Abstract
Background:
This multicenter international cross-sectional observational study characterized vascular and valvular calcification burden and correlations with pulse pressure, diabetes, hypertension, and cardiovascular diseases in prevalent hemodialysis patients.
Methods:
We enrolled 275 consecutive adults with end-stage renal disease on maintenance hemodialysis for ⩾3 months. Coprimary endpoints were prevalences of: (1) echocardiographic calcification in mitral valve, aortic valve or mitral annulus; (2) aortoiliac tree vascular calcifications by plain lateral lumbar X-ray. Correlations among calcification sites and with demographics and comorbidities were determined. Pulse pressures were determined.
Results:
Subjects’ mean ± standard deviation (SD) age was 56 ± 15.9 years; mean (SD) dialysis duration was 4.5 ± 4.3 years. Overall, 100% of echocardiographically imaged patients (n = 243) had calcification in aortic valve, mitral valve, or mitral annulus; 77.8% of X-rayed patients (n = 248) had abdominal aortic calcification. Radiographic abdominal aortic calcification score correlated significantly with calcification of aortic valve (p < 0.0001) and mitral annulus (p = 0.0001) but not mitral valve. Aortic valve, mitral valve, and mitral annulus calcification correlated significantly among themselves (p < 0.0001). Moderate/severe aortic valve calcification was significantly more prevalent in patients aged ⩾65 years than <65 years, men than women, and Whites than African Americans. Pulse pressure correlated significantly with vascular calcification score (p = 0.0049) but not with valvular calcification at any site.
Conclusions:
Vascular and valvular calcification are highly prevalent in the hemodialysis population. Peripheral vascular calcification correlates significantly with elevated pulse pressure and can be assessed easily using lateral lumbar X-ray. Further studies investigating the interaction between pulse pressure and development or progression of vascular calcification are of interest.
Keywords: chronic kidney disease, echocardiography, hemodialysis, lumbar X-ray, vascular calcification, valvular calcification
Introduction
Up to 40–50% of patients with end-stage renal disease (ESRD) die of cardiovascular disease (CVD) [US Renal Data System, 1998], reflecting their rapidly progressing atherosclerotic burden [Goodman et al. 2000, 2004] and heavy arterial calcification [Schwarz et al. 2000]. Altered calcium, phosphorus, parathyroid hormone (PTH), and vitamin D levels in ESRD induce mediators of bone-like metabolism and mineralization in the vascular tunica media [Moe and Chen, 2008].
Management of chronic kidney disease–mineral and bone disorder (CKD-MBD) appropriately includes assessment of vascular and valvular calci-fication according to the US National Kidney Foundation [KDIGO, 2009]. Although the reference standard diagnostic methods for calcification are electron beam computed tomography (EBCT) and multislice spiral computed tomography [Rumberger et al. 1999; Floege and Ketteler, 2004], clinically accessible first-line methods include echocardiography and lumbar lateral X-ray [Raggi and Bellasi, 2007].
Calcification stiffens vessels and valves and reduces their compliance, contributing to left ventricular hypertrophy (LVH) and increasing all-cause mortality risk. Aortic calcification is associated with high cardiovascular risk in patients without CKD [London, 2003; London et al. 2003] or with CKD [Kauppila et al. 1997; Wilson et al. 2001]. Aortic pulse wave velocity, a marker of arterial stiffness related to sudden cardiac death, is associated with abdominal aortic calcification and borderline associated with thoracic aortic and coronary calcification in hemodialysis patients [Raggi et al. 2007].
Few studies have evaluated both vascular and valvular calcification in the same dialysis patients in relation to mortality. Higher baseline coronary artery and aortic valvular calcification predicted mortality in univariate post hoc analysis of 127 hemodialysis patients receiving sevelamer or calcium-based phosphate binders followed for 3.6 years; calcium-based binder use predicted multivariate mortality risk [Spiegel et al. 2007]. Similarly, peritoneal dialysis patients (n = 102) with both valvular and coronary arterial calcification had higher mortality over 4 years than those with coronary arterial calcification alone [Janicka et al. 2011]. Finally, in ADVANCE, 360 prevalent dialysis patients on calcium binders were randomized to vitamin D sterols with/without cinacalcet [Raggi et al. 2011]. Results showed coronary artery, aortic, and valvular calcification scores increased numerically from baseline in all patients and aortic valve calcification increased significantly less in cinacalcet/low-dose vitamin D recipients than those receiving vitamin D alone.
Valvular and vascular calcifications contribute to cardiovascular mortality risk in dialysis recipients, raising interest in interventions to reduce calcification incidence and progression. The current study determined prevalence of vascular and valvular calcification in prevalent hemodialysis patients using noninvasive imaging (echocardiography and lateral lumbar X-ray) and correlations with pulse pressure, an indicator of vascular elasticity.
Methods
Study design
This multicenter international observational study involved 12 centers in the United States, Canada, Puerto Rico, Spain, and the United Kingdom. Consecutive consenting adults with ESRD on maintenance hemodialysis for ⩾3 months were recruited. The first patient enrolled in November 2004, the last patient’s last visit was in August 2005, and the study closed 5 October 2005. Pregnancy, lactation, or planned pregnancy within 6 months post study, as well as unobtainability of imaging in male or female patients, were exclusionary.
Assessments
Centrally read echocardiography measured left ventricular mass (LVM) and ejection fraction (LVEF) and scored calcification at the mitral valve, mitral annulus, and aortic valve [Raggi et al. 2007]. Data quality was assessed as optimal, readable, unreadable, or data missing; if visualization remained suboptimal on repeat imaging, calcification was not graded. Calcification in scorable images was categorized as none, mild (bright ring), moderate, or severe (dense), and scores were grouped into none/mild and moderate/severe for analysis. The proportion of patients with echocardiographic calcification at any site was scored.
Centrally read plain lateral lumbar X-rays assessed presence of abdominal aortic calcifications [Raggi et al. 2007]. Eight locations were evaluated (anterior and posterior abdominal aortic walls at vertebral segments L1–L4) and assigned Framingham calcification scores (0 = no detectable calcification; 1 = small scattered calcifications over ⩽1/3 of segment; 2 = calcification of 1/3 to <2/3 of the segment’s aortic wall; 3 = calcification of ⩾2/3 of the segment’s aortic wall). The eight measurements were summed for the total calcification score ranging from 0 to 24 [Kauppila et al. 1997]. Scores were grouped into categories of 0, 1–6, 7–12, 13–18, and 19–24. The proportion of patients with any radiographic aortic calcification was also scored.
Laboratory data were recorded for serum phosphorus, calcium, albumin, PTH, triglycerides; cholesterol (total, high-density lipoprotein, and low-density lipoprotein); mean standard weekly Kt/V; and urea reduction ratio (URR). Systolic and diastolic blood pressures were recorded predialysis, and pulse pressures were determined (systolic minus diastolic blood pressure).
Additional exploratory analyses evaluated the relationship of calcification to age, gender (including a subanalysis of women before and after 51 years of age to examine menopausal effects), ethnicity, comorbidities (diabetes, hypertension, cardiac disorders, and other chronic diseases), and kidney history (dialysis duration in patients with or without prior transplantation histories).
Institutional review board or institutional ethics committee approvals were obtained for each participating center (see the online supplement for specific organizations providing approval). All participants granted written informed consent. This study conformed to Good Clinical Practice and the Declaration of Helsinki.
Statistical analysis
Calcification extent and prevalence at aortoiliac segments, aortic and mitral valves, and mitral annulus were examined with descriptive statistics. Spearman correlations determined relationships of pulse pressure, radiographic abdominal aortic calcification score, and echocardiographic valvular calcifications as well as relationships of age with calcifications (adjusted or unadjusted for dialysis duration). Cochran–Mantel–Haenszel chi-squared tests assessed correlations of radiographic aortic and echocardiographic valvular calcification with age, gender, race/ethnicity, dialysis vintage, and diabetes/hypertension. Multivariate analyses were not designed into the study in 2004–2005; resource limitations precluded their retroactive inclusion.
Results
Enrolled were 275 patients (63.3% were male), with a mean (standard deviation [SD]) age of 56 (15.9) years (median, 57 years; range, 19–84 years) and a mean (SD) dialysis duration of 4.5 (4.3) years. Among the 36 previously transplanted patients, the mean (SD) time since transplantation was 10.4 (5.5) years. Other demographics are shown in Table 1. Renal failure etiologies were diabetic nephropathy in 19.8% of patients and hypertension in 32.1%. A total of 96 (35.2%) patients had a major cardiovascular event (myocardial infarction [MI], ischemic heart disease, cardiac arrest, cardiac dysrhythmia, pericarditis, peripheral vascular disease, or cerebrovascular disease); two patients lacked cardiovascular history data (Table 1).
Table 1.
Patient characteristics.
| Variable | n (%) |
|---|---|
| Gender (n = 275) | |
| Male | 174 (63.3) |
| Female | 101 (36.7) |
| Race (n = 274) | |
| White | 150 (54.7) |
| African American | 101 (36.9) |
| Other | 23 (8.4) |
| Kidney transplantation (n = 274) | 36 (13.1) |
| Cause of ESRD (n = 274) | |
| Type 1 diabetes mellitus | 7 (2.6) |
| Type 2 diabetes mellitus | 47 (17.2) |
| GN | 39 (14.2) |
| Secondary GN/vasculitis | 5 (1.8) |
| Interstitial nephritis | 3 (1.1) |
| Hypertension | 88 (32.1) |
| Cystic/congenital/hereditary | 35 (12.8) |
| Neoplasms | 2 (0.7) |
| Other | 48 (17.5) |
| Medical historya | |
| Myocardial infarction | 24 (8.8) |
| Ischemic heart disease | 53 (19.4) |
| Cardiac arrest | 6 (2.2) |
| Cardiac dysrhythmia | 33 (12.0) |
| Pericarditis | 2 (0.7) |
| Peripheral vascular disease | 26 (9.5) |
| Cerebrovascular disease | 22 (8.0) |
| COPD | 21 (7.7) |
| Malignant neoplasm | 29 (10.6) |
| Diabetes mellitus (type 1 or 2) | 74 (27.2) |
| Hypertension | 228 (83.2) |
| Alcohol or drug dependence | 5 (1.8) |
| Tobacco use | 26 (9.5) |
Several subjects had missing medical history, but the sample size was at least 272 in all categories.
COPD, chronic obstructive pulmonary disease; ESRD, end-stage renal disease; GN, glomerulonephritis.
Biochemical values are shown in Table 2. Participants were adequately dialyzed (URR mean ± SD, 72.4 ± 7.5%; median, 73.0%; average weekly Kt/V, 1.5) and had normal serum albumin. Mean serum calcium (8.9 mg/dl) and phosphate (5.2 mg/dl) were within National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative (K/DOQI) dialysis target ranges [National Kidney Foundation, 2003], which were current during the study. Patients had moderate secondary hyperparathyroidism (mean PTH, 348.9 pg/ml). K/DOQI guidelines were met by 31.6% of patients for PTH (150–300 pg/ml), 62.6% for calcium (8.4–9.5 mg/dl), and 46.7% for phosphorous (3.5–5.5 mg/dl).
Table 2.
Biochemical and cardiovascular parameters.
| Variable | Mean ± SD | Median (IQR) |
|---|---|---|
| Serum phosphorus, mg/dl (n = 274) | 5.2 ± 1.8 | 5.1 (4.0–6.3) |
| Serum calcium, mg/dl (n = 273) | 8.9 ± 1.0 | 9.0 (8.5–9.4) |
| Albumin, g/dl (n = 269) | 3.9 ± 0.4 | 3.90 (3.7–4.1) |
| PTH, pg/ml (n = 234) | 348.9 ± 399.5 | 256.8 (128.0–436.1) |
| Triglycerides, mg/dl (n = 132) | 166.4 ± 129.3 | 132.0 (91.5–203.5) |
| Total cholesterol, mg/dl (n = 225) | 155.2 ± 35.7 | 152.0 (131.0–175.0) |
| HDL-C, mg/dl (n = 145) | 47.3 ± 15.2 | 46.0 (36.0–55.0) |
| LDL-C, mg/dl (n = 102) | 86.5 ± 36.1 | 81.0 (60.0–100.0) |
| Kt/V (n = 117) | 1.5 ± 0.4 | 1.4 (1.2–1.8) |
| URR, % (n = 205) | 72.4 ± 7.5 | 73.0 (68.0–76.9) |
| Systolic BP, mmHg (n = 273) | 140.2 ± 24.2 | 140.0 (121.0–155.0) |
| Diastolic BP, mmHg (n = 273) | 76.6 ± 13.6 | 77.0 (69.0–83.0) |
| Pulse pressure, mmHg (n = 273) | 63.6 ± 18.3 | 61.0 (50.0–74.0) |
| LV end diastolic volume, ml (n = 250) | 158.2 ± 55.9 | 150.0 (121.0–181.0) |
| LV end systolic volume, ml (n = 250) | 67.7 ± 38.3 | 59.0 (44.0–80.0) |
| LVEF, % (n = 250) | 59.4 ± 16.7 | 59.5 (53.0–66.0) |
| LV mass, g (n = 234) | 201.2 ± 76.8 | 185.0 (144.0–243.0) |
BP, blood pressure; HDL-C, high-density lipoprotein cholesterol; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol; LV, left ventricular; LVEF, left ventricular ejection fraction; PTH, parathyroid hormone; SD, standard deviation; URR, urea reduction ratio.
Table 2 also shows echocardiographic measurements and predialysis blood pressure values; the latter were within K/DOQI targets. Pulse pressure exceeded 40 mmHg in 91.2% of patients; 95.7% had abnormal LVM (>2 SD from 92 ± 16.0 g/m2), and 49.0% had reduced left ventricular systolic function (LVEF <0.67 ± 0.08).
No patient of the 262 with available echocardiographic or X-ray data was free of calcification at all sites, i.e. the prevalence of calcification visible to at least 1 of the 2 techniques was 100% in our evaluable patient set. The prevalence of echocardiographic valvular calcification was 100% among 243 imaged patients, all of whom had at least mild calcification in at least one site (aortic valve, mitral valve, or mitral annulus). Fewer than 2% of patients had any one site calcification-free; more than 50% had at least mild calcifications. Moderate calcifications affected the mitral annulus in 31.3% (of 217) of patients, the aortic valve in 27.3% (of 198), and the mitral valve in 16.2% (of 167) (Table 3). Mitral valve echocardiographic data were incomplete due to the readability and quality of the scans.
Table 3.
Echocardiographic findings of calcification frequency and degree.
| Calcification sites | Number of patients (%) |
|||
|---|---|---|---|---|
| None | Mild | Moderate | Severe | |
| Aortic valve calcification (n = 198) | 1 (0.5) | 143 (72.2) | 44 (22.2) | 10 (5.1) |
| Mitral valve calcification (n = 167) | 2 (1.2) | 138 (82.6) | 27 (16.2) | 0 (0.0) |
| Mitral annulus calcification (n = 217) | 0 (0.0) | 149 (68.7) | 56 (25.8) | 12 (5.5) |
Radiographic aortic calcification scores were available in 248 patients, with an overall mean (SD) calcification score of 7.7 (7.3) and a median (range) of 6.0 (0–24.0). A total of 55 (22.2%) patients were free of abdominal aortic calcifications. The prevalence of radiographic aortic calcifications was 77.8%, affecting 193 of 248 evaluable patients.
Aortic calcification score correlated significantly to aortic valve (rs = 0.31; p < 0.0001; n = 180) or mitral annulus (rs = 0.27; p = 0.0001; n = 198) calcifications and to pulse pressure (rs = 0.18; p = 0.0049; n = 248), but not to mitral valve calcification.
Calcification and age
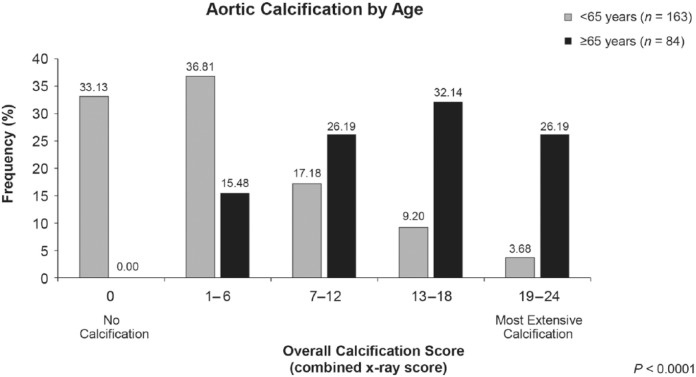
Moderate/severe echocardiographic aortic valve calcification was three times more common in patients aged ⩾65 years than in those younger (46.1% versus 15.7%, respectively; p < 0.0001). Moderate/severe mitral valve calcification prevalence did not differ statistically by age. Moderate/severe mitral annulus calcification was nearly twice as prevalent in older than younger patients (45.0% versus 23.5%, respectively; p = 0.0011); older patients also had a higher prevalence and severity of radiographic abdominal aortic calcification (p < 0.0001; Figure 1). Overall radiographic calcification scores of 19–24 affected 26.2% of older versus only 3.7% of younger patients; conversely, one-third of younger patients but no older patients were aortic calcification-free.
Figure 1.
Relationship between age and overall aortic calcification score (rs = 0.65, p < 0.0001). No patients aged 65 years and older were free of radiographic aortic calcification; overall score categories from 7–12 through 19–24 were more prevalent in this older age group than among those younger than 65 years.
Spearman correlation coefficients showed that radiographic overall abdominal aortic and echocardiographic aortic valve calcifications, respectively, correlated significantly with age with adjustment (rs = 0.65 and rs = 0.39; p < 0.0001 for each) or without adjustment for dialysis duration (rs = 0.67 and rs = 0.3; p < 0.0001 for each). Mitral annulus calcification correlated significantly with age with adjustment (rs = 0.32; p = 0.0003) or without adjustment for dialysis duration (rs = 0.26; p = 0.0001); mitral valve calcification showed no significant correlation with age irrespective of vintage.
Calcification and gender
Moderate/severe calcification at the aortic valve (but not mitral valve or mitral annulus) was significantly more prevalent in men than in women (32.8% versus 17.1%, respectively; p = 0.0182). Proportionately more women than men had zero radiographic abdominal aortic calcification (31.2% versus 16.8%, respectively), whereas men had higher prevalence of moderate abdominal aortic calcification (scores of 7–18).
Female participants were separated into age groups (⩽51 and >51 years) to allow for menopausal effects. The Cochran–Mantel–Haenszel procedure showed a statistically significant association between gender/age groups and overall radiographic abdominal aorta calcification score (p < 0.0001) or echocardiographic aortic valve calcification (p = 0.0120), with older women having the greatest rates of calcification in each modality. Mitral valve or mitral annulus calcification scores were not significantly associated with gender or age groups.
Calcification and ethnicity
Prevalence of moderate/severe aortic valve (p = 0.0202) and mitral annulus (p = 0.0068) calcification differed significantly by ethnicity. Whites had higher prevalence (34.8%) of moderate/severe aortic valve calcification than did African Americans (17.5%) and other races (15.0%). African Americans, meanwhile, had lower prevalence (17.8%) of mitral annulus calcification than did Whites (37.4%) and other ethnic groups (45.0%). In contrast, prevalence of moderate/severe mitral valve calcification did not significantly differ by ethnicity. Radiographically, significantly fewer Whites (9.1%) were calcification-free at the abdominal aorta than African Americans (37.6%) and other races (36.4%; p < 0.0001). Proportionately more Whites had abdominal aortic calcification scores in the most severe category (19–24).
Calcification and dialysis vintage
Calcification data were compared by dialysis vintage (duration of <1 year, 1–3 years, and >3 years; Table 4 summarizes the dialysis characteristics of the population). Echocardiographic data are segmented by dialysis vintage in patients with and without prior transplants in Table 5. Prevalences of moderate/severe calcification at the aortic valve (among 172 evaluable patients) and mitral valve (among 144 evaluable patients) did not differ significantly by vintage. Prevalence of moderate/severe mitral annulus calcification (among 215 evaluable patients) increased significantly with longer dialysis vintage overall (18.4% versus 30.2% versus 36.0% for dialysis duration of <1 year, 1–3 years, and >3 years, respectively; p = 0.0470). After excluding patients with previous transplantations (leaving 189 patients with evaluable mitral annulus data), mitral annulus moderate/severe calcification percentages for the same dialysis duration subsets were 16.7% versus 29.8% versus 34.4%, respectively; the association became borderline significant (p = 0.0581). No other significant associations of calcifications with dialysis durations were observed; radiographic aortic calcifications (Table 6) did not correlate significantly with dialysis duration in patients with (p = 0.3814) or without (p = 0.4304) prior transplants.
Table 4.
Dialysis duration distribution by age groups.
| Renal history demographics | Mean (SD) | Median (IQR) | Range |
|---|---|---|---|
| Dialysis duration, years, overall | 4.5 (4.3) | 3.3 | 0.3–25.0 |
| Dialysis duration, years, patients aged <65 years (n = 179) | 4.8 (4.5) | 3.5 (1.7–6.2) | 0.3–22.9 |
| Dialysis duration, years, patients aged ⩾65 years (n = 93) | 4.0 (3.8) | 3.0 (1.5–5.6) | 0.4–25.0 |
| Time since transplantation, years, in previously transplanted patients (n = 36) | 10.4 (5.5) | 10.7 | 0.2–24.1 |
IQR, interquartile range; SD, standard deviation.
Table 5.
Prevalences of moderate to severe echocardiographic valvular calcification, analyzed by dialysis vintage among patients with and without histories of prior transplantation.
| Patients with moderate/severe echocardiographic calcification, n/N (%)a |
|||
|---|---|---|---|
| Aortic valve | Mitral valve | Mitral annulus | |
| Dialysis duration, including prior transplantees | |||
| <1 year | 8/36 (22.2%) | 7/29 (24.1%) | 7/38 (18.4%) |
| 1–3 years | 18/55 (32.73%) | 7/47 (14.9%) | 19/63 (30.2%) |
| >3 years | 27/105 (25.7%) | 13/89 (14.6%) | 41/114 (36.0%) |
| Total | 53/196 (27.0%) | 27/165 (16.4%) | 67/215 (31.2%) |
| p value (nonzero correlation) | 0.9434 | 0.2941 | 0.0470 |
| Dialysis duration, excluding prior transplantees | |||
| <1 year | 7/34 (20.6%) | 6/27 (22.2%) | 6/36 (16.7%) |
| 1–3 years | 17/50 (34.0%) | 7/41 (17.1%) | 17/57 (29.8%) |
| >3 years | 24/88 (27.3%) | 12/76 (15.8%) | 33/96 (34.4%) |
| Total | 48/172 (27.9%) | 25/144 (17.4%) | 56/189 (29.6%) |
| p value (nonzero correlation) | 0.6755 | 0.4778 | 0.0581 |
Affected = numerator/subgroup total = denominator (%).
Table 6.
Prevalences of radiographic abdominal aortic calcification, analyzed by dialysis vintage among patients with and without histories of prior transplantation.
| Patients with overall X-ray combined scores, n/N (%)a |
|||||
|---|---|---|---|---|---|
| 0 | 1–6 | 7–12 | 13–18 | 19–24 | |
| Dialysis duration, including prior transplantees (246 patients with evaluable data, 29 with missing data) | |||||
| <1 year | 13/41 (31.71%) | 10/41 (24.39%) | 9/41 (21.95%) | 7/41 (17.07%) | 2/41 (4.88%) |
| 1–3 years | 16/80 (20.00%) | 22/80 (27.50%) | 18/80 (22.50%) | 14/80 (17.50%) | 10/80 (12.50%) |
| >3 years | 26/125 (20.80%) | 41/125 (32.80%) | 22/125 (17.60%) | 20/125 (16.00%) | 16/125 (12.80%) |
| Total | 55/246 (22.36%) | 73/246 (29.67%) | 49/246 (19.92%) | 41/246 (16.67%) | 28/246 (11.38%) |
| p value (nonzero correlation) | 0.3814 | ||||
| Dialysis duration, excluding prior transplantees (213 patients with evaluable data, 25 with missing data) | |||||
| <1 year | 12/39 (30.77%) | 10/39 (25.64%) | 9/39 (23.08%) | 6/39 (15.38%) | 2/39 (5.13%) |
| 1–3 years | 14/73 (19.18%) | 20/73 (27.40%) | 16/73 (21.92%) | 14/73 (19.18%) | 9/73 (12.33%) |
| >3 years | 25/101 (24.75%) | 28/101 (27.72%) | 18/101 (17.82%) | 16/101 (15.84%) | 14/101 (13.86%) |
| Total | 51/213 (23.94%) | 58/213 (27.23%) | 43/213 (20.19%) | 36/213 (16.90%) | 25/213 (11.74%) |
| p value (nonzero correlation) | 0.4304 | ||||
Affected = numerator/subgroup total = denominator (%).
Mitral annulus calcification showed a low but significant Spearman correlation with dialysis duration unadjusted for age (rs = 0.13; p = 0.0499). Adjustment for age abrogated significance (rs = 0.14; p = 0.121). No other calcification sites were significantly correlated with dialysis durations.
Calcification and diabetes and hypertension
Echocardiographically, prevalences of moderate/severe calcification of the aortic valve, mitral valve, or mitral annulus did not differ significantly by diabetes or hypertension status. Radiographically, overall abdominal aortic calcification severity distribution did not differ significantly with or without diabetes or hypertension.
Calcification and cardiovascular history
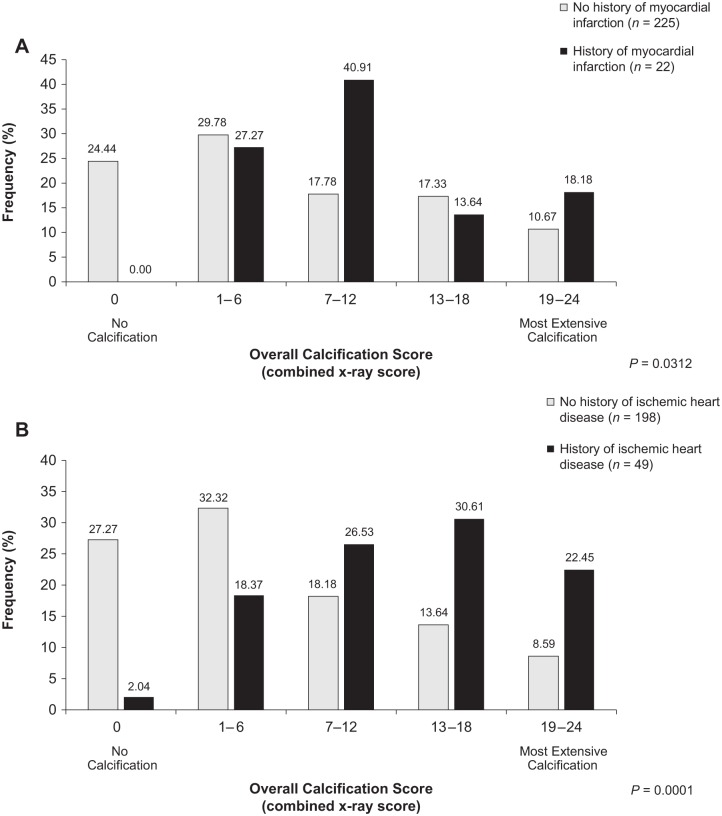
Calcification scores were compared by history or absence of prior cardiovascular events. Figure 2 presents data for MI and ischemic heart disease. History of MI (Figure 2A) was significantly associated with more severe radiographic abdominal aortic calcification (p = 0.03), although only 24 patients reported prior MIs. All patients with prior MIs had abdominal aortic calcification, whereas 24.4% of MI-free patients were also aortic calcification-free. Moderate/severe valvular calcification did not differ by MI history. Patients with ischemic heart disease (Figure 2B) had significantly more severe radiographic abdominal aortic calcification than those without (p < 0.0001). Echocardio-graphically, prevalences of moderate/severe aortic valve and mitral annulus calcification, respectively, were numerically greater among those with ische-mic heart disease (38.6% versus 24.3%; p = 0.0623) than without ischemic heart disease (42.6% versus 28.0%; p = 0.0571). Mitral valve calcification did not differ by ischemic heart disease status.
Figure 2.
Relationship between overall aortic calcification and prior cardiovascular events: (A) myocardial infarction (p = 0.0312) and (B) ischemic heart disease (p < 0.0001). Almost a quarter of patients (24.44%) without myocardial infarction history, but no patients with myocardial infarction history, had an overall aortic calcification score of 0. Patients with histories of ischemic heart disease had significantly more severe aortic calcification than those without.
Patients with cardiac dysrhythmia had significantly greater prevalence of echocardiographic moderate/severe aortic valve than those without dysrhythmia (50.0% versus 23.5%; p = 0.0037), mitral valve (43.5% versus 11.8%; p = 0.0001), and mitral annulus calcification (52.0% versus 28.6%; p = 0.0182). Radiographically assessed overall abdominal aortic calcification scores were significantly more severe among patients with dysrhythmia (p = 0.0008).
Peripheral vascular disease was significantly associated with greater prevalences of moderate/severe aortic valve calcification (45.5% of patients with peripheral vascular disease versus 25.1% of those without; p = 0.0447) and with significantly more severe overall aortic calcification (p = 0.0004). Mitral valve and mitral annulus calcification did not differ significantly in patients with or without peripheral vascular disease.
Calcification and pulse pressure
Pulse pressure correlated significantly to radiographic vascular calcification score (rs = 0.18; p = 0.0049; 248 evaluable patients) but not to echocardiographic valvular calcification at any site. As expected, aortic valve, mitral valve, and mitral annulus calcification correlated significantly with each other (p < 0.0001).
Discussion
Nephrology organizations promote clinically accessible noninvasive imaging of vascular and valvular calcification. The National Kidney Foundation K/DOQI cardiovascular guidelines for dialysis patients [National Kidney Foundation, 2005] emphasize echocardiography; the Foundation’s K/DOQI bone metabolism/disease guidelines [National Kidney Foundation, 2003] and the Kidney Disease Improving Global Outcomes (KDIGO) CKD-MBD Working Group guidelines for CKD-MBD [Moe et al. 2006; KDIGO, 2009] propose radiographic detection of vascular calcification, including abdominal aortic calcification, which allows both scoring for presence and semiquantitative evaluation.
No formal recommendations exist for coronary or peripheral vascular calcification screening in patients on dialysis, despite their 83–92% prevalence of coronary calcification by EBCT [Goodman et al. 2000; Oh et al. 2002; Raggi et al. 2002] and data linking vascular calcification to mortality [Kauppila et al. 1997; Wilson et al. 2001]. KDIGO CKD-MBD guidelines, however, state that if calcification is observed in the abdominal aorta, carotids, iliofemoral axis, or femoropopliteal axis, identification of calcification at another site should be sought [KDIGO, 2009]. The same source [KDIGO, 2009] suggests that patients with CKD and known prevalent vascular or valvular calcification be treated as belonging to the highest category of cardiovascular risk; that the dose of calcium-based phosphate binders be restricted in patients with ESRD and known calcification (and those with persistent hypercalcemia, persistent low PTH, or adynamic bone disease); and that non-calcium-containing phosphate binders should be considered in patients with vascular calcifications at two or more sites. KDIGO acknowledges the need for more research into the role of calcium versus non-calcium binders in the pathogenesis of calcification, but regards limiting binder-associated calcium intake as justified [KDIGO, 2009].
Certain studies associate non-calcium binders with less progression of calcification than calcium-based binders [Chertow et al. 2002; Block et al. 2005; Toussaint et al. 2011; Kalil et al. 2012]. Although a 2011 Cochrane meta-analysis [Navaneethan et al. 2011] found data insufficient to establish superiority of non-calcium to calcium-containing binders for all-cause mortality and cardiovascular outcomes in CKD, a 2012 meta-analysis of 11 randomized studies (n = 4622) reported a significant 22% mortality reduction with non-calcium versus calcium binders [Jamal et al. 2013]. Similarly, the 36-month INDEPENDENT-HD randomized trial in 466 incident dialysis patients observed significantly reduced all-cause and cardiovascular mortality with sevelamer versus calcium carbonate [Di Iorio et al. 2013]. The potential contribution of binder choice to prevention of calcification and its complications merits clinical consideration. A major limitation of our study is the unavailability of data on concomitant medications, including phosphate binders, which were not retrievable from the 2004–2005 archival data.
Calcium and phosphate levels and other CKD-MBD anomalies leading to calcification also contribute to increased pulse wave velocity and pulse pressure, associated with increased mortality in ESRD [Klassen et al. 2002; Blacher et al. 2003]. Ultrasound-measured calcification is proportional to increases in pulse pressure and velocity [Guerin et al. 2000]. In our study, pulse pressure correlated significantly to radiographic overall abdominal aortic calcification score. Whether elevated pulse pressure could serve as a surrogate marker of calcification in dialysis patients requires further study.
We compared and contrasted our findings with those of prior studies relating calcification to hemodialysis patients’ characteristics. Older age was associated with vascular calcification in both our study and its predecessors [Honkanen et al. 2008; Jean et al. 2009]. Abdominal aortic calcification score did not differ significantly by diabetes status among our patients, perhaps because fewer than 20% of our patients had diabetic nephropathy. This finding contrasts with that of Jean and colleagues [Jean et al. 2009], but resembles the Calcification Outcome in Renal Disease (CORD) study (933 European dialysis patients with lumbar X-rays) [Honkanen et al. 2008]. History of CVD in CORD was associated with higher abdominal aortic calcification scores and predicted calcification on multivariate analysis. In our current study, and consistent with CORD, the patients with prior MI, ischemic heart disease, dysrhythmia, or peripheral artery disease had increased abdominal aortic calcification. Furthermore, the proportion of major cardiovascular events among our patients (35.2%) was comparable to combined on-study cardiovascular event rates in the German Diabetes in Dialysis (4D) Study (39 and 33% in 4D placebo and atorvastatin groups, respectively); our prior MI rate (8%) was also comparable to 4D on-study nonfatal MI rates (12% with placebo and 11% with atorvastatin) [Wanner et al. 2005].
Vascular calcification could be expected to increase with dialysis duration. In the study of Goldsmith and colleagues in 38 patients on hemodialysis for 10–25 years, radiographic vascular calcification prevalence increased from 39% at dialysis onset to 92% after an average dialysis duration of 16 years, with a mean onset 9.7 years after starting dialysis [Goldsmith et al. 1997]; calcification severity also increased with vintage. Similarly, in CORD, dialysis duration independently predicted abdominal aortic calcification on multivariate analysis (odds ratio, 1.110 per 1-year increase; p < 0.001) [Honkanen et al. 2008]. In the current study, patients’ radiographic abdominal aortic overall calcification scores did not increase with dialysis vintage, perhaps because most participants were long-time dialysis survivors (0.3–25.0 years). Exclusion of prior transplantees rendered the association of echocardiographic mitral annulus calcification with dialysis vintage borderline significant. Relevantly, the patient population in the current study reflects a lower-risk profile than is typical in prevalent dialysis, with younger age, <20% type 2 diabetes prevalence, and low-risk albumin levels (>3.5 g/dl).
We found no correlation between pulse pressure and calcification of the aortic valve, mitral valve, or mitral annulus. All echocardiographically evaluated patients had at least one calcified site. Mild/moderate mitral valve and aortic valve calcification were reported in 98.8% and 94.4% of patients, respectively, exceeding previously documented prevalences. Echocardiographic prevalences of mitral valve calcification of 45% and aortic valve calcification of 52% occurred in a predominantly White-European patient population [Ribeiro et al. 1998], whereas EBCT investigations observed prevalent mitral valve calcification in 59% of dialysis patients and aortic valve calcification in 55% [Braun et al. 1996].
Cardiovascular calcification predicts cardiovascular morbidity and mortality in general populations and dialysis recipients. In the prospective general-public Framingham Heart Study with 22 years of follow up, radiographic abdominal aortic calcification was associated with increased relative risk of CVD, coronary heart disease, and cardiovascular mortality [Wilson et al. 2001]. Among 205 hemodialysis recipients with EBCT imaging, coronary artery calcification was related to prevalent MI and angina; aortic calcification was related to prevalent peripheral artery disease and aortic aneurysm [Raggi et al. 2002]. Higher peripheral vascular calcification levels on X-rays were associated with a doubled 1-year mortality rate in 161 long-hemodialysis French patients [Jean et al. 2009]. Valvular calcification predicts LVH in hemodialysis recipients, particularly those with both aortic valve and mitral valve calcification [Strozecki et al. 2005]. Mitral annular calcification in hemodialysis patients has been associated with clinical coronary artery disease and increased mortality [Sharma et al. 2007]. Arterial medial calcification in hemodialysis patients was associated with increased mortality but less so than arterial intimal calcification [London et al. 2003]. Pulse pressure predicted 30-month all-cause mortality in peritoneal dialysis patients [Liu et al. 2008].
Limitations of this study include its cross-sectional design, single centralized imaging readers, and unavailability of concomitant medication data and multivariate analyses. Notwithstanding our having collected serum phosphate and calcium laboratory results, data on use of phosphate binders and other CKD-MBD drugs would have been relevant if available. In addition, only 24 of our patients reported prior MI, whose relationship to calcifications was thus determined on a small sample size. Although in an ideal study multivariate analyses would be more informative, these were not performed due to statistical resource limitations for reanalysis of the 2004–2005 data set.
Conclusions
Among 262 imaged hemodialysis patients, 100% had calcification detectable by either valvular echocardiography or abdominal aortic radiography. The prevalence of echocardiographic valvular calcification (at ⩾1 of the 3 sites) was 100% and that of radiographic aortic calcification 77.8%. Peripheral vascular calcification, visible on lateral lumbar aorta X-ray, had a statistically significant, though relatively low correlation (r = 0.18), with elevated pulse pressure. Further studies are needed to evaluate interactions of pulse pressure with vascular calcification and dietary and pharmacologic interventions intended to reduce calcification.
Acknowledgments
The authors recognize additional study investigators: Adeera Levin, James Cotton, Jeffrey Hoggard, Chika Oguagha, Jose Cangiano, Francisco Maduell, Jose Hervas, and Judith Stevens. Participating institutions included: John H. Stroger Jr Hospital, Chicago, IL, USA; Saint Paul’s Hospital, Vancouver, BC, Canada; Tyler Nephrology Associates, Tyler, TX, USA; Eastern Nephrology Associates, Greenville, NC, USA; University of Chicago, Chicago, IL, USA; Nephrology Foundation of Brooklyn, Brooklyn, NY, USA; Jose Cangiano Nephrology, San Juan, Puerto Rico; Hospital General de Castellón, Castellón, Spain; Hospital Clínico Universitario San Cecilio, Granada, Spain; Salford Royal Foundation Trust, Salford, UK; and Queen Alexandra Hospital, Portsmouth, UK. The authors acknowledge the editorial assistance of Kim Coleman Healy, PhD, CMPP, of Envision Scientific Solutions, which was contracted by Sanofi to provide publications support services.
Footnotes
Conflict of interest statement: PK has received lecture fees from Sanofi (formerly known as Genzyme), Shire, Abbott, and Amgen and advisory board fees from Shire, Sanofi, and Amgen; he also reports that his departmental research program has received educational grants from Shire, Sanofi, and Amgen. JH and JM were employed by Sanofi at the time of the study. MAK has received advisory board fees from Alexion. NS has received lecture and advisory board fees from Amgen.
Funding: This work was supported by Sanofi.
Contributor Information
Mark A. Kraus, Division of Nephrology and Hypertension, John H. Stroger Jr Hospital of Cook County, 627 South Wood Street, Suite 621, Chicago, IL 60612, USA
Philip A. Kalra, Renal Unit, Salford Royal Foundation Trust, Salford, UK
John Hunter, Formerly of Biostatistics, Sanofi, Cambridge, MA, USA.
José Menoyo, Formerly of Renal Global Medical Affairs, Sanofi, Cambridge, MA, USA.
Nicole Stankus, Section of Nephrology, Department of Medicine, University of Chicago Medical Center, Chicago, IL, USA.
References
- Blacher J., Safar M., Guerin A., Pannier B., Marchais S., London G. (2003) Aortic pulse wave velocity index and mortality in end-stage renal disease. Kidney Int 63: 1852–1860. [DOI] [PubMed] [Google Scholar]
- Block G., Spiegel D., Ehrlich J., Mehta R., Lindbergh J., Dreisbach A., et al. (2005) Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int 68: 1815–1824. [DOI] [PubMed] [Google Scholar]
- Braun J., Oldendorf M., Moshage W., Heidler R., Zeitler E., Luft F. (1996) Electron beam computed tomography in the evaluation of cardiac calcification in chronic dialysis patients. Am J Kidney Dis 27: 394–401. [DOI] [PubMed] [Google Scholar]
- Chertow G., Burke S., Raggi P. (2002) Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int 62: 245–252. [DOI] [PubMed] [Google Scholar]
- Di Iorio B., Molony D., Bell C., Cucciniello E., Bellizzi V., Russo D., et al. (2013) Sevelamer versus calcium carbonate in incident hemodialysis patients: results of an open-label 24-month randomized clinical trial. Am J Kidney Dis 62: 771–778. [DOI] [PubMed] [Google Scholar]
- Floege J., Ketteler M. (2004) Vascular calcification in patients with end-stage renal disease. Nephrol Dial Transplant 19(Suppl. 5): V59–V66. [DOI] [PubMed] [Google Scholar]
- Goldsmith D., Covic A., Sambrook P., Ackrill P. (1997) Vascular calcification in long-term haemodialysis patients in a single unit: a retrospective analysis. Nephron 77: 37–43. [DOI] [PubMed] [Google Scholar]
- Goodman W., Goldin J., Kuizon B., Yoon C., Gales B., Sider D., et al. (2000) Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med 342: 1478–1483. [DOI] [PubMed] [Google Scholar]
- Goodman W., London G., Amann K., Block G., Giachelli C., Hruska K., et al. (2004) Vascular calcification in chronic kidney disease. Am J Kidney Dis 43: 572–579. [DOI] [PubMed] [Google Scholar]
- Guerin A., London G., Marchais S., Metivier F. (2000) Arterial stiffening and vascular calcifications in end-stage renal disease. Nephrol Dial Transplant 15: 1014–1021. [DOI] [PubMed] [Google Scholar]
- Honkanen E., Kauppila L., Wikstrom B., Rensma P., Krzesinski J., Aasarod K., et al. (2008) Abdominal aortic calcification in dialysis patients: results of the CORD study. Nephrol Dial Transplant 23: 4009–4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal S., Vandermeer B., Raggi P., Mendelssohn D., Chatterley T., Dorgan M., et al. (2013) Effect of calcium-based versus non-calcium-based phosphate binders on mortality in patients with chronic kidney disease: an updated systematic review and meta-analysis. Lancet 382: 1268–1277. [DOI] [PubMed] [Google Scholar]
- Janicka L., Duma D., Grzebalska A., Czekajska-Chehab E., Drop A., Staskiewicz G., et al. (2011) Analysis of some risk factors of coronary and valvular calcification in peritoneal dialysis. Dial Transplant 40: 118–122. [Google Scholar]
- Jean G., Bresson E., Terrat J., Vanel T., Hurot J., Lorriaux C., et al. (2009) Peripheral vascular calcification in long-haemodialysis patients: associated factors and survival consequences. Nephrol Dial Transplant 24: 948–955. [DOI] [PubMed] [Google Scholar]
- Kalil R., Flanigan M., Stanford W., Haynes W. (2012) Dissociation between progression of coronary artery calcification and endothelial function in hemodialysis patients: a prospective pilot study. Clin Nephrol 78: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauppila L., Polak J., Cupples L., Hannan M., Kiel D., Wilson P. (1997) New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: a 25-year follow-up study. Atherosclerosis 132: 245–250. [DOI] [PubMed] [Google Scholar]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group (2009) KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl 76: S1–130. [DOI] [PubMed] [Google Scholar]
- Klassen P., Lowrie E., Reddan D., Delong E., Coladonato J., Szczech L., et al. (2002) Association between pulse pressure and mortality in patients undergoing maintenance hemodialysis. JAMA 287: 1548–1555. [DOI] [PubMed] [Google Scholar]
- Liu J., Chen C., Wang S., Chou C., Liu Y., Kuo H., et al. (2008) Association between pulse pressure and 30-month all-cause mortality in peritoneal dialysis patients. Am J Hypertens 21: 1318–1323. [DOI] [PubMed] [Google Scholar]
- London G. (2003) Cardiovascular disease in chronic renal failure: pathophysiologic aspects. Semin Dial 16: 85–94. [DOI] [PubMed] [Google Scholar]
- London G., Guerin A., Marchais S., Metivier F., Pannier B., Adda H. (2003) Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant 18: 1731–1740. [DOI] [PubMed] [Google Scholar]
- Moe S., Drueke T., Cunningham J., Goodman W., Martin K., Olgaard K., et al. (2006) Definition, evaluation, and classification of renal osteodystrophy: a position statement from kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 69: 1945–1953. [DOI] [PubMed] [Google Scholar]
- Moe S., Chen N. (2008) Mechanisms of vascular calcification in chronic kidney disease. J Am Soc Nephrol 19: 213–216. [DOI] [PubMed] [Google Scholar]
- National Kidney Foundation (2003) K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42(4 Suppl. 3): S1–S201. [PubMed] [Google Scholar]
- National Kidney Foundation (2005) K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis 45: S1–S153. [PubMed] [Google Scholar]
- Navaneethan S., Palmer S., Vecchio M., Craig J., Elder G., Strippoli G. (2011) Phosphate binders for preventing and treating bone disease in chronic kidney disease patients. Cochrane Database Syst Rev 2: CD006023. [DOI] [PubMed] [Google Scholar]
- Oh J., Wunsch R., Turzer M., Bahner M., Raggi P., Querfeld U., et al. (2002) Advanced coronary and carotid arteriopathy in young adults with childhood-onset chronic renal failure. Circulation 106: 100–105. [DOI] [PubMed] [Google Scholar]
- Raggi P., Bellasi A. (2007) Clinical assessment of vascular calcification. Adv Chronic Kidney Dis 14: 37–43. [DOI] [PubMed] [Google Scholar]
- Raggi P., Bellasi A., Ferramosca E., Islam T., Muntner P., Block G. (2007) Association of pulse wave velocity with vascular and valvular calcification in hemodialysis patients. Kidney Int 71: 802–807. [DOI] [PubMed] [Google Scholar]
- Raggi P., Boulay A., Chasan-Taber S., Amin N., Dillon M., Burke S., et al. (2002) Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease? J Am Coll Cardiol 39: 695–701. [DOI] [PubMed] [Google Scholar]
- Raggi P., Chertow G., Torres P., Csiky B., Naso A., Nossuli K., et al. (2011) The ADVANCE study: a randomized study to evaluate the effects of cinacalcet plus low-dose vitamin D on vascular calcification in patients on hemodialysis. Nephrol Dial Transplant 26: 1327–1339. [DOI] [PubMed] [Google Scholar]
- Ribeiro S., Ramos A., Brandao A., Rebelo J., Guerra A., Resina C., et al. (1998) Cardiac valve calcification in haemodialysis patients: role of calcium-phosphate metabolism. Nephrol Dial Transplant 13: 2037–2040. [DOI] [PubMed] [Google Scholar]
- Rumberger J., Brundage B., Rader D., Kondos G. (1999) Electron beam computed tomographic coronary calcium scanning: a review and guidelines for use in asymptomatic persons. Mayo Clin Proc 74: 243–252. [DOI] [PubMed] [Google Scholar]
- Schwarz U., Buzello M., Ritz E., Stein G., Raabe G., Wiest G., et al. (2000) Morphology of coronary atherosclerotic lesions in patients with end-stage renal failure. Nephrol Dial Transplant 15: 218–223. [DOI] [PubMed] [Google Scholar]
- Sharma R., Pellerin D., Gaze D., Mehta R., Gregson H., Streather C., et al. (2007) Mitral annular calcification predicts mortality and coronary artery disease in end stage renal disease. Atherosclerosis 191: 348–354. [DOI] [PubMed] [Google Scholar]
- Spiegel D., Raggi P., Smits G., Block G. (2007) Factors associated with mortality in patients new to haemodialysis. Nephrol Dial Transplant 22: 3568–3572. [DOI] [PubMed] [Google Scholar]
- Strozecki P., Odrowaz-Sypniewska G., Manitius J. (2005) Cardiac valve calcifications and left ventricular hypertrophy in hemodialysis patients. Ren Fail 27: 733–738. [DOI] [PubMed] [Google Scholar]
- Toussaint N., Lau K., Polkinghorne K., Kerr P. (2011) Attenuation of aortic calcification with lanthanum carbonate versus calcium-based phosphate binders in haemodialysis: a pilot randomized controlled trial. Nephrology (Carlton) 16: 290–298. [DOI] [PubMed] [Google Scholar]
- US Renal Data System (1998) Causes of death. Am J Kidney Dis 32: S81–S88. [DOI] [PubMed] [Google Scholar]
- Wanner C., Krane V., Marz W., Olschewski M., Mann J., Ruf G., et al. (2005) Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 353: 238–248. [DOI] [PubMed] [Google Scholar]
- Wilson P., Kauppila L., O’Donnell C., Kiel D., Hannan M., Polak J., et al. (2001) Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation 103: 1529–1534. [DOI] [PubMed] [Google Scholar]