Abstract
Objective
We sought to examine the therapeutic efficacy of motor cortex stimulation (MCS) in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated macaques, and to characterize therapeutic differences with varying modes, frequencies, and durations of stimulation.
Methods
MCS was delivered at currents below motor threshold and at frequencies between 5Hz and 150Hz through epidural electrodes over primary motor cortex. The animals were studied on and off MCS using video analysis, activity logging, and food retrieval tasks. Animals were examined using two different stimulation protocols. The first protocol consisted of one hour of MCS therapy daily. The second protocol exposed the animal to continuous MCS for over 24 hours with at least 2 weeks between MCS therapy.
Conclusions
Daily MCS revealed no consistent change in symptoms, but MCS at two-week intervals resulted in significant increases in activity. Effects of biweekly MCS disappeared, however, within 24 hours of the onset of continuous MCS. In this study, MCS only temporarily reduced the severity of MPTP-induced parkinsonism.
Keywords: Parkinson’s Disease, MPTP, Cortical Stimulation, Non-human primate, Basal Ganglia, Primary Motor Cortex
INTRODUCTION
Long-term medical management of Parkinson’s disease (PD) is limited by reductions over time in drug efficacy and increased incidence of side-effects such as dyskinesias 24. Although surgical treatments such as pallidotomy and deep brain stimulation have proven therapeutic efficacy13,14, these treatments are not recommended for all patients due to their complex and invasive natures11.
The primary motor cortex (PMC) has received recent attention as a potential therapeutic target for PD. Some studies of repetitive transcranial magnetic stimulation (rTMS) of the PMC have reported symptomatic improvement in PD patients20, though this effect has not been substantiated uniformly8. Some case reports of patients undergoing low-frequency MCS have also described clinical improvement3,18,19. Short bouts of high-frequency MCS at two-week intervals have been reported to yield substantial reductions of symptoms in MPTP-treated baboons6. Put together, these observations raise the novel possibility that a global amelioration of parkinsonian symptoms can be produced by unilateral stimulation of a small portion of the PMC at intensities subthreshold to those required to evoke movement. Unfortunately, past animal reports lack details on the long-term effects of MCS and the effects of variations in stimulation polarity and frequency. We sought to clarify the effects of MCS in MPTP-treated primates blinded to treatment, particularly with respect to the mode and duration of MCS.
MATERIALS AND METHODS
Animal Model and Implantation Surgery
Three juvenile monkeys (M. fascicularis, 2.8 kg, 3.6 kg, and 4.5 kg) were used. All procedures involving animals were approved by the Institutional Animal Care and Use Committee.
The overlesioned hemiparkinsonian model2,17 was used to induce bilateral lesions of the dopaminergic (DA) system without completely disabling the animals. In short, animals received initial unilateral intracarotid artery infusions of MPTP (right hemisphere, 0.55 mg/kg), followed by a course of escalating intramuscular (IM) doses. All animals received 6 IM doses spread evenly over two weeks (3 doses at 0.35 mg/kg, followed by 3 at 0.55 mg/kg). In two animals that continued to show relatively mild symptoms, additional doses were administered during the third week (1 and 2 doses each at 0.75 mg/kg). In the months following the course of MPTP administration, the animals displayed stable symptoms of severe parkinsonism including flexed posture, a general paucity of spontaneous movement and frequent freezing (akinesia), bradykinesia, and increased limb rigidity. Due to the unilateral intracarotid artery infusions, these symptoms were more severe in the left limbs. All animals tended to turn in the clockwise direction and all showed evidence of a mild left hemi-neglect consistent with previous reports1. Symptom severity was rated regularly using a scale developed by Schneider et al.23, which includes numerical scores for limb and full body movement, manual dexterity, tremor, and other behaviors associated with parkinsonism in monkeys. All 3 animals had scores indicative of severe parkinsonism throughout the period of testing described here [mean Schneider scores = 37, 38, and 45; on a range from 0 (<10 being considered asymptomatic) to 53 (maximal severity)]. Further indicative of classic parkinsonism, all three animals responded to dopamine replacement therapy (L-DOPA methyl ester, IM at 5 mg/kg, with 10 mg/kg benserazide) with a transient but marked increase in mobility (see below and Fig. 1B), increased limb use, reduced rigidity, and a tendency to turn in the counterclockwise direction.
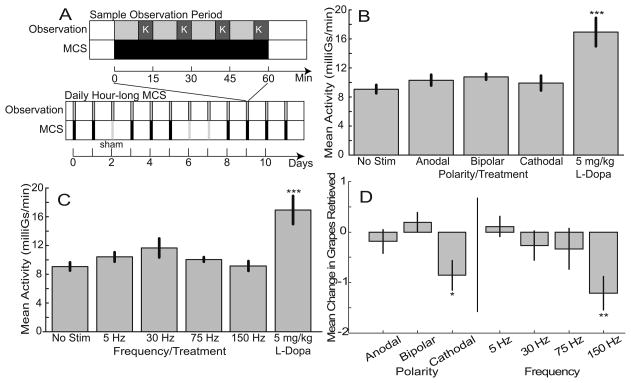
Fig. 1.
Daily MCS failed to improve general akinesia or Klüver task performance. (A) Organization of hour-long observation periods and representative schedule for daily testing. Top timeline illustrates the course of a typical hour-long observation period during which motor cortex stimulation (MCS, black bar) was delivered continuously and the Klüver task (K, dark gray bar) was administered four times. Bottom timeline illustrates how these observation sessions were administered at the same time every day with episodes of MCS at different frequencies and polarities and sham stimulation sessions (Sham, hatched vertical bar) delivered in random order. (B) Mean whole-body activity levels for different MCS polarities (data collapsed across frequencies, n = 19, 8, and 33 sessions for cathodal, bipolar, and anodal MCS, respectively), and off MCS (n = 21) and levodopa (n = 5) control conditions. (C) Mean whole-body activity levels for different MCS frequencies (data collapsed across polarities, n = 23, 13, 7, and 17 sessions for 5 Hz, 30 Hz, 75 Hz, and 150 Hz MCS, respectively), and off MCS (n = 21) and levodopa (n = 5) control conditions. (D) Effects of MCS on Klüver task performance for different MCS polarities and frequencies. Error bars indicate standard error. * p < 0.05, ** p < 0.01, ***p < 0.001 relative to the “No stim” control condition.
Implantation surgeries were performed under isoflurane anesthesia, using aseptic technique. A craniotomy was placed over the arm region of the PMC of the right hemisphere (Horsley-Clark anterior 10, lateral 20, depth 20)29. A two-contact paddle electrode (Northstar Neuroscience, Seattle, WA: 2.5 cm x 1 cm, 0.8 cm between contacts, 2 kΩ at 1 kHz) was implanted on the dura mater overlying the PMC with the two electrode contacts ~1 cm anterior to and parallel with the central sulcus. The bone flap was replaced and secured with bone screws and acrylic cement. A reference electrode was placed under the temporalis muscle. Following the behavioral assessment experiments in two animals, recording chambers were implanted to allow neuronal recording in globus pallidus (GP). A cylindrical titanium chamber (18 mm internal diameter) was implanted with stereotaxic guidance over the burr hole created previously. By necessity, the previously-implanted extradural stimulating electrode was removed during this surgery. The chamber was oriented parallel to the coronal plane at an angle ~35 degrees from vertical. The chamber was fixed to the skull with bone screws and dental acrylic. Bolts were embedded in the acrylic to allow fixation of the head during recording sessions.
MCS variables
MCS was administered using a telemetry-controlled, constant-current pulse generator (Northstar Neuroscience) placed in a backpack on the animal’s back. Stimulus pulses consisted of an initial square wave (width 130μs) followed by an exponentially-decaying opposing deflection to balance charges. MCS polarity was defined as anodal (positive square wave at both cortical electrodes), cathodal (negative at both cortical electrodes), or bipolar (two cortical electrodes used as positive and negative contacts). Pulse frequencies ranged from 5 Hz to 150 Hz.
MCS was administered at 70% of motor threshold (MT), which was established separately for each frequency, polarity, and animal, immediately prior to each MCS session (see below). MT was defined by the lowest current capable of evoking visible muscle contraction reliably in response to 2 seconds of stimulation. The muscles most commonly activated at MT were those of the thenar eminence. MTs ranged between 1.2 and 3.2 mA, with lowest MTs obtained for stimulation at high frequencies. Individual MTs for a given animal, frequency, current, and polarity combination remained nearly constant over the course of 6 to 12 months of testing. Currents above MT consistently evoked arm and leg movement when delivered through medial contacts and arm and orofacial movement for the lateral contacts. The precentral location of implanted electrodes was confirmed postmortem in one animal and during the subsequent chamber implantation surgery in the other two.
Behavioral Assessment
Effects of MCS were evaluated during multiple hour-long observation periods with the animal in a sound-proofed cage under video-tape observation (Fig 1A). An Actitrac (IM Systems) activity logger fixed to the animal’s backpack recorded the total number of whole-body movements (>0.625 mGs) per minute. Manual dexterity was assessed four times (every 15 minutes) per hour-long session, using a Klüver task in which the animal retrieved food from a board with 12 wells (1″ diameter, 0.25″ deep, each holding a grape slice), accessible only to the left arm. The sensitivity of these methods was confirmed by performing identical hour-long observation periods following administration of L-DOPA methyl ester (IM, 5 mg/kg with 10 mg/kg benserazide). Differences in average activity were assessed for significance using the t-test.
One researcher, blinded to therapeutic conditions, reviewed each video-taped observation period, and rated the animal’s level of parkinsonism using both the Schneider scale and a quantitative analysis of the prevalence of nine different behaviors. We found the Schneider scale to be insensitive to small or transient changes in symptoms. Moreover, the video-based assessments prevented inclusion of the following Schneider scale sub-scores: defense reaction, blinking, appetite, and climbing. Quantitative analysis of the videos was adapted from a similar method used by Grabli et al.10. This method reported, as a percent of each hour-long observation session, the prevalence of nine behaviors: food retrieval in the Klüver task, exploring, touching/licking cage bars, chewing, climbing, licking/biting fingers, visually tracking, grooming, and not moving.
All 3 animals were studied for the effects of daily hour-long MCS at different frequencies and polarities (Fig. 1A). One animal was also studied using biweekly long-term MCS at 30 Hz bipolar. For each biweekly stimulation trial, twelve one-hour long observation sessions were distributed over two weeks (Fig. 3A). Following one week of control observations, MCS was delivered continuously for approximately 25 hours, after which the animal’s behavior continued to be measured off MCS. For both treatment schemes, measures obtained under on-MCS and off-MCS conditions were compared using t-tests, similar to the approach used by Drouot et al.10 and Pagni et al.18,19.
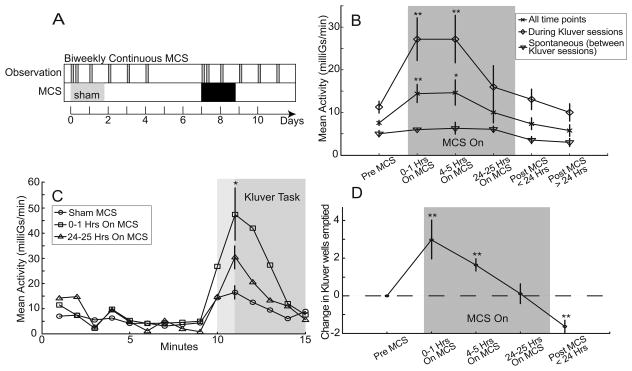
Fig. 3.
Biweekly MCS yielded a transient context-dependent improvement in akinesia and Klüver task performance. (A) Organization of biweekly MCS testing. Following one week of observations during and following sham stimulation, MCS was delivered continuously for about 25 hours. Hour-long observation periods were performed twice on the first day of sham/MC stimulation and then daily for four days. (Conventions follow those of Fig. 1A.) (B) Effects of continuous MCS on mean activity as a function of time since the onset of stimulation. Activity averaged across whole hour-long observation periods (×) showed a significant increase during the first day of MCS. That effect returned close to baseline by the 24th hour of testing. That effect was almost completely accounted for by increased activity during the 5 minute epochs around presentations of the Klüver task (◇). MCS did not affect spontaneous activity duing the 10 minute epochs between Klüver task presentations (▽). (C) Minute-by-minute averages of activity aligned on presentations of the Klüver task illustrate the strong enhancement of activity restricted to the peri-Klüver period (shaded regions). The MCS-induced enhancement was large during the first hour of MCS (□) but attenuated by the 24th hour of MCS (△). (D) Klüver task performance as a function os MCS duration. Each point is an average of three sessions. Error bars indicate standard error. * p < 0.005, ** p < 0.001 relative to the matching “Pre MCS” conditions.
Electrophysiology and Histology
The parkinsonian status of animals was confirmed by single unit recording in the globus pallidus (2 animals) or tyrosine hydroxylase (TH) immunohistochemistry (1 animal). The spontaneous activity of multiple single pallidal neurons was sampled from awake animals at rest using a 4-electrode microdrive, 1 MΩ glass-tungsten electrodes (Alpha Omega, Nazareth, Israel) and a Tucker Davis Technologies (Alachua, FL) acquisition system sampling at 24 kHz. Single unit action potentials were discriminated using Offline Sorter software (Plexon Inc., Dallas TX) and analyzed using custom routines in the Matlab environment. The L-burst statistic was used to determine the prevalence of pathological neuronal activity within the globus pallidus (GP)12,26. Unfortunately, neuronal activity could not be measured during MCS due to significant electrical artifacts from MCS.
At the completion of study, one animal was perfused transcardially using phosphate buffered saline (PBS) with 10% formalin. The tissue was blocked and cut into 40 μM sections in the coronal plane and stained using TH immunohistochemistry. Specifically, a mouse anti-tyrosine monoclonal antibody was incubated with the tissue for 24 hours, followed by 1 hour of incubation with a biotinylated horse anti-mouse antibody (Chemicon international, Temecula, CA, USA). Sections were then incubated with Streptavidin for 1 hour and then revealed using DAB-Vector SK-400 (Vector Laboratries, Burlingame, CA, USA). Histology was unavailable for the other two animals because one died unexpectedly and the other is engaged in an ongoing study.
RESULTS
MPTP Model
Throughout the period of MCS testing, parkinsonism was apparent bilaterally in all animals as evidenced by marked akinesia, bradykinesia, limb rigidity, action tremor, and stooped posture. The left side was more severely affected due to the initial unilateral intracarotid infusion of MPTP. Non-motor effects, such as decreased appetite, were also apparent. A mean Schneider score of 40 was observed during the period of MCS testing (means = 37, 38, and 45 for individual animals) compared with a mean score of 8 prior to systemic MPTP treatment.
Electrophysiological recording in two animals confirmed the presence of pathological neuronal activity within the GP characteristic of parkinsonism (n=80 neurons). Recordings were obtained from animals that were awake and sitting quietly. The firing of globus pallidus neurons was abnormally bursty, as determined by a comparison of the L-statistic for these neurons (6.9 ± 0.25 SEM) versus a large population of pallidal neurons sampled from three normal macaques (5.41 ± 0.09 SEM, n=157, p<0.0001, t-test)26. We have observed similar bursty discharge in the globus pallidus of Parkinson’s disease patients undergoing surgical therapy26. Of 20 cell pairs recorded simultaneously, 35% showed synchronized activity (i.e., significant peaks in crosscorrelograms), similar to the numbers reported previously for parkinsonian monkeys and well in excess of the 6.8% incidence reported for the normal primate pallidum15. Other aspects of the neurophysiological studies in these animals will be reported elsewhere. The postmortem histology available from one animal indicated a widespread DA depletion throughout the striatum and the lateral substantia nigra compacta as measured by TH imunohistochemistry.
Daily One-Hour MCS
MCS applied at varying frequencies (n = 23, 13, 7, and 17 sessions for 5 Hz, 30 Hz, 75 Hz, and 150 Hz MCS, respectively) and polarities (n = 19, 8, and 33 sessions cathodal, bipolar, and anodal MCS, respectively) did not produce significant differences in the rate of whole body accelerations, as measured by the Actitrac, relative to off MCS (n = 21 sessions) (Fig. 1B, C). Manual dexterity of the left arm as assessed by the Klüver task, also did not improve significantly on MCS compared to off MCS (Fig. 1D). Of the frequencies and polarities tested, 30 Hz and bipolar, respectively, showed the greatest increases in activity. Even at these settings, however, the increase in activity did not reach significance (p = 0.173). In contrast, low dosages (5 mg/kg) of L-DOPA resulted in an 89.3% average increase in activity (p < 0.001, n = 5). There was no improvement in Klüver task performance with daily MCS at any frequency or polarity. Klüver task performance deteriorated during cathodal MCS at 150 Hz (p < 0.01, Fig. 1D).
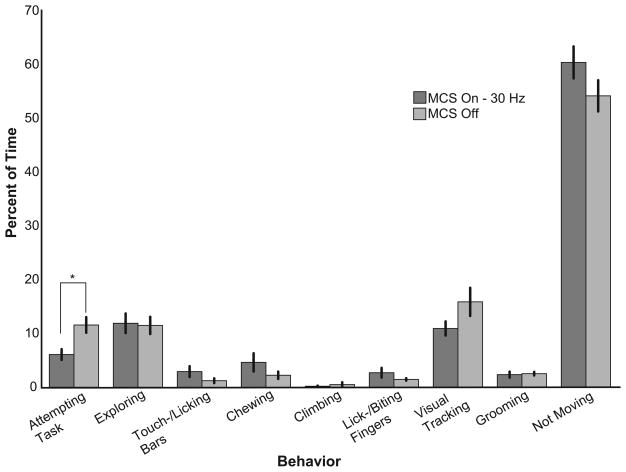
Blinded clinical ratings of video-taped sessions using the Schneider scale provided no evidence of an MCS-induced amelioration of parkinsonian symptoms (data not shown). The quantitative behavioral analysis revealed similar results for daily MCS (representative results for 30 Hz bipolar shown in Fig 2). Eight of the nine behaviors did not differ in prevalence between off-MCS and on 30 Hz MCS (p > 0.05). The one significant effect of 30 Hz MCS was that animals spent slightly less time attempting the Klüver task (p < 0.05).
Fig. 2.
Daily MCS had a minimal effect on clinically-relevant behavioral measures. An observer blinded to stimulation condition quantifed from video tapes the prevalence of nine different behaviors during each hour-long observation session. Representative results are presented comparing sessions off MCS (n = 5 sessions) versus 30 Hz MCS (n = 4 sessions). Error bars indicate standard error. * p < 0.05.
Biweekly Continuous MCS
Biweekly MCS (30 Hz bipolar) resulted in transiently increased activity levels and improved Klüver performance (Fig. 3B, 3D). These increases in activity and Klüver task performance were present for the first 5 hours of continuous MCS, but diminished by the twenty-fourth hour. In the 24-hour period immediately following the end of continuous MCS, the animal’s activity was not different from pre-MCS levels, but Klüver task performance declined below pre-MCS levels (p < 0.001).
The activating effects of MCS were greatest during presentation of the Klüver task. Across all observation sessions, minute-by-minute measures of activity were typically low when the Klüver board was not visible (i.e., when external stimuli were minimized), whereas each animal’s general level of activity was elevated during the 5-minute period when the Klüver board was accessible. Offline video analysis indicated that much of this peri-Klüver activity was attributable to whole-body movement and postural adjustment related to positioning for Klüver task performance. Although peri-Klüver increases in activity were evident even when MCS was off, 30 Hz bipolar MCS accentuated those peri-Klüver increases in activity (p < 0.005, Fig. 3C). The peri-Klüver increase in activity accounted for nearly all of the effects of MCS.
DISCUSSION
Three important results come from this work: (1) we were unable to demonstrate a reliable reduction in parkinsonian symptoms when MCS was delivered daily for an hour; (2) increased activity and improved Klüver performance were observed during the first 24 hours of continuous biweekly MCS; (3) increases in activity were context-specific.
We failed to demonstrate reliable symptom amelioration when MCS was delivered for an hour every day, despite the fact that our battery of behavioral tests was sensitive to small changes in the core symptoms of PD and that we explored a wide range of stimulation modes and frequencies. One might ask why unilateral subthreshold stimulation restricted to a small portion of PMC would be expected to ameliorate parkinsonian symptoms globally, but a number of clinical reports3,18,19 and one primate study6 suggest that this general mode of therapy has value. Using a broad range of stimulation parameters and sensitive quantitative, we were unable to demonstrate significant improvements in response to daily MCS therapy.
It is noteworthy that the optimal frequency found by Drouot et al. was 130 Hz, while we and Pagni et al. found that MCS frequencies between 25 and 30 Hz had the greatest effect (though in our hands, the effects were not statistically significant). The reason for this discrepancy is a matter of speculation.
MCS delivered biweekly produced a significant but transient amelioration of symptoms. Drouot et al. also reported a significant increase in activity during a half hour of MCS when at least one week intervened between MCS administrations6. The mechanism underlying this transitory effect is also open to conjecture. Drouot et al. implicate a mechanism by which MCS disrupts the pathologic firing patterns of basal ganglia output neurons, presumably via cortical inputs to the basal ganglia6. MCS could also work directly at the cortical level to block the abnormal oscillatory and synchronized activity that has been described for cortex in PD and animal models of parkinsonism9,25. Neither of these potential mechanisms, however, explains the apparent transitory nature of the effect. Recent evidence, presented in abstract by Chen et al.[ref???] have noted that the intensity of PMC stimulation necessary to evoke an H-reflex of a fixed size increases continuously over the course of 20 days. These findings may help to explain the reduced efficacy of MCS over the course of continuous 24 hours of stimulation. These results, however, do not explain why daily MCS was relatively ineffective as motor thresholds were re-established prior to each MCS session.
It is also possible that the positive effects of MCS (or rTMS) are mediated by stimulation-evoked release of dopamine (DA) in the striatum. Strafella et al. have shown recently in normal humans that rTMS of the PMC increases DA in the ipsilateral putamen27,28. Ohnishi et al. showed that rTMS over PMC also increases DA release in macaques, here restricted to the mesolimbic ventral striatum16. These effects may be mediated via direct corticostriatal excitation of striatal DA terminals or via multisynaptic pathways5. Such an effect would depend on the presence of residual stores of striatal DA, which have been documented for the mesolimbic striatum of the MPTP-treated primate7,21,22. Daily application of MCS may be so frequent as to leave DA terminals in a perpetually depleted state, and consequently provide no therapeutic benefit. Consistent with this possibility, the only significant effect of daily MCS was a reduction in Klüver task-associated behaviors.
The increased activity associated with biweekly MCS was largely dependent on the presence of the Klüver task. This context sensitivity may be seen as further evidence of a mechanism involving DA. The motivating effects of MCS-induced DA release may account for the animal’s increased effort toward the Klüver task4 in the absence of an effect on spontaneous activity.
CONCLUSIONS
Previous published studies of MCS have reported impressive effects of MCS in both parkinsonian baboons and humans. In contrast, our results show that the positive effects of continuous MCS on mobility do not last beyond a day, and daily MCS, even when delivered in short 1 hour sessions, fails to reduce symptoms reliably. This suggests that MCS, though promising, should be pursued with caution. MCS may eventually prove to be a clinically significant therapy for PD, but it must first overcome the limitations described here.
Acknowledgments
Sources of Support: This research was supported by Northstar Neuroscience, Seattle, WA 98121 and National Institute of Neurological Disorders and Stroke Grant NS044551.
The authors thank Jed Hewitt, Chris Kim, and Valerie Davis for their invaluable assistance in animal care.
References
- 1.Bankiewicz KS, Oldfield EH, Chiueh CC, Doppman JL, Jacobowitz DM, Kopin IJ. Hemiparkinsonism in monkeys after unilateral internal carotid artery infusion of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Life Sci. 1986;39:7–16. doi: 10.1016/0024-3205(86)90431-5. [DOI] [PubMed] [Google Scholar]
- 2.Bankiewicz KS, RS-P, Oiwa Y, Kohutnicka M, Cummins A, Eberling J. Preclinical Models of Parkinson’s Disease. Current Protocols in Neuroscience. 1999;9(supplement):1–32. doi: 10.1002/0471142301.ns0904s09. [DOI] [PubMed] [Google Scholar]
- 3.Canavero S, Paolotti R, Bonicalzi V, Castellano G, Greco-Crasto S, Rizzo L, et al. Extradural motor cortex stimulation for advanced Parkinson disease. Report of two cases. J Neurosurg. 2002;97:1208–1211. doi: 10.3171/jns.2002.97.5.1208. [DOI] [PubMed] [Google Scholar]
- 4.Czernecki V, Pillon B, Houeto JL, Pochon JB, Levy R, Dubois B. Motivation, reward, and Parkinson’s disease: influence of dopatherapy. Neuropsychologia. 2002;40:2257–2267. doi: 10.1016/s0028-3932(02)00108-2. [DOI] [PubMed] [Google Scholar]
- 5.David HN, Ansseau M, Abraini JH. Dopamine-glutamate reciprocal modulation of release and motor responses in the rat caudate-putamen and nucleus accumbens of “intact” animals. Brain Res Brain Res Rev. 2005;50:336–360. doi: 10.1016/j.brainresrev.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Drouot X, Oshino S, Jarraya B, Besret L, Kishima H, Remy P, et al. Functional recovery in a primate model of Parkinson’s disease following motor cortex stimulation. Neuron. 2004;44:769–778. doi: 10.1016/j.neuron.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 7.German DC, Dubach M, Askari S, Speciale SG, Bowden DM. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonian syndrome in Macaca fascicularis: which midbrain dopaminergic neurons are lost? Neuroscience. 1988;24:161–174. doi: 10.1016/0306-4522(88)90320-x. [DOI] [PubMed] [Google Scholar]
- 8.Ghabra MB, Hallett M, Wassermann EM. Simultaneous repetitive transcranial magnetic stimulation does not speed fine movement in PD. Neurology. 1999;52:768–770. doi: 10.1212/wnl.52.4.768. [DOI] [PubMed] [Google Scholar]
- 9.Goldberg JA, Boraud T, Maraton S, Haber SN, Vaadia E, Bergman H. Enhanced synchrony among primary motor cortex neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine primate model of Parkinson’s disease. J Neurosci. 2002;22:4639–4653. doi: 10.1523/JNEUROSCI.22-11-04639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grabli D, McCairn K, Hirsch EC, Agid Y, Feger J, Francois C, et al. Behavioural disorders induced by external globus pallidus dysfunction in primates: I. Behavioural study. Brain. 2004;127:2039–2054. doi: 10.1093/brain/awh220. [DOI] [PubMed] [Google Scholar]
- 11.Houeto JL, Mesnage V, Mallet L, Pillon B, Gargiulo M, du Moncel ST, et al. Behavioural disorders, Parkinson’s disease and subthalamic stimulation. J Neurol Neurosurg Psychiatry. 2002;72:701–707. doi: 10.1136/jnnp.72.6.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaneoke Y, Vitek JL. Burst and oscillation as disparate neuronal properties. J Neurosci Methods. 1996;68:211–223. doi: 10.1016/0165-0270(96)00081-7. [DOI] [PubMed] [Google Scholar]
- 13.Kumar R, Lozano AM, Montgomery E, Lang AE. Pallidotomy and deep brain stimulation of the pallidum and subthalamic nucleus in advanced Parkinson’s disease. Mov Disord. 1998;13 (Suppl 1):73–82. [PubMed] [Google Scholar]
- 14.Limousin P, Krack P, Pollak P, Benazzouz A, Ardouin C, Hoffmann D, et al. Electrical stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med. 1998;339:1105–1111. doi: 10.1056/NEJM199810153391603. [DOI] [PubMed] [Google Scholar]
- 15.Nini A, Feingold A, Slovin H, Bergman H. Neurons in the globus pallidus do not show correlated activity in the normal monkey, but phase-locked oscillations appear in the MPTP model of Parkinsonism. J Neurophysiol. 1995;74:1800–1805. doi: 10.1152/jn.1995.74.4.1800. [DOI] [PubMed] [Google Scholar]
- 16.Ohnishi T, Hayashi T, Okabe S, Nonaka I, Matsuda H, Iida H, et al. Endogenous dopamine release induced by repetitive transcranial magnetic stimulation over the primary motor cortex: an [11C]raclopride positron emission tomography study in anesthetized macaque monkeys. Biol Psychiatry. 2004;55:484–489. doi: 10.1016/j.biopsych.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 17.Oiwa Y, Eberling JL, Nagy D, Pivirotto P, Emborg ME, Bankiewicz KS. Overlesioned hemiparkinsonian non human primate model: correlation between clinical, neurochemical and histochemical changes. Front Biosci. 2003;8:a155–166. doi: 10.2741/1104. [DOI] [PubMed] [Google Scholar]
- 18.Pagni CA, Altibrandi MG, Bentivoglio A, Caruso G, Cioni B, Fiorella C, et al. Extradural motor cortex stimulation (EMCS) for Parkinson’s disease. History and first results by the study group of the Italian neurosurgical society. Acta Neurochir Suppl. 2005;93:113–119. doi: 10.1007/3-211-27577-0_19. [DOI] [PubMed] [Google Scholar]
- 19.Pagni CA, Zeme S, Zenga F. Further experience with extradural motor cortex stimulation for treatment of advanced Parkinson’s disease. Report of 3 new cases. J Neurosurg Sci. 2003;47:189–193. [PubMed] [Google Scholar]
- 20.Pascual-Leone A, Valls-Sole J, Brasil-Neto JP, Cammarota A, Grafman J, Hallett M. Akinesia in Parkinson’s disease. II. Effects of subthreshold repetitive transcranial motor cortex stimulation. Neurology. 1994;44:892–898. doi: 10.1212/wnl.44.5.892. [DOI] [PubMed] [Google Scholar]
- 21.Pessiglione M, Guehl D, Jan C, Francois C, Hirsch EC, Feger J, et al. Disruption of self-organized actions in monkeys with progressive MPTP-induced parkinsonism: II. Effects of reward preference. Eur J Neurosci. 2004;19:437–446. doi: 10.1111/j.0953-816x.2003.03089.x. [DOI] [PubMed] [Google Scholar]
- 22.Rothblat DS, Schroeder JA, Schneider JS. Tyrosine hydroxylase and dopamine transporter expression in residual dopaminergic neurons: potential contributors to spontaneous recovery from experimental Parkinsonism. J Neurosci Res. 2001;65:254–266. doi: 10.1002/jnr.1149. [DOI] [PubMed] [Google Scholar]
- 23.Schneider JS, Gonczi H, Decamp E. Development of levodopa-induced dyskinesias in parkinsonian monkeys may depend upon rate of symptom onset and/or duration of symptoms. Brain Res. 2003;990:38–44. doi: 10.1016/s0006-8993(03)03382-1. [DOI] [PubMed] [Google Scholar]
- 24.Schrag A, Quinn N. Dyskinesias and motor fluctuations in Parkinson’s disease. A community-based study. Brain. 2000;123 (Pt 11):2297–2305. doi: 10.1093/brain/123.11.2297. [DOI] [PubMed] [Google Scholar]
- 25.Silberstein P, Pogosyan A, Kuhn AA, Hotton G, Tisch S, Kupsch A, et al. Cortico-cortical coupling in Parkinson’s disease and its modulation by therapy. Brain. 2005;128:1277–1291. doi: 10.1093/brain/awh480. [DOI] [PubMed] [Google Scholar]
- 26.Starr PA, Rau GM, Davis V, Marks WJ, Jr, Ostrem JL, Simmons D, et al. Spontaneous pallidal neuronal activity in human dystonia: comparison with Parkinson’s disease and normal macaque. J Neurophysiol. 2005;93:3165–3176. doi: 10.1152/jn.00971.2004. [DOI] [PubMed] [Google Scholar]
- 27.Strafella AP, Paus T, Barrett J, Dagher A. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci. 2001;21:RC157. doi: 10.1523/JNEUROSCI.21-15-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strafella AP, Paus T, Fraraccio M, Dagher A. Striatal dopamine release induced by repetitive transcranial magnetic stimulation of the human motor cortex. Brain. 2003;126:2609–2615. doi: 10.1093/brain/awg268. [DOI] [PubMed] [Google Scholar]
- 29.Szabo J, Cowan WM. A stereotaxic atlas of the brain of the cynomolgus monkey (Macaca fascicularis) J Comp Neurol. 1984;222:265–300. doi: 10.1002/cne.902220208. [DOI] [PubMed] [Google Scholar]