Background: Peroxiredoxin II (PrxII) functions as a negative regulator of cellular receptor signaling by efficiently eliminating H2O2 produced upon stimulation of receptors.
Results: PrxII deficiency promotes GPVI-mediated platelet activation through oxidative inactivation of SH2 domain-containing tyrosine phosphatase 2.
Conclusion: PrxII functions as a protective antioxidant against collagen-stimulated platelet activation and thrombosis.
Significance: PrxII is a potential target in thrombovascular diseases.
Keywords: Collagen, Hydrogen Peroxide, Peroxiredoxin, Platelet, Protein-tyrosine Phosphatase (Tyrosine Phosphatase)
Abstract
Collagen-induced platelet signaling is mediated by binding to the primary receptor glycoprotein VI (GPVI). Reactive oxygen species produced in response to collagen have been found to be responsible for the propagation of GPVI signaling pathways in platelets. Therefore, it has been suggested that antioxidant enzymes could down-regulate GPVI-stimulated platelet activation. Although the antioxidant enzyme peroxiredoxin II (PrxII) has emerged as having a role in negatively regulating signaling through various receptors by eliminating H2O2 generated upon receptor stimulation, the function of PrxII in collagen-stimulated platelets is not known. We tested the hypothesis that PrxII negatively regulates collagen-stimulated platelet activation. We analyzed PrxII-deficient murine platelets. PrxII deficiency enhanced GPVI-mediated platelet activation through the defective elimination of H2O2 and the impaired protection of SH2 domain-containing tyrosine phosphatase 2 (SHP-2) against oxidative inactivation, which resulted in increased tyrosine phosphorylation of key components for the GPVI signaling cascade, including Syk, Btk, and phospholipase Cγ2. Interestingly, PrxII-mediated antioxidative protection of SHP-2 appeared to occur in the lipid rafts. PrxII-deficient platelets exhibited increased adhesion and aggregation upon collagen stimulation. Furthermore, in vivo experiments demonstrated that PrxII deficiency facilitated platelet-dependent thrombus formation in injured carotid arteries. This study reveals that PrxII functions as a protective antioxidant enzyme against collagen-stimulated platelet activation and platelet-dependent thrombosis.
Introduction
At sites of vascular injury, the exposure of subendothelial collagen triggers the adhesion and aggregation of platelets, followed by thrombus formation. This process is crucial for normal hemostasis, but the excessive activation of platelets in diseased vessels leads to thrombotic vascular conditions such as myocardial infarction, stroke, and atherothrombosis (1–3). Collagen-induced platelet signaling is mediated by binding to the primary receptor glycoprotein VI (GPVI)2 (4). The GPVI signaling pathway is initiated by Src family kinase-mediated tyrosine phosphorylation on the Fc receptor γ chain (5), followed by the recruitment and activation of the tyrosine kinase Syk and the tyrosine phosphorylation of the linker for the activation of T cells (LAT) (6), which, in turn, leads to the assembly of a signaling complex containing Vav1, Bruton's tyrosine kinase (Btk), and phospholipase C γ2 (PLCγ2). This assembly culminates in tyrosine phosphorylation-based activation of PLCγ2, which promotes 1,2-diacylglycerol and inositol-1,4,5-trisphosphate formation, protein kinase C activation, and calcium mobilization. Such platelet activation results in granule release and inside-out activation of integrin-αIIbβ3 for stable adhesion and aggregation (7). Therefore, protein-tyrosine phosphorylation has been thought to be a central event in the regulation of GPVI signaling in platelets.
Although collagen stimulation profoundly increased reactive oxygen species (ROS) production in platelets, much lower intracellular ROS levels were found in platelets in response to G protein-coupled receptor agonists such as thrombin, ADP, or a thromboxane receptor agonist, U46619 (8–12). In GPVI-stimulated platelets, the production of ROS is required for the propagation of GPVI signaling pathways, including PLCγ2 activation, cytosolic calcium elevation, integrin-αIIbβ3 activation, granule release, and the resultant formation of the platelet aggregate and thrombus (8–11). Evidence indicates that NADPH oxidase (Nox) is largely responsible for collagen receptor-dependent ROS production (9, 10, 13). Nox generates superoxide from O2 via electron transfer from NADPH, and the superoxide then undergoes dismutation to H2O2. Among the best characterized H2O2 targets are protein-tyrosine phosphatases (PTPs). H2O2 specifically oxidizes the catalytic cysteine residue of PTPs and, thereby, inhibits their activity (14–16). We found previously that collagen-induced ROS production triggers the oxidative inactivation of SH2 domain-containing tyrosine phosphatase 2 (SHP-2) and then contributes to the promotion of protein-tyrosine phosphorylation-mediated signal transduction in platelets (12).
Peroxiredoxin II (PrxII), a member of the 2-Cys Prx family, is a cellular peroxidase that effectively eliminates H2O2 produced in response to the stimulation of cells with platelet-derived growth factor, epidermal growth factor, or T cell ligands (17–19). When stimulated with platelet-derived growth factor, cells derived from PrxII-deficient mice have been found to exhibit enhanced tyrosine phosphorylation of platelet-derived growth factor receptor, demonstrating that one function of PrxII is to protect the lipid raft-associated PTPs from oxidative inactivation by removing H2O2 (18). PrxII overexpression has been shown to inhibit the T cell receptor-induced oxidation of SHP-2 and, subsequently, to suppress the tyrosine phosphorylation of key signaling molecules involved in integrin activation and cell adhesion (19). Given that GPVI-stimulated platelet activation is inhibited by antioxidant treatment and that platelet-dependent arterial thrombosis is enhanced in knockout mice lacking antioxidant enzymes, it has been suggested that cellular antioxidants function as negative regulators of GPVI-mediated signaling in platelets (8–12, 20). However, the role of endogenous PrxII in GPVI-stimulated platelets has not been studied.
To explore the involvement of PrxII in GPVI-stimulated platelet activation and platelet-dependent thrombosis, we used PrxII-deficient platelets and mice. We found that PrxII deficiency significantly enhanced GPVI-stimulated platelet activation through the defective elimination of H2O2 and the impaired protection of SHP-2 from oxidative inactivation, which led to increased tyrosine phosphorylation of key components of the GPVI signaling cascade. Interestingly, PrxII-mediated protection of SHP-2 appeared to occur in lipid rafts. PrxII-deficient platelets showed markedly increased adhesion and aggregation activity on collagen in vitro. Finally, we also validated the antithrombotic activity of PrxII in vivo using an arterial injury model.
EXPERIMENTAL PROCEDURES
Antibodies and Reagents
5-(and-6)-carboxy-2′,7′-dichlorofluorescein (CM-H2DCFDA), 3,3′-dihexyloxacarbocyanine iodide, and Fluo-3 acetoxymethyl ester (Fluo-3 AM) were from Molecular Probes (Eugene, OR). Monoclonal antibodies to SHP-2 and anti-FITC-conjugated anti-P-selectin were from BD Biosciences. JON/A-PE was from Emfret Analytics (Würzburg, Germany). Polyclonal antibody to phospho-Vav1 (Tyr174) was from Sigma-Aldrich. Polyclonal antibodies to SHP-2, Btk, and Syk and monoclonal antibodies to α-tubulin and LAT were from Santa Cruz Biotechnology (Santa Cruz, CA). Polyclonal antibody to phospho-Btk (Tyr551) was from BIOSOURCE (Camarillo, CA). Polyclonal antibody to phospho-Syk (Tyr519/520) was from Cell Signaling Technology (Danvers, MA). Monoclonal antibodies to phosphotyrosine antibody (4G10) and Vav1 and polyclonal antibodies to LAT were from Upstate Biotechnology Inc. (Lake Placid, NY). Polyclonal antibodies to PLCγ2, phospho-PLCγ2 (Tyr753) and phospho-PLCγ2 (Tyr759) were a gift from Dr. S. G. Rhee (Yonsei University, Korea). HRP-conjugated streptavidin was from Pierce. Alexa Fluor-conjugated anti-mouse and anti-rabbit antibodies were from Invitrogen. Convulxin was from Alexis Biochemicals (Lausen, Switzerland).
Experimental Animals
PrxII-deficient (PrxII−/−) mice were backcrossed more than 10 times with C57BL/6J mice (21). Wild-type and PrxII−/− C57BL/6J −/−mice were housed under specific pathogen-free conditions at Ewha Womans University. Animal handling and experiments were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC No. 2010-6-4). The mice used in this study were 6–8 weeks old.
Mouse Platelet Preparation
Mouse blood was collected from the abdominal aorta with a syringe containing 1 volume of acid/citrate/dextrose for 10 volumes of blood under isoflurane anesthesia. The blood was diluted with an equal volume of washing buffer containing 2 mm EDTA, 15% of acid/citrate/dextrose solution (0.73% citric acid, 2.2% trisodium citrate, and 2.45% dextrose), 1 μm prostaglandin E1, and Tyrode's buffer (10 mm HEPES (pH 7.4), 129 mm NaCl, 0.8 mm KH2PO4, 8.9 mm NaHCO3, 2.8 mm KCl, 0.8 mm MgCl2 and 5.6 mm glucose). Platelet-rich plasma, which was obtained by centrifugation for 15 min at 50 × g, was centrifuged further for 10 min at 300 × g to concentrate the platelets. The platelet pellet was then suspended in washing buffer and spun once more. Platelets were finally resuspended at a concentration of 5 × 108 platelets/ml in Tyrode's buffer.
Aggregation Study
Washed platelets in Tyrode's buffer containing 0.35% bovine serum albumin were preincubated with 1 mmol/liter CaCl2 for 2 min before adding collagen (Chrono-Log). Platelet aggregation was measured in a siliconized glass cuvette under continuous stirring at 1000 rpm at 37 °C using a four-channel aggregometer (Chrono-Log). Aggregation was assessed turbidometrically and expressed as percent change in light transmission, which, for buffer control, is defined as 100%.
Determination of Intracellular Reactive Oxygen Species and Cytosolic Calcium
Washed platelets suspended in PBS were incubated with 5 μmol/liter CM-H2DCFDA or 1 μmol/liter Fluo-3 AM for 15 min at 37 °C in the dark. Then the excess dye was removed, and the platelets were resuspended in Tyrode's buffer containing 1 mmol/liter CaCl2. After the dye-loaded platelets in fluoro cuvettes were stimulated with 10 μg/ml collagen under continuous stirring at 1000 rpm at 37 °C, the intracellular ROS level at 495 nm excitation and 525 nm emission and the intracellular calcium level at 488 nm excitation and 525 nm emission were measured using a spectrofluorophotometer (Shimadzu).
Immunoblotting
After stimulation, the platelets were lysed in cell extraction buffer (20 mmol/liter HEPES (pH 7.0), 150 mmol/liter NaCl, 1% Triton X-100, 10% glycerol, 1 mmol/liter EDTA, 2 mmol/liter EGTA, 20 mmol/liter β-glycerophosphate, 1 mmol/liter Na3VO4, 1 μg/ml leupeptin, 1 μg/ml aprotinin, and 1 mmol/liter 4-(2-aminoethyl) benzene sulfonyl fluoride hydrochloride (AEBSF)). The cell debris was removed by centrifugation at 12,500 × g for 10 min. Equal amounts of cell lysates were subjected to Western blot analysis using specific antibodies as indicated.
Biotinylation to Detect Oxidized Thiols in SHP-2
As described previously (19), platelets were lysed using the oxygen-free buffer in an anoxic chamber (<1% oxygen). Cell debris was removed by centrifugation. The lysates were incubated with 1 mm EZ-Link PEG2-iodoacetyl-biotin (Pierce) for 3 h under continuous shaking at 800 rpm at room temperature in the dark. SHP-2 was immunoprecipitated with a specific antibody and protein G-Sepharose 4B for 2 h at 4 °C. Biotin incorporation into the immunoprecipitated proteins was detected by immunoblot analysis with horseradish peroxidase-conjugated streptavidin (Pierce).
Lipid Raft Isolation
After stimulation, the platelets were lysed in lipid raft buffer (20 mmol/liter Tris-HCl (pH 8.0), 0.025% Triton X-100, 150 mmol/liter NaCl, 5 mmol/liter NaF, 1 mmol/liter Na3VO4, 1 μg/ml leupeptin, 1 μg/ml aprotinin, and 1 mmol/L AEBSF) (22). Lysates were vortexed and incubated on ice for 30 min. Samples were mixed with equal volumes of 80% w/v sucrose to give 40% w/v final concentration. This was transferred to an ultracentrifuge tube (14 × 89 mm) in which 5 ml of 36% w/v sucrose was layered on top, followed by another 5-ml layer of 5% w/v sucrose. Each sucrose solution also contained 0.025% w/v Triton X-100. Tubes were ultracentrifuged in an Optima LE-80K (Beckman Coulter, Fullerton, CA) at 200,000 × g for 18 h at 4 °C. Sequential 1-ml fractions were collected from the top of each sample and analyzed by immunoblotting.
SHP-2 Activity Assay
Platelets were subjected to the procedure as detailed in Choi et al. (18), with a slight modification. Stimulated platelets were suspended in oxygen-free lysis buffer (50 mm HEPES (pH 6.5), 0.025% Triton X-100, 2 mm EDTA, 5 mm EGTA, 1 μg/ml leupeptin, 1 μg/ml aprotinin, and 1 mm AEBSF) containing 10 mm iodoacteamide and 10 mm N-ethylmaleimide in an anoxic chamber (<1% oxygen). Cells were vortexed, left on ice for 30 min, and then incubated for a further 30 min to achieve complete alkylation of free thiols. Intact platelets were removed by centrifugation at 1500 × g for 10 min at 4 °C. The platelet lysates were then centrifuged at 100,000 × g for 1 h at 4 °C to separate the detergent-soluble and -resistant fractions. The detergent-resistant pellets were dissolved in lysis buffer (50 mm HEPES (pH 6.5), 1% Triton X-100, 150 mm NaCl, 2 mm EDTA, 5 mm EGTA, 1 μg/ml leupeptin, 1 μg/ml aprotinin, and 1 mm AEBSF). The protein concentrations were measured using Bradford reagent. SHP-2 was immunoprecipitated using antibody against SHP-2. To reduce the reversibly oxidized SHP-2, the immunoprecipitated proteins were incubated in 5 mm dithiothreitol-containing reaction buffer for 30 min at room temperature. The protein concentrations were measured using Bradford reagent (Bio-Rad). SHP-2 activity was measured using tyrosine phosphopeptide (RRLIEDAEpYAARG, where pY represents phosphotyrosine) as substrate, according to the protocol of the manufacturer (PTP assay kit 1, EMD Millipore Co., catalog no. 17-125). A serially diluted standard of 1 mm KH2PO4 was used to generate a standard curve. PTP activity was expressed as picomoles of inorganic phosphate released per minute per milligram protein used for the assay.
Flow Cytometry
After being stimulated with convulxin (25 or 250 ng/ml), the platelets were incubated with FITC-conjugated anti-P-selectin (0.5 mg/ml) or JON/A-PE (0.5 mg/ml) for 5 min in the dark. The reaction was stopped by adding ice-cold phosphate-buffered saline. A FACSCalibur flow cytometer (BD Biosciences) was used for all analyses with a minimum of 5 × 104 cells/sample for each measurement. The surface expression of P-selectin and active integrin-αIIbβ3 on the platelets was measured at 530 (FL1) and 585 nm (FL2), respectively. The relative change in fluorescence was analyzed using WinMDI software.
Assessment of Arterial Thrombosis after Ferric Chloride Exposure
Thrombosis was induced in mice using a carotid artery injury model (23). After an intraperitoneal injection containing 1.0 ml/kg Zoletil (Virbac Animal Health Co.) and 0.7 ml/kg Rompun (Bayer Korea Co.) for anesthesia, the left common carotid artery was exposed. Vascular injury was induced by applying a filter paper (1 × 1 mm) that had been saturated with 20% FeCl3 proximal to the carotid artery. The blood volume changes in the carotid artery downstream of the injury site were measured using the photoplethysmography method using a minimized OxiPulse probe (Hurev Inc., Korea) in transmission mode, as described previously (12). The time to thrombotic occlusion was defined as the time required for >90% loss of the initial blood volume.
Ex Vivo Flow Chamber Assay
Washed platelets (3 × 108/ml) were incubated with 1 μmol/liter of the fluorescent dye 3,3′-dihexyloxacarbocyanine iodide (Sigma-Aldrich) for 5 min at 37 °C as described previously (24). A collagen-coated coverslip (Neuvitro) was mounted on a custom-made flow chamber (Chamlide CF, LCI Korea). The fluorescently labeled platelets were then perfused over a matrix of collagen at 1000 s−1 using a syringe pump (Harvard Apparatus Inc., Holliston, MA) (25). Non-adherent platelets in the chamber were washed with PBS. Adherent platelets were fixed with cold 4% paraformaldehyde for 15 min and then washed with PBS. Thrombus formation was visualized with a ×40 long working distance objective for confocal microscopy (Nikon A1R). Flow chamber surface coverage by the thrombi was calculated using ImageJ software.
Statistical Analysis
All of the immunoblot experiments were repeated at least three times. The data in the graphs were analyzed using Student's t test to determine statistical significance (p value). p < 0.05 was considered statistically significant.
RESULTS
PrxII Deficiency Potentiates the Collagen-induced Increase in ROS Levels and the Aggregation of Platelets
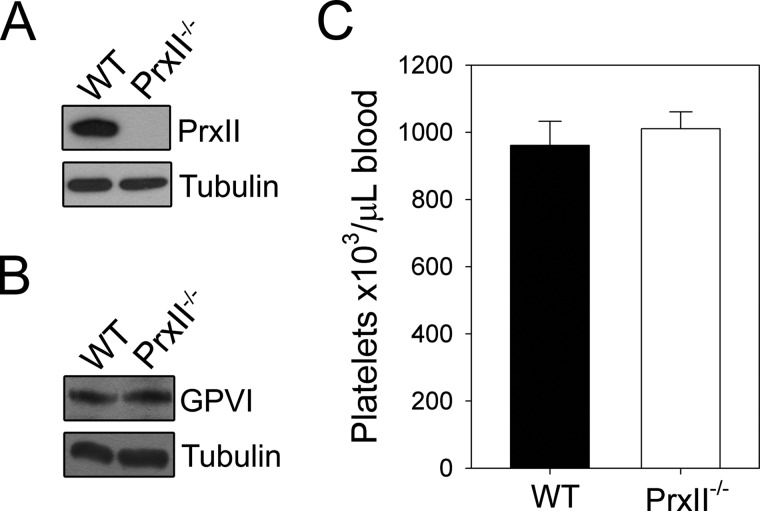
To study the role of PrxII in collagen-stimulated platelet function, we used platelets from PrxII−/− mice. Western blot analysis confirmed the absence of PrxII in PrxII−/− platelets (Fig. 1A). The platelet count and expression level of the collagen receptor GPVI were unaltered compared with the wild-type control (Fig. 1, B and C).
FIGURE 1.
Deletion of PrxII in platelets. A and B, washed platelets from WT and PrxII−/− mice were lysed and immunoblotted with antibodies specific for PrxII (A) or GPVI (B). Expression of tubulin was used as a loading control. The immunoblots shown are representative of three independent experiments. C, peripheral platelet counts from WT and PrxII−/− mice measured with a blood cell counter are depicted. The results are the means ± S.D. of 5 mice/group.
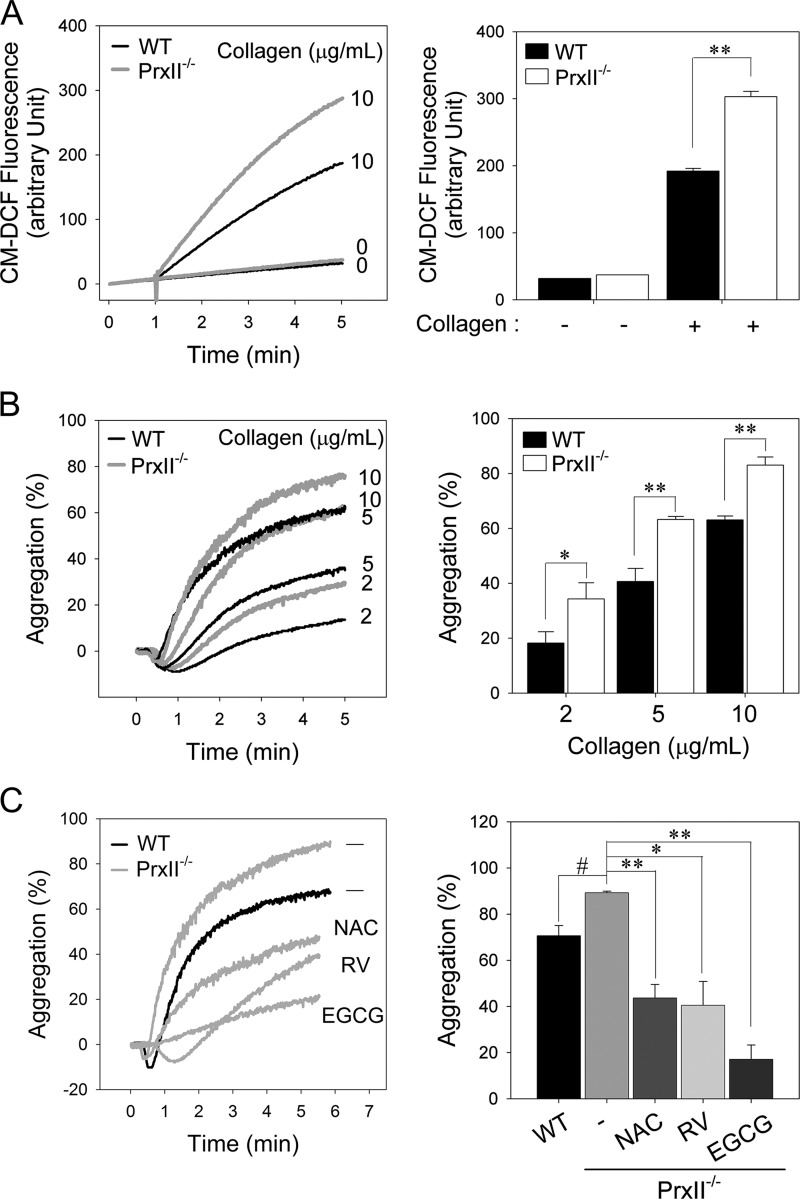
PrxII has a high affinity for H2O2 and efficiently removes the intracellular H2O2 produced upon cell surface receptor stimulation (17–19). Although the intraplatelet ROS levels were markedly increased after collagen stimulation, much lower intracellular ROS levels have been shown in platelets stimulated by other agonists such as thrombin, ADP, or U46619 (8–12). To examine whether PrxII eliminates the H2O2 generated in response to collagen stimulation, we measured the intracellular ROS level in collagen-stimulated platelets using CM-H2DCFDA. Upon collagen stimulation, PrxII−/− platelets exhibited ∼1.5-fold higher ROS production at 5 min than wild-type platelets (Fig. 2A), indicating that, in platelets, PrxII contributes to the elimination of H2O2 generated upon collagen stimulation.
FIGURE 2.
Enhanced intracellular ROS levels and aggregation in PrxII-deficient platelets in response to collagen stimulation. A, CM-H2DCFDA-loaded WT and PrxII−/− platelets were stimulated with collagen (10 μg/ml), and 5-(and-6)-chloromethyl-2′,7′-dichlorofluorescein (CM-DCF) fluorescence was monitored. Representative fluorescence tracings are shown. The quantitative data are mean ± S.D. (n = 4; **, p < 0.01). B, wild-type and PrxII−/− platelets were stimulated with the indicated concentrations of collagen, and platelet aggregation was measured. Representative aggregation peaks are shown. The quantitative data are mean ± S.D. (n = 3; *, p < 0.05; **, p < 0.01). C, washed PrxII−/− platelets were preincubated for 5 min in the presence of dimethyl sulfoxide (−), N-acetyl-l-cysteine (NAC, 5 mm), resveratrol (RV; 0.4 mm), or epigallocatechin gallate (EGCG, 0.1 mm) as indicated and were then stimulated with collagen (10 μg/ml) for 5 min. Platelet aggregation was measured with an aggregometer. The quantitative data are means ± S.D. (n = 3; * and #, p < 0.05, **, p < 0.01).
To investigate the consequences of PrxII deficiency on platelet function, we measured aggregation in response to graded concentrations of collagen (Fig. 2B). The concentrations of collagen that elicited low, intermediate, and high levels of responsiveness from the wild-type platelets evoked a significantly greater response from the PrxII−/− platelets. Previous studies have demonstrated the antiplatelet effects of antioxidants such as N-acetyl-l-cysteine (11, 26), epigallocatechin gallate, and resveratrol (27, 28). To confirm that the increased intracellular ROS in the PrxII−/− platelets promotes platelet activation, we pretreated the platelets with antioxidants before collagen stimulation (Fig. 2C). As expected, the higher aggregation following collagen stimulation in the PrxII−/− platelets was abrogated by antioxidants, indicating that collagen-induced aggregation is dependent on the intracellular ROS level. These observations suggest that PrxII functions as a preventive antioxidant enzyme against collagen-induced platelet aggregation by eliminating ROS.
PrxII Deficiency Up-regulates Protein-tyrosine Phosphorylation-based Signal Transduction in Collagen-stimulated Platelets
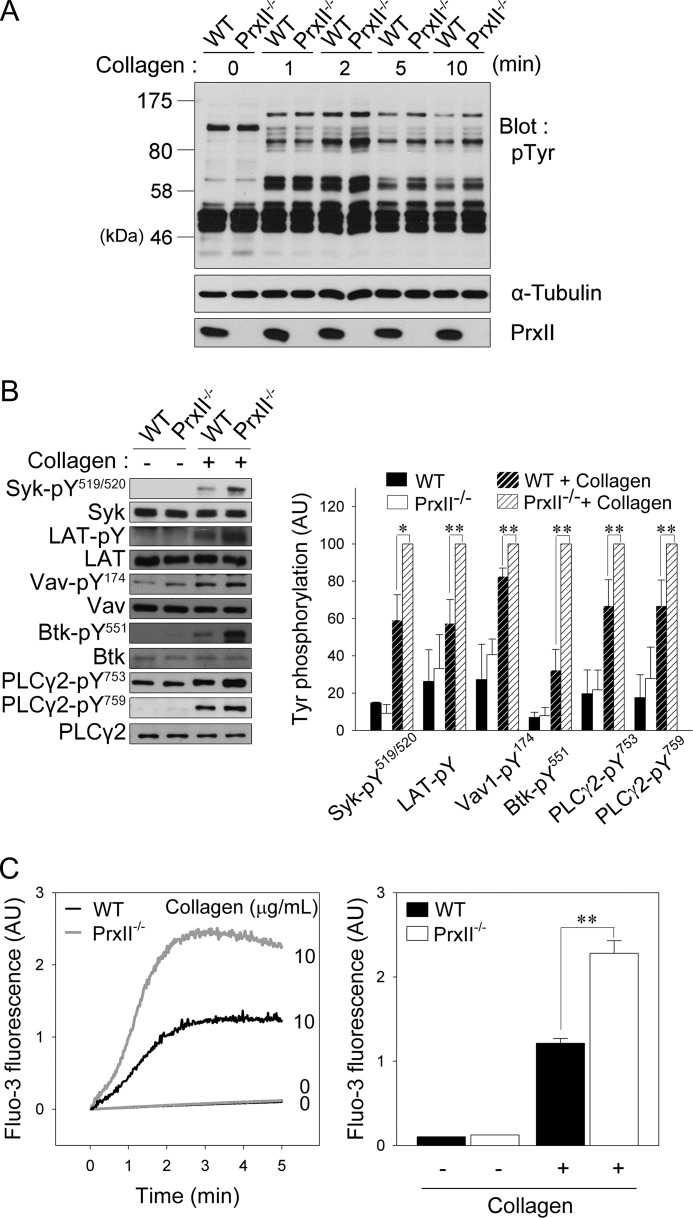
Because intracellular ROS participates in the regulation of protein-tyrosine phosphorylation by oxidizing the catalytic cysteine residue of PTPs (14–16), we next examined the effects of PrxII deficiency on collagen-induced protein-tyrosine phosphorylation in platelets. Stimulation of platelets with collagen caused increased tyrosine phosphorylation of numerous proteins. In the absence of PrxII, the protein-tyrosine phosphorylation response of a few bands was increased (Fig. 3A).
FIGURE 3.
PrxII deficiency up-regulates collagen-induced signal transduction in platelets. A, WT and PrxII−/− platelets were stimulated with collagen (10 μg/ml) for the indicated times. Cell lysates were applied to immunoblot analysis of protein tyrosine phosphorylation with anti-phosphotyrosine antibody (pTyr). Representative immunoblots from three independent experiments are shown. B, wild-type and PrxII−/− platelets were stimulated with collagen (10 μg/ml) for 2 min. Tyrosine phosphorylation of the indicated proteins was analyzed by immunoblotting. Representative immunoblots are shown. The quantitative data are expressed as the mean ± S.D. (n = 3; *, p < 0.05; **, p < 0.01) of arbitrary units (AU). C, Fluo-3 AM-loaded wild-type and PrxII−/− platelets were stimulated with collagen (10 μg/ml) for 5 min, and the Fluo-3 fluorescence expressed in arbitrary units was monitored. Representative fluorescence tracings are shown. The quantitative data are the means ± S.D. (n = 4; **, p < 0.01) for Fluo-3 fluorescence 2 min after stimulation.
To further examine whether PrxII deficiency regulates the activation of the GPVI signaling cascade, we analyzed the collagen-induced phosphorylation of specific tyrosines on key molecules involved in GPVI signaling using phospho-specific antibodies (Fig. 3B). The phosphorylation of Syk at Tyr519/520, which is critical for activating kinase function (29, 30), was strongly increased in PrxII-deficient platelets compared with wild-type platelets. Consistent with the increased Syk activation, the collagen-induced tyrosine phosphorylation of LAT was also significantly increased by PrxII deficiency. The collagen-induced phosphorylation of Tyr174 on Vav1 and Tyr551 on Btk, modifications that are also known to be essential for their activation, was elevated in PrxII−/− compared with wild-type platelets. Given that this tyrosine phosphorylation of Vav1 and Btk has been implicated in the phosphorylation of Tyr753/759 on the downstream target PLCγ2 to increase activation (31–33), we analyzed the phosphorylation of Tyr753/759 in PLCγ2 in collagen-stimulated platelets. In parallel with the increased tyrosine phosphorylation of Vav1 and Btk, the collagen-induced phosphorylation of PLCγ2 at Tyr753 and Tyr759 was also increased in PrxII−/− compared with wild-type platelets (Fig. 3B).
Tyrosine phosphorylation-based activation of PLCγ2 causes inositol-1,4,5-trisphosphate liberation, which, in turn, leads to calcium release from storage sites. Therefore, we examined the effect of PrxII deficiency on the rise in cytosolic calcium by using Fluo-3 AM (Fig. 3C). Concomitant with the change in ROS-dependent PLCγ2 activation, the collagen-induced increase in cytosolic calcium was significantly greater in the PrxII−/− platelets than in the wild-type platelets. Together, these results indicate that PrxII inhibits the tyrosine phosphorylation-based activation of GPVI signaling molecules, which culminates in the suppression of PLCγ2 activity.
PrxII Deficiency Enhances Degranulation-dependent P-selectin Surface Exposure and Integrin-αIIbβ3 Activation in GPVI-stimulated Platelets
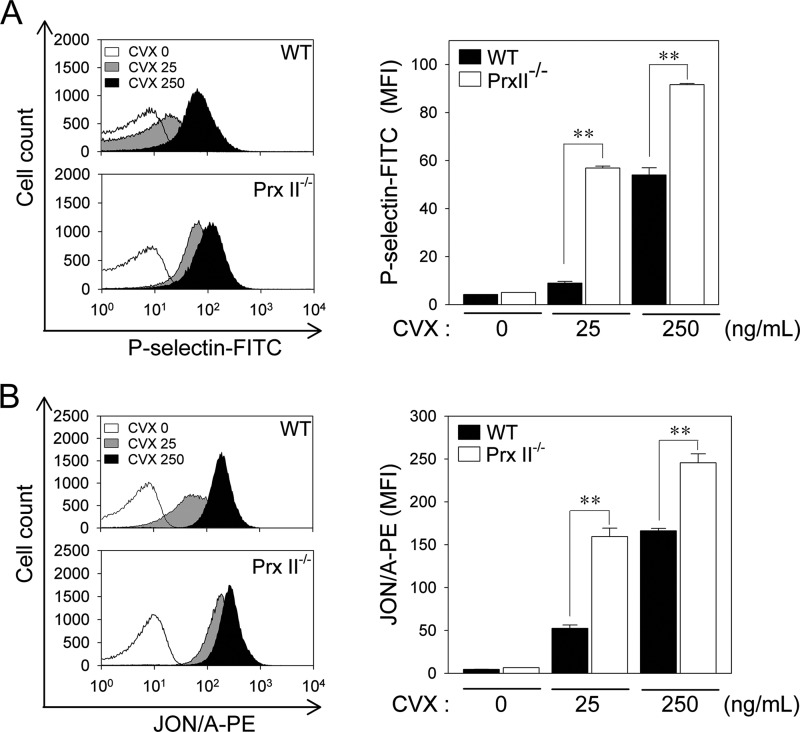
After collagen stimulation, the PLCγ2-mediated generation of 1,2-diacylglycerol and inositol-1,4,5-trisphosphate, which subsequently activate protein kinase C and mobilize calcium, is implicated in degranulation and integrin-αIIbβ3 activation (7). P-selectin appears on the surface of activated platelets that have undergone degranulation of α granules (34). The inside-out activation of integrin-αIIbβ3, which has a high affinity for fibrinogen, is a critical factor for stable adhesion and aggregation (35). Because PrxII deficiency enhances PLCγ2 activity in collagen-stimulated platelets, we next monitored the surface expression of P-selectin and integrin-αIIbβ3 activation using a FITC-labeled antibody specific for P-selectin (P-selectin-FITC) and a PE-labeled antibody specific for activated integrin-αIIbβ3 (JON/A-PE), respectively. Given that collagen can create problems for flow cytometric analysis by cross-linking with itself and aggregating platelets (36), we used convulxin, an activator of GPVI signaling (37). Convulxin increased P-selectin surface expression and integrin-αIIbβ3 activation in a dose-dependent manner (Fig. 4, A and B). The convulxin-induced surface expression of P-selectin from α granules was greater in PrxII−/− platelets than wild-type platelets (Fig. 4A). Integrin-αIIbβ3 activation on the platelet membrane was also significantly greater in platelets from PrxII−/− mice than in those from wild-type mice (Fig. 4B). These results indicate that, in platelets, PrxII negatively regulates degranulation and the inside-out activation of integrin-αIIbβ3 in response to GPVI stimulation.
FIGURE 4.
PrxII deficiency enhances degranulation-dependent P-selectin surface exposure and integrin-αIIbβ3 activation in GPVI-stimulated platelets. A and B, WT and PrxII−/− platelets were incubated with the indicated concentrations of convulxin (CVX) for 5 min in the presence of P-selectin-FITC (A) or JON/A-PE (B). Binding of P-selectin-FITC and JON/A-PE to platelets was analyzed by flow cytometry. Representative histograms are shown. The quantitative data are mean ± S.D. (n = 3; **, p < 0.01) for the mean fluorescence intensity (MFI).
PrxII Relieves SHP-2 from Oxidative Inactivation by Eliminating H2O2 in Lipid Rafts
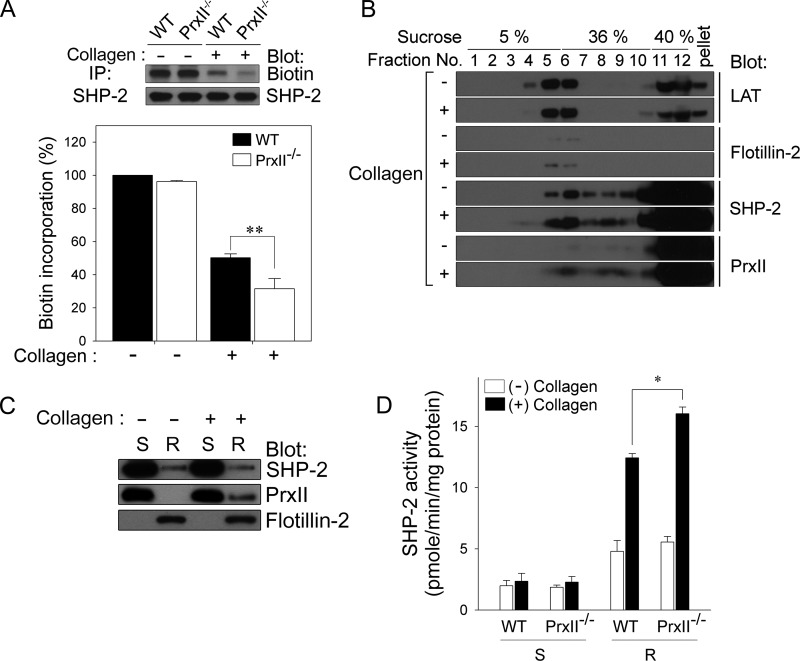
Our recent study revealed that SHP-2 is oxidatively inactivated by collagen-induced ROS in platelets, which promotes platelet activation by up-regulating tyrosine phosphorylation-based signal transduction (12). Therefore, we also examined whether PrxII deficiency influences SHP-2 oxidation in response to collagen. The oxidation of the catalytic cysteine on SHP-2 was analyzed with a thiol-reactive probe, PEG2-iodoacetyl biotin (19). As shown in Fig. 5A, the biotin incorporation into immunoprecipitated SHP-2, which was readily detected in unstimulated platelets, was decreased in response to collagen. Notably, PrxII-deficiency significantly increased the loss of SHP-2 labeling. These data indicate that PrxII functions as a negative regulator of GPVI signaling pathways in platelets by protecting SHP-2 against ROS-dependent oxidative inactivation.
FIGURE 5.
PrxII deficiency increases the oxidative inactivation of SHP-2 in collagen-stimulated platelets. A, WT and PrxII−/− platelets were stimulated with collagen (10 μg/ml) for 2 min. The cell lysates were labeled with PEG2-iodoacetyl biotin. SHP-2 was immunoprecipitated (IP), and its biotinylation was analyzed using HRP-conjugated streptavidin. Representative immunoblots are shown. The data are mean ± S.D. (n = 3; **, p < 0.01). B, after collagen stimulation, platelets were lysed in lipid raft buffer. Lysates were subjected to sucrose fractionation in a sucrose gradient, and each fraction was immunoblotted with specific antibodies as indicated. Sucrose concentrations and fraction numbers are indicated. C, washed platelets were incubated in the absence (−) or presence (+) of collagen, after which detergent-soluble (S) and -resistant (R) fractions were prepared and then subjected to immunoblot analysis with antibodies specific for the indicated proteins. D, after treatment with cysteine-alkylating agents, each fraction was further incubated with dithiothreitol. The activity of SHP-2, which was recovered from oxidation, was determined using SHP-2 immunoprecipitated from detergent-soluble and -resistant fractions as indicated. The data are mean ± S.D. (n = 3; *, p < 0.05). In B and C, representative immunoblots from three independent experiments are shown.
The adaptor protein LAT, present mainly in platelet lipid rafts, is required for the full GPVI signaling responses in platelets (7, 22, 38). Because LAT and SHP-2 have been demonstrated to be associated in platelets, SHP-2 appears to be located in lipid rafts (12). We wondered how the cytosolic peroxidase PrxII protects SHP-2 from oxidative inactivation by eliminating H2O2 in lipid rafts. Given that PrxII is found in the lipid rafts of cancer and vascular endothelial cells (39, 40), we suspected that PrxII is located near SHP-2 in collagen-stimulated platelets. To examine whether PrxII is recruited to lipid rafts following collagen stimulation, we isolated the lipid raft fractions from platelets by sucrose gradient centrifugation (Fig. 5B). LAT was present in lipid raft fractions 4–6, as identified by the marker protein flotillin 2 (41, 42). In resting platelets, SHP-2 was found in both the lipid raft and soluble fractions, whereas a significant amount of PrxII was detected in the soluble fractions. Upon collagen stimulation, PrxII was slightly translocated to the lipid raft fractions in which SHP-2 was colocalized.
It is well known that lipid rafts are distinct submembrane compartments that are resistant to solubilization by nonionic detergents. To examine whether the PrxII-mediated protection of SHP-2 is restricted to a confined area, nonionic detergent-soluble and -resistant fractions were isolated from platelets. Western blot analysis showed that the detergent-resistant fraction containing the lipid raft-associated protein flotillin 2 and the detergent-soluble fraction were isolated from platelets (Fig. 5C). Consistent with our observations, PrxII and SHP-2 were present mostly in the detergent-soluble fractions of resting platelets but were also associated with the detergent-resistant fraction in collagen-stimulated platelets. As shown in Fig. 5D, after reactivating the oxidatively inactivated SHP-2 by dithiothreitol reduction, we measured the activities of recovered SHP-2 in both the detergent-soluble and -resistant fractions of collagen-stimulated wild-type and PrxII−/− platelets using tyrosine phosphopeptide (RRLIEDAEpYAARG) as the substrate. Indeed, collagen-induced oxidative inactivation of SHP-2 occurred mainly in the detergent-resistant fraction and was significantly increased in platelets deficient in PrxII. These results suggest that, in collagen-stimulated platelets, PrxII, which is translocated to a confined area, may protect SHP-2 from oxidative inactivation by eliminating H2O2 in lipid rafts.
PrxII Deficiency Enhances the Adhesion and Aggregation Activity of Platelets on Collagen and Thrombus Formation in Injured Carotid Arteries
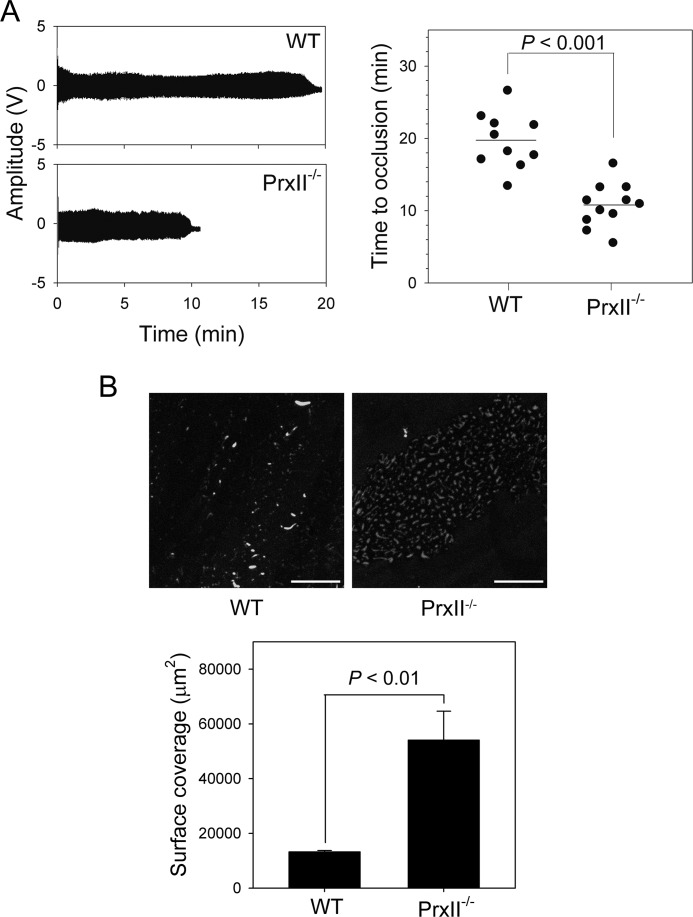
To examine the extent to which the in vitro observed function of PrxII−/− platelets influenced thrombotic events in vivo, we studied occlusive thrombus formation in a model in which the carotid artery is injured with FeCl3 and blood flow is monitored. As shown in Fig. 6A, carotid occlusion in wild-type mice occurred at a mean of 19.4 min, whereas, in PrxII−/− mice, the mean was shortened to 10.8 min (p < 0.001), indicating that PrxII exerts a preventive effect against platelet-mediated plug formation, similar to the findings of a previous study (43).
FIGURE 6.
Accelerated thrombus formation in PrxII-deficient mice and enhanced adhesion and aggregate formation in PrxII-deficient platelets on collagen under flow conditions. A, the left carotid artery of WT and PrxII−/− mice was injured by topical application of 20% ferric chloride. The blood volume changes in the carotid artery downstream of the site of injury were measured. Representative waveforms of blood volume changes are shown. The quantitative data are means ± S.D. representing the time to thrombotic occlusion, and each symbol represents one animal. B, 3,3′-dihexyloxacarbocyanine iodide-labeled wild-type and PrxII−/− platelets were perfused over a collagen-coated surface at a constant shear rate of 1000 s−1. Representative images of platelet adhesion and aggregate formation on collagen after the indicated perfusion time are shown. Scale bars = 50 μm. Surface coverage is presented as mean ± S.D. (n = 3; p < 0.01).
FeCl3-induced injury induces specific damage to the endothelium, creating a site for platelet adhesion and thrombus formation (44), and thrombus formation at the site of vascular injury requires stable shear-resistant platelet adhesion to the extracellular matrix (45). To assess the effect of PrxII deficiency on these processes, we analyzed platelet adhesion and thrombus formation in response to collagen using an ex vivo system with a collagen-coated coverslip in a flow chamber at a constant shear rate. As shown Fig. 6B, at shear rates of 1000 s−1, wild-type platelets simply adhered to collagen without forming thrombi. In sharp contrast, PrxII−/− platelets showed not only a marked increase in adhesion to collagen but also formation of small thrombi on collagen. As a result, both the surface area covered by platelets and the thrombus volume at the end of the perfusion period were markedly increased in PrxII−/− platelets compared with the wild type. These results demonstrate that PrxII down-regulates platelet adhesion and aggregate formation on collagen under flow.
DISCUSSION
Hyperactive platelets underlie the pathophysiology of vascular diseases such as thrombosis and atherosclerosis because endothelial injury leads to the adhesion of platelets to the subendothelial collagen via GPVI, the primary collagen receptor on platelets (4). In GPVI-stimulated platelets, the production of ROS is responsible for the propagation of platelet-activating processes (8–11). Therefore, it has been suggested that cellular antioxidant enzymes act as a physiologically negative regulator of GPVI signaling in platelets. Our study demonstrates for the first time that PrxII functions as a protective antioxidant enzyme against collagen-stimulated platelet activation and platelet-dependent thrombosis.
Accumulating evidence has shown that the oxidative inactivation of PTPs occurs in many cell types in response to ligand-induced activation (15, 46, 47). At the molecular level, the inactivation of PTPs is regulated by H2O2, which induces reversible oxidation of the reactive cysteine at the catalytic site (48). With regard to PrxII function, Choi et al. (18, 19) have demonstrated that PrxII relieves PTPs from oxidative inactivation by eliminating the H2O2 that is produced upon platelet-derived growth factor stimulation and then negatively regulates platelet-derived growth factor receptor signaling. We have now shown that, upon collagen stimulation, PrxII−/− platelets contain more ROS than wild-type platelets. Although we did not directly detect H2O2 in PrxII−/− platelets, the higher level of intracellular ROS seems to be mainly due to an increased amount of H2O2. In accordance with the elevation of ROS levels, PrxII deficiency enhances the total protein tyrosine phosphorylation in collagen-stimulated platelets. These results strongly suggest that PrxII protects PTPs against oxidative inactivation by eliminating H2O2 in GPVI-stimulated platelets. Several PTPs, including low-molecular-weight protein tyrosine phosphatase (LMW-PTP), SHIP-1, phosphatase and tensin homolog (PTEN), and SHP-2, play a negative role in GPVI-mediated platelet activation (12, 49–51) through dephosphorylating multiple substrates. Additionally, we have demonstrated recently that collagen-induced ROS generation causes the oxidative inactivation of SHP-2, which up-regulates the tyrosine phosphorylation of GPVI signaling pathway components in platelets (12). This study shows that PrxII deficiency actually increases the oxidative inactivation of SHP-2 in collagen-stimulated platelets. Because the differences are only modest, oxidative inactivation of SHP-2 alone is not sufficient to explain more pronounced events in PrxII−/− platelets. Therefore, we still cannot completely exclude the possibility that deficiency of PrxII affects other PTPs, which deserve further investigation.
Furthermore, upon collagen stimulation, the tyrosine phosphorylation necessary for the activation of Syk, LAT, Vav1, Btk, and PLCγ2 was elevated in PrxII−/− platelets compared with wild-type platelets. Given that Syk, Btk, and Vav1 have been shown to be substrates for SHP-2 in various cell types (19, 52–54) and collagen-stimulated platelets (12), these data support a model in which PrxII protects SHP-2 against ROS-dependent oxidative inactivation, subsequently inhibiting the activation of Syk, Vav1, and Btk, resulting in decreased PLCγ2 activity.
Another important finding in this study is that the local redox environment is required for SHP-2 inactivation. We showed that, in collagen-stimulated platelets, PrxII colocalizes with SHP-2 in lipid rafts and that SHP-2 oxidation occurs in the nonionic detergent-resistant fraction. This evidence clearly supports the translocation of PrxII from the cytosol to the lipid rafts and this localization of PrxII as a major determinant of localized redox regulation in platelets. This study raises a question about the endogenous source of oxidant causing the SHP-2 oxidation in collagen-stimulated platelets. Because SHP-2 is located in lipid rafts, the best candidate for the oxidant source is Nox. Nox is largely responsible for receptor-dependent ROS production (55, 56). Nox produces superoxide by transferring one electron from NADPH to O2. Superoxide is then dismutated to H2O2. Activated Nox is a multisubunit protein complex assembled within discrete subcellular compartments that are resistant to nonionic detergents, including lipid rafts (55–57). Indeed, Nox2 and its associated subunits (p47phox, p22phox, and p67phox) have been implicated in GPVI signaling in platelets (9, 13). Therefore, in collagen-stimulated platelets, PrxII can translocate to lipid rafts and protect lipid raft-associated SHP-2 from oxidative inactivation by eliminating ROS produced by Nox in the confined area.
In collagen-stimulated platelets, activated PLCγ2 generates 1,2-diacylglycerol and inositol-1,4,5-triphosphate, which promote protein kinase C activation and an increase in cytosolic calcium, respectively, thereby cooperatively up-regulating granule secretion and inside-out integrin-αIIbβ3 activation (7). Our results show that PrxII deficiency enhanced granule secretion and inside-out integrin-αIIbβ3 activation in GPVI-stimulated platelets. Although the molecular mechanism by which ROS activates α granule secretion and integrin activation in GPVI-stimulated platelets is not fully understood, previous studies have shown that these responses can be functionally regulated in a redox-dependent manner (10–12). Because cytosolic calcium plays a key role in actin reorganization, which is required for degranulation and integrin activation (4, 7), our data are partly explained by the increased cytosolic calcium resulting from PLCγ2 activation following the oxidative inactivation of SHP-2 in the absence of PrxII. Platelet α granule secretion leads to the release of soluble fibrinogen, which can bind to activated αIIbβ3-integrin on platelets and form interplatelet bridges. Activated αIIbβ3-integrin also plays a crucial role in platelet adhesion, aggregation, and thrombus formation (35). In parallel with the effects of PrxII deficiency on α granule secretion and integrin activation, platelet adhesion to collagen and the subsequent thrombus formation on collagen-coated plates were markedly increased in the absence of PrxII. Furthermore, PrxII-deficient platelets exhibited enhanced aggregation when stimulated with collagen. These observations indicate that PrxII is a physiologically negative regulator of collagen-induced platelet adhesion, aggregation, and thrombus formation.
In the initial phase after endothelial injury, platelets bind to subendothelial collagen, which leads to granule release and aggregation followed by thrombus formation (1–3). Local application of ferric chloride to the carotid artery serves as an animal model of arterial injury and thrombosis (23, 58). FeCl3-induced injury induces ROS formation and specific damage to the vascular endothelium, generating a site for platelet adhesion and thrombus formation and, thereby, causing widespread endothelial denudation as a result of transendothelial migration of ferric ions (44). Therefore, the accelerated thrombus formation in the carotid artery of PrxII-deficient mice may be partially explained by a reduced capacity to remove the H2O2 produced in response to platelet binding to subendothelial collagen. Because chronic oxidative stress is well recognized as a major factor in platelet-mediated thrombus formation (59, 60), our data suggest that PrxII has a preventive role in the development of thrombotic disease through inhibiting platelet activation.
Although only a handful of studies have demonstrated the involvement of PrxII in cardiovascular disease, some of the evidence is interesting. For example, PrxII has been shown to suppress the platelet-derived growth factor receptor-mediated proliferation and migration of vascular smooth muscle cells (18). Furthermore, the neointimal hyperplasia of vascular smooth muscle cells in an injured carotid artery has been found to be increased in PrxII-deficient mice. Deficiency of PrxII in apolipoprotein E-deficient background mice accelerates plaque formation with increased expression of vascular adhesion molecules, leading to enhanced immune cell adhesion and infiltration into the aortic intima (61). Similar to the findings of this study, PrxII−/− mice have also been shown to exhibit severe thrombosis upon FeCl3-induced carotid artery injury (43). These observations strongly suggest that PrxII-mediated H2O2 elimination may contribute to PrxII's antiplatelet, antithrombotic, and antiatherogenic effects.
In summary, we found that PrxII, an antioxidant enzyme that down-regulates GPVI signaling in platelets, is a potential target for antiplatelet and antithrombotic therapy. PrxII-deficient platelets exhibit markedly increased aggregation and adhesion in response to collagen in vitro. Furthermore, the in vivo data clearly demonstrate that PrxII deficiency accelerates platelet-dependent thrombus formation in injured carotid arteries. These observations suggest that PrxII may be a beneficial target for controlling thrombovascular diseases.
This study was supported by National Research Foundation Grant M10642040002-07N4204-00210 funded by the Korean Ministry of Science, ICT, and Future Planning; by Health Technology R&D Project Grant HI13C1046 funded by the Korean Ministry of Health and Welfare; and by the Brain Korea 21 Plus Program (to J. Y. J., S. B. W., and J. Y. B.) funded by the Korean Ministry of Education.
- GPVI
- glycoprotein VI
- LAT
- linker for the activation of T cells
- ROS
- reactive oxygen species
- Nox
- NADPH oxidase
- PTP
- protein-tyrosine phosphatase
- CM-H2DCFDA
- 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate
- DiOC6
- 3,3′-dihexyloxacarbocyanine iodide
- Fluo-3 AM
- fluo-3 acetoxymethyl ester
- JON/A-PE
- PE-labeled antibody specific for activated integrin-αIIbβ3
- AEBSF
- 4-(2-aminoethyl) benzene sulfonyl fluoride hydrochloride.
REFERENCES
- 1. Bhatt D. L. (2007) Intensifying platelet inhibition: navigating between scylla and charybdis. New Engl. J. Med. 357, 2078–2081 [DOI] [PubMed] [Google Scholar]
- 2. Davì G., Patrono C. (2007) Platelet activation and atherothrombosis. New Engl. J. Med. 357, 2482–2494 [DOI] [PubMed] [Google Scholar]
- 3. Varga-Szabo D., Pleines I., Nieswandt B. (2008) Cell adhesion mechanisms in platelets. Arterioscler. Thromb. Vasc. Biol. 28, 403–412 [DOI] [PubMed] [Google Scholar]
- 4. Nieswandt B., Watson S. P. (2003) Platelet-collagen interaction: is GPVI the central receptor? Blood 102, 449–461 [DOI] [PubMed] [Google Scholar]
- 5. Gibbins J. M. (2004) Platelet adhesion signalling and the regulation of thrombus formation. J. Cell Sci. 117, 3415–3425 [DOI] [PubMed] [Google Scholar]
- 6. Poole A., Gibbins J. M., Turner M., van Vugt M. J., van de Winkel J. G., Saito T., Tybulewicz V. L., Watson S. P. (1997) The Fc receptor γ-chain and the tyrosine kinase Syk are essential for activation of mouse platelets by collagen. EMBO J. 16, 2333–2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Watson S. P., Auger J. M., McCarty O. J., Pearce A. C. (2005) GPVI and integrin αIIbβ3 signaling in platelets. J. Thromb. Haemost. 3, 1752–1762 [DOI] [PubMed] [Google Scholar]
- 8. Pignatelli P., Pulcinelli F. M., Lenti L., Gazzaniga P. P., Violi F. (1998) Hydrogen peroxide is involved in collagen-induced platelet activation. Blood 91, 484–490 [PubMed] [Google Scholar]
- 9. Krötz F., Sohn H. Y., Gloe T., Zahler S., Riexinger T., Schiele T. M., Becker B. F., Theisen K., Klauss V., Pohl U. (2002) NAD(P)H oxidase-dependent platelet superoxide anion release increases platelet recruitment. Blood 100, 917–924 [DOI] [PubMed] [Google Scholar]
- 10. Begonja A. J., Gambaryan S., Geiger J., Aktas B., Pozgajova M., Nieswandt B., Walter U. (2005) Platelet NAD(P)H-oxidase-generated ROS production regulates αIIbβ3-integrin activation independent of the NO/cGMP pathway. Blood 106, 2757–2760 [DOI] [PubMed] [Google Scholar]
- 11. Bakdash N., Williams M. S. (2008) Spatially distinct production of reactive oxygen species regulates platelet activation. Free Radic. Biol. Med. 45, 158–166 [DOI] [PubMed] [Google Scholar]
- 12. Jang J. Y., Min J. H., Chae Y. H., Baek J. Y., Wang S. B., Park S. J., Oh G. T., Lee S. H., Ho Y. S., Chang T. S. (2014) Reactive oxygen species play a critical role in collagen-induced platelet activation via SHP-2 oxidation. Antioxid. Redox Signal. 20, 2528–2540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Seno T., Inoue N., Gao D., Okuda M., Sumi Y., Matsui K., Yamada S., Hirata K. I., Kawashima S., Tawa R., Imajoh-Ohmi S., Sakurai H., Yokoyama M. (2001) Involvement of NADH/NADPH oxidase in human platelet ROS production. Thromb. Res. 103, 399–409 [DOI] [PubMed] [Google Scholar]
- 14. Chiarugi P., Cirri P. (2003) Redox regulation of protein tyrosine phosphatases during receptor tyrosine kinase signal transduction. Trends Biochem. Sci. 28, 509–514 [DOI] [PubMed] [Google Scholar]
- 15. Rhee S. G. (2006) Cell signaling: H2O2, a necessary evil for cell signaling. Science 312, 1882–1883 [DOI] [PubMed] [Google Scholar]
- 16. Tonks N. K. (2005) Redox redux: revisiting PTPs and the control of cell signaling. Cell 121, 667–670 [DOI] [PubMed] [Google Scholar]
- 17. Kang S. W., Chae H. Z., Seo M. S., Kim K., Baines I. C., Rhee S. G. (1998) Mammalian peroxiredoxin isoforms can reduce hydrogen peroxide generated in response to growth factors and tumor necrosis factor-α. J. Biol. Chem. 273, 6297–6302 [DOI] [PubMed] [Google Scholar]
- 18. Choi M. H., Lee I. K., Kim G. W., Kim B. U., Han Y. H., Yu D. Y., Park H. S., Kim K. Y., Lee J. S., Choi C., Bae Y. S., Lee B. I., Rhee S. G., Kang S. W. (2005) Regulation of PDGF signalling and vascular remodelling by peroxiredoxin II. Nature 435, 347–353 [DOI] [PubMed] [Google Scholar]
- 19. Kwon J., Qu C. K., Maeng J. S., Falahati R., Lee C., Williams M. S. (2005) Receptor-stimulated oxidation of SHP-2 promotes T-cell adhesion through SLP-76-ADAP. EMBO J. 24, 2331–2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jin R. C., Mahoney C. E., Coleman Anderson L., Ottaviano F., Croce K., Leopold J. A., Zhang Y. Y., Tang S. S., Handy D. E., Loscalzo J. (2011) Glutathione peroxidase-3 deficiency promotes platelet-dependent thrombosis in vivo. Circulation 123, 1963–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee T. H., Kim S. U., Yu S. L., Kim S. H., Park D. S., Moon H. B., Dho S. H., Kwon K. S., Kwon H. J., Han Y. H., Jeong S., Kang S. W., Shin H. S., Lee K. K., Rhee S. G., Yu D. Y. (2003) Peroxiredoxin II is essential for sustaining life span of erythrocytes in mice. Blood 101, 5033–5038 [DOI] [PubMed] [Google Scholar]
- 22. Lee F. A., van Lier M., Relou I. A., Foley L., Akkerman J. W., Heijnen H. F., Farndale R. W. (2006) Lipid rafts facilitate the interaction of PECAM-1 with the glycoprotein VI-FcR γ-chain complex in human platelets. J. Biol. Chem. 281, 39330–39338 [DOI] [PubMed] [Google Scholar]
- 23. Farrehi P. M., Ozaki C. K., Carmeliet P., Fay W. P. (1998) Regulation of arterial thrombolysis by plasminogen activator inhibitor-1 in mice. Circulation 97, 1002–1008 [DOI] [PubMed] [Google Scholar]
- 24. Murphy A. J., Bijl N., Yvan-Charvet L., Welch C. B., Bhagwat N., Reheman A., Wang Y., Shaw J. A., Levine R. L., Ni H., Tall A. R., Wang N. (2013) Cholesterol efflux in megakaryocyte progenitors suppresses platelet production and thrombocytosis. Nat. Med. 19, 586–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen J., Diacovo T. G., Grenache D. G., Santoro S. A., Zutter M. M. (2002) The α2 integrin subunit-deficient mouse: a multifaceted phenotype including defects of branching morphogenesis and hemostasis. Am. J. Pathol. 161, 337–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Anfossi G., Russo I., Massucco P., Mattiello L., Cavalot F., Trovati M. (2001) N-acetyl-l-cysteine exerts direct anti-aggregating effect on human platelets. Eur. J. Clin. Invest. 31, 452–461 [DOI] [PubMed] [Google Scholar]
- 27. Lill G., Voit S., Schrör K., Weber A. A. (2003) Complex effects of different green tea catechins on human platelets. FEBS Lett. 546, 265–270 [DOI] [PubMed] [Google Scholar]
- 28. Sobotková A., Másová-Chrastinová L., Suttnar J., Stikarová J., Májek P., Reicheltová Z., Kotlín R., Weisel J. W., Malý M., Dyr J. E. (2009) Antioxidants change platelet responses to various stimulating events. Free Radic. Biol. Med. 47, 1707–1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fujii C., Yanagi S., Sada K., Nagai K., Taniguchi T., Yamamura H. (1994) Involvement of protein-tyrosine kinase p72syk in collagen-induced signal transduction in platelets. Eur. J. Biochem. 226, 243–248 [DOI] [PubMed] [Google Scholar]
- 30. Furlong M. T., Mahrenholz A. M., Kim K. H., Ashendel C. L., Harrison M. L., Geahlen R. L. (1997) Identification of the major sites of autophosphorylation of the murine protein-tyrosine kinase Syk. Biochim. Biophys. Acta 1355, 177–190 [DOI] [PubMed] [Google Scholar]
- 31. Atkinson B. T., Ellmeier W., Watson S. P. (2003) Tec regulates platelet activation by GPVI in the absence of Btk. Blood 102, 3592–3599 [DOI] [PubMed] [Google Scholar]
- 32. Pearce A. C., Wilde J. I., Doody G. M., Best D., Inoue O., Vigorito E., Tybulewicz V. L., Turner M., Watson S. P. (2002) Vav1, but not Vav2, contributes to platelet aggregation by CRP and thrombin, but neither is required for regulation of phospholipase C. Blood 100, 3561–3569 [DOI] [PubMed] [Google Scholar]
- 33. Suzuki-Inoue K., Wilde J. I., Andrews R. K., Auger J. M., Siraganian R. P., Sekiya F., Rhee S. G., Watson S. P. (2004) Glycoproteins VI and Ib-IX-V stimulate tyrosine phosphorylation of tyrosine kinase Syk and phospholipase Cγ2 at distinct sites. Biochem. J. 378, 1023–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Palabrica T., Lobb R., Furie B. C., Aronovitz M., Benjamin C., Hsu Y. M., Sajer S. A., Furie B. (1992) Leukocyte accumulation promoting fibrin deposition is mediated in vivo by P-selectin on adherent platelets. Nature 359, 848–851 [DOI] [PubMed] [Google Scholar]
- 35. Bennett J. S. (2005) Structure and function of the platelet integrin αIIbβ3. J. Clin. Invest. 115, 3363–3369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gibbins J. M., Mahaut-Smith M. P. (2004) Platelets and Megakaryocytes, p. 233, Humana Press, Totowa, NJ [Google Scholar]
- 37. Jandrot-Perrus M., Lagrue A. H., Okuma M., Bon C. (1997) Adhesion and activation of human platelets induced by convulxin involve glycoprotein VI and integrin α2β1. J. Biol. Chem. 272, 27035–27041 [DOI] [PubMed] [Google Scholar]
- 38. Locke D., Chen H., Liu Y., Liu C., Kahn M. L. (2002) Lipid rafts orchestrate signaling by the platelet receptor glycoprotein VI. J. Biol. Chem. 277, 18801–18809 [DOI] [PubMed] [Google Scholar]
- 39. Woo H. A., Yim S. H., Shin D. H., Kang D., Yu D. Y., Rhee S. G. (2010) Inactivation of peroxiredoxin I by phosphorylation allows localized H2O2 accumulation for cell signaling. Cell 140, 517–528 [DOI] [PubMed] [Google Scholar]
- 40. Kang D. H., Lee D. J., Lee K. W., Park Y. S., Lee J. Y., Lee S. H., Koh Y. J., Koh G. Y., Choi C., Yu D. Y., Kim J., Kang S. W. (2011) Peroxiredoxin II is an essential antioxidant enzyme that prevents the oxidative inactivation of VEGF receptor-2 in vascular endothelial cells. Mol. Cell 44, 545–558 [DOI] [PubMed] [Google Scholar]
- 41. Vial C., Fung C. Y., Goodall A. H., Mahaut-Smith M. P., Evans R. J. (2006) Differential sensitivity of human platelet P2X1 and P2Y1 receptors to disruption of lipid rafts. Biochem. Biophys. Res. Commun. 343, 415–419 [DOI] [PubMed] [Google Scholar]
- 42. Mairhofer M., Steiner M., Mosgoeller W., Prohaska R., Salzer U. (2002) Stomatin is a major lipid-raft component of platelet α granules. Blood 100, 897–904 [DOI] [PubMed] [Google Scholar]
- 43. Li W., Febbraio M., Reddy S. P., Yu D. Y., Yamamoto M., Silverstein R. L. (2010) CD36 participates in a signaling pathway that regulates ROS formation in murine VSMCs. J. Clin. Invest. 120, 3996–4006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tseng M. T., Dozier A., Haribabu B., Graham U. M. (2006) Transendothelial migration of ferric ion in FeCl3 injured murine common carotid artery. Thromb. Res. 118, 275–280 [DOI] [PubMed] [Google Scholar]
- 45. Dütting S., Bender M., Nieswandt B. (2012) Platelet GPVI: a target for antithrombotic therapy?!. Trends Pharmacol. Sci. 33, 583–590 [DOI] [PubMed] [Google Scholar]
- 46. Monteiro H. P., Arai R. J., Travassos L. R. (2008) Protein tyrosine phosphorylation and protein tyrosine nitration in redox signaling. Antioxid. Redox Signal. 10, 843–889 [DOI] [PubMed] [Google Scholar]
- 47. Salmeen A., Barford D. (2005) Functions and mechanisms of redox regulation of cysteine-based phosphatases. Antioxid. Redox Signal. 7, 560–577 [DOI] [PubMed] [Google Scholar]
- 48. Tonks N. K. (2006) Protein tyrosine phosphatases: from genes, to function, to disease. Nat. Rev. Mol. Cell Biol. 7, 833–846 [DOI] [PubMed] [Google Scholar]
- 49. Mancini F., Rigacci S., Berti A., Balduini C., Torti M. (2007) The low-molecular-weight phosphotyrosine phosphatase is a negative regulator of FcγIIA-mediated cell activation. Blood 110, 1871–1878 [DOI] [PubMed] [Google Scholar]
- 50. Chari R., Kim S., Murugappan S., Sanjay A., Daniel J. L., Kunapuli S. P. (2009) Lyn, PKC-δ, SHIP-1 interactions regulate GPVI-mediated platelet-dense granule secretion. Blood 114, 3056–3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Weng Z., Li D., Zhang L., Chen J., Ruan C., Chen G., Gartner T. K., Liu J. (2010) PTEN regulates collagen-induced platelet activation. Blood 116, 2579–2581 [DOI] [PubMed] [Google Scholar]
- 52. Maeda A., Scharenberg A. M., Tsukada S., Bolen J. B., Kinet J. P., Kurosaki T. (1999) Paired immunoglobulin-like receptor B (PIR-B) inhibits BCR-induced activation of Syk and Btk by SHP-1. Oncogene 18, 2291–2297 [DOI] [PubMed] [Google Scholar]
- 53. Kurosaki T., Maeda A., Ishiai M., Hashimoto A., Inabe K., Takata M. (2000) Regulation of the phospholipase C-γ2 pathway in B cells. Immunol. Rev. 176, 19–29 [DOI] [PubMed] [Google Scholar]
- 54. Wakino S., Hayashi K., Kanda T., Tatematsu S., Homma K., Yoshioka K., Takamatsu I., Saruta T. (2004) Peroxisome proliferator-activated receptor γ ligands inhibit Rho/Rho kinase pathway by inducing protein tyrosine phosphatase SHP-2. Circ. Res. 95, e45–55 [DOI] [PubMed] [Google Scholar]
- 55. Oakley F. D., Abbott D., Li Q., Engelhardt J. F. (2009) Signaling components of redox active endosomes: the redoxosomes. Antioxid. Redox Signal. 11, 1313–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ushio-Fukai M. (2006) Localizing NADPH oxidase-derived ROS. Sci. STKE 2006, re8. [DOI] [PubMed] [Google Scholar]
- 57. Li Q., Harraz M. M., Zhou W., Zhang L. N., Ding W., Zhang Y., Eggleston T., Yeaman C., Banfi B., Engelhardt J. F. (2006) Nox2 and Rac1 regulate H2O2-dependent recruitment of TRAF6 to endosomal interleukin-1 receptor complexes. Mol. Cell. Biol. 26, 140–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kurz K. D., Main B. W., Sandusky G. E. (1990) Rat model of arterial thrombosis induced by ferric chloride. Thromb. Res. 60, 269–280 [DOI] [PubMed] [Google Scholar]
- 59. Freedman J. E. (2008) Oxidative stress and platelets. Arterioscler. Thromb. Vasc. Biol. 28, s11–16 [DOI] [PubMed] [Google Scholar]
- 60. Morrell C. N. (2008) Reactive oxygen species: finding the right balance. Circ. Res. 103, 571–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Park J. G., Yoo J. Y., Jeong S. J., Choi J. H., Lee M. R., Lee M. N., Hwa Lee J., Kim H. C., Jo H., Yu D. Y., Kang S. W., Rhee S. G., Lee M. H., Oh G. T. (2011) Peroxiredoxin 2 deficiency exacerbates atherosclerosis in apolipoprotein E-deficient mice. Circ. Res. 109, 739–749 [DOI] [PMC free article] [PubMed] [Google Scholar]