Abstract
Background
Delay discounting (DD), a decline in the subjective value of reward with increasing delay until its receipt, is an established behavioral model of impulsive choice, a key component of a broader impulsivity construct. Greater DD, i.e. a tendency to choose smaller-immediate over larger-delayed rewards, has been implicated as a potential intermediate phenotype (endophenotype) for addictive disorders and comorbid externalizing psychopathology, particularly in adolescence. However, genetic and environmental origins of DD remain unclear. Accordingly, the goal of the present study was to assess heritability of DD, an important aspect of its utility as an endophenotype.
Methods
A commonly used computerized procedure involving choice between varying amounts of money available immediately and a “standard” amount of $100 presented at variable delays was administered to a population-based sample of twins aged 16 and 18 (n=560, including 134 MZ and 142 DZ pairs). DD was quantified using area under the discounting curve (AUC) and the k coefficient estimated by fitting a hyperbolic model to individual data. Heritability was assessed using linear structural equation modeling of twin data.
Results
The genetic analysis revealed significant heritability of both DD measures (AUC: 46% and 62%; k: 35% and 55% at age 16 and 18, respectively).
Conclusion
The present study provides evidence for heritability of both model-based and model-free DD measures and suggests that DD is a promising intermediate phenotype for genetic dissection of impulsivity and externalizing spectrum disorders.
Keywords: delay discounting, reward, decision making, heritability, endophenotype, twins
Introduction
In their everyday life, humans face numerous decisions where choices have immediate or remote consequences: Go to a party today or stay home and prepare for an exam? Enjoy a nice dessert now or skip it in favor of losing weight in the longer run? Spend money on vacation or put it away for buying a house or retirement? In all of the above examples, decisions involve a choice between a smaller-sooner and larger-later rewards. The subjective value of rewards tends to decline with increasing delay to its receipt, a phenomenon known in behavioral economics as delay discounting (DD). As the temporal distance to larger reward increases, its subjective value decreases until it reaches the subjective value of a smaller but immediate reward (“indifference point”). In more general terms, when the consequences of one's decision are delayed, they are less effective in controlling ongoing behavior (1). Individuals may differ in the degree to which they discount delayed rewards, i.e. how rapidly the value of reward diminishes with increasing time to its delivery.
DD is a ubiquitous phenomenon extensively studied in many living species, including pigeons, mice, rats, and humans. Experimental approaches measuring delay discounting were originally developed in animal studies of operant behavior, where animals chose between e.g. one pellet of food available immediately and two pellets of food delivered after a certain delay (2, 3). In human studies, choice paradigms typically involve hypothetical or real monetary rewards (reviewed in 1). Importantly, DD measures obtained from hypothetical choices correlate strongly with measures obtained from real rewards (4–7).
Studies using repeated measurements of DD in the same individuals have shown that DD measures represent a stable, trait-like characteristic, with estimates of test-retest reliability of DD measures ranging from .55 to .90 (8–14).
Extensive studies in animal models and recent human neuroimaging studies have provided insight into the neural substrates of DD. Animal studies suggest that the choice between immediate and delayed reward critically depends on the orbitofrontal cortex and the core of the nucleus accumbens (reviewed in 15). Human data implicate the left dorsolateral prefrontal cortex (dlPFC), left insula, inferior frontal gyrus (IFG), frontal pole and the anterior cingulate cortex (ACC) as key regions associated with the tendency to choose larger-later rewards (16, 17),while increased ventral striatal activity has been implicated in preference for immediate rewards (18).
Delay discounting is considered a behavioral model of impulsivity, and can be empirically measured using laboratory choice paradigms (19). Substantial evidence indicates that higher propensity to impulsive choice is associated with psychopathology, most notably, addictive disorders, but also with other disorders in which impulsivity is a core behavioral dysfunction, such as externalizing spectrum disorders. In animal studies, preference for smaller-sooner rewards predicted increased susceptibility to substance dependence including alcohol preference and cocaine self-administration (20, 21). Human studies have provided strong evidence for higher rates of DD in individuals with substance use disorders, as is summarized in a recent meta-analysis (22) and several reviews (1, 23–29). Although it remains to be determined whether high DD is a pre-existing risk factor or a consequence of substance abuse, some evidence suggests that heightened DD predates substance use and abuse (30). Furthermore, steeper DD have been shown to predict relapse in smoking cessation trials (31, 32). Indeed, the conceptual relevance of DD to substance abuse is quite straightforward, since the hallmark of the latter is a strong preference for the immediate rewarding effects of drugs and disregard for delayed adverse consequences of one’s decisions. Finally, substantial evidence points to an association between DD and other “unhealthy” behaviors such as overeating, obesity, risky sex, and overall poorer health (reviewed in 33, 34) as well as pathological gambling, compulsive shopping, and financial mismanagement (reviewed in 27).
It has also been suggested that DD may serve as an intermediate phenotype, or endophenotype, in genetic studies of addictive disorders and other externalizing psychopathology in which impulsivity is implicated as a core underlying dysfunction (23, 24, 35, 36). Efforts to identify genes conferring risk for addictive disorders have yielded very modest results so far, which can be attributed largely to the complexity of the phenotype. Shifting the focus of psychiatric genetic research from complex diagnostic phenotypes to relatively discrete and homogenous “component processes” contributing to liability might aid in elucidating the neurobiological and genetic underpinnings of addiction and psychopathology, resolve possible genetic heterogeneity, and clarify the mechanisms of comorbidity. Furthermore, intermediate phenotypes might be helpful in the functional characterization of genetic risk variants being identified in large-scale association studies of psychiatric disorders and thus contribute to bridging the gap between genes and complex phenotypes with which they are associated. Finally, a validation of DD as an intermediate biobehavioral phenotype is directly relevant to the goals of the NIMH-sponsored Research Domain Criteria (RDoC) initiative, the goal of which is to explicate fundamental biobehavioral dimensions that cut across current heterogeneous disorder categories in order to improve the existing classification of psychiatric disorders based on better knowledge of the underlying pathophysiology (37, 38).
Taken together, evidence for the validity of DD as a behavioral model of impulsivity, its test-retest reliability, increasing knowledge of its neural substrates, and evidence for its robust relationship with the externalizing spectrum disorders cited above strongly support the role of DD as an endophenotype for a spectrum of disorders characterized by relative insensitivity to delayed outcomes.
A key requirement for such an intermediate phenotype is its significant heritability. Yet, current knowledge of the relative contribution of genetic factors to inter-individual variation of DD is very limited. Animal studies have demonstrated significant strain differences in DD in rats (39, 40). Furthermore, mice selectively bred for alcohol preference show increased DD rates (41, 42). However, little is known about the heritability of DD in humans. Our recent study of adolescent twins aged 12–14 showed significant genetic influences on DD in humans using a single-choice delay gratification paradigm in which participants were offered a choice between a real amount of $7 available immediately and $10 available in 7 days (35). The heritability of individual differences in the ability to delay gratification was estimated at 30% at age 12 and at 51% at age 14.
However, most clinical studies of DD utilize a different paradigm that provides a quantitative measure of the rate of discounting of hypothetical monetary rewards as a function of their delay. To obtain an individual discounting function, choice options, including both amounts and delay duration, are varied systematically in order to obtain a point of “indifference” between smaller immediate and larger delayed reward at each of the delays. This laboratory procedure has been a standard in DD research, and most of the evidence for association with psychopathology cited above was obtained using this method (1). However, little is known about heritability of this DD measure. Furthermore, generalization of data obtained in younger adolescents to the older age is problematic due to the possibility of significant changes in heritability in the course of development.
Accordingly, the aim of the present study was to estimate the heritability of DD, i.e. the extent to which observed inter-individual variability in the rate of discounting of delayed rewards is determined by genetic factors.
Material and Methods
Sample
Participants were adolescent twins (134 MZ and 142 DZ pairs, n=560, 50.7% females, including 84% Caucasian, 12% Black, and 4% other minorities). One hundred eighty-three participants were first tested at mean age (±s.d.) of 16.6±.26 years, with 126 of them (34 MZ and 28 DZ pairs) retested at a mean age of 18.5±.21years. An additional 377 participants were first tested at mean age of 18.7±.37years. All participants were recruited from the local population using the state birth records database, therefore, the sample is largely representative of the general population with respect to the distribution of socioeconomic status (Hollingshead occupational score for parents, 9-point: M=5.6±1.9) and general intelligence (Raven’s Standard Progressive Matrices: median score 47, corresponding to 50th percentile, IQ=100 according to U.S. norms). Zygosity was determined using a set of 160 DNA markers, an interview administered to the twins' parents, and research assistants' ratings of twins' physical similarity. Subjects with a history of serious head trauma or health conditions precluding a laboratory visit or the ability to perform the experimental tasks (e.g. severe visual impairment or mental retardation) were excluded. The study was approved by Washington University Institutional Review Board, and written informed assent and consent were obtained from adolescent participants and their parents, respectively, after complete description of the study to the subjects and their parents.
Discounting task
We used a computerized delay discounting task described in previous studies (43, 44). In this task, participants were presented with a series of hypothetical choices between varying amounts of money available immediately and a “standard” amount of $100 presented at variable delays. Questions and response options were presented on a computer screen, and participants used the computer mouse to choose a response option. On each of the 138 trials of this task, participants were presented with a question: “At this moment, what would you prefer?” with two choice options displayed underneath the question, e.g. “$100 in 90 days” and “$70 now”. One option was a standard amount of $100 available after one of six delays: 0, 7, 30, 90, 180, or 365 days. The other option was an amount of money varying from $0 to $105 that was available immediately. For each question, a pair of immediate and delayed amounts was selected at random without replacement. The order in which the immediate and delayed amount was presented (first or second in the pair) was varied randomly. Further details of the procedure can be found elsewhere (43).
Discounting measures
For each of the 6 delays of the standard amount ($100), an indifference (switch) point was determined as being midway between the smallest value of the immediate alternative accepted and the largest value of the same alternative that was rejected, i.e. the value of immediate reward at which the participant was indifferent between the immediately available amount and the delayed standard amount (43). This amount may be viewed as the “subjective value” of the delayed standard amount, e.g. indifference point of $65 at 180 days would mean that, for a given individual, $100 to be received in 180 days is subjectively worth only $65. Next, we built an empirical discount curve for each participant by plotting indifference values against the corresponding delays and then computed area under the curve (AUC) as a quantitative measure of DD. Smaller AUC values indicate a steeper discounting function, i.e. greater tendency for preference of smaller immediate rewards over larger but delayed rewards. The advantage of the AUC measure over parametric model-based measures such as hyperbolic or exponential functions is that it does not make any assumptions about the form of the discounting function (45). To maintain continuity with previous literature, we also fit the hyperbolic function to individual subject data points using the following formula: V=A/(1+kD), where V is subjective value of delayed reward, A is the standard amount offered at different delays, and D is delay (46) and used the estimated k coefficient as additional measure of DD in genetic analyses.
Statistical analyses
To estimate heritability, i.e. the relative contribution of genetic and environmental sources to the total phenotypic variance of DD-AUC, we fit linear structural equation models using the Mx package (49), a standard approach in twin genetic research (47, 48). These models assume that phenotypic variance arises from the following factors: additive genetic influences (A), non-additive genetic influences (D) including within-locus allelic interaction (dominance) and between-locus interaction (epistasis), environmental influences shared by family members (C), and individually unique (unshared) environmental influences (E). It is important to note that A, D, and C increase, whereas E decreases, intrapair twin similarity. When using only data from twin pairs reared together, it is only possible to test three of these four components simultaneously, and a decision regarding whether to test an ADE or an ACE model is made based upon the observed twin correlations (see 48).
Heritability was estimated as the percentage of the total variance of the trait attributable to genetic factors. We fit a bivariate Cholesky model (see (47)) with measurements taken at ages 16 and 18 entered as separate variables. In a longitudinal analysis, the bivariate model permits the estimation of both the genetic and environmental influences at each age and the correlations between genetic and environmental factors across ages, which is important for examining whether the same or different genetic and environmental factors influence the trait at different ages.
Path coefficients were estimated using the method of maximum likelihood, and the goodness of model fit was indicated by −2 times the log likelihood (−2LL). As described elsewhere (47, 50), the fit of nested submodels was tested by dropping individual paths from the full model, with the significance of individual paths tested by comparing the fit of the restricted submodel with the fit of the more general model using a χ2 test with degrees of freedom corresponding to the difference in the degrees of freedom between two models (e.g., df=1 if only one parameter is dropped in the restricted model). If dropping a path significantly reduced the goodness of fit (the change in χ2 was significant), the path was retained in the model, otherwise the more parsimonious model was chosen (i.e. the one that accounted for the variance equally well, but with a fewer number of parameters). The fit of the nested submodels was also assessed through Akaike’s Information Criterion (AIC, where AIC=χ2 − 2df; see (47)). Lower AIC values indicate better fit.
Previous studies have shown that some individuals may demonstrate atypical response patterns in DD tasks such as failing to show a monotonous decrease in indifference points with increasing delays or insensitivity to delay (persistently choosing the larger reward), suggesting misunderstanding of instructions, inattention, or low motivation (19). Therefore, we repeated the above analyses after excluding subjects with nonsystematic response patterns following the approach suggested by Johnson and Bickel (19). Individuals were excluded from follow-up analyses if any indifference point was larger than the preceding point by greater than 20% or if the last indifference point was not less than the first one by at least 5% (the criteria were modified to account for substantially shorter time span in the present study).
Results
Indifference points and empirical discounting curves for both age groups are presented in the Supplementary Online Figure. Fitting the hyperbolic function to the group data showed a moderately good fit (mean R2=.78 and .76, with median k-coefficient estimates of .0035 and .003 for ages 16 and 18, respectively). The k values showed a skewed distribution and were therefore log-transformed for further analyses.
The phenotypic correlation between the two DD measurements (test-retest reliability) was r=.67 and .57 for the AUC and log k (both p<.001), respectively, suggesting longitudinal stability of the DD measure.
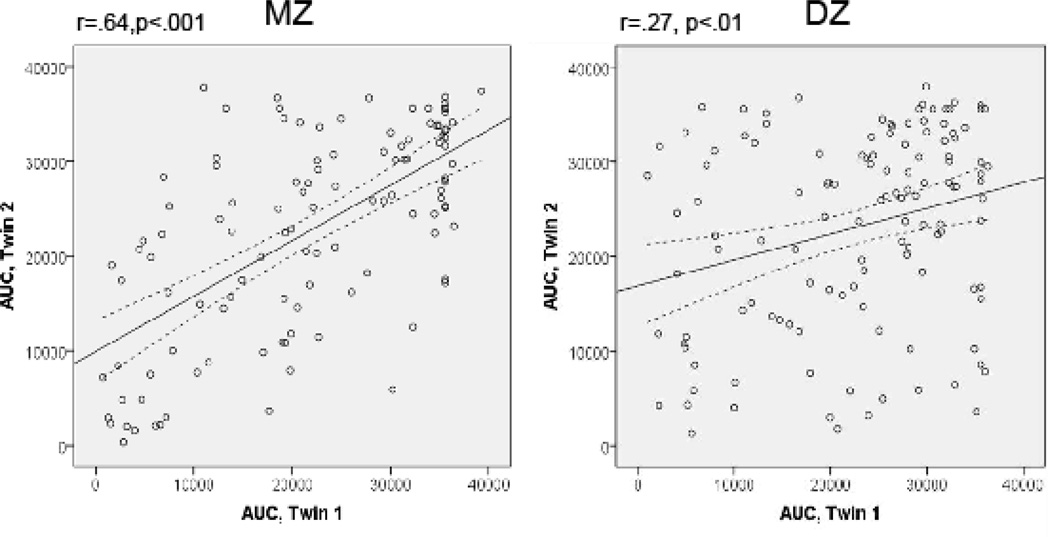
Intrapair twin correlations for DD measures are presented in Table 1 (see also scatterplots in Fig. 1). At both ages and for both DD variables, the DZ correlations were less than one-half of the MZ correlations, suggesting non-additive genetic factors might be important. MZ and DZ twins did not differ significantly with respect to the mean values of AUC and k at any age, consistent with a basic assumption of the twin method. Initially, a full Cholesky ADE model was tested, allowing for additive genetic, non-additive genetic, and non-shared environmental influences at each time as well as overlap across occasions. For the AUC, this model could be reduced to an AE model (Δχ2 = 0.175, 3df, AIC = −5.825, comparing AE to ADE), indicating that non-additive genetic influences were not statistically significant. However a model with no genetic influences (E only) was rejected (Δχ2 = 74.900, 3df, p < .001 AIC = 68.90, comparing E to AE), indicating that DD-AUC is heritable.
Table 1.
Intra-pair twin correlations for delay discounting measures and heritability estimates based on the final bivariate Cholesky model.
| DD Measure | Age | Twin correlations | Proportions of variance | ||
|---|---|---|---|---|---|
| rmz (n) | rdz (n) | a2 (95% CI) | e2 (95% CI) | ||
| AUC | 16 | .45* (50) | .16 (39) | .46 (.28 – .62) | .54 (.38 – .72) |
| 18 | .64* (113) | .27* (125) | .62 (.51 – .70) | .38 (.30 – .49) | |
| log k | 16 | .40* (50) | .03 (38) | .35 (.17 – .52) | .65 (.48 – .83) |
| 18 | .57** (108) | .23* (124) | .55 (.43 – .66) | .45 (.34 – .57) | |
| AUC (Ex) | 16 | .39* (39) | .16 (28) | .35 (.14 – .56) | .65 (.44 – .86) |
| 18 | .64** (69) | .31* (82) | .63 (.50 – .73) | .37 (.27 – .50) | |
| log k (Ex) | 16 | .34 (32) | −.06 (28) | .21 (.05 – .43) | .79 (.57 – .95) |
| 18 | .61** (68) | .27* (82) | .62 (.47 – .73) | .38 (.27 – .53) | |
AUC is area under the discounting curve; log k is log10 of the k-coefficient estimated by fitting a hyperbolic model to individual data; “Ex” indicates re-analysis of data after the exclusion of subjects with “nonsystematic” response patterns. rmz and rdz are intrapair twin correlations showing the degree of twins’ resemblance with respect to DD measures (numbers of twin are shown in brackets); a2=proportion of variance in DD measures attributable to additive genetic influences (heritability); e2=proportion of variance attributable to non-shared environmental influences including measurement error (95% confidence intervals are shown in brackets).
Significance of twin correlations:
p<.05;
p<.01.
Figure 1.
Scatterplots of intra-pair twin correlations for delay discounting in monozygotic (MZ) and dizygotic (DZ) twin pairs. Each circle represents a twin pair, such that delay discounting value of Twin 1 (areaunder the discounting curve) is plotted against the corresponding value of Twin 2. Solid lines indicate linear regression of Twin 1 on Twin 2. Broken lines indicate 95% confidence intervals.
Similar results were observed for the log k measure: the base model was an ADE model, the ADE model could be reduced to an AE model (Δχ2 = .0.122, 3df, AIC = −5.878), and a model with no genetic influences (E only) was rejected (Δχ2 = 54.284, 3df, AIC = 48.284). Thus, these models also indicated that the k measure was heritable.
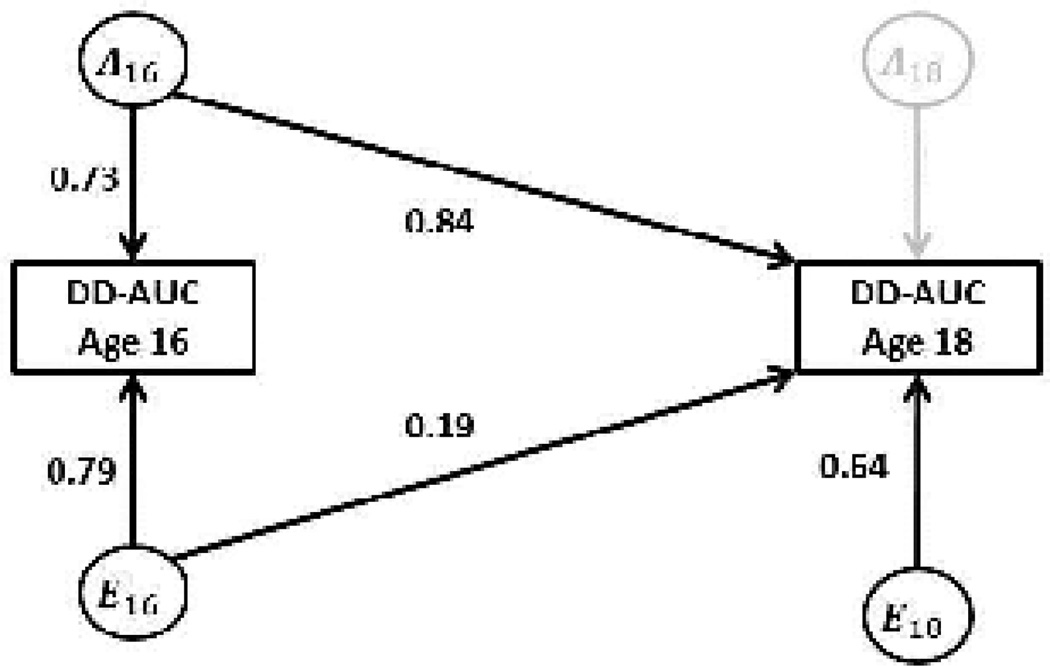
The AE model for AUC could be further reduced by deleting genetic influences specific to age 18, yielding a model with a single genetic factor that contributed to both age 16 and age 18 (i.e., genetic correlation rG=1.00; Δχ2 = 0.000, 1df, AIC = −2.00 for the single factor model compared to the prior AE model). The unstandardized parameter estimates for this model are presented in Figure 2. Estimates of genetic and environmental variance components under this model are presented in Table 1. The results indicate substantial heritability of the DDT-AUC measure: 46% (95% CI: 28–62%) at age 16 and 62% (95% CI: 51–70%) at age 18. In this model, 80% of the test-retest stability was attributable to genetic factors, indicating that most of the “stable” inter-individual variance could be attributed to common genetic factors operating at both ages (Table 2). The AE model for the k measure could also be further reduced to one with a single genetic factor for ages 16 and 18 (rG=1.00; Δχ2 = 0.000, 1df, AIC = −2.00).
Figure 2.
Unstandardized parameter estimates for the final model for delay discounting (area under the curve) at ages 16 and 18 years. A=additive genetic influences, E=nonshared environmental influences. Only one twin is shown for simplicity. Parameter estimates were equated for members of a twin pair, with the additive genetic factors connecting the members of a twin pair. The path connecting MZ twins was fixed to 1.0 and the path connecting DZ twins was fixed to 0.5, per quantitative genetic theory.
Table 2.
Longitudinal cross-age genetic and environmental correlations for delay discounting measures.
| DD Measure | Genetic and environmental correlations |
Proportions of cross-age covariance attributable to |
||
|---|---|---|---|---|
| rG (16–18) | rE (16–18) | A | E | |
| AUC | 1.0 | .29 (.05 – .49) | .80 (.61 – .97) | .20 (.03 – .39) |
| log k | 1.0 | .25 (.03 – .45) | .76 (.53 – .97) | .24 (.03 – .47) |
| AUC (Ex) | 1.0 | .53 (.25 – .73) | .65 (.41 – .85) | .35 (.15 – .59) |
| log k (Ex) | 1.0 | .51 (.22 – .74) | .56 (.27 – .82) | .44 (.18 – .73) |
rG = genetic correlation which is equal to 1.00 because only one genetic factor operating at both ages was retained in the final model, while additional age-specific genetic factors could be dropped without significant deterioration of model fit; rE=non-shared environmental correlation. rG and rE show the extent of the overlap between genetic and environmental influences, respectively, on the DD measures at ages 16 and 18. The last two columns show what proportion of cross-age correlation (i.e. longitudinal stability) can be attributed to genetic or environmental factors operating at both ages. Confidence intervals (95%) are shown in brackets.
AUC showed modest but significant correlations with general cognitive abilities assessed using Raven’s Standard Progressive Matrices (SPM): r=0.17 (p<.05) and r=0.29 (p<.01) at age 16 and 18, respectively.
Discussion
The results indicate a strong heritability of delay discounting (DD) as measured using a hypothetical money choice procedure, a widely utilized laboratory paradigm for the assessment of inter-temporal choice behavior in human studies. Importantly, both model-free (AUC) and model-based (k coefficient) measures showed significant heritability. Furthermore, a complete overlap between genetic factors influencing DD at age 16 and 18 suggests that for both measures the same genes influenced DD at different time points. For the AUC measure, age-specific heritability estimates suggested an increase in the strength of genetic influences over the two-year period (from 46% to 62%), however, it was non-significant in the present sample (Δχ2(1) = 2.943, n. s.). A generalized estimate of heritability for the entire sample was 57% (95% CI: 47–66%), suggesting that most of the observed individual differences in the degree of DD can be attributed to genetic factors. In contrast, for the k coefficient, the model with different heritabilities at ages 16 and 18 showed better fit, suggesting a significant increase in heritability of this model-based measure with age (i.e., although same genes influenced DD at both ages, they accounted for a larger proportion of the variance at age 18 for the log k measure; Δχ2(1) = 3.930, p < 0.05 when k coefficient heritability estimates were equated for ages 16 and 18).
The exclusion of nonsystematic data (19) resulted in a smaller sample size, but did not lead to substantial changes in heritability estimates. Assuming that nonsystematic data represent a random noise, one could expect that the inclusion of participants with atypical response pattern would lead to a marked decrease in heritability. However, this was not the case because twins tended to show resemblance with respect to atypical response patterns. Interestingly, a recent study in patients with schizophrenia suggests that nonsystematic responding can be a clinically relevant feature indicating an executive dysfunction such as inability to maintain the mental representation of previous choices made during the task (51).
The present finding of significant heritability of a quantitative measure of DD in late adolescence is consistent with our previous finding of significant genetic influences on DD assessed using a single-choice, real money test in early adolescence (ages 12 and 14) (35). Although different tests were used to assess DD in these two studies, conceptually, they both map onto the same construct, the propensity to discount delayed rewards. Together, data from these two studies converge to suggest that temporal discounting behavior throughout most of the adolescent period (12–18 years of age) is significantly influenced by genetic factors. These findings are also consistent with evidence obtained in animal studies suggesting genetic influences on DD (39, 41, 42). A recent study using eight inbred rat strains estimated heritability of an AUC measure of DD at 50% (40).
The present results strongly suggest that individual differences in the extent to which individuals tend to discount delayed consequences of their actions in favor of immediate rewards are substantially influenced by genetic factors. This finding has important implications for the understanding of the etiological pathways to a range of abnormal behaviors such as ADHD, conduct disorder, and substance use disorders. These conditions are heritable, show a high degree of comorbidity, and share a common dysfunction, namely, abnormally high levels of impulsivity. Evidence for the heritability of the propensity to impulsive choice in the general population obtained in the present study is consistent with the idea that genetic influences on “externalizing spectrum” disorders can be at least in part mediated by genetically transmitted abnormalities in the neural mechanisms of inter-temporal decision making. Thus, the present results provide further support for the notion that DD may serve as a suitable intermediate phenotype (endophenotype) in genetic studies of addictions and other psychopathology (23, 24, 35).
However, genetic specificity of DD remains an important issue. It is not clear whether strong heritability of DD found in the present study represents genetic influences specific to inter-temporal decision making, or these genetic factors are shared with (or mediated by) other aspects of cognitive function and reward-related processes. In the present study, DD was significantly associated with general intelligence, although the correlations were modest. Increasing evidence suggests that steep DD may be associated with executive dysfunction (51–53). Future genetic studies should directly address the question whether DD and other cognitive functions are influenced by common (overlapping) or specific genetic factors.
Substantial evidence indicates steeper DD in substance abusers (22–24, 36). An important question for future research would be to examine the role of genetic factors and establish the direction of causality in this relationship. Elevated DD can predate substance use and thus constitute a heritable risk factor; conversely, substance abuse, especially during adolescence, may adversely affect the development of brain circuits underlying decision making and lead to higher discounting rates. So far, available evidence tends to support the former explanation (30, 35), however, future research using longitudinal and discordant twin designs should disentangle the causal relationships between impulsive choice, other aspects of cognitive function, and psychopathology.
Behavioral neuroscience research using animal models (15) and neuroimaging studies in humans have identified neural substrates that play a key role in inter-temporal choice behavior including regions sensitive to reward value such as ventral striatum and regions involved in planning and prospective thought including areas of the prefrontal and posterior cingulate cortex (16, 17, 54, 55). Future research using neuroimaging techniques and genetically informative research designs should delineate specific neuroanatomical and neurochemical substrates that mediate genetic influences on individual differences in inter-temporal choice.
Another important question is what specific genes account for heritability of DD. Several recent candidate gene studies (56–59) have reported associations between dopamine-related genetic variants and DD. However, because these studies were based on modest samples (19 to 166 participants), included a mixture of clinical and non-clinical sample population, and yielded conflicting results, these association findings need to be interpreted with caution until they are replicated in larger independent samples.
Several limitations of the present study are worth noting. The present analysis included data for only a limited age range of 16–18 years, and it remains to be seen whether genetic influences on DD might change during the transition from late adolescence to adulthood. The results of bivariate longitudinal analysis must be interpreted with caution because the modest sample size might have limited the power to detect possible age-related changes in heritability.
In conclusion, the present study provides the first evidence for heritability of both parametric and non-parametric (model-free) quantitative measures of DD and suggests that DD is a promising intermediate phenotype for genetic research of impulse-control disorders, including addiction and frequently comorbid conditions such as conduct disorder and ADHD.
Supplementary Material
Acknowledgements
This work was supported by grants DA018899 and DA027096 from the National Institutes of Health (NIH) to A.A. The authors are grateful to Dr. Suzanne Mitchell for her advice on implementation of a computerized test of delay discounting. The authors acknowledge organizational and technical assistance by Tara Tinnin, MSW, Olga Novak, and other project staff. The authors also acknowledge the generous giving of time and effort by the study participants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Reynolds B. A review of delay-discounting research with humans: relations to drug use and gambling. Behav Pharmacol. 2006;17:651–667. doi: 10.1097/FBP.0b013e3280115f99. [DOI] [PubMed] [Google Scholar]
- 2.Ainslie G. Specious reward: a behavioral theory of impulsiveness and impulse control. Psychol Bull. 1975;82:463–496. doi: 10.1037/h0076860. [DOI] [PubMed] [Google Scholar]
- 3.Monterosso J, Ainslie G. Beyond discounting: possible experimental models of impulse control. Psychopharmacology (Berl) 1999;146:339–347. doi: 10.1007/pl00005480. [DOI] [PubMed] [Google Scholar]
- 4.Johnson MW, Bickel WK. Within-subject comparison of real and hypothetical money rewards in delay discounting. J Exp Anal Behav. 2002;77:129–146. doi: 10.1901/jeab.2002.77-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madden GJ, Begotka AM, Raiff BR, Kastern LL. Delay discounting of real and hypothetical rewards. Exp Clin Psychopharmacol. 2003;11:139–145. doi: 10.1037/1064-1297.11.2.139. [DOI] [PubMed] [Google Scholar]
- 6.Madden GJ, Raiff BR, Lagorio CH, Begotka AM, Mueller AM, Hehli DJ, et al. Delay discounting of potentially real and hypothetical rewards: II. Between- and within-subject comparisons. Exp Clin Psychopharmacol. 2004;12:251–261. doi: 10.1037/1064-1297.12.4.251. [DOI] [PubMed] [Google Scholar]
- 7.Matusiewicz AK, Carter AE, Landes RD, Yi R. Statistical equivalence and test-retest reliability of delay and probability discounting using real and hypothetical rewards. Behav Processes. 2013;100:116–122. doi: 10.1016/j.beproc.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker F, Johnson MW, Bickel WK. Delay discounting in current and never-before cigarette smokers: similarities and differences across commodity, sign, and magnitude. J Abnorm Psychol. 2003;112:382–392. doi: 10.1037/0021-843x.112.3.382. [DOI] [PubMed] [Google Scholar]
- 9.Beck RC, Triplett MF. Test-retest reliability of a group-administered paper-pencil measure of delay discounting. Exp Clin Psychopharmacol. 2009;17:345–355. doi: 10.1037/a0017078. [DOI] [PubMed] [Google Scholar]
- 10.Johnson MW, Bickel WK, Baker F. Moderate drug use and delay discounting: a comparison of heavy, light, and never smokers. Exp Clin Psychopharmacol. 2007;15:187–194. doi: 10.1037/1064-1297.15.2.187. [DOI] [PubMed] [Google Scholar]
- 11.Kirby KN. One-year temporal stability of delay-discount rates. Psychon Bull Rev. 2009;16:457–462. doi: 10.3758/PBR.16.3.457. [DOI] [PubMed] [Google Scholar]
- 12.Ohmura Y, Takahashi T, Kitamura N, Wehr P. Three-month stability of delay and probability discounting measures. Exp Clin Psychopharmacol. 2006;14:318–328. doi: 10.1037/1064-1297.14.3.318. [DOI] [PubMed] [Google Scholar]
- 13.Simpson CA, Vuchinich RE. Reliability of a measure of temporal discounting. Psychol Rec. 2000;50:3–16. [Google Scholar]
- 14.Smits RR, Stein JS, Johnson PS, Odum AL, Madden GJ. Test-retest reliability and construct validity of the Experiential Discounting Task. Exp Clin Psychopharmacol. 2013;21:155–163. doi: 10.1037/a0031725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalley JW, Mar AC, Economidou D, Robbins TW. Neurobehavioral mechanisms of impulsivity: fronto-striatal systems and functional neurochemistry. Pharmacol Biochem Behav. 2008;90:250–260. doi: 10.1016/j.pbb.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 16.Luo S, Ainslie G, Pollini D, Giragosian L, Monterosso JR. Moderators of the association between brain activation and farsighted choice. NeuroImage. 2012;59:1469–1477. doi: 10.1016/j.neuroimage.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peters J, Buchel C. The neural mechanisms of inter-temporal decision-making: understanding variability. Trends in cognitive sciences. 2011;15:227–239. doi: 10.1016/j.tics.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Hariri AR, Brown SM, Williamson DE, Flory JD, de Wit H, Manuck SB. Preference for immediate over delayed rewards is associated with magnitude of ventral striatal activity. J Neurosci. 2006;26:13213–13217. doi: 10.1523/JNEUROSCI.3446-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson MW, Bickel WK. An algorithm for identifying nonsystematic delay-discounting data. Exp Clin Psychopharmacol. 2008;16:264–274. doi: 10.1037/1064-1297.16.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verdejo-Garcia A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci Biobehav Rev. 2008;32:777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology (Berl) 2008;200:1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- 22.MacKillop J, Amlung MT, Few LR, Ray LA, Sweet LH, Munafo MR. Delayed reward discounting and addictive behavior: a meta-analysis. Psychopharmacology (Berl) 2011;216:305–321. doi: 10.1007/s00213-011-2229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bickel WK, Koffarnus MN, Moody L, Wilson AG. The behavioral- and neuro-economic process of temporal discounting: A candidate behavioral marker of addiction. Neuropharmacology. 2014;76 Pt B:518–527. doi: 10.1016/j.neuropharm.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mackillop J. Integrating behavioral economics and behavioral genetics: delayed reward discounting as an endophenotype for addictive disorders. J Exp Anal Behav. 2013;99:14–31. doi: 10.1002/jeab.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 26.Crews FT, Boettiger CA. Impulsivity, frontal lobes and risk for addiction. Pharmacol Biochem Behav. 2009;93:237–247. doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamilton KR, Potenza MN. Relations among delay discounting, addictions, and money mismanagement: implications and future directions. Am J Drug Alcohol Abuse. 2012;38:30–42. doi: 10.3109/00952990.2011.643978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogers RD, Moeller FG, Swann AC, Clark L. Recent research on impulsivity in individuals with drug use and mental health disorders: implications for alcoholism. Alcohol Clin Exp Res. 2010;34:1319–1333. doi: 10.1111/j.1530-0277.2010.01216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bickel WK, Yi R. Temporal discounting as a measure of executive function: insights from the competing neuro-behavioral decision system hypothesis of addiction. Adv Health Econ Health Serv Res. 2008;20:289–309. [PubMed] [Google Scholar]
- 30.Audrain-McGovern J, Rodriguez D, Epstein LH, Cuevas J, Rodgers K, Wileyto EP. Does delay discounting play an etiological role in smoking or is it a consequence of smoking? Drug Alcohol Depend. 2009;103:99–106. doi: 10.1016/j.drugalcdep.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacKillop J, Kahler CW. Delayed reward discounting predicts treatment response for heavy drinkers receiving smoking cessation treatment. Drug Alcohol Depend. 2009;104:197–203. doi: 10.1016/j.drugalcdep.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krishnan-Sarin S, Reynolds B, Duhig AM, Smith A, Liss T, McFetridge A, et al. Behavioral impulsivity predicts treatment outcome in a smoking cessation program for adolescent smokers. Drug Alcohol Depend. 2007;88:79–82. doi: 10.1016/j.drugalcdep.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Story GW, Vlaev I, Seymour B, Darzi A, Dolan RJ. Does temporal discounting explain unhealthy behavior? A systematic review and reinforcement learning perspective. Frontiers in behavioral neuroscience. 2014;8:76. doi: 10.3389/fnbeh.2014.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Epstein LH, Salvy SJ, Carr KA, Dearing KK, Bickel WK. Food reinforcement, delay discounting and obesity. Physiol Behav. 2010;100:438–445. doi: 10.1016/j.physbeh.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anokhin AP, Golosheykin S, Grant JD, Heath AC. Heritability of Delay Discounting in Adolescence: A Longitudinal Twin Study. Behav Genet. 2011;41:175–183. doi: 10.1007/s10519-010-9384-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitchell SH. The genetic basis of delay discounting and its genetic relationship to alcohol dependence. Behav Processes. 2011;87:10–17. doi: 10.1016/j.beproc.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 38.Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC medicine. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilhelm CJ, Mitchell SH. Strain differences in delay discounting using inbred rats. Genes Brain Behav. 2009;8:426–434. doi: 10.1111/j.1601-183X.2009.00484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richards JB, Lloyd DR, Kuehlewind B, Militello L, Paredez M, Solberg Woods L, et al. Strong genetic influences on measures of behavioral-regulation among inbred rat strains. Genes Brain Behav. 2013;12:490–502. doi: 10.1111/gbb.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mitchell SH, Reeves JM, Li N, Phillips TJ. Delay discounting predicts behavioral sensitization to ethanol in outbred WSC mice. Alcohol Clin Exp Res. 2006;30:429–437. doi: 10.1111/j.1530-0277.2006.00047.x. [DOI] [PubMed] [Google Scholar]
- 42.Oberlin BG, Grahame NJ. High-Alcohol Preferring Mice Are More Impulsive Than Low-Alcohol Preferring Mice as Measured in the Delay Discounting Task. Alcoholism-Clinical and Experimental Research. 2009;33:1294–1303. doi: 10.1111/j.1530-0277.2009.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitchell SH. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology (Berl) 1999;146:455–464. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- 44.Mitchell SH, Wilson VB. The subjective value of delayed and probabilistic outcomes: Outcome size matters for gains but not for losses. Behav Processes. 2010;83:36–40. doi: 10.1016/j.beproc.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Myerson J, Green L, Warusawitharana M. Area under the curve as a measure of discounting. J Exp Anal Behav. 2001;76:235–243. doi: 10.1901/jeab.2001.76-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mazur JE. An adjusting procedure for studying delayed reinforcement. In: Commons ML, Mazur JE, Nevin JA, Rachlin H, editors. Qualitative Analyses of Behavior. Hillsdale, NJ: Erlbaum; 1987. pp. 55–73. [Google Scholar]
- 47.Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Dordrecht: Kluwer Academic Publishers; 1992. [Google Scholar]
- 48.Rijsdijk FV, Sham PC. Analytic approaches to twin data using structural equation models. Brief Bioinform. 2002;3:119–133. doi: 10.1093/bib/3.2.119. [DOI] [PubMed] [Google Scholar]
- 49.Neale MC, Boker SM, Xie G, Maes HH. Mx:Statistical Modeling. 6th Edition ed. Richmond, VA: Department of Psychiatry; 2002. [Google Scholar]
- 50.Sham P. Statistics in Human genetics. New York: Oxford University Press; 1998. [Google Scholar]
- 51.Weller RE, Avsar KB, Cox JE, Reid MA, White DM, Lahti AC. Delay discounting and task performance consistency in patients with schizophrenia. Psychiatry Res. 2014;215:286–293. doi: 10.1016/j.psychres.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bickel WK, Jarmolowicz DP, Mueller ET, Koffarnus MN, Gatchalian KM. Excessive discounting of delayed reinforcers as a trans-disease process contributing to addiction and other disease-related vulnerabilities: emerging evidence. Pharmacol Ther. 2012;134:287–297. doi: 10.1016/j.pharmthera.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin H, Epstein LH. Living in the moment: effects of time perspective and emotional valence of episodic thinking on delay discounting. Behav Neurosci. 2014;128:12–19. doi: 10.1037/a0035705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- 55.Carter RM, Meyer JR, Huettel SA. Functional neuroimaging of intertemporal choice models: A review. Journal of Neuroscience, Psychology, and Economics. 2010;3:27–45. [Google Scholar]
- 56.Eisenberg DT, Mackillop J, Modi M, Beauchemin J, Dang D, Lisman SA, et al. Examining impulsivity as an endophenotype using a behavioral approach: a DRD2 TaqI A and DRD4 48-bp VNTR association study. Behav Brain Funct. 2007;3:2. doi: 10.1186/1744-9081-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.White MJ, Lawford BR, Morris CP, Young RM. Interaction between DRD2 C957T polymorphism and an acute psychosocial stressor on reward-related behavioral impulsivity. Behav Genet. 2009;39:285–295. doi: 10.1007/s10519-008-9255-7. [DOI] [PubMed] [Google Scholar]
- 58.Boettiger CA, Mitchell JM, Tavares VC, Robertson M, Joslyn G, D'Esposito M, et al. Immediate reward bias in humans: Fronto-parietal networks and a role for the catechol-O-methyltransferase 158(Val/Val) genotype. J Neurosci. 2007;27:14383–14391. doi: 10.1523/JNEUROSCI.2551-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paloyelis Y, Asherson P, Mehta MA, Faraone SV, Kuntsi J. DAT1 and COMT effects on delay discounting and trait impulsivity in male adolescents with attention deficit/hyperactivity disorder and healthy controls. Neuropsychopharmacology. 2010;35:2414–2426. doi: 10.1038/npp.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lagorio CH, Madden GJ. Delay discounting of real and hypothetical rewards III: steady-state assessments, forced-choice trials, and all real rewards. Behav Processes. 2005;69:173–187. doi: 10.1016/j.beproc.2005.02.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.