Abstract
Objective
The purpose of this study was to compare longitudinally sampled maternal angiogenic proteins between singleton and twin pregnancies.
Study Design
Placental growth factor (PlGF), soluble fms-like tyrosine kinase-1 (sFlt-1), and soluble endoglin (sEng) from healthy pregnant women were quantified at 10, 18, 26 and 35 weeks’ gestation (n=91), and during the third trimester (31–39 weeks) and at delivery (33–41 weeks; n=41). Geometric means and 95% confidence intervals were calculated for gestational age adjusted angiogenic protein concentrations and compared between matched twin and singleton pregnancies.
Results
Maternal sFlt-1 concentrations and the sFlt-1/PlGF ratio were higher in twins than singletons across pregnancy and at delivery, with the greatest differences at week 35 [sFlt-1: 36916 vs. 10151 pg/mL; p<0.0001; sFlt-1/PlGF: 168.4 vs. 29.0; p<0.0001]. Maternal concentrations of s-endoglin also were higher in the third trimester and delivery. Maternal PlGF concentrations were lower in twin than singleton pregnancies at week 35 only [219.2 vs. 350.2 pg/mL; p<0.0001]. Placental weight appeared to be inversely correlated with maternal sFlt-1/PlGF ratio at the end of the pregnancy in both twins and singletons.
Conclusions
Higher maternal anti-angiogenic proteins in twin than singleton pregnancies does not appear to be due to greater placental mass in the former, and may be one explanation for the increased risk of preeclampsia in women carrying multiple gestations. Determining whether women with a history of multiple gestations have an altered cardiovascular disease and breast cancer risk, like those with a history of preeclampsia, is warranted.
Keywords: twins, singletons, angiogenic balance, pregnancy, sFlt-1, endoglin
Introduction
Women carrying twins or other higher order multiples are at 2–3 times the risk of developing preeclampsia, a common cause of maternal and fetal morbidity,1 than women with singleton pregnancies.2,3 Preeclampsia is marked by shallow trophoblast invasion into the maternal endometrium resulting in a less extensive vascular network supporting the pregnancy.4 Alterations in angiogenic proteins, as well as inflammatory cytokines and other immune-modulating molecules have been demonstrated in preeclamptic pregnancies,5–13 with elevations in soluble fms-like tyrosine kinase-1 (sFlt-1) and soluble endoglin (s-endoglin), two anti-angiogenic proteins, typically preceding the clinical manifestation of maternal disease.14–17
Maternal angiogenic factors also appear altered in pregnancies involving multiples compared with singletons, with elevated concentrations of sFlt-1 in the former.18–20 The timing of these changes in angiogenic balance may provide insight into the mechanism whereby preeclampsia risk is elevated in women carrying multiple gestations. Therefore, we followed women longitudinally through pregnancy and delivery to examine circulating maternal concentrations of placental growth factor (PlGF), sFlt-1, and s-endoglin in twin and singleton pregnancies.
Materials and Methods
Study subjects
The data for the analysis derive from two sources: the BIRTH cohort,21 and a study of twins at The Geisel School of Medicine (Dartmouth College). The study protocols were approved by institutional review boards at each institution and at the U.S. National Cancer Institute, and written informed consent was obtained from all participating women.
BIRTH Cohort
Participants were enrolled at three U.S. tertiary care academic centers between October 2007 and June 2009. Eligible women initiated routine prenatal care at <15 weeks’ gestation, were >18 years of age, and planned to deliver at the enrolling institution. Women who developed preeclampsia in the index pregnancy or a prior one (gestational hypertension defined as a blood pressure elevation of >140/90 on two occasions with concomitant proteinuria defined as positive urine protein test result >300 mg/24 hours or protein/creatinine >0.20) were excluded from the present analysis. A total of 2,230 singleton and 93 twin gestations were enrolled, and 2,193 and 91 singletons and twins, respectively, met the inclusion criteria for analysis.
Geisel School of Medicine Twin Study
Eligible were pregnant women ≥18 years of age who intended to deliver at the facility. Women carrying twin gestations and presenting for prenatal care or hospitalized for antenatal surveillance from 2003 to 2007 were approached in the third trimester of pregnancy and informed consent was obtained. The next singleton pregnancy that met the eligibility criteria and could be matched to the twin pregnancy on gestational age (within 1 week), parity (nulliparous/parous) and maternal age (+/− 5 years), in that order, was recruited for the study (prenatal controls; n = 40). Another group of women with singleton pregnancies were recruited at admission for labor and delivery (labor controls) and matched to twin mothers according to the criteria above. Five twin pregnancies and three singleton pregnancies were excluded because they developed preeclampsia after enrollment, leaving 41 twins and a total of 62 singleton controls (40 with blood samples in the third trimester and 52 with blood samples at labor and delivery). Placentas were routinely examined by the pathology department.
Biospecimen collection and processing
BIRTH cohort
Maternal blood samples were obtained at the following median (interquartile range) weeks of gestation: 9.7 (8.4–11.6), 17.8 (16.8–18.7), 25.9 (24.8–28.1), and 35.1 (34.6–35.9). Approximately 10 mL of blood was drawn in EDTA plasma tubes; the samples were kept at 4°C until processing for storage within four hours of venipuncture. The specimens were centrifuged for 20 minutes and stored at −80°C. Samples were shipped in batches on dry ice to Abbott Diagnostics (Abbott Park, IL) where they were stored at −80°C.
Geisel School of Medicine Twin Study
Blood samples were collected in the third trimester (31–39 weeks) and at the earliest possible time after admission for labor and before any administration of medication (33–41 weeks). A 10-mL red-top tube of whole blood was collected from the mother. After allowing samples to clot at room temperature, they were centrifuged and the sera were stored at −70°C. Samples were shipped on dry ice to a biorepository in Rockville, MD, where they were stored at −80°C.
Laboratory assays
BIRTH cohort
PlGF and sFlt-1 were measured with prototype ARCHITECT immunoassays (Abbott Laboratories, Abbott Park, IL). The PlGF immunoassay measures the free form of PlGF-1, with a lower limit of detection of 1 pg/mL, and a range up to 1500 pg/mL. The sFlt-1 immunoassay measures both free and bound sFlt-1, with a lower limit of detection of 0.10 ng/mL and a range up to 150 ng/mL. The combined intra- and inter-assay coefficients of variation reported by the laboratory were <7% for PlGF and sFlt-1.
Geisel School of Medicine Twin Study
Serum levels of sFlt-1 and PlGF were determined in a blinded fashion using commercially available ELISA kits (R & D systems, MN) as described elsewhere.5 Interassay CVs for the sFlt1 kit ranged from 7.0–8.1% and for PlGF ranged from 10.9–11.8%. S-endoglin was also measured using commercially available ELISA kits (R&D systems, MN) as) as described elsewhere.5 Interassay CVs for the s-endoglin kit ranged from 6.3–6.7%.
In a subset of BIRTH Cohort mothers with singleton pregnancies, PlGF and sFlt-1 concentrations were measured using both the ARCHITECT immunoassay (used for study samples in the BIRTH cohort) and the R&D systems assay (used for study samples in the Geisel School of Medicine Twin Study) at each time point. Pearson’s correlation was used to describe the concordance between logarithm-transformed values, as well as Cronbach’s alpha, a measure of inter-rater reliability. PlGF and sFlt-1 values measured by the different assays showed high concordance (Table 1). Because absolute values for the angiogenic factors differ between assays, levels between studies could not be directly compared.
Table 1.
Correlations and Cronbach’s Alpha Values between R&D and ARCHITECT Measures of sFlt-1 (soluble fms-like tyrosine kinase-1) and PlGF (placental growth factor)
| Assay | N | Pearson Correlation | Cronbach’s Alpha | |
|---|---|---|---|---|
| Time 1 | PlGF | 639 | 0.79 | 0.88 |
| sFlt-1 | 632 | 0.86 | 0.93 | |
| Time 2 | PlGF | 608 | 0.91 | 0.96 |
| sFlt-1 | 607 | 0.87 | 0.93 | |
| Time 3 | PlGF | 602 | 0.92 | 0.96 |
| sFlt-1 | 602 | 0.86 | 0.93 | |
| Time 4 | PlGF | 568 | 0.95 | 0.98 |
| sFlt-1 | 568 | 0.90 | 0.95 |
Clinical data
BIRTH Cohort
Maternal age, parity, and conception by assisted reproductive technologies (ART), and baby’s birth anthropometrics were abstracted from medical records. The participants completed a brief questionnaire that ascertained information on race/ethnicity, medical history, and history of preeclampsia in a previous pregnancy. Gestational age was confirmed by ultrasound scanning at <15 weeks’ gestation.
Geisel School of Medicine Twin Study
Data on mother’s age, race/ethnicity, parity, and ART conception, and baby’s sex and birth anthropometrics were abstracted from medical records and a form completed at delivery. The pathology report provided data on chorionicity of the twins and placental weight. Gestational age was confirmed by ultrasound.
Statistical methods
Clinical characteristics were compared between twin and singleton pregnancies using Student’s t-test for continuous variables, and chi-square analysis for categorical variables. Z-scores were developed for birth weight values to account for fetal sex and gestational age using an external standard.22 Angiogenic protein values and their ratio were adjusted for gestational week in models with logarithm-transformed angiogenic factor as the dependent variable; geometric means and 95% confidence intervals (CI) were calculated by taking the exponent of the logarithm-transformed mean. Geometric mean values for the angiogenic factors and the ratio of sFlt1/PlGF and 95% confidence intervals (CI) from an analysis of variance model (PROC MIXED) that adjusted for weeks of gestation were plotted by gestational week at blood collection. Statistical analyses were performed using SAS version 9.2.
Results
Mothers of twins were slightly older on average than mothers of singletons in both studies (Table 2). The majority of women in the BIRTH cohort were white as were nearly all mothers in the Dartmouth Twin Study. In the BIRTH cohort, white mothers contributed a greater proportion of twin than singleton pregnancies (68.8% vs. 59%), and African-American mothers contributed a higher proportion of singleton than twin pregnancies (21.0% vs. 9.7%). As expected, gestational week at delivery was earlier among the twin pregnancies in both studies. Approximately a third of the women in both studies were nulliparous, and ART were more likely to have been used among the mothers of twins (BIRTH cohort 68.8%; Geisel School of Medicine study 39.0%) than among the mothers of singletons (6.2% and 8.1%, respectively). Birth weight was lower in the twin pregnancies, even when accounting for gestational age and fetal sex.
Table 2.
Clinical Characteristics1 of Twin and Singleton Pregnancies in the BIRTH Cohort and Geisel School of Medicine Twin Study
| Birth Cohort | Geisel School of Medicine Twin Study | |||||
|---|---|---|---|---|---|---|
| Singletons | Twins | p-value1 | Singletons | Twins | p-value1 | |
| n=2193 | n=91 | n=62 | n=41 | |||
| Maternal and Gestational | ||||||
| Maternal age in years (SD) | 31.4 (5.7) | 35.1 (5.8) | < 0.0001 | 31.0 (5.7) | 33.4 (5.9) | 0.05 |
| Gestational weeks (SD) at delivery | 39.0 (1.8) | 36.3 (2.3) | < 0.0001 | 38.5 (1.8) | 37.1 (2.1) | 0.002 |
| Nulliparous (%) | 648 (29.1) | 27 (29.0) | 0.9957 | 21 (33.9) | 12 (29.3) | 0.62 |
| Artificial reproductive technologies used (%) | 138 (6.2) | 64 (68.8) | < 0.0001 | 5 (8.1) | 16 (39.0) | < 0.0001 |
| Race (%) | ||||||
| White | 1318 (59.1) | 64 (68.8) | 58 (95.1) | 40 (97.6) | ||
| Black | 469 (21.0) | 9 (9.7) | ||||
| Asian | 145 (6.5) | 9 (9.7) | ||||
| Hispanic | 213 (9.6) | 9 (9.7) | ||||
| Other/mixed/unknown | 85 (3.8) | 2 (2.2) | 0.06 | 3 (4.9) | 1 (2.4) | 0.651 |
| Infant2 | ||||||
| Birth weight (g) | 3312 (538) | 2418 (214) | < 0.0001 | 3257 (597) | 2635 (412) | < 0.0001 |
| Z-score for birth weight | 0.09 (0.97) | −0.84 (0.70) | < 0.0001 | 0.20 (1.1) | −0.72 (0.57) | < 0.0001 |
Means are presented for continuous variables, percentages for categorical variables. P-values are from t-tests and chi-square tests, respectively, except for race among the Geisel School of Medicine Study women, which is based on Fisher exact test.
Values are based on mean of twins’ birth weights.
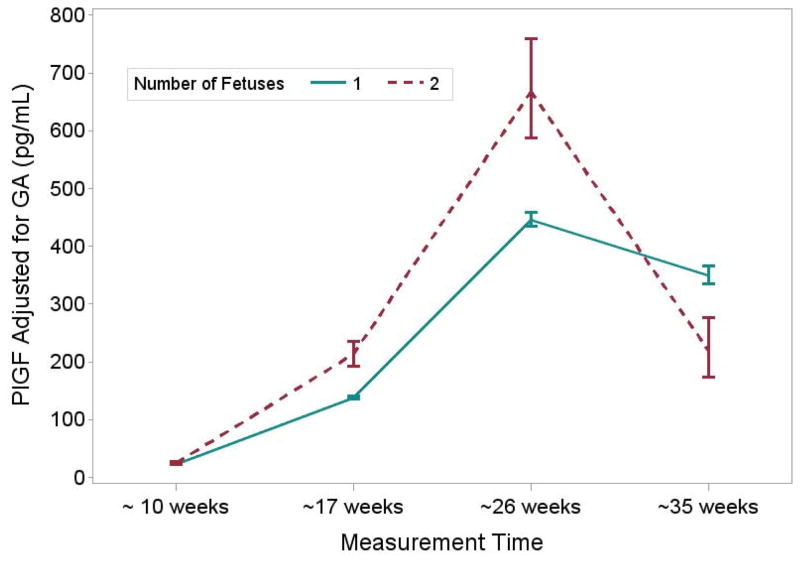
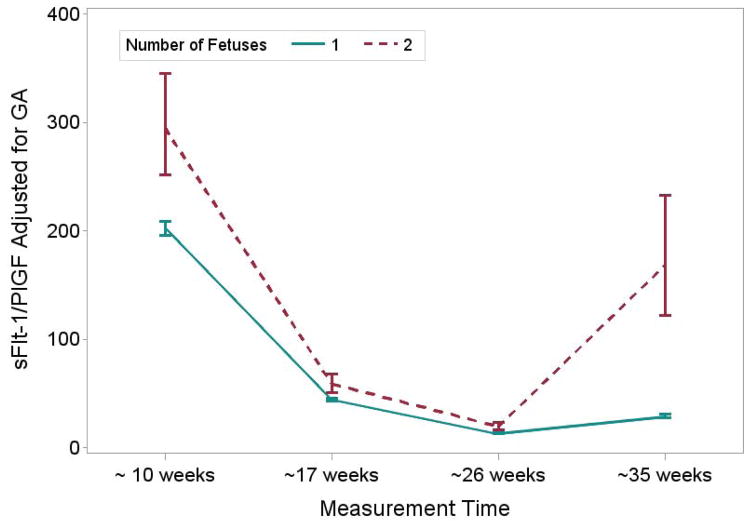
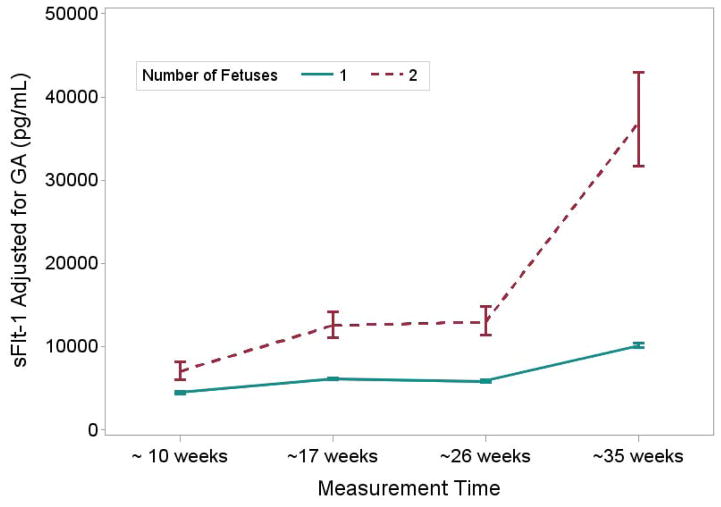
Table 3 shows the values for angiogenic factors at the four time points in pregnancy among women in the BIRTH Cohort. In singleton and twin mothers, PlGF concentrations increased from early to mid-pregnancy and then declined by the third trimester (as noted in the fourth time point), while s-Flt1 concentrations increased throughout the pregnancy. Accordingly, the s-Flt1/PlGF ratio decreased through mid-pregnancy but increased in the third trimester. In twins, maternal PlGF concentrations were higher than in singleton pregnancies until the third trimester, when values became lower than those in singleton pregnancies (Table 3). Maternal sFlt-1 concentrations and the sFlt-1/PlGF ratio were higher in twins compared with singletons throughout the pregnancy, with the greatest differences demonstrated in the third trimester (Table 3). Results for mean concentrations from repeated measures models which accounted for the correlations among angiogenic factors over the pregnancy were similar but the statistical significance of the differences, particularly for PlGF, were attenuated (Figures 1–3). Results were similar with additional adjustment for maternal age, race/ethnicity and parity (data not shown).
Table 3.
Maternal serum angiogenic protein concentrations* at four time points during gestation; BIRTH Cohort
| Gestational weeks mean (range) | 9.7 (8.4–11.6) | 17.8 (16.8–18.7) | ||||
|---|---|---|---|---|---|---|
| Singleton | Twin | p-value | Singleton | Twin | p-value | |
| n=2193 | n=91 | n=2058 | n=89 | |||
| PlGF (pg/ml)* | 22.8 (22.4 – 23.2) | 24.9 (23.0 – 26.9) | 0.0323 | 138.4 (135.6 – 141.3) | 213.5 (193.3 – 235.8) | < 0.0001 |
| sFlt-1 (pg/ml)* | 4485 (4347 – 4628) | 7037 (6031 – 8212) | < 0.0001 | 6131 (5974 – 6291) | 12543 (11074 – 14207) | < 0.0001 |
| sFlt-1:PlGF ratio | 202.4 (196.0 – 209.0) | 294.6 (251.5 – 335.5) | < 0.0001 | 44.3 (42.9 – 45.7) | 58.7 (50.6 – 68.2) | 0.0003 |
| Gestational weeks mean (range) | 25.9 (24.8–28.1) | 35.1 (34.6–35.9) | ||||
|---|---|---|---|---|---|---|
| Singleton | Twin | p-value | Singleton | Twin | p-value | |
| n=2089 | n=91 | n=2064 | n=68 | |||
| PlGF (pg/ml)* | 445.9 (434.1 – 458.1) | 668.0 (587.2 – 759.9) | < 0.0001 | 350.2 (335.6 – 365.5) | 219.2 (173.2 – 277.3) | < 0.0001 |
| sFlt-1 (pg/ml)* | 5898 (5736 – 6065) | 12968 (11347 – 14821) | < 0.0001 | 10151 (9875 – 10435) | 36916 (31714 – 42971) | < 0.0001 |
| sFlt-1:PlGF ratio | 13.2 (12.7 – 13.7) | 19.4 (16.2 – 23.2) | < 0.0001 | 29.0 (27.3 – 30.7) | 168.4 (121.9 – 232.8) | < 0.0001 |
means (95% confidence intervals). Means are adjusted for gestational age from models with logarithm-transformed angiogenic factor as the outcome; presented are geometric means derived from taking the exponent of the adjusted mean. sFlt-1 (soluble fms-like tyrosine kinase-1; PlGF (placental growth factor)
Figure 1.
graph of mean (95% CI) PlGF in twins vs. singletons in BIRTH cohort adjusted for gestational weeks at blood collection and by week of gestation
Figure 3.
graph of mean (95% CI) sFlt-1/PlGF ratio in twins vs. singletons in BIRTH cohort adjusted for gestational weeks at blood collection and by week of gestation
In the Geisel School of Medicine Twin Study, maternal concentrations of sFlt-1, s-endoglin and the sFlt-1/PlGF ratio were higher in twins than singletons in the third trimester and at delivery, while PlGF concentrations did not differ (Table 4). Results were similar when adjusted for the matching factors (gestational age, as well as maternal age and parity; data not shown).
Table 4.
Maternal serum angiogenic protein concentrations* in the third trimester and at delivery; Geisel School of Medicine Twin Study
| Prenatal | Delivery | |||||
|---|---|---|---|---|---|---|
| Singleton | Twin | p-value | Singleton | Twin | p-value | |
| Gestational weeks mean (range) | 32.3 (31.3–38.7) | 32.3 (31.0–33.1) | 38.2 (33.4–41.0) | 37.1 (32.6–40.4) | ||
| N | 40 | 41 | 52 | 41 | ||
| PlGF (pg/ml)* | 467.3 (375.7–581.3) | 386.2 (312.4–477.6) | 0.24 | 152.4 (121.7–190.7) | 147.5 (111.9–194.4) | 0.86 |
| sFlt-1 (pg/ml)* | 2108 (1821–2440) | 6129 (5317–7065) | <0.0001 | 7278 (5860–9039) | 15899 (12179–20756) | <0.0001 |
| s-endoglin (ng/ml) | 4.61 (3.81–5.57) | 13.0 (10.8–15.7) | <0.0001 | 14.5 (11.2–18.6) | 24.8 (18.2–33.8) | 0.01 |
| sFlt-1/PlGF ratio | 4.51 (3.37–6.04) | 15.9 (12.0–21.1) | <0.0001 | 47.8 (34.7–65.7) | 107.8 (72.9–159.5) | 0.003 |
Means (95% confidence intervals); means are adjusted for gestational age from models with logarithm-transformed angiogenic factor as the outcome; presented are geometric means derived from taking the exponent of the adjusted mean. sFlt-1 (soluble fms-like tyrosine kinase-1; PlGF (placental growth factor)
Information on chorionicity of the twin pregnancy as well as twins’ sex was available in the Geisel School of Medicine Twin Study. Thirty-six of the twin pregnancies were dichorionic and 5 were monochorionic. Opposite sex twins accounted for 17 of the twin pregnancies, whereas 8 were female/female and 16 were male/male. Placental weight was higher in dichorionic pregnancies than in monochorionic pregnancies (820 g (SD 158) vs. 743 g (SD 161)). Maternal prenatal concentrations of sFlt-1 (10,721 vs. 6,169 pg/ml, respectively; p=0.03), s-endoglin (26.6 vs.12.3 ng/ml; p=0.001) and the sFLT-1:PlGF ratio (47.0 vs. 15.7 pg/ml; p=0.03)) were higher in monochorionic than dichorionic twins after adjustment for gestational age. At labor, only maternal s-endoglin concentrations (45.8 vs. 23.0 ng/ml, respectively; p=0.005) remained higher in monochorionic than dichorionic twins. There were generally no associations between angiogenic factors and sex of the twins, except for higher prenatal s-endoglin concentrations in same-sex than opposite sex twins (data not shown). Placental weight was positively correlated with maternal PlGF in the third trimester and at delivery in singleton pregnancies (r=0.17 and 0.28, respectively) and in twin pregnancies (r=0.20 and 0.12, respectively), but not with sFlt-1 (r=−0.01 at both time points in singleton pregnancies, and r=0.001 at both time points in twin pregnancies). There was an inverse correlation between placental weight and maternal sFlt-1/PlGF ratio in the third trimester and at delivery in singletons (r=−0.11 and r=−0.22, respectively), and in twins (r=−0.14 and −0.12).
Comment
Analysis of angiogenic factors throughout the pregnancy showed elevated maternal concentrations of sFlt-1, as well as the ratio of sFlt-1 to PlGF among twin compared with singleton pregnancies in the BIRTH Cohort. Analysis of the Geisel School of Medicine data confirmed these findings and showed higher s-endoglin concentrations, as well as elevated sFlt-1 and the sFlt-1 to PlGF ratio in twins compared to singletons at the end of pregnancy and at admission for labor and delivery. PlGF concentrations demonstrated a less consistent pattern in both cohorts with elevated concentrations among twins until mid-pregnancy, followed by higher values in singleton pregnancies in the third trimester, but not at delivery. In addition, angiogenic factor balance was associated with chorionicity, although these results were based on small numbers.
The elevated sFlt-1 concentrations and anti-angiogenic ratio in women carrying twins compared with singletons that we observed are consistent with previous smaller studies with measurements in the first,19 second23 and third trimesters.19,23 While we showed findings for maternal s-endoglin similar to those for sFlt-1 at the end of pregnancy, another study found no differences in the first trimester.20 The etiology of the increases in sFlt1 has been hypothesized to be related to increased placental mass in twins as the placenta is thought to be the major source of sFlt.24 Although the placenta is also the major source of PlGF, dramatic changes in PlGF are not seen with increased placental mass as the commercial assays to measure PlGF only measure the unbound form or free PlGF.14 Our data on placental weight showed no correlation with maternal sFlt-1, a weak positive correlation with maternal PlGF, and a weak inverse correlation with the sFlt-1/PlGF ratio suggesting that greater placental mass does not explain the more antiangiogenic profile in twin compared with singleton pregnancies. The lack of correlation between placental weight and sFlt-1 should be evaluated in other studies. Hypoxia from reduced placental perfusion has been shown to stimulate sFlt-1 production in an animal model.24 The possibility of chronic hypo-perfusion in the placentas of twin pregnancies could also be hypothesized to explain the increased sFlt-1 observed in twin compared with singleton pregnancies.
Our data demonstrating a more anti-angiogenic profile in mothers of twins than singletons are consistent with a few possible explanations. Twinning may share pathological mechanisms hypothesized to occur in preeclampsia: shallow trophoblast invasion of the maternal endometrium and unconverted spiral arteries, resulting in endothelial damage and changes in anti-angiogenic factor concentrations. Preeclampsia cases were excluded from our analysis to determine whether angiogenic factors were altered with twinning itself and not its sequelae. Alternatively, differences in angiogenic balance in mothers of twins and singletons may be due to maternal host factors that result in spontaneous twin pregnancies. Host factors such as age and race/ethnicity did not influence our results, though we cannot exclude the possibility of other, unmeasured or unknown risk factors.
An anti-angiogenic protein balance, i.e. greater concentrations of anti-angiogenic proteins such as sFlt-1 and s-endoglin, is established in preeclampsia. Previous studies comparing women who subsequently develop preeclampsia to those who do not have shown similar or lower first trimester sFlt-1 levels, and increased levels at term. 21, 25 In twin pregnancies, however, patterns of higher sFlt-1 concentrations emerged early in the pregnancy and persisted for the remainder of the gestation. Maternal PlGF in preeclamptic pregnancies is similar or lower in the first trimester and ends the pregnancy lower than levels in uncomplicated pregnancies.21 Low levels of maternal PlGF appeared later in the twin pregnancies in our data, specifically between weeks 26–34. Therefore, greater values for the sFlt-1:PlGF ratio seem to appear earlier in twin compared with preeclamptic pregnancies. A third-trimester increase in sFlt-1 alters angiogenic balance in preeclamptic pregnancies, whereas a decrease in PlGF during the third trimester contributes to this phenomenon in twin pregnancies. These findings support the hypothesis that anti-angiogenic balance plays a causative role in endothelial cell injury and that in most pregnancies, this imbalance is most apparent during the third trimester. Although the placenta is central to the process, it remains unclear; however, what causes sFlt-1 to increase and PlGF to decrease in women with preeclampsia or multiple gestation.26 This study and others27,28 demonstrate that women with twins can have much higher sFlt-1/PlGF levels (compared to singletons) and not develop preeclampsia. It is unclear why but it probably points to the multifactorial aspect of this disease. There are other pro-angiogenic molecules produced by the placenta such as VEGF, and it is possible that in twins, the production of these molecules is also increased. This would counter the effect of sFlt-1 but would not be reflected in the sFlt-1/PlGF ratio.
Pregnancy provides a ‘stress test’ to identify risk for health conditions that occur later in a woman’s life, with investigation of pregnancy characteristics allowing refinement of biological hypotheses. For example, comparing angiogenic factor profiles between singleton, preeclamptic pregnancies and twin pregnancies, with further comparison of pregnancy conditions with risk of diseases that occur later in the mother may help determine a common etiology, or elucidate how pregnancy mediates preexisting host factors. Preeclamptic pregnancies are associated with an increased cardiovascular disease risk29 and continued endothelial dysfunction. The subtle anti-angiogenic balance that persists in women with a history of preeclampsia could shed light on this association;26 it would be interesting to determine if greater sFlt-1, endoglin and sFlt-1/PlGF concentrations persist in women with a twin history as well. Similar anti-angiogenic profiles in preeclamptic and twin pregnancies argue for determining whether women with a history of twin gestations have a similarly elevated cardiovascular disease risk.
Women with a history of preeclampsia may have an altered breast cancer risk as well, as studies have shown some protection,30,31,9 and there is some suggestion of a reduced maternal breast cancer risk after twin pregnancies.32 The biological mechanism to explain protection afforded by a history of preeclampsia is unknown but there has been recent speculation regarding the involvement of angiogenic response.33 Namely, women who mount an anti-angiogenic response when pregnant (as in preeclampsia and multiple gestation) may be more likely to respond similarly in response to tumorigenesis.
Most monozygotic twin gestations result in a monochorionic, diamniotic pregnancy with a single placental mass, while about 20–25% of the time, early post-conception cleavage of the morula will result in a dichorionic, diamniotic pregnancy, with separate placental masses. Maternal anti-angiogenic protein concentrations were greater, as was sFlt-1:PlGF ratio, in monochorionic compared with dichorionic pregnancies in our study. These results are consistent with another small study (n=19) which found some evidence that monochorionic twins had a higher sFlt-1:PlGF ratio. The observation that monochorionic twins have higher anti-angiogenic balance, seems contrary to the hypothesis that sFlt-1 increases with greater placental mass,19 because there are fewer placentas in the former, although one large placenta could have greater mass than two small ones. However, this is consistent with the lack of correlation between placental weight and sFlt-1 demonstrated in our data.
The strengths of our study include its relatively large sample size compared with previous investigations and the inclusion of two independent data sources - one which included longitudinal measurements throughout the pregnancy and women of various ethnic/racial backgrounds, and the other which included an additional time point (labor and delivery), an additional anti-angiogenic protein, s-endoglin which is implicated in preeclampsia, and information on chorionicity and sex of the twins.
In summary, maternal anti-angiogenic factors, as in preeclampsia, were elevated in twin pregnancies compared with singleton pregnancies. These data could indicate a shared physiology, but other explanations cannot be excluded. Determining whether women with a history of twin gestations are at an elevated cardiovascular disease risk, as are women with a history of preeclampsia is warranted.
Figure 2.
graph of mean (95% CI) sFlt-1 in twins vs. singletons in BIRTH cohort adjusted for gestational weeks at blood collection and by week of gestation
Acknowledgments
Grant Support:
This research was supported by federal funds from the Division of Cancer Epidemiology and Genetics and Center for Cancer Training, Cancer Prevention Fellowship Program, National Cancer Institute. The data from the BIRTH cohort was funded by a research grant (9MZ-04-06N03) from Abbott Laboratories.
Footnotes
Condensation: This longitudinal study found higher maternal anti-angiogenic proteins in twin than singleton pregnancies with implications for diseases associated with multiple gestations.
Disclosure Statement: AK is a co-inventor on multiple patents for preeclampsia markers, reports service as a consultant to Siemens Diagnostics, has grant funding from Thermofisher and has financial interest in Aggamin LLC. TM was awarded a research grant by Abbot Laboratories to measure the angiogenic factors in the BIRTH cohort. The remaining authors report no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Walker JJ. Pre-eclampsia. Lancet. 2000:1260–5. doi: 10.1016/S0140-6736(00)02800-2. [DOI] [PubMed] [Google Scholar]
- 2.Sibai BM, Hauth J, Caritis S, et al. Hypertensive disorders in twin versus singleton gestations. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Am J Obstet Gynecol. 2000;182:938–42. doi: 10.1016/s0002-9378(00)70350-4. [DOI] [PubMed] [Google Scholar]
- 3.Krotz S, Fajardo J, Ghandi S, Patel A, Keith LG. Hypertensive disease in twin pregnancies: a review. Twin Res. 2002;5:8–14. [PubMed] [Google Scholar]
- 4.Wang A, Holston AM, Yu KF, et al. Circulating anti-angiogenic factors during hypertensive pregnancy and increased risk of respiratory distress syndrome in preterm neonates. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 25:1447–52. doi: 10.3109/14767058.2011.640368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levine RJ, Lam C, Qian C, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355:992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 6.Levine RJ, Qian C, Maynard SE, Yu KF, Epstein FH, Karumanchi SA. Serum sFlt1 concentration during preeclampsia and mid trimester blood pressure in healthy nulliparous women. Am J Obstet Gynecol. 2006;194:1034–41. doi: 10.1016/j.ajog.2005.10.192. [DOI] [PubMed] [Google Scholar]
- 7.Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–83. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 8.Faupel-Badger JM, Fichorova RN, Allred EN, et al. Cluster analysis of placental inflammatory proteins can distinguish preeclampsia from preterm labor and premature membrane rupture in singleton deliveries less than 28 weeks of gestation. Am J Reprod Immunol. 2011;66:488–94. doi: 10.1111/j.1600-0897.2011.01023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maynard SE, Karumanchi SA. Angiogenic factors and preeclampsia. Semin Nephrol. 31:33–46. doi: 10.1016/j.semnephrol.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rana S, Karumanchi SA, Levine RJ, et al. Sequential changes in antiangiogenic factors in early pregnancy and risk of developing preeclampsia. Hypertension. 2007;50:137–42. doi: 10.1161/HYPERTENSIONAHA.107.087700. [DOI] [PubMed] [Google Scholar]
- 11.Romero R, Nien JK, Espinoza J, et al. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2008;21:9–23. doi: 10.1080/14767050701830480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Venkatesha S, Toporsian M, Lam C, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–9. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 13.Maynard S, Epstein FH, Karumanchi SA. Preeclampsia and angiogenic imbalance. Annu Rev Med. 2008;59:61–78. doi: 10.1146/annurev.med.59.110106.214058. [DOI] [PubMed] [Google Scholar]
- 14.Staff AC, Braekke K, Harsem NK, Lyberg T, Holthe MR. Circulating concentrations of sFlt1 (soluble fms-like tyrosine kinase 1) in fetal and maternal serum during pre-eclampsia. Eur J Obstet Gynecol Reprod Biol. 2005;122:33–9. doi: 10.1016/j.ejogrb.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 15.Thadhani R, Mutter WP, Wolf M, et al. First trimester placental growth factor and soluble fms-like tyrosine kinase 1 and risk for preeclampsia. J Clin Endocrinol Metab. 2004;89:770–5. doi: 10.1210/jc.2003-031244. [DOI] [PubMed] [Google Scholar]
- 16.Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–58. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maynard SE, Venkatesha S, Thadhani R, Karumanchi SA. Soluble Fms-like tyrosine kinase 1 and endothelial dysfunction in the pathogenesis of preeclampsia. Pediatr Res. 2005;57:1R–7R. doi: 10.1203/01.PDR.0000159567.85157.B7. [DOI] [PubMed] [Google Scholar]
- 18.Ruiz-Sacedon N, Perales-Puchalt A, Borras D, Gomez R, Perales A. Angiogenic growth factors in maternal and fetal serum in concordant and discordant twin pregnancies. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2013 doi: 10.3109/14767058.2013.845156. [DOI] [PubMed] [Google Scholar]
- 19.Bdolah Y, Lam C, Rajakumar A, et al. Twin pregnancy and the risk of preeclampsia: bigger placenta or relative ischemia? Am J Obstet Gynecol. 2008;198:428, e1–6. doi: 10.1016/j.ajog.2007.10.783. [DOI] [PubMed] [Google Scholar]
- 20.Sanchez O, Llurba E, Marsal G, et al. First trimester serum angiogenic/anti-angiogenic status in twin pregnancies: relationship with assisted reproduction technology. Hum Reprod. 2012;27:358–65. doi: 10.1093/humrep/der394. [DOI] [PubMed] [Google Scholar]
- 21.McElrath TF, Lim KH, Pare E, et al. Longitudinal evaluation of predictive value for preeclampsia of circulating angiogenic factors through pregnancy. Am J Obstet Gynecol. 2012;207:407, e1–7. doi: 10.1016/j.ajog.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 22.Yudkin PL, Aboualfa M, Eyre JA, Redman CW, Wilkinson AR. New birthweight and head circumference centiles for gestational ages 24 to 42 weeks. Early Hum Dev. 1987;15:45–52. doi: 10.1016/0378-3782(87)90099-5. [DOI] [PubMed] [Google Scholar]
- 23.Maynard SE, Moore Simas TA, Solitro MJ, et al. Circulating angiogenic factors in singleton vs multiple-gestation pregnancies. Am J Obstet Gynecol. 2008;198:200, e1–7. doi: 10.1016/j.ajog.2007.08.042. [DOI] [PubMed] [Google Scholar]
- 24.Bujold E, Romero R, Chaiworapongsa T, et al. Evidence supporting that the excess of the sVEGFR-1 concentration in maternal plasma in preeclampsia has a uterine origin. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2005;18:9–16. doi: 10.1080/14767050500202493. [DOI] [PubMed] [Google Scholar]
- 25.Murphy SR, LaMarca BB, Parrish M, Cockrell K, Granger JP. Control of soluble fms-like tyrosine-1 (sFlt-1) production response to placental ischemia/hypoxia: role of tumor necrosis factor-alpha. American journal of physiology Regulatory, integrative and comparative physiology. 2013;304:R130–5. doi: 10.1152/ajpregu.00069.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Myatt L, Clifton RG, Roberts JM, et al. Can changes in angiogenic biomarkers between the first and second trimesters of pregnancy predict development of pre-eclampsia in a low-risk nulliparous patient population? BJOG: An International Journal of Obstetrics & Gynaecology. 2013;120:1183–91. doi: 10.1111/1471-0528.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sánchez O, Llurba E, Marsal G, Domínguez C, Aulesa C, Sánchez-Durán MA, Goya MM, Alijotas-Reig J, Carreras E, Cabero L. First trimester serum angiogenic/anti-angiogenic status in twin pregnancies: relationship with assisted reproduction technology. Hum Reprod. 2012;27:358–65. doi: 10.1093/humrep/der394. [DOI] [PubMed] [Google Scholar]
- 28.Nevo O, Many A, Xu J, Kingdom J, Piccoli E, Zamudio S, Post M, Bocking A, Todros T, Caniggia I. Placental expression of soluble fms-like tyrosine kinase 1 is increased in singletons and twin pregnancies with intrauterine growth restriction. J Clin Endocrinol Metab. 2008;93:285–92. doi: 10.1210/jc.2007-1042. [DOI] [PubMed] [Google Scholar]
- 29.Powe CE, Levine RJ, Karumanchi SA. Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation. 2011;123:2856–69. doi: 10.1161/CIRCULATIONAHA.109.853127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335:974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aagaard-Tillery KM, Stoddard GJ, Holmgren C, et al. Preeclampsia and subsequent risk of cancer in Utah. Am J Obstet Gynecol. 2006;195:691–9. doi: 10.1016/j.ajog.2006.06.089. [DOI] [PubMed] [Google Scholar]
- 31.Kim JS, Kang EJ, Woo OH, et al. The relationship between preeclampsia, pregnancy-induced hypertension and maternal risk of breast cancer: a meta-analysis. Acta Oncol. 2013;52:1643–8. doi: 10.3109/0284186X.2012.750033. [DOI] [PubMed] [Google Scholar]
- 32.Kim HS, Woo OH, Park KH, et al. The relationship between twin births and maternal risk of breast cancer: a meta-analysis. Breast Cancer Res Treat. 2012;131:671–7. doi: 10.1007/s10549-011-1779-5. [DOI] [PubMed] [Google Scholar]
- 33.Troisi R, Braekke K, Harsem NK, Hyer M, Hoover RN, Staff AC. Blood pressure augmentation and maternal circulating concentrations of angiogenic factors at delivery in preeclamptic and uncomplicated pregnancies. Am J Obstet Gynecol. 2008;199:653, e1–10. doi: 10.1016/j.ajog.2008.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]