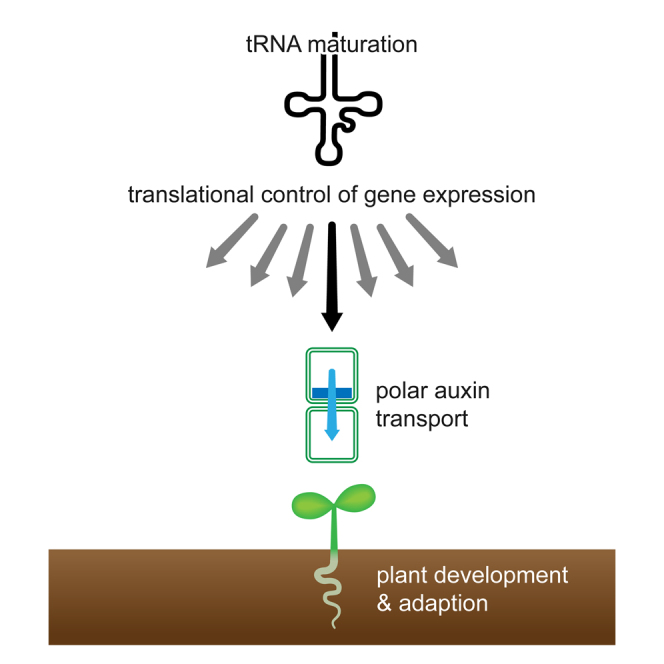
Summary
Polar transport of the phytohormone auxin throughout plants shapes morphogenesis and is subject to stringent and specific control. Here, we identify basic cellular activities connected to translational control of gene expression as sufficient to specify auxin-mediated development. Mutants in subunits of Arabidopsis Elongator, a protein complex modulating translational efficiency via maturation of tRNAs, exhibit defects in auxin-controlled developmental processes, associated with reduced abundance of PIN-formed (PIN) auxin transport proteins. Similar anomalies are observed upon interference with tRNA splicing by downregulation of RNA ligase (AtRNL), pointing to a general role of tRNA maturation in auxin signaling. Elongator Protein 6 (ELP6) and AtRNL expression patterns underline an involvement in adjusting PIN protein levels, whereas rescue of mutant defects by auxin indicates rate-limiting activities in auxin-controlled organogenesis. This emphasizes mechanisms in which auxin serves as a bottleneck for plant morphogenesis, translating common cellular activities into defined developmental readouts.
Graphical Abstract
Highlights
-
•
Arabidopsis Elongator post-transcriptionally modulates PIN protein levels
-
•
Arabidopsis tRNA splicing modulates auxin responses
-
•
Arabidopsis tRNA maturation links basic cellular activities to developmental readouts
Leitner et al. analyze Arabidopsis lines deficient in distinct aspects of tRNA maturation, finding defects in control of auxin transport and signaling events. The occurrence of such auxin-related defects as a result of general deficiencies in translational control underlines a rate-limiting function for auxin in plant development.
Introduction
Sessile plants evolved sophisticated mechanisms to respond to environmental stimuli and developmental cues. Key to several of these processes is the transmission of signals generated by the plant hormone auxin (indole-3-acetic acid, IAA, the predominant form found in plants). Auxin is special among plant hormones as its spectrum of activities requires its active, directional transport throughout the plant body (Peer et al., 2011; Voß et al., 2014; Wabnik et al., 2011). Such polar auxin transport (PAT), together with metabolic control of auxin, defines local variations in hormone levels, which are perceived and transmitted to induce hormonally controlled adjustments in gene expression and activity (Ljung, 2013; Sauer et al., 2013).
Perception of variations in auxin homeostasis and downstream signaling events has been analyzed extensively in the model plant Arabidopsis, which led to establishment of pathways specifically required for the transmission of hormonal signals (Boer et al., 2014; Dharmasiri et al., 2005; Kepinski and Leyser, 2005; Paciorek et al., 2005; Ulmasov et al., 1999; Xu et al., 2010). This involves cellular auxin uptake and efflux proteins with plasma membrane-localized PIN proteins implicated in auxin efflux (Petrásek et al., 2006). Here, we describe components of two distinct Arabidopsis pathways involved in tRNA maturation and characterize their function in auxin distribution and responses. We show that adjustments in PIN auxin transport protein levels are mediated by a plant ortholog of the multifunctional Elongator complex. In eukaryotes, Elongator has been linked to diverse cellular events, including transcriptional elongation, acetylation of chromatin and cytoskeleton components, protein sorting, as well as post-transcriptional modification of tRNAs (Chen et al., 2009; Esberg et al., 2006; Otero et al., 1999; Rahl et al., 2005; Wittschieben et al., 1999). We provide evidence that alterations in Elongator-mediated tRNA maturation in plants give rise to auxin-related growth deficiencies and PIN mis-expression. In addition, Arabidopsis deficient in AtRNL, an RNA-ligase required for splicing of tRNAs that do not represent bona fide ELP substrates, exhibits growth defects resembling those of elp mutants. This suggests a more common requirement of tRNA maturation for morphogenesis and auxin responses. Expression patterns of ELP6 and AtRNL, together with auxin-induced rescue of growth defects associated with diminished tRNA levels, are consistent with scenarios in which tRNA availability modulates auxin responses.
Overall, we demonstrate that deficiencies in Arabidopsis tRNA maturation and availability broadly concern transmission of auxin signals. This underlines rate-limiting roles of the growth regulator in plant morphogenesis and hints at meta-regulatory levels of growth control, by which adjustments in general cellular activities could feed back on defined aspects of plant development via modulation of auxin responses.
Results
Mop Mutants Are Affected in Subunits of an Arabidopsis Elongator Complex
In earlier work, we described two Arabidopsis mutants termed mop2-1 and mop3-1 (for modulator of PIN), which both exhibit developmental and patterning defects reminiscent of mutants deficient in PAT (Malenica et al., 2007). This is indicated by altered expression of an auxin-responsive reporter protein and by reduced abundance of PIN auxin transport proteins. Notably, although PIN protein levels are reduced in both mop mutants, no corresponding changes in PIN transcript levels could be detected, suggesting involvement of both MOP loci in post-transcriptional regulation of PINs (Malenica et al., 2007).
Both mop mutants are derived from a pool of T-DNA insertion lines (Sieberer et al., 2003). For cloning of MOP2, we analyzed F2 populations of mop2-1, outcrossed into Col-0 wild-type, and identified a segregating T-DNA insertion in the second intron of locus At4g10090. This locus represents an Arabidopsis ortholog of Elongator protein 6 (ELP6), a subunit of Elongator holoenzyme found in eukaryotes from baker’s yeast to human (Hawkes et al., 2002; Nelissen et al., 2005; Otero et al., 1999). To test whether mop2-1 mutant phenotypes could result from this T-DNA insertion we analyzed gene expression, demonstrating a loss of ELP6 transcription in mop2-1 (Figure S1B). Transformation of mop2-1 with a VENUS-tagged ELP6 fusion protein expressed by its endogenous promoter (ELP6p::VENUS:ELP6) rescued growth deficiencies, indicating that mop2-1 defects result from a loss of ELP6 (Figures S1C and S1D). We therefore termed the former mop2-1 allele elp6mop2.
The Elongator complex was suggested to be composed of 12 subunits, with two copies of each ELP1, 2, and 3 forming a core sub-complex, and an additional accessory sub-complex, composed of two copies of ELP4, 5, and 6 (Glatt and Müller, 2013; Winkler et al., 2001). Given the resemblance of elp6mop2 and mop3-1 phenotypes, we reasoned that the latter could be affected in another ELP locus and initiated complementation tests between mop3-1 and T-DNA insertion lines affected in the remaining subunits of Arabidopsis Elongator. No complementation of mutant phenotypes was observed in F1 and F2 progeny derived from crosses between mop3-1 and GK_555H06, deficient in ELP3 (At5g50320; ELONGATA 3, HISTONE ACETYLTRANSFERASE OF THE GNAT FAMILY 3). Determination of ELP3 expression in mop3-1 demonstrated a loss of full-length transcript (Figure S1B); however, when analyzing the genomic ELP3 locus in mop3-1, we could not characterize a T-DNA insertion but found evidence for rearrangements at this chromosomal position (Leitner, 2011). Therefore, and to minimize potential effects unrelated to defects in ELP3, all further experiments were performed with line GK_555H06, harboring a characterized T-DNA insertion in the ELP3 locus (elp3; Xu et al., 2012; Figure S1B).
Arabidopsis Elongator Is Involved in Post-transcriptional Control of PIN Protein Levels
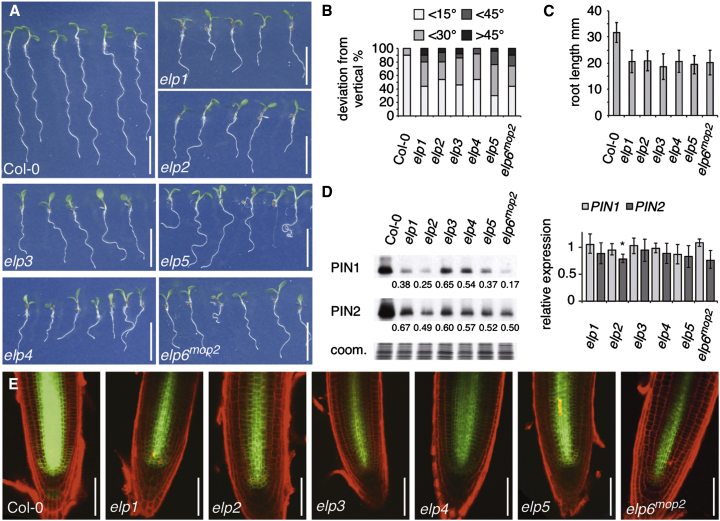
The phenotypic resemblance of elp6mop2, mop3-1, and elp3, suggests that corresponding gene products function as essential subunits of a protein complex, similar to Elongator in other eukaryotes (Glatt and Müller, 2013). We therefore tested mutants defective in the remaining ELP subunits for growth defects (Figure S1B). Homozygous insertion lines for ELP1/ELO2/ABO1 (At5g13680; SALK_005153), ELP2 (At1g49540; SALK_106485), ELP4/ELO1 (At3g11220; SALK_079193), and ELP5 (At2g18410; GK_700A12) were grown along elp3 and elp6mop2. All these mutants exhibited related defects in shoot and root morphology, manifested at the seedling stage as reduced root elongation and deficiencies in directional root growth along the gravity vector, similar to mop alleles (Figures 1A–1C; Malenica et al., 2007). Related developmental defects were observed during later stages of development, affecting leaf and inflorescence morphogenesis (Figure S1A).
Figure 1.
Loss of Elongator Complex in Arabidopsis Interferes with Auxin-Controlled Morphogenesis and Post-transcriptional Regulation of PINs
(A) Comparison of wild-type Col-0 and elp mutant seedlings at 7 DAG.
(B) Orientation of primary root growth of Col-0 and elp seedlings at 9 DAG. A total of 50 seedlings were analyzed for each genotype and plotted as percentage of seedlings displaying <15°, <30°, <45°, and >45° deviation from the vertical growth axes.
(C) Comparison of primary root length of Col-0 and elp seedlings at 5 DAG. 20 seedlings were analyzed for each genotype. SDs are indicated.
(D) Quantification of PIN1 and PIN2 protein (left panels) and mRNA (right panels) in Col-0 and elp seedlings at 7 DAG. Relative signal intensities are indicated below (Col-0 = 1); “coom.” displays Coomassie staining of samples used. qPCR performed with Col-0 (= 1) and elp mutants displaying transcript levels of PIN1 and PIN2. SDs from three biological repeats with three technical replicates are indicated (∗Student’s two-tailed t test; p < 0.05).
(E) Comparison of PIN1::PIN1:GFP signals (green) in Col-0 and elp mutant root meristems at 5 DAG. Propidium iodide staining (PI, red) was used for visualizing cell boundaries.
Size bars in represent 10 mm (A) and 50 μm (E). See also Figures S1 and S2.
Elp phenotypes in patterning and growth responses are reminiscent of mutants in auxin transport and/or signaling (Malenica et al., 2007). In accordance, a comparison of endogenous PIN1 and PIN2 protein levels in all six elp mutants demonstrated reduced abundance of these key regulators of auxin transport, and consistent results were obtained when analyzing expression of the PIN1p::PIN1:GFP translational reporter gene (Figures 1D and 1E). Defects in protein abundance are not restricted to PINs though, exemplified by reduced accumulation of a BOR1:GFP translational fusion, when overexpressed by the 35S promoter in elp6mop2 (Figure S2C; Takano et al., 2005).
We analyzed subcellular distribution of PIN1 in further detail and tested for endogenous PIN1 in soluble elp protein fractions and determined PIN1:GFP localization at higher resolution. No striking differences to wild-type could be observed in these experiments, suggesting that reduced PIN abundance in the membrane fraction is not an immediate consequence of protein mis-localization (Figures S2A and S2B). Evidence for elp deficiencies affecting post-transcriptional regulation of PINs came from analysis of PIN transcript levels in the elp mutants. Specifically, no decrease in PIN1 transcript levels was detectable with qPCR, whereas protein levels were decreased (Figure 1D). A slight decrease in transcript levels was observed for PIN2 in elp mutants, which, however, was significant only for elp2 (Figure 1D).
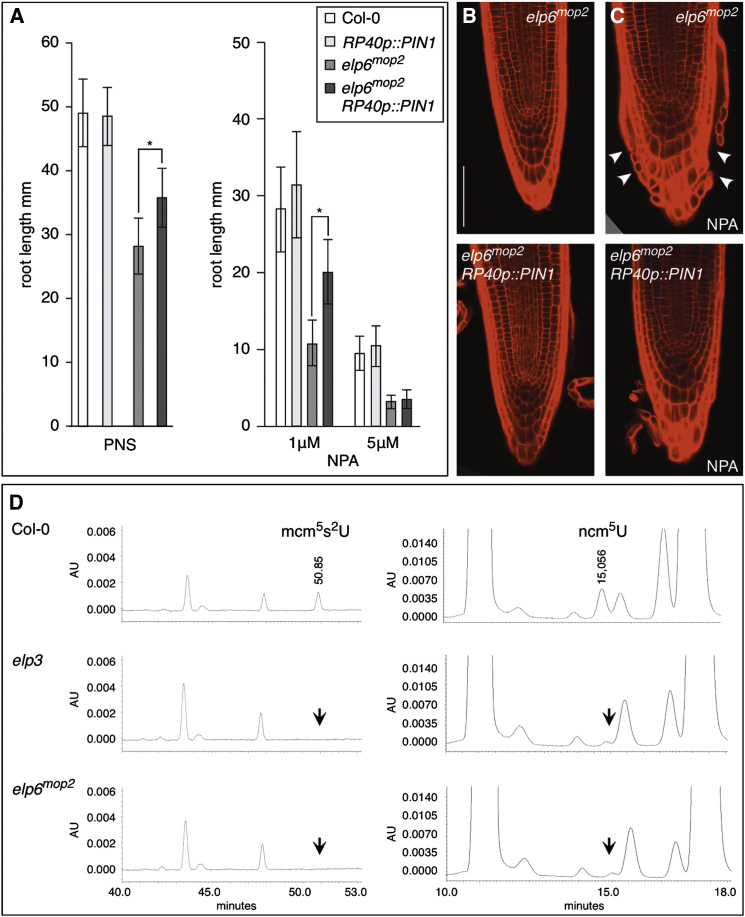
To test for crosstalk between Elongator and PINs, we generated PIN1 overexpression constructs under control of the RP40 ribosomal protein promoter, highly active in meristematic zones (Butt et al., 2014). We made use of this promoter, because attempts to obtain efficient PIN overexpression lines in mop mutants failed, when using the CaMV 35S promoter (Malenica et al., 2007). Expression of RP40p::PIN1 in elp6mop2 resulted in markedly higher PIN1 protein levels, demonstrating that RP40p-driven PIN expression is well suited for this approach (Figure S3A). When analyzing phenotypes, we found that elp6mop2 RP40p::PIN1 primary roots are longer than elp6mop2 roots of identical age whereas no pronounced difference was observed when comparing wild-type and RP40p:PIN1, indicating that reduced abundance of PIN1 contributes to elp6mop2 root growth defects (Figure 2A). We then determined responses of elp6mop2 RP40p::PIN1 to auxin transport inhibitor NPA (1-N-Naphthylphthalamic acid), as elp6mop2 seedlings are overly sensitive to the compound, reflected in reduced root elongation and defects in root meristem patterning (Figures 2A–2C; Malenica et al., 2007). NPA sensitivity is dampened in elp6mop2 RP40p::PIN1, suggestive of a PIN1 dosage-dependent reversion of NPA effects on root elongation (Figure 2A). Consistent with this scenario, NPA-induced ectopic cell proliferation of elp6mop2 root tips is antagonized by PIN1 overexpression, illustrated by reduced ectopic formation of root cap cells (Figures 2B and 2C). However, at the stage of flowering, elp6mop2 RP40p::PIN1 still exhibits marked morphological defects (Figures S3B and S3C). Thus, while PIN1 overexpression is not adequate for a full reversion of elp6mop2 phenotypes, our observations establish a causal link between downregulation of PIN1 and elp6mop2 root growth defects.
Figure 2.
Elp/mop Alleles Are Partially Rescued by PIN1 Overexpression and Characterized by Defects in Ribonucleotide Modifications
(A) Primary root length of Col-0, elp6mop2, RP40p::PIN1 and isogenic elp6mop2RP40p::PIN1 seedlings at 7 DAG, grown on PNS (left) or PNS supplemented with NPA (right). 30–40 seedlings were analyzed for each genotype/condition. SDs are indicated (∗Student’s two-tailed t test; p < 0.001).
(B and C) Comparison of elp6mop2 and elp6mop2RP40p::PIN1 primary root meristems at 14 DAG grown on PNS (B) or on PNS supplemented with 1 μM NPA (C). White arrowheads indicate increased cell proliferation of elp6mop2 in response to the auxin transport inhibitor.
(D) Total tRNA fractions isolated from Col-0, elp3, and elp6mop2 were analyzed by HPLC. Left: parts of chromatograms between retention times 40.0 and 53.0 min are displayed. Arrow indicates the expected retention time of mcm5s2U. Right: parts of chromatograms between retention times 10.0 and 18.0 min: Arrow indicates the expected retention time of ncm5U. Chromatograms were monitored at 314 and 254 nm, respectively.
Size bars represent 50 μm (B and C). See also Figure S3.
ELP-Mediated tRNA Maturation Influences Auxin Responses in Arabidopsis
Arabidopsis Elongator has been associated with diverse processes, revealing widespread roles for the protein complex (Nelissen et al., 2005; Wang et al., 2013b; Xu et al., 2012; Zhou et al., 2009), and phenotypes of the elp mutants point toward a distinctive role in transmitting auxin signals, which earlier has been linked to control of transcriptional elongation (Nelissen et al., 2010). In addition, and analogous to Elongator in yeast, a mutation in Arabidopsis ELP3 was found to be defective in uridine modification of tRNAs, essential for wobble codon recognition during protein translation (Mehlgarten et al., 2010). We analyzed tRNA preparations from elp6mop2 and elp3 and observed a reduction in the occurrence of 5-methoxycarbonyl-methyl-2-thiouridine (mcm5s2U) and of 5-carbamoyl-methyluridine (ncm5U) in both mutant alleles (Figure 2D). This is in accordance with earlier observations and substantiates a role of Arabidopsis Elongator in tRNA maturation at wobble position 34, to improve wobbling accuracy and translational fidelity (Johansson et al., 2008; Mehlgarten et al., 2010).
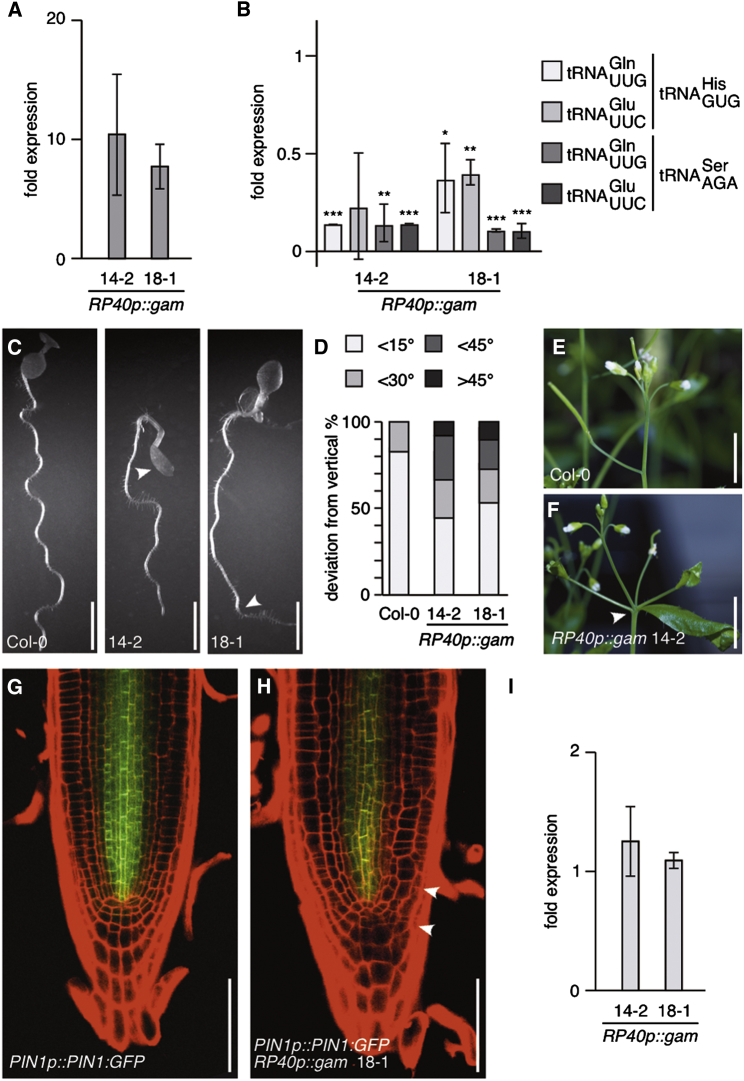
Given that Elongator has been linked to several cellular activities, we asked whether explicitly deficiencies in tRNA maturation could contribute to Elongator mutant phenotypes in Arabidopsis. To this end, we made use of Kluyveromyces lactis γ-toxin, an endonuclease preferentially degrading tRNAs that underwent modification by Elongator (Lu et al., 2005). Assuming that reduced abundance of Elongator-modified tRNAs contributes to elp mutant phenotypes, γ-toxin-mediated degradation of such tRNAs would be expected to give rise to related growth defects. Expression of γ-toxin (gam) under control of the RP40 promoter (Figure 3A) resulted in auxin-related defects, resembling those of elp mutants (13/20 lines analyzed). This involved defects in root growth, cotyledon formation (18/30, 0/30 in controls) and aberrations in lateral organ positioning at inflorescence axes (25/62, 4/50 in controls; Figures 3C–3F). Moreover, PIN1:GFP reporter signals were reduced in RP40p::gam seedlings, while PIN1 transcript levels appeared unaffected, indicating that ectopic gam expression interferes with post-transcriptional control of PIN1 (Figures 3G–3I). To test whether RP40p::gam is affected in tRNA abundance, we determined steady-state levels of potential γ-toxin substrates, namely, tRNAs with a uridine at wobble position 34. In these experiments we normalized amounts of and to levels of or , the latter two lacking a uridine at the wobble position, serving as internal standards (Figure 3B). We detected reduced abundance of and in RP40p::gam lines when compared to wild-type, suggesting that γ-toxin is active in Arabidopsis with substrate specificities similar to those observed in yeast.
Figure 3.
Expression of Kluyveromyces lactis γ-Toxin in Arabidopsis Downregulates Abundance of tRNAs and Causes Defects in Morphogenesis Resembling elp Mutants
(A) qPCR performed with cDNA from RP40p::gam lines 14-2 and 18-1. Transcript levels were normalized to expression of At5g60390 (= 1).
(B) qPCR displaying abundance of and in Arabidopsis seedlings at 5 DAG, after normalization to levels of either or as internal standards (relative tRNA abundance in Col-0 = 1). Results presented are obtained from two biological repeats each with three technical repeats. SDs are indicated (∗t test < 0.05; ∗∗t test < 0.01; ∗∗∗t test < 0.001).
(C) Comparison of Col-0 and RP40p::gam seedlings at 6 DAG. Alterations in cotyledon formation and directional root growth are indicated by white arrowheads.
(D) Orientation of primary root growth of Col-0 and RP40p::gam seedlings at 6 DAG. A total of 30–40 seedlings was analyzed for each data set and plotted as percentage of seedlings displaying <15°, <30°, <45°, and >45° deviation from the vertical growth axes.
(E and F) Comparison of Col-0 and RP40p::gam inflorescences at 35 DAG. White arrowhead: aberrations in inflorescence architecture.
(G and H) Expression of PIN1::PIN1:GFP (green) in Col-0 (G) and RP40p::gam (H) seedlings at 6 DAG. White arrowheads indicate aberrations in root patterning. Roots were stained with PI (red).
(I) qPCR displaying PIN1 transcript levels in RP40p::gam lines 14-2 and 18-1 normalized to Col-0 (= 1). Results from three biological repeats with three technical replicates are shown.
SDs are indicated. Size bars represent 10 mm (C, E, and F) and 50 μm (G and H). See also Figure S4.
Next, we tested for genetic interaction between Elongator and RP40p::gam by crossing RP40p::gam into elp6mop2 followed by analysis of the resulting progeny. Double homozygous elp6mop2 RP40p::gam strongly resembled parental RP40p::gam and elp6mop2 lines, which would be consistent with overlapping tRNA substrate specificities of Elongator and γ-toxin, when expressed in Arabidopsis (Figures S4A–S4F). However, elp6mop2 RP40p::gam appeared delayed in their development, when compared to parental lines (Figure S4G), indicating synergistic interaction between γ-toxin and Elongator, which points to an incomplete overlap of activities of these genetic traits.
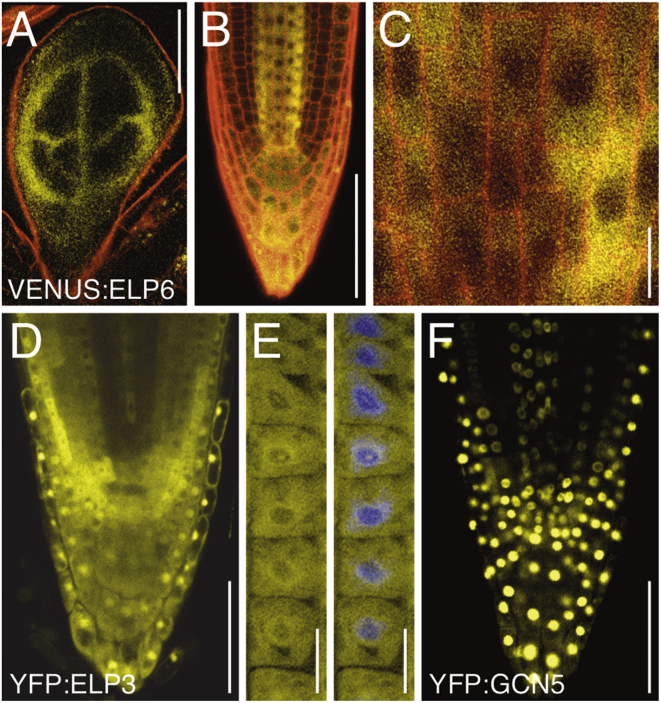
We then tested ELP reporter constructs, stably transformed into Arabidopsis. Signals from functional ELP6p::VENUS:ELP6 expressed in elp6mop2 were pronounced in stele, QC, and columella root cap cells, with weaker signals found in additional parts of the meristem (Figures 4B and 4C). Likewise in areal portions, the reporter appeared most abundant in the vasculature of developing organs (Figure 4A). At cellular resolution, VENUS:ELP6 signals were pronounced in the cytoplasm (Figure 4C). A similar cytoplasmic distribution along with nuclear signals was observed in 35S::YFP:ELP3 overexpression lines, while 35S::YFP:GCN5, overexpressing a bona fide histone acetyltransferase, exclusively exhibited nuclear signals under our experimental conditions (compare Figures 4D–4F; Earley et al., 2007). Thus, analogous to observations made for Elongator in S. cerevisiae, cytoplasmic signals exhibited by Arabidopsis ELP reporter proteins are in agreement with a role of Elongator outside the nucleus and could involve a function in tRNA maturation (Huang et al., 2005; Rahl et al., 2005).
Figure 4.
Expression Analysis of ELP Reporter Genes
(A and B) ELP6p::VENUS:ELP6 expression (yellow) in developing true leaf (A) and primary root meristem (B) at 6 DAG.
(C) Cytoplasmic distribution of ELP6p::VENUS:ELP6 in root meristem stele cells. Areas with reduced signal intensities represent nuclei. Seedlings were stained with PI (red) to visualize cell and organ boundaries.
(D) Expression of 35S::YFP:ELP3 (yellow signals) in 6-day-old Col-0 root meristem cells results in cytoplasmic and additional nuclear signals.
(E) 35S::YFP:ELP3 root meristem epidermis cells (6 DAG) displaying pronounced cytoplasmic signals. To visualize nuclei, seedlings were fixed and counterstained with DAPI (blue).
(F) Expression of 35S::YFP:GCN5, encoding a nuclear-localized protein, in Col-0 root meristem cells under conditions identical to those in (D).
Size bars represent 100 μm (A), 50 μm (B, D, and F), and 10 μm (C and E).
tRNA Processing as a General Determinant of Auxin Responses in Plants
Analysis of elp mutants and RP40p::gam point to a requirement of tRNA maturation for correct auxin responses. We asked whether this effect could be specific for tRNAs modified by Elongator or represents a more general response to alterations in tRNA homeostasis. We therefore analyzed Arabidopsis RNA LIGASE (AtRNL), a locus resembling yeast RNA ligase Trl1, implicated in splicing of tRNAs (Englert and Beier, 2005; Wang et al., 2006). In Arabidopsis, this would predominantly affect maturation of tRNATyr, composed of a gene family of 76 loci, out of which 70 are predicted to contain an intron, whereas only a smaller sub-fraction of tRNAMet (11/24) and tRNASer (2/64) loci contains an intron (http://lowelab.ucsc.edu/GtRNAdb/Athal/). However, none of these tRNAs represents a likely target for Elongator, as they lack a uridine at their wobble position.
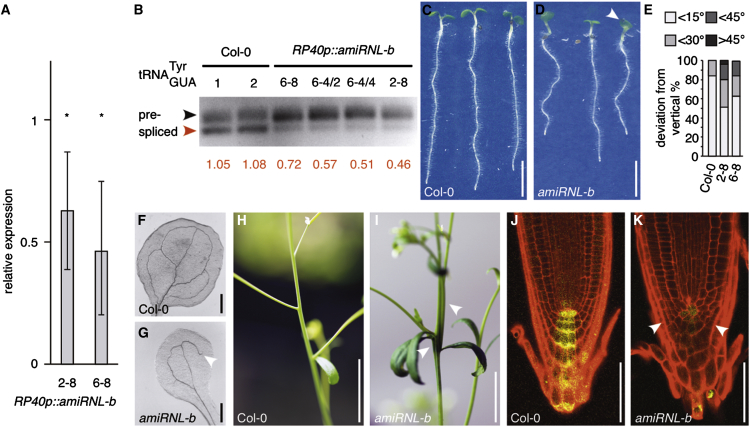
First, we analyzed expression of AtRNLp::GUS reporter transformed into Arabidopsis and found intense GUS staining in root meristems and in the vasculature of developing plantlets as well as in flowers and elongating tissue, pointing to a requirement for tRNA splicing preferentially in proliferating cell files and tissues (Figures S5A–S5G). Next, we sought for insertion mutants but failed to obtain lines with noticeable effects on AtRNL gene expression (see the Supplemental Information). We therefore took another approach and generated artificial microRNA lines (Schwab et al., 2006), in which transcription of endogenous AtRNL was downregulated (Figure 5A; Figures S6A and S6B). When determining splicing of in RP40p::amiRNL-b seedlings at 5 days after germination (DAG), we detected a strong reduction in the amounts of processed tRNA, suggesting that these plants contain only limited quantities of functional tRNATyr due to deficiencies in the splicing reaction (Figure 5B).
Figure 5.
Analysis of AtRNL RNA Ligase Knockdown Lines
(A) qPCR displaying AtRNL transcript levels in RP40p:amiRNL-b lines 2–8 and 6–8 at 6 DAG (Col-0 = 1). Results from three biological repeats with three technical replicates are shown. SDs are indicated (∗Student’s two-tailed t test, p < 0.01).
(B) Quantification of levels in Col-0 and RP40p::amiRNL-b silencer lines by PCR. Upper band corresponds to non-spliced precursor (black arrowhead); lower band corresponds to processed (red arrowhead). Ratios of spliced and precursor band intensities are displayed below.
(C and D) Col-0 (C) and RP40p::amiRNL-b (D) seedlings at 6 DAG. White arrowhead in (D) points to fused cotyledons.
(E) Orientation of primary root growth of Col-0 and RP40p::amiRNL-b lines 2–8 and 6–8 seedlings at 8 DAG. A total of 40–50 seedlings was analyzed for each genotype and plotted as percentage of seedlings displaying <15°, <30°, <45°, and >45° deviation from the vertical growth axes.
(F and G) Cotyledon vasculature in Col-0 (F) and RP40p::amiRNL-b (G). White arrowhead indicates vasculature discontinuity not observed in wild-type.
(H and I) Detail of inflorescence axes of flowering Col-0 (H) and RP40p::amiRNL-b (I) at 28 DAG. White arrowheads indicate fused, fasciated stem.
(J and K) Comparison of wild-type (J) and RP40p::amiRNL-b (K) primary root meristems expressing auxin-responsive DR5rev::GFP (yellow). Roots were stained with PI (red) to visualize cell boundaries. White arrowheads point to alterations in root meristem patterning.
Size bars represent 10 mm (C, D, H, and I), 1 mm (F and G), and 50 μm (J and K). See also Figures S5 and S6.
Morphology and development of RP40p::amiRNL-b resembled those of elp/mop mutants (10/16 lines analyzed). Specifically, RP40p::amiRNL-b seedlings exhibited reduced root elongation, aberrations in cotyledon number (49/91; 1/35 in Col-0) and incomplete cotyledon venation (17/40; 0/35 in Col-0) as well as deficiencies in root meristem morphology and root gravitropism (Figures 5C–5E, 5J, and 5K). At later developmental stages, we observed reduced apical dominance and aberrations in lateral organ positioning at inflorescence stems (27/62; 2/38 in Col-0; Figures 5H and 5I). Related growth defects have been linked to alterations in auxin responses, and we therefore analyzed expression of auxin responsive DR5rev::GFP (Benková et al., 2003). Reporter signals in RP40p::amiRNL-b DR5rev::GFP primary root meristems appeared reduced (Figures 5J and 5K), indicating alterations in auxin distribution and/or signaling that coincide with deficiencies in tRNA splicing.
To validate our observations, we generated another amiRNL construct, expressed under control of the 35S promoter, and targeting a different region of the AtRNL mRNA. Transgenic 35S::amiRNL-a seedlings with reduced abundance of AtRNL transcript exhibited phenotypes resembling those of RP40p::amiRNL-b (Figures S6B–S6F). This corroborates a scenario in which phenotypes observed result from downregulation of AtRNL and associated aberrations in tRNA splicing. Moreover, the resemblance of amiRNL and elp loss-of-function mutants, both predicted to impact on distinct sets of tRNA families, indicates that non-overlapping limitations in tRNA availability give rise to comparable developmental defects.
tRNA Maturation Acts in the Transmission of Instructive Auxin Cues
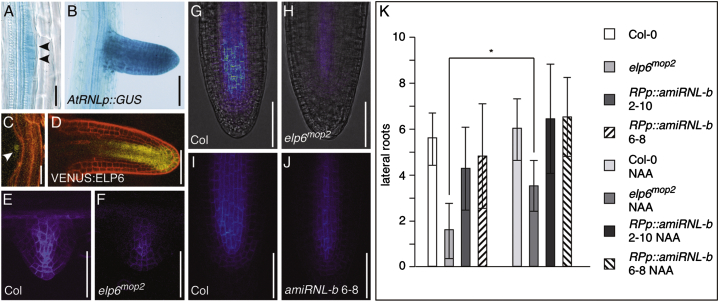
To further define the role of Elongator and AtRNL in auxin-controlled morphogenesis, we analyzed lateral root formation, a process that depends on coordinated distribution of auxin (Benková et al., 2003). When viewing AtRNLp::GUS activity, GUS staining became visible already in stage I lateral root primordia, and strong expression remained detectable during later stages of organ development (Figures 6A and 6B). Likewise, in elp6mop2 ELP6p::VENUS:ELP6 signals became detectable upon primordium development and remained visible during further growth (Figures 6C and 6D). This points to a function for Elongator and AtRNL in lateral root growth, and we next determined expression of PIN1p::PIN1:GFP in lateral roots of wild-type, elp6mop2, and RP40p::amiRNL-b seedlings. PIN1:GFP signals in elp6mop2 turned out to be weaker than in wild-type, and similar findings were made, when analyzing reporter signals in RP40p::amiRNL-b lateral roots (compare Figures 6E–6J). Thus, as observed for primary root meristems (Figure 1; Figures S6G–S6I), PIN1 expression appears reduced in lateral roots upon interference with tRNA maturation.
Figure 6.
Elongator and AtRNL during Lateral Root Formation
(A and B) AtRNLp::GUS activity at early (A) and later (B) stages of lateral root development. Arrowheads point to early-stage lateral root primordium.
(C and D) ELP6p::VENUS:ELP6 expression (yellow) at early (C, white arrowhead) and later (D) stages of lateral root development. Roots were counterstained with PI to visualize cell boundaries.
(E–H) PIN1p::PIN1:GFP signals in Col-0 (E and G) and elp6mop2 (F and H) at early (E and F) and later (G and H) stages of lateral root development.
(I and J) Expression of PIN1p::PIN1:GFP in lateral roots of Col-0 (I) and RP40p::amiRNL-b (J).
(K) Number of lateral roots formed by Col-0, elp6mop2, and RP40p::amiRNL-b at 10 DAG (∗Student’s two-tailed t test; p < 0.001; n = 25–30 for each genotype and condition).
Size bars represent 15 μm (A), 50 μm (B, D, and E–J), and 25 μm (C). See also Figure S6.
We then tested for possible consequences of reduced PIN expression and compared lateral root growth in wild-type and elp6mop2. This revealed a reduction in the number of elp6mop2 lateral roots, when compared to wild-type (Figure 6K). When analyzing RP40p::amiRNL-b, we observed a reduction in lateral root formation as well, but effects in the silencer lines appeared less pronounced and more variable than in elp6mop2 (Figure 6K). When performing root growth experiments in the presence of low concentrations of the auxin analog 1-napthalene acetic acid (NAA) without noticeable effects on lateral root growth in wild-type, we detected rescue of lateral root formation specifically in elp6mop2 (Figure 6K). This is in accordance with limitations in auxin availability and/or distribution causing lateral root growth deficiencies associated with the mutant, and consistent with a role of Elongator in transmission of instructive auxin signals during root morphogenesis. Taken together, our findings link tRNA maturation to root organogenesis, potentially via mechanisms orchestrating PIN activities and associated adjustments in auxin distribution.
Discussion
Recent years have seen characterization of a range of signaling pathways in higher plants, revealing molecular switches that communicate signals essential for plant development. Auxin appears to be transmitted by distinct pathways, with SCFTIR1/AFB-dependent hormone perception predominantly affecting transcriptional control (Dharmasiri et al., 2005; Kepinski and Leyser, 2005). Another pathway, suggested to modulate protein sorting in response to auxin, seemingly requires ROP-GTPase activity (Paciorek et al., 2005; Xu et al., 2010), while involvement of putative auxin receptor auxin binding protein 1 in auxin-controlled development has been seriously challenged very recently (Gao et al., 2015). The identification of components acting in auxin signaling provided insights into principles that might confer specificity to such hormonal responses, but additional reports demonstrated that less specific cellular processes contribute to the orchestration of auxin effects as well (Dhonukshe et al., 2008; Kitakura et al., 2011; Korbei et al., 2013; Rosado et al., 2010, 2012; Wang et al., 2013a). Our findings establish tRNA maturation as a highly general effector of auxin responses, acting via translational control of PIN proteins, and provoking questions on function and specificity of such activities in the regulation of hormonal signaling (Retzer et al., 2014).
Elongator has been linked to diverse cellular activities ranging from gene expression to protein sorting, which seemingly depends on integrity of the protein complex, as loss of individual ELP loci causes essentially identical defects (Chen et al., 2009; Esberg et al., 2006; Frohloff et al., 2001; Huang et al., 2005; Nelissen et al., 2005; Otero et al., 1999; Rahl et al., 2005; Singh et al., 2010; Wittschieben et al., 1999; Xu et al., 2012). Evidence for ELP acting particularly in tRNA modification came from studies in yeast, demonstrating that selective overexpression of two individual tRNAs can bypass major elp mutant phenotypes, presumably via compensating for the loss of translational fidelity that results from defects in Elongator (Esberg et al., 2006). Another link between Elongator and tRNA modifications came from crystallization of the ELP4/5/6 sub-complex (Glatt et al., 2012; Lin et al., 2012). These studies revealed a hexameric ring-shaped structure capable of interacting with the tRNA anticodon loop domain, which presumably represents a topological prerequisite for ELP-mediated ribonucleotide modification (Glatt et al., 2012). Moreover, analysis of protein abundance in an Schizosaccharomyces pombe Δelp3 mutant linked codon usage and Elongator-dependent variations in translational efficiency to discrete growth defects (Bauer et al., 2012). From all that, it seems that tRNA modification represents a major enzymatic function of Elongator (Glatt and Müller, 2013).
Like its fungal counterpart, Arabidopsis Elongator appears to function in diverse processes, including stress response, pathogen defense, and hormonal signaling, which has been attributed to Elongator’s activities in chromatin modification and transcriptional control (Chen et al., 2006; Nelissen et al., 2010; Sanmartín et al., 2011; Wang et al., 2013b; Zhou et al., 2009). Our results now establish links between Elongator-mediated tRNA modification and auxin-controlled pattern formation. Apart from reduced uridine modifications in tRNA preparations of elp/mop mutants, phenotypes observed upon expression of γ-toxin in Arabidopsis further substantiate such a role. Experiments in baker’s yeast demonstrated that tRNAs featuring mcm5s2U or ncm5U at their wobble position represent preferred targets for γ-toxin (Lu et al., 2005), and our results point to related γ-toxin substrate specificities in Arabidopsis. In accordance, we observed comparable phenotypes when analyzing elp/mop mutants and RP40p::gam lines, which is consistent with overlapping tRNA substrate specificities of Arabidopsis Elongator and γ-toxin. Notably, although displaying phenotypes as their parental lines, growth of elp6mop2 RP40p::gam plants turned out to be retarded, when compared to its parents. Such additive phenotypes argue for an incomplete overlap in the activity of Arabidopsis ELP6 and γ-toxin. Alternatively, remaining tRNA modification activities in either elp6mop2 or RP40p::gam could be reduced further in elp6mop2 RP40p::gam plants. Whether these synergistic phenotypes reflect diverging activities in tRNA substrate processing or activities even unrelated to tRNA modifications remains to be addressed.
One might speculate about a function of Elongator in translational control, primarily required for the transmission of auxin signals. Limited PIN abundance in Elongator mutants, together with the finding that efficient overexpression of PIN1 partially rescues elp6mop2 growth defects, is in fact consistent with this model. Analysis of AtRNL, however, argues against such a scenario but rather implies less specific effects of tRNA availability on auxin-controlled growth and differentiation processes. The Arabidopsis family likely represents a major substrate for the AtRNL RNA-ligase, indicated by pronounced deficiencies in tRNATyr splicing in amiRNL lines. In addition, members of the and families represent potential AtRNL substrates, but none of these tRNAs is a likely target of Elongator, as they all lack uridine at wobble position 34. While this implies differing tRNA substrate specificities for Elongator and AtRNL, loss of either ELP or AtRNL still causes similar phenotypes, which points to a requirement of these distinct regulators of translational control in overlapping aspects of plant development.
Broad deficiencies in tRNA biogenesis as apparent in elp/mop and amiRNL should result in major changes in translational control, with reduced abundance of PINs representing only a small fraction of aberrations in protein translation. This would be in agreement with the various functions in signaling and stress responses attributed to Arabidopsis Elongator. In light of such diverse activities, it seems remarkable that several developmental defects associated with elp/mop mutants could be explained essentially by aberrations in auxin-controlled morphogenesis. Another report, linking tRNA function to auxin, described identification of an auxin response mutant, which turned out to be affected in a member of the family (Perry et al., 2005). This mutant is predicted to erroneously and stochastically incorporate valine instead of alanine into growing peptide chains, nonetheless resulting in discrete defects in mediating auxin effects on cell proliferation (Perry et al., 2005). It remains to be determined how altered decoding characteristics of a single tRNA could give rise to such defined growth defects, which is all the more surprising as the Arabidopsis genome is predicted to encode 630 tRNAs, strongly suggestive of extensive functional redundancy (http://lowelab.ucsc.edu/GtRNAdb/Athal/).
The importance of tRNA maturation specifically for auxin responses and cell proliferation is underlined by our study, indicative of a high demand for AtRNL and Elongator activities in metabolically active and proliferating tissue. Limitations in the availability of tRNAs, as is the case for Elongator loss-of-function or AtRNL knockdown alleles, would translate into variations in the abundance of various proteins, including key mediators of plant development and pattern formation. PIN proteins seemingly represent such targets, as interference with tRNA maturation impacts on PIN levels, coinciding with modifications in auxin distribution and developmental reprogramming, which is reflected in major adjustments in proliferative growth. Rescue of elp6mop2 lateral root growth defects by auxin supports such a scenario, as it indicates that aberrations in auxin availability or distribution are indeed accountable for elp/mop growth defects.
A plausible explanation for our observations implies that components of the auxin transport and/or signaling machinery represent rate-limiting determinants for plant morphogenesis. This is underlined by rescue of elp/mop root phenotypes via efficient PIN1 overexpression, likely compensating for suboptimal translation as a result of limiting availability of fully processed tRNAs. Related models have been put forward when studying fundamental aspects of cellular activity, including cytoskeleton function, endocytic protein sorting, and ribosome biogenesis, perturbation of which consistently resulted in phenotypes that could be linked to altered auxin responses (Dhonukshe et al., 2008; Kitakura et al., 2011; Retzer et al., 2014; Rosado et al., 2010; Wang et al., 2013a; Zhou et al., 2010). While none of these processes represents an integral element of auxin signaling pathways, it appears that plant cells have evolved mechanisms, conferring signaling specificity by means of highly general regulatory events. ROP-GTPases modulate intracellular sorting of PIN plasma membrane proteins and hence distribution of auxin (Xu et al., 2010). Moreover, activity of ribosome-associated proteins appears indispensable for translational re-initiation of ARF mRNAs, which contain regulatory uORFs, upstream of their actual Start-ATG (Rosado et al., 2012). However, such translational re-initiation is not restricted to transcriptional regulators of auxin responses, as about one-third of Arabidopsis protein-coding mRNAs is predicted to contain regulatory uORFs (von Arnim et al., 2014). Likewise, ROP activity is assumed to exert general effects on plasma membrane protein sorting not limited to auxin transport components, underpinning the role of PINs as well as additional elements of the auxin signaling machinery as a limiting bottleneck for plant development.
Insights into the mechanisms controlling tRNA maturation in higher plants are just emerging, which should contribute to our understanding of their function in plant development (Chen et al., 2010). Adjustments in tRNA maturation could, for example, arise as a consequence of variations in environmental parameters, causing modifications in translational control. In this scenario, reversible changes in the abundance of PIN auxin transport proteins resulting from fluctuations in translational efficiency would link fundamental cellular activities to defined events in pattern formation and morphogenesis. This would allow for efficient and rapid transmission of morphogenetic cues, simply by exploiting the rate-limiting role of auxin signaling for plant development. Experiments addressing environmental parameters and its effects on translational control are required to further test this hypothesis.
Experimental Procedures
Plant Growth and Lines
Seedlings were cultured and analyzed as described (Korbei et al., 2013), in a 16-hr-light/8-hr-dark regimen at 22°C, unless indicated differently.
Generation of Constructs and Expression Analysis
Construct cloning and plant transformation was performed according to established protocols (Butt et al., 2014). Sample preparation and expression analysis has been described elsewhere (Butt et al., 2014; Korbei et al., 2013).
Microscopy
Seedlings were analyzed on Leica confocal laser scanning microscopes (CLSMs) as described (Korbei et al., 2013). A detailed description of all experimental procedures is provided as Supplemental Information.
Author Contributions
J.L., N.M., R.B., D. L., C.L., and B.K. performed analysis of Elongator mutants and reporter lines. J.L., C.L., and K.R. analyzed γ-toxin lines; K.R. generated and analyzed amiRNL lines. G.J. and A. B. assessed tRNA modifications in elp mutants. A.B and C.L. conceived experiments, and C.L. wrote the manuscript.
Acknowledgments
This work was supported by grants from the Austrian Science Funds (FWF; P19585 and P25931 to C.L.; T477 to B.K.) and a Docfforte fellowship from the Austrian Academy of Sciences to K.R. A.S.B. is supported by grants from the Swedish Cancer Foundation (13 0301), Swedish Research Council (621-2012-3576), and Karin and Harald Silvanders Foundation (223-2808-12). We are indebted to Eva Benkova, Niko Geldner, Toru Fujiwara, and Craig Pikaard for providing published materials.
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Supplemental Information
References
- Bauer F., Matsuyama A., Candiracci J., Dieu M., Scheliga J., Wolf D.A., Yoshida M., Hermand D. Translational control of cell division by Elongator. Cell Rep. 2012;1:424–433. doi: 10.1016/j.celrep.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benková E., Michniewicz M., Sauer M., Teichmann T., Seifertová D., Jürgens G., Friml J. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell. 2003;115:591–602. doi: 10.1016/s0092-8674(03)00924-3. [DOI] [PubMed] [Google Scholar]
- Boer D.R., Freire-Rios A., van den Berg W.A., Saaki T., Manfield I.W., Kepinski S., López-Vidrieo I., Franco-Zorrilla J.M., de Vries S.C., Solano R. Structural basis for DNA binding specificity by the auxin-dependent ARF transcription factors. Cell. 2014;156:577–589. doi: 10.1016/j.cell.2013.12.027. [DOI] [PubMed] [Google Scholar]
- Butt H., Graner S., Luschnig C. Expression analysis of Arabidopsis XH/XS-domain proteins indicates overlapping and distinct functions for members of this gene family. J. Exp. Bot. 2014;65:1217–1227. doi: 10.1093/jxb/ert480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Zhang H., Jablonowski D., Zhou X., Ren X., Hong X., Schaffrath R., Zhu J.K., Gong Z. Mutations in ABO1/ELO2, a subunit of holo-Elongator, increase abscisic acid sensitivity and drought tolerance in Arabidopsis thaliana. Mol. Cell. Biol. 2006;26:6902–6912. doi: 10.1128/MCB.00433-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Tuck S., Byström A.S. Defects in tRNA modification associated with neurological and developmental dysfunctions in Caenorhabditis elegans elongator mutants. PLoS Genet. 2009;5:e1000561. doi: 10.1371/journal.pgen.1000561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P., Jäger G., Zheng B. Transfer RNA modifications and genes for modifying enzymes in Arabidopsis thaliana. BMC Plant Biol. 2010;10:201. doi: 10.1186/1471-2229-10-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmasiri N., Dharmasiri S., Estelle M. The F-box protein TIR1 is an auxin receptor. Nature. 2005;435:441–445. doi: 10.1038/nature03543. [DOI] [PubMed] [Google Scholar]
- Dhonukshe P., Grigoriev I., Fischer R., Tominaga M., Robinson D.G., Hasek J., Paciorek T., Petrásek J., Seifertová D., Tejos R. Auxin transport inhibitors impair vesicle motility and actin cytoskeleton dynamics in diverse eukaryotes. Proc. Natl. Acad. Sci. USA. 2008;105:4489–4494. doi: 10.1073/pnas.0711414105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley K.W., Shook M.S., Brower-Toland B., Hicks L., Pikaard C.S. In vitro specificities of Arabidopsis co-activator histone acetyltransferases: implications for histone hyperacetylation in gene activation. Plant J. 2007;52:615–626. doi: 10.1111/j.1365-313X.2007.03264.x. [DOI] [PubMed] [Google Scholar]
- Englert M., Beier H. Plant tRNA ligases are multifunctional enzymes that have diverged in sequence and substrate specificity from RNA ligases of other phylogenetic origins. Nucleic Acids Res. 2005;33:388–399. doi: 10.1093/nar/gki174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esberg A., Huang B., Johansson M.J., Byström A.S. Elevated levels of two tRNA species bypass the requirement for elongator complex in transcription and exocytosis. Mol. Cell. 2006;24:139–148. doi: 10.1016/j.molcel.2006.07.031. [DOI] [PubMed] [Google Scholar]
- Frohloff F., Fichtner L., Jablonowski D., Breunig K.D., Schaffrath R. Saccharomyces cerevisiae Elongator mutations confer resistance to the Kluyveromyces lactis zymocin. EMBO J. 2001;20:1993–2003. doi: 10.1093/emboj/20.8.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Zhang Y., Zhang D., Dai X., Estelle M., Zhao Y. Auxin binding protein 1 (ABP1) is not required for either auxin signaling or Arabidopsis development. Proc. Natl. Acad. Sci. USA. 2015;112:2275–2280. doi: 10.1073/pnas.1500365112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatt S., Müller C.W. Structural insights into Elongator function. Curr. Opin. Struct. Biol. 2013;23:235–242. doi: 10.1016/j.sbi.2013.02.009. [DOI] [PubMed] [Google Scholar]
- Glatt S., Létoquart J., Faux C., Taylor N.M., Séraphin B., Müller C.W. The Elongator subcomplex Elp456 is a hexameric RecA-like ATPase. Nat. Struct. Mol. Biol. 2012;19:314–320. doi: 10.1038/nsmb.2234. [DOI] [PubMed] [Google Scholar]
- Hawkes N.A., Otero G., Winkler G.S., Marshall N., Dahmus M.E., Krappmann D., Scheidereit C., Thomas C.L., Schiavo G., Erdjument-Bromage H. Purification and characterization of the human elongator complex. J. Biol. Chem. 2002;277:3047–3052. doi: 10.1074/jbc.M110445200. [DOI] [PubMed] [Google Scholar]
- Huang B., Johansson M.J., Byström A.S. An early step in wobble uridine tRNA modification requires the Elongator complex. RNA. 2005;11:424–436. doi: 10.1261/rna.7247705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M.J., Esberg A., Huang B., Björk G.R., Byström A.S. Eukaryotic wobble uridine modifications promote a functionally redundant decoding system. Mol. Cell. Biol. 2008;28:3301–3312. doi: 10.1128/MCB.01542-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepinski S., Leyser O. The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature. 2005;435:446–451. doi: 10.1038/nature03542. [DOI] [PubMed] [Google Scholar]
- Kitakura S., Vanneste S., Robert S., Löfke C., Teichmann T., Tanaka H., Friml J. Clathrin mediates endocytosis and polar distribution of PIN auxin transporters in Arabidopsis. Plant Cell. 2011;23:1920–1931. doi: 10.1105/tpc.111.083030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korbei B., Moulinier-Anzola J., De-Araujo L., Lucyshyn D., Retzer K., Khan M.A., Luschnig C. Arabidopsis TOL proteins act as gatekeepers for vacuolar sorting of PIN2 plasma membrane protein. Curr. Biol. 2013;23:2500–2505. doi: 10.1016/j.cub.2013.10.036. [DOI] [PubMed] [Google Scholar]
- Leitner J. Universitat fur Bodenkultur Wien; Wien: 2011. Ubiquitylation and translational fidelity as regulatory cues for Arabidopsis thaliana PIN proteins. [Google Scholar]
- Lin Z., Zhao W., Diao W., Xie X., Wang Z., Zhang J., Shen Y., Long J. Crystal structure of elongator subcomplex Elp4-6. J. Biol. Chem. 2012;287:21501–21508. doi: 10.1074/jbc.M112.341560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljung K. Auxin metabolism and homeostasis during plant development. Development. 2013;140:943–950. doi: 10.1242/dev.086363. [DOI] [PubMed] [Google Scholar]
- Lu J., Huang B., Esberg A., Johansson M.J., Byström A.S. The Kluyveromyces lactis gamma-toxin targets tRNA anticodons. RNA. 2005;11:1648–1654. doi: 10.1261/rna.2172105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenica N., Abas L., Benjamins R., Kitakura S., Sigmund H.F., Jun K.S., Hauser M.T., Friml J., Luschnig C. MODULATOR OF PIN genes control steady-state levels of Arabidopsis PIN proteins. Plant J. 2007;51:537–550. doi: 10.1111/j.1365-313X.2007.03158.x. [DOI] [PubMed] [Google Scholar]
- Mehlgarten C., Jablonowski D., Wrackmeyer U., Tschitschmann S., Sondermann D., Jäger G., Gong Z., Byström A.S., Schaffrath R., Breunig K.D. Elongator function in tRNA wobble uridine modification is conserved between yeast and plants. Mol. Microbiol. 2010;76:1082–1094. doi: 10.1111/j.1365-2958.2010.07163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelissen H., Fleury D., Bruno L., Robles P., De Veylder L., Traas J., Micol J.L., Van Montagu M., Inzé D., Van Lijsebettens M. The elongata mutants identify a functional Elongator complex in plants with a role in cell proliferation during organ growth. Proc. Natl. Acad. Sci. USA. 2005;102:7754–7759. doi: 10.1073/pnas.0502600102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelissen H., De Groeve S., Fleury D., Neyt P., Bruno L., Bitonti M.B., Vandenbussche F., Van der Straeten D., Yamaguchi T., Tsukaya H. Plant Elongator regulates auxin-related genes during RNA polymerase II transcription elongation. Proc. Natl. Acad. Sci. USA. 2010;107:1678–1683. doi: 10.1073/pnas.0913559107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero G., Fellows J., Li Y., de Bizemont T., Dirac A.M., Gustafsson C.M., Erdjument-Bromage H., Tempst P., Svejstrup J.Q. Elongator, a multisubunit component of a novel RNA polymerase II holoenzyme for transcriptional elongation. Mol. Cell. 1999;3:109–118. doi: 10.1016/s1097-2765(00)80179-3. [DOI] [PubMed] [Google Scholar]
- Paciorek T., Zazímalová E., Ruthardt N., Petrásek J., Stierhof Y.D., Kleine-Vehn J., Morris D.A., Emans N., Jürgens G., Geldner N., Friml J. Auxin inhibits endocytosis and promotes its own efflux from cells. Nature. 2005;435:1251–1256. doi: 10.1038/nature03633. [DOI] [PubMed] [Google Scholar]
- Peer W.A., Blakeslee J.J., Yang H., Murphy A.S. Seven things we think we know about auxin transport. Mol. Plant. 2011;4:487–504. doi: 10.1093/mp/ssr034. [DOI] [PubMed] [Google Scholar]
- Perry J., Dai X., Zhao Y. A mutation in the anticodon of a single tRNAala is sufficient to confer auxin resistance in Arabidopsis. Plant Physiol. 2005;139:1284–1290. doi: 10.1104/pp.105.068700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrásek J., Mravec J., Bouchard R., Blakeslee J.J., Abas M., Seifertová D., Wisniewska J., Tadele Z., Kubes M., Covanová M. PIN proteins perform a rate-limiting function in cellular auxin efflux. Science. 2006;312:914–918. doi: 10.1126/science.1123542. [DOI] [PubMed] [Google Scholar]
- Rahl P.B., Chen C.Z., Collins R.N. Elp1p, the yeast homolog of the FD disease syndrome protein, negatively regulates exocytosis independently of transcriptional elongation. Mol. Cell. 2005;17:841–853. doi: 10.1016/j.molcel.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Retzer K., Butt H., Korbei B., Luschnig C. The far side of auxin signaling: fundamental cellular activities and their contribution to a defined growth response in plants. Protoplasma. 2014;251:731–746. doi: 10.1007/s00709-013-0572-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosado A., Sohn E.J., Drakakaki G., Pan S., Swidergal A., Xiong Y., Kang B.H., Bressan R.A., Raikhel N.V. Auxin-mediated ribosomal biogenesis regulates vacuolar trafficking in Arabidopsis. Plant Cell. 2010;22:143–158. doi: 10.1105/tpc.109.068320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosado A., Li R., van de Ven W., Hsu E., Raikhel N.V. Arabidopsis ribosomal proteins control developmental programs through translational regulation of auxin response factors. Proc. Natl. Acad. Sci. USA. 2012;109:19537–19544. doi: 10.1073/pnas.1214774109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanmartín M., Sauer M., Muñoz A., Zouhar J., Ordóñez A., van de Ven W.T., Caro E., de la Paz Sánchez M., Raikhel N.V., Gutiérrez C. A molecular switch for initiating cell differentiation in Arabidopsis. Curr. Biol. 2011;21:999–1008. doi: 10.1016/j.cub.2011.04.041. [DOI] [PubMed] [Google Scholar]
- Sauer M., Robert S., Kleine-Vehn J. Auxin: simply complicated. J. Exp. Bot. 2013;64:2565–2577. doi: 10.1093/jxb/ert139. [DOI] [PubMed] [Google Scholar]
- Schwab R., Ossowski S., Riester M., Warthmann N., Weigel D. Highly specific gene silencing by artificial microRNAs in Arabidopsis. Plant Cell. 2006;18:1121–1133. doi: 10.1105/tpc.105.039834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieberer T., Hauser M.T., Seifert G.J., Luschnig C. PROPORZ1, a putative Arabidopsis transcriptional adaptor protein, mediates auxin and cytokinin signals in the control of cell proliferation. Curr. Biol. 2003;13:837–842. doi: 10.1016/s0960-9822(03)00327-0. [DOI] [PubMed] [Google Scholar]
- Singh N., Lorbeck M.T., Zervos A., Zimmerman J., Elefant F. The histone acetyltransferase Elp3 plays in active role in the control of synaptic bouton expansion and sleep in Drosophila. J. Neurochem. 2010;115:493–504. doi: 10.1111/j.1471-4159.2010.06892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano J., Miwa K., Yuan L., von Wirén N., Fujiwara T. Endocytosis and degradation of BOR1, a boron transporter of Arabidopsis thaliana, regulated by boron availability. Proc. Natl. Acad. Sci. USA. 2005;102:12276–12281. doi: 10.1073/pnas.0502060102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov T., Hagen G., Guilfoyle T.J. Activation and repression of transcription by auxin-response factors. Proc. Natl. Acad. Sci. USA. 1999;96:5844–5849. doi: 10.1073/pnas.96.10.5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Arnim A.G., Jia Q., Vaughn J.N. Regulation of plant translation by upstream open reading frames. Plant Sci. 2014;214:1–12. doi: 10.1016/j.plantsci.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Voß U., Bishopp A., Farcot E., Bennett M.J. Modelling hormonal response and development. Trends Plant Sci. 2014;19:311–319. doi: 10.1016/j.tplants.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wabnik K., Govaerts W., Friml J., Kleine-Vehn J. Feedback models for polarized auxin transport: an emerging trend. Mol. Biosyst. 2011;7:2352–2359. doi: 10.1039/c1mb05109a. [DOI] [PubMed] [Google Scholar]
- Wang L.K., Schwer B., Englert M., Beier H., Shuman S. Structure-function analysis of the kinase-CPD domain of yeast tRNA ligase (Trl1) and requirements for complementation of tRNA splicing by a plant Trl1 homolog. Nucleic Acids Res. 2006;34:517–527. doi: 10.1093/nar/gkj441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Yan X., Chen Q., Jiang N., Fu W., Ma B., Liu J., Li C., Bednarek S.Y., Pan J. Clathrin light chains regulate clathrin-mediated trafficking, auxin signaling, and development in Arabidopsis. Plant Cell. 2013;25:499–516. doi: 10.1105/tpc.112.108373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., An C., Zhang X., Yao J., Zhang Y., Sun Y., Yu F., Amador D.M., Mou Z. The Arabidopsis elongator complex subunit2 epigenetically regulates plant immune responses. Plant Cell. 2013;25:762–776. doi: 10.1105/tpc.113.109116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler G.S., Petrakis T.G., Ethelberg S., Tokunaga M., Erdjument-Bromage H., Tempst P., Svejstrup J.Q. RNA polymerase II elongator holoenzyme is composed of two discrete subcomplexes. J. Biol. Chem. 2001;276:32743–32749. doi: 10.1074/jbc.M105303200. [DOI] [PubMed] [Google Scholar]
- Wittschieben B.O., Otero G., de Bizemont T., Fellows J., Erdjument-Bromage H., Ohba R., Li Y., Allis C.D., Tempst P., Svejstrup J.Q. A novel histone acetyltransferase is an integral subunit of elongating RNA polymerase II holoenzyme. Mol. Cell. 1999;4:123–128. doi: 10.1016/s1097-2765(00)80194-x. [DOI] [PubMed] [Google Scholar]
- Xu T., Wen M., Nagawa S., Fu Y., Chen J.G., Wu M.J., Perrot-Rechenmann C., Friml J., Jones A.M., Yang Z. Cell surface- and rho GTPase-based auxin signaling controls cellular interdigitation in Arabidopsis. Cell. 2010;143:99–110. doi: 10.1016/j.cell.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D., Huang W., Li Y., Wang H., Huang H., Cui X. Elongator complex is critical for cell cycle progression and leaf patterning in Arabidopsis. Plant J. 2012;69:792–808. doi: 10.1111/j.1365-313X.2011.04831.x. [DOI] [PubMed] [Google Scholar]
- Zhou X., Hua D., Chen Z., Zhou Z., Gong Z. Elongator mediates ABA responses, oxidative stress resistance and anthocyanin biosynthesis in Arabidopsis. Plant J. 2009;60:79–90. doi: 10.1111/j.1365-313X.2009.03931.x. [DOI] [PubMed] [Google Scholar]
- Zhou F., Roy B., von Arnim A.G. Translation reinitiation and development are compromised in similar ways by mutations in translation initiation factor eIF3h and the ribosomal protein RPL24. BMC Plant Biol. 2010;10:193. doi: 10.1186/1471-2229-10-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.