Abstract
The aim of the present study is to determine whether ADAMTS-7 contributes to the onset and severity of joint inflammation in the pathogenesis of inflammatory arthritis. ADAMTS-7 was found to be elevated in the course of collagen-induced arthritis (CIA).. ADAMTS-7 transgenic (TG) mice were more susceptible to the induction of CIA. The onset of CIA was accelerated and the arthritic severity was increased in TG mice compared to wild type mice. The overall incidence was also significantly higher in TG mice. In addition, arthritic TG mice displayed significantly higher clinical and histological arthritis scores. The COMP degradative fragments were significantly elevated in articular cartilage and sera in CIA models of TG mice. Furthermore, the production of tumor necrosis factor-alpha (TNF-α) and interleukin-17 (IL-17) were also increased in serum and draining lymph nodes of arthritic TG mice. Therefore, these data provided the in vivo evidence, suggesting that ADAMTS-7 may play an important role in the pathogenesis of inflammatory arthritis, and that inhibition of ADAMTS-7 may be a potential target to ameliorate the severity of inflammatory arthritis.
Keywords: ADAMTS-7, COMP, transgenic mice, collagen-induced arthritis, TNF-α
Introduction
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease characterized by inflammation of multiple joints and destruction of cartilage and bone [1]. Although the cause of RA remains unclear, increasing evidences indicate that cytokines and proteinases produced from RA synovium play an important role in the erosion of articular cartilage and subchondral bones.
The ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) proteases are enzymes that exert their function through degradation of substrates, including hyalectans, procollagen, and cartilage oligomeric matrix protein (COMP) [2]. The altered regulation of ADAMTS proteases has been implicated in a number of inflammatory pathological conditions. For instance, ADAMTS-5 plays a primary role in aggrecan degradation in murine arthritis [3, 4].
The expression of ADAMTS-7 was found in cartilage, bone, synovium, tendon and ligamentous tissue [5]. COMP, a prominent non-collagenous component of cartilage, plays a critical role in the progression of arthritis and whose degradation has been observed in RA patients [6]. ADAMTS-7 directly associats with and degrads COMP [6]. Moreover, ADAMTS-7 was also found to inhibit endochondral bone formation and involved in coronary atherosclerosis via associating with and degrading COMP [7–9]. But whether ADAMTS-7 also contributes to COMP degradation during inflammatory arthritis has not been elucidated.
Recent studies demonstrated that ADAMTS-7 formed a positive feedback regulatory loop with TNF-α in the progression of surgically-induced osteoarthritis [10]. However, the role of ADAMTS-7 in the initiation and progression of inflammatory arthritis remains unknown. In this study, we examined the expression of ADAMTS-7 in the course of collagen-induced arthritis (CIA) mice model, and established a CIA model in ADAMTS-7 transgenic (TG) mice. We found a higher incidence of CIA and a higher clinical and histological arthritis score with increased COMP degradation, production of TNF-α and interleukin-17 (IL-17) in ADAMTS-7 TG mice. These results indicated the importance of ADAMTS-7 in the maintenance of joint integrity, and inhibition of ADAMTS-7 may be a novel target to control the severity of RA.
Materials and methods
Ethics Statement
All animal studies were performed in accordance with institutional guidelines and with approval by the Institutional Animal Care and Use Committee of New York University (Protocol Number: 130202-01). All efforts were made to minimize the number of animals used and their suffering.
Mice
DBA/1J mice were obtained from Jackson Laboratories. Generation and characterization of ADAMTS-7 TG mice were described recently [10]. The TG mice in FVB/N background were backcrossed onto the DBA/1J mice for 6 generations to produce mice used in experiments.
Induction of CIA
Chicken type II collagen (Chondrex, LLC, Seattle, WA) was emulsified with an equal volume of complete Freund’s adjuvant (CFA) containing 4 mg/ml heat-killed mycobacterium (Chondrex, LLC, Seattle, WA). For male DBA/1J mice (n=9), each mouse (8–10 weeks old) was intradermally immunized at the base of the tail with 100μL of the emulsion. On day 21, a booster immunization is used through injection of 100μL emulsion containing chicken type II collagen and the same volume of incomplete Freund’s adjuvant (IFA).
Clinical assessment of arthritis
Development of arthritis was evaluated every other day by visual observation of paw swelling from experimental 4 weeks and continuing until 14 weeks. Each paw and digit was scored for the degree of inflammation (swelling and erythema) by using a well-established semi quantitative score [11]. The total arthritis score for each mouse was determined by adding the scores from all four paws. Incidence was expressed as the percentage of arthritic mice out of the total number of immunized mice during the experiment.
Histopathological analyses
Serial sections were stained with hematoxylin and eosin (H&E) and the severity of arthritis was determined by the cumulative assessment of three parameters including pannus formation, synovial inflammation, and cartilage/bone erosion by using a score system [12]. All scoring was performed blind with respect to the specific treatment.
Tartrate-resistant acid phosphatase (TRAP) staining was performed for examination of multinucleated osteoclast activity. Sections were also stained with safranin O for detection of cartilage proteoglycans. Safranin O-stained sections were graded using categories of Mankin scores [13].
Radiographic analysis
Prior to histologic processing, paraformaldehyde-fixed samples were evaluated via X-ray (Faxitron) and micro-computed tomography (micro-CT) using a Scanco μ CT 40 scanner (Scanco Medical AG, Basserdorf, Switzerland). X-ray and micro-CT were used to quantify bone erosion within the paws.
Flow cytometry
Fresh isolated draining lymph nodes cells were stained with anti-CD16/32, -CD4, -CD8 (eBioscience). Intracellular staining for TNF-α and IL-17A was conducted according to the manufacturers’ instructions (Fixation and Permeabilization Solution Kit, eBioscience). All results were carried out on a LSRII machine (BD Biosciences) and analyzed with FlowJo (Tree star, Ashland OR)..
Immunohistochemistry
Tissue sections were performed immunohistochemistry (IHC) assay to examine the expression of ADAMTS7, MMP13 and COMP with a standard protocol previously published [10, 14].
Measurement of inflammatory cytokines levels in serum
At the end of the experiments, the blood was centrifuged and serum was obtained for further analysis. The levels of TNF-α and IL-17 in the serum of each group were measured by using mouse TNF-α and IL-17 ELISA kits (eBioscience) in accordance with the manufacturer’s instructions.
Sandwich ELISA for COMP
The following detailed steps were performed according to our previously described COMP fragments specific ELISA [14]. The COMP concentrations in serum were calculated from the linear range of a standard curve.
Quantitative RT-PCR analysis
Quantitative real-time PCR was performed using 7300 Real-time PCR system (Applied Biosystems) and SYBR-Green PCR Master Mix following the manufacturer’s protocol (Applied Biosystems). The sequences of the forward and reverse primers used are listed in Table 1. Data were analyzed by the −2ΔΔCT method normalizing to gapdh.
Table 1.
Mouse primers sequences used for real-time RT-PCR analysis
| Gene Symbol | GenBank Accession α | Primer sequence |
|---|---|---|
| Adamts-7 | NM_001003911 | For 5′ TCACCAGGTTCCTTGACCGTG 3′ |
| Rev 5′ CCAGCTTGGAGTGACAGGTGGT 3′ | ||
| MMP-13 | NM_008607 | For 5′ CATCCATCCCGTGACCTTAT 3′ |
| Rev 5′ GCATGACTCTCACAATGCGA 3′ | ||
| TNF-α | NM_00127860 | For 5′ AGGGTCTGGGCCATAGAACT 3′ |
| Rev 5′ CCACCACGCTCTTCTGTCTAC 3′ | ||
| IL-17 | NM_010552 | For 5′ GCTCCAGAAGGCCCTCAGA 3′ |
| Rev 5′ AGCTTTCCCTCCGCATTGA 3′ | ||
| GAPDH | NM_008084 | For 5′ AGGTCGGTGTGAACGGATTTG 3′ |
| Rev 5′ TGTAGACCATGTAGTTGAGGTCA 3′ |
GenBank is available online at http://www.ncbi.nlm.nih.gov/genbank/.
Statistical analysis
All data were analyzed using SPSS software (SPSS Inc, Chicago, IL). Results represent means ± SD. The group comparisons were analyzed by the Student’s t-test. A value of P < 0.05 was considered to be statistically significant.
Results
ADAMTS-7 expression in the course of collagen-induced arthritis in DBA/1J mice
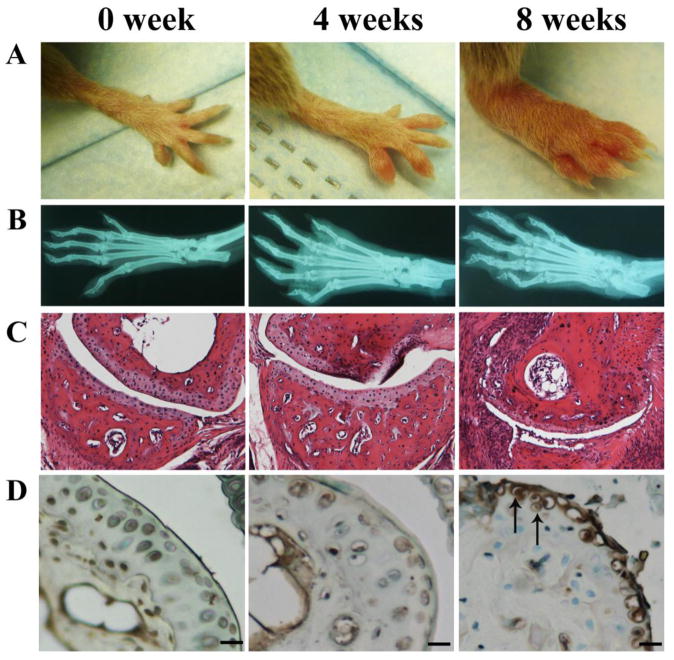
To characterize ADAMTS-7 in CIA mice, we established the CIA model in DBA/1J mice and investigated the alteration of ADAMTS-7 expression during the progression of disease by using IHC. As shown in Fig. 1A, 8 weeks after immunization, DBA/1J mice developed severe joint inflammation evidenced by marked erythema and swelling of forepaws encompassing the wrist and ankle and extended distally through the limb and digits. The radiograph revealed severed narrowing of the joint space and bone erosion around the joints of collagen-induced mice (Fig. 1B). The H&E stained joint sections further confirmed the inflammation and joint destruction during the course of CIA (Fig. 1C). Results of immunohistochemistry showed that the protein level of ADAMTS-7 was significantly upregulated in 8 wk after immunization, in contrast, no or weak staining was observed in 0 wk (non-immune controls) or 4 wk after immunization (Fig. 1D), indicating that ADAMTS-7 was differentially expressed in the course of inflammatory arthritis.
Figure 1.
ADAMTS-7 expression was upregulated in the CIA model in DBA/1J mice (n=9). (A) Representative photographs of forepaws. (B) Representative radiographs of normal and arthritic paws. (C) Representative H&E staining results of joint sections from arthritic mice and control group. (D) IHC for ADAMTS-7 in joint sections. Scale bars, 50μm.
The onset and severity of CIA in hADAMTS-7 transgenic (TG) mice
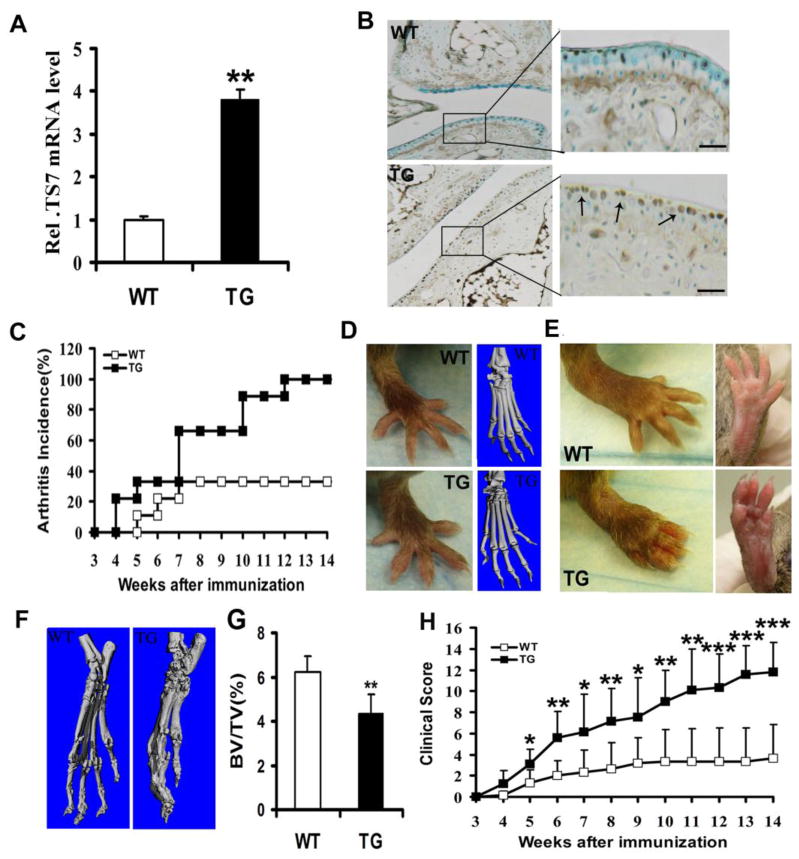
To study the role of ADAMTS-7 in arthritis development using the CIA model, we backcrossed hADAMTS-7 TG mice (FVB/N background) for 6 generations onto the DBA/1J background. As shown in Fig. 2A, the real-time PCR results demonstrated a significant increase in the mRNA level (P < 0.01) in ADAMTS-7 TG mice compared to control mice. Results of IHC further confirmed the expression of ADAMTS-7 was high in TG mice as compared with naïve mice (Fig. 2B). To investigate the effect of ADAMTS-7 on CIA incidence and severity, wild type littermates (n=9) and TG mice (n=9) were immunized with chicken type II collagen and were boosted using chicken type II collagen emulsified with IFA on day 21, and then were observed for up to 14 weeks for the development of clinical arthritis. As indicated in Fig. 2C, arthritis onset was accelerated for 4 week in ADAMTS-7 TG mice, while arthritis in wild type mice appeared on 5 weeks after first immunization and second booster injection. By weeks 4, a significant difference in the incidence of arthritis was observed. On weeks 10, the incidence of ADAMTS-7 TG mice was 89%, in contrast, only 33% wild type mice developed arthritis. By the end of the experiment, the incidence was 100% and 33%, respectively (Fig. 2C). There were slightly difference in hindpaws between TG mice and wild type mice by visual examination at weeks 4, but the images of micro-CT demonstrated that no difference in the joints were observed in the TG group as compared to the wild type mice, as shown in Fig. 2D. However, severe joint inflammation was observed in TG mice by visual examination, including marked swelling and erythema of the hindpaws encompassing the wrist and ankle and extending distally through the limb and digits 14 weeks after collagen induction (Fig. 2E). The hindpaws were further examined by micro-CT at weeks 14, results demonstrated that significant bone erosions in the joints were observed in the TG group as compared to the WT group (Fig. 2F), which confirmed the conclusion of visual examination, and TG mice had a decreased bone volume per total volume (BV/TV) compared to wild type mice (4.0±0.68% vs. 6.3±0.88%, P = 0.0011) (Fig. 2G). ADAMTS-7 TG mice had significantly higher mean clinical scores than wild type mice from weeks 5 after first immunization and booster injection (weeks 8: P = 0.012; weeks 9: P = 0.0016; weeks 10: P = 0.0017; weeks 11: P < 0.001; weeks 12: P < 0.001; weeks 13: P < 0.001; weeks 14: P < 0.001) (Fig. 2H).
Figure 2.
ADAMTS-7 TG mice become more susceptible to CIA. (A) Real-time RT-PCR analysis of ADAMTS-7 mRNA expression from cartilage of TG mice and wide type control. (B) IHC was performed using paw joint sections. Scale bars, 200μm. (C) Total incidence of CIA in TG mice (n=9) compared to wild type littermate mice (n=9). (D) Representative photographs of hindpaws. (E and F) Representative micro-CT images of hindpaws, and BV/TV of bone destruction. (G) Disease severity were scored and recorded by visual examination of mouse paws. All data were expressed as means ±SD from three independent experiments, *P< 0.05, **P<0.01, ***P<0.001.
Histological changes of arthritic joints from wild type and TG mice
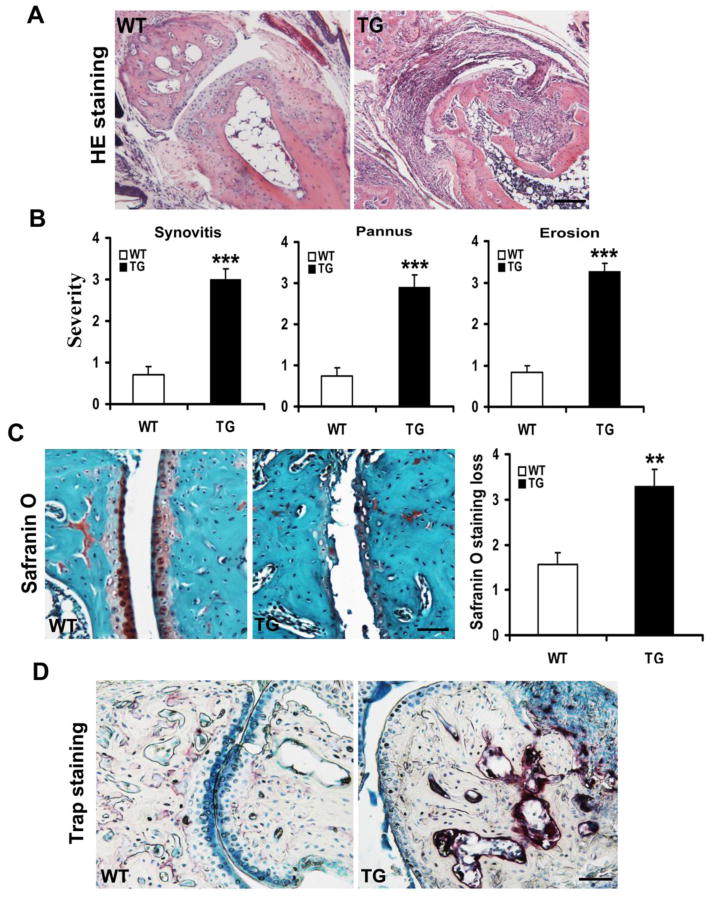
To determine whether clinical arthritis severity was associated with histological changes, we compared paw joint sections between ADAMTS-7 TG mice and wild type mice. The sections of ADAMTS-7 TG mice had more inflammatory cells infiltration and displayed severe loss of joint architecture compared with wild type mice (Fig. 3A). The semi-quantitative histological results demonstrated that ADAMTS-7 TG mice had a statistically significant increase in synovitis, pannus, and erosion score (synovitis severity scores: P < 0.001; pannus scores: P < 0.001; erosion scores: P < 0.001) compared with wild type mice (Fig. 3B). Our safranin O staining results revealed that significantly more loss of safranin O staining was observed in ADAMTS-7 TG group compared with wide type group, 47.5% average increased in scores for proteoglycan depletion in cartilage of TG mice (3.30±0.36 vs. 1.56±0.25, P =0.0012) (Fig. 3C). Moreover, sections from ADAMTS-7 TG mice had more TRAP-positive osteoclast-like cells presented on focal bone erosions than wild type controls (Fig. 3D), suggesting that TG mice developed severe bone erosions.
Figure 3.
Representative results of histological arthritic joint sections from arthritic TG and wild type mice. (A) Representative H&E stained sections of hindpaws joints. Scale bars, 50μm. (B) Histopathological analysis of synovitis, pannus and erosion insections of hindpaws was evaluated using a five-point (0–4) score system. (C) Representative histologic staining with safranin O of hindpaws joints. Scale bars, 50μm. (D) Representative TRAP staining sections showing osteoclast activity. All values are the normalized means±SD, **P<0.01, ***P<0.001, compared with arthritic WT mice. Scale bars, 50μm.
Changes of COMP degradation, MMP13 and inflammatory cytokines in arthritic TG mice
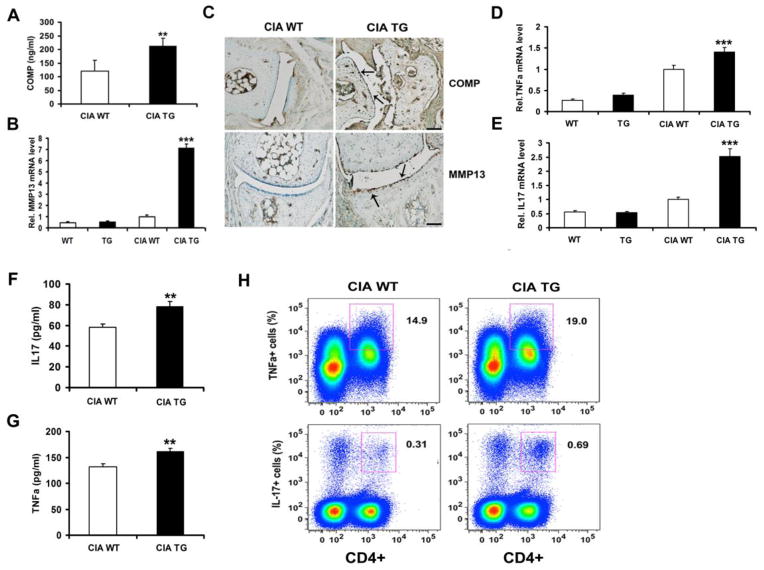
Recent studies revealed that ADAMTS-7 affects the COMP degradation and MMP13 expression in the progression of surgically-induced osteoarthritis [10], promoted us to determine whether overexpression of ADAMTS-7 caused an increase in the degradation of COMP and MMP13 expression in CIA, we thus performed a sandwich ELISA to detect the serum level of COMP degradative fragments [10, 14], and a real-time PCR to measure the levels of MMP13 mRNA in articular cartilage tissues. The serum concentrations of COMP fragments were significantly higher in arthritic ADAMTS-7 TG mice than that of the arthritic wild type mice (P < 0.01) (Fig. 4A). There were significant changes in mRNA expression of MMP13 (P < 0.001) in arthritic TG mice, compared with arthritic wild type controls (Fig. 4B). No significant changes in mRNA expression of MMP13 were found between wild type and TG mice (Fig. 4B). We also performed IHC to examine the levels of protein for COMP fragments and MMP13. As shown in Fig. 4C, COMP fragments and MMP13 were all increased in joint sections of arthritic TG mice, while a lower expression of COMP and MMP13 were detected in arthritic wild type controls.
Figure 4.
Changes of COMP fragments, MMP13 and inflammatory cytokines level in arthritic TG mice. (A) Sandwich ELISA was performed to examine the concentration of COMP fragments in serum. (B) Real-time PCR analysis of MMP13 mRNA expression. (C) Representative IHC sections of hindpaws joints. Scale bars, 50μm. (D) Real-time PCR analysis of TNF-α mRNA expression. (E) Real-time PCR analysis of IL-17 mRNA expression. (F and G) ELISA was performed to examine the levels of IL-17 and TNF-α in serum. (H) Intracellular FACS staining of TNF-α and IL-17 in draining lymph node cells. All data represent three independent results. *P < 0.05, **P<0.01.
Real-time PCR results revealed that the mRNA levels of TNF-α (P =0.0004) and IL-17 (P < 0.001) were significantly increased in arthritic TG mice compared to arthritic wild type controls (Fig. 4D and E). However, there was no significant difference between unchallenged wild type and TG mice (Fig. 4D and E). We also performed ELISA to measure the TNF-α and IL-17 levels in serum, the results demonstrated that the serum levels of TNF-α (P =0.0017) and IL-17 (P =0.002) increased significantly in CIA TG mice as compared with CIA wild type controls (Fig. 4F and G). The TNF-α-producing CD4+ T cells in draining lymph nodes were 19.0 ± 2.66% (P =0.014) in CIA TG mice, in contrast, 14.9 ± 1.03% CD4+ T cells expressed TNF-α in CIA control mice. Furthermore, the fraction of CD4+ IL-17+ cells were significantly increased in draining lymph-node cells of arthritic TG mice (0.69 ± 0.36%, P =0.012), compared with arthritic wild type mice (0.31 ± 0.11%) (Fig. 4H). These findings suggest that ADAMTS-7 overexpression enhances TNF-α and IL-17 production in arthritic TG mice after collagen induction.
Discussion
This was the first study to demonstrate the role of ADAMTS-7 in inflammatory arthritis by using a CIA model, which is characterized by rapid and severe erosions of cartilage and bone. Our results demonstrate that ADAMTS-7 was induced in the course of CIA (Fig 1.D), and this result was in agreement with a previous study revealing that ADAMTS was significantly upregulated in cartilage and synovium samples of RA patients [15], indicating that ADAMTS-7 may be involved in the pathogenesis of inflammatory arthritis.
The notion that ADAMTSs and matrix metalloproteinase (MMP) play an important role in RA has been well-established. In this study, we found that ADAMTS-7 TG mice increased the severity of CIA (Fig. 2D), suggesting that a severe destruction of cartilage and bone results in the arthritic TG mice compared with the arthritic wild type. In arthritis disease conditions, ECM degradation leads to malfunction of articular cartilage and bone. COMP belongs to the subgroup B of the thrombospondin protein family and is a prominent non-collagenous component of cartilage ECM. ADAMTS-7 was isolated as the first metalloproteinase associating with and degrading COMP directly in vitro [7]. To determine whether ADAMTS-7 affects COMP degradation in CIA, we established a novel capture ELISA method which can be used for distinguishing between COMP fragments and intact COMP levels [14]. The sandwich ELISA results demonstrated that concentration of COMP fragments in serum was significantly increased in arthritic TG mice relative to arthritic wild type mice (Fig. 4A). The COMP protein level was also to examine the degradation by using fragments antibody (Fig. 4C), and the level of COMP in TG mice were found to be significantly elevated compared to wild type mice. These findings are in agreement with published studies which propose that monitoring COMP levels may be useful in assessing the progression of arthritis in patients with arthritis and animal disease models [16–18]. In addition, cartilage destruction is mediated in large part through the elaboration of metalloproteinases, whether the severity of inflammation in TG mice related to other MMPs, we examined the mRNA of MMP13 (Fig. 4B) and found that it was elevated in arthritic TG mice compared with wild type mice. The reason may be related to the secondary effect or indirect effect mediated by, for instance TNF-α, where its expression was increased in ADAMTS-7 TG mice (Fig. 4D). Actually, the study also indicated that mRNA levels of TNF-α were significantly induced in ADAMTS-7 treated cartilage cultures relative to controls [10]. It was known that TNF-α induced numerous metalloproteinases [19], including MMP13. Thus, we further examined the protein level of MMP13 (Fig. 4C) and the data in panel 4C was consistent to panel 4B, suggesting that MMP-13 are involved in ADAMTS-7 mediated destruction of cartilage in the CIA model, and MMP-13 mediated collagen II degradation might also be increased in TS7-CIA mice.
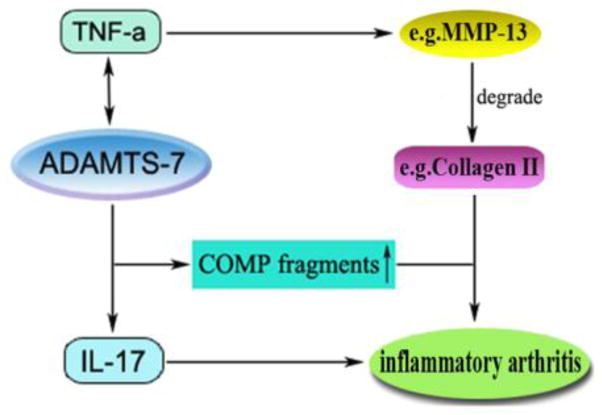
In addition, ADAMTS-7 transgenic mice displayed significantly higher clinical and histological scores after collagen induction (Fig. 2G and Fig. 3B). Safranin O staining results (Fig. 3C) also indicated that arthritic TG mice observed severe destruction of cartilage compared with arthritic wild type mice (P < 0.01). In this study, enhanced osteoclast activity was also examined in arthritic TG mice using TARP staining (Fig. 3D). Bone erosion in arthritis is caused by activated osteoclasts, and TNF-α in addition to T cell-drived IL-17 displayed a strong synergistic effect on the maturation process and the activation of mature osteoclasts [20]. We examined the mRNA levels of TNF-α (P < 0.001) and IL-17 (P < 0.001), and found significantly increase in joint cartilage of TS7-CIA mice. These findings suggest that TNF-α and IL-17 are involved in ADAMTS-7 mediated destruction of cartilage and bone in the CIA model. Overall, ADAMTS-7 may play an important role in the inflammatory arthritis through a) upregulating the proinflammatory cytokines, such as TNF-α and IL-17, and b) degrading COMP, a key early event in arthritic pathology, which may result in proteinase access to the abundant matrix molecules (e.g. Collagen II) leading to significant cartilage loss and arthritic onset (See proposed model in Fig. 5).
Figure 5.
A proposed model for explaining the role and regulation of ADAMTS-7 in the inflammatory arthritis.
The underlying mechanism of RA remains poorly understood. However, inflammatory cytokines are directly implicated in several immune-related diseases including RA [21]. TNF-α clearly plays a critical role in the pathogenesis of RA since inhibition of TNF-α suppresses various arthritis models and overexpression of the TNF-α gene leads to spontaneous inflammatory arthritis [21–23]. However, inhibition of TNF-α alone may not be sufficient to treat all cases of RA [24]. Furthermore, IL-17 plays a key role in the progression of RA and amplifies the inflammation induced by other cytokines, primarily TNF-α [25, 26]. We found a significant increase in production of TNF-α and IL-17 in serum (Fig. 4F and G) and in draining lymph-node cells (Fig. 4H) in arthritic ADAMTS-7 TG mice. TNF-α and IL-17 are involved in destruction of cartilage and bone in TG mice after collagen induction. These findings are consistent with other studies highlighting the inflammatory role of TNF-α and IL-17 in increasing the severity of CIA [27, 28].
Taken together, these results indicate that ADAMTS-7 was induced in the course of CIA, ADAMTS-7 overexpression accelerates the onset and increases the severity of CIA in TG mice and leads to endogenous TNF-α production. Whereas, other studies indicated that the mRNA level of ADAMTS-7 was also higher in TNF-a treated cartilage explants than in the untreated control [19, 29, 30]. In brief, TNF-α induces ADAMTS-7 expression. In our study, we also examined ADAMTS-7 expression by using IHC and found that the protein level of ADAMTS-7 was increased in the TNF-α transgenic mouse which is a spontaneous inflammatory arthritis animal model, compared with the wild type mouse (data not shown). Thus, ADAMTS-7 constitutes feedback regulation with TNF-α in the progression of inflammatory arthritis, as it does in surgically-induced osteoarthritis [10]. In summary, our results demonstrate that importance of ADAMTS-7 in modulating inflammatory arthritis. These findings may provide us with a new intervention strategy in the control of arthritis severity.
Acknowledgments
This work was supported partly by NIH research grants R01AR062207, R01AR061484, R56AI100901, and a Disease Targeted Research Grant from Rheumatology Research Foundation (to C. J. Liu). The founding resources had no involvement in study design, in the collection and analysis of data.
Footnotes
Author’s contributions
YYZ designed and performed the experiments, collected and analyzed data, and wrote and edited the manuscript. FHW collected and analyzed data. CJL supervised this study, and edited the manuscript.
Competing interests
The authors have no financial conflicts of interest.
References
- 1.Feldmann M, Brennan FM, Maini RN. Rheumatoid arthritis. Cell. 1996;85:307–310. doi: 10.1016/s0092-8674(00)81109-5. [DOI] [PubMed] [Google Scholar]
- 2.Jones GC, Riley GP. ADAMTS proteinases: a multi-domain, multi-functional family with roles in extracellular matrix turnover and arthritis. Arthritis Res Ther. 2005;7:160–169. doi: 10.1186/ar1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stanton H, Rogerson FM, East CJ, Golub SB, Lawlor KE, et al. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature. 2005;434:648–652. doi: 10.1038/nature03417. [DOI] [PubMed] [Google Scholar]
- 4.Glasson SS, Askew R, Sheppard B, Carito B, Blanchet T, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434:644–648. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- 5.Liu CJ. The role of ADAMTS-7 and ADAMTS-12 in the pathogenesis of arthritis. Nat Clin Pract Rheumatol. 2009;5:38–45. doi: 10.1038/ncprheum0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu CJ, Kong W, Ilalov K, Yu S, Xu K, et al. ADAMTS-7: a metalloproteinase that directly binds to and degrades cartilage oligomeric matri x protein. FASEB J. 2006;20:988–990. doi: 10.1096/fj.05-3877fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bai XH, Wang DW, Kong L, Zhang Y, Luan Y, et al. ADAMTS-7, a direct target of PTHrP, adversely regulates endochondral bone growth by associating with and inactivating GEP growth factor. Mol Cell Biol. 2009;29:4201–4219. doi: 10.1128/MCB.00056-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L, Zheng J, Bai X, Liu B, Liu CJ, et al. ADAMTS-7 mediates vascular smooth muscle cell migration and neointima formation in balloon -injured rat arteries. Circ Res. 2009;104:688–698. doi: 10.1161/CIRCRESAHA.108.188425. [DOI] [PubMed] [Google Scholar]
- 9.Pu X, Xiao Q, Kiechl S, Chan K, Fg FL, et al. ADAMTS7 cleavage and vascular smooth muscle cell migration is affected by a coronary-artery-disease -associated variant. Am J Hum Genet. 2013;92:366–374. doi: 10.1016/j.ajhg.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai Y, Bai X, Zhao Y, Tian Q, Liu B, et al. ADAMTS-7 forms a positive feedback loop with TNF-alpha in the pathogenesis of osteoarthritis. Ann Rheum Dis. 2012;73:1575–1584. doi: 10.1136/annrheumdis-2013-203561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pierer M, Wagner U, Rossol M, Ibrahim S. Toll-like receptor 4 is involved in inflammatory and joint destructive pathways in collagen -induced arthritis in DBA1J mice. PLoS One. 2011;6:e23539. doi: 10.1371/journal.pone.0023539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blanchet MR, Gold M, Maltby S, Bennett J, Petri B, et al. Loss of CD34 leads to exacerbated autoimmune arthritis through increased vascular permeability. J Immunol. 2010;184:1292–1299. doi: 10.4049/jimmunol.0900808. [DOI] [PubMed] [Google Scholar]
- 13.van der Sluijs JA, Geesink RG, van der Linden AJ, Bulstra SK, Kuyer B, et al. The reliability of the Mankin score for osteoarthritis. J Orthop Res. 1992;10:58–61. doi: 10.1002/jor.1100100107. [DOI] [PubMed] [Google Scholar]
- 14.Lai Y, Yu XP, Zhang Y, Tian Q, Song H, et al. Enhanced COMP catabolism detected in serum of patients with arthritis and animal disease models through a novel capture ELISA. Osteoarthritis Cartilage. 2012;20:854–862. doi: 10.1016/j.joca.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsuzaka K, Itami Y, Takeuchi T, Shinozaki N, Morishita T, et al. ADAMTS5 is a biomarker for prediction of response to infliximab in patients with rheumatoid arthritis. J Rheumatol. 2010;37:1454–1460. doi: 10.3899/jrheum.091285. [DOI] [PubMed] [Google Scholar]
- 16.Misumi K, Vilim V, Hatazoe T, Murata T, Fujiki M, et al. Serum level of cartilage oligomeric matrix protein COMP in equine osteoarthritis. Equine Vet J. 2002;34:602–608. doi: 10.2746/042516402776180205. [DOI] [PubMed] [Google Scholar]
- 17.Mansson B, Carey D, Alini M, Ionescu M, Rosenberg LC, et al. Cartilage and bone metabolism in rheumatoid arthritis. Differences between rapid and slow progression of disease identified by serum markers of cartilage metabolism. J Clin Invest. 1995;95:1071–1077. doi: 10.1172/JCI117753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petersson IF, Boegard T, Svensson B, Heinegard D, Saxne T. Changes in cartil age and bone metabolism identified by serum markers in early osteoarthritis of the knee joint. Br J Rheumatol. 1998;37:46–50. doi: 10.1093/rheumatology/37.1.46. [DOI] [PubMed] [Google Scholar]
- 19.Yamada H, Yoneda M, Inaguma S, Watanabe D, Banno S, et al. Infliximab counteracts tumor necrosis factor-alpha-enhanced induction of matrix metalloproteinases that degrade claudin and occludin in non-pigmented ciliary epithelium. Biochem Pharmacol. 2013;85:1770–1782. doi: 10.1016/j.bcp.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 20.van den Berg WB, van Riel PL. Uncoupling of inflammation and destruction in rheumatoid arthritis: myth or reality? Arthritis Rheum. 2005;52:995–999. doi: 10.1002/art.20981. [DOI] [PubMed] [Google Scholar]
- 21.McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7:429–442. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 22.Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- 23.Keffer J, Probert L, Cazlaris H, Georgopoulos S, Kaslaaris E, et al. Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. EMBO J. 1991;10:4025–4031. doi: 10.1002/j.1460-2075.1991.tb04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar S, Blake SM, Emery JG. Intracellular signaling pathways as a target for the treatment of rheumatoid arthritis. Curr Opin Pharmacol. 2001;1:307–313. doi: 10.1016/s1471-4892(01)00054-6. [DOI] [PubMed] [Google Scholar]
- 25.van den Berg WB, Miossec P. IL-17 as a future therapeutic target for rheumatoid arthritis. Nat Rev Rheumatol. 2009;5:549–553. doi: 10.1038/nrrheum.2009.179. [DOI] [PubMed] [Google Scholar]
- 26.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 27.Notley CA, Inglis JJ, Alzabin S, MsCann FE, McNamee KE, et al. Blockade of tumor necrosis factor in collagen-induced arthritis reveals a novel immunoregulatory pathway for Th1 and Th17 cells. J Exp Med. 2008;205:2491–2497. doi: 10.1084/jem.20072707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ziolkowska M, Koc A, Luszczykiewicz G, Ksiezopolska-Pietrzak K, Klimczak E, et al. High levels of IL-17 in rheumatoid arthritis patients: IL-15 triggers in vitro IL-17 production via cyclosporin A-sensitive mechanism. J Immunol. 2000;164:2832–2838. doi: 10.4049/jimmunol.164.5.2832. [DOI] [PubMed] [Google Scholar]
- 29.Luan Y, Kong L, Howell DR, Ilalov K, Fajardo M, et al. Inhibition of ADAMTS-7 and ADAMTS-12 degradation of cartilage oligomeric matri x protein by alpha-2-macroglobulin. Osteoarthritis Cartilage. 2008;16:1413–1420. doi: 10.1016/j.joca.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo F, Lai Y, Tian Q, Lin EA, Kong L, et al. Granulin-epithelin precursor binds directly to ADAMTS-7 and ADAMTS-12 and inhibits their degradation of cartilage oligomeric matrix protein. Arthritis Rheum. 2010;62:2023–2036. doi: 10.1002/art.27491. [DOI] [PMC free article] [PubMed] [Google Scholar]