Abstract
We have reported a set of electrokinetically pumped sheath flow nanoelectrospray interfaces to couple capillary zone electrophoresis with mass spectrometry. A separation capillary is threaded through a cross into a glass emitter. A side arm provides fluidic contact with a sheath buffer reservoir that is connected to a power-supply. The potential applied to the sheath buffer drives electro-osmosis in the emitter to pump the sheath fluid at nanoliter/minute rates. Our first generation interface placed a flat-tipped capillary in the emitter. Sensitivity was inversely related to orifice size and to the distance from the capillary tip to the emitter orifice. A second generation interface used a capillary with an etched tip that allowed the capillary exit to approach within a few hundred micrometers of the emitter orifice, resulting in a significant increase in sensitivity. In both the first and second-generation interfaces, the emitter diameter was typically 8-μm; these narrow orifices were susceptible to plugging and tended to have limited lifetime. We now report a third-generation interface that employs a larger diameter emitter orifice with very short distance between the capillary tip and the emitter orifice. This modified interface is much more robust and produces much longer lifetime than our earlier designs with no loss in sensitivity. We evaluated the third-generation interface for a 5,000-min (127 runs, 3.5 days) repetitive analysis of bovine serum albumin digest using an uncoated capillary. We observed a 10% relative standard deviation in peak area, an average of 160,000 theoretical plates, and very low carry-over (much less than 1%). We employed a linear-polyacrylamide (LPA) coated capillary for single-shot, bottom-up proteomic analysis of 300 ng of Xenopus laevis fertilized egg proteome digest, and identified 1,249 protein groups and 4,038 peptides in a 110 min separation using an LTQ-Orbitrap Velos mass spectrometer; peak capacity was ~330. The proteome dataset using this third generation interface based CZE-MS/MS is similar in size to that generated using a commercial ultra-performance liquid chromatographic analysis of the same sample with the same mass spectrometer and similar analysis time.
Keywords: Capillary electrophoresis, bottom-up proteomics, Xenopus laevis, developmental proteomics
INTRODUCTION
Capillary zone electrophoresis-electrospray ionization-mass spectrometry (CZE-ESI-MS) is attracting renewed attention as a tool for proteomics research.[1–3] Several innovations have driven this renaissance. In particular, much effort has been devoted to the development of robust and sensitive electrospray interfaces.[4,5] Moini reported a sheathless interface in 2007,[6] which employed a porous capillary tip as the nanospray emitter. That interface system has been used by several research groups for bottom-up (shot-gun) and top-down proteomics.[7–10] As an important example, the Yates group coupled CZE to an Orbitrap Elite mass spectrometer through a sheathless capillary electrophoresis-electrospray ionization interface for top-down profiling of the Pyrococcus furiosus proteome; 134 proteins and 291 proteoforms were identified in 120 min of analysis time, which represents the largest top-down proteome dataset produced to date by CE-MS/MS.[8]
A number of other interfaces have been reported that couple CZE with mass spectrometry. Chen’s group reported a flow-through microvial interface in 2010[11], which has been used for metabolite,[12] glycan,[13] and intact protein[14] analysis. Tang’s group recently reported a sheathless CE-MS interface combining a large inner diameter separation capillary and a detachable small inner diameter porous ESI emitter.[15] This design produced a large sample loading volume and stable nanoESI operation, which significantly improved the concentration detection limit for peptides.
In 2010 and 2013, this group reported two electro-kinetically pumped sheath-flow nanospray CE-MS interfaces, Figure 1.[16,17] In these interfaces, the separation capillary is threaded through a plastic cross into a glass emitter. The emitter is filled with a sheath buffer, and a tube connects a side arm of the cross to a sheath reservoir. The reservoir is held at ~1,000 V potential; this potential drives electroosmotic flow in the glass emitter, pumping the sheath fluid at nanoliters/minute through the interface while also generating an electrospray. We have used the electrokinetically-pumped nanospray interface for the first example of CZE-MS/MS based shot-gun proteomic analysis of a complex proteome.[18] We later reported over 1,250 peptide identifications (IDs) from the E. coli proteome via a single-shot 50-min CZE-MS/MS analysis.[19] We also used this first-generation nanospray system for the first multiple reaction monitoring experiment using CZE-MS;[20] we obtained a ~300-zmole peptide detection limit for a standard peptide spiked in bovine serum albumin tryptic digest background.[20] We also generated confident and reproducible detection of three high abundant proteins from 100 pg of a RAW 264.7 cell proteome digest.[21] Finally, Kelleher’s group used the first-generation interface for the top-down identification of 30 proteins with a mass range of 30–80 kDa from P. aeruginosa whole cell lysate.[22]
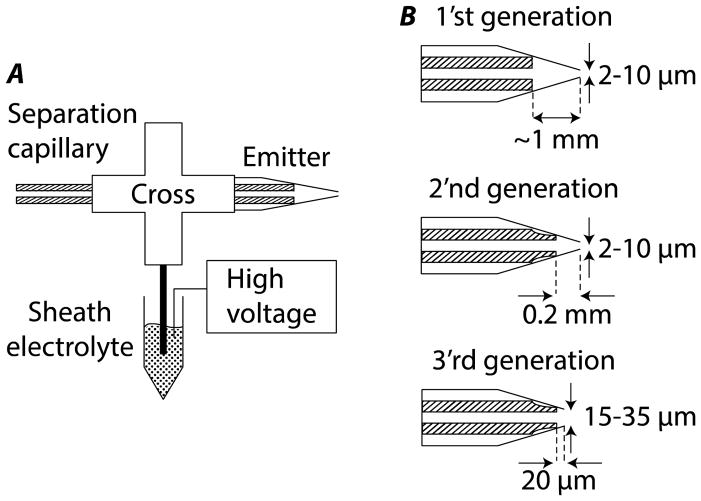
Figure 1.
Comparison of electrokinetically-pumped nanospray CZE interfaces. A. Overall design of the interface. The separation capillary is threaded through a PEEK cross (1.25 mm through hole) into a glass emitter. A tube (~1 mm i.d.) connects a sidearm of the cross to a sheath electrolyte reservoir, which is connected through a platinum electrode to a high voltage power supply. The mass spectrometer inlet is held at ground potential. B. Designs of three generations of interface. The original interface uses a flat separation capillary, which is able to approach within ~1-mm of the emitter orifice; typical orifice diameters are 2- to 10-μm. The second generation uses a separation capillary with an etched tip, which approaches within ~200-μm of the emitter orifice; typical orifice diameters are 2- to 10-μm. The third generation interface also uses an etched separation capillary but with a much larger exit orifice. The etched tip approaches within a few micrometers of the orifice; typical orifice diameters are 15- to 35-μm.
Our first publication to describe the interface included a Comsol model, which predicted that the sensitivity of the interface will increase as the distance from the capillary distal tip to the emitter orifice decreases.[16] That distance was limited to ~1-mm in the original design because the capillary butts against the conical emitter, Figure 1B. In our second-generation design, a few millimeters of the outside of the separation capillary tip were etched with hydrofluoric acid to reduce its outer diameter to ~60 μm, which allowed us to reduce the distance between separation capillary end in the spray emitter and the spray emitter orifice to ~200 μm.[17] The reduced capillary tip – emitter orifice distance significantly reduced the sample diffusion in the spray emitter and improved sensitivity. We used this interface to couple a 10 μm i.d. separation capillary with a Q-Exactive mass spectrometer; this system produced 1 zmole (1 zmol = 10−21 mol = 600 molecules) peptide detection limit (S/N = 3) and over 100 protein IDs based on tandem mass spectra from only 16 pg of E. coli digest.[17] More recently, we collaborated with the Coon group at the University of Wisconsin to couple a high-capacity and wide-separation-window CZE system to a high-speed Orbitrap Fusion mass spectrometer (up to 20 HZ MS/MS acquisition) via our second-generation CE-MS interface.[23] The system produced over 10,000 peptide IDs from a HeLa cell proteome digest in a ~100 min single-shot CZE-MS/MS analysis, which significantly reduced the gap in performance between CZE and single-shot ultra-performance liquid chromatography (UPLC) for shot-gun proteomics.[23]
Our original publication also investigated the effect of the emitter diameter on spray performance.[16] That publication demonstrated that sensitivity improved for smaller emitter diameters (<10 μm). While the narrow emitter diameter provides higher sensitivity, it also is susceptible to plugging.
In this manuscript, we report a third generation interface, Figure 1B, that combines a large emitter orifice with a very short capillary tip-emitter distance. This new system provides very robust operation and high sensitivity detection.
EXPERIMENTAL SECTION
Materials and reagents
Bovine pancreas TPCK-treated trypsin, bovine serum albumin (BSA), urea, ammonium bicarbonate (NH4HCO3), dithiothreitol (DTT), iodoacetamide (IAA), octyl β-D-glucopyranoside, and angiotensin II (human, Asp-Arg-Val-Tyr-Ile-His-Pro-Phe) were purchased from Sigma–Aldrich (St. Louis, MO). Acetonitrile (ACN), acetic acid, formic acid (FA), and hydrofluoric acid (HF) were purchased from Fisher Scientific (Pittsburgh, PA). Methanol and water were purchased from Honeywell Burdick & Jackson (Wicklow, Ireland). Fused silica capillary (30 μm i.d./150 μm o.d.; 158 μm i.d./360 μm o.d.) and linear polyacrylamide (LPA) coated capillary (30 and 50 μm i.d./150 μm o.d.) were purchased from Polymicro Technologies (Phoenix, AZ).
Mammalian Cell-PE LB™ buffer for cell lysis was purchased from G-Biosciences (St. Louis, MO). Complete, mini protease inhibitor cocktail (provided in EASYpacks) was purchased from Roche (Indianapolis, IN).
Sample preparation
Bovine serum albumin (BSA) in 100 mM NH4HCO3 (pH 8.0) containing 8 M urea was denatured at 37 °C for 30 min, followed by standard reduction and alkylation with DTT and IAA. After dilution with 100 mM NH4HCO3 (pH 8.0) to reduce the urea concentration below 2 M, protein digestion was performed at 37 °C with trypsin at a trypsin/protein ratio of 1/30 (w/w) for 12 h. After acidified with FA, the protein digest was desalted with C18-SepPak column (Waters, Milford, MA), and then was lyophilized with a vacuum concentrator (Thermo Fisher Scientific, Marietta, OH). The dried protein digest was stored at −20 °C before use.
The procedure for Xenopus laevis egg collection and fertilization was the same as our previous work.[24] All animal procedures were performed according to protocols approved by the University of Notre Dame Institutional Animal Care and Use Committee. The fertilized Xenopus laevis eggs (a total of 50 eggs) were collected into an Eppendorf tube. After removing the egg culture buffer, 800 μL of mammalian Cell-PE LB™ buffer plus complete protease inhibitors was immediately added into the Eppendorf tube, followed by vortexing for 1 min to preliminarily lyse the eggs. Then, the eggs were homogenized by a PowerGen™ Model 125 homogenizer (Fisher Scientific) for 1 min on ice, and further sonicated for 10 min on ice with a Branson Sonifier 250 (VWR Scientific, Batavia, IL) to lyse the eggs completely. Then, the lysate was centrifuged at 10,000 g for 10 min and three layers were obtained in the Eppendorf tube: top layer (lipid), medium layer (protein), and the pellet. Finally, the protein layer was collected in another Eppendorf tube and a small fraction of the protein solution was subjected to protein concentration measurement with the bicinchoninic acid (BCA) method.[25] The remaining protein solution was subjected to acetone precipitation to purify the proteins; 1 mL cold acetone was added into 200 μL of protein solution (~1 mg of protein) and the mixture was kept at −20 °C for overnight. After centrifugation, the supernatant was removed and the protein pellet was washed with cold acetone again to completely remove the detergent in the lysis buffer. After centrifugation again, the supernatant was removed and the protein pellet was kept in a fume hood at room temperature for ~2 min to dry the sample.
A 1 mg aliquot of proteins from the Xenopus laevis fertilized egg lysate was dissolved in 250 μL of 100 mM NH4HCO3 (pH 8.0) containing 8 M urea via vortex and sonication. Then, the protein solution was kept at 37 °C for 40 min for denaturation, followed by protein reduction with DTT (8 mM) at 37 °C for 40 min and alkylation with IAA (24 mM) at room temperature in the dark for 20 min. 2 μL of 1 M DTT was next added to the protein solution to block the excess IAA at room temperature for 20 min. After dilution with 100 mM NH4HCO3 (pH 8.0) to 1 mL total volume to reduce the urea concentration to ~2 M, protein digestion was performed at 37 °C with trypsin at a trypsin/protein ratio of 1/40 (w/w) for overnight. After acidified with FA, the protein digest was desalted with C18-SepPak column (Waters, Milford, MA), and then was lyophilized with a vacuum concentrator (Thermo Fisher Scientific, Marietta, OH). The dried protein digest was stored at −80 °C before use.
Initial interface optimization
The CE-MS interface optimization experiments were performed with manual operations, and the capillary electrophoresis system was assembled from components reported previously.[16,17] Two Spellman CZE 1000R high-voltage power supplies provided high voltage for separation and electrospray. The electrospray emitter was borosilicate glass capillary (1.0 mm o.d., 0.75 mm i.d., and 10 cm length) pulled with a Sutter instrument P-1000 flaming/brown micropipet puller. Electrophoresis was controlled by LabView software. The injection end of the separation capillary was fixed in an injection block,[26] and a gas line was connected to the injection block to provide pressure for capillary flushing and sample injection. The gas pressure was controlled by a gas regulator. An electrode was also fixed in the injection block to provide high voltage for CE separation; no pressure was used during separation. The base plate of the injection block contains a compartment for a small vial. A set of vials was used that contained a flushing buffer for cleaning the capillary, the sample for injection, or background electrolyte for separation.
The sample was 20 μM of angiotensin II and the separation capillary was 20 μm/150 μm (i.d./o.d.) for all the optimization experiments. The distal tip of the separation capillary was etched with HF to produce a ~5-mm length with a ~30 μm o.d.; details of capillary etching with HF were reported previously.[17] The sheath buffer was 0.5% (v/v) FA in water containing 10% (v/v) methanol.
For the optimization of spray emitter size, the sample buffer and separation buffer were both 10% (v/v) acetic acid. The total separation capillary length was 40 cm and 20 psi for 2 s was used for sample injection. The voltages applied at the injection end and spray emitter were 21.5 kV and 1.5 kV, respectively. The distance between spray emitter orifice and mass spectrometer entrance was ~ 1.2 mm. Four different spray emitter orifice sizes (8, 15, 25, and 35 μm o.d.) were evaluated in the experiment.
For the optimization of the distance between the spray emitter orifice and mass spectrometer entrance, the sample buffer and separation buffer were both 0.5% (v/v) FA containing 10% (v/v) methanol. The total separation capillary length was 60 cm and 30 psi for 1.5 min was used for sample injection. The voltage applied at the injection end was 19.5 kV, and the spray voltage was 1.5 kV for an 8–10 μm o.d. spray emitter. The spray voltage was increased to 2.0 kV for the 25 μm o.d. spray emitter when the distance between spray emitter orifice and mass spectrometer entrance was increased from 0.6 mm to 2.7 mm. Five different distance between the emitter tip and mass spectrometer (0.6 mm, 1.2 mm, 1.7 mm, 2.2 mm, and 2.7 mm) were evaluated.
An LTQ-XL mass spectrometer (Thermo Fisher Scientific) was used for the interface optimization. Only MS1 spectra were acquired. The scan range of ion trap mass analyzer was m/z 400–2000.
Long-term evaluation of the interface
A PrinCE autosampler (Prince Technologies B.V., Netherlands) was used for capillary flushing, sample injection, and separation voltage control. A separate Spellman CZE 1000R high-voltage power supply was used to provide the voltage for electrospray. The third-generation electro-kinetically pumped sheath flow CE-MS interface was used to couple automated CZE to mass spectrometer. A spray emitter with 25/22 μm (o.d./i.d.) orifice was used, and the distance between spray emitter orifice and mass spectrometer entrance was ~1.7 mm for all the experiments.
An uncoated capillary was used for the long-term evaluation. The separation capillary was 30/150 μm i.d./o.d., 80 cm length and its distal end was etched to get ~40 μm outer diameter. The injection end of the separation capillary was threaded through another capillary (158 μm i.d./360 μm o.d., ~13 cm); the assembly was sealed with epoxy. This assembly was required to match the design of the PrinCE autosampler. The distance between the injection end of the separation capillary and the proximal end of the 360 μm o.d. capillary was around 3 mm. The separation buffer was 10% (v/v) acetic acid, and the sheath buffer was 0.5% (v/v) FA in water containing 10% (v/v) methanol. The sample was 0.5 mg/mL of BSA digest in 0.05%FA. For sample injection, 500 mbar for 0.15 min was used. The voltages applied at the injection end and spray emitter were 25 kV and 1.8 kV, respectively.
An LTQ-XL mass spectrometer (Thermo Fisher Scientific) was used, and only MS1 spectra were acquired with the scan range of ion trap mass analyzer as m/z 380–1800.
Electrophoresis conditions for single-shot proteomic analysis
A LPA coated capillary (50/150 μm i.d./o.d.) with 90 cm length was employed, and its distal end was etched to get ~60 μm outer diameter (~5-mm length). The injection end of the separation capillary was threaded through another capillary (158 μm i.d./360 μm o.d., ~13 cm) and the assembly was sealed with epoxy; this step improved compatibility of the capillary with the autosampler. The distance between the injection end of the separation capillary and the proximal end of the 360 μm o.d. capillary was around 1.2 cm.
The separation buffer was 5% (v/v) acetic acid, and the sheath buffer was 0.5% (v/v) FA in water containing 10% (v/v) methanol. The spray emitter with a 25/22 μm (o.d./i.d.) opening size was used. BSA digest (0.35 mg/mL in 30% (v/v) ACN and 0.04% (v/v) FA) and Xenopus laevis fertilized egg proteome digest (~ 3 mg/mL in 30% (v/v) ACN and 0.07% (v/v) FA) were analyzed by the automated system. For sample injection, 250 mbar for 8 s and 500 mbar for 0.2 min were used for BSA digest and Xenopus laevis fertilized egg proteome digest analysis, respectively. The voltages applied at the injection end and spray emitter were 25.5 kV and 1.8 kV, respectively. For BSA digest analysis, after a 70 min separation, 300 mbar pressure was applied at the injection end for 10 min to push the remaining sample from the capillary, and the voltages were applied while applying pressure. For Xenopus laevis fertilized egg proteome digest, after 100 min separation, 150 mbar pressure was applied at the injection end for 20 min.
We also employed a smaller inner diameter LPA coated capillary (30/150 μm i.d./o.d., 90 cm long) with an etched end outer diameter of ~40 μm for automated CZE-MS analysis of Xenopus laevis fertilized egg proteome digest. The spray emitter with ~20/18 μm (o.d./i.d.) opening size was used. Xenopus laevis fertilized egg proteome digest (~ 6 mg/mL) in a solution of 0.07% (v/v) FA containing 30% (v/v) ACN and 0.7% (w/v) octyl β-D-glucopyranoside was analyzed by the system. For sample injection, 500 mbar for 0.12 min was used. After 100 min separation, 500 mbar pressure was applied at the injection end for 10 min while voltages were applied to flush the capillary. The separation buffer, sheath buffer, and voltages for separation and electrospray were the same as those for LPA coated capillary (50/150 μm i.d./o.d.) based experiment.
Mass spectrometry conditions for single-shot proteomic analysis
An LTQ-Orbitrap Velos mass spectrometer (Thermo Fisher Scientific) was used for single-shot proteomic analysis. For the analysis of the Xenopus laevis fertilized egg proteome digest (LPA coated capillary, 50 μm i.d./150 μm o.d.), a top-20 data dependent acquisition method was used. Full MS scans were acquired in the Orbitrap mass analyzer over m/z 350–1500 with 60,000 resolution at m/z 400. The target value was 1.00E+06. The twenty most intense peaks with charge state ≥ 2 were selected for sequencing and fragmented in the ion trap with normalized collision energy of 35%, activation q = 0.25, activation time of 10 ms, isolation window of 2 m/z units, and one microscan. The target value was 1.00E+04. The ion selection threshold was 500 counts, and the maximum allowed ion accumulation times were 500 ms for full scans and 100 ms for collision-induced dissociation (CID). Dynamic exclusion was enabled, and peaks selected for fragmentation more than once within 20 s were excluded from selection for 60 s. For other LTQ-Orbitrap Velos experiments, only MS1 spectra were acquired and no peptide fragmentation was performed. Full MS scans were acquired in the Orbitrap mass analyzer over m/z 350–1500 with resolution 60,000 (m/z 400). The target value was 1.00E+06. The maximum allowed ion accumulation times were 500 ms and one microscan was applied.
UPLC-MS/MS
A nanoACQUITY UltraPerformance LC® (UPLC®) system (Waters, Milford, MA, USA) was used for peptide separation. Buffer A (0.1% FA in water) and buffer B (0.1% FA in ACN) were used as mobile phases for gradient separation. Peptides were automatically loaded onto a commercial C18 reversed phase column (Waters, 100 μm × 100 mm, 1.7 μm particle, BEH130C18, column temperature 40 °C) with 2% buffer B for 10 min at a flow rate of 1 μL/min, followed by 3-step gradient separation, 1 min from 2% to 8%B with flow rate from 1 μL/min to 0.6 μL/min, 84 min to 30% B at flow rate of 0.6 μL/min, 2 min to 80% B with flow rate from 0.6 μL/min to 1 μL/min, and maintained at 80% B for 10 min at 1 μL/min. The column was equilibrated for 12 min with 2% B at 1 μL/min before analysis of the next sample. The eluted peptides from the C18 column were pumped through a capillary tip for electrospray, and analyzed by a LTQ-Orbitrap Velos mass spectrometer (Thermo Fisher Scientific).
The electrospray voltage was 1.8 kV, and the ion transfer tube temperature was 300 °C. The S-Lens RF level was 50.00. A top-20 data dependent acquisition method was used for Xenopus laevis fertilized egg proteome digest analysis. The detailed parameters were the same as those in section 2.3.3.2.
Data Analysis
Raw MS files were analyzed by MaxQuant [27] version 1.3.0.5. MS/MS spectra were searched by the Andromeda search engine [28] against the Xenopus laevis protein database derived from mRNA data [29] (158,428 entries including forward and reverse sequences). The database also included common contaminants. MaxQuant analysis included an initial search with a precursor mass tolerance of 20 ppm, main search precursor mass tolerance of 6 ppm and fragment mass tolerance of 0.5 Da. The search included enzyme was trypsin, variable modifications of oxidation (M), acetylation (K and protein N-terminal) and deamidation (NQ), and fixed modification of carbamidomethyl cysteine. Minimal peptide length was set to six amino acids and the maximum number of missed cleavages was set to two. The false discovery rate (FDR) was set to 0.01 for both peptide and protein identifications. A separate FDR was set to 0.01 for modification sites. The proteins identified by completely same sets of peptides were grouped, and reported as one protein group. The protein and peptide tables were filtered to remove the identifications from the reverse database and common contaminants.
In order to determine the phosphorylated peptide identifications from the CZE-MS/MS and UPLC-MS/MS data, we did another database search with MaxQuant and variable modifications of oxidation (M), deamidation (NQ), and phospho (STY). Other parameters were same as those mentioned above.
Raw MS data were also converted into mzXML files and imported into Matlab for subsequent analysis.
RESULTS AND DISCUSSION
Optimization of the electro-kinetically pumped sheath flow interface
We optimized our second-generation electro-kinetically pumped sheath-flow interface to improve its robustness and sensitivity. Angiotensin II was used as the sample in this set of experiments.
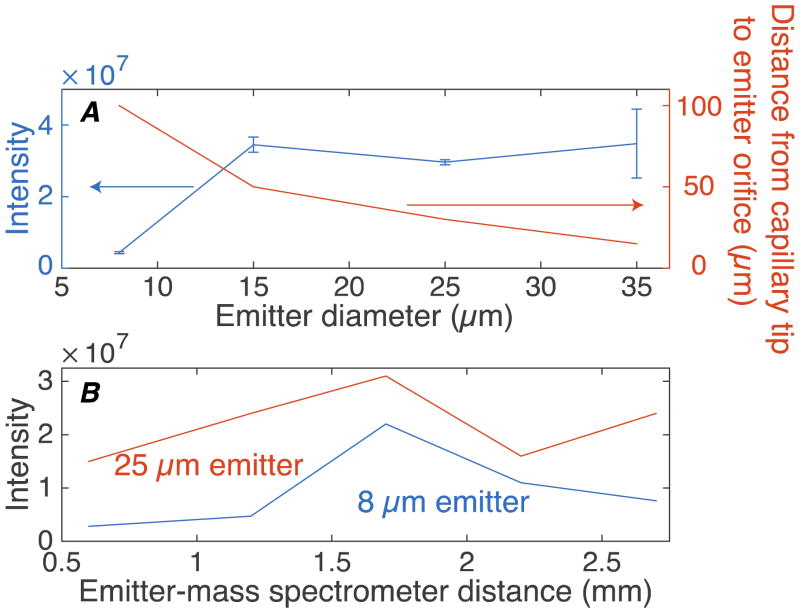
We first evaluated the effect of spray emitter orifice size on the system sensitivity, Figure 2A. Spray emitters with 8, 15, 25, and 35-μm o.d. were evaluated; the distance between spray emitter orifice and mass spectrometer entrance was kept constant (~ 1.2 mm). For each emitter, duplicate CZE-MS analyses of angiotensin II were performed. The intensity of angiotensin II using a 15-μm o.d. spray emitter is nearly 8 times higher than that using an 8 μm o.d. emitter. Within experimental error, the intensity was identical for 15, 25, and 35 μm o.d. spray emitters.
Figure 2.
Optimization data of the 3rd generation CE-MS interface. (A) Intensity of peptide (blue, left) and distance between the etched capillary tip and emitter orifice (brown, right) versus electrospray emitter outer diameter. (B) Intensity of peptide versus distance between spray emitter orifice and MS entrance for an 8-μm and 25-μm diameter orifice. Angiotensin II was used as the sample in these experiments.
This observation appears to be at odds with our initial report, which showed higher sensitivity for smaller emitter size.[16] However, we now use an etched tip on the separation capillary; the larger emitter size allows a decrease in the distance from the capillary tip to the emitter orifice to ~15 μm, Figure 2A. The loss in sensitivity associated with the larger orifice size was recovered by decreasing the capillary tip – emitter orifice distance.
We then varied the distance between the spray emitter orifice and mass spectrometer entrance, Figure 2B. The distance between the spray emitter orifice and mass spectrometer entrance was set at 0.6 mm, 1.2 mm, 1.7 mm, 2.2 mm, and 2.7 mm during a continuous electrophoretic infusion of angiotensin. The signal was recorded for at least 30-s at each distance. Spray emitters with 8- and 25-μm orifice outer diameters were used in the experiment.
The signal maximized at a distance of ~1.7 mm for both 8- and 25-μm o.d. spray emitters, Figure 2B. Unexpectedly, the variance in peptide intensity among different emitter-mass spectrometer entrance distances with the 8-μm emitter is much larger than that with the 25-μm emitter. In addition, the sensitivity of the 25-μm emitter is higher than that of the 8-μm emitter for all distances, due to the shorter distance between the separation capillary tip to the spray emitter orifice. We note that the optimal spray voltage for the 25-μm emitter is slightly higher than that for an 8-μm emitter (1.7–1.8 kV vs. 1.5 kV).
We estimate that the electroosmotic flow (EOF) in the spray emitter under the experimental conditions used in this work is ~ 50 nL/min. The EOF in the separation capillary for the optimization experiments is around 20 nL/min, which is much lower than the sheath buffer flow rate. Therefore, the optimized conditions are demonstrated for CZE-MS experiments with very low EOF in the separation capillary, i.e. very low pH separation buffer (pH 2.5 or lower) or neutrally coated separation capillary. Additional optimizations will be required for CZE-MS experiments with significantly higher EOF in the separation capillary.
Long-term stability of CZE-MS analysis of BSA digest
The higher sensitivity produced by the larger orifice diameter is fortuitous; plugging decreases dramatically for emitters larger than ~20-μm. To evaluate the robustness and lifetime of the third-generation electro-kinetically pumped sheath flow CZE-MS interface, we used a PrinCE autosampler for repetitive injection of a BSA digest onto an uncoated fused silica capillary (80-cm long, 30/150 μm i.d./o.d); an LTQ-XL mass spectrometer was used for data acquisition. ~5 ng of protein digest was loaded for each run. The emitter orifice was 25/22 μm o.d./i.d., and the distance between the spray emitter orifice and the mass spectrometer entrance was ~1.7 mm.
The automated CZE-MS system produced continuous acquisition of BSA digest for over 5,000 min (127 runs) of CZE-MS analysis, Figures S1–S6 in supporting material I. The emitter was in good condition at the end of this period; we terminated the run because of other commitments for the mass spectrometer.
The system was reasonably reproducible. There was a modest drift in migration time over this extended operational period, Figure S7, likely due to contamination of the uncoated capillary wall. There was a ~10% relative standard deviation in peak area across the 127 electropherograms, Figure S8. Separation efficiency started at ~200,000 theoretical plates and slowly drifted to 150,000 plates, corresponding to a 15% increase in peak width over the 5,000 minute run time, Figure S9.
The separation profiles and base peak intensities of BSA digest from CZE-MS are reproducible across the 127 runs, Figure 3. After 127 sequential separations of the BSA digest, we performed a blank run; we observed a featureless background signal that was roughly 0.1% as intense as the sample runs, Figure 3.
Figure 3.
Selected base peak electropherograms generated from ~5 ng of BSA digest. A blank run is also shown. A PrinCE autosampler and a LTQ-XL mass spectrometer were employed to generate this data with an 80-cm long, 30/150 μm i.d./o.d. capillary with etched end outer diameter of ~40 μm. The spray emitter orifice was 25/22 μm o.d./i.d., and the distance between spray emitter orifice and mass spectrometer entrance was ~1.7 mm. NL is the normalization level, which is the most intense signal in the base peak electropherogram.
Automated CZE-MS and MS/MS system with high peak capacity for a complex proteome digest analysis
Our group recently collaborated with the Coon group from the University of Wisconsin.[23] We coupled an LPA coated capillary (50 μm i.d./150 μm o.d.) based CZE system to a state-of-art Orbitrap Fusion mass spectrometer via our second-generation electro-kinetically pumped sheath-flow interface for shot-gun proteomics. The system generated a 90 min separation widow. During this single-shot analysis, over 2,100 protein and 10,000 peptide identifications were produced from a HeLa cell proteome digest, which represents nearly an order of magnitude improvement over earlier CZE-MS/MS studies.[23]
In those earlier studies, capillary flushing, sample injection, buffer change and voltage application were performed manually. In this manuscript, we employed a PrinCE autosampler for automated operation of the CZE system coupled to an LTQ-Orbitrap Velos mass spectrometer. LPA coated capillaries (50/150 μm i.d./o.d. and 30/150 μm i.d./o.d, 90 cm long) with etched end outer diameter ~40 μm (for 30 μm i.d. capillary) and ~60 μm (for 50 μm i.d. capillary) were used for CZE separation of peptides. The emitter orifice was 25/22 μm o.d./i.d. (for 50 μm i.d. capillary) and 20/18 μm o.d./i.d. (for 30 μm i.d. capillary), and the distance between spray emitter orifice and mass spectrometer entrance was ~1.7 mm.
Figure 4A shows the electropherogram from ~10 ng of BSA digest analyzed by automated single-shot CZE-MS with 50/150 μm i.d./o.d LPA coated capillary. ~70 peaks were resolved from the BSA digest electropherogram. The separation window is about 50 min, and is much wider than that from an uncoated capillary (~12 min, Figure 3), due to the significantly reduced electroosmotic flow in the LPA coated capillary. We extracted three peptide peaks from the electropherogram with 5 ppm mass tolerance, Figure S10 in supporting material I. The peaks are reasonably efficient (N = ~100,000).
Figure 4.
Electropherograms from ~10 ng of BSA digest (A) and 300 ng of Xenopus laevis fertilized egg proteome digest (B) analyzed by automated CZE-MS and MS/MS, and chromatogram from 300 ng of Xenopus laevis fertilized egg proteome digest (C) analyzed by UPLC-MS/MS. Conditions of CZE experiments: a PrinCE autosampler, LPA coated capillary (50/150 μm i.d./o.d. 90 cm long) with etched end outer diameter ~60 μm, 25/22 μm o.d./i.d. spray emitter, ~1.7 mm distance between spray emitter orifice and mass spectrometer entrance. An LTQ-Orbitrap Velos mass spectrometer was employed for these experiments.
We then applied the automated CZE-MS/MS system for analysis of a Xenopus laevis fertilized egg proteome digest, Figure 4B. Separation used an LPA coated capillary with 50/150 μm i.d./o.d, and ~300 ng of peptide was injected for analysis. This system produced a > 80 min separation window, which is similar to our recent CZE-MS/MS work with manual operations.[23] This separation window is also similar to the results of an UPLC-MS/MS separation using the same sample, injection amount, and mass spectrometer, Figure 4C.
Single-shot automated CZE-MS/MS generated slightly more MS/MS spectra and peptide-spectrum-matches (PSMs) from the Xenopus laevis fertilized egg proteome than UPLC-MS/MS, but produced slightly fewer peptide and protein IDs (4,038 vs. 4,836 peptide IDs and 1,249 vs. 1,570 protein IDs), Figure 5A. This performance might result because the peak width of relatively late-migrating peptides from CZE is significantly wider than that from UPLC, which results in more MS/MS spectra and PSMs, but lower peptide and protein IDs from CZE.
Figure 5.
Comparison of results using single-shot CZE and UPLC for analysis of the Xenopus laevis fertilized egg proteome. A. Comparison of numbers of protein and peptide IDs. B. Proteome dynamic range. C. Peptide intensity. D. Distributions of molecular weight of peptides.
An earlier study from this group employed an automated CZE-MS/MS system based on our first-generation interface. Our third-generation interface now produces four times higher protein and peptide IDs than the earlier system.[30] This improvement is due to use of the third-generation interface, the use of an acetic acid separation buffer and coated capillary to produce a long separation window, and the use of a more complex sample.
We then evaluated the observed proteome dynamic range from single-shot CZE-MS/MS and UPLC-MS/MS based on the protein intensity information from MaxQuant database search results, Figure 5B. The proteome dynamic range from CZE and UPLC are both around four orders of magnitude. We further compared the peptide intensity generated by CZE and UPLC, Figure 5C. We first summed the peptide intensity from both CZE and UPLC data for peptides identified in both datasets (overlapped peptides) based on MS/MS spectra and used the summed peptide intensity to represent the peptide abundance in the sample. CZE-MS/MS produced higher intensity for almost all the overlapped peptides than LC-MS/MS, and on average the peptide signal from CZE is three times higher than that from LC, which indicates the higher sensitivity of CZE-MS/MS, and is in agreement with previous publications.[9,19] We then evaluated the complementarity of CZE and UPLC for peptide identifications. About 48% of peptide IDs from CZE are covered by data from UPLC. When the CZE and UPLC data were combined, the number of peptide IDs is improved by 44%, suggesting outstanding complementarity of CZE and UPLC for peptide IDs of this complex vertebrate proteome. CZE tends to identify larger peptides compared with UPLC, Figure 5D. We also compared the peptide identifications observed in this work with our previously published multidimensional-LC based large-scale quantitative proteomics data from Xenopus laevis embryos (stages 1, 8, 13 and 22).[24] After reanalyzing the large-scale Xenopus laevis data[24] with MaxQuant software, we observed that ~82% and ~68% of the peptide IDs from UPLC and CZE are covered by the previous large-scale dataset, respectively, which further demonstrates the complementarity of CZE and UPLC for peptide IDs. The complementarity of CZE and UPLC on protein level is not highly significant, and the combination of CZE and UPLC data produces ~10% more protein IDs than UPLC alone, which reasonably agrees with our previous work.[19] The lists of identified proteins and peptides from Xenopus laevis fertilized egg proteome with CZE and UPLC are presented in supporting material II.
Recently, Lindner’s group demonstrated that CE-MS/MS and LC-MS/MS are complementary for characterization of post-translational modifications of histones, especially phosphorylation.[31] To confirm those results, we did another database search for the Xenopus laevis fertilized egg data from CZE-MS/MS and UPLC-MS/MS considering the phosphorylation (STY) modifications to check the difference between the two techniques for phosphorylated peptide identifications from complex proteomes without enrichment. In total, 240 phosphorylated peptides were identified from CZE-MS/MS and UPLC-MS/MS data. CZE-MS/MS produced 171 phosphorylated peptide identifications and UPLC-MS/MS generated 85 phosphorylated peptide identifications. We further analyzed the peptide intensity for 16 phosphorylated peptides identified by both techniques; CZE-MS/MS generated an average of 3.5 times higher intensity compared with UPLC-MS/MS. The data suggests good complementarity between CZE and LC for phosphorylated peptide identifications. The phosphorylated peptide list and phosphorylation site list are presented in supporting material II.
Next, in order to compare our system with Busnel’s sheathless system [10], we employed a smaller inner diameter LPA coated separation capillary (30 μm/150 μm i.d./o.d.)[10], for automated CZE-MS analysis of Xenopus laevis fertilized egg proteome digest, Figure 6. About 50 ng of digest was loaded for analysis. We calculated the peak capacity of the single-shot automated CZE-MS run based on the averaged peak widths at half height of 11 peptides with migration time ranging from ~18 min to ~100 min and the 82-min separation window. The calculated peak capacity is ~330, which is similar to the reported best peak capacity from sheathless interface based single-shot CZE-MS analysis of a complex peptide mixture,[10] representing the best peak capacity from single-shot CZE-MS for peptide separation.
Figure 6.
3D electropherogram from ~50 ng of Xenopus laevis fertilized egg proteome digest analyzed by single-shot automated CZE-MS. Conditions: a PrinCE autosampler, LTQ-Orbitrap Velos mass spectrometer, LPA coated capillary (30/150 μm i.d./o.d. 90 cm long) with etched end outer diameter ~40 μm, 20/18 μm o.d./i.d. spray emitter, ~1.7 mm distance between spray emitter orifice and mass spectrometer entrance.
To improve peptide solubility, 0.7% (w/v) octyl β-D-glucopyranoside (OG) was used as an additive in the sample buffer for the 30 μm/150 μm (i.d./o.d.) LPA coated capillary-based experiment. Because OG is neutral and the electroosmotic flow in the LPA coated capillary under very acidic condition (5% (v/v) acetic acid, pH ~2.4) is extremely low, OG molecules move very slow in the capillary and does not affect peptide detection. In addition, only low nL of the peptide sample was loaded for analysis and the injected OG amount is very small, therefore the contamination of OG on the mass spectrometer can be ignored.
Details on the use of the electro-kinetically pumped sheath-flow CE-MS interface based automated CZE-MS system
For the electro-kinetically pumped sheath-flow CE-MS interface based CZE-MS system, S-Figure 11 in supporting material I, typically one power supply is employed to apply voltage for separation (HV I) and another power supply is used for electrospray (HV II). The power supplies used in our experiments are sources of current, but are unable to sink current.
Under normal conditions, the power supply connected to the interface (HV II) operates as a current source, and that supply’s control circuit holds the interface at the desired voltage. However, operation of the separation capillary under high current conditions (high separation electric field, high ionic strength separation buffer, and large inner diameter capillary) can lead to a situation where the current flowing through the capillary is higher than the expected current determined by HVII. In this case, the electrospray voltage is not controlled by HVII and instead floats to a higher value, where the capillary and electrospray act as voltage dividers. In this case, it will be necessary to either reduce the current flow through the separation capillary or to employ a power supply that can sink current for the interface. Based on our long-term experiences with the CE-MS system, the current across the separation capillary needs to be less than 10 μA in order for the spray potential to be controlled by HV II.
Automated CZE systems fall into two classes. One class is designed for use in DNA sequencing; these capillary array systems tend to use 150-μm OD separation capillaries. Our instruments are descendants from this class of instrument.[32] The second class of instruments employs a single separation capillary; these instruments are typically used for more general analytical applications. Commercial automated CZE systems tend to use 360 μm o.d. capillary. Long-term continuous CZE-MS experiments performed with the150 μm o.d. capillary using an autosampler tend to be unstable because the separation capillary is not stably held in commercial autosamplers.
There are several ways to address this issue. One way is to slide a short piece of 360-μm OD, 160-μm ID fused silica capillary over the 150-μm OD separation capillary; this sleeve can be glued in place to support the separation capillary in the autosampler. A second way is to modify the autosampler’s cartridge to stably hold the 150 μm o.d. separation capillary. The third way is to use 360 μm o.d. capillaies for separation. Our first generation interface is not compatible with large OD capillaries because the distance from the capillary exit to the emitter orifice tends to be quite large, leading to low sensitivity. Our second and third generation interfaces employ an etched capillary tip, which allows use of short distances from the capillary exit to the emitter orifice. We now routinely use both 150 and 360 μm o.d. capillary in these interfaces. We have used our automated CZE-MS system with a 50/360 μm i.d./o.d. capillary (outer diameter of etched end to ~70 μm) coupled to the third generation interface; this system produces over 150 hours continuous analysis of protein digests with good reproducibility (data not shown).
CONCLUSIONS
In this work, we represented a third generation electro-kinetically pumped sheath-flow CE-MS interface with improved lifetime. The third-generation CZE-MS system can be continuously used for over 5,000 min, and produces stable and reproducible peptide separation and detection. Single-shot automated CZE-MS/MS generated over 1,200 protein IDs from a Xenopus laevis fertilized egg proteome in ~100 min analysis duration with an LTQ-Orbitrap Velos mass spectrometer and LPA-coated capillary. This number of protein IDs is 1/4–1/10 of the number of IDs from Xenopus laevis reported recently.[24,29,33] Those studies used off-line LC peptide fractionation followed by online RPLC-MS/MS, and tended to employ ~30 times more mass spectrometer time for analysis. Our results suggests that automated CZE-MS/MS should be able to perform comprehensive shot-gun proteomics using a moderate amount of instrument time by first prefractionating tryptic peptides before CZE analysis. [7,18,23,34]
Three concerns remain for the use of CZE-MS/MS for comprehensive proteome analysis. First, neutral coated capillaries are required to minimize electro-osmosis, which produces long separations, high peak capacities, and a large number of peptide and protein IDs in single run.[10,23] In our experience, commercial LPA coated capillaries suffer from limited lifetime, and an improved coating protocol will be required to generate capillaries with long lifetime.
Second, the injection volume of a standard CZE experiment is at the low nL level, which seriously limits the number of protein and peptide identifications obtained from single-shot analysis. Fortunately, several techniques have been evaluated for improving the loading capacity of CZE-MS for shot-gun proteomics, i.e. stacking [18,19,23,34], pH junction [35] and solid phase microextraction (SPME) [7]. However, these techniques require further optimization to produce robust operation.
Third, although recent reports have demonstrated improved peak capacity in the CZE separation of peptides (~300), this peak capacity remains a factor of 3 lower than the state-of-art UPLC systems.[23] Further optimization will be required to produce CZE separations with similar peak capacity and similar numbers of protein and peptide IDs as high-quality UPLC analyses.
Supplementary Material
Acknowledgments
We thank Olivia F. Cox and Professor Paul W. Huber for generously providing fertilized Xenopus eggs. We also thank Drs. William Boggess and Matthew Champion in the Notre Dame Mass Spectrometry and Proteomics Facility for their help with this project. This project was supported by a grant from the National Institutes of Health (R01GM096767).
Footnotes
Sequential electropherograms from ~5 ng of BSA digest analyzed by automated CZE-MS, Figures S1–S6. Drift in migration time for component with m/z = 489 over the 5000 minute period, Figure S7. Area of the m/z 489 peak for the 127 consecutive runs, Figure S8. Drift in peak efficiency for the m/z = 489 peak, Figure S9. Extracted electropherogram of three peptides from ~10 ng of BSA digest analyzed by automated CZE-MS with 50/150 μm i.d./o.d LPA-coated capillary, Figure S10. Schematic diagram of the electro-kinetically pumped sheath flow interface based CZE-ESI-MS system, Figure S11. Peptide and protein identifications for CZE and UPLC analyses of the Xenopus laevis fertilized egg digest. Supporting Information, this material is available free of charge via the internet http://pubs.acs.org.
References
- 1.Sun L, Zhu G, Yan X, Dovichi NJ. High sensitivity capillary zone electrophoresis-electrospray ionization-tandem mass spectrometry for the rapid analysis of complex proteomes. Curr Opin Chem Biol. 2013;17:795–800. doi: 10.1016/j.cbpa.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun L, Zhu G, Yan X, Champion MM, Dovichi NJ. Capillary zone electrophoresis for analysis of complex proteomes using an electrokinetically pumped sheath flow nanospray interface. Proteomics. 2014;14:622–628. doi: 10.1002/pmic.201300295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heemskerk AA, Deelder AM, Mayboroda OA. CE-ESI-MS for bottom-up proteomics: Advances in separation, interfacing and applications. Mass Spectrom Rev. 2014 doi: 10.1002/mas.21432. [DOI] [PubMed] [Google Scholar]
- 4.Maxwell EJ, Chen DD. Twenty years of interface development for capillary electrophoresis-electrospray ionization-mass spectrometry. Anal Chim Acta. 2008;627:25–33. doi: 10.1016/j.aca.2008.06.034. [DOI] [PubMed] [Google Scholar]
- 5.Zhong X, Zhang Z, Jiang S, Li L. Recent advances in coupling capillary electrophoresis-based separation techniques to ESI and MALDI-MS. Electrophoresis. 2014;35:1214–1225. doi: 10.1002/elps.201300451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moini M. Simplifying CE-MS operation. 2. Interfacing low-flow separation techniques to mass spectrometry using a porous tip. Anal Chem. 2007;79:4241–4246. doi: 10.1021/ac0704560. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Fonslow BR, Wong CC, Nakorchevsky A, Yates JR., 3rd Improving the comprehensiveness and sensitivity of sheathless capillary electrophoresis-tandem mass spectrometry for proteomic analysis. Anal Chem. 2012;84:8505–8513. doi: 10.1021/ac301091m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han X, Wang Y, Aslanian A, Bern M, Lavallée-Adam M, Yates JR., 3rd Sheathless Capillary Electrophoresis-Tandem Mass Spectrometry for Top-Down Characterization of Pyrococcus furiosus Proteins on a Proteome Scale. Anal Chem. 2014;86:11006–11012. doi: 10.1021/ac503439n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faserl K, Sarg B, Kremser L, Lindner H. Optimization and evaluation of a sheathless capillary electrophoresis-electrospray ionization mass spectrometry platform for peptide analysis: comparison to liquid chromatography-electrospray ionization mass spectrometry. Anal Chem. 2011;83:7297–7305. doi: 10.1021/ac2010372. [DOI] [PubMed] [Google Scholar]
- 10.Busnel JM, Schoenmaker B, Ramautar R, Carrasco-Pancorbo A, Ratnayake C, Feitelson JS, Chapman JD, Deelder AM, Mayboroda OA. High capacity capillary electrophoresis-electrospray ionization mass spectrometry: coupling a porous sheathless interface with transient-isotachophoresis. Anal Chem. 2010;82:9476–9483. doi: 10.1021/ac102159d. [DOI] [PubMed] [Google Scholar]
- 11.Maxwell EJ, Zhong X, Zhang H, van Zeijl N, Chen DD. Decoupling CE and ESI for a more robust interface with MS. Electrophoresis. 2010;31:1130–1137. doi: 10.1002/elps.200900517. [DOI] [PubMed] [Google Scholar]
- 12.Lindenburg PW, Ramautar R, Jayo RG, Chen DD, Hankemeier T. Capillary electrophoresis-mass spectrometry using a flow-through microvial interface for cationic metabolome analysis. Electrophoresis. 2014;35:1308–1314. doi: 10.1002/elps.201300357. [DOI] [PubMed] [Google Scholar]
- 13.Jayo RG, Thaysen-Andersen M, Lindenburg PW, Haselberg R, Hankemeier T, Ramautar R, Chen DD. Simple capillary electrophoresis-mass spectrometry method for complex glycan analysis using a flow-through microvial interface. Anal Chem. 2014;86:6479–8646. doi: 10.1021/ac5010212. [DOI] [PubMed] [Google Scholar]
- 14.Zhong X, Maxwell EJ, Ratnayake C, Mack S, Chen DD. Flow-through microvial facilitating interface of capillary isoelectric focusing and electrospray ionization mass spectrometry. Anal Chem. 2011;83:8748–8755. doi: 10.1021/ac202130f. [DOI] [PubMed] [Google Scholar]
- 15.Wang C, Lee CS, Smith RD, Tang K. Capillary isotachophoresis-nanoelectrospray ionization-selected reaction monitoring MS via a novel sheathless interface for high sensitivity sample quantification. Anal Chem. 2013;85:7308–7315. doi: 10.1021/ac401202c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wojcik R, Dada OO, Sadilek M, Dovichi NJ. Simplified capillary electrophoresis nanospray sheath-flow interface for high efficiency and sensitive peptide analysis. Rapid Commun Mass Spectrom. 2010;24:2554–2560. doi: 10.1002/rcm.4672. [DOI] [PubMed] [Google Scholar]
- 17.Sun L, Zhu G, Zhao Y, Yan X, Mou S, Dovichi NJ. Ultrasensitive and fast bottom-up analysis of femtogram amounts of complex proteome digests. Angew Chem Int Ed Engl. 2013;52:13661–13664. doi: 10.1002/anie.201308139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Champion MM, Sun L, Champion PA, Wojcik R, Dovichi NJ. Capillary zone electrophoresis-electrospray ionization-tandem mass spectrometry as an alternative proteomics platform to ultraperformance liquid chromatography-electrospray ionization-tandem mass spectrometry for samples of intermediate complexity. Anal Chem. 2012;84:1617–1622. doi: 10.1021/ac202899p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu G, Sun L, Yan X, Dovichi NJ. Single-shot proteomics using capillary zone electrophoresis-electrospray ionization-tandem mass spectrometry with production of more than 1250 Escherichia coli peptide identifications in a 50 min separation. Anal Chem. 2013;85:2569–2573. doi: 10.1021/ac303750g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Wojcik R, Dovichi NJ, Champion MM. Quantitative multiple reaction monitoring of peptide abundance introduced via a capillary zone electrophoresis-electrospray interface. Anal Chem. 2012;84:6116–6121. doi: 10.1021/ac300926h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun L, Li Y, Champion MM, Zhu G, Wojcik R, Dovichi NJ. Capillary zone electrophoresis-multiple reaction monitoring from 100 pg of RAW 264. 7 cell lysate digest. Analyst. 2013;138:3181–3188. doi: 10.1039/c3an00287j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Compton PD, Tran JC, Ntai I, Kelleher NL. Optimizing capillary electrophoresis for top-down proteomics of 30–80 kDa proteins. Proteomics. 2014;14:1158–1164. doi: 10.1002/pmic.201300381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun L, Hebert AS, Yan X, Zhao Y, Westphall MS, Rush MJ, Zhu G, Champion MM, Coon JJ, Dovichi NJ. Over 10 000 Peptide Identifications from the HeLa Proteome by Using Single-Shot Capillary Zone Electrophoresis Combined with Tandem Mass Spectrometry. Angew Chem Int Ed Engl. 2014;53:13931–13933. doi: 10.1002/anie.201409075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun L, Bertke MM, Champion MM, Zhu G, Huber PW, Dovichi NJ. Quantitative proteomics of Xenopus laevis embryos: expression kinetics of nearly 4000 proteins during early development. Sci Rep. 2014;4:4365. doi: 10.1038/srep04365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 26.Krylov SN, Starke DA, Arriaga EA, Zhang Z, Chan NW, Palcic MM, Dovichi NJ. Instrumentation for chemical cytometry. Anal Chem. 2000;72:872–877. doi: 10.1021/ac991096m. [DOI] [PubMed] [Google Scholar]
- 27.Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol. 2008;26:1367–1372. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- 28.Cox J, Neuhauser N, Michalski A, Scheltema RA, Olsen JV, Mann M. Andromeda: a peptide search engine integrated into the MaxQuant environment. J Proteome Res. 2011;10:1794–1805. doi: 10.1021/pr101065j. [DOI] [PubMed] [Google Scholar]
- 29.Wühr M, Freeman RM, Jr, Presler M, Horb ME, Peshkin L, Gygi SP, Kirschner MW. Deep proteomics of the Xenopus laevis egg using an mRNA-derived reference database. Curr Biol. 2014;24:1467–1475. doi: 10.1016/j.cub.2014.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu G, Sun L, Yan X, Dovichi NJ. Stable, reproducible, and automated capillary zone electrophoresis-tandem mass spectrometry system with an electrokinetically pumped sheath-flow nanospray interface. Anal Chim Acta. 2014;810:94–98. doi: 10.1016/j.aca.2013.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarg B, Faserl K, Kremser L, Halfinger B, Sebastiano R, Lindner HH. Comparing and combining capillary electrophoresis electrospray ionization mass spectrometry and nano-liquid chromatography electrospray ionization mass spectrometry for the characterization of post-translationally modified histones. Mol Cell Proteomics. 2013;12:2640–2656. doi: 10.1074/mcp.M112.024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang J, Voss KO, Shaw DF, Roos KP, Lewis DF, Yan J, Jiang R, Ren H, Hou JY, Fang Y, Puyang X, Ahmadzadeh H, Dovichi NJ. A multiple-capillary electrophoresis system for small-scale DNA sequencing and analysis. Nucleic Acids Res. 1999;27:e36. doi: 10.1093/nar/27.24.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smits AH, Lindeboom RG, Perino M, van Heeringen SJ, Veenstra GJ, Vermeulen M. Global absolute quantification reveals tight regulation of protein expression in single Xenopus eggs. Nucleic Acids Res. 2014;42:9880–9891. doi: 10.1093/nar/gku661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan X, Essaka DC, Sun L, Zhu G, Dovichi NJ. Bottom-up proteome analysis of E. coli using capillary zone electrophoresis-tandem mass spectrometry with an electrokinetic sheath-flow electrospray interface. Proteomics. 2013;13:2546–2551. doi: 10.1002/pmic.201300062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu G, Sun L, Yan X, Dovichi NJ. Bottom-up proteomics of Escherichia coli using dynamic pH junction preconcentration and capillary zone electrophoresis-electrospray ionization-tandem mass spectrometry. Anal Chem. 2014;86:6331–6336. doi: 10.1021/ac5004486. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.