Background: Insulin resistance is an early risk factor for metabolic disease.
Results: Using various insulin resistance models, insulin regulation of glucose metabolism was universally blunted, whereas other actions (protein synthesis and anti-lipolysis) were unimpaired.
Conclusion: Insulin resistance is selective for glucose metabolism in adipocytes.
Significance: Chronic hyperactivation of unaffected insulin action pathways in the context of the metabolic syndrome likely contributes to disease progression.
Keywords: Adipocyte, Glucose Metabolism, Insulin, Insulin Resistance, Lipolysis, Insulin Action, Selective Insulin Resistance, White Adipose
Abstract
Aside from glucose metabolism, insulin regulates a variety of pathways in peripheral tissues. Under insulin-resistant conditions, it is well known that insulin-stimulated glucose uptake is impaired, and many studies attribute this to a defect in Akt signaling. Here we make use of several insulin resistance models, including insulin-resistant 3T3-L1 adipocytes and fat explants prepared from high fat-fed C57BL/6J and ob/ob mice, to comprehensively distinguish defective from unaffected aspects of insulin signaling and its downstream consequences in adipocytes. Defective regulation of glucose uptake was observed in all models of insulin resistance, whereas other major actions of insulin such as protein synthesis and anti-lipolysis were normal. This defect corresponded to a reduction in the maximum response to insulin. The pattern of change observed for phosphorylation in the Akt pathway was inconsistent with a simple defect at the level of Akt. The only Akt substrate that showed consistently reduced phosphorylation was the RabGAP AS160 that regulates GLUT4 translocation. We conclude that insulin resistance in adipose tissue is highly selective for glucose metabolism and likely involves a defect in one of the components regulating GLUT4 translocation to the cell surface in response to insulin.
Introduction
Although most well known for its role in facilitating postprandial glucose disposal, insulin's repertoire of actions in vivo is considerable. For example, in peripheral tissues such as muscle and white adipose, insulin stimulates a variety of synthetic pathways (e.g. glycogen, protein, and lipid synthesis) while simultaneously down-regulating starvation pathways such as lipolysis, autophagy, and FoxO1-dependent transcription. Many actions of insulin are mediated through the PI3K/Akt pathway. Akt is essential for most of the metabolic actions of insulin, and its activity is regulated by phosphorylation at Thr-308 and Ser-473 by PDK1 and mTORC2, respectively (1–3). Akt is a major hub in the insulin signaling network because its activation leads to phosphorylation of numerous intracellular substrates that play key roles in a range of biological processes. For instance, phosphorylation of PRAS40 and TSC2 controls mTORC1 activation, an essential intermediate in regulated protein synthesis; Akt-dependent phosphorylation of phosphodiesterase 3B regulates inhibition of lipolysis (4); and Akt-dependent phosphorylation of the RabGAP TBC1D4/AS160 regulates GLUT4 translocation (5, 6).
Under conditions of chronic nutrient overload (i.e. high fat diet, obesity), peripheral tissues experience a decrease in sensitivity to insulin, such that additional insulin is required both in the fed and fasted states to restore/maintain euglycemia (7, 8). Although the mechanism(s) that contribute to insulin resistance remain controversial, defects within the insulin signaling network, either upstream (insulin receptor, insulin receptor substrate 1) (9–11), downstream (AS160) (6), or at Akt itself (12, 13) have been identified as potential targets. If the defect involves a node in upper insulin signaling or Akt itself, such a derangement should be distributed equally across all Akt-dependent actions of insulin. However, increasing evidence indicates that insulin resistance is selective for specific actions of insulin. For example, mice with hepatic insulin resistance show impaired insulin regulation of hepatic carbohydrate metabolism, whereas insulin regulation of lipogenesis is unperturbed (14). Fibroblasts from patients with polycystic ovary syndrome exhibit defective insulin regulation of glycogen synthesis without any detectable defect in insulin-regulated mitogenesis (15). We recently reported that insulin-dependent regulation of the transcription factor FoxO1 in skeletal muscle of humans with type 2 diabetes (T2D)6 is normal despite impaired insulin regulation of glucose uptake (16). Insulin-mediated FoxO1 translocation to the cytosol was not affected in chronic insulin-treated 3T3-L1 adipocytes at physiological insulin doses, whereas GLUT4 translocation to the cell surface was impaired (17). In this study, evidence is presented suggesting that this might reflect a role for different Akt isoforms in different actions of insulin. Collectively, these studies suggest that insulin resistance does not encapsulate a defect in all of the metabolic actions of insulin, which is inconsistent with a defect upstream in the insulin signaling pathway as being the major contributor to this disorder.
Adipose tissue and the adipocyte itself have emerged as major regulators of whole body insulin action and fuel homeostasis. Targeted defects in insulin action in adipocytes lead to systemic insulin resistance (18). Moreover, defects in adipose insulin action are a common feature of insulin resistance (19–21). Conversely, improvements in fat cell insulin action contribute to enhanced whole body insulin sensitivity despite the fact that adipose tissue itself makes a minor contribution to glucose uptake following a meal, with the majority being consumed by muscle and liver (22). Notwithstanding the key role of the fat cell in whole body insulin action, as to whether insulin resistance in the fat cell represents a global defect or, as in the case of other insulin responsive tissues, it is more selective for specific actions of insulin, is not well understood.
In this study, we have systematically analyzed a range of biological actions of insulin in either 3T3-L1 adipocytes rendered insulin-resistant or fat explants from insulin-resistant mouse models. We observed a consistent reduction in insulin-stimulated glucose uptake in all models of insulin resistance, despite little or no defect in insulin regulation of protein synthesis or lipolysis. Surprisingly, analysis of insulin-stimulated Akt activation, as well as that of several downstream substrates, revealed clear disconnections between various signaling intermediates and functional insulin action. These findings demonstrate a clear selectivity of insulin resistance for glucose metabolism in white adipose tissue.
EXPERIMENTAL PROCEDURES
Cell Culture and Insulin Resistance Treatments
3T3-L1 fibroblasts were cultured in DMEM containing 10% FCS and GlutaMAX and differentiated to adipocytes as previously described (23). Insulin resistance models were induced as previously described (24). Briefly, 3T3-L1 adipocytes were incubated with 10 nm insulin (chronic insulin (CI)) or 1 μm dexamethasone (DEX) in full medium for 16–18 h at 37 °C or with 4 ng/ml TNFα in full medium at 37 °C for 48 h. The medium was replaced twice a day with fresh medium containing TNFα for treated cells and without TNFα for control cells. After insulin resistance treatment, cells were washed and then serum-starved for 2 h prior to insulin stimulation and assessment of insulin-regulated processes. We have previously shown that this protocol is adequate to return the cells to their baseline level of GLUT4 translocation (24).
Western Blotting Analysis
Cells were washed twice with ice-cold PBS and solubilized in 2% SDS/PBS containing phosphatase/protease inhibitors. Proteins were separated by SDS-PAGE. After transferring proteins to polyvinylidene difluoride membranes, membranes were blocked (5% skim milk) and then immunoblotted with the relevant antibodies overnight at 4 °C. After incubation, membranes were washed and incubated with either infrared dye 700- or 800-conjugated secondary antibodies or horseradish peroxidase-labeled secondary antibodies and then detected by Odyssey infrared imaging system software v2.0 (LI-COR Biosciences, Lincoln, NE) or SuperSignal West Pico chemiluminescent substrate, respectively.
2-Deoxyglucose Uptake Assay
3T3-L1 adipocytes in 24-well plates were washed twice and incubated with serum- and bicarbonate-free DMEM containing 20 mm HEPES, pH 7.4, and 0.2% BSA for 2 h. Following serum starvation, cells were washed twice with Krebs-Ringer phosphate buffer (0.6 mm Na2HPO4, 0.4 mm NaH2PO4, 120 mm NaCl, 6 mm KCl, 1 mm CaCl2, 1.2 mm MgSO4, 12.5 mm HEPES, pH 7.4) supplemented with 0.2% BSA. Indicated doses of insulin were added for 20 min, and glucose transport was initiated by the addition of [3H]2-deoxyglucose (PerkinElmer Life Sciences) (0.25 μCi/well, 50 μm unlabeled 2-deoxyglucose) for 5 min. To determine nonspecific glucose uptake, 25 μm cytochalasin B was added prior to the addition of [3H]2-deoxyglucose. Uptake was terminated with three rapid washes in ice-cold PBS, after which the cells were solubilized in 1% Triton X-100 in PBS. Samples were assessed for radioactivity by scintillation counting. Each condition was performed in triplicate.
[3H]Leucine Incorporation Assay for Protein Synthesis
3T3-L1 adipocytes in 24-well plates were washed twice and incubated with leucine-free DMEM (Sigma-Aldrich) supplemented with 0.2% BSA and 20 mm HEPES, pH 7.4, for 2 h. [3H]Leucine (1.25 μCi/well) (PerkinElmer Life Sciences) was added at the same time as indicated doses of insulin for 1 h. To determine background leucine incorporation, 5 μm cycloheximide was added for 10 min before addition of [3H]leucine and insulin. Leucine incorporation was terminated with three rapid washes in ice-cold PBS followed by incubating cells with ice-cold 10% TCA for 10 min to precipitate protein. Pellets were washed three times in ice-cold 10% TCA to remove free [3H]leucine. Pellets were resuspended in 50 nm NaOH with 1% Triton X-100 at 65 °C for 20 min. Samples were assessed for radioactivity, and results were normalized for protein content. Assays were performed in triplicate, and the average of the triplicate was considered as one biological replicate.
Lipolysis Assay
3T3-L1 adipocytes in 24-well plates were washed twice and incubated with Krebs-Ringer phosphate buffer supplemented with 3.5% free fatty acid BSA (Sigma-Aldrich) and 5 mm glucose for 2 h. Serum-starved cells were then treated with or without isoproterenol (1 nm, 5 nm) or indicated doses of insulin for 1 h. Aliquots of media were taken to assay for glycerol content using Sigma glycerol reagent according to the manufacturer's protocol. The cells were next lysed in 2% SDS in PBS and assessed for protein concentration. Glycerol release was normalized to cellular protein content. Each sample was assayed in duplicate, and the average of the duplicates was considered as one biological replicate.
Akt1/2 siRNA Experiments
3T3-L1 adipocytes were electroporated with 200 nm siRNA designed to target Akt1 and Akt2 or scrambled as a control. For Akt1 and Akt2, we utilized pooled siRNAs (four for each) to minimalize any off target effects (25). Glucose uptake, glycerol release, and signaling were assessed as described above 72 h following electroporation. For glycerol release experiments involving MK-2206 and GDC, compounds were added during the 1-h incubation period (DMSO was added to control wells). The following siRNAs were used: Akt1–1, 5′-CAAGAACGATGGCACCTTTTT-3′; Akt1–2, 5′-GGAAAGTGATTCTGGTGAATT-3′; Akt1–3, 5′-GCACAATGCCAGCTGATGATT-3′; Akt1–4, 5′-GAGGTTGCCCACACGCTTATT-3′; Akt2–1, 5′-CCATGAATGACTTCGATTATT-3′; Akt2–2, 5′-GTACTTTGATGACGAGTTTCTT-3′; Akt2–3, CCTGAACAATTTCTCTGTATT-3′; Akt2–4, GATGCGGGCTATCCAGATGTT-3′; and scrambled, 5′-GACTTAACTCATCCAACGATT-3′.
Intraperitoneal Glucose Tolerance Tests
Eight week old male C57BL/6J and ob/ob mice were given chow or high fat (45%) diet for 28 days (only C57BL/6J mice). Intraperitoneal glucose tolerance tests were performed on mice following a 4-h fast. Mice were injected with a 20% (w/v) glucose solution at a final dose of 2 g/kg. Blood glucose was measured by sampling blood from the tail tip with an Accu-Chek II glucometer. Blood insulin was measured via enzyme-linked immune-sorbent assay (Crystal Chem Inc.). Mice that were fasted for the indicated periods were euthanized by cervical dislocation for tissue isolation. Experiments were carried out with approval of Garvan Institute Animal Experimentation Ethics Committee following guidelines issued by the National Health and Medical Research Council of Australia.
Fat Explants and Assays
Epididymal fat depots were obtained from mice. Visible nonparenchymal tissue was removed from the fat pads. Fat pads were immediately transferred to warm DMEM supplemented with 2% BSA and 20 mm HEPES, pH 7.4, and minced into fine pieces. Minced explants were washed twice and incubated in DMEM supplemented with 2% BSA and 20 mm HEPES, pH 7.4, for 2 h. For glucose uptake and signaling in fat explants, explants were rinsed in Krebs-Ringer phosphate buffer supplemented with 2% BSA. Insulin was added for the indicated times, and glucose transport was initiated by addition of [3H]2-deoxyglucose (0.25 μCi/sample, 50 μm unlabeled 2-deoxyglucose) and [14C]mannitol (0.036 μCi/sample) for 5 min. Uptake was terminated with three rapid washes in ice-cold PBS, after which the cells were solubilized in radioimmune precipitation assay buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 1 mm EDTA, and 10% glycerol) supplemented with protease and phosphatase inhibitors. Samples were assessed for radioactivity by scintillation counting using the β-scintillation counter, and the results were normalized for protein content. Samples were also kept for Western blot analysis. Each condition was performed in duplicate. For lipolysis in explants, explants were washed twice and incubated with Krebs-Ringer phosphate buffer supplemented with 3.5% free fatty acid BSA and 5 mm glucose. Explants were then treated with or without isoproterenol (1 or 5 nm) or insulin (100 nm) for 1 h. Aliquots of media were taken to assay for glycerol content using Sigma glycerol reagent according to the manufacturer's protocol. Glycerol release was normalized to protein content.
Statistical Analysis
The data are presented as means ± S.E. Statistical analyses were performed using t tests, one-way analysis of variance, or two-way analysis of variance using GraphPad Prism software and the p values indicated.
RESULTS
Insulin Resistance Is Confined to Glucose Metabolism in 3T3-L1 Adipocytes
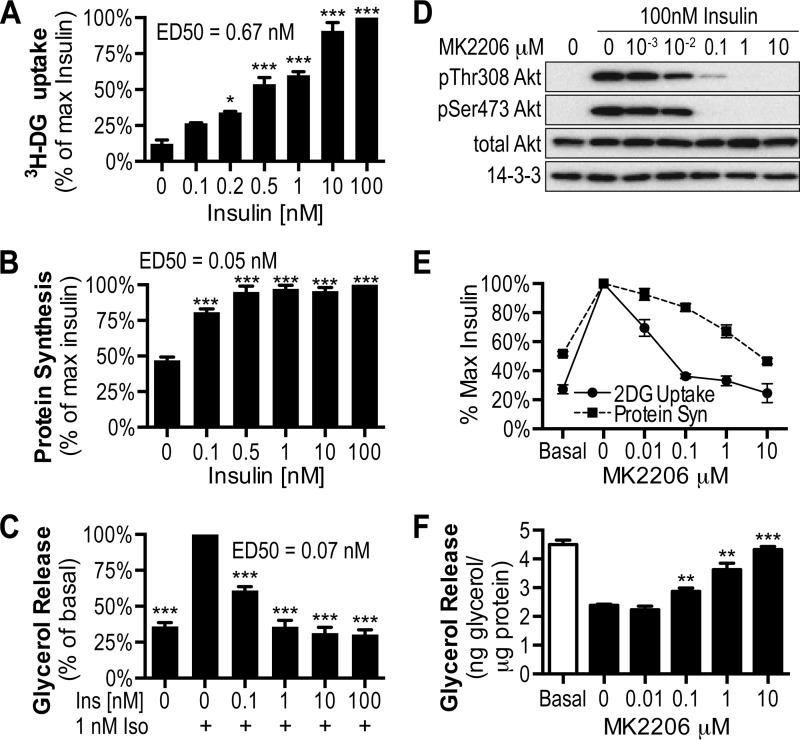
Insulin regulates a variety of cellular processes in adipocytes such as glucose transport, protein synthesis, and lipolysis (26–29). Consistent with this, exposure of 3T3-L1 adipocytes to increasing concentrations of insulin led to a dose-dependent increase in 2-deoxyglucose uptake (Fig. 1A), leucine incorporation into protein (i.e. protein synthesis; Fig. 1B), and inhibition of glycerol release, following β-adrenergic stimulation (Fig. 1C), and 10 or 100 nm insulin resulted in a maximal response in all these processes. Notably, protein synthesis and inhibition of lipolysis displayed higher insulin sensitivity compared with glucose uptake reflected in the difference in their respective ED50 values (Fig. 1, A--C). Each of these processes was blocked by the Akt specific inhibitor MK-2206 (30) (Fig. 1, D–F), suggesting that they are all regulated by the PI3K/Akt pathway. Consistent with its higher ED50, glucose uptake was more sensitive to lower doses of MK-2206 than either protein synthesis or inhibition of lipolysis.
FIGURE 1.
Insulin mediates glucose uptake, protein synthesis, and inhibition of lipolysis in an Akt-dependent manner. A–F, 3T3-L1 adipocytes were stimulated with the indicated dose of insulin and assessed for [3H]2-deoxyglucose (3H-DG) uptake (A) or [3H]leucine incorporation for protein synthesis (B). C and F, lipolysis was assessed via the inhibition of glycerol release in the presence of indicated doses of insulin, following stimulation with indicated doses of isoproterenol (Iso). ED50 doses for insulin are shown. D, 3T3-L1 adipocytes were pretreated for 30 min with the indicated dose of Akt inhibitor MK-2206 prior to treatment of 100 nm insulin for the assessment of phosphorylation of Thr-308 Akt, Ser-473 Akt, total Akt, or pan 14-3-3. E and F, effects of MK-2206 treatment were determined on 100 nm insulin-stimulated glucose uptake and protein synthesis (E) or insulin-mediated inhibition of lipolysis (F). The data are means ± S.E., n = 3–4. *, p < 0.01; **, p < 0.001; ***, p < 0.0001 versus basal.
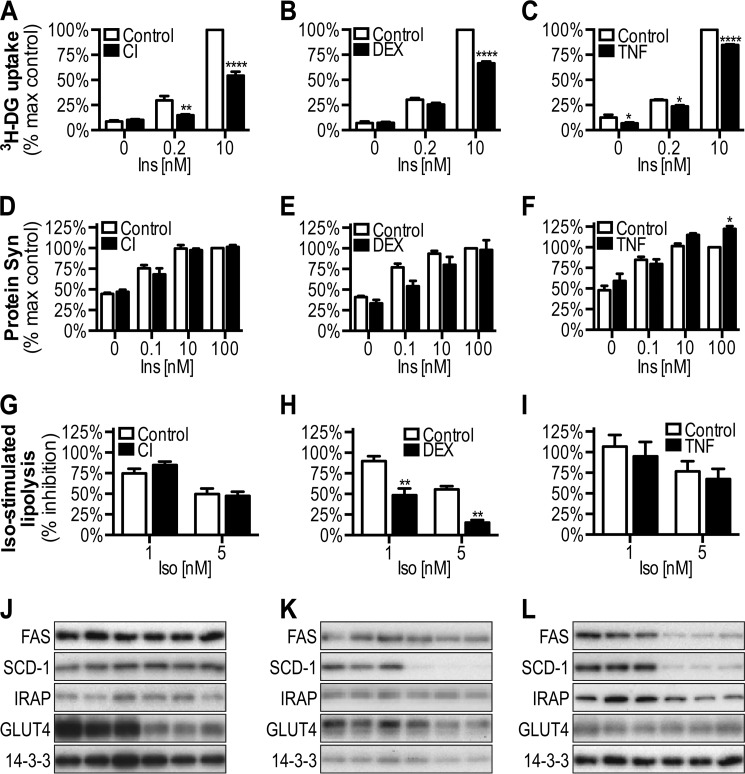
To investigate the potential selectivity of insulin resistance in adipocytes, we first examined a range of insulin-regulated processes in 3T3-L1 adipocytes rendered insulin-resistant via either exposure to high levels of insulin (CI), glucocorticoids (DEX), or inflammatory cytokines (TNFα) (24, 31). In all three of these model systems, insulin-stimulated glucose transport was significantly blunted (Fig. 2, A–C), confirming the presence of insulin resistance. Intriguingly, despite robust impairment in glucose metabolism, insulin-stimulated protein synthesis was unaffected in CI- or DEX-treated cells or enhanced at 100 nm insulin in TNF-treated cells (Fig. 2, D–F). Moreover, insulin-mediated suppression of isoproterenol-stimulated lipolysis was also unchanged, with the exception of cells made insulin-resistant via DEX (Fig. 2, G–I).
FIGURE 2.
Effect of insulin resistance on insulin-stimulated 2-deoxyglucose uptake, protein synthesis, and inhibition of lipolysis in 3T3-L1 adipocytes. 3T3-L1 adipocytes treated with CI (A, D, G, and J), DEX (B, E, H, and K), or TNFα (C, F, I, and L) were washed, serum-starved for 2 h, and then assessed for insulin-stimulated [3H]2-deoxyglucose (3H-DG) uptake (A–C), [3H]leucine incorporation for protein synthesis (D–F), and inhibition of isoproterenol-mediated glycerol release for lipolysis (G–I), and cell lysates were immunoblotted with indicated antibodies (J–L). The data are means ± S.E., n = 3–4. *, p < 0.05; **, p < 0.01; ****, p < 0.0001 versus control (Ctrl).
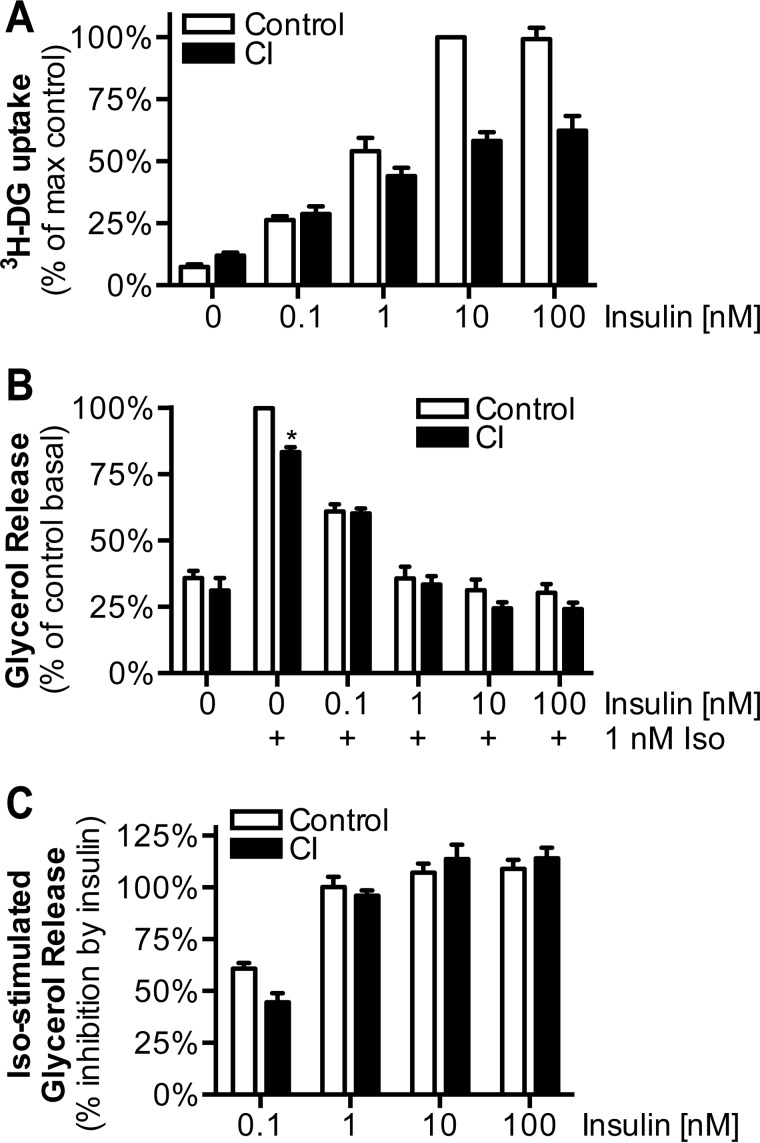
To test whether suppression of lipolysis occurs only at lower insulin doses, we performed an insulin dose-response analysis in control and CI-treated cells. Insulin-mediated suppression of isoproterenol-stimulated lipolysis was not significantly impaired at any insulin dose tested in CI cells, whereas 2-deoxyglucose uptake was significantly reduced at 10 and 100 nm insulin (Fig. 3, A–C). Interestingly, the ED50 for insulin-stimulated 2-deoxyglucose uptake was not significantly different between control and CI-treated cells (data not shown), indicating that insulin resistance does not represent a defect in insulin sensitivity.
FIGURE 3.
Insulin dose-response analysis of chronic insulin induced insulin resistance in adipocytes. A and B, control and CI-treated cells were washed, serum-starved for 2 h, and then stimulated with the indicated doses of insulin and assessed for [3H]2-deoxyglucose (3H-DG) uptake (A) and isoproterenol-stimulated glycerol release (B). C, the percentage of inhibition of isoproterenol-stimulated glycerol release was calculated from data in B. The data are means ± S.E., n = 3–4. *, p < 0.01; **, p < 0.0001 versus control.
We next assessed the expression of fatty acid synthase and stearoyl-CoA desaturase 1, because insulin regulates fatty acid synthesis by regulating the expression of these genes, via sterol regulatory element binding protein-1C (32, 33). With the exception of fatty acid synthase in the TNF model and stearoyl-CoA desaturase 1 in the DEX and TNF models, the levels of these proteins were unaffected by insulin resistance (Fig. 2, J–L).
Reduced GLUT4 protein levels has also been suggested as a mechanism of insulin resistance in adipocytes (34–38). Although GLUT4 protein levels were decreased in CI, there was no significant change in either the DEX or TNF models (Fig. 2, J–L). Insulin-regulated aminopeptidase (IRAP), a GLUT4 vesicle cargo protein, was reduced in TNF but not in the DEX or CI models (Fig. 2, J–L). These data indicate that reduced GLUT4 levels and that of other GLUT4 vesicle cargo proteins is not consistently observed in insulin resistance models and is unlikely to be the sole mechanism underlying insulin resistance in adipocytes. Collectively, these findings demonstrate that insulin resistance is highly selective for glucose metabolism in 3T3-L1 adipocytes.
Akt Signaling in Insulin-resistant 3T3-L1 Adipocytes
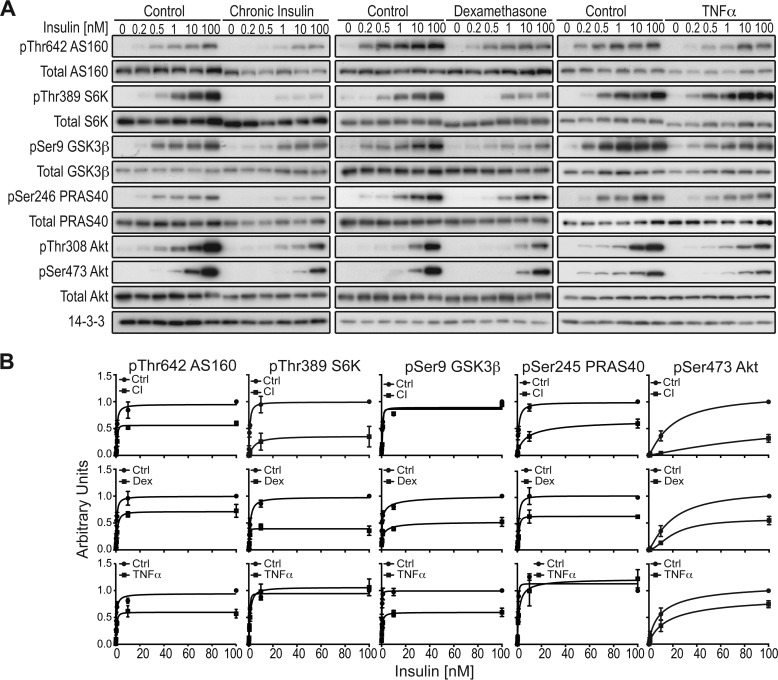
Reduced Akt phosphorylation has been observed in various insulin resistance models (17, 24, 31, 36, 39–41). Consistent with this, insulin-stimulated Akt phosphorylation at Thr-308 and Ser-473 was reduced in all models of insulin resistance (Fig. 4, A and B). It has been shown that Akt phosphorylation at these sites does not always correlate with Akt activity (16), and considerable sparseness was reported for Akt (42). To test this, we also assessed phosphorylation of a range of Akt substrates and the mTORC1 substrate S6K, downstream of Akt. In DEX-treated cells, phosphorylation of all Akt substrates was significantly impaired. In CI-treated cells, all substrates except GSK3β were impaired, whereas in TNFα-treated cells, all substrates aside from PRAS40 and the downstream component S6K were impaired (Fig. 4, A and B).
FIGURE 4.
Phosphorylation of the Akt signaling network in insulin-resistant 3T3-L1 adipocytes. A, control or insulin-resistant 3T3-L1 adipocytes were treated with the indicated doses of insulin. Cell lysates were immunoblotted using indicated phospho and total antibodies. B, quantification of the blots depicted in A of n = 3 independent experiments. Ctrl, control.
siRNA-mediated Knockdown of Akt in 3T3-L1 Adipocytes
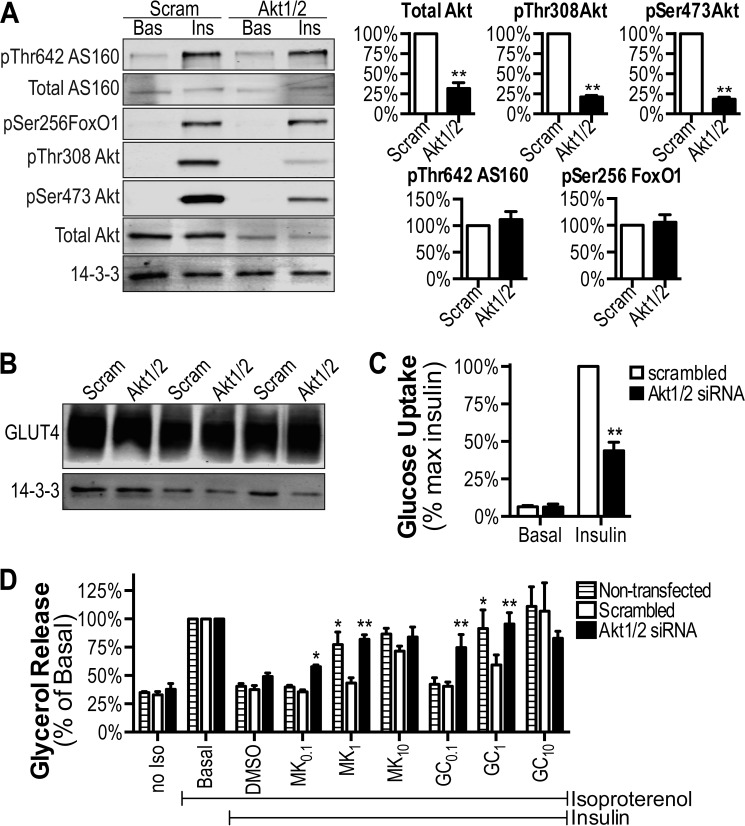
The above data show that in three models of insulin resistance in 3T3-L1 adipocytes, there is a universal defect in glucose uptake and insulin-regulated phosphorylation of Akt and its substrate AS160, whereas other Akt substrates and other actions of insulin were normal under the same conditions. One possible explanation for these data is that a partial reduction in Akt phosphorylation somehow leads to a selective impairment in the phosphorylation of certain substrates (AS160) but not others and that this leads to a selective impairment of glucose uptake. To test this, we next used siRNA to reduce the total levels of Akt1 and Akt2 and in turn the total level of phosphorylation to a similar extent as was observed in the insulin resistance models. Knockdown of either Akt1 or Akt2 alone in 3T3-L1 adipocytes had no significant effect on insulin action (data not shown), presumably because of compensation by the other isoform (43). Combined knockdown of Akt1 and Akt2 resulted in a ∼70% knockdown in total Akt and a >80% reduction in insulin-stimulated phosphorylation of Akt at Ser-308 and Thr-473 (Fig. 5A). Under these conditions, insulin-stimulated 2-deoxyglucose uptake was reduced by 60% (Fig. 5C). Strikingly, insulin-dependent phosphorylation of the Akt substrates FoxO1 and AS160 was unaffected by Akt1/2 knockdown (Fig. 5A), and GLUT4 levels were not altered (Fig. 5B).
FIGURE 5.
siRNA-mediated knockdown of Akt in 3T3-L1 adipocytes. 3T3-L1 adipocytes were electroporated with Akt1/2 siRNA or scrambled as a control. A and B, following serum starvation (2 h), adipocytes were treated with or without 10 nm insulin, and cell lysates were immunoblotted using indicated antibodies. Immunoblots from three independent experiments were quantified. C, insulin (10 nm)-stimulated [3H]2-deoxyglucose uptake. The data are expressed as percentages of maximal insulin for each replicate. D, insulin (100 nm)-mediated inhibition of isoproterenol-stimulated glycerol release was assessed in response to 1 nm isoproterenol. Insulin wells were treated with the indicated doses (μm) of MK-2206 or GDC, and DMSO was added to control wells. The data are presented as percentages of basal for each replicate. The data are means ± S.E., n = 6 except 10 μm GDC (n = 2) and 10 μm MK-2206 (n = 3). *, p < 0.01; **, p < 0.0001 versus scrambled. Norm, normal; Scram, scrambled; Ins, insulin; max, maximum.
Akt1/2 knockdown had no significant effect on the ability of insulin to suppress glycerol release (Fig. 5D), consistent with previous findings (28). One possible explanation for these data is that the difference in insulin sensitivity of glucose uptake compared with suppression of lipolysis (Fig. 1, A and C) confers a differential requirement for Akt activity for these two processes. To test this, we used a combined siRNA-small molecule inhibitor approach in which 3T3-L1 adipocytes transfected with Akt1/2 or scrambled siRNAs were incubated with different doses of two Akt inhibitors (MK-2206 (30) or GDC (44)) that act via discrete mechanisms.
Consistent with our hypothesis, very low doses (0.1 μm) of either MK-2206 or GDC nearly completely inhibited the insulin effect on glycerol release in Akt1/2 knockdown compared with adipocytes transfected with scrambled siRNA or nontransfected cells (Fig. 5D). These findings indicate that it is unlikely that the Akt1/2 knockdown effect on glucose uptake is due to an siRNA off target effect. We also observed a significant effect of both inhibitors at 1 μm in cells transfected with Akt1/2 siRNA but not scrambled siRNA. Curiously, cells transfected with scrambled siRNA were significantly less sensitive to the Akt inhibitors at 1 μm than nontransfected cells (Fig. 5D).
Collectively, these data confirm that glucose transport and anti-lipolysis are both Akt-dependent processes, but they display considerable differences in their Akt sensitivity. Furthermore, partial reduction of Akt led to selective impairment in glucose uptake without any defect in AS160 phosphorylation.
Insulin Resistance in White Adipose Tissue of High Fat-fed Mice Predominantly Affects Glucose Transport
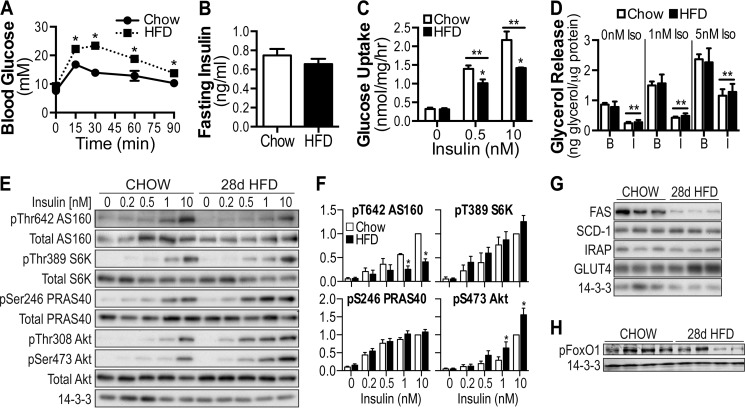
To determine whether insulin resistance is also specific for glucose metabolism in vivo, we performed studies in high fat-fed mice. Consistent with previous studies (36, 45), C57BL/6J mice fed a high fat diet (HFD) for 28 days displayed a significant impairment in whole body glucose tolerance (Fig. 6A) without any change in fasting insulin (Fig. 6B) and a substantial reduction in insulin-stimulated 2-deoxyglucose uptake in fat explants from epididymal fat depots in vitro, confirming insulin resistance in white adipose tissue (Fig. 6C). Conversely, insulin-mediated suppression of lipolysis, in both the absence and the presence of β-adrenergic stimulation, was unaffected by the diet (Fig. 6D). In contrast to that observed in 3T3-L1 cells, insulin-stimulated phosphorylation of Akt was increased, whereas phosphorylation of S6K and PRAS40 were unaffected by the diet (Fig. 6, E and F). Despite increased Akt phosphorylation, AS160 Thr-642 phosphorylation was blunted in HFD-fed mice (Fig. 6, E and F). The level of fatty acid synthase protein was decreased in HFD-fed mice, but stearoyl-CoA desaturase 1, IRAP, and GLUT4 protein levels were unchanged (Fig. 6G).
FIGURE 6.
Selective insulin resistance in adipose tissue of high fat-fed mice. 8-week-old C57BL/6J mice were placed on standard chow diet or HFD for 28 days. A, intraperitoneal glucose tolerance tests were performed on mice at a dosage of 2 g/kg of body weight. B, fasting insulin was assessed via ELISA following a 4-h fast. C, adipose explants were stimulated with the indicated doses of insulin, and [3H]2-deoxyglucose uptake was determined. D, lipolysis was assessed in the absence and presence of insulin (100 nm) following stimulation with 0, 1, and 5 nm isoproterenol. B, basal; I, insulin. E–G, adipose tissue lysates from explants experiments were immunoblotted using indicated antibodies. H, lysates prepared from white adipose tissue collected from 4-h fasted chow, and HFD mice were immunoblotted using antibodies raised against Ser(P)-256 FoxO1 and 14-3-3. The data are means ± S.E., n = 5–8. *, p < 0.05 versus chow; **, p < 0.05 versus 0 insulin condition. Ins, insulin; Iso, isoproterenol.
Analysis of White Adipose Tissue of ob/ob Mice
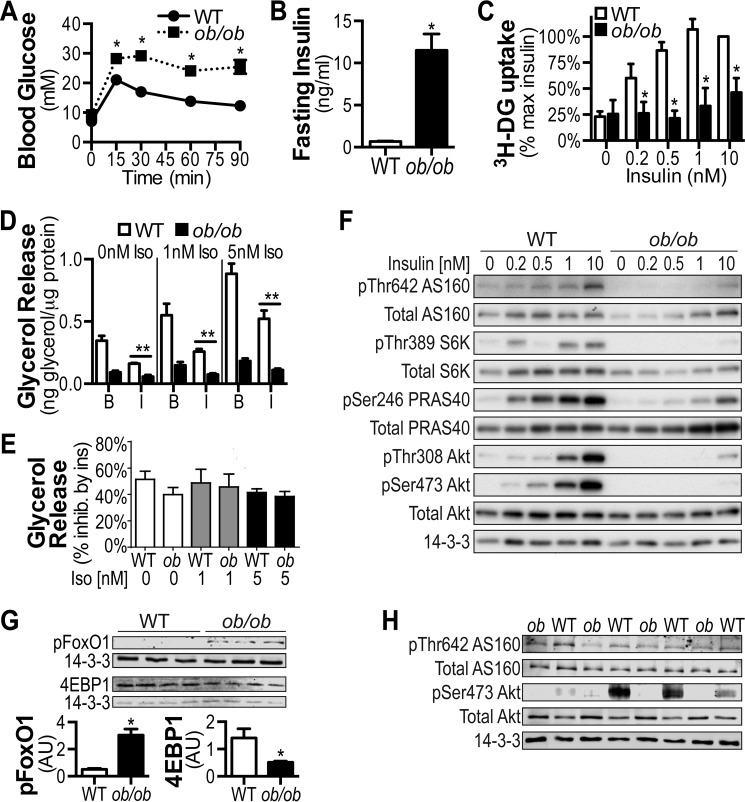
Another model commonly used to study insulin resistance is the ob/ob mouse (46–48). ob/ob mice displayed glucose intolerance (Fig. 7A), reduced insulin-stimulated 2-deoxyglucose uptake into white adipose tissue in vitro (Fig. 7C), and severe fasting hyperinsulinemia (Fig. 7B). The absolute level of lipolysis either in the absence or the presence of β-adrenergic stimulation was markedly reduced in fat explants from ob/ob mice, likely because of the severe hyperinsulinemia present in these mice (Fig. 7D, compare WT and ob/ob in B columns). Despite this reduction in absolute lipolysis, the ability of insulin to suppress lipolysis as a percent change of that observed in the basal state was preserved in fat explants from ob/ob mice (Fig. 7E). Insulin-stimulated phosphorylation of Akt, AS160, S6K, and PRAS40 was severely blunted in ob/ob mice (Fig. 7F), as were protein levels of IRAP and GLUT4 (data not shown).
FIGURE 7.
Selective insulin resistance in adipose tissue of ob/ob mice. A, intraperitoneal glucose tolerance tests were performed on 10-week-old ob/ob mice and age-matched wild-type controls at a dosage of 2g/kg of body weight. B, fasting insulin was assessed via ELISA following a 4-h fast. C, adipose tissue explants were stimulated with the indicated doses of insulin, and [3H]2-deoxyglucose (3H-DG) uptake was determined. The results are presented as a percentage of maximal insulin response. D, lipolysis was assessed in the absence and presence of insulin (100 nm) following stimulation with 0, 1, and 5 nm isoproterenol. B, basal; I, insulin. E, lipolysis data from D are presented as percentage inhibition by insulin to show the intact insulin response in ob/ob mice for all isoproterenol doses. F, adipose tissue lysates from explants experiments were immunoblotted using antibodies raised against the indicated proteins. G and H, lysates prepared from white adipose tissue collected from 4-h fasted WT and ob/ob mice were immunoblotted using antibodies raised against the indicated proteins. The data are means ± S.E., n = 3–6. *, p < 0.05 versus WT; **, p < 0.05 versus 0 insulin condition. Ins, insulin.
Increased basal FoxO1 phosphorylation was recently suggested as a potential consequence of selective insulin resistance because of concomitant hyperinsulinemia (16). Consistent with this, phosphorylation of FoxO1 was significantly increased in white adipose tissue prepared from 4-h fasted ob/ob mice compared with lean controls (Fig. 7G), whereas it was not significantly changed in HFD-fed mice (Fig. 6H), which did not display hyperinsulinemia (Fig. 6B). Moreover, the expression of 4EBP1, a principal transcript regulated by FoxO1, was significantly decreased in hyperinsulinemic ob/ob mice, confirming inactivation of FoxO1 caused by chronic phosphorylation (Fig. 7G). In contrast to FoxO1, phosphorylation of Akt and AS160 were decreased in these same samples from ob/ob mice (Fig. 7H). Together, these findings confirm the presence of selective insulin resistance in vivo and indicate that periods of hyperinsulinemia are associated with global suppression of lipolysis and FoxO1 activity within white adipose tissue.
DISCUSSION
Despite the fact that insulin resistance has been recognized as one of the earliest predictors and causal factors of metabolic disease in humans, its underlying molecular mechanism has remained elusive. The recent observation of selective insulin resistance in certain conditions (14, 16, 17, 49) has added to the complexity of this phenomenon. In view of the central role of the adipocyte in whole body insulin sensitivity (18, 50), in the present study, we have analyzed the selectivity of insulin resistance in various adipose models. Impaired glucose metabolism was a unifying feature of all insulin resistance models, whereas other processes such as lipolysis and protein synthesis were generally not impaired. Although these nondisrupted processes were more insulin-sensitive in control cells, we were unable to observe any defect in them irrespective of the insulin dose studied. Moreover, in regards to the consistent impairment in glucose metabolism, we observed that this was principally due to a reduction in maximum responsiveness rather than reduced sensitivity. Based on these analyses, we conclude that insulin resistance in adipocytes is highly “selective” for glucose metabolism, whereas other actions of insulin remain largely unscathed. In view of the spareness in most components of the upper insulin signaling pathway (i.e. from insulin receptor to the Akt node) (42, 51, 52), we predict that adipocyte selective insulin resistance is not due to a defect in insulin signaling. Consistent with this, although we did observe changes in Akt phosphorylation in most insulin resistance models, this did not coincide with a uniform defect in insulin-regulated phosphorylation of a range of Akt substrates. Interestingly, we observed a defect in AS160 phosphorylation in all models of insulin resistance, and in view of the connectivity between this molecule and glucose uptake, it is tempting to speculate that this represents a major node of insulin resistance. However, it is unlikely that this defect in AS160 phosphorylation is simply due to a defect in Akt activity because our Akt siRNA experiments in which insulin-mediated phosphorylation of Akt was reduced by >80%, a defect that was greater than that observed in most of our insulin resistance models, were not accompanied by reduced AS160 phosphorylation. Hence, additional features of this signaling network that remain to be established must be at play, and until unveiled, we predict the molecular basis for insulin resistance, at least in adipose tissue, will remain unclear. Regardless, the present studies provide justification for a more detailed focus on this node.
This study provides further evidence in support of the emerging view that insulin resistance is highly selective (14, 16, 17, 49, 53) and that this selectivity occurs in all insulin target tissues. In all insulin resistance models, with the exception of DEX-treated 3T3-L1 adipocytes, we observed defective glucose uptake but no defect in insulin-regulated protein synthesis or lipolysis (Table 1). In the case of DEX, insulin-stimulated glucose uptake, as well as suppression of lipolysis, was reduced. However, dexamethasone is known to have profound effects on the expression level and activity of multiple proteins involved in lipolysis, including the Akt substrate phosphodiesterase 3B (54). Hence it is likely that dexamethasone, at least as far as lipolysis is concerned, is not typical of other insulin resistance models including HFD-fed mice. Intriguingly, in control cells the latter two processes display higher insulin sensitivity than glucose uptake with ED50 values in the range of 0.05–0.07 nm compared with 0.7 nm for the latter (Fig. 1 (42, 55)). Similarly, McGraw and co-workers (17) reported defective insulin regulation of GLUT4 translocation in CI-treated adipocytes but no defect in nuclear FoxO1 translocation, and again FoxO1 translocation was more insulin-sensitive than GLUT4 translocation. To ensure that selective insulin resistance was not simply a function of this difference in sensitivity and that all processes display a right shift under insulin resistance conditions, we performed a dose-response analysis of chronic insulin-induced insulin resistance in 3T3-L1 adipocytes. We observed that the glucose uptake defect was principally due to a reduction in the maximal response, whereas in the case of lipolysis, we did not observe a defect at any dose including doses within the physiological range. Hence, this is consistent with selective insulin resistance such that certain processes, like lipolysis, do not exhibit any demonstrable defect even at insulin concentrations within the physiological range.
TABLE 1.
Summary of experimental findings
Increases (↑) or decreases (↓) are indicated with arrows, whereas no change is indicated with ∅. IR, insulin resistance; CI, chronic insulin; DEX, dexamethasone.
| Models | System | Treatment | Glucose uptake | Lipolysis inhibition | pAkt | pAS160 | Other Akt substrates | GLUT4 levels |
|---|---|---|---|---|---|---|---|---|
| IR models | Cell line | CI | ↓ | ∅ | ↓ | ↓ | ∅, ↓ | ↓ |
| DEX | ↓ | ↓ | ↓ | ↓ | ↓ | ∅ | ||
| TNF | ↓ | ∅ | ↓ | ↓ | ∅, ↓ | ∅ | ||
| Mouse | 28-day HFD | ↓ | ∅ | ↑ | ↓ | ∅ | ∅ | |
| ob/ob | ↓ | ∅ | ↓ | ↓ | ↓ | ↓ | ||
| siRNA model | Cell line | Akt1 + 2 siRNA | ↓ | ∅ | ↓ | ∅ | ∅ | ∅ |
Notably, in all insulin resistance models studied, we observed a reduction in glucose uptake using maximum doses of insulin (Table 1). Thus, although we cannot be certain that the same defect causes insulin resistance in each case, it is likely that this represents a convergent defect that is manifested as a change in the maximum response to insulin. Importantly, previous studies in adipocytes and skeletal muscle from humans with T2D or obesity have shown a defect in maximal insulin-stimulated glucose transport and utilization in vitro (56, 57). Moreover, hyperinsulinemic glucose clamp studies in individuals with T2D also reveal a defect in whole body glucose utilization even at maximal insulin concentrations (58). This provides important mechanistic insight into the nature of the defect. Early studies that focused on the insulin receptor as a potential defect in insulin resistance revealed that reduced insulin binding would lead to reduced sensitivity rather than to the observed reduced maximal response in insulin-resistant subjects. This led to the conclusion that the principal defect is downstream of the insulin receptor (52). Subsequent studies showed that other components in upper insulin signaling such as IRS1 and Akt also exhibit substantial spareness, such that maximal biological responses are achieved using a relatively small proportion of the total available pool of each of these molecules (42, 51). Consistent with this, it has been shown that there is considerable redundancy between different isoforms in upper insulin signaling in that deletion of one allele or one isoform can often be compensated for either via the alternate allele or an alternate isoform (59, 60). Beyond Akt, referred to here as lower insulin signaling, there appears to be no spareness, and in fact a 50% reduction in GLUT4 leads to a similar decrease in insulin-regulated glucose uptake, and this defect is observed at all insulin concentrations tested (61, 62). Intriguingly, GLUT4 levels are reduced by ∼50% in adipose tissue of individuals with T2D (63), and so it is conceivable that this is the major reason for selective insulin resistance under these conditions. However, GLUT4 levels are not reduced in skeletal muscle in T2D (64), and so alternate defects must contribute to insulin resistance in this tissue. Similarly in the present study, we observed reduced GLUT4 levels in some models of insulin resistance (CI, ob/ob) but not in others (Table 1). Moreover, we previously showed that insulin-stimulated GLUT4 translocation to the cell surface was impaired in several models of insulin resistance independently of changes in GLUT4 levels (24). Nevertheless, in view of the selectivity of insulin resistance in all cases combined with the fact that in all cases we observed a reduction in the maximum response to insulin, it is tempting to speculate that the defect might involve a component of the machinery that governs GLUT4 trafficking to the cell surface. Intriguingly, in adipocytes expressing Akt1/2 siRNA, we observed reduced insulin-stimulated glucose uptake in the absence of any change in AS160 phosphorylation at Thr-642 (Fig. 5), giving rise to the possibility that this process is likely regulated via multiple Akt substrates, and this warrants future investigation.
The present data highlight the exquisite complexity of the insulin signaling pathway. Overall, we were unable to discern a clear picture concerning the relationship between Akt phosphorylation, Akt activity, and the phosphorylation of a range of Akt substrates. In some insulin resistance models, we observed reduced Akt phosphorylation without any change in the phosphorylation of a range of substrates (e.g. PRAS40 and S6K in TNF or GSK3 in CI), whereas in others, such as the 28-day HFD mouse fat explants, we did not observe any reduction in Akt phosphorylation, yet AS160 phosphorylation was defective (Fig. 6). There are multiple possibilities to account for this complexity. First, we have previously reported considerable heterogeneity in insulin-regulated GLUT4 trafficking at the single cell level in 3T3-L1 adipocytes (65), and so it is conceivable that this complex pattern of signaling at the population level is underpinned by heterogeneity at the single cell level. Second, it is possible that upper components in the insulin signaling pathway operate in a switch-like mechanism so that changes in absolute levels of phosphorylated intermediates may not be an accurate reflection of their biological activity. Third, it is conceivable that changes in phosphorylation of components in upper signaling do not cause but rather are a consequence of insulin resistance. There is an emerging literature, particularly in the cancer field, that metabolism is a key determinant of biological outcome (66), and so this seems feasible. We and others (24, 61) have suggested that a defect in glucose uptake precedes signaling defects in insulin resistance. For example, it is possible that reduced AS160 phosphorylation in insulin resistance is a consequence of reduced trafficking of GLUT4 vesicles because AS160 is thought to be transported to the cell surface on GLUT4 vesicles where it is likely to be phosphorylated (6). However, siRNA-mediated knockdown of Akt1/2 resulted in reduced glucose uptake without any effect on AS160 phosphorylation, suggesting that other pathways that are specific to insulin resistance are responsible for reduced AS160 phosphorylation. Interestingly, as shown in Table 1, defective AS160 phosphorylation was the only consistent defect across all insulin resistance models aside from reduced glucose uptake, and because this was not observed in the Akt1/2 knockdown cells, this defect could not be attributed to reduced Akt phosphorylation, and further studies are required to pinpoint the mechanism of this defect.
These observations, together with several other studies reporting selective insulin resistance in other tissues or pathways (14–17, 49, 53, 67), have significant implications both mechanistically and therapeutically for our understanding of this defect. Physiologically, the observation that only certain actions of insulin are affected in most models of insulin resistance means that in the face of compensatory hyperinsulinemia, which is commonly observed in prediabetic humans or the ob/ob mice (Fig. 7), these unaffected pathways will likely be inappropriately overstimulated. In adipose tissue, for example, it is likely that lipolysis will be chronically repressed and de novo lipogenesis and protein synthesis will be increased. Teleologically, these adaptations are appropriate because in the face of excess nutrient supply, there is reduced demand for endogenous lipids from lipolysis, and there is increased demand for increased fat cell biomass to promote fat cell hypertrophy and proliferation. However, other processes such as the inactivation of FoxO1 might have more deleterious effects because FoxO1 normally regulates pathways that reduce a number of cellular stresses (68). Intriguingly, manipulations that overcome insulin resistance by increasing circulating insulin levels will fuel the obesogenic milieu, thus calling for a potential reconsideration of these strategies.
This work was supported by National Health and Medical Research Council Project Grants GNT1061122 and GNT1047067.
- T2D
- type 2 diabetes
- CI
- chronic insulin
- DEX
- dexamethasone
- HFD
- high fat diet
- IRAP
- insulin-regulated aminopeptidase.
REFERENCES
- 1. Alessi D. R., Andjelkovic M., Caudwell B., Cron P., Morrice N., Cohen P., Hemmings B. A. (1996) Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 15, 6541–6551 [PMC free article] [PubMed] [Google Scholar]
- 2. Alessi D. R., Downes C. P. (1998) The role of PI 3-kinase in insulin action. Biochim. Biophys. Acta 1436, 151–164 [DOI] [PubMed] [Google Scholar]
- 3. Whiteman E. L., Cho H., Birnbaum M. J. (2002) Role of Akt/protein kinase B in metabolism. Trends Endocrinol. Metab. 13, 444–451 [DOI] [PubMed] [Google Scholar]
- 4. Ahmad F., Lindh R., Tang Y., Weston M., Degerman E., Manganiello V. C. (2007) Insulin-induced formation of macromolecular complexes involved in activation of cyclic nucleotide phosphodiesterase 3B (PDE3B) and its interaction with PKB. Biochem. J. 404, 257–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kane S., Sano H., Liu S. C., Asara J. M., Lane W. S., Garner C. C., Lienhard G. E. (2002) A method to identify serine kinase substrates. Akt phosphorylates a novel adipocyte protein with a Rab GTPase-activating protein (GAP) domain. J. Biol. Chem. 277, 22115–22118 [DOI] [PubMed] [Google Scholar]
- 6. Tan S. X., Ng Y., Burchfield J. G., Ramm G., Lambright D. G., Stöckli J., James D. E. (2012) The Rab GTPase-activating protein TBC1D4/AS160 contains an atypical phosphotyrosine-binding domain that interacts with plasma membrane phospholipids to facilitate GLUT4 trafficking in adipocytes. Mol. Cell. Biol. 32, 4946–4959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Samuel V. T., Petersen K. F., Shulman G. I. (2010) Lipid-induced insulin resistance: unravelling the mechanism. Lancet 375, 2267–2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Petersen K. F., Shulman G. I. (2006) Etiology of insulin resistance. Am. J. Med. 119, S10–S16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rice K. M., Turnbow M. A., Garner C. W. (1993) Insulin stimulates the degradation of IRS-1 in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 190, 961–967 [DOI] [PubMed] [Google Scholar]
- 10. Zierath J. R., Houseknecht K. L., Gnudi L., Kahn B. B. (1997) High-fat feeding impairs insulin-stimulated GLUT4 recruitment via an early insulin-signaling defect. Diabetes 46, 215–223 [DOI] [PubMed] [Google Scholar]
- 11. Gao Z., Zuberi A., Quon M. J., Dong Z., Ye J. (2003) Aspirin inhibits serine phosphorylation of insulin receptor substrate 1 in tumor necrosis factor-treated cells through targeting multiple serine kinases. J. Biol. Chem. 278, 24944–24950 [DOI] [PubMed] [Google Scholar]
- 12. Teruel T., Hernandez R., Lorenzo M. (2001) Ceramide mediates insulin resistance by tumor necrosis factor-α in brown adipocytes by maintaining Akt in an inactive dephosphorylated state. Diabetes 50, 2563–2571 [DOI] [PubMed] [Google Scholar]
- 13. Chavez J. A., Knotts T. A., Wang L. P., Li G., Dobrowsky R. T., Florant G. L., Summers S. A. (2003) A role for ceramide, but not diacylglycerol, in the antagonism of insulin signal transduction by saturated fatty acids. J. Biol. Chem. 278, 10297–10303 [DOI] [PubMed] [Google Scholar]
- 14. Biddinger S. B., Hernandez-Ono A., Rask-Madsen C., Haas J. T., Alemán J. O., Suzuki R., Scapa E. F., Agarwal C., Carey M. C., Stephanopoulos G., Cohen D. E., King G. L., Ginsberg H. N., Kahn C. R. (2008) Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab. 7, 125–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Book C. B., Dunaif A. (1999) Selective insulin resistance in the polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 84, 3110–3116 [DOI] [PubMed] [Google Scholar]
- 16. Tonks K. T., Ng Y., Miller S., Coster A. C., Samocha-Bonet D., Iseli T. J., Xu A., Patrick E., Yang J. Y., Junutula J. R., Modrusan Z., Kolumam G., Stöckli J., Chisholm D. J., James D. E., Greenfield J. R. (2013) Impaired Akt phosphorylation in insulin-resistant human muscle is accompanied by selective and heterogeneous downstream defects. Diabetologia 56, 875–885 [DOI] [PubMed] [Google Scholar]
- 17. Gonzalez E., Flier E., Molle D., Accili D., McGraw T. E. (2011) Hyperinsulinemia leads to uncoupled insulin regulation of the GLUT4 glucose transporter and the FoxO1 transcription factor. Proc. Natl. Acad. Sci. U.S.A. 108, 10162–10167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abel E. D., Peroni O., Kim J. K., Kim Y. B., Boss O., Hadro E., Minnemann T., Shulman G. I., Kahn B. B. (2001) Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature 409, 729–733 [DOI] [PubMed] [Google Scholar]
- 19. Lo K. A., Labadorf A., Kennedy N. J., Han M. S., Yap Y. S., Matthews B., Xin X., Sun L., Davis R. J., Lodish H. F., Fraenkel E. (2013) Analysis of in vitro insulin-resistance models and their physiological relevance to in vivo diet-induced adipose insulin resistance. Cell Rep. 5, 259–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Houstis N., Rosen E. D., Lander E. S. (2006) Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 440, 944–948 [DOI] [PubMed] [Google Scholar]
- 21. Adams-Huet B., Devaraj S., Siegel D., Jialal I. (2014) Increased adipose tissue insulin resistance in metabolic syndrome: relationship to circulating adipokines. Metab. Syndr. Relat. Disord. 12, 503–507 [DOI] [PubMed] [Google Scholar]
- 22. Baron A. D., Brechtel G., Wallace P., Edelman S. V. (1988) Rates and tissue sites of non-insulin- and insulin-mediated glucose uptake in humans. Am. J. Physiol. 255, E769–E774 [DOI] [PubMed] [Google Scholar]
- 23. Shewan A. M., van Dam E. M., Martin S., Luen T. B., Hong W., Bryant N. J., James D. E. (2003) GLUT4 recycles via a trans-Golgi network (TGN) subdomain enriched in Syntaxins 6 and 16 but not TGN38: involvement of an acidic targeting motif. Mol. Biol. Cell 14, 973–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hoehn K. L., Hohnen-Behrens C., Cederberg A., Wu L. E., Turner N., Yuasa T., Ebina Y., James D. E. (2008) IRS1-independent defects define major nodes of insulin resistance. Cell Metab. 7, 421–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jackson A. L., Linsley P. S. (2004) Noise amidst the silence: off-target effects of siRNAs? Trends Genet. 20, 521–524 [DOI] [PubMed] [Google Scholar]
- 26. Marshall S. (1989) Kinetics of insulin action on protein synthesis in isolated adipocytes. Ability of glucose to selectively desensitize the glucose transport system without altering insulin stimulation of protein synthesis. J. Biol. Chem. 264, 2029–2036 [PubMed] [Google Scholar]
- 27. Marshall S., Monzon R. (1989) Amino acid regulation of insulin action in isolated adipocytes. Selective ability of amino acids to enhance both insulin sensitivity and maximal insulin responsiveness of the protein synthesis system. J. Biol. Chem. 264, 2037–2042 [PubMed] [Google Scholar]
- 28. Choi S. M., Tucker D. F., Gross D. N., Easton R. M., DiPilato L. M., Dean A. S., Monks B. R., Birnbaum M. J. (2010) Insulin regulates adipocyte lipolysis via an Akt-independent signaling pathway. Mol. Cell. Biol. 30, 5009–5020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fain J. N., Kovacev V. P., Scow R. O. (1966) Antilipolytic effect of insulin in isolated fat cells of the rat. Endocrinology 78, 773–778 [DOI] [PubMed] [Google Scholar]
- 30. Tan S., Ng Y., James D. E. (2011) Next generation Akt inhibitors provide greater specificity-effects on glucose metabolism in adipocytes. Biochem. J. 435, 539–544 [DOI] [PubMed] [Google Scholar]
- 31. Ng Y., Ramm G., James D. E. (2010) Dissecting the mechanism of insulin resistance using a novel heterodimerization strategy to activate Akt. J. Biol. Chem. 285, 5232–5239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang D., Sul H. S. (1998) Insulin stimulation of the fatty acid synthase promoter is mediated by the phosphatidylinositol 3-kinase pathway. Involvement of protein kinase B/Akt. J. Biol. Chem. 273, 25420–25426 [DOI] [PubMed] [Google Scholar]
- 33. Le Lay S., Lefrère I., Trautwein C., Dugail I., Krief S. (2002) Insulin and sterol-regulatory element-binding protein-1c (SREBP-1C) regulation of gene expression in 3T3-L1 adipocytes. Identification of CCAAT/enhancer-binding protein beta as an SREBP-1C target. J. Biol. Chem. 277, 35625–35634 [DOI] [PubMed] [Google Scholar]
- 34. Ikemoto S., Thompson K. S., Takahashi M., Itakura H., Lane M. D., Ezaki O. (1995) High fat diet-induced hyperglycemia: prevention by low level expression of a glucose transporter (GLUT4) minigene in transgenic mice. Proc. Natl. Acad. Sci. U.S.A. 92, 3096–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gnudi L., Tozzo E., Shepherd P. R., Bliss J. L., Kahn B. B. (1995) High level overexpression of glucose transporter-4 driven by an adipose-specific promoter is maintained in transgenic mice on a high fat diet, but does not prevent impaired glucose tolerance. Endocrinology 136, 995–1002 [DOI] [PubMed] [Google Scholar]
- 36. Park S. Y., Cho Y. R., Kim H. J., Higashimori T., Danton C., Lee M. K., Dey A., Rothermel B., Kim Y. B., Kalinowski A., Russell K. S., Kim J. K. (2005) Unraveling the temporal pattern of diet-induced insulin resistance in individual organs and cardiac dysfunction in C57BL/6 mice. Diabetes 54, 3530–3540 [DOI] [PubMed] [Google Scholar]
- 37. Stephens J. M., Lee J., Pilch P. F. (1997) Tumor necrosis factor-α-induced insulin resistance in 3T3-L1 adipocytes is accompanied by a loss of insulin receptor substrate-1 and GLUT4 expression without a loss of insulin receptor-mediated signal transduction. J. Biol. Chem. 272, 971–976 [DOI] [PubMed] [Google Scholar]
- 38. Carvalho E., Jansson P. A., Nagaev I., Wenthzel A. M., Smith U. (2001) Insulin resistance with low cellular IRS-1 expression is also associated with low GLUT4 expression and impaired insulin-stimulated glucose transport. FASEB J. 15, 1101–1103 [PubMed] [Google Scholar]
- 39. Yu Z., Shao W., Chiang Y., Foltz W., Zhang Z., Ling W., Fantus I. G., Jin T. (2011) Oltipraz upregulates the nuclear factor (erythroid-derived 2)-like 2 [corrected](NRF2) antioxidant system and prevents insulin resistance and obesity induced by a high-fat diet in C57BL/6J mice. Diabetologia 54, 922–934 [DOI] [PubMed] [Google Scholar]
- 40. Sabio G., Das M., Mora A., Zhang Z., Jun J. Y., Ko H. J., Barrett T., Kim J. K., Davis R. J. (2008) A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science 322, 1539–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sabio G., Kennedy N. J., Cavanagh-Kyros J., Jung D. Y., Ko H. J., Ong H., Barrett T., Kim J. K., Davis R. J. (2010) Role of muscle c-Jun NH2-terminal kinase 1 in obesity-induced insulin resistance. Mol. Cell. Biol. 30, 106–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tan S. X., Ng Y., Meoli C. C., Kumar A., Khoo P. S., Fazakerley D. J., Junutula J. R., Vali S., James D. E., Stöckli J. (2012) Amplification and demultiplexing in insulin-regulated Akt protein kinase pathway in adipocytes. J. Biol. Chem. 287, 6128–6138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hill M. M., Clark S. F., Tucker D. F., Birnbaum M. J., James D. E., Macaulay S. L. (1999) A role for protein kinase Bβ/Akt2 in insulin-stimulated GLUT4 translocation in adipocytes. Mol. Cell. Biol. 19, 7771–7781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lin K., Lin J., Wu W. I., Ballard J., Lee B. B., Gloor S. L., Vigers G. P., Morales T. H., Friedman L. S., Skelton N., Brandhuber B. J. (2012) An ATP-site on-off switch that restricts phosphatase accessibility of Akt. Sci. Signal. 5, ra37. [DOI] [PubMed] [Google Scholar]
- 45. Turner N., Kowalski G. M., Leslie S. J., Risis S., Yang C., Lee-Young R. S., Babb J. R., Meikle P. J., Lancaster G. I., Henstridge D. C., White P. J., Kraegen E. W., Marette A., Cooney G. J., Febbraio M. A., Bruce C. R. (2013) Distinct patterns of tissue-specific lipid accumulation during the induction of insulin resistance in mice by high-fat feeding. Diabetologia 56, 1638–1648 [DOI] [PubMed] [Google Scholar]
- 46. Mazumder P. K., O'Neill B. T., Roberts M. W., Buchanan J., Yun U. J., Cooksey R. C., Boudina S., Abel E. D. (2004) Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes 53, 2366–2374 [DOI] [PubMed] [Google Scholar]
- 47. Grundleger M. L., Godbole V. Y., Thenen S. W. (1980) Age-dependent development of insulin resistance of soleus muscle in genetically obese (ob/ob) mice. Am. J. Physiol. 239, E363–E371 [DOI] [PubMed] [Google Scholar]
- 48. Godbole V. Y., Grundleger M. L., Thenen S. W. (1980) Early development of lipogenesis in genetically obese (ob/ob) mice. Am. J. Physiol. 239, E265–E268 [DOI] [PubMed] [Google Scholar]
- 49. Li S., Brown M. S., Goldstein J. L. (2010) Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc. Natl. Acad. Sci. U.S.A. 107, 3441–3446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yore M. M., Syed I., Moraes-Vieira P. M., Zhang T., Herman M. A., Homan E. A., Patel R. T., Lee J., Chen S., Peroni O. D., Dhaneshwar A. S., Hammarstedt A., Smith U., McGraw T. E., Saghatelian A., Kahn B. B. (2014) Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell 159, 318–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Clark S. F., Molero J. C., James D. E. (2000) Release of insulin receptor substrate proteins from an intracellular complex coincides with the development of insulin resistance. J. Biol. Chem. 275, 3819–3826 [DOI] [PubMed] [Google Scholar]
- 52. Kahn C. R. (1978) Insulin resistance, insulin insensitivity, and insulin unresponsiveness: a necessary distinction. Metabolism 27, 1893–1902 [DOI] [PubMed] [Google Scholar]
- 53. Glass A. R., Bongiovanni R., Smith C. E., Boehm T. M. (1981) Normal valine disposal in obese subjects with impaired glucose disposal: evidence for selective insulin resistance. Metabolism 30, 578–582 [DOI] [PubMed] [Google Scholar]
- 54. Xu C., He J., Jiang H., Zu L., Zhai W., Pu S., Xu G. (2009) Direct effect of glucocorticoids on lipolysis in adipocytes. Mol. Endocrinol. 23, 1161–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ng Y., Ramm G., Burchfield J. G., Coster A. C., Stöckli J., James D. E. (2010) Cluster analysis of insulin action in adipocytes reveals a key role for Akt at the plasma membrane. J. Biol. Chem. 285, 2245–2257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kashiwagi A., Verso M. A., Andrews J., Vasquez B., Reaven G., Foley J. E. (1983) In vitro insulin resistance of human adipocytes isolated from subjects with noninsulin-dependent diabetes mellitus. J. Clin. Invest. 72, 1246–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dohm G. L., Tapscott E. B., Pories W. J., Dabbs D. J., Flickinger E. G., Meelheim D., Fushiki T., Atkinson S. M., Elton C. W., Caro J. F. (1988) An in vitro human muscle preparation suitable for metabolic studies: decreased insulin stimulation of glucose transport in muscle from morbidly obese and diabetic subjects. J. Clin. Invest. 82, 486–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Revers R. R., Fink R., Griffin J., Olefsky J. M., Kolterman O. G. (1984) Influence of hyperglycemia on insulin's in vivo effects in type II diabetes. J. Clin. Invest. 73, 664–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Taniguchi C. M., Kondo T., Sajan M., Luo J., Bronson R., Asano T., Farese R., Cantley L. C., Kahn C. R. (2006) Divergent regulation of hepatic glucose and lipid metabolism by phosphoinositide 3-kinase via Akt and PKClambda/zeta. Cell Metab. 3, 343–353 [DOI] [PubMed] [Google Scholar]
- 60. Schubert M., Brazil D. P., Burks D. J., Kushner J. A., Ye J., Flint C. L., Farhang-Fallah J., Dikkes P., Warot X. M., Rio C., Corfas G., White M. F. (2003) Insulin receptor substrate-2 deficiency impairs brain growth and promotes tau phosphorylation. J. Neurosci. 23, 7084–7092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Li J., Houseknecht K. L., Stenbit A. E., Katz E. B., Charron M. J. (2000) Reduced glucose uptake precedes insulin signaling defects in adipocytes from heterozygous GLUT4 knockout mice. FASEB J. 14, 1117–1125 [DOI] [PubMed] [Google Scholar]
- 62. Cleasby M. E., Davey J. R., Reinten T. A., Graham M. W., James D. E., Kraegen E. W., Cooney G. J. (2005) Acute bidirectional manipulation of muscle glucose uptake by in vivo electrotransfer of constructs targeting glucose transporter genes. Diabetes 54, 2702–2711 [DOI] [PubMed] [Google Scholar]
- 63. Garvey W. T., Maianu L., Huecksteadt T. P., Birnbaum M. J., Molina J. M., Ciaraldi T. P. (1991) Pretranslational suppression of a glucose transporter protein causes insulin resistance in adipocytes from patients with non-insulin-dependent diabetes mellitus and obesity. J. Clin. Invest. 87, 1072–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Garvey W. T., Maianu L., Hancock J. A., Golichowski A. M., Baron A. (1992) Gene expression of GLUT4 in skeletal muscle from insulin-resistant patients with obesity, IGT, GDM, and NIDDM. Diabetes 41, 465–475 [DOI] [PubMed] [Google Scholar]
- 65. Burchfield J. G., Lu J., Fazakerley D. J., Tan S. X., Ng Y., Mele K., Buckley M. J., Han W., Hughes W. E., James D. E. (2013) Novel systems for dynamically assessing insulin action in live cells reveals heterogeneity in the insulin response. Traffic 14, 259–273 [DOI] [PubMed] [Google Scholar]
- 66. Wellen K. E., Thompson C. B. (2012) A two-way street: reciprocal regulation of metabolism and signalling. Nat. Rev. Mol. Cell Biol. 13, 270–276 [DOI] [PubMed] [Google Scholar]
- 67. Jiang Z. Y., Lin Y. W., Clemont A., Feener E. P., Hein K. D., Igarashi M., Yamauchi T., White M. F., King G. L. (1999) Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. J. Clin. Invest. 104, 447–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. van der Vos K. E., Coffer P. J. (2011) The extending network of FOXO transcriptional target genes. Antioxid. Redox Signal. 14, 579–592 [DOI] [PubMed] [Google Scholar]