-term and long term memory using radial arm water maze (RAWM) memory test.
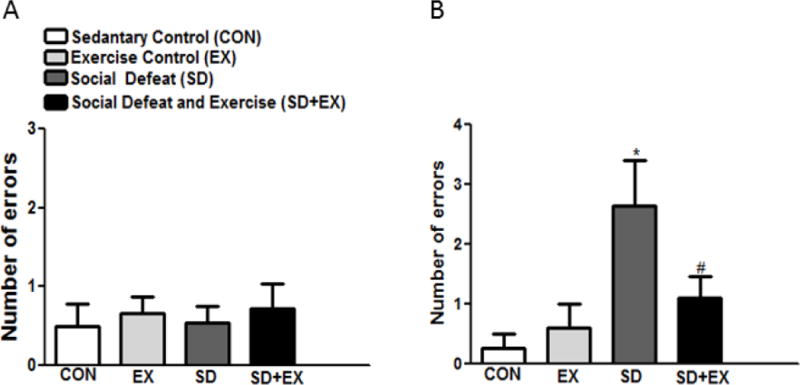
Short term (A) and long term (B) memory was assessed using a series of twelve RAWM trials. Bars are means ± SEM, n = 10 rats/group. *significantly different from control (sedentary and exercise alone) rats (p<0.05), #significantly different from SD rats (p<0.05) using one way ANOVA analysis.
3.4. Markers of oxidative stress and antioxidant enzymes
Protein carbonylation was measured in oxidative stress susceptible brain areas, previously reported to be important for anxiety and learning-memory function [34, 56, 59]. Protein carbonylation significantly increased in the hippocampus of SD rats as compared to the two control groups (CON and EX), while the levels were not altered in the frontal cortex and amygdala (Table. 1). Interestingly, protein carbonylation in the hippocampus of SD+EX rats was significantly lower than SD rats.
Table 1.
Analysis of oxidative stress markers/antioxidant enzymes, proteins involved in neuroprotection and inflammation.
| Markers of Oxidative Stress/Antioxidant Enzymes | Hippocampus | Amygdala | Frontal Cortex | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Con | Ex | SD | SD + Ex | Con | Ex | SD | SD + Ex | Con | Ex | SD | SD + Ex | |
| Prate in Carbonylation (A.U. × 103) | 92.08±4.1 | 90.0±4.1 | 185.88±14.8* | 102.2±12.8# | 102.0±4.08 | 109.0±4.12 | 137.81±14.0* | 100.2±12.8# | 192.0±14.3 | 190.0±12.1 | 195.88±12.7 | 189.2±10.8 |
| GLO-1/β-actin | 1.08±0.09 | 1.11±0.02 | 0.76±0.08* | 1.18±0.1# | 0.98±0.04 | 1.06±0.1 | 0.76±0.05* | 0.88±0.04# | 0.75±0.01 | 0.88±0.03 | 0.75±0.06 | 0.66±0.04 |
| GSR-1/β-actin | 2.21±0.13 | 1.98±0.02 | 1.61±0.11* | 1.93±0.13# | 1.91±0.07 | 1.98±0.02 | 1.91±0.11 | 2.1±0.71 | 1.81±0.13 | 1.66±0.02 | 1.59±0.11 | 1.79±0.13 |
| Mn-SOD/β-actin | 0.77±0.05 | 0.68±0.07 | 0.27±0.02* | 0.65±0.01# | 0.82±0.05 | 0.88±0.05 | 0.83±0.02 | 0.87±0.01 | 0.55±0.01 | 0.43±0.01 | 0.39±0.03 | 0.44±0.06 |
| Cu-Zn SOD/β-actin | 0.52±0.09 | 0.55±0.03 | 0.33±0.04* | 0.45±0.03# | 0.62±0.12 | 0.67±0.12 | 0.53±0.04 | 0.63±0.04 | 0.45±0.08 | 0.44±0.06 | 0.28±0.08 | 0.56±0.08 |
Protein carbonylation was measured in the hippocampus, amygdala and the frontal cortex using the OxyBlot™ Protein Oxidation Detection Kit following manufacturer’s instructions. Protein levels of GLO-1/β-Actin, GSR-1/β-Actin, Cu-Zn SOD/β-Actin and Mn-SOD/β-actin were determined by Western blotting in the hippocampus, amygdala and the frontal cortex. Protein ratios were obtained by normalizing to loading control protein as indicated. Group 1: control (sedentary, CON), group 2: exercised (EX), group 3: social defeat (SD); group 4: social defeat and exercise (SD+EX).
significantly different from control (sedentary and exercise alone) rats (p<0.05),
significantly different from SD rats (p<0.05) using one way ANOVA analysis, n = 4–6 rats/group.
Protein expression levels of GLO-1, GSR-1, Cu-Zn SOD and Mn-SOD were normalized to the internal loading control β-actin and examined in the hippocampus, amygdala and frontal cortex. While Mn-SOD and Cu-Zn SOD protein expression levels decreased only in the hippocampus of SD rats, no change was observed in the frontal cortex and amygdala between all other groups. GLO-1 protein expression levels decreased in the hippocampus and amygdala, but not in the frontal cortex of SD rats, while the levels bounced back in SD+EX group. GSR-1 levels remained unchanged in all groups (Table. 1).
Plasma 8-isoprostane (Fig. 5) significantly increased in SD rats (SD: 41.0 ± 4.2) as compared to CON or EX rats (CON: 28.0 ± 1.8, EX: 28.6 ± 2.2), while SD+EX rats showed significantly reduced plasma 8-isoprostane levels (SD+EX: 24.0 ± 3.2) when compared to SD rats (41.0 ± 4.1). Thus two different markers of oxidative stress indicate that, socially defeated rats have higher oxidative stress than control rats and exercise alleviates this effect.
Fig. 5. Analysis of 8-isoprostane in plasma of rats.
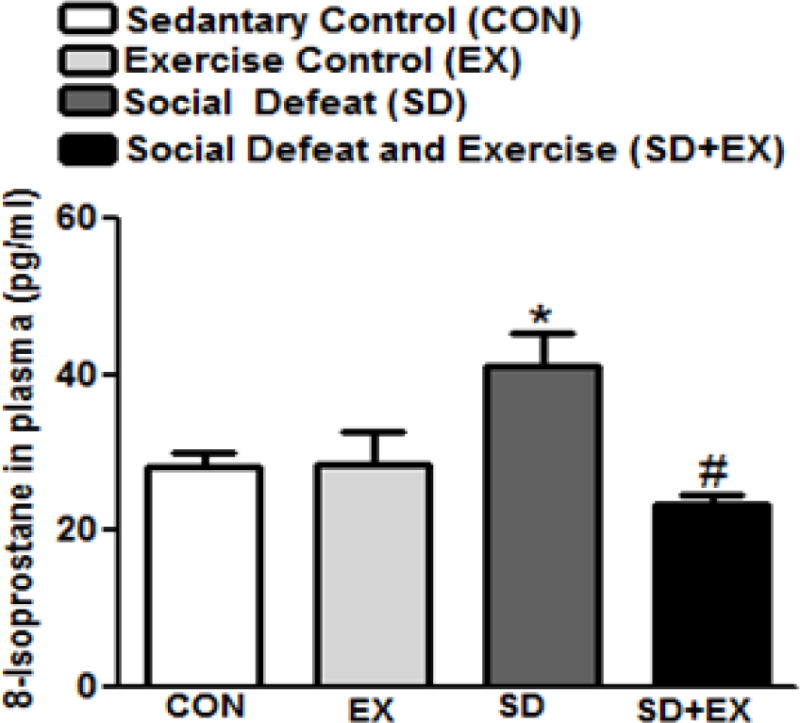
8-isoprostane was measured using EIA kit (516351; Cayman, Ann Arbor, MI). *significantly different from control (sedentary and exercise alone) rats (p<0.05), #significantly different from SD rats (p<0.05) using one way ANOVA analysis, n = 6–10 rats/group.
3.5. Plasma corticosterone
Plasma corticosterone levels significantly increased in SD (66.9 ± 6.4 ng/ml) rats when compared to the control rats (CON: 31.8 ± 3.1 ng/ml, EX: 30.3 ± 3.5 ng/ml), while SD+EX group exhibited significantly reduced levels (54.31 ± 0.2 ng/ml) when compared to SD rats (Fig. 6).
Fig. 6. Analysis of corticosterone levels in plasma of rats.
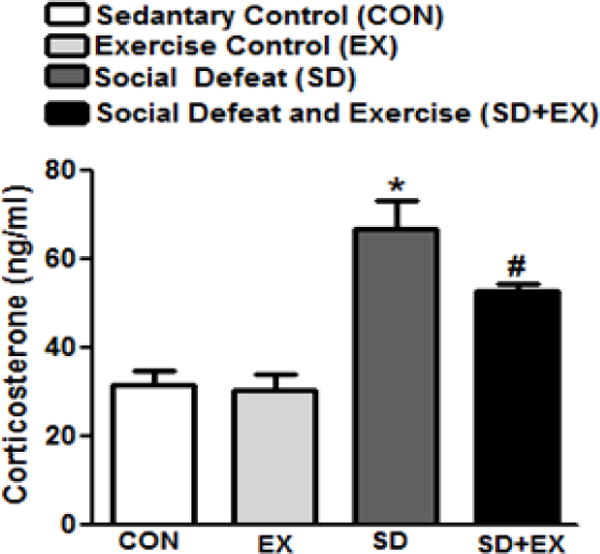
Plasma corticosterone, released in response to stress was measured using an EIA based kit (cat#500651, Cayman Chem. Co., Ann Arbor, MI) per manufacturer’s instructions. *significantly different from control (sedentary and exercise alone) rats (p<0.05), #significantly different from SD rats (p<0.05) using one way ANOVA analysis, n = 10 rats/group.
3.6. Molecules involved in memory consolidation and inflammatory markers
Social defeat significantly decreased the protein levels of BDNF, p-CREB/total CREB and CAMKIV in the hippocampus when compared to control rats (CON and EX). The levels of all three proteins significantly increased in the hippocampus of SD+EX rats when compared to SD rats. The levels of these proteins remained unchanged in the frontal cortex and amygdala (Table 2).
Table 2.
Analysis of proteins involved in neuroprotection and inflammation.
| Molecules involved in Memory consolidation | Hippocampus | Amygdala | Frontal Cortex | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Con | Ex | SD | SD + Ex | Con | Ex | SD | SD + Ex | Con | Ex | SD | SD + Ex | |
| BDNF/β-actin | 1.25+0.03 | 1.37±0.01 | 0.55±0.04* | 1.07+0.01# | 1.31±0.02 | 1.40±0.04 | 0.91±0.02 | 1.10±0.07 | 1.28±0.01 | 1.31±0.02 | 1.22±0.02 | 0.97±0.07 |
| p-CREB/t-CREB | 0.48±0.05 | 0.43±0.03 | 0.22±0.01* | 0.38±0.03# | 0.21±0.03 | 0.33±0.01 | 0.16±0.01 | 0.14±0.02 | 0.34±0.03 | 0.40±0.05 | 0.31±.05 | 0.30±.01 |
| CAMKIV/β-actin | 0.55±0.02 | 0.53±0.02 | 0.20±0.03* | 0.41±0.02# | 0.42±0.02 | 0.43±0.04* | 0.36±0.07 | 0.34±0.02 | 0.51±0.06 | 0.60±0.04 | 0.53±.03 | 0.50±.02 |
| Inflammatory Markers | ||||||||||||
| p-ERK[1/2)/t-ERK(1/2) | 0.23+0.04 | 0.20±0.03 | 0.57±0.04* | 0.38±0.01# | 0.20±0.02 | 0.22+0.03 | 0.60±0.03* | 0.41±0.01# | 0.33±0.02 | 0.30±0.03 | 0.37±0.02 | 0.4±0.01 |
| IL-6/β-actin | 0.44+0.01 | 0.42±0.02 | 0.80±0.01* | 0.58±0.04# | 0.50±0.01 | 0.42±0.02 | 0.60±0.01 | 0.58±0.04 | 0.54±0.02 | 0.42±0.02 | 0.90±0.03* | 0.68±0.03# |
Protein levels of BDNF/β-Actin, p-CREB/t-CREB, CAMKIV/β-Actin, p-ERK1/2/t-ERK1/2 and IL-6/β-Actin were determined by Western blotting in the hippocampus, amygdala and the frontal cortex. Protein ratios were obtained by normalizing to loading control/total protein as indicated. Group 1: control (sedentary, CON), group 2: exercised (EX), group 3: social defeat (SD); group 4: social defeat and exercise (SD+EX).
significantly different from control (sedentary and exercise alone) rats (p<0.05),
significantly different from SD rats (p<0.05) using one way ANOVA analysis, n = 4–6 rats/group.
Induction of social defeat stress caused ERK-1/2 activation (phospho ERK-1/2 normalized to total ERK-1/2 protein) in the hippocampus and amygdala but not in the frontal cortex (Table 2). SD+EX rats showed significantly lesser ERK1/2 activation when compared to SD rats. Moreover, ERK-1/2 activation was associated with upregulation of an inflammatory cytokine IL-6, in the cortex and the hippocampus of SD rats when compared to SD or EX rats (Table 2).
3.7. Histone H3 acetylation and histone deacteylase (HDAC5) levels
The protein levels of histone H3 acetylation and histone deacetylase (HDAC5) were assessed in the hippocampus, amygdala and frontal cortex of rats (Fig. 7). SD rats exhibited significantly reduced levels of H3 as compared to CON or EX rats in the hippocampus. SD+EX rats on the other hand showed significantly greater levels of H3, when compared to SD rats in the hippocampus (A–C). No significant changes were observed in the amygdala or the frontal cortex. HDAC 5 showed significantly higher levels in SD rats, when compared to CON or EX rats while the level was normalized in SD+EX group within the hippocampus. No significant changes were observed in the frontal cortex or amygdala. HDAC 5 showed significantly higher levels in SD rats when compared to CON or EX rats while the level was normalized in SD+EX group within the hippocampus (D–F). No significant changes were observed in the frontal cortex or amygdala.
Fig. 7. Analysis of proteins involved in histone acetylation and deacetylation.
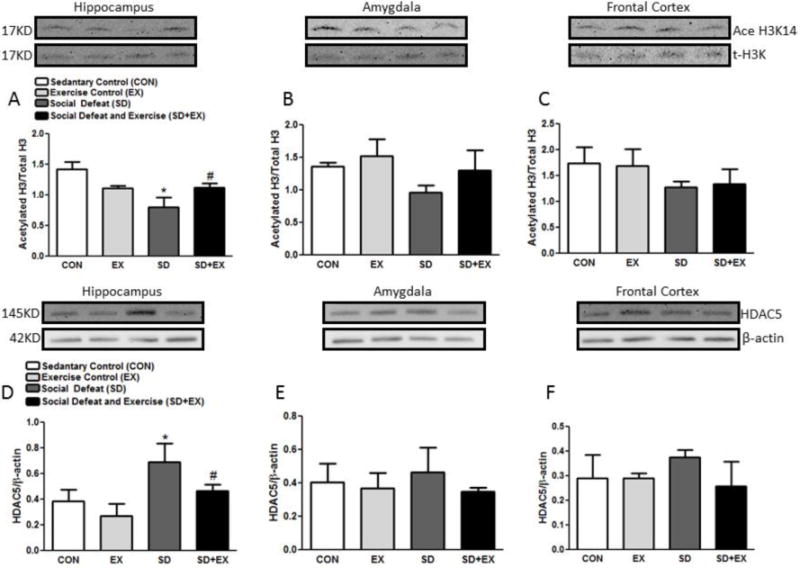
Protein levels of acetyl-H3/t-H3 and HDAC5/β-Actin were determined by Western blotting in the hippocampus, amygdala and the frontal cortex. Protein ratios were obtained by normalizing to loading control/total protein as indicated. *significantly different from control (sedentary and exercise alone) rats (p<0.05), #significantly different from SD rats (p<0.05) using one way ANOVA analysis, n = 4–6 rats/group.
3.8. Methyl-CpG-binding protein (MeCP)-2 levels
SD rats exhibited significantly reduced protein levels of MeCP-2 as compared to CON or EX rats in the hippocampus (Fig. 8). SD+EX rats on the other hand showed significantly greater levels of MeCP-2 when compared to SD rats in the hippocampus as well as in the amygdala (A–C). No significant changes were observed in the amygdala or the frontal cortex in SD rats but both hippocampus and amygdala showed marked increase in MeCP-2 levels.
Fig. 8. The level of methyl-CpG-binding protein (MeCP)-2.
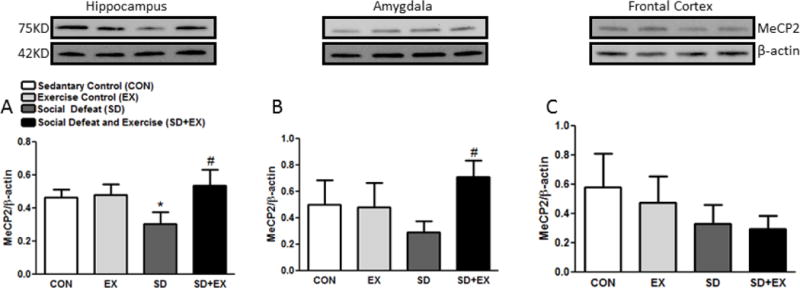
The protein level of MeCP-2/β-Actin was measured in the hippocampus, amygdala and frontal cortex of rats by Western blotting (A–C). Protein ratios were obtained by normalizing to loading control β-Actin as indicated. *significantly different from CON and EX rats, #significantly different from SD rats (p<0.05) one way ANOVA analysis, n = 4–6 rats/group.
4. Discussion
Our results suggest that social defeat-mediated stress increase anxiety-like behavior of rats assessed via LD, EPM and OF behavior tests. Display of increased anxiety-like behavior is indicated by reduced time spent by SD rats in the lit area of the LD box, and reduced time spent in the open-arms of the EPM apparatus. Reduced ambulatory activity, total activity and less distance travelled in the OF arena by SD rats also indicates heightened anxiety-like behavior. Greater number of fecal boli of SD rats also indicates high anxiety-like behavior. Interestingly, SD+EX rats did not show anxiety-like behavior as their anxiety levels were comparable to that of control rats, suggesting that treadmill exercise had a protective effect on anxiety-like behavior. It is possible that SD rats were able to better cope with social defeat-induced stress when subjected to treadmill exercise and their SD-induced anxious phenotype was recused with moderate treadmill exercise intervention. Previously, moderate treadmill exercise regimen used by us, also resulted in prevention of anxiety-like behavior in sleep-deprived rats [34]. Others also have reported anxiolytic effect of treadmill as well as wheel running in rats and several human studies have indicated anxiolytic effects of exercise [60, 61]. However, some have observed either no change or have reported anxiety-inducing behavior with exercise [34, 62, 63]. The divergent results are most likely due to the different regimens of exercise used and different anxiety tests employed and different parameters assessed. Furthermore, social defeat induced long-term memory deficits observed in SD rats also were rescued with moderate treadmill exercise. Beneficial effects of physical exercise on cognitive function in humans as well as in laboratory animals [64–67] are known and impairment of learning and memory processes has been demonstrated by many studies using different stressors [68]. Exposure to chronic restraint stress in rats and psychosocial stress in humans is known to alter cognitive functions and also has been linked to the pathophysiology of many disorders [69]. While protective effect of exercise on stress-induced deficits are convincing, reports that exercise does not have significant protective effects on memory deficit in stressed rats also exist [70, 71].
Overall, our data is in agreement with previous reports which support that exercise improves anxiety [72, 73], depression [74–78], cognitive function [79–83] and overall mental well-being [84, 85].
Consistent with previous findings [28], socially defeated rats did not attain normal rodent weight gain profile and exercise did not contribute to any weight gain in the SD+EX group. All rats consumed comparable amount of diet, but SD and SD+EX rats drank more water. There is no clear consensus on the effect of exercise on body weight gain and food intake, with some studies reporting that exercise promotes reduced body weight gain and decreased food intake [86, 87], while others suggesting no effect of exercise on body weight but report increased food intake as a consequence of exercise [88]. Our studies fit well with that of Applegate et al (1982) and others [86, 87, 89].
Elevated stress indicated by increased plasma corticosterone levels in socially defeated rats was markedly reduced in SD+EX rats, suggesting protective effect of treadmill exercise on SD-induced stress. Earlier, protective effect of treadmill exercise on increased corticosterone levels in sleep-deprived rats has been reported [34]. Furthermore, role of oxidative stress in the protective effects of treadmill exercise is also known [33, 34]. Moderate exercise is known to cause adaptation of brain antioxidant system by increasing its resistance to oxidative stress [33, 90, 91], while exhaustive exercise is reported to enhance lipid peroxidation [56, 92, 93] and known to increase reactive oxygen species, leading to oxidative damage [94–96]. Relevant to this, we observed that increase in oxidative stress in SD rats was reversed when SD rats were subjected to treadmill exercise. The decline in antioxidant enzyme expression including GLO-1 in hippocampus and amygdala and GSR-1, Mn-SOD and Cu/Zn SOD in the hippocampus only, was normalized with exercise. This is in agreement with our recent reports where modulation of these antioxidant enzymes has been observed in specific brain areas including the hippocampus and the amygdala [97–99]. Increased oxidative stress is a result of reduced antioxidant response which most likely occurs due to diminished GLO-1, GSR-1, Mn SOD and Cu/Zn SOD protein expression. It seems reasonable to suggest that reduced levels of these antioxidant enzymes contribute to a failing antioxidant response which leads to an excessive accumulation of reactive oxygen/nitrogen species, leading to inflammation and cytotoxicity. This is in keeping with increased expression of IL-6 and mitogen-activated protein kinase ERK-1/2 observed in the hippocampus and cortex of SD rats. Reduced levels were normalized with exercise treatment. ERK-1/2–mediated increase in inflammatory markers is well known [100]. Exercise also normalized social defeat-induced decrease in the levels of CAMKIV, p-CREB/total CREB and BDNF protein levels in the hippocampus but not in the amygdala or the cortex. These observations are quite significant considering that social defeat has been shown to alter brain-derived neurotrophic factor (BDNF) [25] in the hippocampus [35] and exercise is known to exert a strong influence on brain plasticity and cognition, through epigenetic mechanisms centered on BDNF. Interestingly, treadmill exercise normalized SD-induced decreased histone H3 acetylation and also normalized SD-induced increase in HDAC5 protein levels in the hippocampus only. Furthermore, exercise also normalized SD-induced decreased MeCP2 protein levels in the hippocampus.
5. Conclusions
Present study demonstrates that social defeat stress-induced behavioral and cognitive impairments are rescued by moderate treadmill exercise. Additionally, moderate treadmill exercise activates a pathway which involves suppression of oxidative stress and inflammation. This is enabled perhaps viamodulation of histone H3 acetylation/deacetylation processes, as well as regulation of MeCP-2 and BDNF levels. SiRNA approaches will test the causality of each component in future studies.
Fig. 1. Schematic representation of the experimental plan.

Sprague Dawley rats were assigned into four groups; Group 1: control, group 2: exercised, group 3: social defeat; group 4: social defeat and exercise. At the end of the social defeat or control exposure, one group of socially defeated rats was subjected to treadmill exercise for 2 weeks (1st week – 10m/min for 30 minutes, 2nd week – 15m/min for 30 minutes) whereas the other socially defeated group was handled without the exercise).
Research Highlights.
Social defeat stress-induced behavioral and cognitive impairments are rescued by moderate treadmill exercise.
Moderate treadmill exercise suppresses social defeat-induced activation of oxidative stress and inflammation.
Moderate treadmill via histone H3 acetylation/deacetylation dependent MeCP-2 activation regulates BDNF expression.
Acknowledgments
Funding for this research was provided to S.S. by NIH 1R15MH0939-18-01A1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Statement of Interest
None
References
- 1.Basta M, Chrousos GP, Vela-Bueno A, Vgontzas AN. Chronic Insomnia and Stress System. Sleep medicine clinics. 2007;2:279–91. doi: 10.1016/j.jsmc.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGraw-Hill. Concise dictionary of modern medicine. 2002 [Google Scholar]
- 3.Kessler RC. The effects of stressful life events on depression. Annual review of psychology. 1997;48:191–214. doi: 10.1146/annurev.psych.48.1.191. [DOI] [PubMed] [Google Scholar]
- 4.Post RM. Transduction of psychosocial stress into the neurobiology of recurrent affective disorder. The American journal of psychiatry. 1992;149:999–1010. doi: 10.1176/ajp.149.8.999. [DOI] [PubMed] [Google Scholar]
- 5.Alzoubi KH, Srivareerat M, Aleisa AM, Alkadhi KA. Chronic caffeine treatment prevents stress-induced LTP impairment: the critical role of phosphorylated CaMKII and BDNF. Journal of molecular neuroscience: MN. 2013;49:11–20. doi: 10.1007/s12031-012-9836-z. [DOI] [PubMed] [Google Scholar]
- 6.Arnsten AF. Ameliorating prefrontal cortical dysfunction in mental illness: inhibition of phosphotidyl inositol-protein kinase C signaling. Psychopharmacology. 2009;202:445–55. doi: 10.1007/s00213-008-1274-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnsten AF, Rubia K. Neurobiological circuits regulating attention, cognitive control, motivation, and emotion: disruptions in neurodevelopmental psychiatric disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51:356–67. doi: 10.1016/j.jaac.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Burri A, Maercker A, Krammer S, Simmen-Janevska K. Childhood trauma and PTSD symptoms increase the risk of cognitive impairment in a sample of former indentured child laborers in old age. PloS one. 2013;8:e57826. doi: 10.1371/journal.pone.0057826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devilbiss DM, Jenison RL, Berridge CW. Stress-induced impairment of a working memory task: role of spiking rate and spiking history predicted discharge. PLoS computational biology. 2012;8:e1002681. doi: 10.1371/journal.pcbi.1002681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jonsdottir IH, Nordlund A, Ellbin S, Ljung T, Glise K, Wahrborg P, et al. Cognitive impairment in patients with stress-related exhaustion. Stress. 2013;16:181–90. doi: 10.3109/10253890.2012.708950. [DOI] [PubMed] [Google Scholar]
- 11.Ohman L, Nordin S, Bergdahl J, Slunga Birgander L, Stigsdotter Neely A. Cognitive function in outpatients with perceived chronic stress. Scandinavian journal of work, environment & health. 2007;33:223–32. doi: 10.5271/sjweh.1131. [DOI] [PubMed] [Google Scholar]
- 12.Ronnlund M, Sundstrom A, Sorman DE, Nilsson LG. Effects of perceived long-term stress on subjective and objective aspects of memory and cognitive functioning in a middle-aged population-based sample. The Journal of genetic psychology. 2013;174:25–41. doi: 10.1080/00221325.2011.635725. [DOI] [PubMed] [Google Scholar]
- 13.Schwabe L, Joels M, Roozendaal B, Wolf OT, Oitzl MS. Stress effects on memory: an update and integration. Neuroscience and biobehavioral reviews. 2012;36:1740–9. doi: 10.1016/j.neubiorev.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Shansky RM, Lipps J. Stress-induced cognitive dysfunction: hormone-neurotransmitter interactions in the prefrontal cortex. Frontiers in human neuroscience. 2013;7:123. doi: 10.3389/fnhum.2013.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carek PJ, Laibstain SE, Carek SM. Exercise for the treatment of depression and anxiety. International journal of psychiatry in medicine. 2011;41:15–28. doi: 10.2190/PM.41.1.c. [DOI] [PubMed] [Google Scholar]
- 16.Ruscheweyh R, Willemer C, Kruger K, Duning T, Warnecke T, Sommer J, et al. Physical activity and memory functions: an interventional study. Neurobiology of aging. 2011;32:1304–19. doi: 10.1016/j.neurobiolaging.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Lau YS, Patki G, Das-Panja K, Le WD, Ahmad SO. Neuroprotective effects and mechanisms of exercise in a chronic mouse model of Parkinson’s disease with moderate neurodegeneration. The European journal of neuroscience. 2011;33:1264–74. doi: 10.1111/j.1460-9568.2011.07626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCrate ME, Kaspar BK. Physical activity and neuroprotection in amyotrophic lateral sclerosis. Neuromolecular medicine. 2008;10:108–17. doi: 10.1007/s12017-008-8030-5. [DOI] [PubMed] [Google Scholar]
- 19.Souza LC, Filho CB, Goes AT, Fabbro LD, de Gomes MG, Savegnago L, et al. Neuroprotective Effect of Physical Exercise in a Mouse Model of Alzheimer’s Disease Induced by beta-Amyloid1-40 Peptide. Neurotoxicity research. 2013;24:148–63. doi: 10.1007/s12640-012-9373-0. [DOI] [PubMed] [Google Scholar]
- 20.Horne JA. A review of the biological effects of total sleep deprivation in man. Biological psychology. 1978;7:55–102. doi: 10.1016/0301-0511(78)90042-x. [DOI] [PubMed] [Google Scholar]
- 21.Salmon P. Effects of physical exercise on anxiety, depression, and sensitivity to stress: a unifying theory. Clinical psychology review. 2001;21:33–61. doi: 10.1016/s0272-7358(99)00032-x. [DOI] [PubMed] [Google Scholar]
- 22.Taylor AH. Physical activity and psychological well-being. Newyork: Routledge; 2000. Physical activity, anxiety, and stress; pp. 10–45. [Google Scholar]
- 23.Bjorkqvist K. Social defeat as a stressor in humans. Physiology & behavior. 2001;73:435–42. doi: 10.1016/s0031-9384(01)00490-5. [DOI] [PubMed] [Google Scholar]
- 24.Brousse G, Fontana L, Ouchchane L, Boisson C, Gerbaud L, Bourguet D, et al. Psychopathological features of a patient population of targets of workplace bullying. Occupational medicine. 2008;58:122–8. doi: 10.1093/occmed/kqm148. [DOI] [PubMed] [Google Scholar]
- 25.Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–8. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 26.Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 27.Miczek KA, Yap JJ, Covington HE., 3rd Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacology & therapeutics. 2008;120:102–28. doi: 10.1016/j.pharmthera.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wood SK, Walker HE, Valentino RJ, Bhatnagar S. Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: role of corticotropin-releasing factor. Endocrinology. 2010;151:1795–805. doi: 10.1210/en.2009-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bartolomucci A, Leopardi R. Stress and depression: preclinical research and clinical implications. PloS one. 2009;4:e4265. doi: 10.1371/journal.pone.0004265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu T, Guo M, Garza J, Rendon S, Sun XL, Zhang W, et al. Cognitive and neural correlates of depression-like behaviour in socially defeated mice: an animal model of depression with cognitive dysfunction. The international journal of neuropsychopharmacology/official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2011;14:303–17. doi: 10.1017/S1461145710000945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends in plant science. 2002;7:405–10. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- 32.Ng F, Berk M, Dean O, Bush AI. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. The international journal of neuropsychopharmacology/official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2008;11:851–76. doi: 10.1017/S1461145707008401. [DOI] [PubMed] [Google Scholar]
- 33.Salim S, Sarraj N, Taneja M, Saha K, Tejada-Simon MV, Chugh G. Moderate treadmill exercise prevents oxidative stress-induced anxiety-like behavior in rats. Behavioural brain research. 2010;208:545–52. doi: 10.1016/j.bbr.2009.12.039. [DOI] [PubMed] [Google Scholar]
- 34.Vollert C, Zagaar M, Hovatta I, Taneja M, Vu A, Dao A, et al. Exercise prevents sleep deprivation-associated anxiety-like behavior in rats: potential role of oxidative stress mechanisms. Behavioural brain research. 2011;224:233–40. doi: 10.1016/j.bbr.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 35.Czeh B, Abumaria N, Rygula R, Fuchs E. Quantitative changes in hippocampal microvasculature of chronically stressed rats: no effect of fluoxetine treatment. Hippocampus. 2010;20:174–85. doi: 10.1002/hipo.20599. [DOI] [PubMed] [Google Scholar]
- 36.Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nature neuroscience. 2006;9:519–25. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- 37.Tsankova NM, Kumar A, Nestler EJ. Histone modifications at gene promoter regions in rat hippocampus after acute and chronic electroconvulsive seizures. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:5603–10. doi: 10.1523/JNEUROSCI.0589-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Covington HE, 3rd, Maze I, LaPlant QC, Vialou VF, Ohnishi YN, Berton O, et al. Antidepressant actions of histone deacetylase inhibitors. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:11451–60. doi: 10.1523/JNEUROSCI.1758-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ito K, Hanazawa T, Tomita K, Barnes PJ, Adcock IM. Oxidative stress reduces histone deacetylase 2 activity and enhances IL-8 gene expression: role of tyrosine nitration. Biochemical and biophysical research communications. 2004;315:240–5. doi: 10.1016/j.bbrc.2004.01.046. [DOI] [PubMed] [Google Scholar]
- 40.Kang J, Zhang Y, Chen J, Chen H, Lin C, Wang Q, et al. Nickel-induced histone hypoacetylation: the role of reactive oxygen species. Toxicological sciences: an official journal of the Society of Toxicology. 2003;74:279–86. doi: 10.1093/toxsci/kfg137. [DOI] [PubMed] [Google Scholar]
- 41.Rahman I, Gilmour PS, Jimenez LA, MacNee W. Oxidative stress and TNF-alpha induce histone acetylation and NF-kappaB/AP-1 activation in alveolar epithelial cells: potential mechanism in gene transcription in lung inflammation. Molecular and cellular biochemistry. 2002;234–235:239–48. [PubMed] [Google Scholar]
- 42.Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Current opinion in neurobiology. 2001;11:240–9. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- 43.Nestler EJ, Gould E, Manji H, Buncan M, Duman RS, Greshenfeld HK, et al. Preclinical models: status of basic research in depression. Biological psychiatry. 2002;52:503–28. doi: 10.1016/s0006-3223(02)01405-1. [DOI] [PubMed] [Google Scholar]
- 44.Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barkus C, McHugh SB, Sprengel R, Seeburg PH, Rawlins JN, Bannerman DM. Hippocampal NMDA receptors and anxiety: at the interface between cognition and emotion. European journal of pharmacology. 2010;626:49–56. doi: 10.1016/j.ejphar.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bogdan R, Hariri AR. Neural embedding of stress reactivity. Nature neuroscience. 2012;15:1605–7. doi: 10.1038/nn.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burghy CA, Stodola DE, Ruttle PL, Molloy EK, Armstrong JM, Oler JA, et al. Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nature neuroscience. 2012;15:1736–41. doi: 10.1038/nn.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miczek KA. A new test for aggression in rats without aversive stimulation: differential effects of d-amphetamine and cocaine. Psychopharmacology. 1979;60:253–9. doi: 10.1007/BF00426664. [DOI] [PubMed] [Google Scholar]
- 49.Bhatnagar S, Vining C. Facilitation of hypothalamic-pituitary-adrenal responses to novel stress following repeated social stress using the resident/intruder paradigm. Hormones and behavior. 2003;43:158–65. doi: 10.1016/s0018-506x(02)00011-9. [DOI] [PubMed] [Google Scholar]
- 50.Bhatnagar S, Vining C, Iyer V, Kinni V. Changes in hypothalamic-pituitary-adrenal function, body temperature, body weight and food intake with repeated social stress exposure in rats. Journal of neuroendocrinology. 2006;18:13–24. doi: 10.1111/j.1365-2826.2005.01375.x. [DOI] [PubMed] [Google Scholar]
- 51.Golden SA, Covington HE, 3rd, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nature protocols. 2011;6:1183–91. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bert B, Fink H, Huston JP, Voits M. Fischer 344 and wistar rats differ in anxiety and habituation but not in water maze performance. Neurobiology of learning and memory. 2002;78:11–22. doi: 10.1006/nlme.2001.4040. [DOI] [PubMed] [Google Scholar]
- 53.Aleisa AM, Helal G, Alhaider IA, Alzoubi KH, Srivareerat M, Tran TT, et al. Acute nicotine treatment prevents REM sleep deprivation-induced learning and memory impairment in rat. Hippocampus. 2011;21:899–909. doi: 10.1002/hipo.20806. [DOI] [PubMed] [Google Scholar]
- 54.Alhaider IA, Aleisa AM, Tran TT, Alkadhi KA. Caffeine prevents sleep loss-induced deficits in long-term potentiation and related signaling molecules in the dentate gyrus. The European journal of neuroscience. 2010;31:1368–76. doi: 10.1111/j.1460-9568.2010.07175.x. [DOI] [PubMed] [Google Scholar]
- 55.Paxinos G, W C. The Rat Brain in Stereotaxic Coordinates. (Sixth) 1986 [Google Scholar]
- 56.Salim S, Asghar M, Chugh G, Taneja M, Xia Z, Saha K. Oxidative stress: a potential recipe for anxiety, hypertension and insulin resistance. Brain research. 2010;1359:178–85. doi: 10.1016/j.brainres.2010.08.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patki G, Solanki N, Atrooz F, Allam F, Salim S. Depression, anxiety-like behavior and memory impairment are associated with increased oxidative stress and inflammation in a rat model of social stress. Brain research. 2013;1539:73–86. doi: 10.1016/j.brainres.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arranz L, Guayerbas N, De la Fuente M. Impairment of several immune functions in anxious women. Journal of psychosomatic research. 2007;62:1–8. doi: 10.1016/j.jpsychores.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 59.Salim S, Asghar M, Taneja M, Hovatta I, Chugh G, Vollert C, et al. Potential contribution of oxidative stress and inflammation to anxiety and hypertension. Brain research. 2011;1404:63–71. doi: 10.1016/j.brainres.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Strohle A, Feller C, Onken M, Godemann F, Heinz A, Dimeo F. The acute antipanic activity of aerobic exercise. The American journal of psychiatry. 2005;162:2376–8. doi: 10.1176/appi.ajp.162.12.2376. [DOI] [PubMed] [Google Scholar]
- 61.Strohle A, Graetz B, Scheel M, Wittmann A, Feller C, Heinz A, et al. The acute antipanic and anxiolytic activity of aerobic exercise in patients with panic disorder and healthy control subjects. Journal of psychiatric research. 2009;43:1013–7. doi: 10.1016/j.jpsychires.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 62.Dishman RK, Dunn AL, Youngstedt SD, Davis JM, Burgess ML, Wilson SP, et al. Increased open field locomotion and decreased striatal GABAA binding after activity wheel running. Physiology & behavior. 1996;60:699–705. doi: 10.1016/0031-9384(96)00102-3. [DOI] [PubMed] [Google Scholar]
- 63.Fulk LJ, Stock HS, Lynn A, Marshall J, Wilson MA, Hand GA. Chronic physical exercise reduces anxiety-like behavior in rats. International journal of sports medicine. 2004;25:78–82. doi: 10.1055/s-2003-45235. [DOI] [PubMed] [Google Scholar]
- 64.Ang ET, Gomez-Pinilla F. Potential therapeutic effects of exercise to the brain. Current medicinal chemistry. 2007;14:2564–71. doi: 10.2174/092986707782023280. [DOI] [PubMed] [Google Scholar]
- 65.Berchtold NC, Chinn G, Chou M, Kesslak JP, Cotman CW. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience. 2005;133:853–61. doi: 10.1016/j.neuroscience.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 66.Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends in neurosciences. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- 67.Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends in neurosciences. 2007;30:464–72. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 68.Klenerova V, Kaminsky O, Sida P, Krejci I, Hlinak Z, Hynie S. Impaired passive avoidance acquisition in Sprague-Dawley and Lewis rats after restraint and cold stress. Behavioural brain research. 2002;136:21–9. doi: 10.1016/s0166-4328(02)00093-1. [DOI] [PubMed] [Google Scholar]
- 69.Kim JJ, Yoon KS. Stress: metaplastic effects in the hippocampus. Trends in neurosciences. 1998;21:505–9. doi: 10.1016/s0166-2236(98)01322-8. [DOI] [PubMed] [Google Scholar]
- 70.Radahmadi M, Alaei H, Sharifi MR, Hosseini N. The Effect of Synchronized Forced Running with Chronic Stress on Short, Mid and Long-term Memory in Rats. Asian journal of sports medicine. 2013;4:54–62. doi: 10.5812/asjsm.34532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Radahmadi M, Alaei H, Sharifi MR, Hosseini N. The effect of synchronized running activity with chronic stress on passive avoidance learning and body weight in rats. International journal of preventive medicine. 2013;4:430–7. [PMC free article] [PubMed] [Google Scholar]
- 72.Herring MP, O’Connor PJ, Dishman RK. The effect of exercise training on anxiety symptoms among patients: a systematic review. Archives of internal medicine. 2010;170:321–31. doi: 10.1001/archinternmed.2009.530. [DOI] [PubMed] [Google Scholar]
- 73.Petruzzello SJ, Landers DM, Hatfield BD, Kubitz KA, Salazar W. A meta-analysis on the anxiety-reducing effects of acute and chronic exercise. Outcomes and mechanisms. Sports medicine. 1991;11:143–82. doi: 10.2165/00007256-199111030-00002. [DOI] [PubMed] [Google Scholar]
- 74.Barbour KA, Blumenthal JA. Exercise training and depression in older adults. Neurobiology of aging. 2005;26(Suppl 1):119–23. doi: 10.1016/j.neurobiolaging.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 75.Blumenthal JA, Babyak MA, Doraiswamy PM, Watkins L, Hoffman BM, Barbour KA, et al. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosomatic medicine. 2007;69:587–96. doi: 10.1097/PSY.0b013e318148c19a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Craft LL, Perna FM. The Benefits of Exercise for the Clinically Depressed. Primary care companion to the Journal of clinical psychiatry. 2004;6:104–11. doi: 10.4088/pcc.v06n0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lawlor DA, Hopker SW. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomised controlled trials. Bmj. 2001;322:763–7. doi: 10.1136/bmj.322.7289.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Trivedi MH, Greer TL, Grannemann BD, Chambliss HO, Jordan AN. Exercise as an augmentation strategy for treatment of major depression. Journal of psychiatric practice. 2006;12:205–13. doi: 10.1097/00131746-200607000-00002. [DOI] [PubMed] [Google Scholar]
- 79.Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. The Cochrane database of systematic reviews. 2008:CD005381. doi: 10.1002/14651858.CD005381.pub2. [DOI] [PubMed] [Google Scholar]
- 80.Brisswalter J, Collardeau M, Rene A. Effects of acute physical exercise characteristics on cognitive performance. Sports medicine. 2002;32:555–66. doi: 10.2165/00007256-200232090-00002. [DOI] [PubMed] [Google Scholar]
- 81.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychological science. 2003;14:125–30. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 82.Etnier JL, Sibley BL. Physical activity and hormone-replacement therapy: interactive effects on cognition? Journal of aging and physical activity. 2004;12:554–67. doi: 10.1123/japa.12.4.554. [DOI] [PubMed] [Google Scholar]
- 83.Tomporowski PD. Effects of acute bouts of exercise on cognition. Acta psychologica. 2003;112:297–324. doi: 10.1016/s0001-6918(02)00134-8. [DOI] [PubMed] [Google Scholar]
- 84.Fox KR. The influence of physical activity on mental well-being. Public health nutrition. 1999;2:411–8. doi: 10.1017/s1368980099000567. [DOI] [PubMed] [Google Scholar]
- 85.Windle G, Hughes D, Linck P, Russell I, Woods B. Is exercise effective in promoting mental well-being in older age? A systematic review. Aging & mental health. 2010;14:652–69. doi: 10.1080/13607861003713232. [DOI] [PubMed] [Google Scholar]
- 86.Nance DM, Bromley B, Barnard RJ, Gorski RA. Sexually dimorphic effects of forced exercise on food intake and body weight in the rat. Physiology & behavior. 1977;19:155–8. doi: 10.1016/0031-9384(77)90173-1. [DOI] [PubMed] [Google Scholar]
- 87.Stevenson JA, Box BM, Feleki V, Beaton JR. Bouts of exercise and food intake in the rat. Journal of applied physiology. 1966;21:118–22. doi: 10.1152/jappl.1966.21.1.118. [DOI] [PubMed] [Google Scholar]
- 88.Oscai LB. The role of exercise in weight control. Exercise and sport sciences reviews. 1973;1:103–23. [PubMed] [Google Scholar]
- 89.Applegate EA, Upton DE, Stern JS. Food intake, body composition and blood lipids following treadmill exercise in male and female rats. Physiology & behavior. 1982;28:917–20. doi: 10.1016/0031-9384(82)90214-1. [DOI] [PubMed] [Google Scholar]
- 90.Gessa GL, Pani L, Fadda P, Fratta W. Sleep deprivation in the rat: an animal model of mania. European neuropsychopharmacology: the journal of the European College of Neuropsychopharmacology. 1995;5(Suppl):89–93. doi: 10.1016/0924-977x(95)00023-i. [DOI] [PubMed] [Google Scholar]
- 91.Halliwell B, G J. Free Radicals in Biology and Medicine. Clarendon Press; Oxford: 2006. [Google Scholar]
- 92.Gopalakrishnan A, Ji LL, Cirelli C. Sleep deprivation and cellular responses to oxidative stress. Sleep. 2004;27:27–35. doi: 10.1093/sleep/27.1.27. [DOI] [PubMed] [Google Scholar]
- 93.Silva RH, Abilio VC, Takatsu AL, Kameda SR, Grassl C, Chehin AB, et al. Role of hippocampal oxidative stress in memory deficits induced by sleep deprivation in mice. Neuropharmacology. 2004;46:895–903. doi: 10.1016/j.neuropharm.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 94.Pokk P, Liljequist S, Zharkovsky A. Ro 15-4513 potentiates, instead of antagonizes, ethanol-induced sleep in mice exposed to small platform stress. European journal of pharmacology. 1996;317:15–20. doi: 10.1016/s0014-2999(96)90061-8. [DOI] [PubMed] [Google Scholar]
- 95.Suchecki D, T PA, Tufik S. Hormonal and behavioral responses of paradoxical sleep-deprived rats to the elevated plus maze. Journal of neuroendocrinology. 2002;14:549–54. doi: 10.1046/j.1365-2826.2002.00812.x. [DOI] [PubMed] [Google Scholar]
- 96.van Hulzen ZJ, Coenen AM. Paradoxical sleep deprivation and locomotor activity in rats. Physiology & behavior. 1981;27:741–4. doi: 10.1016/0031-9384(81)90250-x. [DOI] [PubMed] [Google Scholar]
- 97.Allam F, Dao AT, Chugh G, Bohat R, Jafri F, Patki G, et al. Grape powder supplementation prevents oxidative stress-induced anxiety-like behavior, memory impairment, and high blood pressure in rats. The Journal of nutrition. 2013;143:835–42. doi: 10.3945/jn.113.174649. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 98.Chugh G, Asghar M, Patki G, Bohat R, Jafri F, Allam F, et al. A High-Salt Diet Further Impairs Age-Associated Declines in Cognitive, Behavioral, and Cardiovascular Functions in Male Fischer Brown Norway Rats. The Journal of nutrition. 2013 doi: 10.3945/jn.113.177980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Patki G, Allam FH, Atrooz F, Dao AT, Solanki N, Chugh G, et al. Grape powder intake prevents ovariectomy-induced anxiety-like behavior, memory impairment and high blood pressure in female wistar rats. PloS one. 2013 doi: 10.1371/journal.pone.0074522. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shin JA, Lee KE, Kim HS, Park EM. Acute Resveratrol Treatment Modulates Multiple Signaling Pathways in the Ischemic Brain. Neurochemical research. 2012 doi: 10.1007/s11064-012-0858-2. [DOI] [PubMed] [Google Scholar]


