Abstract
Free-feeding animals often face complex nutritional choices that require the balancing of competing nutrients, yet the mechanisms driving macronutrient specific food intake are poorly defined. A large number of behavioral studies indicate that both the quantity and quality of dietary protein can markedly influence food intake and metabolism, and that dietary protein intake may be prioritized over energy intake. This review focuses on recent progress in defining the mechanisms underlying protein-specific feeding. Considering the evidence that protein powerfully regulates both food intake and metabolism, uncovering these protein specific mechanisms may reveal new molecular targets for the treatment of obesity and diabetes while also offering a more complete understanding of how dietary factors shape both food intake and food choice.
Keywords: amino acids, dietary protein, FGF21, GCN2, leucine, macronutrient
Protein as an essential, regulated nutrient
The maintenance of health and fitness requires that organisms procure sufficient nutrition by negotiating a complex nutritional landscape in which food availability and quality can be unreliable. Energy density, macronutrient balance, and procurement cost are often in competition, and organisms must adaptively change behavior and metabolism during periods of nutrient restriction. It is well accepted that an intricate neuroendocrine network detects energy restriction and coordinates adaptive changes in feeding behavior, energy expenditure and metabolism. However, when considered in the context of a natural environment it seems likely that food intake is driven by more than just the number of calories (energy content) in the diet. This review will specifically focus on the hypothesis that dietary protein intake is regulated independently of other dietary macronutrients (carbohydrate and fat) as well as total energy intake. Unlike the regulation of energy homeostasis, there has been little progress in defining a neuroendocrine mechanism governing ‘protein homeostasis’, despite a large and compelling literature indicating that variations in dietary protein or amino acid content produce profound changes in feeding behavior and metabolic health [1].
Behavioral responses to dietary protein
The experimental manipulation of dietary protein substantially alters feeding behavior, metabolism and growth. Studies focusing on the impact of dietary protein on feeding behavior have led to three general conclusions: 1) Diets with severe amino acid imbalance or that are devoid of a single essential amino acid reduce food intake and produce a learned avoidance of the imbalanced diet, 2) High protein (HP) diets tend to suppress food intake acutely, and promote reductions in fat mass but maintenance of lean mass chronically, and 3) Moderately low protein (LP) diets increase food intake and protein selection, while extremely LP diets can reduce food intake. A brief overview of these behavioral responses is provided for perspective, and the reader is referred to several recent reviews which cover this field in more depth [1–6].
Effects of HP and LP diets
HP diets suppress food intake over the short term, with protein being the most satiating macronutrient per calorie [3, 4, 7]. A large number of clinical studies indicate that HP diets promote weight and adiposity loss by reducing food intake, maintaining fat free mass and increasing energy expenditure [8]. For these reasons the maintenance of protein intake but reduction of energy intake is a central focus of many weight loss strategies [4, 9]. Similar data exist in rodents, although some studies describe a waning of the anorectic effect over time due to adaptive increases in amino acid metabolism [10–13].
Fewer studies have focused on the response to a LP diet, and the effect seems to be dependent on the degree of protein restriction and the physiological state of the animal. Rats and mice exhibit hyperphagia in response to moderately LP diets [14–16], but will abandon this approach and spontaneously reduce food intake if the protein content is extremely low [17]. Recent studies have focused on this same question in humans. Interestingly, several studies indicate that moderate restriction of protein triggers adaptive changes in food intake and preference [18–20], whereas other studies involving more severe protein restriction have shown no effect on food intake [4, 21].
Protein selection and amino acid imbalance
There exists a large body of data indicating that a wide range of species will self-select between diets that are high and low in protein to meet protein requirements [1]. Although there is debate as to whether this self-selection produces a precise regulation of protein intake, work utilizing the Geometric Framework to model the interacting effects of all three macronutrients strongly suggests that species as diverse as fish, insects, rodents and pigs seek to consume a specific protein:carbohydrate target, and will prioritize protein over energy [22, 23]. The ability to select for protein also appears to be sensitive to physiological status, as protein selection increases in response to periods of increased protein demand, such as during periods of rapid growth [23, 24].
Evidence also supports selection based on the composition of individual amino acids, not just total protein. Rats rapidly detect and readily avoid diets that are deficient in a single essential amino acid [6], will specifically select the missing amino acid over other non-restricted amino acids in a choice test, and appear to be able to distinguish between minute changes in dietary amino acid content [25, 26]. While diets that are completely devoid of a single amino acid induce aversion and are incompatible with life, a more moderate restriction of a single amino acid increases food intake and actually extends lifespan. The most compelling evidence for this effect comes from work focusing on methionine restriction, which increases food intake and energy expenditure, improves lipid metabolism and insulin sensitivity, and increases lifespan [27–30]. These data suggest that moderate restriction of a single amino acid produces a different physiological response compared to the complete deprivation of that amino acid.
While dietary protein clearly exerts a profound effect on feeding behavior, metabolism and growth, at issue is whether these effects represent a specific, physiologic regulation of protein intake (i.e. protein homeostasis). While the evidence suggests that protein and energy are independently balanced, we currently have a poor understanding of the mechanisms that might contribute to such a protein-specific response. Below we discuss the potential mechanisms underlying protein-dependent regulation of food intake and metabolism.
Potential mechanisms underlying protein intake and selection
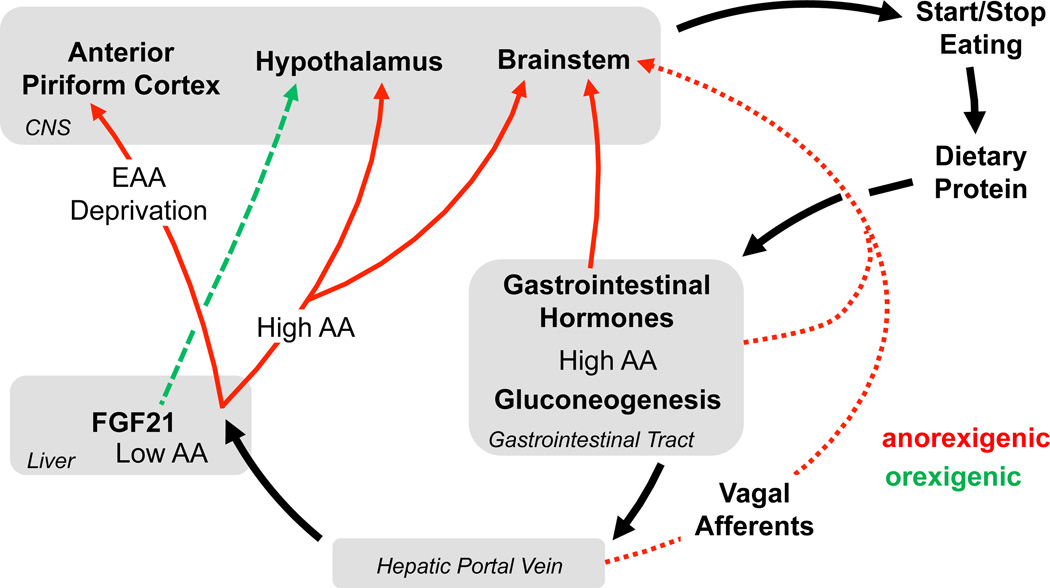
As with any nutrient, the identification, consumption, digestion, absorption and utilization of amino acids is a complex process. Information regarding dietary protein intake or protein status could be transmitted to the brain in a large number of ways, including via taste (umami), neural or endocrine signals from the gastrointestinal (GI) tract, hormones generated by liver or skeletal muscle based on amino acid availability or metabolism, or finally a direct brain effect of circulating amino acids. Delineating the role of these individual pathways is a daunting task, and indeed it seems likely that multiple signals participate in this process. Three mechanisms have been most predominately linked to the response to dietary protein: 1) Direct effects of amino acids in the brain, 2) Gut-derived neural or hormonal signals, and 3) Other endocrine signals, namely fibroblast growth factor 21 (FGF21). We discuss their contribution to the organism’s response to amino acid imbalance, HP and LP diets (Figure 1).
Figure 1. Mechanisms through which changes in dietary protein intake are detected and communicated to the brain.
Dietary protein within the GI tract activates both endocrine and vagal signals, which act in a primarily anorexigenic fashion in the hypothalamus and brainstem. Absorbed amino acids are delivered to the liver via the hepatic portal vein. Reduced amino acid supply to the liver increases hepatic FGF21 secretion, which acts in the brain to increase both food intake and energy expenditure, likely via effects in the hypothalamus. Amino acids are transported out of the liver and into the general circulation, and circulating amino acids can act in both the hypothalamus and brainstem to suppress food intake. Finally, imbalances in dietary or circulating amino acid concentrations are detected in the anterior piriform cortex (APC), with activation of the APC reducing food intake. These various mechanisms allow animals to detect and adaptively respond to diets that are high, low or imbalanced in amino acid content.
Direct effects of amino acids on the brain
As mentioned above, diets that are completely devoid of a single amino acid reduce food intake and induce a learned aversion. There is very strong evidence to suggest that the detection of this imbalance is mediated by the depletion of the limiting amino acid and the resulting activation of the serine/threonine kinase general control nonderepressible-2 (GCN2) within the brain anterior piriform cortex (APC) [6, 31–33]. GCN2 is a conserved amino acid sensor that couples amino acid availability to protein synthesis [34–36], and therefore its activation in the APC provides a molecular mechanism for brain detection of amino acid restriction. However, to date this APC-centric mechanism has not been connected to the regulation of food or protein intake in other settings, although GCN2 has been linked to the metabolic effects of single amino acid deficiency on the liver [36–39]. Whether GCN2 similarly contributes to more moderate restriction of single amino acids or general dietary protein restriction remains unclear, but this distinction is important considering the divergent feeding response to complete deficiency (hypophagia) vs. modest restriction (hyperphagia). Finally, a separate brain signaling system has been implicated in the response to a leucine-devoid diet, although in this case the primary endpoint is the induction of energy expenditure. These studies suggest that leucine deprivation influences ribosomal protein S6 kinase beta-1 (S6K1) signaling and regulates neurons expressing melanocortin receptor (MCR4), corticotropin-releasing hormone (CRH) and thyrotropin-releasing hormone (TRH) in the hypothalamus, leading to an activation of the sympathetic nervous system and increased energy expenditure [40, 41] Currently it is not completely clear whether these effects are mediated by direct amino acid detection within the hypothalamus or instead via a separate organ that relays a signal to the central nervous system (CNS).
In addition to the detection of amino acid imbalance, there is also evidence that the anorexia of HP diets may be driven by the branched-chain amino acid (BCAA) leucine acting locally in the hypothalamus and brainstem [42]. Direct injection of leucine into the brain is sufficient to suppress food intake [43], and leucine can act locally within the mediobasal hypothalamus [44], via activation of mammalian target of rapamycin (mTOR) and/or inhibition of AMP-activated protein kinase (AMPK) signaling [15, 43, 45], or via activation of BCAA metabolism [44]. Leucine can also act locally within the brainstem to suppress food intake, via local induction of both mTOR and ERK signaling [46]. Finally, the anorectic effect of intracerebroventricular leucine injection is not common to other amino acids [47], suggesting that leucine uniquely acts as a signaling molecule in the brain. It should be noted, however, that nearly all of these experiments involve the direct injection of leucine into the CNS, and as such it is less clear whether fluctuations in brain leucine are connected to diet-induced changes in food intake. In support of leucine’s physiological role, several studies suggest that supplementing diets with leucine or BCAAs is sufficient to reproduce the anorectic effects of HP diets [45, 48]. However, in other studies leucine fails to suppress food intake after being administered via the drinking water, gavage, gastric infusion, or intraperitoneal injection [49–52]. Finally, there is also evidence that other amino acids act on the brain, as it was shown that the anorectic effects of both intragastrically or intravenously administered arginine and glutamate required the area postrema [52]. These results collectively indicate that amino acids can alter food intake via direct effects in the brain and support GCN2, mTOR and AMPK as molecular mediators of this effect. However, these data also highlight the need for additional work delineating whether individual amino acids act in functionally unique ways as well as whether combinations of amino acids act in concert to induce effects that are not reproduced by any single amino acid.
While the brain appears to directly sense both amino acid imbalance and amino acid excess, the evidence is weaker regarding whether reductions in brain amino acids contribute to the hyperphagia observed in response to dietary protein restriction. Blood amino acids are buffered by metabolic adaptations that occur within liver and skeletal muscle during protein restriction, and thus LP diets tend to produce only modest, transient reductions in circulating amino acids [47, 53]. It was recently demonstrated that reduced dietary leucine or BCAA content was neither necessary nor sufficient to trigger hyperphagia in rats consuming a LP diet, and that chronic infusion of amino acids into the brain failed to block this LP-induced hyperphagia [47]. These observations are consistent with earlier work demonstrating that elevating BCAA content in a low protein diet had no effect on consumption of that diet in a self-selection paradigm [54], and collectively suggest that reductions in BCAAs alone are insufficient to trigger hyperphagia on a LP diet.
Detection of amino acids in the GI tract and portal system: Protein-induced satiety
HP preloads induce a robust suppression of food intake that is greater than isocaloric loads of either carbohydrate or fat. There is strong support for the hypothesis that this protein-induced satiety is mediated via actions within the GI tract or portal circulation, which are then transmitted to the brain via neural (vagal, spinal) or endocrine (gut peptide) signals [55]. HP meals lead to enhanced activation of the vagus nerve and hindbrain [56] and induce a pattern of hindbrain c-Fos activation that is distinct from that observed with sucrose loads [57]. HP diet also promotes larger increases in glucagon-like peptide-1 (GLP1), peptide YY (PYY), cholecystokinin (CCK) and glucagon levels, and greater reductions in ghrelin levels [58–60], while PYY deficient mice are resistant to HP-induced decreases in food intake and body weight [61]. An additional mechanism to explain the anorectic effects of dietary protein is the induction of intestinal gluconeogenesis and its detection via a portal vein glucose sensor [62]. The functional relevance of this portal mechanism was demonstrated by the fact that denervation of the portal vein blocked the anorexia induced by portal vein glucose infusion [63], while inhibition of intestinal gluconeogenesis blocked the anorexia induced by a HP diet [64].
While the evidence strongly supports the hypothesis that gut-derived signals decrease the consumption of high protein diets, whether these same signals increase the consumption of low protein diets has received much less scrutiny. It is known that the effect of dietary protein on gut hormone secretion is dose dependent, such that LP diets lead to smaller changes in CCK or ghrelin levels relative to control diets [58, 65]. Thus a muted regulation of gut hormone secretion may contribute to LP-induced hyperphagia, although no interventional studies have been conducted to provide compelling support for this hypothesis. Finally, it should also be noted that protein restriction also triggers increased selection for protein [18, 20, 66, 67] and this selection for protein is also apparent in physiological settings of increased protein demand, such as during rapid growth [24]. In other words, regulatory mechanisms detect negative protein balance induced either by reductions in protein intake or increases in protein demand. Currently the neuroendocrine mechanisms underlying this response are completely unclear, as there is no compelling evidence than any of these gut hormones influence macronutrient selection [2] and it is problematic to hypothesize that the gut is a sensor of systemic protein balance. Thus while gut-derived signals are clearly influenced by dietary protein content and contribute to the satiety induced by high protein diets, whether these hormones contribute to the detection of negative protein balance is much less clear.
Homeostatic endocrine signals: FGF21 as a signal of protein restriction
When considering how protein balance might be regulated, it is perhaps worthwhile to consider the mechanisms governing energy balance. While it is well accepted that both glucose and lipids act directly in the brain and that food intake is influenced by gut-derived vagal and endocrine signals, there is consensus that the long-term regulation of energy balance is primarily mediated by hormones such as leptin, an adipose tissue-derived hormone that serves as a signal of the amount of fat (energy) stored in the body. In fact, leptin regulates energy homeostasis in part by interacting with these more short term satiety cues while also coordinating adaptive changes in growth, reproduction, energy expenditure and glucose homeostasis. Just as leptin is a signal of energy status, are there endocrine hormones which are uniquely regulated by protein status and which coordinate behavior and metabolism in response to altered protein intake? Considering that both skeletal muscle and liver represent key sites of amino acid metabolism and protein synthesis, these two organs represent logical sites to look for such a protein specific signal. Skeletal muscle produces a large number of endocrine hormones, many of which can gain access to the CNS [68]. However, to date there has been little progress in identifying a muscle-derived signal that communicates to the brain to regulate food intake, and almost no work has focused on such a signal in the context of altered protein status.
The liver is particularly well suited to act as a sensor of dietary protein intake. The liver is critical for amino acid catabolism and biosynthesis, and protein/amino acid restriction induces significant changes in hepatic metabolism and gene expression [53, 69]. Importantly, very recent research has identified a novel endocrine pathway for the detection of dietary amino acid or protein restriction, with this mechanism centering on FGF21. Initial studies demonstrated that FGF21 is induced by starvation and ketogenic diets [70–72], and that increases in FGF21 influence glucose and lipid metabolism while also increasing energy expenditure, brown adipose tissue activity and the browning of white fat [73–78]. FGF21 appears to induce these metabolic effects by acting in a variety of sites, most notably adipose tissue [79, 80] and brain [81–83].
A connection between FGF21 and protein intake was established when it was shown that hepatic FGF21 expression is induced in vitro and in vivo by the depletion of single amino acids (leucine, histidine or asparagine) via a mechanism involving GCN2-dependent phosphorylation of eukaryotic initiation factor 2α (eIF2α) and activation of activating transcription factor 4 (ATF4) [38, 84–87]. Work focusing on models of dietary methionine restriction indicates that methionine restriction-induced increases in FGF21 are essential for the beneficial metabolic effects seen in the absence of the amino acid [28, 29]. Finally, the role of FGF21 in the behavioral and metabolic responses to general dietary protein restriction was tested recently [14]. These data indicate that dietary protein restriction triggers a rapid and robust increase in circulating FGF21 in rodents and humans, that this effect is not replicated by the restriction of energy alone, and that the increases in food intake and energy expenditure during dietary protein restriction are completely lost in FGF21-deficient mice [14]. These data therefore suggest that FGF21 may be the first known endocrine hormone specifically activated by the restriction of protein or amino acids. Additional work is clearly required to place this new observation within the larger context of FGF21 biology, but it is provocative to hypothesize an important interaction between the protein signal FGF21 and energy signals such as leptin and insulin [88–90]. Taken together, these data identify FGF21 as a key player in a fundamentally new mechanism through which feeding behavior, growth and metabolism are coordinated during periods of reduced dietary protein intake.
Concluding remarks and future perspectives
The consumption of essential amino acids is required for health and survival, and as such it should not be surprising that physiological mechanisms exist to match protein intake to physiological protein demand. Behavioral studies clearly indicate that variations in protein quantity and quality induce marked changes in feeding behavior and metabolism, and substantial progress has been made in identifying potential mechanisms underlying these effects. It is now well established that the learned avoidance of severely imbalanced diets is mediated by activation of GCN2 signaling within the brain APC, that amino acids (particularly leucine) can suppress food intake by acting locally within the hypothalamus and brainstem to regulate mTOR and AMPK signaling, and that high protein diets induce satiety via a robust stimulation of gut hormone and vagal satiety signaling. Finally, recent data implicate the metabolic hormone FGF21 as a critical mediator of adaptive changes in food intake and metabolism during the restriction of individual amino acids or total dietary protein.
Yet despite this progress, substantial questions still remain. Perhaps the most critical is the fact that CNS control of macronutrient selection remains virtually undescribed. We currently have little insight into how the brain specifically regulates protein intake, and how protein intake is balanced against other macronutrients and total energy. Currently no brain area or neuronal population has been compellingly linked to a specific selection for protein, and even though FGF21 appears to be an endocrine signal of protein restriction, it remains unclear if coordinating the physiological response to protein restriction is the primary biological role of FGF21. Finally, it should also be recognized that protein is rarely consumed in isolation, and that the ratio of protein to carbohydrate and fat may be equally as important to physiology as the absolute amount of protein [91].
Recent decades have seen a substantial expansion of our understanding of the neurobiology of feeding behavior, with this work principally focusing on the concept of energy balance. This review argues that food intake may be governed by more than just the consumption of energy, and has specifically emphasized protein as a separately detected nutritional variable. Just as nutritional cues impinge on the brain to coordinate adaptive responses to energy imbalance, additional cues may also coordinate adaptive responses to protein imbalance. Dietary protein exerts a powerful effect on food intake and has been strongly linked to metabolic health and longevity [91, 92]. As such, uncovering and tapping into this mechanism has the potential to produce novel approaches for the treatment of obesity and metabolic disease.
Box 1: Outstanding Questions.
Is dietary protein regulated to a specific, target point in humans as in other species?
What guides macronutrient selection and particularly the selection between low and high protein diets? Do discrete neural circuits govern the consumption of protein?
Are there molecular targets in the brain that uniquely regulate protein-specific feeding?
How is the anorectic effect of GCN activation in the APC during amino acid imbalance integrated within the larger neurobiology of feeding behavior?
Are all amino acids detected equally, or can some amino acids be used to uniquely influence metabolism and behavior?
Is coordinating the metabolic and behavioral response to protein and amino acid restriction a primary biological role of FGF21?
Highlights.
Dietary protein is an essential nutrient that is required for health.
Variations in protein quality or quantity robustly alter food intake.
The intake of protein appears to be regulated independently of other nutrients.
Protein-specific mechanisms offer novel opportunities for the treatment of obesity.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (R01-DK081563) and a research fellowship from the Deutsche Forschungsgemeinschaft (DFG), LA 3042/2-1.
Glossary
- Activating transcription factor 4 (ATF4)
A transcription factor that induces the expression of stress response genes as part of the integrated stress response. ATF4 is downstream of GCN2/eIF2α and is activated by amino acid restriction.
- AMP-activated protein kinase (AMPK)
A kinase that is activated by cellular energy restriction that functions as a metabolic switch to coordinate diverse cellular responses to nutrient restriction.
- Anterior piriform cortex (APC)
An area of the cortical brain classically associated with olfaction, but which is essential for the anorexia induced by deprivation of a single essential amino acid.
- Cholecystokinin (CCK)
A gut derived hormone that reduces food intake in response to food ingestion.
- Corticotropin-releasing hormone (CRH)
A neuropeptide, mainly produced in the hypothalamus, associated with the response to various stressors.
- Eukaryotic initiation factor 2α (eIF2α)
A cellular protein that is phosphorylated by a variety of upstream kinases in response to cellular stress, including GCN2. eIF2α phosphorylation leads to the inhibition of cellular protein synthesis but the specific activation of the integrated stress response.
- Extracellular signal-regulated kinase (ERK)
A kinase that serves as a primary intracellular signaling molecule mediating the cellular response to a variety of growth factors.
- Fibroblast growth factor 21 (FGF21)
A nutritionally regulated hormone which induces a broad range of beneficial metabolic effects.
- General control nonderepressible 2 (GCN2)
A serine/threonine kinase that is activated by essential amino acid restriction and phosphorylates eIF2α to inhibit cellular protein translation and induce a series of cellular stress responses.
- Geometric Framework
A state-space modeling method that has been used to model the interacting effects of macronutrient intake on physiological endpoints.
- Glucagon-like peptide-1 (GLP1)
A gut derived hormone that reduces food intake in response to food ingestion.
- Mammalian target of rapamycin (mTOR)
a kinase that coordinates diverse cellular responses to variations in nutrient availability and growth factor signaling.
- Melanocortin-4 receptor (MCR4)
Receptor expressed on neurons within the brain associated with regulation of body weight, food intake and energy expenditure.
- Peptide YY (PYY)
A gut derived hormone that reduces food intake in response to food ingestion.
- Thyrotropin-releasing hormone (TRH)
A neuropeptide associated with the regulation of thyroid hormone, but which also acts on diverse neural systems.
- Ribosomal protein S6 kinase beta-1 (S6K1)
A kinase which phosphorylates ribosomal protein S6 in response to upstream activation by mTOR, coordinating the effect of growth factors and nutrients on cell growth.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors have nothing to disclose
References
- 1.Morrison CD, et al. Homeostatic regulation of protein intake: in search of a mechanism. Am J Physiol Regul Integr Comp Physiol. 2012;302:R917–R928. doi: 10.1152/ajpregu.00609.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berthoud HR, et al. Neural and metabolic regulation of macronutrient intake and selection. The Proceedings of the Nutrition Society. 2012;71:390–400. doi: 10.1017/S0029665112000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davidenko O, et al. Control of protein and energy intake - brain mechanisms. Eur J Clin Nutr. 2013;67:455–461. doi: 10.1038/ejcn.2013.73. [DOI] [PubMed] [Google Scholar]
- 4.Martens EA, et al. Protein diets, body weight loss and weight maintenance. Curr Opin Clin Nutr Metab Care. 2014;17:75–79. doi: 10.1097/MCO.0000000000000006. [DOI] [PubMed] [Google Scholar]
- 5.Gosby AK, et al. Protein leverage and energy intake. Obes Rev. 2014;15:183–191. doi: 10.1111/obr.12131. [DOI] [PubMed] [Google Scholar]
- 6.Anthony TG, et al. Detection of amino acid deprivation in the central nervous system. Curr Opin Clin Nutr Metab Care. 2013;16:96–101. doi: 10.1097/MCO.0b013e32835b618b. [DOI] [PubMed] [Google Scholar]
- 7.Westerterp-Plantenga MS, et al. Dietary protein, weight loss, and weight maintenance. Annu Rev Nutr. 2009;29:21–41. doi: 10.1146/annurev-nutr-080508-141056. [DOI] [PubMed] [Google Scholar]
- 8.Wycherley TP, et al. Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2012;96:1281–1298. doi: 10.3945/ajcn.112.044321. [DOI] [PubMed] [Google Scholar]
- 9.Soenen S, et al. Normal protein intake is required for body weight loss and weight maintenance, and elevated protein intake for additional preservation of resting energy expenditure and fat free mass. J Nutr. 2013;143:591–596. doi: 10.3945/jn.112.167593. [DOI] [PubMed] [Google Scholar]
- 10.Lacroix M, et al. A long-term high-protein diet markedly reduces adipose tissue without major side effects in Wistar male rats. Am J Physiol Regul Integr Comp Physiol. 2004;287:R934–R942. doi: 10.1152/ajpregu.00100.2004. [DOI] [PubMed] [Google Scholar]
- 11.Bensaid A, et al. A high-protein diet enhances satiety without conditioned taste aversion in the rat. Physiol Behav. 2003;78:311–320. doi: 10.1016/s0031-9384(02)00977-0. [DOI] [PubMed] [Google Scholar]
- 12.Jean C, et al. Metabolic evidence for adaptation to a high protein diet in rats. J Nutr. 2001;131:91–98. doi: 10.1093/jn/131.1.91. [DOI] [PubMed] [Google Scholar]
- 13.Peters JC, et al. Adaptation of rats to diets containing different levels of protein: effects on food intake, plasma and brain amino acid concentrations and brain neurotransmitter metabolism. J Nutr. 1985;115:382–398. doi: 10.1093/jn/115.3.382. [DOI] [PubMed] [Google Scholar]
- 14.Laeger T, et al. FGF21 is an endocrine signal of protein restriction. J Clin Invest. 2014;124:3913–3922. doi: 10.1172/JCI74915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrison CD, et al. Amino acids inhibit Agrp gene expression via an mTOR-dependent mechanism. Am J Physiol Endocrinol Metab. 2007;293:E165–E171. doi: 10.1152/ajpendo.00675.2006.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White BD, et al. Effects of age on the feeding response to moderately low dietary protein in rats. Physiol Behav. 2000;68:673–681. doi: 10.1016/s0031-9384(99)00229-2. [DOI] [PubMed] [Google Scholar]
- 17.Du F, et al. Food intake, energy balance and serum leptin concentrations in rats fed low-protein diets. J Nutr. 2000;130:514–521. doi: 10.1093/jn/130.3.514. [DOI] [PubMed] [Google Scholar]
- 18.Griffioen-Roose S, et al. Protein status elicits compensatory changes in food intake and food preferences. Am J Clin Nutr. 2012;95:32–38. doi: 10.3945/ajcn.111.020503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gosby AK, et al. Testing Protein Leverage in Lean Humans: A Randomised Controlled Experimental Study. PLoS ONE. 2011;6:e25929. doi: 10.1371/journal.pone.0025929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffioen-Roose S, et al. Human protein status modulates brain reward responses to food cues. Am J Clin Nutr. 2014;100:113–122. doi: 10.3945/ajcn.113.079392. [DOI] [PubMed] [Google Scholar]
- 21.Martens EA, et al. Protein leverage effects of beef protein on energy intake in humans. Am J Clin Nutr. 2014;99:1397–1406. doi: 10.3945/ajcn.113.078774. [DOI] [PubMed] [Google Scholar]
- 22.Sorensen A, et al. Protein-leverage in mice: the geometry of macronutrient balancing and consequences for fat deposition. Obesity (Silver Spring) 2008;16:566–571. doi: 10.1038/oby.2007.58. [DOI] [PubMed] [Google Scholar]
- 23.Simpson SJ, et al. Geometric analysis of macronutrient selection in the rat. Appetite. 1997;28:201–213. doi: 10.1006/appe.1996.0077. [DOI] [PubMed] [Google Scholar]
- 24.Roberts TJ, et al. Rats treated with somatotropin select diets higher in protein. J Nutr. 1995;125:2669–2678. doi: 10.1093/jn/125.10.2669. [DOI] [PubMed] [Google Scholar]
- 25.Torii K, et al. Effect of lysine on afferent activity of the hepatic branch of the vagus nerve in normal and L-lysine- deficient rats. Physiol Behav. 2001;72:685–690. doi: 10.1016/s0031-9384(01)00426-7. [DOI] [PubMed] [Google Scholar]
- 26.Hrupka BJ, et al. Small changes in essential amino acid concentrations alter diet selection in amino acid-deficient rats. J Nutr. 1997;127:777–784. doi: 10.1093/jn/127.5.777. [DOI] [PubMed] [Google Scholar]
- 27.Hasek BE, et al. Remodeling the integration of lipid metabolism between liver and adipose tissue by dietary methionine restriction in rats. Diabetes. 2013;62:3362–3372. doi: 10.2337/db13-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lees EK, et al. Methionine restriction restores a younger metabolic phenotype in adult mice with alterations in fibroblast growth factor 21. Aging Cell. 2014;13:817–827. doi: 10.1111/acel.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stone KP, et al. Mechanisms of increased in vivo insulin sensitivity by dietary methionine restriction in mice. Diabetes. 2014;63:3721–3733. doi: 10.2337/db14-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anthony TG, et al. Remodeling of lipid metabolism by dietary restriction of essential amino acids. Diabetes. 2013;62:2635–2644. doi: 10.2337/db12-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maurin AC, et al. The GCN2 kinase biases feeding behavior to maintain amino acid homeostasis in omnivores. Cell Metab. 2005;1:273–277. doi: 10.1016/j.cmet.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 32.Hao S, et al. Uncharged tRNA and sensing of amino acid deficiency in mammalian piriform cortex. Science. 2005;307:1776–1778. doi: 10.1126/science.1104882. [DOI] [PubMed] [Google Scholar]
- 33.Russell MC, et al. The rapid anorectic response to a threonine imbalanced diet is decreased by injection of threonine into the anterior piriform cortex of rats. Nutr Neurosci. 2003;6:247–251. doi: 10.1080/1028415031000151567. [DOI] [PubMed] [Google Scholar]
- 34.Kilberg MS, et al. The transcription factor network associated with the amino acid response in mammalian cells. Adv Nutr. 2012;3:295–306. doi: 10.3945/an.112.001891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wek RC, et al. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans. 2006;34:7–11. doi: 10.1042/BST20060007. [DOI] [PubMed] [Google Scholar]
- 36.Zhang P, et al. The GCN2 eIF2alpha kinase is required for adaptation to amino acid deprivation in mice. Mol Cell Biol. 2002;22:6681–6688. doi: 10.1128/MCB.22.19.6681-6688.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo F, et al. The GCN2 eIF2alpha kinase regulates fatty-acid homeostasis in the liver during deprivation of an essential amino acid. Cell Metab. 2007;5:103–114. doi: 10.1016/j.cmet.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 38.De Sousa-Coelho AL, et al. Activating transcription factor 4-dependent induction of FGF21 during amino acid deprivation. Biochem J. 2012;443:165–171. doi: 10.1042/BJ20111748. [DOI] [PubMed] [Google Scholar]
- 39.Chotechuang N, et al. mTOR, AMPK, and GCN2 coordinate the adaptation of hepatic energy metabolic pathways in response to protein intake in the rat. Am J Physiol Endocrinol Metab. 2009;297:E1313–E1323. doi: 10.1152/ajpendo.91000.2008. [DOI] [PubMed] [Google Scholar]
- 40.Xia T, et al. S6K1 in the central nervous system regulates energy expenditure via MC4R/CRH pathways in response to deprivation of an essential amino acid. Diabetes. 2012;61:2461–2471. doi: 10.2337/db11-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xia T, et al. CREB/TRH pathway in the central nervous system regulates energy expenditure in response to deprivation of an essential amino acid. Int J Obes (Lond) 2015;39:105–113. doi: 10.1038/ijo.2014.65. [DOI] [PubMed] [Google Scholar]
- 42.Schwartz GJ. Central leucine sensing in the control of energy homeostasis. Endocrinology and metabolism clinics of North America. 2013;42:81–87. doi: 10.1016/j.ecl.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cota D, et al. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- 44.Blouet C, et al. Mediobasal hypothalamic leucine sensing regulates food intake through activation of a hypothalamus-brainstem circuit. J Neurosci. 2009;29:8302–8311. doi: 10.1523/JNEUROSCI.1668-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ropelle ER, et al. A central role for neuronal AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) in high-protein diet-induced weight loss. Diabetes. 2008;57:594–605. doi: 10.2337/db07-0573. [DOI] [PubMed] [Google Scholar]
- 46.Blouet C, et al. Brainstem nutrient sensing in the nucleus of the solitary tract inhibits feeding. Cell Metab. 2012;16:579–587. doi: 10.1016/j.cmet.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laeger T, et al. Leucine acts in the brain to suppress food intake but does not function as a physiological signal of low dietary protein. Am J Physiol Regul Integr Comp Physiol. 2014;307:R310–R320. doi: 10.1152/ajpregu.00116.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Newgard CB, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nairizi A, et al. Leucine supplementation of drinking water does not alter susceptibility to diet-induced obesity in mice. J Nutr. 2009;139:715–719. doi: 10.3945/jn.108.100081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koch CE, et al. Effect of central and peripheral leucine on energy metabolism in the Djungarian hamster (Phodopus sungorus) J Comp Physiol B. 2013;183:261–268. doi: 10.1007/s00360-012-0699-y. [DOI] [PubMed] [Google Scholar]
- 51.Zampieri TT, et al. Oral Leucine Supplementation Is Sensed by the Brain but neither Reduces Food Intake nor Induces an Anorectic Pattern of Gene Expression in the Hypothalamus. PLoS ONE. 2013;8:e84094. doi: 10.1371/journal.pone.0084094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jordi J, et al. Specific amino acids inhibit food intake via the area postrema or vagal afferents. J Physiol. 2013;591:5611–5621. doi: 10.1113/jphysiol.2013.258947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kalhan SC, et al. Metabolic and genomic response to dietary isocaloric protein restriction in the rat. J Biol Chem. 2011;286:5266–5277. doi: 10.1074/jbc.M110.185991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anderson SA, et al. Dietary branched-chain amino acids and protein selection by rats. J Nutr. 1990;120:52–63. doi: 10.1093/jn/120.1.52. [DOI] [PubMed] [Google Scholar]
- 55.Fromentin G, et al. Peripheral and central mechanisms involved in the control of food intake by dietary amino acids and proteins. Nutr Res Rev. 2012;25:29–39. doi: 10.1017/S0954422411000175. [DOI] [PubMed] [Google Scholar]
- 56.Tome D, et al. Protein, amino acids, vagus nerve signaling, and the brain. Am J Clin Nutr. 2009;90:838S–843S. doi: 10.3945/ajcn.2009.27462W. [DOI] [PubMed] [Google Scholar]
- 57.Schwarz J, et al. Three-dimensional macronutrient-associated Fos expression patterns in the mouse brainstem. PLoS One. 2010;5:e8974. doi: 10.1371/journal.pone.0008974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Belza A, et al. Contribution of gastroenteropancreatic appetite hormones to protein-induced satiety. Am J Clin Nutr. 2013;97:980–989. doi: 10.3945/ajcn.112.047563. [DOI] [PubMed] [Google Scholar]
- 59.Leidy HJ, et al. Beneficial effects of a higher-protein breakfast on the appetitive, hormonal, and neural signals controlling energy intake regulation in overweight/obese, "breakfast-skipping," late-adolescent girls. Am J Clin Nutr. 2013;97:677–688. doi: 10.3945/ajcn.112.053116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blom WA, et al. Effect of a high-protein breakfast on the postprandial ghrelin response. Am J Clin Nutr. 2006;83:211–220. doi: 10.1093/ajcn/83.2.211. [DOI] [PubMed] [Google Scholar]
- 61.Batterham RL, et al. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab. 2006;4:223–233. doi: 10.1016/j.cmet.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 62.Mithieux G. Nutrient control of hunger by extrinsic gastrointestinal neurons. Trends Endocrinol Metab. 2013;24:378–384. doi: 10.1016/j.tem.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 63.Mithieux G, et al. Portal sensing of intestinal gluconeogenesis is a mechanistic link in the diminution of food intake induced by diet protein. Cell Metab. 2005;2:321–329. doi: 10.1016/j.cmet.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 64.Penhoat A, et al. Protein-induced satiety is abolished in the absence of intestinal gluconeogenesis. Physiol Behav. 2011;105:89–93. doi: 10.1016/j.physbeh.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 65.Brennan IM, et al. Effects of fat, protein, and carbohydrate and protein load on appetite, plasma cholecystokinin, peptide YY, ghrelin, and energy intake in lean and obese men. Am J Physiol Gastrointest Liver Physiol. 2012;303:G129–G140. doi: 10.1152/ajpgi.00478.2011. [DOI] [PubMed] [Google Scholar]
- 66.Deutsch JA, et al. Unlearned specific appetite for protein. Physiol Behav. 1989;46:619–624. doi: 10.1016/0031-9384(89)90341-7. [DOI] [PubMed] [Google Scholar]
- 67.White BD, et al. Protein selection, food intake, and body composition in response to the amount of dietary protein. Physiol Behav. 2000;69:383–389. doi: 10.1016/s0031-9384(99)00232-2. [DOI] [PubMed] [Google Scholar]
- 68.Pedersen BK, et al. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nature reviews Endocrinology. 2012;8:457–465. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 69.Ghosh S, et al. A systems biology analysis of the unique and overlapping transcriptional responses to caloric restriction and dietary methionine restriction in rats. FASEB J. 2014;28:2577–2590. doi: 10.1096/fj.14-249458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Potthoff MJ, et al. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci U S A. 2009;106:10853–10858. doi: 10.1073/pnas.0904187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Badman MK, et al. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 72.Inagaki T, et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007;5:415–425. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 73.Kharitonenkov A, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu J, et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009;58:250–259. doi: 10.2337/db08-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fisher FM, et al. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang Y, et al. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. elife. 2012;1:e00065. doi: 10.7554/eLife.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fisher FM, et al. Integrated regulation of hepatic metabolism by fibroblast growth factor 21 (FGF21) in vivo. Endocrinology. 2011;152:2996–3004. doi: 10.1210/en.2011-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Coskun T, et al. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008;149:6018–6027. doi: 10.1210/en.2008-0816. [DOI] [PubMed] [Google Scholar]
- 79.Ding X, et al. betaKlotho is required for fibroblast growth factor 21 effects on growth and metabolism. Cell Metab. 2012;16:387–393. doi: 10.1016/j.cmet.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Adams AC, et al. The breadth of FGF21's metabolic actions are governed by FGFR1 in adipose tissue. Molecular metabolism. 2012;2:31–37. doi: 10.1016/j.molmet.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sarruf DA, et al. Fibroblast growth factor 21 action in the brain increases energy expenditure and insulin sensitivity in obese rats. Diabetes. 2010;59:1817–1824. doi: 10.2337/db09-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bookout AL, et al. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat Med. 2013;19:1147–1152. doi: 10.1038/nm.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Owen BM, et al. FGF21 Acts Centrally to Induce Sympathetic Nerve Activity, Energy Expenditure, and Weight Loss. Cell Metab. 2014;20:670–677. doi: 10.1016/j.cmet.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schaap FG, et al. Fibroblast growth factor 21 is induced by endoplasmic reticulum stress. Biochimie. 2013;95:692–699. doi: 10.1016/j.biochi.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 85.Kim KH, et al. Metformin-induced inhibition of the mitochondrial respiratory chain increases FGF21 expression via ATF4 activation. Biochem Biophys Res Commun. 2013;440:76–81. doi: 10.1016/j.bbrc.2013.09.026. [DOI] [PubMed] [Google Scholar]
- 86.Keipert S, et al. Skeletal muscle mitochondrial uncoupling drives endocrine cross-talk through the induction of FGF21 as a myokine. Am J Physiol Endocrinol Metab. 2014;306:E469–E482. doi: 10.1152/ajpendo.00330.2013. [DOI] [PubMed] [Google Scholar]
- 87.Wilson GJ, et al. GCN2 is required to increase fibroblast growth factor 21 and maintain hepatic triglyceride homeostasis during asparaginase treatment. Am J Physiol Endocrinol Metab. 2015;308:E283–E293. doi: 10.1152/ajpendo.00361.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Muller TD, et al. Restoration of leptin responsiveness in diet-induced obese mice using an optimized leptin analog in combination with exendin-4 or FGF21. Journal of peptide science : an official publication of the European Peptide Society. 2012;18:383–393. doi: 10.1002/psc.2408. [DOI] [PubMed] [Google Scholar]
- 89.Markan KR, et al. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes. 2014;63:4057–4063. doi: 10.2337/db14-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Emanuelli B, et al. Interplay between FGF21 and insulin action in the liver regulates metabolism. J Clin Invest. 2014;124:515–527. doi: 10.1172/JCI67353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Solon-Biet SM, et al. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 2014;19:418–430. doi: 10.1016/j.cmet.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Levine ME, et al. Low Protein Intake Is Associated with a Major Reduction in IGF-1, Cancer, and Overall Mortality in the 65 and Younger but Not Older Population. Cell Metab. 2014;19:407–417. doi: 10.1016/j.cmet.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]