Abstract
Purpose.
The purpose of this study was to identify the structural and histological effects of a Tano diamond-dusted membrane scraper (DDMS) on the retinal surface after internal limiting membrane (ILM) abrasion in macular hole surgery.
Methods.
Institutional experimental study was performed in 11 eyes. All eyes underwent ILM abrasion in the operating room with a DDMS for macular hole repair as an alternative to traditional ILM peeling. Three human donor eyes underwent an identical procedure in the laboratory. Retinal tissues were removed by ILM abrasion with a DDMS during vitrectomy for macular hole repair and retinal tissues remaining in human donor eyes. Main outcome measures were microscopic and immunohistological characteristics of instrument tip tissues and retinal structure after ILM abrasion.
Results.
The tips of the Tano DDMS showed evidence of cellular membranes and ILM removal. The retinas showed distinct areas of lamellar ILM removal without penetration of the retinal nerve fiber layer (RNFL).
Conclusions.
Application of the Tano DDMS instrument is sufficient to remove membranes from the surface of the ILM and layers of the ILM without disruption of the underlying RNFL. Internal limiting membrane abrasion can be a useful and effective alternative to complete ILM removal for macular surgery.
Keywords: internal limiting membrane abrasion, macular hole, Tano scraper
Internal limiting membrane (ILM) abrasion technique using a diamond-dusted membrane scraper causes disruption and removal of the ILM and its associated constituent elements while achieving macular hole closure rates comparable to those of traditional surgical ILM removal.
Idiopathic macular holes are full-thickness neurosensory retinal defects in the fovea that typically result in moderate to severe vision loss.1–3 Central to the pathogenesis of macular holes is anomalous vitreomacular traction and incomplete posterior vitreous detachment, which often leaves remnants of the vitreous cortex on the internal limiting membrane (ILM) surface.4–6 Vitrectomy is a highly successful procedure for achieving macular hole closure and improved visual acuity.7–11 As surgical methods have evolved to include gas tamponade, epiretinal membrane (ERM) peeling, and ILM peeling, rates of macular hole closure now exceed 90%, with low recurrence rates in most reported series.12–16
Various surgical techniques involve peeling the ILM from the superficial retina. Most use creation of a defect in the ILM, grasping an exposed edge with intraocular forceps, and extending a circumferential tear around the hole. Alternatively, the ILM can be directly grasped with forceps and peeled from the retinal surface. Dissection of the transparent 10-μm-thick ILM is a surgically challenging step and may be facilitated by the use of adjuvant dyes such as indocyanine green to improve visualization and surgical success.17 Adjuvant stains may also alter the ILM structure and assist in creating a cleavage plane between the ILM and underlying retinal layers.18 Application of dyes, however, may be toxic to the retinal cells and/or retinal pigment epithelium, resulting in reduced postoperative visual acuity despite successful hole closure.19
In this report, we describe a technique of ILM abrasion performed to address elements of tangential traction on the retinal surface to achieve successful macular hole closure without complete removal of the ILM. Such a technique may limit the loss of adjacent tissue of the inner retina and eliminate the risk of adjuvant dye toxicity. The technique uses a Tano diamond-dusted membrane scraper (DDMS; Synergetics, Inc., O'Fallon, MO, USA) to abrade the ILM.20 Recently, we showed a 94% success rate in 100 eyes that underwent ILM abrasion with a DDMS as an alternative to traditional ILM peeling at the time of macular hole repair.21 Abrasion of the ILM with a DDMS at the time of pars plana vitrectomy is sufficient to achieve high rates of macular hole closure, and this technique avoids complete removal of the retinal ILM basement membrane and subjacent tissues and provides hole closure rates that are comparable to those in traditional ILM peeling.21 In this study, we used two methods to ascertain the effect of ILM abrasion on the retina, and we describe the immunohistochemical characteristics of retinal tissue removed by the DDMS from surgical specimens with macular holes. We also used electron microscopy to examine the retinal tissue remaining in human donor eyes with macular holes following the same ILM abrasion technique.
Methods
The study protocol was approved by the Institutional Review Board for Human Subjects Research (IRB) at the University of Iowa, and the study adhered to tenets set forth in the Declaration of Helsinki. Experimental study was performed using the instruments for ILM abrasion during macular hole surgery in 11 eyes. Moreover, three human donor eyes with macular holes underwent an identical procedure in the laboratory. Main outcome measures were microscopic and immunohistological characteristics of instrument tip tissues and retinal structure after ILM abrasion. Clinical methods and outcomes are detailed in an additional report.21
We directly analyzed instrument tissues collected during macular hole surgery in order to study the retinal tissues removed by the DDMS in vivo. A standard three-port pars plana vitrectomy was performed, and the DDMS was applied to the superficial inner retinal surface with medium tactile pressure in a systematic fashion (see ref. 21). Diamond-dusted membrane scraper tips were fixed immediately after surgery in 4% paraformaldehyde in 10 mM PBS, and after 10 minutes of fixation, the tips were transferred to PBS. Immunocytochemistry analysis was performed using DDMS tips as described previously,22 except that labeling of tips was performed in a 96-well plate. After samples were blocked for 15 min with 1 mg/mL bovine serum albumin in PBS, they were incubated with antibodies directed against CD45 (2.5 μg/mL, raised in mouse, catalog no. 5554801 BD; Pharmingen, San Jose, CA, USA) and the ILM marker laminin A or collagen type IV (0.5 μg/mL, raised in rabbit, catalog no. AB748; Millipore, Temecula, CA, USA) for 1 hour.23,24 Samples were then washed in PBS and incubated with secondary antibodies (anti-mouse IgG conjugated to Alexa 488 and anti-rabbit IgG conjugated to Alexa 546; Invitrogen, Grand Island, NY, USA). Nuclei were counterstained with 100 μg/mL 4′,6-diamidino-2-phenylindole (DAPI). Following 30 minutes in secondary antibody solution, Tano scraper tips were washed in PBS and cover-slipped in aqueous mounting medium. Samples were viewed using epifluorescence with a model BX41 microscope (Olympus Corp., Center Valley, PA, USA) using FITC, tetramethylrhodamine-isothiocyanate (TRITC), and UV filter sets.
In order to study the underlying retinal tissue that remained following ILM abrasion, we analyzed the retinas in three fresh (less than 4 hours old) human donor (postmortem) eyes with macular holes that underwent ILM abrasion technique in a similar fashion in vitro. Globes were obtained at less than 4 hours from donor death. The anterior segment was dissected at the limbus, removing the cornea and lens but leaving the iris root intact, using subsequent dry Weck-Cel (Beaver-Visitec International, Waltham, MA, USA) vitrectomy. Then, four distinct areas surrounding the macular hole were created and investigated with scanning electron microscopy. The first area was left untreated as a control region. In the second area, a DDMS was brushed toward the hole with lighter tactile pressure than typically used during surgery so that there was no visible underlying movement of the retina. In the third area, medium tactile pressure (i.e., identical to that applied during surgery) was used so that there was visible movement of the underlying retina without creation of a retinal break. Finally, in the fourth area, intentionally excessive tactile pressure (i.e., much greater than that applied during in vivo surgery) was applied to the point of purposely causing retinal break. The extracted tissue was fixed in half-strength Karnovsky's fixative and 1% osmium tetroxide followed by dehydration in a graded series of ethanol. The tissue was incubated in hexamethyldisilazane (HMDS) and allowed to air dry. The sample was mounted on a scanning electron microscopy stub with colloidal silver and sputter coated. The specimen was imaged using a Hitachi S-4800 Scanning Electron Microscope. For transmission electron microscopy, tissue was fixed in half-strength Karnovsky fixative and processed for transmission electron microscopy (TEM), and embedded in Spurrs medium.23 Sections were cut at 90 nm and collected on grids. Transmission electron microscopy images were collected on a model JEM-1230 EM (JEOL USA, Peabody, MA, USA).
Results
To identify tissues removed with the 23-ga Tano DDMS in vivo, instruments were collected after application to the retina during 11 cases of macular hole ILM abrasion surgery. Mean patient age was 68.8 years (range, 54–78 years) with eight females and six right eyes. Median preoperative best-corrected Snellen visual acuity was 20/125, which improved to postoperative visual acuity of 20/80 after 3 months of follow-up.
The 23-ga Tano scratcher tips were immediately fixed in the operating room and examined by light microscopy. All tips showed evidence of semitransparent tissue fragments. Tips were processed for immunohistochemistry analysis. In each case, laminin A and DAPI stains were positive, indicating removal of ILM and cellular membranes from the ILM surface (Fig. 1). Assay of collagen IV immunohistochemistry gave similar results (data not shown). None of the cells were CD45 positive, indicating that cells removed were unlikely to be hyalocytes.25 In some cases, cellular sheets identified prior to processing were lost during processing; however, each tip showed evidence of ILM fragments and cell nuclei interspersed between diamond crystals.
Figure 1.
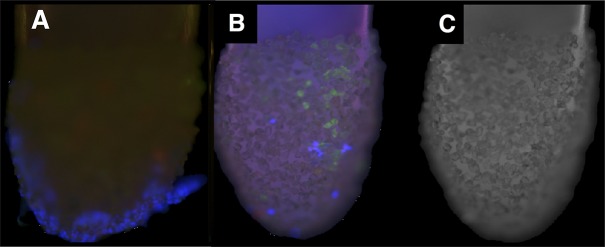
Tano scraper tips show ILM fragments and cells. After they were used for treatment of patient retinas during macular hole surgery, the Tano scratchers were visualized with immunohistochemistry. (A) Numerous cells (blue) can be seen on sheets of tissue attached to the instrument tips. (B) Laminin A–positive tissue fragments (green) indicate ILM and cells (blue) on the Tano tips. (C) Control confocal fluorescence image of an unstained tip.
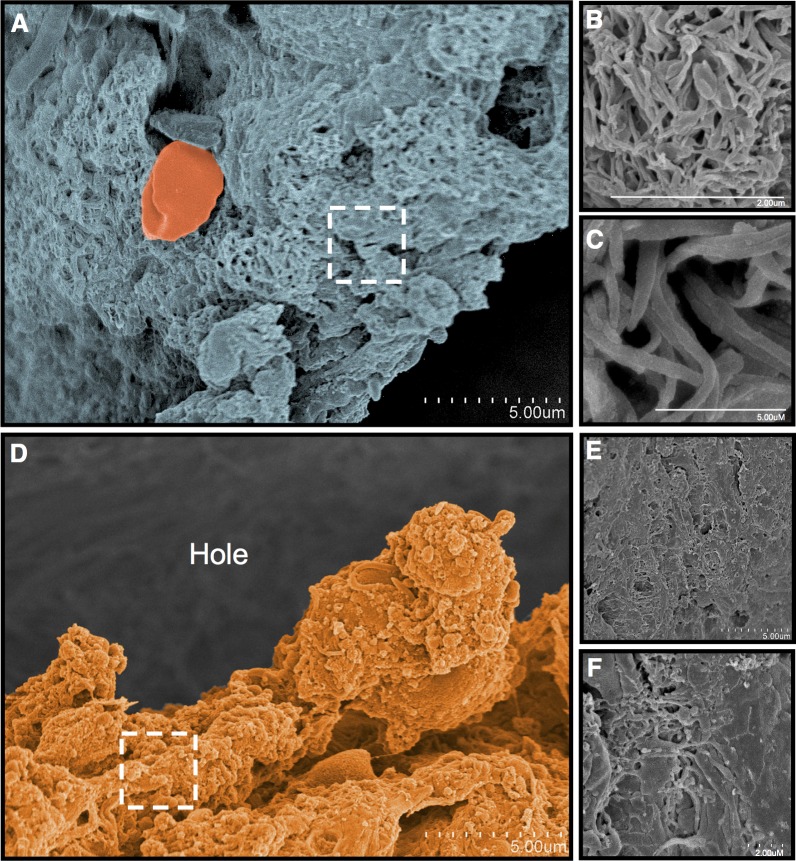
Three donor eyes with macular holes were studied to identify the effect of various tactile pressures (i.e., none, light, medium, heavy) applied with a 23-ga DDMS on the retinal surface. The macula surrounding the hole was visually divided into four areas and brushed with a Tano scraper at various tactile pressures (Fig. 2A). In area 1, no DDMS application was performed. In area 2, light pressure, less than that typically performed during surgery, was applied. In area 3, medium pressure, corresponding to that typically used in patients, was applied. Finally, in area 4, heavy pressure was applied. Low-magnification SEM images of these experimental areas are shown (Fig. 2B).
Figure 2.
Tano scratcher applied to a macular hole in a postmortem eye. (A) Dissecting microscope image of a macular hole. The retina surrounding the macula was divided into four areas (numbered 1–4). (B) A low magnification scanning electron microscopy image of the specimen after treatment with a Tano scratcher. No Tano scratcher was applied to area 1. Light pressure was applied to area 2. Medium pressure was applied to area 3. Heavy pressure was applied to area 4, which shows disruption of the internal limiting membrane and exposure of the nerve fiber layer. Some of the cellular tissue has fallen into the hole.
In area 1, far from the macular hole, where no scraping occurred, SEM showed a structure consistent with a vitreous remnant (Figs. 3A–C). Collagenous elements within this structure showed a mean ± SD of 0.072 ± 0.15 μm. Erythrocytes could be identified in the spaces between these collagen structures. At the edge of the macular hole, a distinct structure consistent with an ERM was identified (Figs. 3D–F). The ERM extended over the edge of the macular hole. Densely packed cellular and various collagenous elements were present.
Figure 3.
Area 1: Macula without Tano scraper application. (A–C) Away from the macular hole are vitreous collagenous elements on the surface of the retina. (D–F) An epiretinal membrane (orange) is present at the edge of the macular hole, where there was no application of the Tano scraper. Higher magnification revealed cellular and collagenous elements, consistent with an epiretinal membrane.
In area 2, light tactile pressure was applied with a Tano scraper. A distinct line could be identified with scanning electron microscopy at the edge of areas 1 and 2. The untreated area had an intact ERM covering the retinal surface, whereas the lightly treated area had nearly complete ERM removal with exposed underlying ILM (Fig. 4A). Most of the ERM had been removed in the scraped area, but there were still scattered cellular elements and collagenous fragments present. (Fig. 4B, 4C).
Figure 4.
Area 2: Macula with light Tano application. (A) After a light application of the Tano, an edge (arrows) of ILM (green) becomes apparent alongside a region where untreated ERM (orange) remains. (B, C) Higher magnification over the scraped area shows that some cell and tissue elements of the epiretinal membrane remain on the surface of the ILM.
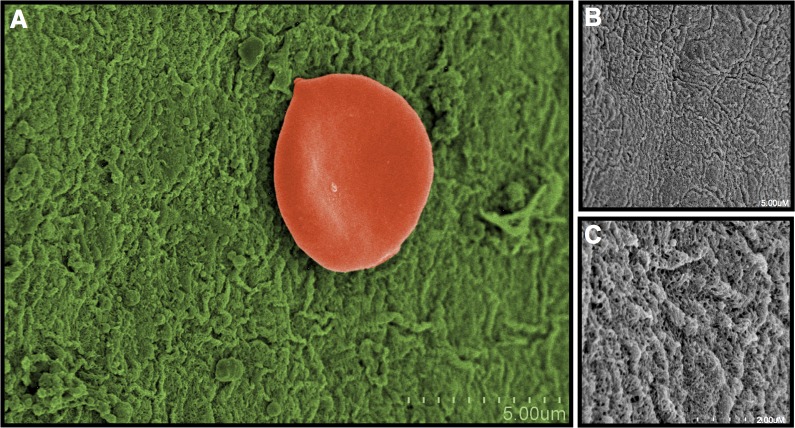
In area 3, where medium tactile pressure was applied, cellular and membranous elements were almost completely absent (Fig. 5). The ILM was visible, but no retinal nerve fiber layer (RNFL) or other retinal cellular elements were seen. The ILM had a homogenous appearance with minimal adherence of cellular constituents.
Figure 5.
Area 3: Macula with medium Tano application. (A) After a medium pressure scrape, nearly all cells and tissues from the epiretinal membrane were removed, revealing the ILM (green). (B, C) The ILM shows no overlying surface cells or tissues at higher magnification.
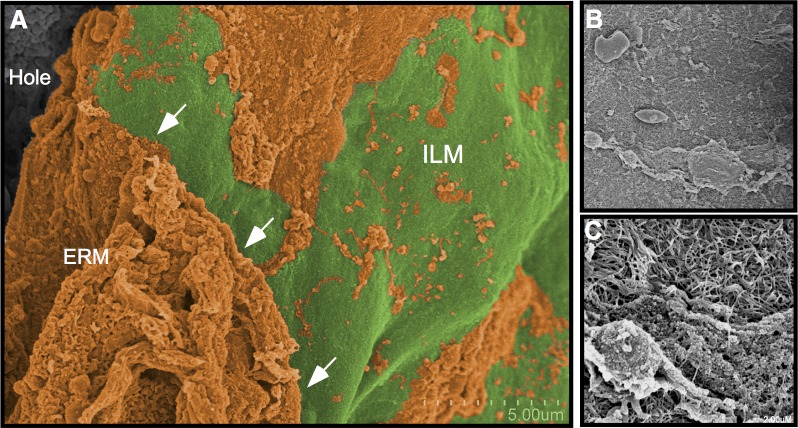
Last, in area 4, where heavy tactile pressure with the Tano scraper was applied, the ILM was clearly disrupted (Fig. 6). The ILM was folded over on itself, and tissue fragments could be identified on its underside. These structures corresponded to RNFL and glial elements that were visibly exposed where the ILM was missing. Distal to the inner retina structures, spherical structures that represented cells could be identified. In contrast to areas 2 and 3 where the light and medium pressures were applied, respectively, the ERM was still present at the edge of the hole. In these cases the membranes were inverted, but several thin string-like elements of the membranes could be seen to tether the membrane to the edge of the macular hole. These string-like elements were intermittently bundled, giving the appearance of a spider web. Examination of the adjacent ILM showed numerous corresponding curvilinear string-like elements on its surface. They appeared thinner than the vitreous collagen, with an average diameter of 30 nm (±6 nm). The appearance of these elements was consistent with elastin, suggesting a contractile element was present between the ERM and ILM.
Figure 6.
Area 4: Macula with heavy Tano application. (A, B) Heavy application of the Tano scraper caused a tear in the ILM (green) and subsequent exposure of the nerve fiber layer (purple) and cellular elements (blue). The underside of the ILM (arrow) shows fragments of the retinal nerve fiber layer (purple) remain attached to the ILM. (C) Fibers consistent with elastin (orange) were found between the ERM and ILM. (D) These fibers remain attached to the ILM and underside of the ERM.
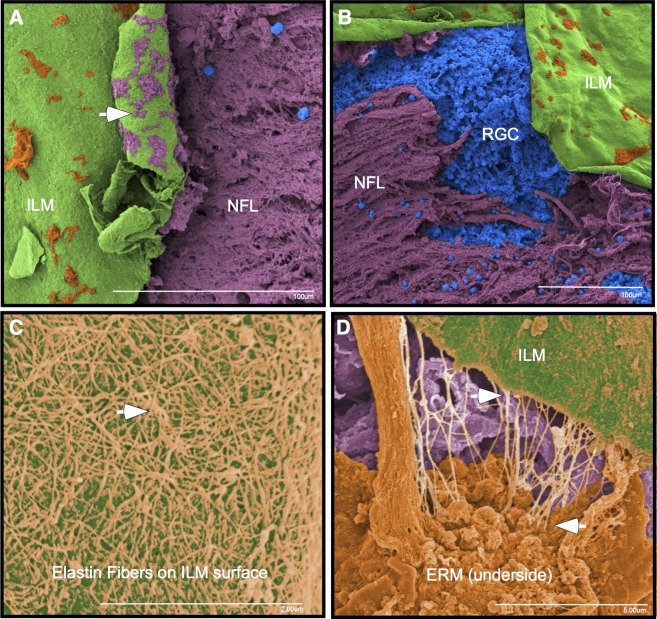
To further examine the ILM following the DDMS application with medium pressure, we sectioned separate postmortem donor retinas for immunohistochemistry and TEM examination. Antibodies against laminin A were used to identify ILM. In both the untreated and the ILM abrasion retinas, ILM was detected (Fig. 7A). None of the sections showed disruption of the RNFL or deeper retinal layers. This indicated that the DDMS might only remove the surface layer of ILM without penetrating the RNFL. Untreated retinas showed Müller glial cell end feet processes at the edge of the ILM and filopodial insertions deeper within the ILM (Fig. 7B). Most of the ILM and the deeper Müller filopodial insertions were removed; however, a thin layer of ILM over the end feet was still present (Fig. 7C).
Figure 7.
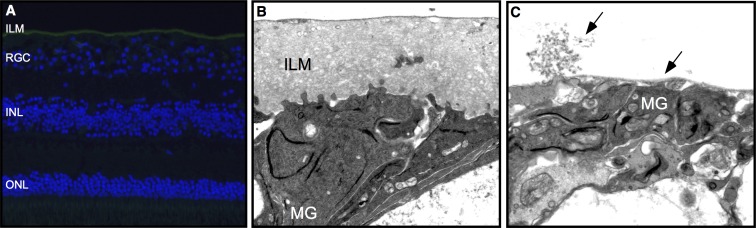
Internal limiting membrane immunohistochemistry and transmission electron microscopy. (A) In human retinas treated with the DDMS, laminin A antibodies (green) identified the ILM. Cell nuclei were stained with DAPI (blue). ONL, outer nuclear layer. (B) Transmission electron microscopy image of untreated retinas showed Müller glial (MG) cell end feet processes at the edge of the ILM and filopodial insertions deeper within the ILM. (C) Treatment of retinas with medium pressure from the Tano resulted in removal of most of the ILM and the deepest Müller filopodial insertions, but preserved a thin layer of ILM over the MG end feet.
Discussion
The pathogenesis of macular hole formation is not completely understood but likely involves a combination of tangential traction, anterior–posterior traction, degenerative changes, and cellular proliferation from abnormal traction at the vitreoretinal interface.26–30 Surgical approaches to address these components involve the induction of a complete posterior vitreous detachment and removal of any remaining cortical vitreous and cellular proliferation on the retinal surface. Removal of the surrounding ILM ensures release of tractional forces and a restoration of retinal compliance. Internal limiting membrane peeling may also activate Müller cells, stimulating the secretion of collagen, basement membrane components, and inflammatory factors which may stimulate glial cell-mediated closure of macular holes; the latter has been suggested in primate studies evaluating histological changes following ILM peeling.31
Although ILM peeling appears to increase rates of macular hole closure, in a recent analysis of ILM and epimacular tissue obtained at the time of macular hole repair, Schumann et al.30 did not find any cells or collagen at the ILM in 43 of 100 eyes, suggesting that complete removal of the ILM may not be necessary to achieve optimal closure rates in all patients with full-thickness macular holes. However, in a subsequent report, the ILM removed during a second macular hole repair of holes that had failed to close without ILM removal demonstrated fibrocellular proliferation on the vitreal side of the ILM in all 16 specimens.31,32 This finding suggests that failure to remove the ILM may have resulted in persistence of fibrocellular proliferation on the retinal surface, impeding hole closure. Despite advancements in imaging of the vitreoretinal interface with optical coherence tomography, it is not possible to clinically distinguish between holes with and without associated proliferation preoperatively, and thus, many surgeons prefer to remove the ILM as part of their standard technique in the surgical repair of macular holes.16,21,33,34
Previous studies evaluating the histopathological characteristics of ILM removed at the time of macular hole repair identified similar contractile elements associated with both epiretinal and ILM.35–37 In some specimens, the ILM was folded over in an accordion fashion, similar to that of our specimen, and we attribute this structural characteristic to the tangential forces present in the ILM of eyes with macular holes.34 Abnormal traction at the vitreoretinal interface of eyes with macular holes would be associated with a structure of low compliance (high Young's modulus), and this could lead to deformations in intrinsic histological architecture as seen in our study.
Results of our study suggest that applying light to medium pressure alone has the effect of removing ERMs and cellular elements on the surface of the ILM. Heavier pressure can peel the entire ILM, but this is associated with the risk of RNFL irregularities and potential damage to the underlying cellular retinal layers.38,39 Heavy pressure, such as accidental instrument touch during surgery, can lead to RNFL loss and damage to underlying inner retina structure. During peeling of the ILM in surgical repair of macular holes, small retinal microhemorrhages may be seen. We hypothesize that these hemorrhages are secondary to damage of the cellular retinal layers and capillaries of the RNFL, which has been shown in eyes with macular holes.30,40,41 We found that with significant heavy tactile pressure with the Tano instrument, collateral damage occurs beyond the ILM. We suspect that similar surgical trauma with any microsurgical instrument could cause similar cellular damage and retinal microhemorrhages.
It has also been suggested that ILM removal can stimulate glial cell-mediated closure of macular holes after ILM peeling, and the Tano scratcher can be used to selectively remove cellular membranes, including only the surface layers of ILM. Creation of shear stress on Müller cells has been shown to stimulate protein expression.42 Indeed, glial cell trauma stimulates a reparative process that includes deposition of collagenous basement membrane, as seen by SEM.43 Müller cell gliosis is characterized by both nonspecific responses (i.e., stereotypic alterations independent of the causal stimulus) and specific responses, which depend on the given pathogenic factor or mechanism. The most sensitive nonspecific response to retinal diseases and injuries, which can be used as a universal early cellular marker for retinal injury, is upregulation of the intermediate filament protein glial fibrillary acidic protein (GFAP).44–46 Nonhuman primate studies show that removal of Müller end-feet stimulate cells to deposit collagen that fills the area where ILM has been removed.47 We found evidence of cellular constituents at the ILM interface which likely contribute to the necessary milieu of this reparative process.
In conclusion, we show that the Tano DDMS instrument removes cellular membranes and only the surface layers of ILM. This technique alone may be sufficient to stimulate microglia that can aid in primary closure of macular holes.
Acknowledgments
Supported by National Institutes of Health Grant K08EY020530, Doris Duke Charitable Foundation, and Research to Prevent Blindness, New York, New York, United States (VBM).
This study adhered to the Declaration of Helsinki and all Federal and State laws; approved by the University of Iowa Research Ethics Board.
Disclosure: D.R.P. Almeida, None; E.K. Chin, None; R.M. Tarantola, None; J.C. Folk, None; H.C. Boldt, None; J.M. Skeie, None; R.F. Mullins, None; S.R. Russell, None; V.B. Mahajan, None
References
- 1.Gass JD.Idiopathic senile macular hole. Its early stages and pathogenesis. Arch Ophthalmol. 1988; 106: 629–639. [DOI] [PubMed] [Google Scholar]
- 2.Gass JD.Risk of developing macular hole. Arch Ophthalmol. 1991; 109: 610–612. [DOI] [PubMed] [Google Scholar]
- 3.Casuso LA,, Scott IU,, Flynn HW, Jr., et al. Long-term follow-up of unoperated macular holes. Ophthalmology. 2001; 108: 1150–1155. [DOI] [PubMed] [Google Scholar]
- 4.Gupta P,, Yee KM,, Garcia P,, et al. Vitreoschisis in macular diseases. Br J Ophthalmol. 2011; 95: 376–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kishi S,, Demaria C,, Shimizu K.Vitreous cortex remnants at the fovea after spontaneous vitreous detachment. Int Ophthalmol. 1986; 9: 253–260. [DOI] [PubMed] [Google Scholar]
- 6.Gandorfer A,, Haritoglou C,, Scheler R,, Schumann R,, Zhao F,, Kampik A.Residual cellular proliferation on the internal limiting membrane in macular pucker surgery. Retina. 2012; 32: 477–485. [DOI] [PubMed] [Google Scholar]
- 7.Kelly NE,, Wendel RT.Vitreous surgery for idiopathic macular holes. Results of a pilot study. Arch Ophthalmol. 1991; 109: 654–659. [DOI] [PubMed] [Google Scholar]
- 8.Wendel RT,, Patel AC,, Kelly NE,, Salzano TC,, Wells JW,, Novack GD.Vitreous surgery for macular holes. Ophthalmology. 1993; 100: 1671–1676. [DOI] [PubMed] [Google Scholar]
- 9.Kim JW,, Freeman WR,, Azen SP,, el-Haig W,, Klein DJ,, Bailey IL.Prospective randomized trial of vitrectomy or observation for stage 2 macular holes. Vitrectomy for Macular Hole study group. Am J Ophthalmol. 1996; 121: 605–614. [DOI] [PubMed] [Google Scholar]
- 10.Freeman WR, Azen SP,, Kim JW,, el-Haig W,, Mishell DR, III, Bailey IL.Vitrectomy for the treatment of full-thickness stage 3 or 4 macular holes. Results of a multicentered randomized clinical trial. The Vitrectomy for Treatment of Macular Hole study group. Arch Ophthalmol. 1997; 115: 11–21. [DOI] [PubMed] [Google Scholar]
- 11.Smiddy WE,, Pimentel S,, Williams GA.Macular hole surgery without using adjunctive additives. Ophthalmic Surg Lasers. 1997; 28: 713–717. [PubMed] [Google Scholar]
- 12.Park DW,, Sipperley JO,, Sneed SR,, Dugel PU,, Jacobsen J.Macular hole surgery with internal-limiting membrane peeling and intravitreous air. Ophthalmology. 1999; 106: 1392–1398; discussion 1397–1398. [DOI] [PubMed] [Google Scholar]
- 13.Margherio RR,, Margherio AR,, Williams GA,, Chow DR,, Banach MJ.Effect of perifoveal tissue dissection in the management of acute idiopathic full-thickness macular holes. Arch Ophthalmol. 2000; 118: 495–498. [DOI] [PubMed] [Google Scholar]
- 14.Brooks HL., Jr.Macular hole surgery with and without internal limiting membrane peeling. Ophthalmology. 2000; 107: 1939–1948; discussion 1948–1949. [DOI] [PubMed] [Google Scholar]
- 15.Sheidow TG,, Blinder KJ,, Holekamp N, et al. Outcome results in macular hole surgery: an evaluation of internal limiting membrane peeling with and without indocyanine green. Ophthalmology. 2003; 110: 1697–1701. [DOI] [PubMed] [Google Scholar]
- 16.Smiddy WE,, Feuer W,, Cordahi G.Internal limiting membrane peeling in macular hole surgery. Ophthalmology. 2001; 108: 1471–1478; discussion 1477–1478. [DOI] [PubMed] [Google Scholar]
- 17.Ando F,, Sasano K,, Ohba N,, Hirose H,, Yasui O.Anatomic and visual outcomes after indocyanine green-assisted peeling of the retinal internal limiting membrane in idiopathic macular hole surgery. Am J Ophthalmol. 2004; 137: 609–614. [DOI] [PubMed] [Google Scholar]
- 18.Haritoglou C,, Gandorfer A,, Gass CA,, Kampik A.Histology of the vitreoretinal interface after staining of the internal limiting membrane using glucose 5% diluted indocyanine and infracyanine green. Am J Ophthalmol. 2004; 137: 345–348. [DOI] [PubMed] [Google Scholar]
- 19.Stanescu-Segall D,, Jackson TL.Vital staining with indocyanine green: a review of the clinical and experimental studies relating to safety. Eye (Lond). 2009; 23: 504–518. [DOI] [PubMed] [Google Scholar]
- 20.Lewis JM,, Park I,, Ohji M,, Saito Y,, Tano Y.Diamond-dusted silicone cannula for epiretinal membrane separation during vitreous surgery. Am J Ophthalmol. 1997; 124: 552–554. [DOI] [PubMed] [Google Scholar]
- 21.Mahajan VB,, Tarantola RM,, Chin EK, et al. Macular hole closure with internal limiting membrane abrasion technique [published online ahead of print March 12, 2015]. JAMA Ophthalmol. doi:http://dx.doi.org/10.1001/jamaophthalmol.2015.204. [DOI] [PubMed]
- 22.Mullins RF,, Skeie JM,, Folk JC,, et al. Evaluation of variants in the selectin genes in age-related macular degeneration. BMC Med Genet. 2011; 12: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mullins RF,, Kuehn MH,, Faidley EA,, Syed NA,, Stone EM.Differential macular and peripheral expression of bestrophin in human eyes and its implication for best disease. Invest Ophthalmol Vis Sci. 2007; 48: 3372–3380. [DOI] [PubMed] [Google Scholar]
- 24.Ponsioen TL,, van Luyn MJ,, van der Worp RJ,, van Meurs JC,, Hooymans JM,, Los LI.Collagen distribution in the human vitreoretinal interface. Invest Ophthalmol Vis Sci. 2008; 49: 4089–4095. [DOI] [PubMed] [Google Scholar]
- 25.Schumann RG,, Eibl KH,, Zhao F, et al. Immunocytochemical and ultrastructural evidence of glial cells and hyalocytes in internal limiting membrane specimens of idiopathic macular holes. Invest Ophthalmol Vis Sci. 2011; 52: 7822–7834. [DOI] [PubMed] [Google Scholar]
- 26.Smiddy WE,, Flynn HW., Jr.Pathogenesis of macular holes and therapeutic implications. Am J Ophthalmol. 2004; 137: 525–537. [DOI] [PubMed] [Google Scholar]
- 27.Gandorfer A,, Scheler R,, Schumann R,, Haritoglou C,, Kampik A.Interference microscopy delineates cellular proliferations on flat mounted internal limiting membrane specimens. Br J Ophthalmol. 2009; 93: 120–122. [DOI] [PubMed] [Google Scholar]
- 28.Hisatomi T,, Enaida H,, Sakamoto T, et al. Cellular migration associated with macular hole: a new method for comprehensive bird's-eye analysis of the internal limiting membrane. Arch Ophthalmol. 2006; 124: 1005–1011. [DOI] [PubMed] [Google Scholar]
- 29.Gandorfer A,, Scheler R,, Haritoglou C,, Schumann R,, Nentwich M,, Kampik A.Pathology of the macular hole rim in flat-mounted internal limiting membrane specimens. Retina. 2009; 29: 1097–1105. [DOI] [PubMed] [Google Scholar]
- 30.Schumann RG,, Schaumberger MM,, Rohleder M,, Haritoglou C,, Kampik A,, Gandorfer A.Ultrastructure of the vitreomacular interface in full-thickness idiopathic macular holes: a consecutive analysis of 100 cases. Am J Ophthalmol. 2006; 141: 1112–1119. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura T,, Murata T,, Hisatomi T, et al. Ultrastructure of the vitreoretinal interface following the removal of the internal limiting membrane using indocyanine green. Curr Eye Res. 2003; 27: 395–399. [DOI] [PubMed] [Google Scholar]
- 32.Schumann RG,, Rohleder M,, Schaumberger MM,, Haritoglou C,, Kampik A,, Gandorfer A.Idiopathic macular holes: ultrastructural aspects of surgical failure. Retina. 2008; 28: 340–349. [DOI] [PubMed] [Google Scholar]
- 33.Mester V,, Kuhn F.Internal limiting membrane removal in the management of full-thickness macular holes. Am J Ophthalmol. 2000; 129: 769–777. [DOI] [PubMed] [Google Scholar]
- 34.Tognetto D,, Grandin R,, Sanguinetti G, et al. Internal limiting membrane removal during macular hole surgery: results of a multicenter retrospective study. Ophthalmology. 2006; 113: 1401–1410. [DOI] [PubMed] [Google Scholar]
- 35.Guyer DR,, Green WR,, de Bustros S,, Fine SL.Histopathologic features of idiopathic macular holes and cysts. Ophthalmology. 1990; 97: 1045–1051. [DOI] [PubMed] [Google Scholar]
- 36.Madreperla SA, McCuen BW,, 2nd,, Hickingbotham D,, Green WR.Clinicopathologic correlation of surgically removed macular hole opercula. Am J Ophthalmol. 1995; 120: 197–207. [DOI] [PubMed] [Google Scholar]
- 37.Yooh HS, Brooks HL, Jr,, Capone A, Jr,, L'Hernault NL,, Grossniklaus HE.Ultrastructural features of tissue removed during idiopathic macular hole surgery. Am J Ophthalmol. 1996; 122: 67–75. [DOI] [PubMed] [Google Scholar]
- 38.Karacorlu M,, Karacorlu S,, Ozdemir H.Iatrogenic punctate chorioretinopathy after internal limiting membrane peeling. Am J Ophthalmol. 2003; 135: 178–182. [DOI] [PubMed] [Google Scholar]
- 39.Haritoglou C,, Gass CA,, Schaumberger M,, Ehrt O,, Gandorfer A,, Kampik A.Macular changes after peeling of the internal limiting membrane in macular hole surgery. Am J Ophthalmol. 2001; 132: 363–368. [DOI] [PubMed] [Google Scholar]
- 40.Funata M,, Wendel RT,, de la Cruz Z,, Green WR.Clinicopathologic study of bilateral macular holes treated with pars plana vitrectomy and gas tamponade. Retina. 1992; 12: 289–298. [DOI] [PubMed] [Google Scholar]
- 41.Smiddy WE,, Michels RG,, de Bustros S,, de la Cruz Z,, Green WR.Histopathology of tissue removed during vitrectomy for impending idiopathic macular holes. Am J Ophthalmol. 1989; 108: 360–364. [DOI] [PubMed] [Google Scholar]
- 42.Campochiaro PA,, Van Niel E,, Vinores SA.Immunocytochemical labeling of cells in cortical vitreous from patients with premacular hole lesions. Arch Ophthalmol. 1992; 110: 371–377. [DOI] [PubMed] [Google Scholar]
- 43.Lewis GP,, Fisher SK.Up-regulation of glial fibrillary acidic protein in response to retinal injury: its potential role in glial remodeling and a comparison to vimentin expression. Int Rev Cytol. 2003; 230: 263–290. [DOI] [PubMed] [Google Scholar]
- 44.Rueger DC,, Huston JS,, Dahl D,, Bignami A.Formation of 100 A filaments from purified glial fibrillary acidic protein in vitro. J Mol Biol. 1979; 135: 53–68. [DOI] [PubMed] [Google Scholar]
- 45.Eisenfeld AJ,, Bunt-Milam AH,, Sarthy PV.Müller cell expression of glial fibrillary acidic protein after genetic and experimental photoreceptor degeneration in the rat retina. Invest Ophthalmol Vis Sci. 1984; 25: 1321–1328. [PubMed] [Google Scholar]
- 46.Bringmann A,, Schopf S,, Faude F,, Reichenbach A.Arachidonic acid-induced inhibition of Ca2+ channel currents in retinal glial (Müller) cells. Graefes Arch Clin Exp Ophthalmol. 2001; 239: 859–864. [DOI] [PubMed] [Google Scholar]
- 47.Trese M,, Chandler DB,, Machemer R.Macular pucker. II. Ultrastructure. Graefes Arch Clin Exp Ophthalmol. 1983; 221: 16–26. [DOI] [PubMed] [Google Scholar]