Key Points
Arginase depletion with BCT-100 pegylated recombinant human arginase is cytotoxic to AML blasts.
Abstract
Acute myeloid leukemia (AML) is one of the most common acute leukemias in adults and children, yet significant numbers of patients relapse and die of disease. In this study, we identify the dependence of AML blasts on arginine for proliferation. We show that AML blasts constitutively express the arginine transporters CAT-1 and CAT-2B, and that the majority of newly diagnosed patients’ blasts have deficiencies in the arginine-recycling pathway enzymes argininosuccinate synthase and ornithine transcarbamylase, making them arginine auxotrophic. BCT-100, a pegylated human recombinant arginase, leads to a rapid depletion in extracellular and intracellular arginine concentrations, resulting in arrest of AML blast proliferation and a reduction in AML engraftment in vivo. BCT-100 as a single agent causes significant death of AML blasts from adults and children, and acts synergistically in combination with cytarabine. Using RNA sequencing, 20 further candidate genes which correlated with resistance have been identified. Thus, AML blasts are dependent on arginine for survival and proliferation, as well as depletion of arginine with BCT-100 of clinical value in the treatment of AML.
Introduction
Treatment of acute myeloid leukemia (AML) has seen significant progress, but overall survival rates have plateaued and significant numbers of patients continue to die of the disease.1,2 New therapeutic approaches are needed that complement standard chemotherapy without increasing the burden of toxicity. Arginine is an amino acid metabolized by cells to provide precursors for cell cycle activity, protein synthesis, and a number of other cell functions. In certain circumstances there can be a high demand for arginine, including rapid growth periods during development, inflammation, organ dysfunction, and tumor growth. Arginine requirements may not be met by synthesis from citrulline alone, thus requiring arginine from the diet, leading to its classification as a semiessential amino acid.3-5 Cancers also pose a unique demand on nutrient requirements, including dependency on arginine supplementation to sustain growth, that is, arginine auxotrophism.6 Thus, control over arginine availability and metabolism represents a potential therapeutic approach that can be exploited.
BCT-100 is a clinical-grade pegylated (PEG) recombinant human arginase that catalyzes the conversion of arginine to ornithine and urea, leading to arginine depletion.7-10 BCT-100 has shown significant benefit against solid tumors in preclinical studies and early-phase clinical trials.8 Here, we characterize the mechanisms of dependence of AML blasts on arginine and the potential for arginine depletion with BCT-100 as a therapeutic approach.
Methods
AML patient samples
Blood samples were obtained from 20 patients with newly diagnosed or newly relapsed AML, before the start of treatment, at the Birmingham Children’s Hospital, University Hospitals Birmingham, or Heartlands Hospital Birmingham (Table 1). The cells were separated from fresh samples as previously described.11 AML samples were investigated within 12 hours of blood sampling from patients and only samples with >98% viability by trypan blue staining were used. Bone marrow samples from 39 newly diagnosed AML patients were obtained from the Chinese Hospital, Hong Kong.
Table 1.
Table of patient characteristics
| Patient ID | Time point | Age, y | Sex | Blast count at diagnosis, ×109/L | Cytogenetics |
|---|---|---|---|---|---|
| P1 | Diagnosis | 1 | M | 92 | ins(X;11) MLL rearrangement |
| P2 | Diagnosis | 74 | F | 72 | Complex, dup 3q, FLT3−, NPM1− |
| P3 | Diagnosis | 73 | F | 16 | Complex, 5q−, FLT3−, NPM1− |
| P4 | Diagnosis | 63 | M | 43 | Normal |
| P5 | Diagnosis | 6 | M | 9 | Inv(16)(p13;1q22) CBFB-MYH11 |
| P6 | Diagnosis | 6 | M | 16 | Monosomy 7 |
| P7 | Relapse | 77 | F | 60 | Del(16), Monosomy 7, FLT3-NPM1− |
| P8 | Diagnosis | 20 | F | 7 | t(8;21) |
| P9 | Diagnosis | 22 | F | 13 | Abnormal 7q and 17p, FLT3−, NPM1− |
| P10 | Diagnosis | 5 | F | 54 | Normal |
| P11 | Diagnosis | 93 | M | 4 | Normal |
| P12 | Diagnosis | 69 | M | 33 | Normal |
| P13 | Diagnosis | 77 | F | 5 | Normal |
| P14 | Relapse | 58 | M | 47 | Normal |
| P15 | Diagnosis | 79 | M | 58 | Normal |
| P16 | Diagnosis | 76 | M | 31 | Normal |
| P17 | Diagnosis | 0.5 | F | 275 | t(9;11)(p22;q23); MLLT3-KMT2A |
| P18 | Diagnosis | 53 | F | 54 | Normal |
| P19 | Relapse | 68 | M | 94 | Trisomy 11, FLT3− |
| P20 | Relapse | 63 | M | 87 | Normal |
MLL, mixed-lineage leukemia.
Cytotoxicity assay
Cell lines or sorted AML blasts from patients were resuspended in complete media and 2 × 105 AML blasts or 0.5 × 105 cell lines were added to each well of 96-well plates. On day 1, BCT-100 was added at final concentrations of 0, 200, 400, 600, 800, 1000, 1500, 2000, or 4000 ng/mL to triplicate wells. The cytotoxicity of cytarabine (500 ng/mL) was also tested in combination with BCT-100. Cells were incubated for a further 72 hours. The effect of arginine deprivation was similarly tested by culturing AML cell lines and patients’ blasts in stable isotope labeling by amino acids in cell culture arginine-free RPMI 1640 (Fisher Scientific), 10% heat-inactivated arginine-free fetal bovine serum (Fisher Scientific), glutamine (1×; Sigma), and sodium pyruvate (1×; Sigma).
Flow cytometric analysis
Cells from cell lines and patient samples were collected and labeled with propidium iodide (PI) to assess viability by flow cytometry. The relative percentage of viable cells at the end of the assay (72 hours) was calculated using the following formula: (mean number of viable blasts recovered in treatment wells/mean number of viable blasts in untreated wells ×100). Apoptosis was estimated by cells being resuspended in 1× annexin binding buffer and labeled with 7-aminoactinomycin D (-) and annexin conjugated to phycocyanin (fluorescein isothiocyanate) (FITC Annexin V Apoptosis Detection Kit I; BD Pharmingen). The cells were analyzed with a Cyan flow analyzer (Beckman Coulter) using FlowJo software (Tree Star Inc). The 50% inhibitory concentration (IC50) is defined as the concentration of BCT-100 that killed 50% of the viable cells at the termination of the assay.
Cell cycle analysis was performed using PI staining and flow cytometry. A total of 1 × 106 cells per well incubated with RPMI 10% with or without BCT-100 (600 ng/mL) in 24-well plates for 72 hours were harvested, washed twice in phosphate-buffered saline, and fixed in cold ethanol for 1 hour at 4°C. Following washing with phosphate-buffered saline, cells were stained with PI solution and 50 µL of RNase A stock solution (10 μg/mL; Invitrogen) at 4°C for 3 hours before analysis with a Cyan flow analyzer in combination with ModFit software.
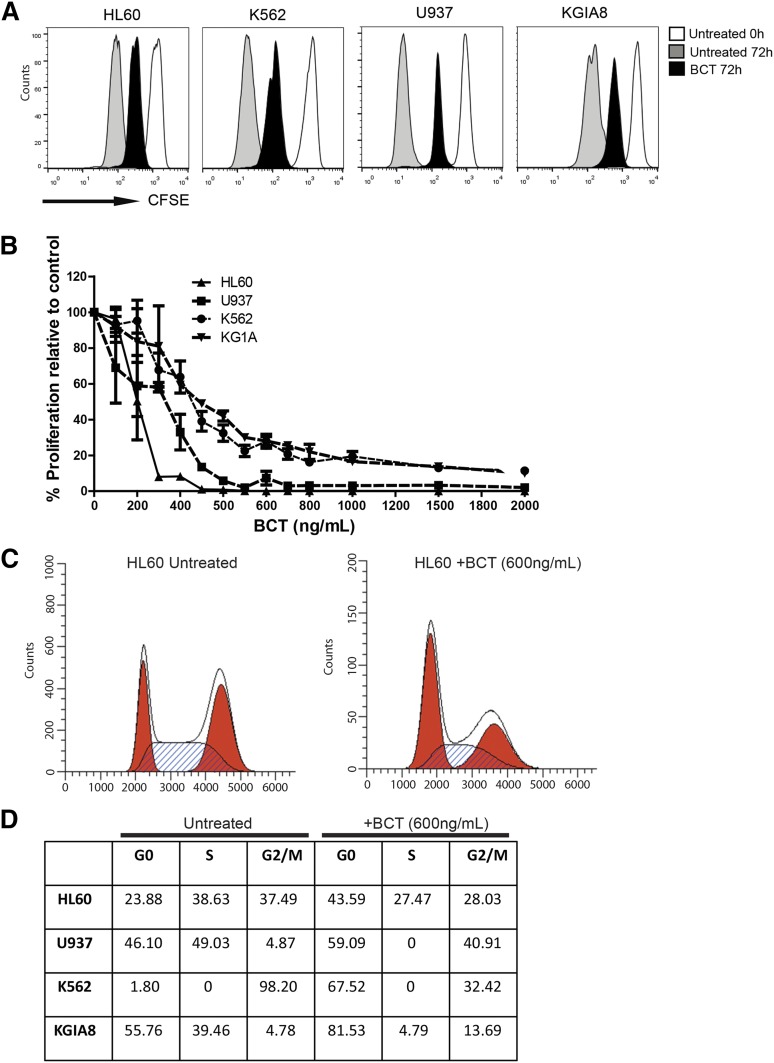
To investigate effects of AML blast proliferation, carboxyfluorescein succinimidyl ester (CFSE)–labeled AML blasts were cultured in the presence or absence of BCT-100 (600 ng/mL) for 72 hours. PI was added to allow viable cells to be gated on flow cytometry. Cell proliferation was determined according to CFSE dilution.
AML murine xenografts
NOD/Shi-scid/IL-2R SCIDγ null (NOG) mice aged 10 to 14 weeks were irradiated with 1.25 Gy. One day later, 5 × 106 HL60 leukemia cells were injected into the tail vein. BCT-100 (5 mg/kg) was injected intraperitoneally (i.p.) twice weekly. A second group of mice was treated with 25 mg/kg cytarabine (i.p. once weekly). Bone marrow was harvested from the leg bones of mice sacrificed after 5 weeks of treatment. AML engraftment was defined by the detection of human CD45+ cells using flow cytometry.
Immunoblotting
Following cell lysis (20 nM Tris-Hcl pH 7.5, 150 nM NaCl, 2 mM EDTA, 1.0% Triton X-100, and protease and phosphatase inhibitors; Roche Applied Science), equal amounts of protein were loaded onto 12% Tris-Glycine sodium dodecyl sulfate–polyacrylamide gel electrophoresis (Bio-Rad) gels and transferred to polyvinylidene difluoride membranes. Hybridization was carried out using antibodies to poly ADP ribose polymerase, caspases-3 and -9, LC3 (Cell Signaling), and actin (Sigma). Horseradish peroxidase–conjugated secondary antibodies, goat anti-rabbit (Cell Signaling), and sheep anti-mouse (GE Healthcare) were used for blots, which were developed with enhanced chemiluminescence substrate (Bio-Rad) and exposed on Kodak film.
Transmission electron microscopy
AML blasts were treated with BCT-100 (600 ng/mL) in culture for 72 hours. Following harvesting, they were fixed in 2.5% glutaraldehyde followed by 1% osmium tetroxide. The samples were dehydrated through ethanol and embedded in propylene oxide/resin mixture at 60°C for 16 hours prior to sectioning at 80 nm in thickness and placement on 300 mesh copper slot grids for examination by transmission electron microscopy.
Immunohistochemistry
Paraffin-embedded tissue sections of bone marrow trephines from AML patients at diagnosis were deparaffinized and rehydrated. Antigens were demasked was performed in 50 mM Tris/2 mM EDTA pH 9.0 using a Philips Whirlpool Sixth Sense microwave on a steaming program. Staining was performed with anti-human argininosuccinate synthase (ASS; Abcam) and anti-human ornithine transcarbamylase (OTC; Abcam) using the Novolink Polymer Detection System (Leica). Primary antibody incubation was performed overnight in a cold room. Sections were counterstained with Gill number 3 hematoxylin (Sigma-Aldrich) and mounted in Aquatex (Merck).
RNA sequencing
RNA was derived from 6 sensitive (P1, P3, P8, P9, P10, P12) and 6 resistant (P11, P6, P4, P5, P7, P13) AML patients’ blasts, as identified by IC50. Samples were prepared with the Illumina TruSeq RNA Sample Preparation kit (version 2; Oxford Gene Technologies). They were sequenced on the Illumina HiSeq2000 platform using TruSeq version 3 chemistry, over 100 cycles. Read files (Fastq) were generated via the manufacturer’s proprietary software. Reads were mapped by their location to the appropriate Illumina iGenomes built using Bowtie version 2.02. Splice junctions were identified using Tophat version 2.0.9. Cufflinks version 2.1.1 was used to perform transcript assembly. Visualization of differential expression results used Cummerbund. RNA-Seq alignment metrics were generated using Picard. A table of arginine-related genes, concerned with arginine recycling and transport and associated pathways, were constructed based on current knowledge of arginine metabolism. Genes were compared with demonstrated differences in fragments per kilobase of transcript per million mapped reads (FPKM) between resistant and sensitive cells and subtracted to demonstrate change and direction in FPKM. Genes of interest were highlighted if the difference in FPKM was ±1.96 (2 standard deviation [SD] from mean).
Statistical analysis
A Wilcoxon rank-sum test was used to determine the statistical significance of the difference in unpaired observations between 2 groups (GraphpPad Prism). Correlations between parameters were evaluated using Spearman rank correlation analyses. P values are 2-tailed and where values were <.05, they were considered statistically significant. For combination studies of BCT-100 with cytarabine, the interaction effect of the 2 drugs was tested in a 2-way analysis of variance (ANOVA).12 Analysis of synergism was assessed according to the Chou and Talalay method, using CompuSyn software (ComboSyn Inc).13 AML blasts from patients were cultured with BCT-100 alone (0, 200, 400, 600, 800, 1000 ng/mL), cytarabine (0, 200, 400, 600, 800, 1000 ng/mL), or both for 72 hours. The percentage of viable cells relative to control after 72 hours was measured by flow cytometry. Using this method, a combination index (CI) at IC50 for individual patient samples was calculated, synergism was defined as CI < 1, while antagonism was CI > 1, and an additive effect was considered as CI = 1.
Study approval
In accordance with the Declaration of Helsinki, patient samples were obtained after written, informed consent prior to inclusion in the study. Regional Ethics Committee (REC number 10/H0501/39) and local hospital trust research approval for the study was granted for United Kingdom hospitals and at the Chinese University Hospital, Hong Kong. The Birmingham Biomedical Ethics Review Subcommittee (BERSC) approved all animal protocols in this study. Procedures were carried out in accordance with UK Home Office guidelines.
Results
AML proliferation is dependent on arginine
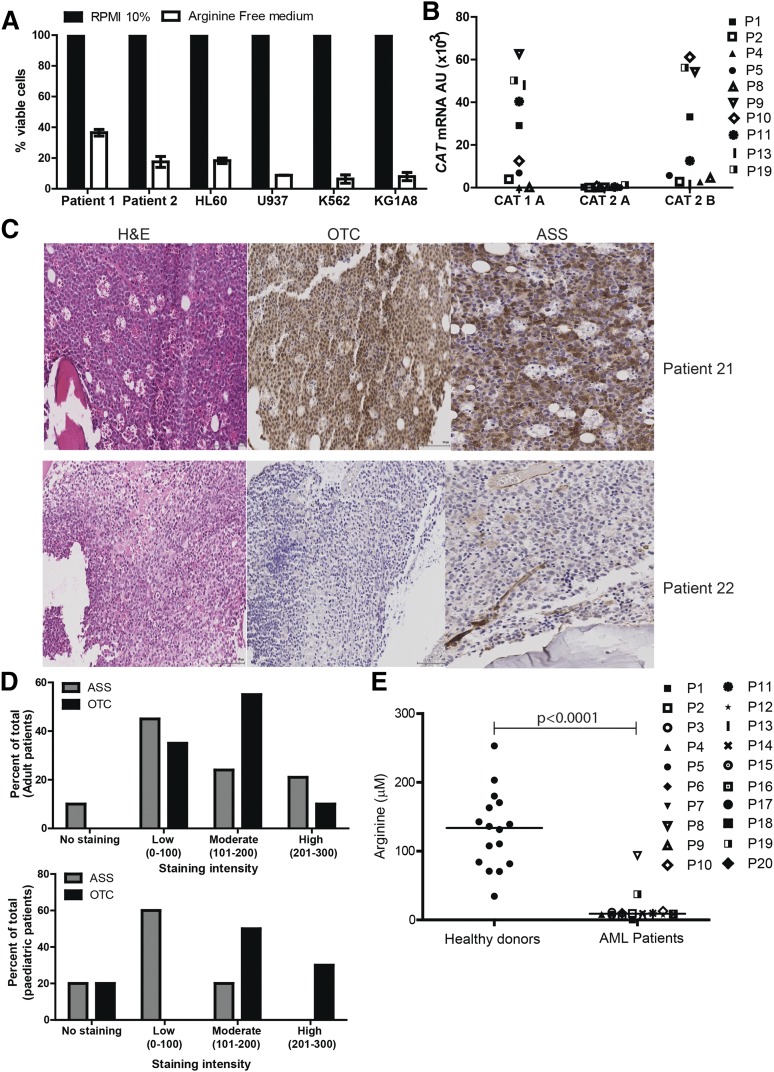
AML blasts create an immunosuppressive microenvironment through arginase activity and release, contributing to AML growth and pathogenesis.11 However, the dependence of AML blasts on arginine for survival has not been reported. Arginine deprivation resulted in a profound decrease in the number of viable AML blasts, providing proof of principle that this amino acid plays a key role in blast viability (Figure 1A).
Figure 1.
AML blasts are auxotrophic for arginine. (A) AML patients’ blasts and AML cell lines were cultured in complete or arginine-depleted media. The viability of AML blasts from patients and cell lines was assessed by flow cytometry after 72 hours. Arginine depletion leads to a decreased percentage of viable blasts. Representative of 2 independent experiments. (B) Expression of CAT-1, CAT-2A, and CAT-2B in blasts from 10 patients was confirmed by quantitative polymerase chain reaction (qPCR). Patients are identified by unique symbols, which are used consistently throughout the manuscript. (C) Staining of 39 bone marrow samples from AML patients at diagnosis with hematoxylin-eosin (left panel), anti-OTC (center panel), and anti-ASS (right panel). Representative marrows from 2 patients showing positive antigen staining (top) and negative antigen staining (bottom). (D) Histoscores of ASS and OTC staining in adult and pediatric AML bone marrow samples. (E) Plasma from 20 AML patients at diagnosis and 16 healthy donors were analyzed for arginine concentration by enzyme-linked immunosorbent assay (ELISA). Plasma arginine levels are significantly lower in newly diagnosed patients (P < .0001).
Blood arginine levels are maintained by dietary consumption, protein turnover, and endogenous synthesis from citrulline through an intestinal-renal arginine cycle.14-17 In mammalian cells, arginine is imported from the microenvironment predominantly by a Na+-independent (System y+) family of transmembrane cationic amino acid transporters (CAT1, CAT2A, CAT2B, CAT3) with tissue-specific expression patterns.18 We identified that AML blasts express CAT-1 and CAT2B, regardless of blast subtype, thus allowing AML blasts to use extracellular arginine (Figure 1B).
To understand whether AML blasts express the key arginine-recycling enzymes ASS and OTC, we examined 39 diagnostic patient samples by immunohistochemistry (Figure 1C-D). Of 29 adult AML samples, 10% had no staining, 45% showed low, 24% showed moderate, and 21% showed high ASS expression, whereas 0% had no staining, 35% showed low, 55% showed moderate, and 10% showed high OTC expression. Of 10 pediatric AML samples, 20% had no staining, 60% had low, and 20% had moderate ASS expression, whereas 20% had no staining, 0% had low, 50% had moderate, and 30% had high OTC expression. One pediatric sample received a 0 score for both ASS and OTC.
AML blasts can therefore lack 1 or both of the important enzymes for endogenous arginine synthesis. As such, their dependence on extracellular arginine levels should become rate-limiting on many metabolic reactions and protein synthesis, thereby preventing proliferation. Proliferation of cultured AML blasts was arrested when arginine fell below 10 μM. Consistent with these findings in newly diagnosed patients, where AML disease burden is highest, plasma arginine concentrations are significantly lower than in healthy controls (mean: 131 μM healthy vs 9.0 μM AML patients; P ≤ .0001) (Figure 1E). Together, these findings highlight the dependency of AML blasts on extracellular arginine–arginine auxotrophism.
BCT-100 reduces the number of AML blasts in vitro and in vivo
BCT-100 is a PEG recombinant human arginase being investigated for the treatment of solid tumors.8 We first demonstrated that BCT-100 catalyzes a dose-dependent reduction in media arginine concentrations in vitro (supplemental Figure 1A, see supplemental Data available on the Blood Web site). Arginine was reduced to <2 μM within 8 hours at concentrations of 600 ng/mL BCT-100 or higher.
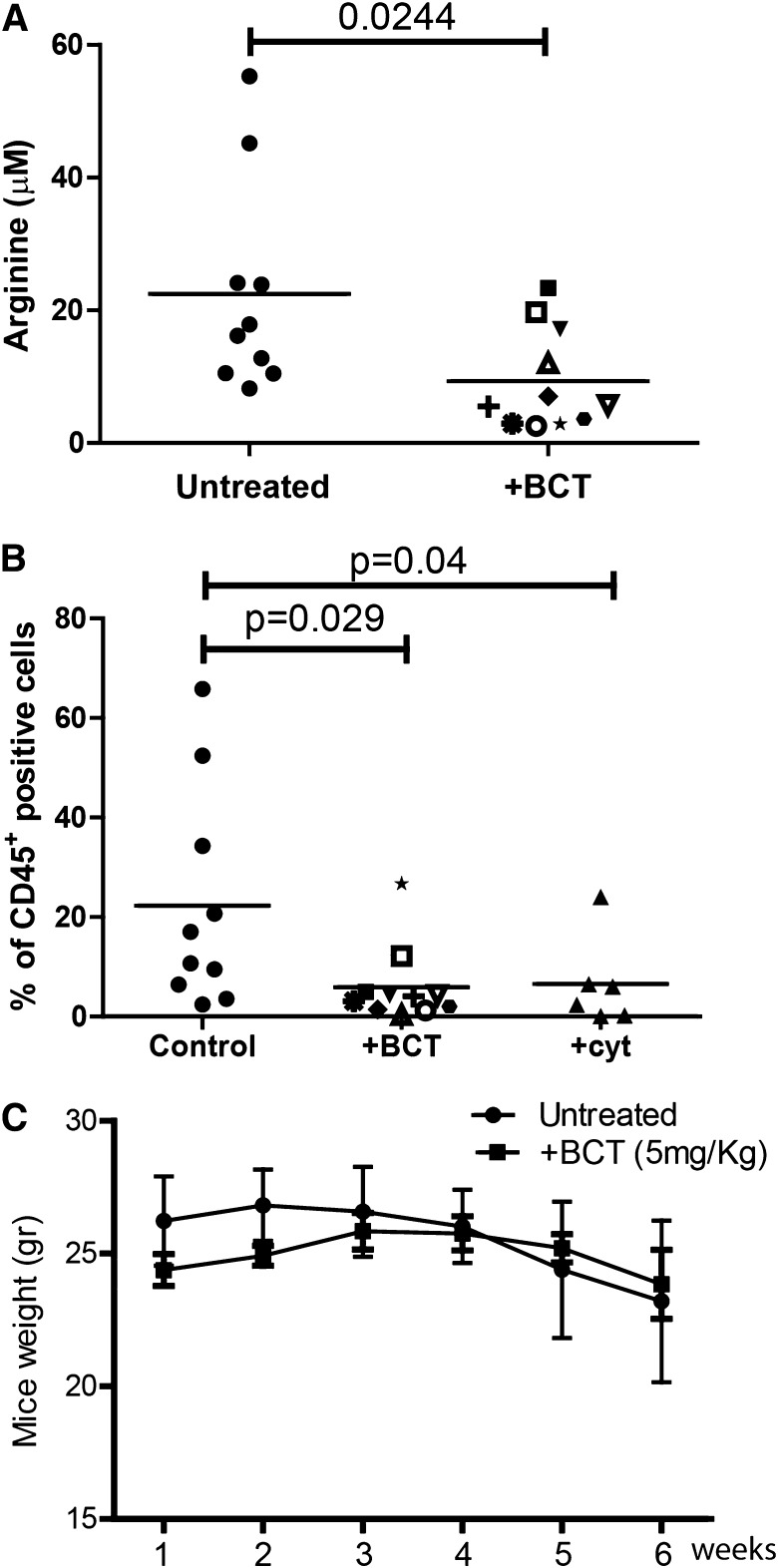
AML cell lines are sensitive to BCT-100 arginine depletion: IC50 ranged between 50 and 180 ng/mL (supplemental Figure 1B). No further decrease in cell number was seen by increasing concentrations of BCT-100 above 600 ng/mL, consistent with the depletion of arginine at this drug concentration and the specificity of drug action. In vivo experiments previously demonstrated that a single BCT-100 dose results in a sustained depletion of plasma arginine levels persisting for 6 days.7 We confirmed that BCT-100 led to a significant decrease in plasma arginine (mean: 20 μM healthy vs 8.5 μM AML mice; P = .0244) (Figure 2A) and treated mice had a significant reduction in AML engraftment (human CD45+ cells: median 21% untreated vs 5% BCT treated, P = .029) (Figure 2B), which was approximately equivalent to 25 mg/kg cytarabine (median 6%). No evidence of toxicity or weight loss was observed (Figure 2C).
Figure 2.
BCT-100 arginine depletion reduces the number of viable AML blasts in vitro and in vivo. (A) Plasma from control and BCT-100 treated NOG mice were collected after 14 days. The concentration of arginine was determined by ELISA. BCT-100 significantly lowers the plasma arginine concentration in vivo (P < .0244). (B) NOG mice were injected with HL-60 AML blasts. BCT-100 (5 mg/kg) or cytarabine (25 mg/kg) was given i.p. injections twice a week. Bone marrow was sampled from the femurs after 5 weeks to assess hCD45+ cells by flow cytometry. BCT-100 leads to significantly lower AML engraftment (P = .029), equivalent to cytarabine treatment. Data are representative of 2 independent experiments. (C) Untreated and BCT-100 NOG mice engrafted with HL-60 AML showed no significant difference in body weight in response to treatment.
BCT-100 demonstrates activity against primary AML blasts from patients
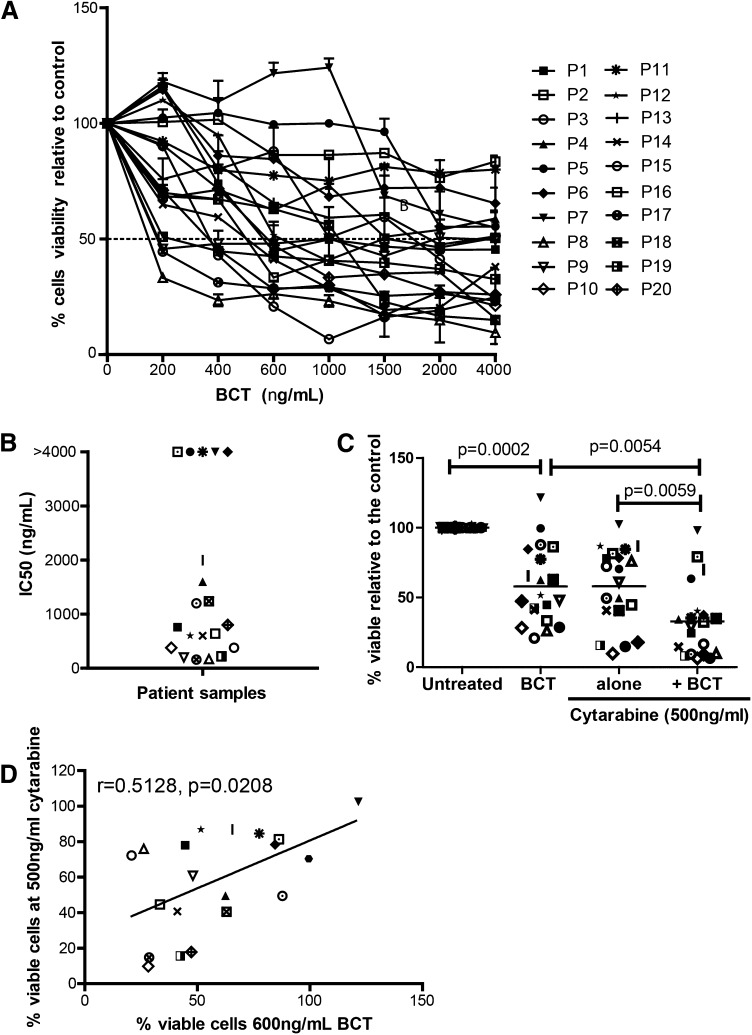
The activity of BCT-100 was tested against sorted, fresh blasts from 20 AML patients. Samples varied in response, ranging from >95% cell death to completely resistant (Figure 3A). IC50 ranged from 100 ng/mL to 2000 ng/mL (Figure 3B). In 5 samples, <50% death occurred, and no increase in activity was seen even with doses up to 16 000 ng/mL, confirming that BCT-100 does not act through off-target toxicity. Sensitivity to BCT-100 did not correlate with clinical characteristics. BCT-100 was also cytotoxic to 3 of the 4 relapse samples (P7, P14, P19, P20). This is the first report of the efficacy of arginine depletion against human AML blasts.
Figure 3.
BCT-100 is cytotoxic against primary blasts from patients. (A) AML blasts from 20 newly diagnosed patients were cultured with BCT-100 (0-4000 ng/mL) for 72 hours. The percentage of viable blasts relative to untreated was determined by flow cytometry. BCT-100 leads to a dose-dependent decrease in AML blast viability. (B) IC50 values for the activity of BCT-100 against AML patient blasts are shown. (C) AML blasts from patients were cultured with 600 ng/mL BCT-100 (OBD) alone, 500 ng/mL cytarabine, or both for 72 hours. The percentage of viable cells relative to control after 72 hours was measured by flow cytometry. BCT-100 cytotoxicity is synergistic in combination with cytarabine (BCT vs combination, P = .0054; cytarabine vs combination, P = .0059; 2-way ANOVA: F(1,57) = 6.405, P < .0001). (D) The percentage of viable cells following treatment with 600 ng/mL BCT-100 and 500 ng/mL cytarabine was correlated. Sensitivity to BCT-100 correlates moderately with sensitivity to cytarabine (r = 0.5128, P = .0208).
BCT-100 synergizes with cytarabine against AML blasts from patients
Cytarabine is a key agent in AML chemotherapy protocols for adults and children.19,20 However, patient AML blasts can develop cytarabine resistance. Therefore, a new drug which resensitizes blasts to cytarabine may play a key role in future therapy.21,22 When BCT-100 was combined with cytarabine, cytotoxicity that is greater than the sum of the 2 individual compounds alone was seen in AML samples (F(1,57) = 6.405, P < .0001) (Figure 3C). Analysis of individual patient samples showed that BCT-100 synergized with cytarabine (CI < 1) for almost all samples (Table 2). BCT-100 sensitivity correlated moderately with sensitivity to cytarabine (r = 0.5182, P = .0280), suggesting complementary mechanisms of activity of these 2 drugs (Figure 3D).
Table 2.
CI calculated according to the Chou-Talalay method
| Patient | Chou-Talalay CI |
|---|---|
| P1 | 0.55 |
| P2 | 0.32 |
| P3 | 0.30 |
| P4 | 0.42 |
| P5 | 0.63 |
| P6 | 0.13 |
| P7 | 0.81 |
| P8 | 0.27 |
| P9 | 0.43 |
| P10 | 0.43 |
| P11 | 0.02 |
| P12 | 0.48 |
| P13 | 0.37 |
| P14 | 0.27 |
| P15 | 0.04 |
| P16 | 0.81 |
| P17 | 0.21 |
| P18 | 0.99 |
| P19 | 0.36 |
| P20 | 0.35 |
CI calculated according to the Chou-Talalay method, using Compusyn software.13
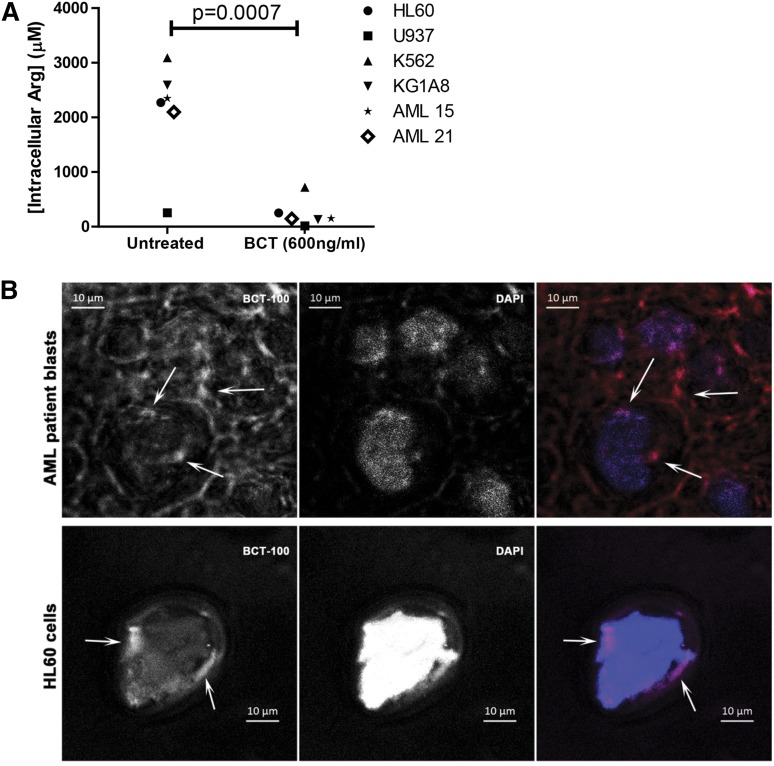
BCT-100 reduces intracellular arginine concentrations
Having demonstrated in supplementary Figures 1 and 2 that BCT-100 reduces local arginine concentrations, we also showed that BCT-100 led to a significant decrease in AML intracellular arginine (P < .0007) (Figure 4A). Although BCT-100 is a relatively large molecule and acts extracellularly, some BCT-100 molecules could also be internalized. Using fluorescently labeled BCT-100, this drug conjugate bound to the cell surface of AML blasts and was internalized (Figure 4B, supplemental Figure 2). The percentage of surface-bound BCT-100 increased moderately over time (supplemental Figure 3). No correlation was identified between the sensitivity of AML blasts and the percentage of internalized BCT-100.
Figure 4.
BCT-100 depletes arginine intracellularly. (A) Cell lines or patient samples were cultured with BCT-100 (600 ng/mL) for 72 hours. Intracellular arginine concentrations were measure by ELISA. BCT-100 causes a depletion of intracellular arginine. Data are representative of 2 independent experiments. (B) Internalization of BCT-100-AF647. AML blasts (top panel) and HL60 cells (bottom panel) were incubated with fluorescently labeled BCT-100 for 8 hours. Unbound drug was removed with stripping buffer and extensive washing. Nucleus was stained with 4,6 diamidino-2-phenylindole (DAPI). Images of representative cells were collected by LSM510 system (Zeiss). Arrows indicate intracellular localization of labeled BCT-100; arrowheads indicate surface-bound drug. Scale, 10 μm. Representative patient sample of 3 different patient samples.
BCT-100 induces cell cycle arrest and death by necrosis in AML blasts
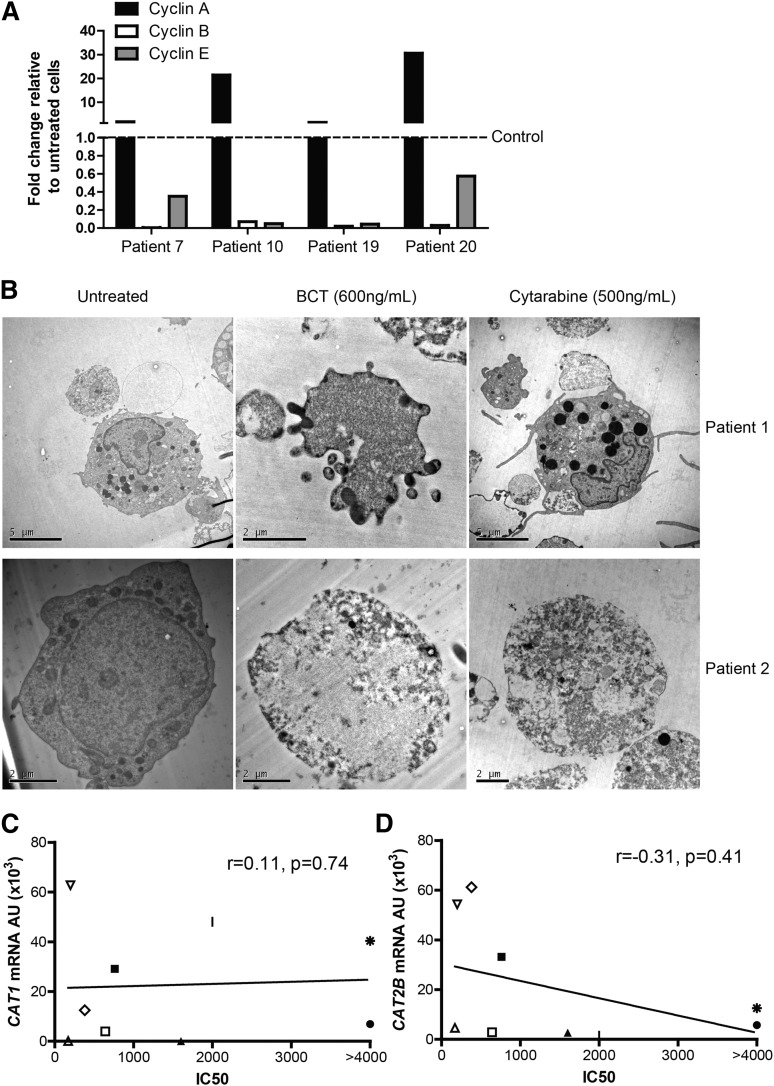
Conventional chemotherapy agents lead to a reduction in leukemia cell numbers through either cell cycle arrest or cell death. To investigate BCT-100 cytotoxicity and its mechanism, we show that arginine depletion leads to a significant arrest of AML proliferation (Figure 5A-B). Cell cycle analysis showed an increase in the percentage of cells in G0/G1, with decreases of those in S phase (Figure 5C-D). G0/G1 arrest was confirmed in the blasts of AML patients treated with BCT-100, by the relative increase in cyclin A expression, and decreases in cyclin B and E, compared with untreated cells (Figure 6A).
Figure 5.
BCT-100 halts proliferation and cell cycle arrest. (A) BCT-100 halts AML cell division. CFSE-labeled cell lines were cultured in the presence of 600 ng/mL BCT-100. Representative histogram plots shown. Independent experiments were performed on 2 separate occasions. (B) Cell lines were cultured with BCT-100 (0-2000 ng/mL) for 72 hours. AML proliferation was measured by 3H-thymidine incorporation after 72 hours. Data are representative of 2 independent experiments. BCT-100 causes a dose-dependent decrease in AML proliferation. (C) AML cell lines were cultured with 600 ng/mL BCT-100. Cell cycle analysis was performed after 72 hours. BCT-100 increases the percentage of cells in G0/G1 arrest. Representative histogram plots for untreated and treated HL-60 shown. Independent experiments were performed on 4 separate occasions. (D) Table showing the relative percentages of cells in G0/G1, S, G2/M based on flow cytometry cell cycle analysis.
Figure 6.
BCT-100 induced cell cycle arrest leads to necrotic cell death. (A) Relative expression of cyclins A, B, E in BCT-100–treated AML patient blasts compared with untreated controls (hashed line) were investigated by qPCR. Representative data of 4 patients shown. (B) AML blasts from patients were treated with BCT-100 (600 ng/mL) or cytarabine (500 ng/mL) for 72 hours. Analysis of cell death was performed by transmission electron microscopy. Representative micrographs of 2 of 5 patients shown. Left panel, Untreated cells. Middle panels, Posttreatment with 600 ng/mL BCT-100. Features consistent with organelle enlargement and cell membrane permeabilization. Right panels, Posttreatment with 500 ng/mL cytarabine. Features consistent with nuclear fragmentation bodies and preserved membrane integrity. Experiments performed on 3 separate occasions. (C) Sensitivity to BCT-100 does not correlate with CAT1 expression (D) and only mildly with CAT-2B expression (r = −0.31, P = .41).
Cell cycle arrest may result in either a steady state of viable cells or in cell death. Examining cell morphology by transmission electron microscopy, BCT-100 caused cell death of AML blasts, with features more consistent with necrosis–including cell membrane permeabilization and organelle enlargement (Figure 6B, supplemental Figure 4 top panel).23 In contrast, cytarabine-treated AML blasts showed nuclear fragmentation bodies characteristic of apoptosis. There was no evidence of cell death in treated normal T cells or monocytes, confirming the low toxicity against nonmalignant hematopoietic cells seen in adult early-phase trials of BCT-100 (supplemental Figure 4).
Nonspecific growth factor withdrawal has been associated with induction of the intrinsic pathway of apoptosis in AML.24 BCT-100 did not induce any significant activation of the requisite effector caspases-9 and -3 or poly ADP ribose polymerase cleavage (supplemental Figure 5A).25 In addition, no significant increase in PI+/annexin+ cells was seen following BCT-100 treatment (supplemental Figure 5B-C). Amino acid deprivation has also been associated with the induction of death by autophagy in AML, identified by the conversion of cytoplasmic LC3-I to autophagosomic LC3-II.26-29 No increase in LC3-II was seen following BCT-100 treatment, confirming the absence of autophagy seen in transmission electron microscopy (supplemental Figure 5D). Amino acid deprivation can induce the rapid production of reactive oxygen species which induce cell death, but no evidence for this was found following BCT treatment (supplemental Figure 6A, data not shown).30
Biomarkers of sensitivity to BCT
Understanding characteristics of AML blasts which correlate with drug sensitivity is important for patient risk stratification. Of the 20 AML samples tested for sensitivity to BCT-100 in vitro, ASS and OTC expression did not correlate with response to BCT-100, consistent with our previous reports in adult solid tumors (supplemental Figure 6B). CAT-1 and CAT-2B expression did not correlate strongly with BCT sensitivity (CAT-1, r = 0.11, P = .74; CAT-2B r = −0.31, P = .41) (Figure 6C-D, supplemental Figure 6C).
As multiple pathways, other than arginine recycling, may be important in AML pathogenesis, we used RNA sequencing to identify genes predictive of BCT-100 response. RNA was isolated from 6 sensitive and 6 resistant patient samples, based on their IC50 values posttreatment with BCT-100 (Figure 3B). We identified 20 genes that were differentially expressed between resistant and sensitive samples (Table 3). The top differentially expressed gene was EREG (epiregulin), which codes for a ligand of the epidermal growth factor family (EGF) capable of binding to the EGF receptor and the ERBB (ERB-B2 avian erythoblastic leukemia viral oncogene homolog) family of tyrosine kinase receptors. Other genes of interest included ZIC5, which have a zinc finger protein known to be an upstream regulator of the Wnt pathway; EN2, a homeobox gene that regulates the forkhead box transcription factor FOXA2; HSPA6, a heat shock–associated protein that seems to be associated with drug sensitivity in AML; and RGL3, a paralog of RASGRF2.
Table 3.
Table of ranked gene expression in arginase-sensitive vs resistant cells as determined via RNA-seq
| Rank | Gene | Ensembl ID | Name | log2 (fold change) | P | q |
|---|---|---|---|---|---|---|
| 1 | EREG | ENSG00000124882 | Epiregulin | 4.61947 | 5 × 10−5 | 0.03 |
| 2 | CCL4 | ENSG00000129277 | Chemokine (C-C motif) ligand 4 | −3.42043 | 5 × 10−5 | 0.03 |
| 3 | ZIC5 | ENSG00000139800 | Zic family member 5 | >30 | 5 × 10−5 | 0.03 |
| 4 | EN2 | ENSG00000164778 | Engrailed homeobox 2 | >30 | 5 × 10−5 | 0.03 |
| 5 | HSPA6 | ENSG00000173110 | Heat shock 70-kDa protein 6 | −4.11747 | 5 × 10−5 | 0.03 |
| 6 | RGL3 | ENSG00000205517 | Ral guanine nucleotide dissociation stimulator-like 3 | >30 | 5 × 10−5 | 0.03 |
| 7 | C17orf98 | ENSG00000214556 | Chromosome 17 open reading frame 98 | >30 | 5 × 10−5 | 0.03 |
| 8 | RP11-65C6.1 | ENSG00000217684 | Ribosomal protein S3a pseudogene | >30 | 5 × 10−5 | 0.03 |
| 9 | EIF3EP2 | ENSG00000224674 | Eukaryotic translation initiation factor 3, subunit E pseudogene 2 | >30 | 5 × 10−5 | 0.03 |
| 10 | RP1-272E8.1 | ENSG00000225066 | X chromosome processed pseudogene | >30 | 5 × 10−5 | 0.03 |
| 11 | AC009313.2 | ENSG00000232337 | Pseudogene | >30 | 5 × 10−5 | 0.03 |
| 12 | AC007386.2 | ENSG00000237638 | Pseudogene | >30 | 5 × 10−5 | 0.03 |
| 13 | DDR1-AS1 | ENSG00000237775 | DDR1 antisense RNA 1 | >30 | 5 × 10−5 | 0.03 |
| 14 | IGKV1-17 | ENSG00000240382 | Immunoglobulin κ variable 1-17 | >30 | 5 × 10−5 | 0.03 |
| 15 | OR10J2P | ENSG00000248642 | Olfactory receptor, family 10, subfamily J, member 2 pseudogene | >30 | 5 × 10−5 | 0.03 |
| 16 | RP11-263I1.1 | ENSG00000248659 | lncRNA | >30 | 5 × 10−5 | 0.03 |
| 17 | CIR1P1 | ENSG00000253146 | Corepressor interacting with RBPJ, 1 pseudogene 1 | >30 | 5 × 10−5 | 0.03 |
| 18 | NF1P1 | ENSG00000258997 | Neurofibromin 1 pseudogene 1 | >30 | 5 × 10−5 | 0.03 |
| 19 | RP11-476D10.1 | ENSG00000260943 | lncRNA | >30 | 5 × 10−5 | 0.03 |
| 20 | RP11-152O14.1 | ENSG00000261749 | lncRNA | >30 | 5 × 10−5 | 0.03 |
lncRNA, long noncoding RNA.
In comparing FPKM between resistant and sensitive cells (Table 4), there were 2 differentially expressed arginine pathway–associated genes. Overexpression of the AKT1 gene (ΔFPKM = 7.1) was observed in sensitive cells, although there was no change in mammalian target of rapamycin (MTOR) gene expression (ΔFPKM = −0.704). Type I arginase (ARG1, ΔFPKM = 8.87) was also overexpressed in sensitive cells as compared with resistant cells. No changes in arginine transporter genes (SLC7A1-4) or ASS and OTC were identified. These findings identify pathways that might be predictive of sensitivity to therapeutic arginine depletion in AML, and shed light on new aspects of AML disease biology.
Table 4.
Table of FPKM for resistant vs sensitive AML lines for arginine-related genes
| Ensembl Gene ID | Gene | Resistant FPKM | Sensitive FPKM | Change in FPKM | Direction |
|---|---|---|---|---|---|
| ENSG00000007171 | NOS2 | 0.000 | 0.000 | 0.000 | — |
| ENSG00000118520 | ARG1 | 1.471 | 10.333 | 8.862 | Over |
| ENSG00000081181 | ARG2 | 0.398 | 0.054 | −0.344 | — |
| ENSG00000126522 | ASL | 2.248 | 2.522 | 0.274 | — |
| ENSG00000130707 | ASS1 | 0.062 | 0.036 | −0.026 | — |
| ENSG00000036473 | OTC | 0.000 | 0.078 | 0.078 | — |
| ENSG00000139514 | SLC7A1 | 0.705 | 0.295 | −0.410 | — |
| ENSG00000003989 | SLC7A2 | 0.028 | 0.309 | 0.281 | — |
| ENSG00000165349 | SLC7A3 | 0.000 | 0.003 | 0.003 | — |
| ENSG00000099960 | SLC7A4 | 0.001 | 0.001 | 0.000 | — |
| ENSG00000142208 | AKT1 | 5.602 | 12.701 | 7.100 | Over |
| ENSG00000198793 | MTOR | 1.560 | 0.856 | −0.704 | — |
| ENSG00000121879 | PIK3CA | 1.327 | 0.741 | −0.586 | — |
Discussion
Normal myeloid cells differ in their requirement for arginine, ranging from maintenance of neutrophil activity, through to the consumption of arginine by myeloid-derived suppressor cells and inflammatory macrophages.31-33 We previously showed that AML blasts have a high arginase activity, creating an immunosuppressive microenvironment.13 However, the mechanism by which arginine is imported from the microenvironment by AML blasts had not been described. We identify that human AML blasts predominantly express the CAT-1 and CAT-2B isoforms. Interrogation of the “R2: microarray analysis and visualization platform” (http://r2.amc.nl) confirms the expression of these transporters across all French-American-British subtypes of AML. The role of the CAT family of proteins in hematopoiesis has only received limited study, identifying CAT-1 and CAT-2B as the main transporters responsible for arginine uptake in in vitro models of nonmalignant myeloid and erythroid cells.34-36
Arginine can be produced within healthy cells, by the ornithine-citrulline-arginine cycle.37,38 We identified that the majority of patients’ blasts are deficient in either ASS or OTC enzymes, strongly suggesting that AML blasts are reliant on extracellular arginine availability. The contribution of bone marrow stroma in producing arginine to support AML expansion is unknown, but analogous mechanisms exist. For example, central nervous system neurons are reliant on astrocyte-derived arginine, and asparaginine is produced by bone marrow stromal cells in cases of acute lymphoblastic leukemia (ALL).39,41 Additionally, it has been shown that ASS has a tumor suppressor function in sarcoma,41,42 perhaps suggesting that ASS may play a more fundamental role in the malignant transformation of blasts.
These findings are consistent with the auxotrophic requirement of arginine by AML blasts. We identified that the need for arginine by AML blasts led to a significant decrease in arginine concentrations both locally and also in the plasma of patients at diagnosis. To our knowledge, this is the first report of a fall in plasma arginine due to a hematologic malignancy and plasma arginine may correlate with AML disease burden. Decreases in plasma arginine concentrations occur in cervical cancer and renal cell carcinoma patients.43,44 Physiological compensation through protein breakdown, contributing to cancer induced cachexia, and arginine recycling by the intestinal-renal axis may try to compensate for lowered arginine levels.45
Because arginine is essential for proliferation and maintenance of AML viability, arginine starvation by the PEG recombinant arginase BCT-100 should be cytotoxic to AML, as in other tumor types. Normal human arginase has limited clinical value because of a short plasma half-life.7 The addition of a 5000-molecular-weight polyethylene glycol molecule significantly increases the plasma half-life of arginase 1 from 10 to 20 minutes to up to 3.5 days in humans with minimal loss of enzyme activity.8
We report for the first time that the majority of AML patients’ blasts from children and adults, including those at relapse, are sensitive to arginine depletion, leading to necrotic cell death. BCT-100 may be internalized by AML blasts, consistent with other PEG molecules in myeloid cells, which would further contribute to low intracellular arginine concentrations.46 BCT-100 can work in combination with cytarabine. Cytarabine in its triphosphorylated form is a substrate for DNA polymerases and is incorporated into phosphodiester linkages in the DNA strand. The addition of subsequent deoxynucleotides is inhibited, resulting in S-phase arrest and cell death.19,20 As BCT-100 may induce G0/G1 arrest, the synergistic effect of the 2 agents may be due to their targeting of different phases of the cell cycle. Similar findings have been described against T-cell acute lymphoblastic leukemia.47 Nonmalignant cells show little cytotoxicity to BCT-100 because the cells tend to become quiescent, a state they can survive in for prolonged periods, due to intact restriction (R) checkpoints.48,49 However, tumor cells are less tolerant of this condition, and metabolic stressors may induce G1 cell cycle arrest and ultimately death by necrosis in tumor cells.50 Tumor-specific arginine requirements and the concurrent use of drugs to drive cell death along a particular mechanistic pathway may explain tumor-specific effects of arginine depletion.51-53
RNA sequencing of sensitive and resistant samples identified 20 genes which predict response to arginine depletion. Pathway analysis confirmed that expression of arginine recycling or transport molecules did not correlate with sensitivity to arginine depletion. Most intriguing was the finding that EREG was differentially expressed, with arginase-sensitive AML overexpressing EREG. Overexpressed EREG has been linked to dysregulation of mitogen-activated protein kinase signaling via the EGF pathway as well as the ERBB pathway, and this overexpression in the sensitive cells may reflect an underlying drive toward the mitogen-activated protein kinase/ERBB pathways.54,55 Conversely, in the arginase-resistant cells, overexpression of the heat shock protein HSPA6 was demonstrated, suggesting that this may be a mechanism by which these cells attain resistance, especially in light of the finding that HSP inhibition is cytotoxic to AML.56 EREG and EGFR signaling, as well as the heat shock protein family, play key roles in solid tumor cell proliferation. Small-molecule inhibitors are currently under development in AML to target these pathways, providing the potential for rational combinations of other drugs with BCT-100 for maximal anti-leukemia effect.57,58
The findings could have important translational consequences for a disease which is in desperate need of new therapies. Although small-molecule arginase pathway inhibitors are available for laboratory use (NG-hydroxy-l-arginine [NOHA] and L-NG-monomethyl arginine [L-NMMA]),11 their clinical application has been limited by the requirement for the molecules to be given together and by off-target toxicity in vivo. An alternative approach is through the depletion of arginine from the microenvironment, thus starving AML blasts of this key amino acid.
Preclinically, BCT-100 has demonstrable activity against hepatocellular, melanoma, and prostate carcinoma. A phase 1 and 2 clinical trial has been completed in adults with refractory hepatocellular carcinoma, in which 1600 U/kg BCT-100 (optimal biological dose) resulted in plasma arginine falling below 8 µM (adequate arginine depletion [ADD]) and can be maintained for up to 5 or more days after a single treatment (the maintenance level of arginine in human blood is ∼40 µM). Interim analysis of patients enrolled in a phase 2 trial in hepatocellular carcinoma suggests that patients experience significant improvements in overall survival, comparable to the standard of care.8 For both pediatric and elderly AML patients in particular, concerns over treatment side-effects limit the type of therapies that can be given safely.59,60 In this study, we show that arginine depletion is not cytotoxic to T cells and monocytes and is well tolerated in vivo. Clinically, this is supported by the excellent toxicity profile of BCT-100 in trial with no evidence of increased patient infections.
Two alternative arginine-depleting enzymes are also undergoing early preclinical and clinical evaluation, but have been subject to a number of limiting toxicities. The first is arginine deiminase (ADI)–polyethylene glycol, a PEG form of mycoplasma-derived arginine deiminase.61 ADI converts arginine into citrulline and ammonia, potentially leading to toxic hyperammonemia, and ensuing neutropenia.61 The bacterial origin of the molecule leads to neutralizing antibody formation and intramuscular injection site hypersensitivity reactions, limiting continued drug administration and a failure to sustain adequately low plasma arginine.62,63 An alternative PEG human arginase has also been described, in which the enzyme cofactor has been replaced with cobalt to increase arginase activity,52 but unfortunately seems in early preclinical studies to be significantly more toxic. Thus, the natural enzyme seems to be the best option.
A similar paradigm of bacterial vs recombinant protein therapy occurred in pediatric ALL, with polyethylene glycol–asparaginase. The bacterial derivative (Erwinia) was eventually superseded by recombinant asparaginase, due to side-effects such as immunogenicity. Recombinant asparaginase has resulted in significant improvements in overall survival for children and its incorporation into upfront treatment protocols.64
The findings of our study highlight a role for arginine in AML pathogenesis and support the ongoing development of BCT-100 and similar arginase preparations in early phase trials for patients with AML.
Acknowledgments
The authors thank the patients and parents who contributed samples to the study. Additionally, the authors thank Jane Cooper and Cay Shakespeare for consent and collection of patient samples, Chen-Li for provision of BCT-100, Paul Stanley and Theresa Morris for technical assistance with electron microscopy, Manoj Raghavan for identifying patients, and Denys Wheatley for review of the manuscript.
This work was supported by the Amber Phillpott Trust, Children with Cancer, the Birmingham Children’s Hospital Research Fund, and Cancer Research UK.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: F.M. and C.D.S. designed the study, performed research, analyzed data, and wrote the manuscript; F.M. additionally secured ethical approval and was chief investigator of the study; S.E. designed and performed research; J.H.-J. performed research; T.P. and P.K. provided access to murine xenografts; A.B. performed RNA-sequencing analysis; E.O. performed confocal microscopy; J.L. and G.P. provided patient samples; A.L. and M.N. provided patient samples and performed immunohistochemistry; and P.C. and K.P.U. provided BCT-100.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Francis Mussai, School of Cancer Sciences, University of Birmingham, Birmingham, United Kingdom; e-mail: francis.mussai@bch.nhs.uk.
References
- 1.Marcucci G, Haferlach T, Dohner H. Molecular genetics of adult acute myeloid leukemia: prognostic and therapeutic implications. J Clin Oncol. 2011;29(5):475–486. doi: 10.1200/JCO.2010.30.2554. [DOI] [PubMed] [Google Scholar]
- 2.Gibson BE, Wheatley K, Hann IM, et al. Treatment strategy and long-term results in paediatric patients treated in consecutive UK AML trials. Leukemia. 2005;19(12):2130–2138. doi: 10.1038/sj.leu.2403924. [DOI] [PubMed] [Google Scholar]
- 3.De Santo C, Serafini P, Marigo I, et al. Nitroaspirin corrects immune dysfunction in tumor-bearing hosts and promotes tumor eradication by cancer vaccination. Proc Natl Acad Sci USA. 2005;102(11):4185–4190. doi: 10.1073/pnas.0409783102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brenner T, Fleming TH, Rosenhagen C, et al. L-arginine and asymmetric dimethylarginine are early predictors for survival in septic patients with acute liver failure. Mediators Inflamm. 2012;2012:210454. doi: 10.1155/2012/210454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Santo C, Salio M, Masri SH, et al. Invariant NKT cells reduce the immunosuppressive activity of influenza A virus-induced myeloid-derived suppressor cells in mice and humans. J Clin Invest. 2008;118(12):4036–4048. doi: 10.1172/JCI36264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wheatley DN. Arginine deprivation and metabolomics: important aspects of intermediary metabolism in relation to the differential sensitivity of normal and tumour cells. Semin Cancer Biol. 2005;15(4):247–253. doi: 10.1016/j.semcancer.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Cheng PN, Lam TL, Lam WM, et al. Pegylated recombinant human arginase (rhArg-peg5,000mw) inhibits the in vitro and in vivo proliferation of human hepatocellular carcinoma through arginine depletion. Cancer Res. 2007;67(1):309–317. doi: 10.1158/0008-5472.CAN-06-1945. [DOI] [PubMed] [Google Scholar]
- 8.Yau T, Cheng PN, Chan P, et al. Preliminary efficacy, safety, pharmacokinetics, pharmacodynamics and quality of life study of pegylated recombinant human arginase 1 in patients with advanced hepatocellular carcinoma [published online ahead of print February 10, 2015]. Invest New Drugs. doi: 10.1007/s10637-014-0200-8. doi:10.1007/s10637-014-0200-8. [DOI] [PubMed] [Google Scholar]
- 9.Lam TL, Wong GK, Chow HY, et al. Recombinant human arginase inhibits the in vitro and in vivo proliferation of human melanoma by inducing cell cycle arrest and apoptosis. Pigment Cell Melanoma Res. 2011;24(2):366–376. doi: 10.1111/j.1755-148X.2010.00798.x. [DOI] [PubMed] [Google Scholar]
- 10.Hsueh EC, Knebel SM, Lo WH, Leung YC, Cheng PN, Hsueh CT. Deprivation of arginine by recombinant human arginase in prostate cancer cells. J Hematol Oncol. 2012;5:17. doi: 10.1186/1756-8722-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mussai F, De Santo C, Abu-Dayyeh I, et al. Acute myeloid leukemia creates an arginase-dependent immunosuppressive microenvironment. Blood. 2013;122(5):749–758. doi: 10.1182/blood-2013-01-480129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slinker BK. The statistics of synergism. J Mol Cell Cardiol. 1998;30(4):723–731. doi: 10.1006/jmcc.1998.0655. [DOI] [PubMed] [Google Scholar]
- 13.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 14.Morris SM., Jr Arginine metabolism: boundaries of our knowledge. J Nutr. 2007;137(6 suppl 2):1602S–1609S. doi: 10.1093/jn/137.6.1602S. [DOI] [PubMed] [Google Scholar]
- 15.Windmueller HG, Spaeth AE. Source and fate of circulating citrulline. Am J Physiol. 1981;241(6):E473–E480. doi: 10.1152/ajpendo.1981.241.6.E473. [DOI] [PubMed] [Google Scholar]
- 16.Ryall J, Nguyen M, Bendayan M, Shore GC. Expression of nuclear genes encoding the urea cycle enzymes, carbamoyl-phosphate synthetase I and ornithine carbamoyl transferase, in rat liver and intestinal mucosa. Eur J Biochem. 1985;152(2):287–292. doi: 10.1111/j.1432-1033.1985.tb09196.x. [DOI] [PubMed] [Google Scholar]
- 17.Levillain O, Hus-Citharel A, Morel F, Bankir L. Localization of arginine synthesis along rat nephron. Am J Physiol. 1990;259(6 Pt 2):F916–F923. doi: 10.1152/ajprenal.1990.259.6.F916. [DOI] [PubMed] [Google Scholar]
- 18.Closs EI, Simon A, Vékony N, Rotmann A. Plasma membrane transporters for arginine. J Nutr. 2004;134(suppl 10):2752S–2759S, discussion 2765S-2767S. doi: 10.1093/jn/134.10.2752S. [DOI] [PubMed] [Google Scholar]
- 19.Major PP, Egan EM, Beardsley GP, Minden MD, Kufe DW. Lethality of human myeloblasts correlates with the incorporation of arabinofuranosylcytosine into DNA. Proc Natl Acad Sci USA. 1981;78(5):3235–3239. doi: 10.1073/pnas.78.5.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Major PP, Egan EM, Herrick DJ, Kufe DW. Effect of ARA-C incorporation on deoxyribonucleic acid synthesis in cells. Biochem Pharmacol. 1982;31(18):2937–2940. doi: 10.1016/0006-2952(82)90266-0. [DOI] [PubMed] [Google Scholar]
- 21.Jabbour E, Daver N, Champlin R, et al. Allogeneic stem cell transplantation as initial salvage for patients with acute myeloid leukemia refractory to high-dose cytarabine-based induction chemotherapy. Am J Hematol. 2014;89(4):395–398. doi: 10.1002/ajh.23655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leonard SM, Perry T, Woodman CB, Kearns P. Sequential treatment with cytarabine and decitabine has an increased anti-leukemia effect compared to cytarabine alone in xenograft models of childhood acute myeloid leukemia. PLoS ONE. 2014;9(1):e87475. doi: 10.1371/journal.pone.0087475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ziegler U, Groscurth P. Morphological features of cell death. News Physiol Sci. 2004;19:124–128. doi: 10.1152/nips.01519.2004. [DOI] [PubMed] [Google Scholar]
- 24.Pallis M, Russell N. P-glycoprotein plays a drug-efflux-independent role in augmenting cell survival in acute myeloblastic leukemia and is associated with modulation of a sphingomyelin-ceramide apoptotic pathway. Blood. 2000;95(9):2897–2904. [PubMed] [Google Scholar]
- 25.Brentnall M, Rodriguez-Menocal L, De Guevara RL, Cepero E, Boise LH. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013;14:32. doi: 10.1186/1471-2121-14-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willems L, Jacque N, Jacquel A, et al. Inhibiting glutamine uptake represents an attractive new strategy for treating acute myeloid leukemia. Blood. 2013;122(20):3521–3532. doi: 10.1182/blood-2013-03-493163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheong H, Lu C, Lindsten T, Thompson CB. Therapeutic targets in cancer cell metabolism and autophagy. Nat Biotechnol. 2012;30(7):671–678. doi: 10.1038/nbt.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taatjes DJ, Sobel BE, Budd RC. Morphological and cytochemical determination of cell death by apoptosis. Histochem Cell Biol. 2008;129(1):33–43. doi: 10.1007/s00418-007-0356-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eskelinen EL, Reggiori F, Baba M, Kovács AL, Seglen PO. Seeing is believing: the impact of electron microscopy on autophagy research. Autophagy. 2011;7(9):935–956. doi: 10.4161/auto.7.9.15760. [DOI] [PubMed] [Google Scholar]
- 30.Brown RD, Burke GA, Brown GC. Dependence of leukemic cell proliferation and survival on H2O2 and L-arginine. Free Radic Biol Med. 2009;46(8):1211–1220. doi: 10.1016/j.freeradbiomed.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Kapp K, Prüfer S, Michel CS, et al. Granulocyte functions are independent of arginine availability. J Leukoc Biol. 2014;96(6):1047–1053. doi: 10.1189/jlb.3AB0214-082R. [DOI] [PubMed] [Google Scholar]
- 32.Mussai F, De Santo C, Cerundolo V. Interaction between invariant NKT cells and myeloid-derived suppressor cells in cancer patients: evidence and therapeutic opportunities. J Immunother. 2012;35(6):449–459. doi: 10.1097/CJI.0b013e31825be926. [DOI] [PubMed] [Google Scholar]
- 33.Chang CI, Liao JC, Kuo L. Macrophage arginase promotes tumor cell growth and suppresses nitric oxide-mediated tumor cytotoxicity. Cancer Res. 2001;61(3):1100–1106. [PubMed] [Google Scholar]
- 34.Fotiadis D, Kanai Y, Palacín M. The SLC3 and SLC7 families of amino acid transporters. Mol Aspects Med. 2013;34(2-3):139–158. doi: 10.1016/j.mam.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 35.Barilli A, Rotoli BM, Visigalli R, Bussolati O, Gazzola GC, Dall’Asta V. Arginine transport in human monocytic leukemia THP-1 cells during macrophage differentiation. J Leukoc Biol. 2011;90(2):293–303. doi: 10.1189/jlb.0910510. [DOI] [PubMed] [Google Scholar]
- 36.Shima Y, Maeda T, Aizawa S, et al. L-arginine import via cationic amino acid transporter CAT1 is essential for both differentiation and proliferation of erythrocytes. Blood. 2006;107(4):1352–1356. doi: 10.1182/blood-2005-08-3166. [DOI] [PubMed] [Google Scholar]
- 37.Evans RW, Fernstrom JD, Thompson J, Morris SM, Jr, Kuller LH. Biochemical responses of healthy subjects during dietary supplementation with L-arginine. J Nutr Biochem. 2004;15(9):534–539. doi: 10.1016/j.jnutbio.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 38.Wu G, Morris SM., Jr Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336(Pt 1):1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiesinger H. Arginine metabolism and the synthesis of nitric oxide in the nervous system. Prog Neurobiol. 2001;64(4):365–391. doi: 10.1016/s0301-0082(00)00056-3. [DOI] [PubMed] [Google Scholar]
- 40.Iwamoto S, Mihara K, Downing JR, Pui C-H, Campana D. Mesenchymal cells regulate the response of acute lymphoblastic leukemia cells to asparaginase. J Clin Invest. 2007;117(4):1049–1057. doi: 10.1172/JCI30235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang HY, Wu WR, Wang YH, et al. ASS1 as a novel tumor suppressor gene in myxofibrosarcomas: aberrant loss via epigenetic DNA methylation confers aggressive phenotypes, negative prognostic impact, and therapeutic relevance. Clin Cancer Res. 2013;19(11):2861–2872. doi: 10.1158/1078-0432.CCR-12-2641. [DOI] [PubMed] [Google Scholar]
- 42.Lan J, Tai HC, Lee SW, Chen TJ, Huang HY, Li CF. Deficiency in expression and epigenetic DNA methylation of ASS1 gene in nasopharyngeal carcinoma: negative prognostic impact and therapeutic relevance. Tumour Biol. 2014;35(1):161–169. doi: 10.1007/s13277-013-1020-8. [DOI] [PubMed] [Google Scholar]
- 43.Hasim A, Aili A, Maimaiti A, Mamtimin B, Abudula A, Upur H. Plasma-free amino acid profiling of cervical cancer and cervical intraepithelial neoplasia patients and its application for early detection. Mol Biol Rep. 2013;40(10):5853–5859. doi: 10.1007/s11033-013-2691-3. [DOI] [PubMed] [Google Scholar]
- 44.Rodriguez PC, Ernstoff MS, Hernandez C, et al. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69(4):1553–1560. doi: 10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buijs N, Luttikhold J, Houdijk AP, van Leeuwen PA. The role of a disturbed arginine/NO metabolism in the onset of cancer cachexia: a working hypothesis. Curr Med Chem. 2012;19(31):5278–5286. doi: 10.2174/092986712803833290. [DOI] [PubMed] [Google Scholar]
- 46.Zhan X, Tran KK, Shen H. Effect of the poly(ethylene glycol) (PEG) density on the access and uptake of particles by antigen-presenting cells (APCs) after subcutaneous administration. Mol Pharm. 2012;9(12):3442–3451. doi: 10.1021/mp300190g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hernandez CP, Morrow K, Lopez-Barcons LA, et al. Pegylated arginase I: a potential therapeutic approach in T-ALL. Blood. 2010;115(25):5214–5221. doi: 10.1182/blood-2009-12-258822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Philip R, Campbell E, Wheatley DN. Arginine deprivation, growth inhibition and tumour cell death: 2. Enzymatic degradation of arginine in normal and malignant cell cultures. Br J Cancer. 2003;88(4):613–623. doi: 10.1038/sj.bjc.6600681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scott L, Lamb J, Smith S, Wheatley DN. Single amino acid (arginine) deprivation: rapid and selective death of cultured transformed and malignant cells. Br J Cancer. 2000;83(6):800–810. doi: 10.1054/bjoc.2000.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Degenhardt K, Mathew R, Beaudoin B, et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006;10(1):51–64. doi: 10.1016/j.ccr.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lam TL, Wong GK, Chong HC, et al. Recombinant human arginase inhibits proliferation of human hepatocellular carcinoma by inducing cell cycle arrest. Cancer Lett. 2009;277(1):91–100. doi: 10.1016/j.canlet.2008.11.031. [DOI] [PubMed] [Google Scholar]
- 52.Morrow K, Hernandez CP, Raber P, et al. Anti-leukemic mechanisms of pegylated arginase I in acute lymphoblastic T-cell leukemia. Leukemia. 2013;27(3):569–577. doi: 10.1038/leu.2012.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zeng X, Li Y, Fan J, et al. Recombinant human arginase induced caspase-dependent apoptosis and autophagy in non-Hodgkin’s lymphoma cells. Cell Death Dis. 2013;4:e840. doi: 10.1038/cddis.2013.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ufkin ML, Peterson S, Yang X, Driscoll H, Duarte C, Sathyanarayana P. miR-125a regulates cell cycle, proliferation, and apoptosis by targeting the ErbB pathway in acute myeloid leukemia. Leuk Res. 2014;38(3):402–410. doi: 10.1016/j.leukres.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Riese DJ, II, Cullum RL. Epiregulin: roles in normal physiology and cancer. Semin Cell Dev Biol. 2014;28:49–56. doi: 10.1016/j.semcdb.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Al Shaer L, Walsby E, Gilkes A, et al. Heat shock protein 90 inhibition is cytotoxic to primary AML cells expressing mutant FLT3 and results in altered downstream signalling. Br J Haematol. 2008;141(4):483–493. doi: 10.1111/j.1365-2141.2008.07053.x. [DOI] [PubMed] [Google Scholar]
- 57.Deangelo DJ, Neuberg D, Amrein PC, et al. A phase II study of the EGFR inhibitor gefitinib in patients with acute myeloid leukemia. Leuk Res. 2014;38(4):430–434. doi: 10.1016/j.leukres.2013.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lancet JE, Gojo I, Burton M, et al. Phase I study of the heat shock protein 90 inhibitor alvespimycin (KOS-1022, 17-DMAG) administered intravenously twice weekly to patients with acute myeloid leukemia. Leukemia. 2010;24(4):699–705. doi: 10.1038/leu.2009.292. [DOI] [PubMed] [Google Scholar]
- 59.Pollyea DA, Kohrt HE, Medeiros BC. Acute myeloid leukaemia in the elderly: a review. Br J Haematol. 2011;152(5):524–542. doi: 10.1111/j.1365-2141.2010.08470.x. [DOI] [PubMed] [Google Scholar]
- 60.Creutzig U, Zimmermann M, Lehrnbecher T, et al. Less toxicity by optimizing chemotherapy, but not by addition of granulocyte colony-stimulating factor in children and adolescents with acute myeloid leukemia: results of AML-BFM 98. J Clin Oncol. 2006;24(27):4499–4506. doi: 10.1200/JCO.2006.06.5037. [DOI] [PubMed] [Google Scholar]
- 61.Glazer ES, Piccirillo M, Albino V, et al. Phase II study of pegylated arginine deiminase for nonresectable and metastatic hepatocellular carcinoma. J Clin Oncol. 2010;28(13):2220–2226. doi: 10.1200/JCO.2009.26.7765. [DOI] [PubMed] [Google Scholar]
- 62.Shawcross DL, Wright GA, Stadlbauer V, et al. Ammonia impairs neutrophil phagocytic function in liver disease. Hepatology. 2008;48(4):1202–1212. doi: 10.1002/hep.22474. [DOI] [PubMed] [Google Scholar]
- 63.Synakiewicz A, Stachowicz-Stencel T, Adamkiewicz-Drozynska E. The role of arginine and the modified arginine deiminase enzyme ADI-PEG 20 in cancer therapy with special emphasis on phase I/II clinical trials. Expert Opin Investig Drugs. 2014;23(11):1517–1529. doi: 10.1517/13543784.2014.934808. [DOI] [PubMed] [Google Scholar]
- 64.Dinndorf PA, Gootenberg J, Cohen MH, Keegan P, Pazdur R. FDA drug approval summary: pegaspargase (oncaspar) for the first-line treatment of children with acute lymphoblastic leukemia (ALL). Oncologist. 2007;12(8):991–998. doi: 10.1634/theoncologist.12-8-991. [DOI] [PubMed] [Google Scholar]