Abstract
Background
Necrotizing enterocolitis (NEC) is an acute neonatal inflammatory disease which may lead to intestinal necrosis, multi-system failure and death. Currently, NEC is diagnosed by a combination of laboratory and radiographic tests conducted a posteriori, i.e. when NEC is already clinically significant. Given the acute onset and rapid progression of NEC, a non-invasive biomarker that allows early detection of patients at risk is required as a matter of urgency. We evaluated whether the high frequency (HF) component of heart rate variability (HRV), a measure of vagal efferent tonic cholinergic activity may be used as a predictive biomarker for NEC-risk before the onset of clinical disease.
Methods
In this prospective study, stable preterm (gestational age 28-35 week) infants had HRV power spectra analyzed from surface electrocardiogram waveforms taken at rest on day 5-8 of life. We used regression modeling to determine the utility of HF-HRV in predicting NEC.
Key Results
HF-HRV power was 21.5±2.7 and 3.9±0.81ms2 in infants that remained healthy and those that later developed stage 2+NEC, respectively (P<0.001). Nine/70 enrolled infants developed NEC. The ROC discriminated a HF-HRV value of 4.68ms2 predictive for developing NEC with a sensitivity and specificity of 89% and 87%, and positive and negative predictive value of 50% and 98%, respectively. With predictive regression modeling, the risk (odds ratio) of developing NEC was 10 per every one SD decrease in HF-HRV.
Conclusions and Inferences
Our preliminary data indicate that HF-HRV may serve as a potential, non-invasive predictive biomarker of NEC-risk in NICU infants.
Keywords: Necrotizing enterocolitis, vagal tone, biomarker
Necrotizing enterocolitis (NEC) is the most prevalent and devastating bowel disease in the neonatal intensive care unit (NICU) affecting 6-10% of preterm infants, with a mortality rate of 15-25%, increasing to 50% in surgically-treated cases (1). NEC primarily affects infants less than 32 weeks postmenstrual age (PMA), and is diagnosed only after clinical observations such as delayed gastric emptying, lethargy, hypotension, abdominal distention and blood in stools become apparent. Often, these symptoms develop suddenly in an otherwise well infant; diagnosis of NEC is then confirmed by laboratory tests and abdominal radiographics, during which time NEC may progress rapidly to pneumatosis intestinalis, intestinal perforation or death (2).
Given multifactorial influences associated with NEC such as, for example, immature GI motility, hypoxia-ischemia, inappropriate bacterial colonization, several, diverse approaches have been proposed to diagnose pre-clinical NEC (3). Many of these approaches are cumbersome with several practical and theoretical flaws, and an authoritative agreement on their use is still lacking; to date there are no strategies for identifying which infants are most likely to develop NEC.
Heart rate variability (HRV) is a non-invasive measure of autonomic nervous system regulation that has become the conventionally accepted term to describe variations of both instantaneous heart rate and R-R interval. Previous studies have identified HRV as an indicator of fetal and neonatal well-being (4) and HRV is altered by several physiological and pathophysiological factors including stress and inflammation. Indeed, there are several studies hypothesizing that HRV may be used as a predictor of morbidity (5-7) and may, potentially, be used as a window into stress and inflammation in preterm infants in the NICU.
Frequency domain analysis of HRV, separates spectral frequencies reflective of the influence and integrity of sympathetic and parasympathetic activity on the cardiovascular system (8). In particular, the high frequency (HF) spectrum provides a reliable reflection of parasympathetic modulation, i.e. vagal tone (9), which is associated with the maintenance of physiological homeostasis. Many studies have shown that HRV and vagal tone decrease in anxiety, stress and inflammation/sepsis(10-16); indeed, the vagus also plays a critical role in the cholinergic anti-inflammatory reflex (17, 18), which has been shown to confer protection against tissue damage in many GI-related inflammatory diseases, including acute pancreatitis, colitis and inflammatory bowel disease (19-21). While HF-HRV provides a direct, non-invasive measure of vagal efferent activity (8); additional potentially useful indirect measures of vagal activity on the cholinergic anti-inflammatory reflex include blood sampling for C-reactive protein and cytokine analysis (18).
It has long been known that vagal efferent outflow regulates motility and secretion of the upper GI tract; and it is well accepted that their main modulator is a tonic cholinergic vagal tone (22, 23). In the preterm infant, immature vagal innervation results in low gastric motility, reduction of anti-inflammatory response and down-regulation of intestinal immune defenses required for cell adhesion and chemotaxis (13, 18). It is well accepted that the pathogenesis of NEC includes an exaggerated inflammatory response resulting in high levels of pro-inflammatory cytokines (2). We surmised that in preterm infants vagal dysregulation with impaired neuroimmune modulation of inflammation, detectable through low HF-HRV power may be a predisposing condition for later development of NEC.
The aim of this study was to test the hypothesis that alterations of the HF component of HRV may be used as a predictive biomarker for NEC-risk before the onset of clinical disease.
Materials and Methods
The study was conducted at the neonatal intensive care unit (NICU) of Penn State Children’s Hospital/The Penn State Milton S. Hershey Medical Center from February 2007 to May 2012. Human study protocols were approved by Penn State Hershey Institutional Review Board. Parental consent was obtained for each infant prior to study enrollment.
Study Sample
Subjects admitted to the NICU were enrolled in two prospective cohort studies, the first cohort (N=30) was recruited from 2007-2008 while the second cohort (N=40) was recruited from 2011-2012. The inclusion criteria were infants born preterm at 28-35 weeks postmenstrual age (PMA) and no longer requiring assisted ventilation by postnatal day 5 of life. Exclusion criteria were congenital (chromosomal, malformational, or deformational) anomalies, known intraventricular-periventricular hemorrhage greater than grade II, administration of narcotics and or sedatives to the infant prior to postnatal day 4 of life, reported maternal use of illicit substances, since these conditions or medications are known to impact HRV, or maternal illness preventing the ability to obtain informed consent. All infants were studied during the immediate intensive care phase between 5 and 8 postnatal days of life and were followed prospectively throughout the duration of their NICU stay. All infants were on some form of enteral feeding by postnatal day 5 of life and were clinically stable without evidence or suspicion of neonatal sepsis at the time of enrollment into the study. Infant characteristics are shown in Table 1.
Table 1.
Infant Characteristics
| No. of patients included (N=70) |
Mean | SE | Min | Max | Cohort Difference (P value) |
|---|---|---|---|---|---|
| Male gender (%) | 55 | - | - | - | 0.54 |
| Apgar at 5 minutes | 8.35 | 0.210 | 7.0 | 10.0 | 0.36 |
| PMA at birth (weeks) | 31.92 | 0.215 | 28 | 35.30 | 0.90 |
| Birth weight (grams) | 1791 | 50.11 | 1070 | 2803 | 0.14 |
| Necrotizing Enterocolitis (%) | 12.90 | - | - | - | 0.91 |
PMA = Postmenstrual Age
Summary of the infant characteristics collected from two cohorts of 30 and 40 patients, respectively. Independent samples t test (right column) did not show differences between the groups, the data were thus merged for subsequent analyses.
Heart Rate Variability (HRV)
Surface electrocardiogram (ECG) waveforms were obtained using standard lead II bipolar chest lead placement. Forty-five to 60 minutes of ECG R-wave data were obtained on day 5-8 of life during periods of quiet/deep sleep; recordings started at 30 minutes post-feeding since feeding, behavioral states, and pain/stress are known to impact HRV measurement (24-26). In addition, to minimize movement artifacts, data acquisition was initiated 30 minutes after the last handling episode and during a time when there was minimal activity around the infant’s crib space.
ECG R-wave data were obtained via a portable calibrated data acquisition system (BioBench, National Instruments, Austin, TX) at a sampling frequency of 1 kHz, and stored in a password protected database file for later HRV spectral analysis using a customized software package as described earlier (27). Each pre-selected 120s-long segment of the R-R wave was reviewed manually for quality and the elimination of ectopic foci and artifacts prior to generation of the spectral frequency output via fast Fourier transform (FFT). Analyses of epochs were then averaged for each infant (N = 8- 20 segments per infant). Because the FFT is known to have a limited accuracy of power estimation in the very low frequency (VLF) range with segments of duration less than 5 minutes, only the low frequency (LF, indicative of both vagal and sympathetic tone) and high frequency (HF, indicative of vagal tone) components were measured (6).
The LF domain was measured in the frequency bandwidth of 0.03 - 0.29 Hz and the HF domain measure, reflective of the effect of respirations on heart rate, was measured in the bandwidth 0.3 - 1.3 Hz. The HF bandwidth was calculated from the mean ± 2SD of the spontaneous resting breath rates (20-80 breaths/min) of preterm infants in our sample population. The sum of HF + LF was calculated to determine the total power (TP) spectra and LF and HF power are expressed as normalized units (nLF = LF/total power × 100% and nHF = HF/total power × 100%) to demonstrate the relative contribution of LF and HF to TP, respectively. The nLF/nHF ratio was calculated to estimate sympathovagal balance.
Morbidity/Outcome Data
Maternal health indicators including: prenatal care, past medical history, gravity/parity, maternal medication use, tobacco and illicit drug use, clinical suspicion for infection, duration of rupture of membranes, and antenatal steroid treatment were recorded from the infant’s electronic medical record (EMR). All mothers of infant subjects received prenatal care and antenatal betamethasone. Infant health indicators including gestational age, gender, mode of delivery, Apgar scores and diagnoses were recorded from EMR.
The Score for Neonatal Acute Physiology (SNAP), a measure of severity of illness (28), was calculated using physiological data from the EMR within 48 hours of birth. Additionally, health outcomes were followed weekly until the infant was discharged from the hospital.
Cases of NEC stage ≥ 2 according to the modified Bell criteria (29) were confirmed by a pediatric radiologist to have pneumatosis intestinalis, portal venous air, and/or pneumoperitoneum, on radiograph(s). Isolated perforation without pneumatosis intestinalis was not diagnosed as NEC.
Statistics
Descriptive statistics (frequencies, means, medians and scatter plots) were computed for key variables and checked for outliers and normality. The t test, ANOVA, or Pearson’s correlation was used to compare continuous parametric data and Mann- Whitney U or Spearman’s correlation was used for non-parametric data. Data were expressed as mean ± SE and analyzed with IBM-SPSS® version 21 software (Armonk, NY). All tests were 2-tailed at a 5% significance level. We used ROC analysis to determine the sensitivity, specificity, positive and negative predictive power of HF as valid predictor of NEC-risk. Due to skewness of the data on tests of normality, HF and LF raw data were transformed using natural log prior to statistical prediction modeling. For hypothesis testing, a 2-step multiple logistic regression analysis was used to determine the effects of increase for each predictor (PMA, SNAP, LF and HF power) on risk of developing NEC. Two predictor variables were entered per step based on sample size.
Results
Patient Population and NEC Outcome
Data were obtained from 70 infants in two separate cohorts of 30 and 40 infants, respectively. At the time when ECG and HRV analysis were conducted, all infants were clinically stable, had no clinical sign of illness and had a low morbidity index (SNAP ≤ 9).
Twelve hours to 20 days after ECG acquisition, 9 of the 70 infants developed clinically diagnosed NEC (4 of 30, i.e. 13.3%, and 5 of 40, i.e. 12% in the two cohorts, respectively; P > 0.05 between groups). The median interval for developing NEC after ECG acquisition was 9 days. Infant characteristics were similar between the cohorts (Table 1). Demographic, antenatal, and early neonatal factors showed no significant association with the development of NEC.
Group Comparisons Analysis
We compared the HRV measures in the group who later developed NEC (i.e. NEC+; N = 9) to those who did not develop NEC (i.e. NEC-; N = 61). Non-parametric, independent samples mean comparisons of HRV indices showed a significant reduction in both LF and HF power spectra in the group of infants that would later develop NEC (P < 0.001 for both comparisons). We found that total power, LF and HF power were approximately 80% lower in the NEC+ group as was the normalized percent of HF (nHF) which represents the contribution of HF to total power. There also was clear evidence of reduced HRV demonstrated by a narrower inter-beat interval (IBI) range in the NEC+ group of infants as compared with NEC- infants that remained ’healthy’ throughout hospitalization.
ECG was also recorded in a group of infants 13-21 days after the first ECG recording, near discharge for healthy patients (N=29) or near the time of NEC diagnosis (N=4). In NEC+ infants, HF power remained significantly lower than in NEC- patients (P<0.05). Representative data are shown in Figure 1, and are summarized in Figure 2 and Table 2.
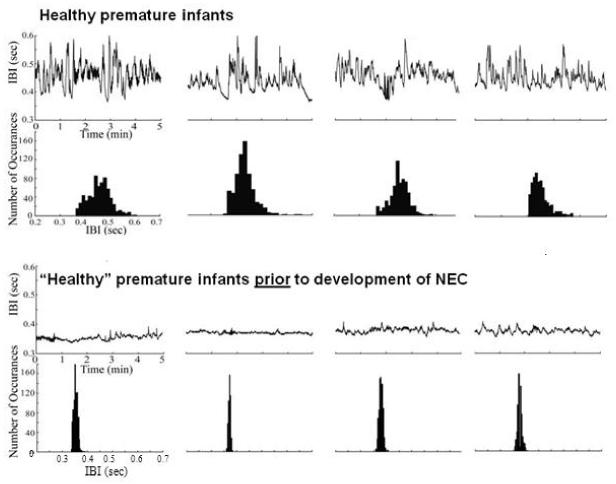
Figure 1.
Raw data (upper) and histogram summary (lower) of cardiac interbeat interval (IBI) in 4 representative healthy premature infants (upper traces) who remained healthy throughout the study duration. The lower traces show the raw data (upper) and summary histogram (lower) of IBI in 4 healthy premature infants who developed NEC (using Bell’s stage II+ criteria) 0.5-20 days later. Note that the healthy premature infants had a larger IBI variability and a broader distribution of events whereas premature healthy infants that went on to develop NEC had much less IBI variability and sharper event distribution even before NEC was clinically diagnosed. All the “healthy” infants which later developed NEC were identified by the lower IBI variability and HF-power. The representative subjects selected for comparison (“healthy” and “healthy prior to NEC”) were matched for gestational age and morbidity index at birth.
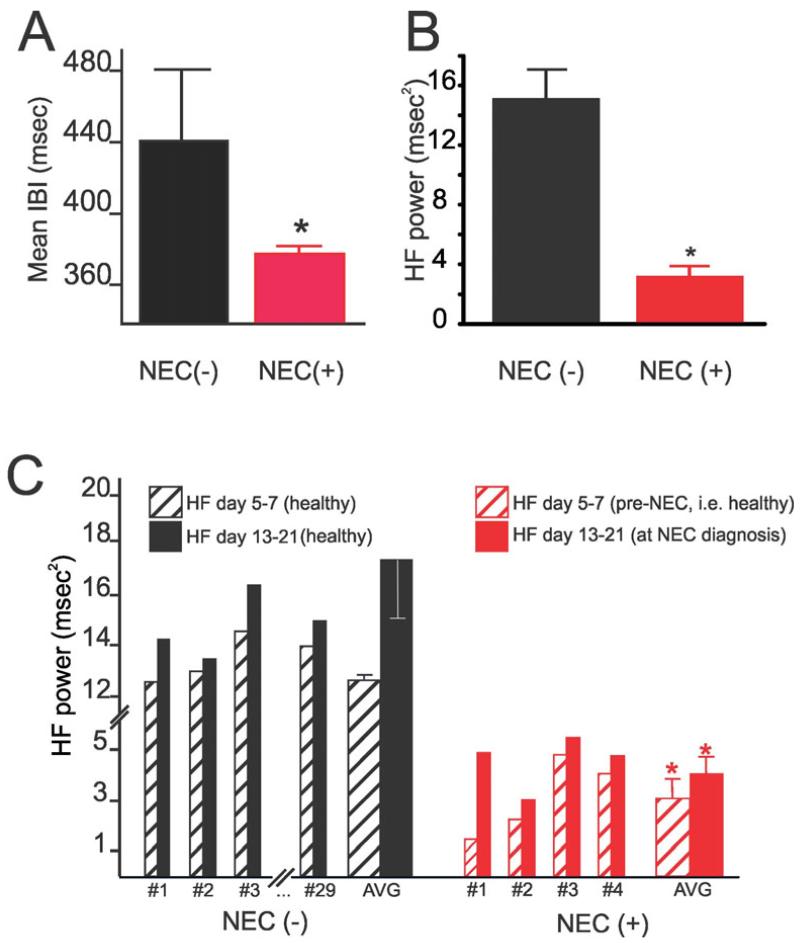
Figure 2.
Graphical summary of the differences in A) time domain (IBI) and B) frequency domain (HF power) analyses of HRV in healthy premature infants who remained healthy throughout the study duration [Healthy, NEC (−), N=61] and “healthy premature infants” prior to the development of NEC [NEC (+), N=9]. Note that even before NEC was clinically diagnosed, susceptible premature infants showed a decrease in both IBI and HF power, *p<0.05, NEC(−) vs. NEC(+). Graphical summary showing measures of HF power taken at 8-14 days intervals (C). Note that HF power in healthy infants increased over time and was higher than in infants that developed NEC; conversely, the HF power in infants that later developed NEC did not increase significantly over time, *p>0.05.
Table 2.
HRV Spectral Power Values
| NEC (−) N = 61 | NEC (+) N = 9 | Group Difference |
|||||
|---|---|---|---|---|---|---|---|
| Variable | Mean | SD | SEM | Mean | SD | SEM | P value |
| Mean HR (bpm) | 145.80 | 11.15 | 2.19 | 163.40 | 4.98 | 2.49 | 0.005+ |
| Mean IBI (msec) | 413.84 | 31.83 | 6.24 | 367.65 | 11.17 | 5.59 | <0.001+ |
| LF Power (ms2) | 87.37 | 107.33 | 13.74 | 16.23 | 10.89 | 3.63 | <0.001* |
| HF Power (ms2) | 21.53 | 21.60 | 2.76 | 3.99 | 12.44 | 0.81 | <0.001* |
| Total Power (ms2) | 108.90 | 123.80 | 15.85 | 20.22 | 13.17 | 4.39 | <0.001* |
| nLF (%) | 75.27 | 12.11 | 1.55 | 79.90 | 5.14 | 1.71 | 0.263+ |
| nHF (%) | 24.73 | 12.11 | 1.55 | 20.09 | 5.14 | 1.71 | 0.263+ |
| nLF/nHF Ratio | 4.32 | 3.41 | 0.437 | 4.28 | 1.40 | 0.47 | 0.971+ |
NEC = necrotizing enterocolitis; HR = heart rate; IBI = interbeat interval; LF = low frequency; HF = high frequency; nLF = normalized low frequency; nHF = normalized high frequency
Summary of the HRV spectral power values at postnatal day 5-8 for infants who remained healthy during hospitalization (NEC (−); N = 61) and those infants who were later diagnosed with NEC (NEC (+); N = 9).
Independent samples t test
Non-parametric, Mann Whitney U
Validity Analysis
HF power was selected as the variable of interest for further testing as HF power represents parasympathetic influence exclusively, conversely we excluded LF power from further analysis since, by representing both sympathetic and parasympathetic influences, its interpretation may be ambiguous (8). Thus, we tested HF power to determine its validity as a predictive biomarker for development of NEC using ROC curve analysis.
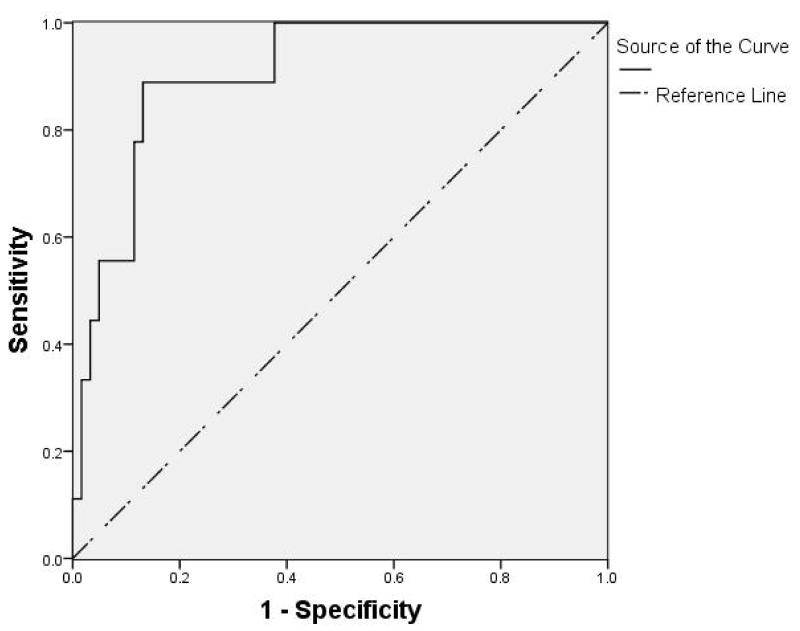
The ROC discriminated a cut off value, i.e. the value representative of the highest sensitivity and specificity for HF power as a predictive test for developing NEC, of 4.68 ms2. The area under the ROC curve (Figure 3) was 0.9 ± 0.04. HF power of 4.68 ms2 identified those healthy infants that later developed NEC with a sensitivity and specificity of 89% and 87%, respectively, and positive and negative predictive value of 50% and 98%, respectively.
Figure 3.
The receiver operating characteristic (ROC) curve was generated for HF-HRV mean values obtained from the two cohorts of infants (28-35 weeks post menstrual age; N=70).The graph represents each cut-off point of sensitivity (true positive rate) on the vertical axis, against 1-specificity (false positive rate) on the horizontal axis. The highest plot on the left represents the point which maximizes the area under the curve (AUC). The optimum cutoff value of HF-HRV for use as a diagnostic criterion of NEC-risk in this cohort is 4.68ms2. The AUC is 0.9 ± 0.04 with asymptotic 95% confidence intervals at 0.81 and 0.992 as lower and higher bound, respectively.
“Sensitivity” represents the proportion of healthy (at the time of the measure) patients who later developed NEC, in these patients an HF power < 4.68 ms2 predicted correctly that 8 of 9 (i.e. 89%) would develop NEC; “Specificity” represents the proportion of those patients who did not develop NEC, in these patients an HF power > 4.68 ms2 predicted correctly that 53 of 61 (i.e. 87%) would not develop NEC. The “Positive predictive value” represents the proportion of patients in whom HF power < 4.68 ms2 correctly predicted they would develop NEC (i.e. 8 of 16; i.e. 50%); the “Negative predictive value”, instead, represents the 53 of 54 (i.e. 98%) proportion of patients in whom HF power > 4.68 ms2 correctly predicted they would not develop NEC.
These data indicate that a value of HF power lower than 4.68 ms2 identifies 89% of the infants that will develop NEC from 0.5 to 20 (median 9) days later. Data are shown in Table 3.
Table 3.
HF power as a Predictive Biomarker for NEC
| NEC(+) | NEC(−) | Total | |
|---|---|---|---|
| HF power < 4.68 ms2 | 8 | 8 | 16 |
| HF power > 4.68 ms2 | 1 | 53 | 54 |
| Total Patients | 9 | 61 | 70 |
Sensitivity = 88.9 % Positive Predictive Value = 50 %
Specificity = 86.9 % Negative Predictive Value = 98.1 %
Multivariate Logistic Regression Analysis
For hypothesis testing, we conducted a step-wise multiple logistic regression to evaluate the contribution each of the predictors: Ln-HF and Ln-LF (Ln represents natural log transformed data), SNAP and PMA had on the dependent variable, defined as the occurrence of ≥stage 2 NEC. This model was fitted to a point estimate at 95% confidence interval for the dichotomous variable of NEC vs. non-NEC.
Our data indicate that Ln-HF was a significant predictor of NEC, with an odds ratio of 0.10; 95% CI: 0.03 - 0.42; P < 0.01, in support of our hypothesis. In fact, the OR and 95% CI translates into 10 (2.56, 33.33) in OR and 95% CI relating 1-SD decrease in HF and the incidence of NEC. Ln-LF and PMA at birth were also significant predictors of NEC while, conversely, SNAP was not a significant predictor of NEC. In the final model while controlling for PMA, Ln-HF and Ln-LF remain significant predictors for the development of NEC. Detailed data are presented in Table 4.
Table 4.
Logistic Regression Model associating increase in key variables and the development of NEC
| Predictor | OR | Model 1 95%CI |
P value | OR | Model 2 95%CI |
P value |
|---|---|---|---|---|---|---|
| PMA | 0.48 | (0.24, 0.95) | 0.04 | - | - | - |
| SNAP | 1.46 | (0.78, 2.74) | 0.24 | 1.34 | (0.69, 2.58) | 0.39 |
| Ln-HF | 0.10 | (0.03, 0.39) | <0.01 | 0.10 | (0.03, 0.42) | <0.01 |
| Ln-LF | 0.04 | (0.01, 0.28) | <0.01 | 0.04 | (0.01, 0.33) | <0.01 |
NEC = Necrotizing enterocolitis
PMA = Postmenstrual Age; SNAP = Score for Neonatal Acute Physiology;
Ln-HF = Natural Log HF; Ln-LF = Natural Log LF
Model 1: Univariate Model.
Model 2: Adjusted for PMA.
Results were expressed as the effects of 1 SD increase in the predictors on risk of developing NEC.
1SD of PMA = 1.79
1SD of SNAP = 2.01
1SD of Ln-HF = 0.98
1SD of Ln-LF = 1.05
Discussion
In a recent study, we supported the use of HRV measurements as an indicator of vagal tone and vagal effects on the gastrointestinal tract by showing, in an anaesthetized rodent model, a positive correlation between HF power and gastric motility (27). This observation implies that vagal dysregulation, assessed by HRV parameters (i.e. low HF power), leads to decreased gastrointestinal motility, decreased acid secretion, and a pro-inflammatory state in the intestinal tract. We hypothesize, therefore, that low vagal tone, which has also been recognized as a biomarker for stress vulnerability in preterm infants (10), further predisposes preterm infants to an increased risk of intestinal injury and NEC (30).
The pathophysiology of NEC is multifactorial and comprises mucosal injury and/or ischemia combined with immature GI motility patterns, inappropriate bacterial colonization and an exaggerated pro-inflammatory response, during which the balance between injury and protection is compromised (31, 32). Interestingly, vagal innervation plays important roles both in the functions of the immature gastrointestinal tract and as modulator of inflammation (18, 33, 34). Of particular interest is the relatively recent description of the “ vagal cholinergic anti-inflammatory pathway” briefly, peripheral inflammation stimulates vagal afferent pathways resulting in the activation of a vagally- mediated neural reflex that, via release of acetylcholine from vagal efferent fibers, inhibits resident tissue macrophages and the release of pro-inflammatory (TNF, IL-1β, IL-18, IL-6), but not anti-inflammatory (IL-10) cytokines (18, 34, 35).
A recent study showed that, in infants with surgical NEC, several pro-inflammatory cytokines were elevated consistently (36), suggesting that perhaps the lower vagal tone observed in high NEC-risk infants may be associated with altered cytokine production and leukocyte trafficking. It could be that the decreased vagal activity, observed lower HF power, not only reduces gut motility but also fails to counteract the increased inflammatory cell recruitment through lack of effective inhibition of pro-inflammatory cytokine production in the intestinal wall (33). In preterm infants, the immature gut is thought to respond to injury with excessive inflammation which may contribute to intestinal barrier damage, via inflammatory leukocytes releasing oxidants and proteases. It is tempting to speculate that, given the importance of the vagal cholinergic anti-inflammatory pathway, low vagal tone such as reported in high NEC-risk infants, may contribute to the pathogenesis of NEC through a decreased anti-inflammatory reflex, reduced gastric acid secretion and peristalsis which would lead to stasis and overgrowth of pathogenic bacteria contributing to the exaggerated inflammatory state seen with clinical NEC.
In the present study, we tested the feasibility that a decrease in the HF power spectrum of HRV obtained from the ECG reflecting diminished vagal cholinergic activity can be used as a non-invasive predictive biomarker for NEC-risk in otherwise healthy NICU infants.
Our clinical data are supported by the observations that in our sample of 70 preterm, stable infants between 5-8 days of age, a decrease in HF power to less than 4.68 ms2 could predict with 89% sensitivity and 50% positive predictive ability those infants that would develop NEC within the next three weeks of life i.e., High NEC-risk. In contrast, an HF power spectrum value higher than 4.68 ms2 was consistent with a very high negative predictive value (98%), indicating that HF power above this critical value during the first week of life can be taken as an assurance of low-risk for the development of NEC i.e., Low NEC-risk. We realize that it is difficult to provide firm conclusions for the predictive utility of a test with ROC analysis given the small number of NEC positive infants (N=9) in our sample. Thus, we did a logistic regression analysis to provide a more robust analysis of HF-HRV as a predictor of NEC. Using this approach, we found the HF power parameter provides a risk-estimate (odds ratio) for developing NEC of more than 10-fold higher in infants in which the HF power was 1SD below the average of age matched infants who did not develop NEC. This finding persists even after controlling for gestational age. It is important to emphasize that the findings in this study provide a relatively simple, economical and non-invasive method to identify patients who are more vulnerable or at high NEC-risk many days or even weeks before they become clinically ill.
The basis of NEC-risk detection in this study is that low HF power, indicative of low vagal tone both to the heart as well as to the viscera (37), may serve as a non-invasive indicator of stress vulnerability of the infant and a very sensitive indicator of compromised health (4). It should be kept in mind, however, that vagal tone increases with gestational age and time post birth (38), so our predictive cut-off value of HF power (i.e. 4.68ms2) can only be applied to infants within a relatively narrow PMA and further studies are needed to provide adequate values for HF power in more immature infants. Our data indicate, however, that the HF power in infants days to weeks prior to NEC onset was significantly lower than that recorded in age-matched infants who remained healthy, thus reinforcing the observation that a decreased HF-HRV power is indicative of a dysregulated vagal cholinergic system.
It should be emphasized that the observation of low HF power (vagal tone), as in the present study, identified infants at NEC-risk early in the postnatal period (between day 5-8 of life) which was many days to weeks in advance of the onset of clinical illness with NEC. This observation indicates that HF power during this early critical window of postnatal development has clinical value for identifying NEC-risk at a time when infants are well and interventions to enhance vagal tone may be employed. In contrast, a proprietary heart rate characteristics (HRC) index, comprised of HR decelerations and reduced HRV showed an association with NEC onset at 6-16 hours before clinical diagnosis of NEC, suggesting detection of subclinical disease just prior to NEC onset (39).
The design and analysis of the present study has several strengths that should be acknowledged: HRV measurements were standardized to time of day, feeding, and sleep state to control for circadian rhythm, feeding, and sleep-state induced variations in autonomic system activity (38). In addition, the timing of the measurement during day 5- 8 of life was selected as a window into the early postnatal period of adaptation to birth, but beyond the immediate influence of the stress of the birth process itself. Hence, this window of measurement provides an opportunity for evaluation of the infant’s autonomic balance in the absence of additional confounders such as stimulatory medications (e.g. caffeine) or therapies (e.g. massage) known to impact autonomic system measurement (40). Finally, each 120-sec epoch of ECG data was filtered manually off-line to eliminate ectopic foci and motion artifacts thus assuring data validity prior to analysis.
Our data have been generated in two prospective cohort studies with a relatively homogenous population of preterm infants where confounding factors such as acuity of illness (SNAP), gestational age and birth weight influences on autonomic system balance were under (relative) control. While our findings from this feasibility study provide significant results, we recognize they may be representative of a relatively limited and well-defined developmental time-frame and caution should be taken when applying the same parameters to the general preterm population. In addition, we recommend further investigation using a large-scale multi-center approach with measures of inflammation (CRP and cytokines) obtained at the time of HRV measurement. This approach would provide a higher number of NEC subjects for analysis and measures of inflammation to support our preliminary findings.
Conclusion
In conclusion, this is the first prospective study designed to evaluate the utility of vagal tone as a predictive biomarker for the development of NEC which we term “NEC- risk” to differentiate from other measures of sub-clinical illness. We put forward the suggestion that low vagal tone (low HF power) in the first week of life in otherwise healthy premature infants may be an important biomarker of vulnerability for subsequent development of NEC (NEC-risk). This relatively simple, economical and non-invasive method offers the opportunity to monitor at-risk infants more closely and test emerging therapies including those that increase the vagal anti-inflammatory reflex and gastrointestinal motility thus attenuating or even preventing altogether the development of NEC. While it is plausible that low vagal tone could contribute to the pathogenesis of NEC; however, the existence and nature of a causal link between low vagal tone, inflammation exposure, and NEC pathogenesis is unknown and deserving of further investigation.
Key messages.
Prognostic identification of infants at risk for necrotizing enterocolitis (NEC) is required as a matter of urgency.
We evaluated whether the high frequency (HF) component of heart rate variability (HRV) representative of vagal tone and the cholinergic anti-inflammatory reflex may be used in infants as a predictive biomarker for NEC-risk before the onset of clinical NEC.
The power spectra of surface electrocardiogram was taken in “healthy” preterm infants at rest on day 5-8 of life and correlated with later diagnosis of NEC.
The risk (odds ratio) of developing NEC 0.5-20 days in advance of clinical symptoms was 10 per every one SD decrease in HF-HRV (vagal tone).
Acknowledgments
Supported by The Children’s Miracle Network and Johnson & Johnson Health Behaviors and Quality of Life (KKD and CP); The National Institutes of Health, NIH-DK 55530 (RAT). None of the funding sources had any role in the design of the study, in the analysis and interpretation of the data, in the decision to submit the manuscript, or in the preparation, review or approval of the manuscript.
The authors acknowledge the medical/nursing staff of Penn State Hershey NICU for assistance with subject recruitment and the parents of infant subjects who gave consent for their infants to participate in the study.
Footnotes
All authors attest to not having any potential conflict-of-interests to disclose.
Competing interests: the authors have no competing interests.
Authors’ contribution
Kim Kopenhaver Doheny and Puneet Jairath performed the research
Kim Kopenhaver Doheny, R. Alberto Travagli and Charles Palmer wrote the paper
Kim Kopenhaver Doheny, Kirsteen N Browning, Charles Palmer and R. Alberto Travagli contributed intellectual inputs to the study
Kim Kopenhaver Doheny, Duanping Liao and Fan He conducted the statistical analyses
References
- 1.Blakely ML, Lally KP, McDonald S, Brown RL, Barnhart DC, Ricketts RR, et al. Postoperative outcomes of extremely low birth-weight infants with necrotizing enterocolitis or isolated intestinal perforation: a prospective cohort study by the NICHD Neonatal Research Network. Annals of surgery. 2005 Jun;241(6):984–9. doi: 10.1097/01.sla.0000164181.67862.7f. discussion 9-94. PubMed PMID: 15912048. Pubmed Central PMCID: 1359076. Epub 2005/05/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma R, Hudak ML. A clinical perspective of necrotizing enterocolitis: past, present, and future. Clinics in perinatology. 2013 Mar;40(1):27–51. doi: 10.1016/j.clp.2012.12.012. PubMed PMID: 23415262. Pubmed Central PMCID: 3575605. Epub 2013/02/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oh S, Young C, Gravenstein N, Islam S, Neu J. Monitoring technologies in the neonatal intensive care unit: implications for the detection of necrotizing enterocolitis. Journal of perinatology: official journal of the California Perinatal Association. 2010 Nov;30(11):701–8. doi: 10.1038/jp.2010.9. PubMed PMID: 20336080. Epub 2010/03/26. [DOI] [PubMed] [Google Scholar]
- 4.Verklan MT, Padhye NS. Spectral analysis of heart rate variability: an emerging tool for assessing stability during transition to extrauterine life. J Obstet Gynecol Neonatal Nurs. 2004 Mar-Apr;33(2):256–65. doi: 10.1177/0884217504263301. PubMed PMID: 15095805. Epub 2004/04/21. [DOI] [PubMed] [Google Scholar]
- 5.Griffin MP, Moorman JR. Toward the early diagnosis of neonatal sepsis and sepsis-like illness using novel heart rate analysis. Pediatrics. 2001 Jan;107(1):97–104. doi: 10.1542/peds.107.1.97. PubMed PMID: 11134441. [DOI] [PubMed] [Google Scholar]
- 6.Rosen H, Craelius W, Curcie D, Hiatt M, Hegyi T. Spectral analysis of heart variability in the newborn infant. Biol neonate. 2000;77:224–229. doi: 10.1159/000014220. [DOI] [PubMed] [Google Scholar]
- 7.Fairchild KD, O’Shea TM. Heart rate characteristics: physiomarkers for detection of late- onset neonatal sepsis. Clinics in perinatology. 2010 Sep;37(3):581–98. doi: 10.1016/j.clp.2010.06.002. PubMed PMID: 20813272. Pubmed Central PMCID: 2933427. Epub 2010/09/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–65. [PubMed] [Google Scholar]
- 9.Porges SW. Cardiac vagal tone: a physiological index of stress. Neurosci Biobehav Rev. 1995;19:225–33. doi: 10.1016/0149-7634(94)00066-a. [DOI] [PubMed] [Google Scholar]
- 10.Porges SW. Vagal tone: a physiologic marker of stress vulnerability. Pediatrics. 1992 Sep;90(3 Pt 2):498–504. PubMed PMID: 1513615. Epub 1992/09/01. [PubMed] [Google Scholar]
- 11.Souza GG, Mendonca-de-Souza AC, Barros EM, Coutinho EF, Oliveira L, Mendlowicz MV, et al. Resilience and vagal tone predict cardiac recovery from acute social stress. Stress. 2007 Nov;10(4):368–74. doi: 10.1080/10253890701419886. PubMed PMID: 17853065. [DOI] [PubMed] [Google Scholar]
- 12.Griffin MP, Lake DE, O’Shea TM, Moorman JR. Heart rate characteristics and clinical signs in neonatal sepsis. Pediatr res. 2007 Feb;61(2):222–7. doi: 10.1203/01.pdr.0000252438.65759.af. PubMed PMID: 17237726. [DOI] [PubMed] [Google Scholar]
- 13.Huston JM, Tracey KJ. The pulse of inflammation: heart rate variability, the cholinergic anti-inflammatory pathway and implications for therapy. Journal of internal medicine. 2011 Jan;269(1):45–53. doi: 10.1111/j.1365-2796.2010.02321.x. PubMed PMID: 21158977. Epub 2010/12/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fairchild KD, Srinivasan V, Moorman JR, Gaykema RP, Goehler LE. Pathogen-induced heart rate changes associated with cholinergic nervous system activation. American journal of physiology: Regulatory, integrative and comparative physiology. 2011 Feb;300(2):R330–9. doi: 10.1152/ajpregu.00487.2010. PubMed PMID: 21068197. Pubmed Central PMCID: 3043803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazurak N, Seredyuk N, Sauer H, Teufel M, Enck P. Heart rate variability in the irritable bowel syndrome: a review of the literature. Neurogastroenterology & motility. 2012;2012:1–11. doi: 10.1111/j.1365-2982.2011.01866.x. [DOI] [PubMed] [Google Scholar]
- 16.Bravi A, Green G, Herry C, Wright HE, Longtin A, Kenny GP, et al. Do physiological and pathological stresses produce different changes in heart rate variability? Frontiers in physiology. 2013;4:197. doi: 10.3389/fphys.2013.00197. PubMed PMID: 23908633. Pubmed Central PMCID: 3726831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metz CNaT KJ. It takes nerve to dampen inflammation. Nat Immunol. 2005;6:756–7. doi: 10.1038/ni0805-756. [DOI] [PubMed] [Google Scholar]
- 18.Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007 Feb;117(2):289–96. doi: 10.1172/JCI30555. PubMed PMID: 17273548. Pubmed Central PMCID: 1783813. Epub 2007/02/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Zanden EP, E Boeckxstaens, G., de Jonge WJ. The vagus nerve as a modulator of intestinal inflammation. Neurogastroenterol Motil. 2009;21:6–17. doi: 10.1111/j.1365-2982.2008.01252.x. [DOI] [PubMed] [Google Scholar]
- 20.Ghia JE, Blennerhassett P, Collins SM. Vagus nerve integrity and experimental colitis. American journal of physiology gastrointestinal and liver physiology. 2007 Sep;293(3):G560–7. doi: 10.1152/ajpgi.00098.2007. PubMed PMID: 17585014. Epub 2007/06/23. [DOI] [PubMed] [Google Scholar]
- 21.Bonaz BL, Bernstein CN. Brain-gut interactions in inflammatory bowel disease. Gastroenterology. 2013 Jan;144(1):36–49. doi: 10.1053/j.gastro.2012.10.003. PubMed PMID: 23063970. [DOI] [PubMed] [Google Scholar]
- 22.Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu Rev Physiol. 2006;68:279–305. doi: 10.1146/annurev.physiol.68.040504.094635. PubMed PMID: 16460274. Pubmed Central PMCID: 3062484. Epub 2006/02/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Browning KN, Travagli RA. Plasticity of vagal brainstem circuits in the control of gastrointestinal function. Autonomic neuroscience: basic & clinical. 2011 Apr 26;161(1-2):6–13. doi: 10.1016/j.autneu.2010.11.001. PubMed PMID: 21147043. Pubmed Central PMCID: 3061976. Epub 2010/12/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veerappan S, Rosen H, Craelius W, Curcie D, Hiatt M, Hegyi T. Spectral Analysis of Heart Rate Variability in Premature Infants with Feeding Bradycardia. Pediatr Res. 2000;47(5):659–62. doi: 10.1203/00006450-200005000-00017. [DOI] [PubMed] [Google Scholar]
- 25.Padhye NS, Williams AL, Khattak AZ, Lasky RE. Heart rate variability in response to pain stimulus in VLBW infants followed longitudinally during NICU stay. Developmental psychobiology. 2009 Dec;51(8):638–49. doi: 10.1002/dev.20399. PubMed PMID: 19739134. Pubmed Central PMCID: 2936240. Epub 2009/09/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evans S, Seidman LC, Tsao JC, Lung KC, Zeltzer LK, Naliboff BD. Heart rate variability as a biomarker for autonomic nervous system response differences between children with chronic pain and healthy control children. Journal of pain research. 2013;6:449–57. doi: 10.2147/JPR.S43849. PubMed PMID: 23788839. Pubmed Central PMCID: 3684221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doheny KK, Tang X, Browning KN, Palmer C, Travagli RA. Correlation between decreased vagal activity and necrotizing enterocolitis (NEC) Gastroenterol. 2012;142:S47–S8. [Google Scholar]
- 28.Richardson DK, Phibbs CS, Gray JE, McCormick MC, Workman-Daniels K, Goldmann DA. Birth weight and illness severity: independent predictors of neonatal mortality. Pediatrics. 1993 May;91(5):969–75. PubMed PMID: 8474818. Epub 1993/05/01. [PubMed] [Google Scholar]
- 29.Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Annals of surgery. 1978 Jan;187(1):1–7. doi: 10.1097/00000658-197801000-00001. PubMed PMID: 413500. Pubmed Central PMCID: 1396409. Epub 1978/01/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin CR, Walker WA. Intestinal immune defences and the inflammatory response in necrotising enterocolitis. Seminars in fetal & neonatal medicine. 2006 Oct;11(5):369–77. doi: 10.1016/j.siny.2006.03.002. PubMed PMID: 16690363. Epub 2006/05/13. [DOI] [PubMed] [Google Scholar]
- 31.Hsueh W, Caplan MS, Qu XW, Tan XD, De Plaen IG, Gonzalez-Crussi F. Neonatal necrotizing enterocolitis: clinical considerations and pathogenetic concepts. Pediatric and developmental pathology: the official journal of the Society for Pediatric Pathology and the Paediatric Pathology Society. 2003 Jan-Feb;6(1):6–23. doi: 10.1007/s10024-002-0602-z. PubMed PMID: 12424605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frost BL, Jilling T, Caplan MS. The importance of pro-inflammatory signaling in neonatal necrotizing enterocolitis. Seminars in perinatology. 2008 Apr;32(2):100–6. doi: 10.1053/j.semperi.2008.01.001. PubMed PMID: 18346533. Pubmed Central PMCID: 2362144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thayer JF. Vagal tone and the inflammatory reflex. Cleveland Clinic journal of medicine. 2009 Apr;76(Suppl 2):S23–6. doi: 10.3949/ccjm.76.s2.05. PubMed PMID: 19376977. Epub 2009/04/25. [DOI] [PubMed] [Google Scholar]
- 34.Rosas-Ballina M, Tracey KJ. Cholinergic control of inflammation. Journal of internal medicine. 2009 Jun;265(6):663–79. doi: 10.1111/j.1365-2796.2009.02098.x. PubMed PMID: 19493060. Epub 2009/06/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000 May 25;405(6785):458–62. doi: 10.1038/35013070. PubMed PMID: 10839541. [DOI] [PubMed] [Google Scholar]
- 36.Benkoe T, Baumann S, Weninger M, Pones M, Reck C, Rebhandl W, et al. Comprehensive evaluation of 11 cytokines in premature infants with surgical necrotizing enterocolitis. PloS one. 2013;8(3):e58720. doi: 10.1371/journal.pone.0058720. PubMed PMID: 23472217. Pubmed Central PMCID: 3589358. Epub 2013/03/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Field T, Diego M. Vagal activity, early growth and emotional development. Infant behavior & development. 2008 Sep;31(3):361–73. doi: 10.1016/j.infbeh.2007.12.008. PubMed PMID: 18295898. Pubmed Central PMCID: 2556849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patural H, Barthelemy JC, Pichot V, Mazzocchi C, Teyssier G, Damon G, et al. Birth prematurity determines prolonged autonomic nervous system immaturity. Clinical autonomic research: official journal of the Clinical Autonomic Research Society. 2004 Dec;14(6):391–5. doi: 10.1007/s10286-004-0216-9. PubMed PMID: 15666067. Epub 2005/01/25. [DOI] [PubMed] [Google Scholar]
- 39.Stone ML, Tatum PM, Weitkamp JH, Mukherjee AB, Attridge J, McGahren ED, et al. Abnormal heart rate characteristics before clinical diagnosis of necrotizing enterocolitis. Journal of perinatology: official journal of the California Perinatal Association. 2013 May 30; doi: 10.1038/jp.2013.63. PubMed PMID: 23722974. Epub 2013/06/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diego MA, Field T, Hernandez-Reif M, Deeds O, Ascencio A, Begert G. Preterm infant massage elicits consistent increases in vagal activity and gastric motility that are associated with greater weight gain. Acta paediatr. 2007 Nov;96(11):1588–91. doi: 10.1111/j.1651-2227.2007.00476.x. PubMed PMID: 17888059. Epub 2007/09/25. [DOI] [PubMed] [Google Scholar]