Background: Maize shows a rapid leaf growth reduction upon salinity, being a thread to global food security.
Results: Exogenous application of Zea mays β-expansin 6 partially restores growth.
Conclusion: ZmEXPB6 improves growth by acting on the capacities of cell walls to extend.
Significance: A strategy to improve growth under saline conditions was shown. This knowledge can be useful for developing crops with a better performance under salinity.
Keywords: Cell Growth, Plant, Plant Biochemistry, Plant Cell Wall, Stress, Zea mays L, Growth Inhibition, Linear Variable Differential Transducer, Real-time Fluorescence Ratio Imaging, Salinity
Abstract
The salt-sensitive crop Zea mays L. shows a rapid leaf growth reduction upon NaCl stress. There is increasing evidence that salinity impairs the ability of the cell walls to expand, ultimately inhibiting growth. Wall-loosening is a prerequisite for cell wall expansion, a process that is under the control of cell wall-located expansin proteins. In this study the abundance of those proteins was analyzed against salt stress using gel-based two-dimensional proteomics and two-dimensional Western blotting. Results show that ZmEXPB6 (Z. mays β-expansin 6) protein is lacking in growth-inhibited leaves of salt-stressed maize. Of note, the exogenous application of heterologously expressed and metal-chelate-affinity chromatography-purified ZmEXPB6 on growth-reduced leaves that lack native ZmEXPB6 under NaCl stress partially restored leaf growth. In vitro assays on frozen-thawed leaf sections revealed that recombinant ZmEXPB6 acts on the capacity of the walls to extend. Our results identify expansins as a factor that partially restores leaf growth of maize in saline environments.
Introduction
Salinity impacts on global agricultural production because, as fertile soils become salinized, vegetative growth and yield of most conventional crops decreases (1–3). Salt stress reduces the rate of leaf growth by shortening the length of the leaf-elongating zone and decreasing the growth intensity in its central and distal portions (4). The physiological mechanisms and perturbations that cause the leaf growth reduction under salt stress, however, are as yet not precisely understood (5, 6).
According to the “acid growth” theory, leaf growth is induced by an auxin-mediated acidification of the leaf apoplast that creates a basal chemical requirement for loosening the load-bearing cell walls (7, 8). The apoplastic pH around growing cells spans ∼4.0 to 5.5 (9, 10), which is the range in which acidification activates expansin activity (11). Expansins are cell wall-located proteins that have been implicated as important factors for mediating acid-induced leaf growth. They facilitate wall loosening in a pH-dependent manner, enabling a turgor-driven wall expansion (12, 13).
Based on a thorough phylogenetic sequence analysis, the expansin superfamily was subdivided into four subfamilies, namely α-expansin, β-expansin, expansin-like A, and expansin-like B (14). A characterization of the structural features of the expansin superfamily revealed a degree of amino acid sequence conservation of ∼20–40% between the sub-families, which is highest in the first of the two domains (14). This first expansin domain is homologous to the catalytic domain of a glycoside hydrolase. The second domain is homologous to a group-2 grass pollen allergen from Phleum pratense, whereas no functions could be ascribed to this second domain, and a biological function of group-2 grass pollen allergen was reported to be unknown (14). Among the expansin superfamily, the members of the α- and β-family have the ability to bind to the cell wall where they induce slippage of cellulose microfibrils, which is a prerequisite for turgor-driven expansion growth. However, the detailed molecular mode of action is not precisely known. Despite the sequence homologies between expansin domain 1 and the catalytic domain of a glycoside hydrolase member, any enzymatic activities that facilitate wall loosening have not yet been assayed (14, 15). Therefore, it is assumed that expansins act non-enzymatically via disrupting non-covalent bonds that tie matrix polysaccharide to cellulose microfibrils (16). With respect to the crop Zea mays that was analyzed in this study, it was found that β-expansins are with eight different maize expansin cDNAs more numerous and more frequently expressed compared with α-expansins with only five different cDNAs. This led to the assumption that β-expansins may be of special importance in the cell wall of maize (17). The different β-expansin isogenes are expressed in more than one organ at different time points, making it difficult to specifically link a certain developmental process during which wall loosening is required to the one or the other β-expansins protein isoform (17). Expansin gene expression is in general associated with expanding organs and meristematic root and leaf tissues but is also implicated in processes such as fruit softening, abscission, or submergence stress (14).
Notably, evidence is increasing for an orchestrated role of expansins in plant stress responses. For example, they have been identified as being involved in the salinity stress response in wheat (18), maize (19), Arabidopsis (20), roses (21), and sorghum (22) or under conditions of water deficit (23–27) or water excess and anoxia (28–31). Recently, genotypic differences in β-expansin abundance have been demonstrated to occur between salt-stressed leaves of two maize cultivars that differ in their ability to grow under saline conditions (19, 32). In leaves of the maize cultivar named Lector that was used in the present study, reduced β-expansin protein abundance has been found to correlate positively with reduced growth. In contrast, a second maize cultivar that almost maintained full shoot growth under salinity surprisingly maintained full β-expansin protein abundance (19). The presented study was designed to elucidate which of the different growth promoting β-expansin isoform had decreased in the growth-inhibited leaves of the maize cultivar Lector upon the 8-day treatment with 100 mm NaCl. In addition, we aimed to test whether this down-regulated isoform can, if present again, restore leaf growth of maize under conditions of salt stress. For this, we used a bacterial expression system to produce this wall loosening protein and assayed its growth-promoting function for the first time in planta after metal chelate affinity chromatography purification.
MATERIALS AND METHODS
Plant Cultivation
Maize (cultivar Lector, Limagrain, Edemissen, Germany) was grown in hydroponic culture in a greenhouse. Seeds were embedded in 1 mm CaSO4 in an aerated solution for 1 day and placed between filter papers moistened with 1 mm CaSO4 for a period of 3 days until germination. Subsequently, the seedlings were transferred to 4.5-liter plastic pots containing ¼-strength nutrient solution. After 2 days of cultivation, the concentration of nutrients was increased to half-strength and, after 4 days of cultivation, to full strength. The nutrient solution had the following composition: 2.5 mm Ca(NO3)2, 1.0 mm K2SO4, 0.2 mm KH2PO4, 0.6 mm MgSO4, 5.0 mm CaCl2, 1 mm NaCl, 1.0 μm H3BO4, 2.0 μm MnSO4, 0.5 μm ZnSO4, 0.3 μm CuSO4, 0.005 μm (NH4)6Mo7O24, 200 μm Fe-EDTA. The solution was changed every second day to avoid nutrient depletion. NaCl treatment was started 2 days after the full-nutrient concentration had been reached by adding 25 mm NaCl and was increased stepwise by 25 mm increments every 2 days to a final concentration of 100 mm (controls without NaCl). The temperature was kept constant at 26 °C during the light period (14 h) and at 18 °C during the dark period (10 h); relative humidity was set to ∼70%. Measurements were performed with leaves that had started to develop for a period of 8 days at 1 day after the full 100 mm NaCl treatment was applied. Leaves used for protein analysis were ground in liquid nitrogen and stored at −80 °C. Leaf sections for LVDT measurements were frozen at −20 °C. Real-time pH measurements were performed non-invasively in planta.
Leaf Thickness Measurements
Leaf thickness was measured on cross-sections. For tissue embedding, leaves were cut into small segments and immediately fixed as described previously (33). Segments were dehydrated in a graded ethanol series and embedded in Technovit 7100 (Heraeus-Kulzer, Hanau, Germany). Subsequently, cross-sections were cut with a microtome (10 μm). Leaf thickness was quantified with an inverted microscope (DMI6000B; Leica Microsystems, Wetzlar, Germany) using the calibrated scale bar provided by LAS AF software (Leica Microsystems).
Two-dimensional Western Blotting (WB) and Labeling of β-Expansin Isoforms
Antibody
For labeling β-expansin proteins in maize, the purified IgG fraction of the polyclonal β-expansin antibody “anti-ZmExpB,” raised in rabbit against the 15-amino acid peptide 152LVARNVIPANWRPNT166, which is conserved in all vegetatively expressed maize, β-expansin isoforms were used (34).
Protein Extraction
Proteins for isoelectric focusing (IEF)2 were extracted from 100 mg of ground leaf material by using a dithiothreitol (DTT)-trichloroacetic acid-acetone precipitation method adopted from Zörb et al. (35). Precipitated proteins were resuspended in 1 ml of protein sample buffer (8 m urea, 2 m thiourea, 0.5% v/v Pharmalyte buffer, pH 3–10 (GE Healthcare), 4% (w/v) CHAPS, 30 mm DTT, 20 mm TRIS, pH 8.8, 5 mm Pefablock (Sigma)) and were solubilized by incubation for 2 h at 33 °C followed by a 10-min incubation in an ultrasonic bath. Samples were centrifuged (18,000 × g; 30 min, 4 °C), and the supernatant was subjected to IEF.
First-dimension IEF
IEF was carried out on a Protean IEF Cell system (Bio-Rad). Proteins (100 μg; two-dimensional QUANT protein determination kit, GE Healthcare) were diluted in a total volume of 200 μl of immobilized pH gradient (IPG) sample buffer (Immobiline pH gradient; GE Healthcare) and loaded on pre-cast (IPG) strips (110 × 3.0 × 0.5 mm, pH 3–11, linear, GE Healthcare). Gels were rehydrated at 20 °C for 12 h at 50 V. IEF was performed for 37,400 volt hours with the initial voltage set to 250 V for 30 min, then a gradient of up to 500 V for 30 min followed by a gradient of up to 3000 V for 30 min and finally separation at 3000 V for 4 h. For the second dimension, proteins were equilibrated in DTT and iodoacetamide as described previously (34).
Two-dimensional PAGE
For achieving high reproducibility, an improved two-dimensional WB procedure according to Geilfus et al. (34) was used. Briefly, all biological replicates of each treatment were simultaneously separated side by side on the identical sodium dodecyl sulfate (SDS) gel. Separation of the proteins according their molecular weight was carried out with a Multi-Gel Electrophoresis unit that enabled all gels to be run in parallel within the identical electrophoretic cycle (Protean 2 Multi-Cell, Bio-Rad). The equilibrated proteins were separated at 30 mA/gel for 6.5 h (120 watts) at 15 °C. The apparent molecular mass of proteins was estimated by using prestained molecular weight standards from 8 to 210 (ColorBurst Electrophoresis Marker; Sigma).
Two-dimensional Blotting Procedures
Gel fragments of ≥40 kDa were not used for the blotting procedure because β-expansin proteins are known to range in size from 20 to 30 kDa. Instead, these gel fragments were used as a loading control because they display a representative proportion of the leaf proteome that was separated under identical conditions on the same gel. By this means, dozens of different protein spots indicated identical protein loading. The smaller gel fragment (∼<40 kDa) was used for blotting and β-expansin detection (Fig. 1 shows a completely Coomassie-stained gel that shows the protein spots in the range smaller than 40 kDa). Proteins were electroblotted onto a PVDF membrane (BioTrace, PALL Life Sciences, Pensacola, FL) by using a semidry transfer system (SemiPhor, Hoefer Pharmacia Biotech, San Francisco, CA) as described previously (34). Briefly, SDS-gel fragments and the PVDF membrane were equilibrated for 15 min in Towbin buffer (25 mm TRIS base, 192 mm glycine, 20% v/v methanol, 0.01% w/v SDS, pH 8.3) and subsequently placed between soaked sheets of filter paper (Whatman Gel Blot paper; 1.2 mm, Sigma). Transfer was conducted for 75 min at 40 V, 0.8 mA/cm2. Efficiency was checked with the prestained protein marker (Sigma).
FIGURE 1.
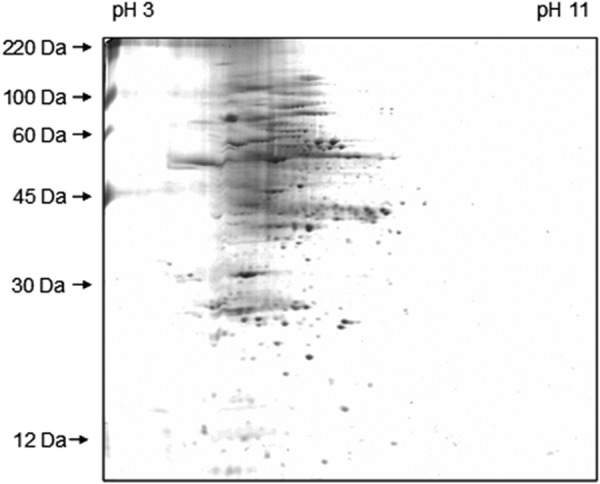
Two-dimensional SDS-PAGE gel of Z. mays L leaf. Separation was achieved by isoelectric focusing (pH 3–11) in the horizontal dimension and by SDS-PAGE in the vertical dimension. The spots were visualized by staining with Coomassie Blue R250.
Immunodetection
For immunostaining, the membrane was saturated in a solution of milk powder in TRIS-buffered saline (TBS) with Tween (TBS-T; 2.5% (w/v) bovine skimmed milk, 0.1% (v/v) Tween) for 2 h at room temperature and then incubated with the β-expansin antibody anti-ZmExpB. The blot was incubated with the purified IgG fraction of the antisera diluted 1/250 in the same TBS-T (0.1 ml/cm2 PVDF membrane) for 2 h and washed 3 times for 5 min with TBS-T. Subsequently, the membrane was incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG as the secondary antibody (Sigma), diluted 1/38,000 in TBS-T, for 1 h at room temperature. Finally, the membrane was washed 3 times for 5 min in TBS-T and once in TBS. Signals were detected by using chemiluminescent peroxidase subtrate-3 (Sigma) on Kodak BioMax light film (Sigma) with an exposure time of 4 min. Because all replicates were analyzed in the same blotting cycle, exposure time was identical and highly comparable (linear range). Negative control with preimmune serum taken before antigen injection did not detect signals (data not shown).
Mass Spectrometry
Peptide identification was carried out as described (36). Briefly, proteins attached to the PVDF blotting membrane that were identified by Western blotting as β-expansin of interest were picked and digested by trypsin, and mass spectra were obtained offline. It was assured that the correct protein was picked for identification by locating its position on the PVDF membrane using the developed x-ray film with the expansin-specific spot signal as a template. For this, the exact position of the x-ray film that covered the PVDF membrane during exposure needed to be marked while the x-ray film was exposed to the β-expansin-derived light signal. This was achieved by making coinages through the ensemble composed of PVDF membrane and x-ray film using a coining punch. These imprintings allowed locating the exact position of the β-expansin of interest on the PVDF membrane once the x-ray film was developed.
Nanospray emitters (Proxeon, Odense, DK) were positioned at a nano-electrospray ionization source, and peptides were analyzed in a Micromass Q-Tof Premier (Waters, Manchester, UK). Amino acid sequences were determined from fragment spectra by utilizing MaxEnt3 within MassLynx 4.1 software by manual interpretation of the b- and y-ion series. Amino acid sequences were used for a similarity search in the NCBI databases (www.ncbi.nlm.nih.gov) for Z. mays.
Expression of Recombinant Z. mays β-Expansin 6 in Escherichia coli
cDNA of Z. mays was amplified with TaqDNA polymerase (Genaxxon Bioscience GmbH, Ulm, Germany) and gene-specific primers that introduced restriction sites for NdeI and XhoI (forward, CATATGGCCACCGCGCTCTCCTTCAAGGCC; reverse, CTCGAGCCAGTACTGGACGAAGGAGC). The resulting 828-bp polymerase chain reaction (PCR) fragment was purified by agarose gel electrophoresis and extracted by using peqGOLD Gel Extraction kit (peqLAB Biotechnologie GmbH, Erlangen, Germany). The fragment was ligated into the expression vector pET23a (Novagen by Merck KGaA) in which the multi cloning site was previously replaced by the XbaI-XhoI-fragment of the vector pET22b (37). The vector coded for a C-terminal hexahistidine (His6) sequence for the subsequent protein purification using nickel-nitrilotriacetic acid metal chelate affinity chromatography. The resulting vector construct was transformed into E. coli TOP 10 (Invitrogen). Positive clones were screened via PCR for the correct insert and then purified for sequencing. The plasmid was transformed into E. coli BL21 (DE3) (Agilent Technologies GmbH, Waldbronn, Germany). The resulting ampicillin-resistant transformants were cultured at 37 °C in LB medium (50 μg/ml ampicillin) overnight and transferred to fresh medium for an additional 1 h. Protein expression was induced with 1 mm isopropyl-β-d-thiogalactoside. Cells were grown for an additional 4 h at 25 °C, and a second dose of ampicillin (12.5 μg/ml) and isopropyl-β-d-thiogalactoside (250 μm) was added 2 h after induction. Cells were harvested by centrifugation and resuspended in lysis buffer for protein purification (50 mm NaH2PO4, 300 mm NaCl, 10 mm imidazole, pH 8.0, with NaOH). Subsequently, cell suspension was lysed by sonification for 5 min in ice water (intervals of 30 s). From the supernatant, His-tagged proteins were purified with nickel nitrilotriacetic acid-agarose affinity chromatography according to the manufacturer's instructions for protein purification (Qiagen, Hilden, Germany). To guarantee low salt content, the elution buffer was subsequently exchanged by using centrifugal filter devices (Amicon Ultra-4 centrifugal filter unit, 10,000 molecular weight cutoff, Merck KGaA) against 25 mm Tris-HCl, pH 5.2, resulting in a final concentration of 0.3 μg·μl−1 ZmEXPB6 (Z. mays β-expansin 6). Protein concentration in the supernatant remained constant after successive centrifugation steps (15,000 × g; 3 min), indicating that proteins did not precipitate. One-dimensional SDS-PAGE revealed the presence of only one protein band in the whole eluate (Fig. 2). To obtain 45 ml of heterologous ZmEXPB6 with a concentration of 0.3 μg/ml, 10 liters of culture were successfully purified.
FIGURE 2.
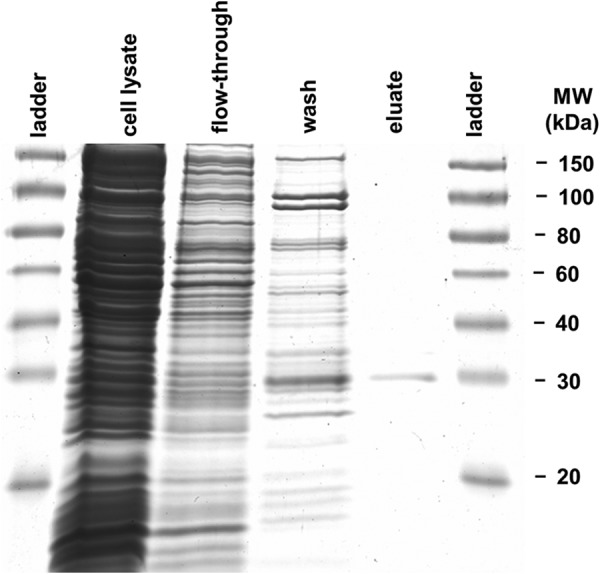
His tag-labeled metal-chelate-affinity chromatography-purified ZmEXPB6 expressed in E. coli. Cell lysate, cells induced with IPTG; wash, third washing step shown; eluate, second elution step shown. Expansin identity in eluate was confirmed by WB (data not shown).
Assaying Growth-mediating Properties of Exogenously Applied ZmEXPB6
To test whether the heterologously expressed ZmEXPB6 improves growth when exogenously applied onto the leaves of salt-stressed plants that lack native ZmEXPB6, plants were divided into the following groups: group A, control-treated plants cultivated without NaCl; group B, salt-treated plants grown for 8 days with 100 mm NaCl; group C, salt-treated plants grown for 8 days with 100 mm NaCl plus exogenous application of heterologous ZmEXPB6; group D, salt-treated plants grown for 8 days with 100 mm NaCl plus exogenous application of heat-inactivated heterologous ZmEXPB6. For inactivation of ZmEXPB6 by heat, proteins were boiled for 5 min. The exogenous application was carried out by brushing the ZmEXPB6 solution (0.3 μg of ZmEXPB6 μl−1) onto the ad- and abaxial leaf sides. To avoid the protein solution dropping off the leaf, 1‰ of the wetting medium Silwet (BASF SE, Ludwigshafen, Germany) was added to the protein solution. In group D, Silwet was added after boiling. To exclude any effects of the solution that harbors the proteins or the surfactant on growth, plants of the groups A and B were also wetted with the same quantity of solution including 1‰ Silwet (without active or inactive ZmEXPB6).
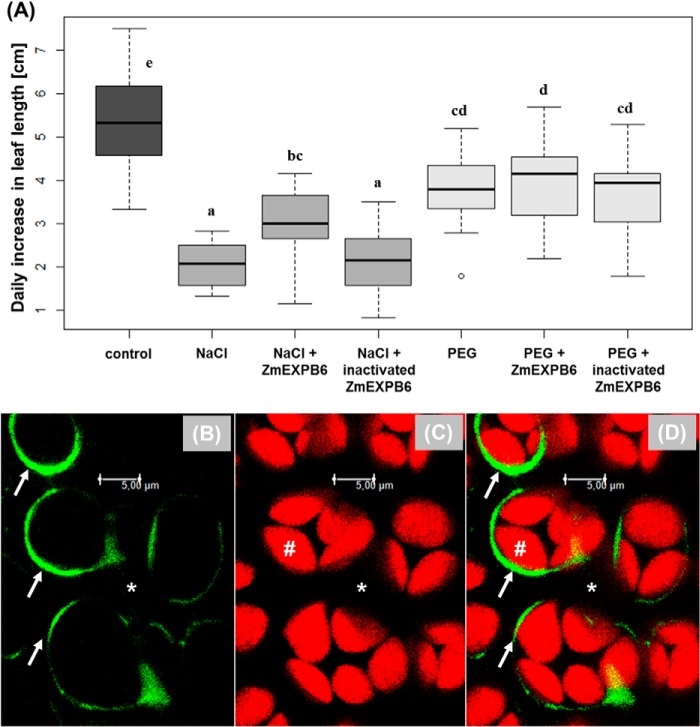
Leaf application treatment was carried out between 8 a.m. and 9 a.m. and was repeated on 4 consecutive days after plants already grew 8 days under the full 100 mm NaCl treatment. Daily increases in leaf length were detected on the following days at 7 a.m. and were compared between the experimental groups A-D. To separate the ionic effects from the osmotic effects of NaCl stress, an additional condition was introduced using 22.5 mm concentrations of the nonionic solute polyethylene glycol (PEG; molecular weight of 6000) instead of NaCl, representing an equivalent effect on the osmotic potential to treatment with 100 NaCl (38). The same control treatments were carried out as above. It is necessary for this experiment that the exogenously applied expansins have access to the cell walls, the site where expansins are active. This prerequisite was exemplary, controlled by monitoring the apoplastic distribution of the fluorescently labeled protein Alexa Fluor 488 goat anti-chicken IgG antibody (Sigma) upon the addition onto the leaf surface. The apoplastic position of the protein was monitored by confocal laser scanning microscopy confocal laser scanning microscopy (CLSM).
CLSM
CLSM imaging was carried out via a Leica TCS SP5 confocal laser scanning system (Leica Microsystems). A plan apochromatic objective (HC PLAN APO 20.0 * 0.70; Leica Microsystems) was used for image collection. The 488-nm beam line of an argon laser was chosen for Alexa Fluor 488 excitation. Emission bandwidth was 498–540 nm. Chloroplast autofluorescence was excited at 633 nm by a helium-neon laser (emission bandwidth: 650–704 nm).
Ratiometric in Planta Measurements of Leaf Apoplastic pH
Non-invasive apoplastic pH measurements were carried out on intact maize leaves using camera-based ratio analysis in combination with the pH-sensitive fluorescence dye Oregon Green 488 (Invitrogen) as described previously in detail (39). The ratiometric dye Oregon Green 488 was conjugated to 10-kDa-sized dextran that guaranteed, due to its size, the exclusive apoplastic localization (see Refs. 9 and 10). In planta pH quantitation was started at 2 h after 25 mm dye was loaded. The fluorescence ratio F490/F440 was calculated on a pixel-by-pixel basis and was converted into pH using an in situ calibration (Fig. 3) as described previously (40).
FIGURE 3.
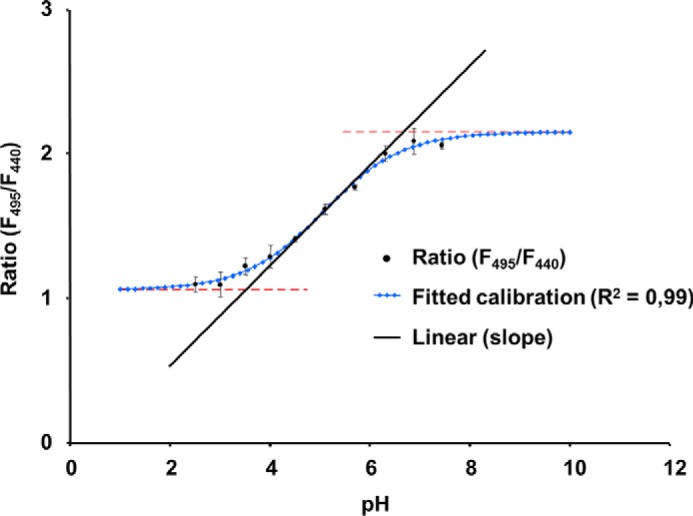
In situ apoplastic pH calibration curve for Oregon Green 488 dextran fluorescence excitation ratio R (490 excitation/440 excitation; 525 emission) in maize leaves. The Boltzmann fit was chosen for fitting sigmoidal curves to in situ calibration ratio data. Fitting resulted in an optimal dynamic range for pH measurements between 3.2 and 6.6 (corresponds to the ratios 0.95 and 2.15). In situ calibration was conducted on 12 different plants.
Linear Variable Differential Transducer Measurements
The potential of recombinant ZmEXPB6 to increase the capacity of cell walls to extend was assayed in vitro by using a linear variable differential transducer (LVDT) as used by Cramer and Bowman (41) following the detailed procedure described by Hu et al. (42). The 20-mm circumference of the LVDT wheel enabled a resolution of 16.7 ohms per mm of leaf extension, whereby 1/100 ohm was displayed by the LVDT. Frozen-thawed basal sections of leaves were connected to the LVDT by a thread that was attached to the leaf tip via a small clamp. The thread was gently tensioned to eliminate oscillations in LVDT output resulting from slipping and friction in the measurement system. Pilot experiments indicated that this force did not affect leaf extension. Extension was induced by continuously increasing the counterweight by adding water into a container that was attached with a clamp and hook to the free end of the thread (flow rate of 500 μl/s). Readings were taken from the transducer at second-by-second intervals. Time course data were stored by a data logger (Delta-T Device, Cambridge, UK). Treatment was started before 10 μl of the ZmEXPBB6-containing solution (25 mm Tris-HCl, 1‰ Silwet; 0.3 μg·μl−1 ZmEXPBB6) were brushed onto the ad- and abaxial surfaces of the leaf segment. Measurements were run until the leaf segments tore (point of breakage of leaf sections). The surfactant Silwet was used to break the surface tension. In addition, the surface of the leaf was gently abraded with the aim to facilitate access of the proteins to the cell wall as described in the protocol given elsewhere (43). As a control measure, a comparable leaf segment was treated with 10 μl of the identical solution that harbored heat-inactivated ZmEXPBB6 (proteins boiled for 5 min; 1‰ Silwet addition after boiling). Comparability between leaf segments was achieved by using the mid-vein as reflecting axes of symmetry; a segment left of the mid rib and the congruent segment right of the mid rib were cut out at the frozen-thawed basal part of the leaf (5-cm length × 1.5-cm width).
Data Evaluation
For statistical evaluation of the biomass data and the pH data, the t test as outlined by Köhler et al. (44) was calculated (with respect to pH data, the t test was calculated based on de-logarithmic pH values). Growth data upon exogenous ZmEXPB6 application were tested using all-pair comparison according to Tukey (45) because a graphical residual analysis (not shown) revealed normal distribution and homoscedasticity.
RESULTS
Salinity-induced Leaf Growth Reduction
The 8-day treatment with 100 mm NaCl resulted in a significant reduction of leaf growth and biomass formation in the maize hybrid Lector. Daily increase in leaf length (Fig. 4A), leaf thickness (Fig. 4B), leaf area (Fig. 4C), and leaf fresh weights (Fig. 4D) were significantly reduced upon salt stress.
FIGURE 4.
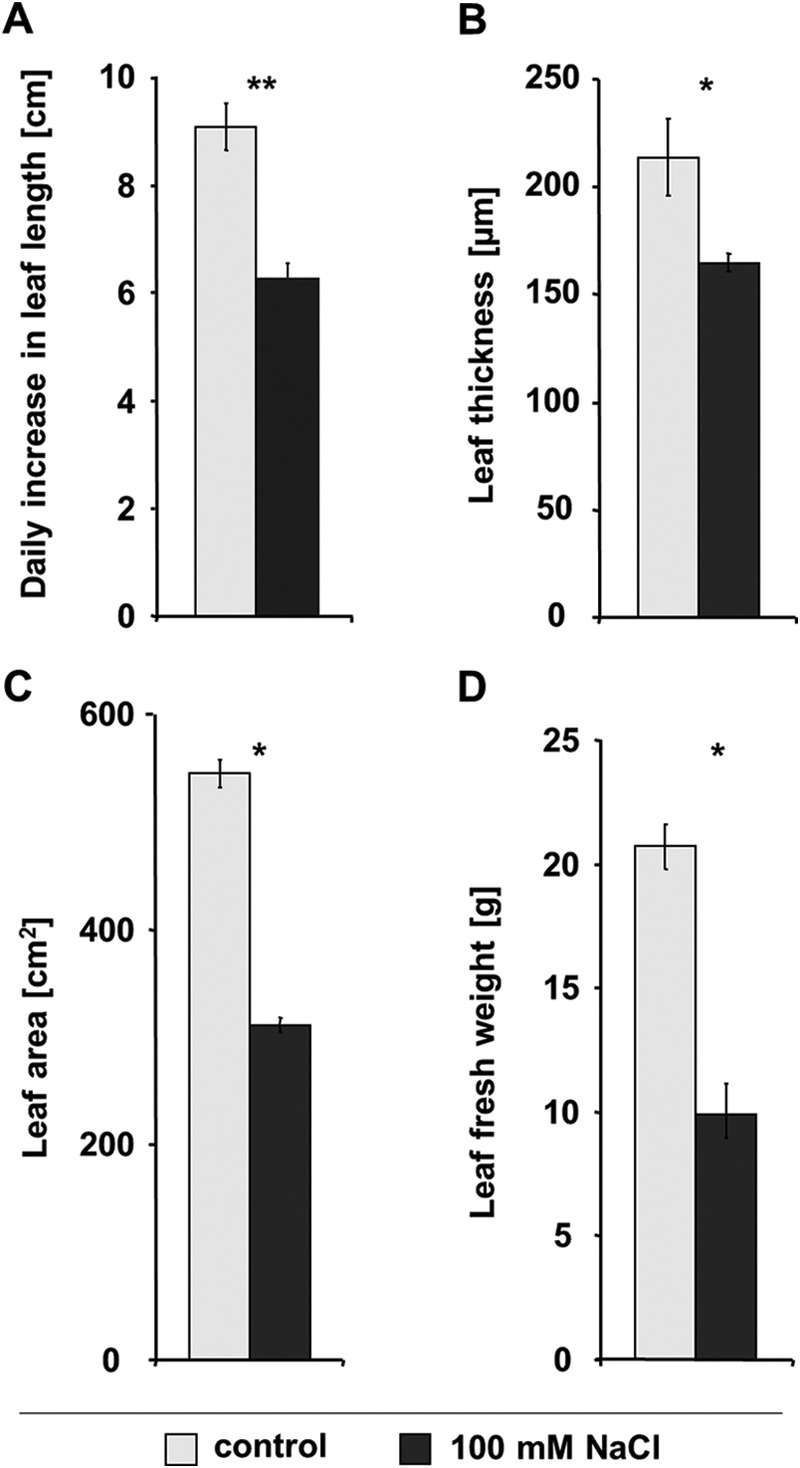
Salinity-induced leaf growth inhibition. Leaf growth and biomass formation with 8-day 100 mm NaCl treatment. Shown are the daily increase in leaf length as averaged over the 8-day salt treatment (A), leaf thickness (B), leaf area (C), and leaf fresh weight (D), each at the last day of the 8-day 100-mm NaCl treatment (A). Light gray, control; dark gray, 100 mm NaCl. All measurements are the means of four biological replicates ± S.E., each replicated in triplicate. Asterisks indicate mean differences betweens salt treatment and control (*, p ≤ 0.05; **, p ≤ 0.01; ns = not significant; t test).
Effect of Salt Treatment on β-Expansin Isoform Pattern in Growth-reduced Leaves
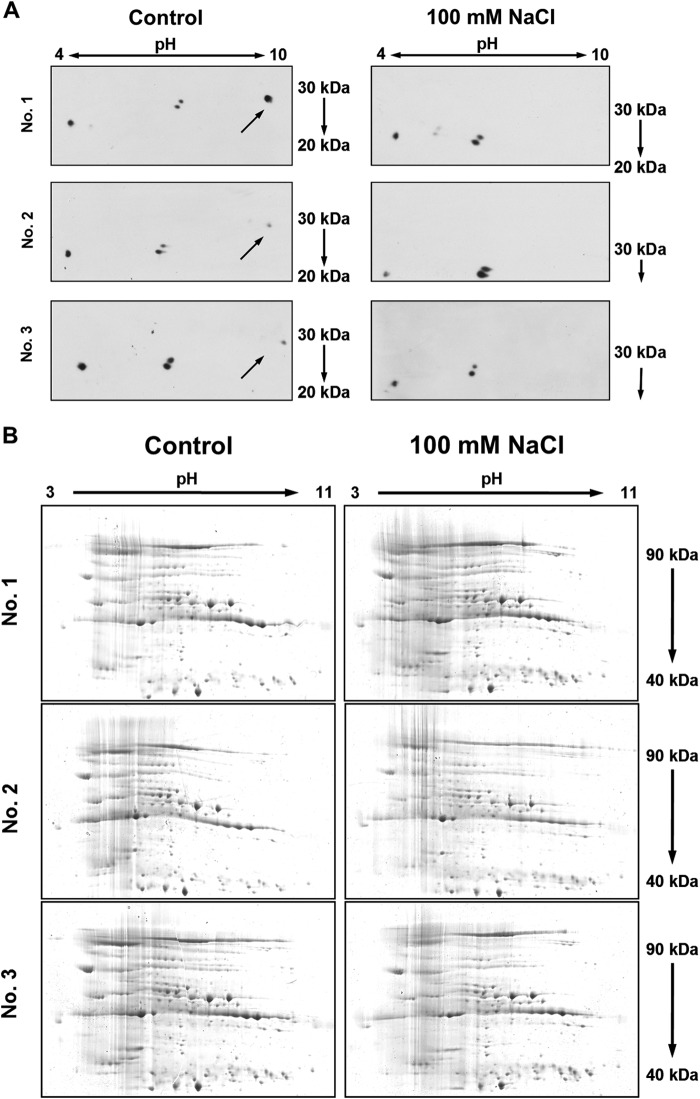
Proteins were isolated from growth-reduced (8-day treatment with 100 mm NaCl) and control treated (no salt) leaves, and β-expansins were labeled by two-dimensional polyacrylamide gel electrophoresis (PAGE)-WB detection. For β-expansin detection in maize leaves, we used the polyclonal β-expansin antibody anti-ZmExpB (34) that was raised against a 15-amino acid peptide that is conserved in all vegetatively expressed maize β-expansin isoforms (see Materials and Methods for more details). Under control conditions, two-dimensional WB revealed the presence of four signal spots with a molecular weight (Mr) ranging from >20 to ∼30 (Fig. 5A). This corresponds to the range that was reported by Wu et al. (46) for vegetatively expressed maize β-expansins and by Li et al. (47) for Zea m 1, the major group 1 allergen of maize pollen being member of the β-expansin family. The same size range was reported by Downes et al. (48) for a cytokinin-inducible β-expansin isoform from soybean (Glycine max cv. Mandarin) named Cim1 that, after post-translational processing, exists in protein forms ranging from 20 to 35 kDa. One particular β-expansin spot signal that appeared under control conditions was not detectable under salt stress conditions (labeled by the arrows in Fig. 5A). For this spot, mass spectrometric analysis supported identity to ZmEXPB6, whereas no sequence homology to other maize β-expansin members was found (Fig. 6).
FIGURE 5.
NaCl decreases abundance of one specific β-expansin protein isoform in size-reduced leaves. For labeling the β-expansin isoforms, two-dimensional WB was performed on whole leaf protein extracts. Gel fragments that contained proteins of <40 kDa were electroblotted onto a PVDF membrane that was subsequently used for immunodetection. Gel fragments that contained proteins of ≥40 kDa were stained to guarantee equal loading of the gels. A, two-dimensional WB showing β-expansin isoforms in control-treated (no NaCl) and salt-stressed (100 mm NaCl) leaves. Arrows indicate the isoform that could not be detected under saline conditions. A negative control with preimmune serum detected no signals (data not shown). B, Coomassie-stained SDS-gels served as loading controls confirming equal loading of proteins in all gels. Numbers 1–3 indicate biological replicates.
FIGURE 6.
Clustal O (1.2.1) multiple amino acid sequence alignment and fragmentation spectrum of ZmEXPB6 peptide. A, alignment between Z. mays (Zm) β-expansin (EXPB) 2 (AF332175), ZmEXPB3 (AF332176), ZmEXPB4 (AF332177), ZmEXPB5 (AF332178), ZmEXPB6 (AF332179), ZmEXPB7 (AF332180), and ZmEXPB8 (AF332181). ZmEXPB6 was identified by the underlined peptide sequence TGAGPLDNG by mass spectrometry analysis of proteins extracted from a PVDF blotting membrane. B, 19 fragmentation spectra of peptide 730.29 (2+) were recorded in positive ion modus with an ion score of 5.12E3, and spectra were combined for peptide sequence analysis.
Leaf Growth-ameliorating Properties of Exogenously Applied Recombinant ZmEXPB6
To investigate the growth-ameliorating properties of ZmEXPB6 proteins when exogenously applied on growth-reduced maize leaves, recombinant ZmEXPB6 protein was produced in E. coli BL21(DE3), His tag-purified by metal chelate affinity chromatography (Fig. 2), and subsequently added onto the leaf surface of NaCl- or PEG-stressed plants. As a result, the application of recombinant ZmEXPB6 protein partially restored daily leaf expansion growth of NaCl-stressed plants but had no growth improving effect when plants were stressed with the nonionic solute PEG6000 (Fig. 7A). To exclude any unspecific proteinogenic effects on growth rates, a further group of salt-stressed plants was treated with heat-inactivated ZmEXPB6; however, growth was not improved (Fig. 7A). Once could now argue that the heat treatment may partially precipitate proteins, whereby physicochemical properties change, and heat-inactivated precipitated proteins possibly only adhere at the leaf surface. For this reason we introduced a “soluble protein control” using bovine serum albumin (BSA). However, the exogenous application of BSA protein did not restore growth (data not shown), excluding unspecific proteinogenic effects and ultimately pointing the activity of heterologously expressed ZmEXPB6. For the feasibility of the presented experiment, it is obligatory that the exogenously applied ZmEXPB6 has access to the cell walls, the site where expansins are active. It must be ensured that the proteins stick not only to the outer side of the leaves. This prerequisite was exemplarily controlled by monitoring the apoplastic distribution of a fluorescently labeled protein that was exogenously applied onto the leaf surface because the fluorescent label allows localization of its position by CLSM. For this, an Alexa Fluor 488-goat anti-chicken IgG antibody was exemplarily chosen. CLSM imaging (Fig. 7, B–D) demonstrated that this protein, when exogenously applied onto the leaf surface, had access to the cell walls even though it was with 144 kDa larger than expansins.
FIGURE 7.
Growth-mediating effects of exogenously applied ZmEXPB6. A, daily length growth of salt-stressed (100 mm NaCl) and osmotic-stressed (22.5 mm PEG 6000) leaves in response to exogenous application of recombinant ZmEXPB6. Stressed plants were divided into the following groups: NaCl- or PEG-treated plants plus exogenous application of heterologous ZmEXPB6; NaCl- or PEG-treated plants plus exogenous application of heat-inactivated heterologous ZmEXPB6; NaCl- or PEG-treated plants without exogenous application of heterologous ZmEXPB6. Control-treated plants were cultivated without NaCl or PEG. The exogenously application was carried out by brushing the ZmEXPB6-containing solution onto the adaxial- and abaxial leaf sides. To keep conditions identical, plants that were not treated with exogenously added expansin were wetted with the identical quantity of brushing solution. Leaf application treatment was repeated on four consecutive days and averaged. Statistical significance (p ≤ 0.01) is indicated by letters (all-pair comparison according to Tukey (45)). Confocal images in b–d show the adaxial view on the leaf apoplast. B, exogenously applied Alexa Fluor 488 goat anti-chicken IgG antibody at 3 h after leaf surface application appears as a green ring labeled by white arrows. C, identical details show autofluorescence of the palisade cell chloroplasts in red, #. D, overlay of B and C. The overlay demonstrates that the fluorescently labeled and exogenously applied protein can enter the apoplast and appears as a ring surrounding the palisade cell walls. *, apoplastic space.
Ratiometric in Planta Measurements of Leaf Apoplastic pH Upon Salt Stress
Plants regulate expansin activity by adjusting the apoplastic pH to ∼4.0–5.5, which is the range in which acidification activates expansin activity (11, 14, 16, 28). Thus, the pH in the leaf apoplastic fluid represents a basic chemical requirement that controls whether the recombinant ZmEXPB6 can become active. For this reason it was checked via microscopy-based in-planta ratio imaging to determine whether the leaf apoplastic proton concentration fulfils the requirement for activating the exogenously applied ZmEXPB6. It turned out that under stress conditions, pH ranged from ∼4.0 (stomatal cavity) to 4.3 (mesophyll apoplast) (Fig. 8).
FIGURE 8.
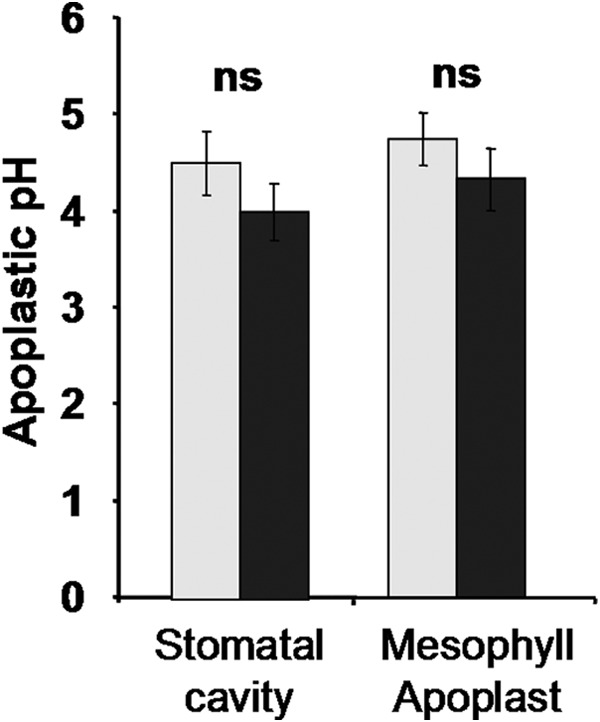
Leaf apoplastic pH. In planta quantitation of apoplastic pH in intact maize leaves under control and NaCl-stress conditions is shown. Leaf apoplastic pH as quantified in the stomatal cavities and in the apoplast that is surrounded by the mesophyll cells. Light gray, control; dark gray, 100 mm NaCl. Measurements were conducted on 45 different plants, with 5 regions of interest quantified for each apoplastic subcompartment. Thus, each mean is based on 225 single data points. Error bars indicate S.D. The t test was calculated based on de-logarithmized pH values.
In Vitro Activity of Recombinant ZmEXPB6
To assay the activity of recombinant ZmEXPB6 in terms of conferring leaf extension, purified ZmEXPB6 was exogenously applied onto frozen-thawed basal leaf sections (5-cm length/1.5-cm width), which were subjected to linear variable differential transducer measurements (Fig. 9). The addition of 10 μl of buffer solution containing 0.3 μg of ZmEXPB6 per μl on the ad- and abaxial leaf surfaces resulted in a higher rate of extension compared with segments that were treated with heat-inactivated ZmEXPB6. Treatment with heat-inactivated ZmEXPB6 represented a control measure for excluding unspecific proteinogenic effects and effects conferred by the buffer solution (Fig. 9). In relation to the total ad- and abaxial leaf surfaces that summed up to 15 cm2, the exogenous addition of 0.2 μg of ZmEXPB6 per cm2 leaf surface elongated the 5-cm-long and 1.5-cm-wide segment by ∼4 mm (∼8%) before it tore (point of breakage of leaf section), whereas the segment treated with heat-inactivated ZmEXPB6 extended only ∼0.5 mm (1%) under increasing tension before it tore (mean difference in the elongation distance between both treatments was significant with p ≤ 0.01; t test). Soluble protein control using BSA instead of heat-inactivated ZmEXPB6 did not facilitate extension of segments (data not shown).
FIGURE 9.
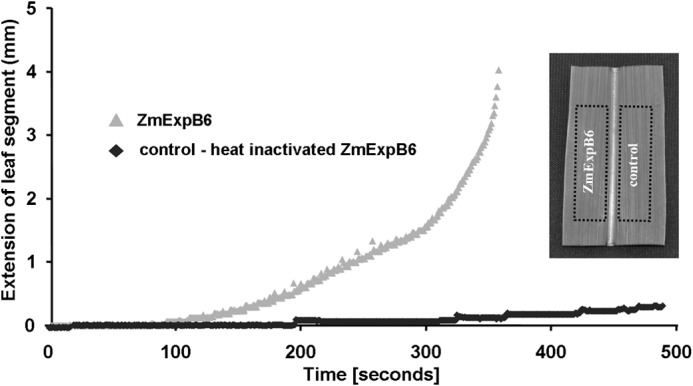
Assaying activity of recombinant ZmEXPB6 proteins in vitro. Extension of frozen-thawed basal leaf sections (5-cm length/1.5-cm width) in response to a force applied to leaf sections treated with recombinant ZmEXPB6 (light gray) or heat-inactivated recombinant ZmEXPB6 protein (dark gray). Extension is in mm, as measured with a linear variable differential transducer until point of breakage of leaf sections, as plotted over time. Extension is in response to application of 0.2 μg of ZmEXPB6 protein per cm2 ad- and abaxial leaf surface. Representative kinetics of equivalent recordings are from independent experiments (n = 14). Inset, comparability between leaf segments was achieved by using a segment left of the mid rib and the congruent segment right of the mid rib.
DISCUSSION
Salt Stress Decreases the Abundance of β-Expansin 6 in Growth-reduced Leaves
Interactions between salt stress and protein-mediated wall-loosening processes were studied in maize leaves to achieve a more comprehensive understanding of mechanisms involved in the salinity-induced leaf growth reduction. The 8-day treatment with 100 mm NaCl significantly reduced leaf growth and biomass formation (Fig. 4). Growth reduction is generally known to occur in the first phase of salt stress, which is dominated by osmotic stress rather than ionic toxicity stress (5, 49). Munns (50) reviewed increasing evidence that over the timescale of days that hormonal signals rather than leaf water relationships control growth in saline soils. In agreement, Fricke and Peters (51) found that leaf and cell elongation rates in barley that was exposed to NaCl-induced osmotic stress were not limited by the magnitude of cell turgor. This led to the idea that salinity somehow modifies the rheological properties of the cell walls, an effect that is thought to impair expansion growth. Cramer and Bowman (41) hypothesized that a modified capacity of the cell walls to irreversibly expand represents a growth-limiting factor under salt stress.
Geilfus et al. (32) recently demonstrated that leaf cell wall extensibility of a maize hybrid that almost maintained full growth was less impaired under salt stress than that of the hybrid Lector that grew stunted. Upon salt stress, the expansin transcripts ZmEXPA1, ZmEXPB2, ZmEXPB6, and ZmEXPB8 proved to be oppositionally regulated within both maize genotypes, viz. down-regulated in the stunted leaves of the maize hybrid Lector (this hybrid was used for this study) but, in contrast, up-regulated in the hybrid that almost maintained full growth (19, 32). This down-regulation of β-expansin transcripts in Lector might contribute to the decrease in the corresponding β-expansin protein abundance previously shown by one-dimensional Western blotting (one-dimensional WB; Ref. 19). Because Geilfus et al. (19) used the technique of one-dimensional WB, it was not possible to elucidate which of the different β-expansin protein isoforms were in particular down-regulated in the size-reduced leaves. The reason was that the one-dimensional SDS-PAGE did not clearly separate all isoforms due to the similar molecular size range. This is where the presented study begins. We used two-dimensional WB to discriminate between the different protein isoforms since two-dimensional SDS-PAGE sufficiently separates all protein isoforms by their isoelectric points. The x-ray film indicated one spot signal to be absent under saline conditions (arrows in Fig. 5A). For this spot, mass spectrometry analysis confirmed the β-expansin nature and supported the identity to ZmEXPB6, whereas no sequence homology to other members of the maize β-expansin family was found (Fig. 6). These data and the knowledge that expansin proteins mediate cell wall enlargement in growing cells (12) suggest that salinity impairs leaf growth in maize by decreasing the abundance of the growth-mediating β-expansin 6.
Exogenously Applied ZmEXPB6 Partially Restores Stress-induced Leaf Growth Inhibition
By inversion of this argument, it is hypothesized that in the case of sufficiently available ZmEXPB6, leaf growth under saline conditions might be partially restored. Exogenous application of heterologously expressed ZmEXPB6 onto salt-stressed leaves that exhibited a growth reduction and lacked native ZmEXPB6 significantly increased growth of size-reduced leaves (Fig. 7A). This response can be ascribed to the activity of ZmEXPB6, because (i) leaf apoplastic pH of salt-stressed Lector was with ∼4.0–4.3 (Fig. 8) in the range in which pH activates expansin activity (52), and (ii) treatment with heat-inactivated ZmEXPB6 did not cause any growth improvement (Fig. 7A). Hence, any unspecific proteinogenic effects can be excluded, emphasizing the functionality of heterologously expressed ZmEXPB6. Heterologous expression of soluble and active ZmEXPB6 for the first time had success after metal chelate affinity chromatography purification from 10 liters of E. coli culture.
The exogenous application of ZmEXPB6 partially restored leaf growth only when plants were stressed with NaCl but not when plants were stress with the nonionic solute PEG6000 (Fig. 7A), proving that this is a specific reaction to the ionic compounds of salt stress.
The in planta assay presented in Fig. 7 demonstrated that the presence of ZmEXPB6, which had disappeared upon the 8-day treatment with 100 mm NaCl, can partially restore growth under saline conditions. The in vitro extension measurements given in Fig. 9 show that the recombinant ZmEXPB6 protein mediates growth by acting on leaf extension, indicative of the proper folding of the His tag-purified protein necessary for mediating turgor-driven cell wall slippage. This conclusion is supported by the fact that frozen-thawed basal sections of leaves were used for the in vitro assay, and hence, the action of the turgor on leaf extension (53) can be excluded because cell membranes were destroyed by the freeze-thaw cycle. The ZmEXPB6 addition on frozen-thawed leaf sections showed immediate effects on leaf extension after ∼1–2 min. This is in agreement with results from Li et al. (47) who have measured extension activity induced by Zea m 1 within the same time frame. Li et al. (47) used not more than 0.05 μg μl−1 of Zea m 1 expansin to detect clear activity that led to a cell wall breakage after ∼10% extension. We used a ZmEXPB6 protein concentration of 0.3 μg μl−1, which led to the breakage of the leaf segment after 8% of extension.
Finally, the question arises as to whether this partial restored growth is a maize-specific phenomenon or whether it is more universal. Because it was assumed (15) that β-expansins specifically interact with structural polysaccharides and proteins that have developed in grasses but not in other land plants (54), the ZmEXPB6-mediated leaf growth improvement of NaCl-stressed plants might be seen as a phenomenon in maize or other grasses in which β-expansin have evolved special functions as proposed elsewhere (17).
Conclusion
Results showed a spatiotemporally correlation between the NaCl-induced leaf growth disturbance and a reduced abundance of cell wall loosening ZmEXPB6 protein in maize. The exogenous application of recombinant ZmEXPB6 on growth-reduced leaves that lack native ZmEXPB6 under salt stress partially restored growth under saline conditions in planta. In vitro assays on frozen-thawed leaf sections demonstrated that the heterologous expressed ZmEXPB6 acts on the capacity of the cell walls to extend, viz. change the rheological properties of the wall.
Acknowledgments
We thank Prof. Dr. Urs Schmidthalter for providing the LVDT device. Privatdozent Dr. Yuncai Hu and Jürgen Plass gave valuable information on using this device. They are also grateful to Brigitte Schemmerling and Stephanie thor Straten for excellent technical assistance. Privatdozent Dr. Christoph Plieth gave advice for fitting sigmoidal pH calibration curves using the Boltzmann fit, and Privatdozent Dr. Mario Hasler calculated the all-pair comparison.
Footnotes
- IEF
- isoelectric focusing
- WB
- Western blot
- LVDT
- linear variable differential transducer
- CLSM
- confocal laser scanning microscopy
- ZmEXPB6
- Z. mays β-expansin 6.
REFERENCES
- 1. Rozema J., Flowers T. (2008) Crops for a salinized world. Science 322, 1478–1480 [DOI] [PubMed] [Google Scholar]
- 2. Cushman J., Tester M. (2010) Special issue on drought and salinity stress. Plant Cell Environ. 4, 453–669 [Google Scholar]
- 3. Hütsch B. W., Saqib M., Osthushenrich T., Schubert S. (2014) Invertase activity limits grain yield of maize under salt stress. J. Plant Nutr. Soil Sci. 177, 278–286 [Google Scholar]
- 4. Bernstein N., Silk W. K., Läuchli A. (1993) Growth and development of sorghum leaves under conditions of NaCl stress: spatial and temporal aspects of leaf growth inhibition. Planta 191, 433–439 [Google Scholar]
- 5. Munns R., Tester M. (2008) Mechanism of salinity tolerance. Annu. Rev. Plant Biol. 59, 651–681 [DOI] [PubMed] [Google Scholar]
- 6. Ismail A., Takeda S., Nick P. (2014) Life and death under salt stress: same players, different timing? J. Exp. Bot. 65, 2963–2979 [DOI] [PubMed] [Google Scholar]
- 7. Hager A., Menzel H., Krauss A. (1971) Versuche und Hypothese zur Primärwirkung des Auxins beim Streckungswachstum. [Experiments and hypothesis concerning the primary action of auxin in elongation growth]. Planta 100, 47–75 [DOI] [PubMed] [Google Scholar]
- 8. Van Volkenburgh E., Boyer J. S. (1985) Inhibitory effects of water deficit on maize leaf elongation. Plant Physiol. 77, 190–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Geilfus C. M., Mühling K. H. (2012) Transient alkalinization in the leaf apoplast of Vicia faba L. depends on NaCl stress intensity: an in situ ratio imaging study. Plant Cell Environ. 35, 578–587 [DOI] [PubMed] [Google Scholar]
- 10. Geilfus C. M., Mühling K. H. (2013) Ratiometric monitoring of transient apoplastic alkalinizations in the leaf apoplast of living Vicia faba plants: chloride primes and PM-H+- ATPase shapes NaCl-induced systemic alkalinizations. New Phytol. 197, 1117–1129 [DOI] [PubMed] [Google Scholar]
- 11. Cosgrove D. J. (2005) Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 6, 850–861 [DOI] [PubMed] [Google Scholar]
- 12. McQueen-Mason S. (1995) Expansins and cell wall expansion. J. Exp. Bot. 46, 1639–1650 [Google Scholar]
- 13. Goh H. H., Sloan J., Dorca-Fornell C., Fleming A. (2012) Inducible repression of multiple expansin genes leads to growth suppression during leaf development. Plant Physiol. 159, 1759–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sampedro J., Cosgrove D. J. (2005) The expansin superfamily. Genome Biol. 6, 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cosgrove D. J., Bedinger P., Durachko D. M. (1997) Group I allergens of grass pollen as cell wall-loosening agents. Proc. Natl. Acad. Sci. U.S.A. 94, 6559–6564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cosgrove D. J. (2000) Loosening of plant cell walls by expansins. Nature 407, 321–326 [DOI] [PubMed] [Google Scholar]
- 17. Wu Y., Meeley R. B., Cosgrove D. J. (2001) Analysis and expression of the α-expansin and β-expansin gene families in maize. Plant Physiol. 126, 222–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Han Y. y., Li A. x., Li F., Zhao M. r., Wang W. (2012) Characterization of a wheat (Triticum aestivum L.) expansin gene, TaEXPB23, involved in the abiotic stress response and phytohormone regulation. Plant Physiol. Biochem. 54, 49–58 [DOI] [PubMed] [Google Scholar]
- 19. Geilfus C. M., Zörb C., Mühling K. H. (2010) Salt stress differentially affects growth-mediating β-expansin in resistant and sensitive maize (Zea mays L.) cultivars. Plant Physiol. Biochem. 48, 993–998 [DOI] [PubMed] [Google Scholar]
- 20. Kwon Y. R., Lee H. J., Kim K. H., Hong S. W., Lee S. J., Lee H. (2008) Ectopic expression of expansin 3 or expansinβ1 causes enhanced hormone and salt stress sensitivity in Arabidopsis. Biotechnol Lett. 30, 1281–1288 [DOI] [PubMed] [Google Scholar]
- 21. Lü P., Kang M., Jiang X., Dai F., Gao J., Zhang C. (2013) RhEXPA4, a rose expansin gene, modulates leaf growth and confers drought and salt tolerance to Arabidopsis. Planta 6, 1547–1559 [DOI] [PubMed] [Google Scholar]
- 22. Buchanan C. D., Lim S., Salzman R. A., Kagiampakis I., Morishige D. T., Weers B. D., Klein R. R., Pratt L. H., Cordonnier-Pratt M. M., Klein P. E., Mullet J. E. (2005) Sorghum bicolor's transcriptome response to dehydration, high salinity and ABA. Plant Mol. Biol. 58, 699–720 [DOI] [PubMed] [Google Scholar]
- 23. Jones L., McQueen-Mason S. (2004) A role for expansins in dehydration and rehydration of the resurrection plant Craterostigma plantagineum. FEBS Lett. 559, 61–65 [DOI] [PubMed] [Google Scholar]
- 24. Li F., Xing S., Guo Q., Zhao M., Zhang J., Gao Q., Wang G., Wang W. (2011) Drought tolerance through over-expression of the expansin gene TaEXPB23 in transgenic tobacco. J. Plant Physiol. 168, 960–966 [DOI] [PubMed] [Google Scholar]
- 25. Zhu J., Alvarez S., Marsh E. L., Lenoble M. E., Cho I. J., Sivaguru M., Chen S., Nguyen H. T., Wu Y., Schachtman D. P., Sharp R. E. (2007) Cell wall proteome in the maize primary root elongation zone. II. Region-specific changes in water soluble and lightly ionically bound proteins under water deficit. Plant Physiol. 145, 1533–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guo W., Zhao J., Li X., Qin L., Yan X., Liao H. (2011) A soybean β-expansin gene GmEXPB2 intrinsically involved in root system architecture responses to abiotic stresses. Plant J. 66, 541–552 [DOI] [PubMed] [Google Scholar]
- 27. Dai F., Zhang C., Jiang X., Kang M., Yin X., Lü P., Zhang X., Zheng Y., Gao J. (2012) RhNAC2 and RhEXPA4 are involved in the regulation of dehydration tolerance during the expansion of rose petals. Plant Physiol. 160, 2064–2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cho H. T., Kende H. (1997) Expansins in deepwater rice internodes. Plant Physiol. 113, 1137–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cho H. T., Kende H. (1998) Tisse localization of expansins in deepwater rice. Plant J. 15, 805–812 [DOI] [PubMed] [Google Scholar]
- 30. Lasanthi-Kudahettige R., Magneschi L., Loreti E., Gonzali S., Licausi F., Novi G., Beretta O., Vitulli F., Alpi A., Perata P. (2007) Transcript profiling of the anoxic rice coleoptile. Plant Physiol. 144, 218–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Magneschi L., Kudahettige R. L., Alpi A., Perata P. (2009) Expansin gene expression and anoxic coleoptile elongation in rice cultivars. J. Plant Physiol. 166, 1576–1580 [DOI] [PubMed] [Google Scholar]
- 32. Geilfus C. M., Zörb C., Neuhaus C., Hansen T., Lüthen H., Mühling K. H. (2011) Differential transcript expression of wall-loosening candidates in leaves of maize cultivars differing in salt resistance. J. Plant Growth Regul. 30, 387–395 [Google Scholar]
- 33. Moll S., Anke S., Kahmann U., Hänsch R., Hartmann T., Ober D. (2002) Cell-specific expression of homospermidine synthase, the entry enzyme of the pyrrolizidine alkaloid pathway in Senecio vernalis, in comparison with its ancestor, deoxyhypusine synthase1. Plant Physiol. 130, 47–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Geilfus C. M., Mühling K. H., Zörb C. (2010) A methodical approach for improving the reliability of quantifiable two-dimensional Western blots. J. Immunol. Methods 362, 89–94 [DOI] [PubMed] [Google Scholar]
- 35. Zörb C., Schmitt S., Neeb A., Karl S., Linder M., Schubert S. (2004) The biochemical reaction of maize (Zea mays L.) to salt stress is characterized by a mitigation of symptoms and not by a specific adaptation. Plant Sci. 167, 91–100 [Google Scholar]
- 36. Plöscher M., Reisinger V., Eichacker L. A. (2011) Proteomic comparison of etioplast and chloroplast protein complexes. J. Proteomics 74, 1256–1265 [DOI] [PubMed] [Google Scholar]
- 37. Kaltenegger E., Eich E., Ober D. (2013) Evolution of homospermidine synthase in the Convolvulaceae: a story of gene duplication, gene loss, and periods of various selection pressures. Plant Cell 25, 1213–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shonjani S. (2002) Salt Sensitivity of Rice, Maize, Sugar Beet, and Cotton during Germination and Early Vegetative Growth. Ph.D. thesis, Justus-Liebig-Universität Giessen [Google Scholar]
- 39. Geilfus C. M., Mühling K. H. (2011) Real-time imaging of leaf apoplastic pH dynamics in response to NaCl stress. Front. Plant. Sci. 2, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Geilfus C. M., Mühling K. H., Kaiser H., Plieth C. (2014) Bacterially produced Pt-GFP as ratiometric dual-excitation sensor for in planta mapping of leaf apoplastic pH in intact Avena sativa and Vicia faba. Plant Methods 10, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cramer G. R., Bowman D. C. (1991) Kinetics of maize leaf elongation. I: Increased yield threshold limits short-term, steady-state elongation rates after exposure to salinity. J. Exp. Bot. 42, 1417–1426 [Google Scholar]
- 42. Hu Y. C., Camp K. H., Schmidhalter U. (2000) Kinetics and spatial distribution of leaf elongation of wheat (Triticum aestivum L.) under saline soil conditions. Int. J. Plant Sci. 161, 575–582 [Google Scholar]
- 43. Cosgrove D. J. (1998) Cell wall loosening by expansins. Plant Physiol. 118, 333–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Köhler W., Schachtel G., Voleske P. (1984) Einführung in die Statistik für Biologen und Agrarwissenschaftler. p. 101, Springer, Berlin [Google Scholar]
- 45. Tukey J. W. (1953) The problem of multiple comparisons. In The Collected Works of John W. Tukey VIII. Multiple Comparisons: 1948–1983, pp. 1–300 Chapman and Hall, New York [Google Scholar]
- 46. Wu Y., Sharp R. E., Durachko D. M., Cosgrove D. J. (1996) Growth maintenance of the maize primary root at low water potentials involves increases in cell wall extensibility, expansin activity and wall susceptibility to expansins. Plant Physiol. 111, 765–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li L. C., Bedinger P. A., Volk C., Jones A. D., Cosgrove D. J. (2003) Purification and characterization of four β-expansins (Zea m 1 isoforms) from maize pollen. Plant Physiol. 132, 2073–2085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Downes B. P., Steinbaker C. R., Crowell D. N. (2001) Expression and processing of a hormonally regulated β-expansin from soybean. Plant Physiol. 126, 244–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sümer A., Zörb C., Yan F., Schubert S. (2004) Evidence of Na+ toxicity for the vegetative growth of maize (Zea mays L.) during the first phase of salt stress. J. Appl. Bot. Food Qual. 78, 135–139 [Google Scholar]
- 50. Munns R. (2002) Comparative physiology of salt and water stress. Plant Cell Environ. 25, 239–250 [DOI] [PubMed] [Google Scholar]
- 51. Fricke W., Peters W. S. (2002) The biophysics of leaf growth in salt-stressed barley. A study at the cell level. Plant Physiol. 129, 374–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rayle D. L., Cleland R. E. (1992) The acid growth theory of auxin-induced cell elongation is alive and well. Plant Physiol. 99, 1271–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Neumann P. M., Van Volkenburgh E., Cleland R. E. (1988) Salinity stress reduces bean leaf expansion and turgor but not cell wall extensibility. Plant Physiol. 88, 233–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Carpita N. C. (1996) Structure and biogenesis of the cell walls of grasses. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47, 445–476 [DOI] [PubMed] [Google Scholar]