Abstract
Tolerance to allograft antigen is the major challenge and final goal of transplant medicine. Our previous study demonstrated that thioredoxin-1 (Trx) priming of donor lung significantly protected allogeneic lung graft. To determine whether Trx priming of donor lung inhibits allograft rejection, extends allograft survival and induces immune tolerance, orthotopic left lung transplantation was performed from Lewis to Sprague-Dawley rats without immunosuppression. Donor lungs were primed with Trx at 4°C for 4 hr prior to transplantation. After up to 37 days post-transplantation, allograft lung morphology, recipient T cell and humoral alloantigen-specific immune responses were examined. We found that Trx-primed lungs exhibited much reduced acute rejection and associated lung injuries resulting in loss of graft functional area at 5-37 days post-transplant in contrast to the control groups. CD4+ T cells from the recipients with Trx-primed grafts responded to the stimulation of dendritic cells (DCs) of donor origin, in contrast to DCs from the third party, with significantly reduced proliferation. Consistent with above findings, we observed that CD4+Foxp3+ regulatory T cells in spleen cells from the recipients with Trx-primed grafts were significantly increased compared to controls, and CD4+ T cells from the recipients with Trx-primed grafts produced much higher levels of immunosuppressive cytokine, IL-10 when stimulated with allogeneic donor DCs. In addition, humoral immune tolerance was also induced as there was no significant increase levels of serum antibodies against donor antigens in Trx-lung recipients when re-challenged with allogeneic donor antigens. Our results demonstrate that one-time Trx-priming of donor lung grafts prior to transplantation significantly prolongs the survival of the grafts through inducing or promoting cellular and humoral alloantigen-specific immune tolerance, which might be associated with the induction of immunosuppressive regulatory T cells.
Introduction
Lung transplantation is the ultimate therapeutic option for end-stage lung diseases. Despite refinement in lung preservation, improvements in surgical techniques, and use of immunosupression regimens, early graft dysfunction and rejection remain a significant cause of morbidity and mortality after lung transplantation. Acute cellular rejection is due to the recipient’s alloreactive T cells which recognize the alloantigen presented in the transplanted tissue and subsequently attack the allograft. Antibody-mediated rejection, on the other hand, is characterized by the development of antibodies to the alloantigen [1]. Although use of immunosuppressive regimens is the current mainstay approach to prevent rejection and promote lung allograft survival, such global immune inhibition leaves the patient susceptible to life-threatening infection, malignancy, organ toxicity, and has limited success in preventing chronic graft rejection [2]. Therefore, selective regulation of the recipient’s immune system to accept the transplanted organ while maintaining normal reactivity against other sources of antigens by alloantigen-specific immune tolerance induction has been the ultimate goal of transplant immunology since the 1950’s [3,4].
We have reported that priming donor lungs with human recombinant thioredoxin-1 (Trx) prior to transplant reduced acute allograft lung injury, transcription factor nuclear factor kappa B (NF-ĸB) activation, and progressive infiltration of inflammatory and cytotoxic CD8+ T cells leading to reduced allograft injury without use of immunosuppressant in a rat model of lung transplantation [5]. Trx, a 12-kDa thiol-disulfide oxidoreductase, is a reactive oxygen species (ROS) scavenger and an essential physiological redox regulator of multiple cellular process including gene regulation and cell proliferation involved in inflammatory and immune responses [6–11]. Although a number of chemical and biological agents have been increasingly used to target the immune cascade to modulate immune responses, the specific effects of Trx on immune modulation in lung transplantation remain to be examined. In the present study, we investigated whether one time Trx priming of donor lung prolongs allograft survival and induces immune tolerance.
Materials and Methods
Ethics statement
Specific, pathogen-free, male Lewis, Sprague-Dawley (SD), and Wistar rats (300∼350 g) obtained from Harlan (Indianapolis, IN) were used in compliance with the Guide for the Care and Use of Laboratory Animals. The study protocol was approved by the Institutional Animal Care and Use Committee (ACORP number: 0013). All animals were survived during the course of this study. Animals were monitored every 5 to 15 min during recovery period followed by twice a day after recovery for first 48 hr, and daily thereafter for total of 37 days. Any change in bleeding, respiratory distress, body weight, food/water consumption, and signs of infection were monitored for potential post-operative complications. Post-operative analgesic, buprenorphine (0.05 mg/kg, s.c.) was administered twice daily for two days. Post-operative care was monitored and recorded under supervision of facility VMO and his staff. Animals were euthanatized using pentobarbital (60 mg/kg, i.p.) at appropriate experimental end point. All animals were survival to the end point of experiment.
Surgical Procedures, Treatment, and Assessment of Allograft Acute Rejection
Orthotopic left lung transplants were performed using Lewis donors and SD recipient rats as described [5]. Briefly, donor rats underwent tracheotomy and were mechanically ventilated with 100% O2 and 3% isoflurane. Continuous mechanically ventilated with 3.0 cm H2O of positive end-expiratory pressure (PEEP) was performed until the end of flushing. The lungs were flushed through the main pulmonary artery with 20 mL of cold (4°C) Perfadex(Vitrolife, Uppsala, Sweden) with or without Trx (4 μg/ml, USB Corporation, Cleveland, OH) at a pressure of 20 cm H2O. When the perfusion is completed the lungs were inflated to 100% of vital capacity and excised. After excision of the heart—lung block, cuffs were attached to the pulmonary artery, pulmonary vein, and bronchus; then, the graft was placed in Perfadex with or without Trx and stored at 4°C for 4 hours. After 4 hours of cold storage, the lung grafts were transplanted into the SD recipients. At sampling, the lung graft was excised and divided into three sections. The upper, middle and lower section of the graft lung, as well as middle section of native lung were embedded in paraffin and used for histological examinations. Hematoxylin and eosin (H&E)–stained lung sections were examined by pathologists to determine the degree of acute cellular rejection (AR) following standardized international grading criteria, 1996 working formulation [12].
Cell isolation
Recipient’s spleen and allograft lung were removed and CD4+ T cells were isolated by positive selection using rat CD4+ isolation kit following instructions from the manufacturer (Miltenyi Biotec, Boston, MA). The purity of CD4+ T cells was in the range of 90–97%. Naïve Lewis (donor) or Wistar (third party control) splenic dendritic cells (DCs) were isolated and purified by positive selection using OX62+ microbeads according to manufacturer’s instructions (Miltenyi Biotec). The purity of OX62+ DC cells was 90%.
Assessment of recipient’s T cell proliferative response in vitro and cytokines assay
Recipient spleen CD4+ T cells (4 × 105) were cultured with splenic DC (4 × 104) from naïve donor or third party rats for four days in a U-bottom 96-well plate for 5 days. 3H-thymidine (1 μCi/well) was added to each culture well for the last 16 h. The cells were harvested and the 3H-thymidine incorporation was measured by scintillation counting. Supernatants were pooled from triplicate cultures and cytokine levels were determined using the Milliplex Map Rat Multi-Cytokine Detection Kit for Luminex xMAP technology following the manufacturer’s instructions (Millipore, Billerica, MA).
Assessment of Foxp3+ regulatory T lymphocytes
Recipient’s splenic and allograft lung CD4+ T cells were incubated with fluorescein isothiocyanate (FITC)-labeled CD4 antibody followed by intracellular staining for phycoerythrin (PE)-conjugated Foxp3 antibodies according to the manufacturer’s instructions (eBiosciences). The number of CD4+Foxp3+ T cells was evaluated by FACS-Fortassa flow cytometer and analyzed by FACSDiva (Becton Dickinson, Palo Alto, CA).
Assessment of in vivo humoral tolerance: Measurement of donor antibodies after donor antigen re-challenge to the recipients
Two weeks after transplant, the recipients (SD rats) of lung grafts (from Lewis rats) with or without Trx-priming (n = 3 for each group) were administered by tail vein injection of naïve Lewis spleen cells (SPC, 1 x 106) to re-challenge the recipients once. Serum samples were collected before SPC, one and two weeks after SPC injection. The development of antibodies against donor was determined by immunofluorescent flow cytometry as previously reported [13]. Briefly, naïve donor (Lewis) rat SPC (1.5 x 105) suspended in 20 μl of PBS containing 0.1 percent sodium azide and 1 percent bovine serum albumin (PBS-azide-BSA) were incubated with 10 μl of recipient’s serum at room temperature for 45 minutes. Cells were washed three times and incubated with 30 μl of 40x diluted FITC-labeled goat anti-rat IgG-Fc antibody (Biolegend) for 30 minutes. After three washes, cells were suspended in 0.5 mL of PBS-azide-BSA and the mean fluorescence intensity (MFI) of 1x104 cells was measured in arbitrary units using a FACS-Fortassa flow cytometer and analyzed by FACSDiva (Becton Dickinson, Palo Alto, CA). A pooled, pre-immune rat serum sample was used as a negative control for each experiment.
Statistical analysis
Experimental results are expressed as mean ± SE. Statistical differences between groups were determined by an unpaired two-tailed Student’s t-test. P values less than 0.05 were considered statistically significant.
Results
Trx priming attenuates acute allograft rejection and prolongs lung allograft survival
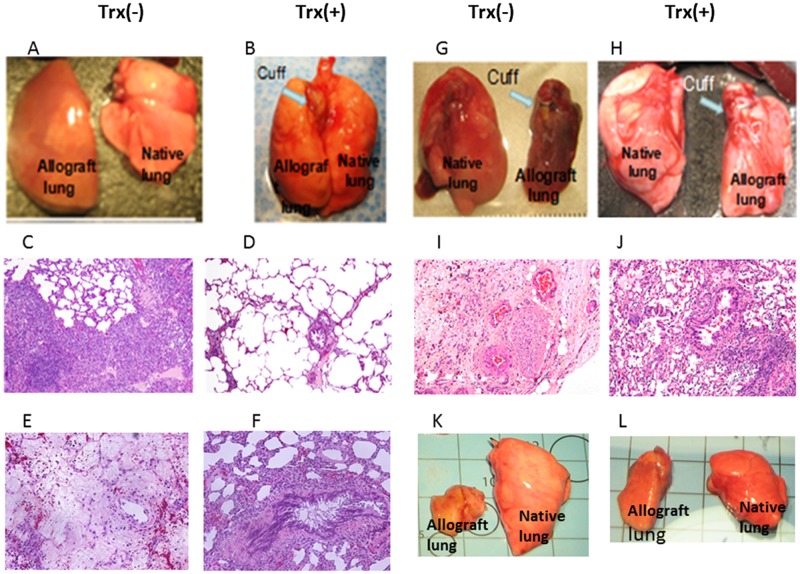
We have reported that one time Trx-priming of donor lung significantly attenuate the allograft lung acute injury accompanied with significant increased oxygen exchange function one and five days post transplantation [5]. To determine the effect of Trx on acute allograft rejection and survival, we extended observation to 37 days post transplantation without any immunosuppressant. As shown in Fig 1, control allograft lungs at 5 days post transplantation are enlarged and edematous (panel A). In contrast Trx-primed allograft lungs were near comparable to native lung (panel B). Histological examination shows moderate to severe perivascular and peribronchial mononuclear cell infiltration in control allografts (acute rejection grade A3–A4 and B2–B3) (panel C). Trx-primed allografts show much reduced perivascular and peribronchial mononuclear cell infiltration (acute rejection grade A1–A3 and B1–B2) (panel D) The difference of acute rejection are significant (n = 7 p<0.05 vs control). Allograft lung tissue necrosis occurred at day 7 post transplantation without Trx-priming (panel E), but not in Trx-primed allograft (panel F) (represented one of three). At day 21, 28, 31, and 37 post-transplant (n = 3 in each group), all control allograft lungs shown markedly reduce in size (panels G and K). Histologic examination showed replacement of the lung parenchyma by granulation tissue and fibrosis in control group (panel I) but viable lung with mild to moderate perivascular and peribronchial infiltrate (acute rejection grade A2–3 and B1–B2) in Trx-primed allograft (panel J). At all-time points lung allograft morphology result consistently indicates that Trx-priming of donor lung prolonged allograft survival without use of immunosuppression.
Fig 1. Trx-priming attenuates acute allograft rejection and prolong allograft survival.
Orthotopic left lung transplantations from Lewis to Sprague-Dawley rats were performed without immunosuppression using with (+) or without (-) Trx-primed donor lungs. Shown are representative gross images of native lung (right) allograft lung (left) of Trx (-) and Trx (+) priming on day 5 (panels A and B), day 31 (panels G and H), and day 37 (panels K and L) post-transplant, respectively. Shown are representative histological images of Trx (-) and Trx (+) allograft lungs on day 5 (panels C and D), day 7 (panels G and H, and day 31 (panels I and J) post-transplant (original magnification 200x), respectively. Images shown are representative of 3–7 transplants in each group.
Trx priming attenuates recipient T cell response to donor alloantigen with increased IL-10 production
To determine whether the improved survival of the Trx-primed allograft is related to T cell tolerance, which is donor-specific low T cell proliferative response to donor alloantigen, purified spleen CD4+ T cells from rats transplanted with Trx-priming or control lungs were stimulated with naïve spleen DCs from Lewis donor or third party control Wistar rats in a mixed lymphocyte reaction (MLR). Following DC stimulation, T cell proliferation and IL-10 level in the DC-T cell co-culture media were determined.
This Trx-priming induced inhibition of T cell response was determined at day 5 and 7 post-transplant. Fig 2A shows the images of T cell proliferating clusters in co-culture of host spleen CD4+ T cells and donor Lewis DC at 3rd day MLR of Trx(-) (left) and Trx(+)(right) groups. There were fewer and smaller T cell clusters formed in Trx-priming group. This donor DC-stimulated recipient spleen T cell response was accompanied by 80% reduction of T cell proliferation and 113% increase in IL-10 production respectively (Fig 2B and 2C). Similar responses to T cell proliferation and IL-10 production in Trx-primed graft to donor DC stimulation were observed at day 5 post-transplant (data not shown). These results suggested that Trx-priming reduces the host’s T cell response to donor alloantigen after lung transplant with consequent induction of alloantigen-specific IL-10-producing Tr1 regulatory cells.
Fig 2. Trx- priming attenuates recipients’ T cell response to the donor type alloantigen accompanied with increased IL-10 production, but not third party control.
Orthotopic left lung transplantations were performed from Lewis to Sprague-Dawley rats without immunosuppression using with (+) or without (-) Trx-primed donor lungs. A: Images of T cell proliferating clusters in the co-culture of 7 day transplant recipients’ CD4+ T cells and naïve donor Lewis DC at day 3 in MLR, image represents Trx (-) and Trx (+) groups respectively. The round dark areas represent T cell clusters. B: 5 day transplant recipients’ spleen CD4+ T cells proliferative response to naive donor DC stimulation in MLR. C: IL-10 concentration in the supernatants of A. D: comparison of 37 day transplanted recipients’ spleen CD4+ T cells proliferative response to the stimulation of donor type antigens from Lewis spleen DC and third party antigens from Wistar spleen DC. E: IL-10 concentration in supernatants of D. Data represent mean ± SE, n = 3. (*p<0.05, **p<0.01 vs Trx (-).
To further address whether this Trx-inhibited T cell response is donor-specific and whether it can be maintained for an extended period, recipients’ splenic CD4+ T cells were isolated 37 days post-transplant and stimulated with naïve DCs from donor or third-party rats. As shown in Fig 2D, Trx-primed donor lung rendered the recipient T cells less proliferative with concomitant increase in IL-10 levels (panel E) in contrast to third party. These results suggest that Trx-priming of allograft induces immune tolerance specifically to donor alloantigen without causing global immunosuppression and this donor-specific tolerance could be maintained for a prolonged period of time in line with 6 weeks post-transplant allograft survival (Fig 1L vs 1K).
Trx priming enhances recipients’ spleen CD4+Foxp3+ regulatory T cells
CD4+Foxp3+ regulatory T lymphocytes play a critical role in maintaining transplant tolerance in lung transplant patients [14]. In order to determine the underlying mechanism of Trx-priming on suppression of acute allograft lung rejection, we examined the CD4+Foxp3+ regulatory T cells levels in the recipient’s spleen and 5 day transplanted allograft lung. We found that Trx-priming resulted in significant increase in the levels of recipient’s spleen CD4+Foxp3+ T cells at days 5, 21 and 37 post lung transplantation compared with control groups (Fig 3). Comparable increase in allograft lung CD4+Foxp3+ regulatory T cells as in spleen were observed at 5 days post transplantation (data not show). These results indicate that Foxp3+ regulatory T lymphocytes may play a critical role in Trx-mediated immune tolerance.
Fig 3. Trx-priming enhances recipients’ spleen CD4+Foxp3+ regulatory cells.
Panels A, B and C shows representative images of one of three separate flow cytometric analysis of spleen CD4 and Foxp3 intracellular staining on day 5, 21 and 37 post-transplant recipients, respectively. Panel D shows quantitatively analysis of host spleen Foxp3+ CD4+ T cells. Data represent mean ± SE, n = 3.*p<0.05 vs Trx (-).
Trx-priming reduces humoral immune response to donor alloantigen
To determine whether the improved survival of the Trx-primed allograft is also related to humoral tolerance, two weeks post-transplant, recipient rats with or without Trx-primed lungs were given tail vein injection of donor (Lewis rats) spleen cells (SPC) for alloantigen re-challenge once. Serum IgG antibody levels against donor antigens before, 1 and 2 weeks after injection were measured using flow cytometry. The quantitative analysis of serum antibody levels (Panel D) in Pre-SPC Trx (+) group was lower but not significantly different than Pre-SPC Trx (-) group. At 1 and 2 weeks of allogeneic SPC injection, serum IgG levels against donor antigen increased 2–3 fold in Trx (-) group compared to Pre-SPC, however, the serum antibody levels of Pre-SPC, 1 week, and 2 weeks SPC injection in Trx (+) groups remained comparable. The response to the donor SPC re-challenge between Trx (-) and Trx (+) of 1 and 2 weeks were significantly different. These results suggest the induction of humoral immune tolerance to donor antigens by Trx-priming of lung allograft.
Discussion
The most significant finding of our study is that one time treatment of donor lungs with Trx prior to transplantation reduced acute rejection and induced immune tolerance leading to extended post-transplant allograft survival in a rat model of allogeneic lung transplantation. Trx priming attenuated the recipient’s T cell response to donor alloantigen without compromising other immune functions. It has been reported that in rat allogeneic transplantation, induction of IL-10-producing CD4+ Tr1 cells is essential for protecting allograft[15–18]. The increased IL-10-producing CD4+ cells in the current study may be involved in the protection of lung graft. CD4+Foxp3+ regulatory T cells has been shown to play an important role in immune tolerance including allogeneic tolerance [19–23]. Our data showed that CD4+Foxp3+ regulatory T cells were significantly increased in the Trx(+) lung recipients, suggesting that this treatment induced the development of Foxp3+ regulatory T cells, which might contribute to the prolonged graft survival through delivering immunosuppressive signals. Humoral immune tolerance is likely to be induced as well because the anti-donor antibodies remain unchanged after donor antigen challenge in vivo (Fig 4). Antibodies against donor antigens play an important role in chronic allograft rejection [24]. The potential humoral immune tolerance induced could explain the prolonged allograft survival (Fig 2, Fig 4). In addition, we previously reported that the infiltration of CD8+ cytotoxic T cells was markedly reduced in the Trx-primed allogeneic lung grafts [5]. Collectively, Trx-priming of lung graft may modulate alloantigen-specific immune responses at different levels. As a result, allograft is protected from immune-mediated destruction.
Fig 4. Trx-priming inhibits the production of antibodies against donor type antigen.
Two weeks post lung transplant recipient rats were injected with SPC from naïve donor to re-challenge the recipients. The levels of antibodies against donor antigen were measured by immunofluorescent flow cytometry and expressed in MFI. The representative levels of one of three separate host antibodies against donor antigens pre-SPC injection, 1 week, and 2 weeks post-SPC injection are shown in panels A, B, and C, respectively. D: The quantitative levels of host antibodies against donor antigen in pre-SPC, SPC-1 week, and SPC-2 weeks groups (n = 3 for each group). # p< 0.05 vs Trx (-) pre-SPC injection group. *p<0.05, **p<0.01 vs Trx (-) 1 week and 2 weeks, respectively.
The precise molecular events associated with Trx priming of donor lung and modulation of the recipient’s immune system leading to extended allograft survival remains to be determined. It is possible that there is a time window, which might be critical for alloantigen-specific immune response to be modulated. Thus, it is speculative in our model that the presence of Trx during the initial contact between recipient immune system and allogeneic lung graft alters the alloantigen-specific immune responses toward protective instead of destructive responses. Increasing evidence supports that reactive oxygen species (ROS) play an essential role in facilitating signal transduction pathway during T cell activation [25,26]. Therefore, Trx-priming may affect alloantigen-specific T cell activation via reduction of ROS by Trx scavenging, or alter T cell differentiation. It has been documented that modulation of ROS production has impact on Th17 cell differentiation [27]. Emerging evidence shows that Th17 plays critical role in lung allograft rejection [28,29]. It is demonstrated that significantly higher immunosuppressive IL-10 is produced by the splenic CD4+ T cells from the recipients receiving Trx-primed lung grafts (Fig 2), suggesting the induction of IL-10-producing Tr1 cells. More importantly, those IL-10-producing Tr1 cells are alloantigen-specific, as the stimulation from third party does not lead to increased IL-10 production (Fig 2E). This finding suggests that Trx-priming of lung graft induces alloantigen-specific immune tolerance without affecting immune response globally. Additionally, we found that there were increased levels of Foxp3+ CD4+ T cells in the recipients receiving Trx-primed allogeneic lung grafts (Fig 3). Those Foxp3+ CD4+ regulatory T cells have not been fully characterized in terms of their antigen reactivity in this study, but they might have contributed considerably to the immune protection of allogeneic lung grafts. In addition, Trx-priming of lung allograft might also interfere with antigen-presenting cells, such as dendritic cells, thereby affecting subsequent immune responses in which the antigen-presenting cells participate. Indeed, our unpublished data demonstrate that Trx can significantly influence dendritic cells immunophenotypically and functionally.
It is worth noting that in our allogeneic lung transplantation model, the recipients did not receive any immunosuppressive agents, which are routinely employed in the clinical settings of allogeneic lung transplantation [2]. Although the mechanism underlying Trx-priming of allogeneic lung graft induced immune modulation remains to be determined, the translational impact of priming donor lungs with Trx prior to transplantation in a rat model is highly significant in context with its potential utility in clinical settings. Such a strategy offers an excellent non-invasive therapeutic approach to prolong graft survival in lung transplant recipients.
Acknowledgments
The authors thank Dr. Zhiwei Xu, Ms. Lin Ai, and Mr. Bert Herrera for surgery and technical assistance.
Data Availability
All relevant data are within the paper.
Funding Statement
This study was supported by the Department of Medicine's Gatorade funding project #00100966 and the Research Service of the VHS. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. McManigle W, Pavlisko EN, Martinu T. Acute cellular and antibody-mediated allograft rejection. Semin Respir Crit Care Med 2013; 34: 320–335. 10.1055/s-0033-1348471 [DOI] [PubMed] [Google Scholar]
- 2. Christie JD, Edwards LB, Kucheryavaya AY, Aurora P, Dobbels F, Aurora P, et al. The Registry of the International Society for Heart and Lung Transplantation: twenty-seventh official adult lung and heart-lung transplant report—2010. J Heart Lung Transplant 2010; 29: 1104–1118. 10.1016/j.healun.2010.08.004 [DOI] [PubMed] [Google Scholar]
- 3. Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. 1953. Nature 172: 603–606. [DOI] [PubMed] [Google Scholar]
- 4. Tolar J, Tolarova S, McGrath JA. On Medawar's 'Actively acquired tolerance of foreign cells'. Exp Dermatol 2014; 23: 97–98. 10.1111/exd.12227 [DOI] [PubMed] [Google Scholar]
- 5. Hu H, Lu L, Mu W, Johnson RJ, Block ER, Patel JM. Priming donor lungs with thioredoxin-1 attenuates acute allograft injury in a rat model of lung transplantation. J Heart Lung Transplant 2008; 27: 1142–1149. 10.1016/j.healun.2008.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hattori I, Takagi Y, Nakamura H, Nozaki K, Bai J, Kondo N, et al. Intravenous administration of thioredoxin decreases brain damage following transient focal cerebral ischemia in mice. Antioxid Redox Signal 2004; 6: 81–87. [DOI] [PubMed] [Google Scholar]
- 7. Shibuki H, Katai N, Yodoi J, Uchida K, Yoshimura N. Lipid peroxidation and peroxynitrite in retinal ischemia-reperfusion injury. Invest Ophthalmol Vis Sci 2000; 41: 3607–3614. [PubMed] [Google Scholar]
- 8. Shibuki H, Katai N, Kuroiwa S, Kurokawa T, Yodoi J,Yoshimura N. Protective effect of adult T-cell leukemia-derived factor on retinal ischemia-reperfusion injury in the rat. Invest Ophthalmol Vis Sci 1998; 39: 1470–1477. [PubMed] [Google Scholar]
- 9. Kasuno K, Nakamura H, Ono T, Muso E, Yodoi J. Protective roles of thioredoxin, a redox-regulating protein, in renal ischemia/reperfusion injury. Kidney Int 2003; 64: 1273–1282. [DOI] [PubMed] [Google Scholar]
- 10. Nakamura T, Nakamura H, Hoshino T, Ueda S, Wada H, et al. Redox regulation of lung inflammation by thioredoxin. Antioxid Redox Signal 2005; 7: 60–71. [DOI] [PubMed] [Google Scholar]
- 11. Glantzounis GK, Yang W, Koti RS, Mikhailidis DP, Seifalian AM, Yodoi J. The role of thiols in liver ischemia-reperfusion injury. Curr Pharm Des 2006; 12: 2891–2901. [DOI] [PubMed] [Google Scholar]
- 12. Yousem SA, Berry GJ, Cagle PT, Chamberlain D, Husain AN, Abrams J. et al. Revision of the 1990 working formulation for the classification of pulmonary allograft rejection: Lung Rejection Study Group. J Heart Lung Transplant 1996; 15: 1–15. [PubMed] [Google Scholar]
- 13. Xia CQ, Kao KJ. Induction of immune tolerance across major histocompatibility complex barrier by transfusion of ultraviolet B-irradiated immature dendritic cells. Transfusion 2005; 45: 181–188. [DOI] [PubMed] [Google Scholar]
- 14. Bhorade SM, Chen H, Molinero L, Liao C, Garrity ER, Vigneswaran WT, et al. Decreased percentage of CD4+FoxP3+ cells in bronchoalveolar lavage from lung transplant recipients correlates with development of bronchiolitis obliterans syndrome. Transplantation 2010; 90: 540–546. 10.1097/TP.0b013e3181e8dabe [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhou F, Ciric B, Zhang GX, Rostami A. Immune tolerance induced by intravenous transfer of immature dendritic cells via up-regulating numbers of suppressive IL-10(+) IFN-gamma(+)-producing CD4(+) T cells. Immunol Res 2013; 56: 1–8. 10.1007/s12026-012-8382-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jeon SG, Kayama H, Ueda Y, Takahashi T, Asahara T, Tsuji H, et al. Probiotic Bifidobacterium breve induces IL-10-producing Tr1 cells in the colon. PLoS Pathog 2012; 8: e1002714 10.1371/journal.ppat.1002714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Andolfi G, Fousteri G, Rossetti M, Magnani CF, Jofra T, Locafaro G, et al. Enforced IL-10 expression confers type 1 regulatory T cell (Tr1) phenotype and function to human CD4(+) T cells. Mol Ther 2012; 20: 1778–1790. 10.1038/mt.2012.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gregori S, Goudy KS, Roncarolo MG. The cellular and molecular mechanisms of immuno-suppression by human type 1 regulatory T cells. Front Immunol 2012; 3: 30 10.3389/fimmu.2012.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Regateiro FS, Chen Y, Kendal AR, Hilbrands R, Adams E, Cobbold SP, et al. Foxp3 expression is required for the induction of therapeutic tissue tolerance. J Immunol 2012; 189: 3947–3956. 10.4049/jimmunol.1200449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tang Q, Bluestone JA, Kang SM. CD4(+)Foxp3(+) regulatory T cell therapy in transplantation. J Mol Cell Biol 2012; 4: 11–21. 10.1093/jmcb/mjr047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bozulic LD, Wen Y, Xu H, Ildstad ST. Evidence that FoxP3+ regulatory T cells may play a role in promoting long-term acceptance of composite tissue allotransplants. Transplantation 2011; 91: 908–915. 10.1097/TP.0b013e31820fafb4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fan H, Wang J, Zhou X, Liu Z, Zheng SG. Induction of antigen-specific immune tolerance by TGF-beta-induced CD4+Foxp3+ regulatory T cells. Int J Clin Exp Med 2009; 2: 212–220. [PMC free article] [PubMed] [Google Scholar]
- 23. Yoon IH, Choi SE, Kim YH, Yang SH, Park JH, Park CS, et al. Pancreatic islets induce CD4(+) [corrected] CD25(-)Foxp3(+) [corrected] T-cell regulated tolerance to HY-mismatched skin grafts. Transplantation 2008; 86: 1352–1360. 10.1097/TP.0b013e31818aa43c [DOI] [PubMed] [Google Scholar]
- 24. Mauiyyedi S, Pelle PD, Saidman S, Collins AB, Pascual M, Tolkoff-Rubin NE, et al. Chronic humoral rejection: identification of antibody-mediated chronic renal allograft rejection by C4d deposits in peritubular capillaries. J Am Soc Nephrol 2001; 12: 574–582. [DOI] [PubMed] [Google Scholar]
- 25. Sena LA, Li S, Jairaman A, Prakriya M, Ezponda T, Hildeman DA, et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 2013; 38: 225–236. 10.1016/j.immuni.2012.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kaminski MM, Sauer SW, Kaminski M, Opp S, Ruppert T, Grigaravičius P, et al. T cell activation is driven by an ADP-dependent glucokinase linking enhanced glycolysis with mitochondrial reactive oxygen species generation. Cell Rep 2012; 2: 1300–1315. 10.1016/j.celrep.2012.10.009 [DOI] [PubMed] [Google Scholar]
- 27. Zhi L, Ustyugova IV, Chen X, Zhang Q, Wu MX. Enhanced Th17 differentiation and aggravated arthritis in IEX-1-deficient mice by mitochondrial reactive oxygen species-mediated signaling. J Immunol 2012; 189: 1639–1647. 10.4049/jimmunol.1200528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lemaitre PH, Vokaer B, Charbonnier LM, Iwakura Y, Estenne M, Goldman M, et al. IL-17A mediates early post-transplant lesions after heterotopic trachea allotransplantation in Mice. PLoS One 2013; 8: e70236 10.1371/journal.pone.0070236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hirayama S, Sato M, Loisel-Meyer S, Matsuda Y, Oishi H, et al. Lentivirus IL-10 gene therapy down-regulates IL-17 and attenuates mouse orthotopic lung allograft rejection. Am J Transplant 2013; 13: 1586–1593. 10.1111/ajt.12230 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.