Abstract
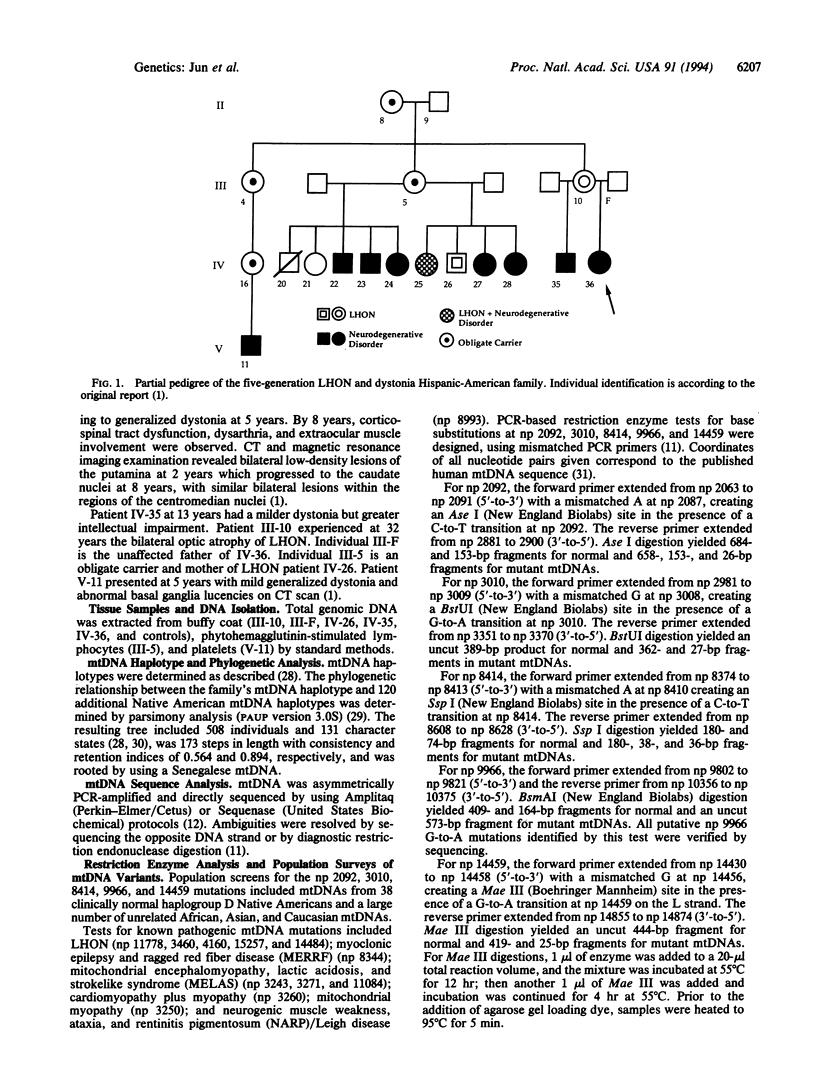
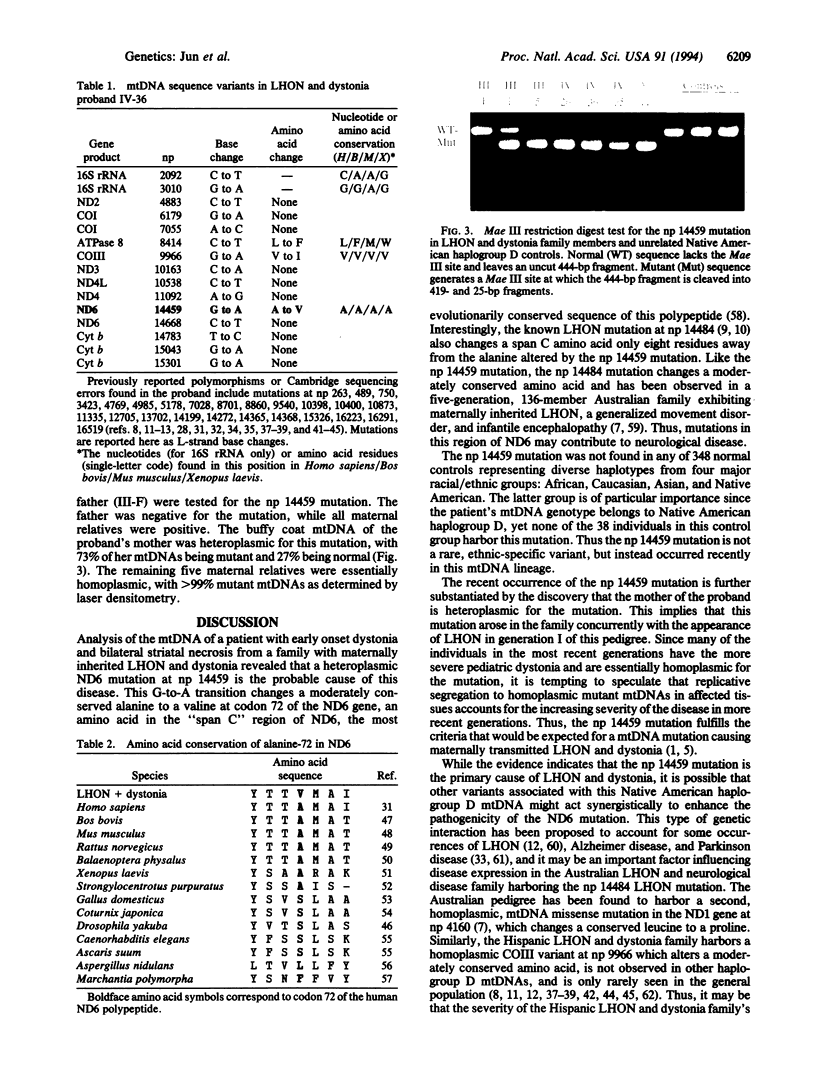
A five-generation Hispanic family expressing maternally transmitted Leber hereditary optic neuropathy and/or early-onset dystonia associated with bilateral basal ganglia lesions was studied. Buffy coat mitochondrial DNA (mtDNA) from a severely affected child was amplified by the polymerase chain reaction and greater than 90% sequenced. The mtDNA proved to be a Native American haplogroup D genotype and differed from the standard "Cambridge" sequence at 40 nucleotide positions. One of these variants, a G-to-A transition at nucleotide pair (np) 14459, changed a moderately conserved alanine to a valine at NADH dehydrogenase subunit 6 (ND6) residue 72. The np 14459 variant was not found in any of 38 Native American haplogroup D mtDNAs, nor was it detected in 108 Asian, 103 Caucasian, or 99 African mtDNAs. Six maternal relatives in three generations were tested and were found to harbor the mutation, with one female affected with Leber hereditary optic neuropathy being heteroplasmic. Thus, the np 14459 G-to-A missense mutation is specific to this family, alters a moderately conserved amino acid in a complex I gene, is a unique mtDNA variant in Native American haplogroup D, and is heteroplasmic, suggesting that it is the disease-causing mutation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Anderson S., de Bruijn M. H., Coulson A. R., Eperon I. C., Sanger F., Young I. G. Complete sequence of bovine mitochondrial DNA. Conserved features of the mammalian mitochondrial genome. J Mol Biol. 1982 Apr 25;156(4):683–717. doi: 10.1016/0022-2836(82)90137-1. [DOI] [PubMed] [Google Scholar]
- Arnason U., Gullberg A., Widegren B. The complete nucleotide sequence of the mitochondrial DNA of the fin whale, Balaenoptera physalus. J Mol Evol. 1991 Dec;33(6):556–568. doi: 10.1007/BF02102808. [DOI] [PubMed] [Google Scholar]
- BRUYN G. W., WENT L. N. A SEX-LINKED HEREDO-DEGENERATIVE NEUROLOGICAL DISORDER, ASSOCIATED WITH LEBER'S OPTIC ATROPHY. I. CLINICAL STUDIES. J Neurol Sci. 1964 Jan-Feb;1(1):59–80. doi: 10.1016/0022-510x(64)90054-1. [DOI] [PubMed] [Google Scholar]
- Ballinger S. W., Schurr T. G., Torroni A., Gan Y. Y., Hodge J. A., Hassan K., Chen K. H., Wallace D. C. Southeast Asian mitochondrial DNA analysis reveals genetic continuity of ancient mongoloid migrations. Genetics. 1992 Jan;130(1):139–152. doi: 10.1093/genetics/130.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benecke R., Strümper P., Weiss H. Electron transfer complex I defect in idiopathic dystonia. Ann Neurol. 1992 Nov;32(5):683–686. doi: 10.1002/ana.410320512. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Brown M. D., Voljavec A. S., Lott M. T., MacDonald I., Wallace D. C. Leber's hereditary optic neuropathy: a model for mitochondrial neurodegenerative diseases. FASEB J. 1992 Jul;6(10):2791–2799. doi: 10.1096/fasebj.6.10.1634041. [DOI] [PubMed] [Google Scholar]
- Brown M. D., Voljavec A. S., Lott M. T., Torroni A., Yang C. C., Wallace D. C. Mitochondrial DNA complex I and III mutations associated with Leber's hereditary optic neuropathy. Genetics. 1992 Jan;130(1):163–173. doi: 10.1093/genetics/130.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruyn G. W., Bots G. T., Went L. N., Klinkhamer P. J. Hereditary spastic dystonia with Leber's hereditary optic neuropathy: neuropathological findings. J Neurol Sci. 1992 Nov;113(1):55–61. doi: 10.1016/0022-510x(92)90265-m. [DOI] [PubMed] [Google Scholar]
- Bruyn G. W., Vielvoye G. J., Went L. N. Hereditary spastic dystonia: a new mitochondrial encephalopathy? Putaminal necrosis as a diagnostic sign. J Neurol Sci. 1991 Jun;103(2):195–202. doi: 10.1016/0022-510x(91)90164-3. [DOI] [PubMed] [Google Scholar]
- Clary D. O., Wolstenholme D. R. The mitochondrial DNA molecular of Drosophila yakuba: nucleotide sequence, gene organization, and genetic code. J Mol Evol. 1985;22(3):252–271. doi: 10.1007/BF02099755. [DOI] [PubMed] [Google Scholar]
- Coles C. J., Edmondson D. E., Singer T. P. Inactivation of succinate dehydrogenase by 3-nitropropionate. J Biol Chem. 1979 Jun 25;254(12):5161–5167. [PubMed] [Google Scholar]
- Desjardins P., Morais R. Nucleotide sequence and evolution of coding and noncoding regions of a quail mitochondrial genome. J Mol Evol. 1991 Feb;32(2):153–161. doi: 10.1007/BF02515387. [DOI] [PubMed] [Google Scholar]
- Desjardins P., Morais R. Sequence and gene organization of the chicken mitochondrial genome. A novel gene order in higher vertebrates. J Mol Biol. 1990 Apr 20;212(4):599–634. doi: 10.1016/0022-2836(90)90225-B. [DOI] [PubMed] [Google Scholar]
- Fearnley I. M., Walker J. E. Conservation of sequences of subunits of mitochondrial complex I and their relationships with other proteins. Biochim Biophys Acta. 1992 Dec 7;1140(2):105–134. doi: 10.1016/0005-2728(92)90001-i. [DOI] [PubMed] [Google Scholar]
- Gadaleta G., Pepe G., De Candia G., Quagliariello C., Sbisà E., Saccone C. The complete nucleotide sequence of the Rattus norvegicus mitochondrial genome: cryptic signals revealed by comparative analysis between vertebrates. J Mol Evol. 1989 Jun;28(6):497–516. doi: 10.1007/BF02602930. [DOI] [PubMed] [Google Scholar]
- Giles R. E., Blanc H., Cann H. M., Wallace D. C. Maternal inheritance of human mitochondrial DNA. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6715–6719. doi: 10.1073/pnas.77.11.6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould D. H., Gustine D. L. Basal ganglia degeneration, myelin alterations, and enzyme inhibition induced in mice by the plant toxin 3-nitropropanoic acid. Neuropathol Appl Neurobiol. 1982 Sep-Oct;8(5):377–393. doi: 10.1111/j.1365-2990.1982.tb00306.x. [DOI] [PubMed] [Google Scholar]
- Holt I. J., Harding A. E., Petty R. K., Morgan-Hughes J. A. A new mitochondrial disease associated with mitochondrial DNA heteroplasmy. Am J Hum Genet. 1990 Mar;46(3):428–433. [PMC free article] [PubMed] [Google Scholar]
- Howell N., Kubacka I., Xu M., McCullough D. A. Leber hereditary optic neuropathy: involvement of the mitochondrial ND1 gene and evidence for an intragenic suppressor mutation. Am J Hum Genet. 1991 May;48(5):935–942. [PMC free article] [PubMed] [Google Scholar]
- Howell N., McCullough D. A., Kubacka I., Halvorson S., Mackey D. The sequence of human mtDNA: the question of errors versus polymorphisms. Am J Hum Genet. 1992 Jun;50(6):1333–1340. [PMC free article] [PubMed] [Google Scholar]
- Huoponen K., Vilkki J., Aula P., Nikoskelainen E. K., Savontaus M. L. A new mtDNA mutation associated with Leber hereditary optic neuroretinopathy. Am J Hum Genet. 1991 Jun;48(6):1147–1153. [PMC free article] [PubMed] [Google Scholar]
- Jacobs H. T., Elliott D. J., Math V. B., Farquharson A. Nucleotide sequence and gene organization of sea urchin mitochondrial DNA. J Mol Biol. 1988 Jul 20;202(2):185–217. doi: 10.1016/0022-2836(88)90452-4. [DOI] [PubMed] [Google Scholar]
- Johns D. R., Heher K. L., Miller N. R., Smith K. H. Leber's hereditary optic neuropathy. Clinical manifestations of the 14484 mutation. Arch Ophthalmol. 1993 Apr;111(4):495–498. doi: 10.1001/archopht.1993.01090040087038. [DOI] [PubMed] [Google Scholar]
- Johns D. R., Neufeld M. J., Park R. D. An ND-6 mitochondrial DNA mutation associated with Leber hereditary optic neuropathy. Biochem Biophys Res Commun. 1992 Sep 30;187(3):1551–1557. doi: 10.1016/0006-291x(92)90479-5. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y., Momoi M. Y., Tominaga K., Shimoizumi H., Nihei K., Yanagisawa M., Kagawa Y., Ohta S. Respiration-deficient cells are caused by a single point mutation in the mitochondrial tRNA-Leu (UUR) gene in mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes (MELAS). Am J Hum Genet. 1991 Sep;49(3):590–599. [PMC free article] [PubMed] [Google Scholar]
- Kohchi T., Shirai H., Fukuzawa H., Sano T., Komano T., Umesono K., Inokuchi H., Ozeki H., Ohyama K. Structure and organization of Marchantia polymorpha chloroplast genome. IV. Inverted repeat and small single copy regions. J Mol Biol. 1988 Sep 20;203(2):353–372. doi: 10.1016/0022-2836(88)90004-6. [DOI] [PubMed] [Google Scholar]
- Lertrit P., Noer A. S., Jean-Francois M. J., Kapsa R., Dennett X., Thyagarajan D., Lethlean K., Byrne E., Marzuki S. A new disease-related mutation for mitochondrial encephalopathy lactic acidosis and strokelike episodes (MELAS) syndrome affects the ND4 subunit of the respiratory complex I. Am J Hum Genet. 1992 Sep;51(3):457–468. [PMC free article] [PubMed] [Google Scholar]
- Marsden C. D., Lang A. E., Quinn N. P., McDonald W. I., Abdallat A., Nimri S. Familial dystonia and visual failure with striatal CT lucencies. J Neurol Neurosurg Psychiatry. 1986 May;49(5):500–509. doi: 10.1136/jnnp.49.5.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzuki S., Noer A. S., Lertrit P., Thyagarajan D., Kapsa R., Utthanaphol P., Byrne E. Normal variants of human mitochondrial DNA and translation products: the building of a reference data base. Hum Genet. 1991 Dec;88(2):139–145. doi: 10.1007/BF00206061. [DOI] [PubMed] [Google Scholar]
- Miyoshi K. Experimental striatal necrosis induced by sodium azide. A contribution to the problem of selective vulnerability and histochemical studies of enzymatic activity. Acta Neuropathol. 1967 Nov 6;9(3):199–216. doi: 10.1007/BF00687931. [DOI] [PubMed] [Google Scholar]
- Moraes C. T., Ciacci F., Bonilla E., Ionasescu V., Schon E. A., DiMauro S. A mitochondrial tRNA anticodon swap associated with a muscle disease. Nat Genet. 1993 Jul;4(3):284–288. doi: 10.1038/ng0793-284. [DOI] [PubMed] [Google Scholar]
- Netzker R., Köchel H. G., Basak N., Küntzel H. Nucleotide sequence of Aspergillus nidulans mitochondrial genes coding for ATPase subunit 6, cytochrome oxidase subunit 3, seven unidentified proteins, four tRNAs and L-rRNA. Nucleic Acids Res. 1982 Aug 11;10(15):4783–4794. doi: 10.1093/nar/10.15.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noer A. S., Sudoyo H., Lertrit P., Thyagarajan D., Utthanaphol P., Kapsa R., Byrne E., Marzuki S. A tRNA(Lys) mutation in the mtDNA is the causal genetic lesion underlying myoclonic epilepsy and ragged-red fiber (MERRF) syndrome. Am J Hum Genet. 1991 Oct;49(4):715–722. [PMC free article] [PubMed] [Google Scholar]
- Novotny E. J., Jr, Singh G., Wallace D. C., Dorfman L. J., Louis A., Sogg R. L., Steinman L. Leber's disease and dystonia: a mitochondrial disease. Neurology. 1986 Aug;36(8):1053–1060. doi: 10.1212/wnl.36.8.1053. [DOI] [PubMed] [Google Scholar]
- Obayashi T., Hattori K., Sugiyama S., Tanaka M., Tanaka T., Itoyama S., Deguchi H., Kawamura K., Koga Y., Toshima H. Point mutations in mitochondrial DNA in patients with hypertrophic cardiomyopathy. Am Heart J. 1992 Nov;124(5):1263–1269. doi: 10.1016/0002-8703(92)90410-w. [DOI] [PubMed] [Google Scholar]
- Okimoto R., Macfarlane J. L., Clary D. O., Wolstenholme D. R. The mitochondrial genomes of two nematodes, Caenorhabditis elegans and Ascaris suum. Genetics. 1992 Mar;130(3):471–498. doi: 10.1093/genetics/130.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa T., Tanaka M., Ino H., Ohno K., Sano T., Wada Y., Yoneda M., Tanno Y., Miyatake T., Tanaka T. Distinct clustering of point mutations in mitochondrial DNA among patients with mitochondrial encephalomyopathies and with Parkinson's disease. Biochem Biophys Res Commun. 1991 Apr 30;176(2):938–946. doi: 10.1016/s0006-291x(05)80276-1. [DOI] [PubMed] [Google Scholar]
- Ozawa T., Tanaka M., Sugiyama S., Ino H., Ohno K., Hattori K., Ohbayashi T., Ito T., Deguchi H., Kawamura K. Patients with idiopathic cardiomyopathy belong to the same mitochondrial DNA gene family of Parkinson's disease and mitochondrial encephalomyopathy. Biochem Biophys Res Commun. 1991 May 31;177(1):518–525. doi: 10.1016/0006-291x(91)92014-b. [DOI] [PubMed] [Google Scholar]
- Prezant T. R., Agapian J. V., Bohlman M. C., Bu X., Oztas S., Qiu W. Q., Arnos K. S., Cortopassi G. A., Jaber L., Rotter J. I. Mitochondrial ribosomal RNA mutation associated with both antibiotic-induced and non-syndromic deafness. Nat Genet. 1993 Jul;4(3):289–294. doi: 10.1038/ng0793-289. [DOI] [PubMed] [Google Scholar]
- Roe B. A., Ma D. P., Wilson R. K., Wong J. F. The complete nucleotide sequence of the Xenopus laevis mitochondrial genome. J Biol Chem. 1985 Aug 15;260(17):9759–9774. [PubMed] [Google Scholar]
- Shoffner J. M., Brown M. D., Torroni A., Lott M. T., Cabell M. F., Mirra S. S., Beal M. F., Yang C. C., Gearing M., Salvo R. Mitochondrial DNA variants observed in Alzheimer disease and Parkinson disease patients. Genomics. 1993 Jul;17(1):171–184. doi: 10.1006/geno.1993.1299. [DOI] [PubMed] [Google Scholar]
- Shoffner J. M., Fernhoff P. M., Krawiecki N. S., Caplan D. B., Holt P. J., Koontz D. A., Takei Y., Newman N. J., Ortiz R. G., Polak M. Subacute necrotizing encephalopathy: oxidative phosphorylation defects and the ATPase 6 point mutation. Neurology. 1992 Nov;42(11):2168–2174. doi: 10.1212/wnl.42.11.2168. [DOI] [PubMed] [Google Scholar]
- Shoffner J. M., Lott M. T., Lezza A. M., Seibel P., Ballinger S. W., Wallace D. C. Myoclonic epilepsy and ragged-red fiber disease (MERRF) is associated with a mitochondrial DNA tRNA(Lys) mutation. Cell. 1990 Jun 15;61(6):931–937. doi: 10.1016/0092-8674(90)90059-n. [DOI] [PubMed] [Google Scholar]
- Shoffner J. M., Watts R. L., Juncos J. L., Torroni A., Wallace D. C. Mitochondrial oxidative phosphorylation defects in Parkinson's disease. Ann Neurol. 1991 Sep;30(3):332–339. doi: 10.1002/ana.410300304. [DOI] [PubMed] [Google Scholar]
- Tatuch Y., Christodoulou J., Feigenbaum A., Clarke J. T., Wherret J., Smith C., Rudd N., Petrova-Benedict R., Robinson B. H. Heteroplasmic mtDNA mutation (T----G) at 8993 can cause Leigh disease when the percentage of abnormal mtDNA is high. Am J Hum Genet. 1992 Apr;50(4):852–858. [PMC free article] [PubMed] [Google Scholar]
- Torroni A., Chen Y. S., Semino O., Santachiara-Beneceretti A. S., Scott C. R., Lott M. T., Winter M., Wallace D. C. mtDNA and Y-chromosome polymorphisms in four Native American populations from southern Mexico. Am J Hum Genet. 1994 Feb;54(2):303–318. [PMC free article] [PubMed] [Google Scholar]
- Torroni A., Schurr T. G., Cabell M. F., Brown M. D., Neel J. V., Larsen M., Smith D. G., Vullo C. M., Wallace D. C. Asian affinities and continental radiation of the four founding Native American mtDNAs. Am J Hum Genet. 1993 Sep;53(3):563–590. [PMC free article] [PubMed] [Google Scholar]
- Torroni A., Schurr T. G., Yang C. C., Szathmary E. J., Williams R. C., Schanfield M. S., Troup G. A., Knowler W. C., Lawrence D. N., Weiss K. M. Native American mitochondrial DNA analysis indicates that the Amerind and the Nadene populations were founded by two independent migrations. Genetics. 1992 Jan;130(1):153–162. doi: 10.1093/genetics/130.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torroni A., Sukernik R. I., Schurr T. G., Starikorskaya Y. B., Cabell M. F., Crawford M. H., Comuzzie A. G., Wallace D. C. mtDNA variation of aboriginal Siberians reveals distinct genetic affinities with Native Americans. Am J Hum Genet. 1993 Sep;53(3):591–608. [PMC free article] [PubMed] [Google Scholar]
- WENT L. N. A SEX-LINKED HEREDO-DEGENERATIVE NEUROLOGICAL DISORDER ASSOCIATED WITH LEBER'S OPTIC ATROPHY. GENETICAL ASPECTS. Acta Genet Stat Med. 1964;14:220–239. doi: 10.1159/000151849. [DOI] [PubMed] [Google Scholar]
- WENT L. N. A SEX-LINKED HEREDO-DEGENERATIVE NEUROLOGICAL DISORDER, ASSOCIATED WITH LEBER'S OPTIC ATROPHY. II. LABORATORY INVESTIGATIONS. J Neurol Sci. 1964 Jan-Feb;1(1):81–87. doi: 10.1016/0022-510x(64)90055-3. [DOI] [PubMed] [Google Scholar]
- Wallace D. C. A new manifestation of Leber's disease and a new explanation for the agency responsible for its unusual pattern of inheritance. Brain. 1970;93(1):121–132. doi: 10.1093/brain/93.1.121. [DOI] [PubMed] [Google Scholar]
- Wallace D. C. Diseases of the mitochondrial DNA. Annu Rev Biochem. 1992;61:1175–1212. doi: 10.1146/annurev.bi.61.070192.005523. [DOI] [PubMed] [Google Scholar]
- Wallace D. C. Mitochondrial genes and disease. Hosp Pract (Off Ed) 1986 Oct 15;21(10):77-87, 90-2. [PubMed] [Google Scholar]
- Wallace D. C., Singh G., Lott M. T., Hodge J. A., Schurr T. G., Lezza A. M., Elsas L. J., 2nd, Nikoskelainen E. K. Mitochondrial DNA mutation associated with Leber's hereditary optic neuropathy. Science. 1988 Dec 9;242(4884):1427–1430. doi: 10.1126/science.3201231. [DOI] [PubMed] [Google Scholar]
- de Vries D. D., van Engelen B. G., Gabreëls F. J., Ruitenbeek W., van Oost B. A. A second missense mutation in the mitochondrial ATPase 6 gene in Leigh's syndrome. Ann Neurol. 1993 Sep;34(3):410–412. doi: 10.1002/ana.410340319. [DOI] [PubMed] [Google Scholar]