Abstract
Current pharmacotherapies for major depressive disorder (MDD) and bipolar depression (BDep) have a distinct lag of onset that can generate great distress and impairment in patients. Furthermore, as demonstrated by several real-world effectiveness trials, their efficacy is limited. All approved antidepressant medications for MDD primarily act through monoaminergic mechanisms, agonists or antagonists with varying affinities for serotonin, norepinephrine and dopamine. The glutamate system has received much attention in recent years as an avenue for developing novel therapeutics. A single subanesthetic dose infusion of the noncompetitive N-methyl-D-aspartate (NMDA) receptor antagonist ketamine has been shown to have rapid and potent antidepressant effects in treatment-resistant MDD and BDep. In a reverse translational framework, ketamine’s clinical efficacy has inspired many preclinical studies to explore glutamatergic mechanisms of antidepressant action. These studies have revealed enhanced synaptic plasticity/synaptogenesis via numerous molecular and cellular mechanisms: release of local translational inhibition of brain-derived neurotrophic factor and secretion from dendritic spines, mammalian target of rapamycin activation and glycogen synthase kinase-3 inhibition. Current efforts are focused on extending ketamine’s antidepressant efficacy, uncovering the neurobiological mechanisms responsible for ketamine’s antidepressant activity in biologically enriched subgroups, and identifying treatment response biomarkers to personalize antidepressant selection. Other NMDA receptor antagonists have been studied both preclinically and clinically, which have revealed relatively modest antidepressant effects compared with ketamine but potentially other favorable characteristics, for example, decreased dissociative or psychotomimetic effects; therefore, there is great interest in developing novel glutamatergic antidepressants with greater target specificity and/or decreased adverse effects.
Keywords: ketamine, glutamate, N-methyl-D-aspartate receptor, NR2B, antagonist, major depressive disorder, bipolar disorder, bipolar depression, treatment-resistant depression
Introduction
Transient mood reactivity often accompanies both rewarding and stressful life events and facilitates adaptive responses to the environment. However, severe mood and behavioral dysregulation, for example, agitation or psychomotor retardation, over a prolonged period can be maladaptive to the individual and, among other possible outcomes, result in the major depressive episodes (MDEs) characteristic of major depressive disorder (MDD) and bipolar depression (BDep). Perhaps the most distressing MDE symptom is suicidal ideation and behaviors; suicide is a leading cause of death among young individuals, for example, the third leading cause of death for subjects between 15 and 24 years old [Centers for Disease Control and Prevention, 2014]. In the first half of 2012, depression and suicide were brought to the forefront of public attention as the number of US Army deaths from suicide outpaced the number of combat casualties [Lineberry and O’Connor, 2012].
More than 60 years ago, the discovery of tricyclic antidepressants and monoamine oxidase inhibitors arose out of serendipitous observations. These medications, designed with structural similarity to first-generation antipsychotics and antituberculosis drugs, respectively, had unintended antidepressant consequences. These first-generation antidepressants altered the reuptake, degradation or receptor pharmacodynamics of biogenic amines: serotonin, norepinephrine and dopamine. Subsequent research and development concentrated primarily on the fine tuning of monoaminergic neurotransmission with agents such as selective serotonin reuptake inhibitors, serotonin and norepinephrine reuptake inhibitors and newer atypical antidepressants. Although these newer agents had better safety and tolerability profiles, they were no more efficacious than the older agents. As a result, there was a clear impetus for research and development of novel pharmacological approaches to treat depression.
Preclinical antidepressant-like effects of the N-methyl-D-aspartate (NMDA) receptor antagonists AP-7 and MK-801 suggested that the glutamate system might be just such a novel experimental therapeutic approach [Trullas and Skolnick, 1990]. Ketamine, a noncompetitive NMDA receptor antagonist, was a reasonable translational candidate due to decades of safety and tolerability in the fields of anesthesia and neurology; nevertheless, there was (and continues to be) concern with the off-label use of ketamine as an antidepressant due to its pharmacological similarity to the potent psychotomimetic drug, phencyclidine (PCP), and its abuse liability, that is, as an hallucinogenic club drug. Over the course of the last 15 years, ketamine’s rapid and robust antidepressant properties in MDD and BDep have been labeled by some leaders in the field as ‘arguably the most important discovery (in depression research) in half a century’ [Duman and Aghajanian, 2012].
In this article, we will briefly review glutamatergic neurotransmission and clinical data supporting ketamine’s antidepressant efficacy in major depression. We will then discuss reverse translational research into ketamine’s mechanism of action in preclinical models of depression and review what is known about the neurobiology of ketamine’s antidepressant efficacy in depressed populations. Finally, we conclude with a discussion of other NMDA receptor antagonists that have been studied in depressive disorders and future areas for exploration.
The glutamate system
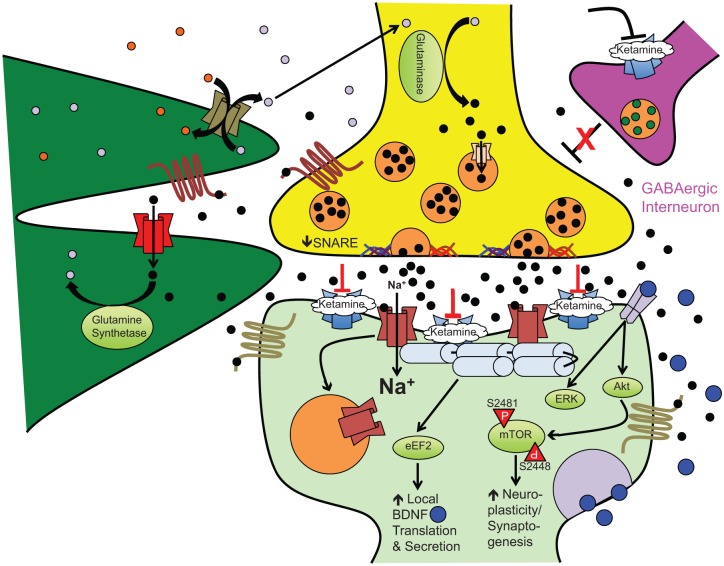
Glutamate is the primary excitatory neurotransmitter in the mammalian central nervous system [Niciu et al. 2012] and, at physiological levels, is essential for dendritic development and neuronal growth [McKinney et al. 1999]. However, in excess, glutamate is excitotoxic to neurons. To prevent neuronal cell death from excessive glutamate, there are a number of receptors and reuptake mechanisms on both neurons and astrocytes to regulate glutamatergic neurotransmission (Figure 1). The NMDA receptor and another ligand (glutamate)-gated ionotropic receptor, the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor, have been postulated to have critical roles in ketamine’s antidepressant efficacy. At rest, the NMDA receptor’s pore is blocked by magnesium ions, and the binding of glutamate releases this inhibition. This permits an influx of extracellular calcium ions and membrane depolarization. NMDA receptor activation and action potential generation stimulates a cascade of potential antidepressant-like effects such as increased brain-derived neurotrophic factor (BDNF) expression [Sanacora et al. 2008]. AMPA receptor activation, however, causes a rapid influx of sodium ions, which leads to membrane depolarization with faster kinetics than NMDA receptor stimulation. AMPA receptor-stimulated depolarization extrudes magnesium from the NMDA receptor pore [Mathew et al. 2012], which may account for the more rapid and delayed pharmacokinetics of AMPA and NMDA receptor-stimulated neuronal depolarization, respectively. The rapid nature of AMPA receptor excitation also results in a refractory desensitization, decreasing the strength of membrane depolarization upon subsequent glutamate binding to their cognate receptors [Sanacora et al. 2008].
Figure 1.
Ketamine-induced synaptoplasticity and rapid-acting antidepressant efficacy. At subnanesthetic doses, ketamine-induced NMDA receptor antagonism on GABAergic interneurons releases outflow (glutamatergic) neuronal inhibition, for example, cortical pyramidal neurons. This results in an acute glutamate ‘surge’ that then preferentially activates AMPA receptors to increase intracellular sodium (Na+) and fast excitatory currents. This stimulates numerous downstream second messenger/signal transduction cascades leading to the following: (1) eEF2K inhibition, (2) GSK-3 inhibition, and (3) mTOR activation. Other postsynaptic cellular and molecular effects stimulated by ketamine include increased AMPA receptor cycling, increased postsynaptic density protein expression, and dendritic spine morphogenesis (from immature filopodia-shaped spines to mature mushroom-shaped spines). The complex interplay of metabotropic glutamate receptors and exchangers on the surface of neurons and astrocytes regulate synaptic and extrasynaptic glutamate levels to facilitate recycling back to the presynaptic neuron and prevent excitotoxic cell damage/death.
Black circles: glutamate; grey circles: glutamine; blue circles: BDNF; maroon channel: NMDA receptor complex; blue channel: AMPA receptor complex; red channel: glial transporter-1/excitatory amino acid transporter 2 (GLT-1/EAAT2); olive channel: system xC-; peach channel: vesicular glutamate transporter; maroon seven-transmembrane receptor: metabotropic glutamate receptor type 2/3; olive seven-transmembrane receptor: metabotropic glutamate receptor type 1/5; grey dimeric receptor: TrkB receptor. Akt, Ak thymoma/protein kinase B; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; eEF2, eukaryotic elongation factor 2; ERK, extracellular signal-regulated kinase; GABA, γ-aminobutyric acid; mTOR, mammalian target of rapamycin; NMDA, N-methyl-D-aspartate; SNARE, soluble NSF attachment protein receptor (superfamily); TrkB, tropomyosin-related kinase B.
Clinical studies of ketamine in MDD and BDep
Single infusion studies
Berman and colleagues reported the first ketamine depression study in 2000 (Table 1). A small sample (n = 7) of unmedicated subjects with major depression were enrolled in a randomized, double-blind, placebo-controlled crossover study with a single subanesthetic dose (0.5 mg/kg) ketamine infusion over 40 minutes [Berman et al. 2000]. Depressive symptoms were measured using two metrics: the Hamilton Depression Rating Scale (HDRS) and the Beck Depression Inventory. HDRS scores significantly decreased with ketamine compared with placebo within 240 min and were maintained for at least 3 days [Berman et al. 2000]. This initial report was then replicated in a larger sample (n = 18) of treatment-resistant MDD [Zarate et al. 2006a]. Again, ketamine improved major depression symptoms within 2 h of infusion, and 71% and 29% met response and remission criteria, respectively, within 1 day. The antidepressant efficacy abated over time but, even a week after a single infusion, 35% still met response criteria [Zarate et al. 2006a]. Valentine and colleagues again demonstrated the rapid and robust antidepressant efficacy of a single subanesthetic dose ketamine infusion in a single-blind, non-counter balanced trial of 10 patients with MDD [Valentine et al. 2011]. One of the major concerns with these placebo-controlled studies, however, was the potential inadequacy of an inert placebo, which may hamper the integrity of the blind. To address this concern, Murrough and colleagues used a psychoactive placebo, intravenous midazolam, to capture the sedative and potentially dissociative effects of intravenous ketamine [Murrough et al. 2013a]. In 2013, they published a two-site randomized controlled trial of ketamine in treatment-resistant MDD (n = 73). In this parallel-design study with a 2:1 randomization scheme (2 ketamine:1 midazolam), ketamine had greater antidepressant efficacy at the primary outcome (24 h post infusion) as best exemplified by a 64% and 28% response rate in subjects randomized to ketamine and midazolam, respectively.
Table 1.
Summary of single subanesthetic-dose ketamine infusion randomized controlled trials for major depression.
| Study | Diagnosis | N | Comorbidity | Dose/route | Adjunctive medications | Response rate at 24 h post infusion | Response rate at 1 week post infusion | Remission rate at 24 h post infusion | Remission rate at 1 week post infusion |
|---|---|---|---|---|---|---|---|---|---|
| Berman et al. [2000] | MDD and BDep (TRD not reported) | 8 | Current anxiety disorder: 13% | 0.5 mg/kg racemic/intravenous | None | 25% (2/8) | N/A (final endpoint at 72 h) | 0% (0/8) (HDRS ⩽ 7) | N/A (final endpoint at 72 h) |
| Zarate et al. [2006a] | MDD/TRD | 17 | Lifetime anxiety disorder: 65% | 0.5 mg/kg racemic/intravenous | None | 71% (12/17) | 38% (6/16) | 29% (5/17) (HDRS ⩽ 7) | 31% (5/16) (HDRS ⩽ 7) |
| Diazgranados et al. [2010a] | BDep TRD | 16 | Lifetime anxiety disorder: 35% | 0.5 mg/kg racemic/intravenous | Li or VPA | 44% (7/16) | 29% (4/14) | 31% (5/16) (MADRS < 10) | 14% (2/14) (MADRS < 10) |
| Valentine et al. [2011] | MDD (TRD not reported) | 10 | Current anxiety disorder: 20% | 0.5 mg/kg racemic/intravenous | None | 20% (2/10) | 20% (2/10) | 20% (2/10) (HDRS ⩽ 7) | 30% (3/10) (HDRS ⩽ 7) |
| Zarate et al. [2012] | BDep TRD | 14 | Lifetime anxiety disorder: 73% | 0.5 mg/kg racemic/intravenous | Li or VPA | 43% (6/14) | 8% (1/12) | 29% (4/14) | 0% |
| Murrough et al. [2013a] | MDD/TRD | 72 | Not reported | Ketamine: 0.5 mg/kg intravenous Midazolam: 0.045 mg/kg intravenous |
None | Ketamine: 64% (30/47) Midazolam: 28% (7/25) |
Ketamine: 45% (21/47) Midazolam: 16% (4/25) |
Not included | Not included |
BDep, bipolar depression; HDRS, Hamilton Depression Rating Scale; Li, lithium; MADRS, Montgomery Åsberg Depression Rating Scale; MDD, major depressive disorder; N/A, not applicable; TRD, treatment-resistant depression; VPA, valproic acid.
In addition to its efficacy in treatment-resistant unipolar depression, our group and others have demonstrated that ketamine has antidepressant efficacy in treatment-resistant BDep. In the first report of 18 treatment-resistant patients with bipolar depression maintained on therapeutic dose lithium or valproate alone and randomized to add-on ketamine or placebo, a single subanesthetic dose of ketamine had rapid (within 40 min) and sustained (up to 3 days) antidepressant efficacy; maximal antidepressant efficacy was observed at day 2 post infusion (d = 0.80) [Diazgranados et al. 2010b]. These results were then replicated in an identical design with a second independent sample (n = 15) with active treatment-resistant BDep [Zarate et al. 2012]. Permoda-Osip and colleagues recently reported the antidepressant efficacy of a single open-label infusion of ketamine in patients with active BDep (n = 42) also maintained on mood stabilizers [Permoda-Osip et al. 2014]. In addition, ketamine does not appear to induce hypo/mania at a greater rate than placebo infusion in susceptible subjects [Niciu et al. 2013c]. Yet, some of the more nonspecific psychotomimetic effects of ketamine, for example, agitation and irritability, may be difficult to distinguish from the activating effects of monoaminergic antidepressants when given to patients with bipolar depression.
Multiple infusion studies
Due to the inconclusive or apparent lack of efficacy of studied maintenance/augmentation strategies, for example riluzole [Mathew et al. 2010; Ibrahim et al. 2012a] and electroconvulsive therapy [Abdallah et al. 2012; Järventausta et al. 2013], repeated-dose ketamine has now emerged as the most promising antidepressant maintenance strategy (Table 2). aan het Rot and colleagues published preliminary data exploring the safety and efficacy of repeated ketamine infusions for treatment-resistant depression (TRD) [aan het Rot et al. 2010], which was followed by a larger trial inclusive of these subjects [Murrough et al. 2013b]. Twenty-four medication-free patients with TRD received open-label ketamine three times a week over a 12-day period, and responders were then followed naturalistically for up to 83 days after their last infusion. After 12 days, the response rate was 70.8%, with a high positive predictive value of 4 h response predicting end-of-trial response. During the naturalistic phase (which allowed for standard psychiatric treatment), the mean time to relapse was 18 days; however, around 30% of responders maintained response. Another repeated-dose ketamine study followed shortly, in which 10 patients with TRD received 0.3 mg/kg ketamine infused over 100 min (to approximately equal the total dose of ketamine received in the 0.5 mg/kg over 40 min infusion paradigms but with potentially fewer side effects) twice weekly until either remission was achieved or the subject received four total infusions [Rasmussen et al. 2013]. These subjects were then followed naturalistically for the following 4 weeks. A total of 10 subjects enrolled. Six patients received the maximum of four doses, and three and five patients were responders and remitters, respectively (leaving two nonresponders). Although firm conclusions on side-effect burden of lower versus higher dose ketamine cannot be drawn (as they were not compared head to head), serial lower-dose ketamine was safe and well tolerated in this repeated-dose paradigm. Finally, in the first ketamine depression study published outside of the United States, an Oxford group reported a repeated-dose ketamine trial of 28 medicated treatment-resistant patients with unipolar and BDep who received either weekly or biweekly 0.5 mg/kg × 40 minute ketamine infusions over 3 weeks for a total of three or six infusions, respectively [Diamond et al. 2014]. This study also assessed patients for cognitive deficits at day 21, as, based on studies in ketamine abusers, cognitive difficulties could limit the utility of repeated-dose ketamine administration [Morgan et al. 2008, 2009, 2010, 2012]. In this study, eight patients responded and four patients remitted (29% and 14%, respectively) without cognitive impairment. Additionally, in a 6-month naturalistic follow up, there was a broad range in time to relapse: 70 days (28–168 days). Finally, there is a case report of a 44-year-old patient with TRD who received more than 40 ketamine infusions over several months [Blier et al. 2012]. Although her depression initially abated and there were no observed cognitive deficits, ketamine was not as efficacious on repeated dosing, potentially indicating tachyphylaxis. Taken together, repeated subanesthetic dose ketamine administration appears to be safe, well tolerated and efficacious in depression research studies; yet, there are currently insufficient data to recommend long-term off-label use in a nonresearch milieu.
Table 2.
Summary of multiple subanesthetic dose ketamine infusion studies for major depression.
| Study | Diagnosis | N | Comorbidity | Dose/route | Number of doses | Adjunctive medications | Response rate after first dose (+24 h) | Response rate after final dose | Remission rate after first dose (+24 h) | Remission rate after final dose |
|---|---|---|---|---|---|---|---|---|---|---|
| aan het Rot et al. [2010] | MDD/TRD | 10 | Current anxiety disorder: 70% | 0.5 mg/kg racemic/intravenous | 6 | None | 90% (9/10) | 100% (9/9) | 10% (1/10) | 89% (8/9) |
| Murrough et al. [2013b] * | MDD/TRD | 24 | Current anxiety disorder: 25% | 0.5 mg/kg racemic/intravenous | 6 | None | Not included | 71% (17/24) | Not included | Not included |
| Rasmussen et al. [2013] | MDD and BDep (TRD reported) | 10 | Not reported | 0.5 mg/kg racemic/intravenous | 4 | Venlafaxine, duloxetine, lithium, lamotrigine, bupropion | 30% (3/10) | 80% (8/10) | 10% (1/10) | 50% (5/10) |
| Diamond et al. [2014] | MDD and BDep (TRD reported) | 28 | Not reported | 0.5 mg/kg racemic/intravenous | 9 | Patients remained on antidepressants | Not included | 29% (8/28) | Not included | 14% (4/28) |
Inclusive of data from prior aan het Rot et al. [2010] study.
BDep, bipolar depression; MDD, major depressive disorder; TRD, treatment-resistant depression.
Alternative modes of administration
While more work is needed to further explore repeated dosing, other studies have examined alternative methods of delivery: oral [Irwin and Iglewicz, 2010; Paslakis et al. 2010; Irwin et al. 2013; De Gioannis and De Leo, 2014], intramuscular [Cusin et al. 2012], sublingual [Lara et al. 2013], and most recently, intranasal [Lapidus et al. 2014], which will be reviewed in detail as follows.
Oral
Oral ketamine has much lower bioavailability (20%) compared to nonparenteral forms. None-theless, the ease of administration, especially for repeated dosing, makes oral ketamine an attractive candidate in select patient populations, for example, hemodialysis patients. After two case series demonstrating preliminary efficacy in major depression [Irwin and Iglewicz, 2010; Paslakis et al. 2010], a systematic oral ketamine study for depression occurred in a hospice/palliative care setting [Irwin and Iglewicz, 2010]. In this open-label study, hospice patients with depression received oral ketamine (0.5 mg/kg) daily for 28 days. Of the eight subjects who completed the study (four subjects withdrew after day 14 due to lack of efficacy, one subject withdrew due to ‘rapid decline in condition unrelated to ketamine’ and one subject withdrew due to ‘mental status changes unrelated to ketamine’), oral ketamine had significant antidepressant and anxiolytic efficacy. Even in this medically compromised patient population, side effects were minor and relatively rare, for example, diarrhea, insomnia and akathisia. This was most recently followed by a case series of two patients with TRD and suicidal ideation, in which escalating-dose oral ketamine (from 0.5 mg/kg to up to 3 mg/kg with boosters) had sustained antidepressant and antisuicidal effects [De Gioannis and De Leo, 2014].
Intramuscular
Intramuscular ketamine has similar bioavailability (93%) to intravenous ketamine. When simulated at equal doses to a slow intravenous infusion, intramuscular ketamine had higher peak exposure (with the additional benefits of not requiring an infusion pump and potential administration in patients with poor intravenous access) [Glue et al. 2011]. To test its antidepressant efficacy, intramuscular open-label ketamine was given to two patients with treatment-refractory depression at ascending doses (0.5, 0.7 and 1.0 mg/kg). A dose-dependent antidepressant response was observed, and, at the highest dose, remission was achieved in one patient at 24 h post injection. A second case series reported on repeated intramuscular ketamine for treatment-resistant bipolar II depression [Cusin et al. 2012]. Both patients with treatment-resistant bipolar II depression received nonintramuscular preparations of ketamine before receiving repeated (from 32 to 100 mg every 3–4 days over several months) intramuscular ketamine. This resulted in depression improvement, and, after months of intramuscular administration, there was an expected side-effect profile (irritability, headaches, nightmares and dissociation) without the medical sequelae seen in ketamine abusers, for example, cystitis. A follow-up case series in two Indian patients with depression also reported rapid antidepressant efficacy to intramuscular ketamine [Harihar et al. 2013]. Finally, in a small (n = 9 in each group) randomized, open-label design, a separate Indian group reported antidepressant noninferiority (up to 3 days) with 0.25 mg/kg intramuscular ketamine compared with both 0.5 mg/kg intramuscular injection and 0.5 mg/kg intravenous infusion [Chilukuri et al. 2014].
Sublingual
Due to its slightly improved bioavailability (~30%) compared with oral ketamine, there is also theoretical interest in sublingual administration. In 27 outpatients with current unipolar and bipolar depression, variable administration (every 2–7 days of add-on escalating (but still subanesthetic) dose sublingual ketamine had antidepressant efficacy in 20 patients (77% of sample) [Lara et al. 2013]. Sublingual ketamine was also well tolerated; the most common side effect was transient lightheadedness. However, unlike intravenous or intramuscular preparations, there was no reported dissociative or psychotomimetic side effects from repeated sublingual dosing.
Intranasal
Finally, in a randomized, double-blind, crossover, placebo-controlled trial, 20 patients with major depression were randomized to either intranasal ketamine (50 mg) or a saline solution with the primary outcome being depression change at 24 h [Lapidus et al. 2014]. There was a significant antidepressant effect of intranasal ketamine at 24 h post infusion [mean Montgomery Åsberg Depression Rating Scale (MADRS) change = 7.6 ± 3.7, 95% confidence interval: 3.9–11.3]. Intranasal ketamine was associated with only minor changes in hemodynamic, dissociative and psychotomimetic parameters, which may distinguish it from intravenous ketamine where dissociative side effects are more prominent (but also positively correlated with ketamine’s antidepressant efficacy [Luckenbaugh et al. 2014]).
Antisuicidal effects
In addition to its antidepressant and anxiolytic effects, ketamine has been reported to have antisuicidal properties. In 33 subjects with TRD who received a single open-label infusion of 0.5 mg/kg ketamine, suicidal thinking (as measured by the Scale for Suicide Ideation) was reduced within 40 min and maintained for up to 4 h post infusion [DiazGranados et al. 2010a]. In addition to the more explicit reduction of suicidal thinking, the Implicit Association Test, an assessment of suicidal cognition and potential predictor of future suicidal behavior, has been studied in the context of ketamine infusion, which has also revealed reductions in implicit suicidal thinking [Price et al. 2009, 2014]. Our group recently published a secondary data analysis of 108 patients with treatment-resistant major depression (both MDD and BDep) who received a single subanesthetic dose infusion of ketamine to study the relationship between improvements in depression/anxiety and suicidality; in this sample, improvements in suicidal thinking were partially related to improvements in neuropsychiatric symptoms, explaining up to 20% of the antisuicidal effect [Ballard et al. 2014]. Interestingly, in another secondary analysis of the same sample, a lack of a lifetime history of suicide attempt predicted improved antidepressant response to ketamine at 1 week post infusion [Niciu et al. 2014b]. Nevertheless, as these studies were not conducted in patients who were acutely suicidal and presenting for intervention, these studies are likely investigating chronic as opposed to acute suicidal ideation with greater potential for suicidal behaviors. In the only published study of patients who were acutely suicidal and encountered in an emergency setting, open-label intravenous ketamine (0.2 mg/kg intravenous push over 1–2 min) had both acute antidepressant and antisuicidal effects in 14 patients with depression [Larkin and Beautrais, 2011]. There are several studies currently listed on ClinicalTrials.gov for the treatment of suicidality with ketamine, including a Janssen-sponsored study investigating repeated-dose intranasal esketamine in emergency departments across the United States [ClinicalTrials.gov identifier: NCT02133001].
Safety and tolerability
As mentioned, ketamine has been used safely and effectively as a dissociative anesthetic since its introduction in the 1960s [Domino, 2010]. Subanesthetic dose ketamine has been used in neuropsychiatric research for more than two decades, initially as provocative challenge in patients with schizophrenia and to induce transient psychotic-like effects in healthy volunteers. Yale’s experience with subanesthetic dose ketamine in neuropsychiatric research with healthy volunteers was collated in 2007 [Perry et al. 2007]. They reported on 450 subjects who received a total of 833 ketamine infusions. Only 10 adverse mental status changes related to ketamine were observed; these effects, for example, excessive sedation and acute dysphoria, were mostly intra infusion and all but one resolved entirely by the end of the testing day. In a subset agreeable to longer follow up, only three patients experienced adverse effects, for example, fatigue, nausea, headache, lightheadedness and nightmares/vivid dreams, in the week after infusion. There were also no observed post-infusion neuropsychiatric sequelae, for example, increased anxiety or cravings for ketamine. After the publication of this report, however, another Yale group reported two cases of next-day dysphoria, anxiety and suicidal ideation in 2 of 10 subjects with treatment-resistant obsessive–compulsive disorder (OCD), a history of MDD and personality vulnerabilities who received open-label subanesthetic dose ketamine for the treatment of OCD [Niciu et al. 2013a].
Mt Sinai and Baylor also recently reported a systematic analysis of their safety and tolerability data inclusive of both their psychoactive placebo (midazolam) and multiple infusion studies [Wan et al. 2014]. Safety, tolerability and acceptability data were pooled from 97 patients with MDD who received a total of 205 intravenous ketamine infusions between 2006 and 2012 with a subset agreeable to longer-term follow up. Four of 205 infusions were discontinued due to adverse events: two with increased blood pressure, one with increased anxiety and one with transient hypotension and bradycardia during venipuncture. The all-cause attrition rate was 3.1%. In this cohort, the most common adverse events were sedation, dizziness, lightheadedness, incoordination, blurry vision and feeling ‘strange or unreal’. Subanesthetic dose ketamine also caused a significant but transient increase in dissociation and hemodynamic vital sign changes. No patients reported any long-term problems from ketamine research participation, and the majority of patients (35/46) considered ketamine infusions at least ‘somewhat acceptable’ (with 22 considering it ‘very acceptable’) on a five-point Likert scale.
Ketamine’s antidepressant mechanisms of action
Pharmacology
As a synthetic derivative of phencyclidine (PCP), ketamine has moderate affinity for the NMDA receptor as a noncompetitive antagonist. By binding to the PCP binding site in the ion pore, ketamine prevents the typical influx of calcium ions in response to binding of the coagonists glutamate and glycine [Mathew et al. 2012]. The R(–) and S(+) enantiomers of ketamine also have differential affinity for the NMDA receptor, with the S(+) having greater binding potential and biological potency. The latter has been demonstrated physiologically by decreased plasma concentrations with successful anesthesia [White et al. 1980]. As described in greater detail below, NMDA receptor blockade leads to increased synaptic glutamate release, which then preferentially favors AMPA receptor activity [Sanacora et al. 2008]. Ketamine is also a µ-opioid receptor agonist at approved anesthetic doses [Mathew et al. 2012]. Despite its superior affinity for NMDA receptors, ketamine’s opioidergic (and potentially its monoaminergic) properties may also be critical for its antidepressant and anxiolytic efficacy.
Preclinical studies
The prevailing cellular and molecular hypotheses for ketamine’s mechanism of action is that NMDA receptor blockade on cortical γ-aminobutyric acid(GABA)ergic interneurons releases the tonic inhibition on major output neurons, for example, cortical pyramidal cells [Moghaddam et al. 1997]. This loss of inhibition then increases acute synaptic glutamate release/cycling [Chowdhury et al. 2012] (although a recent report indicates that ketamine reduces the presynaptic release machinery in the rodent hippocampus) [Müller et al. 2013], increased AMPA to NMDA postsynaptic throughput [Maeng et al. 2008; Andreasen et al. 2013; Koike and Chaki, 2014] and the activation of second messenger/signal transduction cascades [Niciu et al. 2013b]. We will now review some of the molecular players that have been implicated in these processes. An intriguing difference between the mechanisms of ketamine and monoaminergic antidepressant therapies is ketamine and other NMDA receptor antagonists’ rapid (within 24 h) neuroplastic effects, increased synaptic protein expression, dendritic spine morphogenesis (filopodia to mushroom shaped spines) and excitatory postsynaptic currents [Hoeffer and Klann, 2010; Duman et al. 2012], that appear necessary for antidepressant action.
BDNF
BDNF is the most abundant neurotrophin in the brain, and its activation of tropomyosin-related kinase B receptors stimulates neural growth, survival, and plasticity. Ketamine-stimulated translation of BDNF in dendritic spines of hippocampal pyramidal neurons may be essential for ketamine’s rapid-acting antidepressant-like effects in rodents [Autry et al. 2011]. When challenged on the forced swim test, an intraperitoneal injection of subanesthetic-like dose ketamine (3.0 mg/kg) had minimal antidepressant effects in inducible BDNF knockout mice. When C57BL/6 adult male mice were injected with anisomycin, a translational inhibitor, antidepressant-like effects were also not observed. However, antidepressant effects were seen when a separate cohort of C57BL/6 adult male mice were treated with actinomycin D, a RNA polymerase transcriptional inhibitor, suggesting that translation but not transcription is necessary for ketamine’s rapid antidepressant-like action. Monteggia and colleagues then demonstrated that ketamine decreased eukaryotic elongation factor 2 kinase (eEF-2K) activity. eEF-2K normally phosphorylates eEF-2, which halts translation. However, because ketamine inhibits eEF-2K functioning, eEF-2 activity predominates, which results in greater local BDNF translation in dendritic spines [Autry et al. 2011].
Mammalian target of rapamycin
In preclinical models of despair, ketamine activates mammalian target of rapamycin (mTOR), which is a critical hub of cellular growth and proliferation [Hay and Sonenberg, 2004]. Ketamine increased mTOR phosphorylation and other downstream molecular targets critical for transcriptional activation within 1 hour of administration [Li et al. 2010]. Twenty-four hours following ketamine exposure, greater numbers of mature dendritic spines were observed. These molecular and cellular effects were lost if the rodent was pretreated with rapamycin, an mTOR antagonist. Interestingly, rapid antidepressant-like behaviors in rodents were only observed following treatment with low doses (10–20 mg/kg) as opposed to higher anesthetic doses (80 mg/kg), suggesting an inverted ‘U’ dose–effect relationship. In all, these findings suggest that mTOR activation is necessary for ketamine’s rapid-acting antidepressant effects in a rodent model of despair, which may occur through BDNF.
GSK-3
Like lithium, ketamine inhibits glycogen synthase kinase-3 (GSK-3) by increasing serine phosphorylation. This phosphorylation is mediated through Ak thymoma/protein kinase B and is necessary for antidepressant-like effects in model rodents [Beurel et al. 2011; Zhou et al. 2014]. The combination of low (subeffective) dose NMDA receptor antagonists (including ketamine) and lithium also has synergistic antidepressant effects on the forced swim test in rodents [Ghasemi et al. 2010]. Finally, GSK-3 inhibition by lithium and a selective GSK-3 inhibitor (SB 216763) potentiates the antidepressant, synaptogenic and electrophysiological effects of subthreshold dose (1 mg/kg) ketamine, for example, mTOR activation, increased excitatory postsynaptic currents and dendritic spine morphogenesis [Liu et al. 2013].
Clinical studies
We have recently reviewed the growing clinical literature on ketamine’s antidepressant efficacy and treatment response biomarkers [Niciu et al. 2014a, 2014c]. In this section, we will provide an update on additional studies published since those reports.
Clinical predictors
Our group analyzed subject-level data from 108 patients with major depression (both unipolar and BDep) to assess potential correlations between sociodemographic/clinical features and antidepressant efficacy at three time points: 230 minutes (same day), 24 hours (next day) and 1 week (sustained). In a multivariate linear regression, increased body mass index (BMI) correlated with ketamine’s antidepressant action at 230 minutes and 1 day post infusion, a family history of an alcohol use disorder correlated at 1 day and 1 week post infusion and no lifetime personal history of suicide attempt correlated at 1 week only [Niciu et al. 2014b]. Permoda-Osip and colleagues performed a similar factor analysis in 42 patients with bipolar depression. In their sample, ketamine’s antidepressant response was associated with both personal and family histories of an alcohol use disorder [Permoda-Osip et al. 2014].
In our combined sample, dissociative side effects at the end of the 40 minute infusion predicted eventual antidepressant improvement at both 230 minutes and 1 week afterwards; hemodynamic and psychotomimetic side effects, however, did not correlate with ketamine’s antidepressant efficacy at any of the aforementioned time points [Luckenbaugh et al. 2014]. However, in an independent sample of 27 hospitalized inpatients with depression, psychotomimetic side effects (as measured by the total score on the Brief Psychiatric Rating Scale) correlated with depression improvement with maximal effect observed at 1 week post infusion [Sos et al. 2013]. Ketamine-induced dissociative or psychotomimetic effects are, at least in part, reflective of a hypo-(NMDA receptor blockage) or hyper-(acute glutamate surge)glutamatergic state, and, although the adequacy of the blind and subsequent antidepressant expectancies may be called into question, these results suggest that the strength of NMDA receptor antagonism may be a critical component in predicting eventual antidepressant response.
Peripheral measures
Our group and others have examined several candidate peripheral measures as potential correlates of ketamine’s antidepressant response. First, in a bipolar depressed sample of 10 ketamine responders, baseline serum vitamin B12 and vascular endothelial growth factor receptor 1 levels predicted response at 1 week post infusion [Permoda-Osip et al. 2014]. However, in an attempt to replicate the findings of this study, in our 83 patients stratified by diagnostic category and response/nonresponse, baseline peripheral vitamin B12 levels did not correlate with antidepressant efficacy [Lundin et al. 2014].
Next, D-serine is a glial-derived partial agonist at the NMDA receptor glycine site that, at higher doses, acts as a functional antagonist. In 21 patients with TRD, lower baseline peripheral D-serine (and its precursor L-serine) levels correlated with greater antidepressant response to ketamine at 230 minutes post infusion, explaining up to 60% of the variance in antidepressant response [Moaddel et al. 2015]. Interestingly, acute administration of D-serine had antidepressant-like effects in a depression model in rodents [Malkesman et al. 2012], and a homologue, D-cycloserine, had augmenting antidepressant effects in TRD but only at higher doses [Heresco-Levy et al. 2006, 2013].
After initial studies from our group suggested that peripheral BDNF levels do not correlate with ketamine’s antidepressant response without genotypic stratification [Machado-Vieira et al. 2009; Laje et al. 2012], several subsequent studies have reported a positive association between baseline serum BDNF and ketamine’s antidepressant activity. In a sample of 22 subjects with TRD from the ketamine-midazolam study, plasma BDNF levels at 240 minutes were associated with greater antidepressant improvement at the same time point, 24 hours and 72 hours post ketamine infusion [Haile et al. 2014]. Similar associations were not noted when subjects received midazolam. As previously reported by our group, baseline serum BDNF levels did not correlate with antidepressant response in a multivariate linear regression analysis. A neurotrophin panel (including BDNF neurotrophin 3, neurotrophin 4, glial-derived neurotrophic factor, and nerve growth factor) was obtained in 25 patients with bipolar depression receiving mood stabilizers without adjunctive antidepressant medications who received a single subanesthetic dose ketamine infusion [Rybakowski et al. 2013]. At baseline, none of the studied neurotrophins correlated with antidepressant response (n = 13) versus nonresponse (n = 11) at 1 week post infusion. However, serum BDNF alone was significantly reduced at 1 week in bipolar depressed ketamine non-responders.
After demonstrating that SHANK3, a postsynaptic density protein that interacts with NMDA receptors, duplication mice display manic-like behaviors [Han et al. 2013], we investigated peripheral SHANK3 levels as a potential biomarker of ketamine’s antidepressant efficacy in treatment-resistant BDep. Greater baseline SHANK3 levels predicted ketamine’s increased antidepressant efficacy at day 1 and day 3 post infusion [Ortiz et al. 2014].
Neuroimaging
In the past year, there have been several interesting ketamine neuroimaging reports in depression. First, lower baseline left hippocampal volume was a positive predictor of ketamine’s antidepressant improvement in 13 medication-free patients with MDD (r = 0.66, p = .01), but this finding did not survive controlling for total brain volume, handedness, age, sex, height and race (r > 0.6, p = 0.13) [Abdallah et al. 2014]. There was also no correlation between baseline hippocampal volume and antidepressant improvement with midazolam. Next, 21 treatment-resistant patients with bipolar depression received [(18)-F]-fluorodeoxyglucose positron emission tomography scans to assess the metabolic rate of glucose (rMRGlu) after both placebo and ketamine infusions [Nugent et al. 2014]. Increased rMRGlu in the ventral striatum correlated with antidepressant improvement in BDep. In a voxel-wise analysis, left hippocampal rMRGlu decreased after ketamine versus placebo infusion, and increased subgenual anterior cingulate cortical (sgACC) rMRGlu post placebo correlated with antidepressant improvement post ketamine, suggesting sgACC rMRGlu as a potential treatment response biomarker. A subsequent study in the same sample analyzed ketamine’s antianhedonic effects and rMRGlu, which, in addition to a reduction in anhedonia independent of depression improvement, reported a correlation between ketamine’s antianhedonic effects and increased dorsal anterior cingulate cortex and putamen rMRGlu [Lally et al. 2014]. Interestingly, unlike overall depressive symptoms, ketamine’s antianhedonic effects in BDep did not correlate with rMRGlu change in the ventral striatum, a brain area that was a priori hypothesized to correlate with anhedonia. Finally, in addition to the discussed correlation between higher SHANK3 peripheral levels and ketamine’s antidepressant efficacy, peripheral SHANK3 levels also correlated with greater total and right amygdala volumes and increased rMRGlu in the amygdala and hippocampus [Ortiz et al. 2014].
Instead of a strict candidate protein approach, ketamine-associated plasma metabolomic profiles may identify novel associations not previously hypothesized to exist. As a specific example, metabolic profiles were obtained from 22 patients with bipolar depression maintained on therapeutic-dose lithium or valproate. In lithium-maintained ketamine responders at 230 minutes post infusion, baseline plasma levels of lysophosphotidylethanolamines and lysophosphotidylcholines were increased, which suggests that mitochondrial β oxidation of fatty acids may be important for ketamine’s antidepressant effects [Villaseñor et al. 2014]. As a reminder, increased BMI also predicted ketamine’s antidepressant efficacy at this time point, again suggestive of the potential importance of adipose tissue metabolism for ketamine’s antidepressant action.
Other NMDA receptor antagonists in depression
Some major clinical concerns with the use of ketamine as an antidepressant are its acute dissociative and psychotomimetic side effects as well as its potential abuse liability and neurotoxicity with chronic use. As a result, other NMDA receptor antagonists with oral administration or more benign side-effect profiles have been studied in depression. Memantine, another noncompetitive NMDA receptor antagonist approved to treat moderate to severe Alzheimer’s-like dementia, showed preliminary antidepressant-like effects in rodent models of despair [Réus et al. 2010; Quan et al. 2011]. The first clinical report of memantine treatment for depression was an 8-week, double-blind, placebo-controlled study in which 5–20 mg/day memantine did not separate from placebo [Zarate et al. 2006b]. Memantine has also been studied in BDep. In an 8-week randomized, placebo-controlled study of subjects with bipolar depression with poor treatment response to lamotrigine, escalating-dose memantine had better antidepressant success compared with placebo at 4 weeks. However, this effect was not sustained at trial endpoint [Anand et al. 2012]. In 2008, a case study reported antidepressant efficacy with repeated-dose ketamine followed by treatment with memantine; while it should be noted that the patient eventually was placed on seven psychotropic drugs, she remained in remission for 13 weeks [Kollmar et al. 2008]. Finally, Gideons and colleagues investigated mechanistic differences between memantine and ketamine in rodents [Gideons et al. 2014]. In this sample, memantine did not have antidepressant-like effects as detected by both the forced swim test and novelty-suppressed feeding paradigm. At physiological doses of magnesium (Mg2+), ketamine but not memantine inhibited the phosphoryla-tion of eEF-2K and, hence, increased BDNF expression.
AZD6765 is another noncompetitive NMDA receptor antagonist with a Ki (inhibitory constant) similar to ketamine (AZD6765 = 0.56–1.48 μM; ketamine = 0.76 μM) [Mealing et al. 1999], but lower trapping, that is, greater on–off NMDA receptor pharmacodynamics. Because ketamine has greater receptor trapping, AZD6765 may have antidepressant action without psychotomimetic or dissociative adverse effects. A single 150 mg AZD6765 infusion in unmedicated patients with TRD had antidepressant efficacy over placebo infusion without increased psychosis or dissociation; however, the antidepressant response was not as robust or sustained as with ketamine. AZD6765 had lower response rates, lower remission rates, and a shorter duration of effect [Zarate et al. 2013]. In a subsequent 3-week, placebo-controlled trial, patients with TRD received repeated adjunctive AZD6765 (now renamed lanicemine) infusions at two doses (100 and 150 mg); as hypothesized lanicemine had antidepressant efficacy without ketamine-like side effects [Sanacora et al. 2014b]. However, as presented at the 2014 American Society of Clinical Psychopharmacology annual meeting in Hollywood, FL, in a 6-week phase IIb study, adjunctive repeated-dose (50 and 150 mg) AZD6765/lanicemine failed to separate from placebo, potentially due to the large placebo effect (39% placebo response rate at trial end) [Sanacora et al. 2014a].
Like nontrapping antagonists, subtype-specific NMDA receptor antagonists may also have less dissociative and other undesirable adverse effects. In an unpredictable foot shock paradigm, the NR2B antagonist Ro 25-6981 had antidepressant-like behavioral effects [Li et al. 2011]. Ro 25-6981 also increased the expression of postsynaptic and secondary messenger intermediaries (including mTOR) on a similarly rapid time course as ketamine (within 24 hours). Moreover, the antidepressant effects of Ro 25-6981 were negated by pretreatment with rapamycin [Li et al. 2010]. A randomized, double-blind, placebo-controlled study of the intravenous NR2B-selective receptor antagonist CP-101,606 in TRD (n = 30) displayed a 60% response rate (compared with 20% in the placebo group) [Preskorn et al. 2008]. Additionally, 78% of treatment responders maintained their therapeutic response for at least 1 week. Further development of this compound was halted due to potential cardiovascular toxicity, that is, QTc prolongation. Subsequently, a small, randomized, double-blind, placebo-controlled, crossover pilot study was conducted to assess the efficacy of an oral NR2B antagonist MK-0657 in treatment-resistant MDD [Ibrahim et al. 2012b]. Compared with placebo, MK-0657 demonstrated rapid antidepressant action as early as day 5. Although antidepressant improvement was demonstrated with other depression rating scales, no improvement was observed over placebo with the MADRS, the primary outcome measure. MK-0657 is now being developed by Cercor (renamed CERC-301).
Conclusion
2012 was a record year for the previous half decade in terms of new drug approvals; yet, the majority (33% of the list of 39 new drugs) had primary efficacy in oncology whereas neuropsychiatric drugs accounted for just around 5% [Mullard, 2013]. Furthermore, while other areas of medicine have seen significant reductions in morbidity and mortality, the same cannot be said for mental illnesses and suicidality [Insel, 2012]. Yet, the discovery of NMDA receptor antagonists as rapidly acting antidepressants has instilled hope for novel mechanisms of antidepressant action and reinvigorated the otherwise-stagnant ‘me too’ pool of antidepressant drug development. This momentum must continue if novel agents are to advance through the drug development pipeline.
There are many exciting unanswered clinical questions in ketamine depression research. As discussed above, the antidepressant efficacy of subanesthetic intravenous ketamine wanes after several days in most patients, so, as with traditional antidepressants, there is a need for additional studies with repeated dosing to examine the potential for tachyphylaxis. After achieving antidepressant response, the antidepressant effects may be maintained with intermittent dosing similar to ‘booster’ electroconvulsive therapy in the maintenance phase. Some other interesting avenues are continued studies of alternative/nonparenteral modes of administration and ketamine augmentation (including initial concurrent administration with traditional monoaminergic antidepressants to hasten efficacy).
One of the most pressing clinical issues is the continued lack of an antidepressant dose–response curve, especially as numerous preclinical studies have reported loss of antidepressant-like behavioral and molecular effects at anesthetic doses, for example, 80 mg/kg [Li et al. 2010; Chowdhury et al. 2012]. A National Institute of Mental Health-sponsored, multisite study [ClinicalTrials.gov identifier: NCT01920555] will attempt to address this issue with parallel-group, repeated-dose infusions of the psychoactive placebo midazolam, and four doses of ketamine (0.1, 0.2, 0.5 and 1 mg/kg).
Finally, our group and many others remain very interested in translating preclinical findings to patients with depression by elucidating the neurobiological mechanisms underlying ketamine’s rapid and robust antidepressant properties. This includes systematic study of enriched treatment subgroups, for example, patients with treatment-resistant depression and a family history of an alcohol use disorder [Phelps et al. 2009; Niciu et al. 2014b] [Clinical Trials identifier: NCT02122562] or dimensional anxious depression [Ionescu et al. 2014], to develop treatment response biomarkers and other surrogate endpoints for personalized antidepressant treatment selection.
Footnotes
Conflict of interest statement: Dr. Zarate is listed as a coinventor on a patent application for the use of ketamine and its metabolites in major depression. Dr. Zarate has assigned his rights in the patent to the US Government but will share a percentage of any royalties that may be received by the Government. All other authors have no potential conflicts of interest to disclose.
Funding: Funding for this work was supported by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH; NCT00088699, protocol 04-M-0222), by a NARSAD Independent Investigator Award to CAZ, and by a Brain & Behavior Mood Disorders Research Award to CAZ.
Contributor Information
Nicolas D. Iadarola, National Institutes of Health/National Institute of Mental Health, Experimental Therapeutics and Pathophysiology Branch, Bethesda, MD, USA
Mark J. Niciu, National Institutes of Health/National Institute of Mental Health, Experimental Therapeutics and Pathophysiology Branch, Bethesda, MD, USA
Erica M. Richards, National Institutes of Health/National Institute of Mental Health, Experimental Therapeutics and Pathophysiology Branch, Bethesda, MD, USA
Jennifer L. Vande Voort, National Institutes of Health/National Institute of Mental Health, Experimental Therapeutics and Pathophysiology Branch, Bethesda, MD, USA
Elizabeth D. Ballard, National Institutes of Health/National Institute of Mental Health, Experimental Therapeutics and Pathophysiology Branch, Bethesda, MD, USA
Nancy B. Lundin, National Institutes of Health/National Institute of Mental Health, Experimental Therapeutics and Pathophysiology Branch, Bethesda, MD, USA
Allison C. Nugent, National Institutes of Health/National Institute of Mental Health, Experimental Therapeutics and Pathophysiology Branch, Bethesda, MD, USA
Rodrigo Machado-Vieira, National Institutes of Health/National Institute of Mental Health, Experimental Therapeutics and Pathophysiology Branch, Bethesda, MD, USA.
Carlos A. Zarate, Jr, National Institutes of Health/National Institute of Mental Health, Experimental Therapeutics and Pathophysiology Branch, 10 Center Dr., Building 10/CRC, Room 7-5545, Bethesda, MD 20892, USA.
References
- aan het Rot M., Collins K., Murrough J., Perez A., Reich D., Charney D., et al. (2010) Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry 67: 139–145. [DOI] [PubMed] [Google Scholar]
- Abdallah C., Fasula M., Kelmendi B., Sanacora G., Ostroff R. (2012) Rapid antidepressant effect of ketamine in the electroconvulsive therapy setting. J ECT 28: 157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah C., Salas R., Jackowski A., Baldwin P., Sato J., Mathew S. (2014) Hippocampal volume and the rapid antidepressant effect of ketamine. J Psychopharmacol. Epub ahead of print 13 August 2014. doi: 10.1177/0269881114544776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A., Gunn A., Barkay G., Karne H., Nurnberger J., Mathew S., et al. (2012) Early antidepressant effect of memantine during augmentation of lamotrigine inadequate response in bipolar depression: a double-blind, randomized, placebo-controlled trial. Bipolar Disord 14: 64–70. [DOI] [PubMed] [Google Scholar]
- Andreasen J., Gynther M., Rygaard A., Bøgelund T., Nielsen S., Clausen R., et al. (2013) Does increasing the ratio of AMPA-to-NMDA receptor mediated neurotransmission engender antidepressant action? Studies in the mouse forced swim and tail suspension tests. Neurosci Lett 546: 6–10. [DOI] [PubMed] [Google Scholar]
- Autry A., Adachi M., Nosyreva E., Na E., Los M., Cheng P., et al. (2011) NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475: 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard E., Ionescu D., Vande Voort J., Niciu M., Richards E., Luckenbaugh D., et al. (2014) Improvement in suicidal ideation after ketamine infusion: relationship to reductions in depression and anxiety. J Psychiatr Res 58: 161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman R., Cappiello A., Anand A., Oren D., Heninger G., Charney D., et al. (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47: 351–354. [DOI] [PubMed] [Google Scholar]
- Beurel E., Song L., Jope R. (2011) Inhibition of glycogen synthase kinase-3 is necessary for the rapid antidepressant effect of ketamine in mice. Mol Psychiatry 16: 1068–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blier P., Zigman D., Blier J. (2012) On the safety and benefits of repeated intravenous injections of ketamine for depression. Biol Psychiatry 72: e11–e12. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2014) Key Data and Statistics. Injury Prevention and Control. Center for Disease Control and Prevention. Available at: www.cdc.gov/injury/overview/data.html (accessed 18 March 2015).
- Chilukuri H., Reddy N., Pathapati R., Manu A., Jollu S., Shaik A. (2014) Acute antidepressant effects if intramuscular versus intravenous ketamine. Indian J Psychol Med 36: 71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury G., Behar K., Cho W., Thomas M., Rothman D., Sanacora G. (2012) 1H-[13C]-nuclear magnetic resonance spectroscopy measures of ketamine’s effect on amino acid neurotransmitter metabolism. Biol Psychiatry 71: 1022–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusin C., Hilton G., Nierenberg A., Fava M. (2012) Long-term maintenance with intramuscular ketamine for treatment-resistant bipolar II depression. Am J Psychiatry 169: 868–869. [DOI] [PubMed] [Google Scholar]
- De Gioannis A., De Leo D. (2014) Oral ketamine augmentation for chronic suicidality in treatment-resistant depression. Aust N Z J Psychiatry 48: 686. [DOI] [PubMed] [Google Scholar]
- Diamond P., Farmery A., Atkinson S., Haldar J., Williams N., Cowen P., et al. (2014) Ketamine infusions for treatment resistant depression: a series of 28 patients treated weekly or twice weekly in an ECT clinic. J Psychopharmacol 28: 536–544. [DOI] [PubMed] [Google Scholar]
- DiazGranados N., Ibrahim L., Brutsche N., Ameli R., Henter I., Luckenbaugh D., et al. (2010a) Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry 71: 1605–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiazGranados N., Ibrahim L., Brutsche N., Newberg A., Kronstein P., Khalife S., et al. (2010b) A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry 67: 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domino E. (2010) Taming the ketamine tiger. 1965. Anesthesiology 113: 678–684 [DOI] [PubMed] [Google Scholar]
- Duman R., Aghajanian G. (2012) Synaptic dysfunction in depression: potential therapeutic targets. Science 338: 68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman R., Li N., Liu R., Duric V., Aghajanian G. (2012) Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology 62: 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemi M., Raza M., Dehpour A. (2010) NMDA receptor antagonists augment antidepressant-like effects of lithium in the mouse forced swimming test. J Psychopharmacology 24: 585–594. [DOI] [PubMed] [Google Scholar]
- Gideons E., Kavalali E., Monteggia L. (2014) Mechanisms underlying differential effectiveness of memantine and ketamine in rapid antidepressant responses. Proc Natl Acad Sci USA 111: 8649–8654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glue P., Gulati A., Le Nedelec M., Duffull S. (2011) Dose- and exposure-response to ketamine in depression. Biol Psychiatry 70: e9–e10; author reply e11–e12. [DOI] [PubMed] [Google Scholar]
- Haile C., Murrough J., Iosifescu D., Chang L., Al Jurdi R., Foulkes A., et al. (2014) Plasma brain derived neurotrophic factor (BDNF) and response to ketamine in treatment-resistant depression. Int J Neuropsychopharmacol 17: 331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han K., Holder J., Schaaf C., Lu H., Chen H., Kang H., et al. (2013) SHANK3 overexpression causes manic-like behaviour with unique pharmacogenetic properties. Nature 503: 72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harihar C., Dasari P., Srinivas J. (2013) Intramuscular ketamine in acute depression: a report on two cases. Indian J Psychiatry 55: 186–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay N., Sonenberg N. (2004) Upstream and downstream of mTOR. Genes Dev 18: 1926–1945. [DOI] [PubMed] [Google Scholar]
- Heresco-Levy U., Gelfin G., Bloch B., Levin R., Edelman S., Javitt D., et al. (2013) A randomized add-on trial of high-dose D-cycloserine for treatment-resistant depression. Int J Neuropsychopharmacol 16: 501–506. [DOI] [PubMed] [Google Scholar]
- Heresco-Levy U., Javitt D., Gelfin Y., Gorelik E., Bar M., Blanaru M., et al. (2006) Controlled trial of D-cycloserine adjuvant therapy for treatment-resistant major depressive disorder. J Affect Disord 93: 239–243. [DOI] [PubMed] [Google Scholar]
- Hoeffer C., Klann E. (2010) mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci 33: 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim L., Diazgranados N., Franco-Chaves J., Brutsche N., Henter I., Kronstein P., et al. (2012a) Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology 37: 1526–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim L., DiazGranados N., Jolkovsky L., Brutsche N., Luckenbaugh D., Herring W., et al. (2012b) A randomized, placebo-controlled, crossover pilot trial of the oral selective NR2B antagonist MK-0657 in patients with treatment-resistant major depressive disorder. J Clin Psychopharmacol 32: 551–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T. (2012) Next-generation treatments for mental disorders. Sci Trans Med 4: 155–164. [DOI] [PubMed] [Google Scholar]
- Ionescu D., Luckenbaugh D., Niciu M., Richards E., Slonena E., Vande Voort J., et al. (2014) Effect of baseline anxious depression on initial and sustained antidepressant response to ketamine. J Clin Psychiatry 75: e932–e938. [DOI] [PubMed] [Google Scholar]
- Irwin S., Iglewicz A. (2010) Oral ketamine for the rapid treatment of depression and anxiety in patients receiving hospice care. J Palliat Med 13: 903–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin S., Iglewicz A., Nelesen R., Lo J., Carr C., Romero S., et al. (2013) Daily oral ketamine for the treatment of depression and anxiety in patients receiving hospice care: a 28-day open-label proof-of-concept trial. J Palliat Med 16: 958–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järventausta K., Chrapek W., Kampman O., Tuohimaa K., Björkqvist M., Häkkinen H., et al. (2013) Effects of S-ketamine as an anesthetic adjuvant to propofol on treatment response to electroconvulsive therapy in treatment-resistant depression: a randomized pilot study. J ECT 29: 158–161. [DOI] [PubMed] [Google Scholar]
- Koike H., Chaki S. (2014) Requirement of AMPA receptor stimulation for the sustained antidepressant activity of ketamine and LY341495 during the forced swim test in rats. Behav Brain Res 271: 111–115. [DOI] [PubMed] [Google Scholar]
- Kollmar R., Markovic K., Thürauf N., Schmitt H., Kornhuber J. (2008) Ketamine followed by memantine for the treatment of major depression. Aust N Z J Psychiatry 42: 170. [DOI] [PubMed] [Google Scholar]
- Laje G., Lally N., Mathews D., Brutsche N., Chemerinski A., Akula N., et al. (2012) Brain-derived neurotrophic factor Val66Met polymorphism and antidepressant efficacy of ketamine in depressed patients. Biol Psychiatry 72: e27–e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lally N., Nugent A., Luckenbaugh D., Ameli R., Roiser J., Zarate C., Jr (2014) Anti-anhedonic effect of ketamine and its neural correlates in treatment-resistant bipolar depression. Transl Psychiatry 4: e469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidus K., Levitch C., Perez A., Brallier J., Parides M., Soleimani L., et al. (2014) A randomized controlled trial of intranasal ketamine in major depressive disorder. Biol Psychiatry, 76: 970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara D., Bisol L., Munari L. (2013) Antidepressant, mood stabilizing and procognitive effects of very low dose sublingual ketamine in refractory unipolar and bipolar depression. Int J Neuropsychopharmacol 16: 2111–2117. [DOI] [PubMed] [Google Scholar]
- Larkin G., Beautrais A. (2011) A preliminary naturalistic study of low-dose ketamine for depression and suicide ideation in the emergency department. Int J Neuropsychopharmacol 14: 1127–1131. [DOI] [PubMed] [Google Scholar]
- Li N., Lee B., Liu R., Banasr M., Dwyer J., Iwata M., et al. (2010) mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329: 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N., Liu R., Dwyer J., Banasr M., Lee B., Son H., et al. (2011) Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 69: 754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lineberry T, O’Connor S. (2012) Suicide in the US Army. Mayo Clin Proc 87: 871–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R., Fuchikami M., Dwyer J., Lepack A., Duman R., Aghajanian G. (2013) GSK-3 inhibition potentiates the synaptogenic and antidepressant-like effects of subthreshold doses of ketamine. Neuropsychopharmacology 38: 2268–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckenbaugh D., Niciu M., Ionescu D., Nolan N., Richards E., Brutsche N., et al. (2014) Do the dissociative side effects of ketamine mediate its antidepressant effects? J Affect Disord 159: 56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundin N., Niciu M., Luckenbaugh D., Ionescu D., Richards E., Vande Voort J., et al. (2014) Baseline vitamin B12 and folate levels do not predict improvement in depression after a single infusion of ketamine. Pharmacopsychiatry 47: 141–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Vieira R., Yuan P., Brutsche N., DiazGranados N., Luckenbaugh D., Manji H., et al. (2009) Brain-derived neurotrophic factor and initial antidepressant response to an N-methyl-D-aspartate antagonist. J Clin Psychiatry 70: 1662–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng S., Zarate C., Jr, Du J., Schloesser R., McCammon J., Chen G., et al. (2008) Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry 63: 349–352. [DOI] [PubMed] [Google Scholar]
- Malkesman O., Austin D., Tragon T., Wang G., Rompala G., Hamidi A., et al. (2012) Acute D-serine treatment produces antidepressant-like effects in rodents. Int J Neuropsychopharmacol 15: 1135–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew S., Murrough J., aan het Rot M., Collins K., Reich D., Charney D. (2010) Riluzole for relapse prevention following intravenous ketamine in treatment-resistant depression: a pilot randomized, placebo-controlled continuation trial. Int J Neuropsychopharmacol 13: 71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew S., Shah A., Lapidus K., Clark C., Jarun N., Ostermeyer B., et al. (2012) Ketamine for treatment-resistant unipolar depression: current evidence. CNS Drugs 26: 189–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney R., Capogna M., Dürr R., Gähwiler B., Thompson S. (1999) Miniature synaptic events maintain dendritic spines via AMPA receptor activation. Nat Neurosci 2: 44–49. [DOI] [PubMed] [Google Scholar]
- Mealing G., Lanthorn T., Murray C., Small D., Morley P. (1999) Differences in degree of trapping of low-affinity uncompetitive N-methyl-D-aspartic acid receptor antagonists with similar kinetics of block. J Pharmacol Exp Ther 288: 204–210. [PubMed] [Google Scholar]
- Moaddel R., Luckenbaugh D., Xie Y., Villaseñor A., Brutsche N., Machado-Vieira R., et al. (2014) D-serine plasma concentration is a potential biomarker of (R,S)-ketamine antidepressant response in subjects with treatment-resistant depression. Psychopharmacology (Berl) 232: 399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B., Adams B., Verma A., Daly D. (1997) Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci 17: 2921–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C., Duffin S., Hunt S., Monaghan L., Mason O., Curran H. (2012) Neurocognitive function and schizophrenia-proneness in individuals dependent on ketamine, on high potency cannabis (‘skunk’) or on cocaine. Pharmacopsychiatry 45: 269–274. [DOI] [PubMed] [Google Scholar]
- Morgan C., Muetzelfeldt L., Curran H. (2009) Ketamine use, cognition and psychological wellbeing: a comparison of frequent, infrequent and ex-users with polydrug and non-using controls. Addiction 104: 77–87. [DOI] [PubMed] [Google Scholar]
- Morgan C., Muetzelfeldt L., Curran H. (2010) Consequences of chronic ketamine self-administration upon neurocognitive function and psychological wellbeing: a 1-year longitudinal study. Addiction 105: 121–133. [DOI] [PubMed] [Google Scholar]
- Morgan C., Rees H., Curran H. (2008) Attentional bias to incentive stimuli in frequent ketamine users. Psychol Med 38: 1331–1340. [DOI] [PubMed] [Google Scholar]
- Mullard A. (2013) 2012 FDA drug approvals. Nat Rev Drug Discov 12: 87–90. [DOI] [PubMed] [Google Scholar]
- Müller H., Wegener G., Liebenberg N., Zarate C., Jr, Popoli M., Elfving B. (2013) Ketamine regulates the presynaptic release machinery in the hippocampus. J Psychiatr Res 47: 892–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough J., Iosifescu D., Chang L., Al Jurdi R., Green C., Perez A., et al. (2013. a) Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry 170: 1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough J., Perez A., Pillemer S., Stern J., Parides M., aah het Rot M., et al. (2013b) Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry 74: 250-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niciu M., Grunschel B., Corlett P., Pittenger C., Bloch M. (2013a) Two cases of delayed-onset suicidal ideation, dysphoria and anxiety after ketamine infusion in patients with obsessive-compulsive disorder and a history of major depressive disorder. J Psychopharmacol 27: 651–654. [DOI] [PubMed] [Google Scholar]
- Niciu M., Henter I., Luckenbaugh D., Zarate Jr, C., Charney D. (2014a) Glutamate receptor antagonists as fast-acting therapeutic alternatives for the treatment of depression: ketamine and other compounds. Annu Rev Pharmacol Toxicol 54: 119–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niciu M., Ionescu D., Mathews D., Richards E. (2013b) Second messenger/signal transduction pathways in major mood disorders: moving from membrane to mechanism of action, part I: major depressive disorder. CNS Spectr 18: 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niciu M., Kelmendi B., Sanacora G. (2012) Overview of glutamatergic neurotransmission in the nervous system. Pharmacol Biochem Behav 100: 656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niciu M., Luckenbaugh D., Ionescu D., Guevara S., Machado-Vieira R., Richards E., et al. (2014b) Clinical predictors of ketamine response in treatment-resistant major depression. J Clin Psychiatry 75: e417–e423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niciu M., Luckenbaugh D., Ionescu D., Mathews D., Richards E., Zarate C., Jr (2013c) Subanesthetic dose ketamine does not induce an affective switch in three independent samples of treatment-resistant major depression. Biol Psychiatry 74: e23–e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niciu M., Mathews D., Nugent A., Ionescu D., Furey M., Richards E., et al. (2014c) Developing biomarkers in mood disorders research through the use of rapid-acting antidepressants. Depress Anxiety 31: 297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent A., Diazgranados N., Carlson P., Ibrahim L., Luckenbaugh D., Brutsche N., et al. (2014) Neural correlates of rapid antidepressant response to ketamine in bipolar disorder. Bipolar Disord 16: 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz R., Niciu M., Lukkahati N., Saligan L., Nugent A., Luckenbaugh D., et al. (2014) SHANK3 as a potential biomarker of antidepressant response to ketamine and its neural correlates in bipolar depression. J Affect Disord 172C: 307–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paslakis G., Gilles M., Meyer-Lindenberg A., Deuschle M. (2010) Oral administration of the NMDA receptor antagonist S-ketamine as add-on therapy of depression: a case series. Pharmacopsychiatry 43: 33–35. [DOI] [PubMed] [Google Scholar]
- Permoda-Osip A., Dorszewska J., Bartkowska-Sniatkowska A., Chlopocka-Wozniak M., Rybakowski J. (2013) Vitamin B12 level may be related to the efficacy of a single ketamine infusion in bipolar depression. Pharmacopsychiatry 46: 227-228. [DOI] [PubMed] [Google Scholar]
- Permoda-Osip A., Skibińska M., Bartkowska-Sniatkowska A., Kliwicki S., Chłopocka-Woźniak M., Rybakowski J. (2014) [Factors connected with efficacy of single ketamine infusion in bipolar depression]. Psychiatr Pol 48: 35–47. [PubMed] [Google Scholar]
- Perry E., Cramer J., Cho H., Petrakis I., Karper L., Genovese A., et al. (2007) Psychiatric safety of ketamine in psychopharmacology research. Psychopharmacology (Berl) 192: 253–260. [DOI] [PubMed] [Google Scholar]
- Phelps L., Brutsche N., Moral J., Luckenbaugh D., Manji H., Zarate C., Jr (2009) Family history of alcohol dependence and initial antidepressant response to an N-methyl-D-aspartate antagonists. Biol Psychiatry 65: 181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preskorn S., Baker B., Kolluri S., Menniti F., Krams M., Landen J. (2008) An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol 28: 631–637. [DOI] [PubMed] [Google Scholar]
- Price R., Iosifescu D., Murrough J., Chang L., Al Jurdi R., Iqbal S., et al. (2014) Effects of ketamine on explicit and implicit suicidal cognition: a randomized controlled trial in treatment-resistant depression. Depress Anxiety 31: 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price R., Nock M., Charney D., Mathew S. (2009) Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry 66: 522–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan M., Zhang N., Wang Y., Zhang T., Yang Z. (2011) Possible antidepressant effects and mechanisms of memantine in behaviors and synaptic plasticity of a depression rat model. Neuroscience 182: 88–97. [DOI] [PubMed] [Google Scholar]
- Rasmussen K., Lineberry T., Galardy C., Kung S., Lapid M., Palmer B., et al. (2013) Serial infusions of low-dose ketamine for major depression. J Psychopharmacol 27: 444–450. [DOI] [PubMed] [Google Scholar]
- Réus G., Stringari R., Kirsch T., Fries G., Kapczinski F., Roesler R., et al. (2010) Neurochemical and behavioural effects of acute and chronic memantine administration in rats: further support for NMDA as a new pharmacological target for the treatment of depression? Brain Res Bull 81: 585–589. [DOI] [PubMed] [Google Scholar]
- Rybakowski J., Permoda-Osip A., Skibinska M., Adamski R., Bartkowska-Sniatkowska A. (2013) Single ketamine infusion in bipolar depression resistant to antidepressants: are neurotrophins involved? Hum Psychopharmacol 28: 87–90. [DOI] [PubMed] [Google Scholar]
- Sanacora G., Johnson M., Khan A., Atkinson S., Risenberg R., Schronen J., et al. (2014a) Adjunctive lanicemine (AZD6765) in patients with major depressive disorder and a history of inadequate response to antidepressants: primary results from a randomized, placebo-controlled study (PURSUIT). Poster presented on 18 June at the 2014 American Society of Clinical Psychopharmacology Annual Meeting in Hollywood, FL. [Google Scholar]
- Sanacora G., Smith M., Pathak S., Su H., Boeijinga P., McCarthy D., et al. (2014b) Lanicemine: a low-trapping NMDA channel blocker produces sustained antidepressant efficacy with minimal psychotomimetic adverse effects. Mol Psychiatry 19: 978–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G., Zarate C., Jr, Krystal J., Manji H. (2008) Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov 7: 426–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sos P., Klirova M., Novak T., Kohutova B., Horacek J., Palenicek T. (2013) Relationship of ketamine’s antidepressant and psychotomimetic effects in unipolar depression. Neuro Endocrinol Lett 34: 287–293. [PubMed] [Google Scholar]
- Trullas R., Skolnick P. (1990) Functional antagonists at the NMDA receptor complex exhibit antidepressant actions. Eur J Pharmacol 185: 1–10. [DOI] [PubMed] [Google Scholar]
- Valentine G., Mason G., Gomez R., Fasula M., Watzl J., Pittman B., et al. (2011) The antidepressant effect of ketamine is not associated with changes in occipital amino acid neurotransmitter content as measured by [(1)H]-MRS. Psychiatry Res 191: 122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villaseñor A., Ramamoorthy A., Silva dos Santos M., Lorenzo M., Laje G., Zarate C., Jr, et al. (2014) A pilot study of plasma metabolomic patterns from patients treated with ketamine for bipolar depression: evidence for a response-related difference in mitochondrial networks. Br J Pharmacol 171: 2230–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan L., Levitch C., Perez A., Brallier J., Iosifescu D., Chang L., et al. (2014) Ketamine safety and tolerability in clinical trials for treatment-resistant depression. J Clin Psychiatry 2 September (epub ahead of print). [DOI] [PubMed] [Google Scholar]
- White P., Ham J., Way W., Trevor A. (1980) Pharmacology of ketamine isomers in surgical patients. Anesthesiology 52: 231–239. [DOI] [PubMed] [Google Scholar]
- Zarate C., Jr, Brutsche N., Ibrahim L., Franco-Chaves J., Diazgranados N., Cravchik A., et al. (2012) Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry 71: 939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate C., Jr, Mathews D., Ibrahim L., Chaves J., Marquardt C., Ukoh I., et al. (2013) A randomized trial of a low-trapping nonselective N-methyl-D-aspartate channel blocker in major depression. Biol Psychiatry 74: 257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate C., Jr, Singh J., Carlson P., Brutsche N., Ameli R., Luckenbaugh D., et al. (2006a) A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63: 856–864. [DOI] [PubMed] [Google Scholar]
- Zarate C., Jr, Singh J., Quiroz J., De Jesus G., Denicoff K., Luckenbaugh D., et al. (2006b) A double-blind, placebo-controlled study of memantine in the treatment of major depression. Am J Psychiatry 163: 153–155. [DOI] [PubMed] [Google Scholar]
- Zhou W., Dong L., Wang N., Shi J., Yang J., Zuo Z., et al. (2014) Akt mediates GSK-3β phosphorylation in the rat prefrontal cortex during the process of ketamine exerting rapid antidepressant actions. Neuroimmunomodulation 21: 183–188. [DOI] [PubMed] [Google Scholar]