Abstract
Diabetes and hypertension are the leading causes of chronic kidney disease and their incidence is increasing at an alarming rate. Both are associated with impairments in the autoregulation of renal blood flow (RBF) and greater transmission of fluctuations in arterial pressure to the glomerular capillaries. The ability of the kidney to maintain relatively constant blood flow, glomerular filtration rate (GFR) and glomerular capillary pressure is mediated by the myogenic response of afferent arterioles working in concert with tubuloglomerular feedback that adjusts the tone of the afferent arteriole in response to changes in the delivery of sodium chloride to the macula densa. Despite intensive investigation, the factors initiating the myogenic response and the signaling pathways involved in the myogenic response and tubuloglomerular feedback remain uncertain. This review focuses on current thought regarding the molecular mechanisms underlying myogenic control of renal vascular tone, the interrelationships between the myogenic response and tubuloglomerular feedback, the evidence that alterations in autoregulation of RBF contributes to hypertension and diabetes-induced nephropathy and the identification of vascular therapeutic targets for improved renoprotection in hypertensive and diabetic patients.
Keywords: Afferent arteriole, glomerulus, kidney, myogenic response, tubuloglomerular feedback
INTRODUCTION
Renal blood flow (RBF) autoregulation is a vital homeostatic mechanism that protects the kidney from elevations in arterial pressure that would be transmitted to the glomerular capillaries and cause injury. It also allows the kidney to maintain a relatively constant blood flow and glomerular filtration rate (GFR) necessary for the clearance of metabolic wastes while maintaining efficient recovery of filtered electrolytes and nutrients by the renal tubules. Two mechanisms contribute to autoregulation of RBF. The first is the myogenic response of preglomerular arterioles. Elevations in transmural pressure induce contraction of preglomerular arterioles, predominantly at the level of afferent arterioles. The other mechanism is tubuloglomerular feedback which acts in concert with the myogenic response. It senses changes in the concentration of sodium chloride in the tubular fluid reaching the macula densa cells in the distal tubule and adjusts the diameter of the afferent arteriole accordingly [1, 2]. Tubuloglomerular feedback serves as an effective autoregulatory mechanism because the sodium chloride concentration of the fluid reaching the macula densa is dependent on flow rate, which in turn, is related to the GFR and glomerular capillary pressure.
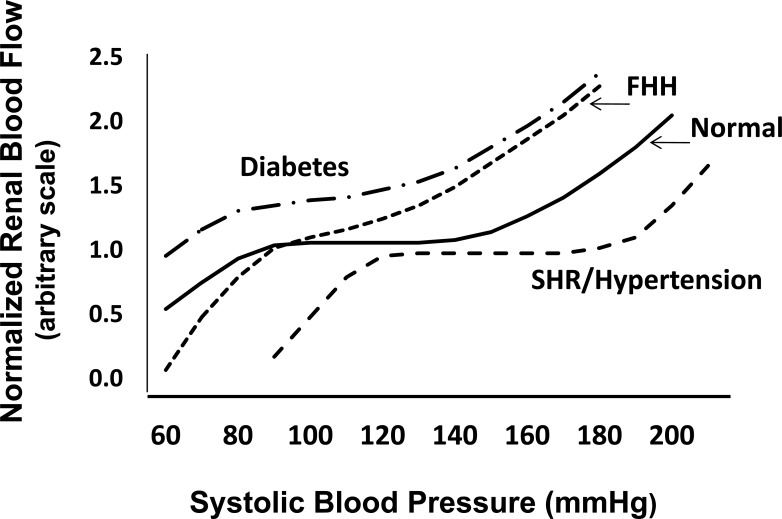
As is presented in (Fig. 1), RBF remains relatively constant in normal rats over a range of mean arterial pressures between 90 and 150 mmHg and from 70 to 130 mmHg in humans. The myogenic response of the afferent arterioles accounts for most of the rapid compensation to changes in arterial pressure in the range of 0.1 to 0.3Hz (3-10 secs).
Fig.(1).
Autoregulation of renal blood flow in normal and hypertensive individuals. Blood flow in normal subjects is maintained nearly constant over a range of arterial pressures from 70 to 120 mm Hg. The autoregulatory range is shifted to higher pressures in mild to moderate hypertensive patients and animal models of hypertension such as SHR and angiotensin II infused hypertensive rodents due to elevations in preglomerular vascular tone and structural changes in the microcirculation. Renal vascular resistance is reduced in diabetes and the efficiency of autoregulation is impaired. The fawn hooded hypertensive (FHH) rat exhibits a lack of a myogenic response in the preglomerular vasculature and impaired autoregulation of RBF and they develop severe glomerulosclerosis. Autoregulation is also impaired in salt sensitive forms of hypertension (Dahl salt sensitive rats, mineralocorticoid hypertension and reduced renal mass) and all are susceptible to glomerulosclerosis.
Tubuloglomerular feedback acts more slowly, and contributes more to the compensation to slow changes in arterial pressure in the range of 0.05Hz (>20 seconds) [3-8]. In general, the available evidence indicates that the myogenic response is most important in protecting the glomerular capillaries against rapid elevations in arterial pressure, while tubuloglomerular feedback is more involved in maintaining RBF and GFR in response to sustained reductions in arterial pressure [9-3].
Elevations in vascular resistance, especially in the renal circulation, are characteristic of hypertension. There is also generalized endothelial dysfunction associated with diminished vasodilatory responses to shear stress and other stimuli. In the spontaneously hypertensive rat (SHR) and angiotensin II dependent models of hypertension and in patients with mild or moderate hypertension, there is an elevation in renal vascular resistance that is in general appropriate for the degree of hypertension, so baseline RBF and GFR remain in the normal range and renal damage is minimal [14, 15]. However, the range of autoregulation of RBF is shifted to higher pressures and the magnitude of the shift is dependent on the severity and duration of the hypertension [16, 17] (Fig. 1), which is largely due to hypertrophy and thickening of the wall of the preglomerular arterioles [18], and potentiation of myogenic responsiveness [19-22]. In patients with moderate hypertension, the shift in the autoregulatory relationship is not severe and arterial pressure can still be lowered into the normotensive range with antihypertensive therapy without compromising renal function. In more malignant forms of hypertension, however, the hypertrophy of the vascular wall narrows the lumen of preglomerular arterioles sufficiently to lower baseline RBF and GFR and it is often not possible to lower blood pressure into the normotensive range without a decline in renal function [23]. Under these conditions, a gradual reduction in blood pressure is recommended.
In contrast to the increase in renal vascular resistance and augmented myogenic autoregulatory responses observed in SHR and in patients with essential hypertension, autoregulation of RBF is impaired in Fawn Hooded Hypertensive (FHH) rats [24, 25], Dahl salt-sensitive rats [26-28], rats with mineralocorticoid induced hypertension [29, 30] and in animals with reduced renal mass [31] (Fig. 1). The impairment of RBF autoregulation leads to increased glomerular capillary pressure and glomerular injury with the development of proteinuria, glomerulosclerosis and renal fibrosis in Dahl salt sensitive [32], FHH [25, 33-35] and in hypertensive rats treated with deoxycorticosterone acetate (DOCA) and a high salt diet. A similar abnormality in glomerular capillary hemodynamics is thought to contribute to the rapid development of glomerulosclerosis in hypertensive African American patients [36] who are four times more likely to develop chronic kidney disease than caucasian patients [37-41]. Alterations in renal hemodynamics is also thought to play a critical role in the initiation and progression of diabetic nephropathy [42]. Hypertension and diabetes now accounts for 67% of new cases of end-stage renal disease with total estimated cost in 2007 of $35.3 billion ($23.9 billion in Medicare costs alone) for the treatment of an estimated 500,000 patients in the US [43]. Thus, new therapies for slowing the development and progression of glomerulosclerosis in diabetic and hypertensive patients is of utmost importance. A better understanding of the molecular mechansims underlying renal autoregulation is key to someday achieving this goal.
MYOGENIC RESPONSE
The myogenic response is an intrinsic property of vascular smooth muscle (VSM) that allows arterioles to constrict in response to elevations in transmural pressure. The myogenic response can be readily demonstrated in de-endothelialized arterioles in vitro devoid of endothelial or parenchymal influences [44-46]. Despite intensive investigation, identification of the mechanosensors responsible for initiating the myogenic response and many aspects of the signaling pathways remain uncertain. The myogenic response involves stretch activation of mechanosensitive ion channels resulting in depolarization of VSM cells and calcium influx through L-type voltage-gated calcium channels (VGCC), Ca2+/calmodulin-dependent phosphorylation of myosin light chain kinase and sensitization of actinomyosin-based contractile mechanisms [47, 48]. There is evidence that the initial mechanotransduction event involves an interaction of cell surface integrins with extracellular matrix proteins such as fibronectin and actin filaments in the cytoskeleton, since blockade of integrins with integrin binding peptide inhibits Ca2+ currents and myogenic tone of skeletal muscle arterioles [47-49]. There is also evidence that β and γ subunits of epithelial sodium channels (ENaC) are expressed in vascular smooth muscle cells and that they play some role in the mechanotransduction event since administration of blockers of ENaC; benzamil and amiloride, impair the myogenic response of isolated vessels [50-53]. Several investigators have identified non-selective cation channels in vascular smooth muscle cells that are activated by membrane stretch [54-56]. These channels were initially thought to be directly activated by membrane stretch to promote Na+ or Ca2+ entry which depolarizes VSM cells and facilitates Ca2+ entry through VGCC to initiate the myogenic response. However, more recent evidence indicates that the stretch-activated cation channels are likely transient receptor potential melastatin 4 (TRPM4) and transient receptor potential canonical 6 (TRPC6) channels that respond to stretch through activation of second messenger signaling pathways rather than direct mechanical activation [57-59]. In support of this view, Early and Brayden have shown that both TRPM4 and TRPC6 channels are expressed in VSM cells and knockdown of the expression of these channels impairs the myogenic response in cerebral arteries [58, 59]. Similar studies in renal afferent arterioles have yet to be performed.
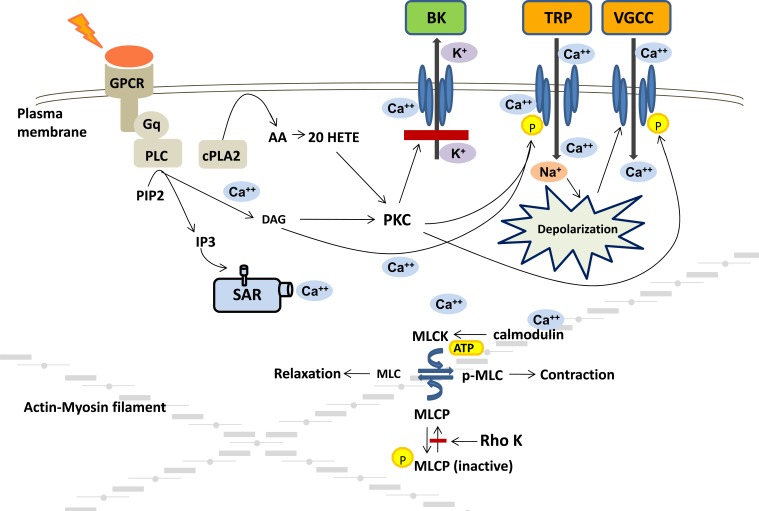
More recent data have indicated that the stretch activation of the TRP channels in cell systems requires co-expression of the channels with Gq dependent receptors, such as the angiotensin type 1, endothelin, vasopressin or histamine receptors [57]. An emerging view summarized in (Fig. 2) is that membrane stretch causes this class of receptors to interact with Gq proteins in the membrane to activate phospholipase C (PLC) and increase the production of diacylglycerol (DAG) and inositol trisphosphate (IP3) [60]. IP3 then promotes the release of Ca2+ from the sarcoplasmic reticulum near the membrane such that local Ca2+ concentration can reach levels sufficient to activate TRPM4 channels to allow Na+ entry and depolarize the membrane [58]. DAG can also directly activate TRPC6 channels [57] and protein kinase C (PKC) which phosphorylates and sensitizes TRPM4 channels to increases in intracellular Ca2+ and enhances the degree of depolarization sufficiently to allow Ca2+ entry through VGCC. The activation of PKC also has additional actions to enhance the depolarization and activation of vascular smooth muscle. In this regard, PKC is known to phosphorylate the VGCC to enhance the voltage sensitivity of this channel to facilitate Ca2+ entry [61]. Indeed, there are studies indicating that inhibitors of PLC [62] and PKC [63, 64] block the myogenic response of renal and cerebral arteries.
Fig.(2).
Mechanisms involved in myogenic response of preglomerular renal arterioles. Stretch activation of Gq dependent receptors activates phospholipase C (PLC) and increases the production of diacylglycerol (DAG) and inositol trisphosphate (IP3). IP3 promotes the release of Ca2+ from the sarcoplasmic reticulum (SR) to increase local Ca2+ concentration sufficiently to activate transient receptor potential melastatin 4 (TRPM4) channels. DAG activates transient receptor potential canonical 6 (TRPC6) channels and protein kinase C (PKC) which phosphorylates and sensitizes TRPM4 channels to depolarize the membrane sufficiently to allow Ca2+ entry through voltage gated calcium channels (VGCC). The activation of PKC also blocks calcium activated large conductance potassium (BK) channel activity to enhance the degree of depolarization and phosphorylates the VGCC to enhance the voltage sensitivity and facilitate Ca2+ entry. The rise in intracellular Ca2+ initiates the contraction and is followed by activation of additional mechanisms that enhance the sensitivity of the contractile mechanism to Ca2+.
Activation of non-selective cation channels (NSCC) such as TRPM4 or TRPC6 and subsequent local increases in intracellular Ca2+ also activate large conductance potassium channels, (BK) [65-67] (Fig. 2). BK channels are also activated by the transient calcium release (Ca2+ sparks) through ryanodine receptors located in the sarcoplasmic reticulum and in close proximity to these channels. In VSM cells, Ca2+ sparks give rise to spontaneous transient outward K+ currents (STOCs) that hyperpolarize the cells [68]. The magnitude and frequency of STOCs can be increased by membrane depolarization, sarcoplasmic reticulum Ca2+ release, and Ca2+ influx through VGCC [69]. The interplay between the activation of NSCC, Ca2+ sparks and activation of BKchannels oppose depolarization of VSM cells and inhibit myogenic responsiveness [65, 66]. Thus, there must be mechanisms in place to blunt the opening of BK channels. In this regard, there is evidence that PKC phosphorylates and inhibits activation of BK channels [65, 66]. There is also considerable evidence that a cytochrome P450 metabolite of arachidonic acid, namely 20-hydroxyeicosatetraenoic acid (20-HETE), plays a key role in augmenting the myogenic response by blocking BK channels [63, 64, 70, 71]. In this regard, the rise in intracellular Ca2+ following stretch of VSM cells activates phospholipase A2 which stimulates the release of arachidonic acid (AA). AA has been shown to be avidly converted to 20-HETE by cytochrome P450 enzymes of the 4A family expressed in renal afferent arterioles [72]. 20-HETE is a potent constrictor of renal arterioles [73, 74] that activates PKC [63, 64, 75], mitogen activated protein kinase [76, 77], src-type tyrosine kinase [78] and rho kinase [79]. 20-HETE potentiates the myogenic response by activating PKC which has been shown to phosphorylate and inhibit BK channels thereby allowing for sustained depolarization of VSM cells [64, 70, 71, 80]. 20-HETE also augments activation of TRPC6 channels enhancing depolarization and increasing activity of L-type calcium channels [81]. Indeed, elevations of transmural pressure increase 20-HETE levels in isolated arterioles [82] and inhibitors of the synthesis of 20-HETE block the myogenic response in isolated renal [83] and cerebral arteries [82] in vitro and impair autoregulation of renal [84] and cerebral blood flow [82] in vivo.
The initial rise in intracellular calcium concentration following activation of the myogenic response is transient, generally falling to levels near or slightly elevated from baseline, whereas the vasoconstrictor response is sustained. This indicates that there must be an increase in the Ca2+ sensitivity of contractile apparatus that plays a key role in the response. As seen in (Fig. 2), the rise in intracellular Ca2+ is thought to activate myosin light chain kinase via a Ca2+-calmodulin dependent process and phosphorylate the 20 kD regulatory subunit of myosin light chain. This permits the myosin light chain to more effectively bind to actin and hydrolyze ATP to enhance cross bridge formation and cycling. Myosin light chain phosphatase normally dephosphorylates the light chain to limit cross bridge formation and allow for diminished tone [85]. Recent studies indicate that the sustained activation of vascular smooth muscle cells is related not only to increased myosin light chain kinase activity but also to inhibition of myosin light chain phosphatase activity. This is due to the increase in PKC activity induced by DAG, [86, 87] and 20-HETE that activate a rho-A sensitive kinase that phosphorylates and inhibits the activity of myosin light chain phosphatase [79]. In summary, the myogenic response involves stretch activation of ion channels, depolarization and Ca2+ influx through VGCC that initiates contraction that is followed by other mechanisms that enhance the Ca2+ sensitivity of the contractile mechanism that are essential for the sustained contractile response.
TUBULOGLOMERULAR FEEDBACK
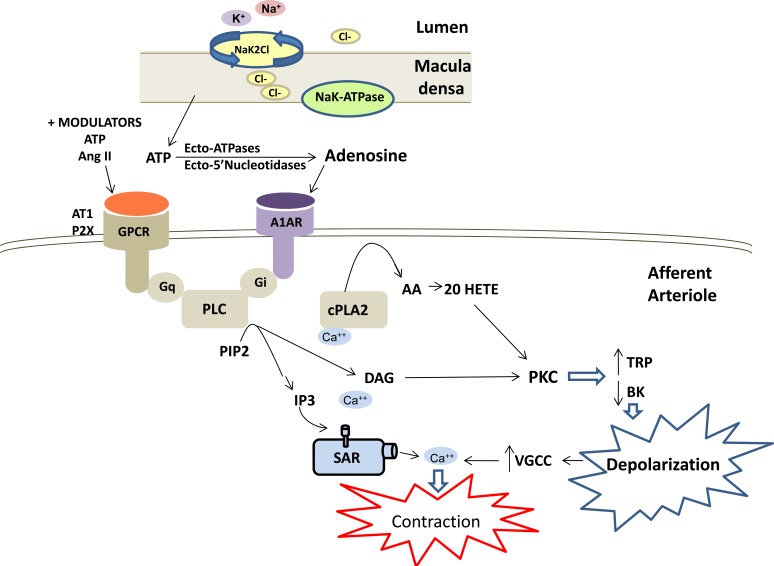
The other mechanism contributing to autoregulation of RBF is tubuloglomerular feedback [1, 2, 88]. This mechanism senses increases in the concentration of sodium chloride (NaCl) in tubular fluid reaching the macula densa cells of the distal nephron which then acts to decrease the diameter of the juxtaposed afferent arteriole. Tubuloglomerular feedback serves as a negative feedback loop to stabilize GFR to enable excretion of metabolic waste and to prevent transmission of elevations in arterial pressure from being transmitted to the glomerular capillaries and damaging the glomerulus. A summary of the proposed mechanism of tubuloglomerular feedback is presented in (Fig. 3). Increases in arterial pressure, that are not fully compensated via the myogenic response, increase glomerular capillary pressure and GFR and inhibit Na+ reabsorption in the proximal tubule via the mechanism of pressure natriuresis [89, 90]. This leads to an increase in the concentration of NaCl delivered to the distal nephron which increases Na+ uptake by the Na-K-2Cl co-transporter in the apical membrane of the macula densa. The essential role of Na+ transport via the Na-K-2Cl cotransporter in triggering tubuloglomerular feedback is based on the observations that inclusion of loop diuretics in the tubular perfusate nearly completely blocks the tubuloglomerular feedback response [91-93] and the observation that targeted knockout of the Na-K-2Cl, A or B genes, impairs the tubuloglomerular feedback response in mice [94, 95]. The uptake of Na+ via the Na-K-2Cl transporter is thought to increase intracellular Na+ concentration and the activity of Na K-ATPase and increase intracellular Ca2+ levels in the macula densa [96]. This is associated with increased release of ATP from the basolateral membrane of macula densa cells through a maxi anion channel [97, 98].
Fig.(3).
Mechanisms involved in tubuloglomerular feedback control of renal vascular tone. Tubuloglomerular feedback is initiated when the filtered load of sodium chloride is increased to the macula densa resulting in increases of; sodium reabsorption via the sodium potassium 2 chloride exchanger (NaK2Cl), intracellular Na+, sodium potassium ATPase (NaK-ATPase) activity and intracellular Ca2+. These events trigger the release of ATP from the macula densa cells. ATP is converted to adenosine extracellularly and then acts on adenosine 1A receptors (A1AR) in the adjacent afferent arteriole to promote vasoconstriction. A1AR activates phospholipase C (PLC) via a GTP binding protein (Gi), the release of inositol trisphosphate (IP3) and diacylglycerol (DAG). The subsequent activation of cytosolic phospholipase A2, formation of arachidonic acid (AA) and 20-HETE, as well as activation of protein kinase C (PKC), alters transient receptor potential channel (TRPC) and large conductance calcium activated potassium channel (BK) activities resulting in depolarization and activation of voltage gated calcium channels (VGCC) and Ca2+ influx. The increase in intracellular Ca2+ initiates contraction of the afferent arteriole, reduction in glomerular capillary pressure and filtered sodium chloride load. Activation of the angiotensin II receptor type I (AT1R) stimulates a similar transduction pathway to that shown in (Fig. 2) for the myogenic response. In addition, ATP and angiotensin II have been shown to be positive modulators of tubuloglomerular feedback through activation of G-protein coupled receptors, specifically purinergic receptor 2X (P2X) and angiotensin 1 receptor (AT1) via Gq to modulate PLC activity.
There still remains some controversy regarding whether the ATP that is released from the macula densa then acts directly on the afferent arteriole to elicit vasoconstriction through stimulation of the ATP P2X receptors or whether it is degraded by ecto-ATPases and nucleotidases to adenosine that constricts the afferent arteriole through activation of the adenosine A1A receptor [99-104]. The evidence supporting the role for ATP in mediating tubuloglomerular feedback is that administration of the P2X1 receptor antagonist, NF-279, impairs the response of afferent arterioles to elevations in perfusion pressure in the isolated perfused juxtamedullary nephron preparation [105]. Similarly, knockout of the P2X1 receptor in mice impairs the response of afferent arterioles to elevations in perfusion pressure in the isolated perfused juxtamedullary nephron preparation [105, 106] and autoregulation of RBF in vivo [107] but tubuloglomerular feedback responses to increases in tubular flow to the macula densa in vivo are not significantly altered [108].
Two independent laboratories have reported that tubuloglomerular feedback responses are completely absent in adenosine A1A receptor knockout mice and this is associated with impaired autoregulation of RBF and GFR especially in response to reductions in renal perfusion pressure [109-111]. Similarly, tubuloglomerular feedback responses are impaired in mice with deletion of the NTPDase1 gene, an extracellular ATPase that dephosphorylates ATP to ADP or ADP to AMP by deletion of ecto-5’-nucleotidase cd73 [99, 102]. Adenosine has a direct effect to constrict isolated perfused afferent arterioles and this effect is blocked by an adenosine A1A receptor antagonist and in vessels obtained from A1R knockout mice [104, 112]. As presented in (Fig. 3), previous studies have indicated that the vasoconstrictor response to adenosine in the afferent arteriole is mediated by Gi-dependent activation of PLC via the bã subunit [113, 114] followed by release of DAG and IP3, the release of Ca2+ from intracellular stores, activation of PKC, depolarization of the membrane and Ca2+ influx in a manner analogous to the activation of the myogenic response by stretch or the vasoconstrictor response to angiotensin II via the angiotensin II receptor type I (AT1R) and Gq dependent pathways. Similarly, there is evidence that ATP, and possibly adenosine as well, stimulate the formation of 20-HETE in the afferent arteriole [115] and that 20-HETE may contribute to the vasoconstrictor response to these agonists by inhibiting BK channels and enhancing calcium influx [70]. In this regard, inhibitors of the formation of 20-HETE have been reported to impair autoregulation of tubuloglomerular feedback responses in the rat in vivo [116].
INTERACTIONS BETWEEN G-PROTEIN COUPLED VASOCONSTRICTORS, MYOGENIC RESPONSE AND TUBULOGLOMERULAR FEEDBACK MECHANISMS IN THE AUTOREGULATION OF RBF AND GLOME-RULAR CAPILLARY PRESSURE
An examination of the pathways summarized in (Figs.2and3) illustrates that elevations in transmural pressure (Fig. 2) and tubuloglomerular feedback (Fig. 3) activate the same second messenger signal transduction cascade characterized by activation of PLC, increases in DAG and IP3, cellular depolarization and influx of intracellular calcium. Moreover, this same pathway is involved in the response of the afferent arteriole to vasoconstrictor agonists acting via the AT1, P2X and endothelin ETA receptors [1, 117-119]. Given the convergence of these second messenger pathways, it is not surprising that the myogenic response, tubuloglomerular feedback and G protein coupled vasoconstrictor pathways interact synergistically to regulate the tone of the afferent arteriole. For example, Inoue et al. [81] recently demonstrated that membrane stress/pressure induced responses were synergistically augmented in A7r5 cells and mesenteric arteries that normally respond poorly to membrane stress/stretch after low level Gq-receptor activation. They further demonstrated that the synergism was linked to increased production of 20-HETE. Other studies have indicated that activation of the angiotenin II AT1 or adenosine A1A receptor augments the myogenic response [104, 110, 114, 120, 121]. Similarly, blockade of the renin angiotensin system with angiotensin converting enzyme (ACE) inhibitors or AT1 receptor blockers (ARBs) attenuate tubuloglomerular feedback responses and shift the relationships between flow to the macula densa and stop flow pressure, and index of glomerular capillary pressure to the right [122, 123]. Although there is little direct data to support this hypothesis, one would also expect that increases in arterial pressure and myogenic tone would increase tubuloglomerular feedback responsiveness. Moreover, elevations in perfusion pressure should potentiate the dose response relationship to vasoconstrictor agonists, and subpressor levels of angiotensin, adenosine and endothelin should potentiate myogenic and tubuloglomerular feedback responsiveness. These types of interactions have been postulated to play a role in the enhanced myogenic responsiveness and elevated renal vascular resistance seen in angiotensin II models of hypertension and in the SHR, in which the kidney is generally protected from the development of glomerular injury until late in the disease process when arterial pressure rises beyond the reset autoregulatory range.
MODULATORS OF MYOGENIC RESPONSE AND TUBULOGLOMERULAR FEEDBACK REPONSIVE-NESS
Shear stress is directly dependent on the velocity of flow in a vessel and is inversely proportional to vascular diameter. Shear stress stimulates the release of endothelial-derived relaxing factors that act as negative modulators of autoregula-tory responses. Following activation of myogenic or tubulo-glomerular feedback, the decrease in the diameter of the afferent arteriole increases shear stress which stimulates the release of vasodilator mediators from the endothelium that oppose further vasoconstriction. The mediators that are released include, nitric oxide, prostaglandin E2, prostacyclin, epoxyeicosatrienoic acids (EETs) which dilate vessels by opening potassium channels which hyperpolarize vascular smooth muscle cells and oppose calcium influx [88, 124-126]. Each uses a different signal transduction pathway. Nitric oxide stimulates the formation of cGMP [127] and binds heme in CYP450 enzymes to inhibit the formation of 20-HETE in vascular smooth muscle cells [71, 128]. Both of these effects open potassium channels to hyperpolarize the cell and diminish calcium entry through voltage sensitive channels [129]. Prostacyclin and prostaglandin E2 act on receptors to promote vasodilation via cAMP-dependent pathway [125]. EETs are metabolites of arachidonic acid formed by cytochrome P450 enzymes of the 2C family that are produced by the endothe-lium. They are potent vasodilators that open BK channels in renal vascular smooth muscle cells by stimulating the formation of cAMP [130, 131] and/or c-AMP ribose [126, 132, 133]. In many vascular beds EETs have been identified as an endothelial derived hyperpolarizing factor that mediates the response to endothelial dependent vasodilators following blockade of nitric oxide sythase (eNOS) and cyclooxygenase [134].
Nitric oxide (NO) and arachidonic acid metabolites also play very important roles in modulating the activity of tubuloglomerular feedback [135]. Neuronal nitric oxide synthase (nNOS) is expressed in macula densa cells and NO is released following activation of tubuloglomerular feedback [96, 136-138]. Blockade of the formation of NO in the macula densa by administration L-NAME or more selective inhibitors of nNOS to the tubular perfusate markedly enhances the tubuloglomerular feedback response.
More recently, Ren et al. and Wang et al. [139, 140], have identified another important pathway that decreases the sensitivity of the tubuloglomerular feedback mechanism. They reported that increased flow in the connecting tubule of the distal nephron stimulates the production and release of both prostaglandin E2 and EETs and that both of these compounds diffuse to the juxtaposed afferent arteriole to promote vasodilation and oppose vasoconstriction Again, the physiologic significance of this modulatory pathway remains to be determined but it is likely a compensatory mechanism that allows the kidney to rapidly increase sodium excretion without reducing GFR following elevations in sodium intake. Moreover, upregulation of the formation of NO by the macula densa or the connecting tubule in diabetes and various forms of hypertension may oppose both myogenic and tubuloglomerular feedback responsiveness, leading to elevations in glomerular capillary pressure, which promotes the development of proteinuria and glomerular injury.
ROLE OF IMPAIRED AUTOREGULATORY MECHA-NISMS IN THE PATHOGENESIS OF GLOMERULOSCLEROSIS AND CHRONIC KIDNEY DISEASE
Although the range of autoregulation may be shifted toward higher pressures, autoregulatory mechanisms are intact in most patients with essential hypertension and they do not develop proteinuria or significant renal injury [141, 142]. Similar renoprotection is seen in the SHR model of hypertension [15] in which the myogenic response of the preglomerular vasculature is enhanced [14, 143] perhaps due to elevated production of 20-HETE [73]. Recent studies have indicated that renal vascular resistance is also markedly elevated in the angiotensin II infused mouse model of hypertension which develops ischemic renal injury to the glomerulus rather than proteinuria, mesangial matrix expansion and focal glomerular sclerosis [144].
In contrast, renal autoregulation is impaired in patients with diabetes, other forms of proteinuric chronic kidney disease (CKD) and in African Americans with low renin forms of hypertension. These patients exhibit increased susceptibility to the development of progressive glomerulosclerosis in response to even modest elevations in pressure [9]. Indeed, this is the reason for the recommendation for strict control of blood pressure in patients with diabetes and CKD and it underlies the rationale for the use of angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) that lower systemic pressure and reduce glomerular capillary pressure.
The increased susceptibility to renal injury observed in patients with diabetes and chronic kidney disease, as well as work done in wide variety of animal models of hypertension and diabetes, suggest that impairments in renal autoregulatory mechanisms leading to elevation in glomerular capillary pressure trigger the development of proteinuria and glomerulosclerosis. Work done in the 5/6 nephrectomy model of chronic kidney disease indicates that the remaining glomeruli hypertrophy and autoregulatory mechanisms are impaired. These animals exhibit hyperfiltration and elevated glomerular capillary pressure [9, 31, 145, 146]. With time these animals develop progressive proteinuria and glomerular sclerosis. Further support for a key role of alterations in renal hemodynamics in the development of renal injury are the findings that administration of calcium channel blockers, that further impair renal autoregulation, markedly accelerate the progression of renal disease in the 5/6 remnant kidney model [147-149].
Increased susceptibility to the development of proteinuria and focal segmental glomerulosclerosis has been observed in other rat models of hypertension that exhibit impaired autoregulation of RBF. These include the DOCA salt model of hypertension [29, 150] the unclipped kidney of 2 kidney 1 clip Goldblatt hypertensive rats [151, 152] and in the FHH rat [35, 153]. The FHH rat in particular develops proteinuria and focal segmental glomerulosclerosis at a relative young age that progresses to end state renal disease. These rats have little or no myogenic response in preglomerular renal arteries but tubuloglomerular feedback responses are intact [28, 35, 154, 155]. Autoregulation of RBF is markedly impaired in these rats and glomerular capillary pressure increases following elevations in renal perfusion pressure [25, 34, 35]. Similarly, Dahl S rats and Brown Norway rats exhibit impaired dynamic autoregulation of RBF related to a defect in the myogenic control of the renal vasculature [26, 156]. Both of these strains rapidly develop proteinuria and progressive glomerular disease following the development of hypertension [32, 157-159]. Glomerular capillary pressure is also thought to be elevated in African Americans [36, 160] with salt-sensitive forms of hypertension and this is thought to underlie the increased susceptibility to the development of proteinuria and chronic kidney disease.
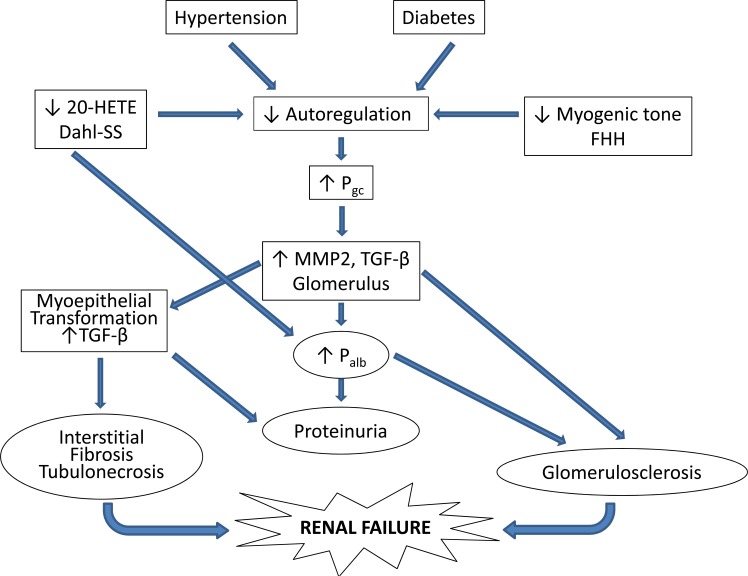
The mechanisms by which impaired autoregulation of RBF in diabetes, salt-sensitive forms of hypertension and chronic kidney disease promote the development of renal disease remain to be determined. A proposed mechanism is presented in (Fig. 4). The available data suggest that impaired autoregulation of RBF leads to an elevation in glomerular capillary pressure, which increases glomerular expression of transforming growth factor beta (TGF-β) through increases in stretch of the glomerular capillaries and adherent mesangial cells and podocytes [161-164] TGF-β is known to increase the production of collagen and fibronectin in cultured mesangial cells and podocytes [165]. There is also an increase in the renal expression of matrix metalloproteinase 2 (MMP2) which has been shown to increase the release of bound forms of TGF-β [166-168], activate the epidermal growth factor pathway and promote epithelial to mesenchymal transformation [169] which is critical to the development of renal fibrosis [170]. TGF-β has been shown to directly increase the permeability of isolated glomeruli to albumin [171, 172]. Damage to the glomerular filtration barrier and increased filtration of macromolecules and/or growth factors may trigger a positive feedback loop that further stimulates podocytes, mesangial cells and renal tubular epithelial cells to upregulate the expression of TGF-β, increasing the production of extracellular matrix. This may be one of the pathways for the development of focal glomerulosclerosis, renal interstitial fibrosis and tubular necrosis in hypertensive and diabetic patients. Ultimately, the sustained overexpression of TGF-β stimulates the production of extracellular matrix and leads to the collapse of glomerular capillaries (glomerulosclerosis), renal interstitial fibrosis, loss of nephrons and chronic renal failure.
Fig.(4).
Proposed mechanisms contributing to the development of diabetic and hypertensive nephropathy. Autoregulation of renal blood flow is impaired in patients with salt sensitive forms of hypertension and diabetes as well as in Dahl salt sensitive (Dahl-SS) and Fawn-hooded hypertensive (FHH) rats. The impaired autoregulation leads to greater transmission of fluctuations in arterial pressure to the glomerulus and increased glomerular capillary pressure (Pgc). Elevated Pgc results in increased expression of matrix metalloproteases and transforming growth factor beta (TGF-β ). Increased TGF-β promotes glomerulosclerosis, increased glomerular permeability to albumin (Palb), myoepithelial transformation, proteinuria and renal interstitial fibrosis.
The mechanism by which TGF-β increases the glomerular permeability to albumin is still unknown. We have reported that TGF-β inhibits the synthesis of 20-HETE in isolated glomeruli and that pretreatment of glomeruli with 20-HETE mimetics opposes the effects of TGF-β to increase the glomerular permeability of albumin [173]. Moreover, inhibitors of the synthesis of 20-HETE mimic the action of TGF-β to increase the albumin permeability of the glomerulus and development of proteinuria [174]. These findings suggest that 20-HETE may also play a role in maintaining glomerular barrier function and that TGF-β may initiate the development of proteinuria and renal disease in part by inhibiting the formation of 20-HETE.
There is also overwhelming evidence that over expression of TGF-β plays a key role in the pathogenesis of renal fibrosis associated with exposure to nephrotoxins, renal ischemia and immune injury, as well as hypertension and diabetic nephropathy. Increased circulating and/or renal concentrations of TGF-β mRNA or protein have been reported in patients with diabetic nephropathy [175-178], following transplant rejection [179] and cyclosporine-induced nephropathy [180] and in various forms of glomerulosclerosis [181, 182]. Increases in renal expression of TGF-β have been reported in every animal model of renal injury examined to date [165]. This includes use of anti-thymocyte or anti-glomerular basement membrane serum and Heyman nephritis[183], cyclosporine and puromycin nephropathies [184, 185], remnant kidney [186, 187], ureteral obstruction[188], chronic allograph rejection [180] and radiation injury [189], as well as animal models of diabetes-induced renal injury including streptozotocin induced rodent models [178, 190], the biobreeding (BB) rat and the non obese diabetic (NOD) mouse model [191]. Hypertension models linked to elevated levels of TGF-β include: L-NAME [192, 193] and angiotensin II treated animals [165, 194], deoxycorticosterone acetate-salt hypertensive rats [150] and genetic models including stroke prone SHR rats [195] and Dahl S rats [172, 196]. Previous studies have shown that treatment of rats and mice with TGF-β2 induces a renal interstitial fibrosis in the outer medulla resembling that seen in patients with hypertension [197, 198]. Transgenic mice that over express TGF-β also develop glomerular lesions and tubulointerstitial renal disease resembling that seen in patients with hypertension and diabetes [199-203].
The most direct evidence for a role for TGF-β in the pathogenesis of renal disease comes from inhibitor studies. These studies have shown that chronic treatment of rats with a TGF-β neutralizing antibody [204], decorin, an endogenous inhibitor of TGF-β [205], or antisense constructs that down regulate the expression of TGF-β or TβRII [193] reduce the degree of glomerulosclerosis in the anti-Thy1 model of glomerulonephritis. Several investigators have reported that knock-down of the production of TGF-β with antisense TGF-β1 oligodeoxynucleotides or by blocking the actions of the TGF-β with a neutralizing antibody also reduces the degree of proteinuria and glomerular damage in diabetic mice [190, 206-208]. Beneficial effects of TGF-β neutralizing antibodies have also been reported in rats with puromycin nephropathy [185] and in mice with cyclosporin-induced nephropathy [209]. These studies indicate that TGF-β levels are elevated in diabetes, hypertension and following renal injury. They also suggest that TGF-β plays a causal role in the pathogenesis of renal fibrosis associated with these conditions. Sustained overproduction of TGF-β [196] also leads to apoptosis of podocytes [210, 211], mesangial cells[212] and tubular epithelial cells [213, 214],damage to the glomerular filtration barrier, proliferation of mesangial cells, increased deposition of extracellular matrix, and collapse of glomerular capillaries. TGF-β also promotes epithelial-mesenchymal transformation, [169, 170, 215, 216] leading to renal interstitial fibrosis, loss of capillaries and tubular necrosis.
POTENTIAL FOR NEW THERAPEUTIC STRATEGIES FOR IMPROVED RENAL PROTECTION
Overall, there is strong evidence that impaired autoregulation of RBF leading to increased transmission of fluctuations in system pressure to the glomerular capillaries markedly increases the susceptibility to the development of proteinuria, glomerulosclerosis and chronic kidney disease. Much of the work using animal models have indicated that this is largely associated with changes in the myogenic component of renal autoregulation. Examination of the signaling pathways involved indicates that the control of the tone of the afferent arteriole by myogenic, tubuloglomerular feedback and vasoconstrictor agonists activate common signaling pathways involving activation of PLC, IP3, DAG, release of intracellular Ca2+, opening of TRP channels, membrane depolarization, Ca2+ entry through VGCC and sensitization of the contractile mechanism to Ca2+. There are several potential intervention points that might restore the efficiency of the myogenic autoregulatory mechanisms to protect the glomerulus from barotrauma. These include; TRP channel agonists, BK channel blockers, 20-HETE agonists or CYP4A inducers, L-type calcium channel agonists and rho kinase agonists. Unfortunately, none of these approaches would be selective for increasing the tone of the afferent arteriole and would likely be associated with unacceptable risks and side effects such as the development of hypertension, cardiac arrhythmias and vasospasm. These same pathways also play a key role in cell growth and proliferation so these drugs may promote the development of vascular hypertrophy and arteriosclerosis or certain forms of cancer. As a result, it appears that the most effective strategy to slow the progression of renal disease for the foreseeable future will remain strict control of diabetes and the associated renal vasodilation and to reduce systemic pressure with ACE inhibitors and ARBs which lower glomerular capillary pressure by dilating the efferent arteriole. This approach may someday be augmented with the emergence of new anti-fibrotic agents that block the actions of TGF-β and matrix metalloproteinase 2. Indeed, recent data have been very promising in experimental animal models of diabetes, hypertension and renal disease. However, none of these compounds have yet entered clinical development for the prevention of hypertension and/or diabetic nephropathy in humans.
CONFLICT OF INTEREST
The authors of this article have no conflict of interest. This work was supported in part by grants HL-36279 and 29547 and predoctoral training grant HL-1T32HL105324-01 from the NIH.
ACKNOWLEDGEMENTS
All authors were involved in writing and editing of this article.
LIST OF ABBREVIATIONS
- ACE =
Angiotensin converting enzyme
- ARB =
Angiotensin receptor blocker
- BK =
Large conductance potassium channel
- DAG =
Diacylglycerol
- DAG =
Diacylglycerol
- DOCA =
Deoxycorticosterone acetate
- EET =
Eicosatetraenoic acid
- ENaC =
Epithelial sodium channels
- FHH rats =
Fawn hooded hypertensive
- GFR =
Glomerular filtration rate
- IP3 =
Inositol trisphosphate
- NOS =
Nitric oxide synthase
- MMP2 =
Matrix metalloproteinase 2
- Palb =
Glomerular permeability to albumin
- PLC =
Phospholipase C
- PKC =
Protein kinase C
- RBF =
Renal blood flow
- SHR =
Spontaneously hypertensive rats
- TGF-β =
Transforming growth factor beta
- TRPC6 =
Transient receptor potential canonical 6
- TRPC6 =
Transient receptor potential canonical 6
- TRPM4
Transient receptor potential melastatin 4
- VGCC
Voltage gated calcium channel
- VSM
Vascular smooth muscle
- 20-HETE
20-hydroxyeicosatetraenoic acid
REFERENCES
- 1.Navar LG, Inscho EW, Majid SA, Imig JD, Harrison-Bernard LM, Mitchell KD. Paracrine regulation of the renal microcirculation. Physiol Rev. 1996;76:425–536. doi: 10.1152/physrev.1996.76.2.425. [DOI] [PubMed] [Google Scholar]
- 2.Schnermann J, Briggs JP. Tubuloglomerular feedback mechanistic insights from gene-manipulated mice. Kidney Int. 2008;74:418–26. doi: 10.1038/ki.2008.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pires SL, Barres C, Sassard J, Julien C. Renal blood flow dynamics and arterial pressure lability in the conscious rat. Hypertension. 201l;38:147–52. doi: 10.1161/01.hyp.38.1.147. [DOI] [PubMed] [Google Scholar]
- 4.Chon KH, Chen YM, Holstein-Rathlou NH, Marsh DJ, Marmarelis VZ. On the efficacy of linear system analysis of renal autoregulation in rats. IEEE Trans Biomed Eng. 1993;40:8–20. doi: 10.1109/10.204766. [DOI] [PubMed] [Google Scholar]
- 5.Abu-Amarah I, Bidani AK, Hacioglu R, Williamson GA, Griffin KA. Differential effects of salt on renal hemodynamics and potential pressure transmission in stroke-prone and stroke-resistant spontaneously hypertensive rats. Am J Physiol Renal Physiol. 2005;289:F305–13. doi: 10.1152/ajprenal.00349.2004. [DOI] [PubMed] [Google Scholar]
- 6.Holstein-Rathlou NH, Wagner AJ, Marsh DJ. Tubuloglomerular feedback dynamics and renal blood flow autoregulation in rats. Am J Physiol. 1991;260:F53–68. doi: 10.1152/ajprenal.1991.260.1.F53. [DOI] [PubMed] [Google Scholar]
- 7.Daniels FH, Arendshorst WJ. Tubuloglomerular feedback kinetics in spontaneously hypertensive and Wistar-Kyoto rats. Am J Physiol. 1990;259:F529–34. doi: 10.1152/ajprenal.1990.259.3.F529. [DOI] [PubMed] [Google Scholar]
- 8.Cupples WA, Novak P, Novak V, Salevsky FC. Spontaneous blood pressure fluctuations and renal blood flow dynamics. Am J Physiol. 1996;270:F82–9. doi: 10.1152/ajprenal.1996.270.1.F82. [DOI] [PubMed] [Google Scholar]
- 9.Bidani AK, Griffin KA, Williamson G, Wang X, Loutzenhiser R. Protective importance of the myogenic response in the renal circulation. Hypertension. 2009;54:393–8. doi: 10.1161/HYPERTENSIONAHA.109.133777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loutzenhiser R, Griffin KA, Bidani AK. Systolic blood pressure as the trigger for the renal myogenic response protective or autoregulatoryκ. Curr Opin Nephrol Hypertens. 2006;15:41–9. doi: 10.1097/01.mnh.0000199011.41552.de. [DOI] [PubMed] [Google Scholar]
- 11.Loutzenhiser R, Bidani A, Chilton L. Renal myogenic response kinetic attributes and physiological role. Circ Res. 2002;90:1316–24. doi: 10.1161/01.res.0000024262.11534.18. [DOI] [PubMed] [Google Scholar]
- 12.Loutzenhiser R, Bidani AK, Wang X. Systolic pressure and the myogenic response of the renal afferent arteriole. Acta Physiol Scand. 2004;181:407–13. doi: 10.1111/j.1365-201X.2004.01312.x. [DOI] [PubMed] [Google Scholar]
- 13.Loutzenhiser R, Griffin K, Williamson G, Bidani A. Renal autoregulation new perspectives regarding the protective and regulatory roles of the underlying mechanisms. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1153–67. doi: 10.1152/ajpregu.00402.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arendshorst WJ, Beierwaltes WH. Renal and nephron hemodynamics in spontaneously hypertensive rats. Am J Physiol. 1979;236:F246–51. doi: 10.1152/ajprenal.1979.236.3.F246. [DOI] [PubMed] [Google Scholar]
- 15.Griffin KA, Churchill PC, Picken M, Webb RC, Kurtz TW, Bidani AK. Differential salt-sensitivity in the pathogenesis of renal damage in SHR and stroke prone SHR. Am J Hypertens. 2001;14:311–20. doi: 10.1016/s0895-7061(00)01282-6. [DOI] [PubMed] [Google Scholar]
- 16.Iversen BM, Sekse I, Ofstad J. Resetting of renal blood flow autoregulation in spontaneously hypertensive rats. Am J Physiol. 1987;252:F480–6. doi: 10.1152/ajprenal.1987.252.3.F480. [DOI] [PubMed] [Google Scholar]
- 17.Roman RJ, Cowley AW ., Jr Abnormal pressure-diuresis-natriuresis response in spontaneously hypertensive rats. Am J Physiol. 1985;248:F199–205. doi: 10.1152/ajprenal.1985.248.2.F199. [DOI] [PubMed] [Google Scholar]
- 18.Folkow B. Myogenic mechanisms in the control of systemic resistance, Introduction and historical background. J Hypertens. 1989;7:S1–4. [PubMed] [Google Scholar]
- 19.Hayashi K, Epstein M, Loutzenhiser R. Enhanced myogenic responsiveness of renal interlobular arteries in spontaneously hypertensive rats. Hypertension. 1992;19:153–60. doi: 10.1161/01.hyp.19.2.153. [DOI] [PubMed] [Google Scholar]
- 20.Ren Y, D'Ambrosio MA, Liu R, Pagano PJ, Garvin JL, Carretero OA. Enhanced myogenic response in the afferent arteriole of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2010;298:H1769–75. doi: 10.1152/ajpheart.00537.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gebremedhin D, Ma YH, Imig JD, Harder DR, Roman RJ. Role of cytochrome P-450 in elevating renal vascular tone in spontaneously hypertensive rats. J Vasc Res. 1993;30:53–60. doi: 10.1159/000158975. [DOI] [PubMed] [Google Scholar]
- 22.Imig JD, Falck Jr, Gebremedhin D, Harder DR, Roman RJ. Elevated renovascular tone in young spontaneously hypertensive rats. Role of cytochrome P-450. Hypertension. 1993;22:357–64. doi: 10.1161/01.hyp.22.3.357. [DOI] [PubMed] [Google Scholar]
- 23.Palmer BF. Impaired renal autoregulation implications for the genesis of hypertension and hypertension-induced renal injury. Am J Med Sci. 2001;21:388–400. doi: 10.1097/00000441-200106000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Van Dokkum RP, Alonso-Galicia M, Provoost AP, Jacob HJ, Roman RJ. Impaired autoregulation of renal blood flow in the fawn-hooded rat. Am J Physiol. 1999;276:R189–96. doi: 10.1152/ajpregu.1999.276.1.R189. [DOI] [PubMed] [Google Scholar]
- 25.Williams JM, Burke M, Lazar J, Jacob HJ, Roman RJ. Temporal characterization of the development of renal injury in FHH rats and FHH 1BN congenic strains. Am J Physiol Renal Physio. 201;300:F330–8. doi: 10.1152/ajprenal.00261.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takenaka T, Forster H, De Micheli A, Epstein M. Impaired myogenic responsiveness of renal microvessels in Dahl salt-sensitive rats. Circ Res. 1992;71:471–80. doi: 10.1161/01.res.71.2.471. [DOI] [PubMed] [Google Scholar]
- 27.Karlsen FM, Andersen CB, Leyssac PP, Holstein-Rathlou NH. Dynamic autoregulation and renal injury in Dahl rats. Hypertension. 1997;30:975–83. doi: 10.1161/01.hyp.30.4.975. [DOI] [PubMed] [Google Scholar]
- 28.Karlsen FM, Leyssac PP, Holstein-Rathlou NH. Tubuloglomerular feedback in Dahl rats. Am J Physiol. 1998;274:R1561–9. doi: 10.1152/ajpregu.1998.274.6.R1561. [DOI] [PubMed] [Google Scholar]
- 29.Moore LC, Schnermann J, Yarimizu S. Feedback mediation of SNGFR autoregulation in hydropenic and DOCA- and salt-loaded rats. Am J Physiol. 1979;237:F63–74. doi: 10.1152/ajprenal.1979.237.1.F63. [DOI] [PubMed] [Google Scholar]
- 30.Hill GS, Heptinstall RH. Steroid-induced hypertension in the rat A microangiographic and histologic study on the pathogenesis of hypertensive vascular and glomerular lesions. Am J Pathol. 1968;52:1–40. [PMC free article] [PubMed] [Google Scholar]
- 31.Bidani AK, Schwartz MM, Lewis EJ. Renal autoregulation and vulnerability to hypertensive injury in remnant kidney. Am J Physiol. 1987;252:F1003–10. doi: 10.1152/ajprenal.1987.252.6.F1003. [DOI] [PubMed] [Google Scholar]
- 32.Williams JM, Sarkis A, Hoagland KM , et al. Transfer of the CYP4A region of chromosome 5 from Lewis to Dahl S rats attenuates renal injury. Am J Physiol Renal Physiol. 2008;295:F1764–77. doi: 10.1152/ajprenal.90525.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simons JL, Provoost AP, Anderson S, Rennke HG, Troy JL, Brenner BM. Modulation of glomerular hypertension defines susceptibility to progressive glomerular injury. Kidney Int. 1994;46:396–404. doi: 10.1038/ki.1994.287. [DOI] [PubMed] [Google Scholar]
- 34.Simons JL, Provoost AP, Anderson S , et al. Pathogenesis of glomerular injury in the fawn-hooded rat early glomerular capillary hypertension predicts glomerular sclerosis. J Am Soc Nephrol. 1993;3:1775–82. doi: 10.1681/ASN.V3111775. [DOI] [PubMed] [Google Scholar]
- 35.van Dokkum RP, Sun CW, Provoost AP, Jacob HJ, Roman RJ. Altered renal hemodynamics and impaired myogenic responses in the fawn-hooded rat. Am J Physiol. 1999;276:R855–63. doi: 10.1152/ajpregu.1999.276.3.R855. [DOI] [PubMed] [Google Scholar]
- 36.Campese VM, Parise M, Karubian F, Bigazzi R. Abnormal renal hemodynamics in black salt-sensitive patients with hypertension. Hypertension. 1991;18:805–12. doi: 10.1161/01.hyp.18.6.805. [DOI] [PubMed] [Google Scholar]
- 37.Toto RB. Hypertensive nephrosclerosis in African Americans. Kidney Int. 2003;64:2331–41. doi: 10.1046/j.1523-1755.2003.00333.x. [DOI] [PubMed] [Google Scholar]
- 38.Freedman BI. Renal microvascular susceptibility in African American pedigrees. Transplant Proc. 1993;25:2423–5. [PubMed] [Google Scholar]
- 39.Freedman BI, Tuttle AB, Spray BJ. Familial predisposition to nephropathy in African-Americans with non-insulin-dependent diabetes mellitus. Am J Kidney Dis. 1995;25:710–3. doi: 10.1016/0272-6386(95)90546-4. [DOI] [PubMed] [Google Scholar]
- 40.Freedman BI, Spray BJ, Tuttle AB, Buckalew VM ., Jr The familial risk of end-stage renal disease in African Americans. Am J Kidney Dis. 1993;21:387–93. doi: 10.1016/s0272-6386(12)80266-6. [DOI] [PubMed] [Google Scholar]
- 41.Freedman BI. End-stage renal failure in African Americans insights in kidney disease susceptibility. Nephrol Dial Transplant. 2002;17:198–200. doi: 10.1093/ndt/17.2.198. [DOI] [PubMed] [Google Scholar]
- 42.Hostetter TH, Rennke HG, Brenner BM. The case for intrarenal hypertension in the initiation and progression of diabetic and other glomerulopathies. Am J Med. 1982;72:375–80. doi: 10.1016/0002-9343(82)90490-9. [DOI] [PubMed] [Google Scholar]
- 43.Collins AJ, Foley RN, Herzog C , et al. Excerpts from the US Renal Data System 2009, Annual Data Report. Am J Kidney Dis. 2010;55:1–420. doi: 10.1053/j.ajkd.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Folkow B. Intravascular pressure as a factor regulating the tone of the small vessels. Acta Physiol Scand. 1949;17:289–310. doi: 10.1111/j.1748-1716.1949.tb00576.x. [DOI] [PubMed] [Google Scholar]
- 45.Folkow B. A study of the factors influencing the tone of denervated blood vessels perfused at various pressures. Acta Physiol Scand. 1952;27:99–117. doi: 10.1111/j.1748-1716.1953.tb00928.x. [DOI] [PubMed] [Google Scholar]
- 46.Harder DR. Pressure-dependent membrane depolarization in cat middle cerebral artery. Circ Res. 1984;55:197–202. doi: 10.1161/01.res.55.2.197. [DOI] [PubMed] [Google Scholar]
- 47.Hill MA, Davis MJ, Meininger GA, Potocnik SJ, Murphy TV. Arteriolar myogenic signalling mechanisms Implications for local vascular function. Clin Hemorheol Microcirc. 2006;34:67–79. [PubMed] [Google Scholar]
- 48.Mogford JE, Davis GE, Platts SH, Meininger GA. Vascular smooth muscle alpha v beta 3 integrin mediates arteriolar vasodilation in response to RGD peptides. Circ Res. 1996;79:821–6. doi: 10.1161/01.res.79.4.821. [DOI] [PubMed] [Google Scholar]
- 49.Martinez-Lemus LA, Crow T, Davis MJ, Meininger GA. alphavbeta3- and alpha5beta1-integrin blockade inhibits myogenic constriction of skeletal muscle resistance arterioles. Am J Physiol Heart Circ Physiol. 2005;289:H322–9. doi: 10.1152/ajpheart.00923.2003. [DOI] [PubMed] [Google Scholar]
- 50.Jernigan NL, Drummond HA. Myogenic vasoconstriction in mouse renal interlobar arteries role of endogenous beta and gammaENaC. Am J Physiol Renal Physiol. 2006;29:F1184–91. doi: 10.1152/ajprenal.00177.2006. [DOI] [PubMed] [Google Scholar]
- 51.Jernigan NL, Drummond HA. Vascular ENaC proteins are required for renal myogenic constriction. Am J Physiol Renal Physiol. 2005;289:F891–901. doi: 10.1152/ajprenal.00019.2005. [DOI] [PubMed] [Google Scholar]
- 52.Drummond HA, Gebremedhin D, Harder DR. Degenerin/epithelial Na+ channel proteins components of a vascular mechanosensor. Hypertension. 2004;44:643–8. doi: 10.1161/01.HYP.0000144465.56360.ad. [DOI] [PubMed] [Google Scholar]
- 53.VanLandingham LG, Gannon KP, Drummond HA. Pressure-induced constriction is inhibited in a mouse model of reduced betaENaC. Am J Physiol Regul Integr Comp Physiol. 2009;297:R723–8. doi: 10.1152/ajpregu.00212.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu X, Davis MJ. Characterization of stretch-activated cation current in coronary smooth muscle cells. Am J Physiol Heart Circ Physiol. 2001;280:H1751–61. doi: 10.1152/ajpheart.2001.280.4.H1751. [DOI] [PubMed] [Google Scholar]
- 55.Davis MJ, Meininger GA, Zawieja DC. Stretch-induced increases in intracellular calcium of isolated vascular smooth muscle cells. Am J Physiol. 1992;263:H1292–9. doi: 10.1152/ajpheart.1992.263.4.H1292. [DOI] [PubMed] [Google Scholar]
- 56.Davis MJ, Donovitz JA, Hood JD. Stretch-activated single-channel and whole cell currents in vascular smooth muscle cells. Am J Physiol. 1992;262:C1083–8. doi: 10.1152/ajpcell.1992.262.4.C1083. [DOI] [PubMed] [Google Scholar]
- 57.Mederos y Schnitzler M, Storch U, Meibers S , et al. Gq-coupled receptors as mechanosensors mediating myogenic vasoconstriction. EMBO J. 2008;27:3092–103. doi: 10.1038/emboj.2008.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Earley S, Waldron BJ, Brayden JE. Critical role for transient receptor potential channel TRPM4 in myogenic constriction of cerebral arteries. Circ Res. 2004;95:922–9. doi: 10.1161/01.RES.0000147311.54833.03. [DOI] [PubMed] [Google Scholar]
- 59.Brayden JE, Earley S, Nelson MT, Reading S. Transient receptor potential (TRP) channels, vascular tone and autoregulation of cerebral blood flow. Clin Exp Pharmacol Physiol. 2008;35:1116–20. doi: 10.1111/j.1440-1681.2007.04855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Narayanan J, Imig M, Roman RJ, Harder DR. Pressurization of isolated renal arteries increases inositol trisphosphate and diacylglycerol. Am J Physiol. 1994;266:H1840–5. doi: 10.1152/ajpheart.1994.266.5.H1840. [DOI] [PubMed] [Google Scholar]
- 61.Gebremedhin D, Lange AR, Narayanan J, Aebly MR, Jacobs ER, Harder DR. Cat cerebral arterial smooth muscle cells express cytochrome P450 4A2 enzyme and produce the vasoconstrictor 20-HETE which enhances L-type Ca2+ current. J Physiol. 1998;507:771–81. doi: 10.1111/j.1469-7793.1998.771bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Osol G, Laher I, Kelley M. Myogenic tone is coupled to phospholipase C and G protein activation in small cerebral arteries. Am J Physiol. 1993;265:H415–20. doi: 10.1152/ajpheart.1993.265.1.H415. [DOI] [PubMed] [Google Scholar]
- 63.Obara K, Koide M, Nakayama K. 20-Hydroxyeicosatetraenoic acid potentiates stretch-induced contraction of canine basilar artery via PKC alpha-mediated inhibition of KCa channel. Br J Pharmacol. 2002;137:1362–70. doi: 10.1038/sj.bjp.0704960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sun CW, Falck Jr, Harder DR, Roman RJ. Role of tyrosine kinase and PKC in the vasoconstrictor response to 20-HETE in renal arterioles. Hypertension. 1999;33:414–8. doi: 10.1161/01.hyp.33.1.414. [DOI] [PubMed] [Google Scholar]
- 65.Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev. 1999;79:387–423. doi: 10.1152/physrev.1999.79.2.387. [DOI] [PubMed] [Google Scholar]
- 66.Faraci FM, Heistad DD. Regulation of the cerebral circulation role of endothelium and potassium channels. Physiol Rev. 1998;78:53–97. doi: 10.1152/physrev.1998.78.1.53. [DOI] [PubMed] [Google Scholar]
- 67.Brayden JE, Nelson MT. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992;256:532–5. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- 68.Nelson MT, Cheng H, Rubart M , et al. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270:633–7. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- 69.Wellman GC, Nelson MT. Signaling between SR and plasmalemma in smooth muscle sparks and the activation of Ca2+-sensitive ion channels. Cell Calcium. 2003;34:211–29. doi: 10.1016/s0143-4160(03)00124-6. [DOI] [PubMed] [Google Scholar]
- 70.Zou AP, Fleming JT, Falck Jr , et al. 20-HETE is an endogenous inhibitor of the large-conductance Ca2+-activated K+ channel in renal arterioles. Am J Physiol. 1996;270:R228–37. doi: 10.1152/ajpregu.1996.270.1.R228. [DOI] [PubMed] [Google Scholar]
- 71.Sun CW, Alonso-Galicia M, Taheri MR, Falck Jr, Harder DR, Roman RJ. Nitric oxide-20-hydroxyeicosatetraenoic acid interaction in the regulation of K+ channel activity and vascular tone in renal arterioles. Circ Res. 1998;83:1069–79. doi: 10.1161/01.res.83.11.1069. [DOI] [PubMed] [Google Scholar]
- 72.Ito O, Alonso-Galicia M, Hopp KA, Roman RJ. Localization of cytochrome P-450 4A isoforms along the rat nephron. Am J Physiol. 1998;274:F395–404. doi: 10.1152/ajprenal.1998.274.2.F395. [DOI] [PubMed] [Google Scholar]
- 73.Imig JD, Zou AP, Stec DE, Harder DR, Falck Jr, Roman RJ. Formation and actions of 20-hydroxyeicosatetraenoic acid in rat renal arterioles. Am J Physiol. 1996;270:R217–27. doi: 10.1152/ajpregu.1996.270.1.R217. [DOI] [PubMed] [Google Scholar]
- 74.Ma YH, Gebremedhin D, Schwartzman ML , et al. 20-Hydroxyeicosatetraenoic acid is an endogenous vasoconstrictor of canine renal arcuate arteries. Circ Res. 1993;72:126–36. doi: 10.1161/01.res.72.1.126. [DOI] [PubMed] [Google Scholar]
- 75.Nowicki S, Chen SL, Aizman O , et al. 20-Hydroxyeicosatetraenoic acid (20 HETE) activates protein kinase C. Role in regulation of rat renal Na+,K+-ATPase. J Clin Invest. 1997;99:1224–30. doi: 10.1172/JCI119279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Muthalif MM, Benter IF, Karzoun N , et al. 20-Hydroxyeicosatetraenoic acid mediates calcium/calmodulin-dependent protein kinase II-induced mitogen-activated protein kinase activation in vascular smooth muscle cells. Proc Natl Acad Sci USA. 1998;95:12701–6. doi: 10.1073/pnas.95.21.12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Muthalif MM, Uddin MR, Fatima S, Parmentier JH, Khandekar Z, Malik KU. Small GTP binding protein Ras contributes to norepinephrine-induced mitogenesis of vascular smooth muscle cells. Prostaglandins Other Lipid Mediat. 2001;65:33–43. doi: 10.1016/s0090-6980(01)00112-5. [DOI] [PubMed] [Google Scholar]
- 78.Toro L, Alioua A, Mahajan A, Nishimaru K, Zarei MM, Stefani E. MaxiK, c-Src and vasoconstriction. J Muscle Res Cell Motil. 2004;25:616–7. [PubMed] [Google Scholar]
- 79.Randriamboavonjy V, Busse R, Fleming I. 20-HETE-induced contraction of small coronary arteries depends on the activation of Rho-kinase. Hypertension. 2003;41:801–6. doi: 10.1161/01.HYP.0000047240.33861.6B. [DOI] [PubMed] [Google Scholar]
- 80.Lange A, Gebremedhin D, Narayanan J, Harder D. 20-Hydroxyeicosatetraenoic acid-induced vasoconstriction and inhibition of potassium current in cerebral vascular smooth muscle is dependent on activation of protein kinase, C. J Biol Chem. 1997;24; 272:27345–52. doi: 10.1074/jbc.272.43.27345. [DOI] [PubMed] [Google Scholar]
- 81.Inoue R, Jensen LJ, Jian Z , et al. Synergistic activation of vascular TRPC6 channel by receptor and mechanical stimulation via phospholipase C/diacylglycerol and phospholipase A2/omega-hydroxylase/20-HETE pathways. Circ Res. 2009;104:1399–409. doi: 10.1161/CIRCRESAHA.108.193227. [DOI] [PubMed] [Google Scholar]
- 82.Gebremedhin D, Lange AR, Lowry TF , et al. Production of 20-HETE and its role in autoregulation of cerebral blood flow. Circ Res. 2000;87:60–5. doi: 10.1161/01.res.87.1.60. [DOI] [PubMed] [Google Scholar]
- 83.Imig JD, Zou AP, Ortiz de Montellano PR, Sui Z, Roman RJ. Cytochrome P-450 inhibitors alter afferent arteriolar responses to elevations in pressure. Am J Physiol. 1994;266:H1879–85. doi: 10.1152/ajpheart.1994.266.5.H1879. [DOI] [PubMed] [Google Scholar]
- 84.Zou AP, Imig JD, Kaldunski M, Ortiz de Montellano PR, Sui Z, Roman RJ. Inhibition of renal vascular 20-HETE production impairs autoregulation of renal blood flow. Am J Physiol. 1994;266:F275–82. doi: 10.1152/ajprenal.1994.266.2.F275. [DOI] [PubMed] [Google Scholar]
- 85.Cole WC, Welsh DG. Role of myosin light chain kinase and myosin light chain phosphatase in the resistance arterial myogenic response to intravascular pressure. Arch Biochem Biophys. 2011;510:160–73. doi: 10.1016/j.abb.2011.02.024. [DOI] [PubMed] [Google Scholar]
- 86.Laher I, Vorkapic P, Dowd AL, Bevan JA. Protein kinase C potentiates stretch-induced cerebral artery tone by increasing intracellular sensitivity to Ca2+ Biochem Biophys Res Commun. 1989;165:312–8. doi: 10.1016/0006-291x(89)91071-1. [DOI] [PubMed] [Google Scholar]
- 87.Hill MA, Falcone JC, Meininger GA. Evidence for protein kinase C involvement in arteriolar myogenic reactivity. Am J Physiol. 1990;259:H1586–94. doi: 10.1152/ajpheart.1990.259.5.H1586. [DOI] [PubMed] [Google Scholar]
- 88.Schnermann J, Levine DZ. Paracrine factors in tubuloglomerular feedback adenosine, ATP, and nitric oxide. Annu Rev Physiol. 2003;65:501–29. doi: 10.1146/annurev.physiol.65.050102.085738. [DOI] [PubMed] [Google Scholar]
- 89.Williams JM, Sarkis A, Lopez B, Ryan RP, Flasch AK, Roman RJ. Elevations in renal interstitial hydrostatic pressure and 20-hydroxyeicosatetraenoic acid contribute to pressure natriuresis. Hypertension. 2007;49:687–94. doi: 10.1161/01.HYP.0000255753.89363.47. [DOI] [PubMed] [Google Scholar]
- 90.Roman RJ, Cowley AW , Jr, Garcia-Estan J, Lombard JH. Pressure-diuresis in volume-expanded rats Cortical and medullary hemodynamics. Hypertension. 1988;12:168–76. doi: 10.1161/01.hyp.12.2.168. [DOI] [PubMed] [Google Scholar]
- 91.Ito S. Characteristics of isolated perfused juxtaglomerular apparatus. Kidney Int. 1998;67:S46–8. doi: 10.1046/j.1523-1755.1998.06709.x. [DOI] [PubMed] [Google Scholar]
- 92.Wright FS, Schnermann J. Interference with feedback control of glomerular filtration rate by furosemide, triflocin, and cyanide. J Clin Invest. 1974;53:1695–708. doi: 10.1172/JCI107721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schlatter E, Salomonsson M, Persson AE, Greger R. Macula densa cells sense luminal NaCl concentration via furosemide sensitive Na+2Cl-K+ cotransport. Pflugers Arch. 1989;414:286–90. doi: 10.1007/BF00584628. [DOI] [PubMed] [Google Scholar]
- 94.Oppermann M, Mizel D, Huang G , et al. Macula densa control of renin secretion and preglomerular resistance in mice with selective deletion of the B isoform of the Na,K,2Cl co-transporter. J Am Soc Nephrol. 2006;17:2143–52. doi: 10.1681/ASN.2006040384. [DOI] [PubMed] [Google Scholar]
- 95.Oppermann M, Hansen PB, Castrop H, Schnermann J. Vasodilatation of afferent arterioles and paradoxical increase of renal vascular resistance by furosemide in mice. Am J Physiol Renal Physiol. 2007;293:F279–87. doi: 10.1152/ajprenal.00073.2007. [DOI] [PubMed] [Google Scholar]
- 96.Bell PD, Lapointe JY, Peti-Peterdi J. Macula densa cell signaling. Annu Rev Physiol. 2003;65:481–500. doi: 10.1146/annurev.physiol.65.050102.085730. [DOI] [PubMed] [Google Scholar]
- 97.Komlosi P, Peti-Peterdi J, Fuson AL, Fintha A, Rosivall L, Bell PD. Macula densa basolateral ATP release is regulated by luminal [NaCl] and dietary salt intake. Am J Physiol Renal Physiol. 2004;286:F1054–8. doi: 10.1152/ajprenal.00336.2003. [DOI] [PubMed] [Google Scholar]
- 98.Bell PD, Lapointe JY, Sabirov R , et al. Macula densa cell signaling involves ATP release through a maxi anion channel. Proc Natl Acad Sci USA. 2003;100:4322–7. doi: 10.1073/pnas.0736323100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huang DY, Vallon V, Zimmermann H, Koszalka P, Schrader J, Osswald H. Ecto-5'-nucleotidase (cd73)-dependent and -independent generation of adenosine participates in the mediation of tubuloglomerular feedback in vivo. Am J Physiol Renal Physiol. 2006;29:F282–8. doi: 10.1152/ajprenal.00113.2005. [DOI] [PubMed] [Google Scholar]
- 100.Castrop H, Huang Y, Hashimoto S , et al. Impairment of tubuloglomerular feedback regulation of GFR in ecto-5'-nucleotidase/CD73-deficient mice. J Clin Invest. 2004;114:634–42. doi: 10.1172/JCI21851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Thomson S, Bao D, Deng A, Vallon V. Adenosine formed by 5'-nucleotidase mediates tubuloglomerular feedback. J Clin Invest. 2000;106:289–98. doi: 10.1172/JCI8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kishore BK, Isaac J, Fausther M , et al. Expression of NTPDase1 and NTPDase2 in murine kidney relevance to regulation of P2 receptor signaling. Am J Physiol Renal Physiol. 2005;288:F1032–43. doi: 10.1152/ajprenal.00108.2004. [DOI] [PubMed] [Google Scholar]
- 103.Osswald H, Hermes HH, Nabakowski G. Role of adenosine in signal transmission of tubuloglomerular feedback. Kidney Int. 1982;12:S136–42. [PubMed] [Google Scholar]
- 104.Vallon V, Muhlbauer B, Osswald H. Adenosine and kidney function. Physiol Rev. 2006;86:901–40. doi: 10.1152/physrev.00031.2005. [DOI] [PubMed] [Google Scholar]
- 105.Inscho EW, Cook AK, Imig JD, Vial C, Evans RJ. Physiological role for P2X1 receptors in renal microvascular autoregulatory behavior. J Clin Invest. 2003;112:1895–905. doi: 10.1172/JCI18499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Inscho EW, Cook AK, Imig JD, Vial C, Evans RJ. Renal autoregulation in P2X1 knockout mice. Acta Physiol Scand. 2004;181:445–53. doi: 10.1111/j.1365-201X.2004.01317.x. [DOI] [PubMed] [Google Scholar]
- 107.Osmond DA, Inscho EW. P2X 1:receptor blockade inhibits whole kidney autoregulation of renal blood flow in vivo . Am J Physiol Renal Physiol. 2010;298:F1360–8. doi: 10.1152/ajprenal.00016.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schnermann J. Maintained tubuloglomerular feedback responses during acute inhibition of P2 purinergic receptors in mice. Am J Physiol Renal Physiol. 2011;300:F339–44. doi: 10.1152/ajprenal.00637.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sun D, Samuelson LC, Yang T , et al. Mediation of tubuloglomerular feedback by adenosine evidence from mice lacking adenosine 1 receptors. Proc Natl Acad Sci USA. 2001;98:9983–8. doi: 10.1073/pnas.171317998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hashimoto S, Huang Y, Briggs J, Schnermann J. Reduced autoregulatory effectiveness in adenosine 1 receptor-deficient mice. Am J Physiol Renal Physiol. 2006;290:F888–91. doi: 10.1152/ajprenal.00381.2005. [DOI] [PubMed] [Google Scholar]
- 111.Vallon V, Richter K, Huang DY, Rieg T, Schnermann J. Functional consequences at the single-nephron level of the lack of adenosine A1 receptors and tubuloglomerular feedback in mice. Pflugers Arch. 2004;448:214–21. doi: 10.1007/s00424-004-1239-8. [DOI] [PubMed] [Google Scholar]
- 112.Osswald H, Nabakowski G, Hermes H. Adenosine as a possible mediator of metabolic control of glomerular filtration rate. Int J Biochem. 1980;12:263–7. doi: 10.1016/0020-711x(80)90082-8. [DOI] [PubMed] [Google Scholar]
- 113.Hansen PB, Castrop H, Briggs J, Schnermann J. Adenosine induces vasoconstriction through Gi-dependent activation of phospholipase C in isolated perfused afferent arterioles of mice. J Am Soc Nephrol. 2003;14:2457–65. doi: 10.1097/01.asn.0000086474.80845.25. [DOI] [PubMed] [Google Scholar]
- 114.Hansen PB, Friis UG, Uhrenholt TR, Briggs J, Schnermann J. Intracellular signalling pathways in the vasoconstrictor response of mouse afferent arterioles to adenosine. Acta Physiol. 2007;19:89–97. doi: 10.1111/j.1748-1716.2007.01724.x. [DOI] [PubMed] [Google Scholar]
- 115.Zhao X, Inscho EW, Bondlela M, Falck Jr, Imig JD. The CYP450 hydroxylase pathway contributes to P2X receptor-mediated afferent arteriolar vasoconstriction. Am J Physiol Heart Circ Physiol. 2001;281:H2089–96. doi: 10.1152/ajpheart.2001.281.5.H2089. [DOI] [PubMed] [Google Scholar]
- 116.Zou AP, Imig JD, Ortiz de Montellano PR, Sui Z, Falck Jr, Roman RJ. Effect of P-450 omega-hydroxylase metabolites of arachidonic acid on tubuloglomerular feedback. Am J Physiol. 1994;266:F934–41. doi: 10.1152/ajprenal.1994.266.6.F934. [DOI] [PubMed] [Google Scholar]
- 117.Inscho EW. P2 receptors in regulation of renal microvascular function. Am J Physiol Renal Physiol. 2001;280:F927–44. doi: 10.1152/ajprenal.2001.280.6.F927. [DOI] [PubMed] [Google Scholar]
- 118.Kohan DE, Rossi NF, Inscho EW, Pollock DM. Regulation of blood pressure and salt homeostasis by endothelin. Physiol Rev. 2011;91:1–77. doi: 10.1152/physrev.00060.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Higuchi S, Ohtsu H, Suzuki H, Shirai H, Frank GD, Eguchi S. Angiotensin II signal transduction through the AT1 receptor novel insights into mechanisms and pathophysiology. Clin Sci (Lond) 2007;112:417–28. doi: 10.1042/CS20060342. [DOI] [PubMed] [Google Scholar]
- 120.Lai EY, Patzak A, Steege A , et al. Contribution of adenosine receptors in the control of arteriolar tone and adenosine-angiotensin II interaction. Kidney Int. 2006;70:690–8. doi: 10.1038/sj.ki.5001650. [DOI] [PubMed] [Google Scholar]
- 121.Weihprecht H, Lorenz JN, Briggs JP, Schnermann J. Synergistic effects of angiotensin and adenosine in the renal microvasculature. Am J Physiol. 1994;266:F227–39. doi: 10.1152/ajprenal.1994.266.2.F227. [DOI] [PubMed] [Google Scholar]
- 122.Traynor T, Yang T, Huang YG , et al. Tubuloglomerular feedback in ACE-deficient mice. Am J Physiol. 1999;276:F751–7. doi: 10.1152/ajprenal.1999.276.5.F751. [DOI] [PubMed] [Google Scholar]
- 123.Schnermann JB, Traynor T, Yang T , et al. Absence of tubuloglomerular feedback responses in AT1A receptor-deficient mice. Am J Physiol. 1997;273:F315–20. doi: 10.1152/ajprenal.1997.273.2.F315. [DOI] [PubMed] [Google Scholar]
- 124.Blantz RC, Deng A, Lortie M , et al. The complex role of nitric oxide in the regulation of glomerular ultrafiltration. Kidney Int. 2002;6:782–5. doi: 10.1046/j.1523-1755.2002.00220.x. [DOI] [PubMed] [Google Scholar]
- 125.Morsing P, Persson AE. Effect of prostaglandin synthesis inhibition on the tubuloglomerular feedback control in the rat kidney. Ren Physiol Biochem. 1992;15:66–72. doi: 10.1159/000173443. [DOI] [PubMed] [Google Scholar]
- 126.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–85. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 127.Cohen RA, Vanhoutte PM. Endothelium-dependent hyperpolariza-tion. Beyond nitric oxide and cyclic GMP. Circulation. 1995;92:3337–49. doi: 10.1161/01.cir.92.11.3337. [DOI] [PubMed] [Google Scholar]
- 128.Alonso-Galicia M, Sun CW, Falck Jr, Harder DR, Roman RJ. Contribution of 20-HETE to the vasodilator actions of nitric oxide in renal arteries. Am J Physiol. 1998;275:F370–8. doi: 10.1152/ajprenal.1998.275.3.F370. [DOI] [PubMed] [Google Scholar]
- 129.Taniguchi J, Furukawa KI, Shigekawa M. Maxi K+ channels are stimulated by cyclic guanosine monophosphate-dependent protein kinase in canine coronary artery smooth muscle cells. Pflugers Arch. 1993;423:167–72. doi: 10.1007/BF00374390. [DOI] [PubMed] [Google Scholar]
- 130.Imig JD, Inscho EW, Deichmann PC, Reddy KM, Falck Jr. Afferent arteriolar vasodilation to the sulfonimide analog of 11, 12-epoxyeicosatrienoic acid involves protein kinase, A. Hypertension. 1999;33:408–13. doi: 10.1161/01.hyp.33.1.408. [DOI] [PubMed] [Google Scholar]
- 131.Imig JD, Dimitropoulou C, Reddy DS, White RE, Falck Jr. Afferent arteriolar dilation to 11, 12-EET analogs involves PP2A activity and Ca2+-activated K+ Channels. Microcirculation. 2008;15:137–50. doi: 10.1080/10739680701456960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li PL, Chen CL, Bortell R, Campbell WB. 11,12-Epoxyeicosatrienoic acid stimulates endogenous mono-ADP-ribosylation in bovine coronary arterial smooth muscle. Circ Res. 1999;85:349–56. doi: 10.1161/01.res.85.4.349. [DOI] [PubMed] [Google Scholar]
- 133.Li PL, Zhang DX, Ge ZD, Campbell WB. Role of ADP-ribose in 11,12-EET-induced activation of K(Ca) channels in coronary arterial smooth muscle cells. Am J Physiol Heart Circ Physiol. 2002;282:H1229–36. doi: 10.1152/ajpheart.00736.2001. [DOI] [PubMed] [Google Scholar]
- 134.Campbell WB, Fleming I. Epoxyeicosatrienoic acids and endothelium-dependent responses. Pflugers Arch. 2010;459:881–95. doi: 10.1007/s00424-010-0804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Peti-Peterdi J, Komlosi P, Fuson AL , et al. Luminal NaCl delivery regulates basolateral PGE2 release from macula densa cells. J Clin Invest. 2003;112:76–82. doi: 10.1172/JCI18018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kovacs G, Komlosi P, Fuson A, Peti-Peterdi J, Rosivall L, Bell PD. Neuronal nitric oxide synthase its role and regulation in macula densa cells. J Am Soc Nephrol. 2003;14:2475–83. doi: 10.1097/01.asn.0000088737.05283.2b. [DOI] [PubMed] [Google Scholar]
- 137.Wilcox CS, Welch WJ, Murad F , et al. Nitric oxide synthase in macula densa regulates glomerular capillary pressure. Proc Natl Acad Sci USA. 1992;89:11993,–7. doi: 10.1073/pnas.89.24.11993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mundel P, Bachmann S, Bader M, Fischer A, Kummer W, Mayer B , et al. Expression of nitric oxide synthase in kidney macula densa cells. Kidney Int. 1992;42:1017–9. doi: 10.1038/ki.1992.382. [DOI] [PubMed] [Google Scholar]
- 139.Ren Y, Garvin JL, Liu R, Carretero OA. Crosstalk between the connecting tubule and the afferent arteriole regulates renal microcirculation. Kidney Int. 2007;71:1116–21. doi: 10.1038/sj.ki.5002190. [DOI] [PubMed] [Google Scholar]
- 140.Wang H, Garvin JL, D'Ambrosio MA, Ren Y, Carretero OA. Connecting tubule glomerular feedback antagonizes tubuloglomerular feedback in vivo. Am J Physiol Renal Physiol. 2010;299:F1374–8. doi: 10.1152/ajprenal.00403.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bidani AK, Griffin KA. Pathophysiology of hypertensive renal damage implications for therapy. Hypertension. 2004;44:595–601. doi: 10.1161/01.HYP.0000145180.38707.84. [DOI] [PubMed] [Google Scholar]
- 142.Bidani AK, Griffin KA. Long-term renal consequences of hypertension for normal and diseased kidneys. Curr Opin Nephrol Hyperten. 2002;11:73–80. doi: 10.1097/00041552-200201000-00011. [DOI] [PubMed] [Google Scholar]
- 143.Bidani AK, Griffin KA, Plott W, Schwartz MM. Renal ablation acutely transforms 'benign' hypertension to 'malignant' nephrosclerosis in hypertensive rats. Hypertension. 1994;24:309–16. doi: 10.1161/01.hyp.24.3.309. [DOI] [PubMed] [Google Scholar]
- 144.Brands MW, Banes-Berceli AK, Inscho EW, Al-Azawi H, Allen AJ, Labazi H. Interleukin 6 knockout prevents angiotensin II hypertension role of renal vasoconstriction and janus kinase 2/signal transducer and activator of transcription 3 activation. Hypertension. 2010;56:879–84. doi: 10.1161/HYPERTENSIONAHA.110.158071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bidani AK, Griffin KA, Picken M, Lansky DM. Continuous telemetric blood pressure monitoring and glomerular injury in the rat remnant kidney model. Am J Physiol. 1993;265:F391–8. doi: 10.1152/ajprenal.1993.265.3.F391. [DOI] [PubMed] [Google Scholar]
- 146.Griffin KA, Picken M, Bidani AK. Method of renal mass reduction is a critical modulator of subsequent hypertension and glomerular injury. J Am Soc Nephrol. 1994;4:2023–31. doi: 10.1681/ASN.V4122023. [DOI] [PubMed] [Google Scholar]
- 147.Griffin KA, Picken MM, Bidani AK. Deleterious effects of calcium channel blockade on pressure transmission and glomerular injury in rat remnant kidneys. J Clin Invest. 1995;96:793–800. doi: 10.1172/JCI118125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Griffin KA, Picken MM, Bakris GL, Bidani AK. Class differences in the effects of calcium channel blockers in the rat remnant kidney model. Kidney Int. 1999;55:1849–60. doi: 10.1046/j.1523-1755.1999.00434.x. [DOI] [PubMed] [Google Scholar]
- 149.Griffin KA, Picken M, Bakris GL, Bidani AK. Comparative effects of selective T- and L-type calcium channel blockers in the remnant kidney model. Hypertension. 2001;37:1268–72. doi: 10.1161/01.hyp.37.5.1268. [DOI] [PubMed] [Google Scholar]
- 150.Kim S, Ohta K, Hamaguchi A , et al. Role of angiotensin II in renal injury of deoxycorticosterone acetate-salt hypertensive rats. Hypertension. 1994;24:195–204. doi: 10.1161/01.hyp.24.2.195. [DOI] [PubMed] [Google Scholar]
- 151.Ploth DW, Schnermann J, Dahlheim H, Hermle M, Schmidmeier E. Autoregulation and tubuloglomerular feedback in normotensive and hypertensive rats. Kidney Int. 1977;12:253–67. doi: 10.1038/ki.1977.110. [DOI] [PubMed] [Google Scholar]
- 152.Schnermann J, Gokel M, Weber PC, Schubert G, Briggs JP. Tubuloglomerular feedback and glomerular morphology in Goldblatt hypertensive rats on varying protein diets. Kidney Int. 1986;29:520–9. doi: 10.1038/ki.1986.30. [DOI] [PubMed] [Google Scholar]
- 153.van Rodijnen WF, van Lambalgen TA, Tangelder GJ, van Dokkum RP, Provoost AP, ter Wee PM. Reduced reactivity of renal microvessels to pressure and angiotensin II in fawn-hooded rats. Hypertension. 2002;39:111–5. doi: 10.1161/hy1201.096817. [DOI] [PubMed] [Google Scholar]
- 154.Verseput GH, Braam B, Provoost AP, Koomans HA. Tubuloglomerular feedback and prolonged ACE-inhibitor treatment in the hypertensive fawn-hooded rat. Nephrol Dial Transplant. 1998;13:893–9. doi: 10.1093/ndt/13.4.893. [DOI] [PubMed] [Google Scholar]
- 155.Lopez B, Ryan RP, Moreno C , et al. Identification of a QTL on chromosome 1 for impaired autoregulation of RBF in fawn-hooded hypertensive rats. Am J Physiol Renal Physiol. 2006;290:F1213–21. doi: 10.1152/ajprenal.00335.2005. [DOI] [PubMed] [Google Scholar]
- 156.Wang X, Ajikobi DO, Salevsky FC, Cupples WA. Impaired myogenic autoregulation in kidneys of Brown Norway rats. Am J Physiol Renal Physiol. 2000;278:F962–9. doi: 10.1152/ajprenal.2000.278.6.F962. [DOI] [PubMed] [Google Scholar]
- 157.Churchill PC, Churchill MC, Bidani AK , et al. Genetic susceptibility to hypertension-induced renal damage in the rat Evidence based on kidney-specific genome transfer. J Clin Invest. 1997;100:1373–82. doi: 10.1172/JCI119657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Moreno C, Williams JM, Lu L , et al. Narrowing a region on rat chromosome 13 that protects against hypertension in Dahl SS-13BN congenic strains. Am J Physiol Heart Circ Physio. 201;300:H1530–5. doi: 10.1152/ajpheart.01026.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mattson DL, Dwinell MR, Greene AS , et al. Chromosome substitution reveals the genetic basis of Dahl salt-sensitive hypertension and renal disease. Am J Physiol Renal Physiol. 2008;295:F837–42. doi: 10.1152/ajprenal.90341.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Aviv A, Hollenberg NK, Weder AB. Sodium glomerulopathy tubuloglomerular feedback and renal injury in African Americans. Kidney Int. 2004;65:361–8. doi: 10.1111/j.1523-1755.2004.00389.x. [DOI] [PubMed] [Google Scholar]
- 161.Endlich N, Kress KR, Reiser J , et al. Podocytes respond to mechanical stress in vitro. J Am Soc Nephrol. 2001;12:413–22. doi: 10.1681/ASN.V123413. [DOI] [PubMed] [Google Scholar]
- 162.Endlich N, Sunohara M, Nietfeld W , et al. Analysis of differential gene expression in stretched podocytes osteopontin enhances adaptation of podocytes to mechanical stress. FASEB J. 2002;16:1850–2. doi: 10.1096/fj.02-0125fje. [DOI] [PubMed] [Google Scholar]
- 163.Ingram AJ, Ly H, Thai K, Kang M, Scholey JW. Activation of mesangial cell signaling cascades in response to mechanical strain. Kidney Int. 1999;55:476–85. doi: 10.1046/j.1523-1755.1999.00276.x. [DOI] [PubMed] [Google Scholar]
- 164.Ingram AJ, James L, Ly H, Thai K, Cai L, Scholey JW. Nitric oxide modulates stretch activation of mitogen-activated protein kinases in mesangial cells. Kidney Int. 2000;58:1067–77. doi: 10.1046/j.1523-1755.2000.00264.x. [DOI] [PubMed] [Google Scholar]
- 165.Noble NA, Border WA. Angiotensin II in renal fibrosis should TGF-beta rather than blood pressure be the therapeutic targetκ. Semin Nephrol. 1997;17:455–66. [PubMed] [Google Scholar]
- 166.Mulder KM. Role of Ras and Mapks in TGFbeta signaling. Cytokine Growth Factor Rev. 2000;11:23–35. doi: 10.1016/s1359-6101(99)00026-x. [DOI] [PubMed] [Google Scholar]
- 167.Wells RG. Fibrogenesis V.; TGF-beta signaling pathways. Am J Physiol Gastrointest Liver Physiol. 2000;279:G845–50. doi: 10.1152/ajpgi.2000.279.5.G845. [DOI] [PubMed] [Google Scholar]
- 168.Iglesias-de la Cruz MC, Ziyadeh FN, Isono M , et al. Effects of high glucose and TGF-beta1 on the expression of collagen IV and vascular endothelial growth factor in mouse podocytes. Kidney Int. 2002;62:901–13. doi: 10.1046/j.1523-1755.2002.00528.x. [DOI] [PubMed] [Google Scholar]
- 169.Cheng S, Lovett DH. Gelatinase A (MMP-2):is necessary and sufficient for renal tubular cell epithelial-mesenchymal transformation. Am J Pathol. 2003;162:1937–49. doi: 10.1016/S0002-9440(10)64327-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yang J, Liu Y. Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am J Pathol. 2001;159:1465–75. doi: 10.1016/S0002-9440(10)62533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sharma R, Khanna A, Sharma M, Savin VJ. Transforming growth factor-beta1 increases albumin permeability of isolated rat glomeruli via hydroxyl radicals. Kidney Int. 2000;58:131–6. doi: 10.1046/j.1523-1755.2000.00148.x. [DOI] [PubMed] [Google Scholar]
- 172.Dahly AJ, Hoagland KM, Flasch AK, Jha S, Ledbetter SR, Roman RJ. Antihypertensive effects of chronic anti-TGF-beta antibody therapy in Dahl S rats. Am J Physiol Regul Integr Comp Physiol. 2002;283:R757–67. doi: 10.1152/ajpregu.00098.2002. [DOI] [PubMed] [Google Scholar]
- 173.Dahly-Vernon AJ, Sharma M, McCarthy ET, Savin VJ, Ledbetter SR, Roman RJ. Transforming growth factor-beta, 20-HETE interaction, and glomerular injury in Dahl salt-sensitive rats. Hypertension. 2005;45:643–8. doi: 10.1161/01.HYP.0000153791.89776.43. [DOI] [PubMed] [Google Scholar]
- 174.Williams JM, Sharma M, Anjaiahh S, Falck Jr, Roman RJ. Role of endogenous CYP450 metabolites of arachidonic acid in maintaining the glomerular protein permeability barrier. Am J Physiol Renal Physiol. 2007;293:F501–5. doi: 10.1152/ajprenal.00131.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Reeves WB, Andreoli TE. Transforming growth factor beta contributes to progressive diabetic nephropathy. Proc Natl Acad Sci USA. 2000;97:7667–9. doi: 10.1073/pnas.97.14.7667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sharma K, McGowan TA. TGF-beta in diabetic kidney disease role of novel signaling pathways. Cytokine Growth Factor Rev. 2000;11:115–23. doi: 10.1016/s1359-6101(99)00035-0. [DOI] [PubMed] [Google Scholar]
- 177.Sharma K, Ziyadeh FN, Alzahabi B , et al. Increased renal production of transforming growth factor-beta1 in patients with type II diabetes. Diabetes. 1997;46:854–9. doi: 10.2337/diab.46.5.854. [DOI] [PubMed] [Google Scholar]
- 178.Yamamoto T, Nakamura T, Noble NA, Ruoslahti E, Border WA. Expression of transforming growth factor beta is elevated in human and experimental diabetic nephropathy. Proc Natl Acad Sci USA. 1993;90:1814–8. doi: 10.1073/pnas.90.5.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Shihab FS, Yamamoto T, Nast CC , et al. Transforming growth factor-beta and matrix protein expression in acute and chronic rejection of human renal allografts. J Am Soc Nephrol. 1995;6:286–94. doi: 10.1681/ASN.V62286. [DOI] [PubMed] [Google Scholar]
- 180.Shihab FS, Andoh TF, Tanner AM , et al. Role of transforming growth factor-beta 1 in experimental chronic cyclosporine nephropathy. Kidney Int. 1996;49:1141–51. doi: 10.1038/ki.1996.165. [DOI] [PubMed] [Google Scholar]
- 181.Yamamoto T, Noble NA, Cohen AH , et al. Expression of transforming growth factor-beta isoforms in human glomerular diseases. Kidney Int. 1996;49:461–9. doi: 10.1038/ki.1996.65. [DOI] [PubMed] [Google Scholar]
- 182.Yoshioka K, Takemura T, Murakami K , et al. Transforming growth factor-beta protein and mRNA in glomeruli in normal and diseased human kidneys. Lab Invest. 1993;68:154–63. [PubMed] [Google Scholar]
- 183.Shankland SJ, Pippin J, Pichler RH , et al. Differential expression of transforming growth factor-beta isoforms and receptors in experimental membranous nephropathy. Kidney Int. 1996;50:116–24. doi: 10.1038/ki.1996.294. [DOI] [PubMed] [Google Scholar]
- 184.Jones CL, Buch S, Post M, McCulloch L, Liu E, Eddy AA. Renal extracellular matrix accumulation in acute puromycin aminonucleoside nephrosis in rats. Am J Pathol. 1992;141:1381–96. [PMC free article] [PubMed] [Google Scholar]
- 185.Ma LJ, Jha S, Ling H, Pozzi A, Ledbetter S, Fogo AB. Divergent effects of low versus high dose anti-TGF-beta antibody in puromycin aminonucleoside nephropathy in rats. Kidney Int. 2004;65:106–15. doi: 10.1111/j.1523-1755.2004.00381.x. [DOI] [PubMed] [Google Scholar]
- 186.Lee LK, Meyer TW, Pollock AS, Lovett DH. Endothelial cell injury initiates glomerular sclerosis in the rat remnant kidney. J Clin Invest. 1995;96:953–64. doi: 10.1172/JCI118143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wu LL, Cox A, Roe CJ, Dziadek M, Cooper ME, Gilbert RE. Transforming growth factor beta 1 and renal injury following subtotal nephrectomy in the rat role of the renin-angiotensin system. Kidney Int. 1997;5:1553–67. doi: 10.1038/ki.1997.214. [DOI] [PubMed] [Google Scholar]
- 188.Kaneto H, Morrissey J, Klahr S. Increased expression of TGF-beta 1 mRNA in the obstructed kidney of rats with unilateral ureteral ligation. Kidney Int. 1993;44:313–21. doi: 10.1038/ki.1993.246. [DOI] [PubMed] [Google Scholar]
- 189.Oikawa T, Freeman M, Lo W, Vaughan DE, Fogo A. Modulation of plasminogen activator inhibitor-1 in vivo a new mechanism for the anti-fibrotic effect of renin-angiotensin inhibition. Kidney Int. 1997;51:164–72. doi: 10.1038/ki.1997.20. [DOI] [PubMed] [Google Scholar]
- 190.Benigni A, Zoja C, Corna D , et al. Add-on anti-TGF-beta antibody to ACE inhibitor arrests progressive diabetic nephropathy in the rat. J Am Soc Nephrol. 2003;14:1816–24. doi: 10.1097/01.asn.0000074238.61967.b7. [DOI] [PubMed] [Google Scholar]
- 191.Sharma K, Ziyadeh FN. Renal hypertrophy is associated with upregulation of TGF-beta 1 gene expression in diabetic BB rat and NOD mouse. Am J Physiol. 1994;267:F1094–01. doi: 10.1152/ajprenal.1994.267.6.F1094. [DOI] [PubMed] [Google Scholar]
- 192.Boffa JJ, Lu Y, Placier S, Stefanski A, Dussaule JC, Chatziantoniou C. Regression of renal vascular and glomerular fibrosis role of angiotensin II receptor antagonism and matrix metalloproteinases. J Am Soc Nephrol. 2003;14:1132–44. doi: 10.1097/01.asn.0000060574.38107.3b. [DOI] [PubMed] [Google Scholar]
- 193.Kashiwagi M, Shinozaki M, Hirakata H , et al. Locally activated renin-angiotensin system associated with TGF-beta1 as a major factor for renal injury induced by chronic inhibition of nitric oxide synthase in rats. J Am Soc Nephrol. 2000;11:616–24. doi: 10.1681/ASN.V114616. [DOI] [PubMed] [Google Scholar]
- 194.Mori T, Cowley AW ., Jr Role of pressure in angiotensin II-induced renal injury chronic servo-control of renal perfusion pressure in rats. Hypertension. 2004;43:752–9. doi: 10.1161/01.HYP.0000120971.49659.6a. [DOI] [PubMed] [Google Scholar]
- 195.Hamaguchi A, Kim S, Ohta K , et al. Transforming growth factor-beta 1 expression and phenotypic modulation in the kidney of hypertensive rats. Hypertension. 1995;26:199–207. doi: 10.1161/01.hyp.26.1.199. [DOI] [PubMed] [Google Scholar]
- 196.Tamaki K, Okuda S, Nakayama M, Yanagida T, Fujishima M. Transforming growth factor-beta 1 in hypertensive renal injury in Dahl salt-sensitive rats. J Am Soc Nephrol. 1996;7:2578–89. doi: 10.1681/ASN.V7122578. [DOI] [PubMed] [Google Scholar]
- 197.Kelly FJ, Anderson S, Thompson MM , et al. Acute and chronic renal effects of recombinant human TGF-beta2 in the rat. J Am Soc Nephrol. 1999;10:1264–73. doi: 10.1681/ASN.V1061264. [DOI] [PubMed] [Google Scholar]
- 198.Ledbetter S, Kurtzberg L, Doyle S, Pratt BM. Renal fibrosis in mice treated with human recombinant transforming growth factor-beta2. Kidney Int. 2000;58:2367–76. doi: 10.1046/j.1523-1755.2000.00420.x. [DOI] [PubMed] [Google Scholar]
- 199.Clouthier DE, Comerford SA, Hammer RE. Hepatic fibrosis, glomerulosclerosis, and a lipodystrophy-like syndrome in PEPCK-TGF-beta1 transgenic mice. J Clin Invest. 1997;100:2697–713. doi: 10.1172/JCI119815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kopp JB, Factor VM, Mozes M , et al. Transgenic mice with increased plasma levels of TGF-beta 1 develop progressive renal disease. Lab Invest. 1996;74:991–1003. [PubMed] [Google Scholar]
- 201.Krag S, Osterby R, Chai Q, Nielsen CB, Hermans C, Wogensen L. TGF-beta1-induced glomerular disorder is associated with impaired concentrating ability mimicking primary glomerular disease with renal failure in man. Lab Invest. 2000;80:1855–68. doi: 10.1038/labinvest.3780196. [DOI] [PubMed] [Google Scholar]
- 202.Mozes MM, Bottinger EP, Jacot TA, Kopp JB. Renal expression of fibrotic matrix proteins and of transforming growth factor-beta (TGF-beta) isoforms in TGF-beta transgenic mice. J Am Soc Nephrol. 1999;10:271–80. doi: 10.1681/ASN.V102271. [DOI] [PubMed] [Google Scholar]
- 203.Wogensen L, Nielsen CB, Hjorth P , et al. Under control of the Ren-1c promoter, locally produced transforming growth factor-beta1 induces accumulation of glomerular extracellular matrix in transgenic mice. Diabetes. 1999;48:182–92. doi: 10.2337/diabetes.48.1.182. [DOI] [PubMed] [Google Scholar]
- 204.Border WA, Okuda S, Languino LR, Sporn MB, Ruoslahti E. Suppression of experimental glomerulonephritis by antiserum against transforming growth factor beta 1. Nature. 1990;346:371–374. doi: 10.1038/346371a0. [DOI] [PubMed] [Google Scholar]
- 205.Border WA, Noble NA, Yamamoto T , et al. Natural inhibitor of transforming growth factor-beta protects against scarring in experimental kidney disease. Nature. 1992;360:361–4. doi: 10.1038/360361a0. [DOI] [PubMed] [Google Scholar]
- 206.Chen S, Iglesias-de la Cruz MC, Jim B, Hong SW, Isono M, Ziyadeh FN. Reversibility of established diabetic glomerulopathy by anti-TGF-beta antibodies in db/db mice. Biochem Biophys Res Commun. 2003;300:16–22. doi: 10.1016/s0006-291x(02)02708-0. [DOI] [PubMed] [Google Scholar]
- 207.Han DC, Hoffman BB, Hong SW, Guo J, Ziyadeh FN. Therapy with antisense TGF-beta1 oligodeoxynucleotides reduces kidney weight and matrix mRNAs in diabetic mice. Am J Physiol Renal Physiol. 2000;278:F628–34. doi: 10.1152/ajprenal.2000.278.4.F628. [DOI] [PubMed] [Google Scholar]
- 208.Ziyadeh FN, Hoffman BB, Han DC , et al. Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-beta antibody in db/db diabetic mice. Proc Natl Acad Sci USA. 2000; 97(5):8015–20. doi: 10.1073/pnas.120055097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ling H, Li X, Jha S , et al. Therapeutic role of TGF-beta-neutralizing antibody in mouse cyclosporin A nephropathy morphologic improvement associated with functional preservation. J Am Soc Nephrol. 2003;14:377–88. doi: 10.1097/01.asn.0000042168.43665.9b. [DOI] [PubMed] [Google Scholar]
- 210.Ding G, Reddy K, Kapasi AA , et al. Angiotensin II induces apoptosis in rat glomerular epithelial cells. Am J Physiol Renal Physiol. 2002;283:F173–80. doi: 10.1152/ajprenal.00240.2001. [DOI] [PubMed] [Google Scholar]
- 211.Schiffer M, Bitzer M, Roberts IS , et al. Apoptosis in podocytes induced by TGF-beta and Smad7. J Clin Invest. 2001;108:807–16. doi: 10.1172/JCI12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lodha S, Dani D, Mehta R , et al. Angiotensin II-induced mesangial cell apoptosis role of oxidative stress. Mol Med. 2002;8:830–40. [PMC free article] [PubMed] [Google Scholar]
- 213.Dai C, Yang J, Liu Y. Transforming growth factor-beta1 potentiates renal tubular epithelial cell death by a mechanism independent of Smad signaling. J Biol Chem. 2003;278:12537–45. doi: 10.1074/jbc.M300777200. [DOI] [PubMed] [Google Scholar]
- 214.Bhaskaran M, Reddy K, Radhakrishanan N, Franki N, Ding G, Singhal PC. Angiotensin II induces apoptosis in renal proximal tubular cells. Am J Physiol Renal Physiol. 2003;284:F955–65. doi: 10.1152/ajprenal.00246.2002. [DOI] [PubMed] [Google Scholar]
- 215.Wang SN, LaPage J, Hirschberg R. Role of glomerular ultrafiltration of growth factors in progressive interstitial fibrosis in diabetic nephropathy. Kidney Int. 2000;57:1002–14. doi: 10.1046/j.1523-1755.2000.00928.x. [DOI] [PubMed] [Google Scholar]
- 216.Yard BA, Chorianopoulos E, Herr D, van der Woude FJ. Regulation of endothelin-1 and transforming growth factor-beta1 production in cultured proximal tubular cells by albumin and heparan sulphate glycosaminoglycans. Nephrol Dial Transplant. 2001;16:1769–75. doi: 10.1093/ndt/16.9.1769. [DOI] [PubMed] [Google Scholar]